BF3·Et2O-mediated intramolecular cyclization of unsaturated amides: convenient synthesis of dihydroquinolin-2-one-BF2 complexes†
Xu Liu,
Qian Zhang,
Xiaoqing Xin,
Rui Zhang,
Ning Zhang*,
Yongjiu Liang and
Dewen Dong*
Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, China. E-mail: ning.zhang@ciac.ac.cn; dwdong@ciac.ac.cn; Fax: +86 431 85262740; Tel: +86 431 85262676
First published on 7th August 2014
Abstract
A facile and efficient synthesis of substituted dihydropyridone–BF2 complexes is developed via intramolecular cyclization of α-acyl acrylamides and α-acyl cinnamamides mediated by BF3·Et2O.
Introduction
Over the past decades, pyridin-2(1H)-ones and their analogues have attracted considerable attention in chemical and biological fields.1,2 These structural motifs can serve as efficient catalysts in a variety of proton-dependent reactions3 and as valuable ligands in coordination chemistry.4 Furthermore, pyridin-2(1H)-ones are versatile intermediates in the synthesis of a wide range of aza-heterocycles, such as pyridines, piperidines, indolizidines, quinolines and quinolizidines.5,6 In particular, pyridin-2(1H)-one is a key unit in numerous natural products and synthetic organic compounds such as elfamycin, cerpegin and camptothecin,7 along with diverse bio-, physio- and pharmacological activities. To date, a variety of synthetic approaches have been well established to access pyridin-2(1H)-ones and their analogues, which comprise the modification of the pre-constructed heterocyclic ring by pyridinium salt chemistry8 and N-alkylation,9 the construction of heterocyclic skeletons from appropriately substituted open-chain precursors via metal-catalyzed sp2 C–H bond amination,10 ring closing metathesis,11 and Diels–Alder reaction.12On the other hand, organoboron compounds have emerged as one of the most important class of organic complexes for their excellent photophysical properties and potential use in molecular sensors,13 biomolecular probes14 and optoelectronic devices.15 Among those reported work, β-dicarbonyl compounds are most used ligands, and the boron difluoride β-diketonates have been extensively investigated and their promising luminescence make them good candidates for optical imaging and sensing applications.16,17
During the course of our studies on the synthesis of heterocycles based on β-oxo amide derivatives, we developed the synthesis of a variety of substituted pyridin-2(1H)-ones under Vilsmeier conditions.18 Most recently, we achieved the synthesis of indeno[2,1-c]quinolin-6(7H)-ones from α-acyl cinnamamides mediated by PPA (eqn (1), Scheme 1),19 divergent synthesis of quinolin-2(1H)-ones (eqn (2), Scheme 1)20 and pyridin-2(3H)-ones (eqn (3), Scheme 1) from 2-acyl penta-2,4-dienamides.21 Encouraged by the previous work, we are interested to examine the reaction behaviors of unsaturated amides toward BF3·Et2O. By this research, we developed a facile and convenient synthesis of dihydropyridone–BF2 complexes under very mild conditions. Herein, we will report our experimental results and present a proposed mechanism involved in the cyclization reactions.
Results and discussion
The substrates, unsaturated amides, were prepared by Knoevenagel condensation of commercially available β-oxo amides with aryl aldehydes in the presence of piperidine and acetic acid in good yields according to reported procedures.19–22 Then, we selected 2-benzylidene-3-oxo-N-phenylbutanamide 1a as a model compound to investigate its reaction behavior in the presence of BF3·Et2O (1.0 equiv.) in CH2Cl2 at room temperature. The reaction could proceed and furnished a product, which was characterized as 2,2-difluoro-4-methyl-5-phenyl-5,10-dihydro-2H-[1,3,2]dioxa-borinino[4,5-b]quinolin-1-ium-2-uide 2a (in 76% yield) on the basis of its spectral and analytical data. A series of experiments revealed that the optimal results were obtained when the reaction of 1a was performed with BF3·Et2O (2.0 equiv.) in CH2Cl2 at room temperature, in which the yield of 2a reached 88% (Table 1, entry 1).| Entry | 1 | R1 | R2 | R3 | R4 | 2 | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reagents and conditions: 1 (2.0 mmol), BF3·Et2O (4.0 mmol), CH2Cl2 (5.0 mL), rt, 2.0–3.0 h.b Isolated yield. | |||||||
| 1 | 1a | H | H | Me | H | 2a | 88 |
| 2 | 1b | 4-Me | H | Me | H | 2b | 81 |
| 3 | 1c | 2-Me | H | Me | H | 2c | 82 |
| 4 | 1d | 3-Me | H | Me | H | 2d | 79 |
| 5 | 1e | 2,4-Me2 | H | Me | H | 2e | 80 |
| 6 | 1f | 4-Cl | H | Me | H | 2f | 83 |
| 7 | 1g | 4-MeO | H | Me | H | 2g | 96 |
| 8 | 1h | 2-MeO | H | Me | H | 2h | 94 |
| 9 | 1i | H | H | Ph | H | 2i | 75 |
| 10 | 1j | H | 4-Me | Me | H | 2j | 87 |
| 11 | 1k | H | 2-Me | Me | H | 2k | 86 |
| 12 | 1l | H | 2-MeO | Me | H | 2l | 85 |
| 13 | 1m | H | 4-Cl | Me | H | 2m | 83 |
| 14 | 1n | H | H | Me | Et | 2n | 81 |
Under the identical conditions as for 2a, a range of reactions of α-acyl N-arylcinnamamides 1b–n were carried out and some of the results are summarized in Table 2. All the reactions of 1b–g bearing various electron-donating and electron-withdrawing substituents R1 on the aryl amides proceeded smoothly to afford the corresponding dihydropyridone–BF2 complexes 2b–g in high yields (Table 1, entries 2–9). In the case of 1d, 2,2-difluoro-4,8-dimethyl-5-phenyl-5,10-dihydro-2H-[1,3,2]dioxaborinino-[4,5-b]quinolin-1-ium-2-uide 2d was exclusively obtained in 79% yield, which suggests that 1d underwent the cyclization reaction in a regioselective manner (Table 1, entry 4). The efficiency of the cyclization proved to be suitable for 1j–m bearing various electron-donating and electron-withdrawing substituents R2 on the benzene ring affording the corresponding substituted dihydropyridone–BF2 complexes 2j–m in very good yields (Table 1, entries 10–13). In the same fashion, the validity of this dihydropyridone–BF2 complex synthesis was further evaluated by performing 1n bearing secondary amide, in which dihydroquinolin-2-one-BF2 complex 2n was obtained in high yield (Table 1, entry 14). The structure of 2g was further confirmed by the X-ray single crystal analysis (Fig. 1). The results shown above demonstrate the efficiency and synthetic interest of the cyclization reaction of variable α-acyl N-aryl cinnamamides 1.
It should be mentioned that when dihydropyridone–BF2 complex 2h was treated with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ, 1.0 equiv.) in CH2Cl2 at room temperature for 1.0 h, 3-acyl-6-methoxy-4-phenylquinolin-2(1H)-one 3h could be obtained in 80% yield (Scheme 2). Therefore, we provided a novel and convenient synthesis of dihydropyridone–BF2 complexes 2 and an alternative synthesis of dihydroquinolin-2-ones 3 as well.
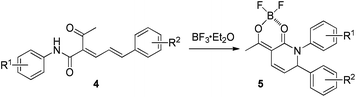
Encouraged by the above results, we intended to explore the reaction of 2-acyl penta-2,4-dienamides under identical reaction conditions as for 2a. However, when 2-acyl-5-phenyl-N-(p-tolyl)penta-2,4-dienamide 4a was subjected to CH2Cl2 in the presence of BF3·Et2O at room temperature for 2.0 h, no reaction was observed. Then, the reaction of 4a was performed in (CH2)2Cl2 under reflux for 1.0 h and furnished a product, which was characterized as 2,2-difluoro-4-methyl-7-phenyl-8-(p-tolyl)-7,8-dihydro-2H-[1,3,2]dioxaborinino[4,5-b]pyridin-1-ium-2-uide 5a (Table 2, entry 1).
Under the identical conditions as for 4a in Table 2 entry 1, a series of reactions of 2-acyl penta-2,4-dienamides 4b–f were carried out in the presence of BF3·Et2O, and some of the results are summarized in Table 2. All the reactions of 4b–f bearing different aryl amide groups for R1 and aryl groups for R2 could proceed smoothly to afford the corresponding dihydropyridin-2(3H)-one-BF2 complexes 5b–f in good yields (Table 2, entries 2–7). The structure of 5d was elucidated by NMR (1H, 13C) spectra and further confirmed by means of the X-ray single crystal analysis (Fig. 2).
In contrast to the conventional acid-catalyzed Knorr quinolin-2(1H)-one synthesis,23 α-acyl N-aryl cinnamamides 1 were found to undergo a distinct intramolecular cyclization in which the nucleophilic addition site was on the β-carbon of the α,β-unsaturated carbonyl compounds 1 instead of their α-acyl groups. On the basis of the results obtained above and the reported literatures, a plausible mechanism for the synthesis of dihydroquinolin-2-one-BF2 complexes 2 is presented in Scheme 3. Mediated by BF3·Et2O, α-acyl N-arylcinnamamide 1 is activated by the formation of BF2-complex intermediate A,17d,e followed by an intramolecular Friedel–Crafts reaction to afford dihydropyridin-2(3H)-one-BF2 complex 2.24 It is most possible that the BF2-complex moiety could not provide enough activation to promote further intramolecular cyclization for 2 under the investigated conditions. As for 2-acyl penta-2,4-dienamides 4, a BF2-complex intermediate B is formed in the same way (Scheme 3). Here, it is worth noting that BF2-complex intermediate B contains a 1-azatriene moiety, which under the investigated conditions may undergo a 6π-azaelectrocyclization reaction21 instead of the Friedel–Crafts reaction as α-acyl N-aryl cinnamamide 1 did. Just like the role of hydrogen bond did in our previous work, the BF2-complex structure provides the driving force to keep the azadiene N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of B in a cis conformation that may favor the subsequent 6π-azaelectrocyclization, and also stabilize the structure of product 5.
C of B in a cis conformation that may favor the subsequent 6π-azaelectrocyclization, and also stabilize the structure of product 5.
Conclusions
In summary, a facile and convenient synthesis of substituted dihydropyridone–BF2 complexes 3 and 5 is developed via intramolecular cyclization of unsaturated amides, α-acyl N-ary lcinnamamides 1 and 2-acyl penta-2,4-dienamides 4, mediated by BF3·Et2O, respectively. The simple execution, readily available substrates, very mild conditions, good yields and wide range of synthetic potential of the products make this protocol much attractive. The extension of the scope of the methodology and its further applications are currently under investigation in our laboratory.Experimental section
General
All reagents were purchased from commercial sources and used without treatment, unless otherwise indicated. The products were purified by column chromatography over silica gel. 1H NMR and 13C NMR spectra were recorded at 25 °C at 300 MHz and 100 MHz, respectively, with TMS as internal standard. IR spectra (KBr) were recorded on FTIR-spectrometer in the range of 400–4000 cm−1. All melting points were determined in open capillary tubes in a Thiele apparatus and are uncorrected.Typical procedure for the synthesis of substituted unsaturated amides 1 (1a as an example)
To a 100 mL round-bottomed flask was added 3-oxo-N-phenylbutanamide (0.89 g, 5.0 mmol), 4-methylbenzaldehyde (0.60 g, 5.0 mmol), piperidine (0.5 mmol), acetic acid (0.5 mmol) and ethanol (30 mL). Then the mixture was stirred for 8.0 h at room temperature. The resulting mixture was slowly poured into saturated aqueous NaCl (100 mL), and extracted with dichloromethane (3 × 30 mL). The combined organic phase was washed with water (3 × 30 mL) and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the crude product was purified by flash chromatography (silica gel, petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate10
ethyl acetate10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 1a as a colorless solid (1.20 g, 86%).
1) to give 1a as a colorless solid (1.20 g, 86%).
Substrates 1a–k and 1n are known compounds (1a and 1j: J. Indian Chem. Soc., 1981, 58, 168, 1c and 1d: Comptes Rendus Hebdomadaires des Seances de I′Academie des Sciences, 1949, 228, 576, 1b, 1e–k and 1n: Org. Lett., 2013, 15, 776.)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 25]. Colorless solid: mp 91–96 °C; 1H NMR (300 MHz, CDCl3) (minor E-isomer): δ 2.45 (s, 3H), 3.86 (s, 3H), 6.94 (d, J = 6.0 Hz, 2H), 7.14–7.16 (m, 1H), 7.28–7.33 (m, 2H), 7.57–7.63 (m, 4H), 8.21 (s, 1H), 9.39 (s, 1H); (major Z-isomer): δ 2.45 (s, 3H), 3.81 (s, 3H), 6.86 (d, J = 6.0 Hz, 2H), 7.16 (t, J = 6.0 Hz, 1H), 7.35 (t, J = 6.0 Hz, 2H), 7.53–7.57 (m, 5H), 7.88 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 13.7, 20.5, 22.2, 26.4, 30.9, 54.8, 59.9, 114.0, 119.7, 124.0, 128.5, 131.2, 131.9, 137.3, 140.5, 145.1, 161.3, 165.8, 195.4; Anal. calcd for C18H17NO3: C, 73.20; H, 5.80; N, 4.74. Found: C, 72.81; H, 5.85; N, 4.69.
25]. Colorless solid: mp 91–96 °C; 1H NMR (300 MHz, CDCl3) (minor E-isomer): δ 2.45 (s, 3H), 3.86 (s, 3H), 6.94 (d, J = 6.0 Hz, 2H), 7.14–7.16 (m, 1H), 7.28–7.33 (m, 2H), 7.57–7.63 (m, 4H), 8.21 (s, 1H), 9.39 (s, 1H); (major Z-isomer): δ 2.45 (s, 3H), 3.81 (s, 3H), 6.86 (d, J = 6.0 Hz, 2H), 7.16 (t, J = 6.0 Hz, 1H), 7.35 (t, J = 6.0 Hz, 2H), 7.53–7.57 (m, 5H), 7.88 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 13.7, 20.5, 22.2, 26.4, 30.9, 54.8, 59.9, 114.0, 119.7, 124.0, 128.5, 131.2, 131.9, 137.3, 140.5, 145.1, 161.3, 165.8, 195.4; Anal. calcd for C18H17NO3: C, 73.20; H, 5.80; N, 4.74. Found: C, 72.81; H, 5.85; N, 4.69.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5]. Colorless solid: mp 122–127 °C; 1H NMR (300 MHz, CDCl3) (minor E-isomer): δ 2.17 (s, 3H), 7.11–7.18 (m, 2H), 7.27–7.35 (m, 2H), 7.47–7.50 (m, 2H), 7.52 (s, 2H), 7.58 (d, J = 6.0 Hz, 2H), 9.28 (s, 1H); (major Z-isomer): δ 2.43 (s, 3H), 7.11–7.18 (m, 1H), 7.20 (d, J = 12.0 Hz, 1H), 7.27–7.35 (m, 4H), 7.40 (d, J = 9.0 Hz, 1H), 7.45 (s, 1H), 7.47–7.50 (m, 2H), 8.11 (d, J = 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 26.4, 31.1, 119.5, 119.9, 124.2, 128.6, 130.5, 135.8, 136.8, 143.1, 160.3, 165.1, 195.4, 206.3; Anal. calcd for C17H14ClNO2: C, 68.12; H, 4.71; N, 4.67. Found: C, 68.44; H, 4.75; N, 4.62.
5]. Colorless solid: mp 122–127 °C; 1H NMR (300 MHz, CDCl3) (minor E-isomer): δ 2.17 (s, 3H), 7.11–7.18 (m, 2H), 7.27–7.35 (m, 2H), 7.47–7.50 (m, 2H), 7.52 (s, 2H), 7.58 (d, J = 6.0 Hz, 2H), 9.28 (s, 1H); (major Z-isomer): δ 2.43 (s, 3H), 7.11–7.18 (m, 1H), 7.20 (d, J = 12.0 Hz, 1H), 7.27–7.35 (m, 4H), 7.40 (d, J = 9.0 Hz, 1H), 7.45 (s, 1H), 7.47–7.50 (m, 2H), 8.11 (d, J = 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 26.4, 31.1, 119.5, 119.9, 124.2, 128.6, 130.5, 135.8, 136.8, 143.1, 160.3, 165.1, 195.4, 206.3; Anal. calcd for C17H14ClNO2: C, 68.12; H, 4.71; N, 4.67. Found: C, 68.44; H, 4.75; N, 4.62.Typical procedure for the synthesis of dihydropyridone–BF2 complexes 2 (2a as an example)
To a 50 mL round bottomed flask was added 1a (530.0 mg, 2.0 mmol), BF3·Et2O (5.0 mmol) and CH2Cl2 (10 mL). The mixture was stirred at room temperature for 1.0 h. After the substrate 1a was consumed completely as indicated by TLC, the mixture was poured into ice water, and then extracted with dichloromethane (3 × 20 mL), the combined organic phase was washed with water (3 × 20 mL), and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the crude product was purified by flash chromatography (silica gel, petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 5
ethyl acetate 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 2a as colorless solid (551.1 mg, 88%).
1) to give 2a as colorless solid (551.1 mg, 88%).
Crystal data for 2g: C18H16BF2NO3, colorless crystal, M = 343.13, monoclinic, C2/c′, a = 26.916(3) Å, b = 8.0515(9) Å, c = 17.954(2) Å, α = 90.00°, β = 123.571(2)°, γ = 90.00°, V = 3241.9(6) Å3, Z = 8, T = 293(2) K, F000 = 1512, R = 0.0474.
The procedure for the synthesis of substituted quinolin-2(1H)-one 3h
To a 50 mL round bottomed flask was added 2 h (686.3 mg, 2.0 mmol), DDQ (3.0 mmol) and CH2Cl2 (10 mL). The mixture was stirred at room temperature for 1.0 h. After the substrate 2g was consumed completely as indicated by TLC, the mixture was poured into ice water, and then extracted with dichloromethane (3 × 20 mL), the combined organic phase was washed with water (3 × 20 mL), and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the crude product was purified by flash chromatography (silica gel, petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 4
ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 3h as colorless solid (469.3 mg, 80%).
1) to give 3h as colorless solid (469.3 mg, 80%).
Typical procedure for the synthesis of dihydropyridone–BF2 complexes 5 (5a as an example)
To a 50 mL round bottomed flask was added 4a (610.7 mg, 2.0 mmol), BF3·Et2O (3.0 mmol) and DCE (10 mL). The mixture was stirred at 80 °C for 2.0 h. After the substrate 4a was consumed completely as indicated by TLC, the mixture was poured into ice water, and then extracted with dichloromethane (3 × 20 mL), the combined organic phase was washed with water (3 × 20 mL), and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the crude product was purified by flash chromatography (silica gel, petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 4
ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 5a as a colorless solid (494.4 mg, 70%).
1) to give 5a as a colorless solid (494.4 mg, 70%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 3). Yellow solid: mp 151–155 °C; 1H NMR (major isomer) (400 MHz, DMSO): δ 2.29 (s, 3H), 3.73 (s, 3H), 5.50 (d, J = 4.0 Hz, 1H), 5.62–5.66 (dd, J1 = 12.0 Hz, J2 = 4.0 Hz, 1H), 6.42 (d, J = 8.0 Hz, 1H), 6.91 (d, J = 12.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 7.21 (t, J = 8.0 Hz, 1H), 7.44 (t, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H); 1H NMR (minor isomer) (400 MHz, DMSO): δ 2.27 (s, 3H), 3.68 (s, 3H), 5.57–5.60 (dd, J1 = 12.0 Hz, J2 = 4.0 Hz, 1H), 5.93 (s, 1H), 6.71 (d, J = 12.0 Hz, 1H), 6.78 (d, J = 12.0 Hz, 2H), 6.99 (d, J = 8.0 Hz, 2H), 7.35–7.39 (m, 2H), 7.49–7.53 (m, 1H), 7.87 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 19.7, 19.8, 55.4, 55.5, 66.0, 67.5, 95.2, 95.3, 113.8, 114.6, 118.0, 118.7, 119.2, 119.3, 128.1, 128.9, 130.4, 131.1, 134.4, 135.1, 159.9, 160.0, 163.8, 164.9, 173.3, 174.4; Anal. calcd for C20H17BClF2NO3: C, 59.52; H, 4.25; N, 3.47. Found: C, 59.91; H, 4.36; N, 3.40.
3). Yellow solid: mp 151–155 °C; 1H NMR (major isomer) (400 MHz, DMSO): δ 2.29 (s, 3H), 3.73 (s, 3H), 5.50 (d, J = 4.0 Hz, 1H), 5.62–5.66 (dd, J1 = 12.0 Hz, J2 = 4.0 Hz, 1H), 6.42 (d, J = 8.0 Hz, 1H), 6.91 (d, J = 12.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 7.21 (t, J = 8.0 Hz, 1H), 7.44 (t, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H); 1H NMR (minor isomer) (400 MHz, DMSO): δ 2.27 (s, 3H), 3.68 (s, 3H), 5.57–5.60 (dd, J1 = 12.0 Hz, J2 = 4.0 Hz, 1H), 5.93 (s, 1H), 6.71 (d, J = 12.0 Hz, 1H), 6.78 (d, J = 12.0 Hz, 2H), 6.99 (d, J = 8.0 Hz, 2H), 7.35–7.39 (m, 2H), 7.49–7.53 (m, 1H), 7.87 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, DMSO): δ 19.7, 19.8, 55.4, 55.5, 66.0, 67.5, 95.2, 95.3, 113.8, 114.6, 118.0, 118.7, 119.2, 119.3, 128.1, 128.9, 130.4, 131.1, 134.4, 135.1, 159.9, 160.0, 163.8, 164.9, 173.3, 174.4; Anal. calcd for C20H17BClF2NO3: C, 59.52; H, 4.25; N, 3.47. Found: C, 59.91; H, 4.36; N, 3.40.Crystal data for 5d: C20H17BClF2NO3, colorless crystal, M = 871.29, P-1, a = 7.643(5) Å, b = 12.269(5) Å, c = 12.840(5) Å, α = 101.954(5)°, β = 104.028(5)°, γ = 98.284(5)°, V = 1118.6(10) Å3, Z = 1, T = 293(2) K, F000 = 450, R = 0.0123.
Acknowledgements
Financial support of this research by the National Natural Science Foundation of China (21172211) and Department of Science and Technology of Jilin Province (201105030 and 201205027) are greatly acknowledged.Notes and references
- (a) A. D. Elbein and R. J. Molyneux, Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Wiley, New York, 1990, vol. 5, pp. 1–54 Search PubMed; (b) G. Jones, Pyridines and Their Benzo Derivatives: Synthesis in Comprehensive Heterocyclic Chemistry II, ed. A. McKillop, Pergamon Press, Oxford, 1996, vol. 5, p. 167 Search PubMed; (c) Q. Li, L. A. Mitscher and L. L. Shen, Med. Res. Rev., 2000, 20, 231 CrossRef CAS.
- (a) E. F. V. Scriven, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, 1984, vol. 2 Search PubMed; (b) G. Jones, Pyridines and Their Benzo Derivatives: Synthesis, in Comprehensive Heterocyclic Chemistry, ed. A. Boulton and A. McKillop, Pergamon Press, Oxford, 1984, p. 395, ch. 2.08 Search PubMed.
- C. B. Fischer, H. Steininger, D. S. Stephenson and H. Zipse, J. Phys. Org. Chem., 2005, 18, 901 CrossRef CAS PubMed.
- J. M. Rawson and R. E. P. Winpenny, Coord. Chem. Rev., 1995, 139, 313 CrossRef CAS.
- (a) M. Torres, S. Gil and M. Parra, Curr. Org. Chem., 2005, 9, 1757 CrossRef CAS; (b) G. M. Strunz and J. A. Findlay, The Alkaloids, ed. A. Brossi, Academic Press, Orlando, 1985, vol. 26, p. 89 Search PubMed; (c) V. P. Litvinov, Russ. Chem. Rev., 2003, 72, 69 CrossRef CAS PubMed.
- (a) L. Jayasinghe, H. K. Abbas, M. R. Jacob, W. H. M. W. Herath and N. P. D. Nanayakkara, J. Nat. Prod., 2006, 69, 439 CrossRef CAS PubMed; (b) P. R. Angibaud, M. G. Venet, W. Filliers, R. Broeckx, Y. A. Ligny, P. Muller, V. S. Poncelet and D. W. End, Eur. J. Org. Chem., 2004, 479 CrossRef CAS PubMed; (c) M. F. Grundon, Nat. Prod. Rep., 1989, 6, 523 RSC; (d) R. Fujita, K. Watanabe, W. Ikeura and Y. H. Ohtake, Heterocycles, 2000, 53, 2607 CrossRef CAS.
- (a) G. B. Fodor and B. Colasanti, Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Wiley, New York, 1985, vol. 3, pp. 49–73 Search PubMed; (b) H. Hong and D. L. Comins, J. Org. Chem., 1996, 61, 391 CrossRef CAS; (c) D. L. Comins and J. M. Nolan, Org. Lett., 2001, 3, 4255 CrossRef CAS PubMed.
- (a) H. Decker, Chem. Ber., 1892, 25, 443 CrossRef PubMed; (b) D. L. Comins and J. K. Saha, J. Org. Chem., 1996, 61, 9623 CrossRef CAS; (c) W. Du, Tetrahedron, 2003, 59, 8649 CrossRef CAS.
- (a) D. L. Comins and J. L. Gao, Tetrahedron Lett., 1994, 35, 2819 CrossRef CAS; (b) T. Hu and C. Li, Org. Lett., 2005, 7, 2035 CrossRef CAS PubMed; (c) H. J. Cristau, P. P. Cellier, J. F. Spindler and M. Taillefer, Chem.–Eur. J., 2004, 10, 5607 CrossRef CAS PubMed.
- (a) M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14058 CrossRef CAS PubMed; (b) K. Inamoto, T. Saito, K. Hiroya and T. Doi, J. Org. Chem., 2010, 75, 3900 CrossRef CAS PubMed.
- (a) C. Fiorelli and D. Savoia, J. Org. Chem., 2007, 72, 6022 CrossRef CAS PubMed; (b) J. H. Lee, S. Shin, J. Kang and S. G. Lee, J. Org. Chem., 2007, 72, 7443 CrossRef CAS PubMed.
- (a) Z. Chen, L. Lin, D. Chen, J. Li, X. Liu and X. Feng, Tetrahedron Lett., 2010, 51, 3088 CrossRef CAS PubMed; (b) J. Itoh, K. Fuchibe and T. Akiyama, Angew. Chem., Int. Ed., 2006, 45, 4796 CrossRef CAS PubMed.
- (a) A. P. De Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. Mccoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed; (b) M. Wang, D. Q. Zhang, G. X. Zhang and D. B. Zhu, Chem. Commun., 2008, 4469 RSC.
- (a) W. Yang, H. He and D. G. Drueckhammer, Angew. Chem., Int. Ed., 2001, 4, 1714 CrossRef; (b) K. Rurack, M. Kollmannsberger and J. Daub, Angew. Chem., Int. Ed., 2001, 40, 385 CrossRef CAS.
- (a) C. T. Chen, Chem. Mater., 2004, 16, 4389 CrossRef CAS; (b) Q. D. Liu, M. S. Mudadu, R. Thummel, Y. Tao and S. N. Wang, Adv. Funct. Mater., 2005, 15, 143 CrossRef CAS PubMed.
- (a) G. T. Morgan and R. B. Tunstall, J. Chem. Soc., Trans., 1924, 125, 1963 RSC; (b) V. A. Reutov and E. V. Gukhman, Russ. J. Gen. Chem., 1999, 1603 CAS; (c) H. Maeda and Y. Ito, Inorg. Chem., 2006, 45, 8205 CrossRef CAS PubMed.
- (a) H. D. Ilge, E. Birckner and D. Fassler, J. Photochem., 1986, 32, 177 CrossRef CAS; (b) A. G. Mirochink, E. V. Fedorenko, G. K. Gizzatulina and V. E. Karasev, Russ. J. Phys. Chem. A, 2007, 81, 1880 Search PubMed; (c) E. Cogné-Laage, J.-F. Allemand, O. Ruel, J.-B. Baudin, V. Croquette, M. Blanchard-Desce and L. Julien, Chem.–Eur. J., 2004, 10, 1445 CrossRef PubMed; (d) G. Zhang, J. Chen, S. J. Payne, S. E. Kooi, J. N. Demas and C. L. Fraser, J. Am. Chem. Soc., 2007, 129, 8942 CrossRef CAS PubMed; (e) G. Zhang, J. Lu, M. Sabat and C. L. Fraser, J. Am. Chem. Soc., 2010, 132, 2160 CrossRef CAS PubMed.
- (a) W. Pan, D. Dong, K. Wang, J. Zhang, R. Wu, D. Xiang and Q. Liu, Org. Lett., 2007, 9, 2421 CrossRef CAS PubMed; (b) D. Xiang, Y. Yang, R. Zhang, Y. Liang, W. Pan, J. Huang and D. Dong, J. Org. Chem., 2007, 72, 8593 CrossRef CAS PubMed; (c) D. Xiang, K. Wang, Y. Liang, G. Zhou and D. Dong, Org. Lett., 2008, 10, 345 CrossRef CAS PubMed; (d) R. Zhang, D. Zhang, Y. Guo, G. Zhou, Z. Jiang and D. Dong, J. Org. Chem., 2008, 73, 9504 CrossRef CAS PubMed.
- X. Liu, Q. Zhang, D. Zhang, X. Xin, R. Zhang, F. Zhou and D. Dong, Org. Lett., 2013, 15, 776 CrossRef CAS PubMed.
- X. Liu, X. Xin, D. Xiang, R. Zhang, S. Kumar, F. Zhou and D. Dong, Org. Biomol. Chem., 2012, 10, 5643 CAS.
- X. Liu, N. Zhang, J. Yang, Y. Liang, R. Zhang and D. Dong, J. Org. Chem., 2013, 78, 3323 CrossRef CAS PubMed.
- A. K. Zarif and S. Y. Amal, J. Indian Chem. Soc., 1981, 58, 168 Search PubMed.
- For Knorr quinolin-2(1H)-one synthesis, see: (a) L. Knorr, Ann, 1886, 236, 69 Search PubMed; (b) F. W. Bergstorm, Chem. Rev., 1944, 35, 157 Search PubMed.
- L.-H. Xie, X.-Y. Hou, Y.-R. Hua, C. Tang, F. Liu, Q.-L. Fan and W. Huang, Org. Lett., 2006, 8, 3701 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectral and analytical data, copies of 1H NMR and 13C NMR spectra for new compounds 1–3 and 5, and CIF files for 2g and 5d. CCDC 922666 and 938933. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra06151a |
| This journal is © The Royal Society of Chemistry 2014 |