Characterisation of a phosphatase-like nanozyme developed by baking cysteine and its application in reviving mung bean sprouts damaged by ash
Yingqiu
Xie†
a,
Ainur
Shaimoldina†
a,
Haiyan
Fan†
 b,
Sandugash
Myrzagali
a,
Guldan
Nazarbek
a,
Arailym
Myrzagalieva
a,
Aliya
Orassay
a,
Amr
Amin
c and
Enrico
Benassi
b,
Sandugash
Myrzagali
a,
Guldan
Nazarbek
a,
Arailym
Myrzagalieva
a,
Aliya
Orassay
a,
Amr
Amin
c and
Enrico
Benassi
 *d
*d
aDepartment of Biology, School of Sciences and Humanities, Nazarbayev University, Nur-Sultan 010000, Kazakhstan
bDepartment of Chemistry, School of Sciences and Humanities, Nazarbayev University, Nur-Sultan 010000, Kazakhstan
cBiology Department, United Arab Emirates University, Al Ain 15551, United Arab Emirates
dFaculty of Natural Sciences, Novosibirsk State University, Novosibirsk 630090, Russian Federation. E-mail: ebenassi3@gmail.com
First published on 24th November 2023
Abstract
Wildfire causes detrimental problems to animals and plants. Nanoparticles with enzymatic activities were applied to repair the damage caused by fire and potentially degrade the produced pollutants. Kinetic studies revealed for the first time an allosteric mechanism of nanozymes. The present work aims to reveal the advantage offered by a phosphatase-like (PL) nanozyme formed by baking L-cysteine to treat mung bean sprouts affected by ashes. The size, morphology, and molecular structure of the nanozyme were characterized using scanning electron microscopy (SEM), transmittance electron microscopy (TEM), and FT-IR spectroscopy combined with quantum mechanical calculations. On the other hand, the morphology and structure of ash along with its interaction with the nanozyme were also studied in detail. Applying the PL nanozyme to plants affected by fire may neutralize the negative impact induced by the ash on germination, rooting, and growth. Thus, plants can grow normally.
Environmental significanceThe catastrophic impact of wildfires on plants, trees and crops is incalculable. The present work focuses on the damage to the germination, rooting, and stem and leaf growth of plants by wood ashes and on how we may take advantage of the powerful enzymatic properties of baked cysteine to remedy such damage. In fact, baked L-cysteine acts as a nanozyme with phosphatase-like activity, with an allosteric mechanism. Such a nanozyme exhibits repairing ability to the damage developed in plant growth by ash at a low cost. More importantly, it does not introduce extra stress to the environment; in contrast, it promotes plant growth when applied alone. |
Introduction
Wildfires cause catastrophic ecosystem perturbation, damaging ca. 3% of the global vegetated land surface.1 The wildfire occurrence rate is predicted to increase, and the devastating effects to intensify this century, especially in semi-arid regions of Europe, the United States and Australia.2 The consequences of wildfire, especially in forests, have adverse impacts on the flora and fauna. Over 1.5 million acres of forest were burned during two large wildfires in California and two in Idaho, Oregon last year.3 One of the cruellest examples is Southern California's “Station Fire” in 2009; it covered a space of 200 miles of the Angeles National Forest,4 leaving a detrimental impact on crops and plants. As the direct product of forest fire, wood ash can modify the soil properties by enriching the nutrient elements on the one hand, but can affect the plant growth by intervening with the nutrient uptake ability on the other hand.5 In addition to the local damage, winds often carry ashes miles away from the wildfire, leading to a wider range of negative impacts on the environment.As nanotechnology penetrates various aspects of our life, its advantages were also considered in agriculture to enhance productivity. Nanoparticles with particular functions were used in the delivery of nano-formulated agrichemicals to detect environmental stress and crop conditions and to develop plant trait protection from environmental stress and diseases.6
Nanoparticles that mimic natural enzyme activities, identified as nanozymes, found wide applications from biosensors7 to antibacterial agents,8 cancer/bone marrow therapy,7 wound healing9 and environmental protection.7 However, the enzymatic activities demonstrated by nanozymes are limited to oxidase,9 peroxidase, catalase10 and superoxide dismutase.11
Liu et al. reported some metal oxide-based nanozymes indicating an antioxidant effect at low concentrations and reducing the detrimental impact of reactive oxygen species (ROS). However, at higher doses, they led to the enhancement of ROS production.12
The impact of metal or metal oxide-based nanoparticles on plant growth depends on the plant species, concentration, morphology and size of nanoparticles used. The applied nanoparticles may impose different effects on plant germination, rooting, and stem and leaf growth by perturbing the biological processes, such as photosynthesis, enzyme activities and N and P cycling.13 Silver nanoparticles are able to promote plant germination, seeding growth, rooting and stem elongation by regulating the plant hormone level, altering membrane permeability and ion homeostasis to increase water and nutrient acquisition, and evolving an antioxidant effect.14 Some stimulating effects of titanium dioxide nanoparticles were identified in plant height, yield of fruit and the number of branches.15 The dose dependence was tested for Al2O3 nanoparticles, in terms of their influence on plant growth, wherein a high dose inhibited plant growth.16
Meanwhile, carbon-based nanoparticles showed their capabilities to enhance plant germination and growth. Nanofertilizers, for instance, help to promote plant growth by increasing the availability of plant nutrients and preventing nutrient loss.17 It was found that at low concentrations, carbon nanoparticles tend to have no effect on or slightly enhance plant growth, whereas, at high concentrations, they generally exert a deleterious effect on plant growth. In general, the positive effects of nanoparticles at low concentrations are: they enhance the water uptake and consequently promote plant growth, whereas, at high concentrations, growth inhibition is induced in response to ROS overproduction under oxidative stress.18 The ash-derived carbon nanofibers were found to enhance tomato seed germination even under salt stress by maintaining moisture, and elevating the level of GABA shunt metabolites, carbohydrates, and total protein.19
Huang et al. discovered that protein tyrosine phosphatase (PTP) plays a critical role in the signalling mechanism that regulates the plant growth,20 and the presence of some heavy metals such as copper may activate the PTP and reduce the protein tyrosine phosphorylation level in rice cells leading to cell death.21 Meanwhile, the study of the negative impacts brought by ash indicated that at high concentration, ash leads to the increase of pH, lack of nitrogen nutrients and high level of heavy metal cytotoxicity.22
The present study is the first attempt to investigate the chemical components, morphology and size of the particles formed in ash produced from the burning of plants, and tests their effect on bean sprout germination, rooting, and leaf and stem growth. On the other hand, the present work aims to develop a nanozyme with PL activity and to test its effect on plant growth. We expect such a nanozyme will not only promote plant growth, but also fight the negative impact of ash from three aspects. Firstly, the PL activity of the nanozyme is expected to adjust the pH fluctuation caused by ash. Secondly, the PL nanozyme may exhaust the heavy metals in ash and prevent them from imposing damage to the plant cell. Thirdly, an affiliation was established between the particles of ash and nanozyme with an altered morphology to provide a reaction centre that optimizes the activities of the nanozyme.
Materials and methods
Preparation of the nanozyme
3 g of L-cysteine crystal was placed into a ceramic crucible and left in the oven for 10 minutes at 200 °C. The treated powder was brought to room temperature to make a sample for XPS and FT-IR measurement. The stock solution was made using this baked powder in deionized water.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 diluted stock solution. 1 μL of the diluted solution was deposited on a carbon type-B support film (200 mesh, Cu).
1000 diluted stock solution. 1 μL of the diluted solution was deposited on a carbon type-B support film (200 mesh, Cu).
Results
Morphological and chemical characterization of ash, baked cysteine and their mixture
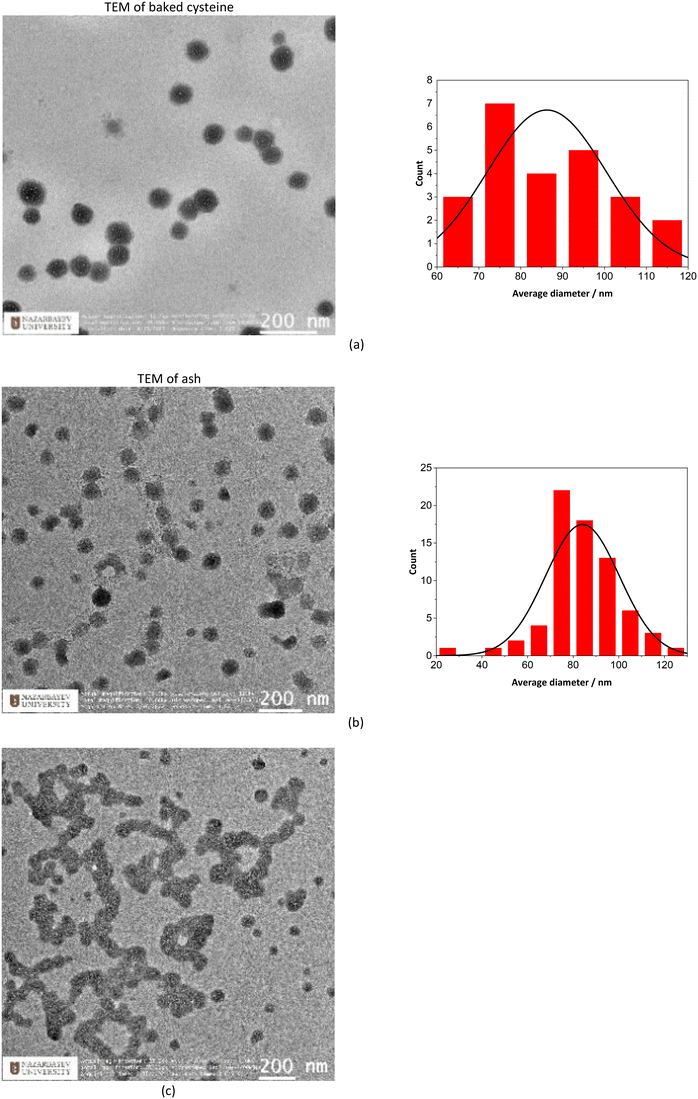
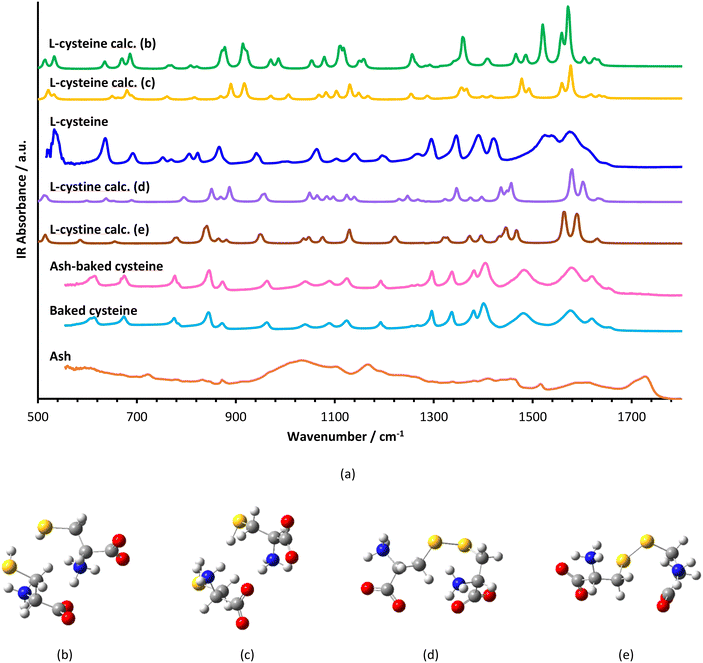
TEM images indicate that both ash and baked cysteine are visualized as some round-shaped particles with an average size of (85 ± 20) nm (Fig. 2a and b). However, as ash and baked cysteine are mixed, the morphology exhibits a significant change, wherein, the individual particles are lined up to form a tube-shaped assembly, according to the TEM image, with the diameter similar to that of individual particles and varied length and overall shapes (Fig. 2c). Such a morphology for the ash–baked cysteine mixture implies the strong affinity between the particles of ash and those of baked cysteine.FT-IR spectra of L-cysteine, baked cysteine, ash–baked cysteine and ash only are presented in Fig. 3a along with the calculated IR spectra of L-cysteine based on the crystal structure for hydrogen bonding dimers 1 (Fig. 3b) and 2 (Fig. 3c). We believe the molecules of L-cysteine are associated with one another through hydrogen bonds; therefore, the experimental FT-IR spectrum of L-cysteine is compared with the calculated IR spectra of hydrogen bonded dimers 1 & 2. The FT-IR spectrum obtained from the commercial L-cysteine finds the best match with the calculated IR spectrum for dimer 1 except for the features in the frequency range from 1330 cm−1 to 1440 cm−1, wherein, the best match occurs between the experimental result and the calculated IR spectrum for dimer 2. Hence, commercial L-cysteine is not a pure crystal, but rather contains a mixture of dimers 1 and 2.
The FT-IR spectrum of ash shows a broad band in the range from 900 cm−1 to 1100 cm−1, representing C–O stretching vibration, typical for cellulose, a few peaks in the range from 1400 cm−1 to 1500 cm−1, an indication of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the aromatic ring, a peak at 1720 cm−1 attributed to the C
C in the aromatic ring, a peak at 1720 cm−1 attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in carboxylic acid and, correspondingly, a broad band centered at 3200 cm−1 representing the O–H stretching in carboxylic acid.
O stretching in carboxylic acid and, correspondingly, a broad band centered at 3200 cm−1 representing the O–H stretching in carboxylic acid.
In order to identify the molecular structure of the baked cysteine, quantum mechanical calculation was performed for the dimer of cysteine connected by a disulphide bond (Fig. 3d and e). The FT-IR spectrum for the baked cysteine is most consistent with the calculated cysteine dimer containing a disulphide bond with the structure presented in Fig. 3e, wherein, the largest shift is reflected in the band centered at 1520 cm−1 for calculated cysteine (indicating –NH3 umbrella bending vibration) shifting to 1442 cm−1 for the calculated cysteine dimer with a disulphide bond. Correspondingly, the band observed in the FT-IR spectrum of L-cysteine centered at 1527 cm−1 shifted to 1478 cm−1 in the FT-IR spectrum of baked cysteine. Therefore, baking cysteine at 200 °C leads to the oxidation to cysteine.24
The FT-IR spectrum of baked cysteine and that of the mixture of ash–baked cysteine are nearly identical, implying that in the mixture, baked cysteine is distributed on the surface. Moreover, the particles of baked cysteine and those of ash have strong affiliation likely due to the intermolecular hydrogen bond between the amino group on baked cysteine and the carboxylic group on ash.
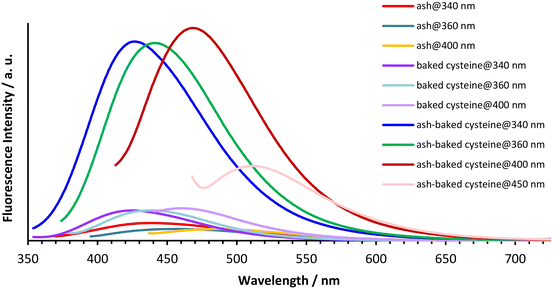
The aqueous solutions of ash, baked cysteine and their mixture all emit fluorescence with dependence on the excitation wavelengths (Fig. 4). The fluorescence peaks at 440 nm, 460 nm and 490 nm are observed for ash solution, whereas the fluorescence peaks at 424 nm, 444 nm and 464 nm are observed for the solution of both baked cysteine and its mixture with ash corresponding to the excitation wavelengths of 340 nm, 360 nm and 400 nm, respectively. In addition, for the solution of the mixture of ash and baked cysteine, the fluorescence at 511 nm was also identified with significant intensity at the excitation wavelength of 450 nm. First of all, at the same concentration, the fluorescence intensity for the solution of the mixture of baked cysteine and ash is much larger than that for the solution of baked cysteine or the solution of ash. Secondly, the fluorophores of the baked cysteine are the same with those of the mixture of baked cysteine and ash due to the structure of the mixture of baked cysteine and ash wherein, the particles of baked cysteine are distributed on the surface. Finally, although the functional groups on the surface of ash are dramatically different from those on the surface of baked cysteine, their fluorescence shows no large difference, meaning that the fluorescence observed for these aqueous solutions is unlikely to be due to the functional groups.
The XPS survey spectrum (Fig. 5) indicates that baked cysteine contains C 1s, N 1s, O 1s and S 1s, and S 2p. The high-resolution XPS spectrum of S 2p was de-convoluted into four peaks at 163.6 eV, 164.8 eV, 167.8 eV, and 169.0 eV representing two sets of doublets (2P3/2 at lower energy and 2P1/2 at higher energy). The dominant component at 163.6–164.8 eV can be assigned as S in the disulphide bonding system –C–S–S–C–,23 which is in agreement with the conclusion from FT-IR spectroscopy and quantum mechanical calculations indicating the formation of disulphide bonds upon baking. The minor component at 167.8–169.0 eV represents oxidized S due to baking. Correspondingly, in the high-resolution XPS spectrum of C 1s, peaks at 288.2 eV and 285.4 eV are observed representing the C in the form of –COO− and –C–S–, respectively. High resolution XPS peaks at 399.1 eV and 400.4 eV for N 1s are identified as N in the form of NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N, respectively.25,26 The peaks at 530.5 eV and 531.6 eV for O 1s are attributed to O in the form of C
O and C–N, respectively.25,26 The peaks at 530.5 eV and 531.6 eV for O 1s are attributed to O in the form of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or NH–C
O or NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COO−, respectively. The XPS spectrum of baked cysteine indicates that its main component is cystine, consistent with the FT-IR spectrum and calculations.
O and COO−, respectively. The XPS spectrum of baked cysteine indicates that its main component is cystine, consistent with the FT-IR spectrum and calculations.
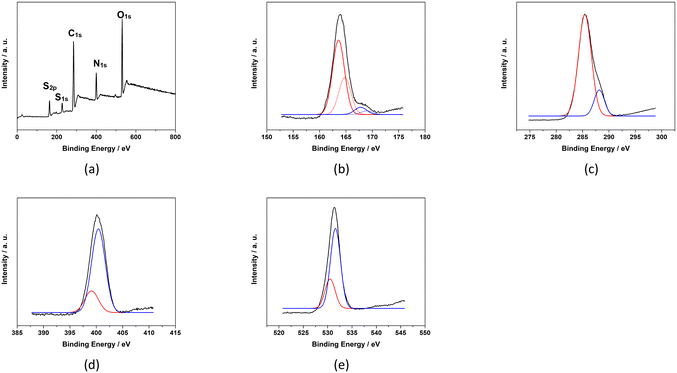 | ||
| Fig. 5 XPS spectra of baked cysteine including the survey spectrum (a), and high resolution spectra of S2p (b), C1s (c), N1s (d) and O1s (e). | ||
PL activity of baked cysteine and its mixture with ash
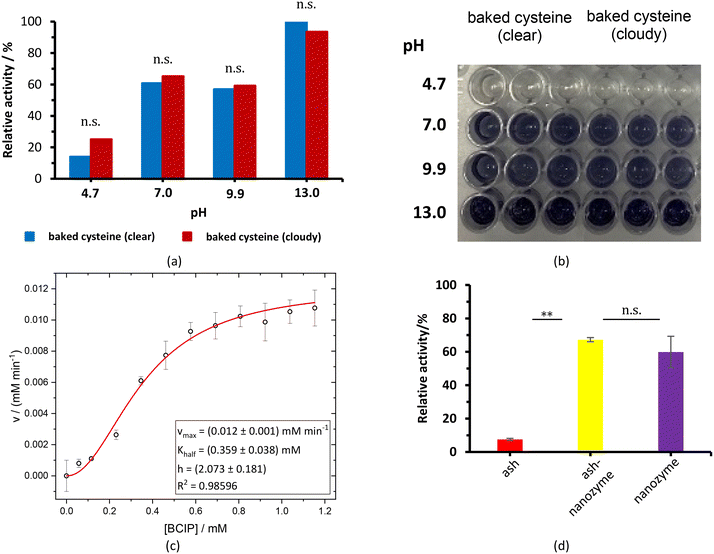
The baked cysteine exhibits phosphatase (PL) activity at pH of 7.0, 9.9, and 13.0 with the NBT/BCIP substrate (Fig. 6a). The relative PL activity for the aqueous solution of baked cysteine with a concentration of 13.5 mg mL−1 (clear solution) shows no difference from that of the saturated aqueous solution of baked cysteine (cloudy looking), which is recognized as the feature of the enzyme. Thus, we will refer to baked cysteine as the PL nanozyme from this point onwards.A quantitative kinetic measurement, i.e. the correlation of the reaction rate with the substrate concentration (Fig. 6c), indicated a large deviation from the Michaelis–Menten mechanism. The enzymatic behavior reflected by the as-prepared nanozyme can be better modelled by evoking an allosteric mechanism (1), wherein a certain cooperation is identified among the active sites on the enzyme.27
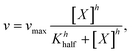 | (1) |
While no PL activity was observed for ash only, the mixture of ash and baked cysteine indicated slightly higher PL activity than the nanozyme itself (Fig. 6d), which may be attributed to two aspects, viz. (i) according to the FT-IR spectrum, nanoparticles of baked cysteine are distributed on the surface and (ii) the mixture exhibits a different morphology, which may potentially enhance the surface to volume ratio, thus increasing the enzymatic activity.
The effect of ash nanoparticles on germination and rooting
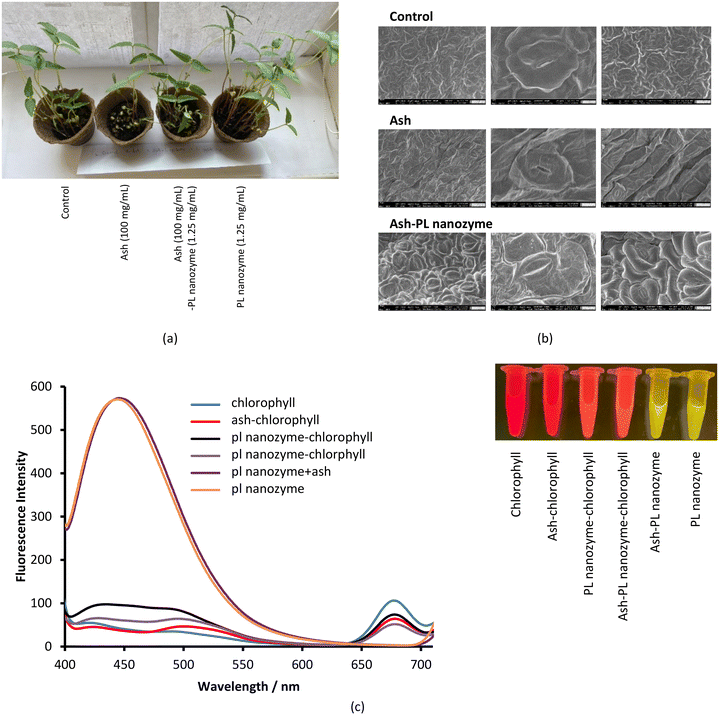
Ash suppresses the germination of mung green beans, wherein, no obvious ash concentration dependence was observed (Fig. 7a). Meanwhile, ash greatly inhibits rooting at a concentration of 50 mg mL−1 and above. On the other hand, the PL nanozyme generally indicates a promotion to both germination and rooting (Fig. 7b), wherein at a concentration of 1.25 mg mL−1, the PL nanozyme achieves the highest germination rate, whereas at a concentration of 0.75 mg mL−1, rooting gains the most degree of promotion. The germination and rooting were compared among the control, 37.5 mg mL−1 ash, 37.5 mg mL−1 ash + 1.25 mg mL−1 nanozyme and 1.25 mg mL−1 nanozyme (Fig. 7c). Treated with 37.5 mg mL−1 ash, the germination rate of mung beans has significantly deteriorated. However, with the addition of 1.25 mg mL−1 nanozyme, the germination rate of ash treated mung beans recovered to almost the same with that of the control. Regardless of the suppression brought by ash (37.5 mg mL−1) in rooting, the addition of 1.25 mg mL−1 nanozyme to the ash treated mung beans indicated better root growth than that of the control. In conclusion, the PL nanozyme was proved to repair or even promote the ability of germination and rooting of mung beans damaged by ash.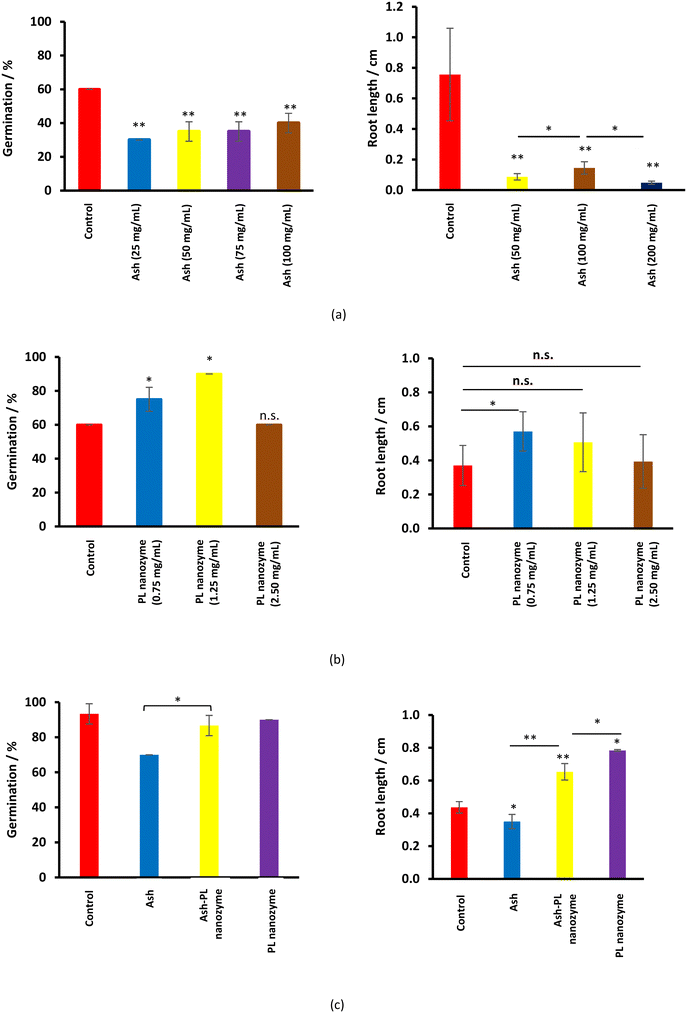 | ||
| Fig. 7 The germination rate and rooting length after the treatment of (a) ash, (b) PL nanozyme, and (c) ash–PL nanozyme mixture. | ||
The PL activity in plant cells linking to the mechanism of the PL nanozyme on repairing the damage induced by ash nanoparticles
To test whether the repairing of the damage of ash nanoparticles to plant rooting induced by ash is linked to phosphatase activities, the enzyme activities were tested using extraction from seedling roots (Fig. 8). The enzymatic activity and the impact on plant growth were also tested using the aqueous solution of pure L-cysteine at the same concentration of the PL nanozyme, and no PL activity was identified, nor an impact on plant growth different from that of watering with tap water was observed. Therefore, the tap water was used as the positive control for the rest of the work. Among the control, ash (37.5 mg mL−1), ash (37.5 mg mL−1) + PL nanozyme (1.25 mg mL−1) and PL nanozyme (1.25 mg mL−1) treated samples, the strongest PL was identified for the sample treated with ash + nanozyme using NBT/BCIP as the substrate. It is, therefore, assumed that the addition of the PL nanozyme activates the phosphatase in the root damaged by ash to render repairing.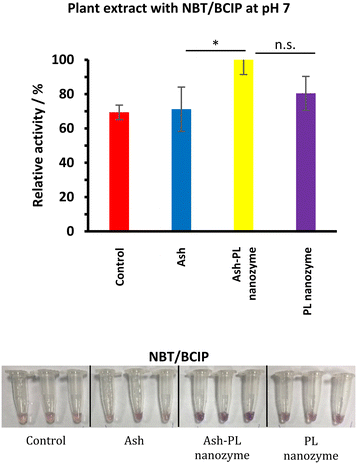 | ||
| Fig. 8 (a) Plants treated with ash (37.5 mg mL−1), ash–PL nanozyme (ash: 37.5 mg mL−1 + PL nanozyme: 5 mg ml−1), PL nanozyme (5 mg mL−1) and water as the control. | ||
The effect of ash nanoparticles on stems and leaves
Plants were sprayed with solutions of ash, PL nanozyme, and their mixture and were observed for 4–5 days, wherein tap water was used as a positive control. The photographs of the plants under different treatments were taken after 6 days. The plants exposed to the PL nanozyme were the strongest (Fig. 9a), whereas the ones treated with ash indicated the least degree of growth. By adding the PL nanozyme (5 mg mL−1) to the ash treated plants, the growth is comparable with that of the control. Such observation indicates that ash inhibits the growth of the stem; however, the inhibition can be relieved by adding the PL nanozyme. Although the average size of the leaves showed not much difference among different groups, the group treated with the PL nanozyme had the largest amount of leaves, whereas the group treated with ash showed the least amount of leaves. Adding the PL nanozyme to the ash treated plant led to more leaves growing, almost comparable with those of the control. The number of stomata on the sample leaf was observed through SEM imaging (Fig. 9b). The leaves treated with ash + PL nanozyme showed more open stomata than those treated with ash and in the control group.In an independent test, the impact of ash and the PL nanozyme on the fluorescence of chlorophyll a was measured (Fig. 9c). Excited at 370 nm, the aqueous solution of chlorophyll a indicated a characteristic fluorescence peak centered at 675 nm. Upon addition of ash and PL nanozyme, the intensity of this emission at 675 nm is deteriorated, and the degree of deterioration is in the order ash + PL nanozyme > ash > PL nanozyme. On the other hand, the aqueous solution of the PL nanozyme and ash + PL nanozyme indicated a strong emission peak centered at 440 nm with a FWHM of ∼100 nm at the excitation of 370 nm. Irradiated by a transilluminator at 470 nm, both the aqueous solution of chlorophyll and ash–chlorophyll indicated red luminescence; both PL nanozyme and ash + PL nanozyme indicated yellow luminescence, whereas the mixture of chlorophyll with either PL nanozyme or ash + PL nanozyme indicated orange-red luminescence.
Discussion
The value of Khalf, obtained from fitting with the allosteric mechanism reflects not only the binding strength between the enzyme and substrate, but also the effect of the occupancy of one substrate binding site to that of the other, whereas the value of h reveals the nature of the cooperation among enzymatic active sites. A positive cooperation is identified, as the value of h is greater than one. In such a case, binding of the substrate with one active site enhances the affinity between the substrate and the other active site. Compared to the values of vmax = (6.38 ± 0.51 μM min−1) and KMM = (0.56 ± 0.15) mM for natural ALP under the same conditions,24 the obtained values of (12 ± 1) μM min−1 and (0.359 ± 0.038) mM for vmax and Khalf, respectively (Fig. 6c), imply a stronger PL activity and binding with the substrate for the PL nanozyme synthesized in the present work than those for ALP.For the very first time, an allosteric mechanism with a positive cooperation was identified for a nanozyme. Such an enzymatic feature is closely related with the molecular structure of baked L-cysteine. We hypothesize that the PL activity of baked cysteine is initiated by a nucleophile, the deprotonated thiol group, like in cysteine-based protein phosphatases, which is well supported by the pH dependence of PL activity. According to the previous literature,28 the thiol group in non-disulphide species always co-exists with the disulphide bond. The described exchange reaction occurring between the thiol group and the disulphide bond demands large accessibility; in other words, the thiol group and disulphide bridge have to stay fairly close to each other. From an enzymatic perspective, the location of one active site determines the location of another active site. Therefore, the thiol–disulphide exchange reaction determines the cooperation among the active sites. On the other hand, since the thiol group acts as the central species in the nanozyme, it can be recovered immediately upon dephosphorization and no bulk containment of thiol is necessary.
The role of phosphatases in plant growth is evident and has contributed to the mineralization of organic phosphorus.29 Through the measurement of acid/alkaline phosphatase activities in the seedling process of S. glauca, Desai et al.30 concluded that both acid and alkaline phosphatases play essential roles during the initial and vigorous stages of seed germination. Moreover, the acid phosphatase activity was found to increase during the first 24 h of maize seed germination.31
Rooting depends much on the abundance and transport of P-containing nutrients. Therefore, it is reasonable to assume that rooting is closely related to the phosphatase activity as evidenced in Fig. 8. Cabugao et al.32 investigated how root and bacterial phosphatases regulated different tree species in Puerto Rico tropical forest to optimize root traits and functions and their interactions with microbial soil communities.
In the study of phosphatase activity in grass with different growth rates, de Oliveira et al.33 discovered that high acidic phosphatase activity enhanced the growth rate of grass by promoting remobilization of P. Such a discovery hints at the importance of phosphatase in leaf and stem growth, which is in agreement with the enhanced stem growth and the amount of leaves of plants upon addition of the PL nanozyme. Besides phosphatase, the photoluminescence of PL nanozyme involved systems centered at 440 nm may also intervene in the photosynthesis and consequently, the growth of leaves. The quenching of the fluorescence of chlorophyll at 675 nm by ash, PL nanozyme and ash + PL nanozyme indicated a potential interaction between chlorophyll and ash and the PL nanozyme. Moreover, addition of the PL nanozyme caused the photoluminescence of chlorophyll to shift to a shorter wavelength, which can affect the total number of leaves.34
Conclusions
Combined with quantum mechanical calculations, both FT-IR spectroscopy and XPS analysis indicated that the main component in the PL nanozyme in the present work is cystine, a molecular dimer with a disulphide bond. Such a nanozyme exhibited a nano-sized morphology and PL activity, and kinetic studies identified for the first time that it exhibits an allosteric mechanism. Such a PL activity reflected in the baked L-cysteine is due to the nano-size morphology and the thiol–disulphide exchange reaction. At the concentration 100 mg mL−1 and above, ash imposed significant inhibition to germination and rooting; however, the addition of the PL nanozyme cancelled the negative impact by ash and even promoted the germination and rooting compared to the control. While the size of leaves was not significantly affected by ash, the stem growth and the number of leaves were greatly suppressed. Such suppression was relieved by the addition of the PL nanozyme. The repairing of ash damaged root and germination by the PL nanozyme was mostly due to PL activity, whereas, the promotion brought by the PL nanozyme to the number of leaves may be also related to the interaction between chlorophyll and baked cysteine, as well as the photoluminescence of PL nanozyme involved systems.List of abbreviations
| HR-AFM | High-resolution atomic-force microscopy |
| FT-IR | Fourier-transform infrared |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| NBT | Nitro-blue tetrazolium chloride |
| BCIP | 5-Bromo-4-chloro-3′-indolyphosphate p-toluidine salt |
| ALP | Alkaline phosphatase |
| NP | Nanoparticle |
| PL | Phosphatase-like |
Author contributions
Haiyan Fan: experiments; data analysis; conceptualization; funding; writing and editing. Ainur Shaimoldina: experiments; data analysis; writing. Yingqiu Xie: conceptualization; funding; experiments. Sandugash Myrzagali: first draft writing. Arailym Myrzagalieva: first draft writing. Guldan Nazarbek: sample preparation. Aliya Orassay: first draft writing. Amr Amin: funding. Enrico Benassi: theoretical modelling; quantum mechanical calculations; data analysis; conceptualization; writing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Nazarbayev University Faculty – Development Competitive Research Grants Funder, with project reference 11022021FD2920 (Yingqiu Xie) and 11022021FD2928 (Haiyan Fan), and by UAEU-AUA Award No. 12R118 (Yingqiu Xie and Amr Amin). Computational resources were kindly provided by Shabyt HPC at Nazarbayev University.References
- L. Giglio, J. T. Randerson, G. R. Van der Werf, P. S. Kasibhatla, G. J. Collatz, D. C. Morton and R. S. DeFries, Biogeosciences, 2010, 7, 1171–1186 CrossRef.
- (a) J. R. Marlon, P. J. Bartlein, M. K. Walsh, S. P. Harrison, K. J. Brown, M. E. Edwards, P. E. Higuera, M. J. Power, R. S. Anderson, C. Briles, A. Brunelle, C. Carcaillet, M. Daniels, F. S. Hu, M. Lavoie, C. Long, T. Minckley, P. J. H. Richard, A. C. Scott, D. S. Shafer, W. Tinner, C. E. Umbanhowar Jr and C. Whitlock, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 2519–2524 CrossRef CAS PubMed; (b) M. Scholze, W. Knorr, N. W. Arnell and I. C. Prentice, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13116–13120 CrossRef CAS PubMed; (c) A. L. Westerling, H. G. Hidalgo, D. R. Cayan and T. W. Swetnam, Science, 2006, 313, 940–943 CrossRef CAS PubMed; (d) A. A. J. Williams, D. Karoly and N. Tapper, Clim. Change, 2001, 49, 171–191 CrossRef CAS.
- (a) National Interagency Fire Center National Fire News, (9th of August, 2022), https://www.nifc.gov/fire-information/nfn Search PubMed; (b) World Health Organization, Health topics: “Wildfires”, https://www.who.int/health-topics/wildfires Search PubMed.
- R. Smith, Wildfires and wilderness life cycles, Salem Press Encyclopedia of Science, 2023 Search PubMed.
- A. Demeyer, J. C. V. Nkana and M. G. Verloo, Bioresour. Technol., 2001, 77, 287–295 CrossRef CAS PubMed.
- B. S. Sekhon, Nanotechnol., Sci. Appl., 2014, 7, 31–53 CrossRef PubMed.
- H. Wang, K. Wan and X. Shi, Adv. Mater., 2019, 31, e1805368 CrossRef PubMed.
- L. Mei, Sh. Zhu, Y. Liu, W. Yin, Zh. Gu and Yu. Zhao, Chem. Eng. J., 2021, 418, 129431 CrossRef CAS.
- B. Xu, H. Wang, W. Wang, L. Gao, S. Li, X. Pan, H. Wang, H. Yang, X. Meng, Q. Wu, L. Zheng, S. Chen, X. Shi, K. Fan, X. Yan and H. Liu, Angew. Chem., Int. Ed., 2019, 58, 4911–4916 CrossRef CAS PubMed.
- S. Singh, Front. Chem., 2019, 7, 46 CrossRef CAS PubMed.
- J. Yang, R. Zhang, H. Zhao, H. Qi, J. Li, J.-F. Li, X. Zhou, A. Wang, K. Fan, X. Yan and T. Zhang, Exploration, 2022, 2, 20210267 CrossRef PubMed.
- Y. Liu, Z. Xiao, F. Chen, L. Yue, H. Zou, J. Lyu and Z. Wang, Sci. Total Environ., 2021, 780, 146578 CrossRef CAS PubMed.
- M. S. Akhtar, A. Khan, M. Swamy and K. Hakeem, Frontiers in Nanoscience and Nanotechnology, 2016, 2, 179–183 Search PubMed.
- P. Landa, Plant Physiol. Biochem., 2021, 161, 12–24 CrossRef CAS PubMed.
- M. S. Khater, Arab J. Nucl. Sci. Appl., 2015, 48, 187–194 Search PubMed.
- A. A. H. A. Latef, A. Zaid, M. F. A. Alhamd and E. A. Khaled, Agronomy, 2020, 10, 681 CrossRef CAS.
- A. K. Bhardwaj, G. Arya, R. Kumar, L. Hamed, H. Pirasteh-Anosheh, P. Jasrotia, P. L. Kashyap and G. P. Singh, J. Nanobiotechnol., 2022, 20, 19 CrossRef CAS PubMed.
- O. Zaytseva and G. Neumann, Chem. Biol. Technol. Agric., 2016, 3, 17 CrossRef.
- N. A. Al-Quraan, M.-A. H. Al-Akhras and A. Ayoub, Turk. J. Bot., 2021, 45, 124–139 CrossRef CAS.
- Y. W. Shih, W. C. Chou, Y. M. Lin, D. D. Huang, Z. H. Liu and H. J. Huang, Plant Growth Regul., 2004, 42, 271–282 CrossRef CAS.
- W. C. Hung, D. D. Huang, P. S. Chien, C. M. Yeh, P. Y. Chen, W. C. Chi and H. J. Huang, Chemosphere, 2007, 69, 55–62 CrossRef CAS PubMed.
- L. Romdhane, L. B. Ebinezer, A. Panozzo, G. Barion, C. D. Cortivo, L. Radhouane and T. Vamerali, Front. Plant Sci., 2021, 12, 661909 CrossRef PubMed.
- L. Nurtay, E. Benassi, F. Nazir, D. Dastan, A. Utupova, A. Dautov, K. Dukenbayev, Y. Xie, T. T. Pham and H. Fan, Discover Nano, 2023, 18, 76 CrossRef PubMed.
- Y. Su, E. P. Hessou, E. Colombo, G. Belletti, A. Moussadik, I. T. Lucas, V. Frochot, M. Daudon, S. Rouzière, D. Bazin, K. Li, P. Quaino and F. Tielens, Amino Acids, 2022, 54, 1123–1133 CrossRef CAS PubMed.
- R. A. Brizzolara, Surf. Sci. Spectra, 1996, 4, 103–107 Search PubMed.
- P. P. Chavan, V. S. Sapner and B. R. Sathe, ChemistrySelect, 2021, 6, 6050–6055 CrossRef CAS.
- S. Lu, Q. Shen and J. Zhang, Acc. Chem. Res., 2019, 2, 492–500 CrossRef PubMed.
- K. Kolšek, C. Aponte-Santamaria and F. Gräter, Sci. Rep., 2017, 7, 9858 CrossRef PubMed.
- V. Jane-Bassett, M. S. A. Blackwell, G. Blair, J. Davies, P. M. Haygarth, M. M. Mezeli and G. Stewart, Soil Biol. Biochem., 2022, 165, 108537 CrossRef.
- N. M. Desai, M. S. Patil, C. Narayankar and D. K. Gaikwad, J. Stress Physiol. Biochem., 2023, 19, 40–45 CAS.
- R. Senna, V. Simonin, M. A. C. Silva-Neto and E. Fialho, Plant Physiol. Biochem., 2006, 44, 467–473 CrossRef CAS PubMed.
- K. G. Cabugao, C. M. Timm, A. A. Carrel, J. Childs, T.-Y. S. Lu, D. A. Pelletier, D. J. Weston and R. J. Norby, Front. Plant Sci., 2017, 8, 1834 CrossRef PubMed.
- L. B. De Oliveira, A. C. R. Marques, L. F. de Quadros, J. G. Farias, R. Piccin, G. Brunetto and F. T. Nicoloso, Oecologia, 2018, 186, 1–11 CrossRef PubMed.
- J. Li, C. Yi, C. Zhang, F. Pan, C. Xie, W. Zhou and C. Zhou, Heliyon, 2021, 7, e06082 CrossRef CAS PubMed.
Footnote |
| † These authors equally contributed. |
| This journal is © The Royal Society of Chemistry 2024 |