 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterization of the first Peacock–Weakley polyoxometalate containing a transplutonium element: curium bis-pentatungstate [Cm(W5O18)2]9−†
Ian
Colliard
*ab and
Gauthier J.-P.
Deblonde
 *ac
*ac
aPhysical and Life Sciences Directorate, Glenn T. Seaborg Institute, Lawrence Livermore National Laboratory, Livermore, California 94550, USA. E-mail: Colliard1@LLNL.gov; Deblonde1@LLNL.gov
bMaterial Sciences Division, Lawrence Livermore National Laboratory, Livermore, California 94550, USA
cNuclear and Chemical Sciences Division, Lawrence Livermore National Laboratory, Livermore, California 94550, USA
First published on 9th May 2024
Abstract
Leveraging microgram-level techniques, we here present the first transplutonium bis-pentatungstate complex: NaCs8Cm(W5O18)2·14H2O (CmW5). Single crystal XRD, Raman, and fluorescence characterization show significant differences relative to analogous lanthanide compounds. The study reveals the unsuspected impact of counterions on fluorescence and vibrational modes of the curium complex and its lanthanide counterparts.
Interests in polyoxometalates can be traced as far back as the 19th century, with the synthesis of ammonium phosphomolybdate (NH4)3(PMo12O40) first reported in 1826.1 Since then, polyoxometalates, POMs, have permeated into various other fields, ranging from drug delivery to microelectronics, catalysts, energy storage, and more.2–6 POMs can also act as metal chelators and, while their chemistry is incredibly diverse, POMs are typically derived from a handful of major structures, with notably, the Peacock–Weakley7e.g., [Mn+(W5O18)2]15−n, the Lindqvist8 (e.g., Nb6O198−), the Keggin9 (e.g., PMo12O40n−), the Well–Dawson10 (e.g., P2W18O626−), the Preyssler11 (e.g., [Mn+P5W30O110]15−n) ions, etc.
Perhaps not surprisingly, compared to other fields, POM chemistry has had little overlap with actinide chemistry thus far, and particularly for heavy actinides (Am, Cm, Bk, Cf…). The first instance of a polyoxometalate compound containing an actinide was reported in 1914, with the synthesis of a thorium(IV) polymolybdate (NH4)8(ThMo12O42).12 However, it is not until after the 1970's that a renewed interest in incorporating actinide elements into polyoxometalate chemistry yielded additional structures. Moreover, while the actinide group consists of fifteen members, most of the data published on actinide POMs is about thorium and uranium. The high specific activity and isotope scarcity are only some of the constraints that have limited studies with transuranic elements. Recently, certain POMs have been leveraged to crystallize their complexes in microgram quantities,13,14 opening the avenue to studying their interactions with elements beyond plutonium (Fig. 1a). However, the number of known actinide POMs with transplutonium elements is still very limited: only one with Am(IV),15 one with americyl(VI),16 and three with Cm(III).13
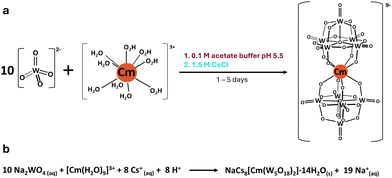 | ||
| Fig. 1 Reaction scheme (a) and equation (b) for forming the curium bis-pentatungstate complex [Cm(W5O18)2]9−. The starting curium(III) aqua ion is considered to have 9 water molecules, as previously reported by Skanthakumar et al.24 | ||
One class of POMs that has historically received strong interest is the bis-pentatungstate–metal complex [Mx(W5O18)2](12+x)−. Intense luminescent and optical properties when complexing lanthanide (Ln) ions, in solution and solid-state,17–20 provide strong motivation to pursue analogous compounds with transplutonium ions, notably Cm3+, as it can undergo fluorescence with a wide variety of ligands.21–23 Unlike the other POM structures that can complex metal ions, such as the Keggin or the Well–Dawson anions, the pentatungstate anion [W5O18]6− (W5), is often considered as the smallest and simplest POM ligand, both in terms of structure and synthesis. Fluorescence studies on aqueous solutions of W5 and Cm3+ previously showed13 a ∼500 times increase in the luminescence of Cm3+ upon complexation to W5, with a shift and splitting of the emission peaks (598.4 to 604.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm with multiple Stark levels). By analogy to the lanthanides, the expected complex formed in solution was [Cm(W5O18)2]9− (CmW5). CmW5 solutions exhibit the longest fluorescence lifetime for a Cm3+ complex reported so far (780 μs), and we hypothesized13 it was due to the complete removal of water molecules from the starting [Cm(H2O)9]3+ aqua ion (Fig. 1b).24 However, the crystal structure of this kind of heavy actinide complex has remained elusive.
nm with multiple Stark levels). By analogy to the lanthanides, the expected complex formed in solution was [Cm(W5O18)2]9− (CmW5). CmW5 solutions exhibit the longest fluorescence lifetime for a Cm3+ complex reported so far (780 μs), and we hypothesized13 it was due to the complete removal of water molecules from the starting [Cm(H2O)9]3+ aqua ion (Fig. 1b).24 However, the crystal structure of this kind of heavy actinide complex has remained elusive.
Herein we report the single crystal XRD structure of CmW5, which represents the first heavy actinide compound of the Peacock–Weakley POM class. The cesium salt of CmW5, fully formulated as NaCs8[Cm(W5O18)2]·14H2O, crystallizes in the anorthic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (V = 2711.36 (6) Å3). We further report the first [Ln(W5O18)2]9− compound with cesium counterions, Na3Cs6[Nd(W5O18)2]·18H2O, which crystallizes in the anorthic space group P
(V = 2711.36 (6) Å3). We further report the first [Ln(W5O18)2]9− compound with cesium counterions, Na3Cs6[Nd(W5O18)2]·18H2O, which crystallizes in the anorthic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (V = 2811.36 (6) Å3). While the only distinguishable feature between the heavy actinide and the lanthanide is a difference in the number of Na+ and Cs+ counterions, the apparent crystallographic divergence preludes the spectroscopic differences observed for these compounds both in solution and solid-state, as discussed below.
(V = 2811.36 (6) Å3). While the only distinguishable feature between the heavy actinide and the lanthanide is a difference in the number of Na+ and Cs+ counterions, the apparent crystallographic divergence preludes the spectroscopic differences observed for these compounds both in solution and solid-state, as discussed below.
A prior attempt to isolate this compound as a rubidium or cesium salt failed,13 but a cesium salt of CmW5 was here successfully isolated as single crystals (Fig. 3) by modifying our recently developed microgram-level POM–metal complex synthesis.13 In this optimized synthesis, we only used 1.2 μg of 247/246/248Cm3+, starting from a HCl solution (see Experimental section in the ESI†). Note that XRD-quality single crystals of CmW5 could only be grown with Cs+ counterions (Fig. 3c and d). Yet, the structure is isomorphic with previously reported sodium and potassium salts of [LnIII(W5O18)2]9− (Table S1, ESI†) and [AnIV(W5O18)2]8− (An = Th, U, and Np).17,25 Briefly, the structure is comprised of two [W5O18]6− ligands coordinating a central Cm3+. The W5 ligand is built upon five edge-sharing octahedral tungstates, four of which corner-share to the central Cm3+ ion, forming an eight-coordinate square antiprismatic coordination environment for curium, with a nearly ideal D4d symmetry. Bond lengths range 2.459(9)–2.508(9) Å for Cm–O, 1.715(6)–1.740(10) Å for W–Oterminal, 2.348–1.776 Å for W–Obridging, and 2.967(10)–3.762(10) Å for Cs–OCm's. See ESI† for additional information on the synthesis and structure of CmW5.
Interestingly, no water molecule is present in the first coordination sphere of the Cm3+ ion, which contrasts with previously studied heavy actinide complexes with organic ligands which are typically 9-coordinated and often have actinide-bound water molecules.24,26,27 Moreover, in all the other W5 reported structures with trivalent lanthanides (Table S1, ESI†), the Na+ counterions bind to several water molecules instead of direct ion-pairing with the POM observed for the Cs–CmW5 structure. The lanthanide structures mainly featured hydrogen bonding interactions between the [LnIII(W5O18)2]9− and the [Na(H2O)n]+ counterions. On the other hand, the CmW5 salt reported herein is the only instance thus far of the W5 complex crystallizing with Cs counterions.
The most noticeable effect of the Cs counterions is the complete removal of the 9 water molecules from the Cm3+ aqua ion,24 and it manifests as increased bonding interactions among Cs+ ions, the POM, and the central actinide ion. Of the eight Cs counterions in the CmW5 structure, five are close to curium (Cs–Cm distance of 4.48–4.54 Å) and bind directly to the bridging O in Cm–O–W (Cs–OCm distance of 2.967(10)–3.762(10) Å). The lone Na+ counterion found in the structure at ∼7 Å from Cm3+ favors binding with solvated water molecules, similar to what is observed in the lanthanide structures, and it only binds with the tungstate POMs via one yl oxygen between two complexes (Fig. 2). The interplay among Cm3+, W5, and Cs+ ion leads to a unique geometry where Cm3+ is completely shielded from solvent molecules and surrounded by eight oxygens at ∼2.48 Å, and five Cs+ at ∼4.51 Å (Fig. 2).
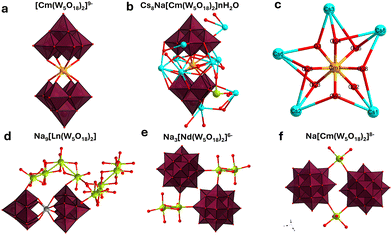 | ||
| Fig. 2 Polyhedral representations of the CmW5 and NdW5 compounds. (a) The [Cm(W5O18)2]9− complex, with counterions and solvated water molecules for clarity. (b) Asymmetric unit of the Cs–CmW5 crystal, fully formulated as NaCs8[Cm(W5O18)2]·14H2O. (c) Closeup view of the [CmO8] square antiprismatic coordination, with five Cs counterions binding to all eight oxygens. (d) Asymmetric unit of the Na9NdW5 structure,13 showing no connectivity between complexes, (e) connectivity between complexes for Na3Cs6[Nd(W5O18)2]. (f) Connectivity between complexes for NaCs8[Cm(W5O18)2]·nH2O. Color code: Cm (orange), Cs (light blue), Na (green), W (maroon octahedra), O (red). | ||
Control experiments were performed with Nd3+ (Fig. 2 and 3), as its size28 is similar to Cm3+, by synthesizing both a cesium salt (Cs–NdW5) and a sodium salt (Na–NdW5). For Cm, a solid sample was also prepared with Na+ counterions (Na–CsW5) but only micro to sub-microcrystals could be obtained (Fig. 3d) so that no single crystal XRD characterization could be performed. Raman microscopy experiments on Na9[LnIII(W5O18)2] previously assigned the characteristics bands13,17: 980–920 cm−1 for the terminal yl’O stretching (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 850–500 cm−1 for the bridging O stretching in W–O–W, <350 cm−1 for various vibrational modes for the entire complex, and lastly peaks at 890 and 200 cm−1 correspond to the bridging O stretching in Ln–O–W. The Raman spectrum of Na–NdW5 is consistent with previously reported spectra.13,17 Interestingly, we observed a clear effect of the Cs+ counterions (Fig. 3b) with combined changes in Raman peak intensities and peak shifting. Although Raman intensities can typically vary slightly from crystal to crystal, significant differences were observed between CmW5 and NdW5. For CmW5, the intensities of the terminal yl oxygens (W
O), 850–500 cm−1 for the bridging O stretching in W–O–W, <350 cm−1 for various vibrational modes for the entire complex, and lastly peaks at 890 and 200 cm−1 correspond to the bridging O stretching in Ln–O–W. The Raman spectrum of Na–NdW5 is consistent with previously reported spectra.13,17 Interestingly, we observed a clear effect of the Cs+ counterions (Fig. 3b) with combined changes in Raman peak intensities and peak shifting. Although Raman intensities can typically vary slightly from crystal to crystal, significant differences were observed between CmW5 and NdW5. For CmW5, the intensities of the terminal yl oxygens (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) increase, which could be due to increased Cs-complex interactions. For Nd–O–W, there is a decrease in intensity for the 890 cm−1 peak and an increase for the 211 cm−1 peak, which also shifts to 223 cm−1. For CmW5, an increase in Raman intensity for the terminal O and bridging O and peak shifting from 211 to 223 cm−1 is also observed (Fig. 3a). However, the 890 cm−1 peak corresponding to Cm–O–W is shifted to higher energy by 35 cm−1 to 925 cm−1, i.e., the opposite of what is observed for the Na/Cs–NdW5 compounds. The blue shifting caused by the Cs on the bridging O in Cm–O–W is a clear indicator of the outsized role Cs+ ions on the [Cm(W5O18)2]9− complex as opposed to its lanthanide analogs. To the best of our knowledge, this represents the first observation of cesium–actinide interactions within a molecular complex.
O) increase, which could be due to increased Cs-complex interactions. For Nd–O–W, there is a decrease in intensity for the 890 cm−1 peak and an increase for the 211 cm−1 peak, which also shifts to 223 cm−1. For CmW5, an increase in Raman intensity for the terminal O and bridging O and peak shifting from 211 to 223 cm−1 is also observed (Fig. 3a). However, the 890 cm−1 peak corresponding to Cm–O–W is shifted to higher energy by 35 cm−1 to 925 cm−1, i.e., the opposite of what is observed for the Na/Cs–NdW5 compounds. The blue shifting caused by the Cs on the bridging O in Cm–O–W is a clear indicator of the outsized role Cs+ ions on the [Cm(W5O18)2]9− complex as opposed to its lanthanide analogs. To the best of our knowledge, this represents the first observation of cesium–actinide interactions within a molecular complex.
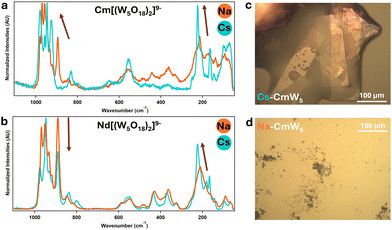 | ||
| Fig. 3 Solid-state Raman microscopy of CmW5 and Nd–W5 (a) [Cm(W5O18)2]9− with sodium (in orange) and cesium (in blue) counterions. (b) Similar experiments with [Nd(W5O18)2]9. (c) CCDC image capture of Cs–CmW5 single crystals. This picture was taken under UV-light – the pink-orange fluorescence highlights the presence of Cm3+. (d) Na–CmW5 solution evaporated onto a microscopy slide. The material was crystalline, but the crystals were too small for XRD analysis. See Fig. S1 (ESI†) for additional crystal pictures. | ||
The impact of Cs counter ions on CmW5 was further investigated via fluorescence spectroscopy (Fig. 4 and 5). We recently reported13 solution-state fluorescence experiments on Na–CmW5. The Cm3+ emission peak is centered at 604.6 nm and the fluorescence lifetime decay of 780 μs is the longest observed for a Cm3+ complex in solution. We performed similar Cm3+ fluorescence experiments with and without cesium counterions, and both in the solid-state (Fig. 4) and solution-state (Fig. 5). Consistent with the Raman spectroscopy results, the fluorescence properties of CmW5 were also found to be impacted by the nature of the counterions. Fig. 4a shows the emission spectrum of single crystals of Cs–CmW5 and a crystalline precipitate of Na–CmW5. Switching the counterions from Na to Cs, the Cm3+ emission peak shifts from 604.6 to 608.0 nm. The structure of the emission peak of Cs–CmW5 is also more pronounced, likely indicative of less disorder in the structure. We also measured the emission spectrum of Na–CmW5 in solution at room temperature and as a flash-frozen solution at liquid-nitrogen temperature (−196 °C). The spectrum of the Na–CmW5 solution (Fig. 4b) at room temperature is consistent with that of the solid (Fig. 4a). Upon cooling, the Na–CmW5 emission peak becomes more structured, and we could confirm the main emission band is at 604.6 nm, consistent with the solid-state data. The results clearly indicate that the nature of the counterion impacts the energy levels of CmW5.
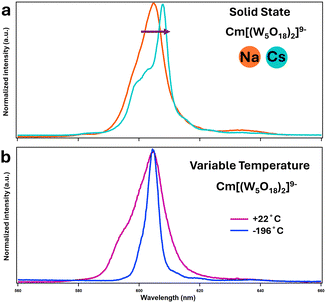 | ||
Fig. 4 (a) Solid-state emission spectra of [Cm(W5O18)2]9− with Na and Cs counterions (b) fluorescence emission of aqueous solutions of Na–CmW5 at room temperature (22 °C – pink curve) and after flash-freezing in liquid nitrogen (−196 °C – blue curve). Solvent: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 vol/vol glycerol 4 vol/vol glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. Excitation wavelength: 271.0 nm. See Fig. S2 and S3 (ESI†) for corresponding excitation spectra. H2O. Excitation wavelength: 271.0 nm. See Fig. S2 and S3 (ESI†) for corresponding excitation spectra. | ||
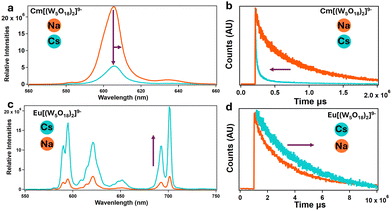 | ||
| Fig. 5 Solution and time-resolved fluorescence. (a) and (b) Emission spectra of solutions of CmW5 with sodium and cesium counterions, showing fluorescence quenching and lifetime shortening. (c) and (d) Similar experiments with NdW5, showing opposite behavior. Both CmW5 and EuW5 complexes are under 100 mM acetate buffer at a pH of 5.5. [EuW5] = 1 mM. [CmW5] = 100 μM (Cs–CmW5 spectra was adjusted to compensate for the dilution for the addition of 3 M CsCl, final concentration of Cs–CmW5 was 50 μM). See Fig. S4 and S5 (ESI†) for corresponding excitation spectra. | ||
To further confirm the results, Fig. 4a shows the emission spectra of a Na–CmW5 solution (consistent with previously reported data13) and the same sample after addition of Cs+ counterions. A significant quenching of the luminescence is observed, with the emission intensity decreasing by an order of magnitude. A shortening of the lifetime to about 515 μs and a peak shifting from 604.6 to 606.0 nm were also observed (Fig. 5a and b). This strongly indicates that Cs+ counterions, typically considered spectator ions, actually influence bonding and energy levels within the CmW5 complex.
The relatively close proximity between Cm3+ and Cs+ ions in the crystal structure (Fig. 2) suggests that Cs+ may provide a relaxation pathway for Cm3+ upon excitation, akin to water molecules, hence leading to shortening of the luminescence lifetime. Another hypothesis is that ion-pairing formation between Cs and W5 is strong enough to change the excited state energy levels of the POM, leading to less efficient fluorescence sensitization of Cm3+ luminescence.
Similar solution-state experiments were performed with Eu3+, due to its relatively similar size to Cm3+ and fluorescence properties (Fig. 5c and d). Solutions of Na–EuW5 exhibit the typical multi-peak emission band of a Eu3+ complex, with main peaks at ∼680, ∼620, ∼650, and ∼700 nm (Fig. 5c). The measured fluorescence lifetime of 2385 μs indicates that no water molecule is present.29 However, contrary to curium, when an excess of Cs+ ions is added, the emission intensity of EuW5 increases significantly, by a factor of ∼10, and the lifetime increases from 2385 to 3431 μs. This, combined with the Cm results indicate the Cs ions impact the energy levels of the POM.
In conclusion, this study reports the first example of a Weakley–Peacock-type polyoxometalate complex with a transplutonium element, namely the curium bis-pentatungstate NaCs8Cm(W5O18)2·14H2O. This compound was synthesized at the microscale and characterized via single crystal XRD, Raman microscopy, as well as solid-state and solution-state fluorescence. Comparative experiments with Nd3+ and Eu3+ analogous compounds revealed a divergence between lanthanide and actinide coordination chemistries. This study further highlights the unsuspected but critical impact that counterions, particularly cesium, have on the fluorescence and vibrational modes of [Cm(W5O18)2]9−. These results show that, even within the same coordination chemistry framework (i.e., 8-coordinated complexes with square antiprismatic geometry), 4f and 5f cations exhibit fundamental chemical differences that can be manifested at long-range and even via interactions with other cations present in the structure. Given the high number of heavy tungsten atoms in the POM compounds, in addition to many oxygens and presence of the heavy actinide, computational work (e.g., density functional theory) to explain the fundamental origin of these experimental observations are currently too expensive. Further compounding this, the results presented herein indicate that computational treatment of f-element POM complexes will require considering the full lattice (not just [Cm(W5O18)2]9−, for example), including all cesium or sodium counterions and water molecules. Future work will focus on expanding the experimental space for actinide–POM compounds in order to provide a consistent framework for future theoretical work on this, so far, overlooked class of actinide compounds.
This material is based upon work supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Element Chemistry program at Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. Release number: LLNL-JRNL-861963.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef.
- C. Busche, L. Vilà-Nadal, J. Yan, H. N. Miras, D.-L. Long, V. P. Georgiev, A. Asenov, R. H. Pedersen, N. Gadegaard, M. M. Mirza, D. J. Paul, J. M. Poblet and L. Cronin, Nature, 2014, 515, 545–549 CrossRef CAS PubMed.
- J.-J. Chen, M. D. Symes and L. Cronin, Nat. Chem., 2018, 10, 1042–1047 CrossRef CAS PubMed.
- A. Gaita-Ariño, F. Luis, S. Hill and E. Coronado, Nat. Chem., 2019, 11, 301–309 CrossRef PubMed.
- N. Gao, H. Sun, K. Dong, J. Ren, T. Duan, C. Xu and X. Qu, Nat. Commun., 2014, 5, 3422 CrossRef PubMed.
- D. Wang, L. Liu, J. Jiang, L. Chen and J. Zhao, Nanoscale, 2020, 12, 5705–5718 RSC.
- R. D. Peacock and T. J. R. Weakley, J. Chem. Soc. A, 1971, 1836–1839 RSC.
- I. Lindqvist, Ark. Kemi, 1953, 5, 247–250 CAS.
- J. F. Keggin and W. L. Bragg, Proc. R. Soc. London, Ser. A, 1997, 144, 75–100 Search PubMed.
- B. Dawson, Acta Crystallogr., 1953, 6, 113–126 CrossRef CAS.
- M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer, Berlin, Heidelberg, 1983 Search PubMed.
- M. T. Pope, in Structural Chemistry of Inorganic Actinide Compounds, ed.S. V. Krivovichev, P. C. Burns and I. G. Tananaev, Elsevier, Amsterdam, 2007, pp. 341–361 Search PubMed.
- I. Colliard, J. R. I. Lee, C. A. Colla, H. E. Mason, A. M. Sawvel, M. Zavarin, M. Nyman and G. J.-P. Deblonde, Nat. Chem., 2022, 14, 1357–1366 CrossRef CAS PubMed.
- C. A. Colla, I. Colliard, A. M. Sawvel, M. Nyman, H. E. Mason and G. J.-P. Deblonde, Inorg. Chem., 2023, 62, 6242–6254 CrossRef CAS PubMed.
- M. N. Sokolova, A. M. Fedosseev, G. B. Andreev, N. A. Budantseva, A. B. Yusov and P. Moisy, Inorg. Chem., 2009, 48, 9185–9190 CrossRef CAS PubMed.
- H. Zhang, A. Li, K. Li, Z. Wang, X. Xu, Y. Wang, M. V. Sheridan, H.-S. Hu, C. Xu, E. V. Alekseev, Z. Zhang, P. Yan, K. Cao, Z. Chai, T. E. Albrecht-Schönzart and S. Wang, Nature, 2023, 616, 482–487 CrossRef CAS PubMed.
- W. P. Griffith, N. Morley-Smith, H. I. S. Nogueira, A. G. F. Shoair, M. Suriaatmaja, A. J. P. White and D. J. Williams, J. Organomet. Chem., 2000, 607, 146–155 CrossRef CAS.
- K. Zheng and P. Ma, Dalton Trans., 2024, 53, 3949–3958 RSC.
- M. Shiddiq, D. Komijani, Y. Duan, A. Gaita-Ariño, E. Coronado and S. Hill, Nature, 2016, 531, 348–351 CrossRef CAS PubMed.
- H. Zhang, X. Li, L. Zhang, Y. Zhou, X. Ren and M. Liu, J. Alloys Compd., 2018, 749, 229–235 CrossRef CAS.
- C. Adam, B. B. Beele, A. Geist, U. Müllich, P. Kaden and P. J. Panak, Chem. Sci., 2015, 6, 1548–1561 RSC.
- S. K. Cary, J. Su, S. S. Galley, T. E. Albrecht-Schmitt, E. R. Batista, M. G. Ferrier, S. A. Kozimor, V. Mocko, B. L. Scott, C. E. Van Alstine, F. D. White and P. Yang, Dalton Trans., 2018, 47, 14452–14461 RSC.
- G. J.-P. Deblonde, J. A. Mattocks, H. Wang, E. M. Gale, A. B. Kersting, M. Zavarin and J. A. Cotruvo, J. Am. Chem. Soc., 2021, 143, 15769–15783 CrossRef CAS PubMed.
- S. Skanthakumar, M. R. Antonio, R. E. Wilson and L. Soderholm, Inorg. Chem., 2007, 46, 3485–3491 CrossRef CAS PubMed.
- P. Villars, K. Cenzual, R. Gladyshevskii, O. Shcherban, V. Dubenskyy, V. Kuprysyuk, I. Savysyuk and R. Zaremba, in Structure Types. Part 11: Space groups (135) P42/mbc – (123) P4/mmm, ed. P. Villars and K. Cenzual, Springer Berlin Heidelberg, Berlin, Heidelberg, 2012, vol. 43A11, pp. 213–214 Search PubMed.
- M. J. Polinski, D. J. Grant, S. Wang, E. V. Alekseev, J. N. Cross, E. M. Villa, W. Depmeier, L. Gagliardi and T. E. Albrecht-Schmitt, J. Am. Chem. Soc., 2012, 134, 10682–10692 CrossRef CAS PubMed.
- S. S. Galley, S. A. Pattenaude, C. A. Gaggioli, Y. Qiao, J. M. Sperling, M. Zeller, S. Pakhira, J. L. Mendoza-Cortes, E. J. Schelter, T. E. Albrecht-Schmitt, L. Gagliardi and S. C. Bart, J. Am. Chem. Soc., 2019, 141, 2356–2366 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., 1976, A32, 751–767 CrossRef CAS.
- J. Bruno, W. D. Horrocks and R. J. Zauhar, Biochemistry, 1992, 31, 7016–7026 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2333195 and 2326544. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc01381f |
| This journal is © The Royal Society of Chemistry 2024 |
