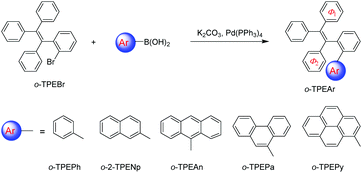
Synthesis, structure and optical properties of tetraphenylethene derivatives with through-space conjugation between benzene and various planar chromophores†
Bairong
He
a,
Han
Nie
a,
Wenwen
Luo
a,
Rongrong
Hu
a,
Anjun
Qin
a,
Zujin
Zhao
*a and
Ben Zhong
Tang
*abc
aState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, China. E-mail: mszjzhao@scut.edu.cn
bDepartment of Chemistry, The Hong Kong University of Science & Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
cHong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, Hong Kong, China
First published on 29th June 2016
Abstract
Through-space conjugation is conducive to efficient multidimensional exciton diffusion and charge transport in optoelectronic functional materials. Herein, a series of tetraphenylethene derivatives with through-space conjugation formed between benzene and various aromatic rings are synthesized and characterized. Their crystal structures, frontier orbital distributions and aggregation-enhanced emission properties are investigated.
π–π interactions, along with cation–π1 and CH–π interactions,2etc., are very important noncovalent weak interactions, which play a vital role in manipulating molecular geometries, ranging over biology, chemistry, and materials.3 When it comes to π–π interactions, the two interplaying aromatic rings can pile up in different manners of parallel face-to-face, parallel offset, and edge-to-face (T-shape),4 resulting in different degrees of interactions. The efficient π–π interaction, generally occurring in the two closely face-to-face stacked aromatic rings, is also referred to as through-space conjugation. Intense electron clouds of the frontier molecular orbitals can delocalize onto the inter-ring region of the two through-space conjugated aromatic rings. The pioneering synthesis of [2.2]paracyclophane,5 where two benzene rings are stacked in an orientation of parallel face-to-face, provided a perfect model for studying the through-space conjugation. Recent reports further demonstrate that through-space conjugation can occur in many molecules that possess well-defined and nonfluxional structures with two chromophores arranged in close proximity,6 and allow for effective multidimensional exciton diffusion and charge transport in optoelectronic functional materials.
In addition to the myriad of [2.2]paracyclophane derivatives including small molecules, oligomers,7 and polymers,8 various other skeletons featuring through-space conjugation have also been developed, including derivatives of naphthalene,9 diarylbiphenylene,10 xanthene,11 bicyclo[4.4.1]undecanone,12 hexaphenylbenzene,13 dibenzofulvene,14 indenofluorene,15 and so forth. However, most studied through-space conjugation is based on two identical aromatic rings, and that which happens in two different kinds of aromatic rings is much rarer, leaving much space for further digging deep through-space conjugated molecules and deciphering the influence of through-space conjugation on molecular properties.
Recently, a novel folded skeleton with intramolecular through-space conjugation has been developed in our study on tetraphenylethene (TPE) derivatives, which is a stereospecific product of McMurry coupling aided by substrate modulation.16 Most TPE derivatives17 exhibit aggregation-induced emission (AIE) characteristics, but the novel folded conformation endows TPE derivatives with another brand new emission experience, and allow for novel optoelectronic functionalities. To further expand the family of through-space conjugated molecules, in this work, we wish to report a series of new TPE derivatives (namely o-TPEAr), where through-space conjugation is formed between benzene and different planar chromophores. These new TPE derivatives were readily obtained by Suzuki–Miyaura coupling of o-TPEBr with the corresponding arylboronic acids as illustrated in Scheme 1. Various aromatic substituents including benzene, naphthalene, anthracene, phenanthrene and pyrene were introduced onto the ortho-position of the phenyl ring in the TPE moiety. The detailed characterization data of NMR and mass spectra are given in the ESI.† The molecular structures of o-TPEPh, o-2-TPENp, o-TPEAn, o-TPEPa and o-TPEPy were further confirmed by X-ray diffraction crystallography analyses on their single crystals, which were grown from THF/ethanol mixtures via slow diffusion and evaporation of the solvents. The parameters for the crystal structures are described in the ESI.† As shown in Fig. 1, the incorporated planar aromatic groups are stacked with the phenyl rings in TPE in a face-to-face, slightly slipped manner. However, different aromatic substituents will give rise to varied stacking choice to the phenyl rings in TPE. For instance, the small-sized benzene and naphthalene rings tend to stack with Φ1 as indicated in Scheme 1, while large anthracene, phenanthrene and pyrene rings prefer to stack with Φ2. The two stacked aromatic rings are not totally parallel. The tilted angles are 9.95°, 15.72°, 12.51°, 25.76°, and 14.40° for o-TPEPh, o-2-TPENp, o-TPEAn, o-TPEPa and o-TPEPy, respectively (Table 1 and Fig. S1–S5†). And they are staggered and overlapped in varied degrees. The shortest distances between the two stacked aromatic rings are in the range of 2.97–3.44 Å, being shorter than the typical π–π stacking interaction distance of 3.5 Å, implying the existence of π–π interactions, that is through-space conjugation.
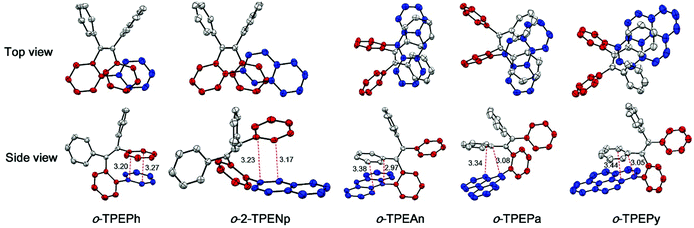 | ||
| Fig. 1 Crystal structures of o-TPEAr with indicated distances (Å) between π–π stacked aromatic rings. | ||
| Compound | Cofacial pairs | Tilted angles (°) |
|---|---|---|
| o-TPEPh | Ph-Φ1 | 9.95 |
| o-2-TPENp | Np-Φ1 | 15.72 |
| o-TPEAn | An-Φ2 | 12.51 |
| o-TPEPa | Pa-Φ2 | 25.76 |
| o-TPEPy | Py-Φ2 | 14.40 |
Intramolecular through-space conjugation is an interesting characteristic of π-electron cloud overlapping between cofacial aromatic rings.7a,16c Density functional theory (DFT) calculations, using the ωB97XD functional with the 6-31g* basis set, were carried out to further verify the occurrence of through-space conjugation in o-TPEAr. The amplitude plots of important frontier molecular orbitals ranging from HOMO−2 to LUMO+2 are displayed in Fig. 2. It can be seen that the transannular π-electron cloud overlapping appears in π-stacked aromatic rings. Despite the slipped and staggered stacking of the two phenyl rings in o-TPEPh (Fig. 1 and S6†), obvious cofacial π-electron cloud overlapping still occurs, as shown in its LUMO+1 orbital. o-2-TPENp and o-TPEAn, which possess large planar chromophores, also show π-electron cloud overlapping in the LUMO+1 orbital and HOMO−2 orbital, respectively. For o-TPEPa and o-TPEPy, these features are not so distinct, probably owing to the unsuitable electronic structures as well as energy levels of phenanthrene and pyrene relative to benzene.
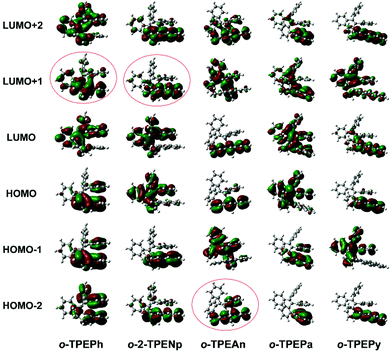 | ||
| Fig. 2 Molecular orbital amplitude plots ranging from HOMO−2 to LUMO+2 of o-TPEAr in the ground state calculated at the ωB97XD/6-31g* level. | ||
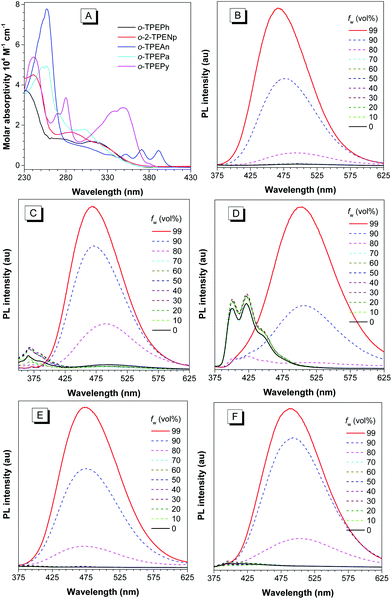
The absorption spectra of o-TPEAr in THF solutions are shown in Fig. 3A. The spectral profiles and maximum absorption peaks varying from 287 to 391 nm (Table 2) are closely related to the type of introduced planar chromophore. The o-TPEAr are nonfluorescent or weakly fluorescent in dilute THF solutions, and their absolute fluorescence quantum yields (ΦF) fall in the range of 0.8–4.6%. With regard to o-TPEAn and o-TPEPy that possess large fluorescent chromophores, they show much lower ΦF values than isolated anthracene (36%) and pyrene (32%).18 These results are well consistent with many TPE derivatives, such as those with aromatic substituents at the para-positions of the phenyl ring in TPE, no matter mono-substitution19 (TPEAr) or bis-substitution.20 It can be interpreted as that intramolecular rotation, which is ubiquitous in many other TPE derivatives,21 activates the nonradiative decay of excitons, and thus leads to fluorescence quenching in the solution state. But their ΦF values are much higher than those of TPEAr (0.019–0.34%), due to lowered rotation by the increased molecular rigidity derived from intramolecular through-space conjugation.
| λ abs (nm) | λ em (nm) |
Φ
F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (%) d (%) |
α AIE | ||||
|---|---|---|---|---|---|---|---|
| Solna | Aggrb | Filmc | Crystal | Solna | Filmc | ||
| a In THF solution (10 μM). b In THF/water mixtures (V/V 1/99). c Film drop-cast on a quartz plate. d Absolute fluorescence quantum yield measured by an integrating sphere. e AIE effect determined as ΦF (Film)/ΦF (Soln). | |||||||
| o-TPEPh | 313 | 466 | 466 | 445 | 1.9 | 55 | 29 |
| o-2-TPENp | 287 | 468 | 467 | 425 | 2.1 | 56 | 27 |
| o-TPEAn | 391 | 504 | 501 | 432 | 4.6 | 61 | 13 |
| o-TPEPa | 303 | 473 | 472 | 465 | 0.8 | 54 | 68 |
| o-TPEPy | 350 | 490 | 484 | 424 | 3.3 | 68 | 21 |
The o-TPEAr are much more fluorescent in the aggregated state. Fig. 3B–F display photoluminescence (PL) spectral changes of o-TPEAr with the addition of water to their THF solutions. Because water is a non-solvent for o-TPEAr, the addition of a large amount of water will make the molecules form aggregates, and thus alter their emission properties. Taking o-TPEAn as an example, it shows an emission spectrum in the blue region with fine structures at around 400 and 422 nm stemming from an anthracene moiety when the water fraction (fw, vol%) is lower than 80%, but when fw increases to 90% or more, the blue emission band disappears and a long-wavelength emission is swiftly enhanced. Similar PL spectral changes can also be observed in o-TPEPh, o-2-TPENp, o-TPEPa and o-TPEPy, except that their blue emission bands (fw < 80%) are relatively weaker. These results clearly demonstrate that these new TPE derivatives have aggregation-enhanced emission (AEE) properties.17 The disappearance of blue emission bands and the appearance of long-wavelength emission bands should result from the restriction of intramolecular rotation, because the aromatic rings that do not form intramolecular π-stacking can rotate freely, and thus impair the electronic coupling between building blocks. When the intramolecular rotation is restricted in the aggregated state, the emission is stemmed from the radiative decay of all the conjugated molecules.
Like many other TPE derivatives, o-TPEAr are strongly fluorescent in solid films. The PL spectra of their solid films are given in Fig. S11A,† and the detailed data are listed in Table 2. The emission peaks of the solid films of o-TPEPh, o-2-TPENp, o-TPEAn, o-TPEPa, and o-TPEPy are located at 466, 467, 501, 472, and 484 nm, respectively, exhibiting variations with the types of incorporated chromophores. These PL peaks are very close to those in the aggregated state, i.e. the corresponding emission peaks are located at 466, 468, 504, 473 and 490 nm, respectively, in THF–water mixtures (fw = 99%) (Table 2). The ΦF values of the solid films are in the range of 54–68%, which are greatly improved with respect to those in solutions. The corresponding values of the AIE effect (αAIE) are in the range of 13–68. The crystals of o-TPEAr show PL emissions peaking at 424–465 nm (Table 2 and Fig. S11B†), which are blue-shifted relative to those of amorphous films. Similar blue-shifted emissions from amorphous films to crystals have also been observed and studied on many AIE-active molecules, which are attributed to the lowered reorganization energy in more rigid crystals,22 with the help of multiple inter- and intramolecular CH–π interactions (Fig. S6–S10†).
In conclusion, a series of TPE derivatives substituted with various planar chromophores at the ortho-position of the phenyl ring in TPE are synthesized and characterized. The crystallography analysis reveals that the introduced chromophores adopt a close cofacial π–π stacking pattern with the phenyl rings in TPE, and theoretical calculation further demonstrates the apparent through-space conjugation characteristic of transannular π-electron cloud overlapping between π-stacked aromatic rings. But different aromatic rings as well as different geometries show significant impacts on the formation of intramolecular through-space conjugation. These TPE derivatives exhibit better PL emission properties than similar TPEAr in the literature, owing to the enhanced molecular rigidity by through-space conjugation. Their PL emissions are further improved in the aggregated state, exhibiting AEE features. These through-space conjugated TPE derivatives are good models for the mechanistic studies of some photophysical phenomena, such as AIE, and will provide deep insights into the structure–property correlation of various luminescent materials.
Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (51273053), the 973 Program (2015CB655004 and 2013CB834702), the Guangdong Natural Science Funds for Distinguished Young Scholar (2014A030306035), the Guangdong Innovative Research Team Program (201101C0105067115), the Natural Science Foundation of Guangdong Province (2016A030312002), the ITC-CNERC14S01 and the Fundamental Research Funds for the Central Universities (2015PT020 and 2015ZY013).References
- A. S. Mahadevi and G. N. Sastry, Chem. Rev., 2013, 113, 2100–2138 CrossRef CAS PubMed.
- (a) L. S. Birchall, S. Roy, V. Jayawarna, M. Hughes, E. Irvine, G. T. Okorogheye, N. Saudi, E. De Santis, T. Tuttle, A. A. Edwards and R. V. Ulijn, Chem. Sci., 2011, 2, 1349–1355 RSC; (b) M. Nishio, Y. Umezawa, J. Fantini, M. S. Weiss and P. Chakrabarti, Phys. Chem. Chem. Phys., 2014, 16, 12648–12683 RSC.
- (a) C. J. Pace and J. Gao, Acc. Chem. Res., 2012, 46, 907–915 CrossRef PubMed; (b) C. D. Sherrill, Acc. Chem. Res., 2013, 46, 1020–1028 CrossRef CAS PubMed; (c) E. M. Perez and N. Martin, Chem. Soc. Rev., 2015, 44, 6425–6433 RSC; (d) L. M. Salonen, M. Ellermann and F. Diederich, Angew. Chem., Int. Ed., 2011, 50, 4808–4842 CrossRef CAS PubMed.
- (a) M. O. Sinnokrot and C. D. Sherrill, J. Am. Chem. Soc., 2004, 126, 7690–7697 CrossRef CAS PubMed; (b) M. Watt, L. K. E. Hardebeck, C. C. Kirkpatrick and M. Lewis, J. Am. Chem. Soc., 2011, 133, 3854–3862 CrossRef CAS PubMed.
- C. J. Brown and A. C. Farthing, Nature, 1949, 164, 915–916 CrossRef CAS.
- G. P. Bartholomew and G. C. Bazan, Acc. Chem. Res., 2001, 34, 30–39 CrossRef CAS PubMed.
- (a) S. Mukhopadhyay, S. P. Jagtap, V. Coropceanu, J. L. Brédas and D. M. Collard, Angew. Chem., Int. Ed., 2012, 51, 11629–11632 CrossRef CAS PubMed; (b) Y. Morisaki, N. Kawakami, T. Nakano and Y. Chujo, Chem. – Eur. J., 2013, 19, 17715–17718 CrossRef CAS PubMed.
- (a) S. P. Jagtap and D. M. Collard, J. Am. Chem. Soc., 2010, 132, 12208–12209 CrossRef CAS PubMed; (b) Y. Morisaki and Y. Chujo, Polym. Chem., 2011, 2, 1249–1257 RSC; (c) Y. Morisaki, S. Ueno, A. Saeki, A. Asano, S. Seki and Y. Chujo, Chem. – Eur. J., 2012, 18, 4216–4224 CrossRef CAS PubMed; (d) S. P. Jagtap and D. M. Collard, Polym. Chem., 2012, 3, 463–471 RSC.
- (a) J. N. Moorthy, S. Mandal and K. N. Parida, Org. Lett., 2012, 14, 2438–2441 CrossRef CAS PubMed; (b) M. Iyoda, T. Kondo, K. Nakao, K. Hara, Y. Kuwatani, M. Yoshida and H. Matsuyama, Org. Lett., 2000, 2, 2081–2083 CrossRef CAS PubMed.
- F. Cozzi, R. Annunziata, M. Benaglia, K. K. Baldridge, G. Aguirre, J. Estrada, Y. Sritana-Anant and J. S. Siegel, Phys. Chem. Chem. Phys., 2008, 10, 2686–2694 RSC.
- (a) Y. Morisaki, T. Sawamura, T. Murakami and Y. Chujo, Org. Lett., 2010, 12, 3188–3191 CrossRef CAS PubMed; (b) T. Nakano, Y. Morisaki and Y. Chujo, Tetrahedron Lett., 2015, 56, 2086–2090 CrossRef CAS.
- (a) K. M. Knoblock, C. J. Silvestri and D. M. Collard, J. Am. Chem. Soc., 2006, 128, 13680–13681 CrossRef CAS PubMed; (b) S. P. Jagtap, S. Mukhopadhyay, V. Coropceanu, G. L. Brizius, J. L. Brédas and D. M. Collard, J. Am. Chem. Soc., 2012, 134, 7176–7185 CrossRef CAS PubMed.
- (a) R. Rathore, C. L. Burns and S. A. Abdelwahed, Org. Lett., 2004, 6, 1689–1692 CrossRef CAS PubMed; (b) V. J. Chebny, R. Shukla and R. Rathore, J. Phys. Chem. A, 2006, 110, 13003–13006 CrossRef CAS PubMed.
- (a) T. Nakano and T. Yade, J. Am. Chem. Soc., 2003, 125, 15474–15484 CrossRef CAS PubMed; (b) H. Qi, J. Chang, S. H. Abdelwahed, K. Thakur, R. Rathore and A. J. Bard, J. Am. Chem. Soc., 2012, 134, 16265–16274 CrossRef CAS PubMed.
- (a) D. Thirion, C. Poriel, F. Barrière, R. Métivier, O. Jeannin and J. Rault-Berthelot, Org. Lett., 2009, 11, 4794–4797 CrossRef CAS PubMed; (b) T. Kowada, T. Kuwabara and K. Ohe, J. Org. Chem., 2010, 75, 906–913 CrossRef CAS PubMed.
- (a) Z. Zhao, J. W. Y. Lam, C. Y. K. Chan, S. Chen, J. Liu, P. Lu, M. Rodriguez, J.-L. Maldonado, G. Ramos-Ortiz, H. H. Y. Sung, I. D. Williams, H. Su, K. S. Wong, Y. Ma, H. S. Kwok, H. Qiu and B. Z. Tang, Adv. Mater., 2011, 23, 5430–5435 CrossRef CAS PubMed; (b) Z. Zhao, B. He, H. Nie, B. Chen, P. Lu, A. Qin and B. Z. Tang, Chem. Commun., 2014, 50, 1131–1133 RSC; (c) L. Chen, Y.-H. Wang, B. He, H. Nie, R. Hu, F. Huang, A. Qin, X.-S. Zhou, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2015, 54, 4231–4235 CrossRef CAS PubMed; (d) B. He, H. Nie, L. Chen, X. Lou, R. Hu, A. Qin, Z. Zhao and B. Z. Tang, Org. Lett., 2015, 17, 6174–6177 CrossRef CAS PubMed.
- (a) J. Huang, M. Yang, J. Yang, R. Tang, S. Ye, Q. Li and Z. Li, Org. Chem. Front., 2015, 2, 1608–1615 RSC; (b) C. Wang, B. Xu, M. Li, Z. Chi, Y. Xie, Q. Li and Z. Li, Mater. Horiz., 2016, 3, 220–225 RSC; (c) J. Huang, N. Sun, J. Yang, R. Tang, Q. Li, D. Ma and Z. Li, Adv. Funct. Mater., 2014, 24, 7645–7654 CrossRef CAS; (d) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- I. B. Berlman, Handbook of Fluorescence Spectra of Aromatic Molecules, Academic Press, New York and London, 1971 Search PubMed.
- Z. Zhao, P. Lu, J. W. Y. Lam, Z. Wang, C. Y. K. Chan, H. H. Y. Sung, I. D. Williams, Y. Ma and B. Z. Tang, Chem. Sci., 2011, 2, 672–675 RSC.
- Z. Zhao, S. Chen, C. Y. K. Chan, J. W. Y. Lam, C. K. W. Jim, P. Lu, Z. Chang, H. S. Kwok, H. Qiu and B. Z. Tang, Chem. – Asian J., 2012, 7, 484–488 CrossRef CAS PubMed.
- Z. Zhao, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem., 2012, 22, 23726–23740 RSC.
- Q. Wu, T. Zhang, Q. Peng, D. Wang and Z. Shuai, Phys. Chem. Chem. Phys., 2014, 16, 5545–5552 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, single crystal data, molecular packing patterns, photoluminescence spectra of o-TPEAr in films and crystals, and 1H NMR spectra. CCDC 955717, 1432503–1432506. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00204h |
| This journal is © the Partner Organisations 2016 |