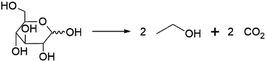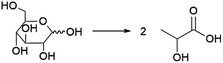Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fermentation for the production of biobased chemicals in a circular economy: a perspective for the period 2022–2050
Tom A.
Ewing
 a,
Niels
Nouse
ab,
Matthijs
van Lint
a,
Jacco
van Haveren
a,
Jeroen
Hugenholtz
ab and
Daan S.
van Es
a,
Niels
Nouse
ab,
Matthijs
van Lint
a,
Jacco
van Haveren
a,
Jeroen
Hugenholtz
ab and
Daan S.
van Es
 *a
*a
aWageningen Food & Biobased Research, Wageningen University & Research, Bornse Weilanden 9, 6708 WG, Wageningen, The Netherlands. E-mail: daan.vanes@wur.nl
bSwammerdam Institute for Life Sciences, University of Amsterdam, Science Park 904, 1098 XH, Amsterdam, The Netherlands
First published on 21st July 2022
Abstract
Chemicals and materials produced from non-renewable, petrochemical feedstocks contribute greatly to the quality of life we currently enjoy. To ensure that quality of life is maintained in the future, it is imperative that we move towards a circular economy, where care is taken to reuse or recycle materials at their end-of-life and new chemicals and materials are sourced from renewable carbon feedstocks such as biomass. To achieve this transition, efficient conversion methods by which biomass-derived feedstocks can be converted to chemicals are required. The high degree of functionalisation (i.e. high content of oxygen atoms) of biomass-derived feedstocks, makes their conversion by microbial fermentation an interesting option. This article provides an overview of currently available fermentation technologies that have the potential to play a role in the production of biobased bulk chemicals in a circular economy in the period up to 2050. Our focus is primarily on technologies that are sufficiently mature to have been implemented on (at least) industrial pilot scale. In addition to an overview of available technologies, we provide a critical assessment of their potential relevance for use in the production of bulk chemicals in a future circular economy. We conclude that seven fermentation processes for the production of (potential) bulk chemicals have already reached a stage of technological maturity where they are ready to be applied in a circular economy. A number of other processes may reach this stage of maturity in the coming years.
Introduction
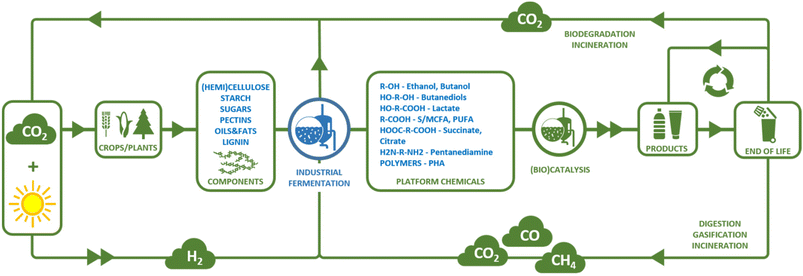
Chemicals and materials produced by the chemical industry are commonplace in our everyday lives and make an important contribution to the standard of living we enjoy today. Currently, the major feedstocks of organic carbon for the chemical industry are fossil-based (petrochemicals). Fossil resources, however, take millions of years to form and therefore cannot be readily replenished in a reasonable timeframe and thus essentially form a finite resource. To ensure a sustainable future for the chemical industry and continued access to chemicals and materials that are essential for our high standard of living, it is imperative that we transition towards a circular economy (see Fig. 1).1 In a circular economy, the consumption of non-renewable resources, such as fossil fuels, for the production of chemicals and materials is eliminated and replaced by the use of renewable resources.1 The most direct way to produce chemicals and materials from renewable resources, is to convert them into new chemicals and materials at their end-of-life by reuse or (mechanical or chemical) recycling.1 However, recycling by itself will be insufficient to provide all starting materials for the chemical industry. A loss of material will always occur as collection and recycling processes will not reach 100% efficiency (see e.g. ref. 2 for an analysis of the maximum recycling rates likely to be achieved for plastic waste in the Netherlands). For some chemicals and materials, collection for recycling will not be feasible, e.g. detergents used in soap or shampoo will always end up in wastewater streams in very low concentrations, making their collection nearly impossible. In addition, the global population is projected to grow to 9.7 billion in 2050, a 23% increase compared to today.3 Together with growing per capita incomes, this growth in population is predicted to lead to an increase in the demand for carbon for chemicals from 450 million metric tons (MMt) per annum in 2021 to 1000 MMt per annum in 2050.4 Thus, the chemical industry's demand for starting materials will exceed the amount of material that can be generated by recycling or reuse alone and other sources of renewable starting materials, in particular sources of organic carbon, will be required. One prominent example of a renewable carbon source is carbon from biomass, which can be replenished within a matter of years to decades using only carbon dioxide and sunlight required for photosynthesis. Therefore, the development of biobased chemicals and materials, and the biorefinery and conversion technologies required to manufacture them has been a topic of extensive research in recent decades.5 Direct capture and use of atmospheric CO2 as a renewable carbon source for chemicals production is also under consideration, but is at an earlier stage of development.6In addition to placing demands on the type of starting materials that can be used for chemicals and materials production, the transition to a circular economy places demands on the properties of the chemicals and materials that we use and how they are produced. Chemicals and materials should be designed such that their active lifetime is as long as possible (e.g. limiting single use products). Care should also be taken to design chemicals and materials in such a way to maximise their potential for reuse or recycling at their end-of-life (see e.g. ref. 7). Incineration or biodegradation should only be applied when the quality of a chemical or material has decreased so much that further reuse or recycling is no longer an option (see Fig. 1). The carbon dioxide released upon incineration or biodegradation serves to replenish the reservoir of renewable carbon available as biomass. In addition to considering these end-of-life options for chemicals and materials that remain within a controllable cycle, we must consider what happens to chemicals and materials that end up in the natural environment, whether due to inadvertent leakage or as an unavoidable side-effect of their manner of use. These chemicals and materials should not accumulate in or harm the natural environment and should therefore biodegrade without releasing toxic components.
The circular economy also places demands on the type of conversion processes that can be employed to convert renewable starting materials to chemicals and materials. Conversion processes should be designed so as to limit the amount of resources consumed and the risk of exposure to toxic chemicals, both for humans and the natural environment. These demands placed on chemical conversion processes in a circular economy are in general well summarised by the twelve principles of green chemistry.8 These principles should be taken into consideration when designing routes for the production of chemicals in a circular economy. In particular in the case of biomass-derived feedstocks, an important consideration is the optimal use of heteroatoms. In contrast to petrochemical feedstocks, that mainly contain hydrogen and carbon atoms, biomass-derived feedstocks typically contain significant amounts of heteroatoms (in particular oxygen and nitrogen). Many end-products produced by the chemical industry also contain heteroatoms, which are currently introduced into the fossil-based hydrocarbon base chemicals. In the production of chemicals from biomass-derived feedstocks in a circular economy, a more efficient use of resources might be achieved by designing routes that maintain the heteroatom contents of the feedstock, rather than routes that rely on the complete defunctionalisation of biomass-derived components (e.g. to the petrochemical drop-in bioethylene) followed by reintroduction of heteroatoms.9 The shorter routes this enables (in terms of chemical steps) can be expected to minimise waste production and energy consumption, leading to more efficient overall processes. Considering the use of renewable, primarily biomass-derived feedstocks and the desire to employ safe conversion technologies that generate limited waste, a conversion technology that has significant potential to be applied in the circular economy is microbial fermentation (see also Fig. 1). The most suitable process by which to achieve a specific chemical conversion must be analysed by comparing techno-economic and life cycle analyses of each available conversion route on a case-by-case basis. Nevertheless, general advantages and disadvantages of different conversion technologies can be defined on a qualitative basis. One advantage of fermentation for the production of chemicals from biomass lies in the multi-step nature of microbial metabolic pathways. This can allow the often highly functionalised (i.e. high content of heteroatoms, in particular oxygen atoms) biomass-derived molecules to be converted to a target chemical in a single process step, which may not always be possible using chemical conversion. In addition, sugars, one of the main biomass-derived chemical feedstocks, are highly water soluble and often heat labile. This makes fermentation, which occurs at mild temperatures in aqueous media, well suited for their conversion, in contrast to many conventional chemical processes, which often require the use of organic solvents and high(er) temperatures. In addition, microorganisms are often capable of converting substrates found in impure or mixed feedstocks, making them well suited for processing complex biomass (-derived) feedstocks (see e.g. volatile fatty acid section below). In certain cases, fermentation can even be used to convert multiple different starting compounds from a chemically heterogeneous biomass source into a single product while still retaining a degree of the functionalisation present in the feedstock, a concept known as “funnelling”. This concept has been demonstrated, for example, by the conversion of mixtures of aromatic compounds that can be obtained from lignin to a single product (muconic acid or a polyhydroxyalkanoate, see e.g. ref. 10 and 11). These advantages provide fermentation with the potential to be a key enabling technology in the transition towards the circular economy. However, fermentation-based processes do come with their drawbacks, which include the consumption of nutrients (other than the main fermentation substrate), the complexity of downstream processing of the fermentation broth, the sensitivity of microorganisms towards toxic compounds in fermentation substrates or products and, compared to conventional chemical processes, the relatively low product and substrate titres and slow reaction kinetics, necessitating large reactor volumes, and thus leading to lower volumetric productivities. Additionally, in many, though not all, fermentation processes, a significant part of the carbon present in the substrate is lost through emission as CO2 (see below).
Recent decades have seen significant advances in the development of fermentative methods for the production of biobased chemicals, from laboratory scale research to industrial development and commercialisation. This review provides an overview on the current status of the industrial application of fermentation for the production of (potential) bulk chemicals from biomass. The goal is to provide an inventory of technologies that are available to assist us in a transition to a fully circular economy in the coming decades, with the transition envisioned to be completed by 2050. In addition to providing an overview of available conversion technologies, we critically discuss the potential of the produced compounds to be converted to chemicals and materials that meet the requirements for application in a circular economy. As we aim to provide an overview of technologies with a high potential to be applied commercially by 2050, the focus is on technologies that have entered industrial application at pilot or demonstration scale, at the minimum. Some particularly promising technologies that are still at the laboratory scale of development are also discussed. For an extensive overview of potential routes to bulk chemicals using biotechnological conversion, including technologies that have not proceeded to commercialisation, see e.g. ref. 12. The focus of this review is on technologies applied for the production of (potential) bulk chemicals, meaning fermentation-based technologies applied for the production of specialty chemicals or pharmaceuticals or in the food industry are excluded, unless the products have potential alternative applications as bulk chemicals.
Biobased feedstocks for fermentation
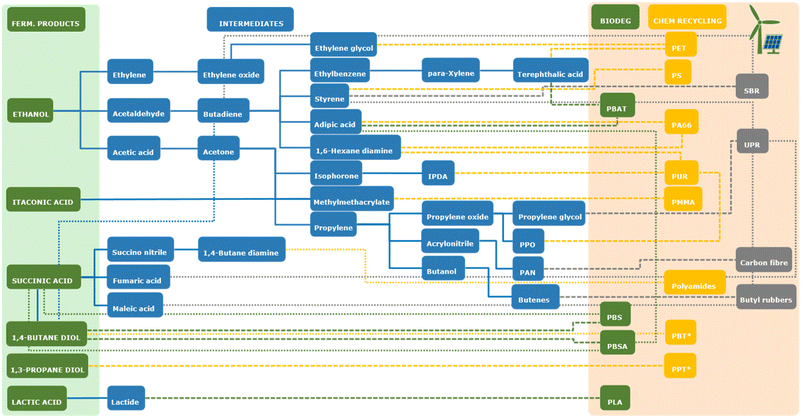
Production of biobased chemicals and materials starts from a biomass-derived feedstock. Biomass feedstocks are typically complex, heterogeneous materials, containing a diverse range of molecules such as carbohydrates, proteins, oils and fats, and lignin. Thus, in cases where a single, homogeneous feedstock is required for conversion into biobased chemicals, biomass must first be pre-treated to obtain the feedstock. The wide range of processing technologies available falls outside the scope of this review. Below, the main feedstocks that are available as a substrate for fermentation are briefly presented.The most commonly used feedstocks for the production of biobased chemicals by fermentation are carbohydrates. Sugars such as glucose and sucrose can be obtained from sucrose- or starch-containing plants. Common sources of sucrose include sugarcane and sugar beet, while common sources of starch are, for example, corn, cassava and sorghum. Collectively, these feedstocks are referred to as first generation biomass feedstocks.13 The use of these feedstocks for the production of biofuels (e.g. bioethanol) has generated some discussion, as they are also suitable for consumption by humans and animals, raising concerns around competition for land use with food and feed production.14 Therefore, the use of sugars from non-edible lignocellulosic biomass residues, such as corn stover, sugarcane bagasse, non-food crops such as silvergrass (Miscanthus) or forestry residues (e.g. bark or wood chips), known collectively as second generation biomass feedstocks has been gathering interest recently.15 Here, the challenge is to develop efficient methods by which to obtain monomeric sugars from these recalcitrant feedstocks and to develop methods for the utilisation of pentose sugars present in hemicellulose in addition to the glucose from cellulose.16,17 Currently, fermentation processes based on second generation feedstocks are mainly in the developmental phase, while most commercial processes for the production of biobased chemicals rely on the use of first generation sugar feedstocks. Although use of first generation feedstocks for the production of biofuels is controversial due to the potential for competition with land use for food production, it should be noted that the lower market volumes associated with the production of chemicals and materials would make the impact of their production from first generation feedstocks on land use lower than that of the production of biofuels (e.g. petrochemicals only account for 16% of current demand for oil in OECD countries).18 Other feedstocks that are, or have been, applied in fermentation processes for the production of chemicals include vegetable oil-derived feedstocks such as glycerol, a by-product of biodiesel production, and fatty acids.19 The use of lignin-derived phenolic compounds as a feedstock for fermentation is also being investigated, although it is still in the developmental stages (e.g. ref. 10). Another class of biomass-derived feedstocks that can be converted to chemicals by fermentation consists of gasses such as methane, syngas (a mixture of H2 and CO) or CO2 that can be obtained by the anaerobic digestion, gasification or incineration of biomass.20 This has the advantage that biomass does not need to be fractionated prior to processing, allowing it to be applied to streams that are not suited for isolation of a single homogeneous feedstock due to their complexity, e.g. mixed waste streams. However, challenges remain in terms of obtaining a gas stream free from inhibiting impurities and overcoming limitations caused by slow gas–liquid phase transfer (see below). Once a suitable feedstock has been obtained from biomass, it can be converted to the desired biobased chemical by fermentation. Products that can be obtained by fermentation include short chain alcohols and diols, (di)acids, diamines, ω-hydroxyamines, triglycerides and polymers (polyhydroxyalkanoates) (Table 1). The processes employed to produce these molecules are the topic of the remainder of this manuscript.
| Chemical | Major application(s)a | Production organism | Feedstock(s) | Typical yield (g product per g feedstock) | Industrial producer(s) | Established capacityb |
|---|---|---|---|---|---|---|
| a (Potential) applications as a bulk chemical intermediate, applications in other domains (e.g. biofuels, food, cosmetics, pharmaceuticals) are not listed. b Estimated production capacity from biobased feedstock by fermentation, based on most recent available estimates. c Value represents an estimate of the actual market volume in the specified year, not total production capacity. d Could not be established from publicly available sources. | ||||||
| Ethanol | Ethylene production | Saccharomyces cerevisiae | Sugarcane juice or molasses, corn starch | 0.45 g/g glucose | Archer Daniels Midland, Flint Hills Resources, Green Plains, POET, Valero Renewable Fuels | 79 MMtc (2020) |
| n-Butanol | Butene, butadiene production | Clostridium acetobutylicum, Clostridium beijerinckii | (Corn) starch, molasses | 0.3–0.35 g product (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone: butanol 1 acetone: butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol by volume) per g glucose ethanol by volume) per g glucose |
None currently confirmed | 103 kt in 2016, then declined due to plant closures |
| Isobutanol | Isobutylene production | Engineered yeasts | Corn starch | Gevo Inc. | 5 kt (2016) | |
| 1,3-Propanediol | Polytrimethylene terephthalate production | Engineered Escherichia coli | Corn starch | 0.51 g/g glucose | Dupont Tate & Lyle Bioproducts | 61 kt (2018) |
| Likely bacteria (e.g. Klebsiella sp.) | Glycerol | Zhangjiagang Glory Biomaterial, Shenghong group | ≥40 kt (2013) | |||
| 1,4-Butanediol | Polybutylene succinate, polybutylene terephthalate, (poly)tetrahydrofuran production | Engineered Escherichia coli | >0.40 g/g glucose | Novamont, BASF | ≥30 kt (2016) | |
| Lactic acid | Polylactic acid production | Lactic acid bacteria | Sugarcane/sugar beet derived sugars, starches | 0.9 g/g glucose | Corbion, Natureworks, Galactic, Henan Jindan Lactic acid | 750 ktc (2014) |
| Succinic acid | Polybutylene succinate production, 1,4-butanediol production | Basfia succiniciproducens, engineered yeasts, engineered Escherichia coli | Starch-derived sugars | 0.9–1.1 g/g glucose | LCY Biosciences, Roquette, Succinity | Reached 64 kT in 2015, then declined due to plant closures |
| Dodecanedioic acid | Polyamide and polyester production | Bacterial strain | Fatty acids, likely from tropical oils | Cathay Industrial Biotech | 70 kt (2018) | |
| Itaconic acid | Production of polyacrylates | Aspergillus terreus | 0.5 g/g glucose | Zhejiang Guogang Biochemistry, Qingdao Kehai Biochemistry | 50 kt (2013) | |
| Citric acid | Production of itaconic acid | Aspergillus niger, Aspergillus wentii | Molasses, starch-derived sugars | 0.7–0.9 g/g glucose | Archer Daniels Midland, Cargill, Citrique Belge, Jungbunzlauer, Tate & Lyle, Weifang Ensign Industry, TTCA, RZBC Group, COFCO Biochemical | >2 MMtc (2018) |
| 1,5-Pentanediamine | Production of polyamides | Likely engineered Escherichia coli | Cathay Industrial Biotech | ≥50 kt (2018) | ||
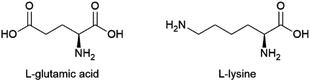
| L-Glutamic acid | To be developed | Corynebacterium glutamicum | Sugars from starch, molasses + ammonium salt | ∼0.6 g/g glucose | Ajinomoto, Evonik, Hefei TNJ Chemical Industry, Global Bio-chem Technology Group Company, Ningxia Yipin Biological Technology, Sichuan Tongsheng Amino Acid, Suzhou Yuanfang Chemical, Kyowa Hakko Bio, Ottokemi | 2.9 MMtc (2014) |
| L-Lysine | Production of polyamide building blocks | (Modified) Corynebacterium glutamicum or Escherichia coli | Sugars from starch, molasses + ammonium salt | 0.55–0.60 g/g glucose | Ajinomoto, Kyowa Hakko Bio, Archer Daniels Midland, Changchun Dacheng, COFCO Biochemical, Global Bio-chem Technology Group Company, Shandong Shouguang Juneng Golden Corn, Sunrise Nutrachem Cheil Jidang, Evonik | 1.9 MMtc (2013) |
| β-Farnesene | Polymer production, lubricants | Engineered Saccharomyces cerevisiae | Sugarcane-derived sugars | 0.2 g/g glucose | DSM (formerly Amyris) | 33 kt (2018) |
| Polyhydroxyalkanoates (PHAs) | Bioplastics | Various bacteria (e.g. Cupriavidis necator) | Plant oils, sugar beet and sugarcane processing waste, sugars from corn or cassava starch, biomass-derived methane | 0.3–0.4 g/g glucose, 0.6–0.8 g/g plant oil | Kaneka, Daminer Scientific, TianAn Biopolymer, Tianjin GreenBio Materials, Newlight Technologies | 36 ktc (2020) |
Short chain alcohols
One class of compounds that can be produced from biomass-derived sugars by fermentation is short chain alcohols. These compounds have gathered interest in recent decades due to their potential use as biofuels (e.g. bioethanol), but are also of interest as feedstocks for the chemical industry as they can be converted into short chain alkenes, such as ethylene, propylene or (iso)butylene by dehydration (see below). These alkenes are one of the primary feedstocks of the current petrochemical industry, making their production from biobased alcohols a potential way to produce “drop-in” feedstocks that can be readily incorporated into existing petrochemical infrastructures.21Ethanol
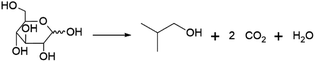
Perhaps the best-known fermentation process for the production of a biobased chemical is the conversion of plant-derived sugars to ethanol. The industrial production of biomass-derived ethanol was widely performed in recent decades, primarily with the goal of using the formed bioethanol as a renewable transportation fuel. However, bioethanol production is also of interest to the chemical industry, as ethanol can be dehydrated to yield ethylene, one of the most important platform chemicals for the current petrochemical industry.22 In 2016, global ethylene production stood at approximately 150 MMt, overwhelmingly from fossil resources, with over 60% of the produced ethylene being converted into the polymer polyethylene.23 In addition to being a precursor of ethylene, ethanol itself has important non-fuel applications, e.g. as a solvent.Ethanol is produced by fermentation from carbohydrate substrates using Saccharomyces cerevisiae (baker's yeast).24 One mole of glucose is converted to two moles of ethanol and two moles of CO2, giving a theoretical yield of 0.51 g ethanol per g glucose (Scheme 1). Currently, commercial production of bioethanol is primarily located in Brazil and the USA, where sugar from sugarcane and corn starch, respectively, are the main feedstocks.13 Global production of bioethanol stood at 100 billion L (or 79 MMt) in 2020.25 Processes for bioethanol production from corn starch typically start with a milling process, by which starch is released from the other components of the grain, followed by the enzymatic depolymerisation of the starch to glucose (Fig. 2). The depolymerisation occurs in two steps: liquefication, during which starch is depolymerised to oligosaccharides by α-amylase at temperatures >80 °C, and saccharification, during which the oligosaccharides produced during liquefication are further hydrolysed by glucoamylase at a slightly lower temperature of around 60 °C to yield glucose. Glucose is subsequently used as the substrate for fermentation. After 2–3 days of fermentation at around 30 °C, a fermentation broth containing 10–12% ethanol is obtained, from which the ethanol is subsequently isolated by distillation.13 In industrial processes, around 90% of the theoretical yield of 0.51 g ethanol per g glucose is typically achieved, yielding 0.45 g ethanol per g glucose (1.8 mol/mol).13
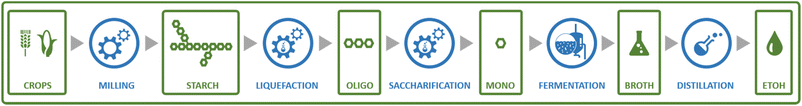 | ||
| Fig. 2 Schematic representation of the steps involved in a typical process for the production of bioethanol from a first generation feedstock: corn starch. | ||
As of 2021, approximately 200 corn-based ethanol plants were operational in the USA, with capacities ranging from approximately 8 million L (6 kt) to 1.4 billion L (1.1 MMt) per annum (with the majority having a capacity between 180 million L (142 kt) and 600 million L (473 kt) per annum).26 Major producers include Archer Daniels Midland, Flint Hills Resources, Green Plains, POET and Valero Renewable Fuels.26 In sugarcane-based ethanol production, the feedstock is crushed to extract a sugar-rich juice that can be used directly as a substrate for fermentation.27 Alternatively, ethanol production can be performed alongside sugar production by fermenting sugarcane molasses, the glucose- and fructose-rich syrup that is obtained after removal of sucrose from sugarcane juice by crystallisation. In 2017, there were 384 plants producing sugarcane-derived ethanol in Brazil, with a total capacity of 40 billion L (32 MMt) per annum, an average of 104 million L (82 kt) per annum per plant.28
Over the last two decades or so, concerns regarding the use of food crops for ethanol production drove efforts to establish production of bioethanol from non-edible 2nd generation lignocellulosic feedstocks such as corn stover or wood chips.17 Production of cellulosic ethanol typically involves a pre-treatment step to enhance the accessibility of the cellulose in the biomass for hydrolysis. Subsequently, the cellulose and hemicellulose are broken down to glucose and pentoses respectively by enzymatic hydrolysis, followed by fermentation of the released sugars to ethanol and isolation of the ethanol by distillation.17 Various companies have attempted the commercialisation of cellulosic bioethanol, with projects for 20 commercial plants with a total production capacity of 1 MMt per annum of ethanol having been initiated in the last 10 years.29 Examples of current commercial activities are the Swedish company SEKAB, that commercialises lignocellulosic ethanol produced from forestry residues for multiple uses,30 and the development by the Swiss company Clariant of a Sunliquid© facility in Romania for bioethanol production from cereal straw at a scale of 250 kt bioethanol per annum.31 However, various technical challenges, e.g. in the development of pre-treatment technologies,32 have hampered the establishment of cost-effective production of ethanol from 2nd generation feedstocks and many of the planned commercialisation projects are lying idle or are on hold.29,33 Estimates of production figures for 2017–2018 indicate that cellulosic ethanol accounted for less than 1% of global bioethanol production.29
Although the future of bioethanol for fuel applications is uncertain due to an increasing trend towards electrically-powered vehicles, its production, at least from 1st generation feedstocks, is well established at multi-MMt per annum scale. Therefore, the technology is in place for bioethanol to be an important feedstock for the chemical industry in future. The desirability of using bioethanol as a “drop-in” replacement for the production of ethylene as a precursor to polyethylene in the circular economy is debatable, as polyethylene does not meet all criteria expected of a truly circular material (in particular due to its poor biodegradability34,35). However, compounds derived from ethanol, such as ethylene, acetaldehyde and acetic acid, have the potential to be precursors to a wide range of other biobased bulk chemicals (see Fig. 3) and therefore ethanol is still a highly interesting compound for the future circular economy.
n-Butanol
Another short chain alcohol that can be produced by fermentation is n-butanol. It has potential applications as a biofuel, but may also be dehydrated to yield 1-butylene, a co-monomer used in the production of certain types of polyethylene, and additionally can be converted to 1,3-butadiene, a precursor of synthetic rubbers.36–38
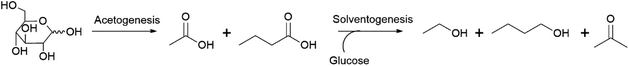
n-Butanol can be produced by anaerobic fermentation using various species of bacteria from the Clostridium genus such as Clostridium acetobutylicum and Clostridium beijerinckii.39,40 These bacteria produce a mixture of acetone, butanol and ethanol from sugar substrates and the associated fermentations are therefore referred to as acetone–butanol–ethanol (ABE) fermentations.39,40 Two phases can be distinguished during these fermentations (Scheme 2).41 In the first, the acetogenic phase, sugar substrates are converted to the organic acids acetic and butyric acid. Subsequently, additional sugar and the formed acids are converted to a mixture of acetone, butanol and ethanol. Conventional ABE fermentations yield product mixtures containing acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butanol
butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol in a ratio of 3
ethanol in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume, with yields of around 0.3–0.35 g product per g glucose typically achieved (= 0.18–0.21 g n-butanol per g glucose, 0.44–0.51 mol/mol).42
1 by volume, with yields of around 0.3–0.35 g product per g glucose typically achieved (= 0.18–0.21 g n-butanol per g glucose, 0.44–0.51 mol/mol).42
ABE fermentation was established as an industrial process in the UK, Canada and the USA in the 1910s, driven in large part by the need to produce acetone for the manufacture of explosives during WWI.42,43 In the period after WWI, emphasis shifted towards the production of n-butanol as a precursor of butyl acetate, which was applied as a solvent for car lacquers. Initially, ABE plants used starch (primarily from corn), which can be hydrolysed to glucose by the employed Clostridium strains, as the feedstock for fermentation. Following the discovery of Clostridium strains capable of metabolising sucrose in the 1930s, sugar-containing molasses became the preferred substrate.42 The rise of the petrochemical industry led to the closure of most ABE plants in the 1950s and 1960s, although industrial ABE fermentation is known to have continued until the 1980s in South Africa and the USSR and the 1990s in China.43–45 The traditional industrial process of ABE fermentation was performed by directly fermenting the feedstock (corn mash or molasses) using Clostridium under a CO2 atmosphere (the employed microorganisms are oxygen sensitive) for 2–3 days at temperatures ranging from approx. 30–40 °C.42,43 Subsequently, the obtained product mixture was separated from the fermentation broth by distillation and fractionated in a second distillation step to yield the pure components. Limitations of the traditional process include the low final concentration of products obtained, which is limited to around 2% w/v due to the toxicity of n-butanol at higher concentrations, and the high energy cost associated with the recovery and fractionation of the product mixtures by distillation.42,43 Interest in the ABE process has recently revived due to potential use of n-butanol as a biofuel. Commercial ABE fermentation from corn starch was re-established in China from 2007 onwards, but soon shut down again as n-butanol prices collapsed after the 2008 financial crisis.46 Lignicell Refining Biotechnologies, in collaboration with UK-based Green Biologics, opened a 40 kt per annum plant producing ABE from lignocellulosic feedstocks in Songyuan, China in 2013.46,47 In the US, Green Biologics established commercial ABE production from corn feedstocks at a retrofitted 79 million L per annum (63 kt per annum) bioethanol plant in Little Falls, Minnesota, in 2016.48 However, the plant closed in 2019, with the company quoting a failure to secure the funding required to scale-up production and sales to a scale where the operation would be financially viable.49 The current status of the Songyuan plant is unclear.
Fermentative methods employing Clostridium strains that yield isopropanol rather than acetone as a product (isopropanol–butanol–ethanol (IBE) fermentations) are also under development, providing a potential feedstock for e.g. biobased propylene production from isopropanol, although this process is yet to reach commercial scale production.50
ABE fermentation has been demonstrated to be feasible at industrial scale in the past. Development of innovative methods for in situ product extraction and recovery and separation of the obtained products, would overcome the main limitations of the process and enhance its potential to be applied in a future circular economy. As is the case for ethanol, the direct application of the butanol-derived alkenes 1-butylene or 1,3-butadiene as (co)-monomers for polymer synthesis is not desirable in a truly circular economy, due to the poor biodegradability of the resulting materials. However, for some of the materials produced from them, in particular synthetic rubbers, no obvious biodegradable alternatives exist and they may therefore still be required for certain applications in the circular economy. Alternatively, 1-butylene and 1,3-butadiene may serve as starting materials for the production of monomers that can be applied in the production of more readily biodegradable polymers. For example, 1,3-butadiene could be converted to 1,4-butanediol, a precursor to biodegradable polyesters such as polybutylene succinate (PBS) or polybutylene adipate-co-terephthalate (PBAT).51 However, such a route would have to compete with the direct fermentative production of 1,4-butanediol from biomass or its production from succinic acid, which can also be produced by fermentation (see below).
Isobutanol
Production of isobutanol is of interest as it can be converted to the alkene isobutylene, a monomer in the production of synthetic butyl rubber and precursor to para-xylene, which can be converted to terephthalic acid used to produce the polyester PET.52Isobutanol can be produced from glucose by yeasts such as Saccharomyces cerevisiae or Kluyveromyces lactis engineered to convert the glycolysis product pyruvate to isobutanol through the introduction of an artificial metabolic pathway (Scheme 3).53 Fermentative production of isobutanol is performed commercially by Gevo Inc. in the USA.54 This occurs at a corn-based bioethanol plant in Luverne, Minnesota, that has been retrofitted to enable isobutanol production in a side-by-side operation alongside ethanol production. Here, corn is initially processed as in conventional processes for bioethanol production, but after release of the fermentable sugars by enzymatic hydrolysis, part of the sugar stream is rerouted to isobutanol production by fermentation with isobutanol-producing engineered yeasts.54 The formed isobutanol is continuously removed from the fermentation broth by flash evaporation in a tank linked to the main fermentor, thus preventing isobutanol from accumulating to levels that would be toxic to the microorganisms.55 Details on the yield of isobutanol achieved using this process are not readily available. Gevo is currently primarily targeting the biobased aviation fuels market, by converting the formed isobutanol to isobutylene, which can subsequently be converted to longer chain hydrocarbons suitable for use as fuels.56 Gevo's retrofitted plant has the capacity to produce 5.7 million L (4.6 kt) isobutanol per annum, alongside 57 million L (44 kt) ethanol.57 Gevo has expressed the ambition to extend the plant so as to incorporate the conversion of the formed isobutanol to hydrocarbon-based fuels on site and increase the isobutanol production capacity to 68 million L (55 kt) per annum.58 In 2020, Gevo announced it was halting ethanol production at its Luverne facility, citing altered economic circumstances due to the Covid-19 pandemic, while announcing their intention to continue to pursue the expansion of their isobutanol production capacity.59 Together with the Indian bioenergy company Praj Industries, Gevo is also developing and commercialising the production of isobutanol from sugarcane and sugarbeet-derived feedstocks.60,61 Butamax, a joint venture between BP and DuPont, has also worked on the development of fermentation technology for the production of isobutanol from sugar feedstocks and in 2017 announced the construction of a retrofitted bioethanol plant for side-by-side isobutanol and ethanol production in Scandia, Kansas, USA.62 The current status of the project is unclear.
The production of biobased isobutanol has been demonstrated to be technologically feasible at a production scale of 4.6 kt per annum in co-production with bioethanol. Developments in the coming years will demonstrate whether isobutanol production at larger scales, and in the absence of co-production of bioethanol, is technologically and economically feasible and can play a role in a future circular economy.
Short chain diols
In addition to the alcohols mentioned above, fermentation processes can be employed for the synthesis of short chain diols such as 1,2-propanediol, 1,3-propanediol, 1,4-butanediol and 2,3-butanediol from sugars. These diols can be applied directly in the synthesis of biobased polymers, e.g. polyesters or polyurethanes.1,2-Propanediol
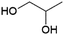
1,2-Propanediol (monopropylene glycol, MPG or 1,2-PDO, Fig. 4) is of interest e.g. as a precursor of polyester resins.63 It is currently produced petrochemically by hydration of propylene oxide, with an estimated market volume of 2 MMt per annum in 2013 and production from biobased feedstocks by the hydrogenolysis of glycerol is also commercially established.63–65 The French biotechnology company Metabolic Explorer has developed engineered strains of the bacteria Escherichia coli capable of producing 1,2-PDO from sugars. A patent application from Metabolic Explorer states a yield of 0.35 g 1,2-PDO per g glucose (0.8 mol/mol).66 In partnership with Finnish forestry company UPM, Metabolic Explorer worked on the industrial demonstration of the production of 1,2-PDO from wood-derived cellulosic sugars.64 However, the project was halted in 2018, with Metabolic Explorer stating only that a bilateral decision had been taken to stop the development.67 From the limited publicly available information, it is difficult to estimate the potential for this route for the production of 1,2-PDO to be developed to the point where it can play a meaningful role in the future circular economy.1,3-Propanediol
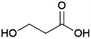
1,3-Propanediol (1,3-PDO, Fig. 5) is of interest as a monomer for the production of polyesters such as polytrimethylene terephthalate (PTT), which is mainly applied in fabrics.68,69 Petrochemical 1,3-PDO can be produced starting either from acrolein or ethylene oxide.70Biobased 1,3-PDO can be produced by fermentation starting either from glucose or glycerol.71 A number of microorganisms are known to convert glycerol to 1,3-PDO using their native metabolic pathways, with the best studied being bacteria from the genera Klebsiella and Clostridium.71 The first commercial efforts to produce 1,3-PDO by fermentation, however, focused on its production from glucose using genetically engineered microorganisms. Dupont Tate & Lyle Bioproducts, a joint venture of Dupont and Tate & Lyle, perform the commercial production of 1,3-PDO at a plant in Loudon, Tennessee, USA.72 Their production process employs a genetically engineered strain of the bacteria Escherichia coli. Nakamura and Whited described the development of such a strain by Dupont and Genencor.73 In this strain, dihydroxyacetone phosphate, which is formed from glucose through E. coli's natural metabolic pathways is converted to glycerol by the action of introduced genes originating from Saccharomyces cerevisiae and subsequently glycerol is converted to 3-hydroxypropionaldehyde by the action of introduced genes originating from the natural 1,3-PDO producer Klebsiella pneumoniae. Finally, 3-hydroxypropionaldehyde is reduced to 1,3-PDO by an enzyme from E. coli.73 A yield of 0.51 g 1,3-PDO per g glucose (1.2 mol/mol) was reported for fermentation at 10 L scale.73 Use of an engineered E. coli strain has the advantage that readily available and cheap feedstocks such as corn starch hydrolysates can be used as a source of glucose. At Dupont & Tate and Lyle Bioproducts’ production plant, corn starch hydrolysate from a nearby bioethanol plant owned by Tate & Lyle is used as a feedstock.73,74 The plant, which has been operating since 2006, is reportedly undergoing expansion to increase the capacity from 61 to 77 kt per annum.75 After the fermentation is complete, downstream processing involves inactivating the engineered E. coli by treatment with hot water, removal of the solid biomass by membrane separation and isolation of pure 1,3-PDO, which involves an ion-exchange step to remove charged species, removal of water by evaporation and finally a distillation process by which pure 1,3-PDO is obtained.76
Commercial production of 1,3-PDO by fermentation starting from glycerol, a by-product of biodiesel production, was established in China by the companies Zhangjiagang Glory Biomaterial and Shenghong Group, with each reportedly producing 1,3-PDO in facilities with a capacity of 20 kt per annum, with plans to increase capacity to 65 and 50 kt per annum respectively, in 2013.77,78 Publicly available information on the processes employed and current production volumes is limited. A patent application from Zhangjiagang Glory Biomaterial describes the use of Klebsiella variicola for the production of 1,3-PDO.79 Production of 1,3-PDO from glycerol is also being commercialised by METEX NØØVISTA, a joint venture between French biotech company Metabolic Explorer and the French Société de Projets Industriels who are building a plant for the production of 5 kt per annum 1,3-PDO (alongside 1 kt per annum butyric acid for application in food and feed).80,81 Although public information on the process and strain employed is limited, Metabolic Explorer has submitted a number of patent applications describing the use of strains of the bacteria Clostridium acetobutylicum, alone or in co-culture with Clostridium sphenoides and Clostridium sporogones, for the production of 1,3-PDO from glycerol, suggesting such a system may be employed in their process.82–85 METEX NØØVISTA has established an agreement with DSM for the distribution of their product as a cosmetics ingredient derived from non-GMO biomass feedstocks.86
Fermentative 1,3-PDO production has been established commercially and operational for more than 15 years. Currently, the main limitation to the scale of production is likely the market for products obtained from 1,3-PDO, e.g. PTT. In order for PTT to meet the criteria for application in a circular economy, the development of commercial production of terephthalic acid from renewable starting materials is a prerequisite.52 Alternatively, 1,3-propanediol may be used as a building block for the production of other biobased polyesters based on diacids that can already be produced from biomass, such as furan-2,5-dicarboxylic acid or succinic acid (e.g. ref. 87 and 88).
1,4-Butanediol
1,4-Butanediol (1,4-BDO, Fig. 6) has interesting applications in the production of biobased and biodegradable polymers, for example in the synthesis of polybutylene succinate (PBS) or polybutylene adipate-co-terephthalate (PBAT).34,89 It can also be dehydrated to yield the important solvent and chemical intermediate tetrahydrofuran, a precursor of the polymer polytetrahydrofuran (PTMEG), which is applied in elastic fibres (e.g. Spandex), or butadiene, a precursor of synthetic rubber.90–921,4-BDO can be obtained from biomass-derived sugars by fermentation using engineered strains of the bacteria Escherichia coli, such as those developed by the American company Genomatica.93 These strains were engineered by introducing a five-step artificial metabolic pathway to convert succinyl-CoA, a natural metabolite of glucose in E. coli, to 1,4-BDO. With an optimised strain, yields of over 0.40 g/g glucose (0.8 mol/mol) are achieved.93 After fermentation, downstream processing involves heat sterilisation of the fermentation fluid, cell removal by microfiltration or centrifugation, membrane filtration steps and ion exchange chromatography to remove charged species and biomacromolecules, evaporation of (most of) the water and finally distillation to obtain pure 1,4-BDO.
The 1,4-BDO production technology developed by Genomatica is employed commercially by Novamont at a dedicated 30 kt per annum 1,4-BDO plant in Bottrighe, Italy.94–96 Novamont uses the produced 1,4-BDO directly in the production of its bioplastics products marketed as Mater-Bi, which are composed of starch and 1,4-BDO-containing polyesters.97–99 BASF, a major producer of petrochemical 1,4-BDO and polytetrahydrofuran, has also taken a license on Genomatica's technology and reported its first production of biobased 1,4-BDO at commercial scale in 2013 and its use to produce biobased polytetrahydrofuran in 2015.100,101 The scale and location of biobased 1,4-BDO production was not disclosed.
Although the production of biobased 1,4-BDO has been shown to be technologically feasible, the disclosed production capacity (30 kt per annum) is a small fraction of the global production capacity of 1,4-BDO, which was estimated at 4.3 MMt per annum in 2018 and biobased 1,4-BDO is used primarily in products that are marketed for their low environmental impact.102 Presumably, the production of biobased 1,4-BDO is currently not cost-competitive with its petrochemical production, but if market conditions and regulation incentivise a shift towards the use of biobased feedstocks, the technology to produce 1,4-BDO is in place. The biodegradable polymers that can be produced using 1,4-BDO as a monomer meet the criteria for use in a circular economy, assuming the other monomers used are produced from renewable resources. This is already the case for e.g. PBS, while routes for the production of the terephthalic acid and adipic acid monomers required for PBT or PBAT production from biobased feedstocks are under development.52,103 It is also interesting to note that 1,4-BDO might in future be produced by the chemical reduction of succinic acid produced by fermentation (see below). The molar yield of succinic acid production from glucose (1.4–1.7 mol/mol) is significantly higher than that achieved for direct fermentative production of 1,4-BDO (0.8 mol/mol) and as chemical reduction of succinic acid in theory yields 1 mole of 1,4-BDO per mole of succinic acid, this route is potentially more efficient than direct fermentation to 1,4-BDO.
2,3-Butanediol
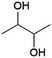
Another diol that can be produced by fermentation is 2,3-butanediol (2,3-BDO, Fig. 7), of interest as a precursor for 1,3-butadiene, which is used to produce synthetic rubbers.37,38,104–106 Some studies have also explored the use of 2,3-butanediol as a building block for polyesters.107–109Fermentative production of 2,3-BDO was extensively investigated during WWII (due to shortages of natural rubber) and production reached industrial pilot scale, but was halted after the development of routes to synthetic rubber starting from petrochemical feedstocks.110 Various bacteria from the genera Klebsiella (K. pneumoniae, K. oxytoca), Enterobacter (E. aerogenes, E. cloacae), and Bacillus (B. subtilis, B. licheniformis) can produce 2,3-BDO from fermentable sugars (glucose, sucrose).110 Natural producers typically produce mixtures of the three possible stereoisomers of 2,3-BDO. Genetic engineering can be used to steer production of strains to a single stereoisomer if required, although this is irrelevant for applications such as the dehydration of 2,3-BDO to butadiene.110 Engineering of non-natural producers such as the bacteria Escherichia coli or Lactobacillus lactis or the yeast Saccharomyces cerevisiae for 2,3-BDO production has also been explored.110 Yields obtained with these strains are typically in the range of 0.4–0.5 g/g glucose (0.8–1.0 mol/mol).
The South Korean company GS Caltex is in the process of commercialising biobased 2,3-BDO produced by fermentation of cassava and sugarcane-derived sugars, operating a demonstration plant with an annual capacity of 300 t.110–112 However, the company is marketing the product for cosmetics and agrochemical applications, not as a bulk chemical. It remains to be seen whether this technology can be scaled to a point where it can be employed for the production of 2,3-BDO for bulk chemical applications in future.
Glycerol
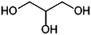
Glycerol (propane-1,2,3-triol, Fig. 8) is of interest as a component of antifreeze and various personal care products and also as a possible precursor of 1,2-PDO (see above). Currently, glycerol is produced commercially from triglycerides (vegetable oil) as a by-product of soap or biodiesel production and the global glycerol market size was estimated at 2.8 MMt in 2020.113,114Glycerol can be produced from sugars by fermentation using the yeast Saccharomyces cerevisiae. The production of glycerol (as opposed to ethanol) is favoured by addition of sulphite to the fermentation broth or by performing the fermentation at a pH of above 7.115 Fermentative production of glycerol was performed industrially in Germany during WWI at a scale of 12 kt per annum and it has been reported that glycerol production by fermentation occurred commercially in China in 2001, at a scale of 10 kt per annum.115,116 Depending on the fermentation conditions used, typical yields for the production of glycerol by yeast fermentation lie in the range of 0.2–0.35 g/g sugar (0.4–0.7 mol/mol).116 The widespread commercial production of glycerol by fermentation would likely only be of interest if significant changes in market conditions were to make its production from vegetable oils unattractive.
Organic acids
Another class of molecules that are interesting building blocks for the production of biobased materials are organic monocarboxylic acids, dicarboxylic acids and hydroxy carboxylic acids. These compounds are of interest as building blocks for biobased polymers such as polyesters or polyamides.Volatile fatty acids
The term volatile fatty acids (VFAs) refers to saturated fatty acids with chain lengths ranging from two to five or six carbon atoms. VFAs have applications as chemical building blocks and as food/feed additives.117 Currently, VFAs are produced petrochemically, with reported production volumes ranging from 90–105 kt per annum for butyric acid to 14–17 MMt per annum for acetic acid.117VFAs can also be produced from biomass, as they are formed as intermediates during the process of anaerobic digestion, where biomass is converted to methane and carbon dioxide by consortia of microorganisms in the absence of oxygen.117,118 This relatively poorly understood process proceeds through the initial conversion of the biomass-derived substrate into VFAs by acidogenic microorganisms, followed by the conversion of VFAs into methane and carbon dioxide by methanogenic microorganisms. By tuning the fermentation conditions, the production of methane by methanogens can be inhibited, causing VFAs to accumulate.117,118 VFA production is of particular interest as it can be applied to relatively impure feedstocks, as opposed to the relatively pure feedstocks that are required for most other processes covered in this manuscript. This makes it well suited for processing highly heterogeneous biomass streams, e.g. municipal organic waste or sewage sludge.117,118 A disadvantage of VFA production is that it leads to a mixture of products, necessitating purification procedures if production of a pure compound is required.
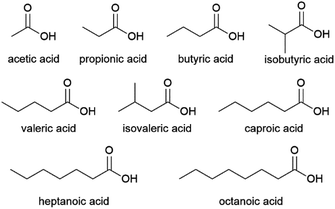
French company Afyren and Dutch company Chaincraft are exploring this technology commercially.119,120 As of 2021, Afyren is offering a range of 7 VFAs (acetic, propionic, butyric, isobutyric, valeric, isovaleric and caproic acid, Fig. 9) at over 99% purity for certain applications.121 The company is in the process of constructing their first commercial production facility, a 16 kt per annum plant in Carling Saint-Avold, France, in a joint venture with investment fund BpiFrance SPI Fund.122 As of 2021, Chaincraft offers 5 fatty acids: the VFAs butyric, valeric and caproic acid, and also the C7 and C8 acids heptanoic and octanoic acid (Fig. 9).123 Production of these longer chain fatty acids is achieved in a two-step process, where short chain VFAs are formed in a first reactor vessel and subsequently subjected to chain elongation in a second vessel.124 Such chain elongation processes involve a mixed culture fermentation, where the addition of an electron donor, e.g. ethanol to the VFAs, leads to the extension of their chain length.125,126 Chaincraft currently operates a demonstration plant in Amsterdam, the Netherlands, with a capacity of 2 kt per annum and is aiming to construct a commercial plant with approximately 20 kt per annum capacity by 2023.127
Production of VFAs by mixed-culture fermentation appears highly promising for applications where the produced VFAs are applied directly as mixtures (e.g. as feed additives). It remains to be demonstrated that the technology can be employed cost-effectively at scale for the production of fatty acid streams of sufficient purity to be employed as bulk chemical building blocks.
The shortest VFA, acetic acid, can also be produced as the sole product of fermentation of sugars. Here, sugars are first fermented to ethanol by Saccharomyces cerevisiae. Subsequently, ethanol is converted to acetic acid by acetic acid bacteria.128,129 Such processes are well known from their application in the production of vinegar in the food industry. Development of efficient downstream processing technologies to obtain concentrated acetic acid from the dilute fermentation broth will be required if such processes are to be employed for production of acetic acid for bulk chemical applications.130 Such a two-step fermentative route would also have to compete with the chemical oxidation of bioethanol produced by fermentation to acetic acid.
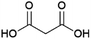
Glycolic acid
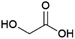
An organic acid that is of interest for the production of polymers is glycolic acid (Fig. 10), the precursor of the biodegradable polyester polyglycolic acid (PGA).131 Glycolic acid can be industrially produced from petrochemical resources through the reaction of formaldehyde and carbon monoxide or of chloroacetic acid and sodium hydroxide.132Biobased glycolic acid can be produced by fermentation using strains of bacteria such as Escherichia coli or Corynebacterium glutamicum or yeasts such as Saccharomyces cerevisiae or Kluyveromyces lactis that are engineered so as to reduce glyoxylic acid, an intermediate in their central carbon metabolism, to glycolic acid.133 The French company Metabolic Explorer, is developing methods for the fermentative production of glycolic acid. After originally halting this development in 2013, Metabolic Explorer announced its intention to pursue it again in 2019.134–137 A patent application from Metabolic Explorer describes the development of engineered E. coli strains capable of producing glycolic acid in a yield of approximately 0.4 g/g glucose (0.9 mol/mol).138 The current status of the development is unclear and it remains to be seen whether the technology is suitable for industrial-scale application.
Lactic acid
Probably the best-known organic acid that is produced commercially by fermentation is lactic acid. Lactic acid is of significant interest as the precursor to polylactic acid (PLA), a biobased and biodegradable polymer.139 Lactic acid currently also finds application as a food additive and cosmetics ingredient.140 Lactic acid is a chiral molecule and an important aspect for its production is the enantiomeric purity of the obtained product. For application in PLA synthesis, this is of importance as the final polymer properties are dependent on the ratio of D- and L-lactic acid used to produce the polymer.139 For food and cosmetics applications, enantiomeric purity is also of importance, as the D-enantiomer is harmful to humans.141 Fermentative processes can yield lactic acid in high enantiomeric purity (of either enantiomer), in contrast to chemical processes that typically yield racemic mixtures.142Lactic acid can be produced from sugars by various species of bacteria, e.g. members of the Lactobacillus genus or Bacillus coagulans.133,141,143 Sugars from various feedstocks can be used, with first generation feedstocks such as sugarcane, sugar beet and corn or cassava starch being applied in industrial practice.144 In contrast to many of the other processes described in this review, certain lactic acid bacteria are capable of producing lactic acid through metabolic pathways where all of the carbon atoms from the sugar substrate end up in the lactic acid product (Scheme 4).141 This allows yields of approximately 0.90 g lactic acid per g glucose (1.8 mol/mol) to be achieved in industrial practice.143 During conventional lactic acid production processes (see Fig. 11), fermentation takes 2–4 days, during which the pH is maintained at 5–6 by addition of calcium hydroxide.143 Downstream processing involves, after removal of biomass by filtration, converting the formed lactate to the acid form by addition of sulphuric acid, leading to the precipitation of calcium sulphate (gypsum) as a by-product.143 Formation of this by-product is undesirable from both the economic and environmental point of view and processes for the isolation of lactic acid that do not involve gypsum formation have been developed.145 To obtain highly pure lactic acid for use in the chemical industry (e.g. for PLA synthesis), the crude product is purified by distillation after esterification with methanol or ethanol.143 The obtained lactate esters are hydrolysed and the alcohol removed by evaporation to obtain pure lactic acid.
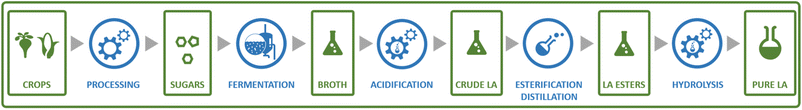 | ||
| Fig. 11 Schematic representation of the steps involved in a typical process for the production of lactic acid from sugars by fermentation. | ||
Lactic acid had an estimated market volume of 750 kt per annum in 2015, with the vast majority (approx. 95%) produced by fermentation.146,147 Major producers include Corbion (headquartered in the Netherlands, with plants in The Netherlands, Brazil, Spain, Thailand and the USA), Natureworks LLC (USA), Galactic (headquartered in Belgium with, plants in Belgium, China and the USA) and Henan Jindan Lactic Acid (China).147–150 In 2020, Corbion announced plans to expand its lactic acid manufacturing facility in Rayong, Thailand from a capacity of 120 kt per annum by building a second plant with a capacity of 125 kt per annum, making it the largest lactic acid production facility in the world.151,152
Industrial production of lactic acid is already well established and is in a position to expand further. PLA, as a biobased and biodegradable polyester is of high interest as a material for the future circular economy. The future growth of lactic acid production for bulk chemical applications will likely depend on the growth in demand for PLA as a substitute for current petrochemical polymers.
3-Hydroxypropionic acid
3-Hydroxypropionic acid (Fig. 12) is of interest as its dehydration leads to the formation of acrylic acid, a precursor of acrylate-based polymers.153 As an ω-hydroxy fatty acid, 3-hydroxypropionic acid also might also be used as a building block for the production of biobased polyesters, e.g. poly(3-hydroxypropionate).1543-Hydroxypropionic acid can be produced from fermentable sugars using engineered strains of bacteria such as Escherichia coli or Corynebacterium glutamicum or from glycerol using engineered strains of the bacteria Klebsiella pneumoniae.155 From glucose, yields of 0.50–0.55 g/g (1.0–1.1 mol/mol) have been reported.155 In an R&D collaboration, BASF, Novozymes and Cargill demonstrated the production of 3-hydroxypropionic acid by fermentation and its conversion to acrylic acid at industrial pilot scale, but the status of the development is unclear as no updates have been released since BASF exited the collaboration in 2015.156,157 From publicly available information, it is hard to evaluate the potential of this technology to be applied at industrial scale.
Malonic acid
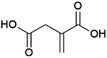
Malonic acid (propanedioic acid, Fig. 13) is a diacid which is of interest as a (precursor to) polymer building block(s) of interest for e.g. coating applications.158 Patents from the American company Lygos describe the production of malonic acid by the fermentation of glucose, employing engineered microorganisms (e.g. the bacteria Escherichia coli or the yeasts Saccharomyces cerevisiae or Pichia kudriavzevii).159,160 These strains were developed by the introduction of a malonyl-CoA hydrolase enzyme that can release malonic acid from malonyl-CoA, which occurs in nature as an intermediate in fatty acid biosynthesis.159,160 Lygos is commercialising the production of malonic acid by fermentation using P. kudriavzevii.161 Pilot-scale production was announced in 2015 and further demonstration of the process under conditions mimicking those for commercial production in 2017.162,163 Lygos’ CEO has stated the company intends to produce malonic acid at a scale of 20 kt per annum by 2024.158 The coming years will be crucial in demonstrating the feasibility of the production of biobased malonic acid by fermentation as a commercial process.Succinic acid
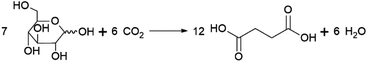
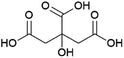
Succinic acid (butanedioic acid) is of interest as a component of biobased polymers, for example polybutylene succinate (PBS), and as a precursor to biobased chemicals such as 1,4-BDO.164Succinic acid can be produced from fermentable sugars using various microorganisms that are natural producers of the compound, such as the bacteria Anaerobiospirillum succiniciproducens, Actinobacillus succinogenes, Mannheimia succiniciproducens and Basfia succiniciproducens.165 These organisms produce succinic acid from fermentable sugars and carbon dioxide under anaerobic fermentation conditions. Succinic acid is produced with a high theoretical yield of 1.12 g/g glucose (with 12 moles of succinic acid being formed from 7 moles of glucose and 6 moles of CO2) (Scheme 5). Strains of the bacteria Escherichia coli and Corynebacterium glutamicum and the yeast Saccharomyces cerevisiae modified to produce succinic acid also exist.165 The actual yield achieved varies depending on the fermentation conditions and producing organism, e.g. with glucose as a substrate, yields of up to 0.9–1.0 g/g (1.4–1.5 mol/mol) are reported for the natural producer A. succiniciproducens, while with engineered E. coli strains yields of up to 1.0–1.1 g/g (1.5–1.7 mol/mol) have been reported.166 Succinic acid production under aerobic fermentation conditions is also possible using engineered E. coli strains (e.g. ref. 167). The choice of host microorganism and fermentation conditions has important implications for the downstream processing of the formed succinic acid. When fermentations are performed at neutral pH, as is required when using natural succinic acid producers or E. coli, the formed succinic acid has to be neutralised by addition of a base during fermentation. Examples of bases that can be applied include calcium hydroxide, ammonia, sodium hydroxide and magnesium hydroxide.165,168 This has the disadvantage that the acid form of succinic acid has to be regenerated from the formed succinate salt during downstream processing, which is achieved either by addition of a mineral acid or by electrodialysis.165,168 Alternatively, engineered yeast strains that are tolerant to low pH can be employed for fermentations under acidic conditions, enabling the direct isolation of succinic acid from the fermentation broth in the acid form.165
Commercial production of biobased succinic acid by the fermentative conversion of sugars was established by a number of companies in the 2010s. (Former) producers include LCY Biosciences (formerly BioAmber) and GC Innovation America (formerly Myriant) in North America and Roquette (formerly Reverdia, a joint venture of DSM and Roquette) and Succinity, a joint venture of BASF and Corbion, in Europe.169–172 Each of these manufacturers established a somewhat different process for succinic acid production, and all established plants with a production capacity of at least 10 kt per annum succinic acid during the 2010s. However, these companies have struggled to establish consistent and economically viable production. BioAmber was formed in 2010 from a joint venture between US-based Diversified Natural Products Green Technology and France-based Agro-Industrie Recherches et Developments. Together, they opened the first biobased succinic acid demonstration plant in Pomacle, France, in 2010, with a capacity of 3 kt per annum.170 BioAmber later applied an engineered, acid-tolerant yeast strain, co-developed with Cargill, in a biobased succinic acid plant with a capacity of 30 kt per annum in Sarnia, Canada, which opened in 2015.170,173 BioAmber went into bankruptcy in 2018, after which the Sarnia plant was acquired by Taiwan-based LCY Biosciences.174 As of 2021, LCY had restarted production of succinic acid at the Sarnia plant, reporting that succinic acid production has reached 18 kt per annum.175 Corn syrup is used as the feedstock for succinic acid production.175 Myriant Technologies was a US-based producer of succinic acid, which employed modified E. coli strains for the production of succinic acid from a sorghum starch feedstock in a 14 kt per annum plant in Lake Providence, Louisiana, that opened in 2013.169 Myriant's Lake Providence plant was closed in 2016, however, and in 2018, Myriant was renamed GC Innovation America and the focus of the company was changed from biobased chemical production to technology scouting for its Thailand-based parent company PTT Global Chemical.176,177 Reverdia was a joint venture between The Netherlands-based DSM and France-Based Roquette that was established in 2012 and operated a 10 kt per annum plant for the production of succinic acid from corn-starch derived sugars in Cassano Spinola, Italy, with a process based on conversion by an engineered, acid-tolerant yeast strain.171,178 In 2019, the dissolution of the Reverdia joint venture was announced, with Roquette taking over full responsibility for succinic acid production, marketing and sales and DSM taking on a role solely as a technology licensor.179 Succinity, a joint venture between German chemical company BASF and The Netherlands-based Corbion, opened a 10 kt per annum succinic acid plant in Montmelo, Spain in 2014.180 In contrast to other succinic acid producers, Succinity applied a natural succinic acid producing organism in their production process, using a proprietary strain of the bacteria Basfia succiniciproducens. As of March 2019, Succinity's succinic acid plant was reportedly not operational.181
Although various companies have established the fermentative production of succinic acid at industrial scale, they appear to have struggled to establish consistent commercial production. The former CEO of Myriant, Cenan Ozmeral, cited the difficulty of competing with petrochemical products as the main reason for the struggles faced by succinic acid producers.181 Nevertheless, the industrial-scale fermentative production of biobased succinic acid has been demonstrated by multiple companies at ≥10 kt per annum scale, indicating that its production at commercial scale is technically feasible if market factors change so as to stimulate the use of products based on it. As a monomer for biobased and biodegradable polyesters, such as PBS, succinic acid has great potential as a building block in the circular economy.
Adipic acid
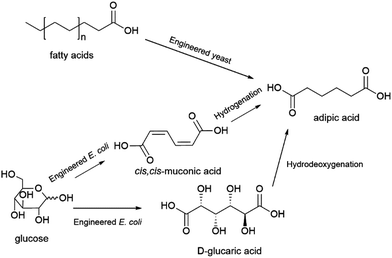
Adipic acid (hexanedioic acid) is another dicarboxylic acid of interest for the production of biobased polymers. Adipic acid is widely applied as a starting material for the production of nylon 6.6, polyurethanes and plasticizers. It is also a starting material for the production of the biodegradable polymers polybutylene adipate-co-terephthalate (PBAT) and polybutylene succinate adipate (PBSA). Adipic acid is produced by oxidation of the petrochemical feedstock ketone–alcohol (KA) oil, a mixture of cyclohexanol and cyclohexanone, with global consumption of adipic acid estimated at approximately 3 MMt in 2016.182Production of biobased adipic acid can be achieved by chemical conversion, where glucose is chemo-catalytically converted to glucaric acid followed by hydrodeoxygenation to adipic acid, direct fermentative conversion of sugars, glycerol or fatty acids to adipic acid, or fermentative conversion of sugars to either cis,cis-muconic acid or D-glucaric acid, both of which can subsequently be chemically converted to adipic acid (Scheme 6).103 Various synthetic metabolic pathways for the direct fermentative production of adipic acid have been explored (see ref. 183 for an overview). Of these, the technology that has been demonstrated at the most advanced scale was developed by the US-based company Verdezyne (see also, dodecanedioic acid) and relied on the production of adipic acid from longer chain fatty acids sourced from plant oils (e.g. palm kernel or coconut oil) using an engineered yeast strain.184 This technology was demonstrated at 300 L pilot scale, prior to Verdezyne entering bankruptcy in 2018 when its main investor decided to withdraw funding.184,185 Fermentative production of precursors of adipic acid, cis,cis-muconic acid or D-glucaric acid, that can be converted chemically to adipic acid has also been explored. Glucose can be converted to cis,cis-muconic acid or D-glucaric acid using engineered strains of the bacteria Escherichia coli or the yeast Saccharomyces cerevisiae, while the production of cis,cis-muconic acid from various lignin-derived aromatic monomers using strains of the bacteria Pseudomonas putida has also been explored.103 It is interesting to note that cis,cis-muconic acid itself has also been proposed to have potential applications as a biobased building block for e.g. terephthalic acid or ε-caprolactam.186
Fermentative production of adipic acid and its precursors is still in the developmental stage. The potential for biobased adipic acid to be used as a substitute for its petrochemical counterpart in the production of polymers that are already on the market makes it a highly interesting development to follow in future.
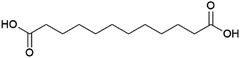
Dodecanedioic acid
The dicarboxylic acid dodecanedioic acid (Fig. 14) is applied in the production of coatings, adhesives and engineering plastics such as the polyamide nylon 6.12.187 It is also a potential monomer for the production of aliphatic polyesters.188 Dodecanedioic acid is currently produced commercially at an estimated volume of 70 kt per annum in 2018, with petrochemical production from butadiene and biobased production from fatty acids both contributing a substantial share of the market.187The Chinese biotechnology company Cathay Industrial Biotech produces dodecanedioic acid from fatty acids using a bacterial strain, with production exceeding 10 kt per annum in 2012.189 Although details of the Cathay process, which was first developed as long ago as the 1990s, are not publicly available, it accounts for a significant portion of the total market volume of dodecanedioic acid, demonstrating that commercial production by fermentation from fatty acids is feasible. According to news reports from Cathay, the company has two facilities in China producing dodecanedioic acid, as well as other long chain diacids: a 40 kt per annum facility in Jinxiang and a 30 kt per annum facility in Wusu (capacity for total diacid production).190 The US-based company Verdezyne also invested in the development of biobased dodecanedioic acid, with patents describing the development of microorganisms capable of producing dodecanedioic acid by fermentation of fatty acid streams containing dodecanoic acid (lauric acid), which is the predominant fatty acid in palm kernel and coconut oils.191–193 This is achieved using strains of the yeast Candida tropicalis engineered so as to block the β-oxidation pathway responsible for fatty acid degradation and include a heterologous pathway for the conversion of fatty acids to diacids through ω-hydroxylation followed by oxidation of the ω-hydroxy functionality to the carboxylic acid. These patents also describe the production of decanedioic acid (sebacic acid) from decanoic acid (capric acid, which also occurs in coconut and palm kernel oils). Yeasts with a partially blocked β-oxidation pathway that enabled the production of shorter diacids such as octanedioic acid (suberic acid) were also described. Verdezyne attempted the commercialisation of dodecanedioic acid production based on this technology, demonstrating ton scale production and announcing the construction of a first commercial plant in 2014.194,195 Construction of this plant started in Nusajaya, Malaysia in 2017.196 The plant had a planned capacity of 14 kt per annum for dodecanedioic acid from palm kernel-oil sourced dodecanoic acid.195 However, with the plant almost complete, Verdezyne went into bankruptcy in 2018, when its largest investor pulled out of the project.197 Reportedly, the equipment installed in the plant was put up for sale, no information as to its final destination was found.198
Production of biobased dodecanedioic acid by fermentation is established as a commercial process. The lack of publicly available details on the process employed, makes it hard to evaluate the potential contribution to the circular economy in 2050. In addition, the use of fatty acids originating from tropical oils is associated with tropical deforestation and therefore the development of alternative feedstocks for this process is to be preferred.
Itaconic acid
Itaconic acid (2-methylidenebutanedioic acid, Fig. 15) is a dicarboxylic acid with potential applications in the production of alternatives to acrylate polymers.153 Itaconic acid also has interesting potential applications as a building block for polyesters.153Itaconic acid is produced by fermentation of glucose by the fungus Aspergillus terreus, with typical yields of around 0.5 g/g glucose (0.7 mol/mol).199 Fermentative production of itaconic acid was first established in the 1960s, with a typical process employed by the pharmaceutical company Pfizer involving fermentation at around 37 °C for 4 days. Currently, commercial itaconic acid production is located primarily in China involving companies such as Zhejiang Guogang Biochemistry and Qingdao Kehai Biochemistry.200,201 Data on the process parameters and production capacity is scarce, though a global production capacity of 50 kt per annum was reported for 2013.202 Future development of biobased itaconic acid production will likely depend on the development of the market for polymers derived from it.
Citric acid
Citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid, Fig. 16) is a tricarboxylic acid that is produced industrially by fermentation of sugars using the fungi Aspergillus niger or Aspergillus wentii. Currently, the major application of citric acid is as an additive to food, drinks or detergents, functioning as a pH-modifier or chelator.203 Citric acid is also of interest as a potential bulk chemical as it can be converted chemically to itaconic acid (see above). Production of citric acid in 2018 exceeded 2 MMt.204The first industrial processes for the production of citric acid, developed in the first half of the 20th century, were based on surface fermentation, but nowadays the majority of citric acid is produced by submerged fermentation processes.205 Typical fermentation processes use sugars from molasses or glucose from starch hydrolysates as a feedstock and yield 0.7–0.9 g citric acid per g glucose (0.7–0.8 mol/mol) after 7 days of fermentation.205 Typical downstream processing involves the precipitation of citrate from the cleared fermentation broth as a calcium citrate salt, followed by regeneration of citric acid by addition of sulphuric acid. Major producers of citric acid include Archer Daniels Midland, Cargill (both USA), Citrique Belge (Belgium), Tate & Lyle (UK), Jungbunzlauer (Switzerland), Gadot Biochemical (Israel), Weifang Ensign Industry, RZBC Group, Huangshi Xinghua Biochemical, and COFCO Biochemical (all China).204
Production of citric acid by fermentation on industrial scale is well established. Its use as a bulk chemical in future will likely be dependent on the availability of techniques for its conversion to itaconic acid that allow this route to be cost-competitive with the direct production of itaconic acid by fermentation and on the development of markets for itaconic acid-derived polymers. As the production of citric acid is already established at industrial scale, other opportunities to convert citric acid to bulk chemical building blocks should also be explored.
Amines
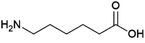
Diamines and ω-hydroxyamines are of interest as building blocks for polyamide and polyimide polymers. A limited number of biobased amines produced by fermentation are already produced commercially or in the process of being commercialised.1,5-Pentanediamine
1,5-Pentanediamine (Fig. 17) has potential applications in the production of polyamides, providing a biobased alternative to the 6-carbon diamine hexamethylenediamine, an important polyamide and polyurethane building block.1,5-Pentanediamine is commercially produced by the Chinese company Cathay Industrial Biotech using a fermentation process starting from glucose.206 Relatively few details of the employed process are publicly available, though a patent application from Cathay describes the use of engineered strains of the bacteria Escherichia coli for 1,5-pentanediamine production.207 In 2016, Cathay announced the construction of a new plant with a production capacity of 50 kt per annum in Wusu, China.208 In addition, a production capacity of “multi-thousands” t per annum is reported for Cathay's initial plant in Jinxiang, China.208 A second news report, from 2018, announces the expansion of Cathay's 1,5-pentanediamine production facility in Wusu from a capacity of 50 kt per annum to 100 kt per annum.190 Cathay uses the produced 1,5-pentanediamine in the production of biobased polyamides marketed as PA5X.209
Although the production of biobased 1,5-pentanediamine seems successful, the lack of publicly available information on the employed process makes it difficult to assess its potential to contribute to the circular economy in 2050.
6-Aminohexanoic acid
6-Aminohexanoic acid (6-aminocaproic acid, Fig. 18) is of interest as it can be converted to ε-caprolactam, which is used to synthesise the polyamide nylon-6 (or PA6).Fermentative production of 6-aminohexanoic acid can be achieved using engineered strains of microorganisms such as the bacteria Escherichia coli, and the American biotech company Genomatica is in the process of commercialising its production. Genomatica holds patents on microorganisms (e.g. E. coli) engineered so as to produce 6-aminohexanoic acid from glucose via a synthetic pathway, where adipoyl-CoA is first formed through a “reverse degradation” pathway (a metabolic pathway naturally employed for degradation of a compound run in reverse to enable its synthesis) and subsequently converted to 6-aminohexanoic acid in two steps, involving conversion to the aldehyde 6-oxo-hexanoic acid, followed by transamination to 6-aminohexanoic acid.210 In the first months of 2020, Genomatica, in collaboration with Italian nylon-6 producer Aquafil, announced the production of nylon-6 from monomers derived from its fermentation process at 1 t scale.211 In November 2020, the partners announced plans to build a demonstration facility for the technology, citing plans to produce 50 t of nylon-6 in the first production runs.212
Commercial production of biobased 6-aminohexanoic acid is yet to be established and thus it remains the question how successful the technology will be at commercial scale. Its potential to offer a direct replacement for nylon-6 precursors currently sourced from petrochemicals, seems highly attractive.
Amino acids
Another class of molecules produced industrially using fermentation is amino acids. Various naturally occurring amino acids can be produced by fermentation using bacteria such as Corynebacterium glutamicum or Escherichia coli.213,214 The total market volume for amino acids was 10.3 MMt in 2021, with their main applications being as food or feed additives.215 Two amino acids are produced by fermentation at a scale of over 1 MMt per annum: L-glutamic acid and L-lysine.L-Glutamic acid
L-Glutamic acid (Fig. 19) is industrially produced by fermentation, with a market volume of 2.9 MMt per annum reported for 2014.216 The main application of L-glutamic acid and its sodium salt monosodium glutamate (MSG) is as flavour enhancing agents in food.217L-Glutamic acid is produced from fermentable sugars using Corynebacterium glutamicum, with yields of up to 0.6 g/g sugar reportedly achieved.218 Following the fermentation, L-glutamic acid is crystallized from the fermentation broth by adjusting the pH to approx. 3, after which it is commonly converted to its monosodium salt by addition of sodium hydroxide.218L-Glutamic acid producers include Ajinomoto, Kyowa Hakko Bio (Japan), Evonik (Germany), Hefei TNJ Chemical Industry, Global Bio-chem Technology Group Company, Ningxia Yipin Biological Technology, Sichuan Tongsheng Amino Acid and Suzhou Yuanfang Chemical (all China), and Ottokemi (India).219
Various potential applications of L-glutamic acid as a chemical building block have been envisioned. For example, L-glutamic acid was listed on the US Department of Energy's list of Top Value Added Chemicals from Biomass in 2004, primarily as a potential precursor to 5 carbon atom diols (1,5-pentanediol) and diacids (1,5-pentanedicarboxylic acid).220 This report also indicated that the chemistry for the conversion of L-glutamic acid to these target chemicals required significant development and it is not apparent that this has successfully been achieved. Routes for the conversion of L-glutamic acid to succinonitrile, a precursor to the polyamide building block 1,4-diaminobutane have also been studied.221L-Glutamic acid can be readily produced at significant scale, but development of relevant applications will be required in order for it to play a major role as a building block in a circular economy.
L-Lysine
L-Lysine (Fig. 19) is produced industrially by fermentation with an annual production volume of 1.9 MMT per annum reported for 2013.222 The main application of L-lysine is as an animal feed additive.L-Lysine is produced from sugars and ammonia by fermentation using modified strains of Escherichia coli or Corynebacterium glutamicum.218 Industrially applied strains reportedly yield 0.55–0.60 g/g sugar substrate.223 Major L-lysine producers include Ajinomoto and Kyowa Hakko Bio (Japan), Archer Daniels Midland (USA), Changchun Dacheng, COFCO Biochemical, Global Bio-chem Technology Group Company, Shandong Shouguang Juneng Golden Corn and Sunrise Nutrachem (all China), Cheil Jidang (South Korea) and Evonik (Germany).224
Lysine is a potential precursor to building blocks for polyamides such as 1,5-pentanediamine, 5-aminovaleric acid or ε-caprolactam.225 It should be noted though that direct fermentative production of 1,5-pentanediamine (see above) and production of ε-caprolactam via fermentative production of 6-aminohexanoic acid (see above) may provide more efficient routes to these compounds. The fermentative production of L-lysine at ∼2 MMt per annum scale is already established, its potential for use in the circular economy likely depends on the development of the market for products derived from it and the development of competing technologies for their production.
Other amino acids
Other amino acids produced at scale by bacterial fermentation include L-threonine (market volume 200 kt per annum in 2009) and L-phenylalanine (market volume 20 kt per annum in 2009).214L-Glutamine, L-arginine, L-valine, L-leucine, L-isoleucine, L-histidine, L-proline, L-serine, L-tyrosine and L-tryptohan are all produced by fermentation with market volumes below 10 kt per annum for 2009.214Isobutylene
Isobutylene (Fig. 20) is a precursor to synthetic butyl rubber and also to para-xylene, which can be converted to terephthalic acid, one of the monomers used in the production of PET.52,226 The global market for petrochemical isobutylene was estimated to be 12 MMt in 2015.227Although a number of naturally occurring microorganisms are capable of producing isobutylene in low amounts, efficient production requires the use of artificial metabolic pathways in engineered microorganisms, such as the bacteria Escherichia coli.228 Theoretical yields of such metabolic pathways range from 0.21–0.31 g/g glucose (0.7–1.0 mol/mol).228
The French company Global Bioenergies is in the process of commercialising the production of isobutylene from sugar feedstocks using engineered E. coli.229 Patent applications from Global Bioenergies describe a number of artificial metabolic pathways for isobutylene production that can be introduced into recombinant microorganisms and it is unclear which pathway is to be employed in the E. coli used in the envisioned commercial process.230–236 Global Bioenergies states that isobutylene formed during the fermentation evaporates into the gaseous phase, preventing accumulation of toxic products in the fermentation broth and simplifying downstream processing.229 Global Bioenergies has operated their isobutylene process in a 10 t per annum pilot plant at Pomacle-Bazancourt, France, since 2015, and at a 100 t per annum demonstration site in Leuna, Germany, since 2017.237,238
The potential for direct fermentative production of isobutylene to play a role in the 2050 circular economy will depend on the success of further scale-up efforts and on whether the technology is competitive with the production of isobutylene by chemical dehydration of isobutanol (see isobutanol section above).
Terpenes
Terpenes are a class of alkenes composed of one or more isoprene units (C5H8, Fig. 21). A well-known and commonly applied terpene is natural rubber (largely consisting of cis-1,4-polyisoprene), which is harvested from the rubber tree (Hevea brasiliensis).239 In addition, to meet the global demand for rubber, some synthetic polyisoprene is produced by polymerisation of isoprene produced from petrochemical feedstocks.239 The total polyisoprene market volume (including both natural and synthetic polyisoprene) stood at approximately 16 MMt in 2020.240Biobased isoprene can be produced from glucose or glycerol by fermentation using engineered strains of the bacteria Escherichia coli or the yeast Saccharomyces cerevisiae, with a maximum theoretical yield of 0.25–0.30 g/g (0.7–0.8 mol/mol) achieved from glucose depending on the production pathway employed.241 The commercial exploitation of such processes has been explored by the US chemical company Dupont, in collaboration with tyre producer Goodyear,242 US biotech company Amyris, in collaboration with the Brazilian chemical company Braskem and tyre producer Michelin,243 Japanese chemical company Ajinomoto, in collaboration with tyre producer Bridgestone244 and US-based GlycosBio, in collaboration with Malaysia-based Bio-XCell.245 No evidence was found that any of these initiatives proceeded beyond the research & development stage. A report from Dupont stated an isoprene yield from their fermentation process of 0.11 g/g glucose (0.3 mol/mol).246
A terpene that has been produced commercially through fermentation of biobased feedstocks is the 15-carbon terpene β-farnesene (Fig. 21).247 β-Farnesene is of interest as a polymer building block and as a base oil for lubricants.248
β-Farnesene can be produced by fermentation from glucose using engineered strains of the yeast Saccharomyces cerevisiae.249 Researchers from the biotechnology company Amyris reported the development of such strains, reporting a yield of approximately 0.2 g/g glucose (0.2 mol/mol) for β-farnese production, corresponding to roughly two-thirds of the maximum theoretical yield for the strain (approx. 0.3 g/g, 0.3 mol/mol).249
Amyris produced β-farnesene commercially at a facility in Brotas, Brazil, using sugarcane-derived sugar as a feedstock.250 Opened in 2013, the Brotas plant has a reported capacity of 33 kt per annum and was sold to Dutch chemical company DSM in 2018, along with Amyris’ IP portfolio related to β-farnese production, with Amyris’ CEO stating the company will focus on the production of smaller volumes of specialty products for the health, personal care and flavour and fragrance markets.251 Considering this development, the future of fermentation-derived β-farnesene as a biobased chemical building block is unclear.
Triglycerides and fatty acids
Triglycerides (oils) provide an important feedstock for the chemical industry as they are important starting points for the production of surfactants, lubricants and medium chain diacids for polymer synthesis. Nowadays, plant oils are the main source of triglycerides for the chemical industry.252A number of oleaginous yeasts such as Yarrowia lipolytica, Cryptococcus curvatus, Lipomyces starkeyi and Rhodosporidium toruloides produce large volumes of triglycerides when grown on fermentable sugars (over 70% of dry cell weight has been reported for engineered strains).253 This provides a potential method for the production of oils that does not necessitate the land use, irrigation and deforestation associated with the production of (tropical) plant oils. Another advantage of using microbial oils, is that the fatty acid composition of the oils can be controlled.254 By varying cultivation conditions of the microbes and by interfering directly in fatty acid-biosynthesis, it is already possible to produce oils containing fatty acids with altered chain-length and altered degrees of saturation.254
Production of triglycerides using oleaginous yeasts is in the early phases of commercialisation by companies such as Switzerland-based Cultivated Biosciences,255 Netherlands-based NoPalm Ingredients256 and US-based C16 Biosciences.257 Though currently in the developmental stages, this technology may provide an important chemical feedstock in future.
Polyhydroxy alkanoates (PHA)
As an alternative to producing small molecules that can serve as the building blocks for polymers, fermentation of a range of biomass-derived substrates (sugars, oils, glycerol) can be applied for the direct production of polymers of the polyhydroxyalkanoate (PHA) class.PHA is accumulated as granules in a number of different bacteria and archaea.258 The PHA can subsequently be isolated and processed into plastic materials. PHA yields vary from 0.3–0.4 g/g glucose from sugar substrates to 0.6–0.8 g/g for plant oil substrates.259 PHA is produced commercially by a number of suppliers, with (former) producers including Kaneka (Japan), Bio-on (Italy), Daminer Scientific (USA), Newlight Technologies (USA, by methane fermentation, see below), Cheil Jedang (South Korea), TianAn Biopolymer (China) and Tianjin GreenBio Materials (China).260–266 Feedstocks used for commercial production of PHA include vegetable oils, sugar beet and sugar cane processing waste, sugars from corn or cassava starch and bio-derived methane. Global production of PHA was estimated at 36 kt per annum in 2020.267,268 The role of PHA production in a future circular economy will likely depend on the degree of market uptake of PHA-based products.
Fermentation of biomass-derived gasses
Most of the fermentation processes described above rely on the use of a relatively well-defined biomass-derived substrate, e.g. sugar-rich syrups or molasses, glycerol or fatty acids, as a starting material for fermentation. For very recalcitrant or heterogeneous biomass-derived feedstocks, the effort required to obtain such a well-defined substrate might be prohibitive. Such feedstocks can, however, be transformed into relatively homogeneous gas streams by processes such as anaerobic digestion (CH4 and CO2), gasification (H2, CO, and CO2), or combustion (CO2). Certain microorganisms can metabolise these gasses and thus can be applied to convert such biomass-derived gas streams into added-value chemicals. In particular, significant effort has been made to establish the fermentative conversion of syngas (mixtures of CO and H2), and in some cases also CO2, into added-value chemicals.Acetogenic bacteria, such as Clostridium autoethanogenum, Clostridium carboxidivorans and Clostridium ljungdahlii, can convert mixtures of CO2 + H2 and/or CO into short to medium chain alcohols and fatty acids, with the product most extensively explored for industrial application being ethanol (Scheme 7).269 These bacteria grow optimally under strictly anaerobic conditions and use the natural Wood-Ljungdahll pathway to fixate CO or CO2 as acetyl-CoA, which is subsequently converted further to the alcohols and/or acids.270 Major challenges for the fermentation of syngas and syngas-related feedstocks are the limited gas–liquid mass transfer of the gas components and thermodynamic limits at which acetogens operate resulting in low growth and product formation rates as well as low biomass and product titres compared to sugar-based fermentations.20,271–273 Reactor designs that facilitate the gas–liquid mass transfer, e.g. trickle bed reactors or pressurised fermenters, cell immobilization, in situ product recovery, and microbial strain engineering have been used to overcome these intrinsic disadvantages.274,275 Another challenge is the relatively low growth rates and low final biomass density obtained with acetogenic bacteria. The composition of the substrate gas and fermentation medium is of importance to steer production towards ethanol and minimize production of acetic acid as a co-product.20,269,275,276 For the production of ethanol, titres of up to 48 g L−1, have been reported for C. ljungdahlii, with a conversion of 90% of CO and 70% of H2 from the gas substrate.277 For C. carboxidivorans P7 titres of up to 24 g L−1 ethanol have been reported, with conversion of 54% of CO and 68% of H2 from the gas substrate.269,278
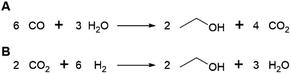 | ||
| Scheme 7 Reaction equations for the production of ethanol from carbon monoxide (A) or mixtures of carbon dioxide and hydrogen (B) by fermentation with acetogenic bacteria. | ||
Extensive efforts have been made to employ such fermentations in industrial processes for the production of ethanol from biomass-derived gas streams. In such processes, an organic feedstock, e.g., waste biomass, is thermochemically gasified, resulting in a crude mixture of mainly CO, CO2, H2 and traces of impurities like methane, hydrogen cyanide, hydrogen sulphide, tars, ethane, ethylene and aromatics.279,280 The heat that is released during gasification can be used to generate electricity, which can be fed back to the power grid or used to operate the plant.279,281 Following gasification, the gas mixture is cooled, compressed, and cleaned before being fed to the fermentation reactor, where it is converted to ethanol.279,280
Attempts at commercialisation of this process started in the early 2000s with a pilot plant in Fayetteville, Arkansas, USA, operated continuously from 2003–2008 by Bioengineering Resources (BRI) on various waste streams, for example biomass or spent tires.282–285 Efficiencies of >284 L (0.224 t) ethanol per dry ton of biomass or >568 L (0.448 t) ethanol per ton of tires or hydrocarbons were achieved, at a plant capacity of about 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L (118 t) ethanol per annum.285,286 Following a takeover of BRI by INEOS, a 30 million L (24 kt) per annum commercial-scale follow up was planned in Vero Beach, Florida, USA, but it would never run as intended due to various troubles upstream of the fermentation.287,288 The presence of 15–200 ppm hydrogen cyanide in the syngas stream initially inhibited the fermentation process and only after installing additional scrubbers to remove it, could sufficiently low concentrations (<5 ppm) be achieved.288,289 In the end, feedstock handling (due to the high water content of the agricultural residues), and syngas clean-up remained bottlenecks for commercialisation.288 In 2016, INEOS announced it was selling its entire ethanol business because of changing market conditions and a revised company strategy, abandoning syngas fermentation entirely.290 During approximately the same period, Coskata used proprietary strains of C. carboxidivorans, Clostridium ragsdalei and a proprietary Clostridium coskatii strain for the production of ethanol from syngas.291 The company operated a demonstration plant for the production of ethanol from syngas from various sources, including wood biomass, sorted MSW and natural gas, for over 15
000 L (118 t) ethanol per annum.285,286 Following a takeover of BRI by INEOS, a 30 million L (24 kt) per annum commercial-scale follow up was planned in Vero Beach, Florida, USA, but it would never run as intended due to various troubles upstream of the fermentation.287,288 The presence of 15–200 ppm hydrogen cyanide in the syngas stream initially inhibited the fermentation process and only after installing additional scrubbers to remove it, could sufficiently low concentrations (<5 ppm) be achieved.288,289 In the end, feedstock handling (due to the high water content of the agricultural residues), and syngas clean-up remained bottlenecks for commercialisation.288 In 2016, INEOS announced it was selling its entire ethanol business because of changing market conditions and a revised company strategy, abandoning syngas fermentation entirely.290 During approximately the same period, Coskata used proprietary strains of C. carboxidivorans, Clostridium ragsdalei and a proprietary Clostridium coskatii strain for the production of ethanol from syngas.291 The company operated a demonstration plant for the production of ethanol from syngas from various sources, including wood biomass, sorted MSW and natural gas, for over 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h from 2009–2011.291,292 Following this, Coskata set out plans for a 61–295 million L (48–232 kt) ethanol per annum commercial plant. The initial intent was to produce syngas from 2/3 wood biomass and 1/3 natural gas, however Coskata switched focus fully to reforming natural gas after noticing that elimination of the biomass-to-syngas production chain would halve the costs and remove the scale-limiting factor of biomass transport.293 No reports have been found that the plant was actually built. In 2016, Coskata became Synata Bio,294 after which no further activities regarding syngas fermentation were reported.
000 h from 2009–2011.291,292 Following this, Coskata set out plans for a 61–295 million L (48–232 kt) ethanol per annum commercial plant. The initial intent was to produce syngas from 2/3 wood biomass and 1/3 natural gas, however Coskata switched focus fully to reforming natural gas after noticing that elimination of the biomass-to-syngas production chain would halve the costs and remove the scale-limiting factor of biomass transport.293 No reports have been found that the plant was actually built. In 2016, Coskata became Synata Bio,294 after which no further activities regarding syngas fermentation were reported.
The experiences of BRI/INEOS and Coskata/Synata Bio highlight the challenges associated with gasification of biomass for the production of a fermentable gas feedstock. The only company that is currently producing ethanol from CO-rich gas streams at commercial scale, LanzaTech, founded in New Zealand in 2005, currently employs non-biomass derived gas streams for their processes.295 Lanzatech operates multiple industrial-scale plants producing ethanol from CO-rich industrial off-gasses from steel mills using a proprietary strain of C. autoethanogenum.20,291 In recent years, LanzaTech demonstrated on a pilot scale that their ethanol platform can be expanded to gas derived from biomass, such as municipal solid waste (in collaboration with Sekisui Chemical) and wood biomass (in collaboration with Aemetis and InEnTec) and higher TRL plants are in development.296,297 Conversion of biomass can also be integrated more indirectly into the gas fermentation process. In an industrial-scale demonstration plant planned for the end of 2022 gas fermentation will be used by LanzaTech and ArcelorMittal to capture off-gasses coming from waste wood bio-coals burned in the blast furnace during steel production.298 This plant is expected to produce 80 million L (63 kt) of ethanol per annum.298 This process demonstrates how gas fermentation, in combination with energy production, can be used to maximize the output of biomass-derived carbon.
In addition to ethanol, acetogenic bacteria have the potential to produce other compounds from gasified biomass either through naturally occurring or genetically engineered metabolic pathways.272,273 Coskata worked on the development of the production of propanol299 and butanol300 from syngas using microbial co-cultures. LanzaTech worked on the commercialisation of the co-production of 2,3-butanediol or 1,3-butadiene, and ethanol from CO-rich waste gas.301 The company also holds many patents on (syn)gas fermentation technology and engineered proprietary bacterial strains to produce chemicals, including isopropanol and acetone.302–305 On a commercial scale however, syngas fermentation is currently only used to produce ethanol for application in fuels or conversion to higher-value chemicals, such as ethylene.306,307
Another development that is of interest in this context is the industrial application of phototrophic microorganisms (i.e. microorganisms capable of photosynthesis) such as algae and cyanobacteria in the production of chemicals. Such processes are being commercialised, for example by the Netherlands-based Photanol, who are employing cyanobacteria for the conversion of CO2 to organic acids such as lactic acid and glycolic acid in a 10 t per annum demonstration plant opened in 2020.308–311 US-based Algenol worked on employing cyanobacteria for the production of ethanol and reported the operation of a 48 t per annum demonstration facility in Florida in 2015.312 Algenol also reported the construction of demonstration facilities in India, in collaboration with India-based Reliance Industries (2015), Mexico, in collaboration with Mexico-based BioFields (2009) and Texas in collaboration with US-based Dow Chemical (2009).313–315 However, no reports were found indicating these plants had reached full operational status. Both Photanol and Algenol currently focus on the use of CO2 extracted from the atmosphere or emitted by traditional industries such as power plants, chemical plants or oil refineries as a feedstock. It will be of interest to discover whether these technologies can be applied for the conversion of CO2 obtained from waste biomass by combustion in future, providing an outlet for heterogeneous waste biomass streams. However, it must first be demonstrated that they can be operated successfully at commercial scale in their current form.
Another approach for the conversion of bio-derived waste gasses by fermentation that is being commercialised is the production of polyhydroxyalkanoates (PHAs, see also the polyhydroxyalkanoates section) through fermentation of methane (biogas).316,317 Newlight Technologies produces polyhydroxybutyrate (PHB) from greenhouse gas (methane) and air with a proprietary methanotroph.318–320 Since 2013, the company started producing on a commercial scale and expanded its production capacity by opening a new commercial-scale plant in California in 2020.316,318,321 Newlight Technologies production capacity reportedly stood at 45 t per annum in 2015, information on current capacity following the opening of their new plant was not found.322 Mango Materials is developing a similar technology by fermenting raw biogas, a mixture of CO2, CH4 and H2S, obtained from a water treatment plant, and is close to PHB production at demonstration scale (5 t PHB per annum).323
Gas fermentation of (biomass-derived) waste gasses is emerging as a commercially viable technology with the commercial scale production of ethanol from CO-rich industrial off-gasses and PHB from methane. Higher TRL plants should start operations soon that will demonstrate whether (syn)gas fermentation can be successfully implemented on a commercial scale for the conversion of highly heterogeneous biomass or organic waste streams into new chemicals. Fermentation of gas feedstocks into chemicals other than ethanol and PHB on a commercial scale is not yet emerging and might be something for a distant future.
Discussion
A range of fermentation technologies could play an important role in the production of bulk chemicals from biomass in the circular economy in 2050. The production of various short chain (di)alcohols (e.g. ethanol, n-butanol, 1,3-PDO, 1,4-BDO), organic (di)acids (e.g. succinic acid, lactic acid, adipic acid, 3-hydroxypropionic acid) and recently also ω-amino acids from biomass-derived feedstocks is already operated on a commercial scale or has been demonstrated at pilot or demonstration scale. Typically, first generation biomass feedstocks have been applied for these processes. It is clear that fermentative processes have significant advantages for the conversion of biomass-derived feedstocks, with the mild reaction conditions and aqueous medium employed making them well suited to the conversion of sugars to added-value chemicals. The retention of heteroatoms present in the biomass-derived substrates makes fermentation an interesting option when considering the production of heteroatom-containing building block molecules such as diols, diacids, or diamines. The polycondensation polymers produced from these building blocks are highly suitable for application in a circular economy as the presence of hydrolysable bonds means they can be selectively converted back to monomers by chemical recycling (e.g. hydrolysis), a feat that is challenging for most non-polycondensation polymers. This provides fermentation with the potential to play a major role in the production of such building blocks in a future circular economy. Nevertheless, efforts to commercialise fermentation processes for bulk chemical production have faced their challenges. The most likely explanation for this seems competition with chemicals derived from fossil resources, both in terms of direct cost-competitiveness, when “drop-in” molecules are targeted, and market development when biobased alternatives to petrochemical products are envisioned. Some fermentation processes also need to compete with chemical processes for production of the target molecules from biobased feedstocks. In future, intelligent combinations of chemical and fermentation technologies may be developed to find the optimal route for production of biobased chemicals.A disadvantage of many of the available fermentation processes is the fact that carbon from the sugar substrates is lost in the form of CO2, limiting the theoretical yield of product in terms of carbon efficiency. In this context, it is of interest to note that of the processes described above, a number of those that have come furthest in the process of commercialisation in recent decades (lactic acid, succinic acid, citric acid) involve processes where loss of carbon from the substrate is limited or absent and where markets are targeted where petrochemical products are not well established (biodegradable polymers) or accepted (food). One obvious exception to this pattern is bioethanol, which is produced commercially at approximately 80 MMt per annum scale, despite the fermentation process displaying a limited theoretical yield and targeting the fuel market, where petrochemical products are well established. However, it is likely that bioethanol production would not have reached such large volumes without assistance from stimulatory regulatory frameworks in e.g. the US, EU and Brazil (see e.g. ref. 324–326). This highlights the role governmental bodies can play in stimulating the adoption of novel technologies required for the transition to a circular economy.
Production of bulk chemicals from second generation biomass feedstocks is attractive from the point of view of enabling the valorisation of currently under-utilised agricultural and forestry waste streams. However, further development efforts are required to overcome technological challenges related to biomass handling and pre-treatment and the co-valorisation of hemicellulose and lignin streams. Fermentation starting from first generation feedstocks has the advantage that fermentable sugars can more easily be extracted from the biomass and issues related to competition with land use for food production may be less problematic for chemicals production than for biofuel production due to the lower associated market volumes. Fermentation using biomass-derived gas streams may also play a role in valorising heterogeneous biomass waste streams.
The fermentation processes described in this review are at different stages of development. Depending on the current level of development, the feedstock used, the yield of product and the availability of competing (chemical) technologies to produce the target compound from biobased feedstocks, we have ranked the discussed processes by their potential to contribute to the circular economy in 2050 (see Table 2). We identified seven fermentation processes that are already sufficiently technologically advanced that they can be readily adopted for the production of bulk chemicals: the production of ethanol, lactic acid, succinic acid, 1,3-PDO, itaconic acid, 1,4-BDO and PHA. These processes vary in the extent that the products produced from them have obvious outlets available in the production of circular chemicals and materials. Lactic acid, succinic acid, 1,3-PDO and 1,4-BDO can be converted into renewable polyesters of high interest for the future circular economy, but which have not yet obtained a large share of the polyester market. Use of these chemicals as building blocks in the circular economy will depend on the development of the market for products derived from them. The same holds true for PHA, which differs from the abovementioned chemicals in that a polymeric product is derived directly from fermentation, but shares the characteristic that future production will likely depend on market development for PHA-based products. The production of itaconic acid by fermentation has been established for decades. However, the market volume remains relatively small (∼50 kt per annum) and further development of the market for itaconic acid-derived polymers is required if it is to play a major role in a future circular economy. Of all the discussed products, ethanol is the molecule currently produced at the largest scale by fermentation (∼80 MMt per annum). This established scale of production has led many to view ethanol as a prime candidate platform molecule for biobased chemicals production. In particular, its conversion to ethylene as a “drop-in” replacement of this fossil-based platform chemical has gathered interest.22 In the opinion of the authors, this development should be viewed critically. Although “drop-in” replacements are attractive from the point of view that we can make use of existing infrastructure for their further processing, transitioning to a circular economy necessitates that we re-evaluate whether the materials we use meet the criteria for circular use. Polyethylene, the main product produced from petrochemical ethylene, performs rather poorly in this sense, as it cannot be easily selectively depolymerised to its monomer (i.e. ethylene), making chemical recycling challenging (polyethylene can be deconstructed by pyrolysis, but this typically yields a complex mixture of products327). Furthermore, polyethylene is not inherently biodegradable. Nevertheless, the scale at which ethanol production has been established and the relative simplicity (and therefore versatility) of the produced molecule, still make it of interest as a platform chemical in the circular economy.
| a Carbon efficiency for the production of n-butanol. If the acetone and ethanol products are also valorised, total carbon efficiency of the ABE process is higher. | ||
|---|---|---|
| Cat I: mature (≥10 kt per annum) technology, high potential for industrial application in circular economy in 2050 | ||
| 1 | Ethanol (from sugar) | Technology mature, ∼80 MMt per annum production primarily for biofuels. Potential precursor to “drop-in” bio-ethylene. Application will depend on market development. Carbon efficiency: 59% |
| 2 | Lactic acid | Technology mature (∼750 kt per annum), development primarily dependent on market for products. Carbon efficiency: 90% |
| 3 | Succinic acid | Technology demonstrated by multiple (former) producers at ≥10 kt per annum, development primarily dependent on market for products. Carbon efficiency: 98% |
| 4 | 1,3-propanediol | Technology mature, ∼100 kt per annum reported capacity. Product mainly used in relatively niche markets. Carbon efficiency: 60% |
| 5 | Itaconic acid | Technology mature, estimated capacity of 50 kt per annum in 2013. Future will depend on market development. Carbon efficiency: 58% |
| 6 | 1,4-Butanediol | Industrially applied at 30 kt per annum scale, which is limited compared to petrochemical counterpart. Potential competition from production of 1,4-butanediol from succinic acid. Carbon efficiency: 53% |
| 7 | Polyhydroxyalkanoates (PHA) | Technology mature, commercially produced by various companies. Estimated production capacity of 36 kt per annum. Future will depend on development of market for PHA-based products. Carbon efficiency from glucose: 56% |
| CAT-II: established at scale for food/feed applications. Bulk chemical applications proposed, but further development of follow-op chemistry required. | ||
| 8 | Citric acid | Technology mature, >2 MMt per annum production, primarily for food applications, not extensively investigated for chemical applications. Carbon efficiency: 84% |
| 9 | L-Lysine | Technology mature, produced at ∼2MMt per annum, mainly for animal feed applications. However, main outlets as a bulk chemical (1,5-pentanediamine and ε-caprolactam) face competition from more direct routes via fermentation (entries 14 and 21). Carbon efficiency: 74% |
| 10 | L-Glutamic acid | Established as industrial process at ∼3 MMt per annum scale, mainly for food applications. Production technology is established, but chemistry for conversion to building blocks needs to be developed. Carbon efficiency: 61% |
| Cat III: industrial-scale demonstration achieved, further development required for commercial application or information required to fully evaluate potential missing | ||
| 11 | n-Butanol | Acetone–butanol–ethanol fermentation established commercially in 20th century, closed-down due to competition from petrochemical sources. Relatively low yield due to toxicity of product, necessitating in situ product removal, presents a challenge for future implementation. Carbon efficiency: 34%a |
| 12 | Dodecanedioic acid | Industrially produced at estimated capacity of 70 kt per annum, relative scarcity of information makes judging potential difficult. Reliance on tropical oil-derived substrates is problematic. Little information available on efficiency. |
| 13 | Isobutanol | Industrial production established at 5 kt per annum capacity alongside ethanol. Scale-up and stand-alone production are the next challenges. Little information available on efficiency. |
| 14 | 1,5-Pentanediamine | Industrially produced at an estimated capacity of at least 50 kt per annum, relative scarcity of information makes judging potential difficult. Little information available on efficiency. |
| 15 | Ethanol (from gas fermentation) | Currently commercially produced from fossil-based off-gasses. Development of production from bio-derived gasses halted after first commercial plants were constructed, impurities in gasses presenting a major problem. Purer bio-derived gas streams may enable it in future. |
| 16 | β-Farnesene | Commercial production in a 33 kt per annum plant established in 2010s. Plant sold in 2018, current status of the development is unclear. Carbon efficiency: 44% |
| Cat IV: currently in developmental stages | ||
| 17 | Volatile fatty acids | Demonstration plants operational, commercial plants planned (36 kt per annum total capacity planned). Promising technology for production of mixtures of volatile fatty acids. Purification is potential challenge for production of pure fatty acids. |
| 18 | Malonic acid | Pilot scale production achieved, commercial scale planned (20 kt per annum by 2024). Little information available on efficiency. |
| 19 | 2,3-Butanediol | Demonstration plant operational, 300 ton per annum. Development currently mainly targeted at cosmetics, agrochemical applications, not bulk chemical applications. Carbon efficiency: 67% |
| 20 | Isobutylene | Production established at 100 ton per annum scale, of interest as direct “drop-in” for petrochemical isobutylene. Competition from production of isobutanol followed by conversion to isobutylene. Little information available on efficiency. |
| 21 | 6-Aminohexanoic acid | Pilot-scale production achieved (1 t). Demonstration plant planned. High potential impact as drop-in alternative to petrochemical analogue in nylon-6 synthesis. Little information available on efficiency. |
| =22 | Triglycerides | High potential as source for chemicals of over 6 carbon atoms, currently in early developmental stages. |
| =22 | Ethanol and organic acids (from CO2 using cyanobacteria) | Demonstration plants (10–50 t per annum) operational. Commercial scale production of bulk chemicals using phototrophic microorganisms yet to be demonstrated. |
| =22 | Glycolic acid | Current status of development is unclear. Carbon efficiency: 32% |
| =22 | Glycerol | Operated industrially during WWI, not currently being explored commercially. Unlikely to be attractive due to competition with production of glycerol from oils. Carbon efficiency: 34% |
| Cat V: halted in developmental stages | ||
| =22 | 1,2-Propanediol | Halted in developmental stages, reasons unclear. Carbon efficiency: 41% |
| =22 | 3-Hydroxypropionic acid | Presumed halted in developmental stages, reasons unclear. Carbon efficiency: 55% |
| =22 | Adipic acid | Halted in developmental stages due to bankruptcy |
| =22 | Isoprene | Halted in developmental stages. Carbon efficiency: 24% |
Some suggestions as to how the chemicals produced using the top 6 fermentation processes identified in this manuscript can be converted to chemical building blocks and materials required in the circular economy are given in Fig. 3. PHA, the product of the fermentation process that completes our top 7, is not included in this scheme as it can be directly used as a material without any further chemical conversion. The produced materials are categorised as biodegradable at end-of-life (e.g. polybutylene succinate, PBS), chemically recyclable to its constituent monomers (e.g. polyethylene terephthalate, PET), and materials that are neither biodegradable nor recyclable yet, but are crucial to various aspects in a circular economy that at end-of-life will enter the long carbon cycle by e.g. incineration.328
Biodegradable materials like PBS, PLA, PBAT and PBSA are preferentially applied in short cycle applications that have a high chance of littering into the environment, e.g. convenience food packaging. Materials that can be efficiently and selectively recycled back to their constituent monomers, such as polyesters and polyamides will form the bulk of materials produced, allowing the majority of carbon used for materials purposes to be kept in controlled, closed-cycle carbon loops. Based on the examples of polymers given in Fig. 3, a wide range of products can be produced, including beverage bottles (PET), textile fibres (PET, PPT, polyamides), foams and cushions (polyurethanes, polystyrene), electrical and electronic components (PBT, polyamides), engineering plastics (PMMA), etc. Materials that are neither biodegradable nor chemically recyclable should only be applied when no suitable alternative exists, e.g. rubbers used for the tyres of electric vehicles (SBR, butyl rubbers), unsaturated polyester resins that are used for the construction of wind turbines, or carbon fibre used for lightweight components of aeroplanes or cars. The chemical conversions shown in Fig. 3 allow the top 6 fermentation products identified in our review to be converted to these materials using conversions that are current or past industrial practice or have at least been proven at an industrial scale.
While currently fossil derived polyolefins, such as polyethylene (PE) and polypropylene (PP) make up more than 50% of the total amount of plastics,329 they are not included in this flowchart as they are neither biodegradable, nor can be chemically recycled to their respective monomers with sufficiently high selectivity (see also above).330 Chemical recycling with high selectivity to the constituent monomers has only been shown for condensation polymers such as polyesters and polyamides (by e.g. hydrolysis or glycolysis) and for certain specific vinyl polymers like polystyrene (PS) and polymethyl methacrylate (PMMA) via pyrolysis.330–334 The focus on back-to-monomer chemical recycling is based on the principle that mechanical recycling options (to initial application quality, such as food packaging) are limited for mixed, polluted, contaminated and or (partially) degraded materials. Chemical recycling allows for (extensive) purification of the monomers, followed by de-novo production of virgin polymer for highly demanding applications, including food packaging.
A further three fermentation processes were identified in our review that are performed at ≥1 MMt/per annum scale for applications other than bulk chemicals production (feed or food): the production of citric acid, L-lysine and L-glutamic acid. Although potential applications for these molecules in the production of bulk chemicals have been proposed, the conversion processes required to convert them into chemical building blocks and/or applications for the obtained building blocks, are not well established. Developing methodologies to convert these molecules into biobased building blocks and to develop applications for them should be an important R&D priority in the coming decades.
The other fermentation processed discussed require further development or validation at industrial scale before they can be deemed mature enough to employ in the future circular economy (or, in a few cases, it is hard to judge their potential due to a lack of publicly available information, see Table 2). In particular, we highlight fermentation using gas streams obtained from the gasification, digestion or incineration of biomass waste as a technology with transformative potential. The ability to convert heterogeneous waste streams of insufficient quality for use in conventional fermentation processes to a single chemical could greatly contribute to the full use of waste materials that is required in a circular economy. It is interesting to note that such a technology could not only be applied to biomass waste, but potentially also to waste streams containing synthetic (biobased) polymers. Thus, this technology may also be a way in which to ensure the use of waste streams where the quality of the polymer material has declined to the extent that mechanical or chemical recycling technologies can no longer process it. Developments in the coming years will demonstrate whether this technology can be successfully applied in a commercial setting.
Further research & development in the coming decades may increase the efficiency of fermentation processes that are currently not viable or lead to novel processes for the production of compounds that are currently not accessible through fermentation. Further development of precision genome editing techniques and high throughput screening technologies combined with increasingly advanced computational tools for the design of improved metabolic pathways and enzymes involved in them will facilitate the development of novel microbial production strains.335,336 Incorporating electrochemistry or microwave irradiation into fermentation processes may increase their efficiency and therefore economic viability.337,338 These technologies may allow new, currently unforeseen, fermentation processes to progress into industrial application and contribute to the circular economy in 2050.
Conclusions
Fermentation processes for the production of bulk chemicals have the potential to play an important role in the future circular economy. The overview presented here demonstrates that seven processes have reached a state of maturity where they are ready to be applied on an industrially relevant scale in such an economy, once market conditions provide sufficient demand for the products obtained from them. The products of these fermentation processes have a number of important (potential) applications that make them highly suited for use in a circular economy, where controlling carbon loops (by cascaded recycling and reducing uncontrolled emissions) and limiting environmental persistence (by designing inherent biodegradability) are crucial. High carbon atom economy and heteroatom retention are necessary to not only make circular products, but also ensure optimal use of the renewable feedstocks. For instance, while lactic acid fermentation is highly efficient, and the end-product polylactic acid (PLA) is safe, recyclable and degradable, ethanol fermentation, despite its limited carbon efficiency, will continue to play a major role due to the scale of operations and the extensive product tree based on ethylene chemistry. In addition to these seven processes, several other promising processes are still in development, and the coming years will be crucial in determining whether these processes can also be applied successfully in an industrial setting at large scale. Considering this, the authors expect that the role of fermentative processes in bulk chemical production will only increase in the coming decades, assuming that the transition towards a circular economy in 2050 leads to a sustained drive towards the use of renewable carbon sources for chemicals production.Conflicts of interest
From January 2022, Jeroen Hugenholtz is CTO of NoPalm Ingredients.Acknowledgements
This research was funded by the Knowledge Base programme “Towards a circular and climate positive society” of Wageningen University & Research (WUR), in the project “Biobased materials and chemicals for relieving and replacing the fossil feedstock system” (KB-34-010-001). The WUR Knowledge Base programme is financed by the Dutch Ministry of Agriculture, Nature and Food Quality. Niels Nouse was further supported by funding from The Netherlands Science Foundation (NWO) under the programme “Closed Cycles” project no. ALWGK.2016.029.Notes and references
- The Ellen MacArthur Foundation, Towards the circular economy: Economic and business rationale for an accelerated transition, 2013 Search PubMed.
- M. T. Brouwer, E. U. Thoden van Velzen, K. Ragaert and R. ten Klooster, Sustainability, 2020, 12, 10021 CrossRef.
- United Nations Department of Economic and Social Affairs, World Population Prospects, 2019 Search PubMed.
- F. Kähler, M. Carus, O. Porc and C. vom Berg, Turning off the tap for fossil carbon, Nova institute, 2021 Search PubMed.
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- A. Al-Mamoori, A. Krishnamurthy, A. A. Rownaghi and F. Rezaei, Energy Technol., 2017, 5, 834–849 CrossRef.
- J. Martínez Leal, S. Pompidou, C. Charbuillet and N. Perry, Sustainability, 2020, 12, 9861 CrossRef.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- Y. Lie, P. Ortiz, R. Vendamme, K. Vanbroekhoven and T. J. Farmer, Ind. Eng. Chem. Res., 2019, 58, 15945–15957 CrossRef CAS.
- D. R. Vardon, M. A. Franden, C. W. Johnson, E. M. Karp, M. T. Guarnieri, J. G. Linger, M. J. Salm, T. J. Strathmann and G. T. Beckham, Energy Environ. Sci., 2015, 8, 617–628 RSC.
- J. G. Linger, D. R. Vardon, M. T. Guarnieri, E. M. Karp, G. B. Hunsinger, M. A. Franden, C. W. Johnson, G. Chupka, T. J. Strathmann, P. T. Pienkos and G. T. Beckham, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12013–12018 CrossRef CAS PubMed.
- A. J. Straathof, Chem. Rev., 2014, 114, 1871–1908 CrossRef CAS PubMed.
- E. Bertrand, L. P. S. Vandenberghe, C. R. Soccol, J.-C. Sigoillot and C. Faulds, in Green Fuels Technology: Biofuels, ed. C. R. Soccol, S. K. Brar, C. Faulds and L. P. Ramos, Springer International Publishing, Cham, CH, 2016, pp. 175–212 Search PubMed.
- J. Tomei and R. Helliwell, Land Use Policy, 2016, 56, 320–326 CrossRef.
- J.-C. Sigoillot and C. Faulds, in Green Fuels Technology: Biofuels, ed. C. R. Soccol, S. K. Brar, C. Faulds and L. P. Ramos, Springer International Publishing, Cham, CH, 2016, pp. 213–239 Search PubMed.
- V. Kumar, P. Binod, R. Sindhu, E. Gnansounou and V. Ahluwalia, Bioresour. Technol., 2018, 269, 443–451 CrossRef CAS PubMed.
- C. G. Liu, Y. Xiao, X. X. Xia, X. Q. Zhao, L. Peng, P. Srinophakun and F. W. Bai, Biotechnol. Adv., 2019, 37, 491–504 CrossRef CAS PubMed.
- https://www.statista.com/statistics/307194/top-oil-consuming-sectors-worldwide/ , (accessed 15-04-2022).
- G. P. da Silva, M. Mack and J. Contiero, Biotechnol. Adv., 2009, 27, 30–39 CrossRef PubMed.
- C. Wu and X. Tu, in Handbook of Biofuels Production, ed. R. Luque, C. Lin, K. Wilson and J. Clark, Woodhead Publishing, Sawston, UK, 2 edn, 2016, pp. 335–357 Search PubMed.
- M. Carus, L. Dammer, Á. Puente, A. Raschka and O. Arendt, Biobased drop-in, smart drop-in and dedicated chemicals, Nova Institute, 2017 Search PubMed.
- A. Mohsenzadeh, A. Zamani and M. J. Taherzadeh, ChemBioEng Rev., 2017, 4, 75–91 CrossRef.
- https://www.coherentmarketinsights.com/market-insight/global-ethylene-market-371 , (accessed 29-05-2020).
- A. J. van Maris, D. A. Abbott, E. Bellissimi, J. van den Brink, M. Kuyper, M. A. Luttik, H. W. Wisselink, W. A. Scheffers, J. P. van Dijken and J. T. Pronk, Antonie Van Leeuwenhoek, 2006, 90, 391–418 CrossRef PubMed.
- https://ethanolrfa.org/statistics/annual-ethanol-production/ , (accessed 20-12-2021).
- https://www.ethanolproducer.com/plants/listplants/US/Operational/All/page:1/sort:plant/direction:asc , (accessed 20-12-2021).
- M. O. de Souza Dias, R. Maciel Filho, P. E. Mantelatto, O. Cavalett, C. E. V. Rossell, A. Bonomi and M. R. L. V. Leal, Environ. Dev., 2015, 15, 35–51 CrossRef.
- Global Agricultural Information Network Report, Biofuels Annual, Brazil, 2018.
- M. Padella, A. O'Connell and M. Prussi, Appl. Sci., 2019, 9, 4523 CrossRef CAS.
- https://www.sekab.com/en/products-services/product/project-nordfuel/ , (accessed 07-12-2021).
- https://www.clariant.com/en/Company/Contacts-and-Locations/Key-Sites/Romania , (accessed 07-12-2021).
- https://ethanolproducer.com/articles/15344/zero-to-10-million-in-5-years , (accessed 29-05-2020).
- https://cen.acs.org/business/biobased-chemicals/POET-DSM-pause-cellulosic-ethanol/97/i46 , (accessed 20-11-2020).
- M. Niaounakis, Biopolymers: Processing and products, Elsevier, Amsterdam, NL, 2015 Search PubMed.
- Y. Tokiwa, B. P. Calabia, C. U. Ugwu and S. Aiba, Int. J. Mol. Sci., 2009, 10, 3722–3742 CrossRef CAS PubMed.
- D. Sun, Y. Li, C. Yang, Y. Su, Y. Yamada and S. Sato, Fuel Process. Technol., 2020, 197, 106193 CrossRef CAS.
- https://www.britannica.com/science/styrene-butadiene-rubber , (accessed 08-04-2022).
- https://www.britannica.com/technology/butadiene-rubber , (accessed 08-04-2022).
- B. O. Abo, M. Gao, Y. Wang, C. Wu, Q. Wang and H. Ma, Environ. Sci. Pollut. Res. Int., 2019, 26, 20164–20182 CrossRef CAS PubMed.
- A. M. López Contreras, W. Kuit, M. A. J. Siemerink, S. W. M. Kengen, J. Springer and P. A. M. Claassen, in Bioalcohol Production: Biochemical conversion of lignocellulosic biomass, ed. K. W. Waldron, Woodhead Publishing, Swanston, UK, 2010, pp. 415–460 Search PubMed.
- S. Y. Lee, J. H. Park, S. H. Jang, L. K. Nielsen, J. Kim and K. S. Jung, Biotechnol. Bioeng., 2008, 101, 209–228 CrossRef CAS PubMed.
- D. Jones and D. Woods, Microbiol. Rev., 1986, 50, 484–524 CrossRef CAS PubMed.
- G. M. Awang, G. A. Jones and W. M. Ingledew, Crit. Rev. Microbiol., 1988, 15(Suppl 1), S33–S67 CrossRef PubMed.
- V. V. Zverlov, O. Berezina, G. A. Velikodvorskaya and W. H. Schwarz, Appl. Microbiol. Biotechnol., 2006, 71, 587–597 CrossRef CAS PubMed.
- Y. Ni and Z. Sun, Appl. Microbiol. Biotechnol., 2009, 83, 415–423 CrossRef CAS PubMed.
- Y. Jiang, J. Liu, W. Jiang, Y. Yang and S. Yang, Biotechnol. Adv., 2015, 33, 1493–1501 CrossRef CAS PubMed.
- https://europe.chinadaily.com.cn/epaper/2015-08/07/content_21525451.htm , (accessed 12-06-2020).
- https://www.biofuelsdigest.com/bdigest/2016/12/13/green-biologics-begins-shipments-from-1st-commercial-plant/ , (accessed 12-06-2020).
- https://biomarketinsights.com/green-biologics-to-shut-its-first-commercial-bio-based-chemicals-plant-in-us/ , (accessed 30-10-2019).
- C. F. dos Santos Vieira, F. Maugeri Filho, R. Maciel Filho and A. P. Mariano, Bioresour. Technol., 2019, 287, 121425 CrossRef CAS PubMed.
- Y. Izawa and T. Yokoyama, in Liquid phase aerobic oxidation catalysis: Industrial applications and academic perspectives, ed. S. S. Stahl and P. L. Alsters, Wiley, Hoboken, USA, 2016, pp. 159–171 Search PubMed.
- D. I. Collias, A. M. Harris, V. Nagpal, I. W. Cottrell and M. W. Schultheis, Ind. Biotechnol., 2014, 10, 91–105 CrossRef CAS.
- Gevo Inc., US 8017375B2, 2011.
- https://gevo.com/wp-content/uploads/2018/02/isobutanol-process.pdf , (accessed 30-10-2019).
- https://gevo.com/wp-content/uploads/2020/05/Gevo-Whitepaper-Isobutanol-Production-Process.pdf , (accessed 9-12-2020).
- https://gevo.com/wp-content/uploads/2020/05/Gevo-Whitepaper-Sustainable-Aviation-Fuel.pdf , (accessed 31-03-2022).
- https://www.chemicalprocessing.com/articles/2016/bio-based-isobutanol-beckons/ , (accessed 9-12-2020).
- https://biomassmagazine.com/articles/16147/gevo-discusses-plans-to-decarbonize-luverne-plant , (accessed 5-12-2019).
- https://bioenergyinternational.com/biofuels-oils/gevo-suspends-ethanol-production-and-pushes-forward-on-project-funding , (accessed 09-12-2020).
- https://investors.gevo.com/news/praj-gevo-joint-development-agreement-to-enter-commercialization-phase , (accessed 05-06-2020).
- https://www.eog-asia.com/gevo-praj-partner-up-to-commercialise-renewable-isobutanol-jet-fuel-in-india/ , (accessed 05-06-2020).
- https://www.butamax.com/wp-content/uploads/2017_04_03_ib_butamax_announce-FINAL.pdf , (accessed 12-06-2020).
- https://www.shell.com/business-customers/chemicals/safe-product-handling-and-transportation/product-stewardship-summaries/_jcr_content/par/expandablelist/expandablesection_1815106617.stream/1525709608519/f9c57aec7f6036a27fb997157740957c77fccf43/mpg-pss-december-2017.pdf , (accessed 31-03-2022).
- https://adhesives.specialchem.com/news/industry-news/metex-and-upm-to-develop-monopropylene-glycol-000175940 , (accessed 20-11-2020).
- https://www.adm.com/products-services/industrials/propylene-glycol , (accessed 13-08-2021).
- Metabolic Explorer, WO2005/073364A2, 2005.
- https://www.metabolic-explorer.com/2018/04/18/discontinuation-of-the-valchem-project-following-the-joint-decision-to-defer-the-mpg-production-project/ , (accessed 20-11-2020).
- J. V. Kurian, J. Polym. Environ., 2005, 13, 159–167 CrossRef CAS.
- https://sorona.com/our-story , (accessed 31-03-2022).
- G. A. Kraus, Clean: Soil, Air, Water, 2008, 36, 648–651 CAS.
- G. Kaur, A. K. Srivastava and S. Chand, Biochem. Eng. J., 2012, 64, 106–118 CrossRef CAS.
- https://www.duponttateandlyle.com/ , (accessed 30-10-2019).
- C. E. Nakamura and G. M. Whited, Curr. Opin. Biotechnol., 2003, 14, 454–459 CrossRef CAS PubMed.
- https://www.postbulletin.com/dupont-tate-lyle-develop-corn-based-polymer/article_44b16782-abc8-5e77-b634-e6c285447c2d.html , (accessed 23-06-2020).
- https://www.areadevelopment.com/newsItems/10-2-2018/dupont-tate–lyle-bio-products-company-distribution-center-loudon-tennessee.shtml , (accessed 05-12-2019).
- Dupont Tate & Lyle Bioproducts, GRAS Exemption claim: 1,3-propanediol, 2009.
- https://issuu.com/ccminternational/docs/glory_biomaterial_s_first_phase_of_biological_pdo_ , (accessed 26-11-2020).
- https://cd.cnchemicals.com/Release_1427.html , (accessed 26-11-2020).
- Zhangjiagang Glory Chemical Ind. Co. Ltd., CN107384975A, 2017.
- https://www.metabolic-explorer.com/2019/11/04/metex-noovista-project-update/ , (accessed 26-11-2020).
- https://www.dsengineers.com/en/references/metex-noovista-propanediol-butyric-acid-production-plant-france/ , (accessed 26-11-2020).
- Metabolic Explorer, WO2010/128070A2, 2010.
- Metabolic Explorer, WO2012/062832A1, 2012.
- Metabolic Explorer, WO2019/025580A1, 2019.
- Metabolic Explorer, WO2018/150023A1, 2018.
- https://www.dsm.com/personal-care/en_US/events-and-news/press-releases/2019/03-12-latest-dsm-and-metex-noovista.html , (accessed 26-11-2020).
- S. S. Umare, A. S. Chandure and R. A. Pandey, Polym. Degrad. Stab., 2007, 92, 464–479 CrossRef CAS.
- S. Thiyagarajan, M. A. Meijlink, A. Bourdet, W. Vogelzang, R. J. I. Knoop, A. Esposito, E. Dargent, D. S. van Es and J. van Haveren, ACS Sustainable Chem. Eng., 2019, 7, 18505–18516 CrossRef CAS.
- B. D. Ahn, S. H. Kim, Y. H. Kim and J. S. Yang, J. Appl. Polym. Sci., 2001, 82, 2808–2826 CrossRef CAS.
- T. Baba and Y. Ono, J. Mol. Catal., 1986, 37, 317–326 CrossRef CAS.
- S. E. Hunter, C. E. S. Ehrenberger and P. E. Savage, J. Org. Chem., 2006, 71, 6229–6239 CrossRef CAS PubMed.
- H. Li, H. Yin, T. Jiang, T. Hu, J. Wu and Y. Wada, Catal. Commun., 2006, 7, 778–782 CrossRef CAS.
- A. Burgard, M. J. Burk, R. Osterhout, S. Van Dien and H. Yim, Curr. Opin. Biotechnol., 2016, 42, 118–125 CrossRef CAS PubMed.
- https://www.genomatica.com/wp-content/uploads/2017/01/Novamont-opens-world%E2%80%99s-first-commercial-plant-for-bio-production-of-a-major-intermediate-chemical.pdf , (accessed 24-06-2020).
- https://apnews.com/377f23f86d934da893adc833b991abc9 , (accessed 24-06-2020).
- https://greenchemicalsblog.com/2016/10/02/first-bio-bdo-commercial-plant-opens/ , (accessed 30-10-2019).
- https://www.novamont.com/public/zip/factsheet_materbiotech_EN_web.pdf , (accessed 24-06-2020).
- https://greenchemicalsblog.com/2013/06/18/novamont-launches-4th-gen-bioplastic/ , (accessed 24-06-2020).
- https://materbi.com/en/ , (accessed 26-04-2020).
- https://greenchemicalsblog.com/2013/12/03/basf-produces-first-bio-bdo-commercial-volumes/ , (accessed 30-10-2019).
- https://plasticsandrubberasia.com/mar2015/materials3.html , (accessed 24-06-2020).
- https://markets.businessinsider.com/news/stocks/global-and-china-1-4-butanediol-bdo-industry-report-2019-2025-1028275091 , (accessed 24-06-2020).
- Y. Deng, L. Ma and Y. Mao, Biochem. Eng. J., 2016, 105, 16–26 CrossRef CAS.
- H. Duan, Y. Yamada and S. Sato, Appl. Catal., A, 2015, 491, 163–169 CrossRef CAS.
- D. Tsukamoto, S. Sakami, M. Ito, K. Yamada and T. Yonehara, Chem. Lett., 2016, 45, 831–833 CrossRef CAS.
- N. T. T. Nguyen, F. Matei-Rutkovska, M. Huchede, K. Jaillardon, G. Qingyi, C. Michel and J. M. M. Millet, Catal. Today, 2019, 323, 62–68 CrossRef CAS.
- E. Gubbels, L. Jasinska-Walc and C. E. Koning, J. Polym. Sci., Part A: Polym. Chem., 2012, 51, 890–898 CrossRef.
- E. Gubbels, L. Jasinska-Walc, B. A. J. Noordover and C. E. Koning, Eur. Polym. J., 2013, 49, 3188–3198 CrossRef CAS.
- X. Hu, X. Shen, M. Huang, C. Liu, Y. Geng, R. Wang, R. Xu, H. Qiao and L. Zhang, Polymer, 2016, 84, 343–354 CrossRef CAS.
- C. W. Song, J. M. Park, S. C. Chung, S. Y. Lee and H. Song, J. Ind. Microbiol. Biotechnol., 2019, 46, 1583–1601 CrossRef CAS PubMed.
- https://www.gscaltex.com/en/Business/RnD , (accessed 24-06-2020).
- https://www.gscaltex.com/downloadfile/2,3-ButaneDiol(en).pdf , (accessed 24-06-2020).
- C. A. G. Quispe, C. J. R. Coronado and J. A. Carvalho Jr., Renewable Sustainable Energy Rev., 2013, 27, 475–493 CrossRef CAS.
- https://www.businesswire.com/news/home/20210115005399/en/Global-Glycerol-Industry-2020-to-2027---Market-Trends-and-Drivers---ResearchAndMarkets.com , (accessed 03-12-2021).
- Z.-X. Wang, J. Zhuge, H. Fang and B. A. Prior, Biotechnol. Adv., 2001, 19, 201–223 CrossRef CAS.
- P. Figard, PhD thesis, Iowa State University, 1949.
- M. Atasoy, I. Owusu-Agyeman, E. Plaza and Z. Cetecioglu, Bioresour. Technol., 2018, 268, 773–786 CrossRef CAS PubMed.
- M. Ramos-Suarez, Y. Zhang and V. Outram, Rev. Environ. Sci. Biotechnol., 2021, 20, 439–478 CrossRef CAS.
- https://afyren.com/en/ , (accessed 04-08-2021).
- https://www.chaincraft.nl/ , (accessed 04-08-2021).
- https://afyren.com/en/solutions/ , (accessed 04-08-2021).
- https://renewable-carbon.eu/news/afyren-and-the-spi-industrial-projects-fund-operated-by-bpifrance-announce-the-creation-of-an-industrial-company-afyren-neoxy/ , (accessed 04-08-2021).
- https://www.chaincraft.nl/project/x-craft-range/ , (accessed 04-08-2021).
- https://www.chaincraft.nl/technology/ , (accessed 04-08-2021).
- T. I. M. Grootscholten, D. P. B. T. B. Strik, K. J. J. Steinbusch, C. J. N. Buisman and H. V. M. Hamelers, Appl. Energy, 2014, 116, 223–229 CrossRef CAS.
- L. T. Angenent, H. Richter, W. Buckel, C. M. Spirito, K. J. J. Steinbusch, C. M. Plugge, D. P. B. T. B. Strik, T. I. M. Grootscholten, C. J. N. Buisman and H. V. M. Hamelers, Environ. Sci. Technol., 2016, 50, 2796–2810 CrossRef CAS PubMed.
- https://www.biofuelsdigest.com/bdigest/2020/09/17/competitive-edge-chaincrafts-technology-converts-waste-into-medium-chain-fatty-acids/ , (accessed 04-08-2021).
- M. Gullo, E. Verzelloni and M. Canonico, Process Biochem., 2014, 49, 1571–1579 CrossRef CAS.
- A. Vidra and Á. Németh, Period. Polytech., Chem. Eng., 2017, 62, 245–256 CrossRef.
- P. Pal and J. Nayak, Sep. Purif. Rev., 2016, 46, 44–61 CrossRef.
- K. Budak, O. Sogut and U. Aydemir Sezer, J. Polym. Res., 2020, 27, 208 CrossRef CAS.
- J. Iglesias, I. Martínez-Salazar, P. Maireles-Torres, D. Martin Alonso, R. Mariscal and M. López Granados, Chem. Soc. Rev., 2020, 49, 5704 RSC.
- J. Becker, A. Lange, J. Fabarius and C. Wittmann, Curr. Opin. Biotechnol., 2015, 36, 168–175 CrossRef CAS PubMed.
- https://www.metabolic-explorer.com/2019/09/27/first-half-2019/ , (accessed 30-10-2019).
- https://www.thefreelibrary.com/Metabolic+Explorer+develops+biological+production+of+glycolic+acid-a0216423311 , (accessed 30-10-2019).
- https://greenchemicalsblog.com/2013/06/12/metex-updates-on-bio-pdo-and-other-projects/ , (accessed 20-07-2020).
- https://www.metabolic-explorer.com/2019/04/16/de-smet-engineers-contractors-selected-to-build-pdo-ab-plant-2/ , (accessed 20-07-2020).
- Metabolic Explorer, US2012/0315682A1, 2012.
- K. Madhavan Nampoothiri, N. R. Nair and R. P. John, Bioresour. Technol., 2010, 101, 8493–8501 CrossRef CAS PubMed.
- F. A. Castillo Martinez, E. M. Balciunas, J. M. Salgado, J. M. Domínguez González, A. Converti and R. P. d. S. Oliveira, Trends Food Sci. Technol., 2013, 30, 70–83 CrossRef.
- R. Alves de Oliveira, A. Komesu, C. E. V. Rossell and R. Maciel Filho, Biochem. Eng. J., 2018, 133, 219–239 CrossRef CAS.
- T. Ghaffar, M. Irshad, Z. Anwar, T. Aqil, Z. Zulifqar, A. Tariq, M. Kamran, N. Ehsan and S. Mehmood, J. Radiat. Res. Appl. Sci., 2019, 7, 222–229 CrossRef.
- R. Datta and M. Henry, J. Chem. Technol. Biotechnol., 2006, 81, 1119–1129 CrossRef CAS.
- https://www.corbion.com/media/550170/corbion_whitepaper_feedstock_sourcing_11.pdf , (accessed 20-07-2020).
- https://www.corbion.com/about-corbion/innovation/gypsum-free-technology , (accessed 12-12-2020).
- https://www.businesswire.com/news/home/20170621005594/en/Global-Lactic-Acid-Market-2017-2025---Growth , (accessed 20-12-2021).
- https://www.corbion.com/about-corbion/corbion-stories/lactic-acid-the-lifeblood-of-our-business/under-the-microscope-lactic-acid , (accessed 08-12-2019).
- https://www.natureworksllc.com/About-NatureWorks , (accessed 08-12-2019).
- https://www.lactic.com/en-us/aboutus/ourhistory.aspx , (accessed 08-12-2019).
- https://www.jindan.eu/company/company-profile/ , (accessed 08-12-2019).
- https://www.ptit.org/InnoBioPlast2016/presentation/Session2/1.pdf , (accessed 20-07-2020).
- https://cen.acs.org/business/biobased-chemicals/Corbion-plans-lactic-acid/98/i10 , (accessed 20-07-2020).
- H. Fouilloux and C. M. Thomas, Macromol. Rapid Commun., 2021, 42, e2000530 CrossRef PubMed.
- D. Zhang, M. A. T. Hillmyer and W. B. Tolman, Macromolecules, 2004, 37, 8198–8200 CrossRef CAS.
- F. de Fouchecour, A. K. Sanchez-Castaneda, C. Saulou-Berion and H. E. Spinnler, Biotechnol. Adv., 2018, 36, 1207–1222 CrossRef CAS PubMed.
- https://www.cargill.com/news/releases/2014/NA31686855.jsp , (accessed 30-10-2019).
- https://www.novozymes.com/en/news/news-archive/2015/01/novozymes-cargill-continue-bio-acrylic-acid-partnership-basf-exits , (accessed 30-10-2019).
- https://cen.acs.org/environment/sustainability/clarity-coming-biobased-chemicals/98/i26 , (accessed 19-02-2021).
- Lygos Inc., WO2015/200545A1, 2015.
- Lygos Inc., WO2013/134424A1, 2013.
- https://www.aiche.org/resources/publications/cep/2016/june/catalyzing-commercialization-fermentation-process-makes-malonates-sugar-not-cyanide , (accessed 19-02-2021).
- https://greenchemicalsblog.com/2015/04/06/lygos-produces-malonic-acid-in-pilot-scale/ , (accessed 19-02-2021).
- https://www.globenewswire.com/news-release/2017/11/07/1176337/0/en/Lygos-Reaches-Major-Technical-Milestone-Scaling-Bio-Malonic-Manufacturing-Process-in-Partnership-with-NREL.html , (accessed 19-02-2021).
- D. P. Minh, M. Besson, C. Pinel, P. Fuertes and C. Petitjean, Top. Catal., 2010, 53, 1270–1273 CrossRef CAS.
- N. Nghiem, S. Kleff and S. Schwegmann, Fermentation, 2017, 3, 26 CrossRef.
- K.-K. Cheng, X.-B. Zhao, J. Zeng and J.-A. Zhang, Biofuels, Bioprod. Biorefin., 2012, 6, 302–318 CrossRef CAS.
- H. Lin, G. N. Bennett and K. Y. San, Metab. Eng., 2005, 7, 116–127 CrossRef CAS PubMed.
- M. L. Jansen and W. M. van Gulik, Curr. Opin. Biotechnol., 2014, 30, 190–197 CrossRef CAS PubMed.
- https://www.chemicals-technology.com/projects/myriant-plant/ , (accessed 30-10-2019).
- https://www.chemicals-technology.com/projects/bioamber-bio-based-succinic-acid-production-ontario/ , (accessed 30-10-2019).
- https://www.chemicals-technology.com/projects/reverdia-bio-based-succinic-acid-plant-italy/ , (accessed 30-10-2019).
- https://www.chemicals-technology.com/projects/succinitys-bio-based-succinic-acid-production-facility-montmel/ , (accessed 30-10-2019).
- https://bionb.org/fr/bioamber-announces-opening-of-worlds-largest-succinic-acid-plant-in-sarnia-2/ , (accessed 16-10-2020).
- https://www.biomassmagazine.com/articles/15729/lcy-chemical-purchases-bioamber-assets-sarnia-biorefinery , (accessed 16-10-2020).
- https://thesarniajournal.ca/a-once-defunct-sarnia-bioplastics-plant-has-risen-from-the-ashes/ (accessed 21-12-2021).
- https://www.theadvocate.com/baton_rouge/news/business/article_7be5c90e-54db-11ea-b161-f323a21a46f9.html , (accessed 16-10-2020).
- https://bioplasticsnews.com/gc-innovation-america/ , (accessed 16-10-2020).
- https://cen.acs.org/articles/90/i35/Italys-Biotech-Surge.html , (accessed 16-10-2020).
- https://www.roquette.com/media-center/press-center/2019-02-22-dsm-and-roquette-take-next-step-in-bio-based-succinic-acid , (accessed 16-10-2020).
- https://www.business-standard.com/content/b2b-chemicals/succinity-starts-commercial-production-of-bio-based-succinic-acid-114031000525_1.html , (accessed 23-10-2020).
- https://cen.acs.org/business/biobased-chemicals/Succinic-acid-once-biobased-chemical/97/i12 (accesed 20-12-2021).
- https://www.grandviewresearch.com/press-release/global-adipic-acid-market (accessed 20-12-2021).
- E. Skoog, J. H. Shin, V. Saez-Jimenez, V. Mapelli and L. Olsson, Biotechnol. Adv., 2018, 36, 2248–2263 CrossRef CAS PubMed.
- T. Beardslee and S. Picataggio, Lipid Technol., 2012, 24, 223–225 CrossRef CAS.
- https://biomarketinsights.com/verdezyne-becomes-latest-victim-of-liquidation-as-investors-withdraw-funding/ , (accessed 30-10-2019).
- I. Khalil, G. Quintens, T. Junkers and M. Dusselier, Green Chem., 2020, 22, 1517–1541 RSC.
- https://www.grandviewresearch.com/industry-analysis/dodecanedioic-acid-ddda-industry , (accessed 19-02-2021).
- G. Barbiroli, C. Lorenzetti, C. Berti, M. Fiorini and P. Manaresi, Eur. Polym. J., 2003, 39, 655–661 CrossRef CAS.
- D. Cyranoski, Nature, 2012, 492, 323 CrossRef CAS PubMed.
- https://bioenergyinternational.com/biochemicals-materials/cathay-industrial-biotech-invests-us500-million-in-bio-based-capacity-expansion , (accessed 19-02-2021).
- Verdezyne Inc., WO2014/100504A2, 2014.
- Verdezyne Inc., WO2013/006733A2, 2013.
- Verdezyne Inc., WO2014/100461A2, 2014.
- https://bioplasticsnews.com/2014/05/01/verdezyne-produces-ddda-from-yeast/ , (accessed 19-02-2021).
- https://www.theedgemarkets.com/article/verdezyne-build-world%E2%80%99s-first-lauric-acid-ddda-plant-malaysia , (accessed 19-02-2021).
- https://bioenergyinternational.com/biochemicals-materials/verdezyne-breaks-ground-worlds-first-bio-based-ddda-plant , (accessed on 19-02-2021).
- https://greenchemicalsblog.com/2018/05/24/verdezyne-exit-confirmed/ , (accessed 19-02-2021).
- https://greenchemicalsblog.com/2020/10/19/verdezyne-biochem-equipment-for-auction/ , (accessed 02-12-2021).
- B. C. Saha, J. Ind. Microbiol. Biotechnol., 2017, 44, 303–315 CrossRef CAS PubMed.
- https://www.kehai.info/en/ , (accessed 06-12-2019).
- https://www.ggbiochem.com/about_en.html , (accessed 06-12-2019).
- M. G. Steiger, M. L. Blumhoff, D. Mattanovich and M. Sauer, Front. Microbiol., 2013, 4, 23 CAS.
- https://www.businesswire.com/news/home/20220408005224/en/Global-Citric-Acid-Market-Outlook-to-2027---Featuring-Cargill-Tate-Lyle-and-Kenko-Among-Others---ResearchAndMarkets.com , (accessed 08-04-2022).
- https://www.prnewswire.com/news-releases/global-citric-acid-markets-report-2011-2018–2019-2024-300814817.html , (accessed 08-12-2019).
- B. Max, J. Salgado, N. Rodríguez, S. Cortés, A. Converti and J. Domínguez, Braz. J. Microbiol., 2010, 41, 862–875 CrossRef CAS PubMed.
- https://greenchemicalsblog.com/2015/12/22/cathay-to-expand-bio-monomers-and-bio-polyamides-capacity/ , (accessed 19-02-2021).
- Shanghai Cathay Biotechnology Co. Ltd., CN110468167A, 2018.
- https://www.prnewswire.com/news-releases/cathay-industrial-biotech-ltd-announces-ground-breaking-and-agreement-signing-for-significant-expansion-in-bio-produced-monomer-and-polyamide-production-300288436.html , (accessed 19-02-2021).
- https://www.cathaybiotech.com/en/newsxq.aspx?ID=36 , (accessed 19-02-2021).
- Genomatica Inc., US7799545B2, 2010.
- https://www.genomatica.com/worlds-first-ton-of-renewable-nylon-intermediate/ , (accessed 4-12-2020).
- https://www.genomatica.com/bio-nylon-scaling-50x-to-support-global-brands/ , (accessed 4-12-2020).
- M. D'Este, M. Alvarado-Morales and I. Angelidaki, Biotechnol. Adv., 2018, 36, 14–25 CrossRef PubMed.
- S. Sanchez and A. L. Demain, in Encyclopedia of Food Microbiology, ed. C. A. Batt and M. L. Tortorello, Academic Press, Cambridge, USA, 2014, pp. 778–784 Search PubMed.
- https://www.imarcgroup.com/amino-acid-technical-material-market-report#:~:text=The%20global%20amino%20acids%20market,4.7%25%20during%202022%2D2027. , (accessed 12-04-2022).
- https://www.mynewsdesk.com/us/global-market-insights/pressreleases/glutamic-acid-and-monosodium-glutamate-msg-market-size-is-likely-to-reach-more-than-4-million-tons-by-2023-1379400 , (accessed 13-04-2022).
- https://www.britannica.com/science/monosodium-glutamate , (accessed 12-04-2022).
- A. Ault, J. Chem. Educ., 2004, 81, 347–355 CrossRef CAS.
- https://www.grandviewresearch.com/industry-analysis/glutamic-acid-market , (accessed 13-04-2022).
- US Department of Energy, Top value added chemicals from biomass: Volume 1 – results of screening for potential candidates from sugars and synthesis gas, 2004 Search PubMed.
- T. M. Lammens, J. Le Notre, M. C. Franssen, E. L. Scott and J. P. Sanders, ChemSusChem, 2011, 4, 785–791 CrossRef CAS PubMed.
- https://www.grandviewresearch.com/press-release/global-lysine-market#:~:text=The%20global%20lysine%20was%201%2C902.3,6.0%25%20from%202014%<?pdb_no 20to?>20to<?pdb END?>%202020 , (accessed 13-04-2022).
- F. Felix, L. A. J. Letti, G. Vinicius de Melo Pereira, P. G. B. Bonfim, V. T. Soccol and C. R. Soccol, Crit. Rev. Biotechnol., 2019, 39, 1031–1055 CrossRef CAS PubMed.
- https://www.marketresearchfuture.com/reports/lysine-market-10103 , (accessed 13-04-2022).
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- https://www.britannica.com/topic/industrial-polymers-<?ccdc_no 468698?>468698<?ccdc END?>/Butyl-rubber-isobutylene-isoprene-rubber-IIR , (accessed 08-04-2022).
- https://www.grandviewresearch.com/press-release/global-isobutene-market , (accessed 20-08-2021).
- B. N. van Leeuwen, A. M. van der Wulp, I. Duijnstee, A. J. van Maris and A. J. Straathof, Appl. Microbiol. Biotechnol., 2012, 93, 1377–1387 CrossRef PubMed.
- https://www.global-bioenergies.com/group/isobutene-process/?lang=en , (accessed 20-08-2021).
- Global Bioenergies/Scientist of Fortune S.A., WO2017/162738A1, 2017.
- Global Bioenergies/Scientist of Fortune S.A., WO2017/085167A3, 2017.
- Global Bioenergies, WO2012/052427A1, 2012.
- Scientist of Fortune S. A./Global Bioenergies, WO2014/064198A1, 2014.
- Global Bioenergies/Scientist of Fortune S.A., WO2015/082447A1, 2015.
- Global Bioenergies, WO2020/188033A1, 2020.
- Global Bioenergies, WO2018/206262A2, 2018.
- https://www.global-bioenergies.com/group/history/?lang=en , (accessed 20-08-2021).
- https://www.global-bioenergies.com/the-construction-of-the-leuna-demo-plant-is-completed/?lang=en , (accessed 20-08-2021).
- https://www.britannica.com/science/polyisoprene , (accessed 08-04-2022).
- https://www.expertmarketresearch.com/reports/polyisoprene-market#:~:text=The%20global%20polyisoprene%20market%20size,21.8%20million%20tons%20by%202026 , (accessed 16-04-2021).
- L. Ye, X. Lv and H. Yu, Metab. Eng., 2016, 38, 125–138 CrossRef CAS PubMed.
- https://notchconsulting.blog/2012/03/07/goodyear-dupont-working-to-develop-bio-based-rubber/ , (accessed 20-12-2021).
- https://investors.amyris.com/2014-09-09-Braskem-Joins-Amyris-and-Michelin-to-Accelerate-the-Industrialization-and-Commercialization-of-Renewable-Isoprene , (accessed 16-04-2021).
- https://www.icis.com/explore/resources/news/2012/06/25/9571887/green-chemicals-ajinomoto-collaborates-with-bridgestone-on-bio-based-isoprene-for-rubber-tires/ , (accessed 16-04-2021).
- https://www.k-online.com/en/News/K_Archive/MALAYSIA_GlycosBio_advances_Malaysian_Bio_Plans_-_Initial_R_D_efforts_focused_on_isoprene , (accessed 16-04-2021).
- G. M. Whited, F. J. Feher, D. A. Benko, M. A. Cervin, G. K. Chotani, J. C. McAuliffe, R. J. LaDuca, E. A. Ben-Shoshan and K. J. Sanford, Ind. Biotechnol., 2010, 6, 152–163 CrossRef CAS.
- https://cen.acs.org/articles/89/i49/Amyris-Hits-Market.html , (accessed 21-04-2021).
- D. McPhee, in Catalytic process development for renewable chemicals, ed. P. Imhof and J. C. van der Waal, Wiley, Hoboken, USA, 2013, pp. 51–79 Search PubMed.
- A. L. Meadows, K. M. Hawkins, Y. Tsegaye, E. Antipov, Y. Kim, L. Raetz, R. H. Dahl, A. Tai, T. Mahatdejkul-Meadows, L. Xu, L. Zhao, M. S. Dasika, A. Murarka, J. Lenihan, D. Eng, J. S. Leng, C. L. Liu, J. W. Wenger, H. Jiang, L. Chao, P. Westfall, J. Lai, S. Ganesan, P. Jackson, R. Mans, D. Platt, C. D. Reeves, P. R. Saija, G. Wichmann, V. F. Holmes, K. Benjamin, P. W. Hill, T. S. Gardner and A. E. Tsong, Nature, 2016, 537, 694–697 CrossRef CAS PubMed.
- https://investors.amyris.com/2013-03-21-Amyris-Welcomes-Proposed-Change-of-Control-in-Its-Sugarcane-Supplier-in-Brazil , (accessed 21-04-2021).
- E4Tech, Report on market and industrial development intelligence for sustainable advanced biofuels, 2018.
- U. Biermann, U. T. Bornscheuer, I. Feussner, M. A. R. Meier and J. O. Metzger, Angew. Chem., Int. Ed., 2021, 60, 20144–20165 CrossRef CAS PubMed.
- J. L. Adrio, Biotechnol. Bioeng., 2017, 114, 1915–1920 CrossRef CAS PubMed.
- I. R. Sitepu, R. Sestric, L. Ignatia, D. Levin, J. B. German, L. A. Gillies, L. A. Almada and K. L. Boundy-Mills, Bioresour. Technol., 2013, 144, 360–369 CrossRef CAS PubMed.
- https://www.cultivated.bio/ , (accessed 15-04-2022).
- https://nopalm-ingredients.com/ , (accessed 07-12-2021).
- https://www.c16bio.com/ , (accessed 07-12-2021).
- M. Koller, Fermentation, 2018, 4, 30 CrossRef.
- J.-Y. Chee, S.-S. Yoga, N.-S. Lau, S.-C. Ling, R. M. M. Abed and K. Sudesh, in Current research, technology and education topics in applied microbiology and microbial biotechnology, ed. A. Méndez-Vilas, World Scientific, Singapore, SG, 2010 Search PubMed.
- https://kanekabiopolymers.com/ , (accessed 26-08-2021).
- https://www.bio-on.it/where.php , (accessed 26-08-2021).
- https://danimerscientific.com/pha-beginning-of-life/ , (accessed 26-08-2021).
- https://www.tianan-enmat.com/# , (accessed 26-08-2021).
- https://www.tjgreenbio.com/en/index.aspx , (accessed 26-08-2021).
- https://www.newlight.com/company , (accessed 26-08-2021).
- https://www.cjbio.net/en/products/cjPha.do , (accessed 01-12-2021).
- https://bioplasticsnews.com/polyhydroxyalkanoates-or-pha/ , (accessed 30-10-2019).
- https://www.european-bioplastics.org/market/ , (accessed 13-08-2021).
- B. Acharya, P. Roy and A. Dutta, Biofuels, 2015, 5, 551–564 CrossRef.
- S. W. Ragsdale and E. Pierce, Biochim. Biophys. Acta, 2008, 1784, 1873–1898 CrossRef CAS PubMed.
- K. Schumann and V. Müller, Nat. Rev. Micrbiol., 2014, 12, 809–821 CrossRef PubMed.
- F. R. Bengelsdorf, M. H. Beck, C. Erz, S. Hoffmeister, M. M. Karl, P. Riegler, S. Wirth, A. Poehlein, D. Weuster-Botz and P. Dürre, Adv. Appl. Microbiol., 2018, 103, 143–221 CAS.
- F. Liew, M. E. Martin, R. C. Tappel, B. D. Heijstra, C. Mihalcea and M. Köpke, Front. Microbiol., 2016, 7, 694 Search PubMed.
- I. K. Stoll, N. Boukis and J. Sauer, Chem. Ing. Tech., 2020, 92, 1226 CrossRef CAS.
- C. A. Vees, C. S. Neuendorf and S. Pflugl, J. Ind. Microbiol. Biotechnol., 2020, 47, 753–787 CrossRef CAS PubMed.
- J. L. Gaddy, D. K. Arora, C.-W. Ko, J. R. Phillips, R. Basu, C. V. Wikstrom and E. C. Clausen, US2003/0211585A1, 2003.
- J. R. Phillips, K. T. Klasson, E. C. Clausen and J. L. Gaddy, Appl. Biochem. Biotechnol., 1993, 39/40, 559–571 CrossRef.
- Y. Shen, R. Brown and Z. Wen, Biochem. Eng. J., 2014, 85, 21–29 CrossRef CAS.
- Y. Richardson, M. Drobek, A. Julbe, J. Blin and F. Pinta, in Recent advances in thermo-chemical conversion of biomass, ed. A. Pandey, M. Stöcker, T. Bhaskar and R. Sukumaran, Elsevier, Amsterdam, NL, 2015, pp. 213–250 Search PubMed.
- J. Bermudez and B. Fidalgo, in Handbook of biofuels production, ed. R. Luque, C. Lin, K. Wilson and J. Clark, Woodhead Publishing, Swanston, UK, 2016, pp. 431–494 Search PubMed.
- T. Schubert, Biofuels, Bioprod. Biorefin., 2020, 14, 845–878 CrossRef CAS.
- The Board of Trustees of the University of Arkansas, US5173429A, 1992.
- Bioengineering Resources Inc., US5821111A, 1998.
- Bioengineering Resources Inc., US6136577A, 2000.
- https://www.greencarcongress.com/2008/07/ineos-bio-to-co.html , (accessed 18-08-2020).
- https://app.ft.com/content/9667a580-568d-11dd-8686-000077b07658 , (accessed 18-08-2020).
- https://ethanolproducer.com/articles/8991/commissioning-under-way-at-florida-cellulosic-ethanol-plant , (accessed 04-08-2021).
- US Department of Energy, INEOS New planet bioenergy technical report final public version, 2016.
- Florida Department of Environmental Protection, INEOS new planet bioenergy: Technical evaluation & preliminary determination, 2014.
- https://biomassmagazine.com/articles/15089/former-ineos-bio-site-purchased-for-conversion-into-eco-district , (accessed 30-06-2020).
- J. Daniell, M. Köpke and S. Simpson, Energies, 2012, 5, 5372–5417 CrossRef CAS.
- https://www.sec.gov/Archives/edgar/data/1536893/000119312511343587/d267854ds1.htm , (accessed 02-12-2021).
- https://www.biofuelsdigest.com/bdigest/2012/07/20/coskata-switches-from-biomass-to-natural-gas-to-raise-100m-in-natgas-oriented-private-placement/ , (accessed 23-07-2020).
- https://www.biofuelsdigest.com/bdigest/2016/01/24/coskatas-technology-re-emerges-as-synata-bio/ , (accessed 01-12-2021).
- https://www.lanzatech.com , (accessed 11-12-2020).
- https://www.sekisuichemical.com/news/2020/1364066_38530.html , (accessed 01-12-2021).
- https://www.biofuelsdigest.com/bdigest/2018/03/08/commercial-time-aemetis-embarks-on-158-million-cellulosic-ethanol-project-in-california/ , (accessed 05-08-2021).
- https://www.biofuelsdigest.com/bdigest/2020/05/18/new-lords-of-circular-carbon-arcelormittal-eu-complete-financing-of-steelanol-project/ , (accessed 21-08-2020).
- R. Datta, M. Enzien, R. Hickey and W. Levinson, US2014/0273120A1, 2014.
- Coskata Inc., WO2014/113209A1, 2014.
- https://www.lanzatech.com/2012/06/04/23-butanediol-bio-based-23-bdo-set-for-2014-sales/ , (accessed 24-08-2020).
- Lanzetech New Zealand Ltd., US2012/0252083A1, 2012.
- Lanzatech New Zealand Ltd., EP2630247B1, 2013.
- Lanzatech New Zealand Ltd., EP2855662B1, 2018.
- Lanzatech New Zealand Ltd., US9994878B2, 2018.
- https://www.technipfmc.com/media/news/2020/07/technipfmcs-hummingbird-ethylene-technology-selected-by-lanzatech? , (accessed 21-08-2020).
- https://www.lanzatech.com/2020/06/02/lanzajet-takes-off/ , (accessed 21-08-2020).
- https://ilbioeconomista.com/2020/07/08/photanol-and-renolit-joined-forces-to-develop-polymers-by-using-co2/ , (accessed 03-12-2021).
- https://innovationorigins.com/en/large-scale-co2-conversion-to-bioplastic-dutch-photanol-factory-in-delfzijl-takes-first-step/ , (accessed 03-12-2021).
- https://photanol.com/ , (accessed 03-12-2021).
- https://www.nouryon.com/news-and-events/features-overview/photanol-shining-praise-for-a-sun-powered-partnership/ , (accessed 03-12-2021).
- https://www.ethanolproducer.com/articles/12629/algenol-ethanol-plant-will-have-capacity-to-produce-18-mmgy , (accessed 03-12-2021).
- https://www.fuelsandlubes.com/algenol-and-reliance-launch-algae-production-module-2/ , (accessed 03-12-2021).
- https://www.ipsnews.net/2009/11/mexico-has-big-plans-for-ethanol-from-algae/ , (accessed 03-12-2021).
- https://www.technologyreview.com/2009/07/16/<?ccdc_no 211566?>211566<?ccdc END?>/dow-to-test-algae-ethanol/ , (accessed 03-12-2021).
- P. Strong, B. Laycock, S. Mahamud, P. Jensen, P. Lant, G. Tyson and S. Pratt, Microorganisms, 2016, 4, 11 CrossRef PubMed.
- L.-Y. Liu, G.-J. Xie, D.-F. Xing, B.-F. Liu, J. Ding and N.-Q. Ren, Environ. Sci. Ecotechnol., 2020, 2, 100029 CrossRef.
- https://www.biofuelsdigest.com/bdigest/2015/07/13/one-billion-pounds-of-carbon-negative-plastic-made-from-greenhouse-gas-and-pure-air/ , (accessed 02-12-2021).
- Newlight Technologies LLC, US10378030B2, 2019.
- Newlight Tech. Inc., US10941426B2, 2021.
- https://www.bioplasticsmagazine.com/en/news/meldungen/20200925-Newlight-Technologies-opens-new-commercial-scale-AirCarbon-production-facility.php , (accessed 02-12-2021).
- https://bioplasticsnews.com/2015/07/29/vinmar-signs-polyhydroxyalkanoates-pha-offtake-deal-with-newlight-technologies/ , (accessed 02-12-2021).
- https://cen.acs.org/business/biobased-chemicals/PHA-biopolymer-whose-time-finally/97/i35 , (accessed 02-12-2021).
- NL Agency, Sustainability requirements for biofuels and biomass for energy in EU and US regulatory frameworks, 2011.
- https://www.epa.gov/renewable-fuel-standard-program , (accessed 08-04-2022).
- R. Stolf and A. P. R. de Oliveira, Eng. Agríc., 2020, 40, 243–248 CrossRef.
- F. Abnisa and P. A. Alaba, J. Environ. Chem. Eng., 2021, 9, 106593 CrossRef CAS.
- P. Quicker, S. Consonni and M. Grosso, Waste Manage. Res., 2020, 38, 481–484 CrossRef PubMed.
- Plastics Europe, Plastics - the facts 2021: An analysis of European plastics production, demand and waste data, 2021 Search PubMed.
- I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. de Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS PubMed.
- S. Thiyagarajan, E. Maaskant-Reilink, T. A. Ewing, M. K. Julsing and J. van Haveren, RSC Adv., 2021, 12, 947–970 RSC.
- G. W. Coates and Y. D. Y. L. Getzler, Nat. Rev. Mater., 2020, 5, 501–516 CrossRef CAS.
- J. Payne and M. D. Jones, ChemSusChem, 2021, 14, 4041–4070 CrossRef CAS PubMed.
- A. F. Sousa, R. Patrício, Z. Terzopoulou, D. N. Bikiaris, T. Stern, J. Wenger, K. Loos, N. Lotti, V. Siracusa, A. Szymczyk, S. Paszkiewicz, K. S. Triantafyllidis, A. Zamboulis, M. S. Nikolic, P. Spasojevic, S. Thiyagarajan, D. S. van Es and N. Guigo, Green Chem., 2021, 23, 8795–8820 RSC.
- M. D. Leavell, A. H. Singh and B. B. Kaufmann-Malaga, Curr. Opin. Biotechnol., 2020, 62, 22–28 CrossRef CAS PubMed.
- L. Wang, S. Dash, C. Y. Ng and C. D. Maranas, Synth. Syst. Biotechnol., 2017, 2, 243–252 CrossRef PubMed.
- I. Calinescu, A. Vlaicu, P. Chipurici, D. Ighigeanu and V. Lavric, Chem. Eng. Process.: Process Intensif., 2018, 126, 16–22 CrossRef CAS.
- J. S. Sravan, S. K. Butti, O. Sarkar and S. V. Mohan, in Microbial Electrochemical Technology, ed. S. V. Mohan, S. Varjani and A. Pandey, Elsevier, Amsterdam, NL, 2019, pp. 723–737 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |