A review on borate bioactive glasses (BBG): effect of doping elements, degradation, and applications
Oluwatosin David
Abodunrin
,
Khalil
El Mabrouk
 * and
Meriame
Bricha
* and
Meriame
Bricha

Euromed Research Centre, Euromed Polytechnic School, Euromed University of Fes, Eco-Campus, Fes-Meknes Road, 30030 Fes, Morocco. E-mail: k.elmabrouk@ueuromed.org
First published on 3rd January 2023
Abstract
Because of their excellent biologically active qualities, bioactive glasses (BGs) have been extensively used in the biomedical domain, leading to better tissue–implant interactions and promoting bone regeneration and wound healing. Aside from having attractive characteristics, BGs are appealing as a porous scaffold material. On the other hand, such porous scaffolds should enable tissue proliferation and integration with the natural bone and neighboring soft tissues and degrade at a rate that allows for new bone development while preventing bacterial colonization. Therefore, researchers have recently become interested in a different BG composition based on borate (B2O3) rather than silicate (SiO2). Furthermore, apatite synthesis in the borate-based bioactive glass (BBG) is faster than in the silicate-based bioactive glass, which slowly transforms to hydroxyapatite. This low chemical durability of BBG indicates a fast degradation process, which has become a concern for their utilization in biological and biomedical applications. To address these shortcomings, glass network modifiers, active ions, and other materials can be combined with BBG to improve the bioactivity, mechanical, and regenerative properties, including its degradation potential. To this end, this review article will highlight the details of BBGs, including their structure, properties, and medical applications, such as bone regeneration, wound care, and dental/bone implant coatings. Furthermore, the mechanism of BBG surface reaction kinetics and the role of doping ions in controlling the low chemical durability of BBG and its effects on osteogenesis and angiogenesis will be outlined.
1. Introduction
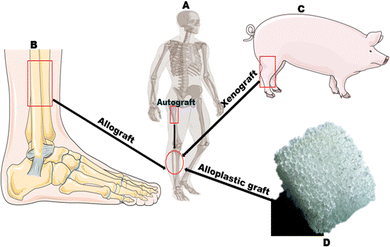
“If you can make a material that will survive exposure to high energy radiation, can you make a material that will survive exposure to the human body?” was a question posed during a cordial dialogue involving professor Larry Hench and a returnee US Army colonel from Vietnam. This question led to the discovery of bioactive glass “45S5’’ trademarked Bioglass®, making it the very first simulated biomaterial to be scientifically confirmed to bond to bones.1,2 The findings sparked a healthcare transformation and set the stage for current biomaterial-driven tissue regeneration, which is utilized to treat bone defects, dental deformities, and wounds as a result of its outstanding biocompatibility, as well as osteoconduction and osteoinduction qualities.3 Iconic Larry Hench Bioglass® is a silicate-based BG comprised entirely of silicon dioxide, sodium dioxide, calcium dioxide, and phosphorus pentoxide that has been known for almost 54 years to bond to bone and soft tissue, as well as being one of the most commonly used materials for tissue regeneration. But even so, a significant focus is already on using borate, phosphate, and other bioactive glass-doped systems as an extracellular matrix for biomedical applications.1,4In orthopedic and maxillofacial applications, bone grafting procedures such as autograft, allograft, and xenograft (see Fig. 1A–C) are generally considered the gold standard for bone tissue engineering (BTE) treatments, as they provide a long-term restorative solution by vascularizing, integrating, and stimulating local bone healing. Despite their advantages, autografts have a fundamental limitation: they cause a structural defect at the donor site, resulting in subsequent severe morbidity and a low supply of autogenous bone. Other therapeutic options, such as allografts and xenografts, also pose a risk of infectious disease transmission, extra costs, ethical issues, and immunological rejection of the bone.5–12 Alternatives to these grafting treatments include three-dimensional porous materials (scaffolds), injectable particles, and pastes made of synthetic and natural biomaterials (see Fig. 1D) that can increase bioactivity and osteo properties while providing mechanical support for bone regeneration.13–15
Silicate-based BGs and glass-ceramics, in particular, have gained a lot of interest for application in bone replacement and repair, along with wound healing processes, due to their inorganic origin, biomechanical strength, and physical attributes that are reasonably near to hard tissues.16 The dissolution of this glass network happens when it comes into contact with the biological environment, resulting in the production of a silica-rich layer on the glass surface, characterized by the development of an amorphous calcium phosphate apatite layer. With the proper adjustments, this sequence of reactions, commonly recognized for silicate BGs, is also applicable for BBGs; however, instead of silica gel, a borate-rich layer emerges.16–18 Since the formation of a calcium phosphate apatite layer is associated with the BG's good adhesion with nearby bony structures and soft tissue. The frequency at which the BG gets converted to the apatite phase offers a threshold for assessing a material's in vitro bioactivity.19 Even though SBF-based in vitro experiments show that BGs have good bioactivity, they are considered inadequate to estimate in vivo conditions.20 Moreover, the requirements of cell culture experiments vary greatly and rely on how BG compositions will be used. Nonetheless, in vitro investigations utilizing various cell types, assays, and in vivo animal models are therefore required to assess the bioactivity of these BGs.21–25
According to their high bioactivity and slow degradation process, silicate-based BGs like 45S5 and 13-93 are slowly absorbed and undergo incomplete transformation to an apatite after implantation.26–28 For instance, the borate equivalent of 45S5 BG designated as 45S5-3B, while immersed in simulated body fluid (SBF), converts swiftly and practically thoroughly around 3–4 days, unlike 45S5, which took many weeks to convert nearly 50% of it into HAP layers. As a result, their in vitro breakdown is relatively slow, which limits their use in biological applications.29–31 On the other hand, the rapid pace of BBG degradation and transformation to HAP can ostensibly impair their ability to match the regrowth of new bone cells. Furthermore, this rapid degradation could weaken the physico-mechanical qualities and have an inefficiently guiding impact on the creation of new bone.32 As a result, by regulating the glass composition, materials with appropriate bioactivity and regulated degradation behavior are needed to match the host bone's replacement of the BG scaffolds through a moderate and complete dissolution of the implanted biomaterial.33,34 Recent findings have shown that a significant amount of research is being conducted to develop borate/borosilicate BG by partial or complete substitution of the silica (SiO2) content with borate (B2O3), leading to decreased chemical durability, a rapid and complete transition of the BG to HAP, and as such easily controlled degradation and bioactivity characteristics.35–40 Furthermore, the body's faster transition of the BG to HAP is considered beneficial because the implant material can be removed entirely by the body system more quicker.41 In addition to replacing SiO2 with B2O3, factors such as BG particle size of different diameters42,43 and incorporation of active ions such as copper,43,44 Aluminium,45 strontium,46 sodium,47 zinc,48 magnesium,49 lithium,50etc. into the BG system contribute to decreased chemical durability and ion release. Furthermore, ions delivered directly off an implant instead of systemically injected drugs have the benefits of being delivered where desired, resulting in better pharmacological efficacy and reduced side effects.51
The synthesis of borate/borosilicate BGs have been the subject of extensive investigation. In addition, multiple studies have emphasized the methods for BBGs production and degradation and their doping with different therapeutic ions, which positively affects angiogenesis and bone remodeling. To highlight a few, Kaur et al.17,52 published an article/book that largely outlined the needs for BGs, their proportions, the structure–property interaction regarding hydroxyapatite deposition, the synthesis of metallic glasses and doped BGs, as well as the methods employed for their manufacture. The processing of BBGs, behavior in vitro, and the impact of boron ion release from BBGs on cell viability were all recently covered by Ege et al.53 in their article. The biological effects of boron and the ability of the bioactive borate glass to regenerate both hard and soft tissues were also discussed by Seiji et al.54 in a recent book chapter. Finally, an assessment of the use of metallic ions in tissue engineering and regenerative medicine is given in another manuscript by Viviana et al.55,56 with a focus on their therapeutic uses and the requirement to develop methods for the release of loaded ions from biomaterial substrates.
Even though the regeneration of both soft and hard tissues has been intensively investigated with bioactive glass, the impacts of dopants on BBGs regarding slowing down the rate of fast degradation have received comparatively less attention. In actuality, the majority of review papers that have been written up to this point only cover a portion of the subject and ignore other vital contributions related to how dopants affect the main osteogenic characteristics of boron-containing BG composition. In light of this, this paper aims to give a general overview of doping ions' function, significance, and impacts on BBGs. This study begins with a description of BBGs, highlighting several characteristics while concentrating on these glasses' chemistry, nomenclature, and structure. Following, we'll talk about the reaction mechanism of BBGs, which is also the bone-bonding property of this sort of BG in physiological fluids. More particularly, the focus will be placed on the mechanism by which these doping ions regulate the BBG's dissolution rate to keep up with bone growth and the dopant impacts on important osteogenic features. Finally, the paper's conclusion outlines BBG's application fields and future potential.
2. Borate bioactive glass (BBGs)
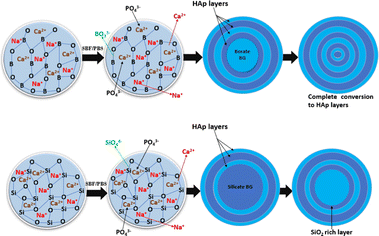
Borate-based glass is a type of inorganic glass with boron element as the network-forming precursor and can be directly absorbed into the shape of various structural elements inside the glass network. On the other hand, Boron is a trace mineral element ranging from 3–20 mg, found in the human body system, that has been shown in studies to play an essential role in wound healing, bone formation, and bone formation maintenance. Similarly, data suggests that boron also regulates steroid hormone development and action, which helps avoid calcium shortage and bone demineralization.57–59 Moreover, boron is used in compositions including borate/borosilicate glass for tissue engineering because of its chemical reactivity and coefficient of thermal expansion (CTE), which prevents strong thermal shock resistance inside the glass network.60 In this regard, boron ions in BG for bone formation and renewal release in the human body are significantly relevant.45S5 silicate compositions have been studied extensively for many years. Still, borate- and borosilicate-based compositions, in which B2O3 replaces a portion of SiO2 as the primary glass-forming agent, have only lately been investigated due to the trivalent electronic structure of boron, which differs from the quadrivalent structure of silicon.62 This incorporation resulted in various physicochemical and biological properties of BBGs, as proven through the fast degradation mechanism (see Fig. 2) and cellular reactions concerning silica-based BG.61 The phenomenon of BO3 triangles and BO4 tetrahedra coordination that constitutes a continuous system of vitreous boric oxide is an exciting feature of the borate glass structure; besides, as more and more of these units assemble, they constitute well-defined and stable borate groups, such as diborate, triborate, and tetraborate, that also form the glass networks.63,64 In contrast to silicate or phosphate glass, adding a certain molar percentage of alkali ions R2O (where R can be Li, Na, or K) to B2O3-based glasses alters the network model by decreasing the boroxol rings. This disrupts the boroxol shape, shifting the boroxol ring vibration from around 790 cm−1 to 806 cm−1 regions in the Raman spectra.65
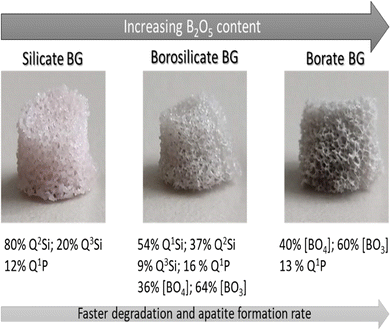 | ||
| Fig. 2 Properties of the silicate, borosilicate, and borate BGs.61 Licensed under a Creative Commons Attribution Non-Commercial 4.0 International (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/). | ||
Consequently, Nuclear magnetic resonance (NMR) measurements reveal that depending on the type and concentration of R2O, the transition of boron coordination from triangular to tetrahedral units takes place at a rate of 35–40 mol% R2O,66,67 with further research indicating that the number of BO4 units increases until it reaches a maximum value of a specific R2O concentration. However, when the addition of R2O is more than 45 mol% concentration, reversal of BO4 to BO3 and characteristics such as increment in the number of non-bridging oxygen (NBO) ions are noticed.68 Furthermore, as the network depolymerizes, ring and chain isomers of trigonal metaborate can be identified, and further depolymerization leads to the formation of pyroborate dimers and, eventually, orthoborate groups (see Fig. 3). This unique characteristic of continuous change in borate properties is referred to as the boron anomaly.17,64,69–73
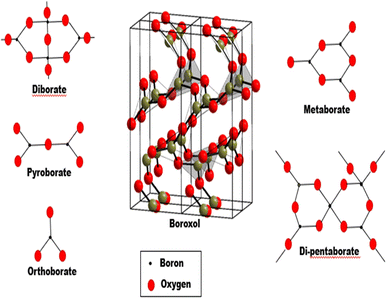 | ||
| Fig. 3 Superstructural units found in borate glass system. “Boroxol structure is adapted from ref. 74, licensed under a Creative Commons Attribution 3.0 Unported License” (https://creativecommons.org/licenses/by/3.0/). | ||
Besides the boron anomalies, vitreous B2O3 has several distinct features from vitreous SiO2 (see Table 1). The same applies to binary R2O–B2O3 and R2O–SiO2 glasses. In particular, the polymerization process affects Borate glass's solubility and thermal characteristics due to an initial network increment from 3 to 4. Glass transition temperature (Tg) increases, and a reversal happens at more significant modifier concentrations, decreasing Tg. This is not the case in a thermally and chemically stable silica glass network.63,64 According to Du et al. study,75 the B–O–Si– bond in typical borosilicate glass with four-coordinated boron can be broken easily as well as considerable phase separation occurs because the bond energy is relatively weak than that of three-coordinated boron. This research shows that when modifier oxides are first introduced, the solubility of borate glass reduces, and the thermal characteristics are also altered. Solubility increases as the modifier content increase due to a more depolymerized borate structure with trigonal BO3 groups.75,76 Omar et al.77,78 Synthesized and evaluated silica-based and BBGs in terms of mechanical properties necessary for their use as metallic coating materials. It was discovered that BBGs have a Coefficient of Thermal Expansion (CTE) that is closer to the substrate's (Ti6Al4V) CTE, resulting in greater mode I significant strain energy release rates of glasses and compressive residual stresses and strains at the coating/substrate interface that perform better than their silica-based glasses equivalents. Another work by Leonie et al.79 provides evidence that borate inclusion increases the BG's mechanical stability and bioactivity. This led to a modulus of 13–16 GPa, which is just marginally lower than that of compact bone, and a hardness of roughly 1 GPa, which is not excessively strong for BTE applications.
| Properties | B2O3 Value | SiO2 Value | Ref. |
|---|---|---|---|
| Average network connectivity | 3 | 4 | 73 |
| Glass mass density, d (g cm−3) | 1.844 | 2.2020 | 64 |
| Crystalline mass density dx (g cm−3) | 2.5544 | 2.3179 | 64 |
| Glass number density ρ0 | 0.01595 | 0.022070 | 64 |
| Crystalline number density ρx0 | 0.022095 | 0.023232 | 64 |
| Network number density ρn0 | 0.03190 | 0.022070 | 64 |
| Oxygen atom number density ρo0 | 0.04785 | 0.044140 | 63,64 |
| Trigonal covalent bond energy/strength (meV) | −5160 | 90 | |
| Tetrahedral covalent bond energy/strength (meV) | −3860 | −4600 | 90 |
| Crystallization from the melt at ambient p | No | Yes | 91 |
| Crystalline melting point, tm (K) | −3.8 | −2.3 | 92 |
| Glass transition temperature, tg (K) | 553 | 1463 | 92 |
| Bulk modulus, k (GPa) | 12.12 | 36.89 | 93 |
| Shear modulus, g (GPa) | 6.85 | 31.03 | 93 |
| Compressive strength | 5–7 | 11 | 38 and 94 |
| Young's modulus, E (GPa) | 35 | 94 | |
| Thermal expansion, α (10−7 K−1; 20–100 °C) | 97–161.6 | 5.35–15.1 | 95–97 |
| Thermal conductivity (Wm−1 K−1) | 0.52 | 1.38 | 98 |
| Specific heat at 295 k, cp (J mol−1 K−1) | 62.38 | 44.07 | 99 |
In addition to their Physico-chemical characteristics, BBGs have also shown excellent bioactive behaviour compared to silicate BGs in terms of biological characteristics.80,81 Using human mesenchymal stem cells (hMSCs) and osteoblasts made from hMSCs (hMSC-Obs), Nicholas et al.82 examined the cytocompatibility of borate glass in in vitro cell culture. The findings showed that these cell lineages survived up to two weeks after being seeded on porous borate glass disks, indicating that porous borate glass is effective at enhancing the expression of the early osteogenic marker while facilitating a favourable environment for cell attachment and proliferation. In a different study by Fabian et al.,83 the viability, proliferation, and osteogenic differentiation of human mesenchymal stromal cells were assessed in vitro using 0106-B1, a borate-based BG, and 45S5-BG. Accordingly, an in vivo test was also performed on scaffolds built from both BGs integrated with stromal cells and infused into mice to analyse osteoid development and angiogenic characteristics. Results demonstrated that there was a comparable effect of 45S5-BG and 0106-B1-BG on osteogenic differentiation, proliferation, and viability. However, in terms of the quantity and maturity of the osteoid created in vivo, 0106-B1-BG-based scaffolds proportionately surpassed 45S5-BG-based scaffolds. Conversely, 0106-B1-BG demonstrated much higher angiogenic gene expression patterns than 45S5-BG, making 0106-B1-BG a more viable choice.
3. Mechanism of degradation process (surface reaction kinetics)
The progressive degradation of implanted biomaterials and simultaneous substitution of the implants by natural bone is required for bone regeneration applications. However, unlike wholly resorbable biomaterials, bioactive silicate glasses do not degrade entirely following implantation. Borate-based BG, on the contrary, due to the reduced network connectivity of trigonal planar [BO3] and tetrahedral [BO4] units in BBGs, has already been proven to have poor chemical stability and transition fast to calcium phosphate in dissolution media.38,84 As a result, developing biomaterials having significant bioactivity and a controlled degradation rate to match the growing pace of bone in vivo is crucial.Upon interaction with a bodily solution, HAP is deposited on the surface of BGs in a series of stages akin to the bone mineral phase.85,86 To explain this phenomenon, the European Society for Biomaterials (in 1987) defined bioactive material as “a material which has been designed to induce specific biological activity”.87 Because of their ability to chemically attach to living bone by creating a bone-like HAP layer at the implant-bone interface, BGs are extremely attractive bone substitutes in bone regeneration.88 However, the ability of a material to be coated by a HAP layer when interacting with physiological fluids has been proposed as a sign of bioactivity, and to evaluate the bioactivity of a BG material, ethics would not permit evaluating every new material directly in animals for experimental research, and it may not be economically sustainable. In this regard, bioactivity tests with blood plasma buffered aqueous solution, and simulated body fluid (SBF) have proven to be an effective way to propose a tangible mechanism that explains the bioactive bond formation, influencing the development of new bioactive materials and allowing for the selection of the best biomaterials to continue development.89
BG behaviors in cells and animals are a more convincing way to demonstrate the bone-bonding phenomenon than buffer solutions, which are also an excellent technique to describe bioactivity. As an illustration, a pertinent work by Miquel et al.100 examined the possible use of a borosilicate BG scaffold as a bone substitute material and demonstrated bioactive behavior in SBF, osteoblast (MC3T3-E1) cell, and animal model. First, two BG scaffolds were pre-treated with SBF to encourage the development of distinct bone-like apatite layers on their surfaces. Based on the surface examination and material characterization, both SBF-treated scaffolds had a layer of carbonated hydroxyapatite (HCA) that was calcium deficient. Following this, MC3T3-E1 cells were seeded onto the scaffolds. Analysis showed that MC3T3-E1 preosteoblasts displayed a more flattened shape and cell proliferation in the untreated scaffold.
On the other hand, SBF-treated samples had longer, more osteoblastic active cells. Results from in vivo experiments using a rabbit calvarial bone deficiency model revealed that SBF pre-treated scaffolds promoted bone growth more effectively than untreated ones. Cui and colleagues101 created an injectable borate bioactive glass cement in their other applicable work. Borate glass was changed into HAP over 25 days when this material was cultured in phosphate-buffered saline. Moreover, the injectable BBG cement developed in this study was incorporated in rabbit tibial defects infected with methicillin-resistant Staphylococcus aureus. The material transformed to HA and promoted new bone formation in the defects within 8 weeks, clearly showing that it is a promising treatment for osteomyelitis and regeneration of bone with defects.
The bioactivity process, first proposed by Hench for silicate glasses, can be thought of as a sort of glass corrosion guided by complex glass–fluid interactions governed by inorganic chemicals (stages 1–5) and biological (stages 6–12) mechanisms.102 This sequence of reactions is commonly recognized for silicate BGs and is also applicable for borate BGs with correct adjustments; nevertheless, instead of silica gel, a borate-rich layer emerges in step 3.39
Primarily, a vast amount of triangle-shaped [BO3] and tetrahedral [BO4] functional units are linked during the formation of the BBG network structure, whereas the modifier M+ (metal) cations inside this glass, such as Na+, K+, and Mg2+, suffice to stabilize the net charge of the bridged oxygen ions. The leaching process begins with the dissolving of Na+ ions and, most certainly, Ca2+ from the glass surface into the solution, while the glass's B–O network is affected by the phosphate solution (see Fig. 4). Cations and OH− levels rise as a result. The reaction equations below show that H+ created during water hydrolysis disrupts the glass network structure, yielding water-soluble B(OH)3 or B(OH)4.30,103
| H2O ⇌ H+ + OH− | (1) |
| B–O–M+ + H+ → B–O–H+ + M+ | (2) |
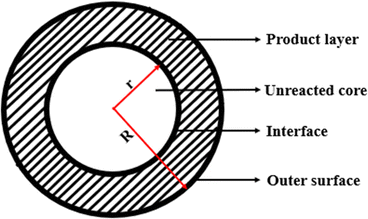
This continual dissolution-precipitation process yields numerous layers of HAP that lower the glass-solution interaction until the aggregate borate glass is transformed into HAP. Moreover, in the case of borate glasses containing silicate as a co-network former, the dissolution-precipitation reaction stops before complete conversion to HAP, leaving a residual or dissolvable SiO2-rich gel layer within reaction products. This subsequently slows the degradation of borosilicate BG.26,104 Typically, for a borate glass like 13-93B3, the conversion is regulated by an interfacial reaction, and the kinetics can be modelled as a 3-D contracting sphere. Therefore, the conversion of a silicate glass, such as 13-93, is somewhat influenced primarily through reactions at the interface (3-D contracting sphere model) and subsequently by ion diffusion to the reaction interface (3-D diffusion model).105 Essentially, a reaction occurs on the surface of the sphere in the contracting sphere model (see Fig. 5), which is usually referred to as a moving interface model. Regarding BG, the particles, whether they are a single particle or a group of particles, have a sphere-shaped core made of unreacted material that gradually decreases in size, encircled by the shell(s) of the product.106,107
Huang et al.'s research30 extensively characterize the conversion of multiple glasses, including a bioactive silicate glass (45S5), a borate equivalent of 45S5 glass with all of the SiO2 in 45S5 glass replaced with B2O3, as well as two intermediate borosilicate glass mixtures. The only principal constituents within the precipitate for the borate equivalent of 45S5 BG comprised oxides of calcium (CaO) and phosphorus (P2O5), showing that the glass was almost wholly converted to HAP. Meanwhile, experimental results show that CaO, P2O5, and SiO2 are the core constituents of the reaction products for the silicate and borosilicate BG. Although, within 4 days, particles of a borate glass could ultimately convert to HAP. Even after 70 days, 45S5 glass particles and two intermediate borosilicate formulations of the same mass were only partially transformed to HAP. As a result, the existence of unreacted SiO2 indicates that the degradation process of BG can be controlled by adjusting the amount of B2O3.30,35 Chandrani et al. conducted a study that further supported the accelerated degradation rate of BBG. According to their findings, the ion exchange activity of BBG after immersion in SBF was attributed to the rapid discharge of Ca2+, (PO4)3, and (BO3)3 ions, as well as the initiation of amorphous calcium phosphate within shells formed upon SBF interaction. This dissolution process shows that the scaffold's percent weight loss was most significant on the first day and intensified with SBF soaking time.108 In a related study, Liu et al. examined the efficacy of using BBG scaffolds to release medications to treat bone infections. More than 90% of the glass in the scaffolds is dissolved after a week to produce poorly crystallized hydroxyapatite, according to an in vitro degradation of the pellets and their transformation to a hydroxyapatite-type in an SBF medium.109
4. Doping ions' role in regulating BBG degradation rate and osteogenic biomarkers
4.1. Copper
Numerous copper (Cu)-containing biomaterials have been researched and created recently due to copper's significant benefits.110–112 Conversely, it has been shown that Cu promotes angiogenesis (see Fig. 6), stimulates endothelial cell development, and makes it easier for vascular endothelial growth factor to be released (VEGF). Besides, research has also shown that incorporating Cu increases the stability of borosilicate and borate glasses, modifying their characteristic features and bioactivity and increasing the antibacterial activity of BG. Therefore, to enhance their applications, it is crucial to understand how Cu, as a dopant, affects the characteristics of BGs. Additionally, understanding how materials degrade is essential for managing the biological performance of BG and its derived scaffolds and determining how released ions interact with cells.44,112–114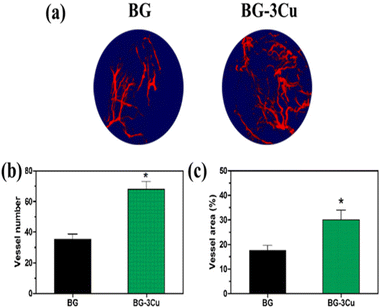 | ||
| Fig. 6 (a) 3D reconstructions of the new blood vessels implanted with Cu-doped BG show higher blood vessel density in the defect implant than with the undoped BG. (b and c) evaluation of Cu ion on the extent and quantity of newly formed blood vessels. Image adapted from ref. 44 with permission from Elsevier. | ||
The phenomenon of the Cu doping impacts on the BBG during the degrading phase can be thought of in terms of the electronegativity, oxygen coordination number, and ionization energy of the dopant cations. Cu ions are therefore integrated into the glass network matrix through octahedral coordination and act as network modifiers in the glass system.115 Wang et al.44 Looked into how copper dopant influenced the borosilicate glass network's structural characteristics and how the derived glass scaffolds' reactivity and degradation behavior changed. The research's findings showed that the pairing of BO2O− units, as well as non-bridging oxygen (NBO), transition to BO4 units. This was enhanced by the doping Cu, which NBO surrounded.
Consequently, as the proportion of Cu doping in the glass system increased, more BO4 groups were formed, creating a more stable structure, which led to a reduced boron ion release rate in the dissolution medium.44 Abdrakhmanov et al.116 have made similar assumptions, asserting that the NBO coordinates Cu ions in BGs and that their influence is visible through the agglutination effect, which is the process by which alkali ions achieve electroneutrality.112,116 However, a different study by Schuhladen et al.117 revealed that the copper added to the borate glass resulted in a lesser release of Ca and B and a marginally reduced release of the other elements. Furthermore, the results discussed above showed that the influence of Cu doping in borosilicate glass led to a more stable glass network connectivity and a lower ions release rate during the degradation process. Again, this was because the Cu ions' agglutination effect and the agglomerate's charge balance in the glass network caused these effects.
Along with other ions found in BGs, the Cu2+ ion also has osteogenic capabilities that considerably enhance this property. Similarly, it has been demonstrated that Cu-doped BGs and scaffolds promote endothelial cell proliferation and increase mesenchymal stem cell (MSC) differentiation levels.118,119 To improve bone healing and anti-tumor therapy via the osteogenic properties of Cu, Libin et al.120 recently doped BBG nanoparticles with Cu. According to an in vitro study, the nanoparticle increased endothelial cell angiogenesis as well as the differentiation potential of bone marrow stromal cells (BMSCs). Analyzing in vivo, restoring the severe bone abnormalities in rats was successfully possible.
More research revealed that the doped BG increased the expression of genes relevant to osteogenesis, including Runt-related transcription factor 2 (RUNX2), Collagen Type I Alpha 1 Chain (COL1A1), and Bone Morphogenetic Protein 2 (BMP2). This indicated that Cu-doped BBG could stimulate osteogenesis. Furthermore, through up-regulating the expression of the relevant osteogenic genes, Cu ions doped with BBG, according to certain additional studies, have been demonstrated to have a considerable impact on the process of secreting Vascular endothelial growth factors (VEGF). Thereby, through a multitude of growth factors, mediating angiogenesis in a multistep, complicated process.44,121 On the other hand, concentration might be a factor in how Cu ions affect tissue regeneration. For example, in BG doped with 2.0 wt% CuO, Lin et al.122 found that bone regeneration was improved along with blood vessels and fibrous tissue; however, the 0.4 and 0.8 wt% groups did not exhibit much angiogenesis in the neo-bone. Consequently, adding Cu to BBGs can endow the glass with outstanding angiogenesis while fostering bone repair and regeneration due to Cu's pro-osteogenic and pro-angiogenic qualities.
4.2. Aluminium
In the presence of high amounts of Al2O3, it has been discovered that BBG exhibits noticeable bioactivity. According to NMR research, the quantity of aluminium ions in the glass matrix determines whether the ions are found in tetrahedral (AlO4) or octahedral (AlO6) structural units in borosilicate glass networks. It is anticipated that SiO4 and BO4 structural units will alternate with AlO4 structural units, preventing the breakdown of the BGs structure.123 Zhao et al.45 conducted research on the impact of substituting varied quantities (0–2.5% mol.) of Al2O3 for B2O3 on the conversion of a borate glass to HAP in an aqueous phosphate solution. According to the study's results, BBG without Al2O3 demonstrated the quickest reaction kinetics, with weight reduction reaching the highest level in only 3–4 days. Likewise, replacing 0.5 and 1.5 mol% B2O3 in the initial glass with the equal mol% of Al2O3 led to a reduction in weight reduction kinetics, with the glass components fully transforming to HAP by 9 and 12 days. In addition, the glass sample that had 2.5 mol% Al2O3 in place of B2O3 demonstrated a significantly delayed conversion rate to HAP, exhibiting weight reduction kinetics that, despite 30 days of reaction, approached roughly 70% of the highest value. Suggesting that Al2O3 could drastically reduce the bioactivity of borate glass.The weight loss of the glass and the change in the pH value of the solution slowed down as the glass' Al2O3 content increased. This is because AlO4 units in the glass limit how easily B3+ and Na+ ions can dissolve from the glass. After some Na+ and BO33− ion dissolution occurs during the conversion of the glasses, a porous Al-rich layer with AlO4 units is produced on the glass' surface. This means that any Ca2+ ions that dissolve from the glass must move via the Al-rich layer to interact with the PO43− in the solution to induce HAP deposition. Given that the structure of the AlO4 units in the glass network is comparable to that of SiO4, the structure of the Al-rich layer is comparable to the Si-rich layer identified by Hench et al. for the transformation of silicate 45S5 glass.124 As a result, the Al-rich layer's inhibitory effects on the solubility and permeability of ions from the glass likely caused the declines in the conversion rate of the glass composition.
Furthermore, because the AlO4 units are negatively charged, they need to be synchronized to cations to preserve the structure's charge neutrality. Due to this, several of the Na+ and Ca2+ ions released from glass would become trapped in the porous Al-rich layer, inhibiting the interaction involving Ca2+ and PO43− to deposit more HAP.45 In short, the conversion rate of the glass reduces dramatically as the number of B2O3 replaced by Al2O3 increases.
Additionally, incorporating Al2O3 into BG functions as a network former, producing more oxygen atoms that bridge with BO3 units, thereby enhancing the material's long-term resilience, crack resistance, and chemical durability.125–127 Even though adding a varying proportion of Al mol% in the BG composition can result in delayed HAP generation and other structural and chemical modifications, up to roughly 1.5 mol% Al2O3 can be introduced into the glass composition without significantly reducing bioactivity.128 Besides this, an experimental investigation by Mohini et al. demonstrates that the HAP occurrence of post-soaked Al-doped BBG sample gradually declines with an increase in Al2O3 content up to 1.9 mol%, indicating a decrease in the glass's bioactivity.123 Meanwhile, numerous investigations on the impact of Al-doped BBGs on their physicochemical and mechanical properties have been conducted.121,123–125,127–133 Besides, a great deal of research has been done on the biological effects of aluminium, which is used in glass ionomers for dental cement and is known to be potentially toxic and to negatively influence biomineralization.134–138 However, a thorough review of the literature reveals that studies on Al-doped BBGs, particularly their effect on critical osteogenic biomarkers and cell differentiation for bone regeneration, are still lacking.
4.3. Strontium
The favorable effects of strontium-containing BGs on bone metabolism, reducing bone resorption, and increasing new tissue formation have recently piqued the interest of academics and scientists, both in vitro and in vivo studies.139,140 Moreover, strontium ranelate, a daily oral prescription for osteoporosis treatment, has recently become recommended.141 According to research, adding strontium has been shown to lower the dissolution of borate glass, resulting in a lower reaction mechanism of boron release. Such behavior, in particular, can be credited to the strontium ion's larger atomic radius of 2.15 Å when contrasted to Mg2+ or Ca+ ions, with 1.6 Å and 1.97 Å respectively; as such, larger radius ions require more space in the glass matrix, which is likely to limit the movement and discharge of the other ions, significantly lowering the dissolution rate of borate glass and potentially improving the glass network's bioactivity.142 Yin et al.143 developed BG scaffolds based on strontium-doped borate for bone tissue engineering (BTE). As per their findings, the presence of strontium in glass has no discernible influence on the structure. More specifically, increasing the strontium concentration within the borate-based glass network amplified the release of Si4+ and Ca2+ ions while significantly reducing the release of B3+ ions, suggesting that when the BG is soaked in SBF for in vitro bioactivity, BBG doped with a certain amount of strontium inhibits the rapid release of boron. Aside from controlling boron degradation and converting the glass surface to a nanosized hydroxyapatite layer in SBF, the influence of strontium ions within those scaffolds had a remarkable capacity to support MG-63 cells proliferation while also reducing the glass scaffolds' cytotoxicity.143 In another study, Pan et al.144 discovered that incorporating strontium into a borate glass matrix could not only regulate the rapid release of boron ions but also trigger the adherence of osteoblast-like cells, significantly boosting the cytocompatibility of borate glass. Furthermore, the production of multilayers of mineral phase with a porous structure suggests that complete breakdown is likely, as well as the proliferation of osteoblast cells overlaid by a new apatite film to create a layered structure may stimulate bone-like tissue synthesis earlier in the process. As a result, such innovative strontium-incorporated borosilicate Sr-BBG may function as a new breed of biomaterial for bone regeneration, delivering strontium to encourage the development of new bones while simultaneously providing boron as a nutritional element for bone health.144Conversely, Sr has been shown to prevent bone loss and encourage bone regeneration. To completely comprehend how strontium facilitates bone regeneration by stimulating osteoblastic activity and suppressing osteoclastic resorption simultaneously, it is crucial to examine the biological response of strontium to BG compositions.145,146 By examining the impacts of Sr on the physicochemical characteristics and osteogenic effectiveness of a bone cement made of BBG and a chitosan matrix, Xu et al.147 could better understand the underlying molecular process that promotes bone regeneration. In vitro testing revealed that Sr substituted BBG cements increased expression of the osteogenic-related genes RUNX2, BSP, and OCN at 7, 14, and 21 days, respectively, with the highest significant increase being seen in the BBG cement with 6 mol% Sr. Furthermore, the cement's ability to promote cell proliferation and function in vitro and to mend bone lesions in vivo was improved at all levels of Sr substitution (0–12 mol% SrO) utilized in the study. Consequently, the creation of a HAP (or HA-type) product was slowed down when Sr was substituted. Interestingly, the outcomes of Xu et al.'s investigation are comparable to those of another study that found that Sr had a stimulating influence on the activity of osteoblastic cells.148,149 Results also showed that Sr inclusion up to 12 mol% (see Fig. 7) in BG composition had no cytotoxic properties.144
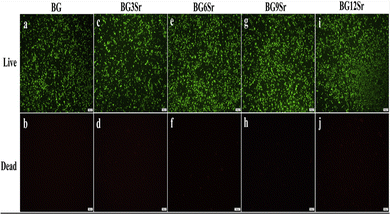 | ||
| Fig. 7 Live–dead assay to evaluate the cytotoxicity of Sr doped BBG cement extracts to hBMSCs.147 licensed under a Creative Commons Attribution Non-Commercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) (https://creativecommons.org/licenses/by-nc-nd/4.0/). | ||
The pathway through which Sr promotes osteogenic gene expression in MSCs (mesenchymal stem cells), alkaline phosphatase activity (ALP), and osteoprotegerin (OPG) secretion in osteoblasts can be conceptualized as OPG expression in osteoblasts that inhibits the relationship between RANK (receptor activator of nuclear factor-B) and its ligand (RANKL, receptor Activator of Nuclear Factor-B Ligand), thereby preventing osteoclastogenesis. Similarly, it has been discovered that Sr increases the production of angiogenic factors, causing a link between angiogenesis and osteogenesis.150–152
Although, in bone regenerative therapies, enhancing and expediting bone recovery are still unmet needs, strontium-doped BGs are auspicious materials to address this issue. However, various borate-based BGs doped with strontium and manufactured in multiple forms have now been created and characterized for treating bone deformities and injuries.140,144,153–159
4.4. Titanium
Materials containing titanium (Ti4+) have been employed extensively in medical applications because of the metal's related bioactivity in vivo. Similarly, studies have demonstrated that titanium dioxide (TiO2) with a concentration lower than 10 ppm is cytocompatible and has no appreciable impact on cell viability. When in contact with bodily fluids, it also promotes the formation of HAP and increases osteoblast differentiation. Previous research has suggested that increasing the proportion of TiO2 in glass can alter its function as a network-forming or network-modifying oxide, which will subsequently affect its solubility and make it easier to adjust the degradation process.78,160–165 One of the study goals in previous work by Romina et al.166 was to evaluate the dissolution rate and the ion release effects of TiO2 incorporation on the morphology of BBG scaffolds. They suggest that the chemical structure of the samples, rather than the wall thickness influence, is the possible source of the ion release effects. As a result, an increase in TiO2 content induces the modification of BO33− with Ti4+ in the B–O–B bond, resulting in the formation of the B–O–Ti structure. More so, TiO2 ions having a modest atomic radius of 1.47 Å and a big electric charge improves network connectivity which may explain why the combined ion dissolution rate of scaffolds decreases with increasing Ti4+ composition. The ion release and pH results reported in this research show that by introducing TiO2 into the glass network of the scaffolds, the dissolution rate of borate-based BG can be controlled.166,167 According to the findings of this research, Ti-doped BBGs scaffolds could be a viable option for treating bone loss during revision total knee arthroplasty (rTKAs). In another work by Omar et al., it was discovered that BBGs with 0 and 15 mol percent of titanium dioxide added had much better antibacterial activities and higher solubility than their silica-based equivalents. Conversely, because there is a lack of study in this area, in vivo studies would be necessary to assess the impact of a dynamic environment on osteogenic cell markers and tissue response to Ti-doped BBGs.5. Applications of BBGs
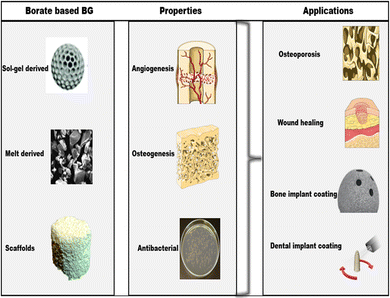
Widespread usage of BGs in biomedical and healthcare applications, as indicated in Fig. 8, has opened the way for contemporary biomaterial-driven regenerative medicine that primarily repairs bone109,168,169 and dental anomalies,170 in addition to wound healing.171 This is because when an implant is placed in a biological environment, a sequence of events creates an apatite layer, which then causes the tissue to bind. Additionally, BG has been demonstrated to promote neocartilage production during the in vitro cultivation of chondrocyte-seeded hydrogels and to act as a subchondral framework for tissue-engineered osteochondral structures.105 Finally, since BBGs can adhere to the bone and even soft tissues, they have found relevance in biomedical applications (see Table 2).| Title | Type of BG | Bone regeneration applications | Remarks | Ref. |
|---|---|---|---|---|
| Evaluation of borate bioactive glass scaffolds as a controlled delivery system for copper ions in stimulating osteogenesis and angiogenesis in bone healing | MQ BBG | Bone healing | Controlled delivery of copper (Cu) ions from borate bioactive glass scaffolds for stimulating angiogenesis and osteogenesis in a rodent calvarial defect model was investigated. Scaffolds doped with 3 wt% CuO showed a significantly better capacity to stimulate angiogenesis and regenerate bone when compared to the undoped glass scaffold. | 43 |
| Bioactive borate glass scaffolds: in vitro and in vivo evaluation for use as a drug delivery system in the treatment of bone infection | MQ BBG | Bone infection treatment | BG scaffolds loaded with the drug were implanted into the right tibiae of rabbits infected with osteomyelitis. HAP formed in vivo, served as a structure to support the growth of new bone and blood vessels | 109 |
| Osteogenic and anti-tumor Cu and Mn-doped borosilicate nanoparticles for syncretic bone repair and chemo dynamic therapy in bone tumor treatment | SG BBG | Bone repair and tumor treatment | Cu and Mn-doped borosilicate nanoparticles (BSNs) were developed for syncretic bone repair and anti-tumor treatment. ions. In vitro study showed that BSNs promoted both osteogenic differentiation of BMSCs and angiogenesis of endothelial cells and consistently, the critical bone defects of rats were efficiently repaired by BSNs through in vivo evaluation. | 120 |
| Mesoporous bioactive glass-coated 3D printed borosilicate bioactive glass scaffolds for improving repair of bone defects | 3D printed BBG scaffold | Bone healing | 3-D scaffolds with mesoporous BBG were fabricated by the 3D printing technique. Biocompatibility of the BG-MBG scaffolds was evaluated by assessing biodegradability, cell proliferation, and alkaline phosphatase (ALP) activity of osteogenic gene expression with human bone marrow stromal cells (hBMSCs). Results showed that the scaffolds possess good biodegradability and stimulated the proliferation and osteogenic differentiation of hBMSCs. In vivo studies showed that the BG-MBG scaffolds could significantly enhance new bone formation in both inner and peripheral scaffolds in defects. | 217 |
| Synthesis and characterization of sol–gel bioactive glass nanoparticles doped with boron and copper | SG BBG | Prior investigation for use as an angiogenic biomaterial in soft TE | The BG nanoparticles can be considered novel promising angiogenic and antibacterial agents and have interesting potential applications in the soft TE field | 218 |
| Novel strontium borate modified Hench's bioglass synthesis and characterization for bone replacement | MQ BBG | Bone replacement | The effect of strontium on the BGs’ structural and biological behavior was investigated. Glass powders were immersed in a simulated fluid body (SBF) solution over varied periods, 1, 2, 3, and 4 weeks, to investigate the bioactivity of prepared samples. Results showed that Sr promotes the formation process of crystalline hydroxyapatite. Thus, it could be one of the best materials for bone regeneration application. | 219 |
| Investigation of the influence of SrO addition on the bioactivity of CaO-Na2O–P2O5–B2O3 glass system | MQ BBG | Bone tissue growth | The role of strontium oxide in the BGs calcium-sodium-borate was studied to evaluate the bioactivity of the system and the rate of the ion release in SBF. Results show that SrO-doped calcium-sodium-borate glasses are materials with a high rate of bioactivity, low cytotoxicity, and reduced dissolution rate of the glasses in an aqueous medium; besides, they induce cell proliferation and bone tissue growth with living tissues. | 220 |
| In vivo bioactivity assessment of strontium-containing soda-lime-borate glass implanted in femoral defect of rat | MQ BBG | Bone regeneration | In vivo bioactivity assessment of strontium-doped borate glass implanted in rat femoral defect was carried out. In vivo bioactivity test showed that implantation of all borate glasses did not demonstrate local or general complications in all rats, and they exhibited nearly complete bone mineralization. | 221 |
| Evaluation of novel injectable strontium-containing borate bioactive glass cement with enhanced osteogenic capacity in a critical-sized rabbit femoral condyle defect model | MQ BBG | Bone regeneration | Strontium-doped borate bioactive glass particles and a chitosan-based bonding phase were prepared and evaluated in vitro and in vivo. Compared to borate glass particles without Sr, the Sr-BBG cement enhanced the proliferation and osteogenic differentiation of hBMSCs in vitro. The Sr-BBG cement showed a better capacity than the BBG cement to regenerate bone at the implant–bone interface at 4- and 8-weeks post-implantation in a critical-sized rabbit femoral condyle defect model | 155 |
| Effects of strontium-doped bioactive glass on the differentiation of cultured osteogenic cells | SG BBG | Bone cell behavior | In vitro effect of a new Sr-doped bioactive glass manufactured by the sol–gel method on osteoblast viability and differentiation. Osteoblast differentiation of fetal mouse calvarial cells was enhanced in the presence of bioactive glass particles containing 5 wt% strontium | 222 |
| Mechanistic study of the bioactivity improvement of Al2O3-doped BBG after dynamic flow treatment | MQ BBG | Bone cell differentiation | The effect of dynamic flow treatment on the biological properties of Al2O3-doped BBG was investigated. Results revealed that the SBF-treated Al2O3-doped BBG exhibits a significantly reduced degradation rate and improved bioactivity. In vitro cell studies suggested that the Al2O3-doped BBG, after being treated by dynamic flow treatment, boosts the proliferation of MC-3T3E1 cell | 223 |
| Title | Type of BG | Wound healing applications | Remarks | Ref. |
|---|---|---|---|---|
| Angiogenesis and full-thickness wound healing efficiency of a copper-doped borate bioactive glass/poly (lactic-co-glycolic acid) dressing loaded with vitamin E in vivo and in vitro | MQ BBG | Wound healing | Cu-doped borate bioactive glass/poly(lactic-co-glycolic acid) loaded with vitamin E (0-3.0 wt% vitamin E) was fabricated to evaluate its efficiency for angiogenesis in cells and full-thickness skin wounds healing in rodents. Results indicated that the Cu-doped biomaterial loaded with vitamin E is effective in stimulating angiogenesis and healing full-thickness skin defects | 224 |
| Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model | MQ BBG | Wound dressing | Results showed that the ionic dissolution product of the biomaterial was not toxic to HUVECs and fibroblasts, promoted HUVEC migration, and secretion of VEGF, as well as stimulating the expression of angiogenic-related genes of the fibroblasts. These results indicate that the Cu-doped borate glass microfibers have a promising capacity to stimulate angiogenesis and heal full-thickness skin defects | 121 |
| In vitro study of improved wound-healing effect of bioactive borate-based glass nano-/micro-fibers | BBG & SIG | Wound healing | Comprehensive material analysis and biocompatibility evaluation of a silicate-based (45S5) and two borate-based (13-93B3 and 1605) nano-/micro-scale bioactive glass fibers were carried out. Evaluation on human skin cell line demonstrated that in comparison with silicate-based fibers, borate-based fibers, under static condition, can significantly stimulate cell growth with higher cell proliferation rate. Moreover, the trace amount doping of metal species within the glass composition, such as copper and zinc, are also potentially important in glass conversion, biocompatibility as well as bioactivity. | 225 |
| In vivo and in vitro studies of borate-based glass micro-fibers for dermal repairing | MQ BBG & SIG | Wound healing | BBG micro-fibers were fabricated and compared with the traditional 45S5 Bioglass® micro-fibers. The BBG micro-fiber wound dressings enhanced the formation of blood vessel, and resulted in a much faster wound size reduction than the SiG micro-fibers, and also than the control groups, after 9 days application. | 226 |
| In situ forming hydrogel based on thiolated chitosan/carboxymethyl cellulose (CMC) containing borate bioactive glass for wound healing | MQ BBG | Wound healing | Thiolated chitosan (tCh)/oxidized carboxymethyl cellulose (OCMC) hydrogel containing Cu-doped borate bioglass (BG) was developed as a wound dressing to improve wound healing in a full-thickness skin defect of mouse animal model. Investigations revealed that the hydrogel containing borate BG had potential enough for wound occlusion in vivo and elevated blood vessel formation throughout the wound repair procedure. | 227 |
| Title | Type of BG | Dental and implant coating applications | Remarks | Ref. |
|---|---|---|---|---|
| Bioactive borate glass coatings for titanium alloys | MQ BBG | Implant coating | BBG coatings was developed for titanium and titanium alloys. The glasses convert to bioactive hydroxyapatite coatings when exposed to simulated body fluid. Also, assays with MC3T3-E1 pre-osteoblastic cells show the borate glasses exhibit in vitro biocompatibility | 97 |
| Borate and silicate bioactive glass coatings prepared by nanosecond pulsed laser deposition | MQ BBG &SIB | Implant coating | Silicate (13-93) and borate (13-93-B3) bioactive glass coatings were deposited on titanium using the nanosecond Pulsed Laser Deposition technique. Cytocompatibility and osteogenic differentiation tests showed that thin films retain the biocompatibility properties of the target silicate and borate glass, respectively. However, no antibacterial activity of the borate glass films was observed, suggesting that ion doping is advisable to inhibit bacterial growth on the surface of borate glass thin film | 228 |
| Borate modified bioglass containing scaffolds for dental tissue engineering applications | SG BBG | Dental TE | The effects of BBG on the odontogenic differentiation of human dental pulp stem cells (hDPSCs) were investigated. All BG groups enhanced odontogenic differentiation of hDPSCs and the presence of borate increased cell viability. Immunohistochemical and histochemical stainings showed that scaffolds positively affected the odontoblastic differentiation of the hDPSCs. | 229 |
5.1. Wound care
By using a three percent boric acid solution to deep scars, boron has substantially revamped wound healing since 1990, cutting the time necessary in critical care by two-thirds. Besides acting on the extracellular environment, the boric acid solution increased the healing of wounds, according to an in vitro study on human fibroblasts.172 Further in vitro research demonstrated that these potential benefits of boron were brought on by its direct interactions with some fibroblast enzymes, notably elastase, trypsin-like enzymes, collagenase, and alkaline phosphatase. These results suggest that boron is essential for wound healing and helps fibroblasts' important enzymes function, which enhances extracellular matrix renewal.58,172–177 Boron's ability to treat deep wounds and chronic diabetic foot ulcers has led to its use as a network former in BBGs, a relatively new development in the field of wound management. Even though the United States Food and Drug Administration (FDA) endorsed the first BG for medicinal use in 1985, a wound management product containing BBG (13-93B3) did not receive FDA approval until 2016.178,179 Demonstrating the importance of conducting additional research on this type of BG has significantly lowered attention from scientists and researchers.30,180A novel borate-based bioactive glass fiber (BBGF) for the treatment of acute and chronic wounds was evaluated for effectiveness and relevance by Donald (2020). Four patients with persistent wounds who had failed various past sophisticated therapies and procedures were treated with MIRRAGEN, a commercialized product made of 13-93B3 glass microfibers. Compared to the average wound duration of 391 days, the results of this treatment with BBGF revealed that all patients experienced effective wound closure in an average of only 55 days. In addition to promoting faster healing and better patient outcomes, BBGF significantly improves the healthcare system by lowering the cost of treating chronic wounds, which was previously estimated to be $87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 per patient, to an average of $3
750 per patient, to an average of $3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 per patient.181 In another study, researchers from several institutes conducted a comparable randomized two-parallel group trial to learn more about the efficacy of MIRRAGEN as a treatment for persistent diabetic foot ulcers compared with the standard of care treatment. After randomization, individuals received treatment for 12 weeks, with the outcome measure being the likelihood of fully healed wounds. Following this therapy time, wound examination revealed that 70% of the MIRRAGEN-treated ulcers had recovered, relative to 25% of those treated solely with standard-of-care (SOC).
564 per patient.181 In another study, researchers from several institutes conducted a comparable randomized two-parallel group trial to learn more about the efficacy of MIRRAGEN as a treatment for persistent diabetic foot ulcers compared with the standard of care treatment. After randomization, individuals received treatment for 12 weeks, with the outcome measure being the likelihood of fully healed wounds. Following this therapy time, wound examination revealed that 70% of the MIRRAGEN-treated ulcers had recovered, relative to 25% of those treated solely with standard-of-care (SOC).
Additionally, compared to the SOC group, the average percentage surface decline for the wounds treated with MIRRAGEN was 79% instead of 37%. Contrary to the SOC group, the MIRRAGEN group experienced no ulcer-related infections.178 These outcomes of infection control measures can be thought to be due to the antibacterial potential of boron in the BG structure, as boron ions alone have been shown to prevent the development of both Gram-positive and Gram-negative bacteria by disrupting the integrity of bacteria's cell membranes, as well as, by suppressing the growth of biofilms.182,183
In addition to effectively treating chronic wounds, BBGs can also be electrospun to form a fibrous structure similar to the extracellular matrix of the skin tissue for a wound dressing. For instance, Schuhladen et al.184 created a fiber mat utilizing methylcellulose and poly(-caprolactone). More so, Manuka honey, unique honey made from the indigenous New Zealand tree Leptospermum scoparium,185 was used to cross-link these fiber mats, which were employed as a biodegradable delivery system for BBG particles. Incorporating BBG into the fiber mats enhanced their bioactivity, according to the team's degradation experiments using simulated bodily fluid. Similarly, cellular biology investigations using human dermal fibroblasts and cells comparable to keratinocytes in humans demonstrated the great potential of the manufactured composite fiber mats as a material for wound dressing, mainly due to their capacity to support wound closure that is typically attributed to the presence of BBG.184 A unique Nano-sized carbonated hydroxyapatite (HCA) covered with BBG was another intriguing approach Chen et al.103 reported to assess its influence on mouse models for wound repair. By dynamically submerging the BBG particles in a flowing buffer medium, Nano-HCA was neo-formed.
In addition to increasing the biocompatibility and maintaining the biodegradability of the BBG, the porous structure was a little more surface-coated with a layer of amorphous HCA. Nano-HCA BBG on wound healing were then assessed and contrasted to those of the Nano-HCA and 45S5® at the in vitro and animal levels. The research's findings showed no negative surgical outcomes and that the proportion of wound scars decreased in all 5 groups. Specifically, wounds administered with Nano-HCA BBG particles virtually entirely healed rapidly during the seventh day (98%), in contrast to wounds left untreated (60%). Additionally, Nano-HA and 45S5® had a somewhat substantial impact on wound healing, as evidenced by the fact that their wound healing rates (61% and 63%, respectively) were essentially in the same range as those of the control group. As a result, the wound healing efficiency of the Nano-HCA BBG group enhances biocompatibility, encourages cell growth, and aids in wound healing in rodent skin deficiencies. Another recent finding on the need for a better wound dressing that possesses the angiogenic capacity for rapid healing of full-thickness skin wounds was developed by Shichang et al.121 In their investigation, copper-doped BBG microfiber materials were developed and tested both in vivo and in vitro. The fibers decayed and changed into hydroxyapatite after being submerged in simulated bodily fluid for around 7 days.
Similarly, in vitro cell culture demonstrated that the fibers’ ionic dissolving product was not hazardous to fibroblasts and human umbilical vein endothelial cells (HUVECs). Instead, it induces the expression of fibroblasts' angiogenic-related genes. At 7 and 14 days after surgery, when used to treat full-thickness skin defects in mice, Cu-doped fibers had a much higher effect of promoting revascularization than the undoped fibers and the untreated defects (control). Comparing the Cu-doped and undoped fiber-treated defects to the untreated defects, the treated defects demonstrated enhanced collagen deposition, maturity, and orientation. These findings indicate a promising ability of the Cu-doped borate glass microfibers to promote angiogenesis and repair full-thickness skin defects.
5.2. Bone tissue engineering
Common diseases like bone defects cause individuals to experience physical pain and can even make them disabled. Biomedical materials research is investigating and attempting to tackle a significant issue: how to correct these faults by creating novel bone repair materials.105,186,187 It is well recognized that boron, which seems frequently found in water and food in modest proportions, is crucial for the nutritional process of developing healthy bones in both humans and animals.188,189 Borate levels in bone are also discovered to be noticeably greater than in other tissues in addition to swiftly dispersing across bodily fluids and still being eliminated via urination. According to reports, 99% of the boric acid from a single intravenous injection got eliminated unaltered in the urine for 120 hours, and no propensity for boron accumulation was seen.57,188–190 These results indicate that more research into the possible use of borate glasses in bone healing and tissue engineering is necessary for bone health.Moreover, chronic bone infections like osteomyelitis are typically accompanied by contamination of the surrounding tissues, poor perfusion, and bone necrosis. Because of this, only a small number of antibiotics given orally or intravenously can effectively treat an infection. Nevertheless, commercially accessible biomaterials, notably poly(methyl methacrylate; PMMA), have been employed in clinical situations as delivery systems for many years. However, once PMMA has finished functioning as a drug carrier, further procedure is needed to eliminate them from the body. Furthermore, polymers like collagen, poly(lactic acid), and polylactides cannot organically bond with bone despite being biodegradable. Nonetheless, inorganic materials such as calcium phosphate cement, hydroxyapatite (HAP), and BBG, including S53P4 with antimicrobial capabilities, tend to attach effectively to bone tissues and may also serve as a medication delivery mechanism for bone healing.89,191–197
Zhang et al.198 conducted a study to show that BBG degrades more quickly and has pro-osteogenic effects than β-TCP, which could greatly enhance huge segmental bone defect healing. In brief, the potential of the BBGs to treat significant segmental bone lesions was assessed using a critical-sized rabbit radius defect model. The effect of BBGs on bone marrow mesenchymal stem cells (BMSCs') osteogenic development was also evaluated. Investigations were also conducted into how the BBGs solubility by-products influenced the bone morphogenetic protein (BMP)/Smad signal pathway activity in BMSCs. In vitro findings show, BBG has better bone regeneration benefits than β-TCP. The analysis also indicates that BBG dissolution products considerably enhance BMSCs' osteogenic development, resulting in the repair and regeneration of bone defects. As a result, the BBG used in this work can be a potential and good bone tissue regeneration material with a wide range of possible applications. The ability of BBG (1393B3 and Cu-doped 13-93B3) to regenerate bone in a rat calvarial defect model was examined in another study by Lianxiang et al.199 They examined the impact of three different microstructures (trabecular, oriented, and fibrous) on this capacity. Histomorphometric analysis and scanning electron microscopy (SEM) was used to monitor the scaffolds for 12 weeks to measure the quantity of new bone growth, mineralization, and blood vessel area. They showed that for the trabecular, oriented, and fibrous microstructures, new bone formed in proportions of 33%, 23%, and 15%, respectively, to the overall defect area. According to subsequent comparisons made by the team, 19% of new bone was generated in implants made of silicate 45S5 BG particles. However, doping the borate glass with 0.4 wt% copper (CuO) had minimal impact on bone regeneration in trabecular and oriented scaffolds. It considerably positively impacted bone regeneration in the fibrous scaffolds, increasing it from 15 to 33 percent. Moreover, blood vessels penetrated all scaffolds, with the average blood vessel area being larger in trabecular scaffolds than in oriented and fibrous scaffolds. According to the team's research, all three scaffold microstructures successfully supported bone regeneration, although the trabecular scaffolds supported more bone growth and may hold the most promise for bone tissue regeneration.
Numerous cell sources have been studied for bone regeneration using doped BBGs, including bone marrow mesenchymal stem/progenitor cells, osteoblasts, cells genetically engineered to express osteogenic factors, embryonic stem cells, and others. Conversely, various studies have indicated that distinct bone growth factors, such as bone morphogenetic protein (BMP), serve essential roles in the progression of bone regeneration, making a significant difference in the differentiation of osteogenic progenitor cells into osteoblasts and the mineralization of bone (see Table 2). Furthermore, new bone repair materials which include hydrogel, nanofiber scaffolds, and 3d-printed scaffolds are being developed.5,200–203 These translational approaches to bone healing based on engineering cells and biomaterial scaffolds promise to impact the future of bone tissue engineering.
5.3. Bone and dental implant coatings
Because BGs can form a strong bond with a living person's bone tissues, they are widely employed in dental and orthopedic implants.204,205 However, their weak mechanical characteristics, particularly their brittleness, are mainly used as coatings over mechanically resilient substrates, such as titanium, alumina, and zirconia. Surface coating is a common and extensively used technique to increase biomaterials' surface bioactivity and biocompatibility. Compared to other metallic materials, BGs are remarkably biocompatible and have a higher probability of integrating into human tissue than the metal implants mentioned earlier. Because of this, BG is an excellent choice for strengthening the biocompatibility and bioactivity of these metals, enhancing the substrates' mechanical characteristics, and providing antibacterial capabilities via the slow release of therapeutic ions. As a result, BGs are widely used in orthopedic, spinal, craniomaxillofacial, and periodontal applications.206–211Antibacterial effects of sodium borate and calcium borate-based polymeric coatings for orthopedic implants were recently reported by Huseyin et al.212 Their goal was to compare the antibacterial and biofilm-degrading characteristics of titanium implants coated with sodium borate and calcium borate minerals to implants without coating and implants coated with alginate. The team discovered that the implant coated with alginate had the highest bacteria load, while biofilm development was also found on the implant without coating. Although, at a low concentration of borate minerals, scaffolds had lower microbial loads than implants without coating. But at high concentrations of 0.5 mg mL−1 and 0.75 mg mL−1, borate minerals showed a potent antibacterial effect on colonization and biofilm formation on the implant surface.212 In further pertinent work, Teicoplanin-loaded BBG implants for treating chronic bone infection in a rabbit tibia osteomyelitis model were investigated by Xin et al.213 Within 12 weeks of being implanted in a rabbit tibia, pellets made of a combination of teicoplanin powder and BBG particles were able to heal a chronic bone infection. Over about 9 days in vivo, the pellets delivered a consistent release of teicoplanin. Furthermore, the BG particles simultaneously broke down in the bodily fluid and transformed into porous HA-type grafts that encouraged the new bone formation and solidly adhered to the neoformed bone, healing the bone defect.
On the other hand, BGs have also been employed clinically in creating dental recovery materials that can remineralize after microbial infections. The BG nanoparticles hasten the production of new bone, which stops gingival and epithelial cells from migrating down the tooth. As such, it is possible to stabilize the junction created between the tooth and the periodontal membrane.207,214 Additionally, BG particles have been utilized in mouthwashes to address oversensitive teeth. These BG particles are believed to adhere to the dentine, so the local pH rises due to released ions. This also causes HCA to precipitate over the tubule end, shielding the tubules from exposure to liquid flow.214–216
6. Conclusion and future outlook
Over the past 50 years, significant effort has been made in the biomedical field to develop BGs, notably those with a 45S5 composition. This created an approach for creating glasses with borate compositions, bringing about new prospects for using BBGs in tissue engineering. Because of their biodegradable properties, as well as doping with several metallic ions, BBGs have shown their ability to support osteogenesis. Likewise, recent work has also demonstrated its angiogenic potential, which may benefit soft tissue repair. In addition, doped BBGs have proved to be effective in promoting osteogenic biomarkers such as BMP, OPG, RUNX2, RANK/L, BSP, etc. Consequently, these BGs have been shown to activate angiogenic growth factors such as VEGF, thus providing unique opportunities for the target delivery of therapeutic ions for wound healing. Furthermore, despite its brittleness, BBG possesses distinct features that include the ability to possess CTE closer to the bio-metallic substrate, thereby resulting in a better performance at coating the substrate interface than their silica-based glasses equivalents.The current literature has clearly shown that doped BBGs can enhance the physico-chemical properties of the glass system as well as stimulate angiogenesis and upregulate key osteogenic biomarkers, which are useful in soft and hard tissue regeneration. However, despite the academic community's curiosity, there is still a lack of understanding of how Al/Ti-doped BBGs affect critical osteogenic biomarkers and cell differentiation for bone regeneration. Conversely, literature has shown that very few previous studies have investigated the synthesis of BBGs using the sol–gel technique. Another area that has been less exploited is additive manufacturing to prepare BBGs scaffolds. In light of these limitations and to support their use in biomedical applications, there is a need for further detailed in vitro and in vivo studies that would be necessary to assess the impact of a dynamic environment on osteogenic cell markers and tissue response to Al/Ti-doped BBGs. Furthermore, researchers must focus on utilizing the sol–gel technique in synthesizing BBGs due to the potential advantages (greater porosity and bioactivity) over the melt-quench method. Similarly, there is a need to expand 3D printing to prepare BBGs scaffolds.
Summarily, this review presents a general overview of the borate-based BG systems, emphasizing their composition, properties, and uses for bone regeneration, wound healing, and coatings for dental and bone implants. The main focus is on the role of doping ions in regulating the degradation process of BBGs and their effects on osteogenic biomarkers that regulate the process of bone healing.
Abbreviations
| Al | Aluminium |
| ALP | Alkaline phosphatase activity |
| BGs | Bioactive glasses |
| BBG | Borate bioactive glass |
| BBGF | Borate-based bioactive glass fiber |
| BMP | Bone morphogenetic protein |
| BMSCs | Bone marrow mesenchymal stem cells |
| BTE | Bone tissue engineering |
| COL1A1 | Collagen type 1 alpha 1 |
| CTE | Coefficient of thermal expansion |
| Cu | Copper |
| FDA | Food and drug administration |
| HAP | Hydroxyapatite |
| HUVECs | Human umbilical vein endothelial cells |
| MQ BBG | Melt quench borate-based bioactive glass |
| NBO | Non-bridging oxygen |
| NMR | Nuclear magnetic resonance |
| OPG | Osteoprotegen |
| PMMA | Poly methyl methacrylate |
| RUNX2 | Runt-related transcription factor 2 |
| SBF | Simulated body fluid |
| SEM | Scanning electron microscopy |
| SG BBG | Sol–gel borate-based bioactive glass |
| SOC | Standard of care |
| Sr | Strontium |
| T g | Glass transition temperature |
| Ti | Titanium |
| VEGF | Vascular endothelial growth factor |
Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
The authors would like to acknowledge the Euro-Mediterranean University of Fes for their support.References
- L. L. Hench, J. Mater. Sci.: Mater. Med., 2006, 17, 967–978 CrossRef CAS PubMed.
- L. L. Hench, R. J. Splinter, W. C. Allen and T. K. Greenlee, J. Biomed. Mater. Res., 1971, 5, 117–141 CrossRef.
- J. Wilson, G. H. Pigott, F. J. Schoen and L. L. Hench, J. Biomed. Mater. Res., 1981, 15, 805–817 CrossRef CAS PubMed.
- E. A. Abou Neel, D. M. Pickup, S. P. Valappil, R. J. Newport and J. C. Knowles, J. Mater. Chem., 2009, 19, 690–701 RSC.
- H. A. Awad, R. J. O’Keefe and J. J. Mao, Bone Tissue Engineering, INC, 2020 Search PubMed.
- H. A. Awad, R. J. O’Keefe, C. H. Lee and J. J. Mao, Bone Tissue Engineering: Clinical Challenges and Emergent Advances in Orthopedic and Craniofacial Surgery, Elsevier, 4th edn, 2013 Search PubMed.
- H. Qu, H. Fu, Z. Han and Y. Sun, RSC Adv., 2019, 9, 26252–26262 RSC.
- A. Ibrahim, 3D bioprinting bone, Elsevier Ltd, 2018 Search PubMed.
- S. A. Brigido, N. M. Protzman, M. M. Galli and S. T. Bleazey, Foot Ankle Spec., 2014, 7, 377–386 CrossRef PubMed.
- G. Zimmermann and A. Moghaddam, Injury, 2011, 42, S16–S21 CrossRef PubMed.
- V. Campana, G. Milano, E. Pagano, M. Barba, C. Cicione, G. Salonna, W. Lattanzi and G. Logroscino, J. Mater. Sci.: Mater. Med., 2014, 25, 2445–2461 CrossRef CAS PubMed.
- R. Dimitriou, G. I. Mataliotakis, A. G. Angoules, N. K. Kanakaris and P. V. Giannoudis, Injury, 2011, 42, S3–S15 CrossRef PubMed.
- S. Ali, A. M. Abdul Rani, Z. Baig, S. W. Ahmed, G. Hussain, K. Subramaniam, S. Hastuty and T. V. V. L. N. Rao, Corros. Rev., 2020, 38, 381–402 CrossRef CAS.
- A. J. Sunija, Biomaterials and biotechnological schemes utilizing TiO2 nanotube arrays-A review, Elsevier Ltd, 2018 Search PubMed.
- S. V. Bhat, Overview of Biomaterials, Springer, 2002, pp. 1–11 Search PubMed.
- F. Baino, M. Tomalino and D. Tulyaganov, PoliTO Springer Series Ceramics, Glass and Glass-Ceramics From Early Manufacturing Steps Towards Modern Frontiers, 2021 Search PubMed.
- G. Kaur, Bioactive glasses-Potential Biomaterials for Future Therapy, 2017 Search PubMed.
- X. Liu, H. Pan, H. Fu, Q. Fu, M. N. Rahaman and W. Huang, Biomed. Mater., 2010, 5, 015005 CrossRef PubMed.
- X. Lu, J. Kolzow, R. R. Chen and J. Du, Bioact. Mater., 2019, 4, 207 CrossRef PubMed.
- M. Bohner and J. Lemaitre, Biomaterials, 2009, 30, 2175–2179 CrossRef CAS PubMed.
- A. A. Zadpoor, Mater. Sci. Eng., C, 2014, 35, 134–143 CrossRef CAS PubMed.
- S. Fujibayashi, M. Neo, H. M. Kim, T. Kokubo and T. Nakamura, Biomaterials, 2003, 24, 1349–1356 CrossRef CAS PubMed.
- E. Jablonská, D. Horkavcová, D. Rohanová and D. S. Brauer, J. Mater. Chem. B, 2020, 8, 10941–10953 RSC.
- T. H. Qazi, S. Hafeez, J. Schmidt, G. N. Duda, A. R. Boccaccini and E. Lippens, J. Biomed. Mater. Res. A, 2017, 105, 2772 CrossRef CAS PubMed.
- F. Westhauser, F. Hohenbild, M. Arango-Ospina, S. I. Schmitz, S. Wilkesmann, L. Hupa, A. Moghaddam and A. R. Boccaccini, Int. J. Mol. Sci., 2020, 21, 1639 CrossRef CAS PubMed.
- A. Yao, D. Wang, W. Huang, Q. Fu, M. N. Rahaman and D. E. Day, J. Am. Ceram. Soc., 2007, 90, 303–306 CrossRef CAS.
- Q. Fu, M. N. Rahaman, B. Sonny Bal, R. F. Brown and D. E. Day, Acta Biomater., 2008, 4, 1854–1864 CrossRef CAS PubMed.
- Q. Fu, M. N. Rahaman, B. S. Bal, W. Huang and D. E. Day, J. Biomed. Mater. Res. A, 2007, 82, 222–229 CrossRef PubMed.
- S. Cavalu, F. Banica, C. Gruian, E. Vanea, G. Goller and V. Simon, J. Mol. Struct., 2013, 1040, 47–52 CrossRef CAS.
- W. Huang, D. E. Day, K. Kittiratanapiboon and M. N. Rahaman, J. Mater. Sci.: Mater. Med., 2006, 17, 583–596 CrossRef CAS PubMed.
- L. Moimas, M. Biasotto, R. Di Lenarda, A. Olivo and C. Schmid, Acta Biomater., 2006, 2, 191–199 CrossRef PubMed.
- L. Xia, W. Ma, Y. Zhou, Z. Gui, A. Yao, D. Wang, A. Takemura, M. Uemura and K. LinY. Xu DOI:10.1155/2019/8961409.
- S. Wei, J. X. Ma, L. Xu, X. S. Gu and X. L. Ma, Mil. Med. Res., 2020, 7, 54 CAS.
- M. N. Rahaman, D. E. Day, B. Sonny Bal, Q. Fu, S. B. Jung, L. F. Bonewald and A. P. Tomsia, Acta Biomater., 2011, 7, 2355–2373 CrossRef CAS PubMed.
- W. Huang, M. N. Rahaman, D. E. Day and Y. Li, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2006, 47, 647–658 CAS.
- S. A. Hussein, R. Roshdy, M. S. A. El-sadek and M. Ezzeldien, Optik, 2022, 250, 1–14 CrossRef.
- R. G. Furlan, W. R. Correr, A. F. C. Russi, M. R. da Costa Iemma, E. Trovatti and É. Pecoraro, J. Sol–Gel Sci. Technol., 2018, 88, 181–191 CrossRef CAS.
- Q. Fu, M. N. Rahaman, H. Fu and X. Liu, J. Biomed. Mater. Res., Part A, 2010, 95, 164–171 CrossRef PubMed.
- X. Liu, M. N. Rahaman and D. E. Day, J. Mater. Sci.: Mater. Med., 2013, 24, 583–595 CrossRef CAS PubMed.
- J. Ning, A. Yao, D. Wang, W. Huang, H. Fu, X. Liu, X. Jiang and X. Zhang, Mater. Lett., 2007, 61, 5223–5226 CrossRef CAS.
- S. Jung, Dr. Diss., 2010, 1–350 Search PubMed.
- A. M. Deliormanli, Ceram. Int., 2013, 39, 8087–8095 CrossRef CAS.
- H. Wang, S. Zhao, J. Zhou, Y. Shen, W. Huang, C. Zhang, M. N. Rahaman and D. Wang, J. Mater. Chem. B, 2014, 2, 8547–8557 RSC.
- H. Wang, S. Zhao, W. Xiao, J. Xue, Y. Shen, J. Zhou, W. Huang, M. N. Rahaman, C. Zhang and D. Wang, Mater. Sci. Eng., C, 2016, 58, 194–203 CrossRef CAS PubMed.
- D. Zhao, W. Huang, M. N. Rahaman, D. E. Day and D. Wang, Acta Biomater., 2009, 5, 1265–1273 CrossRef CAS PubMed.
- R. Zhao, L. Shi, L. Gu, X. Qin, Z. Song, X. Fan, P. Zhao, C. Li, H. Zheng, Z. Li and Q. Wang, J. Appl. Biomater. Funct. Mater., 2021 DOI:10.1177/22808000211040910.
- F. H. Elbatal, M. A. Ouis and H. A. Elbatal, Ceram. Int., 2016, 42, 8247–8256 CrossRef CAS.
- N. Mutlu, F. Kurtuldu, I. Unalan, Z. Neščáková, H. Kaňková, D. Galusková, M. Michálek, L. Liverani, D. Galusek and A. R. Boccaccini, Ceram. Int., 2022, 48, 16404–16417 CrossRef CAS.
- S. Patel, R. K. Samudrala, S. Palakurthy, B. Manavathi, R. Gujjala and A. P. Azeem, Ceram. Int., 2022, 48, 12625–12634 CrossRef CAS.
- A. E. Omar, A. M. Ibrahim, T. H. Abd El-Aziz, Z. M. Al-Rashidy and M. M. Farag, J. Biomed. Mater. Res., Part B, 2021, 109, 1059–1073 CrossRef CAS PubMed.
- S. Adepu and S. Ramakrishna, Molecules, 2021, 26, 5905 CrossRef CAS PubMed.
- G. Kaur, O. P. Pandey, K. Singh, D. Homa, B. Scott and G. Pickrell, J. Biomed. Mater. Res., Part A, 2014, 102, 254–274 CrossRef PubMed.
- D. Ege, K. Zheng and A. R. Boccaccini, ACS Appl. Bio Mater., 2022, 5, 3608–3622 CrossRef CAS PubMed.
- S. Yamaguchi, Bioact. Glas. Glas., 2022, 79–86 Search PubMed.
- A. Hoppe, V. Mouriño and A. R. Boccaccini, Biomater. Sci., 2013, 1, 254–256 RSC.
- V. Mouriñ, J. P. Cattalini and A. R. Boccaccini DOI:10.1098/rsif.2011.0611.
- F. H. Nielsen, Nutr. Rev., 2008, 66, 183–191 CrossRef PubMed.
- L. Pizzorno, Integr. Med. A Clin. J., 2015, 14, 35 Search PubMed.
- R. F. Moseman, Environ. Health Perspect., 1994, 102, 113 CAS.
- M. Hubert and A. J. Faber, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2014, 55, 136–158 CAS.
- K. Schuhladen, U. Pantulap, K. Engel, P. Jeleń, Z. Olejniczak, L. Hupa, M. Sitarz and A. R. Boccaccini, Int. J. Appl. Glas. Sci., 2021, 12, 293–312 CrossRef CAS.
- S. A. R. Coelho, Hybrid Organic-Inorganic Borosilicate Materials by Sol-Gel for Bone Tissue Engineering, Universidade de Aveiro, 2018, https://hdl.handle.net/10773/25818 Search PubMed.
- A. C. Wright, Phys. Chem. Glas., 2010, 51, 1–39 CAS.
- A. C. Wright, G. Dalba, F. Rocca and N. M. Vedishcheva, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2010, 51, 233–265 CAS.
- C. Gautam, A. K. Yadav and A. K. Singh, ISRN Ceram., 2012, 2012, 1–17 CrossRef.
- J. E. Shelby, Introd. to Glas. Sci. Technol., 2005, 72–110 Search PubMed.
- W. C. Lepry and S. N. Nazhat, Mater. Adv., 2020, 1, 1371–1381 RSC.
- J. Wu, J. F. Stebbins and T. Rouxel, J. Am. Ceram. Soc., 2014, 97, 2794–2801 CrossRef CAS.
- A. R. Boccaccini, D. S. Brauer and L. Hupa, Bioactive Glasses Series Editors: Titles in this Series, 2017, pp. 61–88 Search PubMed.
- D. P. Button, R. Tandon, C. King, M. H. Veléz, H. L. Tuller and D. R. Uhlmann, J. Non. Cryst. Solids, 1982, 49, 129–142 CrossRef CAS.
- D. L. Griscom, Borate Glass Structrure, in Borate Glasses: Structure, Properties, Applications, ed. L. D. Pye, V. D. Fréchette and N. J. Kreidl, Plenum Press, New York, 1978 Search PubMed.
- S. Naseri, W. C. Lepry, V. B. Maisuria, N. Tufenkji and S. N. Nazhat, J. Non-Cryst. Solids, 2019, 505, 438–446 CrossRef CAS.
- K. Rao, Structural Chemistry of Glasses, Elsevier, 2002 Search PubMed.
- Kristallstruktur Bortrioxid-Boron trioxide-Wikipedia, https://en.wikipedia.org/wiki/Boron_trioxide#/media/File:Kristallstruktur_Bortrioxid.png, (accessed 28 September 2022).
- W. F. Du, K. Kuraoka, T. Akai and T. Yazawa, J. Mater. Sci., 2000, 35, 4865–4871 CrossRef CAS.
- Y. D. Yiannopoulos, G. D. Chryssikos and E. I. Kamitsos, Phys. Chem. Glas., 2001, 42, 164–172 CAS.
- O. Rodriguez, A. Matinmanesh, S. Phull, E. Schemitsch, P. Zalzal, O. Clarkin, M. Papini and M. Towler, J. Funct. Biomater., 2016, 7, 32 CrossRef.
- O. Rodriguez, D. J. Curran, M. Papini, L. M. Placek, A. W. Wren, E. H. Schemitsch, P. Zalzal and M. R. Towler, J. Non. Cryst. Solids, 2016, 433, 95–102 CrossRef CAS.
- L. Deilmann, O. Winter, B. Cerrutti, H. Bradtmüller, C. Herzig, A. Limbeck, O. Lahayne, C. Hellmich, H. Eckert and D. Eder, J. Mater. Chem. B, 2020, 8, 1456–1465 RSC.
- M. Ojansivu, A. Mishra, S. Vanhatupa, M. Juntunen, A. Larionova, J. Massera and S. Miettinen, PLoS One, 2018, 13, e0202740 CrossRef PubMed.
- S. Decker, M. Arango-Ospina, F. Rehder, A. Moghaddam, R. Simon, C. Merle, T. Renkawitz, A. R. Boccaccini and F. Westhauser, Sci. Rep., 2022, 12, 8510 CrossRef CAS.
- N. W. Marion, W. Liang, G. C. Reilly, D. E. Day, M. N. Rahaman and J. J. Mao, Mech. Adv. Mater. Struct., 2005, 12, 239–246 CrossRef CAS.
- F. Westhauser, B. Widholz, Q. Nawaz, S. Tsitlakidis, S. Hagmann, A. Moghaddam and A. R. Boccaccini, Biomater. Sci., 2019, 7, 5161–5176 RSC.
- K. A. Cole, G. A. Funk, M. N. Rahaman and T. E. McIff, J. Biomed. Mater. Res., Part B, 2020, 108, 1580–1591 CrossRef CAS PubMed.
- D. Zhang, O. Leppäranta, E. Munukka, H. Ylänen, M. K. Viljanen, E. Eerola, M. Hupa and L. Hupa, J. Biomed. Mater. Res., Part A, 2010, 93, 475–483 Search PubMed.
- A. Tilocca, Proc. R. Soc. A Math. Phys. Eng. Sci., 2009, 465, 1003–1027 CAS.
- D. F. Williams, Biomaterials, 2008, 29, 2941–2953 CrossRef CAS PubMed.
- L. L. Hench, Ann. N. Y. Acad. Sci., 1988, 523, 54–71 CrossRef CAS PubMed.
- A. J. Salinas and M. Vallet-Regí, RSC Adv., 2013, 3, 11116 RSC.
- S. A. Bessedina, V. G. Konakov and M. M. Schultz, Rev. Adv.Mater. Sci., 2002, 3, 37–66 CAS.
- F. Kracek and G. Morey, H. M.-A. J. S. A and U. 1938, earth.geology.yale.edu.
- S. Sakka and J. D. Mackenzie, J. Non. Cryst. Solids, 1971, 6, 145–162 CrossRef CAS.
- R. R. Shaw and D. R. Uhlmann, J. Non. Cryst. Solids, 1971, 5, 237–263 CrossRef CAS.
- I. D. Thompson and L. L. Hcnch, Proc. Inst. Mech. Eng., Part H, 2016, 212, 127–136 CrossRef PubMed.
- H. F. Shermer, J. Res. Natl. Bur. Stand., 1934, 56, 73 CrossRef.
- J. M. Gomez-Vega, E. Saiz, A. P. Tomsia, G. W. Marshall and S. J. Marshall, Biomaterials, 2000, 21, 105–111 CrossRef CAS PubMed.
- L. Peddi, R. K. Brow and R. F. Brown, J. Mater. Sci.: Mater. Med., 2008, 19, 3145–3152 CrossRef CAS PubMed.
- O. Nilsson, O. Sandberg and G. Bäckström, Int. J. Thermophys., 1985, 6, 267–273 CrossRef CAS.
- A. K. Varshneya and J. C. Mauro, Fundam. Inorg. Glas., 2019, 273–281 Search PubMed.
- B. San Miguel, R. Kriauciunas, S. Tosatti, M. Ehrbar, C. Ghayor, M. Textor and F. E. Weber, J. Biomed. Mater. Res., Part A, 2010, 94, 1023–1033 Search PubMed.
- X. Cui, C. Zhao, Y. Gu, L. Li, H. Wang, W. Huang, N. Zhou, D. Wang, Y. Zhu, J. Xu, S. Luo, C. Zhang and M. N. Rahaman, J. Mater. Sci.: Mater. Med., 2014, 25, 733–745 CrossRef CAS PubMed.
- C. G. Pantano, A. E. Clark and L. L. Hench, J. Am. Ceram. Soc., 1974, 57, 412–413 CrossRef CAS.
- R. Chen, Q. Li, Q. Zhang, S. Xu, J. Han, P. Huang, Z. Yu, D. Jia, J. Liu, H. Jia, M. Shen, B. Hu, H. Wang, H. Zhan, T. Zhang, K. Ma and J. Wang, Chem. Eng. J., 2021, 426, 130299 CrossRef CAS.
- W. M. Abd-Allah and R. M. Fathy, J. Biol. Inorg. Chem., 2022, 27, 155–173 CrossRef CAS PubMed.
- M. N. Rahaman, D. E. Day, B. Sonny Bal, Q. Fu, S. B. Jung, L. F. Bonewald and A. P. Tomsia, Acta Biomater., 2011, 7, 2355–2373 CrossRef CAS PubMed.
- Y. Gu, W. Xiao, L. Lu, W. Huang, M. N. Rahaman and D. Wang, J. Mater. Sci., 2010, 46, 47–54 CrossRef.
- H. G. Mcllvried and F. E. Massoth, Ind. Eng. Chem. Fundam., 1973, 12, 225–229 CrossRef.
- C. Pramanik, T. Wang, S. Ghoshal, L. Niu, B. A. Newcomb, Y. Liu, C. M. Primus, H. Feng, D. H. Pashley, S. Kumar and F. R. Tay, J. Mater. Chem. B, 2015, 3, 959–963 RSC.
- X. Liu, Z. Xie, C. Zhang, H. Pan, M. N. Rahaman, X. Zhang, Q. Fu and W. Huang, J. Mater. Sci.: Mater. Med., 2010, 21, 575–582 CrossRef CAS PubMed.
- A. Ewald, C. Käppel, E. Vorndran, C. Moseke, M. Gelinsky and U. Gbureck, J. Biomed. Mater. Res., Part A, 2012, 100A, 2392–2400 CAS.
- M. M. Erol, V. Mouriňo, P. Newby, X. Chatzistavrou, J. A. Roether, L. Hupa and A. R. Boccaccini, Acta Biomater., 2012, 8, 792–801 CrossRef CAS PubMed.
- A. Hoppe, R. Meszaros, C. Stähli, S. Romeis, J. Schmidt, W. Peukert, B. Marelli, S. N. Nazhat, L. Wondraczek, J. Lao, E. Jallot and A. R. Boccaccini, J. Mater. Chem. B, 2013, 1, 5659–5674 RSC.
- C. Gérard, L. J. Bordeleau, J. Barralet and C. J. Doillon, Biomaterials, 2010, 31, 824–831 CrossRef PubMed.
- D. Bar-Or, G. W. Thomas, R. L. Yukl, L. T. Rael, R. P. Shimonkevitz, C. G. Curtis and J. V. Winkler, Shock, 2003, 20, 154–158 CrossRef CAS PubMed.
- H. D. Schreiber, B. K. Kochanowski, C. W. Schreiber, A. B. Morgan, M. T. Coolbaugh and T. G. Dunlap, J. Non. Cryst. Solids, 1994, 177, 340–346 CrossRef CAS.
- R. S. Abdrakhmanov, T. A. Ivanova, R. S. Abdrakhmanov and T. A. Ivanova, JMoSt, 1978, 46, 229–244 CAS.
- K. Schuhladen, X. Wang, L. Hupa and A. R. Boccaccini, J. Non. Cryst. Solids, 2018, 502, 22–34 CrossRef CAS.
- J. N. Oliver, O. Akande, M. Ecker, J. N. Oliver, O. Akande and M. Ecker DOI:10.5772/INTECHOPEN.99430.
- Y. Wang, W. Zhang and Q. Yao, J. Orthop. Transl., 2021, 29, 60–71 Search PubMed.
- L. Pang, R. Zhao, J. Chen, J. Ding, X. Chen, W. Chai, X. Cui, X. Li, D. Wang and H. Pan, Bioact. Mater., 2022, 12, 1–15 CrossRef CAS PubMed.
- S. Zhao, L. Li, H. Wang, Y. Zhang, X. Cheng, N. Zhou, M. N. Rahaman, Z. Liu, W. Huang and C. Zhang, Biomaterials, 2015, 53, 379–391 CrossRef CAS PubMed.
- Y. Lin, W. Xiao, B. S. Bal and M. N. Rahaman, Mater. Sci. Eng. C, 2016, 67, 440–452 CrossRef CAS PubMed.
- G. Jagan Mohini, N. Krishnamacharyulu, G. Sahaya Baskaran, P. Venkateswara Rao and N. Veeraiah, Appl. Surf. Sci., 2013, 287, 46–53 CrossRef.
- J. Zhao, X. Xu, X. Chen, Q. Xu, Z. Luo, X. Qiao, J. Du, X. Fan and G. Qian, J. Eur. Ceram. Soc., 2019, 39, 5018–5029 CrossRef CAS.
- G. El Damrawi, A. M. Abdelghany and H. Salaheldin, Bull. Chem. Soc. Ethiop., 2022, 36, 597–606 CrossRef CAS.
- S. Bruns, T. Uesbeck, D. Weil, D. Möncke, L. van Wüllen, K. Durst and D. de Ligny, Front. Mater., 2020, 7, 189 CrossRef.
- A. A. El-Kheshen, F. A. Khaliafa, E. A. Saad and R. L. Elwan, Ceram. Int., 2008, 34, 1667–1673 CrossRef CAS.
- J. R. Jones, E. Gentleman and J. Polak, Elements, 2007, 3, 393–399 CrossRef CAS.
- K. F. Frederiksen, K. Januchta, N. Mascaraque, R. E. Youngman, M. Bauchy, S. J. Rzoska, M. Bockowski and M. M. Smedskjaer, J. Phys. Chem. B, 2018, 122, 6287–6295 CrossRef CAS PubMed.
- K. Bourhis, J. Massera, L. Petit, H. Ihalainen, A. Fargues, T. Cardinal, L. Hupa, M. Hupa, M. Dussauze, V. Rodriguez, C. Boussard-Plédel, B. Bureau, C. Roiland and M. Ferraris, Mater. Res. Bull., 2015, 63, 41–50 CrossRef CAS.
- T. Oey, K. F. Frederiksen, N. Mascaraque, R. Youngman, M. Balonis, M. M. Smedskjaer, M. Bauchy and G. Sant, J. Non. Cryst. Solids, 2019, 505, 279–285 CrossRef CAS.
- N. Mascaraque, K. Januchta, K. F. Frederiksen, R. E. Youngman, M. Bauchy and M. M. Smedskjaer, J. Am. Ceram. Soc., 2019, 102, 1157–1168 CrossRef CAS.
- A. A. Osipov, V. E. Eremyashev, A. S. Mazur, P. M. Tolstoi and L. M. Osipova, Glas. Phys. Chem., 2016, 42, 230–237 CrossRef CAS.
- B. F. Boyce, H. Y. Elder, H. L. Elliot, I. Fogelman, G. S. Fell, B. J. Junor, G. Beastall and I. T. Boyle, Lancet, 1982, 2, 1009–1013 CrossRef CAS PubMed.
- J. G. Joshi, BioFactors, 1990, 2, 163–169 CAS.
- G. Cournot-Witmer, J. Zingraff, J. J. Plachot, F. Escaig, R. Lefèvre, P. Boumati, A. Bourdeau, M. Garabédian, P. Galle, R. Bourdon, T. Drüeke and S. Balsan, Kidney Int., 1981, 20, 375–385 CrossRef CAS PubMed.
- M. C. Blades, D. P. Moore, P. A. Revell and R. Hill, J. Mater. Sci. Mater. Med., 1998, 9, 701–706 CrossRef CAS PubMed.
- C. Exley, Morphologie, 2016, 100, 51–55 CrossRef CAS PubMed.
- A. Hoppe, N. S. Güldal and A. R. Boccaccini, Biomaterials, 2011, 32, 2757–2774 CrossRef CAS PubMed.
- J. Isaac, J. Nohra, J. Lao, E. Jallot, J. M. Nedelec, A. Berdal and J. M. Sautier, Eur. Cells Mater., 2011, 21, 130–143 CrossRef CAS PubMed.
- P. Horák, M. Skácelová and A. Kazi, J. Rheum. Dis. Treat., 2017, 3 DOI:10.23937/2469-5726/1510050.
- P. Balasubramanian, T. Büttner, V. Miguez Pacheco and A. R. Boccaccini, J. Eur. Ceram. Soc., 2018, 38, 855–869 CrossRef CAS.
- H. Yin, C. Yang, Y. Gao, C. Wang, M. Li, H. Guo and Q. Tong, J. Alloys Compd., 2018, 743, 564–569 CrossRef CAS.
- H. B. Pan, X. L. Zhao, X. Zhang, K. B. Zhang, L. C. Li, Z. Y. Li, W. M. Lam, W. W. Lu, D. P. Wang, W. H. Huang, K. L. Lin and J. Chang, J. R. Soc., Interface, 2010, 7, 1025–1031 CrossRef CAS PubMed.
- X. Wu, G. Meng, S. Wang, F. Wu, W. Huang and Z. Gu, Mater. Sci. Eng., C, 2015, 52, 242–250 CrossRef CAS PubMed.
- J. Buehler, P. Chappuis, J. L. Saffar, Y. Tsouderos and A. Vignery, Bone, 2001, 29, 176–179 CrossRef CAS PubMed.
- X. Cui, Y. Zhang, J. Wang, C. Huang, Y. Wang, H. Yang, W. Liu, T. Wang, D. Wang, G. Wang, C. Ruan, D. Chen, W. W. Lu, W. Huang, M. N. Rahaman and H. Pan, Bioact. Mater., 2020, 5, 334–347 CrossRef PubMed.
- C. Capuccini, P. Torricelli, F. Sima, E. Boanini, C. Ristoscu, B. Bracci, G. Socol, M. Fini, I. N. Mihailescu and A. Bigi, Acta Biomater., 2008, 4, 1885–1893 CrossRef CAS PubMed.
- Y. Zhu, Y. Ouyang, Y. Chang, C. Luo, J. Xu, C. Zhang and W. Huang, Mol. Med. Rep., 2013, 7, 1129–1136 CrossRef CAS PubMed.
- S. Peng, G. Zhou, K. D. K. Luk, K. M. C. Cheung, Z. Li, W. M. Lam, Z. Zhou and W. W. Lu, Cell. Physiol. Biochem., 2009, 23, 165–174 CrossRef CAS PubMed.
- P. J. Marie, D. Felsenberg and M. L. Brandi, Osteoporos. Int., 2010, 22, 1659–1667 CrossRef PubMed.
- S. Peng, X. S. Liu, S. Huang, Z. Li, H. Pan, W. Zhen, K. D. K. Luk, X. E. Guo and W. W. Lu, Bone, 2011, 49, 1290–1298 CrossRef CAS PubMed.
- Y. Li, W. Stone, E. H. Schemitsch, P. Zalzal, M. Papini, S. D. Waldman and M. R. Towler, J. Biomater. Appl., 2016, 31, 674–683 CrossRef CAS PubMed.
- Y. Wang, Q. Feng, Y. Chen, F. Wang and R. Song, SSRN Electron. J., 2022 DOI:10.2139/SSRN.4009614.
- Y. Zhang, X. Cui, S. Zhao, H. Wang, M. N. Rahaman, Z. Liu, W. Huang and C. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 2393–2403 CrossRef CAS PubMed.
- J. Lao, J. M. Nedelec and E. Jallot, J. Mater. Chem., 2009, 19, 2940–2949 RSC.
- S. Kargozar, M. Montazerian, E. Fiume and F. Baino, Front. Bioeng. Biotechnol., 2019, 7, 161 CrossRef PubMed.
- Y. C. Fredholm, N. Karpukhina, D. S. Brauer, J. R. Jones, R. V. Law and R. G. Hill, J. R. Soc., Interface, 2012, 9, 880–889 CrossRef CAS PubMed.
- F. G. Neto, R. D. S. Palácios, F. Sato, N. de, S. Fernandes, K. Miyuki, C. V. Nakamura, A. Steimacher and F. Pedrochi, Materialia, 2022, 101582 CrossRef.
- A. W. Wren, A. Coughlan, C. M. Smith, S. P. Hudson, F. R. Laffir and M. R. Towler, J. Biomed. Mater. Res., Part A, 2015, 103, 709–720 CrossRef PubMed.
- L. M. Placek, T. J. Keenan, Y. Li, C. Yatongchai, D. Pradhan, D. Boyd, N. P. Mellott and A. W. Wren, J. Biomed. Mater. Res., Part B, 2016, 104, 1703–1712 CrossRef CAS PubMed.
- J. Y. Wang, B. H. Wicklund, R. B. Gustilo and D. T. Tsukayama, Biomaterials, 1996, 17, 2233–2240 CrossRef CAS PubMed.
- W. Q. Zhu, P. P. Ming, J. Qiu, S. Y. Shao, Y. J. Yu, J. X. Chen, J. Yang, L. N. Xu, S. M. Zhang and C. B. Tang, J. Appl. Toxicol., 2018, 38, 824–833 CrossRef CAS PubMed.
- M. Hashimoto, S. Kitaoka and H. Kanetaka, Adv. Powder Technol., 2016, 27, 2409–2415 CrossRef CAS.
- A. L. Raines, R. Olivares-Navarrete, M. Wieland, D. L. Cochran, Z. Schwartz and B. D. Boyan, Biomaterials, 2010, 31, 4909–4917 CrossRef CAS PubMed.
- R. Shafaghi, O. Rodriguez, S. Phull, E. H. Schemitsch, P. Zalzal, S. D. Waldman, M. Papini and M. R. Towler, Mater. Sci. Eng. C, 2020, 107, 110351 CrossRef CAS PubMed.
- R. Shafaghi, O. Rodriguez, A. W. Wren, L. Chiu, E. H. Schemitsch, P. Zalzal, S. D. Waldman, M. Papini and M. R. Towler, J. Biomed. Mater. Res. A, 2021, 109, 146–158 CrossRef CAS PubMed.
- A. M. Deliormanlı, J. Mater. Sci. Mater. Med., 2015, 26, 1–13 CrossRef PubMed.
- N. Kamboj, A. Ressler and I. Hussainova, Materials, 2021, 14, 5338 CrossRef CAS PubMed.
- M. I. Garchitorena and M. I. Garchitorena, Odontoestomatologia, 2019, 21, 33–43 Search PubMed.
- T. Zhou, B. Sui, X. Mo and J. Sun, Int. J. Nanomedicine, 2017, 12, 3495–3507 CrossRef CAS PubMed.
- J. Borrelly, M. F. Blech, G. Grosdidier, C. Martin-Thomas and P. Hartemann, Ann. Chir. Plast. Esthet., 1991, 36, 65–69 CAS.
- B. Kaymaz, U. H. Gölge, G. Ozyalvaclı, E. Kömürcü, F. Goksel, M. U. Mermerkaya and M. N. Doral, Knee Surgery, Sport. Traumatol. Arthrosc., 2016, 24, 3738–3744 CrossRef PubMed.
- S. Demirci, A. Doğan, S. Aydın, E. Ç. Dülger and F. Şahin, Mol. Cell. Biochem., 2016, 417, 119–133 CrossRef CAS PubMed.
- R. M. Nzietchueng, B. Dousset, P. Franck, M. Benderdour, P. Nabet and K. Hess, J. Trace Elem. Med. Biol., 2002, 16, 239–244 CrossRef CAS PubMed.
- M. Benderdour, K. Hess, M. Dzondo-Gadet, P. Nabet, F. Belleville and B. Dousset, Biochem. Biophys. Res. Commun., 1998, 246, 746–751 CrossRef CAS PubMed.
- M. Benderdour, T. Van Bui, K. Hess, A. Dicko, F. Belleville and B. Dousset, J. Trace Elem. Med. Biol., 2000, 14, 168–173 CrossRef CAS PubMed.
- D. G. Armstrong, D. P. Orgill, R. D. Galiano, P. M. Glat, L. A. DiDomenico, M. J. Carter and C. M. Zelen, Int. Wound J., 2022, 19, 791–801 CrossRef PubMed.
- Borate-based bioactive glass improves treatment of diabetic foot ulcers - The American Ceramic Society, https://ceramics.org/ceramic-tech-today/biomaterials/borate-based-bioactive-glass-improves-treatment-of-diabetic-foot-ulcers, (accessed 20 July 2022).
- H. S. Ryu, J. K. Lee, J. H. Seo, H. Kim, K. S. Hong, D. J. Kim, J. H. Lee, D. H. Lee, B. S. Chang, C. K. Lee and S. S. Chung, J. Biomed. Mater. Res., Part A, 2004, 68, 79–89 CrossRef PubMed.
- D. W. Buck, Adv. Ski. Wound Care, 2020, 33, 1–6 CrossRef PubMed.
- L. Sopchenski, S. Cogo, M. F. Dias-Ntipanyj, S. Elifio-Espósito, K. C. Popat and P. Soares, Appl. Surf. Sci., 2018, 458, 49–58 CrossRef CAS.
- M. Ottomeyer, A. Mohammadkah, D. Day and D. Westenberg, Adv. Microbiol., 2016, 6, 776–787 CrossRef CAS.
- K. Schuhladen, S. N. V. Raghu, L. Liverani, Z. Neščáková and A. R. Boccaccini, J. Biomed. Mater. Res., Part B, 2021, 109, 180–192 CrossRef CAS PubMed.
- K. R. Hixon, T. Lu, M. N. Carletta, S. H. McBride-Gagyi, B. E. Janowiak and S. A. Sell, J. Biomed. Mater. Res. B. Appl. Biomater., 2018, 106, 1918–1933 CrossRef CAS PubMed.
- S. Sadeghzade, R. Emadi and S. Labbaf, Mater. Chem. Phys., 2017, 202, 95–103 CrossRef CAS.
- A. A. El-Rashidy, J. A. Roether, L. Harhaus, U. Kneser and A. R. Boccaccini, Acta Biomater., 2017, 62, 1–28 CrossRef CAS PubMed.
- F. J. Murray, Regul. Toxicol. Pharmacol., 1995, 22, 221–230 CrossRef CAS PubMed.
- F. J. Murray, Biol. Trace Elem. Res., 1998, 66, 331–341 CrossRef CAS PubMed.
- F. H. Nielsen, Environmental Health Perspectives, Environ Health Perspect, 1994, vol. 102, pp. 59–63 Search PubMed.
- J. T. Mader, G. C. Landon and J. Calhoun, Clin. Orthop. Relat. Res., 1993, 295, 87–95 CrossRef.
- J. Rivadeneira and A. Gorustovich, J. Appl. Microbiol., 2017, 122, 1424–1437 CrossRef CAS PubMed.
- K. Kanellakopoulou and E. J. Giamarellos-Bourboulis, Drugs, 2012, 59, 1223–1232 CrossRef PubMed.
- M. T. Cunha, M. A. Murça, S. Nigro, G. B. Klautau and M. J. C. Salles, BMC Infect. Dis., 2018, 18, 1–6 CrossRef PubMed.
- X. Zhang, U. P. Wyss, D. Pichora and M. F. A. Goosen, J. Pharm. Pharmacol., 1994, 46, 718–724 CrossRef CAS PubMed.
- M. P. Ginebra, T. Traykova and J. A. Planell, J. Controlled Release, 2006, 113, 102–110 CrossRef CAS PubMed.
- M. Itokazu, T. Ohno, T. Tanemori, E. Wada, N. Kato and K. Watanabe, J. Med. Microbiol., 1997, 46, 779–783 CrossRef CAS PubMed.
- J. Zhang, J. Guan, C. Zhang, H. Wang, W. Huang, S. Guo, X. Niu, Z. Xie and Y. Wang, Biomed. Mater., 2015, 10, 065011 CrossRef PubMed.
- L. Bi, M. N. Rahaman, D. E. Day, Z. Brown, C. Samujh, X. Liu, A. Mohammadkhah, V. Dusevich, J. D. Eick and L. F. Bonewald, Acta Biomater., 2013, 9, 8015–8026 CrossRef CAS PubMed.
- X. Huang, X. Liu, Y. Shang, F. Qiao and G. Chen, Biomed. Res. Int., 2020, 8787394 CAS.
- J. M. Jukes, S. K. Both, A. Leusink, L. M. T. Sterk, C. A. Van Blitterswijk and J. De Boer, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 6840–6845 CrossRef CAS PubMed.
- S. P. Bruder and B. S. Fox, Clin. Orthop. Relat. Res., 1999, 367, S68–S83 CrossRef PubMed.
- G. Tang, Z. Liu, Y. Liu, J. Yu, X. Wang, Z. Tan and X. Ye, Front. Cell Dev. Biol., 2021, 9, 665813 CrossRef PubMed.
- N. Jafari, M. S. Habashi, A. Hashemi, R. Shirazi, N. Tanideh and A. Tamadon, Biomater. Res., 2022, 26, 1–15 CrossRef PubMed.
- H. R. Fernandes, A. Gaddam, A. Rebelo, D. Brazete, G. E. Stan and J. M. F. Ferreira, Materials, 2018, 11, 2530 CrossRef CAS PubMed.
- E. E. Stroganova, N. Y. Mikhailenko and O. A. Moroz, Glas. Ceram., 2003, 60, 315–319 CrossRef CAS.
- L. L. Hench, J. Am. Ceram. Soc., 1998, 81, 1705–1728 CrossRef CAS.
- M. D. O’Donnell, Acta Biomater., 2011, 7, 2264–2269 CrossRef PubMed.
- R. I. M. Asri, W. S. W. Harun, M. Samykano, N. A. C. Lah, S. A. C. Ghani, F. Tarlochan and M. R. Raza, Mater. Sci. Eng., C, 2017, 77, 1261–1274 CrossRef CAS PubMed.
- S. Minagar, C. C. Berndt, J. Wang, E. Ivanova and C. Wen, Acta Biomater., 2012, 8, 2875–2888 CrossRef CAS PubMed.
- Y. Su, I. Cockerill, Y. Zheng, L. Tang, Y. X. Qin and D. Zhu, Bioact. Mater., 2019, 4, 196–206 CrossRef PubMed.
- H. S. Coskun, L. Kehribar, S. Surucu, M. Aydin and M. Mahirogullari, Cureus, 2022, 14, 1–7 Search PubMed.
- X. Zhang, W. T. Jia, Y. F. Gu, W. Xiao, X. Liu, D. P. Wang, C. Q. Zhang, W. H. Huang, M. N. Rahaman, D. E. Day and N. Zhou, Biomaterials, 2010, 31, 5865–5874 CrossRef CAS PubMed.
- A. C. Profeta, Dent. Mater. J., 2014, 33, 443–452 CrossRef CAS PubMed.
- A. L. B. Maçon, E. M. Valliant, J. S. Earl and J. R. Jones, Biomed. Glas., 2015, 1, 41–50 Search PubMed.
- W. Li and A. R. Boccaccini, Hot Top. Biomater., 2014, 56–68 Search PubMed.
- X. Qi, H. Wang, Y. Zhang, L. Pang, W. Xiao, W. Jia, S. Zhao, D. Wang, W. Huang and Q. Wang, Int. J. Biol. Sci., 2018, 14, 471–484 CrossRef CAS PubMed.
- E. Piatti, E. Verné and M. Miola, Ceram. Int., 2022, 48, 13706–13718 CrossRef CAS.
- A. M. Abdelghany, N. Mahmoud, Y. Abdou and F. El-Husseiny, Biointerface Res. Appl. Chem., 2022, 13, 19 Search PubMed.
- F. G. Neto, R. da, S. Palácios, F. Sato, N. de, S. Fernandes, K. Miyuki, C. V. Nakamura, A. Steimacher and F. Pedrochi, Materialia, 2022, 26, 101582 CrossRef.
- Z. M. Al-Rashidy, A. E. Omar, T. H. A. El-Aziz and M. M. Farag, J. Inorg. Organomet. Polym. Mater., 2020, 30, 3953–3964 CrossRef CAS.
- J. Isaac, J. Nohra, J. Lao, E. Jallot, J. M. Nedelec, A. Berdal and J. M. Sautier, Eur. Cell. Mater., 2011, 21, 130–143 CrossRef CAS PubMed.
- R. Chen, L. Sun, R. Tan, S. Xu, H. Xu, X. Zhao, T. Tao, Q. Zhang, H. Xia, J. Han, C. Liu, Z. Yu, H. Zhan, K. Ma and J. Wang, Ceram. Int., 2022, 49, 773–782 CrossRef.
- H. Hu, Y. Tang, L. Pang, C. Lin, W. Huang, D. Wang and W. Jia, ACS Appl. Mater. Interfaces, 2018, 10, 22939–22950 CrossRef CAS PubMed.
- Q. Yang, S. Chen, H. Shi, H. Xiao and Y. Ma, Mater. Sci. Eng., C, 2015, 55, 105–117 CrossRef CAS PubMed.
- J. Zhou, H. Wang, S. Zhao, N. Zhou, L. Li, W. Huang, D. Wang and C. Zhang, Mater. Sci. Eng. C, 2016, 60, 437–445 CrossRef CAS PubMed.
- A. Mehrabi, A. Karimi, S. Mashayekhan, A. Samadikuchaksaraei and P. B. Milan, Int. J. Biol. Macromol., 2022, 222, 620–635 CrossRef CAS PubMed.
- J. V. Rau, A. De Bonis, M. Curcio, K. Schuhladen, K. Barbaro, G. De Bellis, R. Teghil and A. R. Boccaccini, Coatings, 2020, 10, 1–13 CrossRef.
- R. M. Rad, Borate modified bioglass containing scaffolds for dental tissue engineering applications, PhD thesis, Middle East Technical University, 2018.
| This journal is © The Royal Society of Chemistry 2023 |