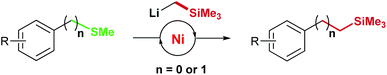
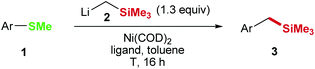Metal catalyzed cross-coupling of aryl and benzyl methyl sulfides: nickel catalyzed Caryl–Csp3 and Csp3–Csp3 bond formations†
Matthias
Leiendecker
,
Adisak
Chatupheeraphat
and
Magnus
Rueping
*
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074, Aachen, Germany. E-mail: Magnus.Rueping@rwth-aachen.de; Fax: +49-241-8092665; Tel: +49-241-8094686
First published on 17th February 2015
Abstract
The functionalization of CAr–SMe and Csp3–SMe bonds by direct exchange of the sulfur atom with an activated sp3-carbon has been developed. Reactions with LiCH2SiMe3 in the presence of a Nickel catalyst proceed with good yields and allow the conversion of aryl and benzyl methyl sulfides to trimethylsilylated products which are valuable precursors for the synthesis of olefins, various alcohols and amines, diols and aromatic carboxylic acids.
Cross-coupling opens the door for a wide variety of molecular transformations due to the ability of transition metal catalysts to activate otherwise inactive bonds and to assist carbon–carbon and carbon–heteroatom bond formation.1 However, there are limitations for both, the applicable substrates and the accessible products and many potentially useful transformations cannot be achieved with our current chemical knowledge. Anisole derivatives are a good example for such a limitation: although they are naturally available in a wide variety and thereby ecologically and economically interesting electrophiles for cross-coupling reactions, the dealkoxylative C–C bond forming cross-coupling of anisoles is limited to few transformations.2–5 In context of a recent natural product synthesis we envisioned a two-step process and developed a synthetic concept to enable diversification of unreactive anisole derivatives.5 During those studies the functionalization of unreactive aryl methyl sulfides (Scheme 1) caught our interest, since the replacement of the SMe group with an activated carbon atom would give access to a variety of products including olefins,6 various alcohols,7 and amines,8 diols,9 and aromatic carboxylic acids10 among others.5
 | ||
| Scheme 1 Nickel catalyzed replacement of the SMe group in aryl and benzyl methyl sulfides with a TMS-activated sp3 carbon nucleophile. | ||
Due to the versatile appearance of sulfur in various oxidation states in thiols, thioethers, thioesters, sulfoxides, sulfones and sulfoximines, a number of C–S bond cleaving protocols are known.11
The coupling of aryl methyl thioethers as the simplest analogs of thiophenols via cleavage of the inactive Csp2–SMe bond is challenging, but at the same time interesting due to its atom economy and naturally unreactive character. Csp2–SMe bond cleaving coupling reactions were first explored by Wenkert et al., who used Grignard reagents in the presence of a NiII catalyst for the direct arylation and methylation of aryl methyl sulfides.12 The addition of bridged phosphine ligands later led to a significant improvement of this method and enabled the coupling with β-hydrogen containing alkyl Grignard reagents.13
Cross-coupling of aryl methyl sulfides in the ortho-position of nitrogen containing heterocycles proceeds especially well and a series of elegant and valuable transformations has been developed by Knochel and others.14 The groups of Willis and Weller reported an elegant Rh(I)-catalyzed carbothiolation of alkynes with aryl methyl sulfides.15 In addition, a very interesting amination reaction applying aniline derivatives as the nucleophiles in the presence of a palladium catalyst with NHC ligands was recently reported by Murakami and Yorimitsu et al.16 A first nickel catalyzed reductive cleavage of aryl methyl sulfides was reported by Martin and proceeds without additional ligand with high yields.17
We started the development of the aryl methyl sulfide functionalization with a reaction of 4-(methylthio)biphenyl (1i) with LiCH2SiMe3 (2) in the presence of Ni(COD)2 as the catalyst.
However, only 22% of the corresponding ArCH2SiMe3 product 3i (Table 1, entries 1 and 2) was obtained. Increasing the temperature to 80 °C led to a significantly higher yield of 75% (entry 3), which could be further improved by the addition of 5 mol% PCy3 or SiPr ligands (entries 4 and 5). Interestingly, the opposite trend concerning PCy3 addition was observed for 2-(methylthio)naphthalene (1a): whereas reactions without ligand yielded 94% at room temperature and 99% at 80 °C, the addition of PCy3 (2 mol%) led to a reduced yield of 79% at 80 °C (entries 7–9). A control reaction without Ni catalyst did not lead to conversion of starting material (entry 6).
| Entry | Ar | Ni(COD)2 [mol%] | Ligand [mol%] | Temp. [°C] | Yielda [%] |
|---|---|---|---|---|---|
| a Yield of isolated product. b Control experiment without Ni catalyst. | |||||
| 1 | 4-Biphenyl | 2.5 | — | r. t. | 22 |
| 2 | 4-Biphenyl | 2.5 | PCy3 (5) | r. t. | 22 |
| 3 | 4-Biphenyl | 2.5 | — | 80 | 75 |
| 4 | 4-Biphenyl | 2.5 | PCy3 (5) | 80 | 88 |
| 5 | 4-Biphenyl | 2.5 | SiPr·HCl (5) | 80 | 79 |
| 6 | 4-Biphenyl | — | — | 80 | —b |
| 7 | 2-Naphthyl | 1 | — | r. t. | 94 |
| 8 | 2-Naphthyl | 1 | — | 80 | 99 |
| 9 | 2-Naphthyl | 1 | PCy3 (2) | r. t. | 79 |
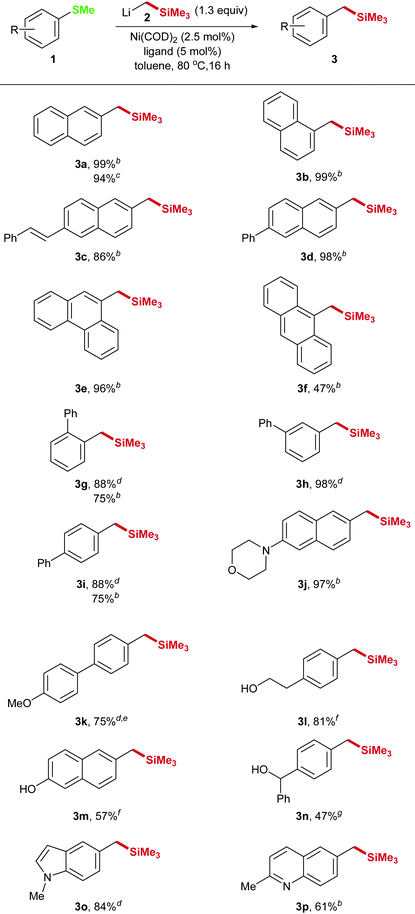
With acceptable reaction conditions in hand we explored the scope of the reaction. The reaction can be applied to a variety of substrates and the products 3a–p were isolated in good yields (Table 2). Different substitution patterns as well as functional groups were tolerated. The selective CAr–S-cleavage in substrate 1k that contains a methylthioether as well as a methoxy group could also be achieved indicating that the C–SMe bond cleaves faster than the C–OMe bond. In this case only 1 equivalent of the nucleophile was applied. In addition, aliphatic, aromatic and benzylic alcohols 1l–n are suitable substrates for our transformation and also nitrogen containing heterocycles 1j, o, p could be converted with good yields.
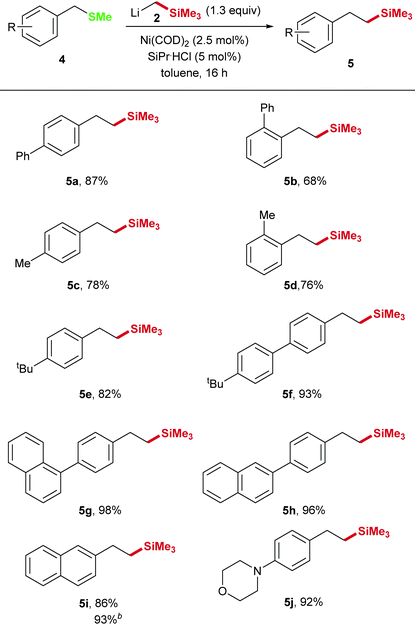
In order to expand the scope we examined the nickel catalyzed Csp3–Csp3 cross coupling using benzyl methyl sulfides. However, with the above optimized conditions, the cleavage of benzylic C–SMe bonds in the synthesis of phenethyl trimethylsilane derivative 5a from benzyl methyl sulfide 4a appeared to be difficult and only traces of the 4-biphenyl product 5a were detected (Table 3, entries 1–3). Since different phosphine ligands (entries 4–9) did not lead to higher conversion, we increased the electron density at the Ni center by addition of the N-heterocyclic carbene (NHC) ligand 1,3-bis(2,6-diisopropylphenyl)imidazolidinium chloride (SiPr·HCl, 5 mol%). Using this catalyst 87% yield were obtained (entry 10).
| Entry | Ar | Ligand [mol%] | Temp. [°C] | Yielda [%] |
|---|---|---|---|---|
| a Yield of isolated product. b Control experiment without Ni catalyst. | ||||
| 1 | 4-Biphenyl | — | r. t. | Traces |
| 2 | 4-Biphenyl | — | 80 | Traces |
| 3 | 4-Biphenyl | PCy3 (5) | 80 | Traces |
| 4 | 4-Biphenyl | PPh3 (5) | 80 | Traces |
| 5 | 4-Biphenyl | Dcype (5) | 80 | Traces |
| 6 | 4-Biphenyl | Dcypp (5) | 80 | Traces |
| 7 | 4-Biphenyl | Dcypb (5) | 80 | Traces |
| 8 | 4-Biphenyl | Dppm (5) | 80 | Traces |
| 9 | 4-Biphenyl | Dppp (5) | 80 | Traces |
| 10 | 4-Biphenyl | SiPr·HCl (5) | 80 | 87 |
| 11 | 4-Biphenyl | — | 80 | —b |
| 12 | 2-Naphthyl | PCy3 (5) | 80 | 93 |
| 13 | 2-Naphthyl | SiPr·HCl (5) | 80 | 86 |
| 14 | 2-Naphthyl | — | r. t. | Traces |
| 15 | 2-Naphthyl | — | 80 | 9 |
A control experiment showed that no conversion was obtained in the absence of a Ni catalyst (entry 11). Interestingly, whereas the 4-biphenyl substrate 4a could not be converted sufficiently when PCy3 was employed as ligand, the transformation of naphthyl derivative 4i proceeded well under these conditions (Table 3, entries 12–15).
In general, these reactions proceed with good yields for various benzyl methyl sulfides 5a–j (Table 4). Similar to the transformation of thioanisole derivatives, benzyl methyl sulfide derivatives with aryl substitution in ortho-, meta-, and para-position could be applied with good yields. In addition, 92% yield was obtained for the transformation of the morpholine derivative 4j into the corresponding phenethyl trimethylsilane.
Conclusions
In summary, we have developed a metal catalyzed cross-coupling of aryl and benzyl methyl sulfides leading to selective Caryl–Csp3 and Csp3–Csp3 bond formations. The direct functionalization of CAr–SMe and Csp3–SMe bonds by exchange of the sulfur atom with an activated sp3-carbon could be achieved for several thioethers using the nickel catalyzed cross coupling and an organolithium nucleophile. The reactions proceed with good yields and allow the conversion to the trimethylsilylated products which are valuable precursors for various products including olefins, alcohols, amines, diols and aromatic carboxylic acids.Experimental
In a typical experiment an oven-dried, argon-flushed Schlenk tube was charged with the aryl or benzyl methyl sulfide (if solid; 0.25 mmol), the ligand and yellow Ni(COD)2. The tube was immediately sealed and again flushed with argon. Subsequently, freshly distilled toluene (1.5 mL), the aryl or benzyl methyl sulfide (if liquid) and a LiCH2SiMe3 solution in pentane (1.0 M) were added and the mixture was stirred overnight at the appointed temperature. Upon purification via column chromatography (elution with n-hexane–EtOAc = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the pure product was obtained after solvent removal.
1) the pure product was obtained after solvent removal.
Acknowledgements
M. L. acknowledges support from the Fonds der Chemischen Industrie and the Studienstiftung des Deutschen Volkes.Notes and references
- (a) Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004, vol. 2 Search PubMed; (b) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed.
- B.-T. Guan, S.-K. Xiang, T. Wu, Z.-P. Sun, B.-Q. Wang, K.-Q. Zhao and Z.-J. Shi, Chem. Commun., 2008, 1437–1439 RSC.
- (a) E. Wenkert, E. L. Michelotti and C. S. Swindell, J. Am. Chem. Soc., 1979, 101, 2246–2247 CrossRef CAS; (b) E. Wenkert, E. L. Michelotti, C. S. Swindell and M. Tingoli, J. Org. Chem., 1984, 49, 4894–4899 CrossRef CAS; (c) J. W. Dankwardt, Angew. Chem., Int. Ed., 2004, 43, 2428–2432 CrossRef CAS PubMed; (d) M. Tobisu, T. Shimasaki and N. Chatani, Angew. Chem., Int. Ed., 2008, 47, 4866–4869 CrossRef CAS PubMed; (e) L.-G. Xie and Z.-X. Wang, Chem. – Eur. J., 2011, 17, 4972–4975 CrossRef CAS PubMed; (f) M. J. Iglesias, A. Prieto and C. Nicasio, Org. Lett., 2012, 14, 4318–4321 CrossRef CAS PubMed; (g) C. Wang, T. Ozaki, R. Takita and M. Uchiyama, Chem. – Eur. J., 2012, 18, 3482–3485 CrossRef CAS PubMed.
- Reviews on cross-coupling of activated and unactivated phenol derivatives: (a) D.-G. Yu, B.-J. Li and Z.-J. Shi, Acc. Chem. Res., 2010, 43, 1486–1495 CrossRef CAS PubMed; (b) G. P. McGlacken and S. L. Clarke, ChemCatChem, 2011, 3, 1260–1261 CrossRef CAS; (c) T. Mesganaw and N. K. Garg, Org. Process Res. Dev., 2013, 17, 29–39 CrossRef CAS; (d) S. I. Kozhushkov, H. K. Potukuchiw and L. Ackermann, Catal. Sci. Technol., 2013, 3, 562–571 RSC; (e) J. Cornella, C. Zarate and R. Martin, Chem. Soc. Rev., 2014, 43, 8081–8097 RSC.
- (a) M. Leiendecker, C.-C. Hsiao, L. Guo, N. Alandini and M. Rueping, Angew. Chem., Int. Ed., 2014, 53, 12912–12915 CrossRef CAS PubMed. For a related study, see: (b) L. Guo, M. Leiendecker, C.-C. Hsiao, C. Baumann and M. Rueping, Chem. Commun., 2015, 51, 1937–1940 RSC.
- (a) D. J. Peterson, J. Org. Chem., 1968, 33, 780–784 CrossRef CAS; (b) D. J. Ager, The Peterson Olefination Reaction in Organic Reactions, Wiley-VCH, Weinheim, 2004, pp. 1–223 Search PubMed.
- M. Das and D. F. O'Shea, Tetrahedron, 2013, 69, 6448–6460 CrossRef CAS PubMed.
- W.-X. Zhang, C.-H. Ding, Z.-B. Luo, X.-L. Hou and L.-X. Dai, Tetrahedron Lett., 2006, 47, 8391–8393 CrossRef PubMed.
- A. B. Smith III and R. Tong, Org. Lett., 2010, 12, 1260–1263 CrossRef PubMed.
- H. Li, Z. Li and Z.-J. Shi, Tetrahedron, 2009, 65, 1856–1858 CrossRef CAS PubMed.
- For review articles, see (a) S. R. Dubbaka and P. Vogel, Angew. Chem., Int. Ed., 2005, 44, 7674–7684 CrossRef CAS PubMed; (b) H. Prokopcov and C. O. Kappe, Angew. Chem., Int. Ed., 2009, 48, 2276–2286 CrossRef PubMed; (c) L. Wang, W. Hea and Z. Yu, Chem. Soc. Rev., 2013, 42, 599–621 RSC; (d) F. Pana and Z.-J. Shi, ACS Catal., 2014, 4, 280–288 CrossRef.
- E. Wenkert, T. W. Ferreira and E. L. Michelotti, J. Chem. Soc., Chem. Commun., 1979, 637–638 RSC.
- S. Kanemura, A. Kondoh, H. Yorimitsu and K. Oshima, Synthesis, 2008, 2659–2664 CAS.
- (a) H. Takei, M. Miura, H. Sugimura and H. Okamura, Chem. Lett., 1979, 1447–1450 CrossRef CAS; (b) A. Metzger, L. Melzig, C. Despotopoulou and P. Knochel, Org. Lett., 2009, 11, 4228–4231 CrossRef CAS PubMed; (c) J.-M. Begouin, M. Rivard and C. Gosmini, Chem. Commun., 2010, 46, 5972–5974 RSC; (d) L. Melzig, A. Metzger and P. Knochel, J. Org. Chem., 2010, 75, 2131–2133 CrossRef CAS PubMed; (e) L. Melzig, A. Metzger and P. Knochel, Chem. – Eur. J., 2011, 17, 2948–2956 CrossRef CAS PubMed.
- J. F. Hooper, A. B. Chaplin, C. González-Rodríguez, A. L. Thompson, A. S. Weller and M. C. Willis, J. Am. Chem. Soc., 2012, 134, 2906–2909 CrossRef CAS PubMed.
- T. Sugahara, K. Murakami, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2014, 53, 9329–9333 CrossRef CAS PubMed.
- N. Barbero and R. Martin, Org. Lett., 2012, 14, 796–799 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00001g |
| This journal is © the Partner Organisations 2015 |