 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface modification of TiO2 nanoparticles with organic molecules and their biological applications
Farid
Hajareh Haghighi
 *a,
Martina
Mercurio
a,
Sara
Cerra
*a,
Martina
Mercurio
a,
Sara
Cerra
 a,
Tommaso Alberto
Salamone
a,
Roya
Bianymotlagh
a,
Cleofe
Palocci
ab,
Vincenzo
Romano Spica
c and
Ilaria
Fratoddi
a,
Tommaso Alberto
Salamone
a,
Roya
Bianymotlagh
a,
Cleofe
Palocci
ab,
Vincenzo
Romano Spica
c and
Ilaria
Fratoddi
 *a
*a
aDepartment of Chemistry, Sapienza University of Rome, Piazzale Aldo Moro 5, 00185, Rome, Italy. E-mail: ilaria.fratoddi@uniroma1.it; farid.hajarehhaghighi@uniroma1.it; Tel: 0039-06 4991 3182
bResearch Center for Applied Sciences to the Safeguard of Environment and Cultural Heritage (CIABC), Sapienza University of Rome, Piazzale Aldo Moro 5, 00185 Rome, Italy
cDepartment of Movement, Health and Human Sciences, University of Rome Foro Italico, Piazza Lauro De Bosis, 15, 00135 Rome, Italy
First published on 27th February 2023
Abstract
In recent years, titanium(IV) dioxide nanoparticles (TiO2NPs) have shown promising potential in various biological applications such as antimicrobials, drug delivery, photodynamic therapy, biosensors, and tissue engineering. For employing TiO2NPs in these fields, their nanosurface must be coated or conjugated with organic and/or inorganic agents. This modification can improve their stability, photochemical properties, biocompatibility, and even surface area for further conjugation with other molecules such as drugs, targeting molecules, polymers, etc. This review describes the organic-based modification of TiO2NPs and their potential applications in the mentioned biological fields. In the first part of this review, around 75 recent publications (2017–2022) are mentioned on the common TiO2NP modifiers including organosilanes, polymers, small molecules, and hydrogels, which improve the photochemical features of TiO2NPs. In the second part of this review, we presented 149 recent papers (2020–2022) about the use of modified TiO2NPs in biological applications, in which specific bioactive modifiers are introduced in this part with their advantages. In this review, the following information is presented: (1) the common organic modifiers for TiO2NPs, (2) biologically important modifiers and their benefits, and (3) recent publications on biological studies on the modified TiO2NPs with their achievements. This review shows the paramount significance of the organic-based modification of TiO2NPs to enhance their biological effectiveness, paving the way toward the development of advanced TiO2-based nanomaterials in nanomedicine.
1. Introduction
With the advent of nanotechnology, numerous nanomaterials have been synthesized and applied for various applications and among them, titanium dioxide nanoparticles (TiO2NPs) are commonly used in the fields of biomedicine,1 food industry,2 wastewater purification,3 and cosmetics4 owing to their unique physicochemical properties such as high chemical stability and photodynamic effects. Regarding their industrial importance, the global market size of TiO2NPs was estimated to be $1.1 billion in 2021, and forecasted to have a compound annual growth rate of 6.5% until 2026, with annual production predicted to reach 2.5 million tons by 2025.5,6 In the biomedical fields, TiO2NPs are frequently studied for photodynamic therapy,1,7 drug delivery,8 antimicrobial applications,3,9–12 biosensors,13 and tissue engineering.14 For these applications, an appropriate surface modification of TiO2NPs is required to improve their physicochemical properties and biological effectiveness and, more importantly, decrease their potential toxicity in mammalian cells.5 The surface modification not only prevents the agglomeration of TiO2NPs but also provides the possibility for further functionalization/conjugation. The modification of TiO2NPs can be achieved using two different approaches: non-covalent and covalent conjugation of organic (or inorganic) species with TiO2NPs. The non-covalent strategy is based on physical interactions (electrostatic, hydrogen bond, van der Waals, and hydrophobic interactions), having benefits of being relatively simple and not changing the structure of the modifiers. However, this type of modification can be easily influenced by different external stimuli, such as temperature, pH, and ionic strength.15 On the other side, the covalent modification (or chemical modification) occurs via covalent bonding of modifiers to the TiO2NP surface which can be performed using various coupling agents such as polymers, organophosphorus molecules, carboxylic acids, and organosilanes, and among them, silane compounds are more common for the surface modification of TiO2NPs.16 In recent years, there has been increasing interest for these two types of modification strategies and this review presents recent publications of organic-based TiO2NP modification, followed by the presentation of those organic modifiers which were used for the biomedical applications with their advantages.2. Surface chemistry of TiO2NPs
Like other types of nanoparticles, TiO2NPs have two distinct atoms: (1) internal and (2) surface atoms. The internal part of TiO2NPs is chemically inert (each Ti atom has four chemical bonds with four neighboring oxygens) and so, the remaining surface atoms are mainly responsible for the interaction of nanoparticles with the environment. The surface atoms are not chemically saturated (they are only attached to the internal atoms), so they should complete their coordination number to gain a stable electronic configuration. Ti and O are considered as hard atoms based on the HSAB concept (hard and soft (Lewis) acids and bases)8 and they tend to interact with the hard atoms. Due to the adsorption of water molecules from the environment, the surface of TiO2NPs has two main OH groups: (1) Ti–OH and (2) Ti–ObrH–Ti (br: bridging) (Fig. 1).17–21 The interaction of TiO2NPs with the surroundings occurs through these two types of OH groups.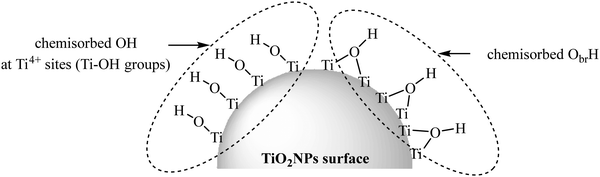 | ||
| Fig. 1 Two different OH groups of the TiO2NP surface. Adapted with permission from ref. 20. Copyright 2014 American Chemical Society. | ||
3. Surface modification of TiO2NPs
Organic stabilizers can bind to the TiO2NP surface by either physical or chemical interactions. The chemical stabilization (or covalent stabilization) is much stronger than the physical one and, in most cases, it results in long-term stability for the modified TiO2NPs.22 In the following sections, the common surface stabilizers of TiO2NPs will be presented into two groups of (1) organofunctional silanes and (2) polymers, small molecules, and hydrogels. In the structure of silane modifiers, there are active functional groups (for example hydrolysable alkoxy groups) which are suitable for the chemical interaction with TiO2NPs.23 On the other hand, physical modification can be carried out by using these organic molecules which are adsorbed on the TiO2NP surface by electrostatic, hydrogen bond, van der Waals, and hydrophobic interactions.243.1. Organofunctional silanes
| Synthesis methods | Advantages | Disadvantages |
|---|---|---|
| Stöber method | Controllable silica shell and TiO2 monodispersity | Lack of understanding of its kinetics and mechanism |
| Microemulsion | Control of the TiO2NP size and high homogeneity | Poor yield, time consuming and large amounts of solvent required |
| Aerosol pyrolysis | Highly productive and suitable for large-scale production | Complex experimental conditions |
| Methods based on sodium silicate solution | Control of the crystallinity and surface area | Depends on the preparation method |
The Stöber method is the most employed method for the silica modification of bare TiO2NPs. In the standard Stöber approach, TiO2NPs are uniformly dispersed in an ethanol solution, followed by the addition of tetraethoxysilane (TEOS) and aqueous ammonia solution (NH3(aq)), respectively.26 Ammonia acts as a basic catalyst to control TEOS hydrolysis and silica thickness to form particles with a regular morphology (see Fig. 2). In this hydrolysis reaction, the –Si–OC2H5 groups of TEOS convert to silanol groups (–Si–OH), and then, the condensation reaction occurs between these –Si–OH groups and the surface –OH groups of TiO2NPs to form chemical Ti–O–Si bonds. This sol–gel reaction results in a 3D silica network around the TiO2NP core. Chen et al. applied the Stöber process to have varying thicknesses of SiO2 onto the surface of anatase and rutile TiO2NPs.27,28 They reported an enhancement of the photocatalytic activity of the modified TiO2NPs when the SiO2 loading weight was lower than 3.25 wt%, while with higher loading percentages, lower photocatalytic activity was observed. Regarding the rutile phase, the complete coverage of TiO2NPs with SiO2 resulted in an enhancement of the photocatalytic activity.
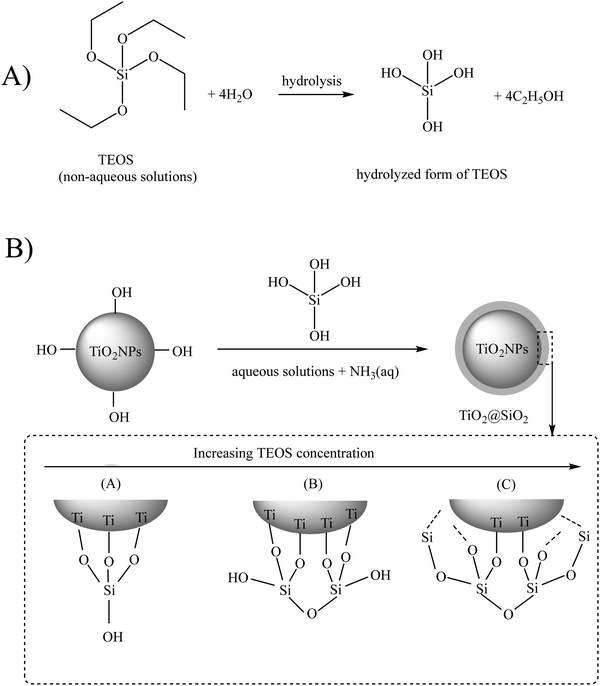 | ||
| Fig. 2 (A) Hydrolysis and (B) condensation reactions of tetraethoxysilane (TEOS). Adapted with permission from ref. 22. Royal Society of Chemistry. | ||
The second approach of silica modification is the microemulsion method, having two different types: (1) water-in-oil (W/O, normal micelles) and (2) oil-in-water (O/W, reverse micelles). Using this method, Xie et al. prepared monodisperse TiO2NPs@SiO2 core–shell particles and showed that the contents of the anatase and rutile crystalline phases of these TiO2NPs were decreased and increased, respectively, when the temperature was increased from 550 °C to 650 °C. In the temperature range of 600–800 °C, the TiO2NPs@SiO2 particles were mainly anatase.29
The third strategy to synthesize TiO2NPs@SiO2 particles is aerosol pyrolysis, considered as an innovative and productive approach, which is usually carried out in a flame environment and can be used for the large-scale production of modified TiO2NP powders. In 2021, Temerov et al. synthesized TiO2NPs@SiO2 (50–70 nm) using a liquid flame spray (LFS) deposition method in a single flame environment.30 They studied the photocatalytic activity of deposited TiO2NPs@SiO2 for oxidation of acetylene into carbon dioxide and they investigated the effect of the silica shell on the photocatalytic activity of these modified TiO2NPs. They reported that the catalytic activity was significantly suppressed when the SiO2 content was increased to 0.5%, 1.0%, 3.0% and 5.0% (33%, 44%, 70% and 100% of suppression, respectively). They mentioned that this suppression might be due to the thick passivating silica layer around the TiO2NP core. Maskrot et al. synthesized a core–shell TiO2NPs@SiO2 composite with different Ti/Si ratios, by the laser pyrolysis of a gas-spray mixture of TEOS and titanium tetra-isopropoxide.31 By increasing the Ti/Si ratio, the color of these modified TiO2NPs@SiO2 composite changes from dark to light blue. Their results showed the correlation between the chemical composition and the size of these TiO2NPs@SiO2 nanoparticles as a function of the Ti/Si ratio.
The fourth route is based on sodium silicate solution as a cheap silica precursor. For instance, Shao et al. used sodium silicate to prepare the TiO2–SiO2 composites using controllable and reproducible approaches to improve the textural properties of the nanostructures.32 The practical photocatalytic application of these TiO2–SiO2 composites was successfully tested for decolorization of methylene blue, as a model pollutant in textile industries.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, epoxy, etc.; n = typically 3), are considered as effective bifunctional silane linkers having two different functional groups in their structures including: (1) –OR moiety (attached to the –Si) and (2) –SH/or –NH2 (or other functional groups) attached to the end of a carbon chain.22 In the general formulation of (RO)3Si–(CH2)n–X, R can be an alkyl, aryl or generally organofunctional group. As shown in Fig. 3, for the surface modification of TiO2NPs, the –OR groups should be hydrolyzed to form silanol groups (–SiOH), followed by the condensation of these silanols with the –OH groups of the bare TiO2 nanosurface, resulting in the formation of a silica network around the TiO2NP core and providing the suitable X functional groups onto the surface. As common organosilane coupling agents, 3-methacryloxypropyltrimethoxysilane (MPS),33 3-aminopropyltriethoxysilane (APTES),34–38 3-glycidoxypropyltrimethoxysilane (GPS),39 and n-propyltriethoxysilane22 are used for different applications. The other recommendations for this type of modification are shown in Table 2.
C, epoxy, etc.; n = typically 3), are considered as effective bifunctional silane linkers having two different functional groups in their structures including: (1) –OR moiety (attached to the –Si) and (2) –SH/or –NH2 (or other functional groups) attached to the end of a carbon chain.22 In the general formulation of (RO)3Si–(CH2)n–X, R can be an alkyl, aryl or generally organofunctional group. As shown in Fig. 3, for the surface modification of TiO2NPs, the –OR groups should be hydrolyzed to form silanol groups (–SiOH), followed by the condensation of these silanols with the –OH groups of the bare TiO2 nanosurface, resulting in the formation of a silica network around the TiO2NP core and providing the suitable X functional groups onto the surface. As common organosilane coupling agents, 3-methacryloxypropyltrimethoxysilane (MPS),33 3-aminopropyltriethoxysilane (APTES),34–38 3-glycidoxypropyltrimethoxysilane (GPS),39 and n-propyltriethoxysilane22 are used for different applications. The other recommendations for this type of modification are shown in Table 2.
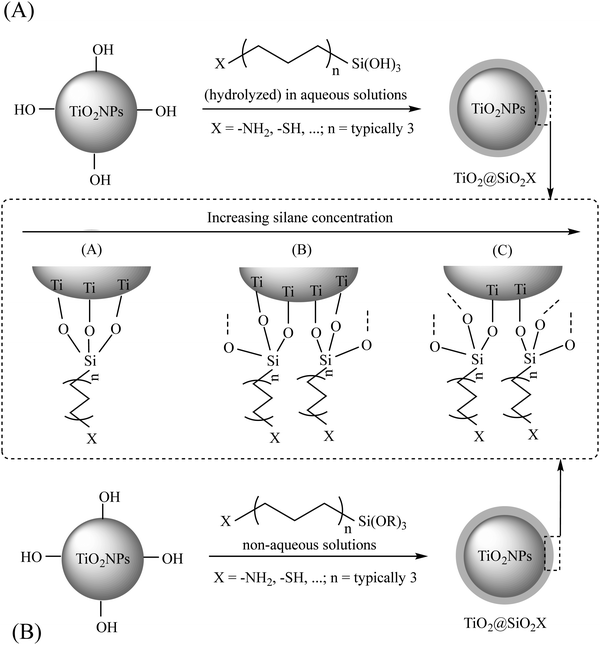 | ||
| Fig. 3 The interaction between a general silane coupling agent and TiO2NPs in aqueous (A) and non-aqueous (B) solutions. Adapted with permission from ref. 22. Royal Society of Chemistry. | ||
| Modifying agent | Structure | Ref. |
|---|---|---|
| (3-Trimethoxysilyl)propyl methacrylate, KH–570 |
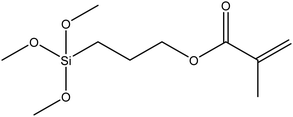
|
40 |
| Fluorosilane | H3Si–F | 41 |
| Glycidyl methacrylate |
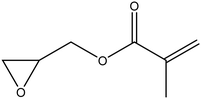
|
42 |
| Bis-(3-triethoxysilylpropyl)tetrasulfide (TESPT) |
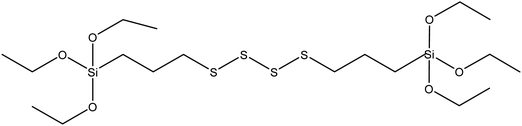
|
43 |
| (3-Aminopropyl)trimethoxysilane |
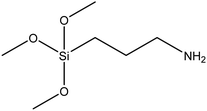
|
44 and 45 |
| (3-Aminopropyl)triethoxysilane (APTES) |
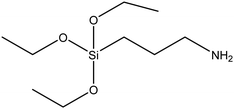
|
46 |
| (3-Mercaptopropyl)triethoxysilane (MPTES) |
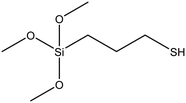
|
47 |
| Hexadecyltrimethoxysilane |
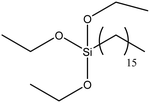
|
48 |
| Vinyltrimethoxysilane (VTMS) |
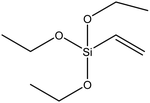
|
49 |
| Ascorbic acid 6-palmitate |
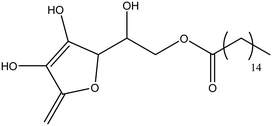
|
50 |
| (3-Methacryloxypropyl)trimethoxysilane |
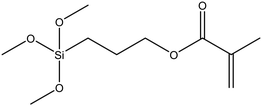
|
51 |
| 3-Isocyanato propyl trimethoxysilane |
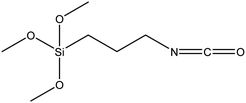
|
52 |
Because of the importance of such bifunctional silane linkers, recent publications on some of these triorganosilanes will be presented as follows:
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C on the TiO2NP surface, and vinyltriethoxysilane (VTES) can be an excellent recommendation in this regard. Aqeel Ashraf et al. successfully used VTES and TEOS to modify TiO2NPs (via a hybrid sol–gel coating) to protect the commercial AZ91 magnesium alloy against corrosion in a 0.05 M NaCl solution.63 Tangchantra et al. modified TiO2NPs with three different silane coupling agents, such as VTES, hexadecyltrimethoxysilane (HTMS), and aminopropyltrimethoxysilane (APTMS).64 Their results demonstrated that the VETS modification could improve the dispersibility and mechanical properties of the TiO2NPs. Yang et al. reported the VTES silanization of TiO2 particles (via a sol–gel method) and their results exhibited the improvement of colloidal stability in tetrachloroethylene solvent.65
C on the TiO2NP surface, and vinyltriethoxysilane (VTES) can be an excellent recommendation in this regard. Aqeel Ashraf et al. successfully used VTES and TEOS to modify TiO2NPs (via a hybrid sol–gel coating) to protect the commercial AZ91 magnesium alloy against corrosion in a 0.05 M NaCl solution.63 Tangchantra et al. modified TiO2NPs with three different silane coupling agents, such as VTES, hexadecyltrimethoxysilane (HTMS), and aminopropyltrimethoxysilane (APTMS).64 Their results demonstrated that the VETS modification could improve the dispersibility and mechanical properties of the TiO2NPs. Yang et al. reported the VTES silanization of TiO2 particles (via a sol–gel method) and their results exhibited the improvement of colloidal stability in tetrachloroethylene solvent.65
Regarding the other types of silanes and non-silane coupling agents, there are several worthwhile publications which are briefly mentioned here. For example, Caris et al. utilized conventional emulsion polymerization to encapsulate TiO2 in poly(methyl methacrylate) (PMMA).66 Weng and Wei studied the radical polymerization of styrene and methyl methacrylate (MMA), initiated at the surface of TiO2 particles by adsorbed hydroperoxide macroinitiators.67 Erdem et al. modified TiO2NPs by the miniemulsion polymerization of styrene and polybutene–succinimide pentamine being used as the stabilizer at the oil/water interface.68 Rong et al. reported the modification of TiO2NPs by (3-trimethoxysilyl)propylmethacrylate, followed by the free-radical copolymerization of styrene with the methacrylate group of 3-methacryloxypropyltrimethoxysilane (MPS).69 Yang and Dan used a similar approach to attach poly(methyl methacrylate) on the modified surface of TiO2NPs.70 Milanesi et al. employed a mixture of isomeric octyltriethoxysilanes (OTESs) to form a hydrophobic layer around TiO2NPs.71 They reported the formation of cross-linked and chemical bonded Ti–O–Si onto the modified TiO2NPs. Xiang et al. used MPS to modify the TiO2NP surface and enhance their compatibility with the poly(butyl acrylate) (PBA) matrix.72 In another study, Qi et al. synthesized hydrophobic TiO2NPs using the acrylonitrile–styrene–acrylate (ASA) terpolymer for cool materials.51 Wang et al. functionalized commercial TiO2NPs with MPS via ultrasonic treatment at room temperature.73 Godnjavec et al. coated TiO2NPs by 3-glycidyloxypropyltrimethoxysilane (GLYMO) as an additive in a clear polyacrylic coating and reported that the modified TiO2NPs improved dispersion, transparency, and UV protection of the clear acrylic coating.74 Dalod et al. modified TiO2NPs with APTES, 3-(2-aminoethylamino)propyldimethoxymethylsilane (AEAPS), and n-decyltriethoxysilane (DTES) using a hydrothermal method and reported that the shape and structure of these nanoparticles depend on the type of silane coupling groups.75
3.2. Polymers, small molecules, and hydrogels
| Polymers | Source/production/preparation25 | Ref. |
|---|---|---|
| Polyethylene glycol (PEG) | Produced by the interaction of ethylene oxide with water, ethylene glycol, or ethylene glycol oligomers | 76–85 |
| Polyvinylpyrrolidone (PVP) | Made from the monomer N-vinylpyrrolidone | 86 |
| Polyethyleneimine (PEI) | Branched PEI: by the ring opening polymerization of aziridine Linear PEI: by the post-modification of other polymers like poly(2-oxazolines) or N-substituted polyaziridines | 87 |
| Polyacrylic acids (PAA) | Polymerization of acrylic acid | 88 and 89 |
| Polyvinyl alcohol (PVA) | Polymerization of vinyl acetate and then the saponification of polyvinyl acetate | 90 and 91 |
| Polydopamine (PDA) | Formed from dopamine at slightly basic pH | 92 |
| Dextran | Produced by lactic acid bacteria | 93 |
| Chitosan | Extracted from shellfish or the fungal cell wall | 94–97 |
| Starch | Produced by green plants | 98 and 99 |
| Alginate | Extracted from brown algae | 100 and 101 |
| Polyphenol | Found in some common plant foods like cocoa, beans, tea, and vegetables | 102 |
| Amino acids | In nature | 103 |
| Flavonoids | Found in some common plant foods like fruits, vegetables, beans, and tea | 104 and 105 |
PEG is a commonly used water-soluble polymer for the surface modification of TiO2NPs, which can enhance the biocompatibility and hydrophilicity of the nanoparticles for biological applications. Recently, several excellent research studies have been reported on the PEG-coated TiO2NPs; for example, in 2022, Connoly et al. compared the bioaccumulation, biodistribution and depuration profile of uncoated TiO2NPs and PEG-modified TiO2NPs in rainbow trout, after 10 days dietary exposure and a 42 day depuration phase.76 Their results showed that PEG modification had an influence on levels of uptake and distributions of the modified TiO2NPs, and a higher uptake of PEG-coated TiO2NPs was observed, compared to the fish exposed to the uncoated TiO2NPs. Tsotetsi et al. synthesized TiO2NPs and then modified their surface with PEG, polyvinylpyrrolidone (PVP), and Pluronic F127 as pore forming agents, to investigate the effects of surface modification on the pore size, morphology, specific surface area, and optical properties of the TiO2NPs.77 All these three modified samples showed porous morphologies with spherical shapes and specific surface areas of ∼69.82, 37.80 and 57.08 m2 g−1 for TiO2-F127, TiO2-PVP and TiO2–PEG, respectively (after calcination at 550 °C). The pore sizes were estimated to be ∼13.01, 10.10 and 8.53 nm for TiO2-F127, TiO2-PVP and TiO2–PEG, respectively. Their results indicated that the surface modification of bare TiO2NPs can improve their photophysical properties to act as an efficient electron transporting layer in solar cell applications. Koushali et al. studied the effects of synthesized TiO2–PEG on the morphological, thermal, and mechanical properties of unsaturated polyester (UPE) nanocomposites.78 The UPE/PEG/TiO2 nanocomposites were prepared by direct mechanical mixing of these three components at different weight ratios of both TiO2NPs and PEG. Their results showed an improvement in the mechanical and thermal characteristics of the nanocomposites containing 0.5 wt% of synthesized TiO2NPs and 10 wt% of PEG, compared to the pristine polyester. Abasifard Dehkordi et al. studied the addition of TiO2/ZnO nanoparticles and PEG (with different molar ratios) to Portland cement to improve the photocatalytic and antibacterial activities of the cement.79 They evaluated the potential ability of this composite for decolorization of an azo dye (as an organic pollutant) and inactivation of E. coli and S. aureus mutants. Their results showed a concentration-dependence antibacterial effect of the modified cement and effective photocatalytic degradation of the dye, which showed promising potential of this modified cement to be used as a self-cleaning and antibacterial coating for urban constructions. Iqbal et al. deposited gold nanoparticles onto TiO2NPs, followed by the PEG modification of the TiO2–Au nanohybrid.106 The authors used this nanohybrid as a novel photosensitizing agent after biodistribution toward the targeted site (cancerous cell injury in the MCF-7 cell line), and showed that the different morphologies of PEG-modified Au-doped TiO2NPs provided various therapeutic effects. Landolsi et al. synthesized TiO2NPs and then modified them with PEG, followed by decoration of Fe2O3NPs onto the surface of modified TiO2NPs.81 The photocatalytic activity of the TiO2NPs–PEG–Fe2O3NPs was studied for the degradation of methylene blue and the significant degradability performance was observed under visible light irradiation (up to 70% after 150 min of irradiation). Bai et al. synthesized ultrasmall iron-doped TiO2 nanodots (Fe–TiO2 NDs) as a type of sonosensitizers to study their potential applications in sonodynamic therapy.82 After PEG modification, Fe–TiO2–PEG NDs showed improved physiological stability and biocompatibility, with efficient tumor retention. Compared to commercial TiO2NPs, this modified TiO2 nanostructure demonstrated higher in vivo therapeutic performance with no long-term toxicity to the treated mice after one month. Birinci et al. used PEG-modified TiO2NPs in the formulation of a novel nano-antioxidant that utilized quercetin-conjugated TiO2NPs (QTiO2, quercetin is a potent antioxidant) for fortifying skin defense against oxidative toxicity.83 The PEG-modified TiO2NPs exhibited better colloidal stability and biocompatibility (compared to the unmodified TiO2NPs), which causes an easy adhesion of these nanosurfaces onto living cells. This nano-antioxidant QTiO2 showed an efficient delivery of Q molecules into mouse fibroblast cells and improved the cellular antioxidant defense system against oxidative toxicity. Wang et al. synthesized TiO2NPs and studied their surface modification with single and mixed stabilizers, such as PEG, cetyltrimethylammonium bromide (CTAB) and carboxamide.107 Their results showed that the surfactants strongly affect the morphology of these TiO2NPs and, for the PEG, they obtained ellipse modified TiO2NPs having an improved photocatalytic activity for the degradation of methyl orange under UV irradiation. Wang et al. synthesized ultrafine titanium monoxide nanorods (TiO1+x NRs) and then modified them with PEG.84 TiO1+x NRs–PEG was used as a new sonodynamic agent and showed much more efficiency for the ultrasound-induced generation of reactive oxygen species (ROS), compared to the conventional sonosensitizer. Interestingly, TiO1+x NRs–PEG could also generate hydroxyl radicals (OH˙−) from endogenous H2O2 in the tumor to enable chemo-dynamic therapy (CDT). For the treated mice, TiO1+x NRs–PEG showed efficient passive retention in tumors post-intravenous injection, with no significant long-term toxicity, indicating the potential ability of this modified TiO2 nanostructure to be used as a sonosensitizer and a CDT agent.
Chitosan (CS) is another frequent hydrophilic polymer for the surface modification of TiO2NPs, which has low toxicity, good biocompatibility and biodegradability.94 Chitosan-modified TiO2NPs (TiO2NPs–CS) have potential applications in various technologies such as photocatalytic nanostructures,108 antibacterial package materials,109 wound healing materials,110,111 wastewater treatment,112 and sensors.113 From a structural point of view, CS has reactive amino and hydroxyl side groups which can interact with TiO2NPs by hydrogen bonding and form stable nanocohybrids.94 For instance, in 2022, Moulahou et al. synthesized TiO2–chitosan nanocomposites (TiO2NPs–CS) and combined them with various metal ions (silver, zinc, copper, and iron) to prepare novel chitosan-based films and improve the physicochemical and biological activities of the individual components.95 The antimicrobial properties of these films were studied against three pathogens (P. aeruginosa, C. albicans, and S. aureus) under different light conditions. Among these nanocomposites, TiO2NPs–CS–Ag showed the best antibacterial activity. Besides, the nanocomposites were tested for the photocatalytic degradation of methylene blue (MB), and the best data were observed for TiO2NPs–CS–Cu with a higher specificity towards MB than the other two tested dyes (methyl orange and bromophenol blue). These results showed that the TiO2NPs–CS–metal ions have great potential as ambient light packaging materials, coating materials, and photocatalysts. In another recent study, Castillo et al. used TiO2NPs–chitosan for the electroanalytical detection of imidacloprid, (a neonicotinoid) that is a systemic insecticide and can accumulate in agricultural products and negatively affect human health.94 The authors showed that the TiO2NPs–chitosan, with a high surface area, served as molecular recognition sites for the imidacloprid detection, with an optimum concentration of 40 wt% of TiO2NPs. Elmehbad et al. prepared two new chitosan derivatives by incorporating salicylhydrazide into a chitosan Schiff base (SCsSB) and chitosan (SCs) to make two nanohybrids, SCs/TiO2-1% and SCs/TiO2-3%.96 The anti-biofilm and antimicrobial activities of these nanostructures were ranked as SCs/TiO2-3% > SCs/TiO2-1% > SCs > SCsSB. Their results showed that the modification of TiO2NPs with the chitosan polymer enhanced the antibacterial performance of the components. SCs/TiO2-3% was biocompatible with normal human cells, indicating the potential ability of this nanocomposite for antimicrobial agents. Majnis et al. integrated chitosan (CS) and ZrO2 into TiO2NPs through a sol–gel fabrication method, to make the CS–ZrO2/TiO2 photocatalyst having a smaller band gap, compared to the unmodified TiO2NPs.97 The photodegradation efficiency of CS–ZrO2/TiO2 was tested (under solar irradiation) using a model dye, malachite green (MG), and the results showed an increase efficiency from 11.87% (for TiO2NPs alone) to 30.72% (with ZrO2 and CS addition in TiO2NPs).
Also, other polymers such as polydopamine (PDA), polysaccharide, polylactic acid (PLA), polyacrylic acid (PAA), alginate (Al), polyvinylidene fluoride, PEI, PVP, and PAMAM (polyamidoamine)114,115 are used for the surface modification of TiO2NPs (see Table 4). For example, Dong et al. used polydopamine (PDA) with excellent hydrophilicity for the surface modification of TiO2NPs.92 Then, these PDA-modified TiO2NPs were combined with hydrophobic graphene (Gr) via the π–π non-covalent interaction to enhance the water dispersion stability of Gr. Regarding practical applications, this nanocomposite was tested for its fire resistance ability inside the intumescent waterborne epoxy coating. The results showed that introducing PDA and TiO2NPs effectively improved the oxidation resistance and stability of the composite. Zhang et al. prepared TiO2NP–PDA hybrid nanoparticles to reduce oxygen vacancies in TiO2NPs and provide better stability for the polymer matrix. TiO2NP–PDA was composited with polylactic acid (PLA) to prepare PLA/TiO2NP–PDA nanocomposites.116 These nanocomposite films showed excellent UV-shielding performance without sacrificing transparency (the transmittance at 550 nm was 85.7%). PLA is very sensitive to UV light; however, due to the shielding effect of TiO2NPs–PDA in this film, the performance of PLA was improved, and it can broaden its application for environmental materials having a wider range of uses with a longer service life.
| Name | Structure | Stability | Modification mechanism (covalent/non- covalent) | Applications | Ref. |
|---|---|---|---|---|---|
| Polysaccharide |
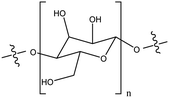
|
Strongly modified the TiO2NP stability by inducing their partial and rapid disagglomeration, by steric effects and electrostatic interactions | N/A | Water purification | 119 |
| Poly lactic acid |
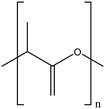
|
Highly dispersible by steric effects | Hydrogen bonding interactions | Antimicrobial against S. aureus, Salmonella and E. coli | 90, 120 and 121 |
| Alginic acid |
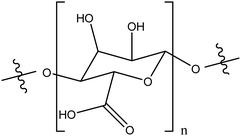
|
Enhanced stability in water, compared to unmodified TiO2NPs through combination of electrostatic repulsion and steric effects | Electrostatic interactions | Environmental applications | 100 and 101 |
| Polyvinylidene fluoride |
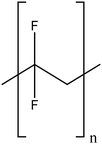
|
Excellent stability of modified TiO2NPs by steric effects | N/A | Increased anti-fouling properties | 122 and 123 |
| Polyethyleneimine (PEI) |
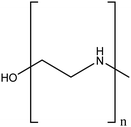
|
Enhanced stability in water, compared to unmodified TiO2NPs by electrostatic repulsion | Physical interactions | Photodegradation of methylene blue | 87 |
| Polyvinylpyrrolidone (PVP) |
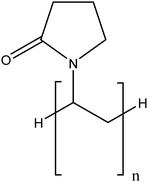
|
Considerable improvement in terms of stability compared to unmodified TiO2NPs via steric hindrance | N/A | Enhancing the TiO2NP dispersion in blood and urine | 86 |
TiO2NPs are also used as food whitening in candies, chocolates, and cakes with a high carbohydrate content. However, there is a little knowledge about the potential interaction between the food carbohydrate and food grade-TiO2NPs. In the case of polysaccharides, Qiaorun et al. studied the interaction between TiO2NPs and seven common carbohydrates (including monosaccharides, disaccharides, and polysaccharides).117 Their results showed that the TiO2NPs can interact with all these tested carbohydrates and enter the body as a food additive, and interact with the food matrix for a series of reactions. The food polysaccharides showed stronger adsorption onto the TiO2NPs than monosaccharides and disaccharides.
Regarding polylactic acid (PLA), Tajdari et al. prepared ZnO–PLA, TiO2–PLA and ZnO/TiO2–PLA nanocomposites with different percentages of nanoparticles and two different types of ZnO morphologies.118 Their results showed the enhanced mechanical and optical properties of PLA when it was combined with the nanoparticles. Also, the antibacterial activity of PLA was improved against Gram-positive L. monocytogenes and Gram-negative bacteria E. coli by incorporating nanoparticles.
Dextran (Dex) is a polysaccharide with excellent biocompatibility and good water solubility, so the modification of TiO2NPs with dextran can improve the physicochemical properties of the nanoparticle. Naghibi et al. compared the in situ and ex situ surface modifications of TiO2NPs using dextran (Dex) and Dex/poly ethylene glycol (PEG), by comparing the colloidal stability of the modified TiO2NPs prepared by these two methods.93 Their results showed that the in situ additions of Dex and PEG, during the synthesis of TiO2NPs (hydrothermal synthesis), resulted in a highly stable colloidal solution (more than 60 days), whereas the ex situ additions of Dex and PEG (after the TiO2NP synthesis) did not significantly impact on the colloidal stability of TiO2NPs.
| Name | Structure | Stability | Modification mechanism | Applications | Ref. |
|---|---|---|---|---|---|
| Oleic acid |
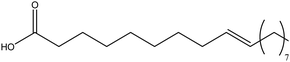
|
Steric hindrance | Chemically bonded with the surface titanium ion (by bidentate linkages) | Healing excision wounds were studied in the rat animal model | 126–128 |
| Cyclodextrin |
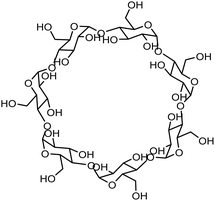
|
Improvement of the colloidal stability, steric hindrance | N/A | Degradation of wastewater pollutants, antibacterials | 129 |
| Lauric acid |
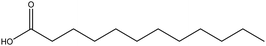
|
The functionalized TiO2NPs exhibited significantly reduced agglomeration, both in dry and in dispersed states (in oily media) | Chemical interaction with silane-functionalized TiO2NPs | UV filtering ability | 130 |
| Dopamine |
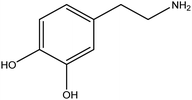
|
Steric stabilization of TiO2NPs when it is polymerized to polydopamine | Probable chemical interactions (determined by computational chemistry) | Drug discovery, diagnostics, environmental applications, and food safety | 131 |
| Dimercaptosuccinic acid (DMSA) |
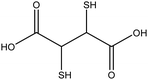
|
Improve dispersity of TiO2NPs in solutions and increase electrostatic repulsion between nanoparticles | N/A | Cytotoxicity on human aortic endothelial cells | 132 and 133 |
However, in some biomedical applications, the use of lipophilic-modified TiO2NPs is greatly limited due to low dispersity of the nanoparticles in biological aqueous solutions. For the medical applications of modified TiO2NPs, the research is more focused on the synthesis of hydrophilic or water dispersible TiO2NPs. In this regard, several small organic molecules such as amino acids, citric acid, cyclodextrin, dopamine, lauric acid, and dimercaptosuccinic acid (DMSA) are often used for the surface modification of TiO2NPs to enhance the hydrophilicity of modified nanoparticles for the biological applications. In the case of citrate (or citric acid), Connolly et al. studied the bioaccumulation of uncoated TiO2NPs and TiO2NPs–citrate in fish to investigate the relationship between surface coating and uptake (biokinetics) in vivo.76 Rainbow trout (Oncorhynchus mykiss) were fed diets spiked with the uncoated and citrate-coated TiO2NPs (100 mg NPs per kg feed) for 10 days and thereafter, fish were allowed to depurate for 42 days. Their results showed that the surface modification affected the uptake and, in some cases, caused slower depuration and distinct distributions. In another research, Liu et al. synthesized citrate-coated Gd-doped TiO2 ellipsoidal nanoparticles (GdTi-SC NPs) to improve the efficiency of gadolinium-based T1 contrast agents (CAs) for magnetic resonance imaging (MRI).124 The in vivo MRI tests on rats demonstrated that the modified TiO2NPs have a high potential ability as high-performance T1 contrast agents for the sensitive imaging of blood vessels and the accurate diagnosis of vascular lesions. Peper et al. studied redox reactions of aqueous colloidal solutions of both citrate capped- and uncapped-TiO2NPs (c-TiO2 and uc-TiO2) and reported the different redox behaviors of these two systems.125
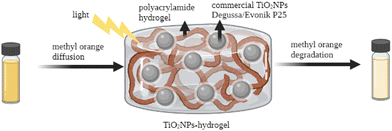 | ||
| Fig. 4 TiO2NP–hydrogel system for the photocatalytic degradation of methyl orange. Adapted with permission from ref. 139. Copyright 2022 Elsevier. | ||
Ulu et al. reported the preparation and characterization of chitosan/PEG/TiO2NP (CH/PEG/TiO2NP) composite hydrogels for antibacterial applications.140 Their results showed that the CH/PEG/TiO2NPs improved the mechanical and thermal properties of the hydrogel due to the presence of TiO2NPs. More importantly, this TiO2 nanocomposite showed potential antimicrobial activity. Yue et al. prepared a novel photocatalytic hydrogel by loading TiO2NPs onto the surface of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized chitin nanofibers (TOCNs), which were further incorporated into the polyacrylamide (PAM) matrix.141 The presence of TiO2NPs enhanced the compressive strength of this hydrogel with excellent stretchability and photocatalytic activity.
In the first part of this review, it can be concluded that the organic-based surface modification of TiO2NPs can result in a significant improvement of their physicochemical properties for preparing much more effective TiO2NP-based nanostructures and decreasing the potential toxicity of TiO2NPs as well. For the following part of this review, the recent biological applications of TiO2-based nanomaterials will be presented. The main aim of this section is to present some specific organic modifiers and polymers for TiO2NPs which have been recently used (2020–2022) in eight main fields of drug delivery, photodynamic therapy, antibacterial, biosensors, antiviral, antifungal, cancer therapy, and tissue engineering. Also, the advantages of these organic modifiers will be discussed.
4. Biological applications of modified TiO2NPs
4.1. Photo-, thermo-, and sonodynamic effects of modified TiO2NPs on cancer cells
According to the World Health Organization, there is an increase in annual cancer cases from 14 to 22 million in 2012–2030 period.142 During recent years, modified TiO2NPs have been studied as promising alternatives for the cancer therapy via different methodologies including photodynamic, photothermal, and sonodynamic therapies.1,7,143–145In the photodynamic therapy, they are regarded as inorganic photosensitizers for anti-cancerous photodynamic therapy (PDT) owing to their unique phototoxic effect upon UV light irradiation. The UV light absorption can excite valence electrons of TiO2NPs to generate electrons and holes on the nanosurface, and consequently, a series of redox reactions are initiated which produce anti-cancerous reactive oxygen species (ROS) such as hydroxyl radicals (HO˙−), superoxide anions (O2˙−), hydrogen peroxide (H2O2), etc.1,7 In spite of their advantages, UV irradiation is not always suitable for PDT due to its limited penetration depth, a lower light content and, more importantly, its harmful side effects for the patients exposed to UV light.146 On this basis, much research has been dedicated to extending the photoresponse of TiO2NPs to the visible light region. In this field, the surface modification of TiO2NPs is focused on the use of biologically active species on the surface of TiO2NPs to enhance the selectivity and therapeutic efficiency of TiO2NPs. Besides, this type of surface modification can reduce the potential toxicity of unmodified TiO2NPs, which is reported in recent publications.5 In the following, some recent publications will be presented in which the TiO2NPs have been modified with such types of organic modifiers to prepare the efficient therapeutic TiO2NPs.
The surface modification of TiO2NPs with organic dyes, especially porphyrins, has attracted growing interest as it can broaden the absorption range of TiO2NPs from the UV region to the visible region.147 Chlorin e6 (Ce6) is a porphyrin-based photosensitizer (PS) with a high sensitizing efficiency,148 which can be conjugated with the TiO2NP surface either by non-covalent or covalent modalities.146 In general, the physical conjugation of PS might suffer from desorption which limit the nanosystem efficiency. Conversely, the covalent attachment of the PS to TiO2NPs can guarantee the stability of the nanosystem, especially when the silane linkers are used which hold a high affinity towards the hydroxyl groups of TiO2NPs. For example, Youssef et al. studied the attachment of Ce6 to TiO2NPs by two approaches: (1) TiO2NPs were encapsulated with silanes (APTES and TEOS) and Ce6 (as PS), followed by polyethylene glycol (PEG) grafting on this shell to obtain TiO2NPs@4Si–Ce6-PEG and (2) for the second approach, the TiO2NPs were first modified only by APTES (as the silane linker) and then Ce6 was covalently attached onto the modified TiO2NPs@APTES via an amide bond to construct TiO2NPs@APTES-Ce6.146In vitro tests on glioblastoma U87 cells were performed to study the cellular uptake, phototoxicity, and dark cytotoxicity of the modified and unmodified TiO2NPs. In contrast to the PEGylated TiO2NPs, the APTES-modified ones showed more PDT efficiency, in which a %89 decrease of U87 viability was observed for 200 μg mL−1 of TiO2NPs@APTES-Ce6, which corresponds to 0.22 μM of Ce6. This surface modification resulted in a change of the absorption profile of the hybridized TiO2NPs from the UV region (for unmodified TiO2NPs) to the visible region. Also, it can enhance the biocompatibility of TiO2NPs and their stability, due to the presence of silane coupling agents on the surface of TiO2NPs.
The modification of TiO2NPs with targeting molecules (such as folic acid (FA)) significantly enhances the selectivity of TiO2NPs to some types of cancer, which is an alternative way to improve the therapeutic efficiency of PDT. The folic acid-modified TiO2NPs can be accumulated in the target sites by increasing the affinity of folic acid-modified NPs to the pathological tissue. Also, this modification can improve cell membrane penetration through folate receptors, which are overexpressed on the surface of some types of cancer cells. For example, Liang et al. synthesized a novel TiO2NPs–folic acid–Al(III) phthalocyanine chloride tetrasulfonic acid (TiO2NPs–FA–Pc) targeting nanosystem for therapy of the folate receptor-positive cancer cells.149 In this system, folic acid (FA) was conjugated with the TiO2NPs as a tumor-targeting agent which enhanced the selectivity of TiO2NPs toward the cancer cells. It should be mentioned that the conventional photosensitizer (Pc) exhibited low selectivity for tumor targeting and low two-photon absorption. The modification of TiO2NPs using this photosensitizer enhanced its two-photon absorption of TiO2NPs–FA–Pc. The in vitro studies of these modified TiO2NPs showed a high PDT efficiency and biocompatibility. Also, it exhibited tumor growth suppression in mice bearing HeLa xenograft tumors with minimal side effects, using low dose of this nanocomposite under low light irradiation.
In another study, Salama et al. investigated the attachment of the epidermal growth factor receptor (EGFR) on PEG-modified TiO2NPs for increasing the PDT effect for epithelial cell carcinoma (A431 cell line).150 The EGFR is vital for cell proliferation and it is highly expressed on many cancer cells, so for this reason, the modification of TiO2NPs with EGF could increase the efficiency and selectivity of TiO2-based PDT. Their results showed that the EGF modification of TiO2NPs–PEG diminished the cell viability of the cancer cells via interrupting DNA synthesis. Also, the PEG modification of the TiO2 core could enhance the stability and bioavailability of this nanosystem.
In spite of the advantage of PDT, the in vivo production of toxic ROS and high photosensitivity of treated patients could limit the PDT technique.7 The development of modified TiO2NPs with a high photothermal conversion efficiency has recently gained much attention, as an efficient and non-invasive method, to destroy target tumor tissues.151 The heat generated by the vibrational relaxation of stimulated TiO2NPs (>42 °C) could trigger several photothermal effects in tumors such as causes necrosis, apoptosis, and necroptosis. The limitation of PTT (photothermal therapy) is the low NIR absorption of cancer cells located far from the tissue surface which can be overcome by modifying TiO2NPs with the organic molecules absorbing long-wavelength visible light or NIR. Behnam et al. used PEG-modified TiO2NPs as PTT agents to increase the water dispersibility and biocompatibility of TiO2NPs.152 Besides, these PEGylated TiO2NPs could escape the reticuloendothelial system (RES) and reach to their target tumors. The in vivo results showed a relatively high PTT efficacy of these TiO2NP–PEG nanosystems on reducing the melanoma tumor size without any symptom of cancer cells in treated cases. Therefore, TiO2NPs–PEG can be utilized as a potent agent with low toxicity in the hyperthermia cancer therapy.
As another advanced technique, combined PDT/PTT approaches have much stronger effects than expected; for instance, Gao et al. synthesized polydopamine-modified TiO2NPs (TiO2-b-P25@PDA NPs) forming a high core–shell structure, as an improved PTT nanosystem.153 They then prepared synergistic nanoprobes (TiO2-b-P25@PDA-Ce6 (Mn)) by combining chlorine e6 (Ce6) and chelating Mn2+ for use in combined PDT/PTT. These modified-TiO2NPs showed high ROS generation and high photothermal conversion efficiency (32.12%). Their in vivo tests on a 4T1 tumor-bearing nude mouse model illustrated a synergistic significant antitumor effect of the nanosystem (under the combination of PDT/PTT with a low-dose laser), compared to the partial tumor inhibition by single PDT and single PTT. So, the co-modification of TiO2NPs with the PTT and PDT agents can dramatically enhance the therapeutic efficacy of modified TiO2NPs, compared to the unmodified structures.
In 2022, Dai et al. modified TiO2NPs with hyaluronan and porphine for the simultaneous PTT/PDT therapies.154 They used these two surface modifiers (hyaluronan and porphine) to mildly reduce the lipid level of RAW 264.7 cells without triggering the harsh cell apoptosis, which is an important strategy for the treatment of chronic cardiovascular diseases. For both PTT alone and PTT + PDT therapies, their result demonstrated a considerable decrease of intracellular lipid load without triggering apoptotic cell death or necrosis, below the 45 °C. Conversely, the PDT modality showed a small decrease in lipid levels and a significant apoptosis or necrosis. These results indicated that the surface modification of TiO2NPs could increase the PTT efficiency and enhance the local temperature to relatively moderate levels (44 °C) after NIR irradiation, which prevented excessive cell apoptosis or necrosis, while PDT resulted in harsh cell death.
Regarding the sonodynamic effect of TiO2NPs, there have been an admirable effort for developing sonodynamic TiO2NPs, as a non-invasive method, having high tissue penetration and spatiotemporal selectivity. In SDT (sonodynamic therapy), the ROS generation is triggered under ultrasound (US) stimulation, resulting in selective tumor targeting with minimal damage to nearby healthy cells.155
Pancreatic cancer is considered as the third-leading cause of death in 2022 because of its increasing cases and mortality rates.156 In the advanced-stage of this cancer, surgical resection is the primary method but only 20–15% of patients can survive and the other types of therapeutic modalities, such as chemotherapy and immunotherapy, show poor response to the majority of clinical treatments.157 Sonodynamic therapy (SDT) has shown to be a promising alternative in this case. However, pancreatic tumors are surrounded by the interstitial fluid pressure (IFP) and hypoxia tumor microenvironment (TME) which decreases the sonosensitizer penetration into the tumor, resulting in low SDT efficiency.158,159 Collagen is the most abundant protein in the ECM (extracellular matrix) of pancreatic cancer160 and so, the modification of the TiO2NP sonosensitizer with collagenase is a promising strategy to improve the SDT efficiency in pancreatic cancer. Recently, Luo et al. synthesized collagenase-modified hollow TiO2NPs (H-TiO2NPs-Co) capable of degrading stromal barriers and producing sufficient ROS.161 The in vivo tests in a patient-derived xenograft (PDX) model showed an enhanced penetration and retention of the TiO2NPs within tumor tissues, due to the presence of Co on the TiO2NP surface. The ultrasonic irradiation caused the controlled release of collagenase which degraded tumor matrix fibers. The attached collagenase (Co) resulted in accumulation of modified TiO2NPs within the tumor which generate abundant ROS under the ultrasound (US) irradiation and dramatically increase the selectivity and therapeutic efficiency of SDT.
In 2021, Wei et al. synthesized newly modified TiO2NPs, functionalized with a malignant melanoma cell membrane (B16F10M) and a targeting aPD-L1 antibody for enhanced sonodynamic tumor therapy.162 Under ultrasound irradiation, these modified TiO2NPs showed a high efficiency to generate ROS (1O2) along with precise targeting effects, high tumor uptake, and intracellular sonocatalytic killing of the B16F10 cells. In this study, the modification of TiO2NPs with the mentioned biomolecules resulted in a dramatic enhancement of biocompatibility, selectivity and therapeutic yield of the modified TiO2NPs.
Lin et al. reported the synthesis of a multifunctional modified TiO2NP sonosensitizer (TiO2NPs-Ce6-CpG, CpG: a targeting oligonucleotide) for highly efficient cancer immunotherapy.163 To improve the biocompatibility and sonotherapeutic ability of these TiO2NPs, they were modified with chlorin e6 (Ce6) and a CpG oligonucleotide (CpG ODN) to enhance the immune response. Ce6 is a hydrophilic porphyrin-type sonosensitizer, which accumulates effectively in tumors, and can generate ROS under the ultrasound activation to induce apoptosis and necrosis of the tumor cells. The CpG ODN oligonucleotide is an immunological adjuvant that can trigger cellular immune responses to enhance the anticancer properties of a variety of cancer treatments. The injected TiO2NPs-Ce6-CpG could induce the release of tumor-associated antigens and demonstrated vaccine-like functions together with the CpG adjuvant, which activated dendritic cells (DCs) and enhanced tumor-infiltrating CD8+ T cells to the tumor tissues, inducing a robust antitumor immunological response.
Lee et al. studied the potential application of SDT against glioblastoma cells using TiO2NPs modified with a targeting molecule, anti-EGFR antibody.164 Their results showed a dramatic enhancement of the selectivity and internalization of modified TiO2NPs toward the target cells, due to the presence of the anti-EGFR antibody on the TiO2NP surface. Under the ultrasound irradiation of modified TiO2NPs, cell viabilities were reduced because of the ROS generation with minimal effects on apoptosis.
In 2021, Yousefi, et al. used porphyrin-loaded TiO2NPs and studied their sonotoxicity on MDA-MB-231 cells.165 To increase the biocompatibility and ultrasound absorption efficiency, the surface of TiO2NPs was modified first with the polyvinyl alcohol (PVA) polymer and then with porphyrin. The in vitro results indicated that these modified TiO2NPs are non-toxic and under the ultrasound radiation they could damage the breast cancer cells.
Pariente et al. synthesized sono-responsive TiO2NPs modified with poly(ethylene oxide)–poly(propylene oxide) (PEO–PPO) copolymers for their potential in sonodynamic applications.166 Their results showed an enhanced biocompatibility of these modified TiO2NPs, due to the modifying copolymer. Upon irradiation with the therapeutic ultrasound, the nanoparticles generated ROS and induced the apoptosis of Rh30 cells. The compatibility and cellular uptake of these modified TiO2NPs were confirmed on the Rh30 cell line, as a model of rhabdomyosarcoma without any significant hemolysis over 24 h treatment.
The other recent publications on the therapeutic effect of TiO2NP-based nanostructures are summarized in Table 6.
| Nanosystem | Applications | Ref. |
|---|---|---|
| Fe2O3–TiO2 nanocomposites, using polyvinylpyrrolidone–polyethylene glycol (PVP–PEG) | Showed remarkable PDT activity in HeLa cell lines via the generation of intracellular ROS | 167 |
| New nanocomposite, TiO2NPs@Ru@siRNA | Remarkable PDT activity on patient-derived xenograft (PDX) and rat oral experimental carcinogenesis models | 168 |
| Folic acid-functionalized TiO2NPs | Modeling of active targeting of tumor cells | 169 |
| Chelate-free gadolinium loaded TiO2NPs coated with transferrin (Tf) | Coating of this TiO2-Gd NPs with Tf stabilized the nanoconstruct and minimized aggregation, showing a dramatic selectivity for the photodynamic targeting of studied cancer cells | 170 |
| TiO2NP–Ag nanohybrid modified with the Pluronic® F-127 polymer, which is permitted by the Food and Drug Administration (FDA) | This polymer improved the biocompatibility of TiO2NP–Ag, tested in 4T1 breast cancer cells and the nanohybrid showed endocytosed by cancer cells produced high intracellular ROS under UV conditions (5.6 mW cm−2), resulting in cancer cell apoptosis | 171 |
| TiO2/Cur@ZIF-8 nano-composite (Cur: chemotherapeutic agent curcumin) | Synergistic photodynamic-chemotherapy and pH-/and NIR-stimulated drug release | 172 |
| N-doped graphene quantum dots (QDs)/titanium dioxide nanocomposites (N-GQDs/TiO2NPs) modified with citric acid | Upon the photo-activation of N-GQDs/TiO2NPs with near-infrared (NIR) light, the nanocomposites generated reactive oxygen species (ROS), mainly singlet oxygen (1O2), which caused more significant cell death in MDA-MB-231 (an epithelial, human breast cancer cells) than in HS27 (human foreskin fibroblast) | 173 |
| Tc-99m-labeled lupulone-conjugated Fe3O4@TiO2 nanocomposite | Lupulone-conjugated Fe3O4@TiO2 nanocomposites showed suitable dispersion and the photodynamic effect on prostate cancer without visible aggregation | 174 |
| Doped TiO2 rhombic nanocomposites modified with Pluronic® F-68 | Mn-TiO2-PF-68 RNCs demonstrated negligible toxicity with physiological stability. Mn3+ doped with photosensitizers (TiO2) also exhibited a great synergistic effect of photo-killing in vitro by developing hydroxyl radicals | 175 |
| Titanium-oxo nanoclusters modified with dopamine and PEG | The introduced dopamine (DA) ligands not only facilitated the water solubility and the photocatalytic properties of the NPs but also involved the tumor-targeting behavior through the binding affinity with DA receptors on cancer cells. Under Cerenkov irradiation, these nanocomposites enable efficient hydroxyl radical generation | 176 |
| C-doped TiO2NPs | They were prepared and tested as a photosensitizer for visible-light-driven photodynamic therapy against cervical cancer cells (HeLa) | 177 |
| Tablet-like TiO2/c nanocomposite with a metal–organic-framework (MOF)-derived carbon structure | This nanocomposite continued to generate ROS in response to repeated ultrasound irradiation and was able to induce tumor cell apoptosis via SDT-induced DNA damage in vitro and in vivo. This TiO2/C nanocomposite also exhibited good biocompatibility and did not induce any apparent toxicity in vitro and in vivo. | 178 |
| Hypoxia-tolerant MOF@TiO2 (MOF, metal–organic framework) | Hypoxia-tolerant type I photodynamic therapy against hypoxic cancer | 179 |
| MnCO@TPP@C-TiO2NPs | MnCO@TPP@C-TiO2NPs selectively localized in the mitochondria of HeLa cells where the overexpressed-H2O2 triggered CO released, resulting in mitochondrial damage | 180 |
Semiconductor quantum dots (CdX, X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = = ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, Te, Se)-TiO2NPs modified with folic acid S, Te, Se)-TiO2NPs modified with folic acid |
Prepared FA-CdX-TiO2NPs (X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = = ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, Se) exhibited excellent cancer-targeting ability during PDT treatment. The optimum PDT efficiency of FA–CdSe–TiO2NPs indicated that the photocatalytic and targeting abilities were much higher than those of the pure TiO2NPs and CdSe- TiO2NPs S, Se) exhibited excellent cancer-targeting ability during PDT treatment. The optimum PDT efficiency of FA–CdSe–TiO2NPs indicated that the photocatalytic and targeting abilities were much higher than those of the pure TiO2NPs and CdSe- TiO2NPs |
181 |
4.2. Drug delivery
Chemotherapy is limited by the uncontrolled distribution of chemodrugs towards both cancerous and healthy cells which results in adverse side effects for the treated patients. TiO2NP-based drug delivery systems have attracted much more interest in recent years to enhance the target specificity of chemotherapy and reduce the systemic side effects. These advanced drug delivery systems benefit various controlled-release mechanisms including pH- and thermo-sensitive, photo-induced, and enzyme-responsive techniques which result in an enhanced specificity of the drugs toward the cancer cells and subsequently, the drug dosage can be significantly minimized while still maintaining the pharmacological effects. Recently, the modified TiO2NPs have been used for the delivery of various anticancer drugs, such as temozolomide, cisplatin, doxorubicin, and daunorubicin.8,182 For instance, Han et al. reported the synthesis of poly(acrylic acid)-calcium phosphate modified TiO2NPs (TiO2NPs@PAA-CaP) for the efficient drug delivery of doxorubicin (DOX).183 This surface modification of TiO2NPs resulted in a significant enhancement of DOX loading and encapsulation up to eight times, compared to that of unmodified TiO2NPs. Due to the pH-responsive surface properties of the PAA-CaP modifying layer, DOX-loaded TiO2NPs@PAA-CaP exhibited much faster cumulative DOX release at acidic pH = 5.2 than at neutral pH = 7.4. TiO2NPs@PAA-CaP(DOX) illustrated an enhanced cellular uptake and a higher cytotoxicity towards MCF-7 tumor cells, compared to that of free DOX. More importantly, this modified nanosystem demonstrated synergistic chemo- and photodynamic therapeutic effects on the target cells.Neuroblastoma is considered as one of the leading causes of cancer-related deaths in children worldwide184 and temozolomide (TMZ) has been widely used to treat neuroblastoma.185,186 The synthesis of TiO2NP–TMZ was studied previously for its potential to treat neuroblastoma;187 however, the clinical application of TiO2NPs is strictly limited by its serious cytotoxicity, inflammation, and brain damage.188 For this reason, alginate was used as a TiO2NP modifier due to the biological advantages of alginate such as biocompatibility, anti-inflammatory effects, antioxidant properties, and easy degradation with little toxicity.189 Zhao et al. reported the modification of TiO2NPs-temozolomide (TiO2NPs-TMZ) with alginate and studied their anti-oxidant, anti-inflammatory, and anti-tumor effects on neuroblastoma.190 Their in vivo results showed that the alginate modification enhanced the cytotoxicity toward neuroblastoma cells and decreased inhibitory activity toward normal neuronal cells. This modification increased the antioxidant, anti-inflammatory, and antitumor activities of TiO2NPs–TMZ and prolonged the survival time of the neuroblastoma model (P < 0.05). The results showed that the alginate modification controlled the TMZ release from the TiO2NPs–TMZ–alginate nanoparticles.
In 2020, Kelin et al. reported a novel drug delivery vehicle of TiO2NPs, encapsulated by bilayer shells that allow the reversible incorporation of hydrophobic drugs.191 In these systems, TiO2NPs were chemically encapsulated by the covalent binding of hydrophobic phosphonic acid, followed by the second surface modification by amphiphilic sodium dodecylbenzenesulfonate via hydrophobic interactions between the dodecylbenzene moiety and the hydrophobic first shell. This two-layer modification makes the hydrophobic surface suitable for the loading of hydrophobic drugs. These modified TiO2NPs were loaded with hydrophobic anticancer drugs 7-amino-4-methylcoumarin and quercetin. The results showed a sustained release of these anticancer drugs into the cytoplasm due to the presence of these modifying layers around the TiO2NP core and induce apoptosis in MCF-7 cancer cells.
Zheng et al. synthesized a novel TiOX (TiOX: oxidized TiO2) nanocomposite modified with PEG, targeting peptide YSA, and an anticancer drug cantharidin (CTD).192 In this nanosystem, PEG could enhance the stability of the nanoplatform and blood circulation time, which increased the tumor accumulation after systemic administration. The YSA peptide, with the YSAYPDSVPMMSK sequence, has been proven to be a targeting motif that mediates drug delivery to tumor cells expressing EphA2. The anticancer drug cantharidin (CTD), as one of the active components of mylabris, was loaded into TiOx for the combination of chemotherapy and PDT. The results showed that this nanosystem could significantly increase ROS production under X-ray exposure and provided a new drug delivery nanocarrier for CTD in combination with PDT to achieve more effective treatment.
In 2020, Kim et al. synthesized modified TiO2NPs using a tumor targeting polymer phenyboronic acid (pPBA) to encapsulate the anticancer drug doxorubicin (DOX) as a sonodynamic chemotherapeutic agent.193 In this system, the self-assembled TiO2NP–DOX was encapsulated into polymeric phenylboronic acid (pPBA) via the formation of phenylboronic esters bonds, which are cleavable by ROS. Also, in this modified TiO2NP nanocomposite, the phenylboronic acid (PBA) moiety acts as a tumor-targeting moiety because of its high affinity toward sialylated epitopes overexpressed on the membrane of various cancers. This polymeric modification also provided an enhanced loading of DOX by the interaction of PBA with 1,3-cis diol of DOX, and it was reported that DOX can bind on the surface of TiO2NPs via the coordination of hydroquinone and the quinone moiety of the DOX structure. The loaded DOX was readily released by the sonodynamic ROS generation of TiO2NPs due to the ROS-cleavable characteristics of the phenylboronic ester bond. Their results confirmed the tumor targeting by the PBA moiety, intracellular ultrasound-generated ROS, and high tumor accumulation of this nanosystem and its efficient anti-tumor effect on tumor-bearing mice.
Yu et al. modified Au@TiO2NPs with poly(lactic-co-glycolic acid) (PLGA) followed by loading of the CPT-11 (irinotecan) drug as the targeting moiety.194 The PLGA modification showed that these Au@TiO2NPs–CPT-11–PLGA have an enhanced antimetastatic activity and reduced cell invasion effects in B-CPAP and FTC-133 thyroid cancer cell lines, with and without NIR irradiation. In this nanosystem, the Au moiety could enhance the NIR absorption of Au@TiO2NPs–CPT-11–PLGA, increasing the anticancer effect.
In 2021, Chen et al. prepared nanocomposites based on polypyrrole-coated mesoporous TiO2NPs with a suitable size distribution for the co-delivery of doxorubicin (DOX) and aspirin prodrugs, with a superior drug loading capacity, due to the presence of the modifier, polypyrrole.195 Also, these modified TiO2NPs showed sonodynamic therapeutic properties and an excellent photothermal conversion efficiency (over 50.8%), with a simultaneous prodrug activation and sustained drug release, under near-infrared (NIR) and ultrasound (US) irradiation. The results showed an enhanced synergistic effect of chemotherapy and photo/sonodynamic effect to suppress the tumor.
As another biologically active polymeric modifier, polypyrrole (PPY) is an ideal photothermal conversion polymer with high photostability which has been successfully used in PTT. He et al. modified TiO2NPs with polypyrrole (PPY), (mTiO2NPs@PPY) to have the synergistic effect of TiO2NPs and this polymer enhanced the photothermal effect of TiO2NPs.196 They used this modified mTiO2NPs@PPY as a drug carrier, a photothermal agent and a sonosensitizer, in a single nanoplatform. They loaded honokiol (HNK), as the model antitumor drug, which demonstrated antitumor efficacy in several cancer types such as breast cancer, pancreatic cancer, prostate cancer, lung cancer, hepatoma, and bladder cancer. The modified mTiO2NPs@PPY showed the suitable size distribution and good biosafety, due to the presence of PPY on the surface of TiO2NPs. The in vitro and in vivo animal experiments demonstrated that mTiO2NPs@PPY-HNK could simultaneously have chemotherapeutic, photothermal, and sonodynamic effects under the laser and ultrasound irradiation. Other recent publications on the drug delivery application of TiO2NPs are summarized in Table 7.
| Nanosystem | Applications | Ref. |
|---|---|---|
| Iron-supplement coated anatase TiO2NPs modified with folic acid | Fe@TiO2NPs showed a controlled pH-sensitive delivery of the loaded imatinib molecules | 197 |
| PLGA–TiO2NPs (PLGA: poly(lactic-co-glycolic acid) | Controlled release of a natural extract (international patent No. PCT/IB2020/061916) | 198 |
| Caffeic acid-mediated synthesis of TiO2NPs (CA-TiO2NPs) | The results indicated that CA-TiO2NPs, as a promising compound with excellent biocompatibility, can be used in healthcare products and clinical and medicinal applications | 199 |
| GO–FA–PEG–TiO2–Avi/Bio | Enhanced water solubility and potential anti-tumor activity and targeted co-delivery of anticancer drug, SN-38 | 200 |
| TiO2NPs–polydopamine | Delivery of loaded icariin (Ica) for the improvement of the osseointegration process | 201 |
| TiO2 and mSiO2 drug delivery systems modified with folic acid | In vitro drug release of DOX experiments, hemolysis experiments, and cytotoxicity experiments on HeLa cell lines confirmed that the drug delivery system has good biocompatibility and GSH concentration-dependent drug release behavior | 202 |
| pH-Sensitive mesoporous bisphosphonate-based TiO2NPs (modified with alendronate sodium trihydrate (AST)) | They were used as nanocarriers for dexamethasone (DEX) drug delivery. The pH-sensitive behavior of the NPs can be attributed to the presence of AST's amine groups in the hybrid nanoparticles | 203 |
4.3. Antibacterials
The widespread overuse of traditional antibiotics has resulted in the emergence of multidrug-resistant bacterial strains and causes serious concerns in different aspects of life such as food safety and human health. In recent years, the research on new antimicrobial substances has focused on metal oxide nanoparticles. Specifically, TiO2 nanostructures are one of the most attractive antimicrobial compounds, mainly due to their photocatalytic effect and chemical stability, low toxicity, and cost-effectiveness.9 Different research studies have shown that modified TiO2NPs can demonstrate excellent antibacterial properties against a broad range of both Gram-positive and Gram-negative bacteria.10,204 This section presents the latest advancements and publications in the antibacterial activity of the modified TiO2NPs. It is worth mentioning that the antibacterial effect of TiO2NPs is due to their ability to absorb light (UV-Vis) to generate reactive oxygen species (ROS) which can be used to damage the chemical structure of microbes. Recently, Diana and Mathew reported the surface modification of TiO2NPs with the alpha-lipoic acid (ALA) functionalized bovine serum albumin (BSA) conjugate, as a biocompatible antibacterial (and anticancer) system. The antibacterial ability of this nanosystem was studied against S. aureus, E. coli, Streptococcus pneumoniae (S. pneumoniae), Candida albicans (C. albicans), and Aspergillus niger (A. niger). The results proved the antimicrobial properties of the developed system and the in vitro cytotoxicity of these modified TiO2NPs showed that the cytotoxicity was selective for cancer cells and negligible for normal cells.205 In another recent study, Maheswari et al. studied the antibacterial and anticancer properties of six TiO2NP systems modified with three plant extracts including: Withania somnifera (Ashwagandha), Eclipta prostrata (Karisalankanni) and Glycyrrhiza glabra (Athimathuram), known as medicinal plants with pharmacological applications.206 The antibacterial features of these six samples were studied against three Gram-negative bacterial strains (E. coli, Klebsiella pneumoniae (K. pneumoniae), and Pseudomonas aeruginosa (P. aeruginosa)) and two Gram-positive bacterial strains (S. aureus and Streptococcus mutans (S. mutans)). Among the modified and unmodified TiO2NP samples, Withania somnifera-Eclipta prostrata modified TiO2NPs showed the good antibacterial nature against the studied bacteria. Also, these modified TiO2NPs exhibited excellent anticancer activities against KB oral cancer cells, among the other bio modified and unmodified TiO2NP samples. These results indicated the improved biological activities of TiO2NPs after surface modification. In 2022, PV et al. synthesized a TiO2/ZnO nanostructure by decorating ZnO nanoparticles over a commercial TiO2 nanosurface.207 The ZnO formed over the anatase TiO2 layer showed excellent antibacterial activity against both S. aureus and E. coli and was found to be non-toxic towards MG-63 osteosarcoma cells. In 2022, Goñi-Ciaurriz and Vélaz prepared polylactic acid (PLA) and cellulose acetate (CA) composite films with β-cyclodextrin-modified TiO2NPs (Fig. 5).129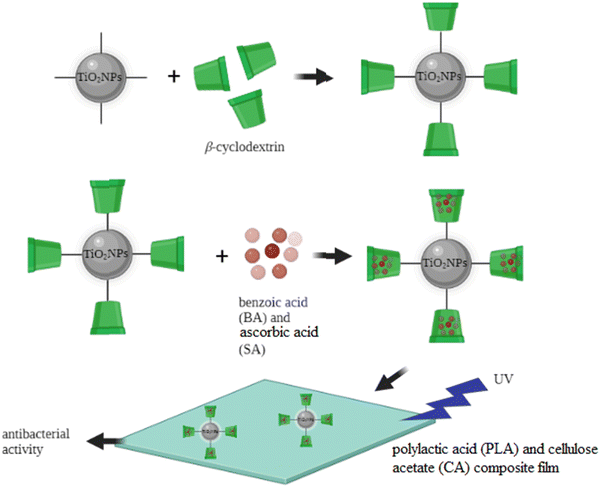 | ||
| Fig. 5 β-Cyclodextrin-modified TiO2NPs for antibacterial applications. Adapted with permission from ref. 129. Copyright 2022 Elsevier. | ||
Benzoic acid (BA) and ascorbic acid (SA) were incorporated into β-cyclodextrin-modified TiO2NPs, and the antibacterial activities of the PLA and CA composite films were successfully tested against E. coli and S. aureus. The highest antibacterial activity was observed with the film containing 5% modified TiO2NPs achieving 71% inhibition of E. coli and 88% inhibition of S. aureus. The modification of the TiO2NP surface with β-cyclodextrin provided an efficient carrier nanosystem and enhanced the therapeutic ability of the TiO2NP core. Previously, these modified TiO2NPs were successfully tested to load and release different food preservatives from the β-cyclodextrin grafted TiO2NPs. The controlled release of therapeutic molecules from the cavity of β-cyclodextrin may extend the antimicrobial effect of the TiO2NPs. Also, it can enhance the thermal stability of the film against volatilization or thermal conversion, when high temperature is applied in the food packing applications. Benzoic acid (BA) and sorbic acid (SA) are known as antimicrobial preservatives in food industry, due to their stable antimicrobial effectiveness against a broad range of microorganisms, including some bacteria, yeasts, and fungi. The authors used the polylactic acid (PLA)/cellulose acetate (CA) film, as a biodegradable polymer matrix, for fixation and stabilization of the modified TiO2NPs, as potential active food packaging. The controlled release of benzoic acid (BA) and sorbic acid (SA) from the β-cyclodextrin-modified TiO2NPs could dramatically improve the antimicrobial characters of the TiO2NPs. These results indicated the great potential of this TiO2NP-based nanocomposite to be used as antimicrobial food packaging.
Sathiyaseelan et al. synthesized modified TiO2NPs using an aqueous extract of the endophytic fungus Paraconiothyrium brasiliense (Pb) to improve the antibacterial activity of common standard antibiotics at a minimum concentration.208 The modification of TiO2NPs with the Pb fungus significantly enhanced the antimicrobial and antioxidant properties, biocompatibility, and stability of the TiO2NPs. The authors used these modified TiO2NPs with standard antibiotics (erythromycin, ampicillin, gentamicin, vancomycin, and tetracycline) to improve the antibacterial properties of these antibiotics without significant adverse effects. Antibacterial studies showed low activity of modified TiO2NPs–Pb at a concentration of 20 μg mL−1. However, a combination of tetracycline hydrochloride (TCH) with TiO2NPs–Pb significantly enhanced the inhibition of the E. coli biofilm. The authors reported the moderate toxicity of TiO2NPs–Pb (100 μg mL−1) on the cell line NIH3T3, red blood cells (RBC), and egg embryos. This research revealed that the antibiotics could be mixed with the modified TiO2NPs–Pb to improve the antibacterial efficiency and minimize antimicrobial resistance and environmental toxicity.
Özdemir et al. prepared modified TiO2NPs using cotton fabric by hydrolysis of the TiCl4 precursor solution over cotton fabric.209 The modified TiO2NPs enhanced the photodegradation of rhodamine B, compared to the cotton fabric alone. The antibacterial activity of this modified TiO2NP was successfully tested against S. aureus (ATCC 6538) and E. coli (ATCC 25922) as representative strains of Gram-positive and Gram-negative bacteria, respectively. In this modified TiO2NP system, the presence of cotton fabric acted as a template to provide active sites for the adsorption of pollutant molecules and microorganisms and more importantly, this template facilitated the transfer of produced ROS to the target molecules for their degradations which resulted in a significant increase of the photocatalytic effect of the TiO2NPs.
Metanawin and Metanawin used the mini-emulsion polymerization of the TiO2NP–polystyrene (TiO2NP–PS) hybrid antibacterial material.210 Triethylene glycol dimethacrylate (TEGDMA) was employed, as a crosslinking agent, to improve the stability/modification efficiency of TiO2NPs and photocatalytic activity. Their results showed an excellent antibacterial effect of these TiO2NPs–PS against both Gram-positive (S. aureus) and Gram-negative (K. pneumoniae) bacteria. Also, the photocatalytic efficiency of TiO2NPs–PS was tested for the photodegradation of methylene blue under UV irradiation. The photocatalytic effect of TiO2NPs–PS was increased in the presence of the crosslinking agent TEGDMA due to the self-organized structure of this hybrid system. These surface modifiers could enhance the surface area of TiO2NPs which has paramount importance to enhance photocatalytic activity during photocatalysis.
Elbarbary et al. synthesized biodegradable poly(PVA/PLA/TiO2NPs) nanocomposite films by combining polyvinyl alcohol (PVA) and polylactic acid (PLA) doped with TiO2NPs.90 The addition of 0.8 wt% of TiO2NPs showed significant enhancement of the thermal stability of the films and the water resistance properties were obtained using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVA
1 PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA ratio. This poly(PVA/PLA/TiO2NPs) nanocomposite displayed an improved antibacterial activity against S. aureus and E. coli strains. The biodegradation tests were performed in soil burial and the results showed a rapid increase of the degradation of the film in the initial 12
PLA ratio. This poly(PVA/PLA/TiO2NPs) nanocomposite displayed an improved antibacterial activity against S. aureus and E. coli strains. The biodegradation tests were performed in soil burial and the results showed a rapid increase of the degradation of the film in the initial 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) weeks with a significant change of morphology. These results suggested the potential application of this biodegradable poly(PVA/PLA/TiO2NP) nanocomposite for developing packaging materials with low environmental impact. The authors used polylactic acid (PLA) because of its thermoplasticity and biodegradability with a wide range of potential industrial applications, such as packaging materials for fresh fruit and vegetables. However, due to the ester group in PLA, it has low mechanical/thermal stability, high rigidity, and poor hydrophilicity. To use PLA in the antibacterial films, it could be combined with synthetic polymers such as polyvinyl alcohol (PVA) to improve the properties of PLA. PVA is considered as a hydrophilic, biodegradable, biocompatible and cost-effective polymer, which is commonly studied in different biological applications such as drug delivery and food packaging. This PLA/PVA film was applied as a template for fixing TiO2NPs, enhance their stability and antibacterial effectiveness.
weeks with a significant change of morphology. These results suggested the potential application of this biodegradable poly(PVA/PLA/TiO2NP) nanocomposite for developing packaging materials with low environmental impact. The authors used polylactic acid (PLA) because of its thermoplasticity and biodegradability with a wide range of potential industrial applications, such as packaging materials for fresh fruit and vegetables. However, due to the ester group in PLA, it has low mechanical/thermal stability, high rigidity, and poor hydrophilicity. To use PLA in the antibacterial films, it could be combined with synthetic polymers such as polyvinyl alcohol (PVA) to improve the properties of PLA. PVA is considered as a hydrophilic, biodegradable, biocompatible and cost-effective polymer, which is commonly studied in different biological applications such as drug delivery and food packaging. This PLA/PVA film was applied as a template for fixing TiO2NPs, enhance their stability and antibacterial effectiveness.
Singh et al. conducted the green and cost-effective synthesis of TiO2NPs using waste leaves of water hyacinth (WH) (Eichhornia crassipes), an aquatic weed, under ambient conditions.211 The antibacterial efficiency of these modified TiO2NPs was tested on a commonly known toilet bacteria, Serratia marcescens. They reported a ∼3.0 cm diameter of the inhibition zone at a 150 μg mL−1 concentration of the nanocomposite which is superior to commercial TiO2NPs and the WH leaf extract. This research showed great potential of the modified TiO2NPs in healthcare industries. In this study, the authors used water hyacinth (WH) as a natural antimicrobial reagent for the TiO2NP modification to improve the stability, biocompatibility, and antimicrobial effect of the TiO2NPs.
Mallakpour and Mohammadi prepared sodium alginate-pectin composite (ALG–PEC CS) and nanocomposite (NC) films, containing different concentrations of TiO2NPs (0.5, 1, and 2 wt%).212 They used CaCl2 and glutaraldehyde (Glu) as cross-linkers, which produce rigid scaffolds for hydroxyapatite (HA) sedimentation. The film containing 2 wt% TiO2NPs exhibited the best bioactivity and biocompatibility on the MG-63 cell line, as well as the best antibacterial effect against S. aureus. The authors modified TiO2NPs with the polymeric matrix to enhance the bioactivity and mechanical properties of implants. Generally, the Ti–OH surface groups facilitated the formation of HA on the composite solid surface. To facilitate the interaction between the implant and surrounding bone tissues, TiO2NPs should be modified/incorporated with a polymeric substrate.
Youssef et al. synthesized TiO2NPs and incorporated them into pure low-density polyethylene (TiO2NPs–LDPE) at different concentrations (0.5, 1, and 2% weight of the polymer) for potential applications in the packaging materials industry.213 Their antibacterial tests revealed the high ability of TiO2NPs to generate the reactive radical species (ROS) which induced the antibacterial activity of LDPE against Gram-negative and Gram-positive bacteria. Among synthetic polymers, low-density polyethylene (LDPE) is commonly used in food packaging due to its thermal stability and flexibility, cost-effectiveness, transparency, ease of processability, and biocompatibility. Most of the antimicrobial food packaging studies have focused on LDPE polymers and for these reasons this polymer can be a good candidate for the surface modification of TiO2NPs, as a biologically active agent to enhance the effectiveness of TiO2NPs and their biocompatibility in the food packing industry.
Makableh et al. investigated the addition of TiO2NPs and ciprofloxacin (CIPRO) to polydimethylsiloxane (PDMS) to enhance the antibacterial activity and hydrophobicity.214 The nanocomposite of PDMS was prepared by combining the TiO2NPs and/or CIPRO with PDMS before the crosslinking step. Various loading concentrations of TiO2NPs (1–5 wt%) were used while the CIPRO concentration was fixed at 0.5 wt%. The antibacterial results revealed the synergistic effect of both TiO2NPs and ciprofloxacin which led to an enhanced antibacterial activity against S. aureus and E. coli. The PDMS polymer has self-healing properties, biocompatibility, cost-effectiveness, high flexibility, and antimicrobial activity. Ciprofloxacin (CIPRO) is also known as a fluoroquinolone drug and considered as one of the most bactericidal agents used widely. So, the modification of TiO2NPs with the PDMS polymer and CIPRO could be a promising strategy to enhance the biological effectiveness of TiO2NPs and decrease their potential toxicity.
Other recent studies on the antibacterial applications of TiO2NP-based nanostructures are summarized in Table 8.
| Nanosystem | Applications | Ref. |
|---|---|---|
| TiO2NPs modified with bio agents: Syzygium aromaticum, Elettaria cardamomum, and Cinnamomum verum | Antibacterial and anticancer (KB oral cancer cell line) | 215 |
| Pure TiO2 nanoparticles and turmeric-, ginger-, garlic-modified TiO2NPs | Antibacterial against five bacterial strains, anticancer activity against the KB oral cancer cell line | 216 |
| Pure TiO2NPs, Aqua Rosa-modified TiO2NPs and protein powder-modified TiO2NPs | Antibacterial against five bacterial strains, anticancer activity against the KB oral cancer cell line | 217 |
| TiO2NP incorporation into the heparin–polyvinyl alcohol nanocomposite | Enhanced in vitro antibacterial activity and care of in vivo burn injury | 218 |
| Polylactic acid/halloysite nanotubes–TiO2NPs | High efficiency to both Gram-positive and Gram-negative bacteria | 219 |
| Nano-natural antimicrobial agent@polymeric microgels–TiO2 hybrid films | Antibacterial on the touch screen panel | 220 |
| Bio-nanocomposite film (polyvinyl alcohol)/TiO2/chitosan/chlorophyll) | Inhibits the growth of both S. aureus and E. coli bacteria under LED light irradiation | 221 |
| In situ coating of the TiO2 surface by plant-inspired tannic acid for the fabrication of thin film nano-composite nano-filtration membranes | Enhanced antibacterial performance | 222 |
| Functionalization of a polyvinylidene fluoride membrane by the biocidal oxine/TiO2 nanocomposite | Anti-biofouling properties | 223 |
| Cellulose acetate/TiO2 nanoparticles | Exhibited good antibacterial activity against E. coli with 55.6% sterilization in 12 h | 224 |
| Preparation of monsonia burkeana plant extracts | The material was found to be selective against E. coli. In real water samples, this material demonstrated remarkable activity | 225 |
| Polylactic acid (PLA)/TiO2 nanocomposite | Antibacterial activities with optimal inhibition zones against S. aureus followed by E. coli | 120 |
4.4. Biosensors
In recent years, there has been an admirable effort to develop TiO2NP-based biosensors,226 specifically for the development of novel biomolecule–TiO2NP systems leading to a dramatic success in the fabrication of bio-nanohybrid devices, such as biomolecule-sensitized solar cells (BSSCs) and photoelectrochemical cells (PECs).227 The high sensitivity of such biosensors can provide opportunities to improve clinical methods in monitoring the patient's response to medical or surgical therapy. A biosensor should have several essential characteristics for the practical applications, including biocompatibility, cost-effectiveness, user-friendliness, low sensitive detection limit/high accuracy, rapid response, and easy manufacturing for the large-scale production.228 In this regard, TiO2-based nanostructures can fulfill the mentioned properties to be used in biosensing applications and there has been an extensive scientific work in this area due to their unique electron-transfer properties.229,230 For instance, in 2022, Feng et al. introduced a new fluorescence method to detect the tyrosine phosphatase 1B protein (PTP1B) using modified TiO2NPs/single-wall carbon nanohorns (TiO2NPs–SWCNHs) (Fig. 6).13 Single-walled carbon nanohorns (SWCNHs) are a new type of carbon nanomaterials which have a large specific surface area, internal space, and fluorescence–quenching ability to construct optical systems, such as SWCNH-based detection systems for biological molecules. In this study, the TiO2NPs were decorated with SCWNHs (TiO2–SWCNHs) for providing the on/off quencher moiety on the TiO2NP surface which enhanced the discrimination difference in SWCNHs between the phosphorylated and nonphosphorylated peptides. This work was reported as the first TiO2NPs–SWCNHs. | ||
| Fig. 6 Modified TiO2NP single-wall carbon nanohorns (TiO2–SWCNHs) for detecting the tyrosine phosphatase 1B protein (PTP1B). Adapted with permission from ref. 13. Copyright 2022 Elsevier. | ||
The resultant TiO2–SWCNH nanocomposite could effectively quench the fluorescence of the phosphorylated-peptide substrate labeled by the fluorophore with a low fluorescence background. In the presence of the target PTP1B protein, dephosphorylation of the attached peptide occurred (due to the PTP1B/peptide reaction), resulting in a detachment of the dye-labeled peptide from the TiO2–SWCNH surface and fluorescence enhancement was observed in the system. This demonstrated a simple and fast approach to detect PTP1B activity, having an ultra-low detection limit of 0.01 ng mL−1 with a linear range of 0–10 ng mL−1. The biosensor can be used in the serum medium using the standard addition method and showed the possibility for screening PTP1B inhibitors.
Tao et al. prepared TiO2NPs modified with graphitized carbon nanofibers (TiO2NPs/GNFs) for the sensitive detection of organophosphorus pesticide residues (OPs).231 The modification of TiO2NPs with GNF resulted in enhanced biocompatibility, catalytic properties, and conductivity, and provided a hydrophilic surface for the effective immobilization of acetylcholinesterase (AChE), as the recognizing moiety. In more detail, the Ti atoms of this nanosurface coordinated with AChE to enhance its stability, and TiO2 has a high tendency for adsorption on OPs. The AChE/TiO2/GNFs/GCE biosensor exhibited a high affinity to acetylthiocholine chloride (ATCh) and demonstrated a low detection limit (3.3 fM) with a wide detection linear range (1.0 × 10−13–1.0 × 10−8 M), for paraoxon detection (a model of OPs). It was successfully tested for the determination of OPs in lake water, showing high anti-interference, long-term stability, and acceptable reproducibility, with great potential for the analysis of OPs in ecological environments.
Hong et al. developed a label-free electrochemical immunosensor for the ultra-sensitive determination of β-lactoglobulin (β-LG), in which they used TiO2NPs, carbon nanochips, and AuNPs on chitosan (as a conducting polymer).232 This biosensor demonstrated a linear relationship between the log β-LG concentration and the square wave voltammetry (SWV) response, with a detection limit of 0.01 pg mL−1. Due to its high stability, reproducibility, and sensitivity, this approach can be applied for the detection of β-LG in real food samples. In this study, TiO2NPs incorporated with chitosan (CS), as a biopolymer having high adhesion ability, biocompatibility and mechanical strength and improve the stability of the electrode surface for the biosensing applications. Due to the low conductivity of CS, they used carbon nanochips and AuNPs to facilitate electron transfer and increase the conductivity of this TiO2NP-based nanosensor.
Shi et al. proposed a novel biosensor for a highly sensitive detection of H9N2 AIV. In fact, the H9N2 subtype avian influenza virus (AIV) is a low-pathogenicity AIV that seriously threatens the healthy development of the poultry industry and public health systems.233 The sensor was constructed by employing a dual-resonance long-period fiber grating (DR-LPFG) modified with TiO2NPs, followed by the chemical attachment of the anti-H9N2 monoclonal antibody (anti-H9N2 MAbs) to the TiO2NPs on the surface of DR-LPFG. The detection limit of this biosensor was estimated to be ∼2.7 ng mL−1 with a high specificity and rapid detection of 96.1%, which is higher than that of a DR-LPFG-based biosensor modified with the Eudragit L100 copolymer. In this system, DR-LPFG provided a stabilizing medium for the TiO2NPs which increased biocompatibility under biological conditions. The attachment of the monoclonal antibody (anti-H9N2 MAbs) to the TiO2NPs resulted in high selectivity of this biosensor to detect H9N2 AIV.
Rajeshwari et al. combined poly(p-phenylenediamine) with TiO2 and a multiwalled carbon nanotube to make a biosensor nanocomposite for the in vivo detection of dopamine, as a biomarker of many mental illnesses.234 The biosensor demonstrated a considerable sensitivity with a linear range of 3.81 × 10−11–4.76 × 10−6 M with a low detection limit of 9.45 × 10−12 M. The incorporation of TiO2NPs with poly(p-phenylenediamine) and carbon nanotubes significantly enhanced the conductivity of the TiO2NPs, along with its stability and biocompatibility.
Zheng et al. used HKUST-1 MOFs (molecular organic frameworks) and its derivative, HKUST-CuO, for incorporation with TiO2NPs to form two resultant composites of HKUST-1/TiO2 and HKUST-CuO/TiO2 to modify the electronic properties of TiO2NPs and make a well-suitable band gap energies (Eg).235 Compared with mono-component HKUST-1 or HKUST-CuO, both TiO2-based composites showed a synergistic photoelectrochemical (PEC) response due to their heterogeneous structure. The HKUST-CuO/TiO2-modified electrode showed a higher photocurrent response which may be due to its hollow structure, greatly enhancing visible light harvesting. Then the authors successfully attached the targeting moiety to this nanohybrid and fabricated the S1 (probe DNA)/HKUST-CuO/TiO2/ITO PEC platform for colitoxin DNA detection without using ascorbic acid (AA) as an electron donor. Compared with S1/HKUST-1/TiO2/ITO, the S1/HKUST-CuO/TiO2/ITO electrode demonstrated a wider linear response range (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
× ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−6–4.0
10−6–4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
× ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−1 nM) with a lower detection limit of 3.73
10−1 nM) with a lower detection limit of 3.73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
× ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−7 nM (S/N
10−7 nM (S/N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Due to its good specificity and stability, this biosensor exhibited a promising strategy for molecular diagnosis in the bio-analysis field.
3). Due to its good specificity and stability, this biosensor exhibited a promising strategy for molecular diagnosis in the bio-analysis field.
Singh et al. developed an electrochemical biosensor for the detection of organophosphorus (OP) pesticides based upon AChE-inhibition, operating in the pM concentration range.236 The synthesized TiO2NPs and molybdenum disulfide nanomaterials were deposited on a screen-printed electrode, followed by modification with chitosan and immobilization of AChE on the modified electrode. The AChE modification of this TiO2-based electrode provided a selectivity for this biosensor. Also, the chitosan modification resulted in an enhanced stability and biocompatibility of the TiO2NPs on the surface of this electrode. The biosensor was successfully tested for the low OP pesticide concentration detection in forensic visceral samples demonstrating a low detection limit of 50 pM. Other recent works on the biosensing applications of modified TiO2NPs are summarized in Table 9.
| Nanosystem | Applications | Ref. |
|---|---|---|
| TiO2NRs and graphene oxide | For detecting dichlorvos (DDVP) | 237 |
| TiO2 nanotubes and AgNPs | Heat shock protein 70 (HSP70) as a potential tumor marker with high diagnostic sensitivity | 238 |
| Gallic acid–TiO2 nano-composites | Detection of DNA | 239 |
| TiO2 nanotube (NT) arrays | Tumor cell detection | 240 |
| 11-Mercaptoundecanoic acid self-assembly and the amidated nano-TiO2 film | For the selective and ultrafast detection of phosphoproteins in food | 241 |
| Nanocomposite graphene/TiO2 | Glucose biosensor | 242 |
| TiO2-graphene composite modified carbon paste electrode | Determination of sufentanil in human plasma and urine | 243 |
4.5. Antifungal
More than 300 million people worldwide are being threatened by severe fungal infections which have caused 1.6 million deaths every year.244 To overcome this issue, many nano-based approaches have been developed, and among them, the photocatalytic deactivation of fungi has become a promising strategy for disinfection of aqueous media to have microbial control.245–248 As a semiconductor material, TiO2NPs have been introduced as a great candidate for the development of advanced antifungal agents.For instance, in 2022, Wang fabricated chitosan/alginate–TiO2NP based bilayer films incorporated with different concentrations of cinnamon essential oil (CEO) to study the effect of this bilayer film on improving the postharvest quality of mangoes.249 In this study, chitosan was used as a packaging material for food preservation due to its biodegradable, antibacterial, and good film-forming properties. The cinnamon essential oil (CEO) is also considered as a natural antioxidant and antibacterial agent and has attracted increasing attention in the field of packaging material. In this study, the film of chitosan/CEO was formed by the interaction of the aldehyde group of CEO with the amino group of the chitosan matrix to improve the hydrophobicity, antibacterial, and antioxidant properties of the chitosan films. To improve the photostability of this film, the modified TiO2NPs–alginate was used as anti-ultraviolet packaging materials, in which the alginate contains –COO− functional groups which can electrostatically interact with cations of the inner chitosan layer to form a bilayer to prevent the volatilization of CEO in the inner layer. The modification of TiO2NPs with alginate improved the antimicrobial performance of the outer part of this film due to their synergistic advantages of TiO2NPs and alginate. This TiO2NP-based film demonstrated an improved mechanical and antimicrobial properties for the film which could be a promising candidate as a multifunctional packaging material to maintain the freshness of harvested mangoes.
Siddiqui et al. fabricated TiO2NPs using 37 strains of cyanobacteria and evaluated their antifungal, antioxidant, antibacterial, and hemolytic activities.250Synechocystis NCCU-370 was introduced as the best strain for the synthesis of TiO2NPs in terms of size (73.39 nm), followed by optimization of the synthesis to obtain smaller nanoparticles (an average grain size of 16 nm). The antifungal activity was studied against Candida albicans (MIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 μg mL−1), Candida glabrata (MIC
125 μg mL−1), Candida glabrata (MIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 μg mL−1), and Candida tropicalis (MIC
500 μg mL−1), and Candida tropicalis (MIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 μg mL−1), and the modified TiO2NPs demonstrated the partial synergistic effect and excellent biocompatibility. The biocompatible nature of these biomodified TiO2NPs is an advantage for their potential in biomedical purposes.
250 μg mL−1), and the modified TiO2NPs demonstrated the partial synergistic effect and excellent biocompatibility. The biocompatible nature of these biomodified TiO2NPs is an advantage for their potential in biomedical purposes.
Sultan et al. fabricated gelatin active packaging films based on nano-sized droplets of coconut oil emulsified by pickering emulsion (PE) and stabilized by chitosan/Arabic gum (CH/AG) nanoparticles, in the presence of TiO2NPs.251 The films showed a significant antifungal activity for all tested microorganisms, such as Bacillus cereus and C. albicans. The antimicrobial and antioxidant packaging materials are frequently produced by embedding natural antimicrobial and antioxidant additives into the natural polymer matrices providing new functionalities to the film and extend shelf life of packaged food. As a natural biopolymer, gelatin shows excellent biodegradability, biocompatibility, and film-forming ability. The coconut oil is another biocompatible candidate for using the packaging biofilm due to its potential antimicrobial and antioxidant characteristics. Chitosan is also considered as an excellent biocompatible, non-toxic, and biodegradable material, showing some important functions such as antibacterial and antifungal properties. Arabic gum (AG) is the last organic component of this film, having amphiphilic polysaccharides with emulsifying properties. These organic polymeric matrices provided a stabilizing biocompatible medium for the TiO2NPs to synergistically enhance the antifungal properties of the individual components of this film.
Mohammad Taghizadeh Kashania et al. synthesized TiO2NPs modified with C. arabicus and studied their effect on improving the biological properties of the dichloromethane fraction (DF) of C. arabicus root smoke (the largest species in the Costaceae plant family).252 The synthesized DF/TiO2NPs (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mg L−1) showed the maximum radical scavenging level up to the IC50 = 8.31 μg mL−1. In this study, the TiO2NPs were modified with the biologically active biomolecule, C. arabicus which is known as a good candidate for the treatment of infectious diseases. The modification of TiO2NPs with this biomolecule can provide the synergistic effect of antifungal for this modified system and can decrease the potential toxicity of TiO2NPs.
mg L−1) showed the maximum radical scavenging level up to the IC50 = 8.31 μg mL−1. In this study, the TiO2NPs were modified with the biologically active biomolecule, C. arabicus which is known as a good candidate for the treatment of infectious diseases. The modification of TiO2NPs with this biomolecule can provide the synergistic effect of antifungal for this modified system and can decrease the potential toxicity of TiO2NPs.
Duan et al. prepared a nanocomposite film made by K-carrageenan (KC), Konjac glucomannan (KGM) and TiO2NPs. The TiO2NPs improved the mechanical and thermal properties of the KC/KGM films.253 Specifically, the film containing 7 wt% of TiO2NPs showed effective photocatalytic antifungal activity (79%) against Penicillium viridicatum after irradiation for 6 h and revealed a protective effect on strawberry storage. The results demonstrated that the nanocomposite films have a broad potential for food preservation and packaging applications. For the food packaging applications, K-carrageenan (KC) is a hydrophilic biomolecule, obtained from red seaweed, with gelling and film-forming properties which allow biodegradable packing films to be produced. Also, Konjac glucomannan (KGM) is a natural polysaccharide which has been widely used to prepare film materials due to its good film-forming ability. Thus, when TiO2NPs are incorporated into these polymer matrices, it will effectively inhibit bacterial growth and food spoilage due to the synergistic effects of these three components to improve the properties of each other. Other studies on the antifungal applications of modified TiO2NPs are summarized in Table 10.
| Nanosystem | Applications | Ref. |
|---|---|---|
| TiO2NPs were produced by Bacillus sp. bacteria | Significant antifungal activities against the oral C. albicans pathogen | 254 |
| Green synthesis of S-doped TiO2NPs using Malva parviflora plant extract | Antimicrobial and antioxidant activities under sunlight illumination | 255 |
| TiO2/Ag nanoparticles | For its activity as an antifungal material for the inhibition of C. albicans in water under visible light irradiation | 256 |
| TiO2-SiO2/chitosan | Enhancement of antifungal capability | 257 |
| TiO2NPs were synthesized by using trianthema portulacastrum, chenopodium quinoa leaf extracts and sol–gel method | Antifungal activities against wheat rust | 258 |
| Cyclodextrin-grafted TiO2NPs | As food preservative carriers | 259 |
| PVA/TiO2-based nanocomposites | Antifungal activity study | 260 |
4.6. Antiviral
Since 2019, with the emergence of pathogenic human coronavirus pandemic, SARS-CoV-2 (COVID-19) has caused serious issues in many aspects of life such as public health and economy, all around the world. Based on the 2019 World Health Organization prediction, the mortality of infection-related diseases will be similar to that of cancer by 2050.11,12,261–263 Modified TiO2NPs have provided some promising candidates for controlling virus-type infections, supported by recent worthwhile publications in this frontier area of nanomedicine.Because of the paramount importance of antiviral modified TiO2NP systems, recent publications in this field are presented; for instance, in 2022, Elsayed et al. studied the condensation of 3-acetylindol, thiophene-2-carbaldehyde and malononitrile in the presence of TiO2NPs, yielded 2-amino-6-(1H-indol-3-yl)-4-(thiophen-2-yl)-4H-pyran-3-carbonitrile derivatives.264 Then, they were reacted with formic acid, formamide, ethyl chloroacetate, chloroacetyl chloride, thiourea and sodium nitrite to form several three-combination systems which were safe, ecologically friendly, and non-toxic. The synthesized compounds were successfully tested for antiviral activity against Vero cells (HAV) and showed an effective activity. In this research, new indoles were synthesized and used for the TiO2NP modification and tested for their antioxidant's outcome. The indole nominees verified their strength as antioxidants, antimicrobial, and anticancer. Specifically, the authors used synthesized contestants containing pyrimidine, pyrazole, pyrane, and pyridine rings. As a crucial nucleobase, pyrimidine derivatives are considered as antioxidant agents in contrast to ROS and reactive nitrogen species (RNS). Similarly, pyridine, pyrazole, and pyrane rings displayed antioxidants properties in their derivatives. The modification of the TiO2NP surface with these compounds could provide opportunity to increase the antiviral effect of TiO2NPs.
Souza et al. developed TiO2-based antiviral hydrophobic cellulose cotton or non-woven fabrics for their potential virucidal effect on Murine Coronavirus (MHV-3) and Human Adenovirus (HAdV-5), under indoor light irradiation.265 In the non-woven fabric, the results demonstrated 90% and 99% reduction of HAdV-5 and MHV-3, respectively, with no reduction of HAdV-5 in cotton fabric. The antiviral activity was assigned to the photocatalytic effects of the modified TiO2 powders, and the hydrophobic properties of fabrics and high surface of the TiO2 particles facilitated their interaction with the viruses, especially MHV-3. These results showed the potential ability of these composite materials as highly effective virucidal agents against MHV-3 and HAdV-5 viruses, particularly for applications in healthcare indoor contaminated environments. In fact, the fabrics used in this study acted as a support for TiO2NPs to significantly promote the interaction between viruses and TiO2NPs. In this regard, cellulose-based fabrics such as cotton and non-woven fabrics have been commonly studied for the application of TiO2 hydrosols to prepare fabrics with photocatalytic and self-cleaning properties. The flexible, porose, and layered surface structures of cotton contributed to the incorporation of TiO2NPs in its structure, providing enhanced antiviral properties.
Regarding SARS-CoV-2 infection, Da Silva et al. developed antimicrobial cotton fabrics based on the Ag/TiO2 nanohybrid and they showed that more than 50% of infectious SARS-CoV-2 survived after direct contact with the nanohybrid under the tested conditions, which indicated that more studies are required on using silver and TiO2 nanostructures as self-disinfecting agents for the prevention of coronavirus transmission.266 In this case, the cotton provided a matrix for the immobilization of Ag/TiO2NPs, which protected the nanosystem against aggregation.
Wang et al. successfully tested the antiviral efficacy of TiO2–chitosan (CS) –AgNP filter for viral aerosols and reported the infection risk reduction and long-term antiviral efficacy of this nanosystem.267 In this study, the TiO2–CS–AgNP filter was synthesized for the removal and deactivation of airborne MS2 bacteriophage particles. In the air purification system, their results showed a 93% removal of the airborne MS2 particles, and more than 95% of MS2 can be efficiently deactivated on the surface of this nanosystem within 20 minutes. The filter could maintain 50% of its original antiviral efficiency after continuous operating for 1 week. The surface modification of TiO2 with the chitosan polymer provided possibility for the AgNP attachment to the surface of TiO2.
Levina et al. used a combination of biocompatible TiO2NPs and immobilized polylysine-containing oligonucleotides (PL) with native (ODN) and partially modified (ODNm) internucleotide to form a new delivery system for the effective attack of oligonucleotides on the viral genome of highly pathogenic H5N1 influenza A virus (IAV) in vivo.268 The intraperitoneal injection of this TiO2·PL–ODN nanocomposite exhibited 65–70% survival of mice, while the intraperitoneal or oral administration of TiO2·PL–ODN was more efficient (∼80% survival). The nanocomposites showed no toxicity on mice under the tested conditions. Interestingly, the TiO2NPs, unbound ODN, and the nanocomposite bearing the random oligonucleotide demonstrated a low protective effect, demonstrating the key role of targeting oligonucleotides in this nanocomposite for the site-specific interaction with complementary RNAs of the target virus. In this antiviral system, the ODN loaded polylysine was non-covalently immobilized onto the TiO2NPs, which enhanced the biocompatibility and enabled the ODN delivery via the cell membrane. More importantly, this modification of TiO2NPs could stabilize and protect ODN against intracellular enzymes, and the carried oligonucleotides were released from the nanocomposites in the cytoplasm or penetrated into the nuclei and bind to the RNA molecules.
León-Gutiérrez et al. studied the modification of TiO2NPs with secondary metabolites implanted to prepare antiviral TiO2NPs (SMNP) and tested them on SARS-CoV-2 infectivity and healthy cells as well. Surprisingly, SMNP showed a considerable reduction of viral infectivity in vitro with minimal toxicity to healthy cells when compared to other commercially available antiseptics (glutaraldehyde, chlorine, chlorhexidine, ethanol, and Lysol™), which indicated this SMNP nanosystem as a safe and effective antiviral against SARS-CoV-2.269 Citrus-derived compounds have shown the clear clinical benefit for viral infections, inducing stimulate immunity. Natural secondary metabolites are considered as antiviral agents due to their inhibitory effect on key metabolic enzymes that influence signaling pathways, cellular function, and gene expression. Conjugation of these therapeutic agents with the TiO2NP surface could provide the synergistic effect for the antiviral ability of the modified TiO2NPs.
4.7. Tissue engineering
Tissue engineering is a multidisciplinary field which includes the fabrication of these biocompatible materials suitable for repairs or replacement of abnormal tissues/organs. It is worth mentioning that the scaffolds, used for different applications in tissue engineering, need to be highly compatible with the cellular matrix inside the body and they should not cause cytotoxic, immunogenic, inflammatory, or any other host reaction. Also, they should exhibit suitable mechanical and physical properties suitable for the biological conditions. Despite many advantages of TiO2NPs in tissue engineering, one of their restrictions is their agglomeration which diminishes their biological effectiveness. Therefore, there is a need for a polymeric matrix to fix and stabilize these TiO2NPs and prevent their leaching out to different parts of the body. In recent years, modified TiO2NPs have attracted significant attention for using in different platforms/scaffolds in the tissue engineering field to promote biological and physicochemical processes of cell/tissue culturing.270,271 In this field, the TiO2NPs are often used as a part of the biocompatible polymeric matrix which can fix and stabilize TiO2NPs, enhancing the biocompatibility and effectiveness of these nanoparticles. Simultaneously, these incorporated TiO2NPs can improve the mechanical and physiochemical properties of the scaffolds.For bone regeneration scaffolds, in 2022, Karbowniczek et al. reported the additions of hydroxyapatite (HA) and TiO2NPs on poly(3-hydroxybuty-rate-co-3-hydroxyvalerate) (PHBV) based fibers and studied the tensile strength, elongation, and toughness of the fibers after this addition.14 It should be mentioned that the biodegradable poly(3-hydroxybuty-rate-co-3-hydroxyvalerate) (PHBV) is a biopolymer synthesized by bacteria, considered as a good alternative for many non-biodegradable synthetic polymers. For tissue engineering purposes, this biopolymer can be processed via electrospinning for the construction of scaffolds. To improve its in vitro cell growth and mechanical properties, PHBV can be combined with ceramic particles such as hydroxyapatite (HA) and antibacterial TiO2NPs. Regarding the effect of HA on the surface properties of TiO2NPs, the authors reported that the presence of HA could decrease the agglomeration of TiO2NPs in the PHBV + HA + TiO2 composite, compared to that in PHBV + TiO2, which is very important for increasing the effectiveness of TiO2NPs. Also, they observed a dramatic improvement in the mechanical strength of the PHBV fibers containing HA nanoparticles, compared with the fibers alone. The homogenous distribution of HA nanoparticles resulted in a 3-time improved tensile strength and a 16-time higher toughness. The authors showed a strategy for tuning mechanical properties by controlling the size and distribution of ceramic fillers (TiO2 and HA) in hybrid scaffolds.
As another surface modifier, poly-ortho-toluidine (POT) has been used as a conductive polymer to stimulate a multitude of cell functions such as attachment, proliferation, migration, and differentiation via the modulation of transferred electrical stimuli from the external support to cell. So, the modification of TiO2NPs with the POT polymer brings several benefits to the TiO2NPs, including promoted cell-material interaction, better antibacterial activity, and excellent biocompatibility. In this regard, Balan et al. synthesized an organic/inorganic nanocomposite poly-ortho-toluidine–TiO2 to construct the POT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2/PCL nanocomposite scaffolds.272 The surface roughness of this nanocomposite provided a great influence on the viability of different cells. Besides, the in vitro antibacterial activities of POT
TiO2/PCL nanocomposite scaffolds.272 The surface roughness of this nanocomposite provided a great influence on the viability of different cells. Besides, the in vitro antibacterial activities of POT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 and POT
TiO2 and POT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2/PCL composite scaffolds were tested on S. aureus and E. coli and the POT
TiO2/PCL composite scaffolds were tested on S. aureus and E. coli and the POT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+ ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2/PCL scaffold demonstrated an improved surface roughness, cell viability and antibacterial activity, indicating economical and effective TiO2NP-based nanocomposites for tissue engineering applications.
TiO2/PCL scaffold demonstrated an improved surface roughness, cell viability and antibacterial activity, indicating economical and effective TiO2NP-based nanocomposites for tissue engineering applications.
Sharaf Saeed et al. prepared a series of poly(ethylene-co-vinyl alcohol)/TiO2NPs (PEVAL/TiO2) nanocomposites containing 1, 2, 3, 4 and 5 weight ratios of TiO2NPs.273 The cell culture tests of these nanohybrids were evaluated on human gingival fibroblast cells (HGFs) in accordance with ISO 10993-5 and ISO 10993-12 standards, with studying the cell viability after 1, 4, and 7 days. The results showed a time-dependent improvement in the cell activity for all systems, and the cell survival for all samples was higher than that of the virgin PEVAL on day 7 (p < 0.002). The bio-SEM results also demonstrated the successful cell adhesion and growth of HGFs on all types of scaffolds (PEVAL/TiO2). In this system, the incorporation of TiO2NPs into the PEVAL polymer matrix exhibited a uniform dispersion of these TiO2 fillers, which are well covered by the copolymer. This could be due to the presence of good affinity between these two components in which the expansion PEVAL macromolecule chain in the solvent promotes the dislocation of the aggregated TiO2, leading to their uniform dispersion in the polymer matrix. This homogeneity and stability could positively affect the biocompatibility of TiO2NPs for the cell culturing purpose.
Alginate is considered as one of the most promising surface modifiers for TiO2NPs in the tissue engineering field, as a marine-based polysaccharide found in brown algae. Compared to synthetic polymers, this biopolymer provides several benefits such as biocompatibility, gel-forming ability at biological pH and temperature, non-toxicity, water solubility, and cost-effectiveness. Alginate-based scaffolds can enhance alkaline phosphatase activity and expressing osteocalcin and mineralization resulting in promoted osteogenesis. In this case, Mallakpour and Naghdi applied a TiO2NPs–alginate nanohybrid to fabricate a bone scaffold to take the benefits of both TiO2NPs and alginate.274 In this study, the alginate provided suitable conditions for the biomineralization process, and it can help better dispersion and fixation of TiO2NPs and decrease their aggregations. This TiO2NP–alginate was incubated in a simulated body fluid at 37 °C for 28 days to evaluate its bioactivity. Cytotoxicity tests were performed on the MG-63 cell line and the scaffold showed no toxicity effects. Besides, this scaffold demonstrated a potent antibacterial effect against the Gram positive strain S. aureus, indicating the great properties of this scaffold to be used for bone tissue engineering applications.
As another well-known polymer in tissue engineering, polyvinyl alcohol (PVA) shows great film-forming properties, high tensile strength, biodegradability, emulsifying characteristics, and water solubility. However, for using scaffolds, it requires to blend this polymer with other polymers and inorganic fillers to improve the mechanical and biological properties.275 Currently, nano-cellulose has been attracting considerable interest due to its strengthening effect, biodegradability, high surface area, non-toxicity, and great mechanical/optical properties. Cellulose can also support cell attachment and facilitate apatite growth. Because of these typical properties, they have been incorporated into nanocomposites and the polymer matrix as reinforcing materials improving the desired properties, which makes it a good choice for biomedical applications.275 TiO2NPs are ideal illustration of inorganic fillers due to their cell adhesion and anti-corrosive properties. Fatima et al. prepared a TiO2NP-doped ternary nanocomposite film (PVA/NC-TiO2) by the fusion of polyvinyl alcohol (PVA), nano-cellulose (NC), and TiO2NPs (0.01 wt%), as well as its binary nanocomposite film (PVA/NC).275 The presence of TiO2NPs in the structure of this film significantly improved the thermo-mechanical stability of the nanocomposite film, which can be due to an enhancement of intermolecular interactions in the PVA/NC-TiO2 film, compared to the PVA/NC binary film. Furthermore, their results showed a higher glass transition temperature and a storage modulus of the PVA/NC-TiO2 film (9131.26 MPa, 74.3 °C) than those of the PVA/NC film (7588 MPa, 64.3 °C). The ternary PVA/NC–TiO2 film showed a more uniform surface layer of the Ca–P mineral than the PVA/NC film, upon incubation in simulated buffer saline. The low percentage of TiO2NPs improved the strength of this PVA/NC–TiO2 film, with a cell viability more than 80% and hemolysis less than 1.7%, demonstrating high potential of this nanocomposite to develop better bone templates for bone regeneration applications. The interaction of PVA and NC with the TiO2NPs resulted in more homogeneity for the particles in this film, compared to the PVA/NC film. Besides, this surface modification improves the biocompatibility of these TiO2NPs.
Nanofiber (NF) scaffolds hold great promise for utilization in bone regeneration purposes and they are interesting stabilizing and modifying matrices for enhancing the efficiency of TiO2NPs used in the scaffolds. Among various biomaterials, poly-ε-caprolactone (PCL), an FDA approved biodegradable polyester, has attracted much attention in this field due to its appropriate biocompatibility and mechanical properties.276 Several works demonstrated that a blend of PCL and gelatin (GEL) can generate a highly biomimetic nanofibrous scaffold with appropriate biodegradability and mechanical stability, and most importantly excellent cytocompatibility. However, PCL/GEL NFs alone are not appropriate for bone regeneration due to the absence of inherent osteoinductive capability. The incorporation of osteoinductive agents or components to these scaffolds can lead to higher mechanical properties and improved biofunctionality of PCL/GEL NFs for bone regeneration. In this regard, nanocomposites based on ceramic nanoparticles such as TiO2NPs have recently drawn considerable attention as bone substitute materials and injectable pastes to fill defects due to their excellent mechanical properties, high chemical stability, and bioactivity.276 In this regard, Ahmadi et al. employed TiO2NPs and metformin (MET)-co-embedded composite electrospun poly-ε-caprolactone/gelatin nanofibers (PCL/GEL NFs) to form scaffolds for the improved osteoblastic differentiation of human adipose-derived stem cells (hADSCs).276 The results showed an improvement of the mechanical properties of this composite due to the presence of TiO2NPs, as well as an enhanced adhesion and growth rate of hADSCs on the nTiO2/MET-loaded NFs. The nanosurface of TiO2NPs and the released metformin could result in a high differentiation capacity toward osteoblasts due to the promoted mineralization content and enhanced expression of osteoblastic markers. This work again demonstrated the high capacity of TiO2NP-based scaffolds for bone tissue regeneration and engineering. The incorporation of TiO2NPs in the polymer/drug matrix can enhance the cell-differentiating abilities of TiO2NPs and prevents their aggregation.
As a soft tissue, skin has potential to repair itself so the key challenge for researchers and clinicians is to find ways to harness this skin regenerative potential to treat cutaneous injuries and diseases. At present, the only gold standard treatment is autologous skin graft for full-thickness skin wounds; however, this treatment also has shortcomings like restricted availability and morbidity of the donor site. Therefore, one of the alternative therapeutic options to treat the skin injuries is tissue-engineered skin (TES) which makes use of natural polymers including proteins which can resolve the autologous donor graft shortcomings and can provide protection against water–electrolyte imbalance and microbial infection as well.277 Silk fibroin (SF) is a protein (FDA approved), mechanically strong, biocompatible, biodegradable, and permeable material which has been used in a wide range of applications of tissue engineering and wound healing.277 Similarly, collagen (CG) is biocompatible and biodegradable protein-based natural polymer. These two biocompatible polymers can be applied as the matrix scaffold useful for the incorporation of TiO2NPs for the tissue engineering studies. Khalid et al. designed silk fibroin/collagen (SF)/(CG) membranes combined with TiO2NPs for skin tissue regeneration applications.277 The membranes exhibited good biocompatibility and antibacterial activity and can be introduced as potential candidates for skin tissue regeneration and wound healing applications.
The natural polymer of chitosan has been used extensively in the field of tissue engineering due to its biocompatibility, biodegradability, non-toxicity and antibacterial properties. Also, bredigite is one of the most well-known silicone biochemicals that has a high potential for the release of silicon ions, which results in the growth of osteoblasts and cellular differentiation. Ghasemi and Ghomi prepared the composite scaffolds of bredigite/TiO2, followed by coating with the chitosan polymer to enhance the mechanical, biological, and antibacterial properties of the scaffold.278 The results showed that the addition of TiO2 to the scaffold of bredigite resulted in reduced porosity and enhancement of the compressive strength of scaffolds from 0.299 to 0.687![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MPa. In addition, the chitosan coating reduced porosity from 83% to 63% and strongly improved the compressive strength from 0.585 to 2.339
MPa. In addition, the chitosan coating reduced porosity from 83% to 63% and strongly improved the compressive strength from 0.585 to 2.339![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MPa. The scaffolds showed antibacterial effects against E. coli (an inhibition zone of 22
MPa. The scaffolds showed antibacterial effects against E. coli (an inhibition zone of 22 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm) and S. aureus (an inhibition zone of 29
mm) and S. aureus (an inhibition zone of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm). Besides, these scaffolds exhibited no toxicity on the MG63 bone cells adjacent to the scaffolds. Regarding the modification effect of chitosan, the results showed that the presence of this modifying polymer could enhance the stability, antibacterial, and biocompatibility of the scaffold containing TiO2NPs.
mm). Besides, these scaffolds exhibited no toxicity on the MG63 bone cells adjacent to the scaffolds. Regarding the modification effect of chitosan, the results showed that the presence of this modifying polymer could enhance the stability, antibacterial, and biocompatibility of the scaffold containing TiO2NPs.
Polyurethane is a synthetic, thermoplastic, elastomeric, biodegradable, and biocompatible polymer having increasing applications in tissue engineering.32 However, the nanofibers made from polyurethane polymers have hydrophobic nature, which makes them undesirable when cultured in the presence of cells and/or implanted in vivo using animal models.33,34 These fibers are efficiently utilized as a bone mineral and modifying the matrix for the TiO2NPs and other antibacterial NPs. This stabilizing matrix could enhance the biocompatibility of the used nanoparticles. Ashraf et al. fabricated nanofibers containing TiO2NPs (as the osteoconductive component) and AgNP (as self-healing) nanostructures.279 The incubation of the nanofibers in the simulated body fluid at 37 °C triggered mineralization on nanofiber scaffolds and resulted in Ca and P crystals’ formation. Also, the scaffolds showed antibacterial activity against E. coli (8.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm) than S. aureus (1.2
mm) than S. aureus (1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm), and the MTT assay on the pre-osteoblasts demonstrated the biocompatibility of both TiO2NPs and AgNPs with the bone-like cells. However, at higher contents of AgNPs (i.e., 0.07
mm), and the MTT assay on the pre-osteoblasts demonstrated the biocompatibility of both TiO2NPs and AgNPs with the bone-like cells. However, at higher contents of AgNPs (i.e., 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M), the scaffold showed cytotoxicity.
M), the scaffold showed cytotoxicity.
Recently, novel hybrid fillers such as TiO2NPs/graphene oxide have been constantly emerging to be used in the structure of polymeric scaffolds to enhance the properties of composites. Since TiO2NPs are antibacterial components, they have been widely used as nanofillers to enhance the performance of the composite scaffold. In this case, recently, Zhang et al. synthesized the reduced graphene oxide/TiO2NP (RGO@TiO2NPs) nanohybrid as a filler to investigate its synergistic effects on electrospun regenerated silk fibroin (RSF) mats (Fig. 7).280 The hybrid filler resulted in an increase in the average diameter of RSF fibers and decrease the content of β-sheet conformation. Interestingly, a 220% increase of the strength of the RSF/RGO@TiO2NP composite mat was detected. Moreover, the growth and expansion of the cells were reported on the composite mat due to the good antibacterial properties of TiO2NPs. In this system, the RGO could bring a stabilizing matrix for the TiO2NPs, protecting them against agglomeration which could result in well dispersion of TiO2NPs in the scaffold and enhanced their antibacterial activity.
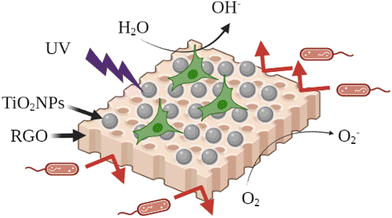 | ||
| Fig. 7 The graphene oxide/titanium dioxide (RGO@TiO2) nanohybrid as a filler to investigate their synergistic effects on electrospun regenerated silk fibroin (RSF) mats. Adapted with permission from ref. 280. Copyright 2021 Elsevier. | ||
For bone tissue engineering, a scaffold with the optimized pore size and interconnected porosity is desirable to establish the tissue response and cell growth. It is essential for cell penetration and migration to effectively vascularize the growth of new tissues to have a maximum porosity (90%) and a suitable pore diameter (minimum 100 μm and maximum 450 μm). The TiO2NPs play a significant role in controlling the pore size and porosity. For bone regeneration, a combination of TiO2NPs with bioceramics (such as hydroxyapatite (HA)) has gained attention, due to the chemical similarity of HA to the mineral phase of natural bone and its extreme biocompatibility. HA can gain optimized microstructures for defected bone by combining with biodegradable polymers (including poly(glycolic acid), chitosan, arabinoxylan, and guar gum). These biodegradable polymers also act as binders that reduce brittleness of HA. Aslam Khan et al. synthesized a TiO2NP-based nanocomposite containing nano-hydroxyapatite (HA NPs), acrylic acid (AA)/guar gum (GG), and optimum graphene oxide (GO) to construct porous scaffolds coated with silver sulfadiazine (as a drug).281 Their results showed that the TiO2NPs and optimized GO improved the physicochemical and microstructural properties of this scaffold and the promising results obtained with mouse pre-osteoblast (MC3T3-E1) cell lines. Also, the organic part of this scaffold provided a stabilizing matrix for the TiO2NPs, increasing their effectiveness.280
Poly(lactic acid) (PLA) is a well-known biocompatible and biodegradable polymer and it is a promising candidate to be used in the bone tissue engineering as the scaffold. It biodegrades to lactic acid without the aid of any enzymes which is innocuous to the body and avoids inflammatory responses. Modification of TiO2NPs with this kind of biodegradable polymer provides a golden opportunity to prepare advanced bone scaffolds; for instance, Mota et al. incorporated TiO2NPs inside the PLA matrix to prepare a modified nanohybrid for its potential ability in bone tissue engineering.282 The cell viability tests on L929 fibroblast confirmed the biocompatibility of this TiO2NP–PLA nanohybrid and its potential for use in the scaffolds. In this system, PLA could provide a biodegradable matrix for the well dispersion and fixation of TiO2NPs against aggregation, enhancing the biocompatibility of the TiO2NPs. Also, other recent articles in this field are summarized in Table 11.
| Nanosystem | Applications | Ref. |
|---|---|---|
| Poly(lactate-co-glycolate) (PLGA)/TiO2 scaffolds | Scaffolds | 283 |
| TiO2/poly(vinyl alcohol) nanocomposite | Bone tissue engineering | 284 |
| Silk fibroin/TiO2 nanocomposite | Bone tissue engineering | 285 |
| Nanostructured chitosan/PLA/HA scaffolds doped with TiO2/Au/Pt NPs | Bone tissue engineering | 286 |
| TiO2-chitosan/sodium alginate blended nanocomposite | Bone tissue engineering | 287 |
| Nanotubular TiO2 with gold nanoparticles | 288 | |
| Poly(lactic acid)/TiO2 nanocomposite | Potential ability of PLA/TiO2 nanocomposites to reduce cutaneous scarring in scaffolds | 289 |
| Alginate/chitosan multilayer films coated on IL-4-loaded TiO2 nanotubes | Alginate/chitosan multilayer films coated on IL-4-loaded TiO2 nanotubes for modulation of the macrophage phenotype | 290 |
5. Conclusion and perspectives
This review demonstrates the paramount importance of the surface modification of TiO2NPs to improve their physicochemical properties and biocompatibility. In this regard, a broad range of organic and organosilane molecules were presented in this review paper which have been used as surface modifiers, according to the final applications of the modified TiO2NPs. The potential biomedical applications of these modified TiO2NPs have been studied in different fields, e.g., photodynamic therapy, drug delivery, antibacterial, tissue engineering, anticancer, antifungals, biosensors, and antivirals. Furthermore, the anti-COVID-19 performance of modified TiO2NPs has attracted growing attention of multidisciplinary groups to develop this field and improve current therapeutic methods. In these fields, surface modifiers were selected from biocompatible and bioactive materials which can improve the therapeutic effectiveness of the TiO2NPs. It is worth mentioning that the clinical trials of the modified TiO2NPs require much more research to fulfill all the requirements of clinical agents in terms of physicochemical and biological properties. For example, much more in vivo studies should be performed for evaluating the toxicity of modified TiO2NPs on mammals. Thus, it is still a frontier research area with worthwhile background knowledge provided by previous research. So, future studies can be more focused on the development of safe, cost-effective, and scalable modified TiO2NPs for the clinical fields, specifically antimicrobial agents. We believe that the modified TiO2NPs will be an important research topic with great vitality and practical potential in biomedical applications.Conflicts of interest
There are no conflicts to declare.References
- N. Rodríguez-Barajas, M. L. Anaya-Esparza, Z. Villagrán-de la Mora, A. J. Sánchez-Burgos and A. Pérez-Larios, Adv. Anticancer Agents Med. Chem., 2022, 22, 2241–2254 CrossRef PubMed.
- X. He, H. Deng and H. Hwang, J. Food Drug Anal., 2019, 27, 1–21 CrossRef CAS PubMed.
- L. M. Margarucci, V. Romano Spica, G. Gianfranceschi and F. Valeriani, Environ. Int., 2019, 133, 105095 CrossRef CAS PubMed.
- A. Morlando, M. Chaki Borrás, Y. Rehman, S. Bakand, P. Barker, R. Sluyter and K. Konstantinov, J. Mater. Chem. B, 2020, 8, 4016–4028 RSC.
- H. Chang, Q. Wang, X. Meng, X. Chen, Y. Deng, L. Li, Y. Yang, G. Song and H. Jia, Chem. Res. Toxicol., 2022, 35, 1435–1456 Search PubMed.
- C. J. Dedman, A. M. King, J. A. Christie-Oleza and G.-L. Davies, Environ. Sci.: Nano, 2021, 8, 1236–1255 RSC.
- S. Sargazi, S. ER, S. Sacide Gelen, A. Rahdar, M. Bilal, R. Arshad, N. Ajalli, M. Farhan Ali Khan and S. Pandey, J. Drug Delivery Sci. Technol., 2022, 75, 103605 CrossRef CAS.
- R. Javed, N. Ul Ain, A. Gul, M. Arslan Ahmad, W. Guo, Q. Ao and S. Tian, IET Nanobiotechnol., 2022, 16, 171–189 CrossRef PubMed.
- F. Valeriani, L. M. Margarucci and V. Romano Spica, Int. J. Environ. Res. Public Health, 2018, 15, 2675 CrossRef CAS PubMed.
- G. Lofrano, F. Ubaldi, L. Albarano, M. Carotenuto, V. Vaiano, F. Valeriani, G. Libralato, G. Gianfranceschi, I. Fratoddi, S. Meric, M. Guida and V. Romano Spica, Nanomaterials, 2022, 12, 2831 CrossRef CAS PubMed.
- L. M. Margarucci, V. Romano Spica, C. Protano, G. Gianfranceschi, M. Giuliano, V. Di Onofrio, N. Mucci, F. Valeriani, M. Vitali and F. Romano, Ann. Ig., 2019, 31, 461–473 CAS.
- L. M. Margarucci, G. Gianfranceschi, V. Romano Spica, G. D’Ermo, C. Refi, M. Podico, M. Vitali, F. Romano and F. Valeriani, Int. J. Environ. Res. Public Health, 2021, 18, 8662 CrossRef CAS PubMed.
- T. Feng, S. Yan, S. Hou and X. Fan, Spectrochim. Acta, Part A, 2022, 280, 121548 CrossRef CAS PubMed.
- J. E. Karbowniczek, D. P. Ura and U. Stachewicz, Composites, Part B, 2022, 241, 110011 CrossRef CAS.
- G. Sanità, B. Carrese and A. Lamberti, Front. Mol. Biosci., 2020, 7, 587012 CrossRef PubMed.
- K. V. Korpany, C. Mottillo, J. Bachelder, S. N. Cross, P. Dong, S. Trudel, T. Friščić and A. S. Blum, Chem. Commun., 2016, 52, 3054–3057 RSC.
- S. Benkoula, O. Sublemontier, M. Patanen, C. Nicolas, F. Sirotti, A. Naitabdi, F. Gaie-Levrel, E. Antonsson, D. Aureau, F.-X. Ouf, S.-I. Wada, A. Etcheberry, K. Ueda and C. Miron, Sci. Rep., 2015, 5, 15088 CrossRef CAS PubMed.
- S. Wendt, R. Schaub, J. Matthiesen, E. K. Vestergaard, E. Wahlström, M. D. Rasmussen, P. Thostrup, L. M. Molina, E. Lægsgaard, I. Stensgaard, B. Hammer and F. Besenbacher, Surf. Sci., 2005, 598, 226–245 CrossRef CAS.
- M.-I. Baraton and L. Merhari, J. Eur. Ceram. Soc., 2004, 24, 1399–1404 CrossRef CAS.
- C. E. Nanayakkara, W. A. Larish and V. H. Grassian, J. Phys. Chem. C, 2014, 118, 23011–23021 CrossRef CAS.
- L. T. Zhuravlev, Colloids Surf., A, 2000, 173, 1–38 CrossRef CAS.
- P. Pallavicini, E. Cabrini, A. Casu, G. Dacarro, Y. Antonio Diaz-Fernandez, A. Falqui, C. Milanese and F. Vita, Dalton Trans., 2015, 44, 21088 RSC.
- E. P. Plueddemann, Nature of Adhesion Through Silane Coupling Agents, Silane Coupling Agents, Springer US, Boston, MA, 1991, p. 115 Search PubMed.
- F. Ahangaran and A. H. Navarchian, Adv. Colloid Interface Sci., 2020, 286, 102298 CrossRef CAS PubMed.
- N. Zhu, H. Ji, P. Yu, J. Niu, M. U. Farooq, M. W. Akram, I. O. Udego, H. Li and X. Niu, Nanomaterials, 2018, 8, 810 CrossRef PubMed.
- R. S. Fernandes, I. M. Raimundo and M. F. Pimentel, Colloids Surf., A, 2019, 577, 1–7 CrossRef CAS.
- C. Chen, W. Wu, W. Z. Xu and P. A. Charpentier, Nanotechnology, 2017, 28, 115709 CrossRef PubMed.
- N. D. Bansod, B. P. Kapgate, C. Das, A. Das, D. Basu and S. C. Debnath, J. Sol-Gel Sci. Technol., 2016, 80, 548–559 CrossRef CAS.
- J. Xie, L. F. Mei, L. B. Liao, G. C. Lv, Z. G. Xia and G. X. Du, Key Eng. Mater., 2014, 602–603, 59–62 CAS.
- F. Temerov, J. Haapanen, J. M. Mäkelä and J. J. Saarinen, Inorganics, 2021, 9, 21 CrossRef CAS.
- H. Maskrot, N. Herlin-Boime, Y. Leconte, K. Jursikova, C. Reynaud and J. Vicens, J. Nanopart. Res., 2006, 8, 351–360 CrossRef CAS.
- G. N. Shao, Y. Kim, S. M. Imran, S. J. Jeon, P. B. Sarawade, A. Hilonga, J.-K. Kim and H. T. Kim, Microporous Mesoporous Mater., 2013, 179, 111–121 CrossRef CAS.
- Y. Zhang, F. Fang, C. Wang, L. Wang, X. Wang, X. Chu, J. Li, X. Fang, Z. Wei and X. Wang, Polym. Compos., 2014, 35, 1204–1211 CrossRef CAS.
- S. Mallakpour and M. Madani, J. Mater. Sci., 2014, 49, 5112–5118 CrossRef CAS.
- S. W. Chong, C. W. Lai, J. C. Juan and B. F. Leo, Sol. Energy, 2019, 191, 663–671 CrossRef CAS.
- H. P. Duong, C.-H. Hung, H. C. Dao, M. D. Le and C.-Y. Chen, New J. Chem., 2018, 42, 8745–8751 RSC.
- D. Meroni, L. Lo Presti, G. Liberto, M. Ceotto, R. G. Acres, K. C. Prince, R. Bellani, G. Soliveri and S. Ardizzone, J. Phys. Chem. C, 2017, 121, 430–440 CrossRef CAS PubMed.
- S. Raqeema, U. Hashim, N. Azizah, S. Nadzirah, M. K. M. Arshad, A. R. Ruslinda and S. C. B. Gopinath, AIP Conf. Proc., 2017, 1808, 20007 CrossRef.
- Y.-L. Liu, Y.-H. Su and J.-Y. Lai, Polymer, 2004, 45, 6831–6837 CrossRef CAS.
- Z.-M. Dang, Y.-J. Xia, J.-W. Zha, J.-K. Yuan and J. Bai, Mater. Lett., 2011, 65, 3430–3432 CrossRef CAS.
- Q. F. Xu, Y. Liu, F.-J. Lin, B. Mondal and A. M. Lyons, ACS Appl. Mater. Interfaces, 2013, 5, 8915–8924 CrossRef CAS PubMed.
- M. M. Rahim-Abadi, A. R. Mahdavian, A. Gharieh and H. Salehi-Mobarakeh, Prog. Org. Coat., 2015, 88, 310–315 CrossRef CAS.
- P. Toh-Ae, B. Junhasavasdikul, N. Lopattananon and K. Sahakaro, Adv. Mater. Res., 2014, 844, 276–279 Search PubMed.
- J. D. Ambrósio, C. V. M. Balarim and G. B. de Carvalho, Polym. Compos., 2016, 37, 1415–1424 CrossRef.
- M. Sabzi, S. M. Mirabedini, J. Zohuriaan-Mehr and M. Atai, Prog. Org. Coat., 2009, 65, 222–228 CrossRef CAS.
- T. P. Selvin, J. Kuruvilla and T. Sabu, Mater. Lett., 2004, 58, 281–289 CrossRef CAS.
- L. Meng, Z. Liu, C. Lan and N. Xu, Catal. Lett., 2022, 152, 912–920 CrossRef CAS.
- P. A. Zapata, H. Palza, K. Delgado and F. M. Rabagliati, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4055–4062 CrossRef CAS.
- V. G. Nguyen, H. Thai, D. H. Mai, H. T. Tran, D. L. Tran and M. T. Vu, Composites, Part B, 2013, 45, 1192–1198 CrossRef CAS.
- E. Džunuzović, M. Marinović-Cincović, J. Vuković, K. Jeremić and J. M. Nedeljković, Polym. Compos., 2009, 30, 737–742 CrossRef.
- Y. Qi, B. Xiang, W. Tan and J. Zhang, Appl. Surf. Sci., 2017, 419, 213–223 CrossRef CAS.
- J. Zhao, M. Milanova, M. M. C. G. Warmoeskerken and V. Dutschk, Colloids Surf., A, 2012, 413, 273–279 CrossRef CAS.
- R. Mokhtari Aghdami, S. R. Mousavi, S. Estaji, R. K. Dermeni, H. A. Khonakdar and A. Shakeri, Polym. Compos., 2022, 43, 4165–4178 CrossRef CAS.
- H. Massoumi, R. Kumar, M. K. Chug, Y. Qian and E. J. Brisbois, ACS Appl. Bio Mater., 2022, 5, 2285–2295 CrossRef CAS PubMed.
- A. Dymerska, B. Zielińska, K. Sielicki, X. Chen and E. Mijowska, Diamond Relat. Mater., 2022, 125, 109027 CrossRef CAS.
- J. Yoo, H. Jeong, S. K. Park, S. Park and J. S. Lee, Biosensors, 2021, 11, 2012 CrossRef PubMed.
- P. Zhang, L. Cao, X. Wang, J. Cui, Z. Lin, S. Ngai, F. Vogel, H. Wang, W. Li, S. Li and Q. Wang, Ceram. Int., 2022, 48, 1731–1739 CrossRef CAS.
- A. Wanag, A. Sienkiewicz, P. Rokicka-Konieczna, E. Kusiak-Nejman and A. W. Morawski, J. Environ. Chem. Eng., 2020, 8, 103917 CrossRef CAS.
- S. Mallakpour and A. Barati, Prog. Org. Coat., 2011, 71, 391–398 CrossRef CAS.
- A. Shakeri, D. Yip, M. Badv, S. M. Imani, M. Sanjari and T. F. Didar, Materials, 2018, 11, 1003 CrossRef PubMed.
- R. Klaysri, T. Tubchareon and P. Praserthdam, J. Ind. Eng. Chem., 2017, 45, 229–236 CrossRef CAS.
- G. Lee, J. Lee and C. Kang, J. Coat. Technol. Res., 2019, 16, 1399–1409 CrossRef CAS.
- M. A. Ashraf, Z. Liu, W.-X. Peng and N. Yoysefi, Prog. Org. Coat., 2019, 136, 105296 CrossRef CAS.
- N. Tangchantra, J. Kruenate, C. Aumnate and T. Sooksomsong, Adv. Mater. Res., 2010, 93–94, 300–303 CAS.
- C. Yang and C. Yang, J. Mater. Sci.: Mater. Electron., 2014, 25, 3285–3289 CrossRef CAS.
- C. H. M. Caris, R. P. M. Kuijpers, A. M. van Herk and A. L. German, Makromol. Chem., Macromol. Symp., 1990, 35–36, 535–548 CrossRef CAS.
- C.-C. Weng and K.-H. Wei, Chem. Mater., 2003, 15, 2936–2941 CrossRef CAS.
- B. Erdem, E. D. Sudol, V. L. Dimonie and M. S. El-Aasser, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4431–4440 CrossRef CAS.
- M. Z. Rong, M. Q. Zhang, H. B. Wang and H. M. Zeng, Appl. Surf. Sci., 2002, 200, 76–93 CrossRef CAS.
- M. Yang and Y. Dan, Colloid Polym. Sci., 2005, 284, 243–250 CrossRef CAS.
- F. Milanesi, G. Cappelletti, R. Annunziata, C. L. Bianchi, D. Meroni and S. Ardizzone, J. Phys. Chem. C, 2010, 114, 8287–8293 CrossRef CAS.
- J. Z. B. Xiang and G. Jiang, Plast., Rubber Compos., 2015, 44, 148–154 CrossRef.
- C. Wang, H. Mao, C. Wang and S. Fu, Ind. Eng. Chem. Res., 2011, 50, 11930–11934 CrossRef CAS.
- J. Godnjavec, B. Znoj, J. Vince, M. Steinbucher, A. Žnidaršič and P. Venturini, Mater. Technol., 2012, 46, 19–24 CAS.
- M.-A. E. Antoine, R. M. Dalod, L. Henriksen and T. Grande, Beilstein J. Nanotechnol., 2017, 8, 304–312 CrossRef PubMed.
- M. Connolly, D. Hernández-Moreno, E. Conde, A. Garnica, J. M. Navas, F. Torrent, I. Rucandio and M. L. Fernandez-Cruz, Environ. Sci. Eur., 2022, 34, 1 CrossRef CAS.
- D. Tsotetsi, M. Dhlamini and P. Mbule, Results Mater., 2022, 14, 100266 CrossRef CAS.
- S. Katebi Koushali, M. Hamadanian, A. R. Ghasemi and M. Ashrafi, J. Nanostruct., 2021, 11, 38–47 Search PubMed.
- B. A. Dehkordi, M. R. Nilforoushan, N. Talebian and M. Tayebi, Mater. Res. Express, 2021, 8, 35403 CrossRef CAS.
- H. M. Yadav, N. D. Thorat, M. M. Yallapu, S. A. M. Tofail and J.-S. Kim, J. Mater. Chem. B, 2017, 5, 1461–1470 RSC.
- Z. Landolsi, I. Ben Assaker, D. Nunes, E. Fortunato, R. Martins, R. Chtourou and S. Ammar, J. Mater. Sci.: Mater. Electron., 2020, 31, 20753–20773 CrossRef CAS.
- S. Bai, N. Yang, X. Wang, F. Gong, Z. Dong, Y. Gong, Z. Liu and L. Cheng, ACS Nano, 2020, 14, 15119–15130 CrossRef CAS PubMed.
- Y. Birinci, J. H. Niazi, O. Aktay-Çetin and H. Basaga, Enzyme Microb. Technol., 2020, 138, 109559 CrossRef CAS PubMed.
- X. Wang, X. Zhong, L. Bai, J. Xu, F. Gong, Z. Dong, Z. Yang, Z. Zeng, Z. Liu and L. Cheng, J. Am. Chem. Soc., 2020, 142, 6527–6537 CrossRef CAS PubMed.
- H. M. Yadav, N. D. Thorat, M. M. Yallapu, S. A. M. Tofail and J.-S. Kim, J. Mater. Chem. B, 2017, 5, 1461–1470 RSC.
- S. Salou, C.-M. Cirtiu, D. Larivière and N. Fleury, Anal. Bioanal. Chem., 2020, 412, 1469–1481 CrossRef CAS PubMed.
- Y. Li, Z. Qin, H. Guo, H. Yang, G. Zhang, S. Ji and T. Zeng, PLoS One, 2014, 9, e114638 CrossRef PubMed.
- V. A. Ortega, D. Boyle, J. W. Hodgkinson, D. B. D. Simmons, M. Belosevic, J. L. Stafford and G. G. Goss, Environ. Sci.: Nano, 2021, 8, 1910–1926 RSC.
- V. A. Ortega, M. S. Bahniuk, S. Memon, L. D. Unsworth, J. L. Stafford and G. G. Goss, Biointerphases, 2020, 15, 51003 CrossRef CAS PubMed.
- H. E. Ali, A. M. Elbarbary, A. M. Abdel-Ghaffar and N. A. Maziad, J. Appl. Polym. Sci., 2022, 139, 52344 CrossRef CAS.
- Z. He, H. Wu, Z. Shi, Z. Kong, S. Ma, Y. Sun and X. Liu, ACS Omega, 2022, 7, 7084–7095 CrossRef CAS PubMed.
- S. Dong, G. Xiao, C. Chen, Z. Yang, C. Chen, Q. Wang and L. Lin, Prog. Org. Coat., 2021, 157, 106291 CrossRef CAS.
- S. Naghibi, H. R. Madaah Hosseini and M. A. Faghihi Sani, Ceram. Int., 2013, 39, 8377–8384 CrossRef CAS.
- B. E. Castillo, E. Prokhorov, G. Luna-Bárcenas and Y. Kovalenko, Polymers, 2022, 14, 1686 CrossRef CAS PubMed.
- H. Moulahoum, F. Ghorbanizamani, S. Sakarya and S. Timur, Prog. Org. Coat., 2022, 169, 106923 CrossRef CAS.
- N. Y. Elmehbad, N. A. Mohamed and N. A. Abd El-Ghany, Int. J. Biol. Macromol., 2022, 205, 719–730 CrossRef CAS PubMed.
- M. F. Majnis, O. C. Yee, M. A. Mohd Adnan, M. R. Yusof Hamid, K. Z. Ku Shaari and N. Muhd Julkapli, Opt. Mater., 2022, 124, 111967 CrossRef CAS.
- O. D. Saliu, M. Mamo, P. Ndungu and J. Ramontja, J. Energy Storage, 2022, 49, 104155 CrossRef.
- F. L. Gomes de Menezes, R. H. de Lima Leite, F. K. Gomes dos Santos, A. I. Aria and E. M. M. Aroucha, Colloids Surf., A, 2021, 630, 127661 CrossRef CAS.
- M. Ren, H. Horn and F. H. Frimmel, Water Res., 2017, 123, 678–686 CrossRef CAS PubMed.
- F. Loosli, L. Vitorazi, J.-F. Berret and S. Stoll, Water Res., 2015, 80, 139–148 CrossRef CAS PubMed.
- M. N. Alomary and M. A. Ansari, Chemistry, 2021, 27, 5817–5829 CrossRef CAS PubMed.
- G. Sarigul, I. Chamorro-Mena, N. Linares, J. García-Martínez and E. Serrano, Adv. Sustainable Syst., 2021, 5, 2100076 CrossRef CAS.
- S. Dessai, M. Ayyanar, S. Amalraj, P. Khanal, S. Vijayakumar, N. Gurav, N. Rarokar, M. Kalaskar, S. Nadaf and S. Gurav, Mater. Lett., 2022, 311, 131639 CrossRef CAS.
- S. Gulla, V. C. Reddy, P. B. Araveti, D. Lomada, A. Srivastava, M. C. Reddy and K. R. Reddy, J. Mol. Struct., 2022, 1249, 131556 CrossRef CAS.
- S. Iqbal, M. Fakhar-e-Alam, K. S. Alimgeer, M. Atif, A. Hanif, N. Yaqub, W. A. Farooq, S. Ahmad, Y.-M. Chu, M. Suleman Rana, A. Fatehmulla and H. Ahmad, Saudi J. Biol. Sci., 2021, 28, 1226–1232 CrossRef CAS PubMed.
- H.-X. Wang, X.-X. Li and L. Tang, Appl. Phys. A: Mater. Sci. Process., 2020, 126, 448 CrossRef CAS.
- A. Razzaz, S. Ghorban, L. Hosayni, M. Irani and M. Aliabadi, J. Taiwan Inst. Chem. Eng., 2016, 58, 333–343 CrossRef CAS.
- A. Babaei-Ghazvini, B. Acharya and D. R. Korber, Polymers, 2021, 13, 2790 CrossRef CAS PubMed.
- V. K. Bui, D. Park and Y.-C. Lee, Polymers, 2017, 9, 21 CrossRef PubMed.
- R. Gobi, P. Ravichandiran, R. S. Babu and D. J. Yoo, Polymers, 2021, 13, 1962 CrossRef CAS PubMed.
- X. Wang, Z. Li, Y. Wu, H. Guo, X. Zhang, Y. Yang, H. Mu and J. Duan, ACS Appl. Mater. Interfaces, 2021, 13, 10902–10915 CrossRef CAS PubMed.
- R. Khan and M. Dhayal, Electrochem. Commun., 2008, 10, 492–495 CrossRef CAS.
- S. A. Matboo, S. Nazari, A. Niapour, M. V. Niri, E. Asgari and S. A. Mokhtari, Water Sci. Technol., 2022, 85, 605–616 CrossRef CAS PubMed.
- A. Maleki, B. Hayati, F. Najafi, F. Gharibi and S. W. Joo, J. Mol. Liq., 2016, 224, 95–104 CrossRef CAS.
- Z. Zhang, Y. Wang, T. Li, P. Ma, X. Zhang, B. Xia, M. Chen, M. Du and W. Dong, Ind. Eng. Chem. Res., 2021, 60, 3999–4008 CrossRef CAS.
- Z. Qiaorun, S. Honghong, L. Yao, J. Bing, X. Xiao, D. Julian McClements, C. Chongjiang and Y. Biao, Food Res. Int., 2022, 159, 111574 CrossRef PubMed.
- A. Tajdari, A. Babaei, A. Goudarzi and R. Partovi, J. Plast. Film Sheeting, 2020, 36, 285–311 CrossRef CAS.
- F. Loosli, P. Le Coustumer and S. Stoll, Sci. Total Environ., 2015, 535, 28–34 CrossRef CAS PubMed.
- S. Li, G. Chen, S. Qiang, Z. Yin, Z. Zhang and Y. Chen, Int. J. Food Microbiol., 2020, 331, 108763 CrossRef CAS PubMed.
- A. Deghiche, N. Haddaoui, A. Zerriouh, S. E. Fenni, D. Cavallo, A. Erto and Y. Benguerba, J. Environ. Chem. Eng., 2021, 9, 106541 CrossRef CAS.
- J. Zhang, M. Zheng, Y. Zhou, L. Yang, Y. Zhang, Z. Wu, G. Liu and J. Zheng, Membranes, 2022, 12, 386 CrossRef CAS PubMed.
- J.-H. Li, Y.-Y. Xu, L.-P. Zhu, J.-H. Wang and C.-H. Du, J. Membr. Sci., 2009, 326, 659–666 CrossRef CAS.
- K. Liu, Z. Cai, X. Chi, B. Kang, S. Fu, X. Luo, Z.-W. Lin, H. Ai, J. Gao and H. Lin, Nano Lett., 2022, 22, 3219–3227 CrossRef CAS PubMed.
- J. L. Peper, N. E. Gentry, B. Boudy and J. M. Mayer, Inorg. Chem., 2022, 61, 767–777 CrossRef CAS PubMed.
- Y.-Z. Lü, S.-N. Zhang, Y.-F. Du, M.-T. Chen and C.-R. Li, J. Inorg. Mater., 2013, 28, 594–598 CrossRef.
- Y. Lv, C. Li, Q. Sun, M. Huang, C. Li and B. Qi, Nanoscale Res. Lett., 2016, 11, 515 CrossRef PubMed.
- M. S. Hanafy, W. M. Desoky, E. M. Hussein, N. H. El-Shaer, M. Gomaa, A. A. Gamal, M. A. Esawy and O. W. Guirguis, J. Biomed. Mater. Res., Part A, 2021, 109, 232–247 CrossRef CAS PubMed.
- L. Goñi-Ciaurriz and I. Vélaz, Int. J. Biol. Macromol., 2022, 216, 347–360 CrossRef PubMed.
- T. Ukmar, A. Godec, U. Maver, O. Planinšek, M. Bele, J. Jamnik and M. Gaberšček, J. Mater. Chem., 2009, 19, 8176–8183 RSC.
- C. Ronchi, D. Selli, W. Pipornpong and C. Di Valentin, J. Phys. Chem. C, 2019, 123, 7682–7695 CrossRef CAS PubMed.
- M. Qi, C. Li, Z. Song and L. Wang, Drug Delivery, 2021, 28, 1785–1794 CrossRef CAS PubMed.
- R. Mohan, J. Drbohlavova and J. Hubalek, Nanoscale Res. Lett., 2013, 8, 503 CrossRef PubMed.
- R. Binaymotlagh, L. Chronopoulou, F. Hajareh Haghighi, I. Fratoddi and C. Palocci, Materials, 2022, 15, 5871 CrossRef CAS PubMed.
- R. Binaymotlagh, A. Del Giudice, S. Mignardi, F. Amato, A. G. Marrani, F. Sivori, I. Cavallo, E. G. Di Domenico, C. Palocci and L. Chronopoulou, Gels, 2022, 8, 700 CrossRef CAS PubMed.
- X. Yang, Y. Wang, W. Qi, R. Xing, X. Yang, Q. Xing, R. Su and Z. He, J. Mater. Chem. B, 2019, 7, 2981–2988 RSC.
- E. Makhado, B. R. Motshabi, D. Allouss, K. E. Ramohlola, K. D. Modibane, M. J. Hato, R. M. Jugade, F. Shaik and S. Pandey, Chemosphere, 2022, 306, 135524 CrossRef CAS PubMed.
- S. Zhao, C. Hou, L. Shao, W. An and W. Cui, Appl. Surf. Sci., 2022, 590, 153088 CrossRef CAS.
- R. R. Mansurov, V. S. Zverev and A. P. Safronov, J. Catal., 2022, 406, 9–18 CrossRef CAS.
- A. Ulu, E. Birhanlı, S. Köytepe and B. Ateş, Int. J. Biol. Macromol., 2020, 163, 529–540 CrossRef CAS PubMed.
- Y. Yue, X. Wang, Q. Wu, J. Han and J. Jiang, J. Colloid Interface Sci., 2020, 564, 99–112 CrossRef CAS PubMed.
- R. F. Bonan, M. F. Mota, R. M. da Costa Farias, S. D. da Silva, P. R. F. Bonan, L. Diesel, R. R. Menezes and D. E. da Cruz Perez, Mater. Sci. Eng., C, 2019, 104, 109876 CrossRef CAS PubMed.
- S. Shiva Samhitha, G. Raghavendra, C. Quezada and P. Hima Bindu, Mater. Today: Proc., 2022, 54, 765–770 CAS.
- N. Lagopati, K. Evangelou, P. Falaras, E.-P. C. Tsilibary, P. V. S. Vasileiou, S. Havaki, A. Angelopoulou, E. A. Pavlatou and V. G. Gorgoulis, Pharmacol. Ther., 2021, 222, 107795 CrossRef CAS PubMed.
- S. Çeşmeli and C. Biray Avci, J. Drug Targeting, 2019, 27, 762–766 CrossRef PubMed.
- Z. Youssef, V. Jouan-Hureaux, L. Colombeau, P. Arnoux, A. Moussaron, F. Baros, J. Toufaily, T. Hamieh, T. Roques-Carmes and C. Frochot, Photodiagn. Photodyn. Ther., 2018, 22, 115–126 CrossRef CAS PubMed.
- Z. Youssef, P. Arnoux, L. Colombeau, J. Toufaily, T. Hamieh, C. Frochot and T. Roques-Carmes, J. Photochem. Photobiol., A, 2018, 356, 177–192 CrossRef CAS.
- P. Huang, C. Xu, J. Lin, C. Wang, X. Wang, C. Zhang, X. Zhou, S. Guo and D. Cui, Theranostics, 2011, 1, 240–250 CrossRef CAS PubMed.
- X. Liang, Y. Xie, J. Wu, J. Wang, M. Petković, M. Stepić, J. Zhao, J. Ma and L. Mi, J. Photochem. Photobiol., B, 2021, 215, 112122 CrossRef CAS PubMed.
- B. Salama, C.-J. Chang, K. Kanehira, E.-S. El-Sherbini, G. El-Sayed, M. El-Adl and A. Taniguchi, Molecules, 2020, 25, 4467 CrossRef CAS PubMed.
- S. Gai, G. Yang, P. Yang, F. He, J. Lin, D. Jin and B. Xing, Nano Today, 2018, 19, 146–187 CrossRef CAS.
- M. A. Behnam, F. Emami, Z. Sobhani and A. R. Dehghanian, Iran. J. Basic Med. Sci., 2018, 21, 1133–1139 Search PubMed.
- Y. Gao, L. Zhang, Y. Liu, S. Sun, Z. Yin, L. Zhang, A. Li, G. Lu, A. Wu and L. Zeng, Nanoscale, 2020, 12, 1801–1810 RSC.
- T. Dai, W. He, S. Tu, J. Han, B. Yuan, C. Yao, W. Ren and A. Wu, Bioact. Mater., 2022, 17, 18–28 CrossRef CAS PubMed.
- Y. Zhang, X. Zhang, H. Yang, L. Yu, Y. Xu, A. Sharma, P. Yin, X. Li, J. S. Kim and Y. Sun, Chem. Soc. Rev., 2021, 50, 11227–11248 RSC.
- R. L. Siegel, K. D. Miller, H. E. Fuchs and A. Jemal, Ca-Cancer J. Clin., 2021, 71, 7–33 CrossRef PubMed.
- A. Vincent, J. Herman, R. Schulick, R. H. Hruban and M. Goggins, Lancet, 2011, 378, 607–620 CrossRef PubMed.
- A. Zinger, L. Koren, O. Adir, M. Poley, M. Alyan, Z. Yaari, N. Noor, N. Krinsky, A. Simon and H. Gibori, ACS Nano, 2019, 13, 11008–11021 CrossRef CAS PubMed.
- K. P. Olive, M. A. Jacobetz, C. J. Davidson, A. Gopinathan, D. McIntyre, D. Honess, B. Madhu, M. A. Goldgraben, M. E. Caldwell and D. Allard, Science, 2009, 324, 1457–1461 CrossRef CAS PubMed.
- A. Neesse, P. Michl, K. K. Frese, C. Feig, N. Cook, M. A. Jacobetz, M. P. Lolkema, M. Buchholz, K. P. Olive and T. M. Gress, Gut, 2011, 60, 861–868 CrossRef PubMed.
- J. Luo, J. Cao, G. Ma, X. Wang, Y. Sun, C. Zhang, Z. Shi, Y. Zeng, T. Zhang and P. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 40535–40545 CrossRef CAS PubMed.
- X. Wei, Z. Feng, J. Huang, X. Xiang, F. Du, C. He, M. Zhou, L. Ma, C. Cheng and L. Qiu, ACS Appl. Mater. Interfaces, 2021, 13, 32810–32822 CrossRef CAS PubMed.
- X. Lin, R. Huang, Y. Huang, K. Wang, H. Li, Y. Bao, C. Wu, Y. Zhang, X. Tian and X. Wang, Int. J. Nanomed., 2021, 16, 1889–1899 CrossRef PubMed.
- G. P. Lee, A. Willis, S. Pernal, A. Phakatkar, T. Shokuhfar, V. Blot and H. H. Engelhard, Nanomedicine, 2021, 16, 523–534 CrossRef CAS PubMed.
- E. Yousefi, S. Javadpour, M. Ansari and H. Eslami, Mater. Technol., 2021, 36, 521–528 CrossRef.
- A. Pariente, E. Peled, I. Zlotver and A. Sosnik, Mater. Today Chem., 2021, 22, 100613 CrossRef CAS.
- P. Magesan, K. I. Dhanalekshmi, J. Prabha, M. J. Umapathy, X. Zhang, N. Punitha, K. Kadambary and K. Sangeetha, Photodiagn. Photodyn. Ther., 2022, 40, 103064 CrossRef CAS PubMed.
- J.-Y. Zhou, W.-J. Wang, C.-Y. Zhang, Y.-Y. Ling, X.-J. Hong, Q. Su, W.-G. Li, Z.-W. Mao, B. Cheng, C.-P. Tan and T. Wu, Biomaterials, 2022, 289, 121757 CrossRef CAS PubMed.
- E. Donadoni, P. Siani, G. Frigerio and C. Di Valentin, Nanoscale, 2022, 14, 12099–12116 RSC.
- L. Fang, H. Huang, J. D. Quirk, J. Zheng, D. Shen, B. Manion, M. Mixdorf, P. Karmakar, G. P. Sudlow, R. Tang and S. Achilefu, Curr. Anal. Chem., 2022, 18, 826–835 CrossRef CAS PubMed.
- Y. Hou, A. Mushtaq, Z. Tang, E. Dempsey, Y. Wu, Y. Lu, C. Tian, J. Farheen, X. Kong and M. Z. Iqbal, J. Sci.: Adv. Mater. Devices, 2022, 7, 100417 CAS.
- X. Wen, N. Liu, J. Ren, X. Jiao, J. Lv, M. H. Akhtar, H. Qi, J. Zhu, C. Yu and Y. Li, New J. Chem., 2022, 46, 6966–6970 RSC.
- P. Ramachandran, B.-K. Khor, C. Y. Lee, R.-A. Doong, C. E. Oon, N. T. K. Thanh and H. L. Lee, Biomedicines, 2022, 10, 421 CrossRef CAS PubMed.
- E. Tutun, V. Tekin, V. Yasakcı, Ö. Aras and P. Ünak, Appl. Organomet. Chem., 2021, 35, e6435 CrossRef CAS PubMed.
- A. Mushtaq, Y. Hou, C. Tian, T. Deng, C. Xu, Z. Sun, X. Kong and M. Zubair Iqbal, Mater. Res. Bull., 2021, 144, 111481 CrossRef CAS.
- J. Li, S. Dai, R. Qin, C. Shi, J. Ming, X. Zeng, X. Wen, R. Zhuang, X. Chen, Z. Guo and X. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 54727–54738 CrossRef CAS PubMed.
- M. Matijević, J. Žakula, L. Korićanac, M. Radoičić, X. Liang, L. Mi, J. F. Tričković, A. V. Šobot, M. N. Stanković, Đ. Nakarada, M. Mojović, M. Petković, M. Stepić and M. D. Nešic, Photochem. Photobiol. Sci., 2021, 20, 1087–1098 CrossRef PubMed.
- J. Cao, Y. Sun, C. Zhang, X. Wang, Y. Zeng, T. Zhang and P. Huang, Acta Biomater., 2021, 129, 269–279 CrossRef CAS PubMed.
- Z. Shi, X. Meng, K. Zhang, S. Tang, C. Zhang, Z. Yang, H. Dong and X. Zhang, ACS Mater. Lett., 2021, 3, 781–789 CrossRef CAS.
- Q. Tang, H.-L. Zhang, Y. Wang, J. Liu and S.-P. Yang, J. Mater. Chem. B, 2021, 9, 4241–4248 RSC.
- Q. Pan, M. Li, M. Xiao, Y. He, G. Sun, T. Xue, Y. Luo, L. Chen, B. Ai and J. Xiong, J. Nanomater., 2021, 2021, 4125350 Search PubMed.
- A. Mansoor, Z. Khurshid, M. T. Khan, E. Mansoor, F. A. Butt, A. Jamal and P. J. Palma, Nanomaterials, 2022, 12, 3670 CrossRef CAS PubMed.
- J. Han, E.-K. Jang, M.-R. Ki, R. G. Son, S. Kim, Y. Choe, S. P. Pack and S. Chung, J. Ind. Eng. Chem., 2022, 112, 258–270 CrossRef CAS.
- J. Cano-Mejia, R. A. Burga, E. E. Sweeney, J. P. Fisher, C. M. Bollard, A. D. Sandler, C. R. Y. Cruz and R. Fernandes, Nanomedicine, 2017, 13, 771–781 CrossRef CAS PubMed.
- L. Chen, F. Pastorino, P. Berry, J. Bonner, C. Kirk, K. M. Wood, H. D. Thomas, Y. Zhao, A. Daga and G. J. Veal, Int. J. Cancer, 2019, 144, 3146–3159 CrossRef CAS PubMed.
- S. G. DuBois, Y. P. Mosse, E. Fox, R. A. Kudgus, J. M. Reid, R. McGovern, S. Groshen, R. Bagatell, J. M. Maris and C. J. Twist, Clin. Cancer Res., 2018, 24, 6142–6149 CrossRef CAS PubMed.
- T. Lopez, J. Sotelo, J. Navarrete and J. A. Ascencio, Opt. Mater., 2006, 29, 88–94 CrossRef CAS.
- F. Grande and P. Tucci, Mini-Rev. Med. Chem., 2016, 16, 762–769 CrossRef CAS PubMed.
- M. Tian, X. Chen, Z. Gu, H. Li, L. Ma, X. Qi, H. Tan and C. You, Carbohydr. Polym., 2016, 144, 522–530 CrossRef CAS PubMed.
- J. Zhao, L. Yao, S. Nie and Y. Xu, Int. J. Biol. Macromol., 2021, 167, 921–933 CrossRef CAS PubMed.
- S. Klein, T. Luchs, A. Leng, L. V. R. Distel, W. Neuhuber and A. Hirsch, Bioengineering, 2020, 7, 1–22 CrossRef PubMed.
- K. Zheng, R. Chen, Y. Sun, Z. Tan, Y. Liu, X. Cheng, J. Leng, Z. Guo and P. Xu, Thorac. Cancer, 2020, 11, 1476–1486 CrossRef CAS PubMed.
- S. Kim, S. Im, E.-Y. Park, J. Lee, C. Kim, T.-I. Kim and W. J. Kim, Nanomedicine, 2020, 24, 102110 CrossRef CAS PubMed.
- T. Yu, L. Tong, Y. Ao, G. Zhang, Y. Liu and H. Zhang, Drug Delivery, 2020, 27, 855–863 CrossRef CAS PubMed.
- W. Chen, J. Wang, L. Cheng, W. Du, J. Wang, W. Pan, S. Qiu, L. Song, X. Ma and Y. Hu, ACS Appl. Bio Mater., 2021, 4, 1483–1492 CrossRef CAS PubMed.
- Y. He, J. Wan, Y. Yang, P. Yuan, C. Yang, Z. Wang and L. Zhang, Adv. Healthcare Mater., 2019, 8, 1801254 CrossRef PubMed.
- S. Bhullar, N. Goyal and S. Gupta, Sci. Rep., 2022, 12, 4600 CrossRef CAS PubMed.
- M. I. Torres-Ramos, M. F. Martín-Marquez, M. D. C. Leal-Moya, S. Ghotekar, J. A. Sánchez-Burgos and A. Pérez-Larios, Int. J. Mol. Sci., 2022, 23, 10755 CrossRef CAS PubMed.
- A. Chahardoli, F. Qalekhani, Y. Shokoohinia and A. Fattahi, J. Mol. Liq., 2022, 361, 119674 CrossRef CAS.
- N. Karki, H. Tiwari, M. Matiyani, R. Bal, M. Pal and N. G. Sahoo, J. Vinyl Addit. Technol., 2022, 28, 474–486 CrossRef CAS.
- A.-M. Negrescu, V. Mitran, W. Draghicescu, S. Popescu, C. Pirvu, I. Ionascu, T. Soare, S. Uzun, S. M. Croitoru and A. Cimpean, J. Funct. Biomater., 2022, 13, 43 CrossRef CAS PubMed.
- X. Zeng, W. Yang, F. X. Song, H. X. Wang and Y. Li, J. Drug Delivery Sci. Technol., 2022, 68, 103120 CrossRef CAS.
- M. Motiei Pour, M. R. Moghbeli, B. Larijani and H. Akbari Javar, Chem. Pap., 2022, 76, 439–451 CrossRef CAS.
- Y. Feng, L. Liu, J. Zhang, H. Aslan and M. Dong, J. Mater. Chem. B, 2017, 5, 8631–8652 RSC.
- E. J. Diana and T. V. Mathew, Colloids Surf., B, 2022, 220, 112949 CrossRef CAS.
- P. Maheswari, S. Harish, M. Navaneethan, C. Muthamizhchelvan, S. Ponnusamy and Y. Hayakawa, Mater. Sci. Eng., C, 2020, 108, 110457 CrossRef CAS PubMed.
- A. M. Mathew, V. I. Chukwuike, K. Venkatesan, S. Raveendran, R. C. Barik and D. K. Pattanayak, Surf. Interfaces, 2022, 33, 102275 CrossRef.
- A. Sathiyaseelan, K. Saravanakumar, K. V. Naveen, K.-S. Han, X. Zhang, M. S. Jeong and M.-H. Wang, Environ. Res., 2022, 212, 113237 CrossRef CAS PubMed.
- A. O. Özdemir, B. Caglar, O. Çubuk, F. Coldur, M. Kuzucu, E. K. Guner, B. Doğan, S. Caglar and K. V. Özdokur, Mater. Chem. Phys., 2022, 287, 126342 CrossRef.
- S. Metanawin and T. Metanawin, Polym. Int., 2022, 71, 777–789 CrossRef CAS.
- T. Singh, D. B. Pal, A. H. Almalki, Y. S. Althobaiti, M. F. Alkhanani, S. Haque, S. Sharma and N. Srivastava, Mater. Lett., 2022, 316, 132012 CrossRef CAS.
- S. Mallakpour and N. Mohammadi, Carbohydr. Polym., 2022, 285, 119226 CrossRef CAS PubMed.
- A. M. Youssef, M. E. Abd El-Aziz and S. M. M. Morsi, Polym. Bull., 2022, 79, 1–15 CrossRef.
- Y. F. Makableh, N. F. Momani, T. Athamneh, R. Al-Abed and I. Alshorman, Polym. Bull., 2022, 79, 1–13 CrossRef.
- P. Maheswari, S. Ponnusamy, S. Harish, C. Muthamizhchelvan and Y. Hayakawa, Mater. Sci. Semicond. Process., 2020, 105, 104724 CrossRef CAS.
- P. Maheswari, S. Ponnusamy, S. Harish, M. R. Ganesh and Y. Hayakawa, Arabian J. Chem., 2020, 13, 3484–3497 CrossRef CAS.
- P. Maheswari, S. Ponnusamy, S. Harish, C. Muthamizhchelvan, M. R. Ganesh and Y. Hayakawa, Appl. Surf. Sci., 2019, 494, 989–999 CrossRef CAS.
- S. Li, J. Zeng, D. Yin, P. Liao, S. Ding, P. Mao and Y. Liu, Mater. Res. Express, 2021, 8, 85012 CrossRef CAS.
- A. M. Alakrach, A. A. Al-Rashdi, T. Alqadi, M. A. Al Saadi, S. S. Ting, O. S. Dahham and N. N. Zulkepli, Mater. Sci. Forum, 2021, 1021, 270–279 Search PubMed.
- X. Wang, X. Li, X. Yang, K. Lei and L. Wang, Colloids Surf., B, 2021, 197, 111410 CrossRef CAS PubMed.
- V. Soltaninejad and A. Maleki, J. Photochem. Photobiol., A, 2021, 404, 112906 CrossRef CAS.
- T. Li, Y. Xiao, D. Guo, L. Shen, R. Li, Y. Jiao, Y. Xu and H. Lin, J. Colloid Interface Sci., 2020, 572, 114–121 CrossRef CAS PubMed.
- R. K. Manoharan, S. Ayyaru and Y.-H. Ahn, New J. Chem., 2020, 44, 807–816 RSC.
- Y. Gao, X. Wang, X. Li and H. Dai, New J. Chem., 2020, 44, 20751–20758 RSC.
- N. M. Ngoepe, M. M. Mathipa and N. C. Hintsho-Mbita, Optik, 2020, 224, 165728 CrossRef CAS.
- S. Janfaza, M. Banan Nojavani, M. Nikkhah, T. Alizadeh, A. Esfandiar and M. R. Ganjali, Microchim. Acta, 2019, 186, 137 CrossRef PubMed.
- B. Mahyad, S. Janfaza and E. S. Hosseini, Adv. Colloid Interface Sci., 2015, 225, 194–202 CrossRef CAS PubMed.
- M.-C. Estevez, M. A. Otte, B. Sepulveda and L. M. Lechuga, Anal. Chim. Acta, 2014, 806, 55–73 CrossRef CAS PubMed.
- Y. Xu, J. Lin, X. Wu, X. Xu, D. Zhang, Y. Xie, T. Pan, Y. He, A. Wu and G. Shao, J. Mater. Chem. B, 2022, 10, 3808–3816 RSC.
- Q. Y. Siew, S. Y. Tham, H.-S. Loh, P. S. Khiew, W. S. Chiu and M. T. T. Tan, J. Mater. Chem. B, 2018, 6, 1195–1206 RSC.
- S. Tao, Y. Guo, S. Wang, F. Xu, X. Zhou and Q. Guo, Anal. Methods, 2022, 14, 2396–2404 RSC.
- S. P. Hong, N. F. Mohd-Naim, N. A. Keasberry and M. U. Ahmed, Electroanalysis, 2022, 34, 684–691 CrossRef CAS.
- S. Shi, Q. Nie, S. Jiang, S. Wu, B. Tang and M. Zhao, Acta Opt. Sin., 2022, 42, 0106001 CrossRef.
- V. Rajeshwari, C. Vedhi and J. Fernando, Mater. Today: Proc., 2022, 68, 287–293 CAS.
- D. Zheng, M. Chen, J. Peng, J. Chen, T. Chen, Y. Chen, L. Huang and W. Gao, Microchim. Acta, 2021, 188, 328 CrossRef CAS PubMed.
- A. P. Singh, S. Balayan, S. Gupta, U. Jain, R. K. Sarin and N. Chauhan, Process Biochem., 2021, 108, 185–193 CrossRef CAS.
- J. Zhang, H. Hu and L. Yang, Microchem. J., 2021, 168, 106435 CrossRef CAS.
- M. Nycz, K. Arkusz and D. G. Pijanowska, Materials, 2021, 14, 3767 CrossRef CAS PubMed.
- B. Baykal, G. Kadikoylu, H. Senturk, Y. O. Donar, A. Sınağ and A. Erdem, J. Electroanal. Chem., 2021, 892, 115262 CrossRef CAS.
- N. Gao, B. Fan, L. Li, X. Sun, X. Wang, H. Ma, Q. Wei and H. Ju, ACS Appl. Bio Mater., 2021, 4, 4479–4485 CrossRef CAS PubMed.
- J. Guo, G. Fang, S. Wang and J. Wang, Food Chem., 2021, 344, 128656 CrossRef CAS PubMed.
- R. H. Sakban, S. M. Abdulalmohsin and M. D. Noori, J. Phys. Conf. Ser., 2021, 1818, 12038 CrossRef CAS.
- M. K. Choińska, I. Šestáková, V. Hrdlička, J. Skopalová, J. Langmaier, V. Maier and T. Navrátil, Biosensors, 2022, 12, 26 CrossRef PubMed.
- J. Geddes-McAlister and R. S. Shapiro, Ann. N. Y. Acad. Sci., 2019, 1435, 57–78 CrossRef PubMed.
- J. You, Y. Guo, R. Guo and X. Liu, Chem. Eng. J., 2019, 373, 624–641 CrossRef CAS.
- X. Zhao, G. Zhang and Z. Zhang, Environ. Int., 2020, 136, 105453 CrossRef CAS PubMed; Y. Rilda, D. Dwiyanti, S. Syukri, A. Agustien and H. Pard, J. Dispers. Sci. Technol., 2021, 42, 784–790 CrossRef.
- X. He, P. Wu, S. Wang, A. Wang, C. Wang and P. Ding, J. Clean. Prod., 2021, 289, 125755 CrossRef CAS.
- P. A. K. Reddy, P. V. L. Reddy, E. Kwon, K.-H. Kim, T. Akter and S. Kalagara, Environ. Int., 2016, 91, 94–103 CrossRef CAS PubMed.
- T. Wang, Z. Yang, C. Zhang, X. Zhai, X. Zhang, X. Huang, Z. Li, X. Zhang, X. Zou and J. Shi, Int. J. Biol. Macromol., 2022, 222, 2843–2854 CrossRef CAS PubMed.
- T. Siddiqui, N. J. Khan, N. Asif, I. Ahamad, D. Yasin and T. Fatma, Environ. Sci. Pollut. Res., 2022, 29, 39052–39066 CrossRef CAS PubMed.
- M. Sultan, H. Elsayed, A. E. F. Abdelhakim and G. Taha, J. Appl. Polym. Sci., 2022, 139, 51442 CrossRef CAS.
- L. Mohammad Taghizadeh Kashani, Shiva Masoudi and M. M. Ahmadian-Attari, Inorg. Nano-Metal Chem., 2022, 52, 297–307 CAS.
- N. Duan, Q. Li, X. Meng, Z. Wang and S. Wu, Food Chem., 2021, 364, 130441 CrossRef CAS PubMed.
- H. Moradpoor, M. Safaei, A. Golshah, H. R. Mozaffari, R. Sharifi, M. M. Imani and M. S. Mobarakeh, Inorg. Chem. Commun., 2021, 130, 108748 CrossRef CAS.
- E. T. Helmy, E. M. Abouellef, U. A. Soliman and J. H. Pan, Chemosphere, 2021, 271, 129524 CrossRef CAS PubMed.
- N. Rahmat, E. T. Wahyuni and A. Suratman, Indones. J. Chem., 2021, 21, 14–23 CrossRef CAS.
- H. P. Yetria Rilda, Dita Dwiyanti, Syukri Syukri and Anthoni Agustien, J. Dispers. Sci. Technol., 2021, 42, 784–790 CrossRef.
- M. A. Irshad, R. Nawaz, M. Z. U. Rehman, M. Imran, J. Ahmad, S. Ahmad, A. Inam, A. Razzaq, M. Rizwan and S. Ali, Chemosphere, 2020, 258, 127352 CrossRef CAS PubMed.
- L. Goñi-Ciaurriz, M. Senosiain-Nicolay and I. Vélaz, Int. J. Mol. Sci., 2021, 22, 2257 CrossRef PubMed.
- R. Chougale, D. Kasai, S. Nayak, S. Masti, A. Nasalapure and A. V. Raghu, Green Mater., 2020, 8, 40–48 CrossRef.
- S. Krishnan, A. Dusane, R. Morajkar, A. Venkat and A. A. Vernekar, J. Mater. Chem. B, 2021, 9, 5967–5981 RSC.
- M. A. Sadique, S. Yadav, P. Ranjan, S. Verma, S. T. Salammal, M. A. Khan, A. Kaushik and R. Khan, J. Mater. Chem. B, 2021, 9, 4620–4642 RSC.
- H. Liu, W. Zhong, X. Zhang, D. Lin and J. Wu, J. Mater. Chem. B, 2021, 9, 7878–7908 RSC.
- D. A. Elsayed, M. G. Assy, S. M. Mousa, G. T. El-Bassyouni, S. M. Mouneir and W. S. Shehab, Bioorg. Chem., 2022, 124, 105805 CrossRef CAS PubMed.
- D. C. S. Souza, S. M. Amorim, R. D. Cadamuro, G. Fongaro, R. A. Peralta, R. M. Peralta, G. L. Puma and R. F. P. M. Moreira, Carbohydr. Polym. Technol. Appl., 2022, 3, 100182 CAS.
- D. J. da Silva, A. G. Souza, G. S. Ferreira, A. Duran, A. D. Cabral, F. L. A. Fonseca, R. F. Bueno and D. S. Rosa, ACS Appl. Nano Mater., 2021, 4, 12949–12956 CrossRef CAS.
- I.-J. Wang, Y.-C. Chen, C. Su, M.-H. Tsai, W.-T. Shen, C.-H. Bai and K.-P. Yu, J. Aerosol Med. Pulm. Drug Delivery, 2021, 34, 293–302 CrossRef CAS PubMed.
- A. Levina, M. Repkova, N. Shikina, Z. Ismagilov, M. Kupryushkin, A. Pavlova, N. Mazurkova, D. Pyshnyi and V. Zarytova, Eur. J. Pharm. Biopharm., 2021, 162, 92–98 CrossRef CAS PubMed.
- G. León-Gutiérrez, C. Cabello-Gutiérrez, M. H. Martínez-Gómez, P. Azuara, B. Madden, J. Shalkow and A. Mejía, J. Nano Res., 2021, 70, 137–145 Search PubMed.
- L. Zhang, H. Forgham, A. Shen, J. Wang, J. Zhu, X. Huang, S.-Y. Tang, C. Xu, T. P. Davis and R. Qiao, J. Mater. Chem. B, 2022, 10, 7473–7490 RSC.
- Z.-Y. Chen, S. Gao, Y.-W. Zhang, R.-B. Zhou and F. Zhou, J. Mater. Chem. B, 2021, 9, 2594–2612 RSC.
- R. Balan and V. Gayathri, Polym. Bull., 2022, 79, 4269–4286 CrossRef CAS.
- W. S. Saeed, D. H. Alotaibi, A.-B. Al-Odayni, A. S. Haidyrah, A. A. Al-Owais, R. Khan, M. A. De Vera, A. Alrahlah and T. Aouak, Int. J. Mol. Sci., 2022, 23, 3449 CrossRef CAS PubMed.
- S. Mallakpour and M. Naghdi, Ceram. Int., 2022, 48, 2045–2057 CrossRef CAS.
- T. Fatima, R. Jolly, M. R. Wani, G. G. H. A. Shadab and M. Shakir, Results Mater., 2021, 12, 100240 CrossRef CAS.
- S. Ahmadi, Y. Pilehvar, N. Zarghami and A. Abri, J. Drug Delivery Sci. Technol., 2021, 66, 102798 CrossRef CAS.
- H. Khalid, H. Iqbal, R. Zeeshan, M. Nasir, F. Sharif, M. Akram, M. Irfan, F. A. Khan, A. A. Chaudhry and A. F. Khan, Polym. Bull., 2021, 78, 7199–7218 CrossRef CAS.
- S. Ghasemi and H. Ghomi, J. Biomater. Appl., 2021, 36, 406–418 CrossRef CAS PubMed.
- R. Ashraf, T. Maqbool, M. A. Beigh, A. H. Jadhav, H. S. Sofi and F. A. Sheikh, J. Appl. Polym. Sci., 2021, 138, 50594 CrossRef CAS.
- C. Zhang, X. Wang, A. Liu, C. Pan, H. Ding and W. Ye, Mater. Lett., 2021, 291, 129563 CrossRef CAS.
- M. U. Aslam Khan, W. S. Al-Arjan, M. S. Binkadem, H. Mehboob, A. Haider, M. A. Raza, S. I. Abd Razak, A. Hasan and R. Amin, Nanomaterials, 2021, 11, 1319 CrossRef PubMed.
- R. C. de Azevedo Gonçalves Mota, L. R. de Menezes and E. O. da Silva, J. Mater. Res., 2021, 36, 406–419 CrossRef.
- S. S. Pelaseyed, H. R. Madaah Hosseini and A. Samadikuchaksaraei, J. Biomed. Mater. Res., Part A, 2020, 108, 1390–1407 CrossRef CAS PubMed.
- N. A. Pattanashetti, C. Hiremath, S. R. Naik, G. B. Heggannavar and M. Y. Kariduraganavar, New J. Chem., 2020, 44, 2111–2121 RSC.
- N. Johari, H. R. Madaah Hosseini and A. Samadikuchaksaraei, Iran. Polym. J., 2020, 29, 219–224 CrossRef CAS.
- J. Radwan-Pragłowska, Ł. Janus, M. Piątkowski, D. Bogdał and D. Matysek, Polymers, 2020, 12, 792 CrossRef PubMed.
- B. K. Shanmugam, S. Rangaraj, K. Subramani, S. Srinivasan, W. K. Aicher and R. Venkatachalam, Mater. Sci. Eng., C, 2020, 110, 110710 CrossRef PubMed.
- X. Zheng, J. Sun, W. Li, B. Dong, Y. Song, W. Xu, Y. Zhou and L. Wang, J. Electroanal. Chem., 2020, 871, 114362 CrossRef CAS.
- M. Kaseem, K. Hamad and Z. U. Rehman, Materials, 2019, 12, 3659 CrossRef CAS PubMed.
- X. Yin, Y. Li, C. Yang, J. Weng, J. Wang, J. Zhou and B. Feng, Int. J. Biol. Macromol., 2019, 132, 495–505 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
