Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient optimization and synthesis of diverse azaarenes via nitrogen atom insertion under continuous flow conditions
Sooyeon
Moon
 a,
Julia C.
Reisenbauer
a,
Julia C.
Reisenbauer
 a,
Ann-Sophie K.
Paschke
a,
Ann-Sophie K.
Paschke
 a,
Alessandro
Scotto
a,
Alessandro
Scotto
 a,
Luca
Terraneo
a,
Luca
Terraneo
 a,
Niklas
Brenner
a,
Niklas
Brenner
 a,
Quentin
Lefebvre
a,
Quentin
Lefebvre
 b,
Thomas C.
Fessard
*b and
Bill
Morandi
b,
Thomas C.
Fessard
*b and
Bill
Morandi
 *a
*a
aETH Zürich, Vladimir-Prelog-Weg 3, HCI, 8093 Zürich, Switzerland. E-mail: morandib@ethz.ch
bSpiroChem AG, 4058 Basel, Switzerland. E-mail: thomas.fessard@spirochem.com
First published on 6th October 2025
Abstract
We report the development of a continuous flow approach to nitrogen atom insertion. The setup is modular, enabling the rapid optimization of reaction conditions for a wide range of substrates. The milder reaction conditions led to an improved substrate scope and functional group tolerance compared to batch conditions. The reactions can also be safely scaled up to preparative scale.
In recent years, novel strategies for the modification of molecular core structures through the insertion, deletion, or transmutation of atoms have provided new approaches for structural diversification, which are especially powerful if applied at a late stage.1–7 Given the importance of nitrogen-containing heterocycles in the pharmaceutical and agricultural industry,8,9 various synthetic methods for the formation of azaarenes via the introduction of nitrogen atoms into ring systems have been developed.10–18 Implementing these strategies in medicinal chemistry campaigns to explore a large array of active substances holds great potential. However, successful streamlining of synthesis often requires individual reaction parameter optimization for diverse starting materials in a rapid fashion.19 This aspect is especially important if a translation of the methodology to similar yet distinctly reactive scaffolds is desired, for instance, diversifying azaindoles instead of indoles. Besides the need to rapidly fine-tune reaction conditions, these synthetic methods also need to tolerate sensitive functional groups and be amenable to preparative scales to enable their efficient adaption across various areas.20,21
Merging the synthetic power of skeletal editing strategies with an enabling flow chemistry set-up was motivated by various advantages that a microfluidic approach would offer compared to traditional batch conditions, which are most commonly used in synthetic chemistry.22–26 Previously, our group has focused on the diversification of indoles and indenes as well as related structures.27–30 Under batch conditions, commercially available hypervalent iodine and a nitrogen atom source, such as ammonium carbamate or ammonium chloride, react in an exothermic fashion, an aspect that often complicates scale-up campaigns. Additionally, side reactions due to the exothermicity and the generation of highly reactive intermediates were observed. At the same time, limitations in substrate scope were identified, which make re-optimization campaigns necessary.27–30 This is a concern, as structurally complex, highly valuable advanced intermediates or active pharmaceutical ingredients (APIs) are usually only available in minute quantities through complex synthetic routes. In this context, flow chemistry, particularly when using miniaturized reactors, offers exceptional advantages over batch processes as high control over reaction parameters, efficient mixing, and enhanced heat and mass transfer are ensured.22 In addition, the miniaturization provided by microreactors significantly reduces reagent consumption, minimizes waste, and facilitates rapid screening and optimization of reaction conditions with only small amounts of valuable starting materials.31 Furthermore, conducting a reaction in tubular microreactor systems under continuous flow conditions allows for the generation and immediate consumption of reactive intermediates, providing better control over the reaction and suppressing side reactions.31 Thus, a microfluidic flow chemical approach was envisioned as an efficient optimization tool to also facilitate subsequent scale-up campaigns, thereby dramatically increasing the applicability of previously developed nitrogen atom insertion reactions.
Here we report the development of a continuous flow approach to nitrogen atom insertion. The setup is modular, enabling the rapid optimization of reaction conditions for a wide range of substrates. The milder reaction conditions, including reduced equivalents of reagents compared to the batch process, led to an improved substrate scope and functional group tolerance. The reactions can also be safely scaled up to preparative scale.
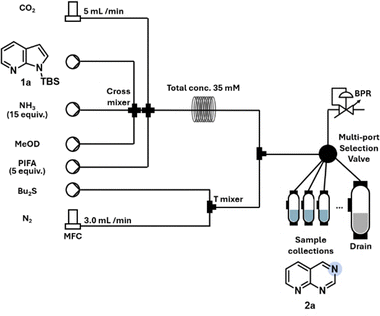
At the outset of this work, we aimed to demonstrate the benefits of flow optimization by developing reaction conditions for medicinally important heterocycles that were hardly converted in batch conditions, such as azaindoles.27 Azaarenes play a pivotal role in medicinal chemistry and material sciences, making efficient synthetic routes to access these essential to further expand the accessible chemical space.8,9 Based on these considerations and the limitations encountered in the batch process,27–29 a continuous flow set-up was tested for this purpose (Table 1). Initially, the direct use of commercially available ammonium carbamate as a nitrogen source was considered. However, due to its low solubility, the direct dosing of solid ammonium carbamate in a uniform fashion in the flow chemistry platform was challenging.32 To prevent solid accumulation, NH3 was introduced into a methanol solution while mixing with CO2 gas, generating ammonium carbamate in situ and creating a Taylor flow.33 The Taylor flow mechanism ensured continuous gas–liquid-solid recirculation, maintaining suspension and controlled reagent mixing. Any solid formed during the reaction was consumed, effectively preventing accumulation.
| Entry | Variation from initial conditions | 1H NMR yield of 2a (%) | 1H NMR conv. of 1a (%) |
|---|---|---|---|
| 1 | None | 33 | 93 |
| 2 | TIPS-7-azaindole | 20 | 57 |
| 3 | TIPS-7-azaindole, 0 °C | 9 | 33 |
| 4 | d 3-MeCN instead of d4-MeOD | <5 | 100 |
| 5 | 3 equiv. PIFA | 29 | 54 |
| 6 | 9 equiv. NH3, 3 equiv. PIFA | 30 | 57 |
| 7 | 9 equiv. NH3, 3 equiv. PIFA, reactor volume: 3 mL | 43 | 79 |
| 8 | 9 equiv. NH3, 3 equiv. PIFA, reactor volume: 7 mL | 55 | 86 |
| 9 | In batch (0.2 mmol scale): 67 mM, 0 °C for 10 min, then rt for 4 h | 12 | 100 |
| 10 | Optimized reaction conditions, 9 equiv. NH3, 3 equiv. PIFA, 3.5 mL min−1 CO2, 3 mL min−1 N2, reactor volume: 17 mL, pressure: 40 psi, temperature: 40 °C, total conc.: 35 mM | 62 | 93 |
We commenced our studies using TBS-7-azaindole (1a) as the model substrate (Table 1). Batch conditions only allowed the identification of trace amounts of the desired product 2a. Initial screening conditions using ammonia in combination with gaseous CO2 and (bis(trifluoroacetoxy)iodo)benzene (PIFA) demonstrated the formation of desired product 2a in deuterated methanol under continuous flow conditions (Table 1). Establishing a triphasic system poses obstacles in both batch as well as continuous flow synthesis. While mass transfer is usually limiting in batch processes, flow chemistry can overcome these challenges by offering the opportunity to precisely control and efficiently mix all reagents. Nevertheless, clogging issues needed to be avoided to ensure the uninterrupted operation of the microfluidic device, which was achieved by adjusting reagent equivalents and the flow rates. This reaction set-up also allowed for the direct preparation of NMR samples for yield determinations. Thus, rapid and efficient monitoring of the reaction was achieved. This implementation drastically improved the optimization workflow as a more time- and resource-efficient optimization was enabled. The concentration of 35 mM was chosen to conserve the limited starting material, while still ensuring a sufficient signal-to-noise (S/N) ratio in the 1H NMR analysis of the crude mixtures. This concentration is not essential for the reaction's efficiency or selectivity, as long as the solubility of the in situ formed ammonium carbamate is considered.
Key to developing a robust and reproducible process was also the incorporation of a thioether, dibutyl sulfide, to quench reactive oxidative species.34 Further evaluation of the reaction parameters revealed that TBS-protected indoles outperformed TIPS-protected indoles (entries 2 and 3). High equivalents of NH3 and PIFA resulted in low yields with significant byproduct formation (entries 5–7). To increase conversion while using lower equivalents of NH3 (9 equiv.) and PIFA (3 equiv.), a longer reaction time was required. Consequently, the reaction conditions were optimized by utilizing a larger reactor volume (17 mL) and higher pressure (40 psi). Unlike the batch reaction, which required cooling to control reactive intermediates, the microfluidic setup achieved optimal performance at 40 °C (entry 10).
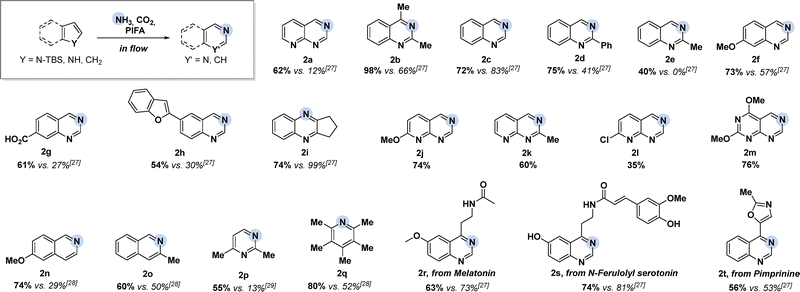
After the successful identification of optimized reaction parameters for the ring expansion of TBS-7-azaindole (1a) to azaarene 2a, we wanted to evaluate other potential substrates (Scheme 1). Using this approach allowed for rapid and substrate-specific optimization of indoles 1b-i. In all cases, comparable or superior reaction yields were obtained compared to the batch reaction based on 1H NMR analysis of the crude reaction mixture.
Drastically improved yields for benzofuran-substituted quinazoline 2h were achieved, as side reactions could be successfully suppressed. It was also demonstrated that the corresponding quinoxaline 2i could be obtained after rapid optimization of the reaction conditions for 2,3-bridged TBS-indoles. The developed reaction set-up was also shown to successfully transform various azaindoles 1j-l into the corresponding ring-expanded products 2j–2l in decent to high yields. Presumably, high control and precise modification of reagent dosing allowed us to overcome these limitations in batch. Pyrimido[4,5-d]pyrimidines 2m are typically synthesized through multistep processes that require specific functional groups to construct the pyrimido[4,5-d]pyrimidine ring systems, often resulting in low yields and multi-step procedures.35 In contrast, the method presented here simplifies the synthesis, achieving higher yields in a single step under milder conditions, offering a more efficient and scalable alternative. The flow conditions could also be further optimized to enable the direct nitrogen atom insertion into indenes to access isoquinolines 2n and 2o in comparable or higher yields compared to the previously established batch procedure.28 Translation to TBS-pyrroles and cyclopentadienes was successfully achieved under the established triphasic continuous flow system yielding product 2p and 2q in 55% and 80% 1H NMR yield, respectively. Together these results show that rapid optimization and fine-tuning of reaction parameters under flow conditions streamlines the method development for a wide range of electronically diverse substrates. We could further demonstrate the robustness of the method by facilitating the late-stage nitrogen atom insertion into pharmaceutically relevant substrates, showcasing the method's tolerance to a diverse range of polar functional groups, such as amides, alcohols, and oxazoles. In this context, quinazolines derived from melatonin (2r), N-ferulolyl serotonin (2s) and pimprinine (2t) were obtained in 63%, 74%, and 56% 1H NMR yield, respectively, through implementing an operationally simple in-line TBS deprotection step to access 2s (see SI for detail).
One significant advantage of continuous flow reactions compared to batch is the inherent scalability.22 To demonstrate this, TBS-indole 1f was transformed into the corresponding quinazoline 2f in an 8-mmol scale reaction (Scheme 2).
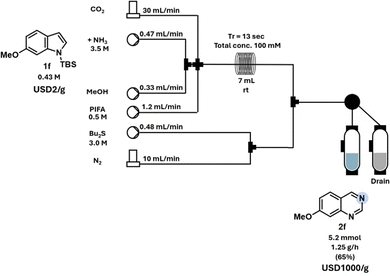 | ||
| Scheme 2 Scale-up (8 mmol) of the nitrogen atom insertion reaction of 1-(tert-butyldimethylsilyl)-6-methoxy-1H-indole (1f). | ||
The scale-up reaction was conducted at room temperature with a higher concentration to minimize solvent usage. The optimized reaction parameters facilitated the production of 1.25 g h−1 of the final desired product 2f. Precise control over mixing and reagent ratios allowed us to perform the reaction under ambient conditions.
In conclusion, the adoption of a flow chemistry process for the synthesis of diverse azaarenes through the nitrogen atom insertion process was achieved. Translation of the initial reaction conditions to various substrate families was accomplished through the implementation of a rapid optimization workflow, ensuring reproducibility and the minimal usage of starting material. At the same time, precise control of reaction parameters overcomes the reproducibility challenges that are usually associated with triphasic reaction systems on both analytical and preparative scales.
We acknowledge funding from Innosuisse (48934.1 IP-LS – Fast-track optimization platform to accelerate drug discovery). ASKP thanks the FCI for a scholarship. We thank the Molecular and Biomolecular Analysis Service (MoBiAS), the X-ray structure service (SMoCC), and the LOC NMR Service at ETH Zürich for technical assistance. We further thank the whole Morandi group for fruitful discussions and critical proofreading of the manuscript.
Conflicts of interest
The authors declare the following competing financial interest(s): Q. L. is an employee, and T. C. F. is CEO of SpiroChem AG, a Innovative Contract Research Organization (iCRO) commercializing products and services.Data availability
The data supporting this article have been included as part of the supplementary information (SI). See DOI: https://doi.org/10.1039/d5cc03194j.Notes and references
- J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong and M. D. Levin, Nat. Synth., 2022, 1, 352–364 CrossRef CAS PubMed.
- B. W. Joynson and L. T. Ball, Helv. Chim. Acta, 2023, 106, e202200182 CrossRef CAS.
- F. Liu, L. Anand and M. Szostak, Chem. – Eur. J., 2023, 29, e202300096 CrossRef CAS PubMed.
- R. Sharma, M. Arisawa, S. Takizawa and M. S. H. Salem, Org. Chem. Front., 2025, 12, 1633–1670 RSC.
- X. Li, J. Xu and Z.-G. Xu, Org. Chem. Front., 2024, 11, 4041–4053 RSC.
- J. Woo, C. Stein, A. H. Christian and M. D. Levin, Nature, 2023, 623, 77–82 CrossRef CAS PubMed.
- N. Kotwal and P. Chauhan, Angew. Chem., Int. Ed., 2024, 63, e202317228 CrossRef CAS PubMed.
- L. D. Pennington and D. T. Moustakas, J. Med. Chem., 2017, 60, 3552–3579 CrossRef CAS PubMed.
- C. M. Marshall, J. G. Federice, C. N. Bell, P. B. Cox and J. T. Njardarson, J. Med. Chem., 2024, 67, 11622–11655 CrossRef CAS PubMed.
- H. Inui, K. Sawada, S. Oishi, K. Ushida and R. J. McMahon, J. Am. Chem. Soc., 2013, 135, 10246–10249 CrossRef CAS PubMed.
- N. P. Gritsan, I. Likhotvorik, M.-L. Tsao, N. Çelebi, M. S. Platz, W. L. Karney, C. R. Kemnitz and W. T. Borden, J. Am. Chem. Soc., 2001, 123, 1425–1433 CrossRef CAS.
- S. Liu and X. Cheng, Nat. Commun., 2022, 13, 425 CrossRef CAS PubMed.
- P. Q. Kelly, A. S. Filatov and M. D. Levin, Angew. Chem., Int. Ed., 2022, 61, e202213041 CrossRef CAS PubMed.
- J. Wang, H. Lu, Y. He, C. Jing and H. Wei, J. Am. Chem. Soc., 2022, 144, 22433–22439 CrossRef CAS PubMed.
- E. Boudry, F. Bourdreux, J. Marrot, X. Moreau and C. Ghiazza, J. Am. Chem. Soc., 2024, 146, 2845–2854 CrossRef CAS PubMed.
- Y. He, J. Wang, T. Zhu, Z. Zheng and H. Wei, Chem. Sci., 2024, 15, 2612–2617 RSC.
- B.-S. Zhang, S. L. Homölle, T. Bauch, J. C. A. Oliveira, S. Warratz, B. Yuan, X.-Y. Gou and L. Ackermann, Angew. Chem., Int. Ed., 2024, 63, e202407384 CrossRef CAS PubMed.
- B. Ghosh, P. Kafle, R. Mukherjee, R. Welles, D. Herndon, K. M. Nicholas, Y. Shao and I. Sharma, Science, 2025, 387, 102–107 CrossRef CAS PubMed.
- A. R. Bogdan and A. W. Dombrowski, J. Med. Chem., 2019, 62, 6422–6468 CrossRef CAS PubMed.
- J. Hughes, S. Rees, S. Kalindjian and K. Philpott, Br. J. Pharmacol., 2011, 162, 1239–1249 CrossRef CAS PubMed.
- L. Zhang and T. Ritter, J. Am. Chem. Soc., 2022, 144, 2399–2414 CrossRef CAS PubMed.
- M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef CAS PubMed.
- J. Luo, Q. Zhou, Z. Xu, K. N. Houk and K. Zheng, J. Am. Chem. Soc., 2024, 146, 21389–21400 CrossRef PubMed.
- G. S. Fleming and A. B. Beeler, Org. Lett., 2017, 19, 5268–5271 CrossRef CAS PubMed.
- B. A. Wright, A. Matviitsuk, M. J. Black, P. García-Reynaga, L. E. Hanna, A. T. Herrmann, M. K. Ameriks, R. Sarpong and T. P. Lebold, J. Am. Chem. Soc., 2023, 145, 10960–10966 CrossRef CAS PubMed.
- A. Gupta, P. Bhatti, J. K. Laha and S. Manna, Chem. – Eur. J., 2024, 30, e202401993 CrossRef CAS PubMed.
- J. C. Reisenbauer, O. Green, A. Franchino, P. Finkelstein and B. Morandi, Science, 2022, 377, 1104–1109 CrossRef CAS PubMed.
- P. Finkelstein, J. C. Reisenbauer, B. B. Botlik, O. Green, A. Florin and B. Morandi, Chem. Sci., 2023, 14, 2954–2959 RSC.
- J. C. Reisenbauer, A.-S. K. Paschke, J. Krizic, B. B. Botlik, P. Finkelstein and B. Morandi, Org. Lett., 2023, 25, 8419–8423 CrossRef CAS PubMed.
- B. B. Botlik, M. Weber, F. Ruepp, K. Kawanaka, P. Finkelstein and B. Morandi, Angew. Chem., Int. Ed., 2024, 63, e202408230 CrossRef CAS PubMed.
- R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502–7519 Search PubMed.
- H. L. D. Hayes and C. J. Mallia, Org. Proc. Res. Dev., 2024, 28, 1327–1354 CrossRef CAS.
- D. S. Hanson, Y. Wang, X. Zhou, E. Washburn, M. B. Ekmekci, D. Dennis, A. Paripati, D. Xiao and M. Zhou, Inorg. Chem., 2021, 60, 5573–5589 CrossRef CAS PubMed.
- L. Degennaro, A. Tota, S. De Angelis, M. Andresini, C. Cardellicchio, M. A. Capozzi, G. Romanazzi and R. Luisi, Eur. J. Org. Chem., 2017, 6486–6490 CrossRef CAS.
- M. Monier, D. Abdel-Latif, A. El-Mekabaty and K. M. Elattar, RSC Adv., 2019, 9, 30835–30867 RSC.
| This journal is © The Royal Society of Chemistry 2025 |