Preparation and performance of antibacterial layer-by-layer polyelectrolyte nanofiltration membranes based on metal–ligand coordination interactions
Hongyan Zhenab,
Tingting Wangb,
Rui Jiab,
Baowei Su*ab and
Congjie Gaoab
aKey Laboratory of Marine Chemistry Theory and Technology (Ocean University of China), Ministry of Education, 238 Songling Road, Qingdao 266100, China. E-mail: subaowei@ouc.edu.cn; Fax: +86 532 66786371; Tel: +86 532 66786371
bCollege of Chemistry & Chemical Engineering, Ocean University of China, 238 Songling Road, Qingdao 266100, China
First published on 29th September 2015
Abstract
The coordination interaction between transition metal Cu2+ ions and polyelectrolyte (PE) ligands is studied to prepare (PEI/PSS(Cu)1/2)n layer-by-layer (LBL) self-assembly (SA) nanofiltration (NF) membranes with unique antibacterial properties. The coordination interaction mechanism has been clearly illustrated by X-ray photoelectron spectroscopy (XPS) analysis of the chemical composition of the active skin layer, and the result reveals that about one third of the Cu2+ ions are involved in the interaction. The prepared (PEI/PSS(Cu)1/2)5 LBL membrane has relatively smooth surface morphology and good separation performance, with a permeation flux of 65 L m−2 h−1 and rejection of about 84% for SO42− at 1.0 MPa. It has an excellent antibacterial rate up to 94.2%. The performance of the LBL membranes could be improved, after cross-linking by glutaraldehyde (GA). This kind of LBL NF membrane shows potential application in the separation of monovalent and divalent anions.
Introduction
Nanofiltration (NF) membranes have been widely used in water treatment applications owing to their unique charge and separation properties. There are many methods to prepare NF membranes, such as phase inversion, interfacial polymerization, chemical cross-linking, layer by layer (LBL) self-assembly (SA), and so on. LBL technology is distinguished by its simplicity, versatility, and efficiency, which allows easy control over the thickness and surface properties.1,2 By alternating adsorption of oppositely charged polyelectrolytes (PEs), the LBL method has gained increasing attention in the field of membrane preparation.Till now, most research on LBL membrane preparation has been focused on the electrostatic interaction between PEs with opposite charges, which was firstly proposed by G. Decher and coworkers in 1991.3 Since then, research on electrostatic SA has been widely carried out, not only in the preparation of PE multilayer membranes such as NF membranes,4 pervaporation (PV) membranes,5 and ion exchange membranes,6 etc., but also in the application of biological molecular SA and in the preparation of inorganic membrane materials with special performance. Wang et al. used electrostatic interaction as the driving force to prepare a PDADMAC/PSS multilayer NF membrane based on a polysulfone substrate, and found that the prepared membrane had good separation performance.7
Alternating adsorption of polyanions and polycations results in stepwise growth of polymer membranes and leads to unique properties due to the electrostatic interaction between oppositely charged molecules. The nanostructure of the LBL membrane can be tuned by the composition and the characteristics of the individual PE constituents. Studies have demonstrated two main steps during the adsorption and deposition of PEs.8 Firstly, PEs undergo fast immobilization through a few sites of their chain segment onto the substrate surface; secondly, the entire PE chain combines with the substrate matrix slowly but closely by adjusting the conformation of the PE segments. Owing to the slow second step, the PE deposition time was mostly selected as 10–20 min to achieve a complete deposition.
In addition to electrostatic interaction, metal–ligand coordination interaction, charge transfer interaction, hydrogen bonding, covalent function, and the synergistic interaction of the above effects can also be used as the driving force. The metal coordination interaction force is much stronger than the electrostatic force, and thus the former is expected to be more stable in water than the latter. The first investigation of using the coordination effect as a film-forming driving force was reported by Mallouk,9 who prepared an alternating deposition multilayer film with Zr4+ and alkyl compounds containing phosphate groups through the coordination effect between metal ions and phosphate. Xiong and Zhang et al. applied the coordination effect between poly(4-vinyl pyridine) (P4VP) and Cd2+ via the LBL method and prepared a PSS(Cd)1/2/P4VP multilayer membrane on PEI modified substrates of quartz, CaF2 and silicon; then they put the SA membranes into a H2S atmosphere for 30 min and obtained polymer-bound CdS hybrid polymer/semiconductor nanoparticles, and demonstrated by infrared spectroscopy that the driving force of this process was the coordination interaction between the pyridine group and cadmium ions.10 South et al. prepared hydrogen-storage SA multilayer films via the coordination effect between PVP and compounds containing Pd(II), and they successfully controlled the nature of the multilayer film by variation of the deposition conditions, solution additives and the polymer molecular weight.11 Greenstein et al. reported an accelerated SA procedure using volatilization under natural conditions to ensure excessive ligands on the substrate surface to combine with metal ions by coordination effect, and the whole process could be controlled within only one minute, hence the membrane preparation time could be greatly shortened.12 Zhang et al. prepared (PSS(Co)1/2/P4VP)2/PEI/PAN multilayer films on planar and 3D PAN substrate surfaces, respectively, using as driving force the coordination interaction between Co2+ and P4VP pyridine groups.13 The prepared PSS(Co)1/2/P4VP multilayer had not only high dehydration performance for solvent–water mixtures, but also high rejection for divalent ions, which proved that it could be used as an NF membrane.
Compared with other driving forces, coordination interaction can introduce transition metal ions into the membrane matrix. A lot of metal–ligand complexes can be used for SA,14,15 since transition metals account for nearly half of the periodic table of the elements. In addition, some transition metals have special properties, for example, both silver and copper have bactericidal performance, thus functional films can be prepared by coordination.16,17
In recent years, more and more research on the preparation of functional membranes with antimicrobial properties using silver or copper ions has been carried out.18–20 Fang et al. prepared ([PSS/PDADMAC]3[PAS/PAH-Ag]3PSS) multilayer NF membranes using LBL technology and coordination interaction, and the prepared NF membrane showed excellent antibacterial properties and the rejection for negative divalent ions reached 93%.21 Zhang et al. prepared a copper-plating carbon nanotube (CNT) film by ion beam assisted deposition (IBAD) and found that the prepared CNT film possessed excellent antibacterial properties.22
To improve the performance of NF membranes regarding higher requirements under more challenging separation systems, a type of novel LBL NF membrane with high flux, excellent stability and antibacterial properties was prepared in this work, using the LBL method with the coordination effect as the driving force. As PEI is a typical water-soluble polyamine macromolecule with many amino functional groups, it was selected as a type of polycationic electrolyte and an ideal polymer ligand for coordination with transition metal copper ions. The coordination mechanism was analyzed through peak fitting of the XPS spectrum. Then, the effects of pH and electrolyte concentration in the PE solutions on the formation of the LBL membranes, as well as on the membrane morphology and performance, were extensively investigated. Finally, the separation performance and the antibacterial performance of the LBL NF membranes were investigated in detail.
Experimental
Materials
Polyacrylonitrile (PAN, MWCO = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dalton (Da)) and polyether sulfone (PES, MWCO = 20
000 dalton (Da)) and polyether sulfone (PES, MWCO = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) ultrafiltration (UF) membranes were purchased from Sepro Co., Xiamen, China. Polyethylenimine (PEI, MW = 60
000 Da) ultrafiltration (UF) membranes were purchased from Sepro Co., Xiamen, China. Polyethylenimine (PEI, MW = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, 50 wt% in water) and polystyrene sulfonic acid sodium (PSS, MW = 70
000 Da, 50 wt% in water) and polystyrene sulfonic acid sodium (PSS, MW = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, 30 wt% in water) were purchased from Sigma-Aldrich Co., USA. Nutrient broth (CM106) was obtained from Beijing Land Bridge Technology Co., Ltd. Nutrient agar, glutaraldehyde (GA), NaOH, NaCl, Na2SO4, MgCl2, MgSO4 and CuCl2 were all analytical reagents (AR) and purchased from Sinopharm Chemical Reagent Co., Ltd.
000 Da, 30 wt% in water) were purchased from Sigma-Aldrich Co., USA. Nutrient broth (CM106) was obtained from Beijing Land Bridge Technology Co., Ltd. Nutrient agar, glutaraldehyde (GA), NaOH, NaCl, Na2SO4, MgCl2, MgSO4 and CuCl2 were all analytical reagents (AR) and purchased from Sinopharm Chemical Reagent Co., Ltd.
Preparation of LBL membranes
A PEI solution containing NaCl, and a PSS solution containing CuCl2 were prepared, respectively. PAN, hydrolyzed PAN (H-PAN) and PES UF membranes were used as substrates, respectively. The H-PAN substrate was obtained by immersing the PAN substrate in aqueous NaOH solution to hydrolyze the –CN group on the PAN substrate surface into –COONa, and followed by immersing the substrate in deionized (DI) water to convert the –COONa into –COOH groups.Prior to SA, the substrate was soaked in DI water for two hours to remove any impurities on its surface. Then it was taken out and immersed in one kind of PE solution, followed by rinsing with DI water. Afterward, it was immersed in the other kind of PE solution, followed by a second rinsing step with DI water. The immersion time in each solution was kept at 15 min, and the rinsing time with DI water between each immersion was 5 min. The described procedure was repeated until the desired bilayer number was achieved.
Factors influencing LBL membrane performance, such as species of substrate membrane, deposition sequence of PEs, concentration of transition metal ions, number of bilayers, as well as pH of the PEI solution, were investigated.
The influence of transition metal Cu2+ concentration on the membrane filtration performance was conducted by preparing (PEI/PSS(Cu)1/2)5 membranes using 0.4% PSS solution with different concentrations of Cu2+ (0, 0.2, 0.4, 0.6, 0.8 M), referred to as PSS/Cu-0, PSS/Cu-0.1, PSS/Cu-0.2, PSS/Cu-0.4, PSS/Cu-0.6, PSS/Cu-0.8, respectively.
The influence of pH on the membrane performance was examined out using five different pH values (4, 6, 8, 10, 12) of the PEI solution.
The influence of bilayer number on the performance of LBL membranes was investigated with five different bilayers (1, 2, 3, 4, 5) prepared using an anionic PE solution of 0.4 M CuCl2 and 0.4% PSS, and a cationic PE solution of 0.5 M NaCl and 0.3% PEI.
Characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and an accelerating voltage of 7.0 kV were used for the observation of the membrane surface, while for the cross-section observation, the magnification ratio was set as 100
000 and an accelerating voltage of 7.0 kV were used for the observation of the membrane surface, while for the cross-section observation, the magnification ratio was set as 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Energy Dispersive X-ray Detection (EDX) was also carried out on the SEM (S-4800, Hitachi Co., Japan).
000. Energy Dispersive X-ray Detection (EDX) was also carried out on the SEM (S-4800, Hitachi Co., Japan).The two-dimensional (2D) and three-dimensional (3D) surface topographies of the prepared LBL NF membranes were visualized by atomic force microscopy (AFM, Nano-3D, Nikon Co., Japan). Quantitative analysis of the membrane surface roughness was conducted based on AFM scans of at least three different areas, and root mean square roughness (RMS) was calculated.
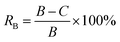 | (1) |
 | (2) |
 | (3) |
Replicates were performed and average values for salt rejections of each procedure were used for discussion.
Results and discussion
Mechanism analysis of the coordinative self-assembly process
Fig. 1 shows the XPS spectrum of the prepared LBL multilayer membrane and partial enlargement spectrum of N and Cu elements. The results indicated the existence of Cu element in the skin layer of the prepared LBL NF membrane, which was also proved by the EDX spectra (Fig. 2). | ||
| Fig. 1 The XPS spectrum of the prepared LBL (PEI/PSS(Cu)1/2)5 membrane (a) and partial enlargement of the XPS spectrum of N (b) and Cu (c). | ||
According to Fig. 1, in the N1s spectrum, the binding energy at 399.9 eV can be assigned to the nitrogen atom in the NH(NH2) groups,25 and there was a chemical shift up to 401.7 eV. In addition, Cu(II)2p3/2 showed a chemical shift from 934.5 eV to 932.7 eV.
The chemical shift in the XPS can be explained by the charge transfer and electrostatic effects.26 Each electron has one or several certain binding energy levels which are due to the strong coulombic force of the atomic nucleus. In addition, the outer electrons have a shielding effect on the inner electrons. When the outer electron cloud density reduces, its shielding effect on the inner electrons weakens, which leads to an increase in the binding energy of the inner electrons. Conversely, an increase in the outer electron cloud density leads to a decrease in the inner electron binding energy.
PEI has a lot of amino N atoms on its linear macromolecular chain (the proportion of primary, secondary, tertiary amine is nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), all the amines are located on the main chain and the branched chain can chelate with transition metal ions (Cu2+, Ni2+, Co2+, Cd2+ etc.).25,27–29 During the coordination interaction between the nitrogen groups of PEI and the Cu atom of PSS(Cu)1/2, the electrons of the imine sites are drawn towards the copper ions, i.e. a pair of unpaired electrons on the N atom of the imine transfers to the empty orbitals of the Cu(II), causing a decrease in the N electron cloud density and an increase in the Cu(II) electron cloud density. Therefore, the binding energy of N1s increases and that of Cu(II) decreases. As shown in Fig. 1, during the SA process, the binding energy of a certain amount of N1s is shifted from 399.9 eV to 401.7 eV, and the binding energy of a certain amount of Cu(II) ions is shifted from 934.5 eV to 932.7 eV, which proves the occurrence of coordination interaction between the transition metal Cu2+ ion of PSS(Cu1/2) and the N atom of PEI.30
1), all the amines are located on the main chain and the branched chain can chelate with transition metal ions (Cu2+, Ni2+, Co2+, Cd2+ etc.).25,27–29 During the coordination interaction between the nitrogen groups of PEI and the Cu atom of PSS(Cu)1/2, the electrons of the imine sites are drawn towards the copper ions, i.e. a pair of unpaired electrons on the N atom of the imine transfers to the empty orbitals of the Cu(II), causing a decrease in the N electron cloud density and an increase in the Cu(II) electron cloud density. Therefore, the binding energy of N1s increases and that of Cu(II) decreases. As shown in Fig. 1, during the SA process, the binding energy of a certain amount of N1s is shifted from 399.9 eV to 401.7 eV, and the binding energy of a certain amount of Cu(II) ions is shifted from 934.5 eV to 932.7 eV, which proves the occurrence of coordination interaction between the transition metal Cu2+ ion of PSS(Cu1/2) and the N atom of PEI.30
In addition, according to the peak fitting data from Fig. 1(b), the peak areas of the binding energy at 399.9 eV and 401.7 eV for N1s were 1379.2 and 232.5, respectively, and the ratio of the areas was about 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. That is to say, about 14.25% of the N atoms had participated in the coordination reaction. Similarly, for Cu2p3, as shown in Fig. 1(c), the peak areas of the binding energy at 934.5 eV and 932.7 eV were 956.6 and 471.3, respectively, and the ratio of the areas was about 2
1. That is to say, about 14.25% of the N atoms had participated in the coordination reaction. Similarly, for Cu2p3, as shown in Fig. 1(c), the peak areas of the binding energy at 934.5 eV and 932.7 eV were 956.6 and 471.3, respectively, and the ratio of the areas was about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This means that about one third of the Cu(II) ions were involved in the coordination reaction. It can be seen from Table 1 that the relative atomic percentage of N1s and Cu2p3 in the prepared membrane were 11.6% and 1.6%, respectively. Thus the relative atomic percentages of N atoms and Cu(II) ions in the coordination complex were calculated to be 1.6% and 0.5% according to the molecular weight of each atoms, and the ratio was approximately 3
1. This means that about one third of the Cu(II) ions were involved in the coordination reaction. It can be seen from Table 1 that the relative atomic percentage of N1s and Cu2p3 in the prepared membrane were 11.6% and 1.6%, respectively. Thus the relative atomic percentages of N atoms and Cu(II) ions in the coordination complex were calculated to be 1.6% and 0.5% according to the molecular weight of each atoms, and the ratio was approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This is closely approaching the four-coordinate ratio, as it is well-known that Cu2+ forms stable complexes having a four-coordinate planar structure.29,31
1. This is closely approaching the four-coordinate ratio, as it is well-known that Cu2+ forms stable complexes having a four-coordinate planar structure.29,31
| Element | C1s | N1s | O1s | S2p | Cu2p3 | Cu LMM |
|---|---|---|---|---|---|---|
| Relative atomic percent (at%) | 66.79 | 11.59 | 15.33 | 4.69 | 1.60 | 0.00 |
| Binding energy (eV) | 284.8 | 399.9, 401.7 | 531.3 | 167.7 | 932.7, 934.5 | 570.7, 572.4 |
Factors influencing LBL membrane performance
| Membrane no. | Substrate | MWCO (Dalton) | Deposition sequence |
|---|---|---|---|
| #1 | PAN | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
PEI/PSS |
| #2 | H-PAN | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
PEI/PSS |
| #3 | PES | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
PSS/PEI |
| #4 | PES | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
PEI/PSS |
 | ||
| Fig. 3 The effect of number of bilayers (n = 1–5) on rejection (a) and flux (b) of the prepared LBL (PEI/PSS(Cu)1/2)n membranes with different substrates and deposition sequences. | ||
It can be seen from Fig. 3 that the rejection of #1 PAN membranes remained essentially unchanged after depositing five bilayers, while that of #2 H-PAN membranes increased with the bilayer number. For #3 and #4 PES membranes, due to the uncharged property of the PES, the deposition of the first layer relied on the hydrophobic effect. When first depositing PSS, i.e. #3 membranes, the rejection was significantly higher than that of #4 membrane; however, the flux of #3 membrane was significantly lower than that of #4 membrane, which illustrated that the deposition sequence of the first layer has a crucial influence on the membrane performance. The reason might be that when first depositing PSS, hydrophobic forces between the PES substrate membrane and PSS had an important effect on the later deposits.
As #2 membrane had essentially the same flux but relatively higher rejection compared with that of #3 membrane, we chose the H-PAN membrane as the substrate in the subsequent experiment, and as it was negatively charged, PEI was deposited first.
 | ||
| Fig. 4 The effect of transition metal concentration on rejection and flux of the prepared LBL (PEI/PSS(Cu)1/2)5 membrane for Na2SO4 solution. | ||
PSS and PEI could combine with each other via electrostatic forces when there were no Cu2+ ions in the PSS solution; therefore, the prepared LBL membrane had some certain rejection. However, the counter-ion concentration in the PSS solution was low in this situation, and as the PSS segments were negatively charged, they were mutually repulsive, causing the extension of the polymer chains. Hence arrangement of the PSS molecules adsorbed on the substrate was not compact, and the following alternate deposition layer of PEs was not dense either, which resulted in a low rejection.
When adding Cu2+, the membrane rejection to divalent ions showed a significant increasing trend as the concentration of Cu2+ increased up to 0.2 M, which clearly demonstrated the coordination effect of Cu2+. However, when the concentration of Cu2+ increased above 0.2 M, the rejection then decreased slightly. This can be attributed to the aggregation effect of PEs at high concentration of Cu2+ ions, which caused the excessive deposition of a PSS mono-molecular layer and resulted in charge mismatch, eventually affecting the LBL process and the performance of the formed multilayer. Therefore, the optimal concentration of Cu2+ was 0.2–0.4 M.
 | ||
| Fig. 6 The effect of pH on rejection and flux of the prepared LBL (PEI/PSS(Cu)1/2)3 membrane for Na2SO4 (a) and NaCl (b) solutions. | ||
It can be seen that the pH of the PEI solution influences the performance of the prepared LBL membranes. PEI is a weak polyelectrolyte, and compared with strong electrolytes, the charge density of weak electrolytes is strongly affected by the solution pH, and the degree of ionization of weak electrolytes in aqueous solution changes with the variation of pH. The charge density of PEs can affect their solution behavior and further affect the performance of the prepared membrane.32 We can see from Fig. 6 that the rejection of both monovalent ions and divalent ions showed an increasing trend as the pH increased from 4 to 6, during which PEI ionization intensity increased. At pH 6, the PEI was strongly charged,32 so that the prepared three-bilayer membrane had the highest rejection due to the stable adsorption of PE molecules. However, when the pH increased even higher, from 6 to 12, the charge density of PEI gradually decreased. Meanwhile, PSS was more soluble in an alkaline environment. Thus adsorption and desorption occurred simultaneously, which led to high permeability of the SA membrane.
Membrane morphology
Surface hydrophilicity
The variation of contact angle with bilayer number is shown in Fig. 9. | ||
| Fig. 9 The effect of number of bilayers (n = 1–5) on the contact angle of the LBL (PEI/PSS(Cu)1/2)n membrane (n = 0 referred to the H-PAN substrate). | ||
It can be seen that the contact angle of the H-PAN substrate membrane was 46.3° (corresponding to the point of n = 0), and that of the unmodified PAN membrane was 60.7° (not shown in Fig. 9), which indicated that alkali modification could improve the hydrophilicity of the substrate. Subsequently, the SA process effectively changed the hydrophilicity of the membrane surface, and the contact angle varied alternately with the alternating deposition of the cationic and anionic PEs. When the outermost skin layer was the same kind of PE, the contact angle showed a decreasing tendency with the increase of the layer number of the same PE, which meant that the hydrophilicity became better. When the outermost layer was PSS, the contact angle of the LBL membrane was maintained at 15–20°; this is mainly due to the excellent hydrophilicity of PSS as a very strong hydrophilic surfactant. Therefore, the prepared LBL membrane had excellent hydrophilicity with the outermost deposition of PSS(Cu)1/2 electrolyte solution.
Antibacterial ability
The antibacterial activity of the UF substrate and the LBL (PEI/PSS(Cu)1/2)n (n = 1–5) membranes is shown in Fig. 10. It can be seen from Fig. 10(a) that there were a large number of bacterial colonies on the nutritional agar medium surface of the substrate. With the increase in the bilayer number, the colonies reduced significantly, as can be seen from Fig. 10(b)–(f), especially for the five-bilayer membrane (Fig. 10(f)) which had only a few sporadic colonies. This demonstrated that the prepared LBL membrane had good antibacterial ability. | ||
| Fig. 10 Photos of antibacterial activity for the PAN substrate (a) and the LBL (PEI/PSS(Cu)1/2)n (n = 1–5) membranes (b–f). | ||
The antibacterial efficiency was calculated and shown in Fig. 11. The larger the bilayer number, the better the antibacterial ability. For the five layers of LBL (PEI/PSS(Cu)1/2)5 membrane, the antibacterial rate could reach 94.2%, which is excellent compared with other reports.35–37
 | ||
| Fig. 11 The effect of number of bilayers (n = 1–5) on the antibacterial ratio of the LBL (PEI/PSS(Cu)1/2)n membranes (n = 0 refers to the H-PAN substrate). | ||
The antibacterial mechanism of Cu2+ ions is attributed to the fact that Cu2+ ions can combine with the plasma membrane by electrostatic attraction, then penetrate through the plasma membrane and combine strongly with intracellular amino acids and proteases, resulting in the degeneration of this intracellular matter and ultimately the denaturation of proteins.38,39 Other studies in the literature had similar explanations.40–42
Filtration
To investigate the filtration performance of the prepared (PEI/PSS(Cu)1/2)5 LBL membranes, Na2SO4, NaCl, MgSO4 and MgCl2 each with a concentration of 2000 mg L−1 in a single solution were used. The rejection and flux are shown in Table 3.| Na2SO4 | NaCl | MgSO4 | MgCl2 | |
|---|---|---|---|---|
| R/% | 84 | 23 | 65 | 19 |
| JV/L m−2 h−1 | 65 | 80 | 75 | 78 |
As can be seen, the rejection by the (PEI/PSS(Cu)1/2)5 membrane of 2–2 valent salt MgSO4 was higher than that of 1–1 valent salt NaCl. This is due to the fact that higher ionic valence results in stronger electrostatic repulsion between ions and membrane surfaces with like charge, hence the membrane will have a higher rejection of higher valent ions having like charge. Similarly, the membrane will have a lower rejection of higher valent ions having opposite charge. Therefore, the attraction interaction between ions and those membrane surfaces with opposite charge can be figured out. As shown in Table 3, the rejection of MgCl2 was very low, and that of Na2SO4 was high, which demonstrated that the surface of the prepared LBL membrane was negatively charged.
Swelling
Hydrophilic PEs could swell after being immersed in aqueous solution, which could affect the performance of the membrane. So we investigated the stability of the (PEI/PSS(Cu)1/2)5 membrane via testing the rejection of the same group of LBL membrane samples for Na2SO4 and NaCl solutions before and after immersion in DI water for 10 days. The results are shown in Fig. 12. | ||
| Fig. 12 Variation of rejection and flux of the prepared LBL (PEI/PSS(Cu)1/2)5 membrane for Na2SO4 and NaCl solutions with immersion time. | ||
From Fig. 12 we can see that the rejection and the permeation flux had only slight fluctuations during the testing days. This indicated that the soak time in DI water did not affect the separation performance of the prepared (PEI/PSS(Cu)1/2)5 membrane and it had good stability and could be preserved for a long time in water.
Glutaraldehyde post-processing
The uncoordinated amino groups of the prepared LBL NF membranes were subjected to GA crosslinking and the result of the separation performance is shown in Table 4.| Types of membrane | R/% | JV/L m−2 h−1 |
|---|---|---|
| (PEI/PSS(Cu)1/2)5 | 84 | 65 |
| (PEI/PSS(Cu)1/2)5/GA | 90 | 53 |
As can be seen, the rejection showed an increasing trend, from 84% to 90% after GA post-processing, while the permeation flux showed a decreasing trend, from 65 to 53 L m−2 h−1, which demonstrated the occurrence of crosslinking.
The effect of crosslinking on the membrane morphology was also investigated. The SEM images of the LBL membrane before and after GA crosslinking are shown in Fig. 13. It can be seen that the membrane surface became rougher and denser after GA crosslinking.
Conclusions
An H-PAN/PEI/PSS composite NF multilayer membrane was prepared successfully based on a combination of the LBL method and metal–ligand coordination interaction, with an inorganic salt (NaCl) introduced into the polycationic electrolyte PEI solution to control the charge density, and transition metal Cu2+ ions introduced into the PSS solution as coordination agent. The optimal preparation conditions were determined, and the performance of the prepared LBL membrane was investigated. The conclusions are as follows:(1) Transition metal Cu2+ ions can be used as metal–ligand coordination agent to prepare LBL functional NF membranes.
(2) The prepared functional LBL NF membrane has excellent antibacterial rate, which could reach over 94% for a five-bilayer LBL NF membrane.
(3) With optimal preparation conditions, the rejection of the five-bilayer NF membrane for Na2SO4 is about 84% and the permeation flux is about 65 L m−2 h−1; the rejection for NaCl is about 23% and the permeation flux is about 80 L m−2 h−1, which means this is a promising membrane for the separation of monovalent and divalent anions.
(4) The prepared functional LBL NF membrane with PSS(Cu)1/2 as outmost skin layer shows very good hydrophilicity, with a contact angle maintaining at 15–20°.
(5) Glutaraldehyde crosslinking could further improve the rejection of the prepared LBL NF membrane, while slightly decreasing the permeation flux.
Acknowledgements
We gratefully acknowledge financial support from National Natural Science Foundation of China (No. 20976170, 21476218). This is MCTL Contribution No. 100.Notes and references
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- X. Shi, M. Shen and H. Möhwald, Prog. Polym. Sci., 2004, 29, 987–1019 CrossRef CAS PubMed.
- G. Decher and J. Hong, Ber. Bunsen-Ges., 1991, 95, 1430–1434 CrossRef CAS PubMed.
- H. Y. Deng, Q. C. Chen, Z. G. Le, B. K. Zhu and Y. Y. Xu, Appl. Mech. Mater., 2013, 302, 204–211 CrossRef.
- Q. Zhao, J. Qian, Q. An and Z. Sun, J. Membr. Sci., 2010, 346, 335–343 CrossRef CAS PubMed.
- G. Liu, D. M. Dotzauer and M. L. Bruening, J. Membr. Sci., 2010, 354, 198–205 CrossRef CAS PubMed.
- Z. Wang, B. Su, X. Gao, S. Han and C. Gao, Mo Kexue Yu Jishu, 2012, 32, 27–32 CAS.
- P. Bertrand, A. Jonas, A. Laschewsky and R. Legras, Macromol. Rapid Commun., 2000, 21, 319–348 CrossRef CAS.
- H. Lee, L. J. Kepley, H. G. Hong, S. Akhter and T. E. Mallouk, J. Phys. Chem., 1988, 92, 2597–2601 CrossRef CAS.
- H. Xiong, Z. Zhou, Z. Wang, X. Zhang and J. Shen, Supramol. Sci., 1998, 5, 623–626 CrossRef CAS.
- C. R. South and M. Weck, Langmuir, 2008, 24, 7506–7511 CrossRef CAS PubMed.
- M. Greenstein, R. Ben Ishay, B. M. Maoz, H. Leader, A. Vaskevich and I. Rubinstein, Langmuir, 2010, 26, 7277–7284 CrossRef CAS PubMed.
- G. Zhang, Z. Ruan, S. Ji and Z. Liu, Langmuir, 2009, 26, 4782–4789 CrossRef PubMed.
- Y. Yan and J. Huang, Coord. Chem. Rev., 2010, 254, 1072–1080 CrossRef CAS PubMed.
- J. Brassinne, C. Mugemana, P. Guillet, O. Bertrand, D. Auhl, C. Bailly, C.-A. Fustin and J.-F. Gohy, Soft Matter, 2012, 8, 4501–4508 RSC.
- V. Marin, E. Holder, R. Hoogenboom and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 618–635 RSC.
- F. S. Han, M. Higuchi and D. G. Kurth, Adv. Mater., 2007, 19, 3928–3931 CrossRef CAS PubMed.
- M. Valodkar, S. Modi, A. Pal and S. Thakore, Mater. Res. Bull., 2011, 46, 384–389 CrossRef CAS PubMed.
- W.-f. Zhang, X.-q. Yan, Z.-h. Wang, L. Sun, Y.-b. Zhao and Z.-j. Zhang, Gongneng Cailiao, 2013, 44, 2156–2161 CAS.
- J. Xu, X. Feng, P. Chen and C. Gao, J. Membr. Sci., 2012, 413–414, 62–69 CrossRef CAS PubMed.
- J.-h. Fang, Z.-y. Cao, T.-m. Lai and L.-y. Shi, Gongneng Gaofenzi Xuebao, 2008, 21, 218–222 CAS.
- H. Zhang, J. Dang, X. Bai, Y. Shen, Y. Zhang, B. Zhang, Y. Zhang, X. Zhang and J. Liu, China Pat., CN101856596A, 2010.
- S. Goh, S. Lee, X. Zhou and K. Tan, Macromolecules, 1998, 31, 4260–4264 CrossRef CAS.
- Z. Han, Y. Wang and J. Wu, Solid State Sci., 2011, 13, 1560–1566 CrossRef CAS PubMed.
- M. Chanda and G. Rempel, React. Polym., 1995, 25, 25–36 CrossRef CAS.
- M. Guittet, J. Crocombette and M. Gautier-Soyer, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 125117 CrossRef.
- W. Bisset, H. Jacobs, N. Koshti, P. Stark and A. Gopalan, React. Funct. Polym., 2003, 55, 109–119 CrossRef CAS.
- M. Chen, L. Deng, X. Li and K. Huang, Guocheng Gongcheng Xuebao, 2001, 1, 218–224 CAS.
- S. Kobayashi, K. Hiroishi, M. Tokunoh and T. Saegusa, Macromolecules, 1987, 20, 1496–1500 CrossRef CAS.
- S. Sun and A. Wang, J. Hazard. Mater., 2006, 131, 103–111 CrossRef CAS PubMed.
- H. Thiele and K. H. Gronan, Makromol. Chem., 1963, 59, 207–221 CrossRef CAS PubMed.
- M. Elzbieciak, S. Zapotoczny, P. Nowak, R. Krastev, M. Nowakowska and P. Warszynski, Langmuir, 2009, 25, 3255–3259 CrossRef CAS PubMed.
- B. Su, T. Wang, Z. Wang, X. Gao and C. Gao, J. Membr. Sci., 2012, 423–424, 324–331 CrossRef CAS PubMed.
- E. M. Vrijenhoek, S. Hong and M. Elimelech, J. Membr. Sci., 2001, 188, 115–128 CrossRef CAS.
- L. Yu, Y. Zhang, B. Zhang, J. Liu, H. Zhang and C. Song, J. Membr. Sci., 2013, 447, 452–462 CrossRef CAS PubMed.
- H. Yu, X. Zhang, Y. Zhang, J. Liu and H. Zhang, Desalination, 2013, 326, 69–76 CrossRef CAS PubMed.
- P. K. S. Mural, A. Banerjee, M. S. Rana, A. Shukla, B. Padmanabhan, S. Bhadra, G. Madras and S. Bose, J. Mater. Chem. A, 2014, 2, 17635–17648 CAS.
- G. Tong, M. Yulong, G. Peng and X. Zirong, Vet. Microbiol., 2005, 105, 113–122 CrossRef CAS PubMed.
- M. Raffi, S. Mehrwan, T. M. Bhatti, J. I. Akhter, A. Hameed, W. Yawar and M. M. ul Hasan, Ann. Microbiol., 2010, 60, 75–80 CrossRef CAS.
- Z. Dan, H. Ni, B. Xu, J. Xiong and P. Xiong, Thin Solid Films, 2005, 492, 93–100 CrossRef CAS PubMed.
- C.-H. Hu and M.-S. Xia, Appl. Clay Sci., 2006, 31, 180–184 CrossRef CAS PubMed.
- T. Liu, H. Tang, X. Zhang, J. Zhao, R. Cui and X. Sun, J. Vac. Sci. Technol., 2007, 27, 286–289 CAS , 295.
| This journal is © The Royal Society of Chemistry 2015 |





