Robust, antibacterial, and fluorescent hybrid films mimicking nacre†
Wentao Haoa,
Xiaomin Wanga,
Songyan Dinga,
Yanyan Caob,
Hongbin Zhangb and
Wen Yang*a
aDepartment of Polymer Materials and Engineering, School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei, Anhui 230009, P. R. China. E-mail: wenyang@hfut.edu.cn
bDepartment of Pharmaceutical Engineering, School of Biomedical Engineering, Hefei University of Technology, Hefei, Anhui 230009, P. R. China
First published on 8th October 2015
Abstract
The preparation of multifunctional nacre-like hybrid films is reported in this article. The copper ions (Cu2+) were introduced into the hybrid films assembled from fluorescent hyperbranched poly(amido amine) (HPAMAM) and clay nanosheets. The “brick and mortar” structure of the hybrid films was proved by the SEM observation and SAXD detection. Due to the strong interactions among the Cu2+ ions and the HPAMAM molecules, the obtained hybrid films showed enormously improved ultimate strength. At the same time, the elongation at break of the Cu2+ modified hybrid films was more than that of the pristine one too. Furthermore, the Cu2+ ions modified hybrid films showed excellent inhibition on the bacterial growth due to the good antibacterial property of the Cu2+. Under UV irradiation, the hybrid films modified with Cu2+ could also emit strong blue fluorescence. We wish this multifunctional nacre-like hybrid film could provide a promising material for biological applications.
1. Introduction
Multifunctional materials are highly desirable in biological research, materials science and engineering.1,2 Very interestingly, it is shown that biomimicking is an effective way in fabrication of multifunctional materials.3–6 For example, conductive or electromagnetic shielding nacre-like composites could be obtained through assembling graphene oxide and poly(vinyl alcohol);7,8 nacre-like composites with light-emitting property could also be fabricated through integrating luminescent inorganic nanoplatelets with polymers, or by assembling fluorescent polymers with clay nanosheets.9–11 However, the two-component systems cannot always meet the increasing demands for multiple functions.Integration of functional nanoparticles into the nacre-like composites is an effective way to acquire multiple functions.12 Through incorporating silver nanoparticles, the artificial nacres were endowed antibacterial property.13,14 After embedding gold nanoparticles or quantum dots inside, the solely high strength nacre-like composites were transferred into catalytic materials or optically tuneable materials.15,16 Nevertheless, after integration of inorganic nanoparticles strength of the obtained artificial nacres may be weakened.15
Incorporation of metal ions can be another effective way in fabricating multifunctional nacre-like composites. The copper ions (Cu2+) are known for their strong ability in inhibiting the growth of bacteria.17–21 More interestingly, it was shown that the metal ions, including Cu2+, could stimulate cell proliferation.22–30 Bio-glass composites integrating Cu2+ have been widely investigated.28–30 Beside of their excellent bioactivity, the metal ions can improve the strength of the nacre-like composites too.31–34 Kotov et al. showed that the tensile strength of artificial nacre could be improved 300% higher after integration of Fe3+ ions.32 The graphene oxide films treated with Ca2+ or Mg2+ showed remarkably improved mechanical stiffness (10–200%) and fracture strength (>50%).33 Therefore, metal ions modification on the nacre-like composites is profitable to construct multifunctional and robust advanced materials. However, the great potential of metal ions in fabrication of multifunctional nacre-like composites has not been fully revealed in the present works.
Here in this report, we prepared a kind of Cu2+ modified hybrid films, which were fabricated from hyperbranched poly(amido amine) (HPAMAM), clay nanosheets and copper ions. The obtained hybrid films were auto-fluorescent and optically transparent. They were much stronger than the pristine samples, which could be attributed to the strong chelation between the HPAMAMs and the Cu2+ ions.35–38 Moreover, the obtained films showed excellent inhibition to the growth of bacteria. Due to the good biocompatibility of HPAMAMs,39–43 this kind of hybrid films is of great potential as bioactive functional material to be applied widely in biological researches.
2. Experimental
2.1 Materials
N,N′-Methylene bisacrylamide (MBA, 97%) and 1-(2-aminoethyl) piperazine (AEPZ, 99%) were purchased from Aladdin Industrial Corporation. CuSO4·5H2O, methanol (≥99.5%) and acetone (≥99.5%) were purchased from Sinopharm chemical reagent Co., Ltd. Clay nanolayers (ADDEZ 128) were purchased from Hangzhou Xihe Chemicals Ltd. Diameter of the clay nanosheets is ∼23 nm and the thickness is ∼1.4 nm. The bacterial strains were kept at −20 °C in a freezer before use.2.2 Synthesis of hyperbranched poly(amido amine) (HPAMAM)
Hyperbranched poly(amido amine)s (HPAMAMs) were synthesized in a similar procedure as the former report.11 Typically, MBA (11.57 g) and AEPZ (9.69 g) were added to a methanol/water mixture (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 150 mL). The solution was heated to 50 °C, and let the monomers to react for 5 days. Later, the solution was poured into cold acetone while stirring vigorously to precipitate the polymers. Finally, the precipitated polymers were dried under vacuum at room temperature. The structural characterization can be found in Fig. S1 and S2.†
3, v/v, 150 mL). The solution was heated to 50 °C, and let the monomers to react for 5 days. Later, the solution was poured into cold acetone while stirring vigorously to precipitate the polymers. Finally, the precipitated polymers were dried under vacuum at room temperature. The structural characterization can be found in Fig. S1 and S2.†
2.3 Preparation of Cu2+-HPAMAM/nanoclay hybrid films
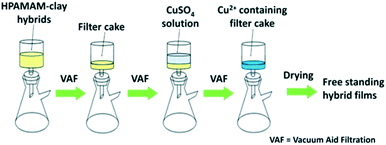
As shown in Fig. 1, the Cu2+-HPAMAM/nanoclay hybrid films were prepared in a similar procedure as indicated in the previous report,11 but with some modification. Typically, the HPAMAM solutions were mixed with the exfoliated clay nanosheets suspensions for a period of time, and then centrifuged to obtain glue-like hybrids. The hybrids were re-dispersed in deionized water and filtrated with the aid of vacuum. During the filtration, 5 mL CuSO4 solutions of 1 mM, 5 mM or 10 mM concentrations were poured onto the wet filter cakes respectively. The Cu2+ modified HPAMAM/nanoclay hybrid films were finally obtained by drying the filter cakes at room temperature. It should be mentioned that the HPAMAM solution concentration was 2 wt%, and that for the clay suspension was 0.5 wt%. Both of them were mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. Composition of the hybrid films is listed in Table 1.
1 volume ratio. Composition of the hybrid films is listed in Table 1.
| HPAMAMa | Claya | Cu2+b | |
|---|---|---|---|
| a Weight ratio of HPAMAM and clay in the hybrid films was determined by TGA (Fig. S3). There is about 7 wt% water in the hybrid films.b The Cu2+ ions were almost all adsorbed in the hybrid films when filtering the CuSO4 solution from the wet filter cake of HPAMAM/nanoclay (Fig. S6). | |||
| Pristine | 0.547 | 0.382 | — |
| 1mM@5mL | 0.547 | 0.382 | 0.84 × 10−3 |
| 5mM@5mL | 0.547 | 0.382 | 4.2 × 10−3 |
| 10mM@5mL | 0.547 | 0.382 | 8.4 × 10−3 |
2.4 Characterizations
The trace amount of Cu2+ in the filtrate was determined by AA-800 atomic adsorption spectrometer (Perkin Elmer).UV-vis spectra were acquired on a UV-vis spectrophotometer (2550, Shimazu).
Fluorescent property was recorded on an F-2700 fluorescent spectrophotometer (Hitachi).
Scanning Electron Microscopy (SEM) images were acquired on Sirion 200 (FEI) under accelerating voltages of 5 kV.
Small-angle X-ray scattering (SAXD) measurements were done using a Rigaku D/max-TTR III diffractometer where the source was a Cu-Kα radiation (λ = 0.154184 nm) with a voltage and current of 40 kV and 30 mA, respectively.
Thermogravimetric analysis (TGA) was performed on a Netsch TG209. The TGA measurement was carried out in a temperature range from 25 °C to 800 °C, with a heating rate of 10 °C min−1. All the tests were performed in air atmosphere.
Mechanical properties of the hybrid films were measured on a universal mechanical testing machine (SANS, China) running in a speed of 10 mm min−1. All the samples were cut into rectangle shape with length of 36 mm and width of 8 mm. The thickness of these samples was about 50 μm.
The antibacterial activity of the pristine hybrid film as well as the Cu2+-HPAMAM/nanoclay hybrid films was investigated using Gram-positive bacteria Staphylococcus aureus (S. aureus) as the model microorganism. Antibacterial activity was checked in nutrient agar media by spread plate method. The fresh shake cultures of the bacteria were diluted as required giving a working concentration 1 × 105 CFU mL−1 for S. aureus before every experiment. Then, 200 μL diluted culture was spread onto the nutrient agar plates inside the laminar hood. After the agar plate became dried in 30 min, the pristine hybrid film as well as the Cu2+-HPAMAM/nanoclay hybrid film (diameter: 8 mm) were placed at the centre of each of the agar plates. Finally, the plates were incubated for 24 h at 37 °C. The experiments were performed in triplicate and were repeated twice.
3. Results and discussion
3.1 Optical property of Cu2+-HPAMAM/nanoclay hybrid films
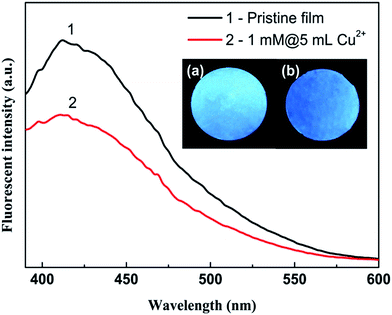
Fig. 2 indicates the transparency of the Cu2+ modified HPAMAM/nanoclay hybrid film. In the visible light range from 450 nm to 700 nm, transparency of the hybrid film can be as high as about 45%–50%. This result suggests a highly ordered alignment of the clay nanosheets inside the films.44,45 It can be seen from inset of Fig. 2 that all the films are smooth and flatten. In addition, they are all transparent. The patterns on the background can be clearly seen. It evidences that the clay nanosheets are in good alignment in the hybrid films.44 Nevertheless, the blue colour is darker as more copper ions were integrated into the hybrid films. It is the result of absorption of Cu2+ ions to the light within a specific wavelength range. It can be also seen that the blue colour is uniform for all the Cu2+ modified hybrid films, suggesting that the Cu2+ are evenly distributed inside the films. Such a speculation was evidenced by the results from energy dispersive X-ray spectroscopy (EDS) analysis (Fig. S7†). The HPAMAM/nanoclay hybrid films are fluorescent.11 Such an excellent property is well preserved by the Cu2+ modified hybrid films. As shown in Fig. 3, the 1mM@5mM Cu2+ solution treated hybrid film can also emit strong blue fluorescence under UV irradiation.3.2 Structural analysis of Cu2+-HPAMAM/nanoclay hybrid films
The cross-section morphology of the pristine hybrid film and the Cu2+ modified hybrid film is shown in Fig. 4. It can be seen that both of them showed oriented multi-layer structure indicating the parallel alignment of clay nanosheets.44,45 However, there is an unique feature for the HPAMAM/nanoclay hybrid films. It can be seen that many compact structures in addition to some voids are on the fractures. Those compact structures are thought to be the dense stacks of clay-rich domains, and those voids might be the results of draw-out of loosely packed polymer-rich domains. These observations suggest that the HPAMAM/nanoclay composites are of a heterogeneous structure. | ||
| Fig. 4 Cross-section morphology of (a) the pristine HPAMAM/nanoclay hybrid film and (b) the Cu2+ modified hybrid film. 5mM@5mL CuSO4 solution was used. The scale bars indicates 500 nm. | ||
When comparing the Cu2+ modified hybrid film and the pristine hybrid film shown in Fig. 4, it can be found that there are fewer voids for the Cu2+ modified one. It became much denser. Besides, thickness measurement also indicates that the Cu2+ modified hybrid films are thinner (Fig. S8†). These results strongly suggest that the Cu2+ ions have tightly bound with the HPAMAM molecules, leading to the contraction of the HPAMAM-rich domain.
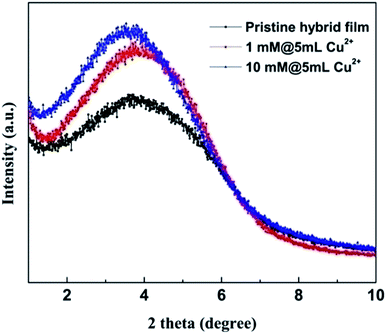
The small angle X-ray diffraction patterns are shown in Fig. 5. It can be found that the diffraction peaks of the Cu2+ modified hybrid films shift a little to smaller angles. This result suggests that the interlayer distances between the clay nanosheets of the Cu2+ modified hybrid films are slightly larger than that of the pristine one (details can be found in the ESI†). Moreover, the full width at half maximum (FWHM) decreases as more Cu2+ ions were integrated into the hybrid films (Fig. S9†). It indicates that the clay nanosheets are more orderly aligned. In another words, the hybrid films are more flattened, which can also be attributed to the contraction of polymer-rich domains.
As indicated in Fig. 6, the thermogravimetric residue of the Cu2+ modified hybrid films is remarkably larger than that of the pristine hybrid film. At 800 °C, the residue weight fraction of the 5mM@5mL sample is about 44.2%, while that for the pristine film is 38.2%. Such a result demonstrates that the Cu2+ modified hybrid films are tight and compact (see ESI†).
In summary, the Cu2+ ions greatly influence the microstructure of the HPAMAM/nanoclay hybrid films. A much denser structure was obtained.
3.3 Mechanical property
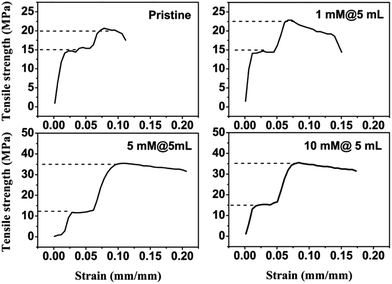
It can be read from Fig. 7 that the stress–strain curves of the Cu2+ modified HPAMAM/nanoclay hybrid films are of similar shape to that of the pristine one. There are three stages on these curves, which are separated by two yielding transitions.The first stage indicates a linear elastic deformation, corresponding to the strain ranging from 0 to 0.025. The first yielding occurs at strain equal to 0.025. It is because the stress and strain of all the hybrid films at this point are almost the same that the first yielding is thought to be the deformation of HPAMAM-rich domain. After this yielding, the HPAMAM segments have to move under stress. Subsequently, the molecules are inevitably extended, and the clay-rich domains begin to slide against each other.
As it can be seen, the second stage is a platform, ranging from strain @ 0.025 to 0.075. In this stage, the stress remains almost constant (∼15 MPa), but the elongation increases gradually. It is similar to the cold-drawing of the crystalline polymers. The rigid clay-rich domains remain their integrity, like those crystallites. Whereas, the flexible HPAMAM-rich domains deform, like those amorphous regions.
At the strain of ∼0.075, the second yielding occurs. The segments of HPAMAM may be extended to its full length at that point. Subsequently, the stress increases drastically within a relative small range of strains. The second yielding is thought to be the destruction of the clay-rich domains because the polymer-rich domain cannot deform any more. It can be found that the yielding strength at this point is not a constant. The yielding strength increases with the Cu2+ amounts. It is much different from that in the first yielding.
As more Cu2+ ions are applied, the second yielding strength is higher. Apparently, the interlocking of Cu2+ on the HPAMAM molecules detains the destruction of the hybrid films, and thus improves the ultimate strength.32,34
The ultimate strength and elongation at break of pristine and the Cu2+ modified hybrid films were collected in Table 2. It can be seen that the ultimate strength of the Cu2+ modified hybrid films is much larger than that of the pristine one. The Cu2+ ions functioned as crosslinkers through binding the HPAMAM molecules together. Subsequently, they effectively improved the strength of the HPAMAM/nanoclay nacre-like composites. Metal ion modification is an efficient way in improving the mechanical performance of the nacre-like composites.32–34
| Tensile strength (MPa) | Standard deviation | Elongation at break (%) | Standard deviation (%) | |
|---|---|---|---|---|
| Pristine | 20.06 | 0.86 | 12.67 | 1.57 |
| 1mM@5mL | 28.44 | 2.72 | 16.27 | 1.07 |
| 5mM@5mL | 36.91 | 3.62 | 14.74 | 5.52 |
| 10mM@5mL | 35.40 | 5.04 | 16.24 | 3.20 |
On the other hand, the elongation at break of the HPAMAM/nanoclay hybrid film is improved by the Cu2+ ions too. In the pristine hybrid films, there are only hydrogen bonds among the HPAMAM molecules. After integration of Cu2+, the interactions among the HPAMAM molecules were remarkably enhanced. Therefore, the destruction of the hybrid films was detained and the strength and the elongation at break were all improved.32 It is unusual for the chemically or physically crosslinked composites, where the crosslinking always blocks the movement of the molecular chains and thus reduced the extensibility.34,46 The elongation at break of the Cu2+ modified HPAMAM/nanoclay hybrid films is about 15%, which is fairly good compared with the other nacre-like composites. Only a few of them could have an extensibility more than 10%.47,48 It can be said that the Cu2+ modified HPAMAM/nanoclay hybrid films are strong and ductile.
3.4 Antibacterial property of the Cu2+ modified hybrid films
Anti-bacterial property of Cu2+ modified HPAMAM/nanoclay hybrid films was tested with S. aureus as model microorganism.49 The pristine hybrid film was used as control. As indicated in Fig. 8a and b, both the pristine HPAMAM/nanoclay hybrid film and the Cu2+ modified hybrid film showed good inhibition ability to growth of the S. aureus. Apparently, the Cu2+ modified hybrid films showed stronger inhabitation effect. | ||
| Fig. 8 Antibacterial properties of (a) the pristine HPAMAM/nanoclay hybrid film and (b) Cu2+ modified hybrid film (10mM@5mL). | ||
It is out of expectation that the pristine HPAMAM/nanoclay hybrid film showed good antibacterial property. The abundant amino groups on the HPAMAM molecules were thought to make a major contribution. The amine groups can be protonated into positively charged NH3+ ions, which are able to bind onto the cell wall of bacteria and inhibit their growth, and eventually kill the micro-organisms.50,51
After the copper ions were integrated in, the anti-bacterial property of the modified HPAMAM/nanoclay hybrid films were greatly enhanced. In Fig. 8b, it can be found that the inhibition zone was apparently larger than that of the pristine film which was shown in Fig. 8a.
The Cu2+ ions have long been known for their excellent anti-bacterial properties, and they were frequently applied in the latest reports.18–21 Even there were only a very small amount of Cu2+ being integrated in the hybrid films (about 0.1 wt%), they effectively inhibited the growth of S. aureus. Additionally, some kind of synergistic effect may be there between the Cu2+ ions and the HPAMAM molecules.
4. Conclusions
In summary, a kind of multi-functional nacre-like hybrid films were fabricated through a multistep integration of HPAMAM, clay nanosheets and Cu2+ ions. The introduction of Cu2+ ions greatly influenced the microstructure of the HPAMAM/nanoclay hybrid films. The hybrid films were much denser and thinner. Subsequently, the hybrid films showed excellent transparency, improved thermostability, stronger mechanical strength and better extensibility. Moreover, the Cu2+ modified HPAMAM/nanoclay hybrid films showed excellent antibacterial property, even there was only 0.094 wt% Cu2+ inside. In addition, the Cu2+ modified hybrid film showed good fluorescent property. Metal ions, not limited to the Cu2+, could play more important roles in development of robust, but multifunctional nacre-like composites.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (No. 21204016) and the Anhui Provincial Natural Science Foundation (No. 1508085ME107).Notes and references
- H. B. Yao, J. Ge, L. B. Mao, Y. X. Yan and S. H. Yu, Adv. Mater., 2014, 26, 163–188 CrossRef CAS PubMed.
- S. Kim and C. B. Park, Adv. Funct. Mater., 2013, 23, 10–25 CrossRef CAS PubMed.
- Y. Z. Wei, G. S. Wang, Y. Wu, Y. H. Yue, J. T. Wu, C. Lu and L. Guo, J. Mater. Chem. A, 2014, 2, 5516–5524 CAS.
- E. Kharlampieva, V. Kozlovskaya, R. Gunawidjaja, V. V. Shevchenko, R. Vaia, R. R. Naik, D. L. Kaplan and V. V. Tsukruk, Adv. Funct. Mater., 2010, 20, 840–846 CrossRef CAS PubMed.
- Y. Q. Shu, P. G. Yin, J. F. Wang, B. L. Liang, H. Wang and L. Guo, Ind. Eng. Chem. Res., 2014, 53, 3820–3826 CrossRef CAS.
- X. Z. Hu, Z. Xu and C. Gao, Sci. Rep., 2012, 2, 767, DOI:10.1038/srep00767.
- L. Kou and C. Gao, Nanoscale, 2013, 5, 4370–4378 RSC.
- B. H. Yuan, C. L. Bao, X. D. Qian, L. Song, Q. L. Tai, K. M. Liew and Y. Hu, Carbon, 2014, 75, 178–189 CrossRef CAS PubMed.
- H. B. Yao, H. Y. Fang, Z. H. Tan, L. H. Wu and S. H. Yu, Angew. Chem., Int. Ed., 2010, 49, 2140–2145 CrossRef CAS PubMed.
- T. W. Lee, O. O. Park, J. Yoon and J. J. Kim, Adv. Mater., 2001, 13, 211–213 CrossRef CAS.
- W. Hao, S. Ding, L. Zhang, W. Liu and W. Yang, ChemPlusChem, 2014, 79, 211–216 CrossRef CAS PubMed.
- D. Zhong, Q. L. Yang, L. Guo, S. X. Dou, K. S. Liu and L. Jiang, Nanoscale, 2013, 5, 5758–5764 RSC.
- Z. Zhang, J. Zhang, B. Zhang and J. Tang, Nanoscale, 2013, 5, 118–123 RSC.
- P. Podsiadlo, S. Paternel, J. M. Rouillard, Z. Zhang, J. Lee, J. W. Lee, E. Gulari and N. A. Kotov, Langmuir, 2005, 21, 11915–11921 CrossRef CAS PubMed.
- H. B. Yao, L. B. Mao, Y. X. Yan, H. P. Cong, X. Lei and S. H. Yu, ACS Nano, 2012, 6, 8250–8260 CrossRef CAS PubMed.
- H. B. Yao, Y. Guan, L. B. Mao, Y. Wang, X. H. Wang, D. Q. Tao and S. H. Yu, J. Mater. Chem., 2012, 22, 13005–13012 RSC.
- A. K. Chatterjee, R. Chakraborty and T. Basu, Nanotechnology, 2014, 25, 135101, DOI:10.1088/0957-4484/25/13/135101.
- L. Huang, E. M. Fozo, T. Zhang, P. K. Liaw and W. He, Mater. Sci. Eng., C, 2014, 39, 325–329 CrossRef CAS PubMed.
- A. Mauerer, B. Lange, G. H. Welsch, F. Heidenau, W. Adler, R. Forst and R. H. Richter, J. Mater. Sci.: Mater. Med., 2014, 25, 813–821 CrossRef CAS PubMed.
- W. Klinkajon and P. Supaphol, Biomed. Mater., 2014, 9, 045008, DOI:10.1088/1748-6041/9/4/045008.
- P. Fernandes, I. Sousa, L. Cunha-Silva, M. Ferreira, B. de Castro, E. F. Pereira, M. J. Feio and P. Gameiro, J. Inorg. Biochem., 2014, 131, 21–29 CrossRef CAS PubMed.
- G. F. Hu, J. Cell. Biochem., 1998, 69, 326–335 CrossRef CAS.
- J. P. Rodríguez, S. Roís and M. González, J. Cell. Biochem., 2002, 85, 92–100 CrossRef PubMed.
- C. K. Sen, S. Khanna, M. Venojarvi, P. Trikha, E. C. Ellison, T. K. Hunt and S. Roy, Am. J. Physiol.: Heart Circ. Physiol., 2002, 282, H1821–H1827 CrossRef CAS PubMed.
- J. Barralet, U. Gbureck, P. Habibovic, E. Vorndran, C. Gerard and C. J. Doillon, Tissue Eng., Part A, 2009, 15, 1601–1609 CrossRef CAS PubMed.
- C. Gérard, L.-J. Bordeleau, J. Barralet and C. J. Doillon, Biomaterials, 2010, 31, 824–831 CrossRef PubMed.
- A. Ewald, C. Kappel, E. Vorndran, C. Moseke, M. Gelinsky and U. Gbureck, J. Biomed. Mater. Res., Part A, 2012, 100, 2392–2400 Search PubMed.
- I. Burghardt, F. Lüthen, C. Prinz, B. Kreikemeyer, C. Zietz, H. G. Neumann and J. Rychly, Biomaterials, 2015, 44, 36–44 CrossRef CAS PubMed.
- C. Wu, Y. Zhou, M. Xu, P. Han, L. Chen, J. Chang and Y. Xiao, Biomaterials, 2013, 34, 422–433 CrossRef CAS PubMed.
- C. Wu, Y. Zhou, W. Fan, P. Han, J. Chang, J. Yuen, M. Zhang and Y. Xiao, Biomaterials, 2012, 33, 2076–2085 CrossRef CAS PubMed.
- Z. Tang, N. A. Kotov, S. Magonov and B. Ozturk, Nat. Mater., 2003, 2, 413–418 CrossRef CAS PubMed.
- P. Podsiadlo, Z. Liu, D. Paterson, P. B. Messersmith and N. A. Kotov, Adv. Mater., 2007, 19, 949–955 CrossRef CAS PubMed.
- S. Park, K. S. Lee, G. Bozoklu, W. Cai, S. T. Nguyen and R. S. Ruoff, ACS Nano, 2008, 2, 572–578 CrossRef CAS PubMed.
- P. Podsiadlo, A. K. Kaushik, B. S. Shim, A. Agarwal, Z. Tang, A. M. Waas, E. M. Arruda and N. A. Kotov, J. Phys. Chem. B, 2008, 112, 14359–14363 CrossRef CAS PubMed.
- M. S. Diallo, L. Balogh, A. Shafagati, J. H. Johnson Jr, W. A. Goddard III and D. A. Tomalia, Environ. Sci. Technol., 1999, 33, 820–824 CrossRef CAS.
- M. S. Diallo, S. Christie, P. Swaminathan, L. Balogh, X. Shi, W. Um, C. Papelis, W. A. Goddard III and J. H. Johnson Jr, Langmuir, 2004, 20, 2640–2651 CrossRef CAS.
- M. L. Tran, L. R. Gahan and I. R. Gentle, J. Phys. Chem. B, 2004, 108, 20130–20136 CrossRef CAS.
- F. Tarazona-Vasquez and P. B. Balbuena, J. Phys. Chem. B, 2004, 108, 15992–16001 CrossRef CAS.
- M. Sun, C. Y. Hong and C. Y. Pan, J. Am. Chem. Soc., 2012, 134, 20581–20584 CrossRef CAS PubMed.
- Y. Chen, L. Zhou, Y. Pang, W. Huang, F. Qiu, X. Jiang, X. Zhu, D. Yan and Q. Chen, Bioconjugate Chem., 2011, 22, 1162–1170 CrossRef PubMed.
- Y. J. Tsai, C. C. Hu, C. C. Chu and T. Imae, Biomacromolecules, 2011, 12, 4283–4290 CrossRef CAS PubMed.
- W. Yang, C. Y. Pan, M. D. Lu and H. B. Zhang, Biomacromolecules, 2010, 11, 1840–1846 CrossRef CAS PubMed.
- W. Yang, C. Y. Pan, X. Q. Liu and J. Wang, Biomacromolecules, 2011, 12, 1523–1531 CrossRef CAS PubMed.
- H. B. Yao, Z. H. Tan, H. Y. Fang and S. H. Yu, Angew. Chem., Int. Ed., 2010, 49, 10127–10131 CrossRef CAS PubMed.
- A. Walther, I. Bjurhager, J.-M. Malho, J. Ruokolainen, L. Berglund and O. Ikkala, Angew. Chem., Int. Ed., 2010, 49, 6448–6453 CrossRef CAS PubMed.
- P. Podsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. S. Shim, J. Xu, H. Nandivada, B. G. Pumplin, J. Lahann, A. Ramamoorthy and N. A. Kotov, Science, 2007, 318, 80–83 CrossRef CAS PubMed.
- L. J. Bonderer, A. R. Studart and L. J. Gauckler, Science, 2008, 319, 1069–1073 CrossRef CAS PubMed.
- X. Wang, H. Bai, Z. Yao, A. Liu and G. Shi, J. Mater. Chem., 2010, 20, 9032–9036 RSC.
- V. Ambrogi, A. Donnadio, D. Pietrella, L. Latterini, F. A. Proietti, F. Marmottini, G. Padeletti, S. Kaciulis, S. Giovagnoli and M. Ricci, J. Mater. Chem. B, 2014, 2, 6054–6063 RSC.
- F. Ferrero, M. Periolatto, C. Vineis and A. Varesano, Carbohydr. Polym., 2014, 103, 207–212 CrossRef CAS PubMed.
- L. Yu, J. Gong, C. Zeng and L. Zhang, Mater. Sci. Eng., C, 2013, 33, 3652–3660 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15570c |
| This journal is © The Royal Society of Chemistry 2015 |