Open Access Article
Open Access ArticleRecent progress in thermally activated delayed fluorescence emitters for nondoped organic light-emitting diodes
Yi-Zhong
Shi†
 ,
Hao
Wu†
,
Kai
Wang
,
Hao
Wu†
,
Kai
Wang
 *,
Jia
Yu
,
Xue-Mei
Ou
and
Xiao-Hong
Zhang
*,
Jia
Yu
,
Xue-Mei
Ou
and
Xiao-Hong
Zhang
 *
*
Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu 215123, PR China. E-mail: xiaohong_zhang@suda.edu.cn; wkai@suda.edu.cn
First published on 22nd February 2022
Abstract
Nondoped organic light-emitting diodes (OLEDs) have drawn immense attention due to their merits of process simplicity, reduced fabrication cost, etc. To realize high-performance nondoped OLEDs, all electrogenerated excitons should be fully utilized. The thermally activated delayed fluorescence (TADF) mechanism can theoretically realize 100% internal quantum efficiency (IQE) through an effective upconversion process from nonradiative triplet excitons to radiative singlet ones. Nevertheless, exciton quenching, especially related to triplet excitons, is generally very serious in TADF-based nondoped OLEDs, significantly hindering the pace of development. Enormous efforts have been devoted to alleviating the annoying exciton quenching process, and a number of TADF materials for highly efficient nondoped devices have been reported. In this review, we mainly discuss the mechanism, exciton leaking channels, and reported molecular design strategies of TADF emitters for nondoped devices. We further classify their molecular structures depending on the functional A groups and offer an outlook on their future prospects. It is anticipated that this review can entice researchers to recognize the importance of TADF-based nondoped OLEDs and provide a possible guide for their future development.
1. Introduction
Organic light-emitting diode (OLED) technology has been extensively applied in high-end solid-state lighting and flat-panel displays owing to its superior advantages of autoluminescence, low driving voltage, flexibility, fast response, and so on.1 According to the spin statistics, electrogenerated singlet and triplet excitons have a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Traditional fluorescent materials with high photoluminescence quantum yields (PLQYs) can only utilize singlet excitons, while the energies of triplet excitons are depleted via nonradiative transition processes. To realize full exciton utilization, phosphorescence and thermally activated delayed fluorescence (TADF) emitters have been proposed.2,3 Phosphorescence emitters can utilize triplet excitons due to the strong spin–orbit coupling (SOC) induced by heavy atoms (e.g., platinum and iridium), which can help to enhance the triplet radiation (i.e., phosphorescence) rate. Although phosphorescence-based OLEDs are now the main commercial OLED technologies, they have several obvious drawbacks, such as high costs and toxicity due to heavy atoms and the absence of stable blue phosphorescence emitters. TADF-based OLEDs are an alternative approach with potential 100% internal quantum efficiencies (IQEs) and have attracted considerable attention within the last decade.4–6
3. Traditional fluorescent materials with high photoluminescence quantum yields (PLQYs) can only utilize singlet excitons, while the energies of triplet excitons are depleted via nonradiative transition processes. To realize full exciton utilization, phosphorescence and thermally activated delayed fluorescence (TADF) emitters have been proposed.2,3 Phosphorescence emitters can utilize triplet excitons due to the strong spin–orbit coupling (SOC) induced by heavy atoms (e.g., platinum and iridium), which can help to enhance the triplet radiation (i.e., phosphorescence) rate. Although phosphorescence-based OLEDs are now the main commercial OLED technologies, they have several obvious drawbacks, such as high costs and toxicity due to heavy atoms and the absence of stable blue phosphorescence emitters. TADF-based OLEDs are an alternative approach with potential 100% internal quantum efficiencies (IQEs) and have attracted considerable attention within the last decade.4–6
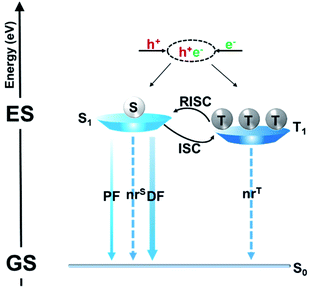
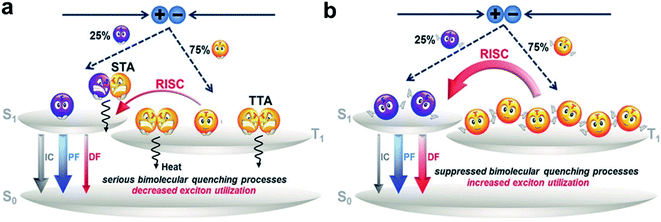
Fig. 1 illustrates a schematic illustration of exciton energy transfer and utilization in a TADF emitter upon electrical excitation. Singlet excitons have three possible energy decay routes: fluorescence radiation, nonradiative transition to the ground state (S0) and intersystem crossing (ISC) from the lowest singlet excited state (S1) to the lowest triplet excited state (T1), while triplet excitons can either be upconverted into singlets via reverse intersystem crossing (RISC) or suffer nonradiative decay (assuming that the phosphorescence process can be ignored in conventional pure organic systems). RISC and fluorescence radiation are the key steps for triplet and singlet exciton harvesting, respectively. To realize a competitive RISC rate (kRISC) to that of the corresponding triplet nonradiative process (kTnr), a small S1–T1 energy splitting (ΔEST) is required. To date, several design strategies have been proposed, such as highly twisted D–A molecular structures,7–10 exciplex systems,11–17 and the recently developed multiple resonance (MR) effect-induced TADF.18–20 A common characteristic of these molecular designs is that they can all restrain the overlaps between the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs), which is essential for small ΔESTs. Singlet–triplet spin–orbit coupling (SOC) is another factor that significantly affects the RISC process. It can be efficiently enhanced via either internal/external heavy atom effects21–24 or excited states with a different nature.25–28 Fluorescence radiation is the ultimate pathway for not only the initial singlet exciton harvest (prompt fluorescence, PF) but also the triplet exciton harvest via delayed fluorescence (DF). Thus, enhancing its rate (kr) is also required to sufficiently surpass the singlet nonradiative decay rate (kSnr). While unlike small ΔESTs, which require the separation of frontier molecular orbitals (FMOs), a high kr requires a sufficient integral between the HOMO and LUMO wavefunctions. Thus, how to strike a balance between small ΔESTs and fast kr becomes key to obtaining optimized performances. PLQY is a parameter that contains both a prompt component that is directly related to the fluorescence process and a delayed component that is related to both the RISC and fluorescence processes. Thus, the PLQY reflects the overall exciton utilization and is typically positively correlated with the corresponding electroluminescent (EL) properties. A high PLQY is a prerequisite for a TADF emitter to realize high performances in OLEDs.
After the rapid development of the past dozen years, considerable progress has been made in developing high-performance TADF-OLEDs, and the reported maximum external quantum efficiencies (EQEs) have been beyond 30%.29–39 In these OLEDs, emitting layers (EMLs) are all obtained by adopting optimized host–dopant configurations. This is mainly to suppress the annoying concentration quenching, especially arising from the long-lived triplet excitons. On the other hand, introducing host matrixes not only complicates device fabrication but also increases the cost of production.40 For mass production, OLEDs with nondoped EMLs would be simpler and more attractive.
In this review, we first summarize the recent progress in the field of TADF emitters toward high-performance nondoped OLEDs, especially the important progress within the last two years. Then, we categorize the TADF emitters according to their different electron-withdrawing units and devote particular emphasis to molecular design strategies, which has not been done in previous reviews. In addition, their physical properties, as well as nondoped device performances, are also summarized. We further discuss the present pros and cons of TADF-based nondoped OLEDs and provide an outlook on their prospects.
2. Molecular design strategies of TADF emitters for nondoped OLEDs
To exploit novel TADF materials toward high-performance nondoped OLEDs, the abovementioned keys of general TADF molecular designs are still essential. In addition, energy loss channels in nondoped EMLs are more complicated than ideal host–guest EMLs and should be carefully blocked. In earlier studies, researchers have recognized that methods of rigidifying organic luminogens through viscous or solid environments or forming J-aggregates can give rise to emission enhancement.41,42 In 2001, Tang et al. accidentally discovered enhanced emission upon aggregation for hexaphenylsilole (HPS) and named the phenomenon “aggregation-induced emission (AIE)”.43,44 Since then, AIE has been widely applied in the fields of optical devices, luminescent sensors, bioimaging, phototherapy, etc. Both theoretical simulation and experimental results suggest that in their aggregate states, molecular vibrations and rotations are evidently suppressed, resulting in decreased kSnr and thus enhanced emission. In particular, some emitters exhibit aggregation-induced delayed fluorescence (AIDF) behaviors (as shown in Fig. 2a).45 Emitters with such AIE/AIDF behaviors are promising candidates for high-efficiency nondoped OLEDs. Many AIE-TADF/AIDF emitters have been reported to show decent performances in nondoped OLEDs.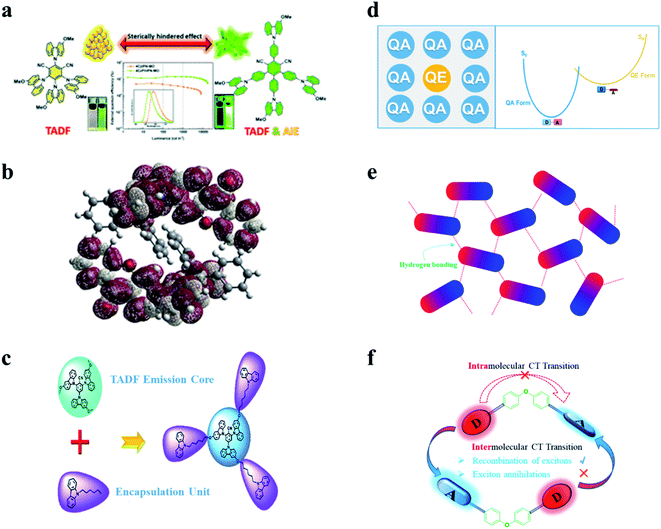 | ||
| Fig. 2 Molecular design strategies of TADF emitters for nondoped OLEDs. (a) The AIE/AIDF mechanism. Reproduced from ref. 45 with permission. Copyright 2020 Wiley-VCH. (b) The mechanism of introducing steric hindrance. Reproduced from ref. 46 with permission. Copyright 2017 Wiley-VCH. (c) Self-host mechanism. Reproduced from ref. 47 with permission. Copyright 2019 the Royal Society of Chemistry. (d) The “self-doping” mechanism. Reproduced from ref. 49 with permission. Copyright 2021 Wiley-VCH. (e) Intermolecular hydrogen bonding mechanism. Reproduced from ref. 50 with permission. Copyright 2019 the Royal Society of Chemistry. (f) D–spacer–A mechanism. Reproduced from ref. 51 with permission. Copyright 2018 Wiley-VCH. | ||
In nondoped OLEDs, intermolecular exciton quenching plays a vital role in causing exciton loss. Many studies have suggested that suppressing the annihilation process associated with long-lived triplet states is key to determining nondoped device performance. The rate constant of triplet-related quenching (kCQ) can be expressed as follows:
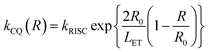 | (1) |
In addition to introducing extra steric groups, integrating the host matrix and guest TADF emitters into new “self-host” materials is also a wise molecular design method for nondoped OLEDs. As displayed in Fig. 2c, introducing carbazoles as steric shields can not only keep the intrinsic TADF feature unchanged but also effectively suppress the exciton quenching caused by intermolecular interactions. Moreover, such “emission-core and host-encapsulation” molecular structures generally meet the requirements of the solution process and are thus usually applied in solution-processed nondoped OLEDs.47,48
It was recently recognized that some organic emitters actually have dual stable conformations. TADF is associated with the highly twisted conformation, which is also known as the “quasi-equatorial (QE)” conformation. On the other hand, the other mildly twisted form, referred to as the “quasi-axial (QA)” conformation, often shows only conventional fluorescence with higher energy instead of TADF. As displayed in Fig. 2d, by using the different characteristics of these conformers, our group proposed a new concept of “self-doping” for realizing high-efficiency nondoped OLEDs.49 Interestingly, this “compositionally” pure film actually behaves as a film with a dopant (QE form) in a matrix (QA form). The concentration-induced quenching that may seriously occur at high concentrations is thus expected to be effectively relieved.
Hydrogen bonds, especially intermolecular bonds, have been widely used to construct rigid intermolecular frameworks to relieve molecular vibrational and rotational motions. Enormous highly efficient chromophores have been exploited based on hydrogen bonds. On this basis, we further proposed that in neat films of TADF emitters with suitable intermolecular hydrogen bonds, continuous and oriented interactions could be achieved. More importantly, in delicate supramolecular frameworks, not only can unimolecular nonradiative transitions be suppressed, but the annoying intermolecular quenching can also be relieved with distanced electron-rich segments (shown in Fig. 2e).50
Intermolecular exciton quenching in OLEDs is dominated by electron-exchange interactions of triplet excitons and is generally serious between TADF emitters based on intramolecular charge-transfer (CT) transitions, while there is another type of TADF emitter based on intermolecular CT transitions, which is an exciplex. Intermolecular mutual collisions in the exciplex favor the recombination of excitons rather than exciton annihilation. In a neat film containing a single material with molecular configurations such as D–spacer–A structures (shown in Fig. 2f), a space-enough and conjugation-forbidden diphenyl ether linkage can effectively suppress the intramolecular charge-transfer (CT) transition while forming an exciplex-type emitter via the intermolecular CT transition.51
3. TADF emitters for nondoped OLEDs
3.1. Cyano-substituted aromatics as acceptors
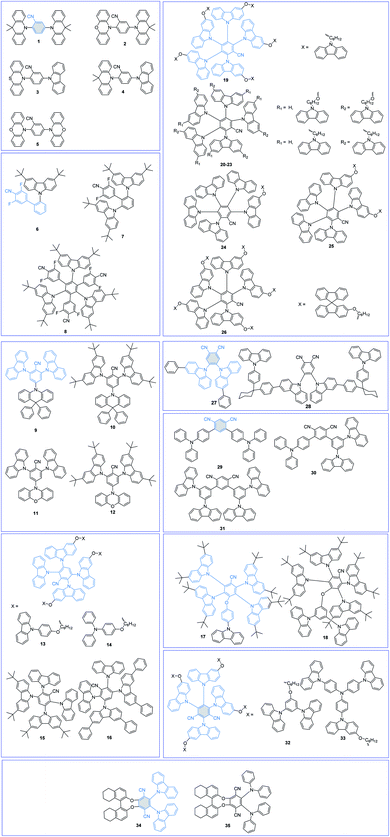
Cyano (CN)-substituted aromatics have been widely used to construct TADF emitters due to their strong electron-withdrawing abilities and high chemical stabilities. The molecular structures of CN-based TADF emitters are shown in Fig. 3, and the corresponding key parameters of their nondoped devices are summarized in Table 1. Grazulevicius et al.52 designed five benzonitrile (BN)-based TADF emitters 1–5 with symmetrical donor–acceptor–donor (D–A–D) and asymmetrical donor–acceptor–donor* (D–A–D*) molecular structures to carefully study the influence of different substitution patterns on device performance. Charge transport tests show that molecular structures significantly affect the charge carrier mobilities, which can be ascribed to the different molecular packings in amorphous states. However, their nondoped devices all show poor results with maximum external quantum efficiencies (EQEs) below 5.0% because their electron-withdrawing core BN moiety is unprotected.| PL | EL | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLQY | τ p [ns] | τ d [μs] | Peak [nm] | V on [V] | CE/PE/EQE [cd A−1/lm W−1/%] | Roll-offb [%] | CIE (x,y) | |||
| Maximum | @103 cd m−2 | |||||||||
| a Turn-on voltage at a luminance of 1 cd m−2. b EQEroll-off = (EQEmax − EQE1000)/EQEmax. | ||||||||||
| 1 | 0.42 | — | — | ∼477 | 3.6 | 5.3/3.6/2.5 | — | — | (0.19, 0.29) | 52 |
| 2 | 0.26 | — | — | ∼525 | 3.4 | 16.3/12.2/5.0 | — | — | (0.31, 0.57) | |
| 3 | 0.11 | — | — | ∼540 | 4.4 | 5.2/3.5/1.6 | — | — | (0.35, 0.52) | |
| 4 | 0.39 | — | — | ∼477 | 4.5 | 8.4/4.9/4.1 | — | — | (0.18, 0.27) | |
| 5 | 0.18 | — | — | ∼534 | 3.6 | 7.7/6.0/2.4 | — | — | (0.34, 0.55) | |
| 6 | 0.42 | 17.0 | — | 466 | 10.1 | 3.6/1.4/2.6 | — | — | (0.16, 0.18) | 53 |
| 7 | 0.19 | 14.1 | — | 466 | 5.7 | 5.0/2.2/3.7 | — | — | (0.16, 0.17) | |
| 8 | 0.76 | 21.3 | — | 484 | 6.3 | 46.4/20.8/21.0 | — | — | (0.19, 0.35) | |
| 9 | 0.57 | 21.4 | 5.5 | 476 | 3.2 | 41.4/35.4/20.0 | — | — | (0.16, 0.26) | 54 |
| 10 | 0.55 | 15.8 | 7.1 | 460 | 5.4 | 10.1/7.1/10.1 | — | — | (0.16, 0.19) | |
| 11 | 0.48 | 18.0 | 0.8 | 568 | 2.4 | 38.1/42.6/13.6 | —/—/12.8 | 5.9 | (0.46, 0.52) | 55 |
| 12 | 0.50 | 21.6 | 1.1 | — | 3.7 | 21.1/14.7/6.6 | —/—/3.9 | 41.0 | (0.36, 0.56) | |
| 13 | 0.63 | 15.0 | 3.3 | — | 2.8 | 45.2/35.5/22.6 | —/—/18.9 | 16.4 | (0.16, 0.29) | 58 |
| 14 | 0.40 | 15.0 | 2.5 | — | — | — | — | — | — | |
| 15 | 0.66 | 15.7 | 9.8 | 470 | 2.7 | 31.1/—/21.6 | 18.9/—/10.8 | 50.0 | (0.17, 0.25) | 59 |
| 16 | 0.45 | 17.4 | 5.1 | 480 | 6.7 | 8.5/—/3.9 | 7.1/—/3.3 | 15.4 | (0.20, 0.33) | |
| 17 | 0.39 | — | — | 496 | 3.7 | 18.4/11.6/5.9 | 12.1/—/— | — | (0.22, 0.39) | 60 |
| 18 | 0.61 | — | — | 484 | 3.5 | 24.9/19.5/8.0 | 14.3/—/— | — | (0.18, 0.31) | |
| 19 | 0.52 | — | — | 510 | 3.1 | 49.7/45.9/17.1 | 46.8/—/— | — | (0.26, 0.52) | 63 |
| 20 | 0.56 | 6.9 | 2.4 | 523 | 2.8 | 40.2/31.5/13.8 | 32.6/—/— | — | (0.37, 0.58) | 64 |
| 21 | 0.77 | 6.3 | 4.2 | 541 | 3.2 | 56.2/45.2/17.4 | 48.6/—/— | — | (0.40, 0.57) | |
| 22 | 0.59 | 8.7 | 3.6 | 516 | 3.0 | 41.7/34.5/14.4 | 39.9/—/— | — | (0.25, 0.55) | |
| 23 | 0.80 | 5.5 | 4.5 | 515 | 3.6 | 63.0/48.7/20.4 | 43.5/—/— | — | (0.25, 0.52) | |
| 24 | 0.38 | 38 | 3.1 | — | 3.4 | 21.9/17.2/7.3 | — | — | (0.28, 0.54) | 65 |
| 25 | 0.46 | 56 | 3.8 | — | 3.2 | 40.7/31.9/13.9 | — | — | (0.27, 0.54) | |
| 26 | 0.70 | 77 | 4.5 | 508 | 3.1 | 58.7/46.2/20.1 | — | — | (0.27, 0.53) | |
| 27 | 0.60 | 24.2 | 6.1 | 524 | 3.6 | 22.3/17.5/7.5 | — | — | (0.31, 0.52) | 66 |
| 28 | 0.89 | 25.2 | 6.9 | 524 | 4.2 | 40.8/28.5/13.4 | — | — | (0.33, 0.54) | |
| 29 | 0.84 | 8.6 | — | 520 | 2.6 | 11.8/10.3/3.5 | —/—/2.7 | 22.8 | (0.20, 0.33) | 67 |
| 30 | 1.00 | 9.1 | — | 520 | 2.8 | 15.7/12.0/4.9 | —/—/3.7 | 24.5 | (0.29, 0.61) | |
| 31 | 0.46 | 13.9 | — | 520 | 3.0 | 26.6/22.4/8.3 | —/—/6.5 | 21.7 | (0.30, 0.58) | |
| 32 | 0.08 | 22.0 | 1.4 | 552 | 4.4 | 1.4/0.9/0.5 | — | — | (0.46, 0.52) | 69 |
| 33 | 0.90 | 16.0 | 1.0 | 548 | 2.7 | 44.5/46.6/16.5 | 30.5/19.1/11.3 | 31.5 | (0.42, 0.55) | |
| 34 | 0.81 | 8.9 | 6.1 | 562 | 3.5 | 47.8/34.6/14.0 | 46.6/—/13.6 | 2.8 | — | 70 |
| 35 | 0.78 | 9.8 | 8.3 | 560 | 3.6 | 23.0/16.8/6.6 | 18.4/—/5.4 | 18.2 | — | 71 |
Yang et al.53 reported a series of sky-blue TADF emitters 6–8 by linking di-tert-butylcarbazole (tBuCz) and difluorocyanobenzene (CNDF) groups on the phenyl bridge simultaneously with mutual ortho-positions. The large steric tBuCz groups not only effectively protect the electron-withdrawing core CNDF moieties but also favor the coexistence of through-space CT and through-bond CT. Compared with the large ΔEST values of 0.24 eV and 0.21 eV for 6 and 7, the highly twisted multi-(D/A) molecular structure of 8 can not only effectively lower its ΔEST (0.03 eV) but also suppress the intermolecular close π–π packing. As a result, the 8 amorphous neat film gives a high PLQY of 0.76. Therefore, the 8-based solution-processed nondoped device realizes sky-blue emission with a peak at 484 nm and a maximum EQE of 21.0%.
Wang et al.54 also proposed a facile and efficient molecular design strategy for shielding the BN moiety with carbazole derivatives (Cz and tBuCz). In their newly designed compounds 9 and 10, 9,9-diphenylacridan (DPAc) and BN were the D and A groups, respectively. Introduction of Cz and tBuCz groups as the protective units can not only effectively isolate the triplet excited states but also enhance the rigidity of the molecular skeletons and decrease molecular vibrations/rotations. Thus, in nondoped OLEDs, 9 and 10 achieved high performance with maximum EQEs of 10.1% and 20.0% and CIE coordinates of (0.16, 0.19) and (0.16, 0.26), respectively. In particular, the inferior performance of the 9-based device can be reasonably attributed to its poorer carrier mobilities. Based on the results, we can notice that a more delicate balance between the protection functions and carrier mobilities is needed. By further replacing the DPAc unit with a more electron-rich phenoxazine (PXZ) unit, two novel TADF emitters 11 and 12 were also synthesized.55 Nevertheless, due to the unprotected rigid and planar PXZ unit, which is prone to close intermolecular packing, the 11- and 12-based nondoped OLEDs show poor performance with maximum EQEs of 6.6% and 13.6%, respectively, which are much lower than those of DPAc-based emitters. These results further demonstrate that both D and A moieties should be well protected to shield potential intermolecular charge exchange.
Since the pioneering work of Adachi et al.3 in 2012, carbazolyl dicyanobenzene (CDCB) derivatives have been carefully studied. As a member of the CDCB family, 2,3,5,6-tetra(9H-carbazol-9-yl)benzonitrile (4CzBN) and its derivatives have been widely used to construct high-performance blue TADF emitters.56,57 By using the self-host molecular design strategy, Sun et al.58 reported two 4CzBN-based TADF emitters 13 and 14 for fully solution-processed blue nondoped devices. Both emitters possess identical emissive cores but different bulky shielding groups. As displayed in Fig. 4, unlike the formation of the 14-based electromer state during electroexcitation, the rigid phenylcarbazole (PCz) unit in 13 can efficiently block such energy leakage. As a result, the 13-based fully solution-processed blue OLED realizes a maximum EQE of 22.6%, which is almost 20 times higher than that of the 14-based OLED. With the same emission core, Tang et al.59 also reported two 4CzBN-based blue TADF emitters 15 and 16 by introducing steric groups. Both emitters exhibit blue emission at 464 nm with a CIEy < 0.2 and an EQE over 20% in doped devices. Meanwhile, compared with the steric tert-butyl (tBu) group in 15, the terminal phenyl group in 16 can effectively weaken the intermolecular π–π packing in the solid-state film and significantly enhance the PLQY. Therefore, the 16-based nondoped device achieves a maximum EQE of 21.6% with an extremely low turn-on voltage (Von) of 2.7 V. Sun et al.60 also synthesized two 4CzBN-based blue TADF emitters 17 and 18 with the host-σ-guest molecular configuration for solution-processed nondoped OLEDs. The oxygen bridge-linked host can not only suppress triplet exciton quenching between guest units but also improve the carrier transport ability.
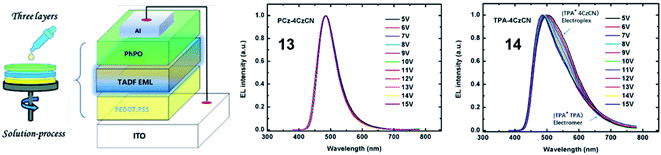 | ||
| Fig. 4 Device structure of the fully solution-processed devices and normalized EL spectra at different voltages. Reproduced from ref. 58 with permission. Copyright 2019 American Chemical Society. | ||
As another representative member of the CDCB family, 2,3,4,5,6-penta(9H-carbazol-9-yl)benzonitrile (5CzBN) is an excellent precursor to exploit TADF emitters for efficient sky-blue nondoped devices.61,62 By applying a self-host molecular design strategy, Sun et al.63 conveniently constructed TADF emitter 19 for solution-processed nondoped OLEDs. The alkyl chain-linked Cz units can sufficiently encapsulate the 5CzBN emission core and effectively suppress intermolecular interaction-induced exciton quenching. Therefore, compared with the low PLQY of 0.21 for precursor 5CzBN, the 19 amorphous neat film exhibits a higher PLQY of 0.52. By further applying 19 as the pure EML, the corresponding solution-processed nondoped device achieves a low Von of 3.1 V and a high maximum EQE of 17.1%. To enhance device performance, they further decorated precursor 5CzBN with different peripheral functional groups (20–23).64 Sufficient Cz decoration can effectively encapsulate the central chromophore and thus prevent these units from being redissolved by isopropanol. Therefore, they can be used in fully solution-processed devices with a device structure of ITO/PEDOT:PSS (40 nm)/EML (40 nm)/PO-T2T (30 nm)/Cs2CO3 (2 nm)/Al (100 nm). Compared with their monosubstituted counterparts (20 and 22), the disubstituted compounds (21 and 23) show superior device performances, which can be ascribed to the well-protected TADF emission core. Tang et al.65 also tried to decorate the 5CzBN core with different numbers of alkyl chain-linked spirobifluorene (SPF) groups (24–26). All these emitters show obvious AIDF characteristics. Moreover, with increasing flexible dendron numbers, intermolecular interactions can be well restricted. Thus, the 26 pristine film shows better resistance to isopropyl alcohol than the others, benefiting the solution fabrication process. The corresponding fully solution-processed nondoped device achieves a maximum EQE of 20.1%. All these results suggest that sufficiently peripheral decorations act as a feasible method to construct TADF emitters for fully solution-processed devices.
To further broaden the luminescence range of CN-based TADF materials, adjusting the numbers and positions of the CN groups is a feasible method. 4,5-di(9H-carbazol-9-yl)phthalonitrile (2CzPN) is a well-known blue TADF emitter; however, due to its poor solubility, it is rarely used in solution-processed devices. To exploit 2CzPN-based materials for solution-processed OLEDs, Choi et al.66 reported a novel AIE and TADF-active triad 28 and a control compound, 4,5-bis(3-phenyl-9H-carbazol-9-yl)phthalonitrile (27). Compared with the parent molecule (27), introducing PCz and nonconjugated cyclohexane units as the host and bridge moieties in 28, respectively, can effectively enlarge the intermolecular distance without affecting the electroluminescence (EL) spectrum. In solution-processed nondoped OLEDs, they exhibit nearly identical green EL emissions with peaks at 524 nm, while the maximum EQE of 28 (13.4%) is significantly better than that of 27 (7.5%).
Wang et al.67 also applied the isophthalonitrile moiety, in which two cyano groups are at the meta-positions, to design D–π–A-type TADF materials 29–31. The nondoped device using Cz-decorated 31 as the EML shows a superior device performance to the others, which can be reasonably ascribed to the following reasons: (1) relatively high hole mobility of the TPA group; (2) the rigid and twisted molecular conformation, which can effectively suppress the aggregation-caused quenching (ACQ) effect. Likewise, 2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile (4CzIPN) is also decorated for nondoped devices due to its excellent device performance. Nevertheless, it shows a high concentration sensitivity and poor nondoped device efficiency due to the tendency of forming Cz-based dimers.68 To achieve 4CzIPN-based high-performance nondoped devices, Jiang et al.69 connected the emission core 4CzIPN with the host matrix via flexible alkyl chains by applying a self-host molecular design strategy. In particular, the peripherally decorated 1,3-di(9H-carbazol-9-yl)benzene/tris(4-(9H-carbazol-9-yl)phenyl)amine (mCP/TCTA) moiety can form a high triplet level interfacial exciplex with the electron-transporting layer (ETL). Due to the extra intramolecular exciplex under electrical stress, the 33-based nondoped device exhibits a much lower device performance than 32.
Circularly polarized luminescence (CPL) has great applications in optical data storage, quantum computing, bioresponsive imaging, liquid crystal displays, and backlights in 3D displays. Pan et al.70,71 developed a series of chiral TADF emitters 34 and 35 by merging chiral (R/S)-octahydro-binaphthyl ((R/S)-OBN) moieties with effective CN-based TADF skeletons. Similar to the abovementioned TPA/Cz-based compounds,67 the rigid and twisted molecular conformation of 34 effectively suppresses triplet exciton quenching.
3.2. Carbonyl-substituted aromatics as acceptors
Aromatic units containing strong electron-withdrawing carbonyl groups in the center, such as benzophenone (BP) and its derivatives, have been widely used as the A unit for designing TADF emitters. The molecular structures of BP-based TADF emitters are shown in Fig. 5, and the corresponding key parameters of the nondoped devices are summarized in Table 2. Adachi et al.72 reported the first D–A–D type BP-based TADF emitter 36 for high-performance nondoped OLEDs. A rigid and steric 9,9-dimethyl-9,10-dihydroacridine (DMAC) moiety can effectively restrain long-lived triplet exciton quenching, and its amorphous neat film thus displays a high PLQY of 0.85 and a short delayed lifetime (τd) of 2.7 μs, guaranteeing efficient nondoped devices. As summarized in Table 2, the 36-based nondoped OLED exhibits a superior performance with a maximum EQE of 18.9% with mild efficiency roll-off at high luminance. Yang et al.73 further modified precursor 36 by encapsulating the DMAC moiety with tBuCz and 3,6-bis-(3,6-di-tert-butylcarbazol-9-yl)-carbazole (tBuCz2) groups and developed two novel TADF dendrimers 37 and 38 for solution-processed nondoped devices. Both dendrimers exhibit good thermal and morphological stabilities, guaranteeing homogeneous and stable amorphous thin films via solution-processed fabrication. Owing to the significantly decreased carrier transport arising from the large steric hindrance of the tBuCz2 unit, the 37-based device exhibits a much lower driving voltage of 4.8 V at a luminance of 10 cd m−2 than that of 38 (7.7 V). Moreover, the nondoped device using 37 as the EML shows a maximum EQE of 12.0%, three times larger than that of the 38-based device. Cheng et al.74 also reported a series of TADF polymers 39 by using conjugated Cz/acridine moieties as the backbone and BP as the pendant A moiety. The solution-processed nondoped device achieves a high device performance with a very mild efficiency roll-off.| PL | EL | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLQY | τ p [ns] | τ d [μs] | Peak [nm] | V on [V] | CE/PE/EQE [cd A−1/lm W−1/%] | Roll-offb [%] | CIE (x,y) | |||
| Maximum | @103 cd m−2 | |||||||||
| a Turn-on voltage at a luminance of 1 cd m−2. b EQEroll-off = (EQEmax − EQE1000)/EQEmax. | ||||||||||
| 36 | 0.85 | — | 2.7 | 510 | 2.6 | —/—/18.9 | —/—/18.0 | 4.8 | (0.26, 0.55) | 72 |
| 37 | 0.77 | — | — | 546 | — | —/—/12.0 | —/—/11.9 | 0.8 | (0.38, 0.56) | 73 |
| 38 | 0.75 | — | — | 522 | — | —/—/5.2 | —/—/4.1 | 21.1 | (0.32, 0.51) | |
| 39 | 0.77 | 22.0 | 1.3 | ∼550 | 2.6 | 60.5/61.2/18.1 | 59.4/50.4/17.8 | 1.6 | (0.40, 0.56) | 74 |
| 40 | 0.65 | 0.45 | 0.6 | 506 | 4.5 | 9.2/—/4.3 | — | — | (0.26, 0.46) | 75 |
| 41 | 0.33 | 10.4 | 2.7 | 500 | 3.4 | 14.0/11.5/5.7 | — | — | (0.26, 0.48) | 76 |
| 42 | 0.21 | 20.8 | 3.4 | 516 | 3.7 | 7.7/5.7/2.9 | — | — | (0.31, 0.50) | |
| 43 | 0.74 | 11.9 | 2.2 | ∼504 | 2.7 | 46.6/40.7/17.0 | —/—/13.8 | 18.8 | (0.27, 0.52) | 77 |
| 44 | 0.24 | 40.8 | 0.9 | ∼541 | 2.7 | 18.9/19.2/6.0 | 18.0/16.1/5.8 | 3.3 | (0.38, 0.55) | 78 |
| 45 | 0.22 | 53.5 | 0.9 | ∼551 | 2.6 | 17.8/20.0/5.9 | 12.0/10.2/4.8 | 18.6 | (0.41, 0.54) | |
| 46 | 0.51 | 46.7 | 0.8 | ∼530 | 2.5 | 20.9/21.8/6.4 | 18.5/17.1/5.4 | 15.6 | — | 79 |
| 47 | 0.44 | 37.3 | 0.5 | ∼530 | 2.5 | 32.3/33.0/9.8 | 31.2/31.9/9.7 | 1.0 | — | |
| 48 | 0.22 | 20.0 | 1.3 | 492 | 4.4 | 9.7/6.1/4.2 | —/—/1.3 | 69.0 | (0.19, 0.36) | 80 |
| 49 | 0.37 | 20.4 | 1.0 | 508 | 4.4 | 24.0/15.6/8.5 | —/—/4.5 | 47.0 | (0.26, 0.50) | |
| 50 | 0.57 | 25.9 | 5.0 | 494 | 3.2 | 34.7/30.1/14.2 | —/—/10.8 | 23.9 | (0.20, 0.39) | |
| 51 | 0.72 | 29.8 | 1.9 | 512 | 3.2 | 56.4/43.5/18.7 | —/—/17.9 | 4.3 | (0.28, 0.53) | |
| 52 | 0.80 | 40.4 | 2.9 | 516 | 2.7 | 43.3/35.7/14.2 | 43.1/33.1/14.2 | 0.0 | (0.26, 0.55) | 81 |
| 53 | 0.38 | 38.3 | 1.8 | 557 | 2.9 | 26.6/27.9/9.2 | 19.6/11.3/6.8 | 26.1 | (0.43, 0.54) | 82 |
| 54 | 0.40 | 27.6 | 1.3 | 563 | 2.7 | 26.5/29.1/9.7 | 23.5/15.4/8.5 | 12.4 | (0.45, 0.53) | |
| 55 | — | — | — | 586 | 5.4 | 37.6/14.8/16.7 | 26.9/10.8/11.8 | 29.3 | — | 83 |
| 56 | 0.69 | — | — | 501 | 2.8 | 42.7/39.8/15.6 | 41.0/27.0/15.0 | 3.8 | (0.25, 0.48) | 84 |
| 57 | 0.37 | — | — | 503 | 3.9 | 18.2/14.1/6.6 | 13.1/5.5/4.8 | 27.3 | (0.26, 0.47) | |
| 58 | 0.45 | 30.2 | 1.4 | ∼560 | 2.7 | 39.9/39.0/13.3 | 37.4/24.0/12.5 | 6.0 | (0.44, 0.54) | 85 |
| 59 | 0.49 | 27.9 | 1.1 | ∼564 | 2.6 | 41.6/45.0/14.3 | 41.4/26.0/14.1 | 1.4 | (0.46, 0.53) | |
| 60 | 0.46 | 27.5 | 1.1 | ∼562 | 2.5 | 36.8/37.9/12.3 | 36.7/28.8/12.2 | 0.8 | (0.46, 0.53) | |
| 61 | 0.47 | 19.1 | 1.0 | 499 | 4.5 | 14.3/6.4/6.7 | — | — | (0.28, 0.47) | 86 |
| 62 | 0.67 | 7.2 | 1.4 | 532 | 4.2 | 35.4/15.9/11.4 | — | — | (0.34, 0.51) | |
| 63 | 0.53 | 12.7 | 1.1 | 544 | 4.3 | 23.8/10.7/9.1 | — | — | (0.39, 0.56) | |
| 64 | 0.33 | 13.5 | 0.9 | 545 | 4.8 | 12.4/4.3/4.8 | — | — | (0.41, 0.55) | |
| 65 | 0.48 | 8.4 | 1.1 | 572 | 4.6 | 21.6/6.8/9.4 | — | — | (0.47, 0.51) | |
| 66 | 0.45 | 19.1 | 5.7 | 502 | 2.7 | 41.6/37.9/15.0 | 41.5/32.6/14.9 | 0.7 | (0.23, 0.49) | 44 |
| 67 | 0.67 | 21.9 | 5.5 | 548 | 2.5 | 59.1/65.7/18.4 | 58.4/49.7/18.2 | 1.1 | (0.40, 0.57) | |
| 68 | 0.58 | 23.5 | 2.1 | 554 | 2.5 | 46.1/55.7/15.3 | 38.4/30.2/12.7 | 17.0 | (0.42, 0.55) | |
| 69 | 0.73 | 22.2 | 0.8 | 540 | 2.8 | 76.6/75.2/21.7 | 69.8/52.2/19.8 | 8.8 | (0.38, 0.58) | 22 |
| 70 | 0.70 | 22.2 | 0.7 | 537 | 2.6 | 72.5/53.5/20.4 | 68.5/43.1/19.4 | 4.9 | (0.37, 0.59) | |
| 71 | 0.73 | 22.1 | 0.4 | 541 | 2.8 | 72.1/65.4/20.6 | 69.0/51.6/19.7 | 4.4 | (0.39, 0.58) | |
| 72 | 0.69 | 22.4 | 2.6 | 548 | 2.5 | 72.9/81.8/22.6 | —/—/22.0 | 2.6 | (0.39, 0.57) | 87 |
| 73 | 0.72 | 21.1 | 2.4 | 546 | 2.5 | 69.0/75.0/21.4 | —/—/20.9 | 2.3 | (0.39, 0.57) | |
| 74 | 0.66 | 23.7 | 2.3 | 542 | 2.5 | 72.3/79.0/22.1 | —/—/22.1 | 0.0 | (0.39, 0.57) | |
| 75 | 0.71 | 23.0 | 2.4 | 542 | 2.5 | 70.4/76.5/21.8 | —/—/21.8 | 0.0 | (0.38, 0.57) | |
| 76 | 0.88 | 22.3 | 2.1 | 540 | 2.6 | 62.2/63.7/19.0 | 60.7/47.5/18.5 | 2.6 | (0.39, 0.57) | 88 |
| 77 | 0.89 | 22.2 | 1.7 | 544 | 2.5 | 61.1/67.4/18.5 | 60.3/60.1/18.2 | 1.6 | (0.38, 0.57) | |
| 78 | 0.40 | 18.9 | 0.9 | 548 | 2.7 | 10.8/8.6/3.3 | 10.7/8.1/3.2 | 3.0 | (0.40, 0.56) | |
| 79 | 0.66 | 12.5 | 0.8 | 535 | 4.2 | 37.2/14.6/12.1 | — | — | (0.38, 0.56) | 89 |
| 80 | 0.31 | 17.5 | 0.4 | 592 | 4.8 | 10.7/4.8/4.9 | 7.2/2.2/3.2 | 34.7 | (0.54, 0.45) | 90 |
| 81 | 0.32 | 15.0 | 0.2 | 606 | 3.0 | 6.5/4.0/3.7 | 6.3/3.3/3.5 | 5.4 | (0.57, 0.42) | |
| 82 | 0.31 | 23.0 | 1.4 | 577 | 3.0 | —/—/7.6 | —/—/∼6.1 | 19.7 | — | 91 |
| 83 | 0.53 | 29.0 | 1.9 | 553 | 3.0 | —/—/11.1 | —/—/∼4.2 | 62.2 | — | |
| 84 | 0.50 | 33.3 | 1.2 | 488 | 3.9 | —/—/5.2 | —/—/2.2 | 57.7 | — | 46 |
| 85 | 0.82 | 23.0 | 2.0 | 497 | 3.6 | —/—/12.6 | —/—/9.5 | 24.6 | — | |
| 86 | 0.92 | 35.0 | 2.2 | 488 | 3.5 | —/—/11.2 | —/—/5.9 | 47.3 | — | |
| 87 | 0.84 | 22.7 | 5.3 | 488 | 3.8 | —/—/14.1 | —/—/10.5 | 25.5 | — | |
| 88 | 0.96 | 43.3 | 1.4 | 526 | 2.9 | 74.0/67.0/21.0 | 74.0/61.0/21.0 | 0.0 | (0.31, 0.61) | 92 |
| 89 | 0.94 | 34.6 | 1.9 | 496 | 3.5 | 55.0/42.0/21.0 | 46.0/27.0/18.0 | 14.3 | (0.22, 0.49) | |
| 90 | — | — | 112 | 520 | — | 69.0/50.0/22.6 | —/—/∼4.0 | 82.3 | — | 93 |
| 91 | 0.76 | 10.0 | 1.7 | 549 | 2.4 | 50.5/60.0/17.1 | 42.7/26.5/13.8 | 19.3 | (0.42, 0.55) | 94 |
| 92 | 0.53 | 25.0 | 2.8 | 462 | 3.6 | —/14.7/12.1 | —/—/7.1 | 41.3 | (0.15, 0.19) | 95 |
| 93 | 0.71 | 33.0 | 2.8 | 478 | 3.3 | —/25.7/15.0 | —/—/11.1 | 26.0 | (0.16, 0.29) | |
| 94 | 0.78 | 24.0 | 3.8 | 478 | 3.0 | —/31.5/16.2 | —/—/12 | 25.9 | (0.17, 0.29) | |
| 95 | 0.47 | 17.9 | 5.3 | 496 | 3.0 | 32.7/34.3/12.9 | 13.8/10.1/4.3 | 66.7 | (0.21, 0.42) | 96 |
| 96 | 0.93 | 24.4 | 2.5 | 504 | 3.1 | 63.7/54.1/22.8 | 61.4/49.9/22.4 | 1.8 | (0.23, 0.50) | |
| 97 | 0.98 | 24.9 | 1.3 | 516 | 3.1 | 65.7/51.6/21.3 | 64/53.4/20.8 | 2.3 | (0.27, 0.56) | |
| 98 | 0.96 | 37.2 | 3.1 | 508 | 2.9 | —/59.7/24.9 | —/46.0/21.7 | 12.8 | (0.24, 0.49) | 97 |
| 99 | 0.55 | — | — | 517 | 3.6 | 17.6/13.9/5.9 | — | — | (0.30, 0.51) | 98 |
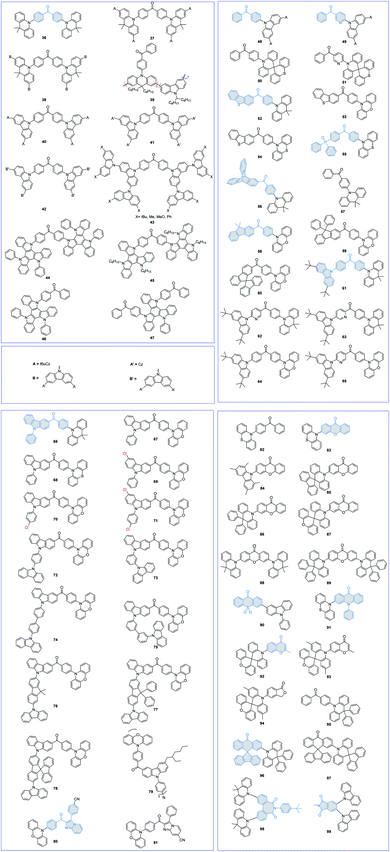
By replacing the DMAC moiety with a soluble tBuCz2 moiety, Sun et al.75 synthesized a novel dendronized TADF emitter (40) for solution-processed nondoped green OLEDs. Although the 40-based neat film exhibits a high PLQY (0.65) and a small ΔEST (0.08 eV), its solution-processed device shows a poor performance with a maximum EQE of 4.3%, which may be ascribed to the decreased bipolar carrier transport.54 Fujita et al.76 also designed a series of BP-based AIDF dendrimers (41 and 42) for solution-processed nondoped devices. By further modulating the terminal groups (43),77 they found that the terminal groups can dramatically alter the photophysical properties of dendritic TADF emitters. The introduction of tBu and phenyl groups favors the AIE character, while the introduction of a methoxy group results in emission quenching. Thus, it is important to choose suitable terminal functional groups for designing TADF emitters for solution-processed nondoped devices.
Triazatruxene (TAT), a planar conjugated system with three fused carbazole fragments that share a benzene ring, acted as a powerful D group to construct TADF emitters. Wang et al.78 first characterized two TAT-based TADF emitters 44 and 45 for solution-processed devices. Compared with the long alkyl-decorated TAT unit in 45, the four peripheral phenyl substituents in 44 can effectively enlarge the distance of adjacent triplet excitons. Therefore, although both emitters realize identical device performances with maximum EQEs of approximately 6.0%, the 45-based device displays a more serious efficiency roll-off at high luminance. To further improve the device performance, they also synthesized two asymmetrical D–A-type AIDF emitters 46 and 47 by using phenyl-substituted TAT and BP as the D and A groups, respectively.79 The 47-based solution-processed nondoped device exhibits a higher device efficiency than 46, possibly due to the balanced carrier transport caused by the introduction of one more BP unit.
Compared with D–A–D-type symmetric molecular structures, BP-based TADF materials with unsymmetrical structures have drawn more attention due to the advantages of effective regulation of molecular packing and bipolar carrier transport. Su et al.80 developed a series of BP-based D–A-type TADF materials 48–51. Compared with the parent BP moiety, the pyridine-containing 3-benzoylpyridine group can significantly enhance the molecular rigidity by forming an intramolecular hydrogen bond. Therefore, pyridine-decorated 49 and 51 exhibit slightly reduced ΔESTs and obviously improved PLQYs compared with their BP-based counterparts (48 and 50). Moreover, the rigid 10H-spiro(acridine-9,9′-thioxanthene) (TXDMAc) group can effectively shield the electron-rich acridine core and restrict concentration quenching. As a result, the nondoped OLEDs obtained by using TXDMAc-based TADF emitters 50 and 51 as the EMLs show superior performances, with maximum EQEs of 14.2% and 18.7%, much higher than those of Cz-based OLEDs (maximum EQEs of 4.2% and 8.5%, respectively, for 48 and 49).
Tang et al.81 also developed a D–A–D′-type AIDF emitter 52 by using DMAC and dibenzothiophene (DBT) moieties as the D and D′, respectively. The DMAC-BP segment is anticipated to form a twisted molecular conformation to realize AIE and TADF properties, while planar DBT may help to increase the charge-transporting ability. Therefore, the 52 neat film displays a high PLQY of 0.80 and a smaller ΔEST of 0.08 eV, benefiting the utilization of electrogenerated excitons. By further replacing the DMAC moiety with electron-rich PXZ and phenothiazine (PTZ) moieties, two analog AIDF emitters 53 and 54 were also synthesized.82 However, since unprotected PXZ/PTZ groups tend to aggregate in solid-states and can induce severe triplet exciton quenching, their nondoped device performance is much worse than that of 52 (maximum EQEs of 14.2%, 9.2%, and 9.7% for 52, 53, and 54, respectively).
By introducing a diphenylphosphoryl (DPO) unit as the A′, Chi et al. designed an unsymmetrical D–A–A′-type TADF emitter 55.83 The corresponding nondoped device demonstrates orange emission with a peak at 586 nm and a maximum EQE of 16.7%, which is among the best performances of TADF-based nondoped OLEDs in the orange-red region.
To enhance the rigidity of the BP skeleton, Lu et al.84 prepared a novel triptycene-fused BP moiety, and a D–A-type TADF emitter 56 was then designed. For comparison, D–A-type emitter 57 was also synthesized by using DMAC and BP as the D and A moieties, respectively. Introducing a rigid and steric triptycene scaffold can not only strengthen the molecular rigidity but also effectively restrict intermolecular interactions in aggregated states. Therefore, the nondoped device using 56 as the EML achieves a maximum EQE of 15.6%, much higher than that of 57 (a maximum EQE of 4.8%).
Tang et al.85 also used steric fluorene derivatives to decorate the precursor BP-PXZ and developed three AIDF emitters 58–60 for nondoped OLEDs. The highly twisted conformation with large sterically hindered fluorene groups can effectively suppress the short-range Dexter energy transfer (DET) process at high exciton concentrations, thus significantly relieving the annihilation of triplet excitons. On this account, the corresponding nondoped OLEDs all exhibit good device performance with maximum EQEs over 12.3%.
Qi et al.86 demonstrated a class of efficient AIDF materials 61–65 bearing pyridine-decorated BP as the A moiety and tBuCz/DMAC/PXZ as the D moieties. The presence of intramolecular hydrogen bonding can effectively suppress nonradiative decay and improve the luminescence efficiency of the aggregated solid-state. Meanwhile, compared with the planar PXZ unit (64 and 65), the steric DMAC group in 61–63 can effectively restrict the intermolecular π–π packing.
By replacing tBuCz with a 9-phenyl-9H-carbazole (PCz) moiety, three unsymmetrical AIDF emitters 66–68 were synthesized by Tang et al.44 As shown in Fig. 6, the PCz moiety can effectively impede close molecular packing and weaken intermolecular interactions. Therefore, concentration-induced emission quenching and exciton annihilation can be greatly suppressed. Meanwhile, the electron-rich PXZ group in 67 favors a smaller ΔEST (0.024 eV) and shorter lifetime τd (2.1 μs) than those of 66 (0.07 eV and 2.7 μs) in neat film states. Therefore, the 67-based nondoped device shows a superior performance with a maximum EQE of 18.4% compared to 66 (maximum EQE of 15.0%). To further explore the methods to alleviate the concentration quenching of PXZ-based TADF emitters, they proposed accelerating the RISC process by introducing heavy atoms (69–71).22 Compared with the precursor AIDF emitter 67, chlorine-modified 69–71 exhibit higher PLQYs and shorter τd values, which can be attributed to the heavy atom effect that greatly increases the SOC values and significantly accelerates the RISC process. As a result, their nondoped OLEDs exhibit outstanding device performances with excellent maximum EQEs over 20.4%. To further improve the device performance, they also proposed a self-host molecular design strategy by grafting the AIDF 4-(phenoxazin-10-yl)benzoyl moiety to common host materials, including 1,4-di(carbazol-9-yl)benzene (DCB), CBP, mCP, and 3,3′-di(carbazol-9-yl)biphenyl (mCBP), and developed materials 72–75.87 The photophysical and electrochemical behaviors of 72–75 are very similar and seem to be mainly determined by the parent 4-(phenoxazin-10-yl)benzoyl fragment. The nondoped OLEDs using these generated luminogens exhibit outstanding performances with excellent maximum EQEs (22.1–22.6%) and high efficiency stability. All these results demonstrate that introducing a host matrix can effectively relieve triplet exciton annihilation without affecting the EL emission. They also decorated the PCz moiety with large steric carbazole-substituted fluorene derivatives (76–78) to study the influences of subtle modulations on the bulkiness and stiffness of fluorene derivatives (9,9-dimethyl-9H-fluorene, 9,9-diphenyl-9H-fluorene, and 9,9′-spirobi[fluorene]) on the PL and EL properties of AIDF materials.88 The results reveal that fluorene derivatives can effectively inhibit intermolecular interactions. On the other hand, the distorted and large steric conformation of the 9,9′-spirobi[fluorene] segment in 78 significantly decreases the electron mobility compared with the others (76 and 77). Eventually, the 78-based nondoped device exhibits a much lower maximum EQE of 3.3% than the others (maximum EQEs of 19.0% and 18.5% for the 76- and 77-based devices, respectively).
 | ||
| Fig. 6 Schematic illustration of the main exciton dynamic processes for (a) a conventional luminogen with delayed fluorescence and (b) an AIDF emitter in nondoped OLEDs. Reproduced from ref. 44 with permission. Copyright 2017 Wiley-VCH. | ||
To improve the solubility of AIDF emitters for solution-processed nondoped OLEDs, Qi et al.89 exploited propeller-like dendritic luminogen 79 by connecting the central triphenylamine (TPA) unit with circumambient AIDF emitter (BP-PXZ derivative) arms. The branched alkyl chains in the Cz unit are beneficial for increasing the free volumes, which are important for improving the film-forming ability and preparing pinhole-free uniform films during the solution process. Using 79 as the nondoped EML in a solution-processed OLED with a device structure of ITO/PEDOT:PSS (40 nm)/79 (40 nm)/TPBi (30 nm)/Cs2CO3 (2 nm)/Al (100 nm) realizes green emission with a peak at 529 nm and a maximum EQE of 12.1%.
Su et al.90 reported two unsymmetrical D–A–A′-type AIDF emitters 80 and 81 by using PXZ, BP, and CN-substituted 3-phenyl-3,8a-dihydroimidazo[1,2-a]pyridine as the D, A, and A′ moieties, respectively. The introduction of strong electron-withdrawing CN groups can effectively redshift the emission to the long wavelength region. Therefore, the 80- and 81-based nondoped devices show ideal orange-red to red emissions with peaks at 592 and 610 nm, CIE coordinates of (0.54, 0.45) and (0.57, 0.42), and maximum EQEs of 4.9% and 3.7%, respectively.
Yasuda et al.91 attempted to improve the IQE of BP-based AIDF emitters by increasing the rigidity of the BP unit. Compared with precursor 82, fixing the BP unit with an oxygen bridge (xanthone (XT)) in 83 can effectively restrict the nonradiative process of the A moiety. Therefore, the 83 amorphous neat film exhibited a higher PLQY value of 0.53 than the 0.31 for 82. They further clarified the actual mechanism of concentration quenching of TADF emitters by replacing the PTZ unit in 83 with rigid and steric 1,3,6,8-tetramethylcarbazole (MCz), 9,9-diphenylacridan (DPAc), spiro[acridan-9,9′-xanthene] (SXAc), and spiro[acridan-9,9′-fluorene] (SFAc) groups (84–87).46 As shown in Fig. 7, they first revealed that the concentration quenching of TADF molecules was dominated by electron-exchange interactions for triplet excitons, and the inhibited intermolecular electron-exchange interactions in their condensed solid-states were the key to exploiting efficient TADF emitters for nondoped OLEDs.
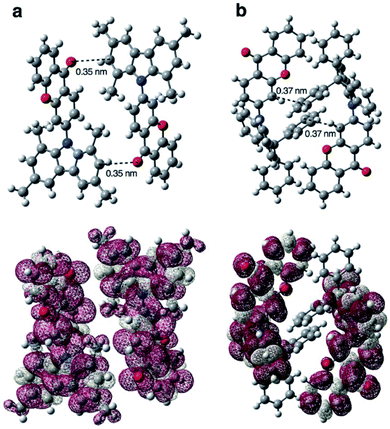 | ||
| Fig. 7 Calculated spin-density distributions of the lowest-excited triplet states of (a) 84 and (b) 86 in the solid-state geometry determined by single-crystal X-ray analysis. Reproduced from ref. 46 with permission. Copyright 2016 Wiley-VCH. | ||
By using XT as the A group, Tang et al.92 also reported two D–A–D-type robust AIDF emitters 88 and 89. Owing to the AIE character and well-restricted intermolecular interactions, both emitters show small ΔESTs of 0.025 and 0.024 eV, and excellent PLQYs of 0.96 and 0.94 in neat films, guaranteeing high-performance nondoped devices. Therefore, the 88- and 89-based nondoped devices emit intense green and sky-blue light with maximum EQEs of approximately 21.0%. Moreover, the 88-based device displays outstanding efficiency stability with no efficiency roll-off (∼0%) when the luminance increases to 1000 cd m−2, which can be ascribed to the high and balanced hole and electron mobilities. However, compared with the performance of optimized doped devices (maximum EQEs of 25.0% and 27.0% for 88 and 89, respectively), the efficiencies of nondoped devices are still less satisfactory, which can be reasonably ascribed to the unprotected electron-rich XT moiety. These results further indicate that both D and A moieties should be well shielded to suppress potential intermolecular packing-induced exciton quenching.
Wang et al.93 reported a special ultrathin EML with a quantum well (QW) structure by using a previously reported TADF emitter 90. The QW structure can effectively confine the charge carriers and excitons, affording high-efficiency and low-efficiency roll-off. A maximum EQE of 22.6% was achieved for the 90-based nondoped device with seven quantum wells without any light outcoupling enhancement. However, the device shows severe efficiency roll-off at high luminance, maintaining only a small EQE of ∼4.0% at a luminance of 1000 cd m−2.
Conformational status is a fundamental issue for organic molecules to determine their overall properties. Wang et al.94 developed TADF emitter 91 with dual stable conformations at the ground state. Both theoretical and physical tests show that the QE conformer with TADF characteristics plays a dominant role at the ground state, guaranteeing effective utilization of the excitons. Therefore, the 91-based yellow nondoped device realizes a maximum EQE of 17.1%, current efficiency (CE) of 50.5 cd A−1, and power efficiency (PE) of 60.0 lm W−1.
To exploit efficient BP-based blue TADF emitters for nondoped OLEDs, Yasuda et al. reported a series of TADF emitters (92–94) combining isobenzofuranone (BF) or chromone (CM) as new A units with SXAc-based D units.95 These materials realize bright blue TADF emissions centered at approximately 460–485 nm, with high PLQYs (0.53–0.78) and short τds (2.8–3.8 μs).
TADF emitters with AIE features are hot candidates for nondoped devices, as they are highly emissive in the solid-state upon photoexcitation. Nevertheless, not every AIDF emitter in the past had guaranteed decent efficiencies in nondoped devices, indicating that the AIE character alone does not necessarily afford ideal nondoped TADF emitters. As shown in Fig. 8, our group designed three AIDF emitters 95–97 to investigate the critical molecular design rules.96 The two new emitters (96 and 97) both retain the electron-donating and electron-withdrawing cores, as in reference compound 95. The BP and DPAc moieties were locked via a rigid fluorene group. Detailed physical measurements prove that these three compounds have similar energy levels as well as characteristics of AIE and TADF, while in neat films, 96 and 97 exhibit significantly higher exciton utilization in PL and EL processes, which can be ascribed to rigid steric fluorenes hindering intermolecular triplet–triplet interactions. Therefore, the nondoped OLEDs based on 96 and 97 display excellent maximum EQEs of 22.8% and 21.3%, respectively, which are evidently higher than that of the 95-based OLED (12.9%). All these results demonstrate a feasible strategy of molecular modification to improve the nondoped EL performance of practical AIDF molecule candidates.
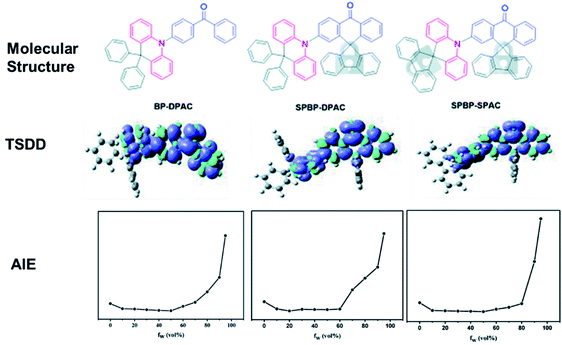 | ||
| Fig. 8 Molecular structures, calculated triplet spin density distribution (TSDD) of the lowest-excited triplet states, and PL intensity versus different water fractions (fw) of 95–97. Reproduced from ref. 96 with permission. Copyright 2020 the Royal Society of Chemistry. | ||
You et al.97 designed a brand-new A moiety of heptagonal diimide (N-(4-(tert-butyl)phenyl)-1,1′-biphenyl-2,2′-dicarboximide (BPI)) and synthesized a D–A–D-type TADF emitter 98. The rigidity of BPI can restrict excessive intramolecular rotation, thus enhancing kr, while moderate rotatability can inhibit close intermolecular π–π packing, reducing exciton quenching in the aggregated state. Therefore, the corresponding nondoped device demonstrates a stable green emission with an emission peak at 511 nm and CIE coordinates of (0.25, 0.51). Moreover, the device achieves a maximum EQE of 24.7% with a small efficiency roll-off. Takano et al.98 also applied phthalimide-based TADF emitter 99 to construct self-host cellulose derivatives for solution-processed nondoped OLEDs.
3.3. Sulfone-substituted aromatics as acceptors
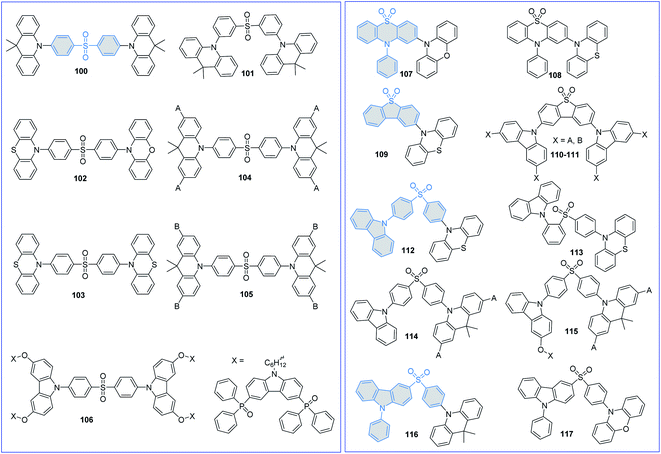
Sulfone-substituted aromatics, such as diphenyl sulfoxide (DPS) and derivatives, have been widely used to construct TADF emitters due to their suitable electron-withdrawing abilities and limited molecular conjugations. The molecular structures of sulfone-based TADF emitters are shown in Fig. 9, and the corresponding key parameters of the nondoped devices are summarized in Table 3. By replacing the BP group in D–A–D-type TADF emitter 36 with a DPS moiety, Adachi et al.72 also reported a novel efficient blue TADF emitter 100. The methyl groups on the DMAC unit can effectively inhibit the intermolecular interactions; thus, the performances of the 100-based devices are nearly concentration-independent. Moreover, in the neat film, 100 displays an even higher PLQY (0.88) than that of the doped film (0.80). To further lower the ΔEST and accelerate the RISC process, Guo et al.99 designed a novel AIDF emitter 101 with a highly twisted zigzag molecular configuration. Compared with the para-positioned control compound 100, connecting the DPS and DMAC units with a meta-position in 101 can effectively decrease the ΔEST and accelerate the RISC process. Therefore, the 101-based pristine film exhibits a smaller ΔEST value of 0.02 eV, and the OLED exhibits a higher maximum EQE of 14.0% than those of 100 (a ΔEST of 0.18 eV and a maximum EQE of 11.2%).| PL | EL | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLQY | τ p [ns] | τ d [μs] | Peak [nm] | V on [V] | CE/PE/EQE [cd A−1/lm W−1/%] | Roll-offb [%] | CIE (x,y) | |||
| Maximum | @103 cd m−2 | |||||||||
| a Turn-on voltage at a luminance of 1 cd m−2. b EQEroll-off = (EQEmax − EQE1000)/EQEmax. | ||||||||||
| 100 | 0.88 | — | — | 480 | 4.3 | —/—/19.5 | —/—/14.6 | 25.1 | (0.16, 0.29) | 72 |
| 101 | 0.72 | 57.9 | 4.3 | 488 | 3.1 | 31.7/28.4/14.0 | — | — | (0.18, 0.32) | 99 |
| 102 | — | — | — | ∼528 | 3.0 | —/—/17.0 | —/—/14.8 | 12.9 | — | 100 |
| 103 | — | — | — | — | 3.0 | —/—/∼14.0 | —/—/∼5.0 | 64.3 | — | |
| 104 | 0.68 | — | — | 502 | 3.6 | 30.6/24.0/12.2 | 11.3/5.1/4.5 | 63.1 | (0.22, 0.44) | 102 |
| 105 | 0.48 | — | — | 478 | 5.2 | 3.8/2.0/2.2 | — | — | (0.18, 0.27) | |
| 106 | 0.61 | — | — | 480 | 5.4 | 12.6/—/7.3 | — | — | (0.18, 0.30) | 103 |
| 107 | 0.62 | 16.1 | 2.5 | 504 | 4.3 | 44.9/32.0/16.4 | — | — | (0.27, 0.50) | 104 |
| 108 | 0.21 | 26.0 | 1870 | 510 | 3.4 | 9.2/8.3/3.3 | 0.8/0.2/0.3 | 90.9 | (0.29, 0.49) | 94 |
| 109 | — | — | — | — | 8.1 | 6.1/2.3/3.1 | — | — | — | 105 |
| 110 | 0.57 | 34.8 | 14.2 | 508 | 4.5 | 15.8/9.9/6.1 | — | — | — | 106 |
| 111 | 0.31 | 85.3 | 8.3 | 522 | 4.6 | 8.3/4.7/3.3 | — | — | — | |
| 112 | 0.97 | 6.3 | 62.2 | 518 | 3.5 | 61.2/38.4/20.7 | —/—/10.0 | 51.7 | — | 107 |
| 113 | 0.92 | 4.4 | 19.1 | 524 | 4.1 | 82.3/51.8/28.7 | —/—/2.8 | 90.2 | — | |
| 114 | 0.88 | 34.0 | 1.5 | 498 | 3.1 | 62.0/51.6/23.3 | — | — | (0.23, 0.42) | 108 |
| 115 | 0.96 | 22.0 | 1.4 | 500 | 3.0 | 65.9/59.2/24.0 | — | — | (0.24, 0.45) | |
| 116 | 0.65 | 7.7 | 2.4 | 484 | 2.9 | 17.0/15.7/9.1 | — | — | (0.16, 0.28) | 109 |
| 117 | 0.52 | 4.0 | 1.0 | 508 | 2.5 | 52.6/62.5/17.9 | — | — | (0.28, 0.52) | |
Lee et al.100 also designed DPS-based asymmetric and symmetric AIDF emitters 102 and 103 to investigate the relationship between molecular configuration and device performance. Since butterfly shaped PTZ groups induce conformational isomerizations,101 PXZ-based asymmetric AIDF emitter 102 thus exhibits superior nondoped device performance with a maximum EQE of 17.0%.
To exploit DPS-based TADF emitters for solution-processed nondoped OLEDs, Yang et al.102 proposed a multicarbazole encapsulation strategy. By introducing carbazole dendrons (tBuCz and tBuCz2) into TADF emissive core 100, two novel solution-processable DPS-based dendrimers 104 and 105 were synthesized. Multicarbazole encapsulation can not only improve the solubility but also minimize the concentration quenching effect, consequently reducing the quenching efficiency in OLEDs. Meanwhile, compared with tBuCz-decorated 104, applying large steric tBuCz2 as the encapsulation group can significantly lower carrier transport. Thus, the 105-based nondoped device exhibits a higher Von of 5.2 V and a lower maximum EQE of 2.2% than those of 104 (a Von of 3.6 V and a maximum EQE of 12.2%).
Sun et al.103 also exploited a self-host dendrimer 106 for solution-processed nondoped OLEDs, in which the bipolar (9H-carbazole-3,6-diyl)bis(diphenylphosphine oxide) moiety was introduced through a long alkyl chain to ensure balanced carrier transport. Peripheral bipolar dendrons can not only improve the morphological stability but also restrain the concentration quenching effect of the TADF emissive core bis[4-(3,6-dimethoxycarbazole)phenyl] sulfone (DMOC-DPS). Therefore, the spin-coated OLED featuring 106 achieves a maximum EQE of 7.3%.
To further reduce exciton annihilation, increasing the rigidity of the DPS group is a feasible method. By oxidizing the sulfur atom in the PTZ unit, Wang et al.104 designed a novel A moiety named 10-phenyl-10H-phenothiazine-5,5-dioxide (2PTO). Further introducing rigid and planar PXZ units as the D moiety, green AIDF emitter 107 with a highly stereoscopic structure was thus synthesized. The 107-based nondoped device exhibits a high efficiency with a maximum EQE of 16.4%, which is comparable with that of the corresponding doped counterpart. They also tried to replace the planar PXZ unit (107) with the butterfly shaped PTZ unit (108).94 Owing to the stable QA conformer at the ground state, 108 shows conventional fluorescence emission. Therefore, compared with the BP-based analog 91, 108 exhibits a lower PLQY and poorer device performance. These results indicate that molecular conformational distributions will significantly influence device performance and need more in-depth research.
Dias et al.105 designed TADF emitter 109 by using butterfly shaped PTZ and rigid dibenzothiophene-S,S-dioxide (DBTO2) as the D and A moieties, respectively. The presence of a suitable 3LE (2.66 eV of the DBTO2 group) close to the CT manifold (2.61 eV of 109) is expected to accelerate the RISC process and facilitate the utilization of triplet excitons. Nevertheless, compared with the doped ones (a maximum EQE of up to 22.0%), its nondoped device only exhibits a very poor performance with a maximum EQE of only 3.1%. This is mainly attributed to the low charge mobilities. By utilizing the dendronization strategy on precursor molecule 2,8-bis(3,6-di-tert-butyl-9H-carbazol-9-yl)dibenzo[b,d]thiophene-5,5-dioxide (tBuCz-DBTO2), Yang et al.106 designed a series of DBTO2-based dendrimers 110–111 for solution-processed nondoped OLEDs. Encapsulation of the parent tBuCz-DBTO2 TADF core with a soluble tBuCz moiety can not only enhance the morphological stabilities but also effectively lower the ΔESTs by decreasing the overlap of the FMOs. As a result, their solution-processed nondoped devices exhibit gradually redshifted EL emissions (456, 508 and 522 nm for tBuCz-DBTO2, 110 and 111, respectively) with increasing generation number of dendrimers. In addition, the 110-based device displays the best EL performance with a maximum EQE of 6.1%, which is nearly double that of the 111-based device (3.3%) and more than 20-fold higher than that of the tBuCz-DBTO2-based device (0.3%).
To regulate the molecular packing and bipolar carrier transport more conveniently, asymmetric DPS-based TADF emitters have been extensively investigated. Chi et al.107 designed two asymmetric D–A–D′-type AIDF emitters 112 and 113 by using PTZ, DPS, and Cz as the D, A, and D′ moieties, respectively. Further introducing an ortho-positioned Cz unit in 113 guarantees the coexistence of intramolecular electrostatic attractions and through-space CT, leading to reduced structural vibrations, suppressed nonradiative decay, and rapid radiative decay to avoid excited state energy loss. As a result, the 113-based nondoped device exhibits a state-of-the-art performance with a maximum EQE of 28.7%. However, at 1000 cd m−2, the EQE of this nondoped device dramatically declined to approximately 2.8%. Such a serious efficiency roll-off can be ascribed to the unbalanced carrier mobilities.
Yan et al. proposed that the poor device performance of DPS-based fully conjugated (104) or fully nonconjugated (106) structures can be attributed to the unwanted degenerate excited states generated by multiple identical dendrons. To suppress the formation of degenerate excited states, two new asymmetrical “half-dendronized” (114) and “half-dendronized–half-encapsulated” (115) emitters have been designed and synthesized.108 Both emitters show distinct AIDF properties, which minimize the exciton quenching in aggregated states, and consequently reduce their efficiency roll-off in their nondoped devices. Moreover, the asymmetric molecular structures successfully limit the unwanted degenerate excited states, favoring the TADF characteristics. As a result, the solution-processed nondoped devices using 114 and 115 as the pure EMLs achieved maximum EQEs of 23.3 and 24.0%, respectively.
Wang et al. also reported two asymmetric AIDF emitters 116 and 117 by using DMAC/PXZ, DPS, and PCz as the D, A, and D′ moieties, respectively.109 Introducing the PCz moiety can effectively restrain the intermolecular interactions in the aggregated states. Therefore, both emitters exhibit typical AIDF characteristics. Moreover, due to the obviously enhanced electron-donating ability of the PXZ unit, the 117 amorphous neat film exhibits a smaller ΔEST of 0.03 eV and shorter τd of 1.0 μs than 116 (ΔEST and τd of 0.07 eV and 2.4 μs, respectively). Therefore, the nondoped OLEDs obtained by using 116 and 117 as the pure EMLs achieve maximum EQEs of 17.9 and 9.1%, respectively.
3.4. Nitrogen-containing heterocycles as acceptors
Nitrogen-containing heterocycles, such as terpyridine, pyrimidine, quinoline, quinoxaline, triazine, and dibenzo[a,c]phenazine, have been widely used to exploit TADF emitters for nondoped OLEDs. Herein, we classify nitrogen-containing heterocycles based on their different electron-withdrawing abilities (exhibited in Fig. 10 and summarized in Table 4). To effectively restrict exciton quenching in the aggregated solid-state, we have proposed a novel strategy of exploiting high-performance nondoped electroluminescence by tuning intermolecular hydrogen bonding.50 Three TADF isomers 118–120 were synthesized by using PXZ and o/m/p-terpyridine as the D and A moieties, respectively. As displayed in Fig. 11, suitable intermolecular hydrogen bonding (119) enables the formation of a 3D supramolecular framework, which can evidently restrict the nonradiative process and suppress triplet exciton quenching caused by π–π packing of triplets. Moreover, such a 3D supramolecular structure also favors horizontal molecular orientations, which can evidently increase the outcoupling efficiency and benefit realization of high device performance.110 Therefore, the 119-based nondoped device exhibits state-of-the-art performance with a maximum EQE of 23.6% with only 7.2% roll-off at 1000 cd m−2. Moreover, it is the first report that the performance of a TADF-based nondoped device can evidently surpass that of its corresponding optimized doped counterpart (maximum EQE = 16.3%), indicating that the intermolecular hydrogen bonding mechanism can not only provide a new pathway for designing TADF emitters for high-performance nondoped devices, but also has great potential in other solid-state luminescence applications. By replacing the peripheral pyridine ring in 119 with a more electron-withdrawing pyrimidine unit, we further synthesized a novel TADF emitter 121.111 Unlike the formation of a 3D framework in 119, the X-ray data show that a 1D chain can be observed in 121. Such a linear chain structure can effectively keep the electron-rich PXZ cores in neighboring molecules apart from each other such that triplet-related exciton quenching can be well suppressed. In addition, it also improves the balance of carrier mobilities and the optical outcoupling from the EML. With these merits, OLEDs using 121 as a dopant emitter, from 10 to 100 wt%, can maintain high EQEs of over 20%. More importantly, the nondoped OLED shows yellow emission and an excellent maximum EQE of 21.8% with a small efficiency roll-off, which is even comparable with that of state-of-the-art yellow OLEDs. These results provide new insight into the role of hydrogen bonding in molecular packing and its subsequent influences on the performance of OLEDs. | ||
| Fig. 10 Molecular structures of nitrogen-containing heterocycle-based TADF emitters for nondoped OLEDs. | ||
| PL | EL | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLQY | τ p [ns] | τ d [μs] | Peak [nm] | V on [V] | CE/PE/EQE [cd A−1/lm W−1/%] | Roll-offb [%] | CIE (x,y) | |||
| Maximum | @103 cd m−2 | |||||||||
| a Turn-on voltage at a luminance of 1 cd m−2. b EQEroll-off = (EQEmax − EQE1000)/EQEmax. | ||||||||||
| 118 | 0.52 | 14.4 | 4.4 | 528 | — | 24.7/20.3/8.2 | 23.6/17.2/7.6 | 7.3 | (0.33, 0.56) | 50 |
| 119 | 0.82 | 19.1 | 7.0 | 532 | — | 79.8/83.8/23.6 | 74.1/72.7/21.9 | 7.2 | (0.34, 0.56) | |
| 120 | 0.64 | 23.8 | 5.2 | 540 | — | 50.4/52.8/16.4 | 43.5/34.1/14.1 | 14.0 | (0.38, 0.55) | |
| 121 | 0.90 | 24.4 | 1.1 | 560 | — | 70.0/90.1/21.8 | 64.5/60.0/20.1 | 7.8 | (0.44, 0.54) | 111 |
| 122 | 0.80 | 13.1 | 17.3 | 548 | 2.6 | 84.1/96.5/25.4 | 78.4/71.9/23.6 | 7.1 | (0.40, 0.56) | 49 |
| 123 | 0.47 | 19.2 | 6.7 | 560 | 2.6 | 44.5/46.2/14.6 | 41.3/36.2/13.6 | 6.8 | (0.45, 0.53) | |
| 124 | 0.80 | 18.5 | 16.0 | 534 | 3.0 | 58.1/57.0/17.4 | —/—/∼7.2 | 58.6 | — | 116 |
| 125 | 0.58 | 57.7 | 2.8 | 570 | 3.2 | 42.4/31.6/14.9 | —/—/∼12.8 | 14.1 | — | |
| 126 | 0.36 | 22.3 | 3.3 | 568 | 3.2 | 36.8/30.4/13.1 | —/—/∼7.4 | 43.5 | — | |
| 127 | 0.74 | 23.0 | 8.3 | 544 | 2.8 | 35.4/32.7/10.1 | 21.2/10.7/6.0 | 40.6 | (0.39, 0.58) | 117 |
| 128 | 0.73 | 32.0 | 2.4 | 608 | 2.4 | 10.5/12.0/5.6 | 9.8//5.2/5.2 | 7.1 | (0.56, 0.43) | |
| 129 | 0.84 | 26.0 | 6.5 | 548 | 2.6 | 41.2/45.4/12.0 | 28.5/17.6/8.3 | 30.8 | (0.40, 0.57) | |
| 130 | 0.76 | 29.0 | 1.9 | 616 | 2.8 | 7.5/6.2/5.3 | 6.2/2.9/4.4 | 17.0 | (0.60, 0.40) | |
| 131 | 0.73 | 57.0 | 3.4 | 584 | 3.4 | 24.3/22.5/10.1 | 14.2/6.3/6.0 | 40.6 | — | 118 |
| 132 | 0.74 | 68.0 | 3.3 | 588 | 3.2 | 21.0/20.6/9.8 | 9.8/4.2/4.7 | 52.0 | — | |
| 133 | — | — | — | 492 | 2.6 | 9.9/12.0/3.6 | 6.1/2.9/2.2 | 38.9 | (0.23, 0.43) | 119 |
| 134 | 0.71 | — | — | 546 | 2.9 | —/—/20.9 | 61.6/41.5/18.0 | 13.9 | — | 120 |
| 135 | 0.14 | 20.8 | 0.8 | 710 | 4.0 | 0.24/0.19/2.1 | — | — | (0.70, 0.29) | 121 |
| 136 | 0.31 | 12.2 | 13.2 | 700 | 3.2 | 0.80/0.78/4.6 | —/—/1.3 | 71.7 | (0.70, 0.30) | 37 |
| 137 | 0.87 | 10.2 | 5.0 | 652 | 7.0 | 8.1/3.6/12.3 | 7.0/1.9/10.7 | 13.0 | (0.67, 0.33) | 122 |
| 138 | 0.16 | — | — | 656 | — | 1.3/0.8/2.2 | — | — | (0.65, 0.35) | 124 |
| 139 | 0.33 | — | — | 680 | — | 2.8/2.3/5.2 | — | — | (0.68, 0.32) | |
| 140 | — | — | — | 626 | 3.0 | 7.3/7.6/5.6 | 2.9/1.5/2.2 | 60.7 | (0.61, 0.38) | 125 |
| 141 | — | — | — | 641 | 3.0 | 2.5/2.6/2.9 | 0.8/0.3/0.9 | 69.0 | (0.64, 0.35) | |
| 142 | 0.83 | — | — | ∼515 | ∼3.0 | 61.1/45.7/20.0 | — | — | — | 126 |
| 143 | 0.52 | 93 | 5.8 | 524 | 3.4 | 15.0/5.8/5.7 | — | — | (0.33, 0.54) | 128 |
| 144 | 0.69 | 59 | 1.8 | — | 3.6 | 45.1/37.3/18.7 | — | — | (0.24, 0.49) | |
| 145 | 0.47 | — | — | 504 | 3.0 | 39.3/37.0/14.7 | 30.0/21.2/11.3 | 23.1 | (0.22, 0.45) | 51 |
| 146 | 0.79 | 1.3 | 229.8 | 524 | 3.3 | 67.4/62.3/20.3 | — | — | (0.31, 0.57) | 129 |
| 147 | 0.44 | 11.1 | — | 498 | 3.5 | 10.3/9.5/3.8 | — | — | (0.20, 0.44) | |
| 148 | 0.70 | 21.8 | 2.8 | ∼478 | 3.3 | 41.1/35.8/20.0 | 21.9/14.9/10.6 | 47.0 | (0.16, 0.30) | 130 |
| 149 | 0.64 | 22.3 | 3.2 | ∼486 | 3.3 | 39.0/33.5/16.5 | 22.8/15.8/9.7 | 41.2 | (0.18, 0.34) | |
| 150 | 0.42 | 55.0 | 0.8 | 576 | 3.4 | 18.0/15.5/7.3 | 14.6/6.3/5.9 | 19.2 | (0.50, 0.49) | 131 |
| 151 | 0.67 | 81.0 | 0.6 | 548 | 3.1 | 45.2/40.0/14.1 | 41.2/26.1/12.8 | 9.2 | (0.42, 0.55) | |
| 152 | 0.20 | — | — | 520 | 3.4 | 11.4/—/3.5 | 9.5/—/3.0 | 14.3 | (0.30, 0.54) | 132 |
| 153 | 0.52 | 48.4 | 4.7 | 521 | 3.1 | 21.4/17.6/6.5 | 19.9/13.0/6.0 | 7.7 | (0.28, 0.58) | 133 |
| 154 | 0.62 | 41.6 | 2.1 | 544 | 2.5 | 41.2/44.9/12.7 | 40.1/31.5/12.3 | 3.1 | (0.39, 0.57) | |
| 155 | 0.36 | 35.9 | 3.5 | 494 | 3.1 | 33.6/32.6/12.8 | 22.9/11.2/8.7 | 32.0 | (0.24, 0.47) | 134 |
| 156 | 0.85 | 19.1 | 2.4 | 467 | 3.1 | 28.6/28.4/15.8 | 7.9/3.4/4.4 | 72.2 | (0.18, 0.27) | |
| 157 | 0.52 | — | — | ∼503 | 3.3 | —/—/2.4 | —/—/∼1.4 | 41.7 | (0.25, 0.49) | 135 |
| 158 | 0.31 | — | — | ∼510 | 3.5 | —/—/3.4 | —/—/∼1.4 | 58.8 | (0.27, 0.49) | |
| 159 | 0.40 | 15.7 | 3.3 | 510 | 3.0 | 26.5/21.5/9.4 | —/—/7.1 | 24.4 | — | 136 |
| 160 | 0.44 | 13.3 | 5.3 | 510 | 3.5 | 25.4/16.1/9.5 | —/—/7.3 | 23.2 | — | |
| 161 | 0.49 | 14.5 | 1.9 | 510 | 3.2 | 23.1/17.3/8.2 | —/—/6.0 | 26.8 | — | |
| 162 | 0.58 | — | — | 490 | 4.0 | 20.0/—/6.5 | ∼4.9 | 24.6 | (0.24, 0.51) | 137 |
| 163 | 0.76 | — | — | 487 | 3.6 | 30.5/—/10.1 | 29.0/—/∼9.0 | 10.9 | (0.24, 0.51) | |
| 164 | 0.56 | — | 0.5 | 490 | 4.0 | 20.0/—/6.5 | — | — | (0.24, 0.51) | 138 |
| 165 | 0.77 | — | 0.8 | 487 | 3.6 | 30.5/—/10.1 | 29.0/—/— | — | (0.24, 0.51) | |
| 166 | 0.69 | 21.7 | 2.9 | 520 | 4.0 | 30.8/24.2/9.5 | — | — | (0.32, 0.57) | 139 |
| 167 | 0.56 | 16.1 | 25.2 | 496 | 4.5 | 20.7/14.5/8.1 | — | — | (0.22, 0.43) | |
| 168 | 0.90 | 11.1 | 2.5 | ∼530 | 2.6 | —/48.5/16.9 | —/34.3/15.6 | 7.7 | (0.35, 0.54) | 140 |
| 169 | 0.89 | 27.9 | 1.1 | ∼545 | 2.6 | 48.7/50.5/15.5 | 45.9/34.3/14.5 | 6.4 | (0.41, 0.55) | 141 |
| 170 | 0.74 | 44.0 | 2.0 | 525 | 3.0 | 50.3/—/16.2 | 40.2/—/13.0 | 19.8 | (0.34, 0.55) | 142 |
| 171 | 0.34 | 53.0 | 0.8 | 568 | 3.2 | 21.1/—/7.8 | 11.9/—/4.4 | 43.6 | (0.46, 0.50) | |
| 172 | 0.06 | 44.0 | 0.9 | 616 | 4.0 | 1.7/—/1.0 | 0.4/—/0.3 | 70.0 | (0.57, 0.41) | |
| 173 | 0.29 | 66.0 | 0.4 | 455 | 3.2 | 10.6/—/7.1 | 5.8/—/3.9 | 45.1 | (0.17, 0.17) | |
| 174 | 0.56 | 44.8 | 2.4 | 469 | 3.2 | 26.1/—/12.9 | 24.8/—/12.3 | 4.6 | (0.19, 0.26) | 143 |
| 175 | 0.81 | 45.4 | 2.5 | 479 | 2.8 | 42.7/—/18.8 | 36.4/—/16.1 | 14.4 | (0.20, 0.31) | |
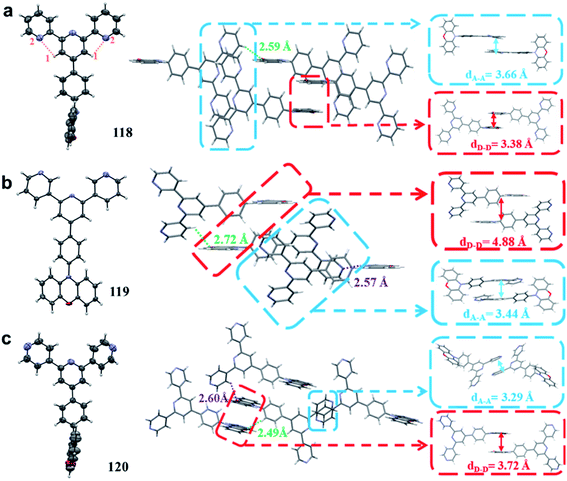 | ||
| Fig. 11 Thermal ellipsoid drawings at the 50% probability level and intermolecular geometries of (a) 118, (b) 119, and (c) 120 in the single crystals determined by X-ray analysis. Reproduced from ref. 50 with permission. Copyright 2020 the Royal Society of Chemistry. | ||
Conformational status is a fundamental issue for TADF emitters to determine their overall properties. Recently, TADF emitters with dual conformations have been widely studied.112–115 However, unlike the detailed investigations between conformational isomerization and excited-state properties in the dilute solution state, their influences in the amorphous solid-state are far from clear due to the lack of a suitable quantitative analysis method. By using temperature-dependent time-resolved photoluminescence spectroscopy, we quantitatively measured the conformational populations for the first time.49 The populations of the quasi-axial (QA) and quasi-equatorial (QE) forms of newly designed TADF emitter 122 in the disordered solid-state were thus measured to be 86 and 14%, respectively. Interestingly, this “compositionally” pure film actually behaves as a film with a dopant (QE form) in a matrix (QA form). In terms of the conformational distribution aspect, we further propose a new concept of “self-doping” for realizing high-efficiency nondoped OLEDs (Fig. 12). The “self-doping” OLED realizes superior device performance with a maximum EQE of 25.4% and neglectable efficiency roll-off. This is similar to those in state-of-the-art OLEDs with “dopant-in-host” systems. On the other hand, as the emitting film employs only one compound, the issues of higher manufacturing cost and potential dopant segregation associated with “dopant-in-host” systems can be effectively avoided.
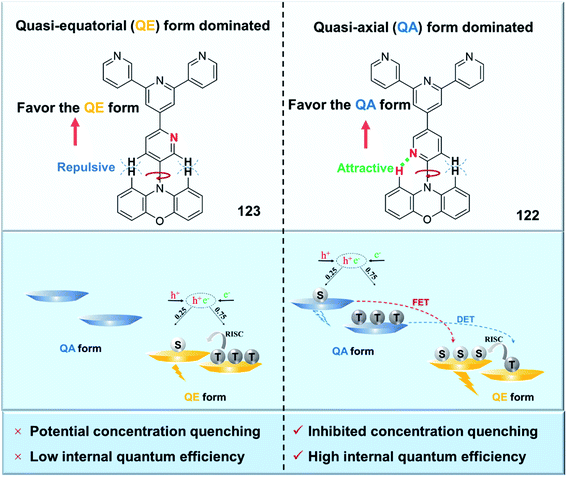 | ||
| Fig. 12 Molecular structures of 122 and 123 and a schematic illustrating the distribution of the conformations at the ground state and corresponding energy transfer at excited states. | ||
Chen et al.116 designed and synthesized three AIDF emitters 124–126 by using the quinoline unit as a new A group. Owing to the highly twisted D–A molecular framework, all three quinoline-based emitters display small ΔEST values (∼0.04 eV), good photoluminescence, and AIDF properties. Meanwhile, the steric DMAC group in 124 can effectively retain the intermolecular mutual interactions and favor the utilization of the excitons. Therefore, the 124-based nondoped device displays a superior performance with a maximum EQE of 17.4%, much higher than that of the others (maximum EQEs of 14.9% and 13.1% for 125 and 126, respectively). To further exploit quinoline-based emitters for high-performance red/orange nondoped OLEDs, Yang et al.117 designed a series of AIDF emitters 127–130 by simultaneously modulating the D units and regulating the degree of ICT characteristics. They also decorated the quinoline unit with the fluorine atom (131 and 132).118 Introducing the fluorine atom can not only slightly enhance the electron-withdrawing ability but also enrich the intermolecular electron coupling capability. The nondoped OLEDs obtained by using 131 and 132 as the EMLs achieve orange emission with EL peaks of 584 and 588 nm and maximum EQEs of 10.1% and 9.8%, respectively.
Tao et al.119 modified the efficient sky-blue precursor 5CzBN by replacing the CN unit with a 1,3,4-oxadiazole (OXD) moiety (133). It exhibits bright light-blue emission, with a maximum EQE of 3.6% of the nondoped device. Moreover, it functions as a highly efficient host for green and yellow TADF OLEDs. Kippelen et al.120 further modified the molecule by introducing two tBu-substituted OXD moieties (134). Due to effectively restricted exciton quenching owing to the steric tBu group, the 134 pristine neat film exhibits a high PLQY of 0.71. The corresponding nondoped OLED yields a maximum EQE of 20.9% and a high luminance of 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2.
000 cd m−2.
By applying TPA and 2,3-dicyanopyrazino phenanthrene (DCPP) as the D and A moieties, respectively, Wang et al.121 synthesized the first V-shaped TADF emitter 135 for near-infrared (NIR) nondoped OLEDs. A large and rigid π-conjugated DCPP moiety can effectively restrain the molecular vibration and rotational processes, while an electron-donating TPA moiety can not only offer excellent hole-transporting capability but also diminish exciton quenching in the aggregated state. Therefore, a small ΔEST and high kr value can be simultaneously obtained, benefiting the utilization of electrogenerated excitons. The corresponding nondoped device realizes NIR emission with a peak at 710 nm and CIE coordinates of (0.70, 0.29). Xu et al.37 also developed a novel NIR TADF emitter by using the CN-decorated quinoline unit as the A moiety (136). The 136-based nondoped device realizes a maximum EQE of 4.6% with NIR emission at a peak of 700 nm.
By modulating the substitution position of the TPA unit from the o- to the p-position, Xu et al.122 developed a novel “T-shaped” deep-red TADF emitter 137 by using the planar dipyridophenazine (DPPZ) moiety as the A. In comparison to the o-substituted precursor,123 the rational spatial arrangement of D and A groups in 137 can not only remarkably improve the carrier transport but also dramatically accelerate the rate of kr by 90-fold without worsening the nonradiative transition process (kSnr) and amplify the thermodynamic advantage of RISC with a nearly unitary efficiency, giving rise to a PLQY as high as 0.87. Therefore, the corresponding nondoped device realizes a record high EQE of 12.3% for the maximum and 10.4% at 1000 cd m−2. All these results demonstrate that it is important to obtain deep insight into the influence of the spatial arrangement of functional groups on the optoelectronic properties.
Based on the DCPP moiety, our group also designed two TADF emitters 138 and 139 to demonstrate the relationship between molecular rigidity and intermolecular packing.124 Although the fused dibenzo[a,c]dipyrido[3,2-h:2′,3′-j]phenazine (BPPZ) moiety can effectively relieve the nonradiative process, a large planar molecular structure induces unavoidable intermolecular π–π interactions, which are detrimental to the performance of nondoped devices. The rotational pyridine unit in 2,3-di(pyridin-3-yl)dibenzo[f,h]quinoxaline (mDPBPZ) can provide suitable steric hindrance against intermolecular π–π packing. The 138-based doped device realizes a remarkably high maximum EQE of 25.2%, while the 139-based nondoped device shows a relatively high EQE of 5.2% with deep red/NIR emission at a peak of 680 nm.
Wang et al.125 designed two AIDF emitters 140 and 141 by using rigid TAT and dibenzo[a,c]phenazine (DBPZ)/fluorine-decorated DBPZ as the D and A moieties, respectively. The large steric hindrance between planar TAT and DBPZ segments can not only restrain the overlap of the FMOs but also suppress aggregation-caused quenching. Therefore, both compounds possess TADF characteristics with small ΔESTs and AIE properties. The 140- and 141-based solution-processable nondoped OLEDs exhibit red and deep-red emissions (CIE coordinates of (0.61, 0.38) and (0.64, 0.35)) with maximum EQEs of 5.6 and 2.9%, respectively.
With three potential modification sites, the triazine (TRZ) group with highly electron-deficient properties is a suitable building block for constructing TADF emitters. By using DMAC/PXZ and TRZ as the D and A groups, respectively, Wu et al. designed two TADF emitters, PXZ-TRZ and 142.126 Compared with PXZ-TRZ, the DMAC unit in 142 can effectively restrict exciton quenching in the solid-state and guarantee that the 142 neat film realizes a high PLQY of 0.83. As a result, a highly efficient nondoped device with a maximum EQE of 20.0% is also realized. Kaji et al.127 further applied 142 for solution-processed nondoped OLEDs. The peripheral phenyl units and large steric methyl units in the TRZ and DMAC moieties, respectively, can effectively suppress the intermolecular interactions. Therefore, the spin-coated 142 neat film displays a high PLQY of 0.84, which is almost identical to that of a vacuum vapour deposited neat film. Hong et al.128 also connected the DMAC and TRZ units via a meta-position and synthesized two TADF emitters 143 and 144 with molecular structures of D–A and D–π–A, respectively. Compared with D–A-type molecule 143, introducing the π group in 144 more efficiently benefits the formation of intermolecular exciplexes. Therefore, the 144-based nondoped device exhibits a superior device performance with a maximum EQE of 18.7%, much higher than that of 143 (5.3%).
To relieve triplet exciton quenching in the aggregated solid-state, our group proposed a novel molecular model to develop TADF emitters radiating via the intermolecular CT transition for nondoped OLEDs.51 As displayed in Fig. 2f, the DMAC and TRZ moieties were connected with a space-enough and conjugation-forbidden linkage (diphenyl ether linkage), which was called the D–spacer–A structure. Such a molecular structure can effectively suppress the intramolecular CT transition while forming an exciplex-type emitter via the intermolecular CT transition. Therefore, 145 exhibits local excited properties in a single-molecule state, as the D–spacer–A molecular backbone strongly suppresses the intramolecular CT transition. With increasing doping concentration, the intermolecular CT transition becomes significant, opening another effective radiation channel and resulting in TADF characteristics. The corresponding devices thus exhibit similar performance under various high doping ratios (40–100 wt%), and the nondoped device retains a high maximum EQE of 14.7%. These results prove that the intermolecular CT transition not only endows emitters with natural TADF but also helps to inhibit the undesired concentration quenching effect. Therefore, the D–spacer–A structure molecule could act as a novel candidate to develop TADF emitters with intermolecular CT transitions for efficient nondoped OLEDs.
At present, the majority of TADF emitters are based on largely twisted aromatic amine-based compounds. To broaden the choice of the D moieties, Su et al.129 designed two TADF emitters 146 and 147 by using thianthrene (TE) and TRZ, respectively, as the D and A groups. Unlike only the existing π-conjugated CT channel in linear 147, ortho-substituted 146 exhibits a twisted D–A backbone with coexistence through the bond and through spatial CT. As a result, the 146 amorphous neat film has a high PLQY of 0.79, which is much higher than that of 147 (0.44). Therefore, the 146-based nondoped device produces a green emission with a maximum EQE of 20.6%, which is comparable to those of the reported aromatic amine-based counterparts. They also designed two novel tri-spiral units, 10H-dispiro[acridine-9,9′-anthracene-10′,9′′-thioxanthene] (TspiroS) and 10H-di acridine-9,9′-anthracene-10′,9′′-fluorene] (TspiroF), with long bar shapes for high-performance nondoped OLEDs.130 By further integrating a strong TRZ acceptor moiety, two linear TADF emitters 148 and 149 were designed and synthesized. Compared with the nonspiral and dual-spiral acridine units, the elongation of the molecular backbone with a large nonconjugated fragment can unambiguously increase the distance between the adjacent luminophores and weaken the intermolecular interactions. Moreover, the long and linear molecular configurations favor the horizontal dipole ratio, which can significantly enhance the outcoupling efficiency of the device.110 As a result, sky-blue nondoped OLEDs realize maximum EQEs of 20.0% and 16.5% for 148 and 149, respectively.
Wang et al.131 designed a novel indole-fused acridine D group donor (13,13-dimethyl-8-phenyl-8,13-dihydro-5H-indolo[3,2-a]acridine, 34AcCz). Then, two AIDF emitters 150 and 151 were designed and synthesized by using TRZ and 2,6-diphenylpyrimidine (PM), respectively, as the A moieties. Through systematic analysis of structure–property correlation, 151 was found to have a more prominent AIE characteristic with a higher PLQY. Thus, the 151-based nondoped device exhibits a maximum EQE of 14.1%, double that of 150 (a maximum EQE of 7.3%).
Hexaarylbenzene (HAB) derivatives, in which six aromatic rings are arranged around the central phenyl ring with a propeller-shaped configuration, can be considered the desired model to carefully investigate the through-space CT process. Wang et al.132 designed a novel AIDF emitter 152 with circularly arrayed DMAC and TRZ units in the periphery of the HAB core. Such a propeller-shaped molecular structure not only favors a reduced ΔEST but also facilitates kr. Nevertheless, the corresponding solution-processed nondoped device can only realize poor device performance with a maximum EQE of 3.5%. Tang et al.133 also decorated the HAB with DMAC/PXZ and TRZ units positioned in close proximity (153 and 154) to ensure that electron clouds of D and A exchange through spatial interactions. Due to the enhanced electron-donating ability of the PXZ unit, the 154 amorphous neat film shows a smaller ΔEST value of 0.02 eV than DMAC-based 153 (0.09 eV). Moreover, the AIE-active HAB moiety can effectively enlarge the intermolecular distance and relieve the ACQ effect. Therefore, the 154-based nondoped device realizes a superior performance with a maximum EQE of 12.7% and a neglectable efficiency roll-off at high luminance.
Chang et al.134 modified the TRZ unit with a long alkyl chain to inhibit unwanted intermolecular D–D/A–A-type π–π interactions and accordingly designed two D–A-type TADF emitters 155 and 156. Owing to the nature of the electron-donating ability of the alkyl chain, the LUMO of the modified TRZ unit can be slightly weakened, generating blueshifted emission. Meanwhile, the crowded molecular architecture favors well-separated FMOs and benefits TADF properties. As a result, blue-emitting nondoped devices with 155 and 156 as the EMLs reveal satisfactory efficiencies of 12.8% and 15.8%, respectively.
Yamamoto et al.135 first designed Cz-decorated TRZ-based TADF dendrimers 157 and 158 by decorating the precursor 2,4,6-tris(4-(9H-carbazol-9-yl)phenyl)-1,3,5-triazine (TCz-TRZ) for solution-processed nondoped OLEDs. Intermolecular interactions between the adjacent emissive cores decrease when the generation number of the Cz unit increases. The second-generation 158-based solution-processable nondoped device exhibits a superior performance compared to 157. Although device performance should be improved by careful optimization, more importantly, the recorded EQE value indicated that the dendrimer emitting layer can harvest triplet excitons. These TRZ-based dendrimers are the first high-molecular-weight TADF materials for solution-processable nondoped OLEDs. To realize fully solution-processed devices, they also tried to decorate precursor 157 by introducing methyl, tBu, and phenyl groups (159–161), respectively.136 All the modified dendrimers exhibit excellent solubility in common organic solvents except alcohol solution, fulfilling the requirements of fully solution-processable devices. As a result, fully solution-processed nondoped OLEDs with a device configuration of ITO/PEDOT-PSS (70 nm)/PVK (poly-vinylcarbazole, 20 nm)/EML (20–25 nm)/TPBi (40 nm)/Ca (10 nm)/Al (80 nm) were fabricated by spin-coating all organic materials. The maximum EQEs are 9.4% (159), 9.5% (160), and 8.2% (161), which are comparable to or even higher than those of the TPBi vacuum evaporated devices. Sun et al.137 also designed self-host dendrimers 162 and 163 by connecting the emission core TCz-TRZ with the peripherally Cz-based host matrix through nonconjugated aliphatic chains. The prevalent concentration quenching effect of TADF materials can thus be effectively restrained by the encapsulation of the emissive core. Moreover, the device performances are found to be greatly dependent on the peripheral dendrons, suggesting the importance of the role in molecular modulation for performance improvement. Using the same self-host strategy, they also decorated the emitting core 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)tris(N,N-diphenylaniline) (TTPA-TRZ) with Cz-based dendrons for high-performance solution-processed nondoped OLEDs (164 and 165).138
Choi et al.139 also designed two dendrimers 166 and 167 by choosing the efficient nondoped green TADF material 142 as the emitting core. Using a methylene group as the linkage can effectively disrupt the conjugation between the peripheral Cz-based dendrons and the emitter core. Thus, the performances of the emitter core are independent of the dendrons. Their nondoped solution-processable OLEDs demonstrate acceptable performance with maximum EQEs of 9.5% for 166 and 8.1% for 167.
In addition to the abovementioned TRZ-based dendrimers, the TRZ unit has also been applied to design novel polymers for solution-processed devices. Cheng et al.140 reported a series of conjugated TADF polymers 168 by using conjugated acridine/carbazole and tBu-substituted TRZ moieties as the backbones and pendant, respectively. The long alkyl branched chains significantly enhance the solubility of the target polymers in common solvents, which is very beneficial for the solution process. Meanwhile, the TADF core can be sparsely incorporated into the poly-3,6-carbazole backbone to realize molecular dispersion, and the distance between the adjacent TADF chromophores can flexibly be regulated through a subtly lengthened carbazole oligomer. Therefore, a high PLQY of 0.90 was realized with the 5% molar content of the TADF emission unit. They also modified 168 by replacing the tBu group with phenyl units on the triazine parent (169).141 Although introducing the Cz group is expected to isolate the TADF emission core and relieve the concentration quenching effect, all these constructed polymers still exhibit high concentration sensitivities, and this needs further in-depth study.
The conjugated acridine/carbazole backbones would lower the triplet excited state, which is detrimental to realizing the TADF characteristic. To realize high-performance TADF polymers, Wang et al.142 proposed exploiting through-space CT polymers 170–173 containing a nonconjugated polystyrene backbone and spatially separated D and A units for solution-processed OLEDs. Such a molecular design strategy can not only avoid the large bathochromic shift of the TADF emission but also limit the electron cloud overlap of the FMOs, which is beneficial for reducing ΔEST and achieving TADF characteristics. By further tuning the CT strengths, an emission color spanning from the deep blue (455 nm) to red (616 nm) region can be achieved. Meanwhile, by incorporating two kinds of D/A pairs into one polymer to create double through-space CT channels, blue and yellow emission can be simultaneously obtained, indicating that white electroluminescence can be realized from a single polymer. They also designed two through-space CT polymers 174 and 175 based on a nonconjugated poly(cis,exo-2,3-diarylnorbornene) backbone with D and A units fixed in edge-to-face/face-to-face alignment patterns.143 Compared to its edge-to-face counterpart 174, the 175 neat film with face-to-face aligned D/A units exhibits a much larger oscillator strength (f) and higher PLQY up to 0.81 owing to the enhanced spatial electron cloud overlaps. Therefore, the 175-based solution-processed nondoped device realizes a maximum EQE of 18.8%, which is the best efficiency for blue TADF polymers thus far.
3.5. Boron-based units as acceptors
Due to the vacant p-orbital of boron atoms, triarylboron-based acceptors have been utilized to exploit emitters for optoelectronics. The molecular structures of boron-based TADF emitters are shown in Fig. 13, and the corresponding key parameters of the nondoped devices are summarized in Table 5. Lu et al.144 reported two efficient blue TADF emitters 176 and 177 bearing triarylboron as the A group and ortho-positioned Cz and tCz, respectively, as the D group. Such ortho-positioned D–A molecular structures favor the coexistence of through-bond and through-space CT processes, guaranteeing a high transition dipole moment and small ΔESTs. Moreover, both highly twisted D/A moieties and steric alkyl substituents can efficiently inhibit aggregation-induced quenching. Meanwhile, compared with Cz-based emitter 176, additional tBu groups in 177 can not only improve the solubility, benefiting solution processing but also restrict the close intermolecular π–π packing. Therefore, solution-processed nondoped OLEDs using 176 and 177 as the EMLs achieve blue EL emission with peaks at 463 nm and 474 nm and maximum EQEs of 8.0% and 19.1%, respectively. By replacing the tBuCz unit in 177 with a butterfly shaped PTZ unit, Lu et al.145 synthesized a novel boron-based TADF emitter 178. Owing to the well-restricted intermolecular interactions, its amorphous neat film achieves a high PLQY of 0.65. The 178-based nondoped device also obtains a high maximum EQE of 19.7%. It should be noted that there is a shoulder emission below 450 nm when 178 is doped in mCP with 5 wt%. The authors believe that this shoulder emission is due to the host material, but the EL emission of mCP cannot reach this position. We suspect that this emission arrives from the QA form of 178 caused by conformational isomerism of the PTZ group.| PL | EL | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLQY | τ p [ns] | τ d [μs] | Peak [nm] | V on [V] | CE/PE/EQE [cd A−1/lm W−1/%] | Roll-offb [%] | CIE (x,y) | |||
| Maximum | @103 cd m−2 | |||||||||
| a Turn-on voltage at a luminance of 1 cd m−2. b EQEroll-off = (EQEmax − EQE1000)/EQEmax. | ||||||||||
| 176 | 0.61 | 52.0 | 15.0 | 463 | 4.4 | 11.6/7.6/8.0 | 3.8/1.5/2.6 | 67.5 | (0.15, 0.17) | 144 |
| 177 | 0.94 | 63.0 | 14.0 | 474 | 3.9 | 37.3/27.6/19.1 | 18.8/8.5/9.7 | 49.2 | (0.15, 0.26) | |
| 178 | 0.60 | — | — | 540 | 2.8 | 62.9/—/19.7 | —/—/17.3 | 12.2 | (0.37, 0.57) | 145 |
| 179 | 0.99 | 7.5 | 1.7 | 487 | 3.4 | 49.9/41.4/22.8 | —/—/19.0 | 16.7 | (0.15, 0.36) | 146 |
| 180 | 0.98 | 27.0 | 2.4 | 474 | 3.5 | 33.6/29.3/21.3 | —/—/8.0 | 62.4 | (0.14, 0.23) | |
| 181 | 0.11 | 1.3 | 1.7 | — | — | — | — | — | — | 147 |
| 182 | 0.74 | 1.0 | 2.3 | 473 | 3.6 | 30.1/23.8/20.9 | —/—/18.2 | 12.9 | (014, 0.20) | |
| 183 | 0.83 | 1.0 | 3.2 | 473 | 4.4 | 25.7/16.9/17.4 | —/—/14.9 | 14.4 | (014, 0.20) | |
| 184 | 0.84 | 50.0 | 0.15 | 452 | 3.1 | 19.3/18.3/22.5 | 10.7/5.9/14.8 | 34.2 | (0.16, 0.09) | 148 |
| 185 | 0.73 | 98.8 | 4.4 | — | — | — | — | — | — | 149 |
| 186 | 0.94 | 62.3 | 1.8 | 516 | 2.7 | 44.3/47.1/14.1 | 39.5/27.6/12.7 | 9.9 | (0.28, 0.54) | |
| 187 | 0.70 | 145.7 | 8.1 | — | — | — | — | — | — | |
| 188 | 0.99 | 13.7 | 4.4 | 424 | 3.5 | 4.0/3.8/9.9 | — | — | (0.17, 0.07) | 150 |
| 189 | 0.93 | 18.5 | 4.7 | 448 | 3.0 | 4.6/4.1/6.1 | — | — | (0.15, 0.08) | |
| 190 | 0.90 | 15.7 | 5.4 | 492 | 2.6 | 15.0/15.7/6.0 | — | — | (0.18, 0.40) | |
| 191 | 0.41 | 16.4 | 1.49 | 416 | 3.7 | 2.2/2.0/8.2 | — | — | (0.17, 0.06) | 151 |
| 192 | 0.52 | 17.1 | 4.06 | 428 | 3.5 | 5.6/5.0/15.8 | — | — | (0.16, 0.05) | |
| 193 | 0.43 | 14.0 | 1.2 | 468 | 3.6 | 12.3/8.9/8.7 | —/—/4.5 | 48.3 | (0.15, 0.17) | 33 |
| 194 | 0.73 | 13.0 | 1.6 | 464 | 3.2 | 40.9/36.0/23.1 | —/—/13.0 | 43.7 | (0.15, 0.20) | |
| 195 | 0.37 | — | — | 451 | 3.8 | 5.8/5.1/3.8 | — | — | (0.18, 0.17) | 152 |
| 196 | 0.48 | — | — | 455 | 3.4 | 10.3/9.5/6.1 | — | — | (0.18, 0.20) | |
| 197 | 0.70 | — | — | 474 | 3.2 | 30.7/30.2/15.0 | — | — | (0.16, 0.27) | |
| 198 | 0.55 | 10.0 | — | 631 | 4.4 | 12.0/7.9/10.1 | — | — | — | 153 |
| 199 | 0.94 | 13.0 | 28.0 | 590 | 4.4 | 19.9/11.2/9.2 | — | — | — | |
| 200 | 0.97 | 15.0 | 27.0 | 586 | 6.3 | 16.7/7.6/11.0 | — | — | — | |
Yasuda et al.146 developed a series of TADF materials with different bridging heteroatoms (sulfur for 179 and oxygen for 180). Highly twisted D–A molecular structures with steric alkyl substituents benefit not only small ΔESTs but also well-restricted concentration quenching effects. Meanwhile, as shown in Fig. 14a, compared with oxygen-linked emitter 180, incorporating a phenothiaborin (PTB) unit into 179 can significantly enhance the SOC effect, thereby accelerating the RISC and shortening the TADF emission lifetime to ∼1 μs, which is among the shortest lifetimes ever reported for efficient TADF emitters and comparable to those of organometallic phosphorescent emitters. The 179- and 180-based nondoped devices attain considerably high EQEs of up to 22.8% and 21.3%, respectively. More importantly, owing to the short τd, the 179-based device can still retain a high EQE of 19.0% at a high luminance of 1000 cd m−2, much higher than that of 180 (an EQE of 8.0%) (shown in Fig. 14c). To further enhance the SOC effect, they also exploited a series of acridan analogues, phenazasiline and phenazagermine, with heavy elements in group 14 (silicon and germanium atoms). By using the same PTB unit as the weak A group, novel blue TADF emitters 181–183 were synthesized.147 Incorporating larger silicon and germanium atoms can effectively enhance the structural flexibility of the D units, leading to conformational heterogeneity as well as dual fluorescence capability. Meanwhile, terminal bulky substituents can effectively suppress the ACQ, and thereby, high PLQY values of 0.74 and 0.83 are obtained for 182 and 183 neat films, respectively. Hence, 182- and 183-based nondoped devices demonstrate excellent EL performances, with maximum EQEs as high as 20.9% and 17.4%, respectively, and suppressed efficiency roll-off at practically high luminance.
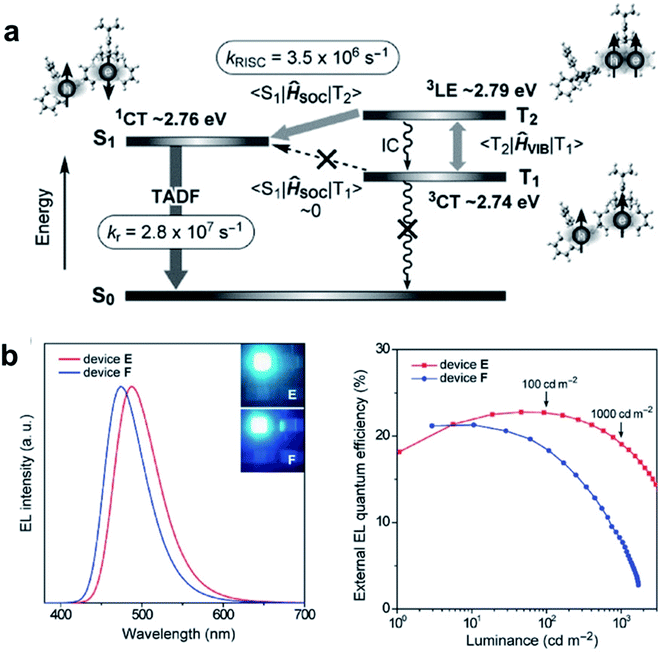 | ||
| Fig. 14 (a) Schematic diagram for plausible RISC and TADF mechanisms involving SOC-induced spin conversion in 179. (b) EL and EQE-L characteristics of the nondoped TADF-OLEDs (devices E and F based on 179 and 180, respectively). Reproduced from ref. 146 with permission. Copyright 2018 Wiley-VCH. | ||
Although TADF-based blue nondoped devices with maximum EQEs exceeding 20% have been achieved, it should be noted that their emissions are mostly in the sky-blue range with peaks at above 470 nm. In contrast, high-performance deep-blue TADF materials (<460 nm) for nondoped devices have been rarely reported. This is because developing them should not only consider the suppression of concentration quenching like with other color ones, but more importantly, it requires suitable D and A pairs that provide wide enough bandgaps. To achieve high-performance deep-blue nondoped OLEDs, Wang et al.148 designed a deep-blue D–π–A-type TADF emitter 184 by combining SFAc and weak electron-withdrawing siprodipenozoborinine (sDBB) as the D and A moieties, respectively. Both the fluorene groups appended on D/A moieties and the bulky isopropyl group on the bridge unit can effectively suppress the intermolecular interactions of neighboring molecules. Therefore, the annoying concentration quenching can be well relieved. Moreover, bulky isopropyl groups can effectively restrict the molecular free rotations between the bridge and the A groups, benefiting a high PLQY and a narrow emission bandwidth. As a result, the corresponding nondoped device realizes a deep-blue EL emission with a maximum EQE of 22.5% and CIEy < 0.1.
Boron-containing polycyclic aromatic hydrocarbons have emerged as very promising candidates for optoelectronic applications by virtue of their unique photophysical properties, good thermal stability, rigid planar structure, and easy structural modification. One typical framework is 5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene (DOBA) (1,3-diphenoxybenzene fused with a boron atom). Wang et al.149 designed and synthesized three isomeric derivatives 185–187 by incorporating two DMAC units at different locations of two of the phenyl rings (wing phenyl groups) in the DOBA unit. The location and orientation of the DMAC unit significantly influence the physical properties of the three isomers. The para-substituted isomer 186 realizes a short τd of 1.8 μs and a higher PLQY value of 0.94 in the neat film state than the others. Therefore, the 186-based nondoped device realizes a green EL emission with a peak at 516 nm, CIE coordinates of (0.28, 0.54), and a maximum EQE of 14.1%.
Choi et al.150 also designed three DOBA-based TADF emitters 188–190 by tethering Cz-, PCz-, and DPA-substituted Cz derivatives (9′H-9,3′:6′,9′′-tercarbazole, 9,9′′-diphenyl-9H,9′H,9′′H-3,3′:6′,3′′-tercarbazole, and N3,N3,N6,N6-tetraphenyl-9H-carbazole-3,6-diamine) with the tBu-substituted DOBA unit (2,12-di-tert-butyl-5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene). All emitters exhibit AIDF features and high PLQY values (>0.90). Consequently, solution-processed nondoped OLEDs fabricated using 188–190 as the EMLs exhibit maximum EQEs of 9.9%, 6.1%, and 6.0%. Moreover, the 188- and 189-based devices exhibit deep-blue EL emission with CIE coordinates of (0.17, 0.07) and (0.15, 0.08), respectively, which approach the NTSC blue standards. They also designed two ultradeep-blue AIDF emitters 191 and 192 by decorating prototype 188 with tBu and tBu phenyl units.151 Owing to the large SOC effect arising from the closely located 1CT state and 3LE states, both emitters exhibit relatively fast kRISC (∼106 s−1), benefiting the upconversion process of the dark triplet excitons. The solution-processed nondoped devices exhibit ultradeep-blue emissions of 416–428 nm, high color purities with FWHMs of 42.2–44.4 nm, and CIE coordinates of (x = 0.16–0.17, y = 0.05–0.06). At the same time, the 192-based nondoped device exhibits a superior performance with a maximum EQE of 15.8%, almost 2-fold higher than that of 191 (8.2%), due to its smaller ΔEST, larger SOC and faster kRISC.
By using DOBA as the A moiety, Yasuda et al.33 also designed two 10,10-diphenylphenazasiline (PASi)-based deep blue TADF emitters 193 and 194. Both emitters exhibit efficient deep blue emission with high PLQYs of 0.81 and 0.98, respectively, along with rather short emission lifetimes of ∼1 μs in their doped films. Therefore, the corresponding doped devices achieve extremely high maximum EQEs of 21.7% and 33.8% with CIE coordinates of (0.14, 0.10) and (0.14, 0.17), respectively, for 193 and 194. Moreover, the 194-based device displays well-relieved concentration quenching, which can be reasonably ascribed to the dual stable conformations of the PASi moiety. In particular, the 194-based nondoped device can still maintain a high maximum EQE of 23.1%, which is among the highest values for nondoped deep blue TADF-OLEDs ever reported.
To exploit DOBA-based polymers for efficient solution-processed nondoped OLEDs, Wang et al.152 developed a series of through-space CT blue polymers 195–197. In these polymers, the DMAC and tBu/H/F-decorated DOBA moieties are linked to the nonconjugated polystyrene backbone as pendants. Such molecular configurations can not only circumvent the influence of the main chain on material properties but also limit the electron cloud overlaps of the FMOs, which is beneficial for reducing the ΔESTs and achieving TADF characteristics. Solution-processed nondoped devices based on polymers 195, 196, and 197 with 10 mol% DOBA content exhibit blue emissions with peaks at 451, 455, and 474 nm and CIE coordinates of (0.18, 0.17), (0.18, 0.20) and (0.16, 0.27), respectively.
In addition to the abovementioned triarylboron derivatives, o-carborane (1,2-closo-dicarbadodecaborane (C2B10H12)) is also known as an electron-deficient icosahedral boron cluster consisting of three-center two-electron bonds and possesses a highly polarizable σ-aromatic character. Based on this unique boron cluster, Yasuda et al.153 developed a series of efficient AIDF emitters 198–200 for orange/red nondoped OLEDs. Due to their AIDF characteristics, all three emitters exhibit small ΔESTs and high PLQYs of up to 97% in solid-state neat films. In nondoped devices, superior performances are realized with maximum EQEs of 10.1%, 9.2%, and 11.0% for 198, 199, and 200, respectively.
4. Conclusion and outlook
Since the pioneering work of Prof. Adachi et al., TADF materials have demonstrated explosive development and gradually expanded from OLEDs to the fields of photocatalysis, biomedical applications, electrochemical cells, etc. As a special category, TADF materials for nondoped OLEDs are a hot research area due to the advantages of high efficiency, structural or process simplicity, and avoidance of potential phase-separation problems faced in host–guest systems. In this review, we mainly discussed TADF emitters for nondoped devices, including the mechanism, exciton leaking channels, molecular design strategies, and molecular structures based on functional A groups. Using materials with AIE/AIDF features or intermolecular hydrogen bonding can effectively suppress nonradiative transitions, while the most important issue for high-performance nondoped OLEDs is to suppress exciton annihilation, especially related to triplet excitons. Based on this, the reported molecular design strategies can be broadly divided into the following two categories: the first is to increase the core distances of the adjacent luminescent materials, such as by introducing large steric hindrance groups or host matrixes with wide bandgaps, applying molecular conformational isomerization, and properly utilizing weak intermolecular interactions (e.g., hydrogen bonding); the other is to design molecules with D–spacer–A molecular skeletons to induce intermolecular CT rather than intermolecular exciton annihilation. By utilizing these molecular design strategies, many efficient nondoped devices with neglectable efficiency roll-off at high luminance have been successfully developed in the past decade. However, their development is still facing some challenges, which need further in-depth investigations. Firstly, compared with the blue and green counterparts that have achieved maximum EQEs of over 20%, the development pace of the red counterparts seriously lags behind due to the inherent narrow bandgaps causing severe exciton losses. Secondly, the operational stability of OLEDs is also an important indicator in determining commercial mass production, but it has seldom been considered in nondoped OLEDs. The few reports of TADF-based nondoped OLEDs that mentioned operational measurements all showed very short operational lifetimes, lagging far behind the development of doped OLEDs. The less satisfactory operational stability of current reported non-doped devices can be mainly attributed to the following two aspects: (i) the employed emitters generally have less stable chemical structures that contain weak bonds; (ii) kRISCs are not high enough, resulting in accumulation and quenching of long-lived triplet excitons particularly at high voltages in the EML. Therefore, exploiting TADF emitters with stable chemical structures and short τds to dilute exciton concentrations should be the main pursuits to improve the operational lifetimes of TADF-based nondoped OLEDs. In addition, MR-TADF emitters have recently attracted tremendous attention due to their excellent color purities and device efficiencies. However, due to their typical rigid and planar molecular structures, precise modulation of doping weight is still necessary to achieve these superior device performances. How to effectively suppress its concentration sensitivity without affecting the light-emission wavelengths and color purity is still unknown and needs further in-depth study. Despite the current challenges, exploiting novel TADF emitters for high-performance nondoped OLEDs remains an important research direction at the current stage.Author contributions
X. H. Zhang and K. Wang conceived the idea of the review and supervised the work. Y. Z. Shi, J. Yu and X. M. Ou designed the structure of the article. Y. Z. Shi and H. Wu conducted the bibliographic research. H. Wu contributed to an early draft, and Y. Z. Shi and K. Wang wrote the manuscript. All the authors participated in the revision of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the National Key Research & Development Program of China (Grant No. 2020YFA0714601, 2020YFA0714604), the National Natural Science Foundation of China (Grant No. 52130304, 51821002, 52003185, 52003186), the China Postdoctoral Science Foundation (Grant No. 2019M661924), Suzhou Key Laboratory of Functional Nano & Soft Materials, Collaborative Innovation Center of Suzhou Nano Science & Technology, the 111 Project, and Joint International Research Laboratory of Carbon-Based Functional Materials and Devices.References
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- K. Goushi, K. Yoshida, K. Sato and C. Adachi, Nat. Photonics, 2012, 6, 253–258 CrossRef CAS.
- Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326–332 CrossRef CAS.
- C. Y. Chan, M. Tanaka, Y. T. Lee, Y. W. Wong, H. Nakanotani, T. Hatakeyama and C. Adachi, Nat. Photonics, 2021, 15, 203–207 CrossRef CAS.
- X. Y. Cai and S. J. Su, Adv. Funct. Mater., 2018, 28, 1802558 CrossRef.
- M. Y. Wong and E. Zysman-Colman, Adv. Mater., 2017, 29, 1605444 CrossRef PubMed.
- Q. Wei, N. Fei, A. Islam, T. Lei, L. Hong, R. Peng, X. Fan, L. Chen, P. Gao and Z. Ge, Adv. Opt. Mater., 2018, 6, 1800512 CrossRef.
- Y. Zou, S. L. Gong, G. H. Xie and C. L. Yang, Adv. Opt. Mater., 2018, 6, 1800568 CrossRef.
- M. Sarma and K. T. Wong, ACS Appl. Mater. Interfaces, 2018, 10, 19279–19304 CrossRef CAS PubMed.
- P. Xiao, J. H. Huang, Y. C. Yu, J. Yuan, D. X. Luo, B. Q. Liu and D. Liang, Appl. Sci., 2018, 8, 1449 CrossRef.
- Q. Wang, Q. S. Tian, Y. L. Zhang, X. Tang and L. S. Liao, J. Mater. Chem. C, 2019, 7, 11329–11360 RSC.
- M. Zhang, C. J. Zheng, H. Lin and S. L. Tao, Mater. Horiz., 2020, 8, 401–425 RSC.
- X. K. Liu, Z. Chen, C. J. Zheng, C. L. Liu, C. S. Lee, F. Li, X. M. Ou and X. H. Zhang, Adv. Mater., 2015, 27, 2378–2383 CrossRef CAS PubMed.
- W. Liu, J. X. Chen, C. J. Zheng, K. Wang, D. Y. Chen, F. Li, Y. P. Dong, C. S. Lee, X. M. Ou and X. H. Zhang, Adv. Funct. Mater., 2016, 26, 2002–2008 CrossRef CAS.
- M. Zhang, W. Liu, C. J. Zheng, K. Wang, Y. Z. Shi, X. Li, H. Lin, S. L. Tao and X. H. Zhang, Adv. Sci., 2019, 6, 1801938 CrossRef PubMed.
- J. M. Ha, S. H. Hur, A. Pathak, J.-E. Jeong and H. Y. Woo, NPG Asia Mater., 2021, 13, 53 CrossRef CAS.
- M. Hirai, N. Tanaka, M. Sakai and S. Yamaguchi, Chem. Rev., 2019, 119, 8291–8331 CrossRef CAS PubMed.
- S. Madayanad Suresh, D. Hall, D. Beljonne, Y. Olivier and E. Zysman-Colman, Adv. Funct. Mater., 2020, 30, 1908677 CrossRef CAS.
- S. Gan, S. Hu, X. L. Li, J. Zeng, D. Zhang, T. Huang, W. Luo, Z. Zhao, L. Duan, S. J. Su and B. Z. Tang, ACS Appl. Mater. Interfaces, 2018, 10, 17327–17334 CrossRef CAS PubMed.
- J. Xu, X. Zhu, J. Guo, J. Fan, J. Zeng, S. Chen, Z. Zhao and B. Z. Tang, ACS Mater. Lett., 2019, 1, 613–619 CrossRef CAS.
- A. Kretzschmar, C. Patze, S. T. Schwaebel and U. H. F. Bunz, J. Org. Chem., 2015, 80, 9126–9131 CrossRef CAS PubMed.
- T. Huang, X. Song, M. Cai, D. Zhang and L. Duan, Mater. Today Energy, 2021, 21, 100705 CrossRef CAS.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680–13686 CrossRef CAS PubMed.
- M. K. Etherington, F. Franchello, J. Gibson, T. Northey, J. Santos, J. S. Ward, H. F. Higginbotham, P. Data, A. Kurowska, P. L. Dos Santos, D. R. Graves, A. S. Batsanov, F. B. Dias, M. R. Bryce, T. J. Penfold and A. P. Monkman, Nat. Commun., 2017, 8, 14987–14997 CrossRef CAS PubMed.
- J. X. Chen, Y. F. Xiao, K. Wang, D. Sun, X. C. Fan, X. Zhang, M. Zhang, Y. Z. Shi, J. Yu, F. X. Geng, C. S. Lee and X. H. Zhang, Angew. Chem., Int. Ed., 2021, 60, 2478–2484 CrossRef CAS PubMed.
- X. C. Fan, K. Wang, Y. Z. Shi, J. X. Chen, F. Huang, H. Wang, Y. N. Hu, Y. Tsuchiya, X. M. Ou, J. Yu, C. Adachi and X. H. Zhang, Adv. Opt. Mater., 2022, 10, 2101789 CrossRef CAS.
- D. R. Lee, B. S. Kim, C. W. Lee, Y. Im, K. S. Yook, S. H. Hwang and J. Y. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 9625–9629 CrossRef CAS PubMed.
- D. Karthik, Y. H. Jung, H. Lee, S. Hwang, B. M. Seo, J. Y. Kim, C. W. Han and J. H. Kwon, Adv. Mater., 2021, 33, 2007724 CrossRef CAS PubMed.
- W. Zeng, H. Y. Lai, W. K. Lee, M. Jiao, Y. J. Shiu, C. Zhong, S. Gong, T. Zhou, G. Xie, M. Sarma, K. T. Wong, C. C. Wu and C. Yang, Adv. Mater., 2018, 30, 1704961 CrossRef PubMed.
- C. Zhang, D. Zhang, Z. Bin, Z. Liu, Y. Zhang, H. Lee, J. H. Kwon and L. Duan, Adv. Mater., 2021, 2103102 CrossRef PubMed.
- I. S. Park, H. Min, J. U. Kim and T. Yasuda, Adv. Opt. Mater., 2021, 9, 2101282 CrossRef CAS.
- M. Kim and J. Y. Lee, Adv. Funct. Mater., 2014, 24, 4164–4169 CrossRef CAS.
- Y. L. Zhang, Q. Ran, Q. Wang, Y. Liu, C. Hanisch, S. Reineke, J. Fan and L. S. Liao, Adv. Mater., 2019, 31, 1902368 CrossRef CAS PubMed.
- C. C. Peng, S. Y. Yang, H. C. Li, G. H. Xie, L. S. Cui, S. N. Zou, C. Poriel, Z. Q. Jiang and L. S. Liao, Adv. Mater., 2020, 32, 2003885 CrossRef CAS PubMed.
- H. Xu, Z. Li, D. Yang, C. Han, B. Zhao, H. Wang, P. Ma, P. Chang and D. Ma, Angew. Chem., Int. Ed., 2021, 60, 14846–14851 CrossRef PubMed.
- M. Ma, J. Li, D. Liu, Y. Mei and R. Dong, ACS Appl. Mater. Interfaces, 2021, 13, 44615–44627 CrossRef CAS PubMed.
- J. X. Chen, K. Wang, Y. F. Xiao, C. Cao, J. H. Tan, H. Wang, X. C. Fan, J. Yu, F. X. Geng, X. H. Zhang and C. S. Lee, Adv. Funct. Mater., 2021, 31, 2101647 CrossRef CAS.
- M. Godumala, S. Choi, M. J. Cho and D. H. Choi, J. Mater. Chem. C, 2019, 7, 2172–2198 RSC.
- D. Mobius, Adv. Mater., 1995, 7, 437–444 CrossRef.
- F. Wurthner, Angew. Chem., Int. Ed., 2020, 59, 14192–14196 CrossRef PubMed.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 18, 1740–1741 RSC.
- J. Huang, H. Nie, J. Zeng, Z. Zhuang, S. Gan, Y. Cai, J. Guo, S. J. Su, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2017, 56, 12971–12976 CrossRef CAS PubMed.
- K. Y. Sun, D. Liu, W. W. Tian, F. Gu, W. X. Wang, Z. S. Cai, W. Jiang and Y. M. Sun, J. Mater. Chem. C, 2020, 8, 11850–11859 RSC.
- J. Lee, N. Aizawa, M. Numata, C. Adachi and T. Yasuda, Adv. Mater., 2017, 29, 1604856 CrossRef PubMed.
- X. Ban, F. Chen, Y. Liu, J. Pan, A. Zhu, W. Jiang and Y. Sun, Chem. Sci., 2019, 10, 3054–3064 RSC.
- S. Wang, H. Zhang, B. Zhang, Z. Xie and W. Y. Wong, Mater. Sci. Eng., A, 2020, 140, 100547 CrossRef.
- Y. Z. Shi, K. Wang, S. L. Zhang, X. C. Fan, Y. Tsuchiya, Y. T. Lee, G. L. Dai, J. X. Chen, C. J. Zheng, S. Y. Xiong, X. M. Ou, J. Yu, J. S. Jie, C. S. Lee, C. Adachi and X. H. Zhang, Angew. Chem., Int. Ed., 2021, 60, 25878–25883 CrossRef CAS PubMed.
- Y. Shi, K. Wang, Y. Tsuchiya, W. Liu, T. Komino, X. Fan, D. Sun, G. Dai, J. Chen, M. Zhang, C. Zheng, S. Xiong, X. Ou, J. Yu, J. Jie, C.-S. Lee, C. Adachi and X. Zhang, Mater. Horiz., 2020, 7, 2734–2740 RSC.
- Y. Z. Shi, K. Wang, X. Li, G. L. Dai, W. Liu, K. Ke, M. Zhang, S. L. Tao, C. J. Zheng, X. M. Ou and X. H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 9480–9484 CrossRef CAS PubMed.
- D. Gudeika, O. Bezvikonnyi, D. Volyniuk and J. V. Grazulevicius, Dyes Pigm., 2020, 172, 107789 CrossRef CAS.
- X. Zheng, R. Huang, C. Zhong, G. Xie, W. Ning, M. Huang, F. Ni, F. B. Dias and C. Yang, Adv. Sci., 2020, 7, 1902087 CrossRef CAS PubMed.
- Z. Cheng, Z. Li, Y. Xu, J. Liang, C. Lin, J. Wei and Y. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 28096–28105 CrossRef CAS PubMed.
- Z. Cheng, J. Liang, Z. Li, T. Yang, C. Lin, X. Mu and Y. Wang, J. Mater. Chem. C, 2019, 7, 14255–14263 RSC.
- D. Zhang, M. Cai, Y. Zhang, D. Zhang and L. Duan, Mater. Horiz., 2016, 3, 145–151 RSC.
- C. Y. Chan, M. Tanaka, H. Nakanotani and C. Adachi, Nat. Commun., 2018, 9, 5036 CrossRef PubMed.
- X. Ban, Y. Liu, J. Pan, F. Chen, A. Zhu, W. Jiang, Y. Sun and Y. Dong, ACS Appl. Mater. Interfaces, 2019, 12, 1190–1200 CrossRef PubMed.
- S. J. Zou, F. M. Xie, M. Xie, Y. Q. Li, T. Cheng, X. H. Zhang, C. S. Lee and J. X. Tang, Adv. Sci., 2019, 7, 1902508 CrossRef PubMed.
- D. Liu, M. Zhang, H. Chen, D. Ma, W. Tian, K. Sun, W. Jiang and Y. Sun, J. Mater. Chem. C, 2020, 9, 1221–1227 RSC.
- H. Noda, H. Nakanotani and C. Adachi, Sci. Adv., 2018, 4, 6910 CrossRef PubMed.
- Y. J. Cho, S. K. Jeon and J. Y. Lee, Adv. Opt. Mater., 2016, 4, 688–693 CrossRef CAS.
- X. Ban, A. Zhu, T. Zhang, Z. Tong, W. Jiang and Y. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 21900–21908 CrossRef CAS PubMed.
- D. Liu, W. Tian, Y. Feng, X. Zhang, X. Ban, W. Jiang and Y. Sun, ACS Appl. Mater. Interfaces, 2019, 11, 16737–16748 CrossRef CAS PubMed.
- J. Y. Wei, D. Liu, W. W. Tian, W. Jiang, Y. M. Sun, Z. Zhao and B. Z. Tang, Chem. Sci., 2020, 11, 7194–7203 RSC.
- H. J. Kim, S. K. Kim, M. Godumala, J. W. Yoon, C. Y. Kim, J. E. Jeong, H. Y. Woo, J. H. Kwon, M. J. Cho and D. H. Choi, Chem. Commun., 2019, 55, 9475–9478 RSC.
- C. Wu, Z. Wu, B. Wang, X. Li, N. Zhao, J. Hu, D. Ma and Q. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 32946–32956 CrossRef CAS PubMed.
- M. K. Etherington, N. A. Kukhta, H. F. Higginbotham, A. Danos, A. N. Bismillah, D. R. Graves, P. R. McGonigal, N. Haase, A. Morherr, A. S. Batsanov, C. Pflumm, V. Bhalla, M. R. Bryce and A. P. Monkman, J. Phys. Chem. C, 2019, 123, 11109–11117 CrossRef CAS PubMed.
- K. Sun, Y. Sun, W. Tian, D. Liu, Y. Feng, Y. Sun and W. Jiang, J. Mater. Chem. C, 2018, 6, 43–49 RSC.
- Z. G. Wu, H. B. Han, Z. P. Yan, X. F. Luo, Y. Wang, Y. X. Zheng, J. L. Zuo and Y. Pan, Adv. Mater., 2019, 31, 1900524 CrossRef PubMed.
- Z. G. Wu, Z. P. Yan, X. F. Luo, L. Yuan, W. Q. Liang, Y. Wang, Y. X. Zheng, J. L. Zuo and Y. Pan, J. Mater. Chem. C, 2019, 7, 7045–7052 RSC.
- Q. Zhang, D. Tsang, H. Kuwabara, Y. Hatae, B. Li, T. Takahashi, S. Y. Lee, T. Yasuda and C. Adachi, Adv. Mater., 2015, 27, 2096–2100 CrossRef CAS PubMed.
- Y. Li, G. Xie, S. Gong, K. Wu and C. Yang, Chem. Sci., 2016, 7, 5441–5447 RSC.
- Y. Yang, S. Wang, Y. Zhu, Y. Wang, H. Zhan and Y. Cheng, Adv. Funct. Mater., 2018, 28, 1706916 CrossRef.
- B. Huang, X. Ban, K. Sun, Z. Ma, Y. Mei, W. Jiang, B. Lin and Y. Sun, Dyes Pigm., 2016, 133, 380–386 CrossRef CAS.
- K. Matsuoka, K. Albrecht, K. Yamamoto and K. Fujita, Sci. Rep., 2017, 7, 41780–41788 CrossRef CAS PubMed.
- K. Matsuoka, K. Albrecht, A. Nakayama, K. Yamamoto and K. Fujita, ACS Appl. Mater. Interfaces, 2018, 10, 33343–33352 CrossRef CAS PubMed.
- Y. Chen, S. Wang, X. Wu, Y. Xu, H. Li, Y. Liu, H. Tong and L. Wang, J. Mater. Chem. C, 2018, 6, 12503–12508 RSC.
- Y. Liu, X. Wu, Y. Chen, L. Chen, H. Li, S. Wang, H. Tian, H. Tong and L. Wang, J. Mater. Chem. C, 2019, 7, 9719–9725 RSC.
- L. Wang, X. Cai, B. Li, M. Li, Z. Wang, L. Gan, Z. Qiao, W. Xie, Q. Liang, N. Zheng, K. Liu and S. J. Su, ACS Appl. Mater. Interfaces, 2019, 11, 45999–46007 CrossRef CAS PubMed.
- J. Guo, X. L. Li, H. Nie, W. Luo, S. Gan, S. Hu, R. Hu, A. Qin, Z. Zhao, S. J. Su and B. Z. Tang, Adv. Funct. Mater., 2017, 27, 1606458 CrossRef.
- J. Guo, X. L. Li, H. Nie, W. Luo, R. Hu, A. Qin, Z. Zhao, S. J. Su and B. Z. Tang, Chem. Mater., 2017, 29, 3623–3631 CrossRef CAS.
- X. Chen, Z. Yang, Z. Xie, J. Zhao, Z. Yang, Y. Zhang, M. P. Aldred and Z. Chi, Mater. Chem. Front., 2018, 2, 1017–1023 RSC.
- Y. Y. Jing, X. D. Tao, M. X. Yang, X. L. Chen and C. Z. Lu, Chem. Eng. J., 2021, 413, 127418 CrossRef CAS.
- J. Guo, J. Fan, L. Lin, J. Zeng, H. Liu, C. K. Wang, Z. Zhao and B. Z. Tang, Adv. Sci., 2019, 6, 1801629 CrossRef PubMed.
- F. Ma, G. Zhao, Y. Zheng, F. He, K. Hasrat and Z. Qi, ACS Appl. Mater. Interfaces, 2019, 12, 1179–1189 CrossRef PubMed.
- H. Liu, J. Zeng, J. Guo, H. Nie, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 9290–9294 CrossRef CAS PubMed.
- H. Liu, H. Liu, J. Fan, J. Guo, J. Zeng, F. Qiu, Z. Zhao and B. Z. Tang, Adv. Opt. Mater., 2020, 8, 2001027 CrossRef CAS.
- F. Ma, X. Zhao, H. Ji, D. Zhang, K. Hasrat and Z. Qi, J. Mater. Chem. C, 2020, 8, 12272–12283 RSC.
- Z. Qiu, W. Xie, Z. Yang, J. H. Tan, Z. Yuan, L. Xing, S. Ji, W. C. Chen, Y. Huo and S. J. Su, Chem. Eng. J., 2021, 415, 128949 CrossRef CAS.
- N. Aizawa, C. J. Tsou, I. S. Park and T. Yasuda, Polym. J., 2016, 49, 197–202 CrossRef.
- J. Chen, J. Zeng, X. Zhu, J. Guo, Z. Zhao and B. Z. Tang, CCS Chem., 2021, 3, 230–240 CrossRef CAS.
- L. Meng, H. Wang, X. Wei, J. Liu, Y. Chen, X. Kong, X. Lv, P. Wang and Y. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 20955–20961 CrossRef CAS PubMed.
- S. Xiang, R. Guo, Z. Huang, X. Lv, S. Sun, H. Chen, Q. Zhang and L. Wang, Dyes Pigm., 2019, 170, 107636 CrossRef CAS.
- J. Lee, N. Aizawa and T. Yasuda, Chem. Mater., 2017, 29, 8012–8020 CrossRef CAS.
- L. Wu, K. Wang, C. Wang, X. C. Fan, Y. Z. Shi, X. Zhang, S. L. Zhang, J. Ye, C. J. Zheng, Y. Q. Li, J. Yu, X. M. Ou and X. H. Zhang, Chem. Sci., 2021, 12, 1495–1502 RSC.
- Z. Huang, Z. Bin, R. Su, F. Yang, J. Lan and J. You, Angew. Chem., Int. Ed., 2020, 59, 9992–9996 CrossRef CAS PubMed.
- M. Shibano, H. Ochiai, K. Suzuki, H. Kamitakahara, H. Kaji and T. Takano, Macromolecules, 2020, 53, 2864–2873 CrossRef CAS.
- J. Li, R. Zhang, Z. Wang, B. Zhao, J. Xie, F. Zhang, H. Wang and K. Guo, Adv. Opt. Mater., 2018, 6, 1701256 CrossRef.
- I. H. Lee, W. Song and J. Y. Lee, Org. Electron., 2016, 29, 22–26 CrossRef CAS.
- H. Tanaka, K. Shizu, H. Nakanotani and C. Adachi, J. Phys. Chem. C, 2014, 118, 15985–15994 CrossRef CAS.
- J. Luo, S. Gong, Y. Gu, T. Chen, Y. Li, C. Zhong, G. Xie and C. Yang, J. Mater. Chem. C, 2016, 4, 2442–2446 RSC.
- X. Ban, W. Jiang, K. Sun, B. Lin and Y. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 7339–7346 CrossRef CAS PubMed.
- S. Xiang, z. huang, S. Sun, X. Lv, L. Fan, S. Ye, H. Chen, R. Guo and L. Wang, J. Mater. Chem. C, 2018, 6, 11436–11443 RSC.
- R. S. Nobuyasu, Z. Ren, G. C. Griffiths, A. S. Batsanov, P. Data, S. Yan, A. P. Monkman, M. R. Bryce and F. B. Dias, Adv. Opt. Mater., 2016, 4, 597–607 CrossRef CAS.
- Y. F. Li, T. H. Chen, M. L. Huang, Y. Gu, S. L. Gong, G. H. Xie and C. L. Yang, J. Mater. Chem. C, 2017, 5, 3480–3487 RSC.
- Z. Yang, Z. Mao, C. Xu, X. Chen, J. Zhao, Z. Yang, Y. Zhang, W. Wu, S. Jiao, Y. Liu, M. P. Aldred and Z. Chi, Chem. Sci., 2019, 10, 8129–8134 RSC.
- C. S. Li, A. K. Harrison, Y. C. Liu, Z. N. Zhao, C. Zeng, F. B. Dias, Z. J. Ren, S. K. Yan and M. R. Bryce, Angew. Chem., Int. Ed., 2021 DOI:10.1002/anie.202115140.
- R. Guo, P. Leng, Q. Zhang, Y. Wang, X. Lv, S. Sun, S. Ye, Y. Duan and L. Wang, Dyes Pigm., 2020, 184, 108781 CrossRef.
- S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lüssem and K. Leo, Nature, 2009, 459, 234–239 CrossRef CAS PubMed.
- Y. Z. Shi, K. Wang, X. C. Fan, J. X. Chen, X. M. Ou, J. Yu, J. S. Jie, C. S. Lee and X. H. Zhang, Adv. Opt. Mater., 2021, 9, 2100461 CrossRef CAS.
- X. Li, S. Shen, C. Zhang, M. Liu, J. Lu and L. Zhu, Sci. China: Chem., 2021, 64, 534–546 CrossRef CAS.
- P. L. dos Santos, M. K. Etherington and A. P. Monkman, J. Mater. Chem. C, 2018, 6, 4842–4853 RSC.
- K. Wang, Y. Z. Shi, C. J. Zheng, W. Liu, K. Liang, X. Li, M. Zhang, H. Lin, S. L. Tao, C. S. Lee, X. M. Ou and X. H. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 31515–31525 CrossRef CAS PubMed.
- K. Wang, C. J. Zheng, W. Liu, K. Liang, Y. Z. Shi, S. L. Tao, C. S. Lee, X. M. Ou and X. H. Zhang, Adv. Mater., 2017, 29, 1701476 CrossRef PubMed.
- L. Zhang, Y. F. Wang, M. Li, Q. Y. Gao and C. F. Chen, Chin. Chem. Lett., 2020, 32, 740–744 CrossRef.
- L. Yu, Z. Wu, G. Xie, W. Zeng, D. Ma and C. Yang, Chem. Sci., 2018, 9, 1385–1391 RSC.
- L. Yu, Z. Wu, G. Xie, C. Zhong, Z. Zhu, D. Ma and C. Yang, Chem. Commun., 2018, 54, 1379–1382 RSC.
- Y. Tao, D. Zhang, X. Cao, Q. Wu, M. Zhang, N. Sun and X. Zhang, J. Mater. Chem. C, 2017, 6, 3675–3682 Search PubMed.
- X. Zhang, M. W. Cooper, Y. Zhang, C. Fuentes-Hernandez, S. Barlow, S. R. Marder and B. Kippelen, ACS Appl. Mater. Interfaces, 2019, 11, 12693–12698 CrossRef CAS PubMed.
- S. Wang, X. Yan, Z. Cheng, H. Zhang, Y. Liu and Y. Wang, Angew. Chem., Int. Ed., 2015, 54, 13068–13072 CrossRef CAS PubMed.
- H. Xu, B. Zhao, H. Wang, C. Han, P. Ma, Z. Li and P. Chang, Angew. Chem., Int. Ed., 2020, 59, 19042–19047 CrossRef PubMed.
- H. Wang, B. Zhao, P. Ma, Z. Li, X. Wang, C. Zhao, X. Fan, L. Tao, C. Duan, J. Zhang, C. Han, G. Chen and H. Xu, J. Mater. Chem. C, 2019, 7, 7525–7530 RSC.
- J. X. Chen, W. W. Tao, W. C. Chen, Y. F. Xiao, K. Wang, C. Cao, J. Yu, S. Li, F. X. Geng, C. Adachi, C. S. Lee and X. H. Zhang, Angew. Chem., Int. Ed., 2019, 58, 14660–14665 CrossRef CAS PubMed.
- Y. Liu, Y. Chen, H. Li, S. Wang, X. Wu, H. Tong and L. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 30652–30658 CrossRef CAS PubMed.
- W. L. Tsai, M. H. Huang, W. K. Lee, Y. J. Hsu, K. C. Pan, Y. H. Huang, H. C. Ting, M. Sarma, Y. Y. Ho, H. C. Hu, C. C. Chen, M. T. Lee, K. T. Wong and C. C. Wu, Chem. Commun., 2015, 51, 13662–13665 RSC.
- Y. Wada, K. Shizu, S. Kubo, T. Fukushima, T. Miwa, H. Tanaka, C. Adachi and H. Kaji, Appl. Phys. Express, 2016, 9, 032102 CrossRef.
- S. Jeong, Y. Lee, J. K. Kim, D. J. Jang and J. I. Hong, J. Mater. Chem. C, 2018, 6, 9049–9054 RSC.
- X. Cai, Z. Qiao, M. Li, X. Wu, Y. He, X. Jiang, Y. Cao and S. J. Su, Angew. Chem., Int. Ed., 2019, 58, 13522–13531 CrossRef CAS PubMed.
- W. Li, B. Li, X. Cai, L. Gan, Z. Xu, W. Li, K. Liu, D. Chen and S. J. Su, Angew. Chem., Int. Ed., 2019, 58, 11301–11305 CrossRef CAS PubMed.
- Q. Zhang, S. Sun, W. Liu, P. Leng, X. Lv, Y. Wang, H. Chen, S. Ye, S. Zhuang and L. Wang, J. Mater. Chem. C, 2019, 7, 9487–9495 RSC.
- X. Wang, S. Wang, J. Lv, S. Shao, L. Wang, X. Jing and F. Wang, Chem. Sci., 2019, 10, 2915–2923 RSC.
- P. Zhang, J. Zeng, J. Guo, S. Zhen, B. Xiao, Z. Wang, Z. Zhao and B. Z. Tang, Front. Chem., 2019, 7, 199 CrossRef CAS PubMed.
- H. Y. Chih, Y. W. Chen, Y. C. Hsieh, W. C. Li, C. W. Liao, C. H. Lin, T. Y. Chiu, W. W. Tsai, C. W. Lu and C. H. Chang, Chem.–Eur. J., 2019, 25, 16699–16711 CrossRef CAS PubMed.
- K. Albrecht, K. Matsuoka, K. Fujita and K. Yamamoto, Angew. Chem., Int. Ed., 2015, 54, 5677–5682 CrossRef CAS PubMed.
- K. Albrecht, K. Matsuoka, D. Yokoyama, Y. Sakai, A. Nakayama, K. Fujita and K. Yamamoto, Chem. Commun., 2017, 53, 2439–2442 RSC.
- X. Ban, W. Jiang, T. Lu, X. Jing, Q. Tang, S. Huang, K. Sun, B. Huang, B. Lin and Y. Sun, J. Mater. Chem. C, 2016, 4, 8810–8816 RSC.
- K. Sun, Y. Sun, T. Huang, J. Luo, W. Jiang and Y. Sun, Org. Electron., 2017, 42, 123–130 CrossRef CAS.
- M. Godumala, S. Choi, H. J. Kim, C. Lee, S. Park, J. S. Moon, K. Si Woo, J. H. Kwon, M. J. Cho and D. H. Choi, J. Mater. Chem. C, 2018, 6, 1160–1170 RSC.
- Y. Zhu, Y. Yang, Y. Wang, B. Yao, X. Lin, B. Zhang, H. Zhan, Z. Xie and Y. Cheng, Adv. Opt. Mater., 2018, 6, 1701320 CrossRef.
- Y. Wang, Y. Zhu, X. Lin, Y. Yang, B. Zhang, H. Zhan, Z. Xie and Y. Cheng, J. Mater. Chem. C, 2018, 6, 568–574 RSC.
- J. Hu, Q. Li, X. Wang, S. Shao, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2019, 58, 8405–8409 CrossRef CAS PubMed.
- Q. Li, J. Hu, J. Lv, X. Wang, S. Shao, L. Wang, X. Jing and F. Wang, Angew. Chem., Int. Ed., 2020, 59, 20174–20182 CrossRef CAS PubMed.
- X. L. Chen, J. H. Jia, R. Yu, J. Z. Liao, M. X. Yang and C. Z. Lu, Angew. Chem., Int. Ed., 2017, 56, 15006–15009 CrossRef CAS PubMed.
- X. Tang, Y. Tao, H. Liu, F. Liu, X. He, Q. Peng, J. Li and P. Lu, Front. Chem., 2019, 7, 373 CrossRef CAS PubMed.
- I. S. Park, K. Matsuo, N. Aizawa and T. Yasuda, Adv. Funct. Mater., 2018, 28, 1802031 CrossRef.
- K. Matsuo and T. Yasuda, Chem. Sci., 2019, 10, 10687–10697 RSC.
- G. Xia, C. Qu, Y. Zhu, J. Ye, K. Ye, Z. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 9598–9603 CrossRef CAS PubMed.
- G. Meng, X. Chen, X. Wang, N. Wang, T. Peng and S. Wang, Adv. Opt. Mater., 2019, 7, 1900130 CrossRef.
- H. J. Kim, M. Godumala, S. K. Kim, J. Yoon, C. Y. Kim, H. Park, J. H. Kwon, M. J. Cho and D. H. Choi, Adv. Opt. Mater., 2020, 8, 1902175 CrossRef CAS.
- H. J. Kim, H. Kang, J. E. Jeong, S. H. Park, C. W. Koh, C. W. Kim, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Adv. Funct. Mater., 2021, 31, 2102588 CrossRef CAS.
- F. Chen, J. Hu, X. Wang, S. Shao, L. Wang, X. Jing and F. Wang, Sci. China: Chem., 2020, 63, 1112–1120 CrossRef CAS.
- R. Furue, T. Nishimoto, I. S. Park, J. Lee and T. Yasuda, Angew. Chem., Int. Ed., 2016, 55, 7171–7175 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |