Highly enantioselective Pd-catalyzed indole allylic alkylation using binaphthyl-based phosphoramidite-thioether ligands†
Bin
Feng
a,
Xiao-Yu
Pu
a,
Zhi-Cheng
Liu
a,
Wen-Jing
Xiao
ab and
Jia-Rong
Chen
*a
aKey Laboratory of Pesticide & Chemical Biology, Ministry of Education; College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei 430079, China. E-mail: chenjiarong@mail.ccnu.edu.cn
bState Key Laboratory of Applied Organic Chemistry Lanzhou University, Lanzhou 730000, China
First published on 28th June 2016
Abstract
A novel type of binaphthyl-based phosphoramidite-thioether ligand has been developed and successfully applied to Pd-catalysed asymmetric C-3 allylic alkylation of indoles, giving the desired products with up to 99% yield and 98% ee.
The privileged chiral indole scaffolds occur frequently in a wide variety of biologically active natural products and medicinally significant compounds.1 As a result, enormous efforts have been directed toward the development of more practical and efficient protocols for their preparation and direct functionalization.2 In this context, the asymmetric Friedel–Crafts alkylation reactions3 and allylic alkylation4 of indoles by using metal or organic catalysts have been established as the predominant approaches for regio- and enantioselective alkylation of indoles at the C-3 position.
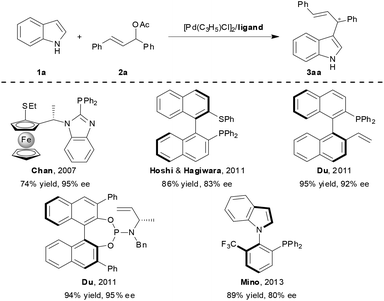
In 2006, the group of Bandini and Umani-Ronchi reported an elegant Pd-catalysed intramolecular enantioselective indole alkylation by allylic carbonates using the Trost ligand.5 Since then, the design of chiral ligands for Pd-catalyzed intermolecular asymmetric allylic alkylation of indoles has attracted considerable attention from the synthetic community (Scheme 1). For example, the Chan research group in 2007 developed a novel class of chiral ferrocenyl P,S-ligands and disclosed the first example of highly enantioselective Pd-catalysed allylic alkylation of indoles with 1,3-diphenyl-2-propenyl acetate.6 Stimulated by this pioneering work, the research groups of Hoshi and Hagiwara,7 Du,8 and Mino9 respectively reported a series of structurally diverse phosphine- or phosphoramidite-based hybrid ligands for this benchmark reaction. Most of these ligands demonstrated generally high reaction efficiency and enantioselectivity. In particular, thioether-containing ligands are of great potential because an additional chirality on the sulfur atom would be generated upon its coordination to the metal, and proved to be beneficial for stereocontrol.10 Therefore, the development of other new, readily tunable and air-stable P,S-type ligands with high stereoselectivity and a broad substrate scope for the indole allylic alkylation is still of great interest.
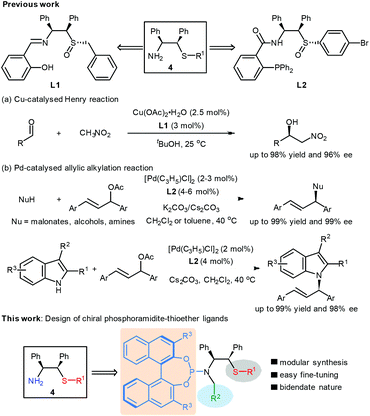
We have recently launched a research program aimed at exploring sulfur-containing chiral ligands based on the strategy of rational incorporation of two privileged backbones into one chiral scaffold.11 Employing the chiral amino alcohol-derived thioether-amines of type 4 as the key intermediates, we have successfully developed a range of chiral ligands, sulfoxide–Schiff bases L1 and sulfoxide–phosphines L2 (Scheme 2). These ligands were found to be highly efficient and enantioselective in the Cu-catalysed Henry reaction12 and Pd-catalysed allylic alkylation.13 In particular, the ligands L2 enabled an efficient Pd-catalyzed direct asymmetric N-allylic alkylation of indoles, giving the corresponding products with generally excellent regio- and enantioselectivities.13c Encouraged by these results, we further explored the expansion of these catalytic systems to the C-3 allylic alkylation of indoles. However, only moderate yields and poor regioselectivity were observed when using ligands L2 (not shown). Inspired by the versatile applications of binaphthyl-based phosphoramidite ligands in asymmetric catalysis,14 we envisioned the development of a new class of phosphoramidite-thioether ligands using thioether-amines 4 and BINOL as the starting materials (Scheme 2). Compared to our previously reported ligands, their modular structure would enable facile construction of a ligand library and easy fine-tuning for improving the regio- and stereoselectivity of indole C3-allylic alkylation reaction. Herein, we wish to report our preliminary results on this subject.
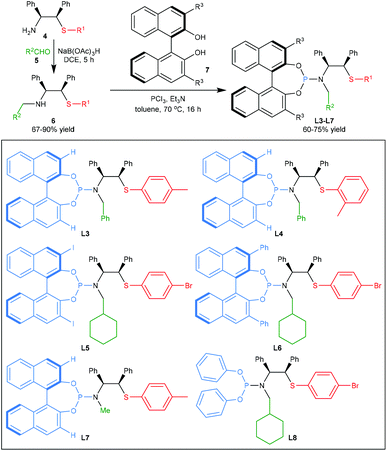
As shown in Scheme 3, the target ligands L3–L7 can be synthesized through two simple steps with good overall yield starting from thioether-amines 4, which are readily prepared on a gram-scale from commercially available (1R,2S)-2-amino-1,2-diphenylethan-1-ol according to our previous procedure.12 Reductive amination of the aldehydes 5 with thioether-amines 4 gave rise to the corresponding secondary amines 6. Then, the reaction of (R)-BINOLs 7 with dichloroaminophosphine, which were in situ-formed by treatment of the amines 6 with phosphorus trichloride in the presence of a base, provided the final binaphthyl-based ligands L3–L7.15 Moreover, we also prepared the ligand L8 without the binaphthyl scaffold.16 Notably, all of these ligands are air- and moisture-stable, and could be manipulated in air. With these ligands in hand, we then attempted to apply them to the Pd-catalysed asymmetric allylic alkylation reaction of N-free indoles with allylic acetates.
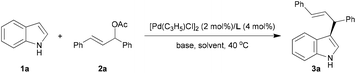
Based on our previous studies,13 we initially screened a series of commonly used bases with ligand L3 in the model reaction of indole 1a and rac-(E)-1,3-diphenylallyl acetate 2a in CH2Cl2 at 40 °C (Table 1).15 To our delight, the reaction with all of the bases gave rise to the corresponding product 3a with the same enantioselectivities and comparable yields; and Cs2CO3 demonstrated somewhat superior reaction efficiency (entries 1–4). With Cs2CO3 as the base, we subsequently examined ligands L4–L8 to investigate the effect of substitution patterns of the ligands on the activity and enantioselectivity. The results of entries 5–9 exhibited that the binaphthyl scaffold, N-substituent, and thioether moiety are all essential for the catalytic performance, with product 3aa being obtained with 60–97% yield and 30–94% ee. Among them, L6 was identified as the optimal ligand in terms of both yield and enantioselectivity (entry 7). With ligand L6, we briefly screened an array of typical solvents to further improve the enantioselectivity. It was found that the reaction proceeded more efficiently in aromatic solvents than that in halogen solvent, with 90% yield and 97% ee being obtained in mesitylene after 5 h (entry 12). By changing the molar ratio of 1a to 2a from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, a slight increase of yield and the same enantioselectivity were observed (entry 13). Accordingly, the optimal reaction conditions were established as follows: 2 mol% of [Pd(C3H5Cl)]2, 4 mol% of ligand L6, and 2.0 equivalents of Cs2CO3 in mesitylene at 40 °C.
1.5, a slight increase of yield and the same enantioselectivity were observed (entry 13). Accordingly, the optimal reaction conditions were established as follows: 2 mol% of [Pd(C3H5Cl)]2, 4 mol% of ligand L6, and 2.0 equivalents of Cs2CO3 in mesitylene at 40 °C.
| Entry | Ligand | Solvent | Base | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, reactions were carried out with 1a (0.9 mmol), 2a (0.3 mmol), [Pd(C3H5)Cl]2 (2.0 mol%), ligand (4.0 mol%), base (0.6 mmol) in solvent (2.0 mL) at 40 °C. b Isolated yield. c The ee values were determined by chiral HPLC analysis, the absolute configuration was established to be S according to ref. 6. d A mixture of BSA (2.0 equiv.) and LiOAc (0.1 equiv.) was added as the base. e A mixture of BSA (2.0 equiv.) and KOAc (0.1 equiv.) was added as the base. f Performed with 1a (0.3 mmol) and 2a (0.45 mmol). | ||||||
| 1 | L3 | CH2Cl2 | Cs2CO3 | 10 | 87 | 71 |
| 2 | L3 | CH2Cl2 | K2CO3 | 18 | 82 | 71 |
| 3d | L3 | CH2Cl2 | BSA + LiOAc | 24 | 85 | 71 |
| 4e | L3 | CH2Cl2 | BSA + KOAc | 24 | 88 | 71 |
| 5 | L4 | CH2Cl2 | Cs2CO3 | 24 | 97 | 89 |
| 6 | L5 | CH2Cl2 | Cs2CO3 | 24 | 84 | 83 |
| 7 | L6 | CH2Cl2 | Cs2CO3 | 24 | 90 | 94 |
| 8 | L7 | CH2Cl2 | Cs2CO3 | 24 | 60 | 30 |
| 9 | L8 | CH2Cl2 | Cs2CO3 | 10 | 85 | 35 |
| 10 | L6 | Toluene | Cs2CO3 | 5 | 88 | 96 |
| 11 | L6 | Xylenes | Cs2CO3 | 5 | 89 | 97 |
| 12 | L6 | Mesitylene | Cs2CO3 | 5 | 90 | 97 |
| 13 | L6 | Mesitylene | Cs 2 CO 3 | 5 | 92 | 97 |
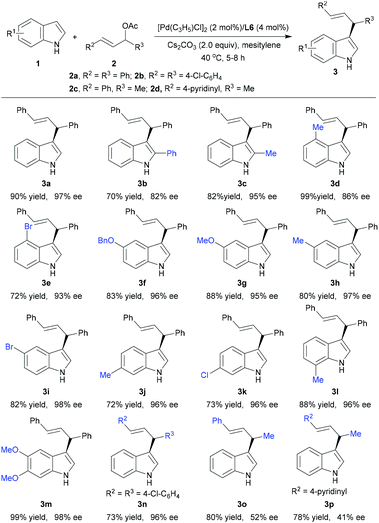
Next, we explored the substrate scope with a variety of indoles 1b–m and several allylic acetates 2 (Table 2). The reaction with 2-substituted indoles 1b–c proceeded smoothly to give the expected products 3b and 3c with 70–82% yield and 82–95% ee. In contrast to the Hoshi and Hagiwara catalytic system,7 it turned out that the electronic properties of the substituents at the phenyl ring of the indoles have no obvious effect on the results. For example, C4-, C5-, C6-, and C7-substituted indoles 1d–l bearing electron-donating (Me, BnO, MeO) or electron-withdrawing (Br, Cl) functional groups were all well tolerated, and the corresponding products 3d–l were obtained in the range of 72–99% yield and 86–98% ee. The multisubstituted indole 1m also underwent the allylic alkylation reaction efficiently to furnish product 3m in 99% yield with 98% ee. Moreover, rac-(E)-1,3-diaryl-2-propenyl acetate 2b could also react with 1a efficiently to give product 3n in 73% yield with 96% ee. In the case of rac-(E)-4-phenylbut-3-en-2-yl acetate 2c and rac-(E)-4-(pyridin-4-yl)but-3-en-2-yl acetate 2d, the reaction still proceeded smoothly to afford products 3o and 3p with good yield and regioselectivity, while with only moderate enantioselectivity (up to 52% ee). In the current catalytic system, however, N-Me- and N-Boc-substituted indoles proved to be not suitable for the reaction (not shown). Remarkably, in all of these reactions, no undesired N-allylic alkylation was observed.17
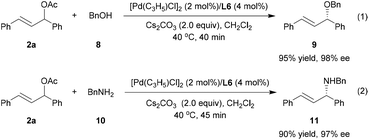
In addition to indole nucleophiles, the current catalytic system could also be successfully extended to enantioselective allylic etherification and amination (Scheme 4). For instance, in the case of benzyl alcohol and benzyl amine as nucleophiles, the reactions completed within 45 minutes to give the corresponding allylic ether 9 and allylic amine 11 with 98% and 97% ee, respectively. These results also demonstrated the importance of incorporation of the privileged BINOL scaffold as an additional stereocontrol element in our newly developed phosphoramidite-thioether ligands.13
In conclusion, we have developed a new class of readily tunable and air-stable binaphthyl-based phosphoramidite-thioether ligands. These ligands can be successfully applied to the Pd-catalysed asymmetric allylic alkylation reaction of indoles at the C3-position, giving the correspondingly products with generally good yields (up to 99%) and enantioselectivities (up to 98% ee). Further exploration of these ligands in other catalytic asymmetric reactions for the formation of carbon–carbon and carbon–heteroatom bonds is ongoing in our laboratory.
We are grateful to the National Natural Science Foundation of China (no. 21272087, 21202053, 21472058, 21472057, and 21232003) and the Youth Chen-Guang Project of Wuhan (no. 2015070404010180) for support of this research.
Notes and references
- For selected reviews, see:
(a) J. E. Saxton, Nat. Prod. Rep., 1997, 14, 559 RSC
; (b) S. E. O'Connor and J. Maresh, Nat. Prod. Rep., 2006, 23, 532 RSC
; (c) M. Ishikura, K. Yamada and T. Abe, Nat. Prod. Rep., 2010, 27, 1630 RSC
; (d) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed
; (e) S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108 CrossRef CAS PubMed
; (f) L. Wang, Y.-Y. Chen and J. Xiao, Asian J. Org. Chem., 2014, 3, 1036 CrossRef CAS
.
- For selected reviews, see:
(a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed
; (b) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed
; (c) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC
; (d) M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed
; (e) R. Dalpozzo, Chem. Soc. Rev., 2015, 44, 742 RSC
.
- For selected reviews, see:
(a) M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550 CrossRef CAS PubMed
; (b) A. Umani-Ronchi, M. Bandini, A. Melloni and S. Tommasi, Synlett, 2005, 1199 Search PubMed
; (c) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed
; (d) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC
.
- For selected reviews, see:
(a) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed
; (b) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed
; (c) Z. Lu and S.-M. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed
; (d) B. M. Trost, T. Zhang and J. Sieber, Chem. Sci., 2010, 1, 427 RSC
.
- M. Bandini, A. Melloni, F. Piccinelli, R. Sinisi, S. Tommasi and A. Umani-Ronchi, J. Am. Chem. Soc., 2006, 128, 1424 CrossRef CAS PubMed
.
- H. Y. Cheung, W.-Y. Yu, F. L. Lam, T.-T. Au-Yeung, Z.-Y. Zhou, T. H. Chan and A. S. Chan, Org. Lett., 2007, 9, 4295 CrossRef CAS PubMed
.
- T. Hoshi, K. Sasaki, S. Sato, Y. Ishii, T. Suzuki and H. Hagiwara, Org. Lett., 2011, 13, 932 CrossRef CAS PubMed
.
-
(a) Z. Liu, Z. Cao and H.-F. Du, Org. Biomol. Chem., 2011, 9, 5369 RSC
; (b) Y. Liu and H.-F. Du, Org. Lett., 2013, 15, 740 CrossRef CAS PubMed
; (c) Z. Cao, Y. Liu, Z. Liu, X. Feng, M. Zhuang and H.-F. Du, Org. Lett., 2011, 13, 2164 CrossRef CAS PubMed
.
- T. Mino, M. Ishikawa, K. Nishikawa, K. Wakui and M. Sakamoto, Tetrahedron: Asymmetry, 2013, 24, 499 CrossRef CAS
.
-
(a)
T. Toru and C. Bolm, Organosulfur Chemistry in Asymmetric Synthesis, Wiley-VCH, Weinheim, 2008 Search PubMed
; (b) H. Pellissier, Chiral Sulfur Ligands: Asymmetric Catalysis, Royal Society of Chemistry, Cambridge, U.K., 2009 Search PubMed
; (c) M. Mellah, A. Voituriez and E. Schulz, Chem. Rev., 2007, 107, 5133 CrossRef CAS PubMed
; (d) F. L. Lam, F. Y. Kwong and A. S. Chan, Chem. Commun., 2010, 46, 4649 RSC
.
-
(a) T. P. Yoon and E. N. Jacobsen, Science, 2003, 299, 1691 CrossRef CAS PubMed
; (b) Q.-L. Zhou, Privileged Chiral Ligands and Catalysts, Wiley-VCH, Weinheim, 2011 Search PubMed
.
-
(a) H.-G. Cheng, L.-Q. Lu, T. Wang, J.-R. Chen and W.-J. Xiao, Chem. Commun., 2012, 48, 5596 RSC
; (b) L.-Y. Chen, J.-R. Chen, H.-G. Cheng, L.-Q. Lu and W.-J. Xiao, Eur. J. Org. Chem., 2014, 4714 CrossRef CAS
.
-
(a) H.-G. Cheng, B. Feng, L.-Y. Chen, W. Guo, X.-Y. Yu, L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Chem. Commun., 2014, 50, 2873 RSC
; (b) B. Feng, H.-G. Cheng, J.-R. Chen, Q.-H. Deng, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2014, 50, 9550 RSC
; (c) L.-Y. Chen, X.-Y. Yu, J.-R. Chen, B. Feng, H. Zhang, Y.-H. Qi and W.-J. Xiao, Org. Lett., 2015, 17, 1381 CrossRef CAS PubMed
.
- For selected reviews, see:
(a) B. L. Feringa, Acc. Chem. Soc., 2000, 33, 346 CrossRef CAS
; (b) L. Eberhardt, D. Armspach, J. Harrowfield and D. Matt, Chem. Soc. Rev., 2008, 37, 839 RSC
; (c) J. F. Teichert and B. L. Feringa, Angew. Chem., Int. Ed., 2010, 49, 2486 CrossRef CAS PubMed
; (d) W.-H. Zhang, S. W. Chien and T. S. A. Hor, Coord. Chem. Rev., 2011, 255, 1991 CrossRef CAS
.
- See the ESI† for more details. We also tried to prepare the diastereomer of ligand L3 using (S)-BINOL and thioether-amines 4a (R1 = Me) as the starting materials. Unfortunately, all the attempts met failure using our own method or previous literature procedures, which was probably because of the steric hindrance.
- Y. Wei, L.-Q. Lu, T.-R. Li, B. Feng, Q. Wang, W.-J. Xiao and H. Alper, Angew. Chem., Int. Ed., 2016, 55, 2200 CrossRef CAS PubMed
.
- M. Bandini, A. Melloni and A. Umani-Ronchi, Org. Lett., 2004, 6, 3199 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00227g |
| This journal is © the Partner Organisations 2016 |