Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emerging trends in nano-based antidiabetic therapeutics: a path to effective diabetes management
Ritika
Sharma
a,
Shikha Jyoti
Borah
b,
Bhawna
c,
Sanjeev
Kumar
c,
Akanksha
Gupta
d,
Vandana
Kumari
e,
Ravinder
Kumar
*f,
Kashyap Kumar
Dubey
g and
Vinod
Kumar
 *b
*b
aDepartment of Biochemistry, University of Delhi, Delhi, India
bSpecial Centre for Nano Science, Jawaharlal Nehru University, Delhi, India. E-mail: kumarv@mail.jnu.ac.in
cDepartment of Chemistry, University of Delhi, Delhi, India
dDepartment of Science and Technology, Delhi, India
eDepartment of Biosciences, Himachal Pradesh University, Shimla, India
fDepartment of Chemistry, Gurukula Kangri (Deemed to be University), Haridwar, Uttarakhand, India. E-mail: ravinder.kumar@gkv.ac.in
gSchool of Biotechnology, Jawaharlal Nehru University, Delhi, India
First published on 28th June 2023
Abstract
Diabetes mellitus is characterized by altered blood sugar homeostasis. Diabetic mortality rates are escalating in developed countries by the day. Currently, no effective treatment and management options are available for diabetic therapy. The popularly used conventional treatment options are expensive and pose several shortcomings. This necessitates immediate development of inexpensive nano-based antidiabetic therapeutics. With the advent of nano-bio research, effective design and development of antidiabetic therapeutics and sensing devices have shown a remarkable future and attracted immense attention from the scientific community. Explicitly, the characteristics of nanoparticles would enhance bioavailability, specificity, absorption, and biocompatibility. Thus, nanocarriers or nanomaterials are a preferred choice. This study primarily focuses on the functionalization and therapeutic implications of nano-based drugs. Herein, synthesis of nanoparticles popularly used in diabetes therapy are explored. This encompasses functionalization of nanoparticles with organic or inorganic molecules, plant extracts, and other moieties. Furthermore, the therapeutic potential of these functionalized nanoparticles was also demonstrated. In this, applications such as nano therapy mediated by nano-based antidiabetic drugs, nano sensors for monitoring and detection of diabetes, and diabetic complications such as wound healing treatment are comprehensively described. Challenges and future trends are also emphasized for effective nanoformulation of antidiabetic drugs as an ultimate therapy.
1. Introduction
People suffering with chronic lifestyle conditions like diabetes, obesity, and hypertension, which are well-known risk determinants for kidney and heart disorders, are seemingly more vulnerable to various infections.1,2 Recently, over 100 million people worldwide have been impacted by the novel coronavirus infection (COVID-19) that resulted in over 2 million fatalities. These have been associated with lifestyle-related variables like obesity and diabetes.3,4 Numerous studies have confirmed that individuals suffering from type I (T1D) and type II (T2D) diabetes face larger mortality threats from COVID-19. Thus, the COVID-19 pandemic has underscored the significance of addressing impacts on individuals with diabetes, necessitating a deeper exploration of advanced science and technology, such as nanomedicine and nanotechnology. This field offers promising solutions but also presents researchers with various challenges to overcome in order to effectively address the current healthcare landscape.Diabetes mellitus (DM) refers to a group of metabolic illnesses marked by an absence of insulin, inhibited insulin secretion, or both, culminating in hyperglycemia.5,6 Diabetes is distinguished by high blood glucose levels (hyperglycemia), extreme thirstiness (polydipsia), and increased appetite (polyphagia).7 The International Diabetes Federation (IDF) predicts that diabetes will affect about 693 million people by 2045.8 According to the WHO, the diabetes mortality rate worldwide is estimated to be 1.5 million every year.9 A sedentary lifestyle and many other factors are the underlying reasons for the escalated mortality in developed countries. If current trends persist, global incidence will reach 366 million by 2030.10 Furthermore, at the current alarming rate of global diabetes increase, the WHO foresees diabetes becoming the 7th leading cause of death by 2030.11 This is mainly due to the various consequential complications that come along with diabetes, rather than diabetes itself. The rapid rise in global diabetes prevalence (Fig. 1) has been attributed to rapid urbanization, significant trends towards sedentary lifestyles, and poor dietary habits.12
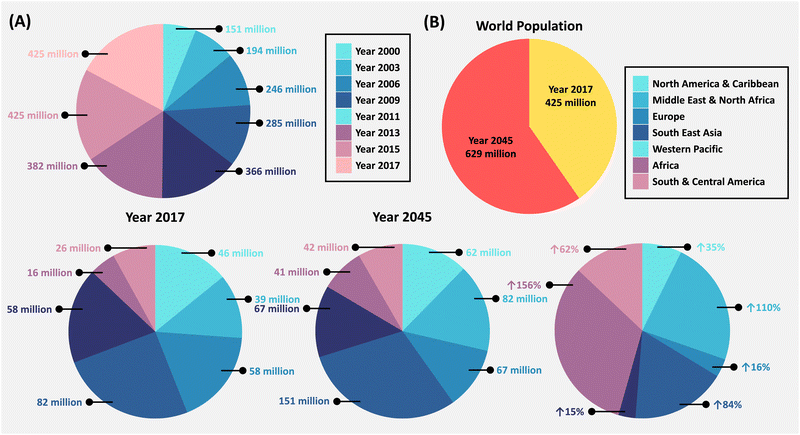 | ||
| Fig. 1 An increasing diabetic population on a global scale. (A) The number of diabetic adults (aged 20–79) from 2000 to 2017, and (B) the projected increase in the diabetic population (aged 20–79) from 2017 to 2045. The data used were sourced from ref. 13. Data courtesy: International Diabetes Federation report, 8th Edition (2017). | ||
The subcategories, T1D and T2D, are based on their respective pathogenesis: T1D is associated with a deficiency of insulin, while T2D is associated with resistance to insulin. Other types of diabetes include gestational diabetes, which develops during pregnancy, and neonatal diabetes, which develops before the age of six months.14,15 The autoimmune annihilation of β-pancreatic cells leads to obstruction of insulin production, giving rise to T1D, also known as insulin-dependent DM (IDDM).16 T2D, contrastingly, arises from a lack of production of sufficient insulin or insulin receptor desensitization, preventing the admission of glucose into the cells.17 T2D is predominantly observed worldwide in about 90–95% of cases, while T1D is mostly seen in children in about 5–10% of cases only. This complexity makes it challenging to establish effective treatment techniques because the etiology varies from case to case. With around 116 million diabetic patients, China has the largest share. India ranks 2nd with 77 million, ensued by the United States of America (USA) with 31 million, suggesting that the USA will be at maximum risk in the future decade.7 While there is no treatment for diabetes, people can lessen disease-related problems by closely monitoring blood glucose levels. This is often achieved without the requirement of laboratory analysis via sensors that rely on electrochemical enzymatic measures with screen printed electrodes, which give quick and precise measurements of blood glucose.18
For the longest duration, treatment and management of diabetes followed the course of conventional pathways through modulation of diet, exercise, and appropriate medication (Fig. 2).19 Sodium-dependent glucose transporters (SGLT-2) and α-glucosidase inhibitors, as well as other well-known antidiabetic medications such as insulin, biguanides, thiazolidinediones, and sulfonylureas, are commercially available.20 Nonetheless, with the increasing number of diabetic patients worldwide, these drugs have repeatedly proven incapable of providing effective results. As a result, new medications with improved drug delivery mechanisms that are precise and have fewer adverse effects are urgently needed. Because of the possible versatility, precision, and intelligently designed nanomaterials (NMs), nanomedicine has emerged as a new hope in biomedicine.
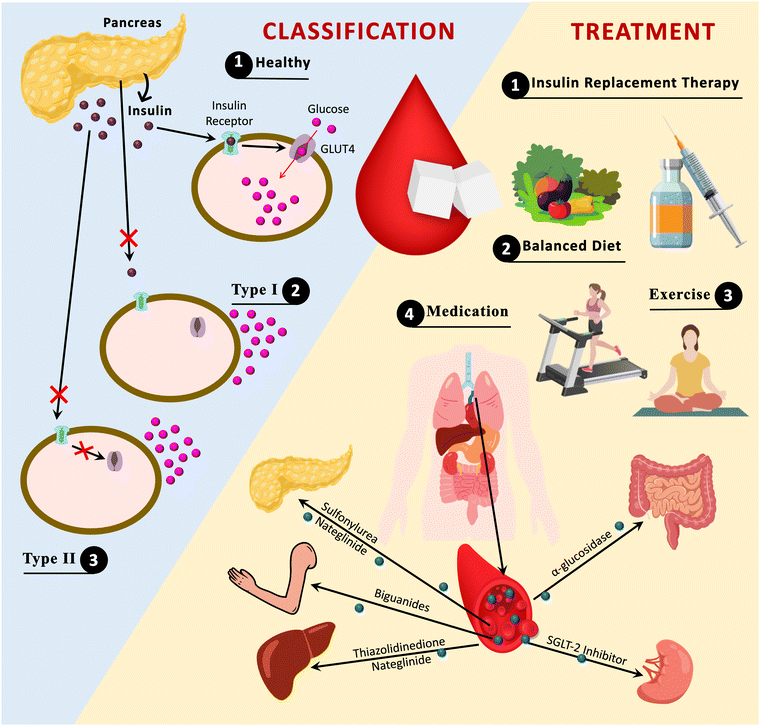 | ||
| Fig. 2 Diabetes classification and approaches for management. GLUT4; glucose transporter 4, SGLT-2; sodium-dependent glucose transporter-2. | ||
However, these methods have certain demerits which have been overcome through the advancements of science and technology made in NMs. Because of their nanoscale dimensions, these materials offer huge benefits, including excellent optical features, a high surface area, and other qualities, resulting in a broad spectrum of NMs in biomedicine.2 Recent literature shows clinical and commercial incorporation of NMs in diabetes, particularly in the context for COVID-19 vaccinations, wound healing, nano sensors, and drug delivery. This seamless integration has reformed the landscape offering unparalleled efficacy and therapeutic outcomes, and has improved treatment for diabetic individuals.21–24 Not just that, a perspective towards the sedentary lifestyle linking to the recent pandemic, and the fatality associated with those are looked upon with the outlook for the prevailing mortality rate in the near future. For instance, the vaccine BNT162b2, developed by the German organization Pfizer-BioNTech, is based on a lipid nanoparticle (NP) formulated, nucleoside-mRNA vaccine.21,25 Similarly, Ag NPs have been determined to show superior characteristics, i.e., chemical, physical, and biological, which could be utilized for wound healing in diabetic patients.26 Additionally, a recent literature review shows that NPs such as polydopamine (PDA)-based NPs have a good scope of application in biosensing, drug delivery, and phototherapy.27
The present work demonstrates a holistic view for the development of nano-based diabetic drugs (Fig. 3). Moreover, it emphasizes the recent trends associated with the emerging field and the rational design of nano-based antidiabetic drugs along with highlighting the NM functionalization. Recent studies offering a wealth of knowledge in this emerging domain have emphasized the antidiabetic nano therapy at the molecular level. However, others are based on several applications of a particular kind of NP, such as PDA NPs, solid NPs, liposomal, micellar, and others.28 The role of NMs in effective delivery of drug molecules is explored and emphasized in the present review. However, major challenges such as cost-effective and large-scale manufacture, biocompatibility, clinical implementation, etc. preclude research from further advancements and, thereby, require in-depth analysis and study.
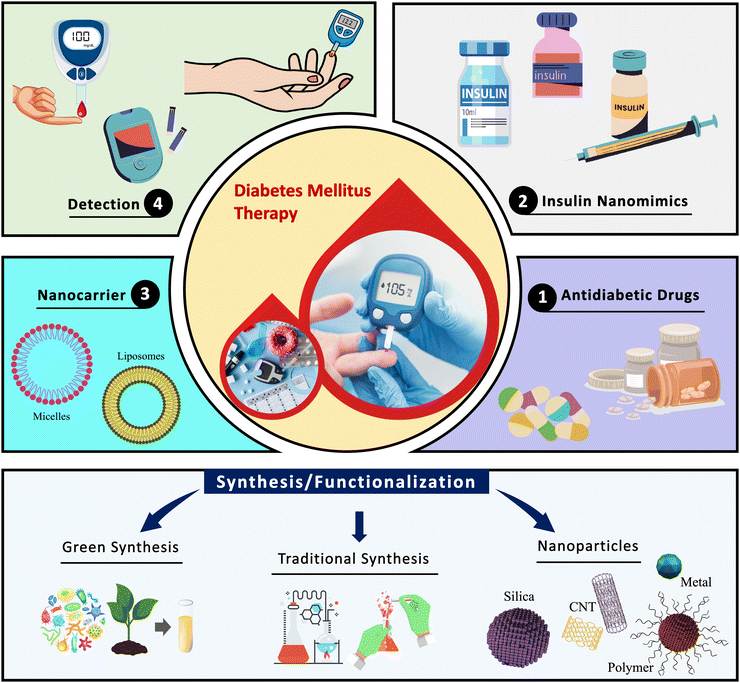 | ||
| Fig. 3 Schematic representation of nanoparticles for diabetes mellitus: synthesis, functionalization, and therapeutic applications. | ||
2. Properties of diabetic nanoparticles
NPs can be categorized into organic and inorganic NPs based on their structure.29 Organic NPs can be further categorized into lipid-based, DNA, protein and polymer-based, while inorganic NPs can be categorized into ceramic and metabolic.19 These are derived from natural life or through chemical synthesis. This class of NPs consists of biogenic macromolecules and synthetic polymers. These NPs are further used to fabricate nanocomposites. Synthetic polymer NPs possess diverse chemical structures (such as spheres, micelles, vesicles, and dendrimers), controlled sizes, functional designability, and a long clearance time, due to which they have high potential for enhancing the therapeutic efficacies of drug delivery systems.30 Additionally, dendrimer NPs can amplify their utility for diabetic wound treatment due to their biocompatibility and water solubility.31On the other hand, inorganic NPs due to their anti-infective and anti-inflammatory properties have been used for treating chronic ulcers, burns, and wound in diabetic patients.32 For combating the risk of infection in unhealed wounds, the antimicrobial properties of inorganic NPs such as metal NPs provide major advantages.33 For instance, Ag NPs exhibit intrinsic resistance towards bacteria thereby inhibiting bacterial growth. This characteristic makes them suitable for serving as a vehicle for drug delivery purposes.34 Besides this, other inorganic NPs have shown the ability to maintain metabolic homeostasis,35 and improve angiogenesis to promote collagen deposition,36 thereby facilitating wound closure.
NMs have also been useful in developing nano sensors for the detection of exhaled breath acetone.37,38 To achieve high sensitivity and quick response rates, a desirable sensing material must have a high specific area and enhanced accessibility of gas molecules, which is often observed in NMs.37 Besides this, NMs with enzyme-like capabilities (nanozymes) have recently received significant study attention in biomedicine due to their simple synthesis, low cost, high activity, and long self-life when compared to natural enzymes.39 Particularly interesting are nanozymes with peroxidase-like enzymatic activity for glucose detection.40,41
NMs incorporated in the treatment of diabetes have been quite effective due to their unique properties. Due to the poor absorption or ineffective distribution within the body, several drugs may not attain the optimum concentration required to elicit the intended pharmaceutical action.42,43 Nanoparticulate systems have the capability to transform bioactive compounds that are poorly soluble or absorbed into medications that can be effectively delivered. Due to their small size, NPs have the ability to penetrate cells and interact with cytoplasmic or genomic molecules in a safer manner. Another benefit of using NMs for antidiabetic drug loading is controlled drug release. Additionally, as nanotechnology has progressed, numerous functional NMs with bio-targeting, anti-oxidation, photoelectric or photothermal properties have been identified.19 These materials may increase the effectiveness of diabetic treatments or expand their range of options.
3. Synthesis and functionalization of diabetic nanomaterials
Nanotechnological breakthroughs have led to the advent of NPs and their functionalization with bio-entities has been beneficial for several biomedical applications.44,45 Several kinds of NPs are being explored, such as metal–organic frameworks (MOFs), polymer-based, silica-based, lipid-based, liposomes, hydrogels, and many others.46 These nanocarriers were developed for smooth delivery of antidiabetic drugs (Fig. 4).12,44,47–49 Furthermore, plant-based substances such as ferulic acid, polydatin, etc. have also been integrated with the former mentioned nano-agents for the formulation of therapeutics for diabetes treatment.50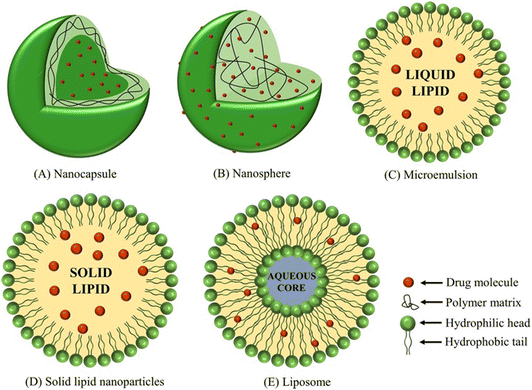 | ||
| Fig. 4 Emerging nanocarrier applications in antidiabetic drug delivery.12 Copyright©2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved. | ||
Inorganic and organic NPs have distinct physical, chemical, and biological features at the nanoscale make them an effective tool for drug discovery. Inorganic NPs are those which do not contain C–H bonds, while organic NPs are nanosized materials originating from natural life or synthesized chemically. Ceramic and metallic NPs are two types of inorganic NPs, with the former having uses such as electrochemical immunosensors and drug administration, while the latter has prominent applications such as antidiabetic, anticancer, antimicrobial, sensing, and so on. Organic NPs can be subcategorized into biogenic NMs (proteins, lipids, and nucleic acids) and synthetic polymers. These organic NPs possess significant biomedical applications because of their biocompatibility and ability to be readily absorbed and to degrade naturally. Synthetic polymer NPs show great promise for improving the therapeutic efficacy of delivery mechanisms because of their benefits such as functional designability, diverse chemical structures, tunable sizes, and more.30,51
In fact, NMs have indeed been introduced in the diabetes sector in recent years, and numerous new applications for diabetes diagnostics and therapeutics, such as blood glucose control, detection of diabetic biomarkers, insulin mimicking, and prevention of diabetes-assisted complications, have been presented based on these NMs. This review sheds light on various NM-assisted diabetes therapeutics. Furthermore, it illustrates the possibility of utilizing innovative NMs with distinct functionalities and characteristics for improved diabetes applications.
Based on the literature survey conducted, the detailed classification of different types of NMs that have been utilized for the design and development of antidiabetic drugs has been demonstrated by Liu et al. They have presented a comprehensive elaboration of NPs categorized into organic and inorganic NPs, on the basis of their chemical composition.19 Herein, a classification of diabetic NPs based on their synthetic methods has been conducted and demonstrated comprehensively. Herein, the synthetic approaches were broadly categorized into two types, namely, conventional or traditional methods and green synthesis or biological methods. Each of these encompasses several processes which are explored below. To the best of the author's knowledge, this demonstration of diabetic NPs has not been performed previously. Table 1 summarizes recent progress in diabetic NMs, focusing on their synthesis and therapeutic applications.
| NP | Functionalized with | Synthesis method | Highlights | Ref. |
|---|---|---|---|---|
| Nano therapy | ||||
| ZnO | Hibiscus subdariffa leaf extract | Green synthesis | Induces the activity of Th1, Th2 cells | 56 |
| Promotes expressions of insulin receptors | ||||
| Blood glucose levels were restored in mice upon administration | ||||
| ZnO | Erythrina variegate leaf extract | Green synthesis | Significant effects on blood glucose levels observed | 57 |
| Also exhibited antimicrobial, antioxidant, and anti-inflammatory properties | ||||
| ZnO | CH–NC | Green synthesis | Controlled release of antidiabetic drugs with minimal side effects | 58 |
| Reduced blood glucose levels after 28 day administration | ||||
| Vildagliptin | Albumin | Spray drying | Formation of mucoadhesive NPs | 54 |
| Au | BSA | Chemical synthesis | NP effectively binds to CPM and TBM | 52 |
| Au | Guavanoic acid | Solution casting | Effective antidiabetic potential on L6 rat skeletal muscle cell lines (in vivo assay) | 53 |
| Improved uptake of insulin-dependent glucose | ||||
| Liposomes | BS + rhINS | Reversed-phase evaporation | Effective for cellular uptake, little cytotoxicity and no apoptosis within 24 hours | 59 |
| Liposomes | sterols (Si, St, La, and Er) + rhINS | Reversed-phase evaporation | 50% decrease in blood glucose level. Increased insulin permeability across Caco-2 monolayers | 60 |
| Low toxicity | ||||
| Bilosomes | Soy lecithin | Thin film hydration | Efficient transport of insulin across TR146 cell layers | 61 |
| SDGC/SC/STC/SGC/SDTC | ||||
| Ag | Taverniera couneifolia | Green synthesis | Low blood glucose levels | 62 |
| Improved dyslipidemia status and lipid, liver, and renal profiles | ||||
| ZnO | — | Sol–gel method | Antidiabetic effects of ZnO NPs and ZnO NPs + vildagliptin | 63 |
| Nano Sensor | ||||
| PtM-apo | WO3 NF | Electrospinning | Accurate detection of biomarker molecules in the breath and distinguishable pattern recognition by PCA | 55 |
| Au | MIPs | Polymerization | Successfully distinguished target molecule (acetone) from other gases | 64 |
| Modified brust-schiffrin | ||||
| Au–Ni | AC | Microwave | Early diagnosis of diabetes | 65 |
| High sensitivity towards glucose | ||||
| UC | PDA | Co-precipitation | Significantly selective, sensitive, and high-throughput bioassay for blood glucose detection | 66 |
| UC | MnO2 NS and OA | Ultrasonication | Extremely selective, sensitive and economical nano sensor for detection of glucose levels | 67 |
| Wound healing | ||||
| Ag | PDA and CPH | Supramolecular assembly | Promote blood vessel formation | 68 |
| Accelerate rate of collagen production | ||||
| Suppress growth of bacteria | ||||
| Manage wound infection | ||||
| SCN | Zn2+@GO | CVD | Increased antibacterial efficacy of SCN | 69 |
| Accelerated wound healing | ||||
| HA-SH | PBAE-TA with multi-acrylate and TA end groups | Polymerization | Sustainable hypoxia-inducing capability | 70 |
| Click reaction | Enhances regulation of immune system and vascular regeneration | |||
| Stimulate reconstruction of blood vessels, hair follicles, and dermal collagen matrix | ||||
| rGO | ADM | EDC/NHS cross-linking | Promotes diabetic wound closure with robust vascularization | 71 |
| Collagen deposition within the wound | ||||
| Rapid re-epithelialization of newborn skin | ||||
| Nephropathy | ||||
| Curcumin | — | — | Reduce kidney inflammation and fibrosis in a DN rat model | 72 |
| Improved renal function and histopathological parameters | ||||
| Inhibits NLRP3 inflammasome activity | ||||
| PPP | RH | EDC/NHS and nanoprecipitation | Reduce inflammation and preserved renal function in a DN mouse model | 73 |
| Fe3O4 | PEG | In situ precipitation | Target specific kidney cells | 74 |
| Sensitive detection in a DN rat model using MRI | ||||
| PFC | Lipid monomer | Emulsification | Quantitative mapping of kidney perfusion | 75 |
| Promote drug delivery | ||||
| Treatment of inflammation, angiogenesis, thrombosis, other kidney comorbidities | ||||
| Exhibit both diagnostic and therapeutic role for the management of kidney diseases | ||||
| Graphene | — | — | Renal TE | 76 |
| Promote adhesion, proliferation, and differentiation of renal cells | ||||
| Lower limb amputation | ||||
| PCL | Drugs | Electrospinning | Accelerate wound closure | 77 |
| Enhance tissue regeneration | ||||
| Reduce bacterial infection | ||||
| Cu/TiO2–SnO2 | — | Sol–gel | Shows improvement in the reepithelization of diabetic foot ulcers | 78 |
| ZnO | CA | Sonication | Enhanced tissue regeneration | 79 |
| Wound closure was reported to be 75% | ||||
| Cardiomyopathy | ||||
| PLGA | FD + PLGA | Nanoprecipitation | Antihypertensive effects, involving the conjugation of felodipine, amlodipine, hydrochlorothiazide, and candesartan | 80 and 81 |
| Fe2O4 | Au + Oleic acid | Thermal decomposition | Atherosclerotic plaque and stem cell modification | 82–84 |
| Treat multiple sclerosis and myocardial failure | ||||
| Identification of CD163 (macrophage M2 marker) in atherosclerotic lesions | ||||
| Au | — | Citrate reduction | Detection of scarred cardiac tissues resulting in comprehensive evaluation of myocardial ischemia | 85 |
| Au | HDL + Apo A1 + phospholipids | Facile conjugation | Facilitate replication of HDL and generation of HDL-like molecules | 86 and 87 |
| Act as both therapeutic and diagnostic roles in atherosclerosis | ||||
| Boosts HDL plasma levels | ||||
| Provides a strong atheroprotective effect | ||||
| Ag | — | Turkevich method | Act as a coating on cardiac stents and pacemakers, lowering the risk of infections | 88 and 89 |
| May induce oxidative stress, cell damage and inhibit cell proliferation | ||||
| Retinopathy | ||||
| Au | Folic acid + Sorafenib tosylate + PEG + ginger extract | Green synthesis | Precise delivery of drug Sorafenib tosylate in the VEGF receptors in the retinal neo-vascularization region | 90 |
| Demonstrate diabetic protection against DR and diabetic cataract | ||||
| Oxidative stress | ||||
| Suppress enzymatic function of aldose reductase in the lens | ||||
| Ag | Mulberry leaf extract | Green synthesis | Effective treatment of both diabetic and Al-induced developing retinopathy | 91 |
| BSA | Apa + HA | Cross-linking | In vivo studies on DR rat models illustrated negligible cytotoxicity and enhanced retinal accumulation | 92 |
| PLGA | Feno encapsulation | Emulsification | Reduced retinal vascular leakage | 93 |
| Suppresses retinal leukostasis | ||||
| Reduces overexpression of VEGF and ICAM-1 | ||||
3.1. Conventional synthesis method
Traditional or conventional approaches of NP synthesis comprise a wide array of methods, for example, spray drying, solution casting, electrospinning, and many more. Utilizing a broad spectrum of fabrication methods deciphers and modulates the properties, characteristics as well as behaviour under in vitro or in vivo settings. In a study conducted by Singh and Mitra, they performed Au NP synthesis with conjugation to bovine serum albumin (BSA) via chemical synthesis. In brief, a precursor, i.e., HAuCl4 was heated with stirring, and then a reducing agent, i.e., tri-sodium citrate was added. This led to a faint blue colored solution, which later on changes to red. The alteration of the color indicates the formation of Au NPs. Furthermore, BSA was conjugated with the as-prepared Au NPs via mixing both in desired amounts, resulting in a final concentration of 5 μM and 1.04 nM of BSA and Au NPs, respectively.52 A research group functionalized Au NPs with guavanoic acid via the solution casting method (Fig. 5). To do so, 5 mg and 1 mM of guavanoic acid and HAuCl4 solution, respectively were stirred. As a result, the solution undergoes a color transformation from pale yellow to ruby red, stipulating the formation of guavanoic acid–Au NPs.53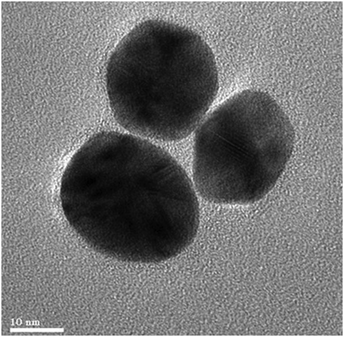 | ||
| Fig. 5 HR-TEM images of guavanoic acid functionalized with Au NPs. Reproduced from ref. 53 with permission from the Royal Royal Society of Chemistry. | ||
In another study, albumin-vildagliptin NPs were designed using the spray-drying method.54 PtM (M = Pd, Rh, Ni) NPs were synthesized, where the metal oxide gets entrapped in the protein cage of apoferritin via the electrospinning method and WO3 as nanofibers (NF) as a mesoporous support (Fig. 6).55
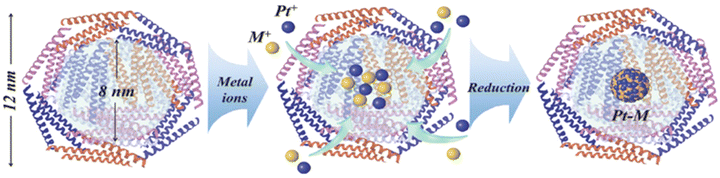 | ||
| Fig. 6 Diagram depicting the methodology of encapsulating PtM within apoferritin to produce PtM-apo NPs.55 Copyright©2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
An acetone nano sensor was designed via polymerization of molecularly imprinted polymers (MIPs). In short, acetonitrile, methacrylic acid (MAA), and acetone were mixed, and then ethylene glycol dimethacrylate (EGDMA) as a cross-linker and azobisisobutyronitrile (AIBN) as an initiator were added. It was then purged in nitrogen gas and then kept in an oil bath at around 70 °C. The formed precipitate was then centrifuged and dried at room temperature. This was followed by MIP conjugation with Au NPs via chemical synthesis (modified Brust–Schiffrin method).64 In a recent investigation, a NP alloy of Au–Ni NPs was synthesized via conjugation to activated carbon (AC) using the microwave synthesis method (Fig. 7).65
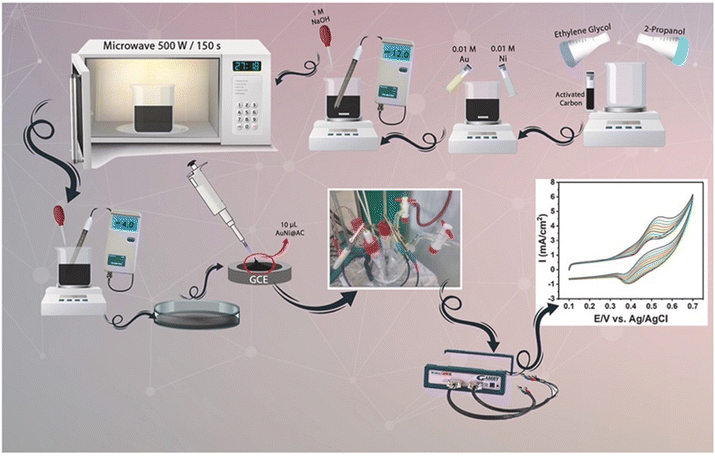 | ||
| Fig. 7 Schematic representation of the microwave assisted synthesis of AuNi@AC NPs.65 Copyright©2021 Elsevier Ltd. All rights reserved. | ||
Liu et al. exemplified the role of NaYF4:Yb/Er upconversion (UC) NPs for precise detection of glucose levels in blood and also determination of other biomolecules generated by H2O2. Firstly, the UCNPs was capped with oleate (1) using the co-precipitation method. Furthermore, (1) was then subjected to acid treatment to obtain ligand-free NaYF4:Yb/Er UCNPs (2). Following this, (2) was then mixed with dopamine (DA) monomers to synthesize PDA-coated NaYF4:Yb/Er UCNPs (3).66 Another investigation conducted by El-Gharbawy et al. synthesized ZnO NPs via sol–gel methods. Briefly, the precursors used are zinc acetate and methanol, which were stirred. This was followed by gelation at 800 °C for a couple of hours, leading to the formation of ZnO NPs with an average size of ∼20 nm.63
Recombinant human insulin (rhINS) loaded into liposomes composed of bile salts (BS) was prepared via reverse-phase evaporation. Briefly, in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio soyabean phosphatidyl choline (SPC) and BS were suspended in absolute ether. Subsequently, rhINS was added in citrate–Na2HPO4 buffer solution. The formed suspension was subjected to ultrasonication leading to water–oil emulsion and the organic solvent was evaporated later on. After that, a homogenous mixture of rhINS-BS liposomes was formed.59 Cui et al. used the above described method for the fabrication of rhINS-liposome with sterols (Si, St, La, and Er), where, Si is β-sitosterol, St is stigmasterol, La is lanosterol, and Er is ergosterol.60
1 molar ratio soyabean phosphatidyl choline (SPC) and BS were suspended in absolute ether. Subsequently, rhINS was added in citrate–Na2HPO4 buffer solution. The formed suspension was subjected to ultrasonication leading to water–oil emulsion and the organic solvent was evaporated later on. After that, a homogenous mixture of rhINS-BS liposomes was formed.59 Cui et al. used the above described method for the fabrication of rhINS-liposome with sterols (Si, St, La, and Er), where, Si is β-sitosterol, St is stigmasterol, La is lanosterol, and Er is ergosterol.60
Bashyal et al. used a thin film hydration approach for the synthesis of bilosomes with soy lecithin and BS activators, namely sodium cholate (SC), sodium taurocholate (STC), sodium glycocholate (SGC), SdeoxyGC (SDGC), or SdeoxyTC (SDTC). These were suspended in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of chloroform and methanol. Furthermore, vacuum evaporation was used to remove organic solvent. After that, the thin dry layer was hydrated with rhINS in a water bath at 35 °C. Lastly, for homogenous distribution, the liposome were ejected from the membrane filter.61 There are many other methods of synthesizing NPs for diabetes therapy, which are not listed here. Each of these approaches has its own pros, and a suitable approach should be opted as per the requirements.
1 mixture of chloroform and methanol. Furthermore, vacuum evaporation was used to remove organic solvent. After that, the thin dry layer was hydrated with rhINS in a water bath at 35 °C. Lastly, for homogenous distribution, the liposome were ejected from the membrane filter.61 There are many other methods of synthesizing NPs for diabetes therapy, which are not listed here. Each of these approaches has its own pros, and a suitable approach should be opted as per the requirements.
3.2. Green synthesis method
Green nanobiotechnology offers several advantages and avoids disadvantages that are imposed by the traditional methods. A few of these disadvantages are toxicity, manufacturing cost, operation, pollution, cytotoxicity, etc. Herein, a brief discussion demonstrating the green synthesis of NPs via bacteria, plants, and fungi takes place. Bala et al. fabricated ZnO NPs and functionalized them with Hibiscus subdariffa extract via the green synthesis method. The Hibiscus subdariffa leaf extract was heated at 50 °C in distilled water. Afterwards, the obtained extract was filtered. Furthermore, for the synthesis of ZnO NP, plant extract was mixed with zinc acetate by adding in a dropwise manner. The mixture was incubated for 30 min approximately and resulted in the formation of zinc hydroxide precipitate. Following this, the reaction mixture was centrifuged and the precipitate was vacuum dried.56 Another study demonstrated the implementation of chitosan (CH)–ZnO–nanocurcumin (NC) NPs via the green synthesis method. In this synthesis, 0.01 M curcumin was dropwise added to an equivalent molar amount of zinc acetate and stirred at 50–60 °C for four hours. Following this, ethanol was used to wash curcumin and then dried in an oven. Then, encapsulation with CH was carried out to form CH–NC NPs.58 A recent investigation reported the use of ZnO NPs via green synthesis using leaf extracts from Erythrina variegate.57 Ul Haq et al. performed green synthesis of Ag NPs via Taverniera couneifolia extract. In their synthesis, a 1 mM solution of AgNO3 was mixed with 100 mg crude extract for the formation of green Ag NPs.62 Another investigation conducted by Govindan et al. used a green synthesis approach of ZnO NPs via Catharanthus roseus extract. In this synthesis, the 30 g of Catharanthus roseus plant extract and 3 g of ZnSO4 were placed into 1 L of distilled water. The prepared solution was then stirred for over 5 days with 500 W Halogen light, following which, the mixture was kept under sunlight for approximately 20 days. Lastly, the obtained powder was dried and calcined for 1 hour at 100 °C.944. Mechanistic aspects of diabetic nanomaterials
In recent times, nanomedicine has shown tremendous potential in revolutionizing the treatment and control of various diseases, like DM. DM is characterized by dysregulated glucose metabolism resulting from insufficient insulin production or impaired insulin action. Traditional treatment modalities, such as oral antidiabetic agents and injectable insulin, often have limitations in terms of efficacy and adverse effects. NMs due to their unique properties, offer exciting opportunities for the development of novel antidiabetic strategies.95–97 Understanding the underlying mechanisms by which these NMs exert their antidiabetic effects is crucial for their successful translation into clinical applications. The mechanistic pathways of diabetic NMs have been comprehensively discussed in the following section. Their role in the regulation of glucose homeostasis, insulin sensitivity and secretion, and inflammation modulation have been exclusively explored to determine their potential as effective antidiabetic drugs.4.1. Insulin secretion and sensitivity
Insulin performs essential functions in the process of glucose metabolism by regulating blood glucose levels and assisting glucose absorption into cells. NMs are capable of boosting the pancreas β cells’ ability to secrete insulin, which subsequently enhances the utilization of glucose. For instance, insulin secretion from β cells can be stimulated by mesoporous silica NPs loaded with insulin-mimetic compounds, mimicking the physiological responses to high glucose levels. This enhanced insulin production encourages glucose absorption by target tissues which lowers blood glucose levels. Moreover, insulin sensitivity can be improved by NMs with surface functionalization that augments binding and activation of insulin receptors on target cells.95 Behzadifar et al. demonstrated the improved insulin secretion and β cell functions of NMs for DM treatment. They illustrated the potential of various NMs including metal, liposomes and polymer, that can stimulate release of insulin and enhance β cell functions.97 In another recent study, various NMs for developing higher insulin sensitivity in DM, their roles in improving glucose uptake and metabolism in target tissues have been explored.954.2. Glucose homeostasis regulation
Effective diabetes management is closely associated with the maintenance of glucose homeostasis. NMs offer unique capabilities for regulating glucose levels through various mechanisms. For example, Au NPs functionalized with glucose oxidase can efficiently convert glucose into gluconic acid, reducing blood glucose levels. This enzymatic conversion occurs selectively when glucose is present, allowing for precise regulation.98,99 Similarly, NM-based glucose-responsive insulin delivery systems have garnered significant attention. These can release insulin when glucose levels are elevated, ensuring timely and targeted insulin administration to maintain normoglycemia. By integrating glucose-responsive elements within NMs, such as nanogels or NPs, researchers have achieved remarkable glucose-responsive release of insulin profiles.100 As an example, Volpatti et al. explored NMs for glucose-responsive insulin delivery, highlighting their ability to detect fluctuations in glucose levels and subsequently release insulin in response.101 Naikoo et al. investigated NMs for non-enzymatic glucose sensing, which can continue to diabetes management by providing accurate and continuous glucose monitoring.100,1024.3. Inflammation modulation
Persistent, mild inflammation has a notable impact on the development and progression of diabetes as well as its related complications. NMs possess anti-inflammatory properties that can ameliorate diabetic conditions. Polymeric NPs loaded with anti-inflammatory agents can selectively target and suppress inflammatory cells, mitigating inflammation-induced insulin resistance. Furthermore, NMs can modulate pro-inflammatory signalling pathways, that include nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK), leading to reduced production of inflammatory mediators and improved insulin sensitivity. Various recent literature studies have explored NMs for inflammation modulation in DM, particularly emphasizing their anti-inflammatory effects and role in enhancing insulin sensitivity.103,1044.4. Gut microbiota modulation
The composition and activity of the gut microbiota, which is essential for maintaining metabolic health and regulation of glucose, can be influenced by NMs. Certain NMs, such as TiO2, possess the unique ability to selectively inhibit bacterial growth while assisting the growth of beneficial bacteria. This modulation of gut microbiota promotes antidiabetic effects by enhancing glucose metabolism and insulin sensitivity.105,106 Yang et al. determined the impact of TiO2 NPs on the composition and metabolic parameters of gut microbiota in diabetic mice, illustrating enhanced glucose metabolism and insulin sensitivity.1074.5. Oxidative stress reduction
Oxidative stress is caused by an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense mechanism of the body. It is a major factor in the development and progression of DM. NMs possess antioxidant properties which allows them to scavenge ROS and reduce oxidative stress, which subsequently protects pancreatic β cells and enhances insulin sensitivity. For example, NMs such as CeO2 NPs have demonstrated potent antioxidant activity, mitigated oxidative stress-related damage and promoted β cell survival.108,109However, further research and development efforts are required to optimize NM design, efficacy, and safety before their clinical translation. With ongoing advancements in nanotechnology, diabetic NMs have the ability to bring about significant advancements in diabetic care.
5. Therapeutic applications
Nanotechnology's intervention has resulted in an emerging array of ideal drug delivery frameworks.110 The subsection that follows delves into various applications associated with diabetes-related complications. As illustrated in the following subsection, a brief description is provided based on their therapeutic applications for diabetes treatment, management, and related complications. Other complications include wound healing, neuropathy, retinopathy, cardiomyopathy, nephropathy, and so on.1065.1. Nano therapy
Exploring the use of various NMs has been scrutinized for the establishment of better therapeutic nanocarriers. As a result, the therapeutic efficacy of a variety of antidiabetic drugs has been improved. The use of organic NPs such as dextran, gums, chitosan, nano-emulsion, alginate, and many others have emerged as excellent antidiabetic nano-drugs owing to their biocompatibility, non-toxicity, bioavailability, and stability in physiological milieu. Antidiabetic treatment utilizing organic NPs derived from biological sources are attractive due to their abundant availability, notable therapeutic advantages, and minimal adverse effects. Organic NPs play a vital role in the treatment of diabetes by employing four mechanisms to lower blood sugar levels: inhibit breakdown of carbohydrate and absorption of glucose, enable glucose metabolism, improve insulin action and sensitivity, and exhibiting anti-inflammatory and free radical scavenging properties. However, often materials employed in the creation of nano-based delivery techniques are generated from precursors of inorganic or synthetic materials using laborious and time-consuming synthesis methods, which may surely result in considerable toxicity. Additionally, rather than a clinical examination, the majority of investigations are based on cellular and animal prototypes. Therefore, clinical trials are ultimately important since animal toxicological research has limitations.111Recent studies have reported successful implementation of silica NPs in diabetes treatment. Silica-based nanocarriers have been developed with the potential to treat diabetes and specifically regulate blood glucose levels by exploring its properties, including surface chemistry, adjustable particle, pore size, and biocompatibility. Silica NPs can operate as reservoirs due to mesopores having substantial and tunable volume and diameter. Furthermore, they can also improve intestinal permeability. The application of silica NPs is extremely diverse and versatile, allowing for a variety of administration routes and modes of action.112
Broadly, nano-drug delivery practice can be categorized into two depending on the route of drug administration, that is, intravenously or orally.10,12,113 The ZnO NP functionalized with Erythrina variegate (leaf extracts) was analysed for the antidiabetic mechanism. A pancreatic α-amylase assay was also conducted to assess antidiabetic activity. The results demonstrate significant effects on blood glucose levels. Aside from that, it has antimicrobial, antioxidant, and anti-inflammatory properties.57 An Au NP was fabricated with BSA, followed by binding of chlorpropamide (CPM) and tolbutamide (TBM), which have been identified as potent antidiabetic drugs. They demonstrated that the binding strength of the drugs was reduced upon conjugation with BSA along with a better inhibitory effect on the advanced glycated end (AGE).52 In another study, green Au NPs were functionalized and their antidiabetic potential was evaluated by in vivo studies conducted on L6 rat skeletal muscle cell lines and demonstrated improved uptake of insulin-dependent glucose.53
An investigation conducted by Nagaraja et al., fabricated albumin-vildagliptin NPs, which were extended to drug uptake studies, demonstrating constant drug release lasting 12 hours. As a result, mucoadhesive NPs demonstrated a promising role in diabetes therapy.54 The antidiabetic mechanism of green ZnO NPs was characterized using HR-TEM (Fig. 8), which depicted agglomerated NPs with a low ratio of surface to volume for ZnO NP (60 °C) compared with for ZnO NP (100 °C). Further demonstrating the dumbbell-shaped particles with a higher surface:volume ratio. The ZnO NP at 60 °C and 100 °C was then evaluated for its anti-diabetic activity. To do so, the mice were injected with streptozotocin (STZ) to induce hyperglycemia. Upon administration of the green ZnO NP, the blood glucose levels were restored in mice that were treated with STZ. The glucose level was reduced by 59.58% with ZnO (60 °C) and 48.27% with ZnO (100 °C).56
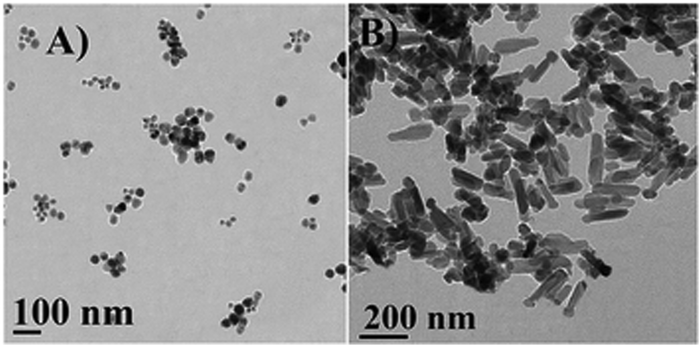 | ||
| Fig. 8 HRTEM images of the synthesized ZnO. (A) Isolated spherical ZnO NPs at 60 °C, with sizes ranging from 12–46 nm at 100 nm scale bar; and (B) dumbbell-shaped ZnO NPs at 100 °C, with lengths ranging from 190–250 nm and widths ranging from 50–60 nm at the 200 nm scale. Reproduced from ref. 56 with permission from the Royal Society of Chemistry. | ||
Chauhan et al. demonstrated the positive effect of the ZnO–CH–NC nanocomplex for controlled release of antidiabetic drugs with minimal side effects. Furthermore, diabetic rats were administered the synthesized NPs daily for 28 days and exhibited reduced blood glucose levels.58 Velsankar et al. also designed green ZnO NPs that were characterized and the HRTEM micrographs (Fig. 9) reveal the shape of the synthesized NP to be hexagonal or rectangular.57
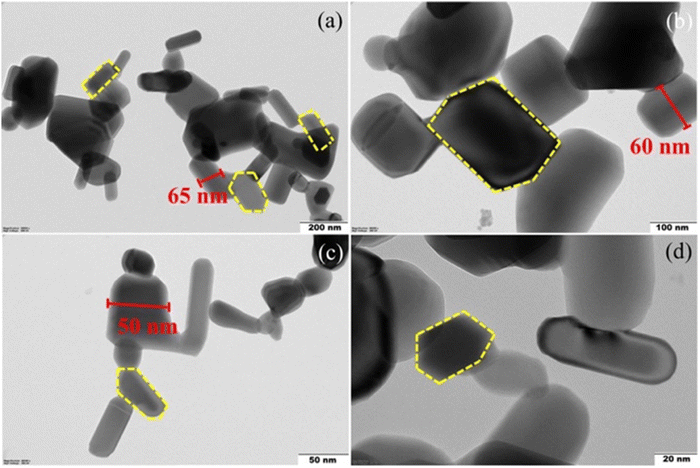 | ||
| Fig. 9 (a)–(d) HRTEM images of ZnO NPs in the 200, 100, 50, and 20 nm scale range.57 Copyright©2022 Elsevier B.V. All rights reserved. | ||
El-Gharbawy et al. fabricated ZnO NPs of average size ∼20 nm for antidiabetic therapy. To investigate their antidiabetic potential, different groups were created. In group I, 10 mg kg−1 per day of vildagliptin was administered. Group II–IV rats was subjected with administration of ZnO NPs of 1, 3, and 10 mg kg−1 per day, respectively. Lastly, Group V was administrated with a combinational therapy of ZnO NPs and vildagliptin. Also, there were control groups of diabetic and non-diabetic rats. This regime was continued for seven days. In all these cases, a marked decrease in the expression of microRNAs (miRNAs), namely, miRNA-103 and miRNA-143 over the diabetic control group. Thus, indicating antidiabetic effects of ZnO NPs alone as well as in combination with vildagliptin. Furthermore, other diabetic indices were also measured that include, glucose tolerance, insulin levels, weight, and others. Their results elucidated an improvement in the above-mentioned indices because of ZnO NPs. However, a synergistic effect of vildagliptin was also observed in the presence of ZnO NPs, thereby further improving the diabetic indices.63
Niu et al. prepared rhINS-BS liposomes via a revere-phased evaporation method. Their results deciphered a significant increase in the bioavailability of rhINS due to BS liposome as a nanocarrier.59 In another investigation, conducted by Cui et al. they determined its potential role as a nanocarrier for effective antidiabetic activity using rhINS-liposome-sterols. In their study they studied four types of sterols, namely, Si, St, La, and Er. However, they demonstrated a markedly improved performance with Er over any other kind of sterol. Therefore, it can be elucidated that substituting cholesterol with Er would improve the oral bioavailability of rhINS.60 Bilosomes with soy lecithin and BS activators were prepared via a thin film hydration approach by Bashyal et al. From their observations, it can be elucidated that SDGC-liposome exhibited a superior antidiabetic performance to enhance insulin bioavailability via the buccal cavity.61
Recent investigation demonstrated the fabrication of 12-tungstophosphoric acid (TPA)-functionalized MCM-48 NPs (nMCM-48) as a carrier for glipizide (GLP) drug, which is a poorly soluble drug. The formation of nMCM-48 was conducted via facile wet chemical synthesis and then functionalized with GLP drug via an impregnation method. Based on the results, the commercial formulation of GLP/TPA/nMCM-48 demonstrated better sustained release of GLP drugs. The cytotoxicity studies showed 15% toxic effects. This opens a new possibility for the further exploration via functionalization with TPA. This could improve the treatment of non-insulin-dependent DM.114
5.2. Nano sensors
Altered blood glucose levels are consistent with DM patients, and therefore, repeated and precise detection is absolutely essential.27,115–117 Therefore, nano sensors provide a suitable platform for selective, accurate, and sensitive detection of blood glucose levels. Exhaled biomarker detection is another criterion for the detection of diabetes. For example, the sensing ability of PtM-apo@WO3 nanocomposite was schematically represented as in Fig. 10. Their results demonstrate Pt/NiO–WO3 NFs showed excellent potential for halitosis breath in all three cases: healthy, simulated diabetic, and simulated halitosis breath. However, their response is relatively low.55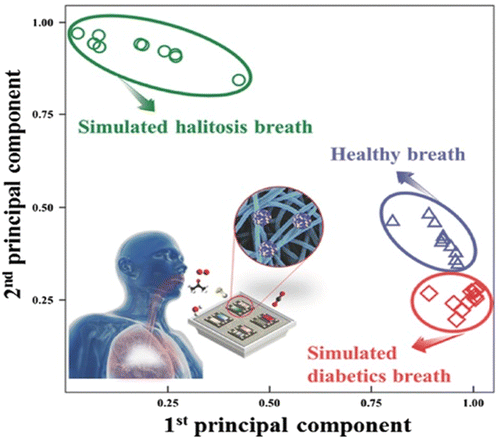 | ||
| Fig. 10 Pattern recognition by principle component analysis using datasets from sensor arrays evaluating real and stimulated (diabetes and halitosis) breath.55 Copyright©2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
A recent investigation focuses on the development of acetone nano sensor MIP@Au NPs (Fig. 11). They optimized the performance of the nano sensor against 100 ppm of acetone gas. Their results demonstrated acetone detection with a detection limit of 66 ppm.64 A study by Arikan et al., elucidated the implementation of Au–Ni@AC NPs to detect glucose levels for early diabetes detection. So, based on their results, the nano sensor's detection level was found to be 0.41 μM. Therefore, it exhibits sensitivity and selectivity and is devoid of enzyme-based detection of glucose.65 In another study, the functionalized UCNPs@PDA nano sensor was used to evaluate sugar levels in biological fluid samples. The TEM and HR-TEM images for each depict a diameter of 23.2 ± 0.6 nm and a spherical shape. Based on the assessment, it can be concluded that the designed nanosystem for glucose detection provides glucose detection that is sensitive and accurate. Therefore, it is a potential sensor for the detection of diabetes.66
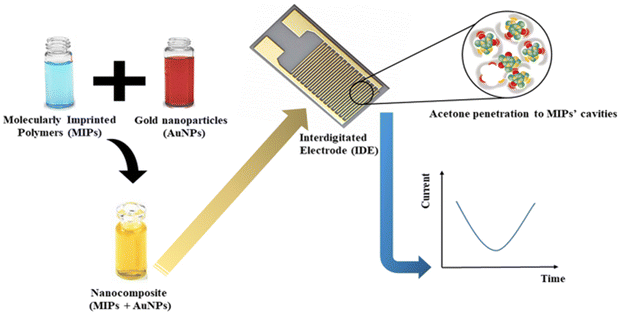 | ||
| Fig. 11 Diagrammatic illustration of MIP@Au nanosensors for the detection of acetone gas.64 Copyright©2022, Iran Polymer and Petrochemical Institute. | ||
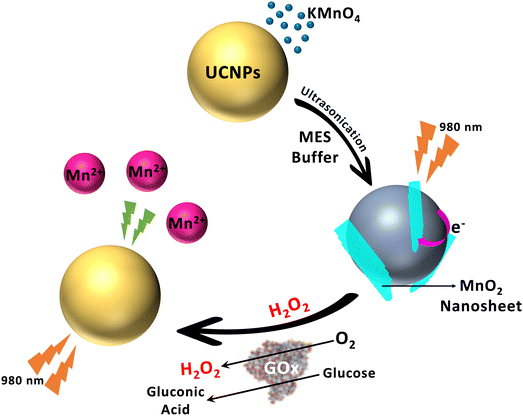
Yuan et al. demonstrated NaYF4:Yb,Tm@NaYF4 core shell UCNPs, functionalized with oleic acid (OA) and MnO2. The TEM images reveal the hexagonal shape of the OA-capped NaYF4:Yb,Tm@NaYF4 UCNPs and amorphous nanosheets (NSs) of MnO2-modified NaYF4:Yb,Tm@NaYF4 nano-assemblies (Fig. 12). Then, the performance of the formed nano-assemblies was then assessed for glucose and H2O2 sensing based on the glucose to H2O2 conversion mediated by the enzyme glucose oxidase (Fig. 13). The results showed improved nano sensor performance and thus makes a significant stride in DM research.67
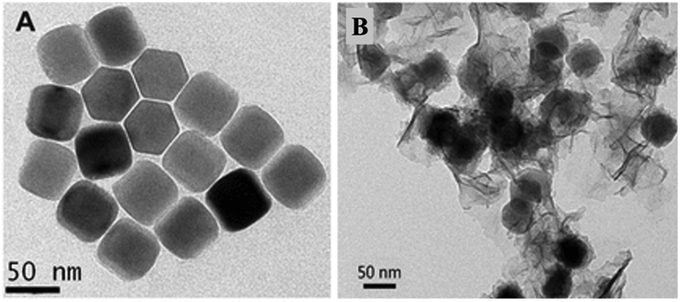 | ||
| Fig. 13 TEM images (A) of OA-capped NaYF4:Yb,Tm@NaYF4 core shell UCNPs, and (B) of MnO2 NS-modified NaYF4:Yb,Tm@NaYF4 UCNP assemblies.67 Copyright©2015, American Chemical Society. | ||
The future of illness diagnosis has been transformed by nano sensors. To counteract market rivalry, innovation is constantly taking place. Nano sensors are incredibly smart devices that can detect even the smallest amounts of potentially dangerous substances, such as low concentrations of a substance that can be intrinsically toxic. Wearable nanosensor apps can even aim to alter home care models that emphasize natural cures and cut down on medical expenses and diagnosis times. Rising demand from the healthcare industry has accelerated the development of nanotechnology through government-sponsored programs. The main requirement for creating glucose sensors is the detection of hypoglycemia in individuals with insulin-dependent diabetes. Nano sensors will enable highly sensitive, fast, precise and thorough detection of disease biomarkers, thereby enhancing diabetes treatment and diagnosis. Besides this, the unique biological, optical, physical, chemical and magnetic properties of NPs make them appropriate candidates for tumor detection, atherosclerotic plaque imaging, etc.118 Furthermore, the modification of these NPs at quantum levels can enhance their diagnostic properties.119 So, the convenience and usefulness of nano sensors in diabetes treatment and diagnosis can provide a real-time analysis of diabetic patients, paving the way for a better healthcare system. However, various disadvantages of nano sensors may also exist. Low detection limits, cytotoxicity of inorganic NPs, toxic precursors for the synthesis of organic NPs, and expensiveness still remain major drawbacks of nano sensors. Despite these drawbacks, nanosensors have tremendous potential, and given the growing need for advanced nanotechnologies, their positive effects on healthcare systems for people cannot be overlooked.
5.3. Wound healing
Prolonged bleeding is most severely associated with diabetic patients. According to the WHO, wound associated co-morbidity has become a public health concern globally. The current data elucidate that prolonged bleeding is reported for 15% of the patients. Of this, 20% undergoes limb amputation. It is evident that the molecular architecture of the localized wound area changes continuously and thus, the traditional therapeutic approaches remain ineffective.120,121 During wound healing, a cascade of sequential events leads to the restoration of damaged tissue.122,123 In a nutshell, diabetic wound healing takes place in four phases, namely, haemostasis, inflammation, proliferation, and remodelling.124–129 As is evident, several events take place in a sequential manner. Therefore, different approaches are needed to treat chronic diabetic wound closure. Fig. 14 describes the nanotechnology associated with the therapy of diabetic wounds.120 Therefore, nano-based conductive materials that can cling to wounds and stop blood flow should be adopted to resolve diabetic wound healing complications.130–134 Herein, the recent advancements of nano-architectured materials for effective diabetic wound healing are explored. | ||
| Fig. 14 A schematic representation illustrating how nanotechnology can be employed in the treatment of diabetic wounds.120 Copyright©2020 Elsevier B.V. All rights reserved. | ||
In a recent review, Liu et al. have thoroughly discussed the various bioactive and non-bioactive materials loaded in nano-drug delivery systems (NNDSs) for diabetic wound healing. The fabrication of NNDSs such as liposomes, nanofibers, and nanohydrogels using bioactive materials have high potential with therapeutic benefits for wound healing. Bioactive molecules including growth factors, genes, peptides, proteins, stem cells, and other natural bio-substances like curcumin can aid wound healing processes due to various properties like antioxidant, anti-inflammatory, and self-renewal ability. On the other hand, non-bioactive materials such as metal NPs can have extensive applications in wound healing due to their strong and long-term antibacterial stability. Nanomedicine and NNDSs have emerged as efficient agents targeted towards diabetic wound healing and great therapeutic results can be anticipated in futuristic diabetic wound management.135
Zhao et al. have synthesized PDA@Ag conductive polymer hydrogels (CPHs) as depicted in Fig. 15. Furthermore, the ability for diabetic wound healing was assessed, demonstrating promising effects, which are achieved by angiogenesis, self-healing ability, and increased deposition of collagen. Therefore, it has exhibited immense potential for wound healing in real time and also epidermal nanosensing.68
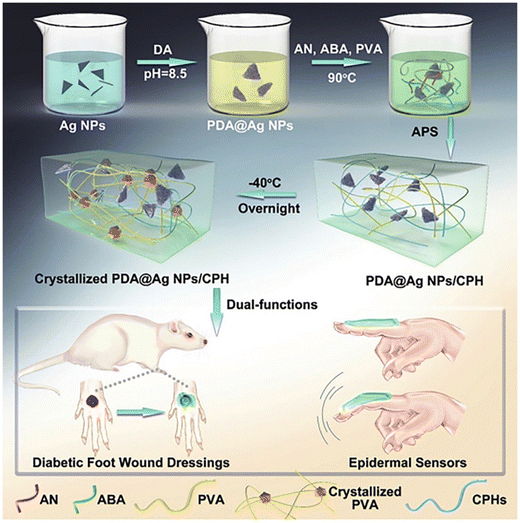 | ||
| Fig. 15 Schematic illustration of PDA for preventing wound infection via PDA@Ag NPs supramolecular assembly.68 Copyright©2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Li et al. functionalized g-C3N4–Zn2+@graphene oxide NSs (SCN-Zn2+@GO) via chemical vapour deposition (CVD). This was prepared by utilizing electrostatic bonding and stacking of π–π interactions. Furthermore, they exhibited the potential role of the synthesized NSs under exposure of light at wavelengths of 660 nm and 808 nm, to kill bacteria. This in turn assists in healing of wounds. Therefore, the efficacy of hybrid NSs exhibiting enhanced photothermal-assisted antimicrobial activity as well as wound healing therapy was investigated.69 Another study towards non-healing wounds because of DM was conducted by Fu et al. In their study, reduced GO (rGO) NPs were incorporated with acellular dermal matrix (ADM) via 1-ethyl-3-(3-(dimethylamino) propyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) cross-linking. These scaffolds demonstrated better stability, an excellent milieu for stem cells adhesion and proliferation, and admirable mechanical properties. The wound healing ability was assessed via in vitro analysis. Briefly, the scaffold was cultured with stem cells. Afterwards, a streptozotocin (STZ) induced diabetes model was transplanted with the culture of scaffold and stem cells. Deposition of collagen, vascularization, and re-epithelialization were observed. Thus, it was determined to be an excellent engineered material for diabetic wound healing.71 In a recent investigation by Li et al., injectable hydrogels that are induced by hypoxia were explored. During synthesis, thiolated hyaluronic acid (HA-SH) was cross-linked with hyperbranched poly(β-amino ester)-tetraaniline (PBAE-TA), which was achieved by a click reaction of thiol-ene showing rapid polymerization. Contrastingly, gelatin grafted on vanillin (Geln-Van) was cross-linked at a slow rate with laccase (Lac). Furthermore, both the injectable hydrogels were injected into stem cells derived from encapsulated adipose. The results depict improved recovery and skin function in skin wounds of diabetic rats.70
In a recent investigation, Ag NPs were incorporated to fabricate a nanocomposite using polyvinyl alcohol (PVA) and eugenol microemulsion (EuME). This process was achieved via electrospinning and resulted in the formation of nanofibrils. Furthermore, this nanofibril was found to exhibit the highest antimicrobial potential against Staphylococcus aureus. The presence of Ag ions under controlled release yields sustainable surroundings for wound healing applications.136
5.4. Nephropathy
Diabetic nephropathy (DN) is responsible for a substantial incidence of end-stage renal disease (ESRD), necessitating transplantation or dialysis for a considerable number of patients.137,138 The development of DN is closely linked to uncontrolled diabetes, particularly when blood glucose levels remain consistently high.138 Although the exact mechanisms are not fully understood, several factors contribute to its onset. Key among them is persistently elevated blood sugar levels, which adversely affect the tiny blood vessels within kidneys.During the early stages, DN often presents no noticeable symptoms. However, as the disease progresses, various symptoms may indicate kidney damage. These symptoms can include proteinuria, edema, fatigue, weakness, high blood pressure, and anemia.139 Early diagnosis and intervention are crucial for effectively managing DN. DN is mainly characterized by chronic inflammation, oxidative stress, and impaired kidney function, resulting in the progressive loss of kidney structure and function. Current treatment strategies primarily focus on blood glucose control, blood pressure management, and the use of certain medications to slow disease progression.137–139 However, these approaches may have limitations and may not fully halt the progression of DN.
Due to its target specificity, therapeutic agent delivery and outstanding treatment efficiency, nanotechnology offers unique advantages for DN treatment. Several nanotechnology-based strategies have demonstrated promising results in preclinical studies, highlighting their substantial potential for clinical translation. Therefore, it holds revolutionizing prospects and treatment landscape for DN conditions. Furthermore, engineered NPs can be developed for direct delivery of therapeutic agents such as antioxidants, anti-inflammatory drugs or renoprotective compounds to affected kidney cells. These nanocarriers can strengthen drug stability, increase drug uptake by target cells and lessen adverse off-target side effects. NP-based drug delivery systems in targeting DN have been reported by various studies in the last decade. For instance, Lu et al. demonstrated that targeted delivery of curcumin-loaded NPs in a DN rat model showed significantly reduced kidney inflammation and fibrosis which lead to improved renal functions and histopathological parameters. Moreover, these NPs also inhibit NLRP3 inflammasome activity.72 Likewise, in another study, Chen et al. reported targeted delivery of anti-inflammatory drug-loaded polymeric NPs to the kidneys of a DN mouse model which showed reduced inflammation and preserved renal function. The polymeric NPs were synthesized through EDC/NHS, which is followed by a nanoprecipitation method. To summarize the synthesis method, polyethyleneglycol-co-polycaprolactone-co-polyethylenimine (PPP) was prepared first, followed by Rhein (RH) loading onto PPP to fabricate PPP-RH NPs.73
The framework of sustained drug release enabled nanotechnology over an extended interval of time. This guarantees a uniform effect of therapeutics, thus decreases the dose of administration, and improves compliance. A study utilized a nanogel-based system for the sustained release of an angiotensin-converting enzyme inhibitor in a DN rat model. The controlled release of the drug significantly reduced proteinuria, oxidative stress, and renal fibrosis compared to conventional therapy.137,138 A study by Chen et al. exhibited emulsification of perfluorocarbon (PFC) NPs utilizing surfactant, safflower oil, glycerin, water, and perfluorooctyl bromide. From this, a final NP comprising of a core of PFC, which is encapsulated with lipid monomer, was achieved. These PFC NPs showed quantitative mapping of kidney perfusion. The synthesized NPs can serve as a carrier for drug delivery and demonstrated potential utilization in the treatment of inflammation, angiogenesis, thrombosis, and other associated kidney comorbidities. Thus, exhibit both diagnostic and therapeutic roles for the management of kidney diseases.75
NPs can be engineered with imaging agents to enable precise detection and monitoring of kidney damage. Advanced imaging techniques, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), can be utilized to visualize kidney abnormalities and assess treatment response. A study by Gómez-Vallejo et al. developed Fe3O4 NPs with a coating of a copolymer of poly(4-vinylpyridine) and PEG via in situ precipitation and targeted specific kidney cells. These NPs enabled sensitive detection in a DN rat model using MRI.74 Moreover, NMs can be utilized to create scaffolds and matrices for tissue engineering (TE) applications. These biomarkers provide structural support and promote the regeneration of damaged kidney tissue, potentially restoring kidney function. A study demonstrated a three-dimensional (3D) graphene-based scaffold for renal TE. This scaffold promoted the adhesion, proliferation, and differentiation of renal cells, offering a promising approach for kidney tissue regeneration.76 These findings highlight that nanotechnology offers innovative solutions to address the complex challenges associated with DN.
5.5. Lower limb amputation
Diabetic lower limb amputation occurs when diseases associated with neuropathy, foot ulcers, arterials disorder, lead to inadequate healing. This is an outcome of long-lasting diabetes. This mainly results from improper blood flow to lower extremities and nerve damage to the peripheral region. These result in impaired sensation, which when combined with foot ulcers could lead to a considerable issue not only to patients, but also to healthcare providers.Different types of NMs, including nanofibers, NPs, nanocomposites, and others exhibit immense avenues in diabetic lower limb amputations and wound healing. Scaffolds can be generated by utilizing these NMs, which demonstrate the potential to the extracellular matrix. This could improve cell growth and regeneration of tissues. Furthermore, assimilation of growth factors and other biomolecules into these scaffolds, could enable healing of wounds, alleviate infection, and also reduces the probability of amputation. Lanno et al. carried out an investigation utilizing scaffolds of nanofibers to establish the efficacy in the healing process of a diabetic wound. To achieve this, scaffolds were fabricated by electrospinning polycaprolactone (PCL), which were then instilled with therapeutics and antimicrobial agents. Their results illustrate better regeneration of tissues, fast-track closure of wounds, and diminished bacterial infection, when compared to conventional dressings.77
The formation of bioactive dressings was enabled by nanotechnology, which could foster wound healing and alleviate infection. They provide meticulous regulation of drug release and thus enable long-term management and expedite the healing process. Recently, Loera-Valencia et al. carried out synthesis of ZnO NPs using sonication. This was subsequently followed by the assimilation of a dressing composed of calcium alginate (CA). These NPs illustrated enhanced regeneration of tissue, and implied potential improvement in wound healing. Their results demonstrated 75% wound closure upon treatment.79 In another investigation, the researchers synthesized Cu/TiO2–SnO2 NPs using a sol–gel method. After successful synthesis, these NPs showed notable elevation in the reepithelization of foot ulcers caused due to diabetes.78
In spite of the immense potential of nanotechnology and advancements, several limitations still persist. For example, the production cost of NMs, regulatory authorization, and inclusion into the operational healthcare system, etc. are domains that demand immediate attention. Furthermore, ensuring the safety and biocompatibility of NMs is crucial to their successful implementation. Thus, further research is needed to optimize NMs for diabetic lower limb amputation applications and ensure their long-term efficacy and safety. By harnessing the power of NMs and nanodevices, we can create a future where diabetic lower limb amputation becomes a rarity rather than a common occurrence, improving the well-being of countless individuals afflicted with diabetes and enhancing their overall quality of life.
5.6. Cardiomyopathy
In comparison to diabetes per se, complications related to diabetes stand as the primary factor contributing to patient mortality. The severity and duration of poorly controlled diabetes when it coexists with other comorbid conditions has consequences such as retinopathy and cardiovascular disease events. Cardiovascular illnesses, including heart failure, coronary heart disease, myocardial infarction, and inflammatory heart disease, are the leading causes of death worldwide.140 Despite a large number of treatment medicines being available for the cardiovascular population, their performance has been constrained by their capacity to penetrate the target tissue. NMs are significant because they avoid quick renal elimination and stay in the bloodstream for long periods of time.141 This distinctive property enhances extravasation through the circulatory system and permits NPs to aggregate and distribute well in the desired tissue or organs, hence attaining optimum therapeutic efficacy at minimal medication dosages.141 Numerous preclinical and clinical studies showing site-specific drug delivery, fewer side effects, and improved treatment outcomes demonstrate how nanomedicines overcome the inadequacies of conventional therapy. Since precise NPs may be designed as carriers for the regulated and focused delivery of therapeutic chemicals to the region of interest, nanomedicine is uniquely adapted to address the primary issues associated with a number of diseases.Management of angiogenic, inflammatory, ischemic, and metabolic illnesses such atherosclerosis, hyperlipidemia, and hypertension can be accomplished through the use of nanomedicine.142,143 The most prevalent condition in the cardiovascular diseases cohort is high blood pressure, also known as hypertension.144 A number of significant cardiovascular diseases, including stroke, myocardial infarction, and peripheral artery disease, are greatly influenced by blood pressure.145
The physiological environment and the therapeutic goal of NP use are taken into consideration while choosing the appropriate class of NPs. Bioavailability, permeability and gut pH highly influence the activity of drugs.146 For example, organic NPs have been shown to be highly biodegradable and biocompatible delivery systems.147 These have garnered considerable interest owing to benefits such as ease of fabrication and higher biological stability. For instance, polymeric NPs showing antihypertensive effects have been reported by various studies involving the conjugation of felodipine (FD), amlodipine, hydrochlorothiazide, and candesartan with poly(lactide-co-glycolide) (PLGA) NPs. The PLGA NPs were synthesized via nanoprecipitation of PLGA and FD.80,81
On the other hand, inorganic NPs can be identified by their electronic properties, which enable magnetically aided therapeutic delivery and diagnostic imaging. They allow for optimal tissue penetration and disintegrate quickly. Iron oxide (Fe2O4) NPs are used predominantly in stem cell and atherosclerotic plaque modification. Their biocompatibility, chemical and magnetic characteristics, and biostability, opens up a wide range of biological uses. They have been used to treat debilitating illnesses including multiple sclerosis and myocardial failure.82,83 Recent studies have demonstrated that Fe2O4/Au/Oleic NPs are involved in the radiological identification of atherosclerotic plaques and identification of CD163 which is a macrophage M2 marker in atherosclerotic lesions.84 However, iron-derived NPs may possess toxicity which may be reduced by fabrication of biocompatible and biodegradable coating agents such as PEG, PDA, and CS, etc.148,149
Other metal NPs such as Au NPs have been highly investigated due to outstanding bio-optical features. For instance, the detection of scarred cardiac tissues has been successfully carried out, allowing for a more comprehensive evaluation of myocardial ischemia.85 Other cardioprotective properties because of anti-oxidative and anti-hypertrophic impacts can also cause downregulation of β-adrenoceptors which are key factors for enhancing heart failure mortality.150 Additionally, Au NPs facilitate the efficient replication of HDL and the generation of HDL-like molecules, which can play therapeutic as well as diagnostic roles in the control of atherosclerosis.86 The primary biological components of HDL, apolipoprotein A1 and phospholipids, are combined with Au NPs to create these synthetic molecules.87 As a result, increasing the production of these molecules with HDL-like biological characteristics boosts the plasma levels of HDL, which in turn provides a strong atheroprotective effect.87 Besides Fe2O4 and Au NPs, Ag NPs have also emerged as important nanomaterials in the field of medical disciplines. The antimicrobial activities of Ag NPs allow its application as a coating on cardiac stents and pacemakers, thereby ensuring lowered risk of infections.88 However, recently it has been observed that Ag NPs may induce oxidative stress, cell damage and inhibit cell proliferation, thus their application still receives critical skepticism in the biomedical field.89
5.7. Retinopathy
Diabetic retinopathy (DR) is the predominant factor behind vision loss and blindness, characterized primarily by damage to the microvascular, blood vessel enlargement, and leakage of fluid. The incidence of DR is rising as more diabetic people live longer and at higher rates. If left untreated, DR can lead in complications such as vitreous hemorrhage, retinal detachment, and glaucoma.19Vascular endothelial growth factor (VEGF) is abundantly present in the intraocular regions of individuals with DR, playing a significant role as a potent activator for DR. Thus, various investigations have been carried out to suppress the expression of VEGF. For instance, investigations were carried out to determine the effectiveness of Au NPs, extracted from ginger extract, which underwent subsequent modification with FA-b-PEG block copolymer. The drug Sorafenib tosylate underwent targeted delivery to the VEGF receptors in the region of retinal neo-vascularization using a FA-b-PEG. The ginger extract demonstrates diabetic protection against DR and diabetic cataracts. Additionally, the mechanism is likely oxidative stress and the inhibition of aldose reductase activity in the lens.90 In another similar study, the administration of Au NPs derived from resveratrol was used to study the amelioration effects on rats. Au NP administration led to a reduction in the expression levels of VEGF mRNA in the retina, which included Tumor Necrosis Factor (TNFα), Monocyte Chemotactic Proteins-1 (MCP-1), Intercellular Adhesion Molecule-1 (ICAM-1), and Interleukin (IL)-6 and IL-1β. Besides this, it was also found that Ag NPs are also effective in treating both diabetic and Al-induced developing retinopathy.91
Natural polymers with unique mucoadhesive and viscoelastic properties have also been explored due to their ability to actively target cells in the retina. For instance, Apatinib (Apa), which acts a novel anti-VEGF, was incorporated to develop Apa-loaded bovine serum albumin (BSA) NPs, which were further coated with HA. In vivo studies on DR rat models illustrated negligible cytotoxicity and enhanced retinal accumulation.92 In another in vivo study, therapeutic effects of fenofibrate (Feno) encapsulated into PLGA NP (Feno-PLGA NP) was observed in diabetic rats. These rats exhibited significant reductions in retinal vascular leakage, inhibited retinal leukostasis, and decreased the overexpression of VEGF and ICAM-1. These effects were observed after a single intravitreal (IVT) injection and were sustained for 8 weeks.93 However, even though research has escalated from conventional techniques to nanomedicine for DR treatment, challenges still persist due to blood retinal barriers which cause hindered permeability.151
6. Critiques, toxicity concerns and outlook
6.1. Critiques and toxicity concerns
The first line of treatment that is popularly used for diabetes treatment is the subcutaneous injection of insulin analogues or other hypoglycemic agents. However, this mode of treatment is expensive, causes other infections due to daily administration of insulin injections, people with trypanophobia (fear of needles) cannot take the treatment, and many other factors.27,152 Additionally, several other limitations, such as improper dosage, reduced specificity, or drug metabolism, might impact potency.44,113 Therefore, there is an urgent entail for a novel outlook regarding detection and therapy of diabetes and its complications.153 Moreover, nanotechnology has emerged as a safe and effective strategy for therapeutic interventions. Nanocargo-based drug delivery systems such as liposomes, micelles, nanopeptides, or other NPs have demonstrated amazing results with reduced dosage frequency.110Additionally, recent studies have also elucidated the implementation of nano-oral drug delivery systems and demonstrated amazing results.110,154,155 It is also capable of evading enzymatic degradation mediated by proteases in the gastrointestinal tract.12 Furthermore, drug administration via the oral mode is much more challenging due to several barriers, i.e., physical, chemical, and enzymatic barriers.47,156,157 The uptake of the nano-drug complex by the cells is dependent on several factors such as the amount, viscosity, molecular weight, and charge. Also, there are several limitations linked to the penetration of NPs through the mucous and epithelial cells.158–160 Chemical degradation of the drug molecule in the stomach lumen causes a reduction in the bioavailability of the oral drug molecule and proteolysis of protein-based nano-oral drugs is mediated by the digestive enzymes in the stomach and small intestinal mucous.161–163 Enzyme susceptibility is still a huge concern, but the nano-interface protects the drug molecule from the belligerent pH environment.164–166
The development of nano-biosensors based on polymeric materials imposes certain limitations. Because they have a higher affinity for the substrate, a lower conductivity, and a lower current flow, they are unstable and not suitable for in vivo biosensing applications.167 In general, in vivo assays are absolutely crucial for diabetes therapy, unlike the detection of diabetes. Therefore, modulating NMs for showcasing effective antidiabetic drugs is very important and could effectively and safely lead to potent antidiabetic mechanistic action as well as epigenetic modulation imposed by diabetic NPs.19 These shortcomings open up new doors for future research and there is a dire need for these challenges to be addressed.
Other materials, such as metallic or magnetic NPs functionalized with biomolecules to prevent degradation or clumping in vivo, are critical. Other than glucose levels, several other biomarkers such as microalbumin should also be targeted for accurate detection and therapy.27 Last but not least, the toxicity of NPs in the in vivo environment is a major drawback that limits the intervention of nanotechnology in biomedical sciences.168 A class of biodegradable NMs should be utilized for effective delivery of antidiabetic drugs and would result in reduced or low toxicity.169 The NPs have the tendency to become accumulated in the reticulocyte-endothelial system that comprises the liver, spleen, and lymph nodes. This occurs due to NP recognition and phagocytosis by cells. It is a major limitation associated with setbacks in the new design and development of antidiabetic drugs.
6.2. Future outlook
Countless studies should focus on using tight junctions to deliver targeted nanocarriers. Additionally, controlled release of NPs is of utmost importance for designing novel antidiabetic NPs. Another possibility is using combinatorial therapy for diabetes treatment.Using different ligands that can be targeted for effective diabetic therapy can also be a possibility. There is a need to focus on the future opportunities for rational design of novel nano-agents as an ultimate solution to diabetes detection and therapy. As reflected in the breakthroughs and obstacles of the recent research associated with diabetes detection and therapy, it is crucial to not dwell upon a smaller number of approvals by the Food and Drug Administration (FDA).
7. Conclusions
The present sedentary lifestyle of many and the associated health drawbacks have led to a major shift in everyone's life. In fact, the World Health Organization has forecasted that diabetes will emerge as the primary cause of mortality by 2030. Today, management and treatment of diabetes is of great significance. Therefore, immediate attention is of utmost importance. The different functionalized NPs for efficient delivery of antidiabetic drugs have been highlighted in the present work. Furthermore, emphasis was put on the different synthesis methods that can be utilized for effectively synthesizing diabetic NPs. As illustrated, the nexus of NMs with drug discovery underlie insights to innovate new strategies. With the advent of new approaches, a few demerits remain for their promising implementation in the future, and some of them are reflected upon, followed by future trends. Moreover, the potential uses of NPs were highlighted with the manifestation of their attributes along with an optimistic outlook for the ultimate therapy for diabetes complications. In the future, it will be crucial to conduct clinical trials in order to validate the safety and long-term effects of antidiabetic nanomedicine.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
SJB thank Jawaharlal Nehru University, New Delhi for financial support, respectively. Bhawna and Sanjeev Kumar thank the University Grant Commission and the Council of Scientific & Industrial Research (File no. 08/694(0004)/2018-EMR-I), respectively, for the Senior Research Fellowship.References
- Q. Cai, F. Chen, T. Wang, F. Luo, X. Liu, Q. Wu, Q. He, Z. Wang, Y. Liu and L. Liu, Diabetes Care, 2020, 43, 1392–1398 CrossRef CAS PubMed.
- A. V. A. Mariadoss, A. S. Sivakumar, C.-H. Lee and S. J. Kim, Biomed. Pharmacother., 2022, 151, 113134 CrossRef CAS PubMed.
- A. Gupta, S. Kumar, R. Kumar, A. K. Choudhary, K. Kumari, P. Singh and V. Kumar, ChemistrySelect, 2020, 5, 7521–7533 CrossRef CAS PubMed.
- J. Wang, T. Sato and A. Sakuraba, Ann. Med., 2021, 53, 1531–1536 CrossRef PubMed.
- L. Rochette, M. Zeller, Y. Cottin and C. Vergely, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840, 2709–2729 CrossRef CAS PubMed.
- K. Crawford, Nurs. Clin., 2017, 52, 621–663 Search PubMed.
- S. Alam, M. K. Hasan, S. Neaz, N. Hussain, M. F. Hossain and T. Rahman, Diabetology, 2021, 2, 36–50 CrossRef.
- N. H. Cho, J. Shaw, S. Karuranga, Y. Huang, J. da Rocha Fernandes, A. Ohlrogge and B. Malanda, Diabetes Res. Clin. Pract., 2018, 138, 271–281 CrossRef CAS.
- WHO, Global Reports on Diabetes, https://www.who.int/news-room/fact-sheets/detail/diabetes, (accessed 16 June, 2022).
- E. B. Souto, S. B. Souto, J. R. Campos, P. Severino, T. N. Pashirova, L. Y. Zakharova, A. M. Silva, A. Durazzo, M. Lucarini and A. A. Izzo, Molecules, 2019, 24, 4209 CrossRef CAS PubMed.
- C. D. Mathers and D. Loncar, PLoS Med., 2006, 3, e442 CrossRef PubMed.
- S. Uppal, K. S. Italiya, D. Chitkara and A. Mittal, Acta Biomater., 2018, 81, 20–42 CrossRef CAS PubMed.
- International Diabetes Federation. IDF Diabetes Atlas, Brussels, Belgium, 10th edn, 2021, available at: https://www.diabetesatlas.org.
- M. Saeedi, Y. Cao, H. Fadl, H. Gustafson and D. Simmons, Diabetes Res. Clin. Pract., 2021, 172, 108642 CrossRef PubMed.
- M. B. Lemelman, L. Letourneau and S. A. W. Greeley, Clin. Perinatol., 2018, 45, 41–59 CrossRef PubMed.
- M. P. Morran, A. Vonberg, A. Khadra and M. Pietropaolo, Mol. Aspects Med., 2015, 42, 42–60 CrossRef CAS PubMed.
- T. Reinehr, World J. Diabetes, 2013, 4, 270 CrossRef PubMed.
- J. Wang, Chem. Rev., 2008, 108, 814–825 CrossRef CAS.
- Y. Liu, S. Zeng, W. Ji, H. Yao, L. Lin, H. Cui, H. A. Santos and G. Pan, Adv. Sci., 2022, 9, 2102466 CrossRef CAS PubMed.
- S. Sen and R. Chakraborty, Mini Rev. Med. Chem., 2015, 15, 1132–1133 CrossRef CAS PubMed.
- R. Sharma, A. Gupta, R. Kumar, P. Singh and V. Kumar, SMC Bull., 2020, 11, 88–96 Search PubMed.
- R. Sharma, S. Kumar, A. Gupta, N. Dheer, P. Jain, P. Singh and V. Kumar, Tissue Eng. Regener. Med., 2022, 1–34 Search PubMed.
- S. Kumar, R. Sharma, Bhawna, A. Gupta, P. Singh, S. Kalia, P. Thakur and V. Kumar, ACS Omega, 2022, 7, 22073–22088 CrossRef CAS PubMed.
- R. Sharma, K. Bhawna, P. Singh, A. Gupta and V. Kumar, Nano LIFE, 2021, 11, 2130008 CrossRef CAS.
- Y. M. Bar-On, Y. Goldberg, M. Mandel, O. Bodenheimer, L. Freedman, S. Alroy-Preis, N. Ash, A. Huppert and R. Milo, N. Engl. J. Med., 2021, 385, 2421–2430 CrossRef CAS PubMed.
- F. Paladini and M. Pollini, Mater., 2019, 12, 2540 CrossRef CAS PubMed.
- H. Li, D. Yin, W. Li, Q. Tang, L. Zou and Q. Peng, Colloids Surf., B, 2021, 199, 111502 CrossRef CAS PubMed.
- S. Maity, A. Acharyya and A. S. Chakraborti, Eur. Polym. J., 2022, 111455 CrossRef CAS.
- M. Jesus and V. Grazu, Nanobiotechnology: inorganic nanoparticles vs organic nanoparticles, Elsevier, 2012 Search PubMed.
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12, 908–931 CrossRef CAS.
- X. Deng, X. Li, W. Chen, T. Zhao, W. Huang and H. Qian, Med. Chem. Res., 2017, 26, 580–586 CrossRef CAS.
- T. Kang, Y. G. Kim, D. Kim and T. Hyeon, Coord. Chem. Rev., 2020, 403, 213092 CrossRef CAS.
- A. Hussain, M. F. Alajmi, M. A. Khan, S. A. Pervez, F. Ahmed, S. Amir, F. M. Husain, M. S. Khan, G. M. Shaik and I. Hassan, Front. Microbiol., 2019, 10, 8 CrossRef PubMed.
- R. Salomoni, P. Léo, A. Montemor, B. Rinaldi and M. Rodrigues, Nanotechnol., Sci. Appl., 2017, 10, 115 CrossRef CAS PubMed.
- M. Kaushik, R. Niranjan, R. Thangam, B. Madhan, V. Pandiyarasan, C. Ramachandran, D.-H. Oh and G. D. Venkatasubbu, Appl. Surf. Sci., 2019, 479, 1169–1177 CrossRef CAS.
- J. Xiao, Y. Zhu, S. Huddleston, P. Li, B. Xiao, O. K. Farha and G. A. Ameer, ACS Nano, 2018, 12, 1023–1032 CrossRef CAS PubMed.
- M. Righettoni, A. Amann and S. E. Pratsinis, Mater. Today, 2015, 18, 163–171 CrossRef CAS.
- H. Gong, C. Zhao, G. Niu, W. Zhang and F. Wang, Research, 2020, 2020 DOI:10.34133/2020/2196063.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- S. Cai, Z. Fu, W. Xiao, Y. Xiong, C. Wang and R. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 11616–11624 CrossRef CAS PubMed.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- R. Rani, S. Dahiya, D. Dhingra, N. Dilbaghi, K.-H. Kim and S. Kumar, Eur. J. Pharm. Sci., 2017, 106, 220–230 CrossRef CAS PubMed.
- N. Mirazi, J. Shoaei, A. Khazaei and A. Hosseini, Eur. J. Drug Metab. Pharmacokinet., 2015, 40, 343–348 CrossRef CAS PubMed.
- P. Kesharwani, B. Gorain, S. Y. Low, S. A. Tan, E. C. S. Ling, Y. K. Lim, C. M. Chin, P. Y. Lee, C. M. Lee and C. H. Ooi, Diabetes Res. Clin. Pract., 2018, 136, 52–77 CrossRef CAS PubMed.
- V. Sagar, R. R. Patel, S. K. Singh and M. Singh, Mater. Adv., 2023, 4, 1831–1849 RSC.
- G. Chakrapani, M. Zare and S. Ramakrishna, Mater. Adv., 2022, 3, 7757–7772 RSC.
- C. Y. Wong, H. Al-Salami and C. R. Dass, J. Controlled Release, 2017, 264, 247–275 CrossRef CAS PubMed.
- K. San Tang, Life Sci., 2019, 239, 117011 CrossRef PubMed.
- S. I. Othman, A. M. Alturki, G. M. Abu-Taweel, N. G. Altoom, A. A. Allam and R. Abdelmonem, Int. J. Biol. Macromol., 2021, 190, 417–432 CrossRef CAS PubMed.
- A. S. D. Wickramasinghe, P. Kalansuriya and A. P. Attanayake, Curr. Ther. Res., 2022, 96, 100672 CrossRef PubMed.
- Y. Ma, P. He, X. Tian, G. Liu, X. Zeng and G. Pan, ACS Appl. Mater. Interfaces, 2019, 11, 23948–23956 CrossRef CAS PubMed.
- I. R. Singh and S. Mitra, J. Fluoresc., 2020, 30, 193–204 CrossRef CAS PubMed.
- K. Govindaraju and K. U. Suganya, RSC Med. Chem., 2020, 11, 814–822 RSC.
- S. H. Nagaraja, B. E. Al-Dhubiab, R. K. Tekade, K. N. Venugopala, R. V. Ghorpade, G. Meravanige and A. Alqadheeb, Indian J. Pharm. Educ. Res., 2019, 53, S43–S49 CrossRef CAS.
- S. J. Kim, S. J. Choi, J. S. Jang, H. J. Cho, W. T. Koo, H. L. Tuller and I. D. Kim, Adv. Mater., 2017, 29, 1700737 CrossRef PubMed.
- N. Bala, S. Saha, M. Chakraborty, M. Maiti, S. Das, R. Basu and P. Nandy, RSC Adv., 2015, 5, 4993–5003 RSC.
- K. Velsankar, A. Venkatesan, P. Muthumari, S. Suganya, S. Mohandoss and S. Sudhahar, J. Mol. Struct., 2022, 1255, 132420 CrossRef.
- P. Chauhan, S. Mahajan and G. Prasad, J. Drug Delivery Sci. Technol., 2019, 52, 738–747 CrossRef CAS.
- M. Niu, Y. n Tan, P. Guan, L. Hovgaard, Y. Lu, J. Qi, R. Lian, X. Li and W. Wu, Int. J. Pharm., 2014, 460, 119–130 CrossRef CAS PubMed.
- M. Cui, W. Wu, L. Hovgaard, Y. Lu, D. Chen and J. Qi, Int. J. Pharm., 2015, 489, 277–284 CrossRef CAS PubMed.
- S. Bashyal, J.-E. Seo, T. Keum, G. Noh, Y. W. Choi and S. Lee, Int. J. Nanomed., 2018, 13, 5173 CrossRef CAS PubMed.
- M. N. Ul Haq, G. M. Shah, F. Menaa, R. A. Khan, N. A. Althobaiti, A. E. Albalawi and H. M. Alkreathy, Nanomaterials, 2022, 12, 1035 CrossRef CAS PubMed.
- R. M. El-Gharbawy, A. M. Emara and S. E.-S. Abu-Risha, Biomed. Pharmacother., 2016, 84, 810–820 CrossRef CAS PubMed.
- A. Jahangiri-Manesh, M. Mousazadeh and M. Nikkhah, Iran. Polym. J., 2022, 1–9 Search PubMed.
- K. Arikan, H. Burhan, E. Sahin and F. Sen, Chemosphere, 2022, 291, 132718 CrossRef CAS PubMed.
- Y. Liu, D. Tu, W. Zheng, L. Lu, W. You, S. Zhou, P. Huang, R. Li and X. Chen, Nano Res., 2018, 11, 3164–3174 CrossRef CAS.
- J. Yuan, Y. Cen, X.-J. Kong, S. Wu, C.-L. Liu, R.-Q. Yu and X. Chu, ACS Appl. Mater. Interfaces, 2015, 7, 10548–10555 CrossRef CAS PubMed.
- Y. Zhao, Z. Li, S. Song, K. Yang, H. Liu, Z. Yang, J. Wang, B. Yang and Q. Lin, Adv. Funct. Mater., 2019, 29, 1901474 CrossRef.
- Y. Li, X. Liu, L. Tan, Z. Cui, X. Yang, Y. Zheng, K. W. K. Yeung, P. K. Chu and S. Wu, Adv. Funct. Mater., 2018, 28, 1800299 CrossRef.
- X. Jin, Y. Shang, Y. Zou, M. Xiao, H. Huang, S. Zhu, N. Liu, J. Li, W. Wang and P. Zhu, ACS Appl. Mater. Interfaces, 2020, 12, 56681–56691 CrossRef CAS PubMed.
- J. Fu, Y. Zhang, J. Chu, X. Wang, W. Yan, Q. Zhang and H. Liu, ACS Biomater. Sci. Eng., 2019, 5, 4054–4066 CrossRef CAS PubMed.
- M. Lu, N. Yin, W. Liu, X. Cui, S. Chen and E. Wang, BioMed Res. Int., 2017, 2017, 1516985 Search PubMed.
- D. Chen, S. Han, Y. Zhu, F. Hu, Y. Wei and G. Wang, Int. J. Nanomed., 2018, 13, 3507 CrossRef CAS PubMed.
- V. Gómez-Vallejo, M. Puigivila, S. Plaza-García, B. Szczupak, R. Piñol, J. L. Murillo, V. Sorribas, G. Lou, S. Veintemillas and P. Ramos-Cabrer, Nanoscale, 2018, 10, 14153–14164 RSC.
- J. Chen, H. Pan, G. M. Lanza and S. A. Wickline, Adv. Chronic Kidney Dis., 2013, 20, 466–478 CrossRef PubMed.
- S. R. Shin, Y.-C. Li, H. L. Jang, P. Khoshakhlagh, M. Akbari, A. Nasajpour, Y. S. Zhang, A. Tamayol and A. Khademhosseini, Adv. Drug Delivery Rev., 2016, 105, 255–274 CrossRef CAS PubMed.
- G.-M. Lanno, C. Ramos, L. Preem, M. Putrins, I. Laidmae, T. Tenson and K. Kogermann, ACS Omega, 2020, 5, 30011–30022 CrossRef CAS PubMed.
- T. López-Goerne, P. Ramírez-Olivares, L. A. Pérez-Dávalos, J. A. Velázquez-Muñoz and J. Reyes-González, Curr. Nanomed., 2020, 10, 290–295 CrossRef.
- R. Loera-Valencia, R. E. Neira, B. P. Urbina, A. Camacho and R. B. Galindo, Biomed. Pharmacother., 2022, 155, 113708 CrossRef CAS PubMed.
- U. Shah, G. Joshi and K. Sawant, Mater. Sci. Eng., C, 2014, 35, 153–163 CrossRef CAS PubMed.
- A. Arora, N. Shafiq, S. Jain, G. Khuller, S. Sharma and S. Malhotra, PLoS One, 2015, 10, e0128208 CrossRef PubMed.
- Jasmin, G. T. de Souza, R. A. Louzada, P. H. Rosado-de-Castro, R. Mendez-Otero and A. C. Campos de Carvalho, Int. J. Nanomed., 2017, 779–793 CrossRef CAS PubMed.
- S. M. Dadfar, K. Roemhild, N. I. Drude, S. von Stillfried, R. Knüchel, F. Kiessling and T. Lammers, Adv. Drug Delivery Rev., 2019, 138, 302–325 CrossRef CAS PubMed.
- C. Tarin, M. Carril, J. L. Martin-Ventura, I. Markuerkiaga, D. Padro, P. Llamas-Granda, J. A. Moreno, I. García, N. Genicio and S. Plaza-Garcia, Sci. Rep., 2015, 5, 17135 CrossRef CAS PubMed.
- D. Danila, E. Johnson and P. Kee, Nanomed. Nanotechnol. Biol. Med., 2013, 9, 1067–1076 CrossRef CAS PubMed.
- N. K. Younis, J. A. Ghoubaira, E. P. Bassil, H. N. Tantawi and A. H. Eid, Nanomed. Nanotechnol. Biol. Med., 2021, 36, 102433 CrossRef CAS PubMed.
- A. J. Luthi, P. C. Patel, C. H. Ko, R. K. Mutharasan, C. A. Mirkin and C. S. Thaxton, Trends Mol. Med., 2010, 16, 553–560 CrossRef CAS PubMed.
- J. Talapko, T. Matijević, M. Juzbašić, A. Antolović-Požgain and I. Škrlec, Microorganisms, 2020, 8, 1400 CrossRef CAS PubMed.
- J. Shi, X. Sun, Y. Lin, X. Zou, Z. Li, Y. Liao, M. Du and H. Zhang, Biomaterials, 2014, 35, 6657–6666 CrossRef CAS PubMed.
- V. Dave, R. Sharma, C. Gupta and S. Sur, Colloids Surf., B, 2020, 194, 111151 CrossRef CAS PubMed.
- L. Xu, W. Li, Q. Shi, H. Li, Z. Yang, D. Liao, L. Li, X. Yang and J. Zhang, J. Photochem. Photobiol., B, 2019, 196, 111502 CrossRef CAS PubMed.
- S. E.-S. Radwan, A. El-Kamel, E. I. Zaki, S. Burgalassi, E. Zucchetti and R. M. El-Moslemany, Int. J. Nanomed., 2021, 16, 4481 CrossRef PubMed.
- F. Qiu, T. Meng, Q. Chen, K. Zhou, Y. Shao, G. Matlock, X. Ma, W. Wu, Y. Du and X. Wang, Mol. Pharmaceutics, 2019, 16, 1958–1970 CrossRef CAS PubMed.
- N. Govindan, K. Vairaprakasam, C. Chinnasamy, T. Sivalingam and M. K. Mohammed, Mater. Adv., 2020, 1, 3460–3465 RSC.
- O. Veiseh, B. C. Tang, K. A. Whitehead, D. G. Anderson and R. Langer, Nat. Rev. Drug Discovery, 2015, 14, 45–57 CrossRef CAS PubMed.
- M. Ikram, B. Javed, N. I. Raja and Z.-u-R. Mashwani, Int. J. Nanomed., 2021, 249–268 CrossRef PubMed.
- Y. V. Simos, K. Spyrou, M. Patila, N. Karouta, H. Stamatis, D. Gournis, E. Dounousi and D. Peschos, Asian J. Pharm. Sci., 2021, 16, 62–76 CrossRef PubMed.
- L. Zhao, L. Wang, Y. Zhang, S. Xiao, F. Bi, J. Zhao, G. Gai and J. Ding, Polymers, 2017, 9, 255 CrossRef PubMed.
- A. R. Deshmukh, H. Aloui and B. S. Kim, Chem. Eng. J., 2021, 421, 127859 CrossRef CAS.
- K. J. Cash and H. A. Clark, Trends Mol. Med., 2010, 16, 584–593 CrossRef CAS PubMed.
- L. R. Volpatti, M. A. Matranga, A. B. Cortinas, D. Delcassian, K. B. Daniel, R. Langer and D. G. Anderson, ACS Nano, 2019, 14, 488–497 CrossRef PubMed.
- G. A. Naikoo, H. Salim, I. U. Hassan, T. Awan, F. Arshad, M. Z. Pedram, W. Ahmed and A. Qurashi, Front. Chem., 2021, 9, 748957 CrossRef CAS PubMed.
- P. Díaz-Pozo, F. Canet, A. Grirrane, S. Lopez-Domenech, J. R. Herance, N. Apostolova, C. Luna-Marco, S. Rovira-Llopis, M. Marti and C. Morillas, Antioxidants, 2022, 11, 2297 CrossRef PubMed.
- K. Samrat, T. Krishna Murthy, G. Divyashri, R. Hari Krishna and M. Chandraprabha, Nanomaterials for Sustainable Development: Opportunities and Future Perspectives, Springer, 2023, pp. 235–263 Search PubMed.
- R. M. DiSanto, V. Subramanian and Z. Gu, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2015, 7, 548–564 CAS.
- Y. He, A. Al-Mureish and N. Wu, J. Diabetes Res., 2021, 2021, 1–11 Search PubMed.
- C. Yang, Y. Tan, F. Li, H. Wang, Y. Lin, F. Lu and H. Zhao, Foods, 2022, 11, 1696 CrossRef CAS PubMed.
- M. Khan, Z.-u-R. Mashwani, M. Ikram, N. I. Raja, A. H. Mohamed, G. Ren and A. A. Omar, Nanomaterials, 2022, 12, 2117 CrossRef CAS PubMed.
- A. Lopez-Pascual, A. Urrutia-Sarratea, S. Lorente-Cebrián, J. A. Martinez and P. González-Muniesa, Oxid. Med. Cell. Longev., 2019, 2019, 2695289 Search PubMed.
- R. Sharma, S. J. Borah, Bhawna, S. Kumar, A. Gupta, P. Singh, V. K. Goel, R. Kumar and V. Kumar, ACS Omega, 2022, 7(41), 36092–36107 CrossRef CAS PubMed.
- V. Natesan and S.-J. Kim, Biomol. Ther., 2023, 31, 16–26 CrossRef CAS PubMed.
- D. Marinheiro, F. Martel, B. J. Ferreira and A. L. Daniel-da-Silva, Bioengineering, 2023, 10, 40 CrossRef CAS PubMed.
- K. Subramani, S. Pathak and H. Hosseinkhani, Dig. J. Nanomater. Biostructures, 2012, 7, 85–95 Search PubMed.
- D. Dasgupta and A. Patel, Mater. Adv., 2022, 3, 8220–8228 RSC.
- H. Khosravi Ardakani, M. Gerami, M. Chashmpoosh, N. Omidifar and A. Gholami, Biochem. Res. Int., 2022, 2022, 2964705 CrossRef PubMed.
- J. Jeevanandam and M. K. Danquah, Nanofabrication for Smart Nanosensor Applications, Elsevier, 2020, pp.187–228 Search PubMed.
- K. Pathak, R. Saikia, H. Sarma, M. P. Pathak, R. J. Das, U. Gogoi, M. Z. Ahmad, A. Das and B. A. A. Wahab, J. Diabetes Metab. Disord., 2023, 1–15 Search PubMed.
- C. L. Ventola, Pharm. Ther., 2012, 40, 525 Search PubMed.
- S. Gul, S. B. Khan, I. U. Rehman, M. A. Khan and M. Khan, Front. Mater., 2019, 6, 179 CrossRef.
- H. Choudhury, M. Pandey, Y. Q. Lim, C. Y. Low, C. T. Lee, T. C. L. Marilyn, H. S. Loh, Y. P. Lim, C. F. Lee and S. K. Bhattamishra, Mater. Sci. Eng., C, 2020, 112, 110925 CrossRef CAS PubMed.
- A. Firmanda, F. Fahma, K. Syamsu, J. Cabral, D. Pletzer and M. Gustiananda, Mater. Adv., 2022, 3, 7463–7483 RSC.
- I. Pastar, O. Stojadinovic, N. C. Yin, H. Ramirez, A. G. Nusbaum, A. Sawaya, S. B. Patel, L. Khalid, R. R. Isseroff and M. Tomic-Canic, Adv. Wound Care, 2014, 3, 445–464 CrossRef PubMed.
- A. Atala, D. J. Irvine, M. Moses and S. Shaunak, MRS Bull., 2010, 35, 597–606 CrossRef CAS PubMed.
- N. K. Rajendran, S. S. D. Kumar, N. N. Houreld and H. Abrahamse, J. Drug Delivery Sci. Technol., 2018, 44, 421–430 CrossRef CAS.
- M. Pakyari, A. Farrokhi, M. K. Maharlooei and A. Ghahary, Adv. Wound Care, 2013, 2, 215–224 CrossRef PubMed.
- T. J. Koh and L. A. DiPietro, Expert Rev. Mol. Med., 2011, 13, e23 CrossRef PubMed.
- J. K. Ho and B. M. Hantash, Expert Rev. Dermatol., 2013, 8, 639–658 CrossRef CAS.
- G. Han and R. Ceilley, Adv. Theory, 2017, 34, 599–610 CrossRef PubMed.
- N. D. Evans, R. O. Oreffo, E. Healy, P. J. Thurner and Y. H. Man, J. Mech. Behav. Biomed. Mater., 2013, 28, 397–409 CrossRef PubMed.
- Q. Bai, K. Han, K. Dong, C. Zheng, Y. Zhang, Q. Long and T. Lu, Int. J. Nanomed., 2020, 15, 9717 CrossRef CAS PubMed.
- S. K. Nethi, S. Das, C. R. Patra and S. Mukherjee, Biomater. Sci., 2019, 7, 2652–2674 RSC.
- M. M. Mihai, M. B. Dima, B. Dima and A. M. Holban, Mater., 2019, 12, 2176 CrossRef CAS PubMed.
- I. Kalashnikova, S. Das and S. Seal, Nanomed., 2015, 10, 2593–2612 CrossRef CAS PubMed.
- A. Barroso, H. Mestre, A. Ascenso, S. Simões and C. Reis, Nano Select, 2020, 1, 443–460 CrossRef.
- M. Liu, X. Wei, Z. Zheng, Y. Li, M. Li, J. Lin and L. Yang, Int. J. Nanomed., 2023, 1537–1560 CrossRef PubMed.
- L. Sethuram, J. Thomas, A. Mukherjee and N. Chandrasekaran, Mater. Adv., 2021, 2, 2971–2988 RSC.
- N. Raval, A. Kumawat, D. Kalyane, K. Kalia and R. K. Tekade, Drug Discovery Today, 2020, 25, 862–878 CrossRef CAS PubMed.
- C. Liu, K. Wu, H. Gao, J. Li and X. Xu, Diabetes, Metab. Syndr. Obes.: Targets Ther., 2022, 2653–2673 CrossRef CAS PubMed.
- R. Thiruvengadam, B. Venkidasamy, R. Samynathan, R. Govindasamy, M. Thiruvengadam and J. H. Kim, Chem.-Biol. Interact., 2023, 110535 CrossRef CAS PubMed.
- M. Iafisco, A. Alogna and D. Catalucci, Nanomed., 2019, 14(18), 2391–2394 CrossRef CAS PubMed.
- A. P. Singh, A. Biswas, A. Shukla and P. Maiti, Signal Transduction Targeted Ther., 2019, 4, 33 CrossRef PubMed.
- V. M. Martín Giménez, D. E. Kassuha and W. Manucha, Ther. Adv. Cardiovasc. Dis., 2017, 11, 133–142 CrossRef PubMed.
- S. Katsuki, T. Matoba, J.-I. Koga, K. Nakano and K. Egashira, Front. Cardiovasc. Med., 2017, 4, 87 CrossRef PubMed.
- N. Moradifar, A. A. Kiani, A. Veiskaramian and K. Karami, Curr. Cardiol. Rev., 2022, 18, 89–100 Search PubMed.
- T. Alam, S. Khan, B. Gaba, M. F. Haider, S. Baboota and J. Ali, Drug Delivery, 2017, 24, 358–369 CrossRef CAS PubMed.
- M. Koziolek, M. Grimm, D. Becker, V. Iordanov, H. Zou, J. Shimizu, C. Wanke, G. Garbacz and W. Weitschies, J. Pharm. Sci., 2015, 104, 2855–2863 CrossRef CAS PubMed.
- C. Fan, J. Joshi, F. Li, B. Xu, M. Khan, J. Yang and W. Zhu, Front. Bioeng. Biotechnol., 2020, 8, 687 CrossRef PubMed.
- G. Kania, M. Sternak, A. Jasztal, S. Chlopicki, A. Błażejczyk, A. Nasulewicz-Goldeman, J. Wietrzyk, K. Jasiński, T. Skórka and S. Zapotoczny, Nanomed. Nanotechnol. Biol. Med., 2018, 14, 131–140 CrossRef CAS PubMed.
- X. Li, Z. Wei, H. Lv, L. Wu, Y. Cui, H. Yao, J. Li, H. Zhang, B. Yang and J. Jiang, Int. J. Nanomed., 2019, 14, 573 CrossRef CAS PubMed.
- S. Chatterjee, G. Biondi-Zoccai, A. Abbate, F. D’Ascenzo, D. Castagno, B. Van Tassell, D. Mukherjee and E. Lichstein, BMJ, 2013, 346, f55 CrossRef PubMed.
- G. Aylward, Eye, 2005, 19, 1115–1118 CrossRef CAS PubMed.
- Y. Li, W. Zhang, R. Zhao and X. Zhang, Bioact. Mater., 2022, 15, 392–408 CrossRef CAS PubMed.
- V. K. Rai, N. Mishra, A. K. Agrawal, S. Jain and N. P. Yadav, Drug Delivery, 2016, 23, 2371–2390 CrossRef CAS PubMed.
- C. Y. Wong, H. Al-Salami and C. R. Dass, Int. J. Pharm., 2018, 549, 201–217 CrossRef CAS PubMed.
- P. Sona, Dig. J. Nanomater. Biostructures, 2010, 5, p411–418 Search PubMed.
- R. Zhao, Z. Lu, J. Yang, L. Zhang, Y. Li and X. Zhang, Front. Bioeng. Biotechnol., 2020, 8, 880 CrossRef PubMed.
- B. L. Zaric, M. Obradovic, E. Sudar-Milovanovic, J. Nedeljkovic, V. Lazic and E. R. Isenovic, Curr. Pharm. Des., 2019, 25, 166–173 CrossRef CAS PubMed.
- M. Yang, S. K. Lai, Y. Y. Wang, W. Zhong, C. Happe, M. Zhang, J. Fu and J. Hanes, Angew. Chem., Int. Ed., 2011, 123, 2645–2648 CrossRef.
- L. M. Ensign, C. Schneider, J. S. Suk, R. Cone and J. Hanes, Adv. Mater., 2012, 24, 3887–3894 CrossRef CAS PubMed.
- J. Mašek, D. Lubasova, R. Lukáč, P. Turanek-Knotigova, P. Kulich, J. Plockova, E. Mašková, L. Prochazka, Š. Koudelka and N. Sasithorn, J. Controlled Release, 2017, 249, 183–195 CrossRef PubMed.
- U. Armbrecht, J. Jensen, S. Eden and R. Stockbrügger, Scand. J. Gastroenterol., 1986, 21, 669–677 CrossRef CAS PubMed.
- S. Davis, J. Hardy, M. Taylor, D. Whalley and C. Wilson, Int. J. Pharm., 1984, 21, 331–340 CrossRef.
- C. Wilson, G. Parr, J. Kennerlev, M. Taylor, S. Davis, J. Hardy and J. Rees, Int. J. Pharm., 1984, 18, 1–8 CrossRef CAS.
- P. Langguth, V. Bohner, J. Heizmann, H. Merkle, S. Wolffram, G. Amidon and S. Yamashita, J. Controlled Release, 1997, 46, 39–57 CrossRef CAS.
- Y. Aoki, M. Morishita and K. Takayama, Int. J. Pharm., 2005, 297, 98–109 CrossRef CAS PubMed.
- F. P. Guengerich, AAPS J., 2006, 8, E101–E111 CrossRef CAS PubMed.
- S. Kumar, R. Sharma, Bhawna, A. Gupta, P. Singh, S. Kalia, P. Thakur and V. Kumar, ACS Omega, 2022, 7(26), 22073–22088 CrossRef CAS PubMed.
- V. Kumar, A. K. Choudhary, P. Kumar and S. J. N. Sharma, Nanotechnol., 2019, 9, 64–78 CAS.
- M. Anwar, F. Muhammad and B. Akhtar, J. Drug Delivery Sci. Technol., 2021, 64, 102638 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |