Kostiantyn O. Marichev and Michael P. Doyle
Org. Biomol. Chem., 2019,17, 4183-4195
DOI:
10.1039/C9OB00478E,
Review Article
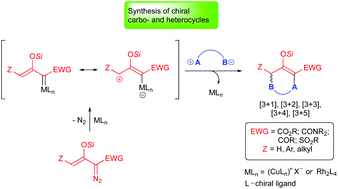
This review describes catalytic asymmetric cycloaddition reactions of silyl-protected enoldiazo compounds for the construction of highly functionalized carbo- and heterocycles which possess one or more chiral center(s). The enoldiazo compound or its derivative, donor–acceptor cyclopropene, form electrophilic vinylogous metal carbene intermediates that combine stepwise with nucleophilic dipolar reactants to form products from [3 + 1]-, [3 + 2]-, [3 + 3]-, [3 + 4]-, and [3 + 5]-cycloaddition, generally in high yield and with exceptional stereocontrol and regioselectivity.