Mechanistic analysis of viscosity-sensitive fluorescent probes for applications in diabetes detection
M. M.
Sreejaya
a,
Vineeth
M Pillai
a,
Ayesha
A
a,
Maanas
Baby
a,
Manoranjan
Bera
b and
Moumita
Gangopadhyay
 *a
*a
aDepartment of Chemistry, Amrita Vishwa Vidyapeetham, Amritapuri, Kollam, Kerala 690525, India. E-mail: orgchemist87@gmail.com; moumita@am.amrita.edu
bClark University, 950 Main Street, Worcester, MA 01610, USA
First published on 19th February 2024
Abstract
Diabetes is one of the most detrimental diseases affecting the human life because it can initiate several other afflictions such as liver damage, kidney malfunctioning, and cardiac inflammation. The primary method for diabetes diagnosis involves the analysis of blood samples to quantify the level of glucose, while secondary diagnostic methods involve the qualitative analysis of obesity, fatigue, etc. However, all these symptoms start showing up only when the patient has been suffering from diabetes for a certain period of time. In order to avoid such delay in diagnosis, the development of specific fluorescent probes has attracted considerable attention. Prominent biomarkers for diabetes include abundance of certain analytes in blood serum, e.g., glucose, methylglyoxal, albumin, and reactive oxygen species; high intracellular viscosity; alteration of enzyme functionality, etc. Among these, high viscosity can greatly affect the fluorescence properties of various chromophores owing to the environment sensitivity of fluorescence spectra. In this review article, we have illustrated the application of some prominent fluorophores such as coumarin, BODIPY, xanthene, and rhodamine in the development of viscosity-dependent fluorescent probes. Detailed mechanistic aspects determining the influence of viscosity on the fluorescent properties of the probes have also been elaborated. Fluorescence mechanisms that are directly affected by the high-viscosity heterogeneous microenvironment are based on intramolecular rotations like twisted intramolecular charge transfer (TICT), aggregation-induced emission (AIE), and through-bond energy transfer (TBET). In this regard, this review article will be highly useful for researchers working in the field of diabetes treatment and fluorescent probes. It also provides a platform for the planning of futuristic clinical translation of fluorescent probes for the early-stage diagnosis and therapy of diabetes.
Introduction
Diabetes is considered one of the most chronic diseases in the world. It is related to the metabolic disorder leading to disruption in the formation of the hormone, insulin. The primary symptom of diabetes is the elevated level of glucose in blood serum, which is often termed as hyperglycemia. Diabetes can eventually affect the regular functioning of the kidneys, nervous system, heart, etc., thus increasing the chances of other severe diseases. The modes of diabetes monitoring presently in use are analysing blood glucose levels and observing other symptoms such as polyphagia, obesity, and fatigue. However, all these modalities detect diabetes only after a certain period of the development of the disease. Therefore, methods for the early detection and treatment of diabetes require the utmost attention.Fluorescence imaging has been proved to be one of the handiest and fastest processes for the selective detection and quantification of any biological analyte. There are several biological analytes found in early-stage diabetic patients, e.g., rise in the cellular content of methylglyoxal, acetone, glycated haemoglobin, albumin, reactive oxygen species (ROS), and reactive sulfur species (RSS), along with enhanced intracellular viscosity and malfunctioning of several enzymes.1 Since fluorescence is largely affected by the surrounding microenvironment, the selective monitoring of diabetes is possible by viscosity-responsive fluorophores. Fluorophores, used for the detection of various bio-analytes, mainly function based on diverse energy transfer processes involving donor–acceptor interactions.2 For a viscosity-dependent probe, restricted rotation between the donor and acceptor is a pivotal mechanism. There are a few fluorescence mechanisms that solely rely on intramolecular rotation, e.g., twisted intramolecular charge transfer (TICT), aggregation-induced emission (AIE), and through-bond energy transfer (TBET). TICT involves the formation of a perpendicular conformation owing to intramolecular bond-rotation.3–5 Such molecular rotation leads to the formation of a local excited state (LE) and a twisted charge transfer state (TICT) denoted by two peaks in the fluorescence spectra at a short and a longer wavelength, respectively. Generally, the LE state shows strong emission; however, the TICT state is rather non-emissive.6 Recent findings indicate that the regulation of TICT mostly depends on the nature of the electron donating group, extended π-conjugation, rigid cyclic structures, etc.7–9 Aggregation-induced emission (AIE) occurs because of the cessation of all the non-radiative pathways upon the aggregation of rigid perpendicular conformation.10 All these energy transfer mechanisms are found to be greatly affected by the polarity and viscosity of the medium. In a highly viscous environment, the intramolecular rotation gets restricted owing to the formation of bright fluorescent emissive conformations. TBET, on the other hand, depends on the modification of the energy gap between the donor group and acceptor group via chemical reaction.11 TBET is superior to other energy transfer processes as this does not depend on the spectral overlap of the donor and acceptor. This leads to a plethora of combinations of donor and acceptor.
As depicted in Scheme 1, in the present review, we have discussed several fluorescence mechanisms that are affected by the viscosity of the surrounding medium, e.g., TICT, AIE, and TBET. Further, we have also elaborated some of the prominent fluorescent probes used for viscosity-sensitive fluorescence, e.g., derivatives of coumarin, BODIPY, and xanthene. Finally, we have elaborated the application of these fluorescent probes in the early detection of diabetes. Although there are a few review articles describing viscosity-responsive fluorescent probes, however, there has not been any detailed discussion on the use of viscosity-responsive probes for the treatment of diabetes. Thus, in the present article, we have focussed mainly on the recent applications of viscosity probes for the early detection of diabetes.
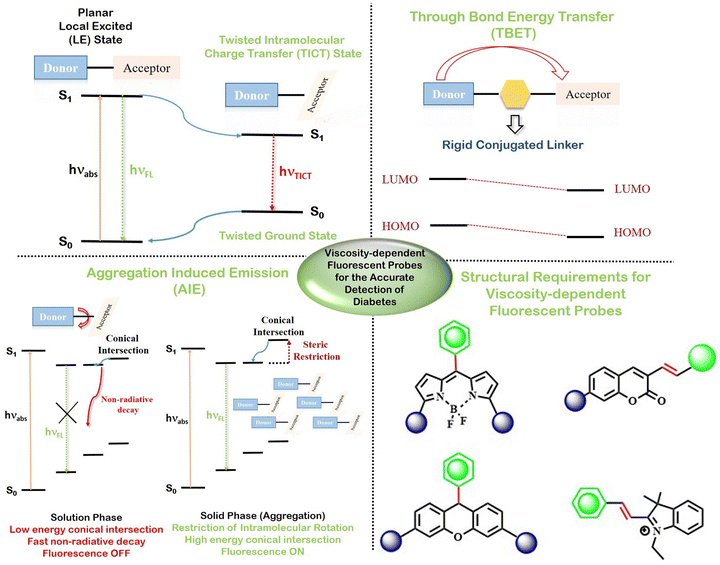 | ||
| Scheme 1 Mechanistic details and basic structural requirements for the development of viscosity-sensitive fluorescent probes for early-stage diabetes detection. | ||
Mechanism of viscosity-dependent fluorescent probes
Fluorescent probes typically comprise of three key components: a fluorophore, a linker, and a recognition group. Traditional sensing mechanisms include diverse energy and charge transfer processes like intramolecular charge transfer (ICT), photoinduced electron transfer (PET) and fluorescence resonance energy transfer (FRET) between a donor and an acceptor group. However, the viscosity of the medium greatly affects intramolecular rotation. Thus, the fluorescent properties stemming from the regulation of bond rotation in any molecule can be employed to selectively detect the viscous nature of the medium. Energy transfer processes that count on intramolecular bond rotation, e.g., twisted intramolecular charge transfer (TICT), aggregation-induced emission (AIE), and through-bond energy transfer (TBET), are exploited for the construction of viscosity-dependent applications probes.Twisted intramolecular charge transfer (TICT)
Twisted intramolecular charge transfer (TICT) serves as a frequently employed mechanism for signal transmission. In 1960, Lippert et al. observed this phenomenon for the first time in 4-dimethylaminobenzonitrile (DMABN) exhibiting solvent-dependent dual fluorescence.12 The discovery of TICT has significant implications in understanding the photophysical properties of organic molecules in different microenvironments. In TICT, a fluorophore undergoes transformation from a quasi-planar emissive state, which can be either a locally excited (LE) state or intramolecular charge transfer (ICT) state, into a non-emissive state marked by charge separation (TICT state) via significant intramolecular rotations. The TICT state often loses its fluorescence compared to the bright fluorescence of the planar LE state owing to the disrupted planarity of the probe. The introduction of a bulky ortho group or an isomerizable double bond can greatly influence the twisting of the fluorescent probe, which in turn can lead to the manipulation of the preference for LE and CT states. The bright fluorescence of the planar LE state stems from the mixing of donor–acceptor orbitals to a great extent, leading to reduced HOMO–LUMO gap. The twisting of the probe increases the HOMO–LUMO gap, thereby weakening the fluorescence. Such molecular rotation results in a near perpendicular conformation.6 In contemporary research, the primary emphasis lies in investigating the sensitivity of TICT fluorophores with a predominant focus directed towards electron-donating groups like dialkylamino groups.13 The electron-donating groups act as rotor groups facilitating TICT process, thus contributing significantly to the intricate dynamics of these fluorophores. Since the TICT mechanism largely depends on the intramolecular rotation, it is widely utilised as a handy tool to differentiate between viscous environments. Following this principle, several TICT-guided viscosity-dependent fluorescent probes have been constructed.Xingzong Wang et al. engineered a unique class of dual mode fluorescent probes capable of simultaneously responding to both pH and viscosity. They synthesised two probes for cancer-lysosome cells, namely, CL-1 and CL-2.14 In both the probes, the benzoindole moiety and phthalhydrazide moiety acted as the donor and acceptor, respectively. The probes’ sensitivity to viscosity was achieved by restraining the intramolecular charge transfer mechanism (TICT) under conditions of high viscosity. In another report, Wei Liu et al. developed an exceptionally viscosity-sensitive NIR fluorescent probe utilising the TICT mechanism (Fig. 1).15 The TICT effect was facilitated by the intramolecular rotation between the tetraphenylethylene and pyridine groups. In high-viscosity conditions, the probe showed strong fluorescence due to restricted rotation. Due to limited solubility in water, aggregation occurred in environments with high water content. This aggregation constrained intermolecular rotation, leading to the manifestation of the AIE effect.
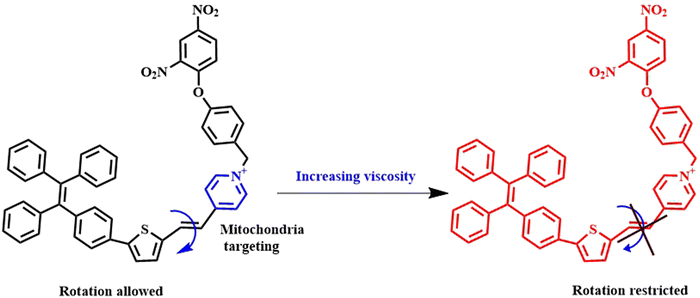 | ||
| Fig. 1 Demonstration of the mechanism of the tetraphenylethylene-pyridine conjugated TICT-induced viscosity probe.15 [Reproduced from Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 304, Wei Liu, Tengfei Wang, Liwen Wang, Yan Wang, Sheng Hu, Dating Tian, A novel near-infrared fluorescent probe for the ultrasensitive and visual detection of mitochondrial viscosity, 123329, Copyright (2023), with permission from Elsevier]. | ||
Qi Zan et al. developed a dual sensing fluorescent probe for the concurrent detection of H2S and environmental viscosity/polarity.16 The probe had a donor–π–acceptor design with N,N-dimethylamino unit as the donor as well a free molecular rotor and quinolone cationic group as the acceptor linked by a double-vinyl bridge. The 2,4-dinitrophenyl unit functioned as the HS- recognition unit. The probe showed the recovery of fluorescence when the intramolecular rotation was restricted in high-viscosity environments.
Yijia Liu et al. synthesised a TICT fluorescent probe that could simultaneously detect peroxynitrite and viscosity.17 The probe had a benzoquinolizine coumarin unit as the electron donor and a quinolinium unit as the electron acceptor (Fig. 2). These units were linked by a vinyl bridge. Low-viscosity environments caused fluorescence quenching due to the free rotation of the coumarin and quinolinium units; on the other hand, in high-viscosity environments, the probe showed increased fluorescence due to restricted intramolecular rotation.
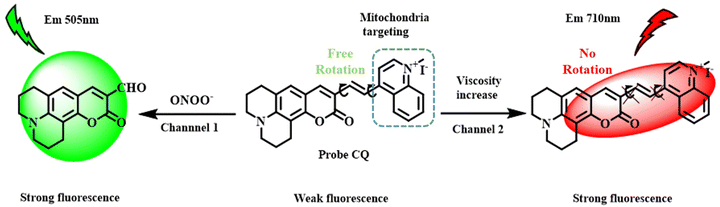 | ||
| Fig. 2 Mechanism of viscosity sensitivity of coumarin-quinolinium probe owing to restricted rotation.17 [Reproduced (adapted) with permission from Liu Y, Feng S, Gong S, Feng G. Dual-channel fluorescent probe for detecting viscosity and ONOO− without signal crosstalk in nonalcoholic fatty liver. Anal Chem. 2022 Dec 20;94(50):17439–17447. Doi: 10.1021/acs.analchem.2c03419. Epub 2022 Dec 7. PMID: 36475623. Copyright 2022 American Chemical Society.]. | ||
Aggregation-induced emission (AIE)
AIE is a phenomenon that contradicts our conventional understanding of how luminescent molecules behave. Unlike the typical observation that molecules become less luminescent when they aggregate, in line with aggregation-induced quenching (ACQ), AIE demonstrates that some molecules become more luminescent when they are in the aggregated form. AIE was introduced by Tang et al. in 2001.18 The underlying mechanism of AIE lies in the restriction of intramolecular rotations (RIR) and restricted molecular vibration (RIV) in the aggregated state, preventing non-radiative decay pathways. Precisely, compounds containing multiple aromatic rings are preferred for AIE application. This can be attributed to their free rotation across the rings in a dilute solution triggering non-radiative relaxation pathways. However, in highly concentrated solution or solid state, the free rotation is ceased, thereby blocking all non-radiative pathways and encouraging radiative de-excitation processes. This leads to the bright fluorescence in the solid phase or upon aggregation. Therefore, restricted molecular movement can induce AIE in any molecule. The incorporation of AIE-active fluorophore in any ACQ molecule can easily lead to ACQ to AIE transition. AIE has found a great deal of advantage in biomedicine in terms of identification of specific pathogens, interaction with specific biomolecules, and electrochemical biosensing. Different classes of AIE-based probes have been developed and exploited till date including charged AIE-probes, AIE-based bioconjugates, and amphiphilic AIE-probes for protein binding.19–23Triphenyl amine (TPA) is a well-known AIE-unit owing to the presence of three non-planar benzene ring rotors.24 One of the three phenyl rings of TPA goes out of plane, hindering π–π stacking, which in turn prevents ACQ. Additionally, TPA units can display prominent electron donating abilities. These TPA units have been extensively exploited in developing AIE-probes. Recently, Wei Liu et al. synthesised an AIE molecule that simultaneously detected the superoxide anion and viscosity via the restricted molecular motion of the probe.25 In their work, they conjugated a diphenyl phosphinic acid group, an oxidizable group by superoxide ion with a triphenyl amine-thiophene derivative containing a pyridinium moiety (Fig. 3). The pyridine cation was used as the electron acceptor and the triphenylamine thiophene unit acted as the electron donor. The restricted rotation between the pyridine and thiophene units facilitated the viscosity sensing. Monitoring the normal functioning of specific organelles like mitochondria, lipid droplet, etc., is highly important in order to maintain proper vitals for liver.
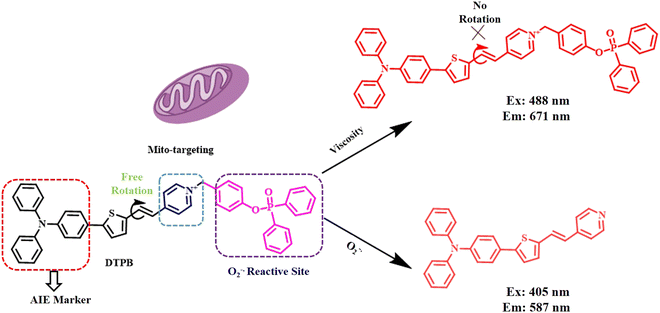 | ||
| Fig. 3 Representative scheme of viscosity responsiveness by the triphenylamine-based AIE probe.25 [Reproduced from Analytica Chimica Acta, 1253, Wei Liu, Yan Wang, Tengfei Wang, Liwen Wang, Sheng Hu, Dating Tian, A versatile AIE probe with mitochondria targeting for the dual-channel detection of superoxide anion and viscosity, 341099., Copyright (2023), with permission from Elsevier]. | ||
Huili Wang et al. synthesised a triphenylamine-based AIE fluorescent probe for the specific bio-imaging of lipid droplets.26 The probe, comprised of triphenyl amine as the electron donor and benzopyran unit as the electron-acceptor, was highly sensitive to the viscosity of lipid droplets. The AIE-probe also displayed enhanced fluorescence intensity upon internalization into the highly viscous lipid droplet owing to the restricted rotation, as discussed earlier. Apart from prominent biomedical applications AIE-probes have found significant application in food additive detections. Several commercially available liquids, e.g., juices, sauces, and soups, contain edible thickeners to maintain their consistency. The quantification of these thickening agents and monitoring their thickening activity is of utmost importance in the food industry. Viscosity-induced AIE-probes can be great tools for the analysis of thickening agents. Recently, Lingfeng Xu et al. designed and developed an AIE molecular sensor utilising the strong electron donating effect of triphenylamine and acceptor property of indanedione.27 The donor and acceptors were connected through the thiophene or benzene unit, leading to a rotatable structure. The sensor was used to monitor viscosity variations due to the addition of food thickeners. This is associated with the molecule's ability to freely rotate and aggregate in response to changes in solvent composition.
Another widely utilized fluorescent probe for AIE is tetraphenyleneethylene (TPE). The non-planar propeller-shaped arrangement of four phenyl rings connected by a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond results in highly emissive solid aggregates of TPE. The restricted rotation of the phenyl rings makes them a brilliant class of AIE probes. TPE has been diversely utilized for transforming ACQ moieties into proficient AIE-probes. Gang Nie et al. developed two AIE fluorescent probes targeting cell membrane and lipid droplets by decorating a dicyanomethylene derivative and benzophenone derivatives with TPE, respectively.28 The TPE-based probe was used to study the microscopic changes happening in the cell membrane fluid and liquid droplets of patients with alcoholic liver disease. The increased cholesterol levels increased the viscosity of these structural components in our body.
C double bond results in highly emissive solid aggregates of TPE. The restricted rotation of the phenyl rings makes them a brilliant class of AIE probes. TPE has been diversely utilized for transforming ACQ moieties into proficient AIE-probes. Gang Nie et al. developed two AIE fluorescent probes targeting cell membrane and lipid droplets by decorating a dicyanomethylene derivative and benzophenone derivatives with TPE, respectively.28 The TPE-based probe was used to study the microscopic changes happening in the cell membrane fluid and liquid droplets of patients with alcoholic liver disease. The increased cholesterol levels increased the viscosity of these structural components in our body.
Jinyin Ge et al. developed an NIR-II fluorescent AIE probe that facilitated the early diagnosis of liver injury (Fig. 4).29 The probe was designed by linking a benzindole sulfonate skeleton as the acceptor and the molecular rotor, with an electron-donating diphenylxanthine group. The extended conjugation in diphenylxanthine prevents them from π-stacking, thereby preventing any ACQ. This probe is part of a group of fluorophores characterised by their non-planar structure with multiple rotors. This probe was utilized to determine the extent of liver damage caused by the overdose of acetaminophen, leading to elevated viscosity level inside the liver.
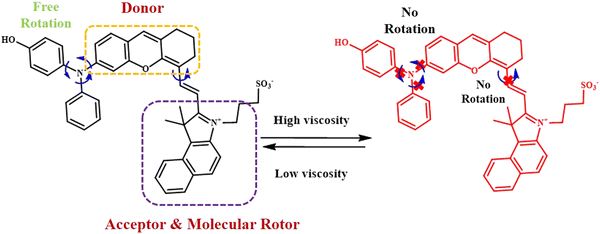 | ||
| Fig. 4 Reversible response to viscosity of benzindole sulfonate-substituted TPA-based fluorophore.29 [Reproduced from Biomaterials, 300, Jinyin Ge, Wenwen Cai, Niu Niu, Yating Wen, Qian Wu, Lei Wang, Dong Wang, Ben Zhong Tang, Ruiping Zhang, Viscosity-responsive NIR-II fluorescent probe with aggregation-induced emission features for the early diagnosis of liver injury, 122190, copyright (2023) with permission from Elsevier]. | ||
Although a great amount of research has been conducted in the field of AIE, most of the probes are derivatives of either TPE or TPA, which often involves crucial synthetic steps. Hence, there is an urgent need of developing new AIE probes that can show intriguing fluorescence properties in their solution phase and powder form.
Through-bond energy transfer (TBET)
TBET-based fluorescent chemosensors consist of three essential components: an energy donor, an energy acceptor, and a rigid conjugated linker.30 Here, energy transfer mainly occurs via conjugated bonds from the donor group to the acceptor. Attributed to bond-mediated energy transfer, the TBET-probes involve a large difference in the emission wavelengths of the donor and acceptor, leading to reduced signal to noise ratio, high resolution images, and efficient energy transfer compared to other energy transfer processes. The rigid conjugated linker between the donor and acceptor enables these probes to attain a non-planar structure, which can be extensively exploited to construct viscosity-sensitive probes. The major advantage of TBET-based viscosity probes lies in the appearance of two different emission peaks at two different wavelengths, which can improve the accuracy of the probe.Shuyang Zhai et al. developed a fluorescent probe for assessing lysosomal viscosity employing the TBET mechanism.31 This probe incorporated a dicyanomethylene isophorone-conjugated styrene derivative connected to a two-photon N,N-diethylcoumarin unit as the donor via a rigid conjugated alkyne bond containing benzene ring. The stryrene moiety also served as the viscosity-sensitive unit. Upon an increase in the viscosity of the medium, the energy gap between the donor and acceptor changed remarkably owing to the rigid planar structure of the probe; however, under dilute condition, free rotation across the alkyne bond was possible. As the surrounding viscosity increased, a noticeable increase in the red emission of the probe was observed (Fig. 5a). Following a similar line of approach, Shengze Su et al. designed a viscosity-sensitive fluorescent probe, which relied on a combination of two fundamental phenomena, TBET and AIE.32 The TBET system was constructed using a coumarin derivative as the energy donor and quinolone nitrile derivatives as acceptors linked by a phenyl bond as the conjugated linker (Fig. 5b).
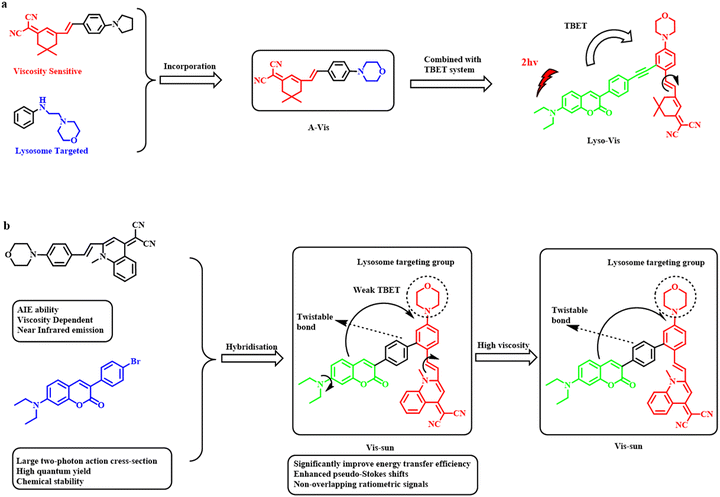 | ||
| Fig. 5 Design and mechanism of lysosome-targeting viscosity-responsiveness of isophorone-coumarin probe.31 [Reproduced from Biosensors and Bioelectronics, 213, Shuyang Zhai, Wei Hu, Weibo Wang, Li Chai, Qian An, Chunya Li, Zhihong Liu, Tracking autophagy process with a through bond energy transfer-based ratiometric two-photon viscosity probe, 114484, Copyright (2022), with permission from Elsevier]. (b) Schematic diagram of the design and response mechanism of viscosity-responsive coumarin-quinolone–based probe.32 [Reproduced (adapted) with permission from Anal. Chem. 2022, 94, 43, 15146–15154. Copyright {2022} American Chemical Society.]. | ||
To develop an organelle-specific viscosity probe, Linlin Zhu and his co-workers designed a TBET fluorescent probe for studying blood viscosity in lipid droplets.33 The probe had a conjugated structure with tetraphenylethylene (TPE) as the energy donor as well as free rotor and Nile red, a phenoxazone dye, as the energy acceptor. Nile red could show promising lipid droplet localization and intense NIR emission property. The extended conjugation between TPE and Nile red was responsible for fluorescence enhancement under viscous environment of lipid droplets. The probe exhibited a prominent blue shift from the NIR region (660 nm) to 480 nm owing to the attainment of planarity between Nile red and TPE under high viscosity.
Thus, several examples of TBET-based probes have been proved to be superior to existing energy-transfer based probes, e.g., fluorescence resonance energy transfer (FRET). The FRET probes primarily depend on the spectral overlap between the donor and the acceptor, which limits its resolution, accuracy, and possible combinations of donor and acceptor. However, TBET does not require any energy overlap; rather, it involves the occurrence of a prominent energy gap between the donor and acceptor, resulting in a large difference in the emission peaks. This change in the energy gap is brought about by the interaction with the analyte.
Other mechanisms
Apart from the mechanisms discussed above, there are a few other fluorescence mechanisms, which can be influenced by the change in viscosity of the medium. One such example is found in the employment of through-space energy transfer (TSCT).34 TSCT is most often observed in case of macrocyclic fluorophores like bottlebrush polymers.35 Such polymers obtain a cylindrical structure, bringing the donor and acceptor ends close to one another; however, upon swelling, the TSCT process ceases, thereby resulting in a significant shift in the emission property of the polymer. Regulated TSCT is observed by modifying the tail end of the polymer, which often serves as the acceptor. A relatively less prominent effect is observed in porphyrin dimers known as triplet energy transfer (TET).36 This process generally relies on the Coulombic interaction between donor and acceptor. The energy transfer here largely depends on the electronic coupling between the donor and acceptor, which in turn is guided by their relative orientation. Thus, TET processes are also highly influenced by the viscosity of the surrounding media. In one of the newer findings, Shen et al. established the effect of intramolecular hydrogen bonding on regulating the excited state rotational properties.37 In their study, they functionalized 4-hydroxybenzylidene-imidazolinone to attain effective hydrogen bonding between the imidazole ring nitrogen and an ortho hydroxyl group. The energy barrier for rotation was found to increase proportionally with increasing strength of hydrogen bonding, resulting in minimum effect of viscosity. They also studied the influence of the electron density regulating group, e.g., extended π-conjugation on the rotational barrier of the fluorescent probe by introducing the indole moiety. Extended conjugation further increased the activation energy for rotation. Thus, Shen and his colleagues successfully demonstrated the effect of hydrogen bonding and extended conjugation on the viscosity sensitivity of the fluorescent probes. For practical implementation, they used a series of hydroxybenzylideneimidazole derivatives to monitor protein aggregation in the HEK cell line.These examples indicate that there are several unexplored energy transfer processes that can find a great deal of superiority in selective response to viscous heterogeneous medium.
Examples of prominent viscosity probes
The development of viscosity-based probes is one of the most relevant research fields these days. It has been observed from various recent studies that the monitoring of intracellular viscosity level is of utmost significance owing to the high viscosity related to different diseases like cancer, diabetes, inflammation, and metabolic disorders. The regulated fluorescence properties of the probes can be handy tools for the estimation of viscosity of the medium. Intramolecular rotational effects can largely be dictated by the viscosity of the surrounding environment. Intramolecular rotational restrictions can be introduced by means of two main structural attributes; (i) connecting donor and acceptor moieties of the fluorophore via a vinyl bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond) or (ii) curating a unique biphenyl-type structure by connecting two aromatic rings with ortho steric hindrance. In the following section, few of the most prominent fluorescent probes with respect to viscosity have been elaborated.
C bond) or (ii) curating a unique biphenyl-type structure by connecting two aromatic rings with ortho steric hindrance. In the following section, few of the most prominent fluorescent probes with respect to viscosity have been elaborated.
Coumarin-based probes
Coumarins represent a well-explored group of compounds characterized by the presence of the 1-benzopyran-2-one structure.38 Chemosensors designed with the coumarin platform are favoured because of their strong fluorescence, compatibility with biological systems and versatile structural adaptability. Coumarin derivatives have been used extensively in the field of chemosensors ranging from physiologically significant anions,39–43 pH,44–46 reactive nitrogen species,47–49 to metal ions.50–52 Such versatile application of coumarin derivatives as fluorescent sensors is achieved by the facile functionalization of the 7th and 3rd positions of the chromene skeleton.Tian-Zhen et al. developed a dual-response coumarin-hemicyanine-based fluorescent probe with favorable water solubility capable of simultaneously detecting both SO2 and intracellular viscosity. The interaction of the fluorescent probe with SO2 derivatives induced significant changes in the blue/red fluorescence ratio and high viscosity triggered pronounced alterations in red fluorescence.53 Increasing the viscosity resulted in a significant increase in fluorescence intensity at 625 nm, while the fluorescence intensity at 487 nm remained relatively unchanged. The change in the emission property in highly viscous medium was attributed to the FRET process from N,N-diethylcoumarin donor to hemicyanine acceptor unit via the suppression of the rotation of the pi-conjugated backbone.
Following a similar approach, Feng-Ting Liu and his co-workers designed a novel FRET and ICT dual responsive fluorescent probe with improved NIR absorption wavelength for the simultaneous detection of SO2 derivatives and viscosity changes.54 Coumarin moiety acted as the donor; however, the acceptor unit consisted of both an electron-donating julolidine group and an electron-withdrawing quinolinium unit, leading to inherent ICT (Fig. 6). With the increase in the viscosity, the fluorescence intensity of the probe increased. The increase in the viscosity caused the hindering of rotation of the diethylamino group in the donor component and the rotation of the π-conjugated backbone in the acceptor component.
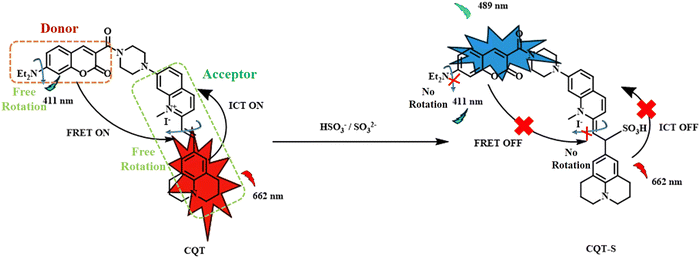 | ||
| Fig. 6 Dual effect of ICT and FRET on coumarin-julolidine probe with respect to SO2 and viscosity.54 [Reproduced from Dyes and Pigments, 210, Feng-Ting Liu, Wen-Wen Han, Hui Ren, Wan-Jing Yang, Ruo-Nan Wang, Jun-Ying Miao, Bao-Xiang Zhao, Zhao-Min Lin, A ratiometric lysosome-targeted fluorescent probe for imaging SO2 based on the coumarin-quinoline-julolidine molecular system, 111017, Copyright (2023), with permission from Elsevier.]. | ||
Wei Pan et al. developed a dual-responsive NIR fluorescent probe with a substantial Stoke's shift.55 This probe was developed by modifying N,N-diethylaminocoumarin with a benzindolium unit through conjugated double bonds. As the viscosity increased, the rotation of the benzindolium moiety across the double bond was restricted, and the probe moved to the unexcited TICT state from the excited state with enhanced fluorescence emission. The probe was capable of the real-time monitoring of the variations in mitochondrial viscosity.
Majority of the coumarin-based viscosity probes are mitochondria-targeted owing to the crucial role of mitochondria in liver functioning. However, other organelles like lysosome maintain cellular homeostasis. Keeping this in mind, Huili Wang synthesised a multi-organelle targeting viscosity-sensitive probe by combining a hemicyanine group with a 7-pyrrolidine coumarin derivative.56 In this design, the pyrrolidine-modified coumarin behaved as the electron donor, while the cyanogen group on hemicyanine acted as the electron-withdrawing component. This configuration enabled the probe to exhibit sensitivity to changes in viscosity through a TICT mechanism. This probe could be localized within multiple organelles and thus could be used to determine viscosity in lysosomes as well as mitochondria. It represented a promising approach for crafting fluorescence probes that are responsive to lysosomal viscosity under varying pH conditions.
In a rare attempt to develop photoinduced electron transfer (PET)-based viscosity probes, a range of phenyl-rhodol compound featuring a fluorophore-quencher arrangement were formulated and synthesized by Yong Gao et al.57 In the present study, rhodol was employed as the stable fluorophore component, while a series of mono and disubstituted benzenes were incorporated as the movable quencher element. Through systematic alterations of the substituent groups on the phenyl rings, the connection between the switchable photoinduced electron transfer (PET) and the responsiveness of these derivatives to changes in viscosity was investigated. The benzene substituent having an electron withdrawing nitro group showed an appropriate energy gap between the fluorophore and quencher highest occupied molecular orbitals for an efficient PET, and it also displayed restricted rotation across the single bond connecting rhodol and benzene ring in the lysosome as well as the mitochondria.
Xinya Hao et al. designed and developed a two-photon fluorescent probe tailored for the detection of variations in the intracellular micro-viscosity in a highly sensitive manner.58 The probes contained diethylaminocoumarin as donor connected to a quinolinium moiety as the acceptor via a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unit (Fig. 7). The key factor of this probe was its improved hydrophilicity by the introduction of a carboxylic side chain. The quinolinium moiety was strategically introduced as an electron acceptor, extending the π-electron conjugation system, improving biocompatibility, and serving as a molecular rotor. In low-viscosity solutions, molecules rotated freely, leading to fluorescence quenching, while in high-viscosity solutions, intramolecular rotation was restricted, resulting in increased fluorescence intensity.
C unit (Fig. 7). The key factor of this probe was its improved hydrophilicity by the introduction of a carboxylic side chain. The quinolinium moiety was strategically introduced as an electron acceptor, extending the π-electron conjugation system, improving biocompatibility, and serving as a molecular rotor. In low-viscosity solutions, molecules rotated freely, leading to fluorescence quenching, while in high-viscosity solutions, intramolecular rotation was restricted, resulting in increased fluorescence intensity.
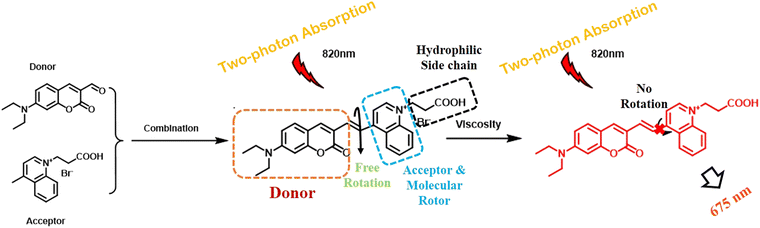 | ||
| Fig. 7 Mechanism of viscosity sensing by coumarin-quinolinium probe under two-photon excitation.58 [Reproduced from Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 284, Xinya Hao, Jingting Zhan, Chen Geng, Weiying Lin, Discriminating normal and inflammatory mice models by viscosity changes with a two-photon fluorescent probe, 121807, Copyright (2023), with permission from Elsevier.]. | ||
BODIPY-based probes
The synthetic fluorescent dye BODIPY, also known as boron-dipyrromethene, is widely used in scientific research as a fluorophore, particularly as the building block for fluorescent probes that are used to detect and visualise particular molecules and cellular processes in areas like cell biology, chemistry, and biochemistry. The versatile functionalization of the meso position of the BODIPY moiety has given rise to a wide range of fluorophores with intriguing emission properties.59–61 The introduction of a biphenyl system at the meso position of BODIPY can lead to a great deal of restricted intramolecular rotation, promoting a non-radiative de-excitation pathway. Such BODIPY derivatives can serve as prominent viscosity responsive fluorophores.Following this approach, Kun Dou and colleagues developed an ensemble of viscosity-activated NIR-II emissive probes (WD-X) consisting of meso-phenyl BODIPY connected to a benzoindolium unit via a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (Fig. 8).62 The benzoindolium salt served as both molecular rotors and electron-withdrawing groups, extending the BODIPY's conjugated chain and shifting its emission to NIR-II. Under low viscous medium, free rotation is possible across the vinyl bond and C–N single bond in BODIPY, reducing its fluorescence. However, as the viscosity rises, intramolecular rotation is restricted, leading to the restoration of fluorescence. A series of BODIPY fluorophores with ortho-substituents ranging from electron donating p-N,N-dimethyl benzene to electron withdrawing p-nitrobenzene have been reported in the present study for the detection of subtle viscosity changes in diabetes-induced liver injury, marking a significant advance in NIR-II emissive viscosity-activatable probes. The nitro-substituted probe displayed maximum response against viscosity owing to its very high dihedral angle of about 47.3°, which was much higher than the other electron donating groups like methoxy and N,N-dimethylbenzene.
C bond (Fig. 8).62 The benzoindolium salt served as both molecular rotors and electron-withdrawing groups, extending the BODIPY's conjugated chain and shifting its emission to NIR-II. Under low viscous medium, free rotation is possible across the vinyl bond and C–N single bond in BODIPY, reducing its fluorescence. However, as the viscosity rises, intramolecular rotation is restricted, leading to the restoration of fluorescence. A series of BODIPY fluorophores with ortho-substituents ranging from electron donating p-N,N-dimethyl benzene to electron withdrawing p-nitrobenzene have been reported in the present study for the detection of subtle viscosity changes in diabetes-induced liver injury, marking a significant advance in NIR-II emissive viscosity-activatable probes. The nitro-substituted probe displayed maximum response against viscosity owing to its very high dihedral angle of about 47.3°, which was much higher than the other electron donating groups like methoxy and N,N-dimethylbenzene.
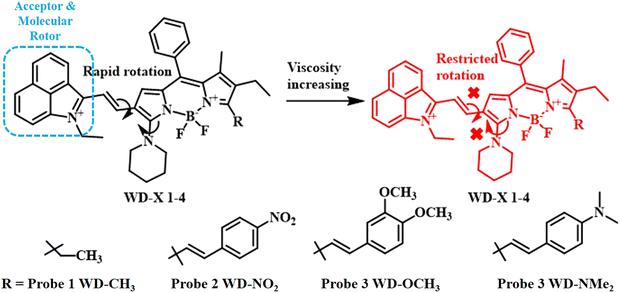 | ||
| Fig. 8 Molecular structures of BODIPY-based fluorescent probes with a series of electron donating and electron withdrawing groups.62 [Reproduced (adapted) with permission from {Anal. Chem. 2020, 92, 6, 4177–4181}. Copyright {2020} American Chemical Society.]. | ||
In the broader landscape of fluorescent probe development, researchers have been exploring innovative strategies to enhance their sensitivity to viscosity changes. This quest for more effective probes has led to the emergence of novel meso-substituted BODIPY derivatives, such as those featuring meso-CF3 substituent,63,64 phenyl groups,65–67 benzothiazole,68 and keto groups,69,70 reflecting a transition from traditional rotors like C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or C
C or C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds. In this context, Wen-Jing Shi et al. explored four BODIPY derivatives with small five-membered heterocyclic ring attached to the meso position of BODIPY.59 The meso-thiazole-based probe demonstrated both viscosity-responsiveness and AIE properties suitable for cellular viscosity imaging in both mitochondria and lysosomes. The best viscosity-responsiveness was observed with the heterocycle ring containing a saturated thiophene and an unsaturated nitrogen atom, preventing any sort of photoinduced electron transfer to the BODIPY core. The large size of the saturated sulfur with diffused d-orbitals resulted in strong steric restriction for the rotation of the BODIPY derivative, which led to their prominent response in a highly viscous medium.
C bonds. In this context, Wen-Jing Shi et al. explored four BODIPY derivatives with small five-membered heterocyclic ring attached to the meso position of BODIPY.59 The meso-thiazole-based probe demonstrated both viscosity-responsiveness and AIE properties suitable for cellular viscosity imaging in both mitochondria and lysosomes. The best viscosity-responsiveness was observed with the heterocycle ring containing a saturated thiophene and an unsaturated nitrogen atom, preventing any sort of photoinduced electron transfer to the BODIPY core. The large size of the saturated sulfur with diffused d-orbitals resulted in strong steric restriction for the rotation of the BODIPY derivative, which led to their prominent response in a highly viscous medium.
Acute kidney damage caused by nephrotoxicity is a major medical problem, prompting the development of novel diagnostic and treatment tools. In this context, a novel near-infrared (NIR) polarity-sensitive p-N,N-dimethylamino-functionalized BODIPY fluorescent probe (LD-B) was developed by Junlan Zhang et al. to target lipid droplets. The probe utilized a rational donor–π–acceptor configuration, displaying noticeable (TICT) characteristics.71 In this study, the hydrophobicity of the BODIPY ring was exploited to construct a selective tool for the quantification of viscosity in physiological hydrophobic pockets like lipid droplets (LD). This probe's unique properties, including NIR emission and photostability, enabled the efficient visualization of the lipid droplet and marked the first study to investigate LD polarity changes in contrast-induced acute kidney injury (CI-AKI) disease models, offering potential applications in fundamental and clinical research. In the context of lysosomal autophagy, where minor changes in viscosity occur, the solution lies in the meticulous design of a series of NIR fluorescent probes with significantly low detection limit. Dongyang Li et al. developed a novel NIR fluorescent probe by incorporating a p-N,N-dimethylaminostyrene-based rotor moiety.72 The uniqueness of this probe stemmed from the introduction of a piperizine unit, which could undergo efficient hydrogen bonding interactions. The piperizine unit was protonated under acidic lysosomal pH, leading to a greater extent of hydrogen bonding, which helped in detecting even a trace amount of viscosity. This probe was found to be a handy tool due to its exceptional sensitivity in visualizing low viscosity and detecting even the subtlest changes in viscosity. Dongyang Li and his colleagues employed commercial localization probes, LysoTracker Green and MitoTracker Green, to examine the targeting ability of the probe towards lysosomes and mitochondria, respectively. The results demonstrated that the probe had a significant lysosome-targeting capacity.
A wide range of protein molecules guide different physiological processes in human. Any alteration in the structure of the protein molecule can hinder the normal functioning of these processes, leading to detrimental diseases like Alzheimer's and Parkinson's. Owing to the hydrophobic pockets present in the α-helical or β-sheet structure of protein, they can control the intramolecular rotation of any probe inside the pockets. Following this line of approach, Baoxing Shen and his group designed a series of BODIPY derivatives with varied steric hindrance.73 Such hydrophobic BODIPY derivatives connected to another substituted aromatic ring could undergo efficient intramolecular rotation in normal folded protein structure; however, upon interaction with the aggregated protein, these probes ceased to rotate. Such sterically-hindered rotation led to a >10-fold increase in fluorescence emission.
The identification of lipid phases in the plasma membrane is of utmost importance as this controls cardiovascular functioning, host-pathogen signalling, immune signalling, etc. Lipid phases are mainly of two types, namely, highly viscous ordered and low viscous disordered phases. Therefore, molecular rotors discussed so far can be advantageous in distinguishing the type of lipid present in the plasma membrane depending on the difference in their viscosities. In this line, Arturas Polita and his co-workers synthesized a novel phospholipid-mimicking BODIPY derivative decorated with hydrophilic sulfonate tail group.60 Due to the combination of the hydrophobic BODIPY core and the hydrophilic sulfonate part, this probe easily intercalated through the lipid bilayer. Polita et al. monitored the distinctly different fluorescent lifetime values for the probe upon interaction with different phases of lipid in the plasma membrane. These promising findings indicated that this BODIPY probe can successfully replace other commercially available biological strainers.
To summarize, fluorescent probes based on BODIPY have become crucial instruments in a variety of scientific fields because they can be used to detect and visualise intricate biological processes and molecules. Notably, these probes have found use in the study of diabetes, in the diagnosis of nephrotoxicity, and in the highly accurate monitoring of lysosomal autophagy. Overall, the development of BODIPY-based probes highlights their critical contribution to improving our comprehension of cellular processes and shows great promise for further study and therapeutic use. However, the inherently hydrophobic nature of the BODIPY molecule is a matter of serious concern and needs further attention.
Xanthene-based probes
Xanthenes are a class of compound consisting of three benzene rings fused together with a bridging oxygen atom strengthening the conjugation further. The general chemical formula for xanthene is C13H10O. There are mainly three main classes of xanthene derivatives: (i) rhodamines having two amine groups on the two terminal benzene rings, (ii) fluorescein derivatives containing two phenol rings on either end, and (iii) rhodols having amine group on one terminal benzene ring and an OH group on the other.74–76 Due to their intriguing photophysical properties, xanthene derivatives have found applications in diverse fields including analytical chemistry, medical diagnostics and materials science.77,78 Some of the xanthene-based dyes are even commercialized as trackers in the realm of biology, e.g., mitochondria-selective dye MitoTracker®.79 The flexible equilibrium between the spirocyclic structure of these xanthene dyes and the ring-opened structure renders these dyes with regulated emission properties. The extended and rigid conjugation between the aromatic rings via the bridging oxygen atom is the source of prominent fluorescence in the xanthene dyes. Advantages like the (a) easy modification of the terminal free amine or hydroxyl group to introduce targeting moieties for target-specific drug delivery,80,81 (b) pH-responsiveness,82,83 (c) moderate to good hydrophilicity, (d) rotational restriction84 across the spirocyclic ring structures make these xanthene dyes excellent choices for designing probes with respect to pathological microenvironments like pH, oxidative stress, and viscosity.Recently, Chao et al. synthesised a quinoline-xanthene dye (QX-V)-based NIR-active fluorescent probe for the selective identification and quantification of mitochondrial viscosity.85 A powerful electron-donating diethylamino group on the xanthene ring underwent strong intramolecular charge transfer (ICT) to the electron-accepting quinolone moiety. In low viscosity healthy cells, the ICT process was hindered due to the facile rotation of the molecular rotor, quinolone ring quenching the fluorescence of the probe; however, with increased viscosity in cancer cells or fatty liver tissues, rotation is restricted, resulting in enhanced fluorescence.
In the same line of approach, Liu et al. developed a ratiometric and dual-responsive NIR fluorescence probe (Mito-HC-TZ) using an N,N-diethyl xanthene derivative attached to an N-ethylbenzothiazole group via a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond for selective response to viscosity as well as the reactive nitrogen species (RNS), peroxynitrite (ONOO−) (Fig. 9).86 The benzothiazole ring played the dual role of molecular rotor and mitochondria targeting unit owing to the presence of positively charged nitrogen atom. Peroxynitrite ions can easily oxidize the C
C bond for selective response to viscosity as well as the reactive nitrogen species (RNS), peroxynitrite (ONOO−) (Fig. 9).86 The benzothiazole ring played the dual role of molecular rotor and mitochondria targeting unit owing to the presence of positively charged nitrogen atom. Peroxynitrite ions can easily oxidize the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond connecting the benzothiazole and xanthene rings, resulting in the formation of N,N-ethylformyl xanthene derivative, reducing the fluorescence intensity at the 760 nm region with concomitant enhancement of a blue-shifted peak at 630 nm. Similarly, under a highly viscous medium, the emission at 760 nm increased to a large extent. The UV/vis spectral study with a variety of solvents having different polarities revealed no prominent alteration in the spectra, emphasizing on the sole effect of viscosity on the change in the fluorescence spectra of the xanthene-benzothiazole probe. Cell imaging experiments disclosed the selective accumulation of the probe inside the mitochondria in HepG2 cells.
C bond connecting the benzothiazole and xanthene rings, resulting in the formation of N,N-ethylformyl xanthene derivative, reducing the fluorescence intensity at the 760 nm region with concomitant enhancement of a blue-shifted peak at 630 nm. Similarly, under a highly viscous medium, the emission at 760 nm increased to a large extent. The UV/vis spectral study with a variety of solvents having different polarities revealed no prominent alteration in the spectra, emphasizing on the sole effect of viscosity on the change in the fluorescence spectra of the xanthene-benzothiazole probe. Cell imaging experiments disclosed the selective accumulation of the probe inside the mitochondria in HepG2 cells.
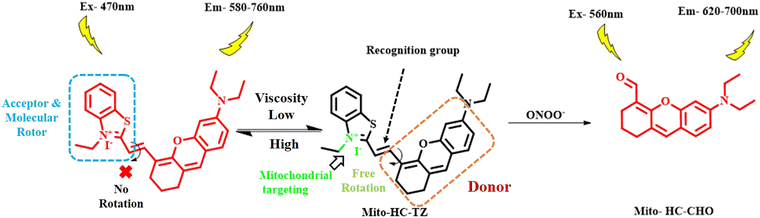 | ||
| Fig. 9 Schematic of independent detection of viscosity and peroxynitrite by the xanthene-benzothiazole probe.86 [Reproduced from Dyes and Pigments, 210, Qingqing Liu, Chao Dong, Jing Zhang, Bo Zhao, Yongqing Zhou, Chunhua Fan, Zhengliang Lu, A mitochondria-targeted ratiometric NIR fluorescent probe for simultaneously monitoring viscosity and ONOO− based on two different channels in living HepG2 cells, 111045, Copyright (2023), with permission from Elsevier.]. | ||
Gao et al. fabricated a wide variety of phenyl-rhodol derivatives constituted of fluorophore-quencher combinations.57 They connected a rhodol unit to a diverse range of phenyl rings comprising a range of substituents from a strongly electron-donating one like the methoxy group to a strongly electron-withdrawing one like the nitro group (Fig. 10). The phenyl rings here behaved as the quenching rotor unit, whereas the rhodol unit was designated as the acceptor unit. It was observed that the PET process was the most prominent when the energy gap between the highest occupied molecular orbitals (HOMOs) of the acceptor and the quencher was <0.6 eV. In a combined solvent system having glycerol and water, these fluorophores exhibited a sharp increase in fluorescence intensity at 570 nm with an increase in the glycerol content. The probe with strongest quencher group, i.e., p-nitrophenyl-rhodol, showed the most promising results and was utilized to study mitochondrial viscosity variation under mitochondrial pH shift and viscosity variation in living zebrafish, indicating its great physiological applications.
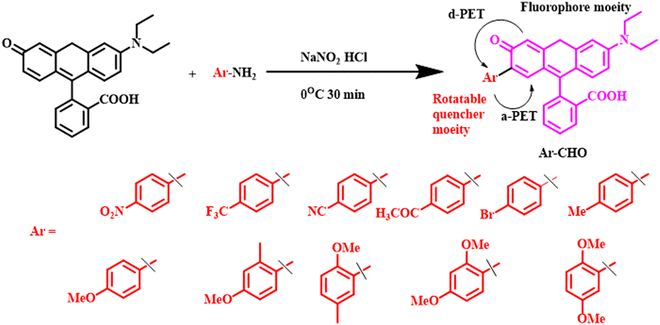 | ||
| Fig. 10 Syntheses and molecular structures of phenyl rhodol derivatives containing a series of aromatic rotatable quencher moieties.57 [Reproduced from Chemical Engineering Journal, 466, Yong Gao, Jianwen Qiu, Meng Liu, Xinyi Xiong, Hu Zhu, A guideline for design of photoinduced electron transfer-based viscosity probe with rigid fluorophore-quencher configuration, 143100, Copyright (2023), with permission from Elsevier.]. | ||
Varied substituted xanthene derivatives have shown promising application in viscosity sensing with the help of flexible intramolecular rotation. However, the versatile structure of xanthene also provides a means of developing a rigid planar structure, avoiding any influence of viscosity. One such example was put forth by Zhang et al. Zhang and co-workers rigidified a rhodamine B structure by fusing two rings by an oxygen bridge.87 This rhodamine-fused xanthene derivative was found to be highly susceptible to fluorescence off–on mechanism in the presence of both peroxynitrate and nitroreductase enzyme, which are crucial biomarkers to quantify the hypoxic nature of cancer cells. The new xanthene core-based fluorophore showed excellent quantum yield (16.1%). NIR fluorescence (maxima emission at 770 nm), along with a controllable fluorescence off–on nature upon interaction with a specific analyte, leads to better signal-to-noise ratio.
Cyanine-based probes
Cyanine dyes are compounds that are well known for their vibrant and intense colors. They have found applications in the interface of chemistry, biology, and materials science. These dyes have a backbone chain of sp2-hybridized carbon atoms with two positively charged nitrogen atoms at the two tails.88 Owing to such extended conjugation, cyanine dyes can absorb at least at 100 nm longer wavelength than the other dyes.88 These properties enable the cyanine derivatives to exhibit strong absorption in the visible or NIR region, which is highly beneficial for biomedical applications. Such strong absorption in the visible or NIR window leads to bright colour and distinct fluorescence emission properties for various cyanine derivatives. The fluorescence properties vary with the number of methane groups and the nature of the heteroatoms. Cyanines have found extensive use in the development of fluorescent probes for various biomarkers and as prominent photo-switches. In addition to such promising fluorescence properties, the molecular rotor-type conjugated sp2-carbon backbone of cyanine dyes make them greatly sensitive to the viscosity of the surroundings.89 Therefore, scientists have exploited various cyanine dyes in formulating viscosity-sensitive probes.90,91 In the following section, we have highlighted few of the recent works on cyanine-based viscosity-dependent fluorescent probes from a critical point of view.Recently, Si-Lin Xu et al. synthesized a mitochondria-targeting chromene-hemicyanine-based probe (Probe 1) for the concurrent detection of hypochlorous acid (HClO−) and viscosity in Zebra fish (Fig. 11).92 They constructed the hemicyanine probe by introducing a methoxy chromene ring as the donor to the acceptor indoleamine ring carrying a positively charged nitrogen atom via a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unit. The C
C unit. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C served the dual purpose of a detecting unit for HClO− and simultaneously led to the restricted rotation of hemicyanine derivatives. Probe 1 emitted a deep red emission at 628 nm owing to intramolecular charge transfer (ICT) and a weak emission at 493 nm upon excitation at 380 nm. The red emission was subsequently quenched with consecutive enhancement of the peak at 493 nm. This could be attributed to the reaction of C
C served the dual purpose of a detecting unit for HClO− and simultaneously led to the restricted rotation of hemicyanine derivatives. Probe 1 emitted a deep red emission at 628 nm owing to intramolecular charge transfer (ICT) and a weak emission at 493 nm upon excitation at 380 nm. The red emission was subsequently quenched with consecutive enhancement of the peak at 493 nm. This could be attributed to the reaction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C with ClO− inhibiting the ICT. The viscosity response was examined using a mixed solution of glycerol and HEPES buffer (50 mM, pH 7.4) at various volume ratios. When compared to HEPES buffer (50 mM, pH 7.4), the fluorescence intensity at 628 nm in 99% glycerol was about 20 times higher owing to limited rotation in the presence of the highly viscous solvent glycerol. Additionally, the red fluorescence gradually increased when exposed to a 365 nm UV lamp. The detection limit for hypochlorous ion was calculated to be 0.082 μM. Although this work involved the selective detection of hypochlorous ion and mitochondrial viscosity, the detection processes are not independent.
C with ClO− inhibiting the ICT. The viscosity response was examined using a mixed solution of glycerol and HEPES buffer (50 mM, pH 7.4) at various volume ratios. When compared to HEPES buffer (50 mM, pH 7.4), the fluorescence intensity at 628 nm in 99% glycerol was about 20 times higher owing to limited rotation in the presence of the highly viscous solvent glycerol. Additionally, the red fluorescence gradually increased when exposed to a 365 nm UV lamp. The detection limit for hypochlorous ion was calculated to be 0.082 μM. Although this work involved the selective detection of hypochlorous ion and mitochondrial viscosity, the detection processes are not independent.
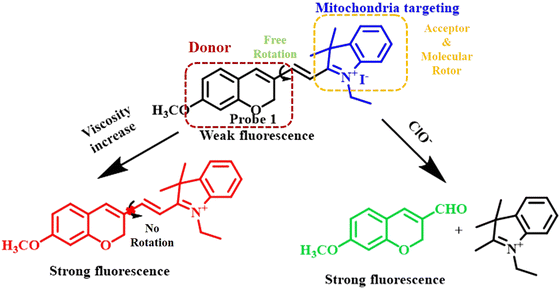 | ||
| Fig. 11 Mechanistic presentation of concerted response to viscosity and hypochlorous ion showing a sharp rise in the fluorescence intensity with a gradual increase in the viscosity of the mixed solution of HEPES-glycerol.92 [Open access]. | ||
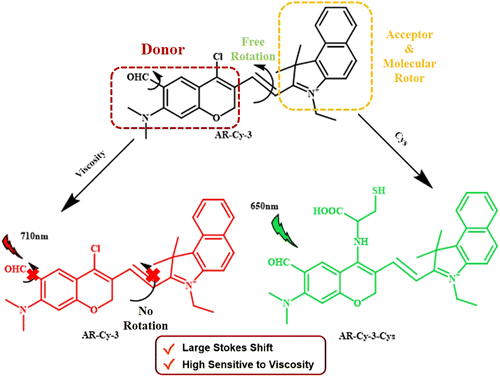
In another recent study, Yaun Ye and his co-workers synthesized an NIR probe for the dual sensing of mitochondrial cysteine and viscosity during the ferroptosis of kidney (Fig. 12).93 The main importance of their work lied in the independent detection mechanism of cysteine and viscosity. Such an independent mechanism helps in reducing signal crosstalk, enhancing selectivity, fast response, avoiding the use of multiple probes, etc. In the present work, Ye and his co-workers developed a series of chloro-N,N-dimethyl merocyanine-type derivatives, which exhibited excellent absorption at about 700 nm with a large Stoke's shift as high as 208 nm in polar solvent methanol, resulting in a prominent rise in the signal-to-noise ratio. Notably, DFT calculations and spectroscopic measurements showed that the additional electron withdrawing rotor endowed by the introduction of the aldehyde group through a modified Vilsmeier–Haack reaction was crucial for enhancing the sensing performance towards both mitochondrial viscosity and oxidative stress.
 | ||
| Fig. 12 Development of a merocyanine-derivative for the independent detection of viscosity and intracellular level of cysteine during the ferroptosis process.93 [Reproduced from Sensors and Actuators B: Chemical, 396, Yuan Ye, Mengye He, Liping Wang, Xing-Can Shen, Hua Chen, Unexpected dual-functional cyanine fluorophores: NIR multifunctional fluorescent probes for simultaneous monitoring of mitochondrial cysteine/viscosity during kidney Ferroptosis in vivo, 134600, Copyright (2023), with permission from Elsevier.]. | ||
Fluorescent probes with emission in the NIR-II window have been proved to have pronounced advantages over the shorter wavelength regions in terms of deeper tissue penetration, higher resolution, lower scattering, and photoabsorption. Keeping this in mind, four non-symmetrical cynanine dyes were designed by Yang Tian and his colleagues.94 The novelty of their system relied on the dual activation nature under acidic (4–5.5) and highly viscous medium. At the lysosomal pH, dyes with pKa less than 4 exhibited a transition from non-fluorescent to weak NIR fluorescence. By virtue of the characteristic free rotation in heptamethine chains, the dyes could selectively respond to the highly viscous tissues. Another promising aspect of Tian's work was the high photothermal conversion up to 59.5%, which enabled the dyes to show profound photothermal therapy in vivo. Since multimodal therapy is one of the promising approaches to circumventing multidrug-resistant cancers, Tian et al. incorporated an anticancer drug in the cyanine derivatives via a disulphide linker. The disulphide linker was utilized to have controlled release of the anticancer drug triggered by the oxidative stress of cancer cells.
Rhodamine-based probes
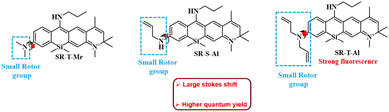
Rhodamine derivatives, renowned for their outstanding photophysical and photostability characteristics, serve as crucial elements in the development of fluorescent probes. Widely used in biotechnology, these dyes play a pivotal role as fluorescent markers, enabling the detection of biomolecules and offering a potent tool for visualising living systems. The crucial properties of rhodamines that bestow them with prominent sensing abilities under biological conditions include extended conjugation, leading to absorption and emission in the NIR region, regulated solubility, bright fluorescence with low signal to noise ratio, significant pH-sensitivity, low detection limit, diverse energy/charge transfer processes, and high quantum yield.95–98 The introduction of aromatic rings into the structure of rhodamine dye opens up the possibility of restricted rotation. Hence, rhodamine dyes can be molecules of interest to construct viscosity-sensitive probes.Very recently, Jun-Mei Li et al. synthesised a unique class of silicon-rhodamine-based viscosity-sensitive NIR fluorescent probe (Fig. 13).99 They synthesized three probes by varying the amount of alkylation at the nitrogen atom at the third position of rhodamine. Among these, the di-allyl substituted probe showed the most significant electron donating ability and was chosen for further study. These probes were quite stable as their fluorescence intensity remained unaffected by changes in environmental polarity, pH, ROS, etc. Instead, their fluorescence intensity was solely influenced by changes in environmental viscosity, showing the probes’ excellent viscosity-sensing capabilities. The study employed an TICT mechanism to monitor the changes in intracellular viscosity. In low viscosity environment, the unrestricted rotation of Si-rhodamine molecules causes the non-radiative dissipation of energy from the excited state. This led to a decrease in the fluorescence intensity. As the environmental viscosity steadily increased, the intramolecular rotation is restricted, leading to a substantial increase in fluorescence intensity.
 | ||
| Fig. 13 Molecular structures of Si-Rhodamine-based probes containing small molecular rotor moieties.99 [Open access]. | ||
Peisheng Tang et al. developed a novel terpyridyl-functionalized rhodamine dye.100 In the molecular design, they incorporated a positively charged semi-rhodamine for mitochondria targeting functionalized with the terpyridyl moiety for enhanced viscosity sensitivity. The introduction of the terpyridyl unit was helpful in modulating the Stoke's shifts via increased molecular asymmetry. The increased viscosity hindered the rotation of the terpyridyl group, reducing non-radiative decay and indicating an ICT process during excitation, which led to a substantially large Stoke's shifts, resulting in enhanced fluorescence.
In another recent report, Chuang Liu et al. synthesised a viscosity-responsive NIR fluorophore via a facile Knoevenagel condensation between cinnamaldehyde and rhodamine.101 The probe demonstrated the real-time monitoring of liquid droplet viscosity variations with increased viscosity, leading to enhanced fluorescent intensity both in vitro and in vivo. Under low-viscosity conditions, the fluorescence was quenched due to unrestricted intramolecular rotations. An increase in environmental viscosity restricted intramolecular rotations, leading to enhanced fluorescence.
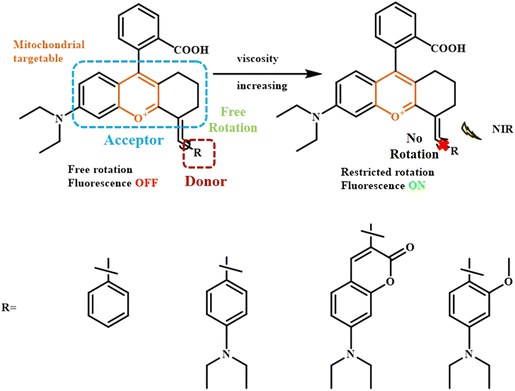
Qian Chen et al. developed a series of NIR fluorescent probes by functionalizing rhodamine dyes with additional aromatic moieties like benzene, N,N-diethylbenzene, coumarin, and 2-methyl-N,N-diethylbenzene (Fig. 14).102 The rhodamine unit acted as the electron withdrawing group while the aromatic units acted as the electron-donating group. In low-viscosity conditions, such biphenyl structures exhibited spontaneous rotation across the C–C bond between the donor and acceptor, leading to fluorescence quenching, while under high viscosity environment, the fluorescence is enhanced due to restricted rotation.
 | ||
| Fig. 14 Response mechanism of the rhodamine-based biphenyl probes to viscosity.102 [Reproduced from Analytica Chimica Acta, 1231, Qian Chen, Xiao-Fan Zhang, Teng Wang, Xiao-Qun Cao, Shi-Li Shen, A sensitive NIR mitochondria-targeting fluorescence probe for visualizing viscosity in living cells and mice, 340443, Copyright (2022), with permission from Elsevier.]. | ||
From the discussion above, it is evident that diverse research efforts have been invested in yielding innovative viscosity-sensitive fluorescent probes with distinct molecular designs and mechanisms. These studies collectively underscore the versatility and potential applications of viscosity-sensitive fluorescent probes across various scientific domains. As can be derived from the reports of viscosity-specific fluorophores mentioned above, the basic structural requirement for viscosity probes is restricted intramolecular rotation. Besides the prominent fluorophores mentioned in the sections above, small hydrocarbon-based highly-fluorescent molecules like pyrene, naphthalene, and thiophene have also been functionalized appropriately to construct viscosity-sensitive probes.103,104 Although there has been extensive research lately in this field, issues like critical synthesis, lack of specificity, limited bioavailability, and inadequate cellular internalization impede the clinical translation of these probes. Nevertheless, researchers are putting in constant effort in this field to formulate novel viscosity probes involving diverse combinations of fluorophores.
Applications of viscosity probes in diabetes treatment
Diabetes is a one of the most crucial global health concerns these days. Diabetes stands very close to cardiac diseases, cancer, respiratory infection, etc., in terms of detrimental effects. Proper monitoring of diabetes is imperative to prevent severe kidney problems, heart problems, eye disease, liver malfunctions, etc. Therefore, the early detection of diabetes is of paramount importance for the betterment of general public healthcare. There are specific biomarkers overexpressed in diabetic patients, e.g., glycated haemoglobin,105 glycated albumin,106 reactive oxygen species,107–109 high viscosity,110,111 and volatile organic compounds like acetone, aldehyde, etc. These biomarkers have been proved to be of great benefit for the early diagnosis of diabetes. Fluorescent probes have been widely exploited for the detection of various diseases owing to their non-invasiveness, spatial, and temporal control. The fluorescent properties of any molecule are greatly affected by the adjacent medium. Hence, intracellular viscosity can be a favorable biomarker for designing specific probes to detect early-stage diabetes.Recently, Dai et al. developed a viscosity-responsive xanthene-quinolinium-based fluorescent probe, NI-VD (Fig. 15).112 It contained a methoxyxanthene group as the basic fluorophore core attached with a quinolinium moiety via a vinyl bond. Rotation across the vinyl bond between the donor methoxyxanthene to the acceptor quinolinium unit gave rise to a planar local excited (LE) sate, whereas in the highly viscous diabetic cell, the restriction in molecular rotation resulted in a significant enhancement in fluorescence following the typical TICT mechanism. The positively charged quinolinium unit facilitated the internalization of the probe through the negatively charged mitochondrial membrane. NI-VD showed low fluorescence in a lower viscosity medium due to the rotation of the bond, whereas in a high viscosity medium inside the mitochondrial cavities, an enhancement in fluorescence was observed due to restricted rotation. Diabetic cells are more viscous than normal cells; thus, this probe can be used to differentiate between diabetes cells and healthy cells. This probe was particularly useful for diagnosing and treating diabetic nephropathy.
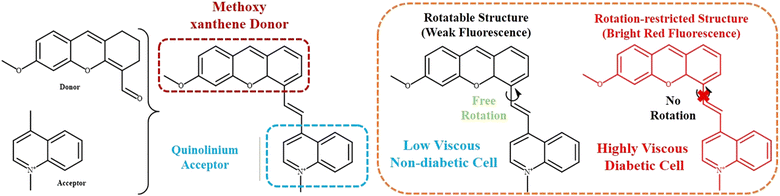 | ||
| Fig. 15 Mechanism of xanthene-quinolinium-based viscosity-responsive fluorescent probe for the treatment of diabetic mice.112 [Reproduced from Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 254, Lixuan Dai, Mingguang Ren, Weiying Lin, Development of a novel NIR viscosity fluorescent probe for visualizing the kidneys in diabetic mice, 119627, Copyright (2021), with permission from Elsevier.]. | ||
Diabetes is largely guided by insulin metabolism. Peroxynitrite-based ROSs disrupt the receptors of insulin in the cell membrane. With this concern, Shi et al. developed a dually-locked fluorescent probe for the concurrent detection of peroxynitrite (ONOO−) and viscosity of the diseased cells (Fig. 16).113 To achieve viscosity sensitivity, Shi and his co-workers curated the probe with a molecular rotor isophorone moiety containing electron-withdrawing cyanide groups. A boronate ester unit was introduced to selectively detect the oxidising peroxynitrite group to the donor N,N-dimethylphenol. Owing to the significant TICT process from the donor to the acceptor isophorone, the probe remained non-fluorescent. Upon exposure to a large concentration of ONOO− and high viscous medium in the diabetic patients, the fluorophore exhibited a bright increase in its fluorescence property. This probe was found to be able to distinguish between diabetic and healthy cells.
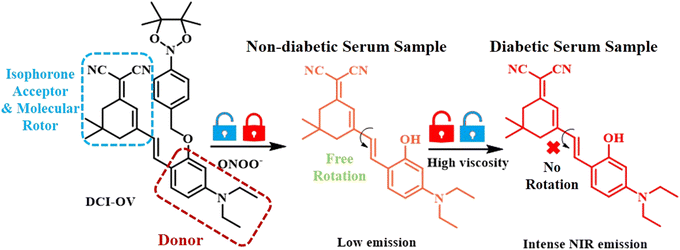 | ||
| Fig. 16 Mechanistic details of viscosity responsive probe for the detection of diabetes using human diabetic serum samples.113 [Reproduced from Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 278, Baotang Shi, Huiling Wang, Xingxia Wan, Yu Guo, Shi-Yu Liu, Quan Gong, A novel “dual-locked” fluorescent probe for ONOO− and viscosity enables serum-based rapid disease screening, 121375, Copyright (2022), with permission from Elsevier.]. | ||
Following these lines of approach, several research groups are presently focussing more on the early detection of diabetes.114,115 As discussed in previous sections, most of the common probes utilized like BODIPY, xanthene, Nile red, and rhodamine face severe solubility issues, relatively smaller Stoke's shift, restricted bioavailability, and non-specificity. Therefore, the development of new generation viscosity probes is the need of the hour.
Lipid droplets are the key location for the metabolism of lipids. Lipid droplets tend to undergo unusual accumulation in case of diabetic patients, which leads to severe metabolic disorders. Hence, in addition to viscosity sensitivity, the real-time visualization of lipid droplets is also a prominent treatment modality in early-stage diabetes patients. Recently, Wang et al. developed a benzopyran-based NIR probe clubbed with an AIE agent TPA for the simultaneous monitoring of lipid droplet accumulation and viscosity of the medium (Fig. 17).26 In the present study, triphenylamine not only served as the AIE-inducing donor component but also as the lipid droplet-targeting moiety. Positively charged benzopyran unit helped internalization into the mitochondria. Therefore, the present probe under consideration with its noteworthy push–pull effect showed negligible fluorescence in its planar form in non-viscous healthy liver; however, under diabetic viscous medium, the TICT state was attained with restoration of bright fluorescence. Aggregation-induced emission (AIE) features of the compound helped the probe detect viscosity, and this feature can be applied in models of diabetes.
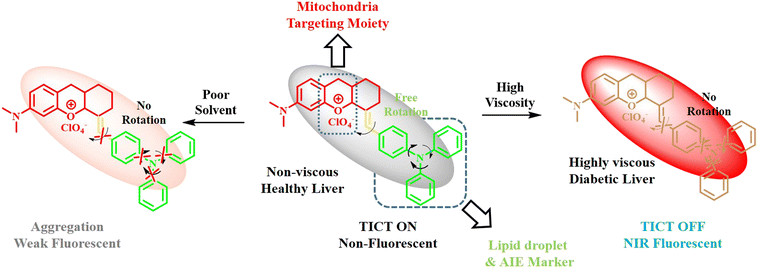 | ||
| Fig. 17 Analysis of viscosity-responsive probe for the treatment of damaged liver in diabetic mice.26 [Reproduced from Sensors and Actuators B: Chemical, 394, Huili Wang, Hongyong Zheng, Wenjing Zhang, Lei Yang, Mingming Yu, Zhanxian Li, A near-infrared aggregation-induced emission probe for imaging lipid droplet and in vivo visualization of diabetes-related viscosity variations, 134347, Copyright (2023), with permission from Elsevier.]. | ||
The aggregation of lipid droplets, as discussed above, often facilitates advancement of diabetic cataract, which is a severe form compared to regular cataract in terms of lack of surgical methods owing to retinopathy, iris neovascularization, slow wound healing, etc. Therefore, the development of drugs targeting the lipid droplets is highly necessary. In a recent report, Guo and his colleagues fabricated a xanthene-boronate-ester-based probe functionalized with a quinolinium unit via a vinyl bond.116 The substitution of the quinolinium unit on the otherwise hydrophobic phenothiazine molecule not only induced polarity in the probe but also made the probe highly responsive to the strong viscosity of the lipid droplets. Owing to the significant viscosity response, this phenothiazine probe found great application in discriminating between regular cataract and diabetic cataract and helped in early treatment. Recently, Feng et al. also reported another xanthene-quinoline-based viscosity-responsive fluorescent probe for the visualization of lysosomal autophagy.117 In another attempt by Jin Zhao's group, a novel viscosity sensitive probe based on 3-bromonitrostyryl derivative combined with dicyanomethylene group was introduced.118 This probe was found to be highly responsive to the changes in lipid droplet viscosity brought about by the adverse side-effects of radiocontrast imaging using an iodinated agent (iodinated contrast media).
From the above discussion, it is evident that the viscosity-responsive probes are of tremendous advantage in the early detection of diabetes. Since the emission properties of the viscosity-responsive probes are solely affected by the viscous heterogeneous microenvironment of the cell, their response remains nearly unaffected by other biologically relevant analytes, thus avoiding any undesired signal.
Conclusions and future outlook
To summarize, in this review, we have presented mechanistic analysis and basic structural requirements for the development of viscosity-sensitive probes. Viscosity-sensitive probes have shown significant use in the early detection of various detrimental diseases like diabetes, cancer, and inflammation. For all these cases, the high viscosity medium of the diseased tissues played a pivotal role. The high viscosity of the tissues was found to disrupt the rotations and vibrations in the molecule. Therefore, researchers have focused on formulating fluorescent probes that can show a prominent change in their fluorescent properties in terms of large Stoke's shift, significant rise in intensity, etc. upon change in molecular rotation. As per the examples discussed in this article, there are mainly two kinds of chromophores that can show restricted rotation upon increase in viscosity: (i) biphenyl-type system connecting two bulky aromatic rings via a rotatable single bond and (ii) rigid double bond connecting donor and acceptor units. Of late, several research groups are focusing on such viscosity-sensitive probes. They mainly follow coupling a donor and an acceptor unit by means of a rigid linker. There are certain fluorophores that have been extensively utilized in the development of probes for a diverse range of biomarkers. In these articles, some of the most important fluorophores have been elaborated, e.g., coumarin-based, BODIPY-based, hemicyanine-based, xanthene-based, and rhodamine-based. Due to their extensive π-conjugation, easy functionalization, absorption in the longer wavelength, and NIR emission with deep penetration, the above-mentioned fluorophores have been exploited to a great extent. Therefore, all these fluorophores can be modified by incorporating rigid linkers and proper donor–acceptor combinations in order to respond to high viscosity medium. Present methods of monitoring diabetes involve measurements of glucose level in the blood serum, obesity, fatigue, etc. All these parameters can be monitored only after a certain period of diabetes progression. However, the use of fluorescent probes and viscosity as the biomarker paves the way for the early and facile detection of diabetes.With tremendous focus on diabetes treatment lately, several other biomarkers have also been identified for diabetes, which include volatile organic compounds (VOCs) like acetone, ethanol, and formaldehyde. Keeping this in mind, future diabetes research is mainly shifted towards the analysis of breath samples of patients rather than glucose monitoring. Such breath analysis is superior to regular blood sample analysis in terms of non-invasiveness, cost, and time effectiveness. Steps towards omitting the extraction of blood samples have already been implemented by the clinical translation of glucose sensors into various wearable devices. Such wearable devices are highly efficient in monitoring real-time glucose level in patients throughout the day. The fast and accurate responses of such wearable devices have already fetched FDA approval and several of them are already in the market.
Considering such futuristic implications of diabetes sensors, the present article is highly relevant in the sense that it provides all the requisite mechanistic and structural requisites for the construction of a proficient sensor for diabetic patients.
Author contributions
Conceptualization and writing original draft: M M Sreejaya; data curation and writing original draft: Vineeth M; writing original draft: Ayesha A, Maanas Baby; supervision: Dr Manoranjan Bera; supervision and writing – review & editing: Dr Moumita Gangopadhyay.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors are grateful to the Director, Amrita Vishwa Vidyapeetham, Amritapuri, Kerala, India for providing all requisite support. M M Sreejaya is thankful to Amrita Vishwa Vidyapeetham, Amritapuri for her fellowship. Moumita would like to thank the Science and Engineering Research Board (SERB) for funding under the SRG/2023/001109 scheme.Notes and references
- T. T. Jia, Y. Li and H. Niu, Chemosensors, 2022, 10, 280 CrossRef CAS.
- X. Yang, D. Zhang, Y. Ye and Y. Zhao, Coord. Chem. Rev., 2022, 453, 214336 CrossRef CAS.
- C. Wang, W. Chi, Q. Qiao, D. Tan, Z. Xu and X. Liu, Chem. Soc. Rev., 2021, 50, 12656–12678 RSC.
- C. Chen, R. Huang, A. S. Batsanov, P. Pander, Y. T. Hsu, Z. Chi, F. B. Dias and M. R. Bryce, Angew. Chem., Int. Ed., 2018, 57, 16407–16411 CrossRef CAS PubMed.
- K. Stallhofer, M. Nuber, F. Schüppel, S. Thumser, H. Iglev, R. de Vivie-Riedle, W. Zinth and H. Dube, J. Phys. Chem. A, 2021, 125, 4390–4400 CrossRef CAS PubMed.
- C. Wang, W. Jiang, D. Tan, L. Huang, J. Li, Q. Qiao, P. Yadav, X. Liu and Z. Xu, Chem. Sci., 2023, 14, 4786–4795 RSC.
- S. Ye, H. Zhang, J. Fei, C. H. Wolstenholme and X. Zhang, Angew. Chem., Int. Ed., 2021, 60, 1339–1346 CrossRef CAS PubMed.
- C. H. Wolstenholme, H. Hu, S. Ye, B. E. Funk, D. Jain, C. H. Hsiung, G. Ning, Y. Liu, X. Li and X. Zhang, J. Am. Chem. Soc., 2020, 142, 17515–17523 CrossRef CAS PubMed.
- M. S. Michie, R. Götz, C. Franke, M. Bowler, N. Kumari, V. Magidson, M. Levitus, J. Loncarek, M. Sauer and M. J. Schnermann, J. Am. Chem. Soc., 2017, 139, 12406–12409 CrossRef CAS PubMed.
- H. Wang, Q. Li, P. Alam, H. Bai, V. Bhalla, M. R. Bryce, M. Cao, C. Chen, S. Chen, X. Chen, Y. Chen, Z. Chen, D. Dang, D. Ding, S. Ding, Y. Duo, M. Gao, W. He, X. He, X. Hong, Y. Hong, J. J. Hu, R. Hu, X. Huang, T. D. James, X. Jiang, G. I. Konishi, R. T. K. Kwok, J. W. Y. Lam, C. Li, H. Li, K. Li, N. Li, W. J. Li, Y. Li, X. J. Liang, Y. Liang, B. Liu, G. Liu, X. Liu, X. Lou, X. Y. Lou, L. Luo, P. R. McGonigal, Z. W. Mao, G. Niu, T. C. Owyong, A. Pucci, J. Qian, A. Qin, Z. Qiu, A. L. Rogach, B. Situ, K. Tanaka, Y. Tang, B. Wang, D. Wang, J. Wang, W. Wang, W. X. Wang, W. J. Wang, X. Wang, Y. F. Wang, S. Wu, Y. Wu, Y. Xiong, R. Xu, C. Yan, S. Yan, H. B. Yang, L. L. Yang, M. Yang, Y. W. Yang, J. Yoon, S. Q. Zang, J. Zhang, P. Zhang, T. Zhang, X. Zhang, X. Zhang, N. Zhao, Z. Zhao, J. Zheng, L. Zheng, Z. Zheng, M. Q. Zhu, W. H. Zhu, H. Zou and B. Z. Tang, ACS Nano, 2023, 17, 14347–14405 CrossRef CAS PubMed.
- W. Hu, T. Qiang, L. Chai, T. Liang, L. Ren, F. Cheng, C. Li and T. D. James, Chem. Sci., 2022, 13, 5363–5373 RSC.
- E. Lippert, W. Lüder, F. Moll, W. Nägele, H. Boos, H. Prigge and I. Seibold-Blankenstein, Angew. Chem., 1961, 73, 695–706 CrossRef CAS.
- L. Ma, Y. Geng, G. Zhang, Z. Hu, T. D. James, X. Wang and Z. Wang, Adv. Healthcare Mater., 2023, 12, 2300733 CrossRef CAS PubMed.
- X. Wang, L. Wang, T. Jin, K. Sun and J. Yang, Sens. Actuators, B, 2023, 375, 132935 CrossRef CAS.
- W. Liu, T. Wang, L. Wang, Y. Wang, S. Hu and D. Tian, Spectrochim. Acta, Part A, 2024, 304, 123329 CrossRef CAS PubMed.
- Q. Zan, L. Fan, L. Ma, Q. Yang, K. Zhao, Y. Huang, C. Dong and S. Shuang, Sens. Actuators, B, 2023, 397, 134596 CrossRef CAS.
- Y. Liu, S. Feng, S. Gong and G. Feng, Anal. Chem., 2022, 94, 17439–17447 CrossRef CAS PubMed.
- Y. Li, D. Zhang, Y. Yu, L. Zhang, L. Li, L. Shi, G. Feng and B. Z. Tang, ACS Nano, 2023, 17, 16993–17003 CrossRef CAS PubMed.
- J. Mei, Y. Huang and H. Tian, ACS Appl. Mater. Interfaces, 2018, 10, 12217–12261 CrossRef CAS PubMed.
- J. Qi, C. Sun, A. Zebibula, H. Zhang, R. T. K. Kwok, X. Zhao, W. Xi, J. W. Y. Lam, J. Qian and B. Z. Tang, Adv. Mater., 2018, 30, 1706856 CrossRef PubMed.
- Y. Yu, C. Feng, Y. Hong, J. Liu, S. Chen, K. M. Ng, K. Q. Luo and B. Z. Tang, Adv. Mater., 2011, 23, 3298–3302 CrossRef CAS PubMed.
- R. T. K. Kwok, J. Geng, J. W. Y. Lam, E. Zhao, G. Wang, R. Zhan, B. Liu and B. Z. Tang, J. Mater. Chem. B, 2014, 2, 4134–4141 RSC.
- X. Gu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Biomaterials, 2017, 146, 115–135 CrossRef CAS PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- W. Liu, Y. Wang, T. Wang, L. Wang, S. Hu and D. Tian, Anal. Chim. Acta, 2023, 1253, 341099 CrossRef CAS PubMed.
- H. Wang, H. Zheng, W. Zhang, L. Yang, M. Yu and Z. Li, Sens. Actuators, B, 2023, 394, 134347 CrossRef CAS.
- L. Xu, F. Xiong, M. Kang, Y. Huang and K. Wu, Analyst, 2022, 147, 4132–4140 RSC.
- G. Nie, J. Che, Y. Feng, W. Liang, D. Chen and H. Wang, Dyes Pigm., 2023, 219, 111535 CrossRef CAS.
- J. Ge, W. Cai, N. Niu, Y. Wen, Q. Wu, L. Wang, D. Wang, B. Z. Tang and R. Zhang, Biomaterials, 2023, 300, 122190 CrossRef CAS PubMed.
- D. Cao, L. Zhu, Z. Liu and W. Lin, J. Photochem. Photobiol., C, 2020, 44, 100371 CrossRef CAS.
- S. Zhai, W. Hu, W. Wang, L. Chai, Q. An, C. Li and Z. Liu, Biosens. Bioelectron., 2022, 213, 114484 CrossRef CAS PubMed.
- S. Su, L. Chai, Q. An, W. Hu, L. Wang, X. Li, H. Zhang and C. Li, Anal. Chem., 2022, 94, 15146–15154 CrossRef CAS PubMed.
- L. Zhu and W. Lin, Sens. Actuators B, 2022, 352, 131042 CrossRef CAS.
- J. Hu, Q. Li, X. Wang, S. Shao, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2019, 58, 8405–8409 CrossRef CAS PubMed.
- J. R. Caine, P. Hu, A. T. Gogoulis and Z. M. Hudson, ACC Mater. Res., 2023, 4, 879–891 CrossRef CAS.
- J. Andréasson, A. Kyrychenko, J. Mårtensson and B. Albinsson, Photochem. Photobiol. Sci., 2002, 1, 111–119 CrossRef PubMed.
- B. Shen, L. Liu, Y. Huang, J. Wu, H. Feng, Y. Liu, H. Huang and X. Zhang, Aggregate, 2023, 00, e421 Search PubMed.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Chem. Rev., 2019, 119, 10403–10519 CrossRef CAS PubMed.
- N. Ahmed, W. Zareen, Z. Shafiq, S. F. de Alcântara Morais, M. Khalid, A. A. C. Braga, K. S. Munawar and Y. Yong, Spectrochim. Acta, Part A, 2023, 286, 121964 CrossRef CAS PubMed.
- M. Arooj, M. Zahra, M. Islam, N. Ahmed, A. Waseem and Z. Shafiq, Spectrochim. Acta, Part A, 2021, 261, 120011 CrossRef CAS PubMed.
- W. Bouali, M. Yaman, N. Seferoğlu and Z. Seferoğlu, J. Photochem. Photobiol., A, 2024, 448, 115227 CrossRef CAS.
- E. Kandemir, M. Özkütük, B. Aydıner, N. Seferoğlu, H. Erer and Z. Seferoğlu, J. Mol. Struct., 2022, 1249, 131593 CrossRef CAS.
- Z. Aydin, M. Keskinates, B. Yilmaz, M. Durmaz and M. Bayrakci, Anal. Biochem., 2022, 654, 114798 CrossRef CAS PubMed.
- A. Orte, J. M. Alvarez-Pez and M. J. Ruedas-Rama, ACS Nano, 2013, 7, 6387–6395 CrossRef CAS PubMed.
- W. Luo, H. Jiang, X. Tang and W. Liu, J. Mater. Chem. B, 2017, 5, 4768–4773 RSC.
- M. Yahya, R. Metin, B. Aydıner, N. Seferoğlu and Z. Seferoğlu, Anal. Sci., 2023, 39, 829–842 CrossRef CAS PubMed.
- K. Sunwoo, K. N. Bobba, J. Y. Lim, T. Park, A. Podder, J. S. Heo, S. G. Lee, S. Bhuniya and J. S. Kim, Chem. Commun., 2017, 53, 1723–1726 RSC.
- G. Wu, X. Tang, W. Ji, K. W. C. Lai and Q. Tong, Methods Appl. Fluoresc., 2017, 5, 015001 CrossRef PubMed.
- X. Zhou, Y. Kwon, G. Kim, J. H. Ryu and J. Yoon, Biosens. Bioelectron., 2015, 64, 285–291 CrossRef CAS PubMed.
- P. Srisuwan, A. Sappasombut, W. Thongyod, T. Jantarat, V. Tipmanee, N. Leesakul and D. Sooksawat, J. Photochem. Photobiol., A, 2022, 427, 113841 CrossRef CAS.
- M. Wu, Y. Yu, Y. Liu, J. You, W. Wu and B. Liu, Chin. J. Org. Chem., 2022, 42, 803 CrossRef CAS.
- G. Kaur, I. Singh, R. Tandon and N. Tandon, Inorg. Chem. Commun., 2023, 158, 111480 CrossRef CAS.
- T. Z. Liu, S. Wang, J. R. Xu, J. Y. Miao, B. X. Zhao and Z. M. Lin, Talanta, 2023, 256, 124302 CrossRef CAS PubMed.
- F.-T. Liu, W.-W. Han, H. Ren, W.-J. Yang, R.-N. Wang, J.-Y. Miao, B.-X. Zhao and Z.-M. Lin, Dyes Pigm., 2023, 210, 111017 CrossRef CAS.
- W. Pan, L. Han, X. Cao, S. Shen, X. Pang and Y. Zhu, Food Chem., 2023, 407, 135163 CrossRef CAS PubMed.
- H. Wang, Y. Sun, X. Lin, W. Feng, Z. Li and M. Yu, Chin. Chem. Lett., 2023, 34, 107626 CrossRef CAS.
- Y. Gao, J. Qiu, M. Liu, X. Xiong and H. Zhu, Chem. Eng. J., 2023, 466, 143100 CrossRef CAS.
- X. Hao, J. Zhan, C. Geng and W. Lin, Spectrochim. Acta, Part A, 2023, 284, 121807 CrossRef CAS PubMed.
- W. J. Shi, X. H. Yan, J. Yang, Y. F. Wei, Y. T. Huo, C. L. Su, J. W. Yan, D. Han and L. Niu, Anal. Chem., 2023, 95, 9646–9653 CrossRef CAS PubMed.
- A. Polita, M. Stancikaitė, R. Žvirblis, K. Maleckaitė, J. Dodonova-Vaitkūnienė, S. Tumkevičius, A. P. Shivabalan and G. Valinčius, RSC Adv., 2023, 13, 19257–19264 RSC.
- Y. Mei, Z. Li, K. Rong, Z. Hai, W. Tang and Q. H. Song, Chem. Commun., 2023, 59, 12775–12778 RSC.
- K. Dou, W. Huang, Y. Xiang, S. Li and Z. Liu, Anal. Chem., 2020, 92, 4177–4181 CrossRef CAS PubMed.
- W. J. Shi, J. Yang, Y. F. Wei, X. T. Li, X. H. Yan, Y. Wang, H. Leng, L. Zheng and J. W. Yan, Chem. Commun., 2022, 58, 1930–1933 RSC.
- W.-J. Shi, Y.-F. Wei, J. Yang, H.-Z. Li, Q.-H. Wan, Y. Wang, H. Leng, K. Chen and J. Yan, Sens. Actuators, B, 2022, 359, 131594 CrossRef CAS.
- S. Toliautas, J. Dodonova, A. Žvirblis, I. Čiplys, A. Polita, A. Devižis, S. Tumkevičius, J. Šulskus and A. Vyšniauskas, Chem. – Eur. J., 2019, 25, 10342–10349 CrossRef CAS PubMed.
- C. Liu, L. Zhou, L. Xie, Y. Zheng, H. Man and Y. Xiao, Chin. Chem. Lett., 2022, 33, 2537–2540 CrossRef CAS.
- X. Zhang, L. Wang, N. Li and Y. Xiao, Chin. Chem. Lett., 2021, 32, 2395–2399 CrossRef CAS.
- W. J. Shi, R. Chen, J. Yang, Y. F. Wei, Y. Guo, Z. Z. Wang, J. W. Yan and L. Niu, Anal. Chem., 2022, 94, 14707–14715 CrossRef CAS PubMed.
- C. Yu, Z. Huang, W. Gu, Q. Wu, E. Hao, Y. Xiao, L. Jiao and W.-Y. Wong, Mater. Chem. Front., 2019, 3, 1823–1832 RSC.
- H. Zhu, J. Fan, M. Li, J. Cao, J. Wang and X. Peng, Chem. – Eur. J., 2014, 20, 4691–4696 CrossRef CAS PubMed.
- J. Zhang, W. Han, X. Zhou, X. Zhang, H. Zhang, T. Li, J. Wang, Y. Yuan, Y. He and J. Zhou, Anal. Chem., 2023, 95, 11785–11792 CrossRef CAS PubMed.
- D. Li, T. Shen, X. Xue, W. Chen, W. Tao, W. Chi, S. H. Liu, Y. Tan, X. Liu and J. Yin, Sci. China: Chem., 2023, 66, 2329–2338 CrossRef CAS.
- B. Shen, K. H. Jung, S. Ye, C. A. Hoelzel, C. H. Wolstenholme, H. Huang, Y. Liu and X. Zhang, Aggregate, 2023, 4, e301 CrossRef CAS.
- M. Rajasekar, J. Mol. Struct., 2021, 1224, 129085 CrossRef CAS.
- M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410–2433 RSC.
- Y. M. Poronik, K. V. Vygranenko, D. Gryko and D. T. Gryko, Chem. Soc. Rev., 2019, 48, 5242–5265 RSC.
- K. Prabakaran, H. Oh, R. Manivannan, S. H. Hyeong Park and Y. A. Son, Spectrochim. Acta, Part A, 2022, 279, 121437 CrossRef CAS PubMed.
- S. Kamino and M. Uchiyama, Org. Biomol. Chem., 2023, 21, 2458–2471 RSC.
- Y. Wu, J. Wu and W. Y. Wong, Biomater. Sci., 2021, 9, 4843–4853 RSC.
- A. Méndez-Ardoy, J. J. Reina and J. Montenegro, Chem. – Eur. J., 2020, 26, 7516–7536 CrossRef PubMed.
- S. Nath, M. A. Saad, M. Pigula, J. W. R. Swain and T. Hasan, Cancers, 2019, 11, 1887 CrossRef CAS PubMed.
- R. Tachibana, M. Kamiya, S. Suzuki, K. Morokuma, A. Nanjo and Y. Urano, Commun. Chem., 2020, 3, 82 CrossRef CAS PubMed.
- L. Wang, M. S. Frei, A. Salim and K. Johnsson, J. Am. Chem. Soc., 2019, 141, 2770–2781 CrossRef CAS PubMed.
- S. Feng, S. Gong and G. Feng, Chem. Commun., 2020, 56, 2511–2513 RSC.
- J. J. Chao, H. Zhang, Z. Q. Wang, Q. R. Liu, G. J. Mao, D. H. Chen and C. Y. Li, Anal. Chim. Acta, 2023, 1242, 340813 CrossRef CAS PubMed.
- Q. Liu, C. Dong, J. Zhang, B. Zhao, Y. Zhou, C. Fan and Z. Lu, Dyes Pigm., 2023, 210, 111045 CrossRef CAS.
- X.-F. Zhang, L. Shen, S. Wang, Q. Chen, X.-Q. Cao, S.-L. Shen and X. Li, Chem. Eng. J., 2023, 472, 145065 CrossRef CAS.
- K. Ilina and M. Henary, Chem. – Eur. J., 2021, 27, 4230–4248 CrossRef CAS PubMed.
- X. Z. Yang, B. Xu, L. Shen, R. Sun, Y. J. Xu, Y. L. Song and J. F. Ge, Anal. Chem., 2020, 92, 3517–3521 CrossRef CAS PubMed.
- B. Chen, S. Mao, Y. Sun, L. Sun, N. Ding, C. Li and J. Zhou, Chem. Commun., 2021, 57, 4376–4379 RSC.
- B. Chen, C. Li, J. Zhang, J. Kan, T. Jiang, J. Zhou and H. Ma, Chem. Commun., 2019, 55, 7410–7413 RSC.
- S.-L. Xu, F.-F. Guo, Z.-H. Xu, Y. Wang and T. D. James, Sens. Actuators, B, 2023, 383, 133510 CrossRef CAS.
- Y. Ye, M. He, L. Wang, X.-C. Shen and H. Chen, Sens. Actuators, B, 2023, 396, 134600 CrossRef CAS.
- Y. Tian, Z. Chen, S. Liu, F. Wu, W. Cao, D. Pang and H. Xiong, Angew. Chem., Int. Ed., 2023, 62, e202309768 CrossRef CAS PubMed.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
- H. Moon, J. Park and J. Tae, Chem. Rec., 2016, 16, 124–140 CrossRef CAS PubMed.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- R. Lalitha and S. Velmathi, J. Fluoresc., 2024, 34, 15, DOI:10.1007/s10895-023-03231-1.
- J.-M. Li, F.-F. Xiang, D.-H. Zhou, J.-X. Xu, H. Zhang, Y.-Z. Liu, Q.-Q. Kong, X.-Q. Yu and K. Li, Chem. Biomed. Imaging, 2024, 2, 126–134, DOI:10.1021/cbmi.3c00071.
- P. Tang, Q. Wang, Q. Tan, K. Huang, B. Du and L. Liang, Microchem. J., 2023, 194, 109296 CrossRef CAS.
- C. Liu, S. Ye, D. Zhang, J. Chen, H. Xiao, J. Qu and R. Liu, SSRN Electron. J., 2022, 31, DOI:10.2139/ssrn.4046029.
- Q. Chen, X. F. Zhang, T. Wang, X. Q. Cao and S. L. Shen, Anal. Chim. Acta, 2022, 1231, 340443 CrossRef CAS PubMed.
- Y. L. Mu, L. Pan, Q. Lu, S. Xing, K. Y. Liu and X. Zhang, Spectrochim. Acta, Part A, 2022, 264, 120228 CrossRef CAS PubMed.
- P. Ghosh and P. Roy, Chem. Commun., 2023, 59, 5174–5200 RSC.
- A. Syreeni, N. Sandholm, J. Cao, I. Toppila, D. M. Maahs, M. J. Rewers, J. K. Snell-Bergeon, T. Costacou, T. J. Orchard, M. L. Caramori, M. Mauer, B. E. K. Klein, R. Klein, E. Valo, M. Parkkonen, C. Forsblom, V. Harjutsalo and A. D. Paterson, DCCT/EDIC Research Group, P. H. Groop and FinnDiane Study Group, Diabetes, 2019, 68, 858–867 CrossRef CAS PubMed.
- N. Wang, Z. Xu, P. Han and T. Li, Diabetes/Metab. Res. Rev., 2017, 33, e2843 CrossRef PubMed.
- J. Chen, S. E. Stimpson, G. A. Fernandez-Bueno and C. E. Mathews, Antioxid. Redox Signaling, 2018, 29, 1361–1372 CrossRef CAS PubMed.
- S. K. Panigrahy, R. Bhatt and A. Kumar, J. Drug Targeting, 2017, 25, 93–101 CrossRef CAS PubMed.
- H. M. Lim and S. H. Park, Crit. Rev. Food Sci. Nutr., 2023, 63, 5911–5936 CrossRef CAS PubMed.
- E. Rodriguez-Boulan and I. G. Macara, Nat. Rev. Mol. Cell Biol., 2014, 15, 225–242 CrossRef CAS PubMed.
- Z. Yang, Y. He, J. H. Lee, N. Park, M. Suh, W. S. Chae, J. Cao, X. Peng, H. Jung, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2013, 135, 9181–9185 CrossRef CAS PubMed.
- L. Dai, M. Ren and W. Lin, Spectrochim. Acta, Part A, 2021, 254, 119627 CrossRef CAS PubMed.
- B. Shi, H. Wang, X. Wan, Y. Guo, S. Y. Liu and Q. Gong, Spectrochim. Acta, Part A, 2022, 278, 121375 CrossRef CAS PubMed.
- J. Cheng, Z. Li, L. Nong, P. Huang and W. Lin, Anal. Chim. Acta, 2022, 1221, 340104 CrossRef CAS PubMed.
- L. Chai, Y. Li, H. Yang, Y. Wang, R. Huang, Z. Wei and Z. Zhan, Sens. Actuators, B, 2023, 393, 134345 CrossRef CAS.
- S. Guo, C. Li, L. Lian, Z. Le, Y. Ren, Y.-X. Liao, J. Shen and J.-T. Hou, ACS Sens., 2023, 8, 3882–3891 CrossRef CAS PubMed.
- B. Feng, Y. Ma, F. Zheng, X. Huang, X. Feng, K. Zhang, L. Liu, F. Chen and W. Zeng, Chem. Eng. J., 2023, 464, 142554 CrossRef CAS.
- N. Ding, X. Liu, A. Meng, X. Zhao, G. Ma, W. Han, P. Dong, J. Li and J. Zhou, Chin. Chem. Lett., 2023, 34, 107745 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |