Ting Tang and Andrew M. Harned
Org. Biomol. Chem., 2018,16, 8249-8252
DOI:
10.1039/C8OB01652F,
Communication
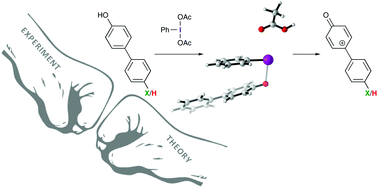
Iodine(III)-based oxidants are commonly used reagents for the oxidative dearomatization of phenols. Having a better understanding of the mechanism through which these reactions proceed is important for designing new iodine(III)-based reagents, catalysts, and reactions. We have performed a Hammett analysis of the oxidative dearomatization of substituted 4-phenylphenols. This study confirms that iodine(III)-mediated oxidative dearomatizations likely proceed through cationic phenoxenium ions and not the direct addition of a nucleophile to an iodine-bound phenol intermediate.