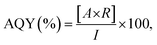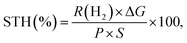Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Long-wavelength photoresponsive gallium zinc oxynitride for efficient oxygen evolution and Z-scheme water splitting reactions†
Natsutogi
Iwasa‡
a,
Hiroka
Sandaiji‡
b,
Swarnava
Nandy
 c,
Mamiko
Nakabayashi
d,
Tsuyoshi
Takata
c,
Takashi
Hisatomi
c,
Mamiko
Nakabayashi
d,
Tsuyoshi
Takata
c,
Takashi
Hisatomi
 *cef and
Kazunari
Domen
*cef and
Kazunari
Domen
 *cfgh
*cfgh
aDepartment of Science and Technology, Graduate School of Medicine, Science and Technology, Shinshu University, Nagano 380-8553, Japan
bDepartment of Engineering, Graduate School of Science and Technology, Shinshu University, Nagano 380-8553, Japan
cResearch Initiative for Supra-Materials (RISM), Shinshu University, Nagano 380-8553, Japan. E-mail: hisatomi@shinshu-u.ac.jp; domen@shinshu-u.ac.jp
dInstitute of Engineering Innovation, School of Engineering, The University of Tokyo, Tokyo 113-8656, Japan
ePrecursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency (JST), Nagano 380-8553, Japan
fInstitute for Aqua Regeneration, Shinshu University, Nagano 380-8553, Japan
gOffice of University Professors, The University of Tokyo, Tokyo 113-8656, Japan
hDepartment of Chemistry, Kyung Hee University, Seoul, 130-701, Republic of Korea
First published on 11th July 2024
Abstract
Long-wavelength photoresponsive GaN:ZnO, a solid solution of GaN and ZnO, can be obtained by reacting Ga2O3 and Zn3N2 in the presence of ZnX2 (X = halogen) in a sealed evacuated tube. However, the activity of GaN:ZnO for overall water splitting via one-step excitation under visible light remains low due to the small particle size, which leads to uncontrollable aggregation and lack of suitable cocatalysts. In this study, well-dispersed particulate GaN:ZnO with a ZnO concentration of 66 mol%, optical absorption up to about 600 nm, and anisotropic crystalline facets was obtained using δ-Ga2O3 as a raw material and adding Zn to the starting material. The resulting GaN:ZnO exhibited an apparent quantum yield of 11.9% at 420 nm in the oxygen evolution reaction when loaded with an IrOx cocatalyst. It also served as an oxygen evolution photocatalyst in a photocatalyst sheet for Z-scheme water splitting, in combination with La5Ti2Cu0.9Ag0.1O7S5, whose absorption edge wavelength was about 700 nm, as a hydrogen evolution photocatalyst. This work, which demonstrates the activation of long-wavelength photoresponsive GaN:ZnO, expands the possibilities of constructing photocatalytic systems that effectively utilize long-wavelength visible light for water splitting.
Introduction
Large-scale implementation of solar fuel production is desired in order to address increasingly serious energy and environmental problems.1 Sunlight-driven water splitting using particulate semiconductor photocatalysts has been studied as a means of green hydrogen production on a large scale.2–4 To produce green hydrogen in a practical manner, it is indispensable to develop photocatalytic materials that can efficiently split water into hydrogen and oxygen under long-wavelength visible light.5 Various semiconducting (oxy)nitrides, oxysulphides and metal-doped oxides have been extensively studied as visible-light responsive photocatalysts.6–9 Despite having a narrower bandgap than the corresponding pure oxides, many such materials have a band structure suitable for splitting water into hydrogen and oxygen under visible-light irradiation. This is due to a negative shift of the valence band edge potential induced by nitride and sulphide ions or impurity levels associated with dopants, while the conduction band edge potential is largely unaffected.GaN:ZnO is one of the earliest oxynitride photocatalysts shown to be capable of splitting water into hydrogen and oxygen under visible light.10 The bandgap energy of GaN:ZnO can be controlled by the concentration of ZnO. With increasing ZnO concentration up to 90 mol%, the valence band maximum shifts negatively while the conduction band minimum shifts positively.11 In 2010, an apparent quantum yield (AQY) of 5.1% at 410 nm was achieved in the overall water splitting reaction using GaN:ZnO with a ZnO concentration of 13 mol% and an absorption edge wavelength of about 480 nm.12 More recently, in 2022, GaN:ZnO with an absorption edge wavelength of around 550 nm and activity in the water splitting reaction was synthesised by applying NH4Cl as a solid nitrogen source in a sealed evacuated tube.13 The activity of the resultant long-wavelength responsive GaN:ZnO for overall water splitting was rather low, with a solar-to-hydrogen energy conversion efficiency (STH efficiency) on the order of 1 × 10−2%. Nevertheless, GaN:ZnO could be effectively applied to visible-light-driven water splitting by constructing a Z-scheme system using two-step excitation of a hydrogen evolution photocatalyst (HEP) and an oxygen evolution photocatalyst (OEP). Namely, GaN:ZnO effectively functioned as the OEP in combination with Rh-doped SrTiO3 as the HEP in the presence of Fe3+/Fe2+ as the redox mediator, and the resulting Z-scheme system split water with a higher STH efficiency of 3.7 × 10−2%. The use of long-wavelength photoresponsive GaN:ZnO allowed the wavelength range of light applicable to Z-scheme water splitting reactions to be significantly extended, because most previous work had used WO3 and BiVO4 as OEPs for Z-scheme water splitting, whose absorption edge wavelengths were rather short, at 470 and 525 nm, respectively.14,15
Further improvements in efficiency can be expected by optimizing the performance of GaN:ZnO. At the same time, it is still essential to explore new long-wavelength HEP materials, because Rh-doped SrTiO3 can utilise visible light only up to 520 nm, and its visible-light absorption induced by the Rh impurity level is rather weak.16
In our recent study, GaN:ZnO absorbing visible light up to 580 nm was synthesized by reacting crystalline Ga2O3 and Zn3N2 in the presence of ZnX2 (X = halogen) in sealed evacuated tubes.17 The use of hydrogen-free Zn3N2 as a nitriding reagent allowed gram-scale synthesis of GaN:ZnO that was active for overall water splitting via one-step photoexcitation. However, the AQY remained on the order of 1 × 10−2% at a wavelength of 430 nm. This low activity is due to the uncontrolled particle size and morphology of GaN:ZnO, resulting in severe aggregation, which makes it difficult to refine the cocatalyst loading.
In general, the properties of the products of solid-state reactions are affected by the particle size and crystallinity of the reactants.18 Therefore, refinement of the raw materials is expected to lead to improved morphology control and photocatalytic activity of GaN:ZnO obtained using Zn3N2 as a solid nitrogen source. In the present study, well-dispersed crystalline GaN:ZnO particles with a high ZnO concentration were synthesised using δ-Ga2O3 as a raw material and adding Zn to the starting materials. The particles absorbed visible light up to 600 nm and exposed hexagonal crystalline facets. The resulting GaN:ZnO drove the oxygen evolution reaction with an AQY of 11.9% at 420 nm when loaded with an IrOx cocatalyst. When used in combination with La5Ti2Cu0.9Ag0.1O7S5 (LTCA) as a HEP, it also enabled a Z-scheme system consisting only of non-oxide photocatalysts with an absorption edge wavelength equal to or longer than 600 nm, but still active in visible light-driven water splitting.
Results and discussion
Synthesis of GaN:ZnO
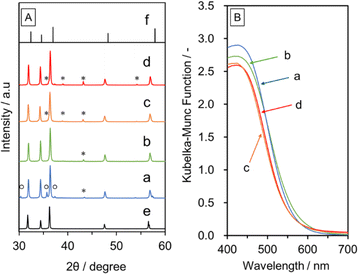
Fig. S1A in ESI† shows XRD patterns for the δ- and K-Ga2O3 samples. The pattern for the δ-Ga2O3 sample is consistent with that reported in the literature.19 The synthesised δ-Ga2O3 particles were aggregated to form irregularly shaped secondary particles (Fig. S1B in ESI†). TGA measurements revealed that the δ-Ga2O3 sample contained about 8 wt% water, as indicated by the weight loss associated with endothermic signals during the heating process (Fig. S2 in ESI†). On the other hand, the K-Ga2O3 was composed of a mixture of α-Ga2O3 and β-Ga2O3. The K-Ga2O3 particles had a high-aspect-ratio rectangular parallelepiped shape with a length of several micrometers and a thickness of less than 1 μm. The K-Ga2O3 contained a negligible amount of water. Fig. 1A and B respectively show XRD patterns and UV-vis DRS data for GaN:ZnO synthesised using δ- and K-Ga2O3 as a starting material with and without addition of Zn after washing with distilled water. The washing step was performed to investigate byproducts of the synthesis of GaN:ZnO. Single-phase GaN:ZnO was obtained from K-Ga2O3, while a small amount of ZnGa2O4 was generated as an impurity phase, as confirmed by the diffraction peak at 36.8°, when δ-Ga2O3 was used. This is due to oxidation of Zn3N2 by the moisture contained in δ-Ga2O3. The addition of metallic Zn to the precursor mixture prevented the formation of ZnGa2O4, because Zn reacted with moisture to form ZnO and H2 in the sealed quartz tube. On the other hand, unreacted Zn remained when Zn was added to the synthesis using K-Ga2O3. GaN:ZnO(δ) prepared with Zn addition had similar crystallinity to GaN:ZnO(K), but exhibited diffraction peaks at lower angles closer to those for ZnO than GaN, indicating the production of GaN:ZnO with a higher ZnO concentration. In fact, the ZnO concentration estimated from the Zn/Ga ratio measured by EDS analysis was higher for GaN:ZnO(δ) prepared with Zn addition (63 mol%) than for GaN:ZnO(K) prepared with or without Zn addition (58 mol% for both). The higher ZnO concentration in the former case indicates that ZnO generated from Zn and moisture was partially incorporated in the GaN:ZnO(δ) product. In accordance with this, the GaN:ZnO(δ) sample prepared with Zn absorbed visible light up to 600 nm, which was approximately 30 nm longer than the absorption edge for GaN:ZnO(K). Note that when commercial submicron ZnO particles were added instead of Zn for the synthesis of GaN:ZnO(δ), the XRD peak for the product was not shifted relative to that for conventional GaN:ZnO(K), and instead XRD peaks attributable to ZnO were generated, indicating that externally added ZnO was not incorporated into GaN:ZnO (Fig. S3†).Fig. 2 shows SEM images of GaN:ZnO(δ) and GaN:ZnO(K) synthesised with and without Zn addition. The morphology and size of the GaN:ZnO(K) particles are not very clear or uniform regardless of the addition of Zn during synthesis. In contrast, hexagonal prismatic particles reflecting the wurtzite-type crystal structure of GaN:ZnO are observed when δ-Ga2O3 is used because Ga2O3 can be more smoothly converted into GaN:ZnO. This effect is enhanced by the addition of Zn to the starting material, and allows GaN:ZnO(δ) particles to grow. This is probably related to suppression of oxidation and hydrolysis of the starting Zn3N2 and the intermediate Zn2NI, as described later.
 | ||
| Fig. 2 SEM images of (a and b) GaN:ZnO(K) and (c and d) GaN:ZnO(δ) prepared by heating at 1173 K for 10 h (a and c) without and (b and d) with Zn addition. | ||
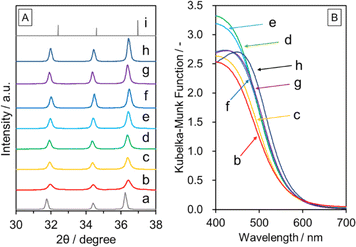
Fig. 3 shows XRD and DRS data for GaN:ZnO(δ) prepared at different temperatures. All the synthesized samples were attributed to single-phase wurtzite-type GaN:ZnO. It can be seen that the diffraction peaks become sharper as the synthesis temperature is increased, indicating an improvement in crystallinity. Namely, the full width at half maximum (FWHM) values for the 101 diffraction peak for GaN:ZnO(δ) synthesised at 923 and 1123 K are 0.35° and 0.17°, respectively. At temperatures above 1123 K, the FWHM is largely unchanged. The onset of optical absorption observed in the DRS data was around 600 nm regardless of the synthesis temperature, and was approximately 30 nm longer than that for GaN:ZnO(K). This reflects a higher ZnO concentration in the GaN:ZnO(δ) samples due to the incorporation of ZnO generated in situ from Zn and H2O during synthesis. In fact, elemental analysis revealed that the ZnO concentration in the GaN:ZnO(δ) samples was around 65 mol% (Table 1), while that for GaN:ZnO(K) reported in our previous work was 58 mol%.17
| Synthesis temperature/K | Ga/wt%a | Zn/wt%a | N/wt%b | O/wt%b | Total/wt% | Composition | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Measured by ICP-AES. b Measured by N/O combustion analysis. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1073 | 28.3 | 49.6 | 5.8 | 13.0 | 96.7 | Ga0.35Zn0.65N0.35O0.67 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1123 | 28.4 | 50.1 | 5.7 | 12.8 | 97.0 | Ga0.35Zn0.65N0.34O0.66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1173 | 28.5 | 50.8 | 5.7 | 12.9 | 97.9 | Ga0.34Zn0.66N0.34O0.66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1223 | 28.0 | 49.6 | 5.8 | 13.2 | 96.6 | Ga0.35Zn0.65N0.33O0.67 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SEM images of GaN:ZnO(δ) synthesized at different temperatures are shown in Fig. 4. GaN:ZnO(δ) synthesised at 923 K was composed of particles smaller than 100 nm without noticeable crystal facets. Increasing the synthesis temperature led to the growth of particles with some crystal facets, and well-dispersed hexagonal prismatic particles reflecting the crystal structure and hundreds of nanometers in size were generated at 1073 K and above. Note that GaN:ZnO with such anisotropic particle shapes can also be synthesised using K-Ga2O3 instead of δ-Ga2O3 when Zn2NI is used as the zinc and nitride ion source.17 In fact, Zn2NI is generated in situ from Zn3N2 and ZnI2 during the synthesis of GaN:ZnO. However, Zn3N2 is prone to oxidation and hydrolysis. In addition, Zn2NI starts to decompose at 873 K to Zn, N2, and ZnI2, which are no longer reactive enough to form GaN:ZnO.20 The use of δ-Ga2O3, which is more reactive than K-Ga2O3, is therefore preferable for the growth of hexagonal prismatic particles of GaN:ZnO, as the decomposition of Zn2NX into inert byproducts prior to the formation of GaN:ZnO can be suppressed. Notably, Pt and MnOx were reductively and oxidatively photodeposited respectively on the hexagonal and lateral planes of hexagonal prismatic particles of GaN:ZnO (Fig. S4 in ESI†). This indicates a spontaneous polarisation of GaN:ZnO like GaN and ZnO.21–23
 | ||
| Fig. 4 SEM images of GaN:ZnO(δ) synthesised with addition of Zn at (a) 923, (b) 973, (c) 1023, (d) 1073, (e) 1123, (f) 1173, and (g) 1223 K. | ||
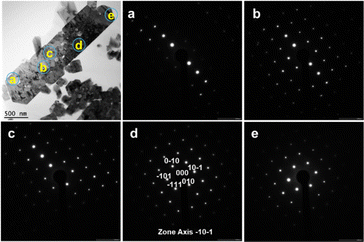
A transmission electron microscopy (TEM) image and electron diffraction patterns for GaN:ZnO(δ) synthesised at 1173 K are shown in Fig. 5. The electron diffraction patterns obtained at different positions in the particle shown in the TEM image are similar, indicating that the GaN:ZnO(δ) particle is a single crystal. Considering the results of the elemental analysis (Table 1), the GaN:ZnO(δ) material is also stoichiometric. Nevertheless, as seen in the TEM image, the GaN:ZnO(δ) particle contains internal voids suggesting the presence of local defects and distortions.
Photocatalytic water splitting reaction
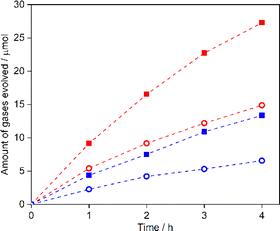
Fig. 6 shows the oxygen evolution activity under visible light in an aqueous AgNO3 solution for GaN:ZnO(δ) synthesised at 973–1223 K and loaded with IrOx (0.5 wt% Ir). The activity is seen to increase as the synthesis temperature is raised from 973 to 1173 K due to improvements in crystallinity. However, the activity for the sample synthesised at 1223 K decreases even though there is no appreciable deterioration in crystallinity or bulk composition. This may be related to a change in the surface properties of GaN:ZnO(δ). In fact, when GaN:ZnO was synthesized at high temperature using NH4Cl as a nitriding reagent, a decrease in the surface Zn/Ga ratio was observed, and the photocatalytic activity was eventually degraded.13 XPS analysis of GaN:ZnO(δ) was therefore carried out. Fig. S5 and Table S1 in ESI† show the surface composition of GaN:ZnO(δ) prepared at different temperatures. GaN:ZnO(δ) synthesised at 1223 K showed a higher Zn/Ga molar ratio on the surface than the other GaN:ZnO samples, probably due to the heating at too high a temperature in an oxygen-free atmosphere for this material. The large deviation between the bulk and surface compositions would disturb the band structure and carrier transfer, which could lower the photocatalytic activity. As seen in Fig. S6 in ESI,† the oxygen evolution activity for GaN:ZnO(δ) decreases to about 20% in the absence of an IrOx cocatalyst, indicating that the IrOx acts as an efficient oxygen evolution cocatalyst as reported previously.13 The oxygen evolution activity is almost constant for an Ir loading of 0.2 wt% or greater. The wavelength dependence of the AQY for the most active IrOx-GaN:ZnO(δ) sample, which was synthesised at 1173 K and loaded with 0.5 wt% of Ir, for the oxygen evolution reaction is presented in Fig. 7. As can be seen, the AQY at a wavelength of 420 nm is 11.9%, which is comparable to that for GaN:ZnO synthesised using NH4Cl as a solid nitrogen source, and outstanding among GaN:ZnO with high ZnO concentration (Table S2 in ESI†). Notably, the GaN:ZnO(δ) sample maintains a reasonably high AQY of 1% at a wavelength of 560 nm, but its activity becomes negligible under irradiation with monochromatic light at 600 nm, which represents the absorption edge. This suggests that the photocatalytic reaction proceeds through bandgap transitions of GaN:ZnO(δ) and that visible light absorption up to 600 nm can indeed contribute to the photocatalytic activity. On the other hand, GaN:ZnO(δ) prepared at 1123 K showed negligible hydrogen evolution activity from aqueous methanol solution although it generated hydrogen from aqueous ascorbic acid solution (Fig. S7 in ESI†). In previous studies, the conduction band minimum and valence band maximum for GaN:ZnO with a ZnO concentration of 50–68 mol% have been estimated to be in the range of −0.58 to −0.08 V vs. RHE and +1.80 to +2.47 V vs. RHE, respectively, with large variations probably due to differences in stoichiometry and homogeneity of the materials.11,13,24 However, as the ZnO concentration increases, the energy offset between the conduction band minimum and the hydrogen evolution reaction potential decreases more than the energy offset between the valence band minimum and the oxygen evolution reaction potential, which may explain the low hydrogen evolution activity of GaN:ZnO(δ) in this work.Since IrOx-GaN:ZnO(δ) exhibited reasonably high activity in the oxygen evolution reaction in response to visible light with relatively long wavelengths, its applicability as an OEP for Z-scheme water splitting was investigated using a LTCA/Au/IrOx-GaN:ZnO sheet. After photodeposition of Cr2O3/Rh cocatalysts, the sheet evolved H2 and O2 stoichiometrically under visible light from water free from sacrificial reagents or additives (Fig. 8). A sheet based on GaN:ZnO(δ) without IrOx modification exhibited lower activity, indicating the critical role of the IrOx cocatalyst in activating GaN:ZnO(δ). The STH efficiency for the sheet was 0.05% at 5 kPa and 310 K, which was an improvement on a previously reported SrTiO3:Rh/Fe3+/2+/GaN:ZnO suspension system, indicating the potential of achieving higher STH efficiency by using GaN:ZnO as the OEP in a sheet system.
Experimental
Preparation of GaN:ZnO
δ-Ga2O3 and Zn3N2 were synthesised as starting materials for GaN:ZnO in the laboratory. δ-Ga2O3 was prepared by calcining Ga(NO3)3·xH2O (Kojundo Chemical Laboratory Co., Ltd, 99.9%) at 573 K for 18 h in air.19 The obtained white powder was crushed and again calcined at 573 K for 2 h. Zn3N2 was synthesized by heating commercial metallic Zn powder (Kojundo Chemical Laboratory Co., Ltd, particle size <75 μm, 99.9%) at 873 K for 10 h under an NH3 flow at a rate of 100 mL min−1.17 δ-Ga2O3, Zn3N2, ZnI2 (Sigma-Aldrich, 98%), and Zn were blended at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a N2-filled glove box (dew point < 197 K, O2 < 1 ppm), where Zn was added to prevent unwanted oxidation of the sample during heating. Commercially available Ga2O3 (Kojundo Chemical Laboratory Co., Ltd, 99.9%), denoted as K-Ga2O3, was also used to synthesise GaN:ZnO for comparison. The precursor mixture was transferred to a quartz tube, sealed under evacuation, and heated at 923–1273 K for 10 h. After heating, the sample was allowed to cool naturally to room temperature. The obtained GaN:ZnO powder was washed using 0.5 M HNO3 for 1 h and thoroughly rinsed with distilled water to remove excess Zn and ZnI2. The GaN:ZnO powder was recovered by filtration and dried overnight at 313 K in an oven. The sample was subsequently calcined in air at 873 K for 1 h to reduce the density of defects in the material and improve its photocatalytic activity.25 The GaN:ZnO samples synthesized from δ-Ga2O3 and K-Ga2O3 are denoted as GaN:ZnO(δ) and GaN:ZnO(K), respectively.
1 in a N2-filled glove box (dew point < 197 K, O2 < 1 ppm), where Zn was added to prevent unwanted oxidation of the sample during heating. Commercially available Ga2O3 (Kojundo Chemical Laboratory Co., Ltd, 99.9%), denoted as K-Ga2O3, was also used to synthesise GaN:ZnO for comparison. The precursor mixture was transferred to a quartz tube, sealed under evacuation, and heated at 923–1273 K for 10 h. After heating, the sample was allowed to cool naturally to room temperature. The obtained GaN:ZnO powder was washed using 0.5 M HNO3 for 1 h and thoroughly rinsed with distilled water to remove excess Zn and ZnI2. The GaN:ZnO powder was recovered by filtration and dried overnight at 313 K in an oven. The sample was subsequently calcined in air at 873 K for 1 h to reduce the density of defects in the material and improve its photocatalytic activity.25 The GaN:ZnO samples synthesized from δ-Ga2O3 and K-Ga2O3 are denoted as GaN:ZnO(δ) and GaN:ZnO(K), respectively.
The IrOx cocatalysts (0.5 wt%) were loaded onto the surface of GaN:ZnO as an oxygen evolution cocatalyst by a solvothermal reaction using a microwave reactor (Monowave 200, Anton-Paar). GaN:ZnO was dispersed in ethylene glycol containing Na3IrCl6·nH2O (Kanto Chemical, 99.5%), followed by heating at 423 K for 10 min.7,13 The sample was washed with distilled water and recovered by filtration. The IrOx-loaded GaN:ZnO powder was collected by filtration and dried at 313 K.
Rh was loaded onto IrOx/GaN:ZnO by the photodeposition for the measurement of hydrogen evolution activity. The IrOx/GaN:ZnO was suspended in a aqueous 10 mM ascorbic acid solution. To the suspension, Na3RhCl6·nH2O (Mitsuwa Chemicals, 18.3 wt% Rh) was added so that the fraction of Rh became 0.5 wt% with respect to the photocatalyst sample. The suspension was evacuated to completely remove dissolved air and then exposed to visible light (λ ≥ 420 nm) from a Xe lamp equipped with a dichroic mirror and a cut-off filter.
To investigate the possibility of the facet-oriented electron/hole migration, Pt and MnOx were reductively and oxidatively photodeposited on GaN:ZnO, respectively. Firstly, Pt (0.5 wt%) was loaded by reductive photodeposition. GaN:ZnO was dispersed in 10 vol% methanol solution containing H2PtCl6·6H2O (Kanto Chemical Co., Inc., >98.5%), followed by irradiation with visible light (λ ≥ 420 nm). Subsequently, MnOx (Mn 0.5 wt%) was loaded by oxidative photodeposition. Pt-loaded GaN:ZnO was dispersed in 10 mM NaIO3 solution containing Mn(NO3)2·6H2O (Fujifilm Wako Pure Chemical Co., 98.0%), followed by irradiation with visible light (λ ≥ 420 nm). The reactant solution was evacuated to remove air, and 10 kPa Ar was added prior to photodeposition.
Preparation of LTCA
Mg- and Al-codoped LTCA was synthesized by a conventional solid-state reaction in a sealed quartz tube similarly to a previous report.26 TiO2 (rutile, Kanto Chemical Co. Inc., 99.99%) was first impregnated with Mg(NO3)2·6H2O (Fujifilm Wako Pure Chemical Co., 99.5%) and Al(NO3)3·9H2O (Fujifilm Wako Pure Chemical Co., 99.5%) as Mg and Al dopant sources, respectively, and calcined at 1073 K for 1 h in air. The Mg (Mg−1 + Al + Ti) and Al/(Mg + Al + Ti) molar ratios were both 1%. The resulting material is denoted as Mg- and Al-codoped TiO2. Before mixing with other chemicals, La2O3 (Kanto Chemical Co., Inc., 99.99%) was calcined in air at 1273 K for 10 h to remove moisture and carbonates. La2O3, La2S3 (Kojundo Chemical Laboratory Co., Ltd, 99.9%), Mg- and Al-codoped TiO2, Cu2S (Kojundo Chemical Laboratory Co., Ltd, 99%), Ag2S (Kojundo Chemical Laboratory Co., Ltd, 99%) and sulfur (Kojundo Chemical Laboratory Co., Ltd, 99.99%) were mixed at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 in a N2-filled glovebox. The precursor mixture was sealed in an evacuated quartz tube and heated from room temperature to 473 K in 9 min, from 473 to 673 K in 100 min, and from 673 to 1323 K in 53 h, and then maintained at 1323 K for 96 h. After the annealing process, the resulting solid chunk was ground into powder. The obtained Mg- and Al-codoped LTCA is denoted as LTCA for simplicity.
0.5 in a N2-filled glovebox. The precursor mixture was sealed in an evacuated quartz tube and heated from room temperature to 473 K in 9 min, from 473 to 673 K in 100 min, and from 673 to 1323 K in 53 h, and then maintained at 1323 K for 96 h. After the annealing process, the resulting solid chunk was ground into powder. The obtained Mg- and Al-codoped LTCA is denoted as LTCA for simplicity.
Fabrication of photocatalyst sheet
Photocatalyst sheets employing LTCA and GaN:ZnO as the HEP and OEP, respectively, were fabricated by the particle transfer method.27,28 Briefly, LTCA and IrOx-loaded GaN:ZnO (denoted as IrOx-GaN:ZnO) (10 mg each) were suspended in 0.5 mL of 2-propanol. After sonication for a few minutes, the solution was dropcast onto a 3 × 3 cm glass plate. After the plate was dried at room temperature, an Au layer was deposited by vacuum evaporation (VFR-200 M/ERH, ULVAC) to a thickness of 0.3 μm. The plate was then heated at 523 K for 10 min in air to reduce the contact resistance between the particles and the Au layer. The primary glass plate was then attached to a second glass plate using adhesive carbon tape to peel off the powder/Au assembly, and then sonicated for a few seconds to remove the loosely bound particles to obtain a working photocatalyst sheet. To conduct Z-scheme water splitting reactions, the resulting sheet, denoted as LTCA/Au/IrOx-GaN:ZnO, was successively loaded with Rh (0.1 μmol) and Cr2O3 (0.2 μmol as Cr) by photodeposition under visible light irradiation (λ ≥ 420 nm) for 1 h each.Characterisation of materials
The GaN:ZnO samples were analysed after washing with 0.5 M HNO3 and calcining in air at 873 K, unless otherwise noted. X-ray diffraction (XRD) patterns were acquired using a Rigaku MiniFlex 300 with Cu Kα radiation operating at 30 kV and 10 mA. UV-vis diffuse reflectance spectroscopy (DRS) data were obtained using a spectrometer (V-670, JASCO) equipped with an integrating sphere. Scanning electron microscopy (SEM) images were captured using a Hitachi SU8000 system. TEM images and selected area electron diffraction (SAED) patterns were recorded using a JEOL JEM-2800. The cross-sectional sample for TEM observation was made by Ar ion milling using a JEOL EM-09100IS ion Slicer. Thermogravimetric analysis (TGA) measurements were performed using a Rigaku Thermo Plus TG 8120. The measurements were conducted by raising the temperature from room temperature to 1173 K at 10 K min−1 in ambient air. Energy-dispersive X-ray spectroscopy (EDS) was performed using an analytical system integrated with a desktop SEM instrument (Phenom Pharos, ThermoFisher Scientific). XPS analysis was carried out using a PHI Quantera II spectrometer with an Al Kα X-ray source. The binding energy was calibrated using the C 1s peak (285.0 eV) The bulk composition of the samples was analysed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES, ICPS-8100, Shimadzu) and oxygen/nitrogen analysis (EMGA-620W, Horiba).Photocatalytic reactions
Water splitting reactions were carried out in a Pyrex top-irradiation-type reaction vessel connected to a closed gas circulation system. The oxygen evolution reaction was performed using 0.1 g of IrOx-loaded GaN:ZnO powder dispersed in an aqueous 30 mM AgNO3 solution, while the hydrogen evolution reaction was performed using 0.1 g of Rh- and IrOx-coloaded GaN:ZnO dispersed in an aqueous ascorbic acid (10 mM) or methanol (10 vol%) solution. The reactant solution was evacuated to remove air and irradiated with visible light (λ ≥ 420 nm) from a 300 W Xe lamp equipped with a dichroic mirror and a cut-off filter after introduction of Ar (10 kPa) into the reaction system. The reactor temperature was maintained at 283 K by circulating cooling water in the reactor jacket. The amount of evolved gases was analysed using a gas chromatograph (GC-8A, Shimadzu) equipped with Molecular Sieve 5A columns and a thermal conductivity detector.The overall water splitting reaction was carried out using the same reaction system. Distilled water (40 mL) was added to the reactor, and a Cr2O3/Rh-loaded photocatalyst sheet was immersed. After degassing the reaction system thoroughly, the background pressure was adjusted to 5 kPa by introducing Ar. The photocatalyst sheet was illuminated with visible light (λ ≥ 420 nm) from a 300 W Xe lamp or simulated sunlight generated by a solar simulator (HAL-320, Asahi Spectra Co., Ltd).
Apparent quantum yield measurement
The AQY for the oxygen evolution reaction was calculated aswhere A, R and I denote the number of electrons involved in the reaction, the number of gas molecules generated, and the number of incident photons in a given time span, respectively. In the oxygen evolution reaction from water, the value of A is 4 because it is a four-electron reaction. The oxygen evolution reaction was carried out in a 10 mM AgNO3 aqueous solution and under irradiation from a Xe lamp equipped with a series of bandpass filters to produce monochromatic light. The number of incident photons was determined using a LS-100 spectroradiometer (EKO Instruments Co., Ltd). In the Z-scheme water splitting reaction using LTCA/Au/IrOx-GaN:ZnO sheet, A = 4 and R is the rate of H2 evolution. The coefficient of 4 reflects a two-step photoexcitation process to drive the hydrogen evolution reaction, which is a two-electron reaction. Z-scheme water splitting was carried out under the same conditions described in the previous section except for the use of a bandpass filter (λ = 420 nm) instead of a cut-off filter.
Solar-to-hydrogen energy conversion efficiency measurement
The STH efficiency for the photocatalyst sheet during Z-scheme water splitting was measured using the same experimental apparatus but with illumination from a solar simulator (HAL-320, Asahi Spectra Co., Ltd). The STH efficiency was calculated aswhere R(H2), ΔG, P, and S are the rate of hydrogen evolution from the Z-scheme water splitting system, the change in Gibbs free energy that accompanies water splitting, the energy intensity of the solar light irradiation, and the irradiation area (∼6.25 cm2), respectively. A value of 237 kJ mol−1 was used for ΔGf, corresponding to the standard condition at 298 K. The energy intensity of the simulated sunlight (P) was adjusted to match that of Air Mass 1.5 Global (AM1.5 G) irradiance in the wavelength range of 350–800 nm and was assumed to be 100 mW cm−2.
Conclusions
Porous but well-dispersed single crystal GaN:ZnO particles absorbing visible light up to 600 nm and exposing hexagonal crystal facets were synthesised in a sealed evacuated tube by using poorly crystalline δ-Ga2O3 as a raw material and adding metallic Zn to the starting materials. The Zn reacted with moisture generated from δ-Ga2O3 to form ZnO in situ, preventing oxidation of the nitriding reagents during the synthesis of GaN:ZnO. The in situ generated ZnO was incorporated into the GaN:ZnO material, increasing the ZnO content to about 65 mol%, which allowed extension of the visible light absorption edge to 600 nm. Moreover, the use of δ-Ga2O3 favored the growth of hexagonal prismatic GaN:ZnO particles by suppressing decomposition of the reactants and the associated production of inert byproducts.The obtained GaN:ZnO evolved oxygen from an aqueous AgNO3 solution with an AQY of 11.9% at 420 nm when loaded with an IrOx cocatalyst. Moreover, IrOx-GaN:ZnO acted as an OEP in a photocatalyst sheet for Z-scheme water splitting in combination with doped LTCA as a HEP and an Au thin film as a conductor layer. The resulting photocatalyst sheet exhibited a STH efficiency that was superior to that for a previously-reported Z-scheme suspension system using IrOx-GaN:ZnO. There is still much room for clarification and optimization of the properties of GaN:ZnO and photocatalyst sheets containing it. This is expected to lead to improvements in the activity of photocatalytic sheets based on narrow bandgap nonoxide materials such as GaN:ZnO and LTCA.
Data availability
The data supporting this article have been included as part of the ESI.† Data are also available upon request from the authors.Author contributions
T. H. and K. D. designed and supervised the research. N. I., H. S. and S. N. carried out the experiments. N. I., H. S., S. N., M. N., T. T., T. H. and K. D. discussed the results. N. I., S. N., T. H. and K. D. wrote the manuscript with contributions from the other authors.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This research was supported by JST, PRESTO, Japan (grant no. JPMJPR20T9), the Artificial Photosynthesis Project (ARPChem) of the New Energy and Industrial Technology Development Organization (NEDO), Advanced Research Infrastructure for Materials and Nano-technology in Japan (ARIM)” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (Grant Number JPMXP1223UT0004), research equipment shared in MEXT Project for promoting public utilization of advanced research infrastructure (program for supporting construction of core facilities, grant number JPMXS0441000024), and Mitsubishi Chemical Corporation. The authors thank Ms. Keiko Kato of The University of Tokyo and Ms. Michiko Obata of Shinshu university for their assistance with ICP-AES and XPS measurements.Notes and references
- M. R. Shaner, H. A. Atwater, N. S. Lewis and E. W. McFarland, A comparative technoeconomic analysis of renewable hydrogen production using solar energy, Energy Environ. Sci., 2016, 9, 2354–2371 RSC.
- S. Nandy, S. A. Savant and S. Haussener, Prospects and challenges in designing photocatalytic particle suspension reactors for solar fuel processing, Chem. Sci., 2021, 12, 9866–9884 RSC.
- Q. Wang, C. Pornrungroj, S. Linley and E. Reisner, Strategies to improve light utilization in solar fuel synthesis, Nat. Energy, 2022, 7, 13–24 CrossRef.
- H. Nishiyama, T. Yamada, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Photocatalytic solar hydrogen production from water on a 100-m2 scale, Nature, 2021, 598, 304–307 CrossRef CAS PubMed.
- T. Hisatomi and K. Domen, Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts, Nat. Catal., 2019, 2, 387–399 CrossRef CAS.
- Z. Wang, Y. Inoue, T. Hisatomi, R. Ishikawa, Q. Wang, T. Takata, S. Chen, N. Shibata, Y. Ikuhara and K. Domen, Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles, Nat. Catal., 2018, 1, 756–763 CrossRef CAS.
- K. Chen, J. Xiao, J. J. M. Vequizo, T. Hisatomi, Y. Ma, M. Nakabayashi, T. Takata, A. Yamakata, N. Shibata and K. Domen, Overall Water Splitting by a SrTaO2N-Based Photocatalyst Decorated with an Ir-Promoted Ru-Based Cocatalyst, J. Am. Chem. Soc., 2023, 145, 3839–3843 CrossRef CAS.
- Q. Wang, M. Nakabayashi, T. Hisatomi, S. Sun, S. Akiyama, Z. Wang, Z. Pan, X. Xiao, T. Watanabe, T. Yamada, N. Shibata, T. Takata and K. Domen, Oxysulfide photocatalyst for visible-light-driven overall water splitting, Nat. Mater., 2019, 18, 827–832 CrossRef CAS PubMed.
- R. Asai, H. Nemoto, Q. Jia, K. Saito, A. Iwase and A. Kudo, A visible light responsive rhodium and antimony-codoped SrTiO3 powdered photocatalyst loaded with an IrO2 cocatalyst for solar water splitting, Chem. Commun., 2014, 50, 2543–2546 RSC.
- K. Maeda, T. Takata, M. Hara, N. Saito, Y. Inoue, H. Kobayashi and K. Domen, GaN:ZnO Solid Solution as a Photocatalyst for Visible-Light-Driven Overall Water Splitting, J. Am. Chem. Soc., 2005, 127, 8286–8287 CrossRef CAS PubMed.
- Y. Li, L. Zhu, Y. Yang, H. Song, Z. Lou, Y. Guo and Z. Ye, A Full Compositional Range for a (Ga1-xZnx)(N1-xOx) Nanostructure: High Efficiency for Overall Water Splitting and Optical Properties, Small, 2015, 11, 871–876 CrossRef CAS.
- K. Maeda and K. Domen, Photocatalytic Water Splitting: Recent Progress and Future Challenges, J. Phys. Chem. Lett., 2010, 1, 2655–2661 CrossRef CAS.
- K. Liu, B. Zhang, J. Zhang, W. Lin, J. Wang, Y. Xu, Y. Xiang, T. Hisatomi, K. Domen and G. Ma, Synthesis of Narrow-Band-Gap GaN:ZnO Solid Solution for Photocatalytic Overall Water Splitting, ACS Catal., 2022, 12, 14637–14646 CrossRef CAS.
- R. Abe, Development of a New System for Photocatalytic Water Splitting into H2 and O2 under Visible Light Irradiation, Bull. Chem. Soc. Jpn., 2011, 84, 1000–1030 CrossRef CAS.
- H. Kato, Y. Sasaki, N. Shirakura and A. Kudo, Synthesis of highly active rhodium-doped SrTiO3 powders in Z-scheme systems for visible-light-driven photocatalytic overall water splitting, J. Mater. Chem. A, 2013, 1, 12327–12333 RSC.
- R. Konta, T. Ishii, H. Kato and A. Kudo, Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation, J. Phys. Chem. B, 2004, 108, 8992–8995 CrossRef CAS.
- N. Iwasa, Z. Teng, G. Ma, T. Hisatomi and K. Domen, Synthesis of narrow bandgap gallium zinc nitride oxide solid solutions for photocatalytic water splitting under visible light, Chem. Mater., 2024, 36, 2917–2924 CrossRef CAS.
- K. Maeda and K. Domen, Solid Solution of GaN and ZnO as a Stable Photocatalyst for Overall Water Splitting under Visible Light, Chem. Mater., 2010, 22, 612–623 CrossRef CAS.
- A. Sharma, M. Varshney, H. Saraswat, S. Chaudhary, J. Parkash, H. J. Shin, K. H. Chae and S. O. Won, Nano-structured phases of gallium oxide (GaOOH, α-Ga2O3, β-Ga2O3, γ-Ga2O3, δ-Ga2O3, and ε-Ga2O3): fabrication, structural, and electronic structure investigations, Int. Nano Lett., 2020, 10, 71–79 CrossRef CAS.
- X. Liu, C. Wessel, F. Pan and R. Dronskowski, Synthesis and single-crystal structure determination of the zinc nitride halides Zn2NX (X=Cl, Br, I), J. Solid State Chem., 2013, 203, 31–36 CrossRef CAS.
- Z. Li, L. Zhang, Y. Liu, C. Shao, Y. Gao, F. Fan, J. Wang, J. Li, J. Yan, R. Li and C. Li, Surface-Polarity-Induced Spatial Charge Separation Boosts Photocatalytic Overall Water Splitting on GaN Nanorod Arrays, Angew. Chem., Int. Ed., 2020, 59, 935–942 CrossRef CAS.
- M. Huang, J. Lian, R. Si, L. Wang, X. Pan and P. Liu, Spatial Separation of Electrons and Holes among ZnO Polar {0001} and {10
![[1 with combining overline]](https://www.rsc.org/images/entities/char_0031_0305.gif) 0} Facets for Enhanced Photocatalytic Performance, ACS Omega, 2022, 7, 26844–26852 CrossRef CAS.
0} Facets for Enhanced Photocatalytic Performance, ACS Omega, 2022, 7, 26844–26852 CrossRef CAS. - Y. Chen, H. Zhao, B. Liu and H. Yang, Charge separation between wurtzite ZnO polar {001} surfaces and their enhanced photocatalytic activity, Appl. Catal., B, 2015, 163, 189–197 CrossRef CAS.
- M. W. Liao, H. T. Jeng and T. P. Perng, Formation Mechanism and Bandgap Reduction of GaN–ZnO Solid-Solution Thin Films Fabricated by Nanolamination of Atomic Layer Deposition, Adv. Mater., 2023, 35, 2207849 CrossRef CAS.
- K. Maeda, K. Teramura and K. Domen, Effect of post-calcination on photocatalytic activity of (Ga1−xZnx)(N1−xOx) solid solution for overall water splitting under visible light, J. Catal., 2008, 254, 198–204 CrossRef CAS.
- S. Nandy, T. Hisatomi, M. Nakabayashi, H. Li, X. Wang, N. Shibata, T. Takata and K. Domen, Oxide layer coating enabling oxysulfide-based photocatalyst sheet to drive Z-scheme water splitting at atmospheric pressure, Joule, 2023, 7, 1641–1651 CrossRef CAS.
- Q. Wang, Y. Li, T. Hisatomi, M. Nakabayashi, N. Shibata, J. Kubota and K. Domen, Z-scheme water splitting using particulate semiconductors immobilized onto metal layers for efficient electron relay, J. Catal., 2015, 328, 308–315 CrossRef CAS.
- T. Minegishi, N. Nishimura, J. Kubota and K. Domen, Photoelectrochemical properties of LaTiO2N electrodes prepared by particle transfer for sunlight-driven water splitting, Chem. Sci., 2013, 4, 1120–1124 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta03576c |
| ‡ These authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |