Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Glycan-induced fluorescence enhancement using a molecular rotor–boronic acid conjugate
Mandana
Oloub
 a,
Alen
Koçak
a,
Alen
Koçak
 a,
Cihan
Baydaroğlu
b,
Amal K.
Homer
a,
Florian
Telschow
c,
Victor M.
Loyo-Cruz
a,
Cihan
Baydaroğlu
b,
Amal K.
Homer
a,
Florian
Telschow
c,
Victor M.
Loyo-Cruz
 c,
Sebastian
Trunschke
a,
Gaël M.
Vos
c,
Sebastian
Trunschke
a,
Gaël M.
Vos
 d,
Kevin
Pagel
d,
Kevin
Pagel
 d,
Michael
Gradzielski
d,
Michael
Gradzielski
 b,
Ulrike
Alexiev
b,
Ulrike
Alexiev
 c and
Oliver
Seitz
c and
Oliver
Seitz
 *a
*a
aInstitute of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, 12489 Berlin, Germany. E-mail: oliver.seitz@chemie.hu-berlin.de
bInstitute of Chemistry, Technische Universität Berlin, Strasse des 17. Juni 124, 10623 Berlin, Germany
cInstitute of Physics, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany
dInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Altensteinstrasse 23A, 14195 Berlin, Germany
First published on 8th August 2025
Abstract
Fluorescent molecular rotors (FMRs) are known for their sensitivity to changes in viscosity and confinement. Here, we show that a boronic acid conjugate of the rotor dye CCVJ exhibits strong enhancements in fluorescence upon binding to complex glycans. Glycan-induced fluorescence enhancement (GIFE) operates at low and high viscosity.
Fluorescent molecular rotors (FMRs) are a special class of dyes that respond to their microenvironment, in particular viscosity and steric confinement.1–3 In their excited state, FMRs undergo twisting motions around a bond connecting two π-systems. Excited FMRs will return to the ground state by non-radiative relaxation, unless constraints hinder twisting, resulting in fluorescence enhancement. This property has led to the development of fluorescent probes that report the confinement resulting from biomolecular interactions with proteins through an effect referred to as protein-induced fluorescence enhancement (PIFE).4–6 The concept has been further extended to nucleic acid-induced fluorescence enhancement (NAIFE, and related approaches).7–9 PIFE has been used to study protein–nucleic acid interactions, providing insights into binding affinities, kinetics, and conformational changes.10 Other applications include imaging of proteins in living cells and monitoring of drug–protein interactions.11,12
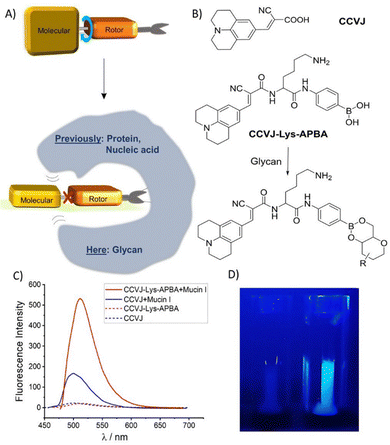
During our work with boronic acid-modified fluorescent probes, we realized that FMR dyes could also exhibit enhanced fluorescence when brought into close proximity of glycans. Sugars are kosmotropic and as such bind water tightly. The large hydration shells could help to reduce the degrees of rotational freedom available to a glycan-bound FMR. Notably, glycan-induced fluorescence enhancement (GIFE) has not been previously reported. We envisioned that GIFE could be useful for rapidly measuring the integrity of glycan-based materials, which can suffer from degradation upon exposure to chemicals, heat or (microbial) enzymes. Here, we investigate the fluorescence response of an archetypic molecular rotor, 9-(2-carboxy-2-cyanovinyl) julolidine (CCVJ).2,13 We show that a CCVJ-boronic acid conjugate exhibits a fluorogenic response upon interaction with polysaccharides and glycoproteins.
The interaction of boronic acids with cis-1,2- or 1,3-diols in carbohydrates is the basis of a number of glycan sensors14–16 and was used here to bring the rotor dye into the glycan's vicinity and explore the feasibility of GIFE (Fig. 1A). The FMR dye CCVJ was conjugated with aminophenylboronic acid (APBA) through a lysine tether (Fig. 1B and Scheme S2 for synthesis). A comparison with a glycine derivative showed that the free amino group of lysine was required to increase the solubility in aqueous buffer (Fig. S1). As expected, a 10 μM solution of CCVJ-Lys-APBA in Tris buffer showed only weak fluorescence (Fig. 1C). However, addition of 2% Mucin I (high molecular weight glycoprotein from bovine submaxillary glands rich in O-glycosidically bound oligosaccharides containing N-acetylgalactosamine, N-acetylglucosamine, fucose, galactose, and sialic acid, Fig. S2) induced a strong enhancement of fluorescence (Fig. 1C), which was visible by the naked eye (Fig. 1D). At the emission maximum, intensity was increased by a factor of 25. A 2% suspension of Mucin I has higher viscosity than the Tris buffer. Therefore, viscosity-induced increases of CCVJ emission are to be expected. To distinguish between a viscosity- and a confinement-induced fluorescence response, we measured CCVJ lacking the boronic acid module. In this case, the fluorescence increase was weaker (i.e. 7.5-fold). Therefore, the particularly strong enhancement of fluorescence from CCVJ-Lys-APBA suggests that interactions of the boronic acid with sugar hydroxyl groups play an important role.
It is interesting to note that the fluorescence of CCVJ-Lys-APBA responded strongly to Mucin I despite the presence of 1 M Tris. The triol compound Tris has been reported to act as a competitor that shields boronic acids from low affinity interactions with protein side chains but can be displaced by glycans offering cis-1,2- or 1,3-diols.17,18 In fact, a Tris adduct (most likely a boronic-Tris ester) was detected via MS analysis (Fig. S3). The fact that stronger fluorescence increases were observed when CCVJ-Lys-APBA and Mucin I interacted in the absence of competing Tris (Fig. S4) can be seen as an indication that the boronic acid is required to localize the CCVJ fluorophore to an environment that restricts degrees of rotational freedom. For further analysis, stained Mucin I pellets obtained after centrifugation were washed and resuspended in PBS at pH 7.4. The remaining fluorescence confirmed that CCVJ-Lys-APBA binds Mucin I whereas the boronate-free CCVJ can be washed away (Fig. S5B).
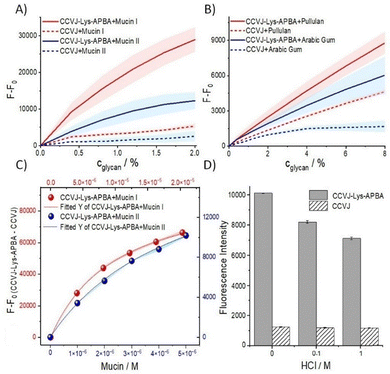
Next, we evaluated the fluorescence response in titration experiments. The fluorescence enhancement was found to increase in correlation with the incremental rise in the concentration of Mucin I (Fig. 2A). This increase in fluorescence was weak for CCVJ. The spread between the fluorescence responses provided by CCVJ-Lys-APBA and CCVJ was higher when measurements were performed in PBS instead of 1 M Tris buffer (Fig. S6A). During titrations with Mucin II (large, highly O-glycosylated glycoprotein from porcine stomach), CCVJ-Lys-APBA exhibited, again, a higher degree of fluorescence enhancement compared to the non-conjugated CCVJ (Fig. 2A). With human milk mucin, even small amounts of this high molecular weight glycoprotein were found to induce substantial increases in the fluorescence of CCVJ-Lys-APBA (Fig. S7). The titration data were used to plot saturation binding curves (Fig. 2C and Fig. S8). The viscosity response of the FMR in CCVJ-Lys-APBA was removed by subtracting the “CCVJ-only” signal. CCVJ-Lys-APBA was found to bind the highly multivalent Mucin I and II with low micromolar affinity (apparent Kd = 10 μM and 5 μM, respectively), which is potentially useful for an application in assays. Given the varied uses of glycan-based materials (e.g. in drug delivery systems, tissue engineering and wound healing), we hypothesized that the fluorescence response could provide information about the integrity of glycan chains, which are essential for their functional properties. To investigate the impact of glycan degradation, Mucin II was subjected to acidic conditions. Probes were added after the pH was adjusted to 7.4. The fluorescence intensity of CCVJ remained unchanged, indicating that viscosity was not altered. However, the fluorescence of CCVJ-Lys-APBA decreased when the mucin was treated with acid (Fig. 2D), which suggests that glycans were partially cleaved.
We tested whether the fluorescence response of CCVJ-Lys-APBA also extends to other classes of polysaccharides/glycoproteins that have little similarity with mammalian mucins. Pullulan is a polysaccharide composed of α1,6-linked maltotriose units (Fig. S9A) used for example for food packaging and cosmetics. Arabic gum is a tree gum, also used in foods, which is composed of a mixture of glycoproteins and polysaccharides rich in arabinogalactans (Fig. S9B). Compared to the boronic acid-free CCVJ, CCVJ-Lys-APBA exhibited significantly stronger fluorescence enhancements with both pullulan and Arabic gum (Fig. 2B). The fluorescence response was, again, higher in the PBS buffer (Fig. S6B).
The observed enhancement in the fluorescence of CCVJ-Lys-APBA could potentially be ascribed to aggregation-induced emission (AIE) when the fluorophores stack upon binding to adjacent positions of the glycan.19 In such a case, a red-shifted fluorescence emission would be expected upon addition of glycans.20 However, this was not observed. The absorption spectra of the CCVJ probes were also found not to change in the presence of glycan (Fig. S10), supporting the conclusion that an AIE effect is unlikely. Absorbance of CCVJ and CCVJ-Lys-APBA at 437 and 463 nm, respectively (Fig. S11) does not change upon addition of Arabic gum or pullulan (which, in contrast to the mucins samples, do not contribute to the absorbance at this wavelength), ruling out the most trivial explanation i.e. a glycan-induced solubilization. According to an alternative explanation, the boronic acid functional group could act as an internal quencher of fluorescence. Yet, fluorescence quantum yields of CCVJ and CCVJ-Lys-APBA in the buffer are similar indicating that the boronic acid module is not quenching fluorescence (Fig. S12). In further control experiments, we investigated whether the boronic acid functionality in CCVJ-Lys-APBA could be altering the viscosity of the glycan solutions. Measurements of the shear rate-dependent viscosity of mucins were performed by using a Couette rheometer. No discernible differences were observed between solutions containing CCVJ-Lys-APBA and those containing the boronic acid-free CCVJ (Fig. S13). With pullulan and Arabic gum available in abundance, further viscosity measurements were performed by using both a Couette rheometer and a capillary viscosimeter. Both, shear-rate dependent viscosity (Fig. S14) and the capillary measurements (Fig. S15 and Table S1) showed little difference between the two probes, both in Tris and PBS buffer. We conclude that the high degree of fluorescence enhancement observed with CCVJ-Lys-APBA cannot be attributed to viscosity increases. Instead, we consider it more likely that GIFE of CCVJ-Lys-APBA is caused by a steric confinement upon binding to the clustered glycan structures. The differences in fluorescence response could be due to structural properties of the glycans.
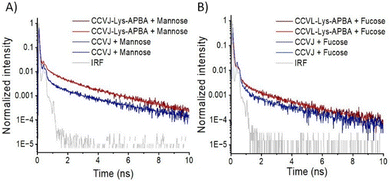
To examine the possibility of sugar-specific fluorescence responses, CCVJ or CCVJ-Lys-APBA were allowed to interact with the saccharides glucose (Glc), mannose (Man), fucose (Fuc), galactose (Gal), N-acetylgalactosamine (GalNAc), and fructose (Fru) at 2 M concentration. MS analysis was performed for representative examples and provided evidence for the formation of the respective boronic acid esters (Fig. S16). The fluorescence intensities were measured and fluorescence lifetime signatures were assessed by time-correlated single photon counting (TCSPC). In buffered solutions of fructose, GalNAc, and glucose there was little difference between the fluorescence properties of CCVJ and CCVJ-Lys-APBA (Fig. S17 and S18). A clear difference in fluorescence decay was observed in solutions of mannose and fucose (Fig. 3). Steady state measurements also showed a small, yet significant increase in fluorescence from CCVJ-Lys-APBA compared to CCVJ (Fig. S17). A four-component fit was necessary to adequately describe the decays (Table 1). Due to the limitations given the instrument's response function, the fastest component (τ1 = 1–17 ps) was neglected in the analysis. The remaining decay processes were classified as fast (τ2 = 0.05 ns), medium (τ3 = 0.59–0.77 ns) and slow (τ4 = 2.93–3.31 ns). There was no difference between CCVJ and CCVJ-Lys-APBA in the fast component except that the population of molecules undergoing this decay process (i.e. values in brackets in Table 1) was slightly reduced for CCVJ-Lys-APBA, particularly on mannose. This sugar caused notable differences in the medium decay process. For CCVJ-Lys-APBA, the population of molecules that decayed in this manner was 4-fold higher than for CCVJ, and the lifetime was prolonged (τ3 = 0.77 ns vs. 0.69 ns). Lifetimes in this range have been reported for CCVJ conjugated with antibodies.6 We hypothesize that this process reflects the decay through partially restricted intramolecular rotations. The longest lifetime components appear surprisingly long. We attribute these components to intrinsic features/contaminations of the mannose solution, as the autofluorescence and the dye decay show similar decay characteristics after 1.5 ns (Fig. S19).
| 2 M D-mannoseb | 2 M L-fucoseb | |
|---|---|---|
| a Measured by time-correlated photon counting, λex = 460 nm, 1–2 ps pulse width, instrument response function <38 ps. b All solutions in 1 M Tris, pH 7.4, 25 °C. c Values in brackets give the relative amplitude of the decay component. | ||
| Fast, τ2/ns (a)c | ||
| CCVJ-Lys-APBA | 0.05 ± 0.01 (93.4 ± 1.0) | 0.05 ± 0.01 (98.3 ± 0.2) |
| CCVJ | 0.05 ± 0.01 (98.4 ± 0.3) | 0.05 ± 0.01 (99.4 ± 0.2) |
| Medium, τ3/ns (a)c | ||
| CCVJ-Lys-APBA | 0.77 ± 0.04 (4.5 ± 0.7) | 0.66 ± 0.08 (1.1 ± 0.2) |
| CCVJ | 0.69 ± 0.03 (1.1 ± 0.1) | 0.59 ± 0.10 (0.4 ± 0.1) |
| Slow, τ4/ns (a)c | ||
| CCVJ-Lys-APBA | 2.94 ± 0.06 (2.1 ± 0.4) | 2.98 ± 0.10 (0.5 ± 0.1) |
| CCVJ | 3.31 ± 0.04 (0.5 ± 0.1) | 2.93 ± 0.12 (0.2 ± 0.1) |
In previous work, p-aminoacylphenylboronic acid was found to bind sugars with associations constants KA = 101–103 M−1 at pH 7.4.21 Therefore, association between the boronic acid conjugate and the diol structures should be quantitative at concentrations larger than 1 M sugar. Accordingly, any differences in the boronic acid probe's affinity for specific sugar structures are likely to be negligible at 2 M sugar. It should be considered that highly concentrated monosaccharides not only interact with water but also with other sugar molecules. While the structure of the resulting supramolecular complexes will be ill-defined, it is plausible to assume that this can influence the rotational degrees of freedom available to the sugar-bound CCVJ dye to different degrees. In control experiments, we ruled out that the boronic acid in CCVJ-Lys-APBA is altering the rheological properties of the sugar solutions (Table S1). We infer that structural features of a monosaccharide such as fucose can alter the fluorescence properties of CCVJ-Lys-APBA by increasing the likelihood of the rotor localizing to crowded environments formed by sugar–sugar interactions in aggregates.
In conclusion, we propose glycan-induced fluorescence enhancement (GIFE) as a novel glycan sensing paradigm that operates via a mechanism distinct from that of previously reported fluorescence glycan sensors14–16,22 based on boronic acids. The julolidine fluorophore in CCVJ-Lys-APBA is an archetypical FMR dye that responds not only to increases in viscosity but also to confinement,2,6,13,23 when its twisting motions are restricted by specific interactions with proteins,24 nucleic acids,25 membranes,26 and, according to our findings, with glycans. Considering a report on wash-free glycocalyx imaging upon conjugation with cell surface sialic acids,27 we anticipate that GIFE could be extended to other FMR dyes such as Cy3 or thiazole orange (TO), which emit brighter fluorescence. We believe that the detection of monosaccharides using FMR-Lys-APBA probes may be challenging. This is due to the low affinity of the probes and, perhaps more importantly, because interactions between monosaccharides at high concentrations are required to cause crowding in the FMR microenvironment. However, the glycan structures of naturally occurring glycoconjugates (in glycoproteins, peptidoglycans, polysaccharides) are more complex, multivalent, often branched and characterized by large hydration shells, resulting in dissociation constants in the lower micromolar range. Our data shows that FMR-based glycan sensing is feasible in such cases and can be used, for example, to monitor glycan degradation/remodelling. Further improvements to GIFE-based glycan sensing are anticipated with brighter FMR dyes and by integrating recognition units that offer increased sugar binding affinity.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information. Supplementary information: procedures used for synthesis, characterization data and measurement protocols. See DOI: https://doi.org/10.1039/d5cc02827b.Notes and references
- M. Paez-Perez and M. K. Kuimova, Angew. Chem., Int. Ed., 2024, 63, e202311233 CrossRef CAS PubMed.
- M. A. Haidekker and E. A. Theodorakis, Org. Biomol. Chem., 2007, 5, 1669–1678 RSC.
- S. C. Lee, J. Heo, H. C. Woo, J. A. Lee, Y. H. Seo, C. L. Lee, S. Kim and O. P. Kwon, Chem. – Eur. J., 2018, 24, 13706–13718 CrossRef CAS PubMed.
- E. Ploetz, B. Ambrose, A. Barth, R. Börner, F. Erichson, A. N. Kapanidis, H. D. Kim, M. Levitus, T. M. Lohman and A. Mazumder, Methods Appl. Fluoresc., 2023, 12, 012001 CrossRef PubMed.
- S. Myong, S. Cui, P. V. Cornish, A. Kirchhofer, M. U. Gack, J. U. Jung, K.-P. Hopfner and T. Ha, Science, 2009, 323, 1070–1074 CrossRef CAS PubMed.
- T. Iwaki, C. Torigoe, M. Noji and M. Nakanishi, Biochemistry, 1993, 32, 7589–7592 CrossRef CAS PubMed.
- F. D. Steffen, R. K. Sigel and R. Börner, Phys. Chem. Chem. Phys., 2016, 18, 29045–29055 RSC.
- M. J. Morten, S. G. Lopez, I. E. Steinmark, A. Rafferty and S. W. Magennis, Nucleic Acids Res., 2018, 46, 11618–11626 CrossRef CAS PubMed.
- A. Feklistov, B. Bae, J. Hauver, A. Lass-Napiorkowska, M. Kalesse, F. Glaus, K.-H. Altmann, T. Heyduk, R. Landick and S. A. Darst, Science, 2017, 356, 863–866 CrossRef CAS PubMed.
- H. Hwang and S. Myong, Chem. Soc. Rev., 2014, 43, 1221–1229 RSC.
- S. Tang, E. H. G. Zadeh, B. Kim, N. T. Toomey, M. V. Bondar and K. D. Belfield, Org. Biomol. Chem., 2017, 15, 6511–6519 RSC.
- V. Kumar, P. K. C. Lakshman, T. K. Prasad, K. Manjunath, S. Bairy, A. S. Vasu, B. Ganavi, S. Jasti and N. Kamariah, Heliyon, 2024, 10, e23864 CrossRef CAS PubMed.
- S. Sawada, T. Iio, Y. Hayashi and S. Takahashi, Anal. Biochem., 1992, 204, 110–117 CrossRef CAS PubMed.
- S. Tommasone, F. Allabush, Y. K. Tagger, J. Norman, M. Köpf, J. H. Tucker and P. M. Mendes, Chem. Soc. Rev., 2019, 48, 5488–5505 RSC.
- N. A. Siddiqui, N. Billa and C. J. Roberts, J. Biomater. Sci., Polym. Ed., 2017, 28, 781–793 CrossRef CAS PubMed.
- X. Huang, Y. Han, J. Li, M. Tang and G. Qing, Anal. Bioanal. Chem., 2023, 415, 4061–4077 CrossRef CAS PubMed.
- J. M. De Guzman, S. A. Soper and R. L. McCarley, Anal. Chem., 2010, 82, 8970–8977 CrossRef PubMed.
- Y. Li, E. L. Larsson, H. Jungvid, I. Y. Galaev and B. Mattiasson, J. Chromatogr. A, 2001, 909, 137–145 CrossRef CAS PubMed.
- M. Oloub, R. Hosseinzadeh, M. Tajbakhsh and M. Mohadjerani, RSC Adv., 2022, 12, 26201–26205 RSC.
- T. Mori, H. Komatsu, N. Sakamoto, K. Suzuki, J. P. Hill, M. Matsumoto, H. Sakai, K. Ariga and W. Nakanishi, Phys. Chem. Chem. Phys., 2018, 20, 3073–3078 RSC.
- W. L. Brooks, C. C. Deng and B. S. Sumerlin, ACS Omega, 2018, 3, 17863–17870 CrossRef CAS PubMed.
- I. B. Sivaev and V. I. Bregadze, Boron-Based Compounds: Potential and Emerging Applications in Medicine, 2018, pp. 174–204 Search PubMed.
- N. Chakraborty, A. Silswal and A. L. Koner, Sens. Diagn., 2024, 3, 585–598 RSC.
- W.-T. Yu, T.-W. Wu, C.-L. Huang, I.-C. Chen and K.-T. Tan, Chem. Sci., 2016, 7, 301–307 RSC.
- T.-K. Kha, Q. Shi, N. Pandya and R.-Y. Zhu, Chem. Sci., 2024, 15, 5009–5018 RSC.
- Q. Xu, T. Zhao and Z. Sun, Analyst, 2016, 141, 4676–4684 RSC.
- A. Koçak, A. K. Homer, A. Feida, F. Telschow, J. L. G. López, C. Baydaroğlu, M. Gradzielski, C. P. Hackenberger, U. Alexiev and O. Seitz, Chem. Commun., 2024, 60, 4785–4788 RSC.
| This journal is © The Royal Society of Chemistry 2025 |