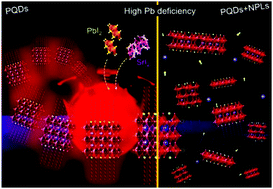
The Pb substitution in quantum dots (PQDs) with lesser toxic metals has been widely searched to be environmentally friendly, and be of comparable or improved performance compared to the lead-perovskite. However, the chemical nature of the lead substitute influences the incorporation mechanism into PQDs, which has not been explored in depth. In this work, we analyzed Sr-doping-induced changes in FAPbI3 perovskites by studying the optical, structural properties and chemical environment of FAPb1−xSrxI3 PQDs. The substitution of Pb by 7 at% Sr allows us to achieve FAPb1−xSrxI3 PQDs with 100% PLQY, high stability for 8 months under a relative humidity of 40–50%, and T80 = 6.5 months, one of the highest values reported for halide PQDs under air ambient conditions. FAPb0.93Sr0.07I3 PQDs also exhibit photobrightening under UV illumination for 12 h, recovering 100% PLQY at 15 days after synthesis. The suppression of structural defects mediated by Sr-doping decreases the non-radiative recombination mechanism. By attempting to increase the Sr content in PQDs, a mixture of 2D nanoplatelets/3D nanocubes has emerged, caused by a high Pb deficiency during the FAPb1−xSrxI3 synthesis. This contribution gives a novel insight to understand how the suitable/poor Pb substitution achieved through Sr-doping dictates the photophysical properties of PQDs that may be potentially applicable in optoelectronics.