Photoinduced drug release from complexes of liposome and fluorescent silver nanoparticles
Junlin Lia,
Xueqin An*ab,
Zhengfeng Pana and
lianmei Suna
aJiangsu Key Laboratory of Biofunctional Materials, College of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu 210046, China
bSchool of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai, 200237, China. E-mail: anxueqin@ecust.edu.cn; Fax: +86-021-64252012; Tel: +86-021-64252012
First published on 27th January 2014
Abstract
Fluorescent silver nanoparticles (AgNPs) were embedded in the bilayer of liposomes and acted as a photothermic switch for photoinduced drug release. AgNPs in the liposome could absorb light energy, convert optical energy into localized heat, induce phase transition of liposome and release drug. The drug released from the AgNPs–liposome could be controlled by the irradiation time and AgNPs concentration.
Liposomes are spherical vesicles consisting of phospholipid bilayers surrounding an aqueous cavity that offer several advantages as lipoidal drug-delivery vehicles, and there are several liposome-mediated drug delivery products approved for clinical trials.1–4 One of the main challenges for the modern pharmaceutical research is the controlled drug release at the target site.5 Liposomes can be modified for controlled and triggered drug release to maximize the release at the desired site while minimizing it elsewhere.5 The modified liposomes include thermosensitive,6,7 pH-sensitive,8,9 photosensitive,10,11 and electromagnetic field-triggered12,13 liposomes. The success of the approaches requires high selectivity of the activating mechanism thus rendering the liposome susceptible to the signal while leaving the cell membranes unaffected.14 The phase transition temperature of gel-to-liquid crystalline in the liposome is called lower critical solution temperature (LCST) and the drug in the liposome can be release above LCST.14 However, it is difficult to control the LCST using traditional methods, because the LCST of liposome is easily influenced by type of drug, additive and medium pH value. The main challenges of current drug release remain to be the spatial and temporal control of the release.15
The noble metal nanoparticles (NPs) with various sizes have been used for many novel applications in biolabeling and luminescent tagging in biological areas.16 Ag nanoparticles (AgNPs) can exhibit surface plasmon resonance, and thereby they absorb energy at a distinctive wavelength in UV-vis region. Most of the absorbed energy is converted into localized heat producing a selective photothermal effect, while part of it is emitted as fluorescence. AgNPs encapsulated in the liposome are used as functional material because AgNPs have both properties of photo thermal conversion and fluorescence. The photo thermal conversion can be used to control release drug from liposome, and the fluorescence can be used in drug tracing. Nonetheless, it is essential to determine their potential toxicological effects in vivo, prior to fully using them in living organisms, and the potential toxicological effects of AgNPs was estimated in vivo.17 According to the newest research on the toxicity of AgNPs,18 AgNPs show toxicity to mammals when the particle size was greater than 12 nm.18 In this work, the small size AgNPs (about 3.5 nm) as functional material were encapsulated in the liposome, and the AgNPs concentration in liposome was no more than 81 μM. Therefore, the AgNPs potential toxicological effects in vivo may be neglected.
There are many disadvantages in the traditional liposome preparation methods, such as low drug encapsulation, poor stability and residue organic solvent and so on. Supercritical carbon dioxide fluids (scCO2) has been used as a green and safe method in the preparation of liposomes,12,16,19 owing to its high density, high solubility, high mass transfer rate, moderate critical pressure and non-toxicity. There are some advantages in the preparation of liposome, such as high encapsulation, good monodispersity, high stability, without any residual organic solvent and so on.20 The high pressure of scCO2 may promote the drug encapsulation, the high mass transfer rate may accelerate the hatching process of liposome, and the high solubility could be used to remove the residual organic solvent in the liposome.
Berberine is an alkaloid that has been reported to exhibit inhibitory and antitumor effects on esophageal cancer cells (ECCs) and liver cancer cell line HepG2.16
In this work, a thermosensitive liposome with embedded fluorescent silver nanoparticles (AgNPs) in the bilayer of the liposome was prepared by the supercritical carbon dioxide (scCO2) method and berberine as a model drug was encapsulated in the central aqueous compartment of the AgNPs–liposome. The drug encapsulated in AgNPs–liposome was released by UV light irradiation in a short time, where the AgNPs acted as a photothermic nano-switch for controlled drug release both spatially and temporally.
AgNPs were prepared by microemulsion method.21 The AgNPs–liposomes were synthesized through film – scCO2 hatching process.19 The AgNPs were relatively monodisperse spherical nanoparticles (Fig. 1a). The sizes of AgNPs were measured by dynamic light scattering method (Fig. 1b) and the average diameter of AgNPs was 3.5 nm. The morphology of AgNPs–liposome was obtained by transmission electron microscope (TEM) (Fig. 1c). The relation between berberine encapsulation efficiency and AgNPs concentration in the liposome was shown in Fig. 1d. It revealed that the encapsulation efficiency of berberine was gradually reduced with addition of AgNPs because the additive AgNPs occupied part of the space of the liposome and resulted in decrease of useful space for berberine.22
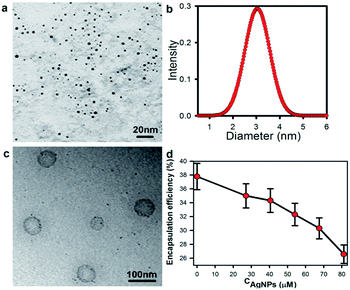 | ||
| Fig. 1 (a) TEM image of AgNPs, (b) the size and size distribution of AgNPs, (c) TEM image of AgNPs–liposome and (d) the encapsulation efficiency of the berberine in liposome with various CAgNPs. | ||
LCST of the liposome obtained from differential scanning calorimetry (DSC) was about 41.37 °C (Fig. 2a). The berberine in AgNPs–liposome was released at various temperatures for 10 min (Fig. 2b). The berberine released slowly below temperature of 41 °C, but it released quickly above temperature of 42 °C. It revealed that the AgNPs–liposome was thermosensitive, and drug release temperature was about 41.5 °C, which accorded with LCST of liposome obtained from DSC (Fig. 2a).
 | ||
| Fig. 2 (a) The LCST of AgNPs–liposome measurement (DSC) and (b) the berberine released from thermosensitive AgNPs–liposome at various temperatures for 10 min. | ||
To explore the effect of the photothermal effect of AgNPs on release drug of the liposome, the berberine in the liposomes with various AgNPs concentrations (CAgNPs) were released at room temperature by irradiation of UV light (250 nm) (Fig. 3a). It demonstrated that the berberine almost cannot be released from the liposome without AgNPs (Fig. 3a, CAgNPs = 0 μM), but it was released very quickly (Fig. 3a) from the AgNPs–liposome by UV irradiation (250 nm) at room temperature. The berberine release rate increased with the AgNPs concentration in the AgNPs–liposome (Fig. 3a). Nearly 70% berberine was released from the AgNPs–liposome (CAgNPs = 81 μM) in 5 min (black line in Fig. 3a) because the AgNPs embedded in the bilayer of the liposome had a photothermal effect due to light irradiation, and it resulted in phase transition of gel-to-liquid crystalline, and the berberine was released from liposome by photothermal effect.16 It suggested that drug was released by photoinduction using AgNPs as a photothermal switch. It also revealed that the amount and the rate of the released berberine could be controlled by altering the AgNPs concentration in the AgNPs–liposome and irradiation time.
As further proof of AgNPs as a photothermic switch in the AgNPs–liposome, repetitious release of the berberine encapsulated in AgNPs–liposome with various CAgNPs was undertaken by commutative irradiation with UV light (3 min) and visible light (3 min) as shown in Fig. 3b. An important feature was that the berberine was markedly released by UV light irradiation, but it could not be released by visible light irradiation, and this phenomenon can be repeated. It proved that berberine release from the AgNPs–liposome was due to the photothermic effects inducing phase transition of gel-to-liquid crystalline in the liposome rather than destruction of the bilayer of the liposome. Therefore the controlled release in the AgNPs liposome could be achieved by the use of AgNPs as a photothermic switch.
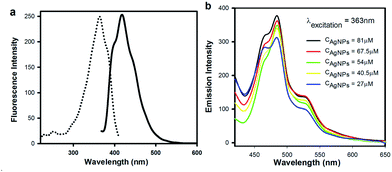
AgNPs could show fluorescence (excitation wavelength and emission wavelength were 363 nm and 420 nm, respectively) as shown in Fig 4a. The fluorescence emission spectra of AgNPs–liposome with various CAgNPs were shown in Fig. 4b at excitation wavelength of 363 nm. As we all know, liposome can't show fluorescence in general. However, AgNPs–liposome display fluorescence with emission wavelength of 482 nm. Even though the AgNPs–liposome emission wavelength is a bit redshift, it would be easy to believe that the fluorescence of AgNPs–liposome comes from AgNPs. The redshift of emission wavelength was probably due to the interaction between the positive charges of the inner surface of the liposome bilayer and the negative charges on the free terminals of surfactant molecules of the AgNPs surface.23
In conclusion, a novel fluorescent AgNPs–liposome was prepared by the supercritical CO2 method and it was possessed of a structure of the AgNPs embedded in the bilayer and drug encapsulated in the polarity area of the liposome. The drug encapsulation efficiency of the AgNPs–liposome decreased with incremental AgNPs concentration. The AgNPs–liposome can absorb light energy and release drug by photothermal effect. The AgNPs in the liposome acted as a photothermic switch for drug release. The drug release can be controlled by altering the AgNPs concentration in the liposome and irradiation time. The repetitious release of AgNPs–liposome by commutative irradiation with UV light and visible light suggested that drug release from the AgNPs–liposome was due to the photothermic effect inducing phase transition of the liposome rather than destroying the bilayer of the liposome. The result demonstrated that the fluorescent nanoparticle was successfully encapsulated into liposome. The part of the absorbed energy in the AgNPs–liposome was converted into localized heat and produced a selective photothermal effect, while part of it was emitted as fluorescence. It was the possibility and potential for AgNPs acted as a new class of fluorescent probe in biolabeling application.
Experimental
Materials
Sodium bis-(2-ethylhexyl) sulfosuccinate (AOT, 96.0%) was purchased from Alfa Aesar. AgNO3 (99.9%) was provided by Hubei Xinyin Noble Metal Co. Ltd. Soybean lecithin was purchased from GengBen Biotechnology Shanghai Co., Ltd. and cholesterol was provided by Sinopharm Chemical Reagent Co., Ltd. N2H4·H2O, cyclohexane (99.5%), toluene (99.5%), chloroform (99%), and methanol (99.5%) obtained from commercial sources were used as received. All other chemicals were analytic grade reagents without further purification.Preparation of AgNPs
AgNPs were synthesized in a water-in-oil microemulsion consisting of cyclohexane as the continuous oil phase and AOT as the surfactant.21 A metallic precursor (AgNO3, 0.1 M) and a reducing agent (N2H4·H2O, 0.3 M) were separately loaded into 0.1 M AOT/cyclohexane solution followed by intensely stirring. The molar ratio of water-to-oil was fixed at 3. The AgNO3-dispersed microemulsion and the N2H4·H2O-dispersed microemulsion were mixed under vigorous stirring until the color changed to dark yellow. The AgNPs were stabilized by AOT surfactant molecules. Then, methanol as extractant was added into the AgNPs-dispersed microemulsion to remove the excrescent AOT surfactants by extraction method. After this, the AOT modified AgNPs (AgNPs/AOT) were dispersed in toluene, followed by sonication for 30 min. The whole process was carried out at the room temperature.Preparation of AgNPs–liposome
The AgNPs–liposome was synthesized by the supercritical CO2 method.19 The soybean lecithin (0.1 g) and cholesterol (0.0333 g) (mass ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) together with a certain amount of hydrophobic AgNPs were dissolved in methanol–chloroform (15 ml, volume ratio of 1/2) solution. The mixture solution was evaporated in rotating vacuum evaporator, and a bilayer membrane with AgNPs was formed and the organic solvents were removed. Then the membrane was dissolved in berberine aqueous solution (10 ml, 1 mg ml−1) and transferred to a high pressure cell. Supercritical CO2 fluid was introduced into the cell and the incubation process was performed with a magnetic stirrer at a certain high pressure (16 MPa) and incubation temperature (42 °C) for 30 min. Finally the CO2 was released slowly and the transparent AgNPs–liposome aqueous solutions were obtained.
1) together with a certain amount of hydrophobic AgNPs were dissolved in methanol–chloroform (15 ml, volume ratio of 1/2) solution. The mixture solution was evaporated in rotating vacuum evaporator, and a bilayer membrane with AgNPs was formed and the organic solvents were removed. Then the membrane was dissolved in berberine aqueous solution (10 ml, 1 mg ml−1) and transferred to a high pressure cell. Supercritical CO2 fluid was introduced into the cell and the incubation process was performed with a magnetic stirrer at a certain high pressure (16 MPa) and incubation temperature (42 °C) for 30 min. Finally the CO2 was released slowly and the transparent AgNPs–liposome aqueous solutions were obtained.
Characterization
Morphology of AgNPs and AgNPs–liposome was obtained by transmission electron microscope (TEM, Hitachi H-7650, 120 kV). The particle size analysis of AgNPs was performed by dynamic light scattering (DLS). The LCST of the AgNPs–liposome was measured by the differential scanning calorimetry (DSC, Diamond DSC, Perkin-Elmer). The fluorescence spectra of AgNPs and AgNPs–liposome were recorded using a PerkinElmer LS-50B fluorescence spectrometer with fixed slit width of the raster (2.5 nm).The drug encapsulation efficiency of AgFNPs–liposome
After berberine encapsulated in the liposome, unencapsulated berberine (Wunencapsulated) was separated by dialysis process, and Wunencapsulated was determined by UV-vis spectrophotometer (Agilent 8453). The encapsulation efficiency (Eencapsulation) of the berberine in the liposome was calculated as follows:16,19| Eencapsulation = (Wencapsulated)/(Wtotal) × 100% = (Wtotal − Wunencapsulated)/(Wtotal) × 100% | (1) |
The drug release from AgFNPs–liposome
After drug release, the released berberine in the liposome solution was separated through dialysis process. The released berberine amount (Wrelease) was determined using UV-vis spectrometry. The release efficiency (Erelease) of the berberine in the liposome was calculated as follows:16,19| Erelease = (Wrelease/Wencapsulated) × 100% | (2) |
Acknowledgements
This work is financially supported by the National Nature Science Foundation of China (21273073 and 21073063) and the Fundamental Research Funds for the Central Universities, China (no. WK0913002).Notes and references
- N. A. Peppasa, P. Buresa, W. Leobandunga and H. Ichikawa, Eur. J. Pharm. Biopharm., 2000, 50, 27–46 CrossRef
.
- L. Zhang, L. Hong, Y. Yu, S. C. Bae and S. Granick, J. Am. Chem. Soc., 2006, 128, 9026–9027 CrossRef CAS PubMed
.
- V. Torchilin, Adv. Drug Delivery Rev., 2006, 58, 1532–1555 CrossRef CAS PubMed
.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145–160 CrossRef CAS PubMed
.
- L. Paasonen, T. Sipilä, A. Subrizi, P. Laurinmäki, S. J. Butcher, M. Rappolt, A. Yaghmur, A. Urtti and M. Yliperttula, J. Controlled Release, 2010, 147, 136–143 CrossRef CAS PubMed
.
- L. H. Lindner, Clin. Cancer Res., 2004, 10, 2168–2178 CrossRef CAS
.
- L. H. Lindner, M. Hossann, M. Vogeser, N. Teichert, K. Wachholz, H. Eibl, W. Hiddemann and R. D. Issels, J. Controlled Release, 2008, 125, 112–120 CrossRef CAS PubMed
.
- S. Simoes, Adv. Drug Delivery Rev., 2004, 56, 947–965 CrossRef PubMed
.
- I.-Y. Kim, Y.-S. Kang, D. S. Lee, H.-J. Park, E.-K. Choi, Y.-K. Oh, H.-J. Son and J.-S. Kim, J. Controlled Release, 2009, 140, 55–60 CrossRef CAS PubMed
.
- W. J. Egan and G. Lauri, Adv. Drug Delivery Rev., 2002, 54, 273–289 CrossRef
.
- B. Bondurant, A. Mueller and D. F. O'Brien, Biochim. Biophys. Acta, 2001, 1511, 113–122 CrossRef
.
- D. Qiu and X. An, Colloids Surf., B, 2013, 104, 326–329 CrossRef PubMed
.
- L. Zhu, Z. Huo, L. Wang, X. Tong, Y. Xiao and K. Ni, Int. J. Pharm., 2009, 370, 136–143 CrossRef CAS PubMed
.
- L. Paasonen, T. Laaksonen, C. Johans, M. Yliperttula, K. Kontturi and A. Urtti, J. Controlled Release, 2007, 122, 86–93 CrossRef CAS PubMed
.
- L. Anderson, E. Hansen, H. Lukianova, J. Hafner and D. Lapotko, J. Controlled Release, 2010, 144, 151–158 CrossRef CAS PubMed
.
- X. An, F. Zhang, Y. Zhu and W. Shen, Chem. Commun., 2010, 46, 7202–7205 RSC
.
- L. Browning, K. Lee, T. Huang, P. Nallathamby, J. Lowman and X. Xu, Nanoscale, 2009, 1, 138–152 RSC
.
- S. Yu, Y. Yin and J. Liu, Environ. Sci.: Processes Impacts, 2013, 15, 78–92 CAS
.
- X. An, F. Zhan and Y. Zhu, Langmuir, 2013, 29, 1061–1068 CrossRef CAS PubMed
.
- H. Wakayama and Y. Fukushima, Ind. Eng. Chem. Res., 2006, 45, 3328–3331 CrossRef CAS
.
- J. Li, X. An and Y. Zhu, J. Nanopart. Res., 2012, 14, 1325–1333 CrossRef
.
- G. Piel, M. Piette, V. Barillaro, D. Castagne, B. Evrard and L. Delattre, Int. J. Pharm., 2006, 312, 75–82 CrossRef CAS PubMed
.
- J.-Y. Wang, J.-F. Zhao, P.-N. Wang, W.-L. Yang and J.-Y. Chen, J. Fluoresc., 2011, 21, 1635–1642 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |