Dehydrogenation of sodium borohydride and ammonia borane over cobalt-based catalysts: advances and prospects
Xiugang
Li
 ab,
Longhua
Yao
b,
Qilu
Yao
b,
Jianhui
Xia
b and
Zhang-Hui
Lu
ab,
Longhua
Yao
b,
Qilu
Yao
b,
Jianhui
Xia
b and
Zhang-Hui
Lu
 *b
*b
aCollege of Material and Chemical Engineering, Tongren University, Tongren, 554300, China
bKey Laboratory of Green Catalysis of Jiangxi Education Institutes, College of Chemistry and Materials, Jiangxi Normal University, Nanchang 330022, China. E-mail: luzh@jxnu.edu.cn
First published on 15th April 2025
Abstract
Chemical hydrogen storage is acknowledged as a promising approach for hydrogen storage, offering numerous advantages, such as high energy density, enhanced safety, and environmental adaptability, as well as potential economic benefits. Among the chemical hydrogen storage materials that have been reported, sodium borohydride and ammonia borane have attracted considerable scholarly interest due to their capacity to release hydrogen conveniently via solvolysis processes, such as hydrolysis and methanolysis, under ambient temperature conditions. Cobalt-based nanocatalysts, as representatives of non-noble metals, have been extensively investigated as cost-effective and efficient catalysts for hydrogen evolution from the solvolysis of sodium borohydride and ammonia borane. Nevertheless, a comprehensive review specifically focusing on cobalt-based catalysts for hydrogen production from sodium borohydride and ammonia borane has yet to be published. In this review, we provide a comprehensive summary of the historical development and recent advancements in cobalt-based catalysts for hydrogen generation from sodium borohydride and ammonia borane, encompassing synthesis methods, notable performances, and potential catalytic mechanisms. Our objective is to establish a reliable structure–property relationship and offer guidance for the future design of catalysts for hydrogen evolution from sodium borohydride and ammonia borane.
1. Introduction
The advancement of green sources of energy has emerged as a primary focus for the future, among which hydrogen, as the most abundant renewable energy source on Earth, exhibits significant potential for commercial utilizations.1–5 Hydrogen has garnered unprecedented attention within the scientific community due to its numerous advantages, such as cleanliness, the absence of pollution, high calorific value upon combustion, and widespread accessibility.6,7 In particular, the emergence of hydrogen fuel cells greatly broadens the range of potential uses of hydrogen by enabling the direct conversion of hydrogen energy into electrical energy.8–10 The increasing utilization of hydrogen energy in portable electronic devices and vehicles underscores the need for the development of high-efficiency, secure, and portable hydrogen storage materials and hydrogen production technology.11–14Among the various available methods, chemical hydrogen storage presents notable advantages in terms of safety, convenience, and efficiency, thus rendering it the most promising option for extensive practical implementation on a large-scale.15,16 In recent years, some promising hydrogen storage materials, for instance sodium borohydride (NaBH4)17–21 and ammonia borane (NH3BH3),22–26 have garnered significant interest for potential applications in fuel cell vehicles owing to their high hydrogen content and good chemical stability (Fig. 1a). Sodium borohydride and ammonia borane are capable of generating hydrogen via pyrolysis.27,28 Nevertheless, the elevated pyrolysis temperatures required for hydrogen production and the incomplete release of hydrogen significantly constrain their practical utility in fuel cells. An alternative method for the release of hydrogen from sodium borohydride and ammonia borane involves solvolysis in specific protic solvents, such as water (hydrolysis) and methanol (methanolysis). Given that solvolysis can occur at ambient temperature, this process has been considered as the most probable method for hydrogen production from sodium borohydride and ammonia borane. In order to facilitate the liberation of H2 from these storage materials, exploiting appropriate catalysts is imperative. Although noble metal catalysts have exhibited conspicuous activities, scarcity and outrageous prices severely restrict their practical applications. Therefore, developing cost-efficient nanocatalysts is crucial for facilitating the rapid release of hydrogen from the solvolysis of sodium borohydride and ammonia borane in the liquid phase. With these points in mind, many recent works have focused on non-noble metal nanocatalysts, for instance Fe,29–31 Co,32,33 Ni34,35 and Cu.36,37 It is noteworthy that Co-based nanocatalysts with distinctive structures demonstrate superior catalytic properties and stability to those of other metals.38–43 To date, a wide range of Co-based nanocatalysts, including monometallic Co, Co-based alloy, and Co X-ides (X = phosph, sulf, nitr, and carb) have been thoroughly studied as economical and efficient catalysts for hydrogen evolution from sodium borohydride and ammonia borane (Fig. 1b). However, to the best of our knowledge, no special reviews have been published on Co-based catalysts for hydrogen production from these hydrogen storage materials.
This review aims to offer a comprehensive overview of recent advancements of Co-based nanocatalysts for catalytic hydrogen generation from sodium borohydride and ammonia borane. Firstly, different Co-based nanocatalysts, for instance monometallic Co, Co alloys, as well as Co X-ides, are systematically discussed with a focus on their application in catalytic hydrogen generation from the solvolysis of sodium borohydride and ammonia borane. Meanwhile, the interrelationships between synthesis method, material structure, composition, and catalytic activity are thoroughly analyzed. Then, the dehydrogenation mechanisms of these hydrogen storage materials over Co-based nanocatalysts are critically summarized. Finally, the challenges and potential opportunities of Co-based nanocatalysts are discussed for designing efficient Co-based catalysts for hydrogen generation from sodium borohydride and ammonia borane.
2. Performance evaluation methods
2.1 Hydrogen generation rate (HGR)
The hydrogen production rate (HGR) was firstly utilized as a metric for assessing the hydrolytic or methanolytic hydrogen production of sodium borohydride.44,45 Presently, the HGR value has emerged as the predominant indicator for evaluating the hydrogen production efficacies of catalysts. The HGR value can be calculated based on the linear range of hydrogen evolution curves. The calculation formula is as follows:where V(H2) represents the volume number of hydrogen, m denotes the total weight of the catalyst or metal, and t signifies the hydrogen production time. The unit of HGR can be denoted as mL min−1 gCatalyst−1 or mL min−1 gmetal−1. Generally, a higher HGR value indicates increased catalytic performance. It is important to emphasize that researchers are advised to maintain consistency in the quantity of NaBH4 and experimental temperature when comparing the catalytic activity.
2.2. Turnover frequency
Turnover frequency (TOF) is an essential measure for evaluating the efficiency of hydrogen production from hydrogen storage materials.46–49 The determination of the TOF value is contingent upon the linear segment of the hydrogen production curve, with the calculation formula outlined as follows:where n(H2) signifies the molar quantity of liberated hydrogen, n(metal) denotes the molar quantity of the metal employed, and t represents the duration of the reaction process. The unit of TOF is usually expressed in molH2 molmetal−1 min−1 or molH2 molmetal−1 h−1, which can be abbreviated as min−1 or h−1. The properties of heterogeneous nanocatalysts are primarily attributed to the surface atoms of metal NPs. Quantifying the exact number of metal surface atoms is challenging due to the variability in particle size of metal NPs. Therefore, it is imperative to further investigate a more comprehensive method for evaluating the metal in order to accurately determine TOF values for various catalysts and compare their catalytic activities.
3. Performance of Co-based nanocatalysts
Increasing attention on hydrogen energy has prompted an escalation in research on the dehydrogenation of sodium borohydride and ammonia borane since 2006. In the last 20 years (2005–2024), a total of 7519 articles have been published on catalytic hydrogen generation from these hydrogen storage materials with an upward trend year by year, highlighting the research hotspots in this field (Fig. 2a). Co-based nanocatalysts, as representatives of non-noble metals, have been the subject of extensive research due to their abundant reserves, low cost, and good catalytic properties for hydrogen generation from the solvolysis of sodium borohydride and ammonia borane. As illustrated in Fig. 2b, over the past five years, the number of scholarly articles addressing hydrogen production from sodium borohydride and ammonia borane over Co has markedly exceeded that of articles focused on Ni, Cu, and Fe, thereby underscoring the superiority of Co in facilitating hydrogen evolution from these hydrogen storage materials.3.1. Sodium borohydride
Sodium borohydride (NaBH4, SB) has garnered predominant interest as an exemplary hydrogen storage material, owing to its high hydrogen storage ability (10.8 wt%), exceptional stability, and non-toxic property.8,25,50 Sodium borohydride can release hydrogen through hydrolysis (eqn (1)) or methanolysis (eqn (2)).| NaBH4 + 2H2O → NaBO2 + 4H2 | (1) |
| NaBH4 + 4CH3OH → NaB(CH3O)4 + 4H2 | (2) |
The hydrolysis and methanolysis of NaBH4 each present distinct advantages for hydrogen production. The hydrolysis of NaBH4 is widely recognized as a sustainable and cost-effective approach for hydrogen production due to the high hydrogen purity, controlled hydrogen production rate, and the exclusion of organic solvents.51,52 On the other hand, the methanolysis of NaBH4 exhibits unique characteristics, including enhanced reaction kinetics, reduced activation energy, and hydrogen production at temperatures below freezing.53,54 NaBH4 is capable of spontaneously liberating hydrogen via methanolysis at ambient temperature. Consequently, alkalis, for instance LiOH, NaOH, and KOH, are commonly utilized to impede the self-methanolysis of NaBH4.55 The development of suitable nanocatalysts is imperative for augmenting the efficiency of hydrogen production via the hydrolysis and methanolysis of NaBH4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023 mL gCo−1 min−1 based on the total number of atoms within Co NPs at 303 K (Fig. 3b). Mahmood et al. fabricated a two-dimensional nitrogenated network polymer, which was used to encapsulate Co-oxide (Co@C2N) through an in situ solvothermal method (Fig. 3c and d).67 Highly crystalline Co oxide NPs exhibited a remarkable dehydrogenation property for NaBH4 hydrolysis, achieving a maximum HGR value of 8903 mL min−1 gCatalyst−1 at 303 K. Furthermore, Co@C2N can reduce nitro compounds to aniline in situ with NaBH4 as the reducing agent. The improved catalytic property and durability of Co@C2N was attributed to the robust interactions between Co oxide NPs and the nitrogen-enriched C2N framework. Sun et al. encapsulated Co NPs in a nitrogen-doped mesoporous graphitic carbon (NMGC) to fabricate core–shell structured Co@NMGC by carbonizing ethylenediaminetetraacetic acid (Fig. 3e).68 Among the exploratory catalysts, Co@NMGC-500 demonstrated the optimum catalytic property (3575 mL min−1 gCatalyst−1) for NaBH4 hydrolysis at 298 K. Based on theoretical calculations, the prominent dehydrogenation property of Co@NMGC was ascribed to the redistribution of the electron potential within Co@NMGC, which enhanced the adsorption and dissociation of H atoms on Co NPs.
023 mL gCo−1 min−1 based on the total number of atoms within Co NPs at 303 K (Fig. 3b). Mahmood et al. fabricated a two-dimensional nitrogenated network polymer, which was used to encapsulate Co-oxide (Co@C2N) through an in situ solvothermal method (Fig. 3c and d).67 Highly crystalline Co oxide NPs exhibited a remarkable dehydrogenation property for NaBH4 hydrolysis, achieving a maximum HGR value of 8903 mL min−1 gCatalyst−1 at 303 K. Furthermore, Co@C2N can reduce nitro compounds to aniline in situ with NaBH4 as the reducing agent. The improved catalytic property and durability of Co@C2N was attributed to the robust interactions between Co oxide NPs and the nitrogen-enriched C2N framework. Sun et al. encapsulated Co NPs in a nitrogen-doped mesoporous graphitic carbon (NMGC) to fabricate core–shell structured Co@NMGC by carbonizing ethylenediaminetetraacetic acid (Fig. 3e).68 Among the exploratory catalysts, Co@NMGC-500 demonstrated the optimum catalytic property (3575 mL min−1 gCatalyst−1) for NaBH4 hydrolysis at 298 K. Based on theoretical calculations, the prominent dehydrogenation property of Co@NMGC was ascribed to the redistribution of the electron potential within Co@NMGC, which enhanced the adsorption and dissociation of H atoms on Co NPs.
 | ||
| Fig. 3 (a) Synthetic route to MNPs@ZIF-8. (b) The corresponding hydrogen production curve from NaBH4 hydrolysis over MNPs@ZIF-8. Reproduced with permission.66 Copyright 2019, Wiley-VCH. (c and d) Morphology analysis of Co@C2N nanocomposite. Reproduced with permission.67 Copyright 2015, American Chemical Society. (e) Schematic illustration of Co@NMGC nanocomposite. Reproduced with permission.68 Copyright 2020, Elsevier. (f) Schematic illustration of Co–CeOx/NCNS. Reproduced with permission.69 Copyright 2021, Royal Society of Chemistry. | ||
In 2021, our group synthesized nitrogen-doped carbon nanosheets (NCNS) via carbonizing premixed ZIF-8 and potassium citrate.69 Then Co–CeOx nanocomposite was deposited on NCNS to fabricate the efficient and economical catalyst Co–CeOx/NCNS for NaBH4 hydrolysis (Fig. 3f). The optimized catalyst demonstrated an ultrahigh HGR value of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 mL min−1 gCo−1 for NaBH4 hydrolysis. The remarkable hydrogen evolution performance of the catalyst derived from the diminutive dimensions of the Co–CeOx nanocomposite, electron-rich Co NPs, and the robust synergistic interplay between Co–CeOx and NCNS.
410 mL min−1 gCo−1 for NaBH4 hydrolysis. The remarkable hydrogen evolution performance of the catalyst derived from the diminutive dimensions of the Co–CeOx nanocomposite, electron-rich Co NPs, and the robust synergistic interplay between Co–CeOx and NCNS.
Monometallic Co catalysts have also been used to produce hydrogen via methanolysis of sodium borohydride. Caglar et al. synthesized activated carbon supported Co for the methanolysis of NaBH4.70 Optimal Co/AC with a metal loading of 1 wt% exhibited a HGR value of 7120.7 mL min−1 gCatalyst−1 at 303 K. Demirci et al. fabricated TiO2 immobilized Co nanocatalysts, which were employed for hydrogen evolution from the methanolysis of NaBH4.71 1 wt% Co/TiO2 achieved the highest catalytic performance for the methanolysis of NaBH4 (664![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL min−1 gCo−1) at 298 K. Wang's group prepared a composite consisting of ZIF-67 and graphene oxide (GO) and then a Co-based catalyst was obtained for the methanolysis of NaBH4 after the reduction of Co2+ in ZIF-67.72 ZIF-67@GO-2 with 6.0 wt% GO addition achieved the maximum HGR of 3200 mL min−1 gCatalyst−1 for NaBH4 methanolysis at 273 K.
000 mL min−1 gCo−1) at 298 K. Wang's group prepared a composite consisting of ZIF-67 and graphene oxide (GO) and then a Co-based catalyst was obtained for the methanolysis of NaBH4 after the reduction of Co2+ in ZIF-67.72 ZIF-67@GO-2 with 6.0 wt% GO addition achieved the maximum HGR of 3200 mL min−1 gCatalyst−1 for NaBH4 methanolysis at 273 K.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 mL min−1 gRu−1, highlighting the significantly promoted effect of Co. Zabihi et al. prepared a magnetic activated carbon using wood as the precursor (MWAC). Then they deposited bimetallic Co–Ni on MWAC to fabricate alloy nanocatalysts via a co-deposition–precipitation method.76 They found that the catalysts exhibited varying catalytic activities for NaBH4 hydrolysis, with the order of Co/Ni/MWAC > Ni/Co/MWAC > Co–Ni/MWAC (prepared with various permutations of addition). Distinctly, Co/Ni/MWAC demonstrated the highest HGR value of 740.7 mL min−1 gCatalyst−1 for NaBH4 hydrolysis. Han and coworkers synthesized Co–Mo alloy NPs on three dimensional graphene oxide (3DGO) (Fig. 4a).77 The resulting Co–Mo/3DGO catalyst with a Co
700 mL min−1 gRu−1, highlighting the significantly promoted effect of Co. Zabihi et al. prepared a magnetic activated carbon using wood as the precursor (MWAC). Then they deposited bimetallic Co–Ni on MWAC to fabricate alloy nanocatalysts via a co-deposition–precipitation method.76 They found that the catalysts exhibited varying catalytic activities for NaBH4 hydrolysis, with the order of Co/Ni/MWAC > Ni/Co/MWAC > Co–Ni/MWAC (prepared with various permutations of addition). Distinctly, Co/Ni/MWAC demonstrated the highest HGR value of 740.7 mL min−1 gCatalyst−1 for NaBH4 hydrolysis. Han and coworkers synthesized Co–Mo alloy NPs on three dimensional graphene oxide (3DGO) (Fig. 4a).77 The resulting Co–Mo/3DGO catalyst with a Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo molar ratio of 0.7
Mo molar ratio of 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 demonstrated optimal catalytic performance (7023.3 mL min−1 gCo−1) for NaBH4 hydrolysis. Guo's group impregnated Co and Zr precursors onto porous sheet-like activated carbon to obtain bimetal Zr–Co alloy NP catalysts by an impregnation–chemical reduction method.78 They confirmed that the incorporation of Zr into Co resulted in heightened interactions between Co and the substrate. As a result, the Zr/Co/C catalyst demonstrated an enhanced dehydrogenation performance compared to that of Co/C for NaBH4 hydrolysis.
0.3 demonstrated optimal catalytic performance (7023.3 mL min−1 gCo−1) for NaBH4 hydrolysis. Guo's group impregnated Co and Zr precursors onto porous sheet-like activated carbon to obtain bimetal Zr–Co alloy NP catalysts by an impregnation–chemical reduction method.78 They confirmed that the incorporation of Zr into Co resulted in heightened interactions between Co and the substrate. As a result, the Zr/Co/C catalyst demonstrated an enhanced dehydrogenation performance compared to that of Co/C for NaBH4 hydrolysis.
 | ||
| Fig. 4 (a) Synthesis of the nanocatalyst Co–Mo/3DGO. Reproduced with permission.77 Copyright 2019, Elsevier. (b and c) Morphology characterization of hollow Co–B nanospheres. Reproduced with permission.84 Copyright 2008, Elsevier. (d) Synthetic route to Co–Ce–B/Chi-C. Reproduced with permission.60 Copyright 2018, Elsevier. (e) Preparation of Fe–CoP/Ti and schematic illustration of hydrogen generation from NaBH4 and H2O. (f–j) Morphology features of the Fe–CoP/Ti nanoarray. Reproduced with permission.86 Copyright 2016, Wiley-VCH. | ||
For the methanolysis of NaBH4, Figen et al. synthesized Ru–Co alloy NPs via a sol–gel method with citric acid as the gelling agent.79 The resulting Ru–Co NPs provided a HGR of 1260 mL min−1 gcatalyst−1 for the methanolysis of NaBH4 at 293 K. Wang's group fabricated a carbon nanotube-immobilized Ru–Co alloy nanocatalyst via microwave-assisted heating electroless plating.80 The resulting Ru–Co/CNT catalyst demonstrated exceptional catalytic activity for NaBH4 methanolysis (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 mL min−1 gcatalyst−1).
190 mL min−1 gcatalyst−1).
Saka et al. employed the ZnCl2-treated spirulina microalgal strain (SSMS-ZnCl2) as the support to immobilize Co–B NPs.87 The optimized SSMS-ZnCl2–CoB gave maximal HGR values of 9266 and 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 mL min−1 gcatalyst−1 at 303 K and 333 K for NaBH4 methanolysis. Zhu et al. deposited Co–P NPs on the dandelion-like CNT–Ni foam via a chemical vapor deposition (CVD) method.88 At pH = 11 and NaH2PO2·H2O concentration of 1.2 M in the plating solution, Co–P/CNT–Ni exhibited the highest catalytic performance (2430 mL min−1 gcatalyst−1) for the methanolysis of NaBH4. The hydrogen evolution performances of NaBH4 over different Co-based nanocatalysts are shown in Table 1.
366 mL min−1 gcatalyst−1 at 303 K and 333 K for NaBH4 methanolysis. Zhu et al. deposited Co–P NPs on the dandelion-like CNT–Ni foam via a chemical vapor deposition (CVD) method.88 At pH = 11 and NaH2PO2·H2O concentration of 1.2 M in the plating solution, Co–P/CNT–Ni exhibited the highest catalytic performance (2430 mL min−1 gcatalyst−1) for the methanolysis of NaBH4. The hydrogen evolution performances of NaBH4 over different Co-based nanocatalysts are shown in Table 1.
| Catalysts | Active sites | Temp. (K) | Solvent | NaOH (wt%) | HGR (mL min−1 g−1) | Cycles, times and retained activity (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a Based on Co metal weight. b Based on the catalyst weight. c Based on Ru metal weight. | |||||||
| Co | Co | 295 | H2O | 8 | — | — | 89 |
| Co/γ-Al2O3 | Co | 303 | H2O | 5 | 220b | — | 90 |
| Co film/Cu foil | Co | 298 | H2O | 0.8 | 1270a | 10, 90 | 43 |
| p(HEMA)–Co | Co | 303 | H2O | 5 | 1596a | 5, 57.7 | 91 |
| p(SPM)–Co | Co | 303 | H2O | 2.5 | 1288a | — | 92 |
| Co NPs@ZIF-8 | Co | 303 | H2O | 1.6 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023a 023a |
— | 66 |
| Co/PGO | Co | 298 | H2O | 1 | 5955a | 5, 73 | 93 |
| Co@C2N | Co | 303 | H2O | 5 | 8903b | 5, 56 | 67 |
| Co@C | Co | 303 | H2O | 6 | 1680b | 5, 93 | 94 |
| Co@NMGC | Co | 298 | H2O | 1 | 3575b | 20, 82.5 | 68 |
| Co–CeOx/NCNS | Co | 303 | H2O | 1.6 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410a 410a |
5, 12.5 | 69 |
| Co-BTC@PUS | Co | 303 | H2O | 3 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640b 640b |
6, 82.4 | 95 |
| (Co-Ver)cat | Co | 303 | H2O | 7 | 1991.4a | 10, 91.8 | 96 |
| Co3O4-SCQD | Co | 303 | H2O | 4 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 555b 555b |
5, 30.6 | 97 |
| Co/Al2O3 | Co | 298 | CH3OH | — | 2500b | — | 98 |
| Co/AC | Co | 303 | CH3OH | — | 7120.7b | — | 70 |
| Co/TiO2 | Co | 298 | CH3OH | — | 664![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a 000a |
— | 71 |
| ZIF-67@GO-2 | Co | 273 | CH3OH | — | 3200b | 5, 99 | 72 |
| Ca0.8Co0.2CO3 | Co | 300 | CH3OH | — | 6666b | 5, 93 | 99 |
| Co–Ru/C | CoRu | 303 | H2O | 0.4 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700c 700c |
5, 80 | 74 |
| Co–Fe/CQD | CoFe | 308 | H2O | 4 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220b 220b |
4, 70 | 100 |
| Ni–Co@3DG | CoNi | 298 | H2O | — | 2041.5b | 5, 95.9 | 75 |
| CoNi/Fe3O4@GO | CoNi | 308 | H2O | 4 | 8200b | 5, 44.3 | 101 |
| Co/Ni/MWAC | CoNi | 303 | H2O | 5 | 740.7b | 6, 58.1 | 76 |
| Co–Mo/3DGO | CoMo | 298 | H2O | 1 | 7023.3a | 5, 56.3 | 77 |
| ZnxCo1–Co@NC | ZnCo | 303 | H2O | 7.3 | 1807a | 5, 32.5 | 102 |
| Zr/Co/carbon | ZrCo | 298 | H2O | 2 | 1708b | — | 78 |
| Ru–Co | Ru–Co | 293 | CH3OH | 10 | 1260b | 5, 50.8 | 79 |
| Ru–Co/CNTs | Ru–Co | 298 | CH3OH | 7 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190b 190b |
5, 75 | 80 |
| Co–P | CoP | 303 | H2O | 1 | 1647.9b | 11, 61 | 103 |
| Co–P/Cu | CoP | 303 | H2O | 1 | 954b | — | 104 |
| Co–P/Cu sheet | CoP | 303 | H2O | 10 | 5965b | 5, 40.8 | 105 |
| Co–P/Cu sheet | CoP | 303 | H2O | 1 | 1846b | 10, 66.8 | 106 |
| Co–P/Ni foam | CoP | 298 | H2O | 5 | 1490b | — | 107 |
| Fe–CoP/Ti | CoP | 298 | H2O | 1 | 6060b | 5, 99 | 86 |
| Co–Ni–P/Pd–TiO2 | CoNiP, Pd | 328 | H2O | 10 | 3780b | 5, 86.4 | 108 |
| NiCoP/Ni foam | CoNiP | 298 | H2O | 1 | 3986b | 5, 76 | 109 |
| Ni–Co–P/γ-Al2O3 | CoNiP | 328 | H2O | 4 | 6599.6b | 10, 37.3 | 110 |
| Co–Ni–P/Cu sheet | CoNiP | 303 | H2O | 10 | 3636b | — | 111 |
| Co–W–P/Cu | CoWP | 303 | H2O | 10 | 5000b | 5, 49 | 112 |
| NiCoP/Ti | NiCoP | 303 | H2O | 1 | 8010b | 8, 93 | 113 |
| Co–W–P/CC | CoWP | 303 | H2O | 2 | 4379b | 5, 60 | 114 |
| Cu–Co–P/γ-Al2O3 | CuCoP | 298 | H2O | 5 | 1115b | 6, 66 | 115 |
| CoNiFeP | CoNiFeP | 303 | H2O | 1 | 1128b | 10, 70 | 116 |
| Co–B–10CNTs | CoB | 298 | H2O | 5 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
5, 64 | 117 |
| Co–B/carbon | CoB | 298 | H2O | 8 | 1128b | — | 118 |
| Co–B/HPCM | CoB | 298 | H2O | 5 | 3083.1b | 5, 91.7 | 119 |
| Co–B/Ni foam | CoB | 298 | H2O | 5 | 1640b | — | 120 |
| Co–B/Ni foam | CoB | 303 | H2O | 5 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
— | 121 |
| Co–B/Cu sheet | CoB | 303 | H2O | 5 | 7935b | 5, 80 | 122 |
| Co–B | CoB | 303 | H2O | 5 | 2750b | — | 123 |
| Co–B hollow spheres | CoB | 298 | H2O | 8 | 3200b | — | 84 |
| Mesoporous Co–B | CoB | 298 | H2O | — | 3350b | — | 82 |
| CoB/TiO2 | CoB | 303 | H2O | 3.8 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 503b 503b |
— | 124 |
| Co–B/attapulgite clay | CoB | 298 | H2O | 10 | 3350b | 9, 68.5 | 125 |
| GO-modified Co–B | CoB | 303 | H2O | 5 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340b 340b |
5, 81.5 | 126 |
| CoB/sepiolite clay | CoB | 303 | H2O | 1 | 1486b | 5, 31.6 | 127 |
| Co2B–CoPOx | CoB, CoP | 298 | H2O | 0.4 | 7716.7b | 5, 63.3 | 128 |
| Co–Cu–B | CoCuB | 293 | H2O | 7 | 2120b | — | 129 |
| Co–Cu–B | CoCuB | 298 | H2O | 1 | 3554b | — | 130 |
| Co–Cr–B | CoCrB | 298 | H2O | 0.1 | 3400b | — | 131 |
| Co–Ni–B | CoNiB | 301 | H2O | 15 | 2608b | — | 132 |
| Co–Ce–B | CoCeB | 303 | H2O | 5 | 4760b | 5, 81.4 | 60 |
| Co–Mn–B | CoMnB | 293 | H2O | 7.5 | 1440b | — | 133 |
| Co–W–B/Ni foam | CoWB | 303 | H2O | 5 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
5, 91 | 134 |
| SSMS–ZnCl2–CoB | CoB | 303 | CH3OH | — | 9266b | 5, 28.6 | 87 |
| Co–P/CNT–Ni foam | CoP | 298 | CH3OH | 1 | 2430b | 8, 65 | 88 |
3.2. Ammonia borane
Ammonia borane (NH3BH3, AB) has garnered more and more attention as a prominent hydrogen storage material; this is attributed to the non-toxic property, extended stability, and notable gravimetric hydrogen density (19.6 wt%).135,136 The initial discovery of ammonia borane's capacity to generate hydrogen via thermal decomposition upon melting was documented in 1987.137,138 Nevertheless, the practical application of ammonia borane has been hindered by the need for high dehydrogenation temperatures and the formation of undesired byproducts of pyrolyzation.139 An alternative method for releasing hydrogen is solvolysis, including hydrolysis (eqn (3)) or methanolysis (eqn (4)).140–145| NH3BH3 + 2H2O → NH4BO2 + 3H2 | (3) |
| NH3BH3 + 4CH3OH → NH4B(CH3O)4 + 3H2 | (4) |
Numerous nanocatalysts have been explored for AB solvolysis, with noble metal-based nanocatalysts being recognized as the leading-edge in the field.146–152 However, achieving the practical application of this system necessitates the development of cost-effective nanocatalysts to augment the kinetic performance. Numerous studies have been conducted on AB solvolysis over non-noble metal catalysts, among which Co-based NP catalysts, including monometallic Co NPs, Co-based alloy, and Co X-ides, exhibit higher catalytic activity than other non-noble metals.153–155
 | ||
| Fig. 5 (a) Illustration of the sustainable synthesis of Co NPs and their application in catalytic hydrogen production from AB. Reproduced with permission.157 Copyright 2013, Elsevier. (b) Illustration of the synthesis of KIT-6-confined Co NPs via single reducing agent, double reducing agent, and thermal reduction methods. Reproduced with permission.161 Copyright 2018, Elsevier. (c) Illustration of the synthesis of amorphous Co NP catalysts Co/MIL-101-1-U and Co/MIL-101-2-U via an in situ reduction method, and crystalline Co NP catalysts Co/MIL-101-1 and Co/MIL-101-2 via an ex situ reduction method. Reproduced with permission.164 Copyright 2017, American Chemical Society. (d) Schematic illustration of photoactive Co/MIL-101(Cr)-NH2 and the catalytic hydrolysis of AB under visible light irradiation. Reproduced with permission.165 Copyright 2017, Elsevier. (e) The synthetic route to Co-g-C3N4/rGO. Reproduced with permission.166 Copyright 2016, American Chemical Society. | ||
In recent decades, various supports including porous carbon, silica, metallic oxide, and molecular sieves have been developed for the dispersion of Co-based metal NPs for the dehydrogenation of AB.8,16,158 Xu et al. synthesized diverse non-noble NP catalysts utilizing SiO2, γ-Al2O3, porous carbon, and MOFs as the support.159 Notably, Co NPs immobilized on porous carbon exhibited optimal efficacy for AB hydrolysis. Conversely, porous carbon-supported Ni and Cu displayed low catalytic activities, while porous carbon-immobilized Fe NPs demonstrated negligible catalytic performance. Sullivan et al. synthesized SBA-15-immobilized Co NPs by a deposition impregnation method, and confirmed that the hydrogen evolution property of Co-SBA-15 for AB hydrolysis was influenced by counterions in the metal precursor, and the duration of activation for the reaction depended on the specific counterion.160 The catalyst Co-SBA-15 derived from the reduction of Co(NO3)2 exhibited a prolonged activation period and lower activity energy for AB hydrolysis compared to those derived from the reduction of Co(BF4)2 or Co(CH3COO)2. Kao et al. effectively encapsulated ultra-small Co NPs in the mesopores of cubic silica KIT-6 through a straightforward chemical reduction method utilizing NaBH4 and NH3BH3 as dual reducing agents (Fig. 5b).161 In comparison with the single reducing agent NaBH4, Co@KIT-6 synthesized with double reducing agents exhibited superior activity, as evidenced by a TOF and activation energy of 20.05 min−1 and 32.6 kJ mol−1, respectively.
In recent years, organic porous polymers, for instance metal organic frameworks (MOFs), porous coordination cages (PCCs), porous aromatic frameworks (PAFs), covalent triazine frameworks (CTFs), covalent organic frameworks (COFs), and their derived porous carbons, are widely utilized as the carrier to stabilize metal NPs for the dehydrogenation of AB, owing to the large specific surface areas and abundant anchoring sites.162,163 Gu et al. developed two preparation techniques for synthesizing amorphous or crystalline Co/MIL-101, focusing on the control and enhancement of catalyst performance (Fig. 5c).164 Among them, Co/MIL-101 fabricated through an ultrasound-assisted in situ reduction strategy exhibited superior properties for AB hydrolysis, providing a TOF of 51.4 min−1. The excellent hydrogen evolution property derived from the ultrafine and amorphous Co NPs. In the same year, the group synthesized a photoactive MOF material though the NH2-functionalization of MIL-101, which was used to anchor Co NPs (Fig. 5d).165 The resulting Co/MIL-101(Cr)-NH2 catalyst demonstrated a conspicuous dehydrogenation property (117.7 min−1) for AB hydrolysis under visible light irradiation. Li et al. fabricated graphitic carbon nitride (g-C3N4)-encapsulated Co NPs (Co@g-C3N4), and subsequently deposited this composite onto reduced graphene oxide (rGO) (Fig. 5e).166 The g-C3N4 shells and rGO effectively inhibited the aggregation and leaching of Co NP cores. The optimized Co@g-C3N4–rGO catalyst demonstrated exceptional catalytic activity (15.4 min−1) for AB hydrolysis.
He's group synthesized covalent triazine framework (CTF-1) immobilized Co NPs through an impregnation method and the optimal Co/CTF catalyst exhibited a notable dehydrogenation property (42.3 min−1) for AB hydrolysis.167 Zhou et al. fabricated porous coordination cage (PCC) encapsulated Co nanoclusters (NCs) via the Co2+ ion capture method (Fig. 6a).168 The higher net charges of PCC-2a facilitated the dispersion of Co2+ cations, leading to the in situ formation of ultra-small Co NCs. In contrast, larger Co NPs were observed in PCC-2b with low net charges. Consequently, Co NCs supported on PCC-2a exhibited a superior hydrogen evolution property for AB hydrolysis to those supported on PCC-2b. Yang's group fabricated hierarchically porous carbon-confined Co NPs (Co/HPC) using Co/Zn-MOF-74 as the precursor via selective atom evaporation–isolation methods (Fig. 6b).169 The inclusion of Zn atoms within Co/Zn-MOF-74 was found to be a significant factor in the generation of superfine Co NPs. The optimal Co/HPC-900 catalyst demonstrated a favorable hydrogen evolution property for AB hydrolysis with a TOF of 2.9 min−1. Tao's group synthesized nitrogen-doped nanowires (NPCNW) through high-temperature calcination of the Co-MOF precursor. Ultrafine Co NPs immobilized on NPCNW (Co/NPCNW) demonstrated enhanced hydrogen evolution efficiency (12.2 min−1) for AB hydrolysis, and maintained 94.6% of their initial property after ten cycles.65
 | ||
| Fig. 6 (a) Synthetic route to Co nanoclusters encapsulated by PCCs. Reproduced with permission.168 Copyright 2017, Wiley-VCH. (b) Schematic illustration of the fabrication of Co/HPC via the pyrolysis of Co-MOF. Reproduced with permission.169 Copyright 2019, American Chemical Society. (c) The synthesis of multilayer benzidine-functionalized GO (M-BZ-GO), parallel nanocomposite (P-PAF-GO), and vertical nanocomposite (V-PAF-GO). Reproduced with permission.170 Copyright 2023, Wiley-VCH. | ||
Monometallic Co nanocatalysts also demonstrated significant catalytic activities in the methanolysis of AB. Our group developed a controllable step-growth strategy for fabricating vertical porous aromatic frameworks on graphene oxide (V-PAF-GO) (Fig. 6c). Subsequently, ultrafine Co NPs were encapsulated in the slit pores of the vertical nanocomposite.170 The resulting Co/V-PAF-GO catalyst demonstrated an exceptional hydrogen evolution property (47.6 min−1) for AB methanolysis. Experimental and theoretical studies demonstrated that electron transfer between Co and PAF contributed to the low activation energy of methanol decomposition, resulting in the preeminent catalytic performance. Oh's research team encapsulated ultrafine Co NPs in hierarchical Ni metal–organic framework (Ni-MOF) nanocolumn arrays (NCAs), which were subsequently utilized as catalysts for AB methanolysis.171 Attributed to the synergy between Co and Ni-MOF NCAs, Co@Ni-MOF NCA/NF displayed an outstanding catalytic activity for AB methanolysis with a TOF of 20.5 min−1.
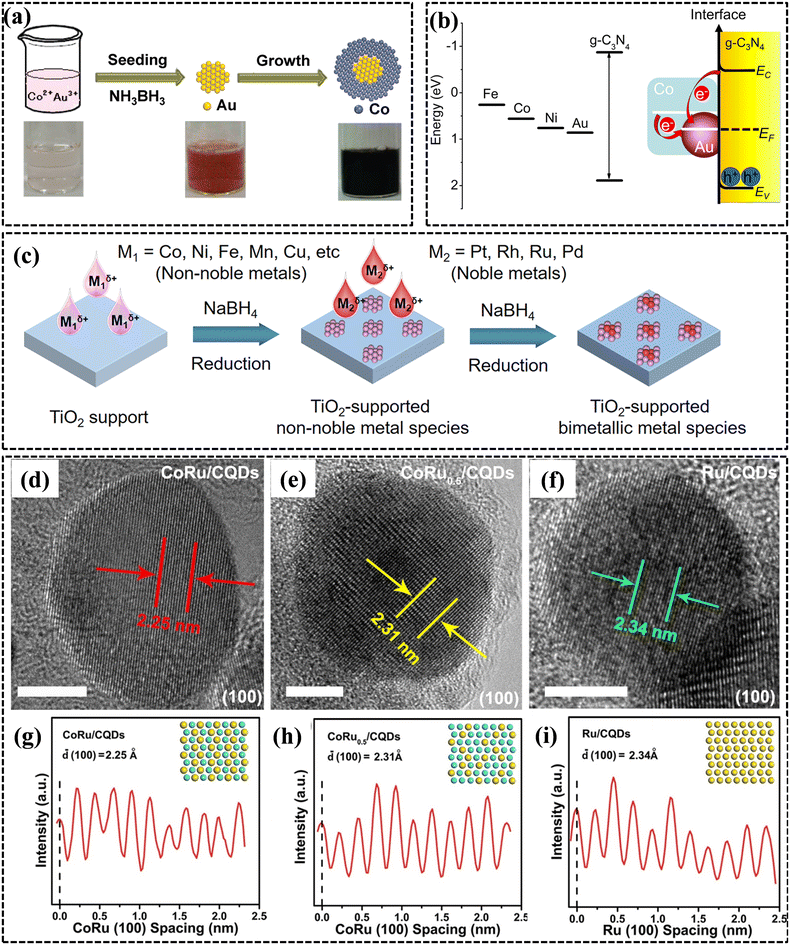 | ||
| Fig. 7 (a) Schematic representation and color transformation during the fabrication of the Au@Co catalyst. Reproduced with permission.172 Copyright 2009, American Chemical Society. (b) Work functions and band structures of g-C3N4 and representative metals, and schematic illustration of the Mott–Schottky type contact of Au–Co alloy NPs. Reproduced with permission.173 Copyright 2014, American Chemical Society. (c) Fabrication of TiO2-supported bimetallic nanocatalysts. Reproduced with permission.174 Copyright 2023, American Chemical Society. (d–f) Morphology characterization of carbon quantum dot-supported Ru and CoRu alloy NPs. (g–i) Integrated pixel intensities for the lattices of carbon quantum dot-supported Ru and CoRu alloy NPs. Blue spheres and yellow spheres represent Co and Ru, respectively. Reproduced with permission.176 Copyright 2021, Wiley-VCH. | ||
Xu's group successfully synthesized MIL-101-confined CuCo alloy NPs though a double-solvent strategy.177 The resulting CuCo@MIL-101 catalyst displayed a superior catalytic property to that of monometallic Cu@MIL-101 and Co@MIL-101, because of the synergistic interactions between Cu and Co. In a follow-up study, the researchers found that another efficient means of averting particle agglomeration was to dope with inactive transition metals, for instance Mo and W. These metals exhibited high effectiveness in both metallic and oxidized states, with even low atomic concentrations leading to a substantial enhancement in catalytic surface area by preventing the agglomeration of metal NPs. Our group successfully synthesized noble metal-free CuCoMo alloy NPs using a straightforward co-reduction method, without the need for additional surfactants or carriers (Fig. 8a).178 The optimal trimetallic CuCoMo NPs displayed notably improved catalytic performance compared to their bi-/monometallic counterparts. Furthermore, subsequent research demonstrated that the addition of NaOH prominently promoted the dehydrogenation rate of AB hydrolysis over CuCoMo NPs, achieving a nearly threefold increase in TOF compared to dehydrogenation without NaOH. Zhao et al. synthesized porous nitrogen-doped carbon immobilized NiCo alloy NPs using bimetallic ZnCo zeolitic imidazole frameworks as the precursor (Fig. 8b).179 The enhanced catalytic property of the NiCo-NC nanocatalyst was attributed to its high nitrogen content and strong synergistic effect between the alloy components, which was evidenced by the elevated TOF value of 35.2 min−1 achieved under alkaline conditions. Ma's group synthesized nearly atomically dispersed CoNi alloy on α-MoC and ascertained the interactions between Co and Ni utilizing real-space chemical mapping techniques at the atomic scale (Fig. 8c and d).180 The Fourier transformed k2-weighted extended X-ray absorption fine structure (EXAFS) indicates that Co and Ni predominantly associate with C within the α-MoC matrix, as opposed to Mo. Furthermore, these spectra corroborate that Co and Ni are highly dispersed within the α-MoC structure without aggregating to form nanoparticles. 1.5Co1.5Ni/α-MoC demonstrated an optimal hydrogen evolution performance (321 min−1) for AB hydrolysis than other Co/Ni ratios (Fig. 8e and f). Mechanistic investigations demonstrated that the enhanced catalytic performance derived from the collective abilities of α-MoC to facilitate water splitting and the synergistic interaction of the bimetallic species.
 | ||
Fig. 8 (a) The enhanced catalytic property of AB hydrolysis with the assistance of a base over the CuCoMo catalyst. Reproduced with permission.178 Copyright 2018, Royal Society of Chemistry. (b) Illustration of the synthesis of NiCo-NC. Reproduced with permission.179 Copyright 2021, Elsevier. (c) Topography analysis of CoNi/α-MoC. (d) X-ray absorption near-edge characterization of CoNi/α-MoC. (e and f) Dehydrogenation curve and the corresponding TOF values for AB hydrolysis over CoNi/α-MoC with diverse molar ratios of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni. Reproduced with permission.180 Copyright 2021, American Chemical Society. Ni. Reproduced with permission.180 Copyright 2021, American Chemical Society. | ||
Li et al. synthesized magnetically recyclable PVP-stabilized Co–Ni alloy nanocatalyst, and Co0.7Ni0.3 exhibited the highest hydrogen generation activity for AB methanolysis.181 Moreover, the catalyst retained 91.2% of its initial catalytic activity after eight cycles, demonstrating excellent stability. Sun's group fabricated monodisperse CoPd alloy NPs via the co-reduction of Co and Pd precursors in oleylamine and trioctylphosphine.182 Among all the tested catalysts, Co48Pd52 supported on C exhibited the highest catalytic activity for catalyzing AB methanolysis, providing a TOF of 27.7 min−1. Rakap et al. synthesized a series of hydroxyapatite-immobilized Rh–M (RhCo, RhCu, RhFe) nanoclusters.183 Rh51Co49@HA displayed the highest catalytic performance for AB methanolysis among all the catalysts examined.
 | ||
| Fig. 9 (a) Illustration of the synthesis of the rGO/CoNi–N nanocomposite. Reproduced with permission.185 Copyright 2021, Elsevier. (b) Preparation of the CoNiP/GO nanocatalyst. Reproduced with permission.186 Copyright 2021, Elsevier. (c) Preparation of the atomic-bridge structured B–Co–P complex. Reproduced with permission.187 Copyright 2022, Elsevier. | ||
Jagirdar et al. synthesized Co–Co2B, Ni–Ni3B and Co–Ni–B nanocomposites via the reduction of metal salts with ammonia borane.189 The Co–Ni–B nanocomposite exhibited enhanced activity (10 min−1) relative to the individual metal–metal boride nanocomposites. Kalu's group prepared Co–Ni–P composites using Pd-activated Al2O3 as the support.190 Co–Ni–P/Pd-Al2O3 (Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 86.4
Ni = 86.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.6) emerged as a promising candidate for highly efficient hydrogen generation from AB methanolysis due to the high activity, stability, and reusability. The primary Co-based nanocatalysts for hydrogen evolution from AB and their corresponding efficiencies are presented in Table 2.
13.6) emerged as a promising candidate for highly efficient hydrogen generation from AB methanolysis due to the high activity, stability, and reusability. The primary Co-based nanocatalysts for hydrogen evolution from AB and their corresponding efficiencies are presented in Table 2.
| Catalyst | Active sites | Temp. (K) | Solvent | TOF (min−1) | Cycles, times and retained activity (%) | Ref. |
|---|---|---|---|---|---|---|
| a Based on the moles of metal Co. b Based on the moles of alloy. c Based on the moles of all metals. | ||||||
| Co/γ-Al2O3 | Co | 298 | H2O | 2.3a | — | 159 |
| In situ Co NPs | Co | 298 | H2O | 44.1a | 10, 100 | 191 |
| PSMA-Co | Co | 298 | H2O | 25.7a | — | 156 |
| Co/PEI-GO | Co | 298 | H2O | 39.9a | 5, 65.6 | 192 |
| Co/MIL-101-1-U | Co | 298 | H2O | 51.4a | 5, 49.6 | 164 |
| Co/NPCNW | Co | 298 | H2O | 12.2a | 10, 94.6 | 193 |
| Co@N–C-700 | Co | 298 | H2O | 5.6a | 10, 97.2 | 194 |
| Co/HPC | Co | 323 | H2O | 2.9a | 12, 90 | 169 |
| Co/CTF | Co | 298 | H2O | 42.3a | 5, 54.2 | 167 |
| Co NCs@PCC-2a | Co | 298 | H2O | 90.1a | — | 168 |
| Co@C–N@SiO2-800 | Co | 298 | H2O | 8.4a | 5, 99 | 154 |
| G6-OH(Co60) | Co | 298 | H2O | 10.0a | 4, 46.2 | 195 |
| Co–(CeOx)0.91/NGH | Co | 298 | H2O | 79.5a | 5, 87 | 196 |
| CoCl2 | Co | 298 | CH3OH | 3.7a | — | 197 |
| Co/PAF-GO-V | Co | 298 | CH3OH | 47.6a | 5, 53.6 | 170 |
| Co@Ni-MOF NCA/NF | Co | 298 | CH3OH | 20.5a | 5, 60.3 | 171 |
| HCSS-Co3O4@CuO–NiO | Co | 298 | CH3OH | 67.1a | 8, 83 | 198 |
| RuCo/γ-Al2O3 | RuCo | 338 | H2O | 32.9b | 4, 21.2 | 199 |
| AuCo@MIL-101 | AuCo | 298 | H2O | 23.5b | 5, 71.4 | 200 |
| AgCo/PAMAM | AgCo | 298 | H2O | 15.8b | 5, 60 | 201 |
| Co35Pd65/C | CoPd | 298 | H2O | 22.7b | 5, 75 | 202 |
| Pd@Co@MIL-101 | Pd, Co | 303 | H2O | 51.0b | 5, 72.3 | 203 |
| Fe0.3Co0.7 alloy | FeCo | 293 | H2O | 13.9b | — | 204 |
| CuCo/graphene | CuCo | 293 | H2O | 9.18b | 3, 93 | 205 |
| Cu0.2Co0.8/PDA–rGO | CuCo | 303 | H2O | 51.5b | 5, 31.6 | 206 |
| Cu0.5Co0.5@SiO2 | CuCo | 298 | H2O | 4.3b | 10, 93 | 207 |
| Cu0.3Co0.7@MIL-101 | CuCo | 298 | H2O | 19.6b | 5, 81.8 | 177 |
| Cu0.72Co0.18Mo0.1 | CuCo | 298 | H2O | 119.0b | — | 178 |
| Cu0.72Co0.18Mo0.1 | CuCo | 298 | H2O | 46.0b | 3, 45.5 | 178 |
| CuCo@MIL-101-1-U | CuCo | 298 | H2O | 51.7b | 5, 46.2 | 164 |
| Cu0.8Co0.2O–GO | CuCo | 298 | H2O | 70.0b | 6, 94.7 | 208 |
| PVP-stabilized Co0.7Ni0.3 | CoNi | 298 | CH3OH | 35.3b | 8, 91.2 | 181 |
| Co48Pd52/C | CoPd | 298 | CH3OH | 27.7b | 8, 98 | 182 |
| Rh51Co49@HA | RhCo | 298 | CH3OH | 193.7 | 5, 90 | 183 |
| CoP@CNF | CoP | 298 | H2O | 165.0a | 4, 90 | 188 |
| Br1-CoP@C | CoP | 298 | H2O | 67.3a | 5, 35 | 209 |
| Co2P-CDs/mHNTs | CoP | 298 | H2O | 178.0a | 5, 73 | 210 |
| Fe–CoP@C | Fe–CoP | 298 | H2O | 183.5a | 5, 68 | 211 |
| B–Co–P | B–Co–P | 303 | H2O | 37.0a | 5, 30 | 187 |
| CoP–CoO/NCDS | CoP, CoO | 298 | H2O | 89.6a | 5, 32.6 | 212 |
| Ni0.23Co0.19P0.58@NHPC900 | NiCoP | 298 | H2O | 125.2c | 5, 75 | 213 |
| rGO/CoNi–N | CoNiN | 298 | H2O | 126.0c | 10, 40 | 185 |
| Co–Co2B | Co, CoB | 298 | CH3OH | 7.5a | 10, 99 | 189 |
| Co–Ni–B | CoNiB | 298 | CH3OH | 10.0c | 10, 99 | 189 |
| Co–Ni–P | CoNiP | 303 | CH3OH | 4.3c | 5, 65 | 190 |
3.3. Other borohydrides and dimethylamine borane
Other borohydrides such as potassium borohydride, lithium borohydride, and the borane adduct dimethylamine borane are also employed for hydrogen production. However, due to their scarcity, there are few reports on their hydrogen production over Co-based catalysts. Büyükkanber's group synthesized carbon sphere-immobilized Co for the hydrolysis of KBH4. The optimum catalyst, 10%Co/90%CS, gave a HGR of 7149.2 mL min−1 gcat−1 for the hydrolysis of 1 wt% KBH4 with 7 wt% KOH at 303 K.214 The lack of micropores improved the performance by mitigating mass transfer limitations, particularly those associated with internal diffusion. Saka et al. fabricated caffeine carbon dot-immobilized Co catalysts in ethanol for the hydrolysis of KBH4. The resulting Co@MOF-CQD (ethanol) catalyst displayed an ultrahigh HGR of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 mL min−1 gcat−1.215 Oxygen atoms present on the catalyst surface promoted the generation of active sites on the cobalt substrate through the formation of hydrogen bonds with water molecules, consequently augmenting the catalytic activity.
081 mL min−1 gcat−1.215 Oxygen atoms present on the catalyst surface promoted the generation of active sites on the cobalt substrate through the formation of hydrogen bonds with water molecules, consequently augmenting the catalytic activity.
Liu et al. synthesized a series of MoS2 nanosheet-supported non-noble metal NPs, and found Co/MoS2 was the most efficient catalyst for the hydrolysis of dimethylamine borane (DMAB), providing a TOF of 6.3 min−1. Sen's group prepared RuCo alloy nanomaterials using functionalized multiwalled carbon nanotubes (MWCNTs) as the support via an ultrasonic double reduction strategy.216 The RuCo@G-MWCNT catalyst obtained exhibited a superhigh hydrogen generation performance for the DMAB dehydrogenation reaction, accompanied by great reusability. Rakap synthesized hexadecyltrimethyl ammonium bromide-immobilized RhCo alloy nanoclusters; the optimal Rh0.63Co0.37@CTAB catalyst exhibited high efficiency and stability for the hydrolysis of DMAB, providing a TOF of 142.9 min−1.217
4. Investigation of the catalytic mechanism
Comprehending the catalytic mechanism holds paramount significance in the design of the catalyst and for the improvement of the catalytic properties.14,26,218 The study of catalytic mechanisms involves the breaking and formation of bonds, the adsorption of substrates, the dissociation of product hydrogen, and the energy barrier of the transition states. Isotope experiments and theoretical calculations are frequently utilized to explore the catalytic mechanism.4.1. Mechanism of hydrogen production from NaBH4
In 2019, Astruc's group first proposed an oxidative addition mechanism for NaBH4 hydrolysis over CoNPs@ZIF-8.66 They believed that the hydrogen bonding interactions between H2O and borohydride effectively reduced the electron density of the O–H bond in H2O, thus promoting the challenging oxidative addition of O–H bonds (Fig. 10a). Then, they investigated the isotopic effects of NaBH4 hydrolysis using deuterium oxide (D2O) instead of H2O. A significant kinetic isotope effect was observed when utilizing D2O (kH/kD = 6.85), suggesting that the cleavage of the O–H bonds in H2O was the key to the rate-determining step (RDS) of NaBH4 hydrolysis (Fig. 10b). | ||
| Fig. 10 (a) Oxidative addition mechanism of NaBH4 hydrolysis over CoNPs@ZIF-8. (b) Hydrogen release of NaBH4 in H2O and D2O over CoNPs@ZIF-8. Reproduced with permission.66 Copyright 2019, Wiley-VCH. (c) Potential energy diagram associated with the dehydrogenation of NaBH4 over Co/C. Reproduced with permission.219 Copyright 2022, Elsevier. (d) Free energy diagram of NaBH4 hydrolysis over Co@NMGC. (e) Electronic potential of Co@NMGC. Reproduced with permission.68 Copyright 2020, Elsevier. | ||
Dou et al. synthesized Co-inlaid carbon sphere catalysts and investigated the process of NaBH4 hydrolysis.219 They proposed that the initial formation of hydrogen molecules originated from NaBH4, while the dissociation of water occurred during subsequent hydrogen molecule formation. The formation of NaOBO + H + H + 3H2 exhibited the highest activation barrier (1.88 eV) among the reaction processes, suggesting that it served as the RDS of the hydrolysis reaction (Fig. 10c). Regarding the competing reaction, the energy barriers for the cleavage of the first and fourth B–H bonds in sodium borohydride exceed that of the O–H bond cleavage in water. Conversely, the energy barriers for the cleavage of the second and third B–H bonds are lower than that of the O–H bond cleavage in water. Sun's group investigated the adsorption behavior of BH4− on the surface of Co@NMGC, Co, as well as graphene, and calculated the Gibbs free energy of hydrogen adsorption (ΔGH*).68 As shown in Fig. 10d, metallic Co exhibited a higher hydrogen adsorption energy compared to the graphene shell. After the introduction of encapsulated Co clusters, a significant reduction in ΔGH* of Co@NMGC was noted from 1.99 eV to 0.07 eV. Additionally, during the second step, Co@NMGC displayed the lowest activation barrier (0.22 eV), indicating a notable enhancement in hydrogen evolution efficiency. Analysis of the electronic properties of Co@NMGC indicated that the graphene shells displayed a higher proton affinity following adsorption compared to pristine graphene. The redistribution of electron potential within Co@NMGC, facilitated by the transfer of charges from the Co atom to the C atom, significantly improved the adsorption and dissociation of the H atom on the Co surface.
4.2. Mechanism of hydrogen production from NH3BH3
The initial reaction mechanism for the dehydrogenation of AB was primarily formulated through conjecture regarding the underlying reaction processes. Three potential mechanisms, the nucleophilic substitution mechanism, the activation of H2O mechanism, and the oxidative addition mechanism, have been posited.26,220,221 Lu's group presented a nucleophilic substitution mechanism for AB hydrolysis over Co–Co3O4/CDs.221 They proposed that H2O molecules adsorbed onto Co3O4 first dissociated to form OH* radicals (Fig. 11a). Consequently, the accumulation of OH* radicals on the Co surface facilitated the advancement of SN2 reactions and enhanced the reaction rate of AB hydrolysis. Then, Li's group proposed the H2O activation mechanism for AB hydrolysis (Fig. 11b–d).153,222 They believed that the activation of B–H bonds of NH3BH3 took place on the Co–Co bonds of Co NPs, while H–O bonds in H2O were activated by Co–O bonds. Ultimately, two activated hydrogen atoms combined to produce a hydrogen molecule. The activation process was pervasive within the whole reaction system, serving as a robust kinetic basis for achieving heightened catalytic activity. Astruc's group investigated the process of AB hydrolysis over Ni/ZIF-8 and proposed that water activation via the oxidative addition of O–H bonds resulted in the formation of adsorbed –OH and –H species (Fig. 11e).223 | ||
| Fig. 11 (a) Nucleophilic substitution mechanism of AB hydrolysis over Co–Co3O4/CDs. Reproduced with permission.221 Copyright 2019, Elsevier. (b and c) The activation mechanism of H2O for AB hydrolysis catalyzed by Co-NC/NF600. Reproduced with permission.222 Copyright 2022, American Chemical Society. (d) The activation mechanism of H2O for AB hydrolysis catalyzed by Co–CoOx@NCS-II. Reproduced with permission.153 Copyright 2019, American Chemical Society. (e) Oxidative addition mechanism for AB hydrolysis over Ni/ZIF-8. Reproduced with permission.223 Copyright 2017, American Chemical Society. | ||
The breaking of B–N, B–H, and O–H bonds is involved in AB solvolysis. Isotope experiments serve as a valuable approach for elucidating the kinetics of these processes. Yu et al. performed measurements on the kinetic isotope effect during AB hydrolysis utilizing Pt0.1%Co3%/TiO2 as the catalyst.174 The dehydrogenation of NH3BH3 in D2O exhibits an evident slow rate compared to that in H2O, revealing the involvement of the breaking of O–H bonds in the RDS of AB hydrolysis (Fig. 12a). Astruc's group studied the kinetic isotope effect of hydrogen production from NH3BH3 and NH3BD3 in H2O, and NH3BH3 in D2O over Ni/ZIF-8 (Fig. 12b).223 The hydrolysis of NH3BD3 in H2O over Ni/ZIF-8 exhibited a reduced reaction rate, and the kinetic isotope effect (KIE) was determined as 1.33, suggesting dehydrogenation behavior similar to that observed for NH3BH3 in H2O. Conversely, a significant KIE value of 2.49 was detected during hydrogen generation in the NH3BH3–D2O system, suggesting that the cleavage of O–H bonds in water could potentially serve as a slow step for the hydrolysis of AB. Our group thoroughly analyzed the kinetic isotope effects of AB methanolysis through the substitution of CH3OH with CD3OD and NH3BH3 with NH3BD3 and 15NH3BH3 (Fig. 12c).170 The kinetic isotope effect constants were determined to be 1.05 for NH3BD3, 1.03 for 15NH3BH3, and 6.32 for CD3OD, confirming the involvement of O–H bond breaking in the RDS.
 | ||
| Fig. 12 (a) H2 generated from AB in H2O and D2O over Pt0.25%Co3%/TiO2. Reproduced with permission.174 Copyright 2023, American Chemical Society. (b) Hydrogen production from NH3BH3 and NH3BD3 in H2O, and NH3BH3 in D2O over Ni/ZIF-8. Reproduced with permission.223 Copyright 2017, American Chemical Society. (c) Isotopic experiments for AB methanolysis catalyzed by Co/V-PAF-GO. (d) Gibbs free-energy profiles and active energy diagrams of CH3OH and NH3BH3 over Co, Co/GO, and Co/BZ-PAF. Reproduced with permission.170 Copyright 2023, Wiley-VCH. (e) Spin-polarized partial density of states (PDOS) and energy diagrams of AB hydrolysis over CoRu0.5/CQDs and bulk CoRu0.5/CQDs. Reproduced with permission.176 Copyright 2021, Wiley-VCH. (f) Energy profiles for the dissociation of H2O and AB on Pt(111) and Pt4@CoO(111). Reproduced with permission.174 Copyright 2023, American Chemical Society. | ||
Our group studied the adsorption and activation of AB and methanol molecules on GO-supported Co(111) (Co/GO), BZ-PAF-stabilized Co(111) (Co/BZ-PAF), and supported-free Co(111) by DFT calculations (Fig. 12d).170 The activation barriers for CH3OH molecules on the three catalysts were evidently higher than those for NH3BH3, thereby substantiating that breaking of the O–H bonds in CH3OH served as the RDS of AB methanolysis. Lu's group investigated the PDOS and energy diagrams for AB hydrolysis over CoRu0.5/CQDs and bulk CoRu0.5/CQDs to provide a more comprehensive understanding of the reaction mechanism (Fig. 12e).176 According to the partial density of states (PDOS) of H2O adsorbed on CoRu0.5/CQDs, they confirmed a higher degree of orbital overlap at the Ru sites compared to the Co sites in the presence of free H2O. This observation indicated that Ru played a crucial part in the initial activation of H2O on CoRu0.5/CQDs. Furthermore, the PDOS analysis of relevant species during H2O adsorption provided further evidence supporting the crucial role of Ru in facilitating electron transfer. The reaction pathway exhibited a consistent downward trend in the production of H2, aligning with electronic structure calculations. The dissociation energy barrier of OH* to O* was calculated to be 0.43 eV, representing a critical determinant in the potential reaction. Additionally, a significant energy decrease of 3.69 eV was noted during the dissociation of AB and its subsequent combination with O*, highlighting the stronger binding of B–O compared to B–OH. Yu et al. constructed a Pt4 cluster supported on CoO(111) (Pt4@CoO(111)) and calculated the energy profiles for the dissociation of water and AB on the surface of Pt4@CoO(111) and Pt(111) (Fig. 12f).174 The dissociation of B–H bonds in AB on Pt4@CoO(111) and Pt(111) exhibited low activation energies of 0.35 eV and 0.09 eV, suggesting a facile step for B–H bond dissociation. A significantly higher activation barrier (0.79 eV) for H2O dissociation on Pt(111) was observed compared to that on Pt4@CoO(111). The enhanced dehydrogenation property of Pt4@CoO(111) for facilitating water dissociation was ascribed to the synergistic effects between Pt4 and CoO(111).
The catalytic pathways for AB solvolysis catalyzed by Co-based X-ide catalysts are also investigated to explore the nature of the catalytic reactions. Li et al. constructed Ni3N (110), Co–Ni3N (110), Ni3N (111), and Co–Ni3N (111) modes to probe the reaction pathways of AB hydrolysis.212 According to Fig. 13a, the calculated Eads(H2O) values for Ni3N (110), Co–Ni3N (110), Ni3N (111), and Co–Ni3N (111) were −0.186, −0.315, −0.291, and −0.418 eV, respectively. These results indicated that Co–Ni3N exhibited a greater propensity for H2O adsorption than Ni3N, suggesting that Co doping effectively enhanced the binding affinity of H2O. The results of the energy profiles for H2O dissociation clearly demonstrated a decrease in activation energy (Ea) for H2O dissociation on the surface of Co–Ni3N (110) and Co–Ni3N (111) by introducing Co species. This provided evidence that the incorporation of Co species in Ni3N significantly enhanced the cleavage of H2O, thereby remarkably enhancing the catalytic property of AB hydrolysis. Li's group investigated the adsorption and dissociation of AB and H2O on CoP, Co3B–CoP, and Co3B–CoP/h-BN to reveal the potential mechanism of the B–Co–P site (Fig. 13b).187 They confirmed that Co3B–CoP/h-BN had the lowest dissociation barrier for AB, and the Co–P site was more efficient at activating the AB molecule than the Co–B site. In addition, the Co–P/Co–B sites on Co3B–CoP/h-BN exhibited a lower energy barrier for H2O dissociation than those on CoP, Co3B, and Co3B–CoP. Furthermore, the H2O molecule proved to be more easily activated on the Co–B site than the Co–P site. Eventually, they revealed a possible mechanism for AB hydrolysis on B–Co–P sites (Fig. 13c). NH3BH3 and H2O were first adsorbed on dual-active sites of atomic-bridge structured B–Co–P to form NH3BH3–[Co–P]* and H2O–[Co–B]* species. Then, the H2O–[Co–B]* species were dissociated to OH–[Co–B]* and H–[Co–B]*. On the other hand, NH3BH3–[Co–P]* cleaved to form NH3BH2–[Co–P]* and H–[Co–P]*. The combination of H–[Co–P]* and H–[Co–B]* resulted in the production of the H2 molecule, which was subsequently liberated from the B–Co–P sites. The reaction continued until two additional H2 molecules were generated through a similar reaction mechanism. Recently, the same group synthesized a Co4N–Co3O4 interface structure catalyst for hydrogen generation from AB (Fig. 13d).224 They constructed Co4N, Co3O4, and Co4N–Co3O4 models and calculated the density of states of three catalysts and energy profiles for the adsorption and dissociation of AB and H2O on these catalysts. The activation energy barriers for AB and H2O on Co3O4–Co4N (311) were significantly lower than those of Co4N, Co3O4, and Co3O4–Co4N. It was demonstrated that the construction of an interface structure between Co3O4 and Co4N was crucial for enhancing the adsorption and activation of water and AB. An analysis of the aforementioned mechanism reveals that the energy barriers for O–H bond dissociation in water and methanol exceed that of B–H bond dissociation in AB during hydrolysis and methanolysis. This suggests that the B–H bond exhibits a higher propensity to dissociate in comparison with the O–H bond during AB solvolysis. Furthermore, the adsorption affinity of AB on the cobalt catalyst surface is greater than that of water and methanol, indicating the preferential adsorption of AB on the surface of cobalt-based catalysts.
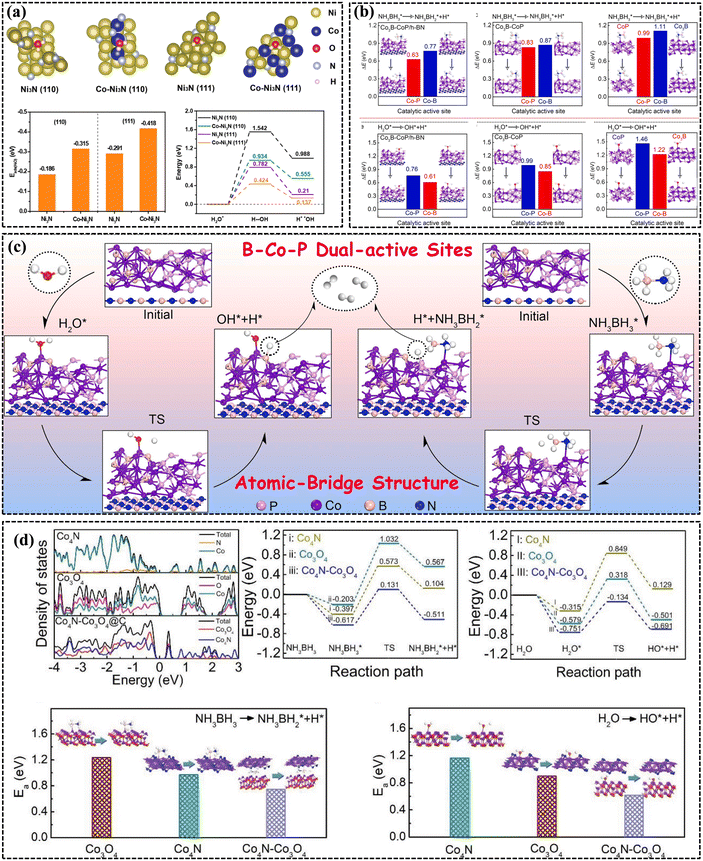 | ||
| Fig. 13 (a) The optimized structure modes for H2O adsorption, and the corresponding Eads(H2O), and activation energy diagram of water on the surface of Ni3N, Co–Ni3N, Ni3N, and Co–Ni3N.212 Copyright 2021, Elsevier. (b) Activation energy of AB and H2O dissociation on Co–B and Co–P sites of CoP, Co3B, Co3B–CoP, and Co3B–CoP/h-BN. (c) The proposed catalytic mechanism of AB hydrolysis over Co3B–CoP/h-BN. Reproduced with permission.187 Copyright 2022, Elsevier. (d) Density of states of Co4N, Co3O4, and Co4N–Co3O4 and the corresponding active energies of AB and H2O adsorption and dissociation on these catalysts. Reproduced with permission.224 Copyright 2022, Wiley-VCH. | ||
5. Conclusions and perspectives
In this review, we first introduced the characteristics of each chemical hydrogen storage material and proposed evaluation methods for their hydrogen evolution performance, followed by a conclusion on the catalytic performance of different Co-based nanocatalysts for the dehydrogenation of sodium borohydride and ammonia borane over the last 20 years. Then, we utilized numerous examples to demonstrate the benefits of different supports for catalyst design and their impact on the active center. Finally, we outlined the mechanisms and catalytic pathways of hydrogen production from these hydrogen storage materials, underscoring the significance of mechanistic investigations for catalyst design. Fig. 14 offers a thorough summary and future perspective on the dehydrogenation of sodium borohydride and ammonia borane with Co-based nanocatalysts. However, there remain several questions for both theoretical investigation and practical implementation of these hydrogen storage materials that require further attention. The following enumeration outlines the challenges and proposes potential predictions for future progress. | ||
| Fig. 14 Summary and outlook of dehydrogenation from sodium borohydride and ammonia borane over Co-based nanocatalysts. | ||
5.1. Comparison of different hydrogen storage materials
Hydrogen production from sodium borohydride and ammonia borane has exhibited numerous advantages, including excellent stability, high safety for storage and transportation, as well as efficient and fast hydrogen production.225–227 Nevertheless, there is limited research on the comparison of characteristics among these hydrogen storage materials, such as price, storage capacity, environmental stability, and regeneration performance. In light of this, a comprehensive analysis of these diverse hydrogen storage materials is provided in Table 3. The production of sodium borohydride, with sodium hydride as a raw material, is associated with high production costs.228 On the other hand, ammonia borane has not yet been produced on a large-scale, resulting in a high price.226,229 For environmental storage, ammonia borane demonstrated greater stability as an ionic compound. Sodium borohydride undergoes deliquescence in the presence of air, resulting in the release of hydrogen.228 Regarding the hydrogen storage capacity, ammonia borane demonstrates the highest mass hydrogen storage density (19.4 wt%) among its counterparts. Nevertheless, hydrogen contained within the amino group remains unreleased during the processes of hydrolysis and methanolysis.| Hydrogen storage materials | Advantages | Disadvantages |
|---|---|---|
| NaBH4 | • Ambient operation temperature | • Expensive |
| • Good kinetics | • Self-solvolysis | |
| • High hydrogen storage density (10.6 wt%) | • Hygroscopic | |
| • Good regenerability | ||
| NH3BH3 | • Ambient operation temperature | • Expensive and scarce |
| • Long-term stability | • Poor regenerability | |
| • High hydrogen storage density (19.4 wt%) | • Unreleased hydrogen from the NH3 group |
In terms of hydrogen production, sodium borohydride demonstrates superior kinetic properties, undergoing complete spontaneous methanolysis in methanol and 10% spontaneous hydrolysis in water at room temperature. Consequently, the addition of a base is frequently required to prevent spontaneous methanolysis or hydrolysis and facilitate consistent hydrogen generation within the fuel cell.50,230 Ammonia borane can undergo hydrolysis or methanolysis at ambient temperature with appropriate catalysts, suggesting favorable kinetic behavior.
Additionally, the regeneration of sodium borohydride and ammonia borane is one of the factors that increases their cost. The regeneration methods for hydrogen storage materials are demonstrated in Table 4. The hydrolyzed derivative of sodium borohydride (NaBO2) can be regenerated directly through the reduction of MgH2, whereas the methanolysis derivative (NaB(OMe)4) must first undergo hydrolysis to form NaBO2 before being regenerated through the reaction with MgH2.231 The hydrolysis product of ammonia borane also yields NaBO2, which can be subsequently reduced to NaBH4 and then regenerated through reaction with an ammonium salt.232 The methanolysis by-product, NH4B(OMe)4, can be directly regenerated through reduction with LiAlH4.197
| Hydrogen storage materials | Regeneration method |
|---|---|
| NaBH4 | • NaBO2 + 2MgH2 → NaBH4 + 2MgO |
| • NaB(OMe)4 + 2H2O → NaBO2 + 4MeOH | |
| • NaBO2 + 2MgH2 → NaBH4 + 2MgO | |
| NH3BH3 | • NH4BO2 + NaOH → NaBO2 + NH3 + H2O |
| • NaBO2 + 2MgH2 → NaBH4 + 2MgO | |
| • 2NaBH4 + (NH4)2SO4 → 2NH3BH3 + Na2SO4 + 2H2 | |
| • NH4B(OMe)4 + NH4Cl + LiAlH4 → NH3BH3 + Al(OMe)3 + MeOH + H2 + LiCl + NH3 |
5.2. Comparison of different Co-based nanocatalysts
A number of Co and Co-containing nanocatalysts have been exploited for liquid phase catalytic hydrogen generation from sodium borohydride and ammonia borane. Co-based catalysts exhibit cost-effectiveness and superior catalytic performance, rendering them a prominent avenue for the advancement of these hydrogen storage materials in portable electronic devices. Advantages and disadvantages of the previously discussed Co-based catalysts for the dehydrogenation of chemical storage materials are summarized in Table 5.| Co-based catalysts | Hydrogen production application | Advantages | Disadvantages |
|---|---|---|---|
| Monometallic Co NPs or NCs | NaBH4, NH3BH3 | • Facile fabrication | • Easy to aggregate |
| • High dispersion | • Easily oxidized | ||
| • Good property | • Poor durability and recyclability | ||
| • Viable mass production | |||
| Co alloy NPs or NCs | NaBH4, NH3BH3 | • Synergistic effect | • Preparation complexity |
| • Good stability | • Uneven dispersion | ||
| • Enhanced property | |||
| Co SACs | NaBH4, NH3BH3 | • Ultrahigh atomic dispersion | • Preparation complexity |
| • Ultrahigh atomic utilization | • Poor durability and recyclability | ||
| • Unique adsorption | • Low metal loading | ||
| • Ultrahigh property | • Need for stabilizer | ||
| Co X-ides | NaBH4, NH3BH3 | • High thermal stability | • Preparation complexity |
| • High oxidation stability | • Poor durability and recyclability | ||
| • Unique adsorption | • Discharge of nitrogen and phosphoric wastewater | ||
| • Good property |
Monometallic Co NPs or NCs have been extensively utilized in the solvolysis of sodium borohydride and ammonia borane for hydrogen production, owing to their high dispersity and facile preparation, underscoring the effectiveness of Co NPs or NCs in the breaking of B–H bonds. Nevertheless, these Co NPs and NCs exhibit a propensity to aggregate and oxidize due to inadequately stable ligands or confined protection, leading to a rapid deterioration in cycling stability. Compared to monometallic Co, Co alloy NPs or NCs displayed enhanced catalytic properties for the dehydrogenation of these hydrogen storage materials due to the alloying effect, which modified the electrical configuration and shifted the d-band center of the metals.233,234 In addition, alloy NPs or NCs demonstrated better cycling stability than that of monometallic NPs or NCs, which was attributed to the partitioning effect between distinct metal components. However, the preparation process for alloy NPs or NCs is complex and it is necessary to prevent the inhomogeneity caused by segregation. Single-atom catalysts maximize the utilization of metal atoms, with each individual metal atom serving as a distinct active site for catalytic reactions.235–238 Single-atom Co catalysts exhibit significant catalytic activity in the solvolysis of sodium borohydride and ammonia borane for hydrogen generation that is attributed to ultrahigh atomic utilization and unique adsorption forms.239,240 Nevertheless, the elevated atomic dispersion rate induces a surge in surface energy, leading to a reduction in stability. In addition, this ultrahigh dispersion also results in the low metal loading of single-atom catalysts. Consequently, constructing specialized coordination groups or atoms to stabilize single atoms is an intricate and nuanced process. Co X-ide catalysts, such as borides, phosphides, and nitrides, are also used for hydrogen evolution from these hydrogen storage materials, due to excellent thermal stability, oxidation resistance, and unique adsorption and activation sites different from monometallic Co and its alloys.241–243 After analyzing the properties of different Co X-ides, Co phosphates (Co–P) and nitrides (Co–N) were found to be more active than Co borides (Co–B);244–246 this can potentially be attributed to more electron transfer, which is expected to be further investigated. Furthermore, Co X-ides demonstrated low cycling stability and complexity of preparation.88,247 Therefore, the development of new supports or suitable synthesis methods to anchor or confine Co X-ides with high stability and durability will be a future development direction.
The primary objective of catalyst design is to facilitate their application in hydrogen production from chemical hydrogen storage materials. This necessitates the consideration of large-scale catalyst production, environmentally friendly synthesis, and industrial feasibility. In the context of Co-based catalysts, monometallic and alloy NPs are the most straightforward to produce industrially via a wet chemistry method, with the production process discharging only minimal amounts of B-containing wastewater, thereby largely achieving the aims of green synthesis. In contrast, metal clusters and single-atom catalysts demand stringent control over production conditions, posing challenges for large-scale production. Furthermore, the synthesis of Co X-ides involves lengthy preparation processes, requiring multiple reaction steps, and is associated with the discharge of nitrogenous and phosphorus wastewater.
5.3. Exploration of catalytic mechanisms and reaction pathways
The structure, composition, and physical characteristics of the support significantly influence the activity of metal active sites.248–250 Investigating the influence of the support on the active sites is essential for the development of highly efficient and durable Co-based nanocatalysts. The high specific surface area of the support facilitates the dispersion of metal NPs, and the pore structure is conducive to the confinement of metal NPs.251,252 The presence of heteroatoms and defects in the support can serve as anchoring sites for metal NPs, leading to a decrease in particle size and improvement in catalytic properties.253–255 Furthermore, the specific crystal face of metal NPs significantly affects their properties, warranting further investigation.The electronic structure of the support and the electronic interaction between the support and metal can also affect the hydrogen generation performance of NaBH4 and NH3BH3 solvolysis.256 An electron-rich support (e.g. pyridine-rich, hydroxy-rich, and porphyrin-rich polymers) can interact with the metal precursor, enhancing the dispersibility of the metal, reducing its particle size, and consequently improving the catalytic activity.257,258 In addition, the sharing of electrons between the electron-rich support and the vacant orbitals of Co leads to an accumulation of electrons on the surface of Co NPs, thereby creating numerous electron-rich sites that facilitate the activation of B–H.66,170 On the other hand, an electron-deficient support (e.g. g-C3N4, MoO3, and polymers containing nitro and cyano groups) facilitates the transfer of electrons from metallic Co to the support, resulting in the formation of electron-deficient Co. This electron-deficient state of Co subsequently promotes the adsorption of water on the Co surface, ultimately weakening the O–H bond. The influence of electron transfer between the support and Co on the adsorption and dissociation of B–H and O–H bonds remains unclear, meriting additional investigation by scholars.
Acid–base sites are crucial in the solvolysis of sodium borohydride and ammonia borane for hydrogen production. Brønsted acids have been demonstrated to catalyze the hydrolysis of sodium borohydride, facilitating the production of hydrogen in the absence of metal catalysts.259,260 However, the solvolysis of sodium borohydride facilitated by Lewis acids has not been previously documented. Furthermore, it has been established that alkalis can inhibit the solvolysis process of sodium borohydride.50 Research by Linares et al. demonstrated that both Lewis and Brønsted acid sites in zeolites facilitated the hydrolysis of ammonia borane, leading to enhanced hydrogen generation performance.85 Specifically, Lewis acid sites slightly enhance the hydrogen production rate, while Brønsted acid sites increase it by 50%. On the other hand, the introduction of p-phenylenediamine base sites significantly enhances catalytic activity, with the effect being amplified as the base content increases. This finding suggests that base sites more effectively improve the hydrolytic hydrogen production of ammonia borane compared to acid sites. Our research group further substantiated that Brønsted bases, including NaOH, KOH, and Na2CO3, could significantly enhance the hydrolysis of ammonia borane, thereby facilitating hydrogen production.178 Therefore, in-depth studies of the mechanism of acid and base promoted solvolysis of sodium borohydride and ammonia borane, and the strategic design of catalysts incorporating appropriate acid and base sites are expected to significantly enhance the performance of cobalt-based catalysts.
Based on statistical analysis, it was determined that Co exhibited superior performance for the solvolysis of NaBH4 and NH3BH3 in comparison with Ni, Fe, and Cu.256 Subsequent investigations revealed that Co possessed the ability to decrease the energy barrier associated with the breaking of B–H bonds in NaBH4 and NH3BH3 and O–H bonds in H2O and CH3OH.68,170,261 Therefore, an in-depth investigation of the selectivity of Co in relation to the dehydrogenation of sodium borohydride and ammonia borane from an electronic structure perspective is crucial for the advancement of high-performance Co-based hydrogen production catalysts.
5.4. Prediction of future progress for hydrogen evolution from sodium borohydride and ammonia borane
Co-based supported nanocatalysts have attracted more and more attention for facilitating the dehydrogenation of sodium borohydride and ammonia borane, due to their unique metal active centers, strong metal–support interactions, and the promoting effects of specific sites in the support. The introduction of a support containing heteroatoms, such as N, B, O, S, and P, can enhance the interaction and electron transfer between the metal and the support, leading to a reduction in the size of metal NPs, alteration of the electronic structure of the metal, and enhancement of the catalytic performance of the catalyst.262–266 Investigating the promoting effect of different heteroatoms on metal active sites is beneficial for designing metal nanocatalysts with high activity and stability. Based on the confinement effect of micropores and enhanced diffusion of substrates through mesopores and macropores, the hierarchical pore structure of the support can effectively increase the stability of metal NPs and improve their catalytic properties.158,267 Furthermore, certain sites in the support can enhance the adsorption of substrates and improve their dehydrogenation performance. For instance, strongly acidic sites can improve the adsorption of sodium borohydride and ammonia borane.268–270 Hence, the exploitation of a novel support to enhance the effectiveness of metal nanocatalysts holds significant importance in the development of these hydrogen storage materials.Currently, the predominant focus of mechanistic research in the field of chemical hydrogen storage materials pertains to sodium borohydride and ammonia borane. In previous reports, research on the hydrogen production mechanism of sodium borohydride and ammonia borane primarily concentrated on the initial step of dehydrogenation, with limited exploration of the subsequent dehydrogenation process. Furthermore, investigations into the hydrogen production mechanism of sodium borohydride and ammonia borane predominantly relied on DFT calculations. The current model fails to accurately depict the catalyst structure, particularly in regard to the establishment of metal clusters, resulting in discrepancies between the atomic number and crystal structure in comparison with the actual metal structure. Therefore, it is imperative to investigate the mechanism from the perspective of theory and experiments, taking into consideration the actual structure of the catalyst.
The catalytic process consistently operates within a dynamic non-equilibrium state, posing challenges for directly observing the catalyst morphology and ascertaining the relationship between structure and activity. Operando characterization techniques can monitor the activity of catalysts in real time and help researchers understand the changes that occur during the reaction.271–274Operando techniques such as in situ transmission electron microscopy (TEM) and scanning electron microscopy (SEM) can be utilized to observe morphological changes to the catalysts, while in situ X-ray diffraction (XRD) can be employed to analyze the crystal phase structure and grain size alterations. Additionally, in situ mass spectrometry (MS), nuclear magnetic resonance (NMR), and Fourier transform infrared spectroscopy (FTIR) are valuable tools for characterizing the changes to intermediates during catalytic reactions. In situ characterization technology enables a comprehensive understanding of the structure, composition, reaction mechanism, activity, and lifespan of catalysts, thereby offering a theoretical foundation for catalyst design and optimization. In addition, X-ray absorption fine structure (XAFS) spectroscopy is a valuable technique for characterizing the valence state of Co, elucidating its binding interactions with the carrier material, and analyzing electron transfer dynamics, with a particular emphasis on the single-atom structural configuration. Spherical aberration corrected transmission electron microscopy (AC-TEM) serves as a powerful tool for identifying subnanometer Co clusters and Co single atoms, thereby elucidating the correlation between metal dispersion and catalytic activity. Surface enhanced Raman spectroscopy (SERS) provides valuable insights into dynamic surface phenomena, facilitating the monitoring of Co-based catalyst structures, adsorbent interactions of sodium borohydride and ammonia borane, as well as the reaction kinetics with exceptionally high spatial and temporal resolution.
In the process of developing catalysts to address urgent environmental concerns and energy demands, conventional design and optimization approaches frequently fall short of meeting the necessary criteria due to the intricate and vast nature of the catalyst parameters. The emergence of artificial intelligence (AI) has introduced a new epoch in catalyst optimization, presenting prospective solutions for the limitations of traditional methodologies.275–277 Based on large language models (LLM), Bayesian optimization, and machine learning (ML), AI is anticipated to be employed in the screening of hydrogen production catalysts for chemical hydrogen storage materials, the optimization of catalytic hydrogen production processes, and the investigation of mechanisms.
The final industrialization goal of hydrogen generation systems based on sodium borohydride and ammonia borane over Co-based catalysts is the portable hydrogen storage system. The utilization of liquid hydrogen storage technology in the portable power supply system holds significant economic implications and research merit.278 This technology is expected to have broad applications in various industries, for instance fuel cell mopeds, hydrogen-powered drones, and large hydrogen-powered buses.
Data availability
All relevant data are within the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 22162014, 22162013, and U24A2044), the Guizhou Provincial Basic Research Program (Natural Science) (No. MS [2025]094), the Doctoral Talents Project of Tongren City (No. [2023]7), and the Doctoral Research Foundation Project of Tongren University (No. trxyDH2204).References
- J. A. Turner, Sustainable hydrogen production, Science, 2004, 305, 972–974 CrossRef CAS PubMed.
- Z. Abdin, A. Zafaranloo, A. Rafiee, W. Mérida, W. Lipiński and K. R. Khalilpour, Hydrogen as an energy vector, Renewable Sustainable Energy Rev., 2020, 120, 109620 CrossRef CAS.
- Y. Kojima, Hydrogen storage materials for hydrogen and energy carriers, Int. J. Hydrogen Energy, 2019, 44, 18179–18192 CrossRef CAS.
- Z.-X. Yang, X.-G. Li, Q.-L. Yao, Z.-H. Lu, N. Zhang, J. Xia, K. Yang, Y.-Q. Wang, K. Zhang, H.-Z. Liu, L.-T. Zhang, H.-J. Lin, Q.-J. Zhou, F. Wang, Z.-M. Yu and J.-M. Ma, 2022 roadmap on hydrogen energy from production to utilizations, Rare Met., 2022, 41, 3251–3267 CrossRef CAS.
- D. Guan, B. Wang, J. Zhang, R. Shi, K. Jiao, L. Li, Y. Wang, B. Xie, Q. Zhang and J. Yu, Hydrogen society: From present to future, Energy Environ. Sci., 2023, 16, 4926–4943 RSC.
- J. O. Abe, A. Popoola, E. Ajenifuja and O. M. Popoola, Hydrogen energy, economy and storage: Review and recommendation, Int. J. Hydrogen Energy, 2019, 44, 15072–15086 CrossRef CAS.
- W. Lubitz and W. Tumas, Hydrogen: an overview, Chem. Rev., 2007, 107, 3900–3903 CrossRef CAS PubMed.
- Q. Sun, N. Wang, Q. Xu and J. Yu, Nanopore–supported metal nanocatalysts for efficient hydrogen generation from liquid–phase chemical hydrogen storage materials, Adv. Mater., 2020, 32, 2001818 CrossRef CAS PubMed.
- S. Z. Al Ghafri, S. Munro, U. Cardella, T. Funke, W. Notardonato, J. M. Trusler, J. Leachman, R. Span, S. Kamiya and G. Pearce, Hydrogen liquefaction: a review of the fundamental physics, engineering practice and future opportunities, Energy Environ. Sci., 2022, 15, 2690–2731 RSC.
- M. R. Usman, Hydrogen storage methods: Review and current status, Renewable Sustainable Energy Rev., 2022, 167, 112743 CrossRef CAS.
- L. Li, Y. Huang, C. An and Y. Wang, Lightweight hydrides nanocomposites for hydrogen storage: Challenges, progress and prospects, Sci. China Mater., 2019, 62, 1597–1625 CrossRef.
- C. He, R. Yu, H. Sun and Z. Chen, Lightweight multilayer composite structure for hydrogen storage tank, Int. J. Hydrogen Energy, 2016, 41, 15812–15816 CrossRef CAS.
- L. Van Hoecke, L. Laffineur, R. Campe, P. Perreault, S. W. Verbruggen and S. Lenaerts, Challenges in the use of hydrogen for maritime applications, Energy Environ. Sci., 2021, 14, 815–843 Search PubMed.
- C. Wang and D. Astruc, Recent developments of nanocatalyzed liquid-phase hydrogen generation, Chem. Soc. Rev., 2021, 50, 3437–3484 RSC.
- U. B. Demirci, Ammonia borane, a material with exceptional properties for chemical hydrogen storage, Int. J. Hydrogen Energy, 2017, 42, 9978–10013 Search PubMed.
- Z. Chen, K. O. Kirlikovali, K. B. Idrees, M. C. Wasson and O. K. Farha, Porous materials for hydrogen storage, Chem, 2022, 8, 693–716 Search PubMed.
- J. H. Türkcan, H. Elçiçek and O. K. Özdemir, Optimization of synthesis parameters for catalytic performance of Ni-B catalysts using response surface methodology, Int. J. Hydrogen Energy, 2021, 46, 7903–7911 CrossRef.
- C. Zhang, J. Liu, Y. Ye, Q. Chen and C. Liang, Encapsulation of Co-based nanoparticle in N-doped graphitic carbon for efficient oxygen reduction reaction, Carbon, 2020, 156, 31–37 CrossRef CAS.
- C. Huff, J. M. Long, A. Heyman and T. M. Abdel-Fattah, Palladium nanoparticle multiwalled carbon nanotube composite as catalyst for hydrogen production by the hydrolysis of sodium borohydride, ACS Appl. Energy Mater., 2018, 1, 4635–4640 CrossRef CAS.
- A. Paksoy, S. F. Kurtoğlu, A. K. Dizaji, Z. Altıntaş, S. Khoshsima, A. Uzun and Ö. Balcı, Nanocrystalline cobalt-nickel-boron (metal boride) catalysts for efficient hydrogen production from the hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2021, 46, 7974–7988 CrossRef CAS.
- A. Salehabadi, E. A. Dawi, D. A. Sabur, W. K. Al-Azzawi and M. Salavati-Niasari, Progress on nano-scaled alloys and mixed metal oxides in solid-state hydrogen storage; an overview, J. Energy Storage, 2023, 61, 106722 Search PubMed.
- N. Wang, Q. Sun, T. Zhang, A. Mayoral, L. Li, X. Zhou, J. Xu, P. Zhang and J. Yu, Impregnating subnanometer metallic nanocatalysts into self-pillared zeolite nanosheets, J. Am. Chem. Soc., 2021, 143, 6905–6914 Search PubMed.
- Z. Li, T. He, D. Matsumura, S. Miao, A. Wu, L. Liu, G. Wu and P. Chen, Atomically dispersed Pt on the surface of Ni particles: Synthesis and catalytic function in hydrogen generation from aqueous ammonia–borane, ACS Catal., 2017, 7, 6762–6769 CrossRef CAS.
- J.-K. Sun, W.-W. Zhan, T. Akita and Q. Xu, Toward homogenization of heterogeneous metal nanoparticle catalysts with enhanced catalytic performance: Soluble porous organic cage as a stabilizer and homogenizer, J. Am. Chem. Soc., 2015, 137, 7063–7066 Search PubMed.
- Q. Yao, Y. Ding and Z.-H. Lu, Noble-metal-free nanocatalysts for hydrogen generation from boron-and nitrogen-based hydrides, Inorg. Chem. Front., 2020, 7, 3837–3874 Search PubMed.
- S. Guan, Y. Liu, H. Zhang, R. Shen, H. Wen, N. Kang, J. Zhou, B. Liu, Y. Fan and J. Jiang, Recent advances and perspectives on supported catalysts for heterogeneous hydrogen production from ammonia borane, Adv. Sci., 2023, 10, 2300726 CrossRef CAS PubMed.
- A. Staubitz, A. P. Robertson and I. Manners, Ammonia-borane and related compounds as dihydrogen sources, Chem. Rev., 2010, 110, 4079–4124 Search PubMed.
- D. Santos and C. Sequeira, Sodium borohydride as a fuel for the future, Renewable Sustainable Energy Rev., 2011, 15, 3980–4001 Search PubMed.
- J. Guo, Y. Hou and B. Li, Facile synthesis of a hollow Ni–Fe–B nanochain and its enhanced catalytic activity for hydrogen generation from NaBH4 hydrolysis, RSC Adv., 2018, 8, 25873–25880 RSC.
- J. M. Yan, X. B. Zhang, S. Han, H. Shioyama and Q. Xu, Iron-nanoparticle-catalyzed hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage, Angew. Chem., Int. Ed., 2008, 47, 2287–2289 CrossRef CAS PubMed.
- M. Dinç, Ö. Metin and S. Özkar, Water soluble polymer stabilized iron(0) nanoclusters: A cost-effective and magnetically recoverable catalyst in hydrogen generation from the hydrolysis of sodium borohydride and ammonia borane, Catal. Today, 2012, 183, 10–16 CrossRef.
- N. Patel, R. Fernandes, S. Gupta, R. Edla, D. C. Kothari and A. Miotello, Co-B catalyst supported over mesoporous silica for hydrogen production by catalytic hydrolysis of Ammonia Borane: A study on influence of pore structure, Appl. Catal., B, 2013, 140, 125–132 Search PubMed.
- L. Wang, Y. Liu, S. Ashraf, J. Jiang, G. Han, J. Gao, X. Wu and B. Li, Pitaya pulp structural cobalt–carbon composite for efficient hydrogen generation from borohydride hydrolysis, J. Alloys Compd., 2019, 808, 151774 Search PubMed.
- P. Z. Li, A. Aijaz and Q. Xu, Highly dispersed surfactant-free nickel nanoparticles and their remarkable catalytic activity in the hydrolysis of ammonia borane for hydrogen generation, Angew. Chem., Int. Ed., 2012, 51, 6753–6756 Search PubMed.
- K. Guo, H. Li and Z. Yu, Size-dependent catalytic activity of monodispersed nickel nanoparticles for the hydrolytic dehydrogenation of ammonia borane, ACS Appl. Mater. Interfaces, 2018, 10, 517–525 Search PubMed.
- K. Güngörmez and Ö. Metin, Composition-controlled catalysis of reduced graphene oxide supported CuPd alloy nanoparticles in the hydrolytic dehydrogenation of ammonia borane, Appl. Catal., A, 2015, 494, 22–28 Search PubMed.
- H. Yen, Y. Seo, S. Kaliaguine and F. Kleitz, Role of metal–support interactions, particle size, and metal–metal synergy in CuNi nanocatalysts for H2 generation, ACS Catal., 2015, 5, 5505–5511 CrossRef CAS.
- M. Bechelany, A. A. Chaaya, F. Frances, O. Akdim, D. Cot, U. B. Demirci and P. Miele, Nanowires with controlled porosity for hydrogen production, J. Mater. Chem. A, 2013, 1, 2133–2138 Search PubMed.
- R. Fernandes, N. Patel, A. Miotello and L. Calliari, Co–Mo–B–P alloy with enhanced catalytic properties for H2 production by hydrolysis of ammonia borane, Top. Catal., 2012, 55, 1032–1039 CrossRef CAS.
- L. Wei, X. Cao, M. Ma, Y. Lu, D. Wang, S. Zhang and Q. Wang, Co3O4 nanowires as efficient catalyst precursor for hydrogen generation from sodium borohydride hydrolysis, Funct. Mater. Lett., 2018, 11, 1850018 CrossRef CAS.
- X. Wang, J. Liao, H. Li, H. Wang, R. Wang, B. G. Pollet and S. Ji, Highly active porous Co–B nanoalloy synthesized on liquid-gas interface for hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2018, 43, 17543–17555 CrossRef CAS.
- T. Liu, K. Wang, G. Du, A. M. Asiri and X. Sun, Self-supported CoP nanosheet arrays: a non-precious metal catalyst for efficient hydrogen generation from alkaline NaBH4 solution, J. Mater. Chem. A, 2016, 4, 13053–13057 Search PubMed.
- H. Li, J. Liao, X. Zhang, W. Liao, L. Wen, J. Yang, H. Wang and R. Wang, Controlled synthesis of nanostructured Co film catalysts with high performance for hydrogen generation from sodium borohydride solution, J. Power Sources, 2013, 239, 277–283 Search PubMed.
- H. Schlesinger, H. C. Brown, A. Finholt, J. R. Gilbreath, H. R. Hoekstra and E. K. Hyde, Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen1, J. Am. Chem. Soc., 1953, 75, 215–219 CrossRef CAS.
- S. C. Amendola, S. L. Sharp-Goldman, M. S. Janjua, N. C. Spencer, M. T. Kelly, P. J. Petillo and M. Binder, A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst, Int. J. Hydrogen Energy, 2000, 25, 969–975 CrossRef CAS.
- S. Özkar and M. Zahmakıran, Hydrogen generation from hydrolysis of sodium borohydride using Ru (0) nanoclusters as catalyst, J. Alloys Compd., 2005, 404, 728–731 CrossRef.
- S. C. Amendola, S. L. Sharp-Goldman, M. S. Janjua, M. T. Kelly, P. J. Petillo and M. Binder, An ultrasafe hydrogen generator: aqueous, alkaline borohydride solutions and Ru catalyst, J. Power Sources, 2000, 85, 186–189 CrossRef CAS.
- S. Akbayrak, Y. Tonbul and S. Özkar, Ceria supported rhodium nanoparticles: superb catalytic activity in hydrogen generation from the hydrolysis of ammonia borane, Appl. Catal., B, 2016, 198, 162–170 Search PubMed.
- Q. Yao, Z.-H. Lu, W. Huang, X. Chen and J. Zhu, High Pt-like activity of the Ni–Mo/graphene catalyst for hydrogen evolution from hydrolysis of ammonia borane, J. Mater. Chem. A, 2016, 4, 8579–8583 Search PubMed.
- H. N. Abdelhamid, A review on hydrogen generation from the hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2021, 46, 726–765 CrossRef CAS.
- O. Netskina, E. Tayban, I. Prosvirin, O. Komova and V. Simagina, Hydrogen storage systems based on solid-state NaBH4/Co composite: Effect of catalyst precursor on hydrogen generation rate, Renewable Energy, 2020, 151, 278–285 Search PubMed.
- O. Netskina, E. Tayban, V. Rogov, A. Ozerova, S. Mukha, V. Simagina and O. Komova, Solid-state NaBH4 composites for hydrogen generation: Catalytic activity of nickel and cobalt catalysts, Int. J. Hydrogen Energy, 2021, 46, 5459–5471 Search PubMed.
- S. Demirci, A. K. Sunol and N. Sahiner, Catalytic activity of amine functionalized titanium dioxide nanoparticles in methanolysis of sodium borohydride for hydrogen generation, Appl. Catal., B, 2020, 261, 118242 CrossRef CAS.
- T. Wang, T. Jiang, H. Zhang and Y. Zhao, Advances in catalysts for hydrogen production by methanolysis of sodium borohydride, Int. J. Hydrogen Energy, 2022, 47, 14589–14610 Search PubMed.
- A. Hojjati-Najafabadi, A. Aygun, R. N. E. Tiri, F. Gulbagca, M. I. Lounissaa, P. Feng, F. Karimi and F. Sen, Bacillus thuringiensis based ruthenium/nickel Co-doped zinc as a green nanocatalyst: Enhanced photocatalytic activity, mechanism, and efficient H2 production from sodium borohydride methanolysis, Ind. Eng. Chem. Res., 2023, 62, 4655–4664 Search PubMed.
- A. Balbay and C. Saka, The effect of the concentration of hydrochloric acid and acetic acid aqueous solution for fast hydrogen production from methanol solution of NaBH4, Int. J. Hydrogen Energy, 2018, 43, 14265–14272 Search PubMed.
- L. Semiz, N. Abdullayeva and M. Sankir, Nanoporous Pt and Ru catalysts by chemical dealloying of Pt-Al and Ru-Al alloys for ultrafast hydrogen generation, J. Alloys Compd., 2018, 744, 110–115 Search PubMed.
- D. D. Tuan and K.-Y. A. Lin, Ruthenium supported on ZIF-67 as an enhanced catalyst for hydrogen generation from hydrolysis of sodium borohydride, Chem. Eng. J., 2018, 351, 48–55 Search PubMed.
- G. Bozkurt, A. Özer and A. B. Yurtcan, Development of effective catalysts for hydrogen generation from sodium borohydride: Ru, Pt, Pd nanoparticles supported on Co3O4, Energy, 2019, 180, 702–713 Search PubMed.
- Y. Zou, Y. Yin, Y. Gao, C. Xiang, H. Chu, S. Qiu, E. Yan, F. Xu and L. Sun, Chitosan-mediated Co–Ce–B nanoparticles for catalyzing the hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2018, 43, 4912–4921 Search PubMed.
- D. D. Tuan and K.-Y. A. Lin, ZIF-67-derived Co3O4 rhombic dodecahedron as an efficient non-noble-metal catalyst for hydrogen generation from borohydride hydrolysis, J. Taiwan Inst. Chem. Eng., 2018, 91, 274–280 Search PubMed.
- O. Baytar, Investigation of high-activity activated carbon-supported Co-Cr-B catalyst in the generation of hydrogen from hydrolysis of sodium borohydride, Acta Chim. Slovaca, 2018, 65, 407–415 CrossRef CAS PubMed.
- C. Wu, J. Guo, J. Zhang, Y. Zhao, J. Tian, T. T. Isimjan and X. Yang, Palladium nanoclusters decorated partially decomposed porous ZIF-67 polyhedron with ultrahigh catalytic activity and stability on hydrogen generation, Renewable Energy, 2019, 136, 1064–1070 Search PubMed.
- Y. Wang and X. Liu, Catalytic hydrolysis of sodium borohydride for hydrogen production using magnetic recyclable CoFe2O4-modified transition-metal nanoparticles, ACS Appl. Nano Mater., 2021, 4, 11312–11320 Search PubMed.
- M. R. Nabid, Y. Bide and B. Kamali, Hydrogen release from sodium borohydride by Fe2O3@ nitrogen-doped carbon core-shell nanosheets as reasonable heterogeneous catalyst, Int. J. Hydrogen Energy, 2019, 44, 25662–25670 Search PubMed.
- C. Luo, F. Fu, X. Yang, J. Wei, C. Wang, J. Zhu, D. Huang, D. Astruc and P. Zhao, Highly efficient and selective Co@ZIF–8 nanocatalyst for hydrogen release from sodium borohydride hydrolysis, ChemCatChem, 2019, 11, 1643–1649 Search PubMed.
- J. Mahmood, S.-M. Jung, S.-J. Kim, J. Park, J.-W. Yoo and J.-B. Baek, Cobalt oxide encapsulated in C2N-h2D network polymer as a catalyst for hydrogen evolution, Chem. Mater., 2015, 27, 4860–4864 CrossRef CAS.
- J. Li, X. Hong, Y. Wang, Y. Luo, P. Huang, B. Li, K. Zhang, Y. Zou, L. Sun and F. Xu, Encapsulated cobalt nanoparticles as a recoverable catalyst for the hydrolysis of sodium borohydride, Energy Storage Mater., 2020, 27, 187–197 CrossRef.
- L. Yao, X. Li, W. Peng, Q. Yao, J. Xia and Z.-H. Lu, Co-CeOx nanoparticles anchored on a nitrogen-doped carbon nanosheet: a synergistic effect for highly efficient hydrolysis of sodium borohydride, Inorg. Chem. Front., 2021, 8, 1056–1065 Search PubMed.
- A. Caglar, S. Kaya, C. Saka, D. Yildiz and H. Kivrak, Investigation of electrooxidation and methanolysis of sodium borohydride on activated carbon supported Co catalysts from poplar sawdust, Int. J. Hydrogen Energy, 2024, 75, 171–178 CrossRef CAS.
- J. Hannauer, U. Demirci, G. Pastor, C. Geantet, J. Herrmann and P. Miele, Hydrogen release through catalyzed methanolysis of solid sodium borohydride, Energy Environ. Sci., 2010, 3, 1796–1803 Search PubMed.
- P. Dai, Y. Yao, E. Hu, D. Xu, Z. Li and C. Wang, Self-assembled ZIF-67@ graphene oxide as a cobalt-based catalyst precursor with enhanced catalytic activity toward methanolysis of sodium borohydride, Appl. Surf. Sci., 2021, 546, 149128 Search PubMed.
- H. Zhong, K. Chen, C. Qin, C. Lang, J. Liu, H. Wang, J. Zhang and L. Ouyang, Enhancing NaBH4 regeneration using an Al-rich alloy, J. Alloys Compd., 2024, 976, 173160 Search PubMed.
- W. Huang, F. Xu and X. Liu, Superior hydrogen generation from sodium borohydride hydrolysis catalyzed by the bimetallic Co–Ru/C nanocomposite, Int. J. Hydrogen Energy, 2021, 46, 25376–25384 Search PubMed.
- O. Karaman, Three-dimensional graphene network supported nickel-cobalt bimetallic alloy nanocatalyst for hydrogen production by hydrolysis of sodium borohydride and developing of an artificial neural network modeling to forecast hydrogen production rate, Chem. Eng. Res. Des., 2022, 181, 321–330 Search PubMed.
- M. Soltani and M. Zabihi, Hydrogen generation by catalytic hydrolysis of sodium borohydride using the nano-bimetallic catalysts supported on the core-shell magnetic nanocomposite of activated carbon, Int. J. Hydrogen Energy, 2020, 45, 12331–12346 CrossRef CAS.
- Y. Li, X. Hou, J. Wang, X. Feng, L. Cheng, H. Zhang and S. Han, Co-Mo nanoparticles loaded on three–dimensional graphene oxide as efficient catalysts for hydrogen generation from catalytic hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2019, 44, 29075–29082 CrossRef CAS.
- X. Zhang, Z. Wei, Q. Guo and H. Tian, Kinetics of sodium borohydride hydrolysis catalyzed via carbon nanosheets supported Zr/Co, J. Power Sources, 2013, 231, 190–196 CrossRef CAS.
- B. C. Filiz and A. K. Figen, Insight into the role of solvents in enhancing hydrogen production: Ru-Co nanoparticles catalyzed sodium borohydride dehydrogenation, Int. J. Hydrogen Energy, 2019, 44, 28471–28482 CrossRef.
- L. Kong, T. Yu, J. Zhan, Y. Zhang, G. Li, H. Wang, X. Wang, L. Cheng and F. Wang, Study on the performance of NaBH4 using Ru-Co/CNTs catalyst to catalyze alcoholysis to produce hydrogen, Fullerenes, Nanotubes Carbon Nanostruct., 2020, 28, 891–899 Search PubMed.
- N. Patel and A. Miotello, Progress in Co–B related catalyst for hydrogen production by hydrolysis of boron-hydrides: A review and the perspectives to substitute noble metals, Int. J. Hydrogen Energy, 2015, 40, 1429–1464 Search PubMed.
- S. Gupta, N. Patel, R. Fernandes, D. C. Kothari and A. Miotello, Mesoporous Co–B nanocatalyst for efficient hydrogen production by hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2013, 38, 14685–14692 Search PubMed.
- B. Liu and Q. Li, A highly active Co-B catalyst for hydrogen generation from sodium borohydride hydrolysis, Int. J. Hydrogen Energy, 2008, 33, 7385–7391 Search PubMed.
- H. Ma, W. Ji, J. Zhao, J. Liang and J. Chen, Preparation, characterization and catalytic NaBH4 hydrolysis of Co-B hollow spheres, J. Alloys Compd., 2009, 474, 584–589 Search PubMed.
- Q. Li, W. Yang, F. Li, A. Cui and J. Hong, Preparation of CoB/ZIF-8 supported catalyst by single step reduction and its activity in hydrogen production, Int. J. Hydrogen Energy, 2018, 43, 271–282 Search PubMed.
- C. Tang, R. Zhang, W. Lu, L. He, X. Jiang, A. M. Asiri and X. Sun, Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation, Adv. Mater., 2017, 29, 1602441 Search PubMed.
- M. Kaya, M. Bekiroğullari and C. Saka, Highly efficient CoB catalyst using a support material based on Spirulina microalgal strain treated with ZnCl2 for hydrogen generation via sodium borohydride methanolysis, Int. J. Energy Res., 2019, 43, 4243–4252 Search PubMed.
- F. Wang, Y. Zhang, Y. Wang, Y. Luo, Y. Chen and H. Zhu, Co-P nanoparticles supported on dandelion-like CNTs-Ni foam composite carrier as a novel catalyst for hydrogen generation from NaBH4 methanolysis, Int. J. Hydrogen Energy, 2018, 43, 8805–8814 CrossRef CAS.
- C. M. Kaufman and B. Sen, Hydrogen generation by hydrolysis of sodium tetrahydroborate: effects of acids and transition metals and their salts, Dalton Trans., 1985, 307–313 Search PubMed.
- W. Ye, H. Zhang, D. Xu, L. Ma and B. Yi, Hydrogen generation utilizing alkaline sodium borohydride solution and supported cobalt catalyst, J. Power Sources, 2007, 164, 544–548 Search PubMed.
- F. Seven and N. Sahiner, Superporous P(2-hydroxyethyl methacrylate) cryogel-M (M:Co, Ni, Cu) composites as highly effective catalysts in H2 generation from hydrolysis of NaBH4 and NH3BH3, Int. J. Hydrogen Energy, 2014, 39, 15455–15463 Search PubMed.
- N. Sahiner, S. Yildiz, M. Sahiner, Z. A. Issa and H. Al-Lohedan, Macroporous cryogel metal nanoparticle composites for H2 generation from NaBH4 hydrolysis in seawater, Appl. Surf. Sci., 2015, 354, 388–396 CrossRef CAS.
- H. Zhang, X. Feng, L. Cheng, X. Hou, Y. Li and S. Han, Non-noble Co anchored on nanoporous graphene oxide, as an efficient and long-life catalyst for hydrogen generation from sodium borohydride, Colloids Surf., A, 2019, 563, 112–119 Search PubMed.
- D. Xu, X. Zhang, X. Zhao, P. Dai, C. Wang, J. Gao and X. Liu, Stability and kinetic studies of MOF–derived carbon–confined ultrafine Co catalyst for sodium borohydride hydrolysis, Int. J. Energy Res., 2019, 43, 3702–3710 CrossRef CAS.
- F. Alkhatib, S. F. Ibarhiam, A. A. A. Sari, N. M. Alatawi, N. D. Alkhathami, M. M. Aljohani and F. Shaaban, Effective hydrogen production from sodium borohydride using reusable cobalt based metal-organic framework supported polyurethane sponge, J. Mol. Struct., 2025, 1331, 141467 CrossRef CAS.
- Ş. Karakaya, E. Pehlivan and A. A. Ceyhan, Preparation of an efficient and reusable cobalt doped vermiculite ore catalyst for hydrogen production from sodium borohydride, Int. J. Hydrogen Energy, 2024, 73, 282–293 Search PubMed.
- E. Onat, M. S. Izgi, Ö. Şahin and C. Saka, Robust carbon quantum dots supported cobalt oxide composite particles prepared by hydrothermal process for green hydrogen generation via sodium borohydride hydrolysis, Fuel, 2024, 378, 132900 Search PubMed.
- B. G. Gang and S. Kwon, The proton exchange membrane fuel cell systems using methanolysis of sodium borohydride as a hydrogen source with cobalt catalysts, Int. J. Green Energy, 2016, 13, 1224–1231 CrossRef CAS.
- F. Abdulaziz, S. Latif and T. A. M. Taha, Preparation of Co–CaCO3 catalysts for improved hydrogen production from sodium borohydride, Int. J. Hydrogen Energy, 2024, 56, 271–279 CrossRef CAS.
- F. Mirshafiee and M. Rezaei, Synergistic catalytic performance of Co–Fe/CQD nanocatalyst for rapid hydrogen generation through NaBH4 hydrolysis, Int. J. Hydrogen Energy, 2024, 79, 139–149 CrossRef CAS.
- F. Mirshafiee and M. Rezaei, Bifunctional CoNi/Fe3O4@GO catalyst for hydrogen generation through NaBH4 in different solvolytic environments: The effect of sequential metal introduction, Int. J. Hydrogen Energy, 2024, 68, 1108–1118 Search PubMed.
- Z. Gao, C. Ding, J. Wang, G. Ding, Y. Xue, Y. Zhang, K. Zhang, P. Liu and X. Gao, Cobalt nanoparticles packaged into nitrogen-doped porous carbon derived from metal-organic framework nanocrystals for hydrogen production by hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2019, 44, 8365–8375 Search PubMed.
- Y. Wang, K. Qi, S. Wu, Z. Cao, K. Zhang, Y. Lu and H. Liu, Preparation, characterization and catalytic sodium borohydride hydrolysis of nanostructured cobalt–phosphorous catalysts, J. Power Sources, 2015, 284, 130–137 Search PubMed.
- K. W. Cho and H. S. Kwon, Effects of electrodeposited Co and Co–P catalysts on the hydrogen generation properties from hydrolysis of alkaline sodium borohydride solution, Catal. Today, 2007, 120, 298–304 Search PubMed.
- Y. Guo, Q. Feng, Z. Dong and J. Ma, Electrodeposited amorphous Co–P catalyst for hydrogen generation from hydrolysis of alkaline sodium borohydride solution, J. Mol. Catal. A: Chem., 2013, 378, 273–278 CrossRef CAS.
- X. Zhang, J. Zhao, F. Cheng, J. Liang, Z. Tao and J. Chen, Electroless-deposited Co–P catalysts for hydrogen generation from alkaline NaBH4 solution, Int. J. Hydrogen Energy, 2010, 35, 8363–8369 Search PubMed.
- T. H. Oh and S. Kwon, Effect of manufacturing conditions on properties of electroless deposited Co–P/Ni foam catalyst for hydrolysis of sodium borohydride solution, Int. J. Hydrogen Energy, 2012, 37, 15925–15937 CrossRef CAS.
- M. Rakap, E. E. Kalu and S. Özkar, Cobalt–nickel–phosphorus supported on Pd-activated TiO2 (Co–Ni–P/Pd-TiO2) as cost-effective and reusable catalyst for hydrogen generation from hydrolysis of alkaline sodium borohydride solution, J. Alloys Compd., 2011, 509, 7016–7021 CrossRef CAS.
- A. M. Pornea, M. W. Abebe and H. Kim, Ternary NiCoP urchin like 3D nanostructure supported on nickel foam as a catalyst for hydrogen generation of alkaline NaBH4, Chem. Phys., 2019, 516, 152–159 Search PubMed.
- Z. Li, H. Li, L. Wang, T. Liu, T. Zhang, G. Wang and G. Xie, Hydrogen generation from catalytic hydrolysis of sodium borohydride solution using supported amorphous alloy catalysts (Ni–Co–P/γ-Al2O3), Int. J. Hydrogen Energy, 2014, 39, 14935–14941 Search PubMed.
- Y. Guo, Q. Feng and J. Ma, The hydrogen generation from alkaline NaBH4 solution by using electroplated amorphous Co–Ni–P film catalysts, Appl. Surf. Sci., 2013, 273, 253–256 CrossRef CAS.
- Y. Guo, Z. Dong, Z. Cui, X. Zhang and J. Ma, Promoting effect of W doped in electrodeposited Co–P catalysts for hydrogen generation from alkaline NaBH4 solution, Int. J. Hydrogen Energy, 2012, 37, 1577–1583 CrossRef CAS.
- K. Li, M. Ma, L. Xie, Y. Yao, R. Kong, G. Du, A. M. Asiri and X. Sun, Monolithically integrated NiCoP nanosheet array on Ti mesh: An efficient and reusable catalyst in NaBH4 alkaline media toward on-demand hydrogen generation, Int. J. Hydrogen Energy, 2017, 42, 19028–19034 Search PubMed.
- Y. Wei, X. Huang, J. Wang, H. Yu, X. Zhao and D. Cheng, Synthesis of bifunctional non-noble monolithic catalyst Co-WP/carbon cloth for sodium borohydride hydrolysis and reduction of 4-nitrophenol, Int. J. Hydrogen Energy, 2017, 42, 25860–25868 Search PubMed.
- Z. Li, L. Wang, Y. Zhang and G. Xie, Properties of CuCoP/γ-Al2O3 catalysts for efficient hydrogen generation by hydrolysis of alkaline NaBH4 solution, Int. J. Hydrogen Energy, 2017, 42, 5749–5757 CrossRef CAS.
- D. H. Kim, S. Jo, J. Kwon, S. Lee and K. Eom, Effect of iron content on the hydrogen production kinetics of electroless-deposited CoNiFeP alloy catalysts from the hydrolysis of sodium borohydride, and a study of its feasibility in a new hydrolysis using magnesium and calcium borohydrides, Int. J. Hydrogen Energy, 2019, 44, 15228–15238 CrossRef CAS.
- L. Shi, Z. Chen, Z. Jian, F. Guo and C. Gao, Carbon nanotubes-promoted Co–B catalysts for rapid hydrogen generation via NaBH4 hydrolysis, Int. J. Hydrogen Energy, 2019, 44, 19868–19877 Search PubMed.
- J. Zhao, H. Ma and J. Chen, Improved hydrogen generation from alkaline NaBH4 solution using carbon-supported Co–B as catalysts, Int. J. Hydrogen Energy, 2007, 32, 4711–4716 Search PubMed.
- S. Li, S. Qiu, Y. S. Chua, Y. Xia, Y. Zou, F. Xu, L. Sun and H. Chu, Co–B supported on waxberry-like hierarchical porous carbon microspheres: An efficient catalyst for hydrogen generation via sodium borohydride hydrolysis, Mater. Chem. Phys., 2024, 319, 129399 Search PubMed.
- P. Krishnan, S. G. Advani and A. K. Prasad, Thin-film CoB catalyst templates for the hydrolysis of NaBH4 solution for hydrogen generation, Appl. Catal., B, 2009, 86, 137–144 CrossRef CAS.
- S. S. Muir, Z. Chen, B. J. Wood, L. Wang, G. M. Lu and X. Yao, New electroless plating method for preparation of highly active Co–B catalysts for NaBH4 hydrolysis, Int. J. Hydrogen Energy, 2014, 39, 414–425 CrossRef CAS.
- Y. Wang, T. Li, S. Bai, K. Qi, Z. Cao, K. Zhang, S. Wu and D. Wang, Catalytic hydrolysis of sodium borohydride via nanostructured cobalt–boron catalysts, Int. J. Hydrogen Energy, 2016, 41, 276–284 CrossRef CAS.
- S. Jeong, R. Kim, E. Cho, H.-J. Kim, S.-W. Nam, I.-H. Oh, S.-A. Hong and S. H. Kim, A study on hydrogen generation from NaBH4 solution using the high-performance Co-B catalyst, J. Power Sources, 2005, 144, 129–134 Search PubMed.
- Y.-C. Lu, M.-S. Chen and Y.-W. Chen, Hydrogen generation by sodium borohydride hydrolysis on nanosized CoB catalysts supported on TiO2, Al2O3 and CeO2, Int. J. Hydrogen Energy, 2012, 37, 4254–4258 Search PubMed.
- H. Tian, Q. Guo and D. Xu, Hydrogen generation from catalytic hydrolysis of alkaline sodium borohydride solution using attapulgite clay-supported Co-B catalyst, J. Power Sources, 2010, 195, 2136–2142 CrossRef CAS.
- L. Shi, W. Xie, Z. Jian, X. Liao and Y. Wang, Graphene modified Co–B catalysts for rapid hydrogen production from NaBH4 hydrolysis, Int. J. Hydrogen Energy, 2019, 44, 17954–17962 CrossRef CAS.
- N. Selvitepe, A. Balbay and C. Saka, Optimisation of sepiolite clay with phosphoric acid treatment as support material for CoB catalyst and application to produce hydrogen from the NaBH4 hydrolysis, Int. J. Hydrogen Energy, 2019, 44, 16387–16399 Search PubMed.
- L. Shi, K. Zhu, Y. Yang, Q. Liang, Q. Peng, S. Zhou, T. T. Isimjan and X. Yang, Phytic acid-derivative Co2B-CoPOx coralloidal structure with delicate boron vacancy for enhanced hydrogen generation from sodium borohydride, Chin. Chem. Lett., 2024, 35, 109222 CrossRef CAS.
- X.-L. Ding, X. Yuan, C. Jia and Z.-F. Ma, Hydrogen generation from catalytic hydrolysis of sodium borohydride solution using cobalt–copper–boride (Co–Cu–B) catalysts, Int. J. Hydrogen Energy, 2010, 35, 11077–11084 Search PubMed.
- Y. Wang, K. Zou, D. Zhang, Z. Cao, K. Zhang, Y. Xie, G. Li and S. Bai, Cobalt–copper–boron nanoparticles as catalysts for the efficient hydrolysis of alkaline sodium borohydride solution, Int. J. Hydrogen Energy, 2020, 45, 9845–9853 CrossRef CAS.
- N. Patel, R. Fernandes and A. Miotello, Promoting effect of transition metal-doped Co–B alloy catalysts for hydrogen production by hydrolysis of alkaline NaBH4 solution, J. Catal., 2010, 271, 315–324 CrossRef CAS.
- J. Ingersoll, N. Mani, J. Thenmozhiyal and A. Muthaiah, Catalytic hydrolysis of sodium borohydride by a novel nickel–cobalt–boride catalyst, J. Power Sources, 2007, 173, 450–457 CrossRef CAS.
- X. Yuan, C. Jia, X.-L. Ding and Z.-F. Ma, Effects of heat-treatment temperature on properties of Cobalt–Manganese–Boride as efficient catalyst toward hydrolysis of alkaline sodium borohydride solution, Int. J. Hydrogen Energy, 2012, 37, 995–1001 CrossRef CAS.
- H. Dai, Y. Liang, P. Wang, X. Yao, T. Rufford, M. Lu and H. Cheng, High-performance cobalt–tungsten–boron catalyst supported on Ni foam for hydrogen generation from alkaline sodium borohydride solution, Int. J. Hydrogen Energy, 2008, 33, 4405–4412 CrossRef CAS.
- Q. Xu and M. Chandra, A portable hydrogen generation system: Catalytic hydrolysis of ammonia-borane, J. Alloys Compd., 2007, 446, 729–732 CrossRef.
- H. L. Jiang, S. K. Singh, J. M. Yan, X. B. Zhang and Q. Xu, Liquid–phase chemical hydrogen storage: catalytic hydrogen generation under ambient conditions, ChemSusChem, 2010, 3, 541–549 CrossRef CAS PubMed.
- A. Gutowska, L. Li, Y. Shin, C. M. Wang, X. S. Li, J. C. Linehan, R. S. Smith, B. D. Kay, B. Schmid, W. Shaw, M. Gutowski and T. Autrey, Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane, Angew. Chem., Int. Ed., 2005, 44, 3578–3582 CrossRef CAS PubMed.
- V. Sit, R. Geanangel and W. Wendlandt, The thermal dissociation of NH3BH3, Thermochim. Acta, 1987, 113, 379–382 Search PubMed.
- Z. Li, G. Zhu, G. Lu, S. Qiu and X. Yao, Ammonia borane confined by a metal-organic framework for chemical hydrogen storage: enhancing kinetics and eliminating ammonia, J. Am. Chem. Soc., 2010, 132, 1490–1491 CrossRef CAS PubMed.
- Q. Yao, W. Shi, G. Feng, Z. H. Lu, X. Zhang, D. Tao, D. Kong and X. Chen, Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: Highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane, J. Power Sources, 2014, 257, 293–299 CrossRef CAS.
- H. L. Jiang, T. Akita, T. Ishida, M. Haruta and Q. Xu, Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework, J. Am. Chem. Soc., 2011, 133, 1304–1306 CrossRef CAS PubMed.
- J. K. Sun, Z. Kochovski, W. Y. Zhang, H. Kirmse and J. Yuan, A general synthetic route towards highly dispersed metal clusters enabled by poly(ionic liquid)s, J. Am. Chem. Soc., 2017, 139, 8971 Search PubMed.
- Q. Yao, Z.-H. Lu, Y. Yang, Y. Chen, X. Chen and H.-L. Jiang, Facile synthesis of graphene-supported Ni-CeOx nanocomposites as highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane, Nano Res., 2018, 11, 4412–4422 Search PubMed.
- C. Y. Peng, L. Kang, S. Cao, Y. Chen, Z. S. Lin and W. F. Fu, Nanostructured Ni2P as a robust catalyst for the hydrolytic dehydrogenation of ammonia–borane, Angew. Chem., 2015, 127, 15951–15955 Search PubMed.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Applications of metal–organic frameworks in heterogeneous supramolecular catalysis, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- W. Wang, Z.-H. Lu, Y. Luo, A. Zou, Q. Yao and X. Chen, Mesoporous carbon nitride supported Pd and Pd-Ni nanoparticles as highly efficient catalyst for catalytic hydrolysis of NH3BH3, ChemCatChem, 2018, 10, 1620–1626 CrossRef CAS.
- L. Zhang, L. Zhou, K. Yang, D. Gao, C. Huang, Y. Chen, F. Zhang, X. Xiong, L. Li and Q. Xia, Pd Ni nanoparticles supported on MIL-101 as high-performance catalysts for hydrogen generation from ammonia borane, J. Alloys Compd., 2016, 677, 87–95 Search PubMed.
- C. Yao, L. Zhuang, Y. Cao, X. Ai and H. Yang, Hydrogen release from hydrolysis of borazane on Pt- and Ni-based alloy catalysts, Int. J. Hydrogen Energy, 2008, 33, 2462–2467 CrossRef CAS.
- X. Wang, D. Liu, S. Song and H. Zhang, Graphene oxide induced formation of Pt-CeO2 hybrid nanoflowers with tunable CeO2 thickness for catalytic hydrolysis of ammonia borane, Chemistry, 2013, 19, 8082–8086 Search PubMed.
- L. Wang, H. Li, W. Zhang, X. Zhao, J. Qiu, A. Li, X. Zheng, Z. Hu, R. Si and J. Zeng, Supported rhodium catalysts for ammonia-borane hydrolysis: Dependence of the catalytic activity on the highest occupied state of the single rhodium atoms, Angew. Chem., Int. Ed., 2017, 56, 4712–4718 Search PubMed.
- D.-C. Zhong, Y.-L. Mao, X. Tan, P. Zhong and L.-X. Liu, RhNi nanocatalyst: Spontaneous alloying and high activity for hydrogen generation from hydrous hydrazine, Int. J. Hydrogen Energy, 2016, 41, 6362–6368 CrossRef CAS.
- S. Wang, S. Li, Y. Yu, T. Zhang, J. Qu and Q. Sun, Cobalt Phosphide–supported single–atom Pt catalysts for efficient and stable hydrogen generation from ammonia borane hydrolysis, Small Methods, 2024, 8, 2400376 Search PubMed.
- H. Zhang, Y. Fan, B. Liu, Y. Liu, S. Ashraf, X. Wu, G. Han, J. Gao and B. Li, Birdcage-type CoOx-carbon catalyst derived from metal–organic frameworks for enhanced hydrogen generation, ACS Sustainable Chem. Eng., 2019, 7, 9782–9792 Search PubMed.
- M. Chen, R. Xiong, X. Cui, Q. Wang and X. Liu, SiO2-encompassed Co@N-doped porous carbon assemblies as recyclable catalysts for efficient hydrolysis of ammonia borane, Langmuir, 2019, 35, 671–677 CrossRef CAS PubMed.
- Z. C. Fu, Y. Xu, S. L. Chan, W. W. Wang, F. Li, F. Liang, Y. Chen, Z. S. Lin, W. F. Fu and C. M. Che, Highly efficient hydrolysis of ammonia borane by anion ((-)OH, F(-), Cl(-))-tuned interactions between reactant molecules and CoP nanoparticles, Chem. Commun., 2017, 53, 705–708 RSC.
- Ö. Metin and S. Özkar, Water soluble nickel(0) and cobalt(0) nanoclusters stabilized by poly(4-styrenesulfonic acid-co-maleic acid): Highly active, durable and cost effective catalysts in hydrogen generation from the hydrolysis of ammonia borane, Int. J. Hydrogen Energy, 2011, 36, 1424–1432 Search PubMed.
- Z.-L. Wang, J.-M. Yan, H.-L. Wang and Q. Jiang, Self-protective cobalt nanocatalyst for long-time recycle application on hydrogen generation by its free metal-ion conversion, J. Power Sources, 2013, 243, 431–435 Search PubMed.
- S.-H. Li, X.-R. Song, Y.-T. Li, Y.-Q. Zhao and X.-C. Zheng, Efficient hydrolytic dehydrogenation of ammonia borane over ultrafine Ru nanoparticles supported on biomass-derived porous carbon, Int. J. Hydrogen Energy, 2021, 46, 27555–27566 CrossRef CAS.
- Q. Xu and M. Chandra, Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia–borane at room temperature, J. Power Sources, 2006, 163, 364–370 CrossRef CAS.
- R. Herron, C. Marchant and J. A. Sullivan, Co-SBA-15 catalysts in the hydrolysis of NH3BH3-Influences of Co precursors and catalyst pre-treatment, Catal. Commun., 2018, 107, 14–17 CrossRef CAS.
- M.-H. Lee, J. R. Deka, C.-J. Cheng, N.-F. Lu, D. Saikia, Y.-C. Yang and H.-M. Kao, Synthesis of highly dispersed ultra-small cobalt nanoparticles within the cage-type mesopores of 3D cubic mesoporous silica via double agent reduction method for catalytic hydrogen generation, Appl. Surf. Sci., 2019, 470, 764–772 CrossRef CAS.
- Q. Yao, H. Du and Z.-H. Lu, Catalytic hydrolysis of ammonia borane for hydrogen production, Prog. Chem., 2021, 32, 1930 Search PubMed.
- Y.-T. Li, S. Ullah, Z. Han, X.-C. Zheng and G.-P. Zheng, Hierarchical porous CuNi-based bimetal-organic frameworks as efficient catalysts for ammonia borane hydrolysis, Catal. Commun., 2020, 143, 106057 CrossRef CAS.
- P. Liu, X. Gu, K. Kang, H. Zhang, J. Cheng and H. Su, Highly efficient catalytic hydrogen evolution from ammonia borane using the synergistic effect of crystallinity and size of noble-metal-free nanoparticles supported by porous metal-organic frameworks, ACS Appl. Mater. Interfaces, 2017, 9, 10759–10767 Search PubMed.
- J. Song, X. Gu, J. Cheng, N. Fan, H. Zhang and H. Su, Remarkably boosting catalytic H2 evolution from ammonia borane through the visible-light-driven synergistic electron effect of non-plasmonic noble-metal-free nanoparticles and photoactive metal-organic frameworks, Appl. Catal., B, 2018, 225, 424–432 CrossRef CAS.
- S. Duan, G. Han, Y. Su, X. Zhang, Y. Liu, X. Wu and B. Li, Magnetic Co@g-C3N4 core–shells on rGO sheets for momentum transfer with catalytic activity toward continuous-flow hydrogen generation, Langmuir, 2016, 32, 6272–6281 CrossRef CAS PubMed.
- Z. Li, T. He, L. Liu, W. Chen, M. Zhang, G. Wu and P. Chen, Covalent triazine framework supported non-noble metal nanoparticles with superior activity for catalytic hydrolysis of ammonia borane: from mechanistic study to catalyst design, Chem. Sci., 2017, 8, 781–788 RSC.
- Y. Fang, Z. Xiao, J. Li, C. Lollar, L. Liu, X. Lian, S. Yuan, S. Banerjee, P. Zhang and H.-C. Zhou, Formation of a highly reactive cobalt nanocluster crystal within a highly negatively charged porous coordination cage, Angew. Chem., Int. Ed., 2018, 57, 5283–5287 CrossRef CAS PubMed.
- X.-L. Zhang, D.-X. Zhang, G.-G. Chang, X.-C. Ma, J. Wu, Y. Wang, H.-Z. Yu, G. Tian, J. Chen and X.-Y. Yang, Bimetallic (Zn/Co) MOFs-derived highly dispersed metallic Co/HPC for completely hydrolytic dehydrogenation of ammonia–borane, Ind. Eng. Chem. Res., 2019, 58, 7209–7216 CrossRef CAS.
- X. Li, Q. Yao, R. Shi, M. Huang and Z. H. Lu, A step–growth strategy to grow vertical porous aromatic framework nanosheets on graphene oxide: Hybrid material–confined Co for ammonia borane methanolysis, Carbon Energy, 2023, 5, e357 Search PubMed.
- D. R. Kumar, S. Prabu, K. Y. Chiang and T. H. Oh, Methanolysis of ammonia borane using binder–free hierarchical Co@Ni metal–organic framework nanocolumn arrays catalyst for hydrogen generation, Int. J. Energy Res., 2022, 46, 18134–18145 Search PubMed.
- J.-M. Yan, X.-B. Zhang, T. Akita, M. Haruta and Q. Xu, One-step seeding growth of magnetically recyclable Au@Co core−shell nanoparticles: Highly efficient catalyst for hydrolytic dehydrogenation of ammonia borane, J. Am. Chem. Soc., 2010, 132, 5326–5327 CrossRef CAS PubMed.
- L.-T. Guo, Y.-Y. Cai, J.-M. Ge, Y.-N. Zhang, L.-H. Gong, X.-H. Li, K.-X. Wang, Q.-Z. Ren, J. Su and J.-S. Chen, Multifunctional Au–Co@CN nanocatalyst for highly efficient hydrolysis of ammonia borane, ACS Catal., 2015, 5, 388–392 Search PubMed.
- Y. Meng, Q. Sun, T. Zhang, J. Zhang, Z. Dong, Y. Ma, Z. Wu, H. Wang, X. Bao, Q. Sun and J. Yu, Cobalt-promoted noble-metal catalysts for efficient hydrogen generation from ammonia borane hydrolysis, J. Am. Chem. Soc., 2023, 145, 5486–5495 CrossRef CAS PubMed.
- Z.-H. Lu, H.-L. Jiang, M. Yadav, K. Aranishi and Q. Xu, Synergistic catalysis of Au-Co@SiO2 nanospheres in hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage, J. Mater. Chem., 2012, 22, 5065 Search PubMed.
- W. Li, Y. Zhao, Y. Liu, M. Sun, G. I. Waterhouse, B. Huang, K. Zhang, T. Zhang and S. Lu, Exploiting Ru–induced lattice strain in CoRu nanoalloys for robust bifunctional hydrogen production, Angew. Chem., Int. Ed., 2021, 133, 3327–3335 CrossRef.
- J. Li, Q.-L. Zhu and Q. Xu, Non-noble bimetallic CuCo nanoparticles encapsulated in the pores of metal–organic frameworks: synergetic catalysis in the hydrolysis of ammonia borane for hydrogen generation, Catal. Sci. Technol., 2015, 5, 525–530 Search PubMed.
- Q. Yao, K. Yang, X. Hong, X. Chen and Z.-H. Lu, Base-promoted hydrolytic dehydrogenation of ammonia borane catalyzed by noble-metal-free nanoparticles, Catal. Sci. Technol., 2018, 8, 870–877 RSC.
- L. Zhao, Q. Wei, L. Zhang, Y. Zhao and B. Zhang, NiCo alloy decorated on porous N-doped carbon derived from ZnCo-ZIF as highly efficient and magnetically recyclable catalyst for hydrogen evolution from ammonia borane, Renewable Energy, 2021, 173, 273–282 CrossRef CAS.
- Y. Ge, X. Qin, A. Li, Y. Deng, L. Lin, M. Zhang, Q. Yu, S. Li, M. Peng, Y. Xu, X. Zhao, M. Xu, W. Zhou, S. Yao and D. Ma, Maximizing the synergistic effect of CoNi catalyst on α-MoC for robust hydrogen production, J. Am. Chem. Soc., 2021, 143, 628–633 CrossRef CAS PubMed.
- X. Su and S. Li, PVP-stabilized Co–Ni nanoparticles as magnetically recyclable catalysts for hydrogen production from methanolysis of ammonia borane, Int. J. Hydrogen Energy, 2021, 46, 14384–14394 Search PubMed.
- D. Sun, V. Mazumder, N. Metin and S. Sun, Methanolysis of ammonia borane by CoPd nanoparticles, ACS Catal., 2012, 2, 1290–1295 CrossRef CAS.
- B. Abay and M. Rakap, Rh–M (M: Co, Cu, and Fe) nanoclusters as highly efficient and durable catalysts for the methanolysis of ammonia borane, Catal. Sci. Technol., 2020, 10, 7270–7279 Search PubMed.
- E. I. Epelle, K. S. Desongu, W. Obande, A. A. Adeleke, P. P. Ikubanni, J. A. Okolie and B. Gunes, A comprehensive review of hydrogen production and storage: A focus on the role of nanomaterials, Int. J. Hydrogen Energy, 2022, 47, 20398–20431 Search PubMed.
- P. Li, R. Chen, S. Zhao, W. Li, Y. Lin and Y. Yu, Architecture control and electronic structure engineering over Ni-based nitride nanocomposite for boosting ammonia borane dehydrogenation, Appl. Catal., B, 2021, 298, 120523 CrossRef CAS.
- Y. Chen, K. Feng, G. Yuan, Z. Kang and J. Zhong, Highly efficient CoNiP nanoboxes on graphene oxide for the hydrolysis of ammonia borane, Chem. Eng. J., 2022, 428, 131219 CrossRef CAS.
- H. Zhang, Y. Liu, H. Wei, C. Wang, T. Liu, X. Wu, S. Ashraf, S. Mehdi, S. Guan, Y. Fan, X. Yue, B. Liu, Y. Zhang, H. Cao and B. Li, Atomic-bridge structure in B-Co-P dual-active sites on boron nitride nanosheets for catalytic hydrogen generation, Appl. Catal., B, 2022, 314, 121495 CrossRef CAS.
- C.-C. Hou, Q.-Q. Chen, K. Li, C.-J. Wang, C.-Y. Peng, R. Shi and Y. Chen, Tailoring three-dimensional porous cobalt phosphides templated from bimetallic metal–organic frameworks as precious metal-free catalysts towards the dehydrogenation of ammonia-borane, J. Mater. Chem. A, 2019, 7, 8277–8283 Search PubMed.
- S. B. Kalidindi, A. A. Vernekar and B. R. Jagirdar, Co-Co2B, Ni-Ni3B and Co-Ni-B nanocomposites catalyzed ammonia-borane methanolysis for hydrogen generation, Phys. Chem. Chem. Phys., 2010, 40, 770–775 Search PubMed.
- K. O. Amoo, E. N. Onyeozili, E. E. Kalu, J. A. Omoleye and V. E. Efeovbokhan, Activity of varying compositions of Co-Ni-P catalysts for the methanolysis of ammonia borane, Int. J. Hydrogen Energy, 2016, 30, 1–15 Search PubMed.
- J.-M. Yan, X.-B. Zhang, H. Shioyama and Q. Xu, Room temperature hydrolytic dehydrogenation of ammonia borane catalyzed by Co nanoparticles, J. Power Sources, 2010, 195, 1091–1094 CrossRef CAS.
- J. Hu, Z. Chen, M. Li, X. Zhou and H. Lu, Amine-capped Co nanoparticles for highly efficient dehydrogenation of ammonia borane, ACS Appl. Mater. Interfaces, 2014, 6, 13191–13200 CrossRef CAS PubMed.
- L. Zhou, J. Meng, P. Li, Z. Tao, L. Mai and J. Chen, Ultrasmall cobalt nanoparticles supported on nitrogen-doped porous carbon nanowires for hydrogen evolution from ammonia borane, Mater. Horiz., 2017, 4, 268–273 Search PubMed.
- H. Wang, Y. Zhao, F. Cheng, Z. Tao and J. Chen, Cobalt nanoparticles embedded in porous N-doped carbon as long-life catalysts for hydrolysis of ammonia borane, Catal. Sci. Technol., 2016, 6, 3443–3448 RSC.
- K. Aranishi, Q.-L. Zhu and Q. Xu, Dendrimer-encapsulated cobalt nanoparticles as high-performance catalysts for the hydrolysis of ammonia borane, ChemCatChem, 2014, 6, 1375–1379 Search PubMed.
- Y. Men, J. Su, C. Huang, L. Liang, P. Cai, G. Cheng and W. Luo, Three-dimensional nitrogen-doped graphene hydrogel supported Co-CeOx nanoclusters as efficient catalysts for hydrogen generation from hydrolysis of ammonia borane, Chin. Chem. Lett., 2018, 29, 1671–1674 CrossRef CAS.
- P. V. Ramachandran and P. D. Gagare, Preparation of ammonia borane in high yield and purity, methanolysis, and regeneration, Inorg. Chem., 2007, 46, 7810–7817 CrossRef CAS PubMed.
- J. Liao, Y. Li, J. Tian, Y. Feng, Q. Liu and H. Li, Morphology engineering of hollow core@shell structured Co3O4@CuO-NiO for fast hydrogen release from ammonia borane methanolysis, J. Colloid Interface Sci., 2025, 680, 78–87 CrossRef CAS PubMed.
- G. P. Rachiero, U. B. Demirci and P. Miele, Bimetallic RuCo and RuCu catalysts supported on γ-Al2O3: A comparative study of their activity in hydrolysis of ammonia-borane, Int. J. Hydrogen Energy, 2011, 36, 7051–7065 Search PubMed.
- J. Li, Q. L. Zhu and Q. Xu, Highly active AuCo alloy nanoparticles encapsulated in the pores of metal–organic frameworks for hydrolytic dehydrogenation of ammonia borane, Chem. Commun., 2014, 50, 5899–5901 Search PubMed.
- D. Ke, Y. Li, J. Wang, L. Zhang, J. Wang, X. Zhao, S. Yang and S. Han, Facile fabrication of poly(amidoamine) (PAMAM) dendrimers-encapsulated Ag–Co bimetallic nanoparticles for highly efficient dehydrogenation of ammonia borane, Int. J. Hydrogen Energy, 2016, 41, 2564–2574 Search PubMed.
- D. Sun, V. Mazumder, Ö. Metin and S. Sun, Catalytic hydrolysis of ammonia borane via cobalt palladium nanoparticles, ACS Nano, 2011, 5, 6458–6464 CrossRef CAS PubMed.
- Y.-Z. Chen, Q. Xu, S.-H. Yu and H.-L. Jiang, Tiny Pd@Co core–shell nanoparticles confined inside a metal–organic framework for highly efficient catalysis, Small, 2015, 11, 71–76 CrossRef CAS PubMed.
- F. Y. Qiu, Y. J. Wang, Y. P. Wang, L. Li, G. Liu, C. Yan, L. F. Jiao and H. T. Yuan, Dehydrogenation of ammonia borane catalyzed by in situ synthesized Fe–Co nano-alloy in aqueous solution, Catal. Today, 2011, 170, 64–68 CrossRef CAS.
- J. M. Yan, Z. L. Wang, H. L. Wang and Q. Jiang, Rapid and energy-efficient synthesis of a graphene–CuCo hybrid as a high performance catalyst, J. Mater. Chem., 2012, 22, 10990–10993 RSC.
- F.-Z. Song, Q.-L. Zhu, X.-C. Yang and Q. Xu, Monodispersed CuCo nanoparticles supported on diamine-functionalized graphene as a non-noble metal catalyst for hydrolytic dehydrogenation of ammonia borane, ChemNanoMat, 2016, 2, 942–945 CrossRef CAS.
- Q. Yao, Z.-H. Lu, Y. Wang, X. Chen and G. Feng, Synergetic catalysis of non-noble bimetallic Cu–Co nanoparticles embedded in SiO2 nanospheres in hydrolytic dehydrogenation of ammonia borane, J. Phys. Chem. C, 2015, 119, 14167–14174 Search PubMed.
- K. Feng, J. Zhong, B. Zhao, H. Zhang, L. Xu, X. Sun and S. T. Lee, CuxCo1−xO nanoparticles on graphene oxide as a synergistic catalyst for high–efficiency hydrolysis of ammonia–borane, Angew. Chem., Int. Ed., 2016, 55, 11950–11954 CrossRef CAS PubMed.
- C. Yuan, T. Xu, M. Guo, Y. Sun, T. Zhang and X. Yu, Unveiling the bifunctional modulation evoked by bromine doping of CoP towards efficient hydrolytic hydrogen generation, Appl. Catal., B, 2024, 343, 123562 CrossRef CAS.
- Y. Zhao, S. Guo, X. Xue, C. Xiong, X. Gao and B. Zhang, Halloysite nanotubes supported Co2P bridged by carbon dots for enhanced hydrogen evolution from ammonia borane, Chem. Eng. J., 2024, 483, 149332 Search PubMed.
- W. Xu, W. Li, M. Liu, X. Guo, H. Wen and B. Li, P-bridged Fe-X-Co coupled sites in hollow carbon spheres for efficient hydrogen generation, J. Colloid Interface Sci., 2024, 660, 792–799 Search PubMed.
- H. Wu, Y. Cheng, B. Wang, Y. Wang, M. Wu, W. Li, B. Liu and S. Lu, Carbon dots-confined CoP-CoO nanoheterostructure with strong interfacial synergy triggered the robust hydrogen evolution from ammonia borane, J. Energy Chem., 2021, 57, 198–205 CrossRef CAS.
- W. Wang, Z. Dai, R. Jiang, Q. Li, X. Zheng, W. Liu, Z. Luo, Z. Xu and J. Peng, Highly phosphatized magnetic catalyst with electron transfer induced by quaternary synergy for efficient dehydrogenation of ammonia borane, ACS Appl. Mater. Interfaces, 2020, 12, 43854–43863 CrossRef CAS PubMed.
- Ö. Şahin, G. Y. Keleş and K. Büyükkanber, Cobalt-doped carbon spheres: Impact on potassium borohydride hydrolysis, Int. J. Hydrogen Energy, 2024, 90, 1227–1236 CrossRef.
- E. Onat, M. S. Izgi, Ö. Şahin and C. Saka, Highly active hydrogen production from hydrolysis of potassium borohydride by caffeine carbon quantum dot-supported cobalt catalyst in ethanol solvent by hydrothermal treatment, Int. J. Hydrogen Energy, 2024, 51, 362–375 CrossRef CAS.
- B. Sen, S. Kuzu, E. Demir, S. Akocak and F. Sen, Highly monodisperse RuCo nanoparticles decorated on functionalized multiwalled carbon nanotube with the highest observed catalytic activity in the dehydrogenation of dimethylamine-borane, Int. J. Hydrogen Energy, 2017, 42, 23292–23298 CrossRef CAS.
- N. Tunç and M. Rakap, Surfactant-aided synthesis of RhCo nanoclusters as highly effective and recyclable catalysts for the hydrolysis of methylamine borane and dimethylamine borane, Catal. Sci. Technol., 2020, 10, 7865–7874 RSC.
- S. Xie, S. Tian, J. Yang, N. Wang, Q. Wan, M. Wang, J. Liu, J. Zhou, P. Qi and K. Sui, Synergizing Mg single atoms and Ru nanoclusters for boosting the ammonia borane hydrolysis to produce hydrogen, Angew. Chem., Int. Ed., 2025, e202424316 CAS.
- L. Sun, Y. Meng, X. Kong, H. Ge, X. Chen, C. Ding, H. Yang, D. Li, X. Gao and J. Dou, Novel high dispersion and high stability cobalt-inlaid carbon sphere catalyst for hydrogen generation from the hydrolysis of sodium borohydride, Fuel, 2022, 310, 122276 CrossRef CAS.
- Y. Wang, Z. Hu, W. Chen, S. Wu, G. Li and S. Chou, Non–noble metal–based catalysts applied to hydrogen evolution from hydrolysis of boron hydrides, Small Struct., 2021, 2, 2000135 CrossRef CAS.
- H. Wu, M. Wu, B. Wang, X. Yong, Y. Liu, B. Li, B. Liu and S. Lu, Interface electron collaborative migration of Co–Co3O4/carbon dots: Boosting the hydrolytic dehydrogenation of ammonia borane, J. Energy Chem., 2020, 48, 43–53 CrossRef.
- S. Mehdi, Y. Liu, H. Wei, H. Wen, R. Shen, Z. Peng, H. Zhang, X. Wu, C. Wang, S. Guan, T. Liu and B. Li, Co-based nanoparticles fabricated on Ni foams for efficient hydrogen generation from ammonia borane, ACS Appl. Nano Mater., 2022, 5, 5064–5074 Search PubMed.
- C. Wang, J. Tuninetti, Z. Wang, C. Zhang, R. Ciganda, L. Salmon, S. Moya, J. Ruiz and D. Astruc, Hydrolysis of ammonia-borane over Ni/ZIF-8 nanocatalyst: High efficiency, mechanism, and controlled hydrogen release, J. Am. Chem. Soc., 2017, 139, 11610–11615 CrossRef CAS PubMed.
- S. Guan, Y. Liu, H. Zhang, H. Wei, T. Liu, X. Wu, H. Wen, R. Shen, S. Mehdi, X. Ge, C. Wang, B. Liu, E. Liang, Y. Fan and B. Li, Atomic interface-exciting catalysis on cobalt nitride-oxide for accelerating hydrogen generation, Small, 2022, 18, 2107417 CrossRef CAS PubMed.
- Q.-L. Zhu and Q. Xu, Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage, Energy Environ. Sci., 2015, 8, 478–512 Search PubMed.
- Q. Yao, X. Zhang, Z.-H. Lu and Q. Xu, Metal-organic framework-based catalysts for hydrogen production from liquid-phase chemical hydrides, Coord. Chem. Rev., 2023, 493, 215302 Search PubMed.
- J. Li, T. Cai, Y. Feng, X. Liu, N. Wang and Q. Sun, Subnanometric bimetallic Pt–Pd clusters in zeolites for efficient hydrogen production and selective tandem hydrogenation of nitroarenes, Sci. China: Chem., 2024, 67, 2911–2917 Search PubMed.
- M. Liu, Z. Yao, J. Gu, C. Li, X. Huang, L. Zhang, Z. Huang and M. Fan, Issues and opportunities facing hydrolytic hydrogen production materials, Chem. Eng. J., 2023, 461, 141918 Search PubMed.
- J. Huo, K. Zhang, H. Wei, L. Fu, C. Zhao, C. He and X. Hu, A review on hydrogen production from ammonia borane: Experimental and theoretical studies, Chin. Chem. Lett., 2023, 34, 108280 CrossRef CAS.
- D. Xu, Y. Zhang and Q. Guo, Research progress on catalysts for hydrogen generation through sodium borohydride alcoholysis, Int. J. Hydrogen Energy, 2022, 47, 5929–5946 Search PubMed.
- C. Lang, Y. Jia and X. Yao, Recent advances in liquid-phase chemical hydrogen storage, Energy Storage Mater., 2020, 26, 290–312 CrossRef.
- M. Nagyházi, G. Turczel, P. T. Anastas and R. Tuba, Highly efficient ammonia borane hydrolytic dehydrogenation in neat water using phase-labeled CAAC-Ru catalysts, ACS Sustainable Chem. Eng., 2020, 8, 16097–16103 CrossRef.
- Y. Liu, K. Zhang, K. Wang, M. Wang, Y. Liu, J. Jiang, T. Liu, E. Liang and B. Li, Out-of-plane CoRu nanoalloy axially coupling CosNC for electron enrichment to boost hydrogen production, Appl. Catal., B, 2022, 318, 121890 CrossRef CAS.
- G. Li, N. Wei and Y. Wang, Active clusters ensemble effect of bimetallic RuCo alloys for efficient hydrogen production from ammonia borane, Appl. Surf. Sci., 2023, 610, 155459 Search PubMed.
- A. R. Poerwoprajitno, L. Gloag, J. Watt, S. Cheong, X. Tan, H. Lei, H. A. Tahini, A. Henson, B. Subhash and N. M. Bedford, A single-Pt-atom-on-Ru-nanoparticle electrocatalyst for CO-resilient methanol oxidation, Nat. Catal., 2022, 5, 231–237 Search PubMed.
- P. Zhu, X. Xiong and D. Wang, Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction, Nano Res., 2022, 15, 5792–5815 CrossRef CAS.
- X. Liang, N. Fu, S. Yao, Z. Li and Y. Li, The progress and outlook of metal single-atom-site catalysis, J. Am. Chem. Soc., 2022, 144, 18155–18174 Search PubMed.
- X. Zheng, B. Li, Q. Wang, D. Wang and Y. Li, Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis, Nano Res., 2022, 15, 7806–7839 Search PubMed.
- P.-C. Poon, Y. Wang, W. Li, D. W.-S. Suen, W. W. Y. Lam, D. Z. J. Yap, B. L. Mehdi, J. Qi, X.-Y. Lu and E. Y. C. Wong, Synergistic effect of Co catalysts with atomically dispersed CoN x active sites on ammonia borane hydrolysis for hydrogen generation, J. Mater. Chem. A, 2022, 10, 5580–5592 Search PubMed.
- J. Wang, Z. Huang, L. Lu, Q. Jia, L. Huang, S. Chang, M. Zhang, Z. Zhang, S. Li and D. He, Colloidal Co single-atom catalyst: a facile synthesis strategy and high catalytic activity for hydrogen generation, Green Chem., 2020, 22, 1269–1274 RSC.
- N. Patel, A. Kale and A. Miotello, Improved dehydrogenation of ammonia borane over Co-P-B coating on Ni: A single catalyst for both hydrolysis and thermolysis, Appl. Catal., B, 2012, 111, 178–184 CrossRef.
- X. Jia, Z. Sang, L. Sun, F. Xu, H. Pan, C. Zhang, R. Cheng, Y. Yu, H. Hu and L. Kang, Graphene-modified Co-B-P catalysts for hydrogen generation from sodium borohydride hydrolysis, Nanomaterials, 2022, 12, 2732 CrossRef CAS PubMed.
- M. Shi, Y. Hou, X. Du, J. Guo, B. Li and Y. Li, 2D/2D-Ni–Co–B/rGO nanolayers prepared by galvanic replacement for hydrogen generation through NaBH4 hydrolysis, ACS Appl. Nano Mater., 2023, 6, 6798–6809 Search PubMed.
- H. Guo, X. Liu, Y. Hou, Q. Xie, L. Wang, H. Geng and D.-L. Peng, Magnetically separable and recyclable urchin-like Co–P hollow nanocomposites for catalytic hydrogen generation, J. Power Sources, 2014, 260, 100–108 CrossRef CAS.
- R. Fernandes, N. Patel, A. Miotello, R. Jaiswal and D. Kothari, Dehydrogenation of ammonia borane with transition metal-doped Co–B alloy catalysts, Int. J. Hydrogen Energy, 2012, 37, 2397–2406 CrossRef CAS.
- C. Peng, T. Li, Y. Zou, C. Xiang, F. Xu, J. Zhang and L. Sun, Bacterial cellulose derived carbon as a support for catalytically active Co–B alloy for hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2021, 46, 666–675 CrossRef CAS.
- R. Singh, Reversible chemical hydrogen storage in borohydrides via thermolysis and hydrolysis: Recent advances, challenges, and perspectives, Int. J. Hydrogen Energy, 2022, 47, 26549–26573 CrossRef CAS.
- Y. Guo, M. Wang, Q. Zhu, D. Xiao and D. Ma, Ensemble effect for single-atom, small cluster and nanoparticle catalysts, Nat. Catal., 2022, 5, 766–776 CrossRef CAS.
- Z. Liu, Y. Du, P. Zhang, Z. Zhuang and D. Wang, Bringing catalytic order out of chaos with nitrogen-doped ordered mesoporous carbon, Matter, 2021, 4, 3161–3194 CrossRef CAS.
- C. Xie, Z. Niu, D. Kim, M. Li and P. Yang, Surface and interface control in nanoparticle catalysis, Chem. Rev., 2019, 120, 1184–1249 CrossRef PubMed.
- L. Liu and A. Corma, Confining isolated atoms and clusters in crystalline porous materials for catalysis, Nat. Rev. Mater., 2021, 6, 244–263 CrossRef CAS.
- T. Pu, W. Zhang and M. Zhu, Engineering heterogeneous catalysis with strong metal–support interactions: characterization, theory and manipulation, Angew. Chem., Int. Ed., 2023, 62, e202212278 CrossRef CAS PubMed.
- C. Dong, Y. Li, D. Cheng, M. Zhang, J. Liu, Y.-G. Wang, D. Xiao and D. Ma, Supported metal clusters: fabrication and application in heterogeneous catalysis, ACS Catal., 2020, 10, 11011–11045 CrossRef CAS.
- Y. Liu, Q. Wang, J. Zhang, J. Ding, Y. Cheng, T. Wang, J. Li, F. Hu, H. B. Yang and B. Liu, Recent advances in carbon–supported noble–metal electrocatalysts for hydrogen evolution reaction: Syntheses, structures, and properties, Adv. Energy Mater., 2022, 12, 2200928 CrossRef CAS.
- A. Kumar, P. Raizada, A. Hosseini-Bandegharaei, V. K. Thakur, V.-H. Nguyen and P. Singh, C-, N-Vacancy defect engineered polymeric carbon nitride towards photocatalysis: viewpoints and challenges, J. Mater. Chem. A, 2021, 9, 111–153 RSC.
- C. Wan, G. Li, J. Wang, L. Xu, D. Cheng, F. Chen, Y. Asakura, Y. Kang and Y. Yamauchi, Modulating electronic metal–support interactions to boost visible–light–driven hydrolysis of ammonia borane: nickel–platinum nanoparticles supported on phosphorus–doped titania, Angew. Chem., Int. Ed., 2023, 62, e202305371 CrossRef CAS PubMed.
- X. Li, C. Zhang, M. Luo, Q. Yao and Z.-H. Lu, Ultrafine Rh nanoparticles confined by nitrogen-rich covalent organic frameworks for methanolysis of ammonia borane, Inorg. Chem. Front., 2020, 7, 1298–1306 RSC.
- X. Li, Q. Yao, Z. Li, H. Li, Q.-L. Zhu and Z.-H. Lu, Porphyrin framework-derived N-doped porous carbon-confined Ru for NH3BH3 methanolysis: the more pyridinic-N, the better, J. Mater. Chem. A, 2022, 10, 326–336 RSC.
- H. J. Kim, K.-J. Shin, H.-J. Kim, M. K. Han, H. Kim, Y.-G. Shul and K. T. Jung, Hydrogen generation from aqueous acid-catalyzed hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2010, 35, 12239–12245 CrossRef CAS.
- S. Murugesan and V. Subramanian, Effects of acid accelerators on hydrogen generation from solid sodium borohydride using small scale devices, J. Power Sources, 2009, 187, 216–223 CrossRef CAS.
- L. Kang, C. Liu, J. Ye, W. Niu, X. Cui, Y. Zhu, L. Xue, J. Zhang, L. Zheng and Y. Li, Polypyrrole regulates active sites in Co–based catalyst in direct borohydride fuel cells, ChemSusChem, 2024, e202301622 Search PubMed.
- Q. Wei, S. Qiu, C. Yin, J. Liu, Y. Xia, X. Wen, Y. Zou, F. Xu, L. Sun and H. Chu, Nitrogen-doped carbon encapsulated Ru-decorated Co2P supported on graphene oxide as efficient catalysts for hydrogen generation from ammonia borane, J. Alloys Compd., 2022, 921, 166207 CrossRef CAS.
- S. Song, Y. He, G. Fan and X. Yu, Boron-doping-induced modulation of structural parameters of pristine commercial carbon black for promoting Ru-catalyzed OH bond activation toward hydrogen evolution, Appl. Surf. Sci., 2023, 639, 158250 CrossRef CAS.
- L. Shi, K. Zhu, Y. Yang, Y. Liu, S. Xu, T. T. Isimjan and X. Yang, Oxygen-vacancy-rich Ru-clusters decorated Co/Ce oxides modifying ZIF-67 nanocubes as a high-efficient catalyst for NaBH4 hydrolysis, Int. J. Hydrogen Energy, 2022, 47, 37840–37849 Search PubMed.
- C. Saka, Phosphorus and sulphur-doped microalgae carbon as a highly active metal-free catalyst for efficient hydrogen release in NaBH4 methanolysis, Fuel, 2022, 309, 122183 Search PubMed.
- C. Saka, Highly active and durable hydrogen release in NaBH4 methanolysis reaction with sulphur and phosphorus-doped metal-free microalgal carbon nanoparticles, Appl. Catal., B, 2021, 292, 120165 Search PubMed.
- Y. Yang, L. Zhao, X. Gao and Y. Zhao, Constructing ultrafine monodispersed Co2P/(0.59-Cu3P) on Cu doped CoZn-ZIF derived porous N-doped carbon for highly efficient dehydrogenation of ammonia borane, Nano Res., 2023, 16, 6687–6700 Search PubMed.
- Y. Feng, X. Zhang, Y. Shao, X. Chen, H. Wang, J. Li, M. Wu, H. Dong, Q. Liu and H. Li, Modulating the acidic properties of mesoporous Mox–Ni0.8Cu0.2O nanowires for enhanced catalytic performance toward the methanolysis of ammonia borane for hydrogen production, ACS Appl. Mater. Interfaces, 2022, 14, 27979–27993 Search PubMed.
- O. J. Metters, S. R. Flynn, C. K. Dowds, H. A. Sparkes, I. Manners and D. F. Wass, Catalytic dehydrocoupling of amine–boranes using cationic zirconium(IV)–phosphine frustrated lewis pairs, ACS Catal., 2016, 6, 6601–6611 CrossRef CAS.
- Y.-X. Luo, W. Nie, Y. Ding, Q. Yao, G. Feng and Z.-H. Lu, Robust hydrogen production from additive-free formic acid via mesoporous silica-confined Pd-ZrO2 nanoparticles at room temperature, ACS Appl. Energy Mater., 2021, 4, 4945–4954 Search PubMed.
- J. Li and J. Gong, Operando characterization techniques for electrocatalysis, Energy Environ. Sci., 2020, 13, 3748–3779 Search PubMed.
- X. Li, X. Yang, J. Zhang, Y. Huang and B. Liu, In situ/operando techniques for characterization of single-atom catalysts, ACS Catal., 2019, 9, 2521–2531 CrossRef CAS.
- Q. Meyer, Y. Zeng and C. Zhao, In situ and operando characterization of proton exchange membrane fuel cells, Adv. Mater., 2019, 31, 1901900 CrossRef CAS PubMed.
- H. Topsøe, Developments in operando studies and in situ characterization of heterogeneous catalysts, J. Catal., 2003, 216, 155–164 Search PubMed.
- J. G. Freeze, H. R. Kelly and V. S. Batista, Search for catalysts by inverse design: artificial intelligence, mountain climbers, and alchemists, Chem. Rev., 2019, 119, 6595–6612 Search PubMed.
- Z.-K. Han, D. Sarker, R. Ouyang, A. Mazheika, Y. Gao and S. V. Levchenko, Single-atom alloy catalysts designed by first-principles calculations and artificial intelligence, Nat. Commun., 2021, 12, 1833 Search PubMed.
- A. Mazheika, Y.-G. Wang, R. Valero, F. Viñes, F. Illas, L. M. Ghiringhelli, S. V. Levchenko and M. Scheffler, Artificial-intelligence-driven discovery of catalyst genes with application to CO2 activation on semiconductor oxides, Nat. Commun., 2022, 13, 419 CrossRef CAS PubMed.
- I. Hassan, H. S. Ramadan, M. A. Saleh and D. Hissel, Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives, Renewable Sustainable Energy Rev., 2021, 149, 111311 CrossRef CAS.
| This journal is © the Partner Organisations 2025 |






