Recent advances in electrospinning supramolecular systems
Hailong
Che
 *a and
Jinying
Yuan
*a and
Jinying
Yuan
 b
b
aDepartment of Chemical Engineering, School of Environmental and Chemical Engineering, Shanghai University, 200444, Shanghai, China. E-mail: hche@shu.edu.cn
bKey Lab of Organic Optoelectronics and Molecular Engineering of Ministry of Education, Department of Chemistry, Tsinghua University, 100084, Beijing, China
First published on 18th November 2021
Abstract
Electrospinning is one of the simple, versatile, and convenient techniques for producing nanofibers that have found numerous applications in the fields of biomedical engineering, surface materials, and catalysis. Despite the great achievements, the electrospinning compounds are still limited to the utilization of polymers with high molar mass which are regarded as an indispensable element for the production of nanofibers. It is found that electrospinning chemicals based on supramolecular systems can avoid the use of high molecular weight polymers, and it is emerging as a powerful route to generate fibers in the nano-scale size. The presence of strong intermolecular interactions that function as chain entanglements allows for the formation of nanofibers during the process of electrospinning. This article provides recent impressive developments concerning nanofiber preparation made by the combination of electrospinning and supramolecular chemistry, which enables easy access to tailor-made nanofibers. Electrospinning supramolecular systems consisting of phospholipids, surfactants, crown ether derivatives as well as cyclodextrins will be highlighted in this review. Moreover, we will pay particular attention to the functionalities of electrospun nanofibers obtained from supramolecular systems.
1. Introduction
Nanostructured materials have attracted considerable research attention because of their unique and interesting physical, chemical and biological properties. Among these nanocomponents with different structures and dimensionalities, one dimensional (1D) nanomaterials such as wires, rods, belts, and tubes have aroused much more research interest.1–3 As a cost-effective, powerful and convenient method for generating continuous 1D nanofibers, electrospinning has been intensively investigated to prepare nanofibers from polymer solutions and melts since their discovery in the 1970s.4–10 Owing to their high porosity and large surface-to-volume ratio, electrospun nanofibers are finding many applications in plenty of areas including tissue engineering, drug delivery, sensors, energy, filters, etc.11–16The primary setup for electrospinning is made up of three major components: a high-voltage power supply, a collector (a grounded conductor), and a spinneret (a metallic needle). Electrospinning occurs when a charged polymer solution or melt is placed in a region of high electric field. Specifically, the charged solution passes slowly through a needle with the high voltage increase, and the polymer droplet generates a jet which follows a chaotic, whip-like trajectory towards a grounded collection plate.17 In general, to obtain unbroken nanofibers, several factors should be taken into consideration, including voltage, polymer concentrations, environment temperature, flow rate, receive distance, etc.18–21 The chain overlap and entanglements are vital factors in the production of nanofibers and high molar mass polymer solutions or melts are indispensable in the process of electrospinning. Sufficient viscosities are indispensable to sustain electrostatic forces of the charged electrospun jet.22 At low polymer concentrations, undesirable spherical droplets of material instead of fibers are acquired owing to the high forces experienced by the jet. When the concentrations are above the critical entanglement limit (Ce) for the polymer, the polymer chains and entanglements are formed and consequently stretched and orientated. At the same time, the solvent rapidly evaporates, yielding continuous nanofibers.23 Long and his co-workers correlated the morphology and diameter of the electrospun nanofibers with the degree of chain entanglements and chain overlap in solution.24 Wnek et al. brought forward a semi-empirical model to predict the fiber morphology with respect to the polymer concentration, the weight average molar mass (Mw), and the entanglement molar mass (Me).23 In this regard, high molecular weight polymer seems to be one of the necessary factors during electrospinning.
However, nanofibers can also be obtained from low molecular weight polymers or small molecules via the electrospinning technique. Supramolecular chemistry is a new growing branch in the field of chemistry, and the structure and function of the supramolecular entities can be converted into highly complex chemical systems.25–29 Compared with molecular chemistry based on the covalent bonds, intermolecular bonds in the supramolecular system are non-covalent interactions consisting of host–guest interactions, hydrogen bonding, π–π interactions, and electrostatic interactions, which bestow on them excellent and interesting properties.30–35 A broad range of components can spontaneously self-assemble into a specific structures with defined organization and features such as layers, vesicles, micelles, mesomorphic phases, etc.36–39
The special properties of supramolecular assemblies raise the question of whether it is possible to acquire nanofibers from supramolecular systems via electrospinning. It seems that electrospinning supramolecular systems is feasible since enough assemblies of supramolecular systems can act similarly to the high molecule weight of polymers when the concentrations of the molecular solution are high enough. As a result, it enables access to the formation of nanofibers despite the absence of high molecular weight polymers. The idea of electrospinning nanofibers based on the supramolecular system can combine the dynamic nature of supramolecular chemistry and versatile feature of electrospinning, which can endow the produced nanofibers with specific characters, expanding the application of electrospinning nanofibers. In this aspect, the study of electrospinning supramolecular systems will undoubtedly be a very fascinating research direction, and it will provide an innovative strategy to develop nanostructured scaffolds or membranes with more intriguing structures and functions than those produced from macromolecules.
Although there is a vast array of excellent reviews on the topic of electrospinning, a comprehensive review regarding electrospun nanofibers based on supramolecular systems has not been reported. In this review, we focus on recent progress in nanofibers from electrospinning supramolecular compounds. We describe the supramolecular systems such as phospholipids, gemini surfactants, supramolecular polymers, crown ethers, and cyclodextrins for electrospinning and discuss the electrospun nanofiber formation process of these systems (Fig. 1). Finally, we will give a further discussion on the functional supramolecular-based nanofibers and highlight their potential applications and challenges.
2. Electrospun nanofibers based on supramolecular systems
2.1 Electrospun nanofibers from a phospholipid and a gemini surfactant
Phospholipids can assemble into a bilayer structure, which takes part in the construction of cell membranes due to their amphiphilic chemical properties. As a natural mixture of phospholipids and neutral lipids, lecithin has the capability to form cylindrical or wormlike reverse micelles in nonaqueous solutions.40 However, the micellar structures of lecithin assemblies could undergo a reversible transition from spherical to cylindrical when increasing the lecithin concentrations. Moreover, the cylindrical micelles overlapped and entangled in a similar way to polymers at higher concentrations.41 It is the hydrogen bonds between neighboring phospholipids that play an important part in bridging the phosphate head groups in water and other polar molecules solutions.42Long and his co-workers reported that self-assembly of small phospholipids allows for the formation of nanofibers using the electrospinning technique.43 The lecithin amphiphiles assembled into spherical micelles at the critical micelle concentration (CMC), and a significant evolution from spheric micelles to worm-like micelles was observed when the lecithin concentration is higher than entanglement concentration (Ce). At low phospholipid concentrations (C < Ce), droplets other than nanofibers were observed due to the absence of chain entanglements in the supramolecular structure. Fewer fibers with substantial beaded structures were obtained with the concentration increasing to entanglement concentration. As the concentration C > Ce, the electrospinning jet could be stabilized by enough entanglements between the wormlike micelles that prevent the formation of its breakup. As a result, continuous fibers were formed with diameters on the order of 1 to 5 micrometers. Without the use of high molar mass polymers, the successfully electrospun phospholipid fibers offer one new convenient method for the direct preparation of biologically based, high-surface-area nanofibers webs. It will certainly endow the exceptional promise of phospholipid with a great diversity of biomedical applications.
The initial discovery of electrospun phospholipid nanofibers was limited to organic solvents. Long's group studied the possibility of electrospinning an ammonium gemini surfactant, N,N′-dodecyl-N,N,N′,N′-tetramethyl-N,N′-ethanediyldiammonium dibromide (12-2-12) (Fig. 2).44 Because of their low CMC and interesting supramolecular assemblies in solution, gemini surfactants are regarded as an intriguing class of amphiphiles.45,46 The quaternary ammonium gemini surfactants assemblies possess the spacer group which may influence the microstructure of ammonium gemini in different solutions. Increasing concentration in water, surfactant assemblies could change from spherical micelles to threadlike micelles, which is a viscoelastic, entangled, and highly branched threadlike network.47,48 Both in water and water/methanol, 12-2-12 supramolecular microstructure at high concentration could be formed to provide the possibility for electrospinning gemini surfactant fibers. The high entanglements of the molecules give rise to the formation of polymer-like solution which can help the formation of fibers in the absence of polymers.
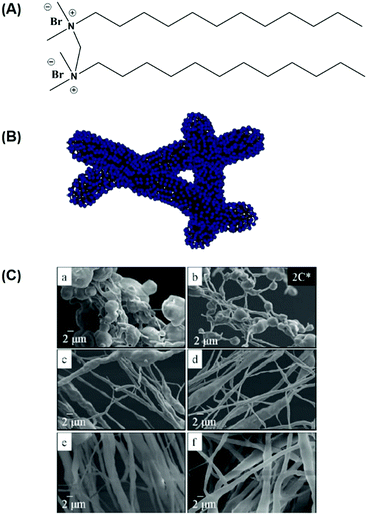 | ||
| Fig. 2 (A) N,N′-didodecyl-N,N,N′,N′-tetramethyl-N,N′-ethanediyl-diammonium dibromide (12-2-12) gemini surfactant. (B) Cartoon of gemini surfactant entangled threadlike micelle in a polar solvent. (C) FESEM of electrospun fibers of 12-2-12, electrospun from (a) 20, (b) 22, (c) 28, (d) 30, (e) 42, and (f) 44 wt% 12-2-12 gemini surfactant solutions in water/methanol (C* = 11 wt%). Reproduced from ref. 44 with permission from the American Chemical Society, copyright 2009.44 | ||
Except for solution electrospinning, melt electrospinning is another technique used to produce continuous fibers in the submicron-to-nanometer diameter range. Importantly, applying a polymer melt to generate nanofibers can avoid the use of organic solvents, which is friendly to the organism when it comes to the biomedical applications such as drug delivery, cancer therapy, and tissue engineering.49,50 Based on the work of electrospinning nanofibers from lecithin amphiphiles, Long et al successfully obtained nanofibers from a low molecular weight amphiphilic phospholipid by making use of melt electrospinning.51 A well-defined phospholipid named i-palmitoyl-2-oleoyl-sn-glycerol-3-phosphoethanolamine (POPE) (Fig. 3) could form long-range aggregates in the melt state. It was flowed into a stable electrospinning jet when the melt was electrospun at 200 °C and amphiphilic fibers were deposited. This is the first example of melt electrospinning of low molecular weight amphiphiles, and this new finding provides a unique tool for preparing biologically compatible phospholipid membranes. More recently, the Schmidt Group systematically studied the structure–property relationships concerning melt electrospinning of small molecules.52 They found that 1,3,5-benzene- and 1,3,5-cyclohexanetrisamides are readily spun into nanofibers due to the existence of three strong hydrogen bonds while mainly electrospraying occurred when bisamides based on benzene, naphthyl, and cyclohexane were used, which demonstrates the significance of supermolecular interactions during the melt electrospinning.
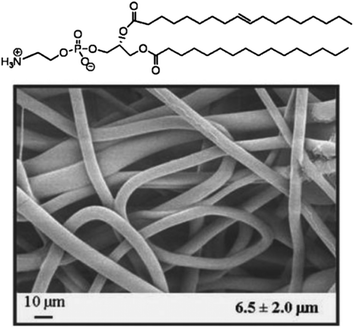 | ||
| Fig. 3 Chemical structure and melt electrospun nanofibers of the POPE phospholipid. Reproduced from ref. 51 with permission from John Wiley & Sons Inc, copyright 2008.51 | ||
2.2 Electrospun nanofibers from crown ethers and their derivatives
The supramolecular systems based on host–guest interactions are one of the research focus in the field of supramolecular chemistry.53–58 Among them, crown ether and its derivatives have been one kind of the most widely used hosts in supramolecular chemistry since Pedersen firstly discovered crown ether compounds in 1967. To date, crown ether supramolecular systems with host–guest interaction are extensively used in many fields such as materials science, life science, and information science.59–62A linear supermolecular polymer can be obtained from a heteroditopic A-B monomer 1, which is consisted of benzo-21-crown-7(B21C7) and its guest dialkylammonium salt (Fig. 4A).63 Moreover, B21C7 can bind dialkylammonium salts and form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 threaded structure with secondary dialkylammonium salts.64 On this basis, nanofibers were successfully acquired via electrospinning from this supramolecular polymer solution. Two factors are facilitating the formation of nanofibers in the course of electrospinning: (1) the flexible long alkyl chain promotes the entanglement of the self-assembled supramolecular polymer and (2) the good interaction between B21C7 and dialkylammonium salt moieties in chloroformcan give rise to the formation of high viscosity of the supramolecular polymer solution contributing to the generation of a continuous nanofiber when an electric filed was applied to the supramolecular polymer solution. Representative SEM and TEM images of the electrospun supramolecular polymer nanofibers are shown in Fig. 4B.65 Droplets other than fibers were acquired when electrospinning a solution of monomer 1 from acetonitrile at an even higher molar concentration (500 mM) which demonstrated the significance of the solvent because both the binding strength between the monomers and the solution viscosity depend on the type of solvent. To explore the surface morphology of the microstructures, atomic force microscopy (AFM) was used to observe the cross-section profile of the supramolecular polymer nanofibers which are shown in Fig. 5C. The successful electrospinning of supramolecular polymers based on low molecular weight crown ethers opens up a variety of new interesting feasibilities for the development of nanofibers based on multifunctional supramolecular polymer.
1 threaded structure with secondary dialkylammonium salts.64 On this basis, nanofibers were successfully acquired via electrospinning from this supramolecular polymer solution. Two factors are facilitating the formation of nanofibers in the course of electrospinning: (1) the flexible long alkyl chain promotes the entanglement of the self-assembled supramolecular polymer and (2) the good interaction between B21C7 and dialkylammonium salt moieties in chloroformcan give rise to the formation of high viscosity of the supramolecular polymer solution contributing to the generation of a continuous nanofiber when an electric filed was applied to the supramolecular polymer solution. Representative SEM and TEM images of the electrospun supramolecular polymer nanofibers are shown in Fig. 4B.65 Droplets other than fibers were acquired when electrospinning a solution of monomer 1 from acetonitrile at an even higher molar concentration (500 mM) which demonstrated the significance of the solvent because both the binding strength between the monomers and the solution viscosity depend on the type of solvent. To explore the surface morphology of the microstructures, atomic force microscopy (AFM) was used to observe the cross-section profile of the supramolecular polymer nanofibers which are shown in Fig. 5C. The successful electrospinning of supramolecular polymers based on low molecular weight crown ethers opens up a variety of new interesting feasibilities for the development of nanofibers based on multifunctional supramolecular polymer.
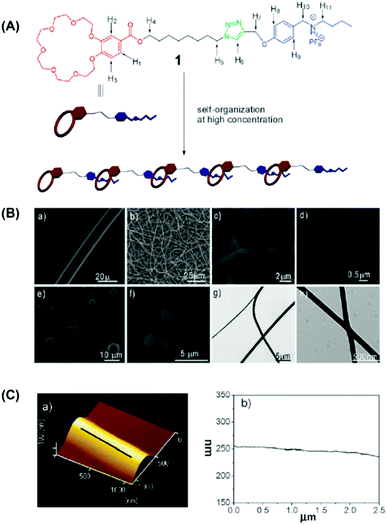 | ||
| Fig. 4 (A) Cartoon representation of the formation of a linear supramolecular polymer from the self-organization of monomer 1. (B) SEM images of the electrospun benzo-21-crown-7 (B21C7) and its complementary guest dialkylammonium salt. (C) (a) AFM image and (b) fiber axis cross-section profile of the electrospun supramolecular nanofibers. Reproduced from ref. 65 with permission from the Royal Society of Chemistry, copyright 2009.65 | ||
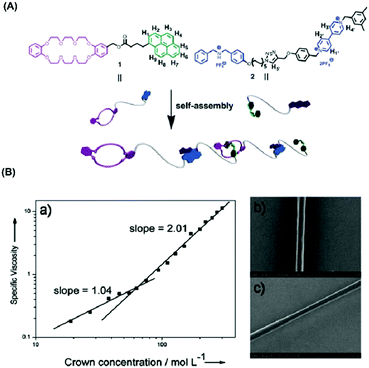 | ||
| Fig. 5 (A) Schematic representation of the formation of a supramolecular polymer via self-assembly of monomers 1 and 2. (B) (a) Log–log plot of specific viscosity of equimolar solutions of monomers 1 and 2 versus the monomer concentration at 298 K; (b and c) scanning electron micrography of (gold-coated) fibers obtained from a high concentration solution of 1/2 in CH3CN. Reproduced from ref. 66 with permission from the Royal Society of Chemistry, copyright 2013.66 | ||
Based on this report, this group discovered a new kind of supramolecular polymer composed of two heteroditopic monomers which are constructed by integrating crown ether and charge-transfer molecular recognition, and they can be used to prepare electrospun nanofibers via electrospinning.66 In this system, an AB-type heteroditopic monomer (A: dibenzo[24]crown-8 (DB24C8), B: dimethylammonium salt (DBA)) can form a linear supramolecular polymer via the host–guest interactions between DB24C8 and DBA.67 As shown in Fig. 5A, in the low concentration range, the main components in the solution are cyclic oligomers or monomers; while high molecular weight polymers emerged as the concentration increased. This type of supramolecular polymers has the potential to electrospun nanofibers. Notably, the surfaces of nanofibers were quite smooth, demonstrating an excellent electrospinning processability of this unique supramolecular polymer based on host-guest/charge transfer.
Recently, Li and co-workers constructed a kind of multi-stimuli responsive supramolecular nanofiber via the solution electrospinning of benzo-21-crown-7 (B21C7) host units and secondary ammonium salt guest moieties (Fig. 6).68 The B12C7 host units containing poly(ε-caprolactone) (PCL) chain can form a supramolecular polymer with secondary ammonium salts (guest units) via host–guest interactions. They found that the supramolecular polymer showed cation-, pH-, anion-, and thermoresponsive behavior which can give rise to self-assembly and disassembly of the system. Interestingly, this stimuli-responsive supramolecular polymer can be converted into nanofibers utilizing solution electrospinning, and the prepared nanofibers demonstrated improved degradation behaviors upon stimulation of K+, Cl−, Et3N, or heating. These electrospun supramolecular nanofibers show potential in a variety of applications in the field of smart drug delivery and tissue engineering.
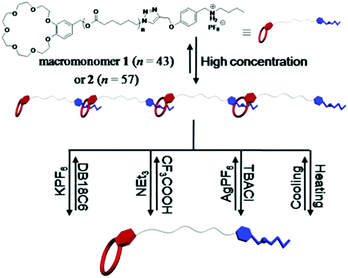 | ||
| Fig. 6 Representation of the formation of supramolecular polymer from the self-organization of heteroditopic macromonomers based on the benzo-21-crown-7/secondary ammonium salt recognition motif and their multi-responsive processes. Reproduced from ref. 68 with permission from the Royal Society of Chemistry, copyright 2016.68 | ||
2.3 Electrospun nanofibers from cyclodextrins and their derivatives
Cyclodextrins (CDs) are cyclic oligosaccharides including six α-cyclodextrin, seven β-cyclodextrin, eight γ-cyclodextrin, or more glucopyranose units linked by α-(1,4) bonds which were first discovered in 1891. CD has a hydrophilic outsider and a hydrophobic cavity described as a ‘micro heterogeneous environment’. As is well-known, cyclodextrins are capable of self-assembling and forming aggregates efficiently acting as chain entanglements in the presence of intermolecular hydrogen bonding in their solutions. They can self-assemble into intriguing supramolecular structures by forming host–guest inclusion complexes with a diverse set of small molecules and macromolecules. Due to their interesting properties, CDs can be used in many fields such as pharmaceuticals, foods, sensor devices, molecular machines, or other systems.69–72 Thus, cyclodextrins and their derivatives are well candidates to acquire electrospun nanofibers from CD solutions.73–75 Moreover, chain entanglements based on hydrogen bonding between the CD molecules facilitate the formation of the stable jet to stretch fully and result in uniform nanofibers. Some reviews have already discussed the electrospun nanofibers based on CD and their derivatives;76–78 here we will highlight the representative examples of electrospun CD nanofibers.Uyar and his co-workers first obtained CD nanofibers via electrospinning without the utilization of a carrier of the polymer matrix (Fig. 7).79 In the process of electrospinning, with the increase of the concentration and/or viscosity of the CD solution, the electrospun structures witnessed the transition from beads to beaded nanofibers and finally bead-free nanofibers.80 This interesting phenomenon can also be found in the electrospinning of MβCD in water. As for 100% (w/v) MβCD water solution, elongated beaded structures instead of continuous nanofibers were produced thanks to the insufficient amount of CD aggregates which enable them to form stable jets during the electrospinning process. Very fine fibers with some undesirable beads were obtained at 120% (w/v) MβCD. At 140% and 160% (w/v) MβCD, bead-free nanofibers with diameters ranging from 20 nm to 100 nm were formed because the hydrogen bonds between the CD molecules can be destroyed in the presence of urea which can even lead to notable depression of the self-association of the CD molecules.81 Fibers were not obtained from the solution 140% (w/v) MβCD containing urea because of the destruction of the electrospinning jet. This result also suggests the importance of the presence of adequate CD aggregates based on hydrogen bonding in the electrospinning of MβCD into nanofibers. The surfaces of fibers obtained from water and DMF solutions are further tested by AFM. The fibers obtained from water are rougher than those from DMF due to the use of volatile solvents.
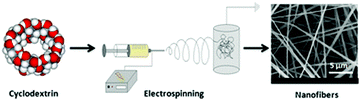 | ||
| Fig. 7 Electrospun nanofibers from cyclodextrin. Reproduced from ref. 79 with permission from the Royal Society of Chemistry, copyright 2010.79 | ||
Despite the electrospinning of CD solutions, MβCD nanofibers have low mechanical property in contrast to electrospun polymer nanofibers because of the existence of small molecules. Additionally, when MβCD nanofibers are heated up to 175 °C, which is very close to the melting point of MβCD (180–185 °C), the nanofibers will be out of shape and at 200 °C, the MβCD nanofibers were melted and their nanofiber structures could completely vanish. Recently, to address this issue, this group developed the electrospun nanofibers from hydroxypropyl-β-cyclodextrin (HPβCD) and its inclusion complexes with triclosan (HPβCD/triclosan-IC) without the use of polymeric carriers (Fig. 8).80 Two HPβCD/triclosan-IC solutions containing different stoichiometries were prepared to explore the possibility of electrospinning. As expected, bead-free nanofibers were obtained from HPβCD/triclosan with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The diameter of bead-free nanofibers acquired from HPβCD/triclosan-IC (1
1 molar ratio. The diameter of bead-free nanofibers acquired from HPβCD/triclosan-IC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 molar ratio) is smaller than those having a 1
1.3 molar ratio) is smaller than those having a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio because of the lower solution viscosity of the former. However, it needs to be pointed out that the mechanical integrity of HPβCD and HPβCD/triclosan-IC electrospun nanofibers was predicted to be weak compared to electrospun polymer nanofibers.
1 molar ratio because of the lower solution viscosity of the former. However, it needs to be pointed out that the mechanical integrity of HPβCD and HPβCD/triclosan-IC electrospun nanofibers was predicted to be weak compared to electrospun polymer nanofibers.
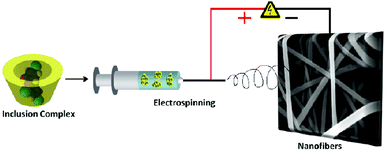 | ||
| Fig. 8 Representation electrospinning process from cyclodextrin inclusion complexes. Reproduced from ref. 80 with permission from the American Chemical Society, copyright 2011.80 | ||
To further investigate the consequence of solvent on the formation of CD electrospun nanofibers, Uyar and his co-workers further studied the polymer-free electrospun nanofibers from three different CD derivatives, hydroxypropyl-β-cyclodextrin (HPβCD), hydroxypropyl-γ-cyclodextrin (HPγCD) and methyl-β-cyclodextrin (MβCD) in pure water, dimethylformamide (DMF) and dimethylacetamide (DMAc), respectivelly.75 The results suggest that the solvent type and the solution conductivity are non-ignorable factors to the electrospinning CD and their derivatives nanofibers.
Recently, the relationships of the physical–chemical properties of HPCD-based formulations of electrospun nanofibers were explored by Diao and his co-workers.82 The shear rate dependence of apparent viscosity for HPCD solutions demonstrated that all HPCD solutions at different concentrations showed Newtonian behavior over the range of shear rates. Simultaneously, the zero shear rate viscosity (η0) increased from 202.70 to 1737.04 cP as the concentration increased from 51.4 to 61.4 wt%. When the HPCD concentration increased, the hydrogen binding between CD became strong enough to induce the formation of HPCD self-assembled aggregates. As a result, enough hydrogen bonding interactions function as the driving force for the formation of supramolecular system, which causes the generation of HPCD electrospun nanofibers. The authors also explored the excellent adsorption capacity neutral red (NR) on HPCD nanofibrous membranes. The adsorption process can be interpreted as the pseudo-second-order kinetic model.
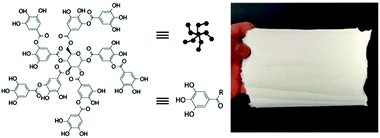
The guest–host interaction of adamantane (Ad) and β-cyclodextrin (CD) make them associate strongly with each other because of the hydrophobic interactions. Burdick and co-workers synthesized two hyaluronic acids (HA) derivatives, one modified with Ad (Ad-HA) and the other with CD (CD-HA)83 and prepared CD/Ad electrospun nanofibers (Fig. 9).84 Using Ad-HA as a supramolecular glue, it can bond opposing layers of CD-modified nanofibers which is ascribed to macroscale assembly. The multi-layered supramolecular electrospun nanofibers provide a unique tool for the preparation of self-assembling nanofibers for cell culture or tissue engineering. Combined with the high biocompatibility of CD, these studies will expand the applications of this type of membrane such as organic pollutants, tissue engineering and drug delivery.85,86
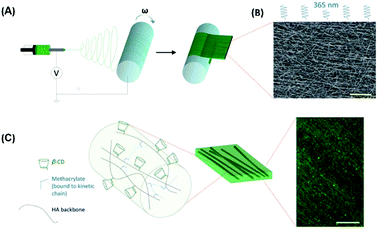 | ||
| Fig. 9 (A) Electrospinning was used to deposit CD-MeHA as aligned nanofibers on a rotating mandrel. (B) After crosslinking under UV light, the fibers were imaged by SEM to assess alignment (scale bar: 20 mm). (C) Within the fiber, HA polymers (black) modified with b-CD (green) are crosslinked through methacrylate groups (blue) which polymerize to form kinetic chains (dashed blue). (scale bar: 100 mm). Reproduced from ref. 84 with permission from the Royal Society of Chemistry, copyright 2014.84 | ||
Recently, Schmidt and his co-workers obtained nanofibers by combining the technique of electrospinning and molecular self-assembly (Fig. 10).87 1,3,5-Cyclohexane and 1,3,5-benzenetrisamides can self-aggregate into supramolecular structures such as fibers and networks because of the strong hydrogen bonds among three amide groups.88–90 In this research, the general electrospinnability of one 1,3,5-cyclohexane- (1) and two types of 1,3,5,-benzenetri-amides (2 and 3) were studied. Electrospinning a derivative of cyclohexane-cis, cis-1, 3, 5-tricarboxylic acid, was not successful at 200 °C due to the high viscosity of this compound which prevent the elongation and deformation of the droplet at the tip. However, because of the lower viscosity of the Nc phase, electrospun nanofibers were formed successfully at 300 °C and also from the optical isotropic melt at 330 °C. As for compound 2, having a broad isotropic phase of around 170 K which is most suit for melt electrospinning even at 310 °C only spheres are observed. Based on 1, 3, 5-triaminobenzene, compound 3 showed only a melting point at 183 °C other than any LC mesophases. It suggested that during fiber formation from the optical isotropic melt, the existence of a mesophase at lower temperatures is not an indispensable factor.
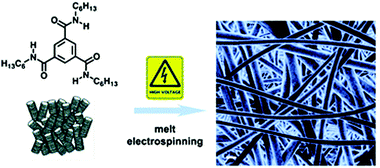 | ||
| Fig. 10 Representative of melt electrospinning 1,3,5-cyclohexane- and 1,3,5-benzenetrisamides into nanofibers. Reproduced from ref. 87 with permission from the Royal Society of Chemistry, copyright 2012.87 | ||
2.4 Other electrospun supramolecular nanofibers
Except for the above-mentioned electrospun nanofibers from small molecules, other kinds of electrospun nanofibers can also be acquired in the presence of supramolecular interactions, such as peptides, liquid-crystalline (LC) dyes, polyphenol, etc. For example, the Stupp group reported the successful formation of peptide-based electrospun nanofibers without any carrier polymer because of the inherent amphiphilic nature of peptides which can self-assemble into functional supramolecular polymers (Fig. 11).91 They found that the electrospinning can occur even the peptide is at very low concentrations (<4 wt%). More importantly, by optimizing the viscosity, conductivity, and surface tension of the solution, a peptide with low molecular weights (<1 kDa) could be electrospun into nanofibers. These peptide-based nanofibers show promise as bioactive interfacial materials for implantable devices and tissue regenerative tissues.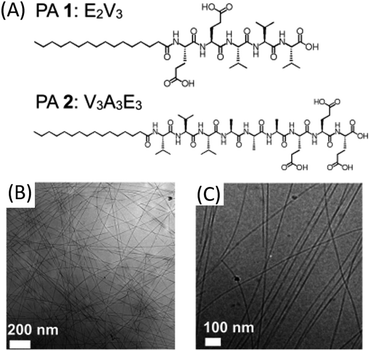 | ||
| Fig. 11 (A) Molecular structure of peptide amphiphiles 1 and 2. (B) Cryo-TEM image of PA 1 illustrating a twisted ribbon morphology of nanostructures. (C) Cryo-TEM image of PA 2 illustrating fibrous morphology of nanostructures. Reproduced from ref. 91 with permission from the American Chemical Society, copyright 2014.91 | ||
Recently, Davis and co-workers electrospun low molecular weight self-assembling supramolecular polyurethanes (1–4) into fibers and investigated the effect of hydrogen bonding strength of the end-group on the production of nanofibers by electrospinning supermolecular systems.92 In solutions, these polyurethanes can self-assemble into supramolecular polymers by hydrogen bonding. The electrospun nanofibers can be obtained from polyurethanes 3 and 4 using THF as the solvent while in the cases of polyurethanes 1 and 2, fiber formation was not observed at similar concentrations. The results clearly showed hydrogen bonding plays a vital role in fiber production and these studies help to predict the value for the equilibrium constant Ka which can be used to optimize the process of electrospinning. The researchers suggested that small but significant increases in the effective degree of polymerization in the concentrated solutions resulted from the increasing binding constant for the end-groups. In all factors which influence the electrospinning, the monomer concentrations and the value of the association equilibrium constant Ka may be the most crucial.
Dankers et al. have realized electrospun bioactive supramolecular polymer based on quadruple-hydrogen-bonding ureido-pyrimidinone (UPy) moieties due to their perfect bioactive properties such as low-temperature processability, favorable degradation, and biocompatible behavior.93 Enough (association constant, Kass = 106–107 J mol−1) non-covalent quadruple-hydrogen-bonding among 2-ureido-4[1H]-pyrimidinone (UPy) moieties makes it possible to bridge the gap between covalent functionalization and non-covalent supramolecular polymers with bioactive species.94–96 In this research, biofunctional UPy-modified polycaprolactone (PCLdiUPy) by reaction of hydroxy telechelic low-molecular-weight polycaprolactone (PCL; Mn = 2100 g mol−1) with the UPy-isocyanate-synthon, 2(6-isocyThePCLdiUPy anatohexylamino-carbonyl anatohexylaminocarbonyl-amino)-6-methyl-4[1H]-pyrimidinone, is selected as the supramolecular polymer monomer. The PCLdiUPy electrospun nanofiber membrane could be easily obtained because of the reversible nature of the hydrogen bonds. Due to the existence of strong, but reversible binding of UPy-based polymers and oligopeptides, the UPypeptide dissolved slowly in water. The good reversibility of the hydrogen bonds provides a modular strategy to controlling the cellular behavior and activity both in vitro and in vivo. The authors have also carried out in vivo experiments to test the important influence of oligopeptides on the signaling of cells.
More recently, Schlatter and coworkers demonstrated that tannic acid in water/ethanol mixture or pure water can also be electrospun to nanofibers without using a carrier polymer (Fig. 12).97 A supramolecular interconnected network of tannic acid aggregates was observed when the concentration of tannic acid was above the critical concentration, which is strong enough to the occurrence of electrospinning to form continuous and regular nanofibers. Moreover, they showed that the resulting nanofiber membranes have high mechanical and flexible properties. Another interesting feature of the tannic acid nanofibers is that they can be cross-linked in water upon treatment with sodium periodate or FeIII by the oxidative reaction. Compared with other electrospun nanofibers from small molecules, the cross-linked tannic acid nanofibers represent many advantages such as biocompatible, easy preparation, and nontoxic solvents use. Given the chelation capacity of tannic acid with a diverse set of metals, this type of nanofibers is envisaged for applications in the field of water purification.
 | ||
| Fig. 12 Tannic acid molecule structures and a picture of electrospun tannic acid nanofiber web. Reproduced from ref. 97 with permission from the Royal Society of Chemistry, copyright 2018.97 | ||
Diketopyrrolopyrrole (DPP) dyes are finding increasing use in red colour pigments and organic electronic devices. In an attempt to shape and orient the DPP self-assembled materials in a specific direction, Hecht and coworkers recently reported the successful electrospinning liquid-crystalline DPP, which allows for the production of micrometric fibers with an anisotropic DPP alignment.98 They noticed that the hydrogen bonding and π–π interactions among DPP in solution enable access to a supermolecular polymer, which appears to be crucial for electrospinning.
3. Functional electrospun supramolecular nanofibers
Considering the dynamic properties of supramolecular chemistry, the introduction of supramolecular interactions into electrospun nanofibers emerges as a powerful route to developing a wider variety of functional materials. However, in the pursuit of electrospun supramolecular nanofibers, how to impart functionalities to the system remains a great challenge. In supramolecular systems, their dynamic and reversible properties such as light, pH, carbon dioxide (CO2), and voltage responsiveness have been widely studied.99–102 Using these responsive supramolecular systems to develop polymer-free membranes via electrospinning would make it possible to endow supramolecular-based electrospun nanofibers with a variety of functions.It has been found that light-responsive azobenzene is capable of forming ICs with CDs in its trans state, while no binding of CD with its cis form is seen.104 Such a supramolecular system has been widely investigated due to its light responsive character.105–107 Chen's group reported the first electrospun UV-responsive supramolecular nanofibers from ICs of HPβCD with 4-aminoazobenzene (AAB) and explored the UV response of ICs before, during, and post electrospinning (Fig. 13).103 The UV light-induced trans–cis isomerization of azobenzene resulted in a significant change in the AAB molecular geometry and subsequently caused the dissociation from the ICs. During the process of electrospinning, UV irradiation light gave rise to interruption of the ICs and consequently generated inhomogeneous diameter distributions. Interestingly, post-spinning UV irradiation triggered controllable topography and adhesion force regulation of the electrospun membrane surfaces. This interesting finding regarding supramolecular nanofibers from a cyclodextrin–azobenzene inclusion complex may enrich their great potential in biomedical engineering, biosensors, and display devices. Recently, photo-responsive supramolecular nanofibers based on the polypeptide hybrids were developed by Moretto's group.108 The final 3D structures and macroscopic properties of the materials are accompanied by the conformational changes of a single azobenzene amino acid. These polypeptide-based electrospun hybrid membranes are promising materials in the field of light-controlled releasers of biologically relevant molecules.
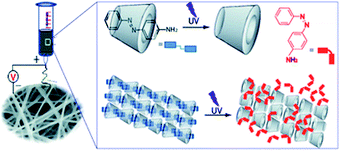 | ||
| Fig. 13 Schematic representation of electrospun UV-responsive supramolecular nanofibers from ICs of HPβCD with 4-aminoazobenzene (AAB). Reproduced from ref. 103 with permission from the Royal Society of Chemistry, copyright 2013.103 | ||
Except for light-responsive supramolecular-based electrospun nanofibers, multi-responsive supramolecular assemblies are good candidates for electrospinning. Yang and co-workers have fabricated supramolecular polymer nanofibers from a pillar[5]arene-based receptor via electrospinning (Fig. 14).109 The supramolecular system demonstrated reversible multi-responsive (pH, counter ion, and solvent composition) transition between supramolecular polymers and disassembled monomers. This approach provides a new way to construct novel organic nanofiber membranes with multi-responsiveness.
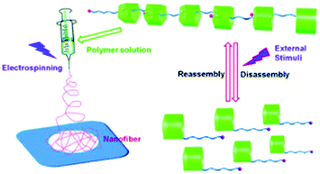 | ||
| Fig. 14 Schematic representation of electrospinning multi-responsive supramolecular assemblies constructed from a pillar[5]arene-based receptor. Reproduced from ref. 109 with permission from the Royal Society of Chemistry, copyright 2013.109 | ||
Nanofiber membranes prepared from the electrospinning possess a high volume-to-surface ratio and high porosities, which allows them to be exploited as sorbent materials for removing heavy metal ions and organic molecules.111–114 Chang and co-workers reported a smart photo-cross-linkable supramolecular electrospun nanofiber comprising a uracil-containing poly[1-(4-vinylbenzyl uracil)] (PVBU) PVBU for Hg2+ removal (Fig. 15).110 The supramolecular nanofibers can be transformed into a hybrid network by the selective binding of Hg2+ with a pair of uracil–uracil mismatches. The nanofibers represented significantly higher Hg2+ removal capacity (543.9 mg g−1) and high Hg2+ removal efficiency (10 mg g−1). This new kind of nanofiber membrane will provide a robust strategy for the construction of functional membranes for the selective removal of Hg2+ from aqueous environments. Another example was reported by Uyar's group where they fabricated γ-CD electrospun nanofibers without using a carrier polymeric matrix and the prepared nanofibrous membranes are of great use to entrapping volatile organic molecules (aniline and toluene) by inclusion complexation (Fig. 16).115 This can be ascribed to the non-covalent host–guest inclusion complexes between CD and various organic molecules, which render CD nanofiber membranes a good candidate for the removal of hazardous organic molecules. They found that the surface area of the γ-CD nanofiber web was three times higher than γ-CD powder which plays a key role in entrapping aniline and toluene by inclusion complexation.
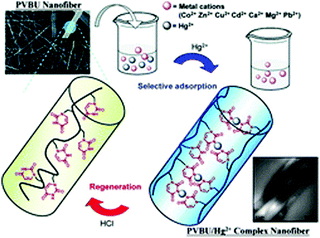 | ||
| Fig. 15 Schematic representation of electrospinning supramolecular nanofiber for Hg2+ removal. Reproduced from ref. 110 with permission from the Royal Society of Chemistry, copyright 2013.110 | ||
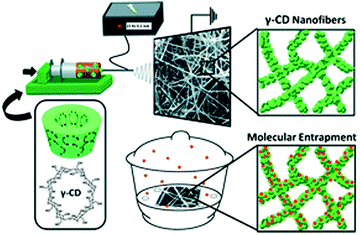 | ||
| Fig. 16 Schematic representation of electrospinning γ-CD nanofibers for the entrapment of volatile organic compounds. Reproduced from ref. 115 with permission from the Royal Society of Chemistry, copyright 2013.115 | ||
Another critical issue in human health is to develop smart sensors with highly sensitive and rapid detection of organic vapor at low concentrations. Lu and co-workers successfully developed a type of colorimetric sensor based on supramolecular nanofibers composed of polydiacetylene (PDA) acid through electrospinning (Fig. 17).116 During electrospinning, PDA was embedded into polymer matrix copoly (4-vinylpyridine) (PS-co-P4VP) via hydrogen bonding. This hydrogen bond could be partially destroyed upon exposure to organic amine vapor. In the meantime, the stress to the backbone of PDA was amplified, resulting in a color change of the nanofiber from blue to red. More importantly, an obvious blue-to-red color change was observed at 100 ppb of dipropylamine, propylamine, or diethylamine vapor. The fast and reliable spot test based on this new type of supramolecular nanofibers would be particularly useful in the area of the colorimetric sensor.
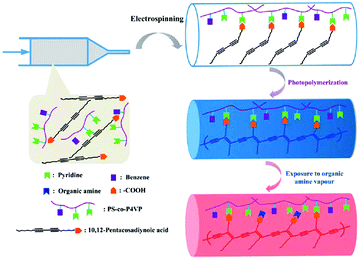 | ||
| Fig. 17 Schematic representation of the preparation of the PDA-embedded supramolecular electrospun nanofibers. Reproduced from ref. 116 with permission from the Royal Society of Chemistry, copyright 2013.116 | ||
It is well known that ureido-pyrimidinone (UPy) moieties are self-complementary with a relatively strong but reversible interaction among fourfold hydrogen bonding.95 Besides, UPy dimers can stack via π–π interactions which can be further enhanced by adjacent urea linkers.117 Recently, Danker's group constructed an electrospun nanofibers from UPy-based supramolecular system which is made up of UPy units, UPy-functionalized polymers and UPy-modified bioactive peptides (Fig. 18).118 This supramolecular approach in the formation of electrospun scaffolds involved noncovalent synthesis. Besides, the excellent properties of the membranes including mechanical stability, prevention of cell adhesion, and adaptive regulation of cell adhesion are attributed to the separate supramolecular building. The single electrospun bilayered scaffold comprising both cell-adhesive and non-cell adhesive characters offers a versatile toolbox for tissue engineering and regenerative medicine applications.
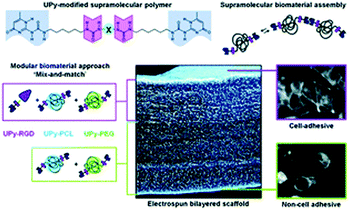 | ||
| Fig. 18 Schematic representation of supramolecular bilayered scaffolds with tailorable properties. Reproduced from ref. 118 with permission from the Royal Society of Chemistry, copyright 2014.118 | ||
4. Conclusions and perspective
Electrospun nanofibers from supramolecular systems provide an innovative approach to developing high surface area scaffolds or membranes. This novel method to construct electrospun nanofibers breaks the traditional point of view that polymer solution or melt with high molar mass is one of the indispensable factors in the process of electrospinning. In this review, we described the formation of electrospun nanofibers from a number of small molecules based on supermolecular chemistry. Small molecules such as phospholipids and surfactants can form supramolecular systems owing to the existence of enough hydrogen bonds which can facilitate the production of continuous nanofibers via electrospinning. Crown ethers and cyclodextrins are two vital host molecules in the field of supramolecular chemistry. Successful formation of electrospun nanofibers from these types of supramolecular systems has expanded the applications of crown ethers and cyclodextrins. Electrospun nanofibers based on supramolecular systems can fully make use of the advantages of supramolecular chemistry and pave new pathways forward for the applications of electrospun nanofibers in many areas including biotechnology, filters, sensors, electronics, energy and optics.Despite the significant advances in electrospun supermolecular nanofibers, this research theme still faces several challenges. For example, weak supermolecular interactions can inevitably lead to low mechanical stability when it comes to specific applications. Additionally, the stimuli-responsive electrospun nanofibers based on supermolecular systems are still in their infancy, and a great deal of effort is needed to establish the feasibility of these materials. With these synthetic challenges in mind, we, for the next step, need to focus our attention on the construction of new types of electrospinning supermolecular systems and explore their real applications which are key to developing electrospinning as an ideally compelling technology.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by start-up funding (N.13-G210-21-307) from Shanghai University, and the National Natural Science Foundation of China (51573086).Notes and references
- Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- J. Hu, T. W. Odom and C. M. Lieber, Acc. Chem. Res., 1999, 32, 435 CrossRef CAS.
- Z. L. Wang, Adv. Mater., 2000, 12, 1295 CrossRef CAS.
- R. Langer and D. A. Tirrell, Nature, 2004, 428, 487 CrossRef CAS PubMed.
- D. Li and Y. Xia, Adv. Mater., 2004, 16, 1151 CrossRef CAS.
- W. Wang, X. Lu, Z. Li, J. Lei, X. Liu, Z. Wang, H. Zhang and C. Wang, Adv. Mater., 2011, 23, 5109 CrossRef CAS.
- J. T. McCann, M. Marquez and Y. Xia, J. Am. Chem. Soc., 2006, 128, 1436 CrossRef CAS PubMed.
- M. Wang, K. Wang, Y. Yang, Y. Liu and D.-G. Yu, Polymers, 2020, 12, 103 CrossRef CAS.
- J. Zhang, F. Zhang, J. Song, L. Liu, Y. Si, J. Yu and B. Ding, J. Mater. Chem. A, 2019, 7, 20075 RSC.
- J. Xue, T. Wu, Y. Dai and Y. Xia, Chem. Rev., 2019, 119, 5298 CrossRef CAS.
- X. Lu, W. Zhang, C. Wang, T.-C. Wen and Y. Wei, Prog. Polym. Sci., 2011, 36, 671 CrossRef CAS.
- A. Greiner and J. H. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670 CrossRef CAS PubMed.
- J. P. F. Lagerwall, J. T. McCann, E. Formo, G. Scalia and Y. Xia, Chem. Commun., 2008, 5420 RSC.
- W. Wang, Z. Li, X. Xu, B. Dong, H. Zhang, Z. Wang, C. Wang, R. H. Baughman and S. Fang, Small, 2011, 7, 597 CrossRef CAS PubMed.
- Y. Yu, L. Gu, C. Zhu, P. A. van Aken and J. Maier, J. Am. Chem. Soc., 2009, 131, 15984 CrossRef CAS PubMed.
- S. Liu, Y. Zhao, X. Li, J. Yu, J. Yan and B. Ding, Adv. Mater., 2021, 33, 2008084 CrossRef CAS PubMed.
- D. H. Reneker and I. Chun, Nanotechnology, 1996, 7, 216 CrossRef CAS.
- W. Liu, S. Thomopoulos and Y. Xia, Adv. Healthcare Mater., 2012, 1, 10 CrossRef CAS.
- C. Huang, S. J. Soenen, J. Rejman, B. Lucas, K. Braeckmans, J. Demeester and S. C. De Smedt, Chem. Soc. Rev., 2011, 40, 2417 RSC.
- M. Bognitzki, T. Frese, M. Steinhart, A. Greiner, J. H. Wendorff, A. Schaper and M. Hellwig, Polym. Eng. Sci., 2001, 41, 982 CrossRef CAS.
- A. J. Mieszawska, R. Jalilian, G. U. Sumanasekera and F. P. Zamborini, Small, 2007, 3, 722 CrossRef CAS PubMed.
- G. C. Rutledge and S. V. Fridrikh, Adv. Drug Delivery Rev., 2007, 59, 1384 CrossRef CAS PubMed.
- P. Gupta, C. Elkins, T. E. Long and G. L. Wilkes, Polymer, 2005, 46, 4799 CrossRef CAS.
- M. G. McKee, G. L. Wilkes, R. H. Colby and T. E. Long, Macromolecules, 2004, 37, 1760 CrossRef CAS.
- J. Lehn, Science, 1993, 260, 1762 CrossRef CAS PubMed.
- G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, Angew. Chem., Int. Ed., 2007, 46, 2366 CrossRef CAS PubMed.
- T. Park, S. C. Zimmerman and S. Nakashima, J. Am. Chem. Soc., 2005, 127, 6520 CrossRef CAS PubMed.
- G. Vantomme and E. W. Meijer, Science, 2019, 363, 1396 CrossRef CAS PubMed.
- X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971 CrossRef CAS PubMed.
- K. Liu, Y. Kang, Z. Wang and X. Zhang, Adv. Mater., 2013, 25, 5530 CrossRef CAS.
- S. I. Stupp, V. LeBonheur, K. Walker, L. S. Li, K. E. Huggins, M. Keser and A. Amstutz, Science, 1997, 276, 384 CrossRef CAS.
- J. Ruokolainen, R. Mäkinen, M. Torkkeli, T. Mäkelä, R. Serimaa, G. T. Brinke and O. Ikkala, Science, 1998, 280, 557 CrossRef CAS PubMed.
- B. Rybtchinski, ACS Nano, 2011, 5, 6791 CrossRef CAS.
- E. Krieg and B. Rybtchinski, Chem. – Eur. J., 2011, 17, 9016 CrossRef CAS PubMed.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38 CrossRef CAS.
- Y. Tanaka, H. Katagiri, Y. Furusho and E. Yashima, Angew. Chem., Int. Ed., 2005, 117, 3935 CrossRef.
- D. J. Cram and J. M. Cram, Science, 1974, 183, 803 CrossRef CAS PubMed.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 7017 CrossRef CAS.
- C. J. Pedersen, Angew. Chem., Int. Ed. Engl., 1988, 27, 1021 CrossRef.
- P. Schurtenberger, R. Scartazzini, L. J. Magid, M. E. Leser and P. L. Luisi, J. Phys. Chem., 1990, 94, 3695 CrossRef CAS.
- Y. A. Shchipunov and H. Hoffmann, Langmuir, 1999, 15, 7108 CrossRef CAS.
- Y. A. Shchipunov, E. V. Shumilina and H. Hoffmann, Colloid Polym. Sci., 1998, 276, 368 CrossRef CAS.
- M. G. McKee, J. M. Layman, M. P. Cashion and T. E. Long, Science, 2006, 311, 353 CrossRef CAS.
- M. P. Cashion, X. Li, Y. Geng, M. T. Hunley and T. E. Long, Langmuir, 2009, 26, 678 CrossRef.
- F. M. Menger and J. S. Keiper, Angew. Chem., Int. Ed., 2000, 39, 1906 CrossRef.
- R. Zana, Adv. Colloid Interface Sci., 2002, 97, 205 CrossRef CAS.
- D. Danino, Y. Talmon and R. Zana, Langmuir, 1995, 11, 1448 CrossRef CAS.
- A. Bernheim-Groswasser, R. Zana and Y. Talmon, J. Phys. Chem. B, 2000, 104, 4005 CrossRef CAS.
- P. D. Dalton, K. Klinkhammer, J. Salber, D. Klee and M. Möller, Biomacromolecules, 2006, 7, 686 CrossRef CAS PubMed.
- C. Mota, D. Puppi, M. Gazzarri, P. Bártolo and F. Chiellini, Polym. Int., 2013, 62, 893 CrossRef CAS.
- M. T. Hunley, A. S. Karikari, M. G. McKee, B. D. Mather, J. M. Layman, A. R. Fornof and T. E. Long, Macromol. Symp., 2008, 270, 1 CrossRef CAS.
- J. C. Singer, A. Ringk, R. Giesa and H.-W. Schmidt, Macromol. Mater. Eng., 2015, 300, 259 CrossRef CAS.
- J.-M. Lehn, Polym. Int., 2002, 51, 825 CrossRef CAS.
- J. Gao, Y. He, F. Liu, X. Zhang, Z. Wang and X. Wang, Chem. Mater., 2007, 19, 3877 CrossRef CAS.
- T. F. A. de Greef and E. W. Meijer, Nature, 2008, 453, 171 CrossRef CAS PubMed.
- M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398 CrossRef CAS PubMed.
- P. Wei, X. Yan and F. Huang, Chem. Soc. Rev., 2015, 44, 815 RSC.
- Y.-H. Liu, Y.-M. Zhang, H.-J. Yu and Y. Liu, Angew. Chem. Int. Ed., 2021, 60, 3870 CrossRef CAS PubMed.
- L. J. Twyman, A. S. H. King and I. K. Martin, Chem. Soc. Rev., 2002, 31, 69 RSC.
- F. Huang and H. W. Gibson, Prog. Polym. Sci., 2005, 30, 982 CrossRef CAS.
- C. Lu, M. Zhang, D. Tang, X. Yan, Z. Zhang, Z. Zhou, B. Song, H. Wang, X. Li, S. Yin, H. Sepehrpour and P. J. Stang, J. Am. Chem. Soc., 2018, 140, 7674 CrossRef CAS PubMed.
- L. Wang, L. Cheng, G. Li, K. Liu, Z. Zhang, P. Li, S. Dong, W. Yu, F. Huang and X. Yan, J. Am. Chem. Soc., 2020, 142, 2051 CrossRef CAS PubMed.
- A. Harada, A. Hashidzume and Y. Takashima, in Supramolecular Polymers Polymeric Betains Oligomers, Springer, Berlin, Heidelberg, 2006, vol. 201, ch. 56, pp. 1 Search PubMed.
- C. Zhang, S. Li, J. Zhang, K. Zhu, N. Li and F. Huang, Org. Lett., 2007, 9, 5553 CrossRef CAS PubMed.
- X. Yan, M. Zhou, J. Chen, X. Chi, S. Dong, M. Zhang, X. Ding, Y. Yu, S. Shao and F. Huang, Chem. Commun., 2011, 47, 7086 RSC.
- S. Dong, L. Gao, J. Chen, G. Yu, B. Zheng and F. Huang, Polym. Chem., 2013, 4, 882 RSC.
- S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed.
- J. Chen, S. Zhang, F. Sun, N. Li, K. Cui, J. He, D. Niu and Y. Li, Polym. Chem., 2016, 7, 2947 RSC.
- J. Szejtli, Chem. Rev., 1998, 98, 1743 CrossRef CAS PubMed.
- J. Araki and K. Ito, Soft Matter, 2007, 3, 1456 RSC.
- G. Chen and M. Jiang, Chem. Soc. Rev., 2011, 40, 2254 RSC.
- X. Liao, G. Chen and M. Jiang, Polym. Chem., 2013, 4, 1733 RSC.
- A. Celebioglu and T. Uyar, J. Mater. Sci., 2020, 55, 404 CrossRef CAS.
- W. Zhang, M. Chen, B. Zha and G. Diao, Phys. Chem. Chem. Phys., 2012, 14, 9729 RSC.
- A. Celebioglu and T. Uyar, Nanoscale, 2012, 4, 621 RSC.
- A. Dodero, G. Schlatter, A. Hébraud, S. Vicini and M. Castellano, Carbohydr. Polym., 2021, 264, 118042 CrossRef CAS PubMed.
- A. Costoya, A. Concheiro and C. Alvarez-Lorenzo, Molecules, 2017, 22 Search PubMed.
- A. Celebioglu and T. Uyar, J. Colloid Interface Sci., 2013, 404, 1 CrossRef CAS PubMed.
- A. Celebioglu and T. Uyar, Chem. Commun., 2010, 46, 6903 RSC.
- A. Celebioglu and T. Uyar, Langmuir, 2011, 27, 6218 CrossRef CAS PubMed.
- M. Messner, S. V. Kurkov, M. E. Brewster, P. Jansook and T. Loftsson, Int. J. Pharm., 2011, 407, 174 CrossRef CAS PubMed.
- W. Zhang, M. Chen, B. Zha and G. Diao, Phys. Chem. Chem. Phys., 2012, 14, 9729 RSC.
- C. B. Rodell, A. L. Kaminski and J. A. Burdick, Biomacromolecules, 2013, 14, 4125 CrossRef CAS PubMed.
- C. B. Highley, C. B. Rodell, I. L. Kim, R. J. Wade and J. A. Burdick, J. Mater. Chem. B, 2014, 2, 8110 RSC.
- A. Celebioglu and T. Uyar, J. Colloid Interface Sci., 2013, 404, 1 CrossRef CAS PubMed.
- Y. Ahn, Y. Kang, M. Ku, Y.-H. Yang, S. Jung and H. Kim, RSC Adv., 2013, 3, 14983 RSC.
- J. C. Singer, R. Giesa and H.-W. Schmidt, Soft Matter, 2012, 8, 9972 RSC.
- M. P. Lightfoot, F. S. Mair, R. G. Pritchard and J. E. Warren, Chem. Commun., 1999, 1945, 10.1039/A905245C.
- A. Bernet, M. Behr and H.-W. Schmidt, Soft Matter, 2011, 7, 1058 RSC.
- M. M. J. Smulders, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2007, 130, 606 CrossRef PubMed.
- A. S. Tayi, E. T. Pashuck, C. J. Newcomb, M. T. McClendon and S. I. Stupp, Biomacromolecules, 2014, 15, 1323 CrossRef CAS PubMed.
- D. Hermida-Merino, M. Belal, B. W. Greenland, P. Woodward, A. T. Slark, F. J. Davis, G. R. Mitchell, I. W. Hamley and W. Hayes, Eur. Polym. J., 2012, 48, 1249 CrossRef CAS.
- P. Y. W. Dankers, M. C. Harmsen, L. A. Brouwer, M. J. A. Van Luyn and E. W. Meijer, Nat. Mater., 2005, 4, 568 CrossRef CAS PubMed.
- R. P. Sijbesma, F. H. Beijer, L. Brunsveld, B. J. Folmer, J. K. Hirschberg, R. F. Lange, J. K. Lowe and E. Meijer, Science, 1997, 278, 1601 CrossRef CAS PubMed.
- F. H. Beijer, R. P. Sijbesma, H. Kooijman, A. L. Spek and E. W. Meijer, J. Am. Chem. Soc., 1998, 120, 6761 CrossRef CAS.
- B. J. B. Folmer, R. P. Sijbesma, R. M. Versteegen, J. A. J. van der Rijt and E. W. Meijer, Adv. Mater., 2000, 12, 874 CrossRef CAS.
- M. Allais, D. Mailley, P. Hébraud, D. Ihiawakrim, V. Ball, F. Meyer, A. Hébraud and G. Schlatter, Nanoscale, 2018, 10, 9164 RSC.
- M. Hecht, B. Soberats, J. Zhu, V. Stepanenko, S. Agarwal, A. Greiner and F. Würthner, Nanoscale Horiz., 2019, 4, 169 RSC.
- L. Peng, A. Feng, M. Huo and J. Yuan, Chem. Commun., 2014, 50, 13005 RSC.
- Q. Yan, J. Yuan, Z. Cai, Y. Xin, Y. Kang and Y. Yin, J. Am. Chem. Soc., 2010, 132, 9268 CrossRef CAS PubMed.
- Q. Yan, H. Zhang and Y. Zhao, ACS Macro Lett., 2014, 3, 472 CrossRef CAS.
- Q. Yan, Y. Xin, R. Zhou, Y. Yin and J. Yuan, Chem. Commun., 2011, 47, 9594 RSC.
- M. Chen, S. R. Nielsen, T. Uyar, S. Zhang, A. Zafar, M. Dong and F. Besenbacher, J. Mater. Chem. C, 2013, 1, 850 RSC.
- Y. Liu, Y.-L. Zhao, Y. Chen and D.-S. Guo, Org. Biomol. Chem., 2005, 3, 584 RSC.
- X. Liao, G. Chen, X. Liu, W. Chen, F. Chen and M. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4409 CrossRef CAS PubMed.
- K. Peng, I. Tomatsu and A. Kros, Chem. Commun., 2010, 46, 4094 RSC.
- G.-D. Fu, L.-Q. Xu, F. Yao, G.-L. Li and E.-T. Kang, ACS Appl. Mater. Interfaces, 2009, 1, 2424 CrossRef CAS PubMed.
- D. Mazzier, M. Maran, O. Polo Perucchin, M. Crisma, M. Zerbetto, V. Causin, C. Toniolo and A. Moretto, Macromolecules, 2014, 47, 7272 CrossRef CAS.
- K. Wang, C.-Y. Wang, Y. Wang, H. Li, C.-Y. Bao, J.-Y. Liu, S. X.-A. Zhang and Y.-W. Yang, Chem. Commun., 2013, 49, 10528 RSC.
- Y.-S. Wang, C.-C. Cheng, J.-K. Chen, F.-H. Ko and F.-C. Chang, J. Mater. Chem. A, 2013, 1, 7745 RSC.
- Y. Che, X. Yang and L. Zang, Chem. Commun., 2008, 1413 RSC.
- J. Lv, C. Ouyang, X. Yin, H. Zheng, Z. Zuo, J. Xu, H. Liu and Y. Li, Macromol. Rapid Commun., 2008, 29, 1588 CrossRef CAS.
- X. Liu, C. Qi, T. Bing, X. Cheng and D. Shangguan, Anal. Chem., 2009, 81, 3699 CrossRef CAS PubMed.
- Y.-S. Wang, C.-C. Cheng, Y.-S. Ye, Y.-C. Yen and F.-C. Chang, ACS Macro Lett., 2011, 1, 159 CrossRef.
- A. Celebioglu and T. Uyar, RSC Adv., 2013, 3, 22891 RSC.
- J. Wu, X. Lu, F. Shan, J. Guan and Q. Lu, RSC Adv., 2013, 3, 22841 RSC.
- H. Kautz, D. J. M. van Beek, R. P. Sijbesma and E. W. Meijer, Macromolecules, 2006, 39, 4265 CrossRef CAS.
- B. B. Mollet, M. Comellas-Aragones, A. J. H. Spiering, S. H. M. Sontjens, E. W. Meijer and P. Y. W. Dankers, J. Mater. Chem. B, 2014, 2, 2483 RSC.
| This journal is © The Royal Society of Chemistry 2022 |