A highly sensitive and water soluble fluorescent probe for rapid detection of hydrogen sulfide in living cells†
Xin Li*a,
Yanling Gonga,
Kai Wub,
Steven H. Liangc,
Ji Caoa,
Bo Yanga,
Yongzhou Hua and
Yifeng Han*b
aCollege of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China. E-mail: lixin81@zju.edu.cn
bDepartment of Chemistry, Zhejiang Sci-Tech University, Hangzhou, 310018, China. E-mail: hanyf@zstu.edu.cn
cDivision of Nuclear Medicine and Molecular Imaging, Massachusetts General Hospital and Department of Radiology, Harvard Medical School, 55 Fruit St., Boston, MA 02114, USA
First published on 6th August 2014
Abstract
A highly sensitive and specific fluorescent probe has been developed for the rapid detection of H2S in living cells. This probe exhibits the unique qualities of quick response, low detection limit, excellent water solubility and good membrane permeability. Its potential for biological applications has been demonstrated by imaging H2S in living cells.
Hydrogen sulfide (H2S) is traditionally viewed as a toxic gas with the characteristic odor of rotten-eggs. However, more recent studies have established it as the third gaseous transmitter after nitric oxide (NO) and carbon monoxide (CO).1 H2S at physiological levels exerts fine modulatory control over cellular functions by signaling an array of processes such as neuromodulation in the brain and smooth muscle relaxation in the vascular system.2,3 Endogenous H2S is synthesized in mammalian tissues in a controlled fashion and dysregulation of H2S production or metabolism is reported to be linked to a variety of disease phenotypes including Alzheimer's disease,4 diabetes,5 hypertension,6 etc. To date, many details on the physiological and pathological roles of H2S are still under active investigation. Therefore, it is important to have robust assays for H2S detection in its native environment to facilitate detailed biological function studies.
Current methods available for the detection of H2S, including colorimetric, electrochemical,6 and gas chromatography assays,7 are limited by low resolution, complicated requirements for sample preparation and poor biocompatibility, which forbid the real time monitoring of H2S production, trafficking, and consumption in living cells, tissues, and whole organisms. As such, fluorescent molecular probes offer an appealing approach for the detection of H2S attributed to their nondestructive attribute and high sensitivity.8,9 A number of fluorescent probes have recently been developed to measure H2S in living cells or in whole blood. These probes, judiciously designed by making use of the nucleophilic or reducing properties of H2S, undergo chemoselective reactions with H2S in the native cellular environment and their fluorescence properties are altered as a consequence.10,11 However, most of these probes are limited by two substantial challenges. One is their sluggish reaction kinetics with H2S, as exemplified by our previous probe ZS1, which utilized an aldehyde–acrylate group to trap H2S and required 50 minutes to reach its highest fluorescent response.12 The other is their poor water-solubility. Being poor water-soluble, most probes can only be used in mixed solvents containing a large portion of non-biocompatible organic solvents for fluorescent spectrometry analysis, which greatly limits their application. Given the diffusive and highly reactive nature of H2S, more sensitive probes with faster response and improved water-solubility are still highly desirable.
In the present work, we are interested in devising new probes by taking advantage of the extremely strong affinity and facile kinetic profile between S2− and Cu2+. Indeed, several fluorescent probes have been developed employing this strategy, including HSip-1 from Nagano group, L1Cu from Zeng group and Cu-1 from Shen group (Fig. 1).13 However, they are limited by their poor membrane permeability or poor water solubility.13 Herein we disclose a highly sensitive and selective H2S probe, namely ZS2-Cu, with good water solubility and excellent membrane permeability. The probe was assembled by complexing Cu2+ with the BODIPY-based ligand ZS2 (Fig. 1). Our design of ZS2-Cu was based on the following facts: (1) BODIPY fluorophore represents a desirable fluorescent scaffold for the design of live-organism imaging probes owning to its prominent photophysical properties and excellent membrane permeability;14 (2) N, N-bis(2-pyridylmethyl)ethylenediamine may serve as a coordinating group for Cu2+ to quench the fluorescence of ZS2 after binding Cu2+ because of the paramagnetic character of the cation;15 (3) in the presence of S2−, Cu2+ would be rapidly de-coordinated from the probe to release ligand ZS2 and therefore restore the strong fluorescence of the system, yielding a quick and sensitive response towards S2−; (4) in addition to its function to chelate Cu2+, N, N-bis(2-pyridylmethyl)ethylenediamine also helps to improve the water solubility of the probe. Besides, fluorescent ligand ZS2 is readily accessible due to the well documented nucleophilic reaction between α-chloroBODIPY and N-centered nucleophiles.16
With all these considerations, ZS2 and ZS2-Cu were synthesized as outlined in Scheme 1. Nucleophilic substitution of α-chloro-BODIPY (1) with N, N-bis(pyridin-2-ylmethyl)ethane-1,2-diamine (2), both of which were prepared according to literature procudeures,17,18 readily yielded ZS2. Treatment of ZS2 with copper(II) chloride in THF in the dark gave ZS2-Cu quantitatively. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the complex was confirmed by both mass spectrometry and a Job's plot (Fig. S1†). The nitrogen atom directly attached to the BODIPY core in ZS2 was speculated being involved in the chelation by UV-vis spectra analysis. ZS2 alone in PBS showed an absorption band with the maximum peak centered at 494 nm (ε 17
1 stoichiometry of the complex was confirmed by both mass spectrometry and a Job's plot (Fig. S1†). The nitrogen atom directly attached to the BODIPY core in ZS2 was speculated being involved in the chelation by UV-vis spectra analysis. ZS2 alone in PBS showed an absorption band with the maximum peak centered at 494 nm (ε 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150), which can be assigned to the lowest-lying π–π* transition of the BODIPY core, while the addition of Cu2+ caused a blue-shift of 18 nm (λmax 476 nm, ε 17 940), presumably due to the decreased donation effect of the lone pair electrons on the N atom to the π system of BODIPY after chelation (Fig. S2†).
150), which can be assigned to the lowest-lying π–π* transition of the BODIPY core, while the addition of Cu2+ caused a blue-shift of 18 nm (λmax 476 nm, ε 17 940), presumably due to the decreased donation effect of the lone pair electrons on the N atom to the π system of BODIPY after chelation (Fig. S2†).
With these probes in hand, we first measured their water solubility employing the UV spectrometry method. As expected, ZS2-Cu exhibited an improved water solubility of 1.28 ± 0.02 g L−1. This advantage greatly facilitated following cuvette-based experiments which were carried out solely in aqueous solution without the need for organic co-solvent. The fluorescent properties of ZS2 without or with the presence of Cu2+ were then investigated. As shown in Fig. 2, ZS2 alone in PBS exhibited an intense emission centered at 546 nm (λex 480 nm, Φ 0.1040), which was gradually attenuated by the addition of increasing amount of Cu2+, indicating the coordination of ZS2 with Cu2+. The system reached 90% quenching when 1.0 eq. of Cu2+ in total was added, in agreement with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation stoichiometry. Significantly, the fluorescent turn-off response of ZS2 was specific to Cu2+ with other cations including Ca2+, Cd2+, Cr3+, Fe3+, K+, Na+, Ni2+, Pb2+, Sn4+, Zn2+ and Fe2+ triggering no obvious responses (Fig. S3†). Besides, ZS2 was able to respond to Cu2+ even in the presence of the above mentioned metal ions, implying it could be utilized as a Cu2+-selective fluorescent sensor.
1 complexation stoichiometry. Significantly, the fluorescent turn-off response of ZS2 was specific to Cu2+ with other cations including Ca2+, Cd2+, Cr3+, Fe3+, K+, Na+, Ni2+, Pb2+, Sn4+, Zn2+ and Fe2+ triggering no obvious responses (Fig. S3†). Besides, ZS2 was able to respond to Cu2+ even in the presence of the above mentioned metal ions, implying it could be utilized as a Cu2+-selective fluorescent sensor.
Next, probe ZS2-Cu was examined for its ability to sense S2− with NaHS as an aqueous sulfide source. As expected, ZS2-Cu alone in PBS was almost nonfluorescent (Φ 0.004), while the addition of NaHS triggered an immediate increment in fluorescence intensity. A maximum fluorescence enhancement factor of 19 was achieved at 546 nm when 2.0 eq. of NaHS was added. Interestingly, the fluorescent spectrum of the system taken right after the addition of NaHS could overlap with that taken after an incubation time of 30 minutes, verifying rapid response of the probe. Moreover, both the shape and intensity of the emission band of the system resembled those of ZS2, indicating the de-coordination of Cu2+ from the probe and therefore the release of ZS2 from the complex because of the higher affinity between Cu2+ and S2−. Noteworthy, the fluorescence enhancement of the system was linear to the concentration of NaHS up to 20 μM, with the detection limit to be as low as 250 nM, indicating the great potential of ZS2-Cu to quantify H2S (Fig. 3).
Furthermore, the specificity of ZS2-Cu was evaluated by measuring its response after exposure to various common anions and biothiols in PBS buffer. As shown in Fig. 4, among all the anions tested, including bio-relevant F−, Cl−, Br−, I−, H2PO4−, NO3−, CO32−, ClO−, and sulfur oxyanions such as SO32−, SO42−, S2O32−, etc., no species other than S2− was able to induce a fluorescence enhancement. Moreover, common biothiols such as reduced glutathione (GSH) and L-cysteine (L-cys) which are the major interferents jeopardizing the specificity of reported H2S probes, proved to be innocent in this case. Actually, ZS2-Cu exhibited more than 30-fold selectivity towards sulfide over other anions and biothiols. Based on these preliminary results, we next investigated whether the fluorescent response of ZS2-Cu towards NaHS could be affected by the presence of other anions or biothiols. Much to our delight, ZS2-Cu was still able to respond to NaHS with 15–18 fold fluorescence increasement in the coexistence of other anions or biothiols and the resultant fluorescent profile was pretty much the same as that without the presence of other competitive analytes (Fig. S4†). These results taken together demonstrated the high selectivity and sensitivity of ZS2-Cu for H2S and its feasibility to detect H2S in the complex biological samples.
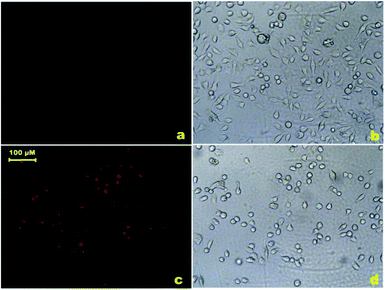
Finally, the ability of ZS-2Cu to visualize H2S in living cells was assessed. HeLa cells were incubated with ZS-2Cu (5 μM) for 15 min and weak fluorescence was observed (Fig. 5a). The medium was then removed and cells were washed with PBS to remove any extracellular probe. The cells were then treated with NaHS (100 μM) for 15 min which resulted in a dramatic increase in intracellular fluorescence (Fig. 5c). These results illustrated the excellent membrane permeability of ZS2-Cu and its ability to detect H2S sensitively in living cells. It is noteworthy that live-cell imaging experiments also demonstrated that ZS2 could act as a dual sensor for both Cu2+ and H2S with the former gave a turn-off response and the latter restoring the strong fluorescent signal (Fig. S5†).
Conclusions
In summary, we have devised a highly specific and sensitive fluorescent probe for the rapid detection of H2S in living cells employing the inorganic chemistry-based principle of precipitating copper with sulfide from the copper-complexing quenched probe and therefore releasing the fluorescent signal of the probe. Representing an appealing tool for H2S analysis in biological samples, this probe exhibited rapid response towards H2S, good water solubility and excellent membrane permeability. Besides, it responded to NaHS with a linear fluorescent enhancement and thus has the potential to be utilized for H2S quantification. Its feasibility to monitor H2S in living cells was also confirmed.Acknowledgements
This work was supported by NSFC (30901858) and the Fundamental Research Funds for the Central Universities (2014FZA7016).Notes and references
- (a) R. Wang, Antioxid. Redox Signaling, 2003, 5, 493 CrossRef CAS PubMed; (b) L. Li and P. K. Moore, Biochem. Soc. Trans., 2007, 35, 1138 CrossRef CAS PubMed; (c) L. Li, P. Rose and P. K. Moore, Annu. Rev. Pharmacol. Toxicol., 2011, 51, 169 CrossRef CAS PubMed.
- K. Qu, S. W. Lee, J. S. Bian, C. M. Low and P. T. H. Wong, Neurochem. Int., 2008, 52, 155 CrossRef CAS PubMed.
- (a) R. Hosoki, N. Matsuki and H. Kimura, Biochem. Biophys. Res. Commun., 1997, 237, 527 CrossRef CAS PubMed; (b) W. Zhao, J. Zhang, Y. Lu and R. Wang, EMBO J., 2001, 20, 6008 CrossRef CAS PubMed; (c) D. J. Elsey, R. C. Fowkes and G. F. Baxter, Cell Biochem. Funct., 2010, 28, 95 CrossRef CAS PubMed.
- K. Eto, T. Asada, K. Arima, T. Makifuchi and H. Kimura, Biochem. Biophys. Res. Commun., 2002, 293, 1485 CrossRef CAS.
- Y. Kaneko, Y. Kimura, H. Kimura and I. Niki, Diabetes, 2006, 55, 1391 CrossRef CAS PubMed.
- G. Yang, L. Wu, B. Jiang, W. Yang, J. Qi, K. Cao, Q. Meng, A. K. Mustafa, W. Mu, S. Zhang, S. H. Snyder and R. Wang, Science, 2008, 322, 587 CrossRef CAS PubMed.
- U. Hannestad, S. Margheri and B. Sörbo, Anal. Biochem., 1989, 178, 394 CrossRef CAS.
- V. S. Lin and C. J. Chang, Curr. Opin. Chem. Biol., 2012, 16, 595 CrossRef CAS PubMed.
- W. Xuan, C. Sheng, Y. Cao, W. He and W. Wang, Angew. Chem., Int. Ed., 2012, 5, 2282 CrossRef PubMed.
- (a) Y. Qian, J. Karpus, O. Kabil, S. Zhang, H. Zhu, R. Banerjee, J. Zhao and C. He, Nat. Commun., 2011, 2, 495 CrossRef PubMed; (b) C. Liu, J. Pan, S. Li, Y. Zhao, L. Y. Wu, C. E. Berkman, A. R. Whorton and M. Xian, Angew. Chem., Int. Ed., 2011, 50, 10327 CrossRef CAS PubMed; (c) C. Liu, B. Peng, S. Li, C. M. Park, A. R. Whorton and M. Xian, Org. Lett., 2012, 14, 2184 CrossRef CAS PubMed.
- (a) A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078 CrossRef CAS PubMed; (b) H. Peng, Y. Cheng, C. Dai, A. L. King, B. L. Predmore, D. J. Lefer and B. Wang, Angew. Chem., Int. Ed., 2011, 50, 9672 CrossRef CAS PubMed; (c) F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 2852 RSC; (d) L. A. Montoya and M. Pluth, Chem. Commun., 2012, 48, 4767 RSC; (e) S. Chen, Z. Chen, W. Ren and H. Ai, J. Am. Chem. Soc., 2012, 134, 9589 CrossRef CAS PubMed; (f) S. K. Das, C. S. Lim, S. Y. Yang, J. H. Han and B. R. Cho, Chem. Commun., 2012, 48, 8395 RSC.
- X. Li, S. Zhang, J. Cao, N. Xie, T. Liu, B. Yang, Q. He and Y. Hu, Chem. Commun., 2013, 49, 8656 RSC.
- (a) K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura and T. Nagano, J. Am. Chem. Soc., 2011, 133, 18003 CrossRef CAS PubMed; (b) F. Hou, L. Huang, P. Xi, J. Cheng, X. Zhao, G. Xie, Y. Shi, F. Cheng, X. Yao, D. Bai and Z. Zeng, Inorg. Chem., 2012, 51, 2454 CrossRef CAS PubMed; (c) X. Qu, C. Li, H. Chen, J. Mack, Z. Guo and Z. Shen, Chem. Commun., 2013, 49, 7510 RSC.
- (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed; (b) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed; (c) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC.
- T. Koike, T. Watanabe, S. Aoki, E. Kimura and M. Shiro, J. Am. Chem. Soc., 1996, 118, 12696 CrossRef CAS.
- T. Rohand, M. Baruah, W. Qin, N. Boens and W. Dehaen, Chem. Commun., 2006, 3, 266 RSC.
- L. Jiao, C. Yu, M. Liu, Y. Wu, K. Cong, T. Meng, Y. Wang and E. Hao, J. Org. Chem., 2010, 75, 6035 CrossRef CAS PubMed.
- K. Kiyose, H. Kojima, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2009, 131, 10077 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for the syntheses of ZS2, ZS2-Cu, NMR traces, biological methods and additional figures. See DOI: 10.1039/c4ra05430j |
| This journal is © The Royal Society of Chemistry 2014 |