Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Liquid electrolyte development for low-temperature lithium-ion batteries
Dion
Hubble
 a,
David Emory
Brown
ab,
Yangzhi
Zhao
a,
Chen
Fang
a,
David Emory
Brown
ab,
Yangzhi
Zhao
a,
Chen
Fang
 a,
Jonathan
Lau
a,
Bryan D.
McCloskey
a,
Jonathan
Lau
a,
Bryan D.
McCloskey
 b and
Gao
Liu
b and
Gao
Liu
 *a
*a
aEnergy Storage and Distributed Resources Division, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720, USA. E-mail: gliu@lbl.gov
bDepartment of Chemical and Biomolecular Engineering, University of California, Berkeley, CA 94720, USA
First published on 14th January 2022
Abstract
Lithium-ion batteries (LIBs) power virtually all modern portable devices and electric vehicles, and their ubiquity continues to grow. With increasing applications, however, come increasing challenges, especially when operating conditions deviate from room temperature. While high-temperature performance and degradation has been extensively studied in LIBs, sub-zero Celsius performance has received less attention, despite being critical for batteries in transportation roles. Although many individual processes contribute to the capacity loss commonly observed in LIBs at low temperatures, most of them are governed to some extent by the non-aqueous liquid electrolyte present throughout the cell interior. Therefore, electrolyte engineering presents an unparalleled opportunity to study and address the fundamental causes of low-temperature failure. In this review, we first briefly cover the various processes that determine lithium-ion performance below 0 °C. Then, we outline recent literature on electrolyte-based strategies to improve said performance, including various additives, solvents and lithium salts. Finally, we summarize these findings and provide some perspectives on the current state of the field, including promising new areas of investigation.
Broader contextThe upcoming switch to renewable energy across the globe will depend heavily on lightweight, reliable energy storage being readily available. As of now, the best candidate for the job is the lithium-ion battery (LIB). Nearly all portable electronics and electric vehicles already contain lithium-ion chemistry in some form, and LIBs have garnered serious consideration for expanded roles in aerospace and power distribution. However, as their applications increase in significance, so too do their drawbacks, including their notorious temperature sensitivity. LIB operating characteristics at elevated temperatures (>40 °C) have been extensively studied, but low-temperature (<0 °C) performance has received much less attention, despite its increasing relevance. For example, widespread adoption of electric vehicles will require them to function during winter just as reliably as during summer. In the course of our research, we have found that LIB operation at low temperature is often governed most strongly by electrolyte composition. Therefore, we present this review on liquid electrolyte design for LIBs under low-temperature conditions. It is our hope that this article will inspire additional advancements in the field so that clean energy will become available to more people in more environments than ever before. |
Introduction
It is difficult to overstate how thoroughly the lithium-ion battery, or LIB, has permeated life in the 21st century thus far. At the time of this writing, nearly all portable electronics and electric vehicles contain some form of LIB, and more recently they have garnered serious consideration for expanded roles in aerospace1 and power distribution.2,3 However, as their applications increase in significance, so too do their drawbacks, including their notorious temperature sensitivity. LIBs function acceptably between 0–40 °C, but they encounter severe problems under harsher conditions.4–6 Performance beyond the high end of this range has been extensively studied, including the mechanisms of high-temperature battery degradation and its consequences, ranging from capacity fade to thermal runaway.7,8 By comparison, the low-temperature performance of LIBs has received considerably less attention, especially in the preceding 15 years. Nonetheless, this topic continues to gain relevance, as emerging applications in energy storage require increasing tolerance to frigid conditions. For example, widespread adoption of electric vehicles will require them to function during winter just as reliably as during summer.Successful LIB operation depends on a complex web of physical and chemical processes that must function harmoniously; as a result, it is hardly possible to change one aspect of battery design without affecting many others. This is especially true for the non-aqueous liquid electrolytes used in LIBs, which contact nearly all internal surface area. Indeed, the early success of lithium-ion chemistry was spurred by the discovery that ethylene carbonate (EC) can form a dimensionally-stable solid electrolyte interphase (SEI) on carbon when included as an electrolyte co-solvent.9 On the other hand, organic electrolyte chemistry may also contribute to capacity loss,10 poor power density11 and flammability,12 among other concerns. Therefore, the prudent battery scientist should view electrolyte design as a “double-edged sword” with the potential to address multiple points of failure, but conversely introduce multiple new complications.
In light of its key role in LIB function, it should be unsurprising that early studies identified electrolyte chemistry as one of the main governing factors in low-temperature performance,13 a consensus that largely remains to this day. In an effort to inspire renewed research in this area, we hereby present this concise review of liquid electrolyte design for low-temperature LIBs. We first summarize the various major processes that determine lithium-ion performance metrics at low temperature (<0 °C) and how the electrolyte may influence them. Then, we review research geared towards improving low-temperature performance using electrolyte engineering (additives, solvents and salts; Fig. 1), focusing primarily on recent reports (2010–now). While the majority of this work covers LIBs with traditional active materials – graphite (Gr) or Li4Ti5O12 (LTO) anodes plus lithium transition–metal oxide or phosphate cathodes – we also include a brief summary of reports related to silicon anodes at low temperature, since this material is a likely candidate for next-generation commercial LIBs. Finally, we conclude with some suggestions as to future directions of this field.
Challenges in low-temperature LIB performance
While the processes that limit low-temperature LIB performance are myriad, their effect is commonly the same: capacity loss. The usable capacity of a fresh lithium–ion cell between predefined voltage limits generally decreases with decreasing temperature – especially below −20 °C – sometimes to less than 25% of its room-temperature value. This capacity loss is usually reversible when the temperature is raised back to normal conditions. On the other hand, irreversible capacity loss may also occur if the cell is charged at low temperature, due to the plating of lithium metal on the anode surface, which is only partially recoverable.14 Both types of capacity loss may be largely attributed to increased internal resistance of the cell at sub-zero temperatures. This internal resistance has many components, each corresponding to a different physical process associated with Li+ transport across the cell (Fig. 2).15 Electrolyte composition can affect many of these processes, as we discuss below.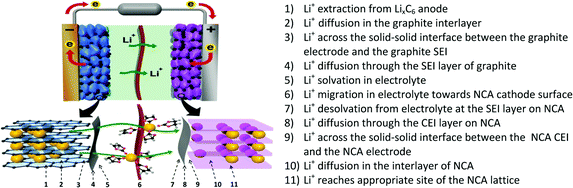 | ||
| Fig. 2 Complete overview of lithium transport pathway in a graphite‖LiNi0.80Co0.15Al0.05O2 full cell. The identity of the rate-limiting step may change with temperature, depending on the active material properties and electrolyte composition. Reprinted with permission from ref. 15. Copyright 2017 American Chemical Society. | ||
Bulk electrolyte considerations
Within the operating range of LIBs, electrolytic conductivity universally decreases with temperature – a trend primarily attributable to viscosity effects. Naturally, this causes the internal resistance to rise. Because viscosity correlates with EC content, it is generally accepted that this solvent should be minimized in cells designed for low temperature; however, this task has proven challenging due to the vital role of EC in low-resistance and stable SEI formation, causing lean-EC electrolytes to commonly suffer from reduced cycling stability and Coulombic efficiency. Additionally, bulk ohmic resistance is rarely the largest component of total battery impedance,† meaning that conductivity alone does not typically predict low-temperature performance with any degree of accuracy.
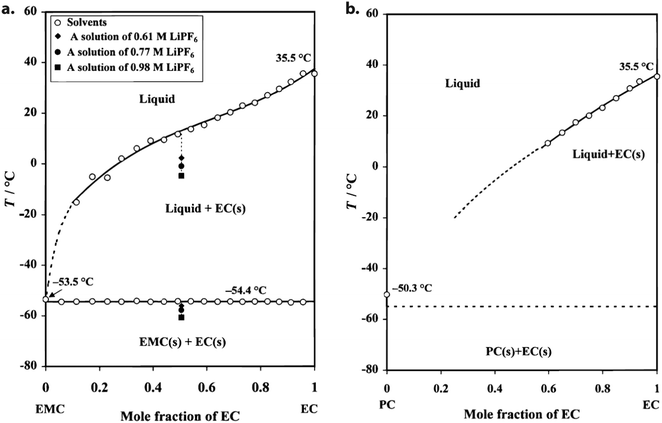 | ||
| Fig. 3 (a) Liquid–solid phase diagram of EMC-EC. The open dots represent measured data from which the solubility curve and the solidus line have been obtained through data fitting. The dotted curve is an estimated extension of the measured liquidus curve. The closed dots represent measured data for three different solutions of LiPF6 in an EMC-EC solvent, plotted to demonstrate the change of the phase transition temperatures with the concentration of a lithium salt. (b) Liquid–solid phase diagram of PC–EC. The dotted solubility curve and the solidus line are both estimated due to the tendency of PC-rich mixtures to supercool, precluding accurate measurement. Compared to EMC, PC reduces liquidus temperature more effectively when present >20 mol% in the mixture. Reprinted from ref. 19, copyright The Electrochemical Society. Reproduced by permission of IOP Publishing Ltd. All rights reserved. | ||
Interfacial considerations
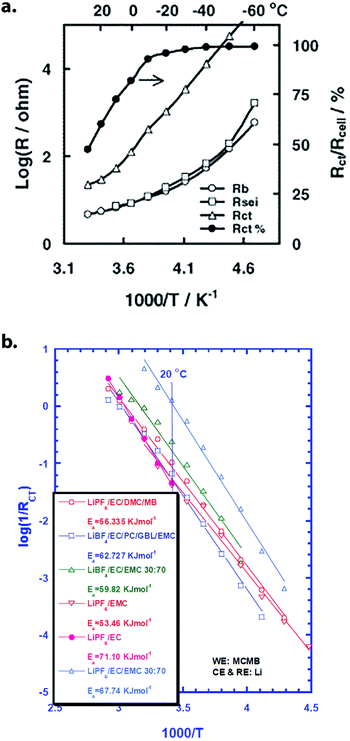 | ||
| Fig. 4 (a) Variation of bulk electrolyte resistance (Rb), SEI resistance (RSEI), charge-transfer resistance (Rct) and the percentage of total resistance made up of Rct (Rct%) with temperature in a commercial Li-ion cell discharged to 3.87 V. Reprinted from ref. 31 with permission from Elsevier. (b) Arrhenius plot of Rctvs. temperature for Li‖MCMB half cells with varying electrolytes. Extracted Ea values (kJ mol−1) are included in the legend. Reprinted from ref. 32, copyright The Electrochemical Society. Reproduced by permission of IOP Publishing Ltd. All rights reserved. | ||
Recent works by various authors have strengthened the conclusion that Li+ desolvation is the rate-limiting process at low temperature (Fig. 5a). Xu and coworkers corroborated previous results by demonstrating that Ea,ct on LTO varies with the electron donicity of the solvent, which is a major factor in solvation strength. These authors obtained values of 40 kJ mol−1 for 1 M LiTFSI in tetrahydrofuran vs. 52.5 kJ mol−1 for 1 M LiPF6 in EC/EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.34 Their report also noted similar resistance trends on graphite anodes, but with values consistently offset upwards by ∼20 kJ mol−1; the authors attributed this to the additional effect of ion transport through a resistive SEI, which appeared at a similar resonant frequency and was difficult to separate out. Similarly, Li et al. studied Li‖Gr and Li‖LiFePO4 half cells with identical electrolytes, finding graphite to be limiting in both charge transfer and interfacial transport (although electrolyte design had a strong effect).35
7.34 Their report also noted similar resistance trends on graphite anodes, but with values consistently offset upwards by ∼20 kJ mol−1; the authors attributed this to the additional effect of ion transport through a resistive SEI, which appeared at a similar resonant frequency and was difficult to separate out. Similarly, Li et al. studied Li‖Gr and Li‖LiFePO4 half cells with identical electrolytes, finding graphite to be limiting in both charge transfer and interfacial transport (although electrolyte design had a strong effect).35
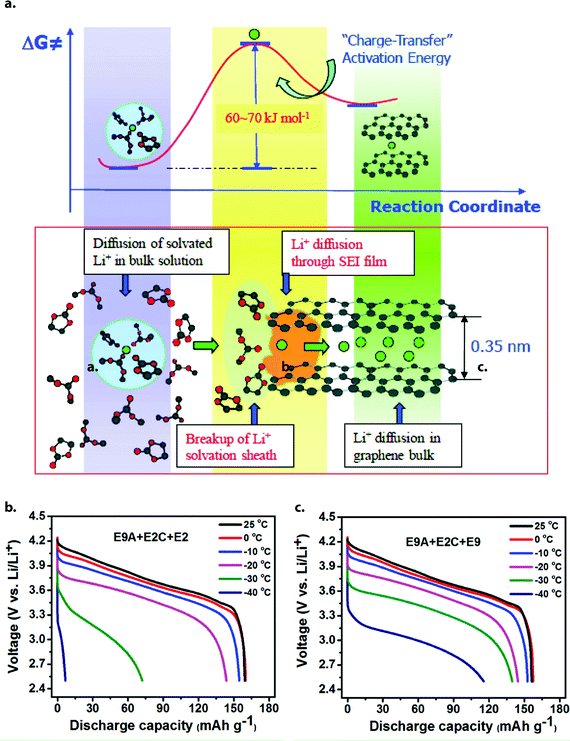 | ||
| Fig. 5 (a) Schematic depicting Li+ desolvation at the graphite interface, widely proposed to be the rate-limiting step in charge transfer at low temperature. Reprinted with permission from ref. 34. Copyright 2010 American Chemical Society. (b and c) Discharge profiles of Gr‖NCA cells containing identical pre-cycled electrodes, but different electrolytes. E9A refers to an anode that has undergone formation cycles in E9 electrolyte (an EC-lean formulation), while E2C refers to a cathode that has undergone formation in E2 (an EC-rich formulation). Despite identical pre-formed interphase layers, major differences in discharge capacity below −20 °C are observed, consistent with lower Rct in the EC-lean electrolyte E9. Reprinted/adapted with permission from ref. 15. Copyright 2017 American Chemical Society. | ||
In 2017, a team from Pacific Northwest National Laboratory systematically compared carbonate electrolytes of varying composition in Gr‖Gr, NCA‖NCA and LTO‖LTO symmetric cells, thereby eliminating any competing effects from Li metal or multiple electrodes.15 Despite the disparate chemical structures of these materials and their interfaces, their mid-frequency EIS responses were nearly identical at −40 °C, implying that ion desolvation – their only common factor – is the rate-limiting process in each case. Furthermore, when graphite anodes were subjected to an SEI-formation process in one electrolyte, then re-assembled into Gr‖NCA full cells with another electrolyte, the discharge capacity of the cells at −20 °C had virtually no correlation with the former electrolyte composition, but correlated strongly with the latter (Fig. 5b and c). The overall lesson appears to be that electrolyte design affects low temperature performance primarily by determining the makeup of Li-ion solvation shells – the breakup of which requires the greatest energy input out of all simultaneous processes during operation.
Why, then, does this misconception persist? One likely reason is that RSEI and Rct can be difficult to distinguish. The impedances associated with interphase ion transport and charge transfer often overlap quite substantially in characteristic frequency, which can cause them to appear as a single semicircle in Nyquist plots generated by EIS (Fig. 6a and b). Attempting to extract separate, accurate resistance values from such a feature becomes an exercise in futility, and it is easier to refer to a single, combined value for “interfacial resistance.” Another possible reason is that RSEI, while not the largest component of total resistance, may still compose a sufficient fraction of it to notably affect performance. An excellent example of this can be found in the recent work of Liu et al., who systematically studied interphase-forming additives, while keeping the base electrolyte constant.38 Under such conditions, Rct should hardly change, but capacity improvements of up to 7% were observed at −40 °C and C/5 rate, which correlated well to changes in interphase chemistry/thickness with different additives.
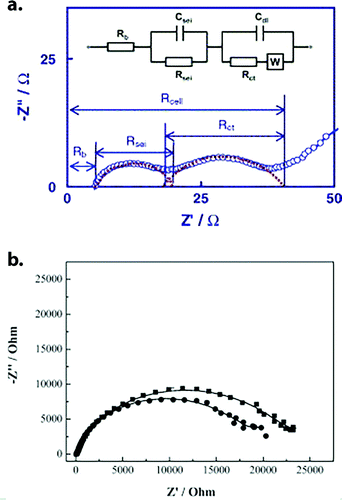 | ||
| Fig. 6 (a) Nyquist plot of Li-ion cell impedance showing relatively well-separated semicircles. In this case, both the individual values of RSEI and Rct can be reasonably determined (although they each represent lumped values for cathode and anode processed). Reprinted from ref. 31 with permission from Elsevier. (b) Nyquist plot of Li‖MCMB half-cell impedance, where only one semicircle is distinguishable. In this case RSEI cannot be separated from Rct, either due to similar time constants for both processes or because one is much larger than the other. Additional context is required to interpret the combined “interfacial resistance.” Reprinted from ref. 66 with permission from Elsevier. | ||
The third possible answer is that interfacial chemistry may not be totally separable from charge-transfer kinetics. After all, if Li+ desolvation at the electrolyte/interphase boundary is responsible for Rct, it follows that surface chemistry may play a role in that process – partially coordinating the ion as its solvation shell strips away, for example. However, such an effect would be very difficult to study, especially given how little we still know about SEI/CEI chemistry on the molecular scale, which is notoriously difficult to characterize.39–41 Nonetheless, the fact remains that changes to SEI composition are often reported to produce outsized effects on low-temperature capacity (vide infra). Therefore, when posed the question “Exactly what role does SEI/CEI play in low-temperature performance?”, we can unfortunately provide no definitive answer other than “It depends.”
That being said, even if RSEI does not ordinarily limit low-temperature capacity, the stabilizing role of the interphase cannot be ignored. In order to successfully cycle a graphite-based LIB at any temperature, the electrolyte must form a dimensionally-stable layer to protect against continued decomposition, as well as exfoliation. Therefore, the issue is often less about optimizing interphase composition for sub-freezing performance directly, and more about adjusting other factors (freezing point, charge transfer resistance, etc.) without compromising the robustness of anode/cathode interphases. This is of special concern for low-liquidus-point electrolytes with little-to-no EC content, which must rely on additives for stable cycling.
Lithium plating
Our discussion thus far has centered on discharge capacity as the primary metric by which low-temperature performance is measured, with the implicit assumption that charging is done at 25 °C or higher. This is because charging a graphite-anode LIB at lower than 25 °C and/or at a high rate introduces significant risk of lithium metal plating, a major safety hazard and cause of premature capacity fade. Much of the work to detect the onset of – and to mitigate – lithium metal deposition on graphite anodes has focused on achieving fifteen-minute charge times at room temperature. This has been well summarized and described in other reviews, including Waldman et al.,42 Liu et al.43 and Tomaszewska et al.;11 the latter review also includes some analysis of work to enable low-temperature LIB operation. While “extreme fast charging” at around 25 °C has numerous issues, low-temperature charging brings many similar challenges associated with undesired lithium metal nucleation and growth, even at relatively slow charging rates.During “typical,” facile charging of a graphite-anode LIB, Li+ ions are reduced at the graphite anode and intercalate between individual graphene layers. However, if the electrochemical potential of the graphite particle dips below 0 V vs. Li/Li+, lithium metal formation becomes thermodynamically possible. Graphite is particularly susceptible to this phenomenon because its operating potential (under open-circuit conditions) is only ∼100 mV vs. Li/Li+.44 When current is applied, anode potential may further drop into the lithium plating regime, given sufficiently-large overpotential at the graphite/electrolyte interface (Fig. 7a). As discussed above, detrimental overpotentials can arise from a number of factors, including ohmic losses—which scale with applied charging current—as well as mass transport and kinetic limitations. Solid-state transport limitations (vide infra) will increase at a high graphite SOC; as the graphite fills with Li, it becomes more difficult to insert additional Li atoms. The large concentration gradients that form at high charging rates, i.e. polarization overpotentials, also contribute to this drop.23,45 Kinetic overpotentials are additionally impacted by such concentration gradients, as charge transfer resistance is dependent on the surface concentration of Li+. Mass transport and kinetic overpotentials will also both increase with decreasing temperature, generally following Arrhenius-type dependencies. Waldmann et al. used a 3-electrode cell to demonstrate this temperature-dependent decrease in the anode potential;46 during low-temperature charging, these overpotentials led to large amounts of Li plating at relatively low charging rates. A fraction of such Li metal deposits cannot be reversibly stripped, instead forming “dead” lithium which includes electronically-isolated metallic Li and graphite, as well as degradation products from the reaction of electrolyte with metallic Li. The formation of dead Li necessarily means a reduction in cyclable Li capacity; this has been quantified as a function of rate and cycle life at room temperature using destructive mass spectrometry titration.47
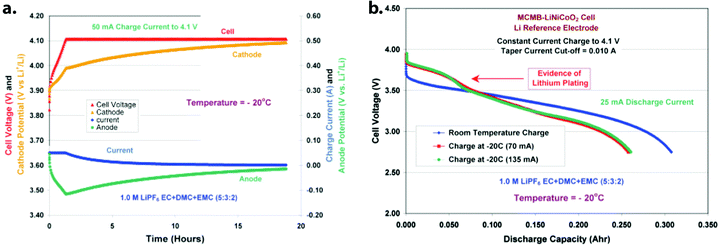 | ||
| Fig. 7 (a) Electrode/cell voltage profiles during −20 °C charge of a MCMB‖LiNi0.8Co0.2O2 cell with a Li reference electrode. Note that the anode potential drops below 0 V vs. Li/Li+ almost immediately and remains there throughout charging, making Li plating possible. (b) Voltage–capacity plot of MCMB‖LiNi0.8Co0.2O2 cells during −20 °C discharge following various charge protocols. A characteristic upper voltage plateau is present in the cells charged at −20 °C, indicating that lithium plating has occurred. Their discharge capacity is also reduced in comparison to the room-temperature-charged cell. Reprinted from ref. 48, copyright The Electrochemical Society. Reproduced by permission of IOP Publishing Ltd. All rights reserved. | ||
While the majority of literature has focused on fast charging at ambient temperatures (>20 °C), there have still been numerous studies characterizing Li plating on graphite during low-temperature operation. Electrochemical methods have been used to detect when Li plating has occurred at low temperature, including observation of a stripping plateau during discharge of spiral-rolled, 3-electrode LIBs (300–400 mA h, Gr‖NCO‖Li Ref.) operated at −40 °C (Fig. 7b),48 along with dV/dQ analysis of discharge curves for LIBs (2.5 A h, 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cylindrical Gr‖LFP) cycled at temperatures down to −30 °C.14,49 The latter technique relies on the presence of the stripping plateau seen in the former. These dV/dQ studies also reveal an impedance rise after low-temperature charging, which the authors attributed to SEI growth from Li metal reacting with the electrolyte. This reaction of plated Li with the electrolyte is also noted by Ng et al. as the primary culprit for significant gas formation in their LIBs (50 A h, Gr‖NMC532 prismatic) cycled at −29 °C. This gas formation was shown to cause detrimental additional stresses on the electrodes, leading to eventual cell failure.50
650 cylindrical Gr‖LFP) cycled at temperatures down to −30 °C.14,49 The latter technique relies on the presence of the stripping plateau seen in the former. These dV/dQ studies also reveal an impedance rise after low-temperature charging, which the authors attributed to SEI growth from Li metal reacting with the electrolyte. This reaction of plated Li with the electrolyte is also noted by Ng et al. as the primary culprit for significant gas formation in their LIBs (50 A h, Gr‖NMC532 prismatic) cycled at −29 °C. This gas formation was shown to cause detrimental additional stresses on the electrodes, leading to eventual cell failure.50
Still, simple optical inspection of the graphite electrode remains the most common technique for Li plating detection upon post-mortem analysis. This necessarily requires enough Li to have plated for visual inspection to be possible. However, certain groups have expanded upon this relatively simple analysis to allow for more robust diagnoses; one of the previously-mentioned studies using dV/dQ and impedance spectroscopy also analyzed the deposited Li layer thickness to quantify the amount of Li plated.14 Unfortunately, this still requires significant Li plating to have already occurred. Waldmann et al. have utilized optical inspection, combined with capacity fade monitoring, to label Li plating as the primary capacity fade mechanism for LIBs (1.5 A h, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cylindrical Gr‖NMC111 + LMO) cycled at temperatures down to −20 °C, relative to cells cycled up to 70 °C.51 Ghanbari et al. used a more specialized technique—glow discharge optical emission spectroscopy—to characterize Li plating in cells, which showed homogeneous Li plating in cells cycled at −20 °C (2.5 A h, 26
650 cylindrical Gr‖NMC111 + LMO) cycled at temperatures down to −20 °C, relative to cells cycled up to 70 °C.51 Ghanbari et al. used a more specialized technique—glow discharge optical emission spectroscopy—to characterize Li plating in cells, which showed homogeneous Li plating in cells cycled at −20 °C (2.5 A h, 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650-format Gr‖LFP) relative to the “island” deposits formed in cells cycled at 45 °C (16 A h, Gr‖NMC).52 It should be pointed out, though, that some of the differences in plating morphology could be attributed to different cell geometries. This optical emission technique can also discriminate between aging related to SEI growth vs. that related to Li plating. Each of these studies seems to suggest that Li plating is especially prevalent at low temperatures, and that it is the primary culprit for capacity fade of LIBs repeatedly charged under such conditions.
650-format Gr‖LFP) relative to the “island” deposits formed in cells cycled at 45 °C (16 A h, Gr‖NMC).52 It should be pointed out, though, that some of the differences in plating morphology could be attributed to different cell geometries. This optical emission technique can also discriminate between aging related to SEI growth vs. that related to Li plating. Each of these studies seems to suggest that Li plating is especially prevalent at low temperatures, and that it is the primary culprit for capacity fade of LIBs repeatedly charged under such conditions.
As others have noted, though, no technique to-date has been able to reliably detect the onset of Li plating in LIBs with high general sensitivity. dV/dQ analysis relies on the presence of a stripping plateau which is not always present, even when lithium has plated,53 and requires a slow discharge immediately after charging. Incidentally, a concise summary of all differential voltage analysis work performed for low temperature LIB applications thus far may be found in ref. 47 above. Optical inspection – perhaps the most universally-used technique – necessarily requires a large amount of Li deposition to have occurred; therefore, the damage of substantial, irreversible capacity loss must already be done. Differential open-circuit voltage analysis has recently shown some promise as a more general method for plating detection,54 but requires further validation. Li plating detection techniques and their limitations, as well as ideas for Li plating mitigation, are more-thoroughly discussed in the previously mentioned fast charging and Li plating review articles. However, it should be emphasized that this current limitation in the field will need to be addressed in order to make low-temperature LIBs operation—where Li plating more readily occurs—a reality.
As a final comment, it should be noted that, under realistic operating conditions, i.e. large-format cells assembled in packs, the situation may be less bleak than it seems. Several authors have proposed tailoring battery pack design and/or charging protocols to take advantage of self-heating, so that overpotential is minimized by the end of charge and lithium plating is mitigated. Details are outside the scope of this review but may be readily found elsewhere.55,56
Non-electrolyte factors
There are, of course, physical processes relevant to low-temperature performance which are altogether divorced from electrolyte choice. While a detailed discussion falls outside the scope of this review, these factors may sometimes play a role in observed performance and, therefore, should be kept in mind. One such process is electron transport, which depends not only on bulk electrical conductivities (often quite low for cathode materials), but also on particle–particle interfacial contact. Luckily, modern electrode designs minimize these problems quite effectively, to the point where internal electrical resistance is generally negligible.57Solid-state properties of the cathode and anode active materials also influence sub-zero behavior, most obviously through the dependence of surface area (and, thus, effective current density) on particle size. Indeed, an early study found that coke anode capacity at ≤−20 °C was improved when average particle size was reduced from 25 μm to 6 μm.58 More subtly, limited solid-state diffusion of lithium naturally causes the surface concentration of lithium to differ from the bulk when current is non-zero, which produces concentration polarization. The associated polarization resistance, although usually mild at room temperature and low rate, may become more significant with dropping temperature due to a decrease in lithium-ion solid-state diffusion coefficient.59 A coupled electrochemical-thermal modeling study performed by Ji, Zhang and Wang has demonstrated that the limiting factors in large-format cells (2.2 A h 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cylindrical) may be significantly different than in smaller cells due to non-isothermal conditions.60 At −20 °C and low-rate discharge (0.001C), their model indeed predicted that interfacial kinetics dominate internal resistance; however, at higher rate (1C), self-heating effects reduced the influence of kinetic factors very early in the discharge process, whereas solid particle resistance at the anode grew continuously, becoming dominant towards the end of discharge. In all cases, accounting for self-heating resulted in higher capacity than predicted by an isothermal model. This result should caution the reader against generalizing any particular low-temperature result to batteries of dissimilar size or geometry.
650 cylindrical) may be significantly different than in smaller cells due to non-isothermal conditions.60 At −20 °C and low-rate discharge (0.001C), their model indeed predicted that interfacial kinetics dominate internal resistance; however, at higher rate (1C), self-heating effects reduced the influence of kinetic factors very early in the discharge process, whereas solid particle resistance at the anode grew continuously, becoming dominant towards the end of discharge. In all cases, accounting for self-heating resulted in higher capacity than predicted by an isothermal model. This result should caution the reader against generalizing any particular low-temperature result to batteries of dissimilar size or geometry.
Progress in low-temperature electrolyte design
When formulating a liquid electrolyte, there are three general components to adjust: additives, solvents and salts. The lines between these descriptors sometimes blur, as a particular chemical species may fall into multiple categories. For the sake of this review, we will consider a “solvent” to be a component with a melting point ≤40 °C that is present at >5 wt% concentration in the final mixture. Likewise, we will consider a “primary salt” to be a dissociable ionic substance containing Li+, which is present at either >0.5 M or >5 wt% in the final mixture. Any reagent that falls outside these guidelines – for example, an ionic substance that is solid at room temperature and does not contain lithium, or a molecular liquid added to merely 2 wt% – would be considered an additive. These guidelines are largely arbitrary and chosen purely for convenience. Note that a single substance could be considered an additive in one study and a primary salt or solvent in another; the difference depends solely on the concentrations used. For the sake of relevance, we exclude LiPF6 and EC/DEC/DMC/EMC from receiving focused discussion, since these materials are all well-studied and used commercially. More background on these standard electrolyte materials can be found in existing reviews.13,61,62Additives
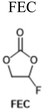
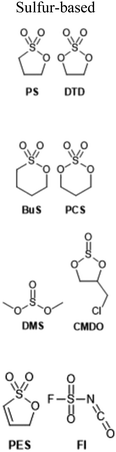
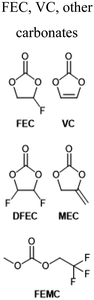
Electrolyte additives, by nature of their broad definition, take many forms and produce a variety of effects, such as reduced gas evolution or overcharge protection.63 Most commonly, however, additives are intended to improve the cycling performance of LIBs, usually by stabilizing electrode interfaces to chemical or morphological changes over time. Given the strong correlation between electrode interfacial chemistry and low temperature performance, it is unsurprising that many additives have been investigated specifically for their effects below 0 °C. Additive formulations used by the references cited here may be found summarized in Table 1 along with their structures.| Additive | Electrolyte | Ref. |
|---|---|---|
|
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w) + 0.05 M CsPF6 + various additives 8 w/w) + 0.05 M CsPF6 + various additives |
38 |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v) + 2 vol% FEC 8 v/v) + 2 vol% FEC |
66 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 1 vol% FEC 3 v/v) + 1 vol% FEC |
67 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 2 vol% FEC 3 v/v) + 2 vol% FEC |
||
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 5 vol% FEC 3 v/v) + 5 vol% FEC |
||
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 5 vol% FEC 1 v/v) + 5 vol% FEC |
69 | |
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 vol% CMDO + 5 vol% FEC 1 v/v) + 2 vol% CMDO + 5 vol% FEC |
||
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 3 vol% EC + 5 vol% FEC 1 v/v) + 3 vol% EC + 5 vol% FEC |
||
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 vol% CMDO + 3 vol% EC + 5 vol% FEC 1 v/v) + 2 vol% CMDO + 3 vol% EC + 5 vol% FEC |
||
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 2 wt% VC + 5 wt% FEC 20 v/v) + 2 wt% VC + 5 wt% FEC |
86 | |
1 M LiPF6, EC/PC/EMC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) + 2 wt% FEC 3 w/w) + 2 wt% FEC |
87 | |
|
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w) + 0.05 M CsPF6 + various additives 8 w/w) + 0.05 M CsPF6 + various additives |
38 |
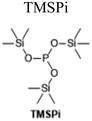
1 M LiPF6, MA/EC/DEC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 1 wt% TMSPi + 1 wt% PCS 1 v/v) + 1 wt% TMSPi + 1 wt% PCS |
74 | |
|
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w) + 0.05 M CsPF6 + various additives 8 w/w) + 0.05 M CsPF6 + various additives |
38 |
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 vol% CMDO 1 v/v) + 2 vol% CMDO |
69 | |
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 vol% CMDO + 5 vol% FEC 1 v/v) + 2 vol% CMDO + 5 vol% FEC |
||
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 vol% CMDO + 3 vol% EC + 5 vol% FEC 1 v/v) + 2 vol% CMDO + 3 vol% EC + 5 vol% FEC |
||
1 M LiPF6, MA/EC/DEC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 1 wt% TMSPi + 1 wt% PCS 1 v/v) + 1 wt% TMSPi + 1 wt% PCS |
74 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) + 1 wt% BuS 3 w/w) + 1 wt% BuS |
75 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 1 vol% BuS 3 v/v) + 1 vol% BuS |
76 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 2 vol% BuS 3 v/v) + 2 vol% BuS |
||
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 5 vol% BuS 3 v/v) + 5 vol% BuS |
||
0.9 M LiDFOB/LiBF4 (5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w), EC/DMS/EMC (1 1 w/w), EC/DMS/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
77 | |
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 0.5 wt% DMS 2 w/w) + 0.5 wt% DMS |
78 | |
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 0.5 wt% DTD 2 w/w) + 0.5 wt% DTD |
||
1 M LiPF6, EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 1 wt% FI 1 v/v) + 1 wt% FI |
80 | |
1 M LiPF6, EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 wt% FI 1 v/v) + 2 wt% FI |
||
1 M LiPF6, EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 5 wt% FI 1 v/v) + 5 wt% FI |
||
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 3 wt% PES 2 w/w) + 3 wt% PES |
79 | |
|
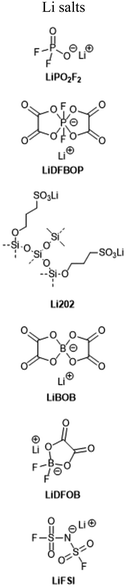
1 M LiPF6, EC/EMC/PC (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) + 1 wt% LiPO2F2 1 w/w) + 1 wt% LiPO2F2 |
83 |
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 2 wt% LiPO2F2 2 w/w) + 2 wt% LiPO2F2 |
84 | |
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 0.25 wt% LiDFBOP 2 w/w) + 0.25 wt% LiDFBOP |
79 | |
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 0.5 wt% LiDFBOP 2 w/w) + 0.5 wt% LiDFBOP |
||
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 1 wt% LiDFBOP 2 w/w) + 1 wt% LiDFBOP |
||
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 2.5 wt% Li202 + 2 wt% VC 20 v/v) + 2.5 wt% Li202 + 2 wt% VC |
85 | |
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 1 wt% Li202 + 2 wt% VC + 5 wt% FEC 20 v/v) + 1 wt% Li202 + 2 wt% VC + 5 wt% FEC |
86 | |
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC 20 v/v) + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC |
||
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 1 wt% Li202 + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC 20 v/v) + 1 wt% Li202 + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC |
||
1 M LiPF6, EC/EMC/MP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v) + 2 wt% [VC or PS] 6 v/v) + 2 wt% [VC or PS] |
104 | |
1 M LiPF6, EC/EMC/MP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v) + 0.1 M [LiBOB, LiDFOB, or LiFSI] 6 v/v) + 0.1 M [LiBOB, LiDFOB, or LiFSI] |
||
| 0.45 M LiTFSI, EMIMFSI + 0.01 M LiBOB | 116 | |
|
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-x w/w) + 0.05 M CsPF6 9-x w/w) + 0.05 M CsPF6 |
15 |
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8-x w/w) + 0.05 M CsPF6 8-x w/w) + 0.05 M CsPF6 |
||
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w) + 0.05 M CsPF6 + various additives 8 w/w) + 0.05 M CsPF6 + various additives |
38 | |
1 M LiPF6, EC/PC/EMC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) + 0.05 M CsPF6 3 w/w) + 0.05 M CsPF6 |
87 | |
1 M LiPF6, EC/PC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-x w/w) + 0.05 M CsPF6 7-x w/w) + 0.05 M CsPF6 |
88 | |
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-x w/w) + 0.05 M CsPF6 9-x w/w) + 0.05 M CsPF6 |
89 | |
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8-x w/w) + 0.05 M CsPF6 8-x w/w) + 0.05 M CsPF6 |
||
More commonly, FEC and other carbonates are used as individual components of a multi-additive mixture. For instance, Liu and coworkers demonstrated a complex, multi-additive system in which 0.5 wt% FEC played a crucial role to stabilize Gr‖NMC111 pouch cells at both −40 °C and 60 °C.38 Another recent report by Wotango et al. demonstrated additive mixtures that included up to 5 wt% FEC.69 Often, this additive is used in large enough concentration to qualify as a “solvent” component; such instances are discussed in the relevant section below.
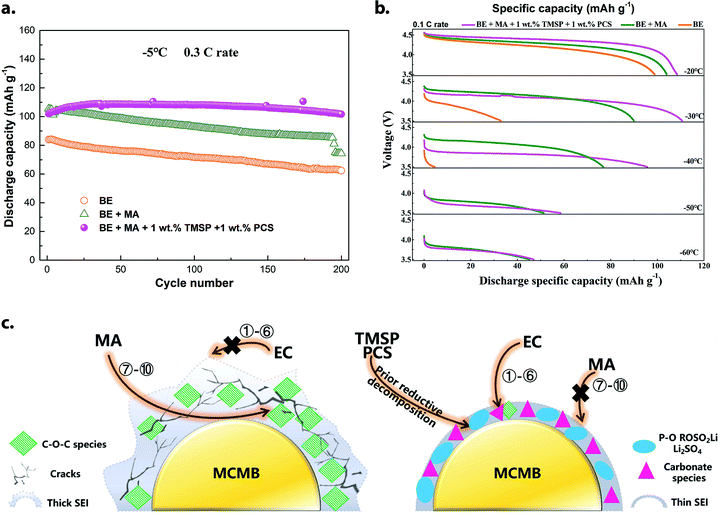 | ||
| Fig. 8 (a) Cycling performance of MCMB‖LiNi0.5Mn1.5O4 full cells (3.5–4.9 V) at −5 °C and 0.3C rate in commercially-available baseline electrolyte (BE), a modified electrolyte containing methyl acetate (BE + MA) and the preceding electrolyte with tris(trimethylsilyl)phosphite and 1,3-propanediol cyclic sulfate additives (BE + MA + 1 wt% TMSP + 1 wt% PCS). (b) Discharge characteristics of the above cell designs at −20 to −60 °C, following a 0.5C charge at room temperature. (c) Proposed working mechanism of the aforementioned phosphite and sulfate additives in this system, based on XPS analysis of cycled MCMB anodes. Formation of a thin, robust SEI prevents continuous decomposition of methyl acetate and resists Li dendrite growth during low-temperature charge. Reprinted/adapted from ref. 74 with permission from Elsevier. | ||
More unique functionalities have also proven useful. Shi and coworkers recently demonstrated fluorosulfonyl isocyanate as a novel sacrificial additive which preferentially reduces to form a low-impedance SEI on graphite.80 The resulting electrodes showed dramatically-improved rate performance in half cells with lithium, especially at low temperatures. Wotango et al. devised a novel chemical derivative of ethylene sulfite: 4-chloromethyl-1,3,2-dioxathiolane-2-oxide, which was used as a one of several additives to significantly improve Li‖MCMB half-cell performance at −10 °C.69
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w improved the relative capacity retention of Gr‖NMC523 cells at −30 °C from 9.6% to 57.9% (Fig. 9).83 Galvanostatic cycling performance was also impacted: cells with control electrolyte experienced rapid capacity fading during repeated 0.5C charge/discharge at −20 °C, while additive-containing cells retained 91% of their initial capacity over 100 such cycles. Most recently, some of the same authors reported 2 wt% LiPO2F2 to improve the temperature range of 4.4 V pouch cells containing an electrolyte of 1 M LiPF6 in EC/EMC 1
1 w/w improved the relative capacity retention of Gr‖NMC523 cells at −30 °C from 9.6% to 57.9% (Fig. 9).83 Galvanostatic cycling performance was also impacted: cells with control electrolyte experienced rapid capacity fading during repeated 0.5C charge/discharge at −20 °C, while additive-containing cells retained 91% of their initial capacity over 100 such cycles. Most recently, some of the same authors reported 2 wt% LiPO2F2 to improve the temperature range of 4.4 V pouch cells containing an electrolyte of 1 M LiPF6 in EC/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w.84 Combined analytical results from both studies indicate that this additive acts as an interface-former on both graphite and NMC surfaces, with the resulting SEI and CEI exhibiting vastly-reduced resistance. Further study may be required to probe the mechanistic underpinnings of this effect. A structurally-related salt, lithium difluorobis(oxolato)phosphate (LiDFBOP), has also been shown to dictate anode and cathode interfacial makeup when added at 1%, with associated full-cell performance improvement at 0 °C.79
2 w/w.84 Combined analytical results from both studies indicate that this additive acts as an interface-former on both graphite and NMC surfaces, with the resulting SEI and CEI exhibiting vastly-reduced resistance. Further study may be required to probe the mechanistic underpinnings of this effect. A structurally-related salt, lithium difluorobis(oxolato)phosphate (LiDFBOP), has also been shown to dictate anode and cathode interfacial makeup when added at 1%, with associated full-cell performance improvement at 0 °C.79
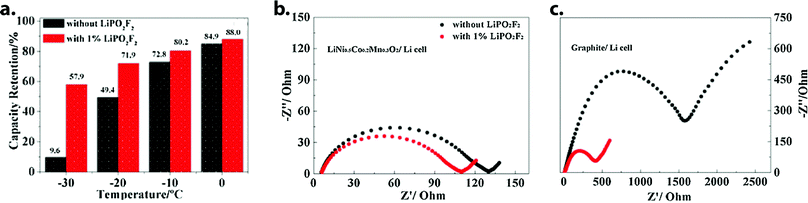 | ||
Fig. 9 (a) Discharge capacity retention of Gr‖NMC523 cells at sub-zero temperatures, relative to room temperature. Addition of 1 wt% LiPO2F2 to the standard electrolyte (1 M LiPF6 in EC/EMC/PC 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) caused significant performance improvement. (b and c) Impedance response of fully-charged cathode and anode half-cells at 0 °C. The additive was found to reduce graphite interfacial resistance more than three-fold, while a comparatively minor improvement was observed for NMC532. Reprinted/adapted from ref. 83 with permission from Elsevier. 1 w/w) caused significant performance improvement. (b and c) Impedance response of fully-charged cathode and anode half-cells at 0 °C. The additive was found to reduce graphite interfacial resistance more than three-fold, while a comparatively minor improvement was observed for NMC532. Reprinted/adapted from ref. 83 with permission from Elsevier. | ||
A particularly unique design comes from Ko et al., who reported on a lithium-modified silica nanosalt (“Li202”) synthesized by treating hydrophobic silica with LiH, followed by 1,3-propane sultone.85 The resulting sulfonate-rich surface allowed the silica to form a stable dispersion (2.5 wt%) with an electrolyte solution of 1 M LiPF6 in EC/PC/EMC/DEC 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v + 2 wt% vinylene carbonate (VC). The presence of Li202 additive slightly improved the cycling performance of Gr‖LiCoO2 coin cells at −20 °C when compared to the electrolyte alone and a non-functionalized silica dispersion. EIS analysis determined this effect to come from reduced RSEI in the presence of the additive, a hypothesis which was corroborated by SEM images showing thinner interfacial layers. Several of the same authors also published a follow-up report showing a synergistic effect of Li202 additive with an acrylate-grafted PDMS additive.86 Altogether, graphite‖LiCoO2 cells with this dual-additive formulation showed improved capacity of up to 110 mA h g−1 at −20 °C and 0.1C discharge rate, as compared to 95 mA h g−1 without the additives. The combination appeared to significantly reduce both interfacial resistance and bulk electrolyte resistance as measured by EIS, although the exact reason was not explored.
20 v/v + 2 wt% vinylene carbonate (VC). The presence of Li202 additive slightly improved the cycling performance of Gr‖LiCoO2 coin cells at −20 °C when compared to the electrolyte alone and a non-functionalized silica dispersion. EIS analysis determined this effect to come from reduced RSEI in the presence of the additive, a hypothesis which was corroborated by SEM images showing thinner interfacial layers. Several of the same authors also published a follow-up report showing a synergistic effect of Li202 additive with an acrylate-grafted PDMS additive.86 Altogether, graphite‖LiCoO2 cells with this dual-additive formulation showed improved capacity of up to 110 mA h g−1 at −20 °C and 0.1C discharge rate, as compared to 95 mA h g−1 without the additives. The combination appeared to significantly reduce both interfacial resistance and bulk electrolyte resistance as measured by EIS, although the exact reason was not explored.
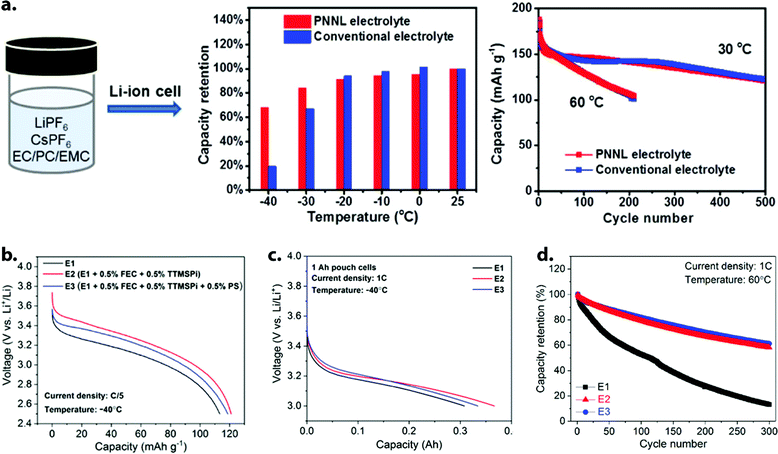 | ||
Fig. 10 (a) Comparison of electrolyte effect on Li-ion full cell performance at sub-zero temperatures (Gr‖NMC111 pouch cells) and room/elevated temperatures (Gr‖NCA coin cells). A small amount (0.05 M) of CsPF6 additive enabled stable cycling in a lean-EC environment (1 M LiPF6 in EC/PC/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w) and vastly improved low-temperature capacity compared to a conventional formulation (1 M LiPF6 in EC/EMC 3 8 w/w) and vastly improved low-temperature capacity compared to a conventional formulation (1 M LiPF6 in EC/EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v). Reprinted/adapted with permission from ref. 89. Copyright 2017 American Chemical Society. (b) Discharge voltage profiles of Gr‖NCA coin cells at −40 °C and C/5 rate in three electrolytes containing CsPF6 additive, where E1 = 1 M LiPF6 in EC/PC/EMC 1 7 v/v). Reprinted/adapted with permission from ref. 89. Copyright 2017 American Chemical Society. (b) Discharge voltage profiles of Gr‖NCA coin cells at −40 °C and C/5 rate in three electrolytes containing CsPF6 additive, where E1 = 1 M LiPF6 in EC/PC/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w + 0.05 M CsPF6. (c) 1C discharge of 1 A h-nominal Gr‖NMC111 pouch cells containing E1, E2, or E3 electrolyte at −40 °C. The electrolyte E2, containing 0.5 wt% each of FEC and TMSPi but no 1,3-propane sultone (PS), produced the best capacity (0.37 A h) under these harsh conditions. (d) Capacity retention of Gr‖NCA coin cells containing E1, E2, or E3 during 1C galvanostatic cycling at 60 °C. The presence of 0.5 wt% PS in electrolyte E3 gives a slight advantage at elevated temperature. Reprinted/adapted with permission from ref. 38. Copyright 2019 American Chemical Society. 8 w/w + 0.05 M CsPF6. (c) 1C discharge of 1 A h-nominal Gr‖NMC111 pouch cells containing E1, E2, or E3 electrolyte at −40 °C. The electrolyte E2, containing 0.5 wt% each of FEC and TMSPi but no 1,3-propane sultone (PS), produced the best capacity (0.37 A h) under these harsh conditions. (d) Capacity retention of Gr‖NCA coin cells containing E1, E2, or E3 during 1C galvanostatic cycling at 60 °C. The presence of 0.5 wt% PS in electrolyte E3 gives a slight advantage at elevated temperature. Reprinted/adapted with permission from ref. 38. Copyright 2019 American Chemical Society. | ||
Solvents
With few exceptions, solvents make up the largest portion of any electrolyte, whether by weight, volume or mole fraction. Their principal job is to dissolve and dissociate the primary salt into free ions, which carry the internal current of the battery. This process is facilitated by solvents with high dielectric permittivity and electron donor number, such as EC, which forms strong solvation complexes with Li+. Unfortunately, this presents a dilemma at low temperatures, where desolvation becomes the rate-limiting step in charge transfer (vide supra). This has driven a large body of research into alternative solvents which can replace or augment EC without sacrificing the favorable ion transport properties it endows. Such efforts are additionally complicated by the secondary role of LIB electrolyte solvents: interfacial compatibility. Solvent blends must be stable to oxidation at high cathode potentials (>4 V) and form sufficiently inert SEI layers at graphite anodes, as discussed previously. Therefore, alternative solvents are often used in combination with passivating additives in order to realize the benefits of low-EC-content electrolytes at sub-zero temperature without sacrificing cycle life. Solvent formulations investigated by the references cited here are summarized in Table 2 along with their structures.| Solvent | Electrolyte | Ref. |
|---|---|---|
|
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-x w/w) + 0.05 M CsPF6 9-x w/w) + 0.05 M CsPF6 |
15 |
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8-x w/w) + 0.05 M CsPF6 8-x w/w) + 0.05 M CsPF6 |
||
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w) + 0.05 M CsPF6 + various additives 8 w/w) + 0.05 M CsPF6 + various additives |
38 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v) + 2 vol% FEC 8 v/v) + 2 vol% FEC |
66 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 1 vol% FEC 3 v/v) + 1 vol% FEC |
67 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 2 vol% FEC 3 v/v) + 2 vol% FEC |
||
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 5 vol% FEC 3 v/v) + 5 vol% FEC |
||
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 5 vol% FEC 1 v/v) + 5 vol% FEC |
69 | |
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 vol% CMDO + 5 vol% FEC 1 v/v) + 2 vol% CMDO + 5 vol% FEC |
||
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 3 vol% EC + 5 vol% FEC 1 v/v) + 3 vol% EC + 5 vol% FEC |
||
1 M LiPF6, PC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 2 vol% CMDO + 3 vol% EC + 5 vol% FEC 1 v/v) + 2 vol% CMDO + 3 vol% EC + 5 vol% FEC |
||
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) + 1 wt% BuS 3 w/w) + 1 wt% BuS |
75 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 1 vol% BuS 3 v/v) + 1 vol% BuS |
76 | |
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 2 vol% BuS 3 v/v) + 2 vol% BuS |
||
1 M LiPF6, EC/PC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) + 5 vol% BuS 3 v/v) + 5 vol% BuS |
||
1 M LiPF6, EC/EMC/PC (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) + 1 wt% LiPO2F2 1 w/w) + 1 wt% LiPO2F2 |
82 | |
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 2.5 wt% Li202 + 2 wt% VC 20 v/v) + 2.5 wt% Li202 + 2 wt% VC |
85 | |
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 2 wt% VC + 5 wt% FEC 20 v/v) + 2 wt% VC + 5 wt% FEC |
86 | |
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 1 wt% Li202 + 2 wt% VC + 5 wt% FEC 20 v/v) + 1 wt% Li202 + 2 wt% VC + 5 wt% FEC |
86 | |
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC 20 v/v) + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC |
||
1 M LiPF6, EC/PC/EMC/DEC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 1 wt% Li202 + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC 20 v/v) + 1 wt% Li202 + 1 wt% PDMS-A + 2 wt% VC + 5 wt% FEC |
||
1 M LiPF6, EC/PC/EMC (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) + 0.05 M CsPF6 3 w/w) + 0.05 M CsPF6 |
87 | |
1 M LiPF6, EC/PC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-x w/w) + 0.05 M CsPF6 7-x w/w) + 0.05 M CsPF6 |
88 | |
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9-x w/w) + 0.05 M CsPF6 9-x w/w) + 0.05 M CsPF6 |
89 | |
1 M LiPF6, EC/PC/EMC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8-x w/w) + 0.05 M CsPF6 8-x w/w) + 0.05 M CsPF6 |
||
1 M LiBF4, PC/EMC/MB/EC (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w) 5 w/w) |
106 | |
1 M LiPF6, THTO/PC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100-x mol/mol) 100-x mol/mol) |
118 | |
1 M LiBF4, PC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
122 | |
1 M LiBOB, PC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
125 | |
1 M LiDFOB, PC/EC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) 4 w/w) |
128 | |
|
1 M LiPF6, EC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w) + 2 wt% VC 7 w/w) + 2 wt% VC |
91 |
1 M LiPF6, [EA or MP]/[VC or FEC] (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
1 M LiPF6, [EA or MP]/[VC or FEC] (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
||
1 M LiPF6, [EA or MP]/[VC or FEC] (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
| 1 M LiPF6, EMC + 1% wt [VC, FEC, DFEC, or MEC] | 92 | |
| 1 M LiPF6, EMC + 2% wt [VC, FEC, DFEC, or MEC] | ||
| 1 M LiPF6, EMC + 3% wt [VC, FEC, DFEC, or MEC] | ||
| 1 M LiPF6, EMC + 4% wt [VC, FEC, DFEC, or MEC] | ||
| 1 M LiPF6, EMC + 5% wt [VC, FEC, DFEC, or MEC] | ||
1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
93 | |
1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 10% VC 1 v/v) + 10% VC |
||
1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 10% FEC 1 v/v) + 10% FEC |
||
1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 10% DFEC 1 v/v) + 10% DFEC |
||
4.2 M LiFSI, FEC/FEMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) 2 v/v) |
94 | |
1.28 M LiFSI, FEC/FEMC/D2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) 7 v/v) |
||
0.7 M LiBETI, FEC/DEC/M3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 v/v) 14 v/v) |
||
1.2 M LiPF6, EC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w) + 10 wt% FEC 7 w/w) + 10 wt% FEC |
95 | |
1 M LiPF6, FEC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) 4 w/w) |
139 | |
1 M LiPF6, EC/DMC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 60% EA + 10% FEC 1 v/v) + 60% EA + 10% FEC |
140 | |
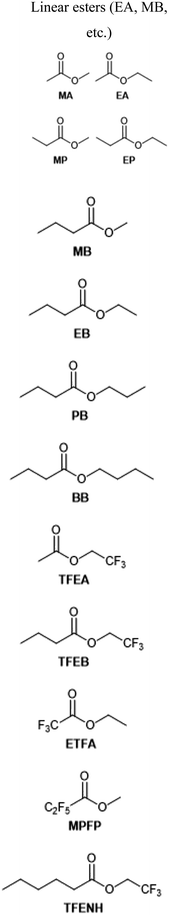
|
1 M LiPF6, MA/EC/DEC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 1 wt% TMSPi + 1 wt% PCS 1 v/v) + 1 wt% TMSPi + 1 wt% PCS |
74 |
1 M LiPF6, [EA or MP]/[VC or FEC] (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
91 | |
1 M LiPF6, [EA or MP]/[VC or FEC] (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
||
1 M LiPF6, [EA or MP]/[VC or FEC] (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
1 M LiPF6, EC/EMC/[MP, EP, MB, EB, PB, or BB] (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) 2 v/v) |
99 | |
1 M LiPF6, EC/EMC/[TFEB, TFEA, ETFA, or MPFP] (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) 2 v/v) |
100 | |
1 M LiPF6, EC/EMC/[TFEB, TFEA, ETFA, or MPFP] (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) 4 v/v) |
||
1 M LiPF6, EC/EMC/TFENH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
101 | |
1 M LiPF6, EC/EMC/TFENH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v) 6 v/v) |
||
1 M LiPF6, EC/EMC/TFENH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) 10 v/v) |
||
1 M LiPF6, EC/EMC/TFENH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
||
1 M LiPF6, EC/EMC/TFENH (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v) 60 v/v) |
||
| 1 M LiPF6, TFENH | ||
1.2 M LiPF6, EC/EMC/DMC/[MP, EA, or MB] (23.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.75 4.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66.5 66.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) + 2 wt% VC 5 v/v) + 2 wt% VC |
102 | |
1.2 M LiPF6, EC/EMC/DMC/[MP, EA, or MB] (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) + 2 wt% VC 20 v/v) + 2 wt% VC |
||
1.2 M LiPF6, EC/EMC/DMC/[MP, EA, or MB] (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v) + 2 wt% VC 40 v/v) + 2 wt% VC |
||
1.2 M LiPF6, EC/EMC/DMC/[MP, EA, or MB] (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v) + 2 wt% VC 60 v/v) + 2 wt% VC |
||
1.2 M LiPF6, [EC/EMC/DMC (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v)]/[MA or MP] (8 70 v/v)]/[MA or MP] (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 2 wt% [VC or FEC] 2 w/w) + 2 wt% [VC or FEC] |
103 | |
1.2 M LiPF6, [EC/EMC/DMC (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v)]/[MA or MP] (6 70 v/v)]/[MA or MP] (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) + 2 wt% [VC or FEC] 4 w/w) + 2 wt% [VC or FEC] |
||
1 M LiPF6, EC/EMC/MP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v) + 2 wt% [VC or PS] 6 v/v) + 2 wt% [VC or PS] |
104 | |
1 M LiPF6, EC/EMC/MP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v) + 0.1 M [LiBOB, LiDFOB, or LiFSI] 6 v/v) + 0.1 M [LiBOB, LiDFOB, or LiFSI] |
||
1.2 m LiPF6, [EC/EMC/DMC (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 w/w)]/[MA, EA, MP, or MB] (4 70 w/w)]/[MA, EA, MP, or MB] (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
105 | |
1 M LiBF4, PC/EMC/MB/EC (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w) 5 w/w) |
106 | |
1 M LiPF6, EC/EMC/EA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) 4 v/v) |
||
| 2 M LiTFSI, EA | 107 | |
1 M LiDFOB, GBL/MB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
109 | |
|
1 M LiDFOB, GBL/MB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
109 |
| 1 M LiBOB, GBL | 110 | |
1 M LiBOB, GBL/DMC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
||
1 M LiBOB, GBL/F-EPE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
||
1 M LiDFOB, GBL/F-EPE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
111 | |
1 M LiBF4, EC/DMC/GBL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
125 | |
| See patent text | 126 | |
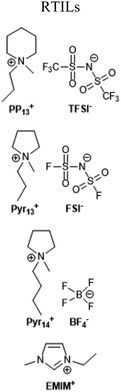
|
0.1 m LiTFSI, PP13TFSI | 112 |
| 0.2 m LiTFSI, PP13TFSI | ||
| 0.3 m LiTFSI, PP13TFSI | ||
| 0.4 m LiTFSI, PP13TFSI | ||
0.4 m LiTFSI, PP13TFSI/DEC (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) 2 w/w) |
||
0.4 m LiTFSI, PP13TFSI/DEC (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) 4 w/w) |
||
| Pyr14TFSI | 114 | |
Pyr13FSI/Pyr14TFSI (0.248![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.752 mol/mol) 0.752 mol/mol) |
||
Pyr13FSI/Pyr14TFSI (0.568![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.432 mol/mol) 0.432 mol/mol) |
||
Pyr13FSI/Pyr14TFSI (0.836![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.164 mol/mol) 0.164 mol/mol) |
||
| Pyr13FSI | ||
| 0.45 M LiTFSI, EMIMFSI | 116 | |
| 0.45 M LiTFSI, EMIMFSI + 0.01 M LiBOB | ||
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 0.5 wt% EMIMBF4 2 w/w) + 0.5 wt% EMIMBF4 |
117 | |
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 1 wt% EMIMBF4 2 w/w) + 1 wt% EMIMBF4 |
||
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 2 wt% EMIMBF4 2 w/w) + 2 wt% EMIMBF4 |
||
1 M LiPF6, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) + 5 wt% EMIMBF4 2 w/w) + 5 wt% EMIMBF4 |
||
|
0.9 M LiDFOB/LiBF4 (5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w), EC/DMS/EMC (1 1 w/w), EC/DMS/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
77 |
1.28 M LiFSI, FEC/FEMC/D2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) 7 v/v) |
94 | |
0.7 M LiBETI, FEC/DEC/M3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 v/v) 14 v/v) |
||
1 M LiBOB, GBL/F-EPE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
110 | |
1 M LiDFOB, GBL/F-EPE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
111 | |
1 M LiPF6, THTO/PC (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100-x mol/mol) 100-x mol/mol) |
118 | |
0.1 M LiTFSI, CH3F/CO2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
119 | |
0.2 M LiTFSI, CH3F/CO2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
0.3 M LiTFSI, 0.3 M THF, CH3F/CO2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
120 | |
1.2 M LiTFSI, 1 M AN, CH3F/CO2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
121 | |
| 21 m LiTFSI, H2O | 131 | |
| 21 m LiTFSI, H2O + 9.25 m LiTFSI, DMC | ||
15.3 m LiTFSI, H2O/AN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol) 1 mol/mol) |
||
| Composition series, see text | 132 | |
| 5.2 m LiTFSI, H2O | 133 |
For instance, as mentioned previously, Xu and coworkers have published a series of reports on CsPF6 additive, the original purpose of which was to stabilize graphite anodes in a majority-PC electrolyte.15,38,87–89 In one study from 2017, Li et al. thoroughly compared the thermal properties and ionic conductivity of electrolytes containing varying ratios of EC, PC and EMC with constant salt concentrations (1.0 M LiPF6 and 0.05 M CsPF6).89 Addition of PC was found to significantly reduce both thermodynamic liquidus temperature and the kinetic precipitation point, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 blend exhibiting values of −58.4 °C and −67.2 °C, respectively (Fig. 11a). Moreover, conductivity uniformly increased with decreasing EC content at temperatures below −20 °C, with values as high as 1 mS cm−1 at −40 °C being obtainable for the 1
8 blend exhibiting values of −58.4 °C and −67.2 °C, respectively (Fig. 11a). Moreover, conductivity uniformly increased with decreasing EC content at temperatures below −20 °C, with values as high as 1 mS cm−1 at −40 °C being obtainable for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 blend (Fig. 11b). This relatively simple ternary mixture enabled both Gr‖NCA coin cells and Gr‖NMC111 pouch cells to discharge >65% of their normal capacity at −40 °C and C/5 discharge rate (Fig. 11c).
8 blend (Fig. 11b). This relatively simple ternary mixture enabled both Gr‖NCA coin cells and Gr‖NMC111 pouch cells to discharge >65% of their normal capacity at −40 °C and C/5 discharge rate (Fig. 11c).
 | ||
Fig. 11 (a) Differential scanning calorimetry results for electrolytes containing 10 wt% PC and either 30 wt% EC (E6), 20 wt% EC (E7), or 10 wt% EC (E8), with the balance being EMC. Reduction of EC content in the presence of PC allows the liquid range of the electrolyte to be extended below −50 °C. (b) Temperature-dependent conductivity of 10% PC electrolytes with varying EC content. Conductivity varies inversely with %EC below −20 °C, although the overall variation is small. (c) C/5 discharge capacity of Gr‖NCA coin cells at varying temperatures as a function of EC content. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 EC/PC 8 EC/PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC blend performs best under sub-zero conditions. Reprinted/adapted with permission from ref. 89. Copyright 2017 American Chemical Society. EMC blend performs best under sub-zero conditions. Reprinted/adapted with permission from ref. 89. Copyright 2017 American Chemical Society. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 FEC/EMC electrolyte produced much lower Rct in Gr‖NMC442 full cells compared to 5
95 FEC/EMC electrolyte produced much lower Rct in Gr‖NMC442 full cells compared to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 VC/EMC.92 While these later results were obtained at room temperature, they may have implications for low-temperature performance as well, given that Rct increases in relative importance with decreasing temperature. A striking example of electrolyte design using FEC comes from Fan et al., who recently published a report on electrolytes with high fluorine content utilizing FEC as the primary high-dielectric solvent component.94 These authors dissolved a high concentration of fluorinated salts LiFSI or LiBETI salts in 1
95 VC/EMC.92 While these later results were obtained at room temperature, they may have implications for low-temperature performance as well, given that Rct increases in relative importance with decreasing temperature. A striking example of electrolyte design using FEC comes from Fan et al., who recently published a report on electrolytes with high fluorine content utilizing FEC as the primary high-dielectric solvent component.94 These authors dissolved a high concentration of fluorinated salts LiFSI or LiBETI salts in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixtures of FEC with methyl (2,2,2-trifluoroethyl) carbonate or DEC, respectively. The concentrated electrolytes were then further diluted into inert fluorinated solvents, which cannot ordinarily dissolve lithium salts, but are miscible with the pre-solvated blends. The resulting localized high-concentration electrolytes remained liquid below −120 °C (Fig. 12a), an astonishing low-temperature limit for any organic fluid. Their conductivities also showed unexpectedly weak thermal dependence, varying merely two orders of magnitude (10−5–10−3 S cm−1) from −80 °C to 25 °C (Fig. 12b). Moreover, due to the high ratio of salt to (coordinating) solvent molecules, the average solvation energy of Li+ – and thus, indirectly, the charge transfer resistance – was predicted to be significantly reduced (although it was not directly measured). Altogether, this combination of favorable electrolyte properties allowed the authors to successfully operate Li‖NCA cells from −95 to 70 °C (Fig. 12c and d). To our knowledge, this is the widest temperature range ever reported for a lithium-based battery with a liquid electrolyte. It is also notable that the authors reported Coulombic efficiencies >99% for a large number of anode materials (lithium metal, Gr, LTO) and cathode materials (NMC811, NCA, LiNMO, LiCoMnO4) with this electrolyte, demonstrating the universality of the design approach.
3 mixtures of FEC with methyl (2,2,2-trifluoroethyl) carbonate or DEC, respectively. The concentrated electrolytes were then further diluted into inert fluorinated solvents, which cannot ordinarily dissolve lithium salts, but are miscible with the pre-solvated blends. The resulting localized high-concentration electrolytes remained liquid below −120 °C (Fig. 12a), an astonishing low-temperature limit for any organic fluid. Their conductivities also showed unexpectedly weak thermal dependence, varying merely two orders of magnitude (10−5–10−3 S cm−1) from −80 °C to 25 °C (Fig. 12b). Moreover, due to the high ratio of salt to (coordinating) solvent molecules, the average solvation energy of Li+ – and thus, indirectly, the charge transfer resistance – was predicted to be significantly reduced (although it was not directly measured). Altogether, this combination of favorable electrolyte properties allowed the authors to successfully operate Li‖NCA cells from −95 to 70 °C (Fig. 12c and d). To our knowledge, this is the widest temperature range ever reported for a lithium-based battery with a liquid electrolyte. It is also notable that the authors reported Coulombic efficiencies >99% for a large number of anode materials (lithium metal, Gr, LTO) and cathode materials (NMC811, NCA, LiNMO, LiCoMnO4) with this electrolyte, demonstrating the universality of the design approach.
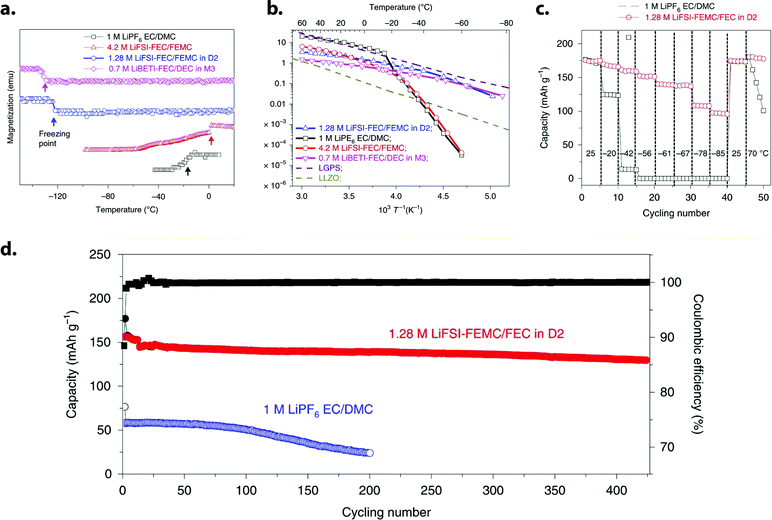 | ||
| Fig. 12 (a) Freezing points of highly-fluorinated FEC-based electrolytes as measured by a superconducting quantum interference magnetometer device. (b) Temperature-dependent conductivity of several electrolytes, with two common solid electrolytes as references (dashed lines). The highly-fluorinated electrolytes diluted with inert solvents exhibit conductivities >10−3 S cm−1 at room temperature and remain above 10−5 S cm−1 down to −80 °C. (c) Variable-temperature C/15 cycling of Li‖NCA half cells containing a highly-fluorinated electrolyte vs. a standard formulation. The fluorinated electrolyte enables cell function between −95 to 70 °C, whereas EC/DMC electrolyte produces negligible capacity below −20 °C and experiences rapid capacity fade at 70 °C. (d) C/3 cycling of Li‖NCA at −20 °C, which shows excellent discharge capacity and coulombic efficiency in the fluorinated electrolyte with minimal capacity fade. Reprinted by permission from Springer Nature: ref. 94, copyright 2019. | ||
Several design strategies have emerged to balance the advantages and drawbacks of ester (co-)solvents. Structure/blend optimization is perhaps the simplest solution, as demonstrated by the comprehensive screening published by Smart et al. in 2010.99 Amongst the many formulations studied by these authors, methyl propionate (MP) was found to be most effective (Fig. 13a), as an electrolyte of 1 M LiPF6 in EC/EMC/MP 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v enabled nominal 7 A h Gr|LiNi0.8Co0.2O2 cells to retain over 5 A h down to −60 °C. Another recurring concept involves partial fluorination, as in the case of 2,2,2-trifluoroethyl butyrate100 and 2,2,2-trifluoroethyl n-caproate101 among others, which generally tends to improve high-voltage stability and modify SEI composition. The most common recent development, however, has seen ester-based formulations paired with interface-modifying additives, including VC,91,102–104 FEC103,105 and others.74,104 For instance, Jones et al. tested a series of additives for their ability to inhibit lithium plating during low-temperature charge in a MP-rich electrolyte, finding 0.1 M LiFSI to be most effective.104 In at least one case, additives have made it possible to eliminate EC from the electrolyte entirely, with a MP
2 v/v enabled nominal 7 A h Gr|LiNi0.8Co0.2O2 cells to retain over 5 A h down to −60 °C. Another recurring concept involves partial fluorination, as in the case of 2,2,2-trifluoroethyl butyrate100 and 2,2,2-trifluoroethyl n-caproate101 among others, which generally tends to improve high-voltage stability and modify SEI composition. The most common recent development, however, has seen ester-based formulations paired with interface-modifying additives, including VC,91,102–104 FEC103,105 and others.74,104 For instance, Jones et al. tested a series of additives for their ability to inhibit lithium plating during low-temperature charge in a MP-rich electrolyte, finding 0.1 M LiFSI to be most effective.104 In at least one case, additives have made it possible to eliminate EC from the electrolyte entirely, with a MP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VC 95
VC 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w electrolyte allowing acceptable capacity retention during 40 °C cycling of Gr‖NMC111 pouch cells and enabling significantly-improved rate performance at −14 °C compared to an EC/EMC/VC mixture.91 Inclusion of such additives also frees up the design space to include more-reactive, but less-viscous and higher-polarity esters like methyl acetate.103 Shorter esters have also proven valuable to the performance of LIBs without graphite: Chen and coworkers tested LTO‖lithium manganese oxide cells with a variety of electrolytes and found an ethyl acetate-containing commercial blend to produce the lowest total impedance, with resulting cells able to pass the USABC cold cranking test at −30 °C.106 Similarly, an all-organic battery design has been demonstrated at −70 °C using an electrolyte with ethyl acetate as the sole solvent component.107
5 w/w electrolyte allowing acceptable capacity retention during 40 °C cycling of Gr‖NMC111 pouch cells and enabling significantly-improved rate performance at −14 °C compared to an EC/EMC/VC mixture.91 Inclusion of such additives also frees up the design space to include more-reactive, but less-viscous and higher-polarity esters like methyl acetate.103 Shorter esters have also proven valuable to the performance of LIBs without graphite: Chen and coworkers tested LTO‖lithium manganese oxide cells with a variety of electrolytes and found an ethyl acetate-containing commercial blend to produce the lowest total impedance, with resulting cells able to pass the USABC cold cranking test at −30 °C.106 Similarly, an all-organic battery design has been demonstrated at −70 °C using an electrolyte with ethyl acetate as the sole solvent component.107
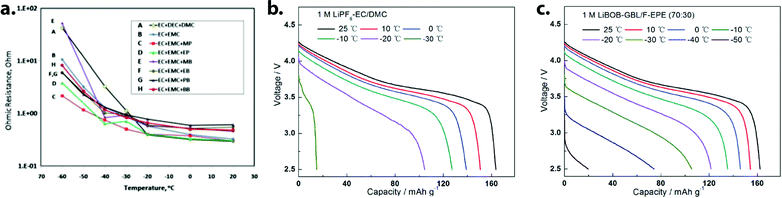 | ||
Fig. 13 (a) Temperature-dependent series resistance of MCMB‖LixNiyCo1−yO2 pouch cells containing electrolytes of 1 M LiPF6 in EC (20 vol%), EMC (60 vol%) and various esters (20 vol%). Methyl propionate (MP) produced the best results. Reprinted from ref. 99, copyright The Electrochemical Society. Reproduced by permission of IOP Publishing Ltd. All rights reserved. (b and c) 0.1C discharge of Gr‖NMC111 coin cells at various temperatures. The control electrolyte of 1 M LiPF6 in EC/DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v caused rapid capacity loss below −20 °C, with the cell becoming inoperable at −30 °C. On the other hand, a carbonate-free electrolyte of 1 M LiBOB in 70 wt% gamma-butyrolactone (GBL) and 30 wt% hydrofluoroether (F-EPE) enabled the cell to retain nearly half its capacity down to −40 °C, while matching the performance of the control at 25 °C. Reproduced and adapted from ref. 110. 1 v/v caused rapid capacity loss below −20 °C, with the cell becoming inoperable at −30 °C. On the other hand, a carbonate-free electrolyte of 1 M LiBOB in 70 wt% gamma-butyrolactone (GBL) and 30 wt% hydrofluoroether (F-EPE) enabled the cell to retain nearly half its capacity down to −40 °C, while matching the performance of the control at 25 °C. Reproduced and adapted from ref. 110. | ||
We would be remiss to conclude our discussion of ester electrolytes without mentioning lactones. Unlike linear esters, which generally play a similar role to linear carbonates in electrolyte design, cyclic lactones are polar enough to replace EC/PC partially or entirely. The best-studied member of this class, gamma-butyrolactone (GBL), has a relative dielectric constant of 42 at room temperature108 – around half that of EC, but still large enough to permit effective ionization of Li+ salts. It also remains liquid over a wider range (−44 to 204 °C) and possesses lower viscosity (1.7 cP at 25 °C) than EC, both valuable qualities for a low-temperature electrolyte solvent. Like other esters, however, it is unable to form a passivating SEI on graphite,13 a discovery that all-but-killed research into GBL electrolytes early in the development of LIBs. Yet recent progress may provide a way forward, as the responsibility for interfacial stability shifts away from solvents and towards salts/additives. For instance, Lazar and Lucht reported successful cycling of Gr‖NCA coin cells in an all-ester electrolyte of GBL and methyl butyrate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol) containing 1 M lithium difluoro(oxalato)borate (LiDFOB).109 Shi and coworkers also developed a similar electrolyte of 1 M lithium bis(oxalato)borate (LiBOB) in a novel blend of GBL with inert hydrofluoroether.110 This unique combination proved effective across a wide temperature spectrum (−40 to 60 °C) in Gr‖NMC111 cells, which could still deliver 74 mA h g−1 at the low end of this range, but did not function at all with a conventional electrolyte (Fig. 13b and c). A follow-up study by the same group also demonstrated excellent safety and processability characteristics for a similar electrolyte containing LiDFOB.111 Given these promising early results and the wealth of modern additive choices, it may be time to revisit GBL-based electrolytes as a serious option for next-generation LIBs.
1 vol) containing 1 M lithium difluoro(oxalato)borate (LiDFOB).109 Shi and coworkers also developed a similar electrolyte of 1 M lithium bis(oxalato)borate (LiBOB) in a novel blend of GBL with inert hydrofluoroether.110 This unique combination proved effective across a wide temperature spectrum (−40 to 60 °C) in Gr‖NMC111 cells, which could still deliver 74 mA h g−1 at the low end of this range, but did not function at all with a conventional electrolyte (Fig. 13b and c). A follow-up study by the same group also demonstrated excellent safety and processability characteristics for a similar electrolyte containing LiDFOB.111 Given these promising early results and the wealth of modern additive choices, it may be time to revisit GBL-based electrolytes as a serious option for next-generation LIBs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PP13TFSI
1 PP13TFSI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC blend with 0.4 mol kg−1 LiTFSI allowed Li‖LiCoO2 half cells to operate at −10 °C with 102 mA h g−1 capacity at C/10 rate – 72% of its room-temperature value.
DEC blend with 0.4 mol kg−1 LiTFSI allowed Li‖LiCoO2 half cells to operate at −10 °C with 102 mA h g−1 capacity at C/10 rate – 72% of its room-temperature value.
An additional challenge facing RTILs is their general instability at low voltages. In order to intercalate and deintercalate lithium reversibly, low-potential anodes, i.e. graphite or lithium metal must form a dimensionally-stable SEI that prevents continuous decomposition. For graphite, ionic liquids based on the TFSI anion cannot accomplish this. However, many researchers have partially addressed the issue by adding significant amounts of the structurally-similar bis(fluorosulfonyl)imide (FSI) anion, which degrades rapidly to form inorganic protecting layers.113 This strategy also tends to suppress crystallization and lower viscosity. Kunze et al. demonstrated that mixtures of N-butyl-N-methylpyrrolidinium (Pyr14) TFSI and N-methyl-N-propylpyrrolidinium (Pyr13) FSI have liquidus temperatures far below that of either parent compound (−18 °C and −8 °C, respectively).114 The (Pyr13FSI)0.836(Pyr14TFSI)0.164 intermediate blend maintained a conductivity >10−3 S cm−1 down to −20 °C, while (Pyr13FSI)0.568(Pyr14TFSI)0.432 remained liquid with conductivity 10−4 S cm−1 at −40 °C. These values are comparable to state-of-the-art organic electrolytes, although the relevance of this data may be limited, since none of the reported blends contained lithium and the lithium transference numbers of RTIL blends are generally quite low.115 Yamagata et al. tested a mixed electrolyte of 0.45 M LiTFSI in 1-ethyl-3-methylimidazolium (EMIM) FSI in Li‖Gr half-cells, comparing the performance to a standard electrolyte (1 M LiPF6 in EC/DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v).116 The IL electrolyte outperformed the standard at rates up to 5C and temperatures from 25–60 °C, but was initially inoperable at 0 °C. However, a small amount of LiBOB additive dramatically improved the performance, allowing these cells to pass 67% of their room-temperature capacity after 25 cycles at 0 °C (C/10 rate).
1 v/v).116 The IL electrolyte outperformed the standard at rates up to 5C and temperatures from 25–60 °C, but was initially inoperable at 0 °C. However, a small amount of LiBOB additive dramatically improved the performance, allowing these cells to pass 67% of their room-temperature capacity after 25 cycles at 0 °C (C/10 rate).
Overall, the concept of low-temperature electrolytes based purely on ILs appears to have some potential, but more focused study would be required to realize competitive performance. When the additional drawbacks of IL electrolytes are factored in, such as high cost and low t+, the outlook becomes discouraging, at least for this application space. We suggest that additional research might focus on IL/organic solvent mixtures rather than pure ILs. At least one existing study has validated this approach: Wang and coworkers found that 1 wt% EMIMBF4 added to a standard EC/EMC electrolyte could nearly double the discharge capacity of Gr‖NMC523 pouch cells at −30 °C.117 Furthermore, it remains to be seen whether the general trends discussed in this review still apply to a majority-ionic system, e.g. is charge transfer still the limiting step at low temperature, and is solvation structure still predictive of Rct when the coordinating species are themselves charged? Further research into these fundamental questions is encouraged.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w), the mixed electrolyte 1 M LiPF6 in THTO/PC 15
1 w/w), the mixed electrolyte 1 M LiPF6 in THTO/PC 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 mol/mol roughly doubled the capacity of Gr‖NMC111 cells at 0 °C and 1C rate.
85 mol/mol roughly doubled the capacity of Gr‖NMC111 cells at 0 °C and 1C rate.
One extraordinarily unique low-temperature system comes from Rustomji et al., who reported electrolytes based on liquefied gases.119 Using a pressurized cell, these authors were able to dissolve 0.1 M LiTFSI in fluoromethane, a gas with a boiling point of −78 °C at 1 atm. Due to the extreme low viscosity of this material (owing to the weak interactions between molecules), the resulting electrolyte displayed high conductivity ∼10−3 S cm−1 across all temperatures from −60 °C to 25 °C, despite the comparatively low salt concentration. Addition of 5% CO2 enabled formation of a stable SEI on lithium metal and allowed more salt to be dissolved. Li‖LiCoO2 half cells cycled perfectly well in a liquefied gas electrolyte (0.2 M LiTFSI in fluoromethane/CO2 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) down to −60 °C (60.6% capacity compared to room temperature) and performed similarly at 25 °C to 1 M LiPF6 in EC/DEC 1
1) down to −60 °C (60.6% capacity compared to room temperature) and performed similarly at 25 °C to 1 M LiPF6 in EC/DEC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w. The same group recently followed up on this work using either tetrahydrofuran120 or acetonitrile121 as solvating additives to achieve higher salt concentrations and improved performance with lithium metal. It should be noted, of course, that the vapor pressures of these liquified gas solvents are multiple MPa under ordinary conditions, creating an inherent challenge to any practical application of this system.
1 w/w. The same group recently followed up on this work using either tetrahydrofuran120 or acetonitrile121 as solvating additives to achieve higher salt concentrations and improved performance with lithium metal. It should be noted, of course, that the vapor pressures of these liquified gas solvents are multiple MPa under ordinary conditions, creating an inherent challenge to any practical application of this system.
Primary salts
No LIB can function without a lithium-containing electrolyte to transport Li+ across the cell interior and, when it comes to sources of dissociable lithium, LiPF6 is the undisputed first choice. It has been said that LiPF6 earned this position not due to any one outstanding property, but rather due to its lack of any crippling disadvantages.13 That being said, non-PF6− salts can – and have – been utilized in application-specific LIBs, where they may provide certain upside. For example, different salts may have better solubility in non-typical solvents or participate in the passivation of electrode interfaces. These characteristics can be leveraged to the benefit of low-temperature performance. Salt formulations investigated by the references cited here may be found summarized in Table 3 along with their structures.| Primary salt | Electrolyte | Ref. |
|---|---|---|
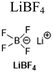
|
0.9 M LiDFOB/LiBF4 (5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w), EC/DMS/EMC (1 1 w/w), EC/DMS/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
77 |
1 M LiBF4, PC/EMC/MB/EC (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w) 5 w/w) |
106 | |
1 M LiBF4, PC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
122 | |
1 M LiBF4, EC/DMC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
123 | |
1 M LiBF4, EC/DMC/GBL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
125 | |
1 M LiDFOB/LiBF4 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol), DMC/EC/EMC (1 1 mol/mol), DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
129 | |
1 M LiDFOB/LiBF4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol), DMC/EC/EMC (1 1 mol/mol), DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
1 M LiDFOB/LiBF4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mol/mol), DMC/EC/EMC (1 4 mol/mol), DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
1 M LiBF4, DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
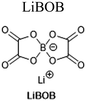
|
1 M LiBOB, GBL | 110 |
1 M LiBOB, GBL/DMC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
||
1 M LiBOB, GBL/F-EPE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
||
1 M LiBOB, EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
125 | |
1 M LiBOB, PC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
||
| See patent text | 126 | |
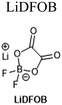
|
0.9 M LiDFOB/LiBF4 (5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w), EC/DMS/EMC (1 1 w/w), EC/DMS/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
77 |
1 M LiDFOB, EC/DMC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
109 | |
1 M LiDFOB, GBL/MB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
||
1 M LiDFOB, GBL/F-EPE (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
111 | |
1 M LiDFOB, PC/EC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) 4 w/w) |
128 | |
1 M LiDFOB, DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
129 | |
1 M LiDFOB/LiBF4 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol), DMC/EC/EMC (1 1 mol/mol), DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
1 M LiDFOB/LiBF4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol), DMC/EC/EMC (1 1 mol/mol), DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
1 M LiDFOB/LiBF4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mol/mol), DMC/EC/EMC (1 4 mol/mol), DMC/EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
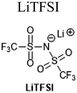
|
2 M LiTFSI, EA | 107 |
0.1 M LiTFSI, CH3F/CO2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
119 | |
0.2 M LiTFSI, CH3F/CO2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
||
0.3 M LiTFSI, 0.3 M THF, CH3F/CO2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
120 | |
0.9 M LiTFSI, EC/DMC/EMC (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 w/w) 48 w/w) |
130 | |
| 21 m LiTFSI, H2O | 131 | |
| 21 m LiTFSI, H2O + 9.25 m LiTFSI, DMC | ||
15.3 m LiTFSI, H2O/AN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol) 1 mol/mol) |
||
| Composition series, see text | 132 | |
| 5.2 m LiTFSI, H2O | 133 | |
|
0.7 M LiBETI, FEC/DEC/M3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 v/v) 14 v/v) |
94 |
|
4.2 M LiFSI, FEC/FEMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) 2 v/v) |
94 |
1.28 M LiFSI, FEC/FEMC/D2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) 7 v/v) |
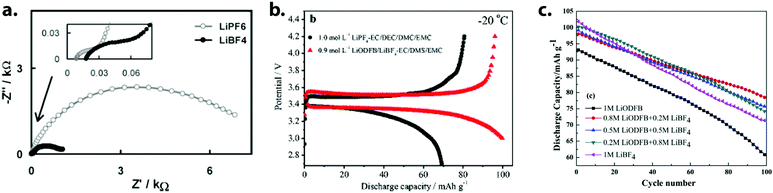 | ||
Fig. 14 (a) Impedance spectra at −20 °C of fully-charged Li-ion cells containing 1 molal LiPF6 in EC/DMC/DEC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, compared to 1 molal LiBF4 in the same solvent mixture. Charge-transfer resistance is significantly reduced in the LiBF4-based electrolyte. Reprinted by permission from Springer Nature: ref. 123, copyright 2002. (b) Initial −20 °C charge/discharge curves of Li‖LiFePO4 cells with a mixed LiDFOB/LiBF4 electrolyte versus a standard mixture. The discharge rate is 0.5C. Reprinted by permission from Springer Nature: ref. 77, copyright 2014. (c) Cycling performance of Gr|LiNi0.5Mn1.5O4 cells at −20 °C in electrolytes containing varying ratios of LiDFOB to LiBF4, with 1 M total salt in EC/DMC/EMC 1 1 w/w, compared to 1 molal LiBF4 in the same solvent mixture. Charge-transfer resistance is significantly reduced in the LiBF4-based electrolyte. Reprinted by permission from Springer Nature: ref. 123, copyright 2002. (b) Initial −20 °C charge/discharge curves of Li‖LiFePO4 cells with a mixed LiDFOB/LiBF4 electrolyte versus a standard mixture. The discharge rate is 0.5C. Reprinted by permission from Springer Nature: ref. 77, copyright 2014. (c) Cycling performance of Gr|LiNi0.5Mn1.5O4 cells at −20 °C in electrolytes containing varying ratios of LiDFOB to LiBF4, with 1 M total salt in EC/DMC/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w. A combination of 0.8 M LiDFOB and 0.2 M LiBF4 strikes the best balance between initial capacity and retention. Reprinted from ref. 129 with permission from Elsevier. 1 w/w. A combination of 0.8 M LiDFOB and 0.2 M LiBF4 strikes the best balance between initial capacity and retention. Reprinted from ref. 129 with permission from Elsevier. | ||
Unfortunately, LiBF4 has not gained widespread popularity as a primary salt due to the inadequate SEI formed in its electrolyte solutions, which precludes extended cycling. On the other hand, lithium bis(oxolato)borate (LiBOB) has received steady investigation precisely because of its SEI-forming abilities, which may reduce or eliminate the need for EC solvent.124 Naturally, this has attracted interest for low-temperature electrolyte designs,110,125,126 where it is desirable to minimize EC content. However, LiBOB comes with its own set of drawbacks, including poorer solubility and conductivity than LiPF6, which limit its applications as well. Kang Xu has explored the topic of LiBOB electrolyte design in more detail elsewhere.127
An appropriate compromise can be found in lithium difluoro(oxolato)borate (LiDFOB), which shares the SEI-forming characteristics of LiBOB, but with improved solubility and ion dissociation characteristics. This salt has proven to be suitable for a wide range of electrolyte designs,128 including lactone-based formulations.109,111 Several groups have reported that a mixed-salt approach – using LiDFOB together with LiBF4 – can maximize the advantages of both compounds at low temperature. For instance, Li et al. investigated an EC/DMS/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v electrolyte containing 0.9 M total of LiDFOB and LiBF4 in a 5.365
3 v/v electrolyte containing 0.9 M total of LiDFOB and LiBF4 in a 5.365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio.77 These authors found the ionic conductivity of this mixture to be similar to that a traditional LiPF6-based blend across the temperature range −40 to 20 °C. Importantly, the electrolyte was found to be compatible with both graphite and LiFePO4 in half-cell tests, enabling drastically-higher capacities than the control electrolyte at −20 °C (Fig. 14b). Zhou and coworkers conducted a detailed study of similar electrolytes (1 M salt in EC/DMC/EMC 1
1 mass ratio.77 These authors found the ionic conductivity of this mixture to be similar to that a traditional LiPF6-based blend across the temperature range −40 to 20 °C. Importantly, the electrolyte was found to be compatible with both graphite and LiFePO4 in half-cell tests, enabling drastically-higher capacities than the control electrolyte at −20 °C (Fig. 14b). Zhou and coworkers conducted a detailed study of similar electrolytes (1 M salt in EC/DMC/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) with varying DFOB/BF4 ratio across the range −20 to 60 °C.129 Pure LiDFOB performed universally best at room temperature and higher, producing the best conductivity and discharge capacity in Gr‖LMNO cells, but this trend was exactly reversed at −20 °C, with blends showing intermediate properties (Fig. 14c). An ideal balance was struck at 0.8 M LiDFOB and 0.2 M LiBF4, since even a relatively small amount of LiBF4 was found to reduce cell Rct from 482.6 Ω to 346.3 Ω at −20 °C, without compromising the effective passivation of LiDFOB. As a result, cells containing this blend showed excellent capacity retention across all temperatures.
1 w/w) with varying DFOB/BF4 ratio across the range −20 to 60 °C.129 Pure LiDFOB performed universally best at room temperature and higher, producing the best conductivity and discharge capacity in Gr‖LMNO cells, but this trend was exactly reversed at −20 °C, with blends showing intermediate properties (Fig. 14c). An ideal balance was struck at 0.8 M LiDFOB and 0.2 M LiBF4, since even a relatively small amount of LiBF4 was found to reduce cell Rct from 482.6 Ω to 346.3 Ω at −20 °C, without compromising the effective passivation of LiDFOB. As a result, cells containing this blend showed excellent capacity retention across all temperatures.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 w/w) across various temperatures.130 Most interestingly, the LiTFSI electrolyte showed nearly an order-of-magnitude reduction in charge-transfer resistance on lithium metal at 30 °C, although no measurements were done at lower temperatures. Since charge-transfer resistance is generally the limiting factor in LIB cells under sub-zero conditions, this observation could prove relevant. Li‖LiNi0.8Co0.2O2 half cells and Gr‖LiNi0.8Co0.2O2 full cells were also demonstrated to operate well in the LiTFSI-containing electrolyte, with the half cells remaining functional down to −40 °C. More recently, several independent studies have reported “water-in-salt” electrolytes based on lithium sulfonylimides with low-temperature operability as a key selling point.131–133 Overall, LiTFSI and its related compounds appear to be underutilized in low-temperature LIB literature, and it is our opinion that the field could benefit by adding this class of materials to its toolbox. Potential investigators should be warned, however, that these salts may cause severe corrosion to Al current collectors unless additional passivating strategies are employed.134
48 w/w) across various temperatures.130 Most interestingly, the LiTFSI electrolyte showed nearly an order-of-magnitude reduction in charge-transfer resistance on lithium metal at 30 °C, although no measurements were done at lower temperatures. Since charge-transfer resistance is generally the limiting factor in LIB cells under sub-zero conditions, this observation could prove relevant. Li‖LiNi0.8Co0.2O2 half cells and Gr‖LiNi0.8Co0.2O2 full cells were also demonstrated to operate well in the LiTFSI-containing electrolyte, with the half cells remaining functional down to −40 °C. More recently, several independent studies have reported “water-in-salt” electrolytes based on lithium sulfonylimides with low-temperature operability as a key selling point.131–133 Overall, LiTFSI and its related compounds appear to be underutilized in low-temperature LIB literature, and it is our opinion that the field could benefit by adding this class of materials to its toolbox. Potential investigators should be warned, however, that these salts may cause severe corrosion to Al current collectors unless additional passivating strategies are employed.134
A note on silicon anodes
Although graphite has been the anode of choice for virtually the entire commercial history of LIBs, future designs are likely to rely on some combination of silicon and carbon due to the former's high specific capacity, earth abundance and mature commercial production. The various characteristics and challenges of silicon and Si/C composite anodes under “ordinary” operating conditions have been covered elsewhere extensively.64,135–137 Curiously, however, we were only able to locate a handful of reports that directly address silicon performance at low temperatures. One of the earliest reports came in 2005 from Kasavajjula and Wang, who developed a composite anode containing graphite and nano-Si in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, along with a solid electrolyte filler and PEO-LiClO4 binder.138 In half cells with lithium metal and an electrolyte of 1 M LiPF6 in EC/DMC/DEC/EMC 1
1 ratio, along with a solid electrolyte filler and PEO-LiClO4 binder.138 In half cells with lithium metal and an electrolyte of 1 M LiPF6 in EC/DMC/DEC/EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, this electrode was found to reversibly store 321 mA h g−1 at 25 °C, with moderate retention of 277 mA h g−1 at −10 °C and 222 mA h g−1 at −20 °C. Further lowering the temperature caused more dramatic losses, with 160 mA h g−1 available at −30 °C and only 84 mA h g−1 at −40 °C. Careful impedance analysis led the authors to conclude that increasing charge-transfer resistance was responsible for the capacity loss below −20 °C, although the inclusion of solid electrolyte helped this situation significantly compared to a control electrode without it.
3, this electrode was found to reversibly store 321 mA h g−1 at 25 °C, with moderate retention of 277 mA h g−1 at −10 °C and 222 mA h g−1 at −20 °C. Further lowering the temperature caused more dramatic losses, with 160 mA h g−1 available at −30 °C and only 84 mA h g−1 at −40 °C. Careful impedance analysis led the authors to conclude that increasing charge-transfer resistance was responsible for the capacity loss below −20 °C, although the inclusion of solid electrolyte helped this situation significantly compared to a control electrode without it.
Several more articles have been published on this topic within the past five years as silicon has inched closer to widespread adoption. Markevitch, Salitra and Aurbach compared the performance of monolithic amorphous silicon (a-Si) anodes (capacity-limited to 600 mA h g−1 lithiation) to graphite counterparts of identical areal capacity, using half cells with 1 M LiPF6 in FEC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) electrolyte.139 Following lithiation at 30 °C, the delithiation capacity of a-Si exhibited virtually no temperature dependence down to −30 °C; furthermore, the silicon anodes could be charged and discharged under such conditions with no observable capacity drop, while graphite lost capacity rapidly when charged at 0 °C and below. Haruta et al. compared the effects of several common electrolyte additives on Si nanoflake anodes over a wide temperature range (Fig. 15),93 finding 10 wt% FEC to be the most effective at −5 °C and moderately effective at 60 °C. In contrast, 10 wt% VC produced slightly better capacity at high temperature, but drastically reduced capacity at sub-zero temperature.
4 w/w) electrolyte.139 Following lithiation at 30 °C, the delithiation capacity of a-Si exhibited virtually no temperature dependence down to −30 °C; furthermore, the silicon anodes could be charged and discharged under such conditions with no observable capacity drop, while graphite lost capacity rapidly when charged at 0 °C and below. Haruta et al. compared the effects of several common electrolyte additives on Si nanoflake anodes over a wide temperature range (Fig. 15),93 finding 10 wt% FEC to be the most effective at −5 °C and moderately effective at 60 °C. In contrast, 10 wt% VC produced slightly better capacity at high temperature, but drastically reduced capacity at sub-zero temperature.
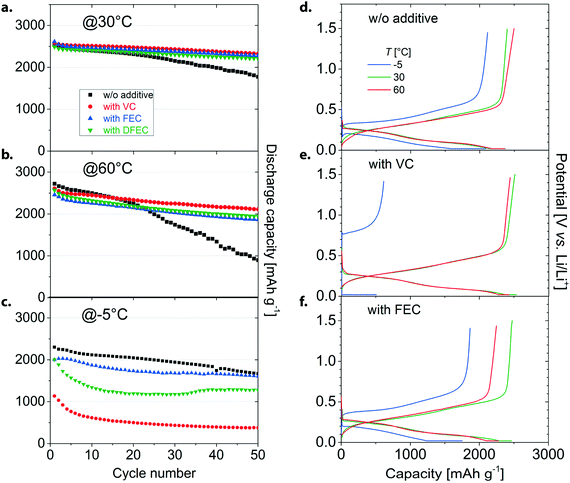 | ||
Fig. 15 (a–c) Cycling performance of Si nanoflake powder half-cells containing various electrolytes at varying temperatures. The base electrolyte was 1 M LiPF6 in EC/DEC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, with 10 wt% “additive” compounds as indicated. FEC appears to offer the best trade-off between high-temperature stability and low-temperature discharge capacity. (d and e) 10th cycle charge and discharge curves for the above cells at different temperatures. While VC-added electrolyte produces the best capacity (by a small amount) at 25 °C and 60 °C, it creates extreme polarization in the cell at −5 °C. Both FEC and VC additives appear to increase cell resistance at sub-zero conditions relative to the base electrolyte. Reprinted from ref. 93 with permission from Elsevier. 1 v/v, with 10 wt% “additive” compounds as indicated. FEC appears to offer the best trade-off between high-temperature stability and low-temperature discharge capacity. (d and e) 10th cycle charge and discharge curves for the above cells at different temperatures. While VC-added electrolyte produces the best capacity (by a small amount) at 25 °C and 60 °C, it creates extreme polarization in the cell at −5 °C. Both FEC and VC additives appear to increase cell resistance at sub-zero conditions relative to the base electrolyte. Reprinted from ref. 93 with permission from Elsevier. | ||
Notably, all of the above studies derived their conclusions from half cells with lithium metal. In contrast, two articles published just months prior to this writing examined commercial large-format cells containing Si or Si/Gr anodes paired with NCA cathodes. Subburaj and coworkers studied 1 A h pouch cells made from sputter-deposited Si anodes and a conventional electrolyte blend (1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC
DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC 1
DEC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v).140 These cells retained an impressive 65.3% of their 20 °C discharge capacity at −40 °C; however, capacity faded rapidly after 40 low-temperature charge/discharge cycles. Notably, the authors identified cathode structural transformation as the cause of this degradation, rather than lithium plating or other anode processes. Richter and coworkers took a different approach, examining minor-Si-content (3.5 wt%) anodes in 18
1 v/v).140 These cells retained an impressive 65.3% of their 20 °C discharge capacity at −40 °C; however, capacity faded rapidly after 40 low-temperature charge/discharge cycles. Notably, the authors identified cathode structural transformation as the cause of this degradation, rather than lithium plating or other anode processes. Richter and coworkers took a different approach, examining minor-Si-content (3.5 wt%) anodes in 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 format cylindrical cells with operando neutron diffraction.141 This experiment revealed that, under low-temperature charge conditions, graphite was lithiated more rapidly than silicon despite its lower potential, indicating a kinetic limitation that may be a target for future optimization.
650 format cylindrical cells with operando neutron diffraction.141 This experiment revealed that, under low-temperature charge conditions, graphite was lithiated more rapidly than silicon despite its lower potential, indicating a kinetic limitation that may be a target for future optimization.
Doubtless, many questions remain about the characteristics of silicon anodes below 0 °C, indicating that this area is ripe for further investigation. We observe that – generally speaking – silicon has been reported to resist low-temperature capacity loss better than graphite, an advantage also shared by the related alloy-type material tin.142 However, it remains to be seen whether this is an intrinsic benefit, or merely a result of different particle shapes/sizes, electrode fabrication methods and testing protocols. We strongly encourage additional research in this area. Results from the various references cited here are summarized in Table 4.
| Cathode‖anode | Electrolyte | Temperature (οC) | Discharge capacity (mA h g−1) | Capacity retention (% of RT) | Rate | Notes | Ref. |
|---|---|---|---|---|---|---|---|
| a-Si nanoflakes‖Li | 1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
−5 | 2100 | 84% | n/a | Capacity estimated after 10 cycles at low temperature | 93 |
1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 10% VC 1 v/v) + 10% VC |
600 | 25% | |||||
1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 10% FEC 1 v/v) + 10% FEC |
1900 | 79% | |||||
1 M LiPF6, EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 10% DFEC 1 v/v) + 10% DFEC |
1300 | 54% | |||||
| Si/Gr‖Li | 1 M LiPF6, EC/DEC/DMC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
−10 | 277 | 86% | 5 mA g−1 | 10% nano-Si, polymer/ceramic composite binder | 138 |
| −20 | 222 | 69% | |||||
| −30 | 160 | 50% | |||||
| −40 | 84 | 26% | |||||
| a-Si nanopillars‖Li | 1 M LiPF6, FEC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) 4 w/w) |
0 | 600 | 100% | 0.24 mA c−1 m−2 | Capacity-limited to 600 mA h g−1 charge | 139 |
| −10 | 600 | 100% | |||||
| −20 | 600 | 100% | |||||
| −30 | 600 | 100% | |||||
| NCA‖Si | 1 M LiPF6, EC/DMC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + 60% EA + 10% FEC 1 v/v) + 60% EA + 10% FEC |
−40 | 707 (mA h) | 65% | C/10 | Sputtered Si, pouch cell | 140 |
| NCA‖Si/Gr | n/a | −21 | 2.03 (A h, charge) | 81% | 0.1C | 3.5 wt% Si, pouch cell, mechanistic study | 141 |
| 1.71 (A h, charge) | 69% | 0.5C | |||||
| 1.16 (A h, charge) | 47% | 0.75C | |||||
| Nano-Sn/Gr‖Li | 1 M LiPF6, EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
−20 | 195 | 30% | 65 mA g−1 | Sn nanoparticles embedded in expanded graphite | 142 |
| 130 | 20% | 130 mA g−1 |
Summary and outlook
The future of lithium-ion batteries (LIBs) will require them to fill more roles than ever before and do so across a wider range of operating temperatures. It is well-known that the available discharge capacity of lithium-ion cells (even when charged at room temperature) tends to drop off considerably below −20 °C, which poses a significant challenge for applications in aerospace and transportation where sub-zero ambient conditions are common. This is largely due to a massive increase to internal resistance as the temperature is lowered. Solving the issue of low-temperature performance requires careful consideration of the many simultaneous chemical processes occurring within a lithium-ion cell, each of which contributes an associated impedance. Often, the best way to address this complex problem is by way of the one component which affects nearly every internal process: the non-aqueous liquid electrolyte.In this review, we first summarized the many individual sources of impedance that are associated with liquid electrolytes and the role that they play in low-temperature performance. A typical lithium-ion electrolyte consists of 1 M LiPF6 dissolved in a liquid mixture of ≤50% ethylene carbonate (EC) along with linear carbonates (DMC, DEC, EMC) and a few wt% of additives (e.g. FEC, VC). While the bulk physical properties of these formulations, e.g. ionic conductivity, have been well-studied due to their importance at moderate-to-high temperatures and/or high currents, low-temperature performance does not generally correlate with bulk conductivity. If anything, the most relevant physical property is instead liquidus point, where solids begin to block electrode pores and reduce ion access to active surfaces. However, by far the biggest limiting factor in sub-zero LIB operation is charge-transfer resistance, a process that has been linked by several studies to Li+ desolvation at the electrolyte/active material interface. These large desolvation energies and high freezing points are both largely due to the use of EC, which is a high-melting compound (36 °C) that possesses the large dielectric permittivity (ε = 90 at 40 °C) necessary to dissociate lithium salts from their counterions. EC is also indispensable because of its ability to passivate graphite anode surfaces upon reductive breakdown, forming a solid–electrolyte interphase (SEI) that stops further electrolyte decomposition and stabilizes the carbon structure to exfoliation upon repeated cycling. While the SEI (and its thinner cathode counterpart, the CEI) do indeed contribute some impedance to the cell, evidence suggests that interphasial ion transport is not the primary limit on low-temperature performance. Rather, the importance of SEI comes from its ability to prevent capacity loss via parasitic corrosion current, which is critical at all temperatures and becomes especially challenging when attempting to reduce EC content in the electrolyte. Attempting to charge a LIB under sub-zero conditions also introduces new capacity loss mechanisms associated with lithium deposition at low anode potentials.
Just as the causes of low-temperature capacity loss are multifaceted, so too are the potential strategies to address them via electrolyte engineering. We have concisely summarized recent developments in this area, which can be broken down into three primary research thrusts: additives, solvents and salts. Successful electrolyte additives generally assist in the formation of robust SEI/CEI layers with low resistance to Li+ transport, which reduces reliance on EC as a main solvent component. Studied additives include molecular compounds like fluoroethylene carbonate (FEC), tris(trimethylsilyl)phosphite (TMSPi) and sulfur compounds in a variety of oxidation states, e.g. dimethyl sulfite (DMS) and 1,3,2-dioxathiolane-2,2-dioxide (DTD). Ionic compounds may also fill this role, including novel lithium salts like LiPO2F2 or even non-lithium-containing salts such as CsPF6. Alternately or in combination with additives, many authors have sought to augment or replace traditional solvents as well. One of the simplest, but most effective, solvent components at low temperature is propylene carbonate (PC), which shares many characteristics of EC, but with a lower melting point (−49 °C). Modern additive developments have made it possible to partially or entirely replace EC with PC without causing undesirable breakdown of graphite, as known from the early days of LIB research. FEC and vinylene carbonate (VC) have also been explored as major solvent components. However, many researchers have turned to another class of solvents entirely: esters, which possess low melting points and low viscosities, while being of moderately polar character. On the other hand, low-molecular-weight esters are often detrimentally unstable at low anode potentials; an exhaustive study has found methyl propionate (MP) to possess the best trade-off in properties, although protective additives or higher-potential anodes like Li4Ti5O12 (LTO) can eliminate stability concerns. The cyclic ester gamma-butyrolactone (GBL) has also shown great potential as a replacement for chemically-similar EC. Other creative solvents include room-temperature ionic liquids (RTILs) and liquified gases, i.e. fluoromethane contained under pressure. Finally, other investigators have turned away from the ubiquitous LiPF6 in favor of other lithium salts. In particular, borates have received significant attention due to the discovery of vastly-reduced charge-transfer resistance in LiBF4-based electrolytes at low temperatures. Recently, lithium bis(oxalate)borate (LiBOB) and lithium difluoro(oxalato)borate (LiDFOB) have been characterized for sub-zero applications and been found to possess intriguing advantages. Other salts like lithium bis(trifluoromethane)sulfonylimide (LiTFSI), while well-characterized in other contexts, have received comparatively little investigation at low temperature.
Finally, we would like to conclude by pointing out several emerging areas of research for low-temperature LIB electrolytes which deserve greater attention. The most obvious of these is in silicon-based anode materials, which have seen increasing commercial attention in combination with graphite and are likely to entirely replace graphite at some point in the future. Despite the explosion of silicon research over the past decade, there have been remarkably few published reports on Si anodes at low temperatures. It remains to be seen how the “conventional wisdom” enumerated in this review will apply to Si materials systems, especially since the anode is generally considered to be the dominating factor when it comes to sub-zero battery operation. Additionally, there are many electrolyte components which, for one reason or another, were deemed unsuitable during the early days of LIB research, despite marked advantages at low temperature. A perfect example is PC, which cannot form a protective SEI on its own, but is now enjoying a revival due to the advent of sacrificial additives. GBL, on the other hand, has yet to experience such renewed interest, despite several promising recent results, especially in combination with borate salts like LiDFOB for which GBL seems to exhibit a particular synergy. It should also be mentioned that fluorine-containing solvents, especially hydrofluoroethers, have (deservedly) enjoyed recent popularity as electrolyte components for a variety of applications.94,110,143–145 While a handful of these reports have addressed sub-zero performance, this angle remains underexplored given their tendencies towards wide liquid range and low Li+ solvation energy. In general, while incremental progress remains possible, the most exciting developments in low-temperature LIB performance have often come from the most novel design strategies, such as highly-fluorinated electrolytes, liquefied gases or non-lithium metallic salt additives. With a greater commitment to push the envelope on electrolyte development, we can expect a bright future for batteries that will carry us down the road, across the sky, through space and to the limits of human imagination.
List of abbreviations
| AN | Acetonitrile |
| BB | Butyl butyrate |
| BETI | Bis(pentafluoroethanesulfonyl)imide |
| BOB | Bis(oxalato)borate |
| BuS | Butyl sultone |
| CEI | Cathode-electrolyte interphase |
| CMDO | 4-Chloromethyl-1,3,2-dioxathiolane-2-oxide |
| D2 | Tetrafluoro-1-(2,2,2-trifluoroethoxy)ethane |
| DEC | Diethyl carbonate |
| DFEC | Difluoroethylene carbonate |
| DMC | Dimethyl carbonate |
| DMS | Dimethyl sulfite |
| DFBOP | Difluorobis(oxolato)phosphate |
| DFOB | Difluoro(oxalato)borate |
| DTD | 1,3,2-Dioxathiolane-2,2-dioxide |
| EA | Ethyl acetate |
| EB | Ethyl butyrate |
| EC | Ethylene carbonate |
| EIS | Electrochemical impedance spectroscopy |
| EMC | Ethyl methyl carbonate |
| EMIM | 1-Ethyl-3-methylimidazolium |
| EP | Ethyl propionate |
| ETFA | Ethyl trifluoroacetate |
| FEC | Fluoroethylene carbonate |
| FEMC | 2,2,2-Trifluoroethyl methyl carbonate |
| F-EPE | 1,1,2,2-Tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether |
| FI | Fluorosulfonyl isocyanate |
| FSI | Bis(fluorosulfonyl)imide |
| GBL | Gamma-butyrolactone |
| Gr | Graphite |
| IL | Ionic liquid |
| LIB | Lithium-ion battery |
| LTO | Li4Ti5O12 |
| M3 | Methoxyperfluorobutane |
| MA | Methyl acetate |
| MB | Methyl butyrate |
| MCMB | Mesocarbon microbead (graphite) |
| MEC | Methylene ethylene carbonate |
| MP | Methyl propionate |
| MPFP | Methyl pentafluoropropionate |
| NCA | LiNi0.8Co0.15Al0.05O2 |
| NMCxyz | LiNi0.xMn0.yCo0.zO2 |
| PB | Propyl butyrate |
| PC | Propylene carbonate |
| PCS | 1,3-Propanediol cyclic sulfate |
| PES | Prop-1-ene-1,3-sultone |
| PP13 | N-Methyl-N-propylpiperidinium |
| PS | 1,3-Propane sultone |
| Pyr13 | N-Methyl-N-propylpyrrolidinium |
| Pyr14 | N-Butyl-N-methylpyrrolidinium |
| R bulk | Bulk electrolyte ionic resistance |
| R ct | Charge-transfer resistance |
| R SEI | SEI ionic resistance |
| SEI | Solid–electrolyte interphase |
| TDI | 2-Trifluoromethyl-4,5-dicyanoimidazole |
| TFEA | 2,2,2-Trifluoroethyl acetate |
| TFEB | 2,2,2-Trifluoroethyl butyrate |
| TFENH | 2,2,2-Trifluoroethyl n-caproate |
| TFSI | Bis(trifluoromethanesulfonyl)imide |
| THF | Tetrahydrofuran |
| THTO | Tetrahydrothiophene-1-oxide |
| TMSPi | Tris(trimethylsilyl)phosphite |
| VC | Vinylene carbonate |
| XPS | X-ray photoelectron spectroscopy |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.References
- M. C. Smart, B. V. Ratnakumar, R. C. Ewell, S. Surampudi, F. J. Puglia and R. Gitzendanner, Electrochim. Acta, 2018, 268, 27–40 CrossRef CAS.
- T. Chen, Y. Jin, H. Lv, A. Yang, M. Liu, B. Chen, Y. Xie and Q. Chen, Trans. Tianjin Univ., 2020, 26, 208–217 CrossRef.
- L. Trahey, F. R. Brushett, N. P. Balsara, G. Ceder, L. Cheng, Y.-M. Chiang, N. T. Hahn, B. J. Ingram, S. D. Minteer, J. S. Moore, K. T. Mueller, L. F. Nazar, K. A. Persson, D. J. Siegel, K. Xu, K. R. Zavadil, V. Srinivasan and G. W. Crabtree, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12550–12557 CrossRef CAS PubMed.
- M. Chen, Y. Zhang, G. Xing, S. L. Chou and Y. Tang, Energy Environ. Sci., 2021, 14, 3323–3351 RSC.
- T. M. Bandhauer, S. Garimella and T. F. Fuller, J. Electrochem. Soc., 2011, 158, R1 CrossRef CAS.
- M.-T. F. Rodrigues, G. Babu, H. Gullapalli, K. Kalaga, F. N. Sayed, K. Kato, J. Joyner and P. M. Ajayan, Nat. Energy, 2017, 2, 17108 CrossRef CAS.
- T. Ould Ely, D. Kamzabek and D. Chakraborty, Front. Energy Res., 2019, 7, 71 CrossRef.
- P. V. Chombo and Y. Laoonual, J. Power Sources, 2020, 478, 228649 CrossRef CAS.
- R. Fong, U. von Sacken and J. R. Dahn, J. Electrochem. Soc., 1990, 137, 2009–2013 CrossRef CAS.
- P. Jehnichen, K. Wedlich and C. Korte, Sci. Technol. Adv. Mater., 2019, 20, 1–9 CrossRef CAS.
- A. Tomaszewska, Z. Chu, X. Feng, S. O’Kane, X. Liu, J. Chen, C. Ji, E. Endler, R. Li, L. Liu, Y. Li, S. Zheng, S. Vetterlein, M. Gao, J. Du, M. Parkes, M. Ouyang, M. Marinescu, G. Offer and B. Wu, eTransportation, 2019, 1, 100011 CrossRef.
- S. Hess, M. Wohlfahrt-Mehrens and M. Wachtler, J. Electrochem. Soc., 2015, 162, A3084–A3097 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303–4417 CrossRef CAS PubMed.
- M. Petzl, M. Kasper and M. A. Danzer, J. Power Sources, 2015, 275, 799–807 CrossRef CAS.
- Q. Li, D. Lu, J. Zheng, S. Jiao, L. Luo, C. M. Wang, K. Xu, J. G. Zhang and W. Xu, ACS Appl. Mater. Interfaces, 2017, 9, 42761–42768 CrossRef CAS PubMed.
- R. Payne and I. E. Theodorou, J. Phys. Chem., 1972, 76, 2892–2900 CrossRef CAS.
- B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554–4581 CrossRef PubMed.
- M. S. Ding, K. Xu, S. S. Zhang, K. Amine, G. L. Henriksen and T. R. Jow, J. Electrochem. Soc., 2001, 148, A1196 CrossRef CAS.
- M. S. Ding, K. Xu and T. R. Jow, J. Electrochem. Soc., 2000, 147, 1688 CrossRef CAS.
- J. M. Tarascon and D. Guyomard, Solid State Ionics, 1994, 69, 293–305 CrossRef CAS.
- E. J. Plichta and W. K. Behl, J. Power Sources, 2000, 88, 192–196 CrossRef CAS.
- K. B. Oldham and J. C. Myland, Fundamentals of Electrochemical Science, Academic Press, San Diego, CA, 1994 Search PubMed.
- K. M. Diederichsen, E. J. McShane and B. D. McCloskey, ACS Energy Lett., 2017, 2, 2563–2575 CrossRef CAS.
- P. Barai, K. Higa and V. Srinivasan, Phys. Chem. Chem. Phys., 2017, 19, 20493–20505 RSC.
- A. Ehrl, J. Landesfeind, W. A. Wall and H. A. Gasteiger, J. Electrochem. Soc., 2017, 164, A826–A836 CrossRef CAS.
- I. Villaluenga, D. M. Pesko, K. Timachova, Z. Feng, J. Newman, V. Srinivasan and N. P. Balsara, J. Electrochem. Soc., 2018, 165, A2766–A2773 CrossRef CAS.
- J. Landesfeind, H. A. Gasteiger, J. E. Soc and J. Landesfeind, J. Electrochem. Soc., 2019, 166, A3079 CrossRef.
- L. O. Valoen and J. N. Reimers, J. Electrochem. Soc., 2005, 152, A882 CrossRef CAS.
- A. Nyman, M. Behm and G. Lindbergh, Electrochim. Acta, 2008, 53, 6356–6365 CrossRef CAS.
- H. Lundgren, M. Behm and G. Lindbergh, J. Electrochem. Soc., 2015, 162, A413–A420 CrossRef CAS.
- S. S. Zhang, K. Xu and T. R. Jow, Electrochim. Acta, 2004, 49, 1057–1061 CrossRef CAS.
- R. Jow, S. S. Zhang, K. Xu and J. Allen, ECS Transactions, ECS, 2007, vol. 3, pp. 51–58 Search PubMed.
- D. P. Abraham, J. R. Heaton, S.-H. Kang, D. W. Dees and A. N. Jansen, J. Electrochem. Soc., 2008, 155, A41 CrossRef CAS.
- K. Xu, A. Von Cresce and U. Lee, Langmuir, 2010, 26, 11538–11543 CrossRef CAS PubMed.
- J. Li, C. F. Yuan, Z. H. Guo, Z. A. Zhang, Y. Q. Lai and J. Liu, Electrochim. Acta, 2012, 59, 69–74 CrossRef CAS.
- A. Lewandowski, M. Biegun, M. Galinski and A. Swiderska-Mocek, J. Appl. Electrochem., 2013, 43, 367–374 CrossRef CAS.
- M. C. Smart, J. Electrochem. Soc., 1999, 146, 486 CrossRef CAS.
- B. Liu, Q. Li, M. H. Engelhard, Y. He, X. Zhang, D. Mei, C. Wang, J. G. Zhang and W. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 21496–21505 CrossRef CAS PubMed.
- X. Yu and A. Manthiram, Energy Environ. Sci., 2018, 11, 527–543 RSC.
- Y. Chu, Y. Shen, F. Guo, X. Zhao, Q. Dong, Q. Zhang, W. Li, H. Chen, Z. Luo and L. Chen, Electrochem. Energy Rev., 2020, 3, 187–219 CrossRef.
- C. Fang, Z. Liu, J. Lau, M. Elzouka, G. Zhang, P. Khomein, S. Lubner, P. N. Ross and G. Liu, J. Electrochem. Soc., 2020, 167, 020506 CrossRef CAS.
- T. Waldmann, B.-I. Hogg and M. Wohlfahrt-Mehrens, J. Power Sources, 2018, 384, 107–124 CrossRef CAS.
- Y. Liu, Y. Zhu and Y. Cui, Nat. Energy, 2019, 4, 540–550 CrossRef.
- K. G. Gallagher, D. W. Dees, A. N. Jansen, D. P. Abraham and S.-H. Kang, J. Electrochem. Soc., 2012, 159, A2029–A2037 CrossRef CAS.
- C. Uhlmann, J. Illig, M. Ender, R. Schuster and E. Ivers-Tiffée, J. Power Sources, 2015, 279, 428–438 CrossRef CAS.
- T. Waldmann, B.-I. Hogg, M. Kasper, S. Grolleau, C. G. Couceiro, K. Trad, B. P. Matadi and M. Wohlfahrt-Mehrens, J. Electrochem. Soc., 2016, 163, A1232–A1238 CrossRef CAS.
- E. J. McShane, A. M. Colclasure, D. E. Brown, Z. M. Konz, K. Smith and B. D. McCloskey, ACS Energy Lett., 2020, 5, 2045–2051 CrossRef CAS.
- B. V. Ratnakumar and M. C. Smart, ECS Trans., 2010, 25, 241–252 CAS.
- M. Petzl and M. A. Danzer, J. Power Sources, 2014, 254, 80–87 CrossRef CAS.
- B. Ng, P. T. Coman, E. Faegh, X. Peng, S. G. Karakalos, X. Jin, W. E. Mustain and R. E. White, ACS Appl. Energy Mater., 2020, 3(4), 3653–3664 CrossRef CAS.
- T. Waldmann, M. Wilka, M. Kasper, M. Fleischhammer and M. Wohlfahrt-Mehrens, J. Power Sources, 2014, 262, 129–135 CrossRef CAS.
- N. Ghanbari, T. Waldmann, M. Kasper, P. Axmann and M. Wohlfahrt-Mehrens, J. Phys. Chem. C, 2016, 120, 22225–22234 CrossRef CAS.
- I. D. Campbell, M. Marzook, M. Marinescu and G. J. Offer, J. Electrochem. Soc., 2019, 166, A725–A739 CrossRef.
- Z. M. Konz, E. J. Mcshane and B. D. Mccloskey, ACS Energy Lett., 2020, 15, 41 Search PubMed.
- C. Vidal, O. Gross, R. Gu, P. Kollmeyer and A. Emadi, IEEE Trans. Veh. Technol., 2019, 68, 4560–4572 Search PubMed.
- Y. Ji and C. Y. Wang, Electrochim. Acta, 2013, 107, 664–674 CrossRef CAS.
- S. Hein, T. Danner, D. Westhoff, B. Prifling, R. Scurtu, L. Kremer, A. Hoffmann, A. Hilger, M. Osenberg, I. Manke, M. Wohlfahrt-Mehrens, V. Schmidt and A. Latz, J. Electrochem. Soc., 2020, 167, 013546 CrossRef.
- C.-K. Huang, J. S. Sakamoto, J. Wolfenstine and S. Surampudi, J. Electrochem. Soc., 2000, 147, 2893 CrossRef CAS.
- T. L. Kulova, A. M. Skundin, E. A. Nizhnikovskii and A. V. Fesenko, Russ. J. Electrochem., 2006, 42, 259–262 CrossRef CAS.
- Y. Ji, Y. Zhang and C.-Y. Wang, J. Electrochem. Soc., 2013, 160, A636–A649 CrossRef CAS.
- K. Xu, Chem. Rev., 2014, 114, 11503–11618 CrossRef CAS PubMed.
- G. Zhu, K. Wen, W. Lv, X. Zhou, Y. Liang, F. Yang, Z. Chen, M. Zou, J. Li, Y. Zhang and W. He, J. Power Sources, 2015, 300, 29–40 CrossRef CAS.
- A. M. Haregewoin, A. S. Wotango and B. J. Hwang, Energy Environ. Sci., 2016, 9, 1955–1988 RSC.
- Z. Xu, J. Yang, H. Li, Y. Nuli and J. Wang, J. Mater. Chem. A, 2019, 7, 9432–9446 RSC.
- C. Wang, Y. S. Meng and K. Xu, J. Electrochem. Soc., 2019, 166, A5184–A5186 CrossRef CAS.
- L. Liao, P. Zuo, Y. Ma, Y. An, G. Yin and Y. Gao, Electrochim. Acta, 2012, 74, 260–266 CrossRef CAS.
- L. Liao, X. Cheng, Y. Ma, P. Zuo, W. Fang, G. Yin and Y. Gao, Electrochim. Acta, 2013, 87, 466–472 CrossRef CAS.
- X. B. Cheng, R. Zhang, C. Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- A. S. Wotango, W. N. Su, A. M. Haregewoin, H. M. Chen, J. H. Cheng, M. H. Lin, C. H. Wang and B. J. Hwang, ACS Appl. Mater. Interfaces, 2018, 10, 25252–25262 CrossRef CAS PubMed.
- J. Wang, F. Lin, H. Jia, J. Yang, C. W. Monroe and Y. Nuli, Angew. Chem., Int. Ed., 2014, 53, 10099–10104 CrossRef CAS PubMed.
- X. Qi, L. Tao, H. Hahn, C. Schultz, D. R. Gallus, X. Cao, S. Nowak, S. Röser, J. Li, I. Cekic-Laskovic, B. R. Rad and M. Winter, RSC Adv., 2016, 6, 38342–38349 RSC.
- Y. K. Han, J. Yoo and T. Yim, J. Mater. Chem. A, 2015, 3, 10900–10909 RSC.
- T. Yim and Y. K. Han, ACS Appl. Mater. Interfaces, 2017, 9, 32851–32858 CrossRef CAS PubMed.
- G. Xu, S. Huang, Z. Cui, X. Du, X. Wang, D. Lu, X. Shangguan, J. Ma, P. Han, X. Zhou and G. Cui, J. Power Sources, 2019, 416, 29–36 CrossRef CAS.
- M. Xu, Acta Phys. Chim. Sin., 2006, 22, 335–340 CrossRef CAS.
- L. Liao, T. Fang, X. Zhou, Y. Gao, X. Cheng, L. Zhang and G. Yin, Solid State Ionics, 2014, 254, 27–31 CrossRef CAS.
- S. Li, X. Li, J. Liu, Z. Shang and X. Cui, Ionics, 2015, 21, 901–907 CrossRef CAS.
- R. Guo, Y. Che, G. Lan, J. Lan, J. Li, L. Xing, K. Xu, W. Fan, L. Yu and W. Li, ACS Appl. Mater. Interfaces, 2019, 11, 38285–38293 CrossRef CAS PubMed.
- B. Liao, H. Li, M. Xu, L. Xing, Y. Liao, X. Ren, W. Fan, L. Yu, K. Xu and W. Li, Adv. Energy Mater., 2018, 8, 1800802 CrossRef.
- J. Shi, N. Ehteshami, J. Ma, H. Zhang, H. Liu, X. Zhang, J. Li and E. Paillard, J. Power Sources, 2019, 429, 67–74 CrossRef CAS.
- C. Wang, L. Yu, W. Fan, J. Liu, L. Ouyang, L. Yang and M. Zhu, ACS Appl. Energy Mater., 2018, 1, 2647–2656 CrossRef CAS.
- K.-E. Kim, J. Y. Jang, I. Park, M.-H. Woo, M.-H. Jeong, W. C. Shin, M. Ue and N.-S. Choi, Electrochem. Commun., 2015, 61, 121–124 CrossRef CAS.
- B. Yang, H. Zhang, L. Yu, W. Z. Fan and D. Huang, Electrochim. Acta, 2016, 221, 107–114 CrossRef CAS.
- Q. Lei, T. Yang, X. Zhao, W. Fan, W. Wang, L. Yu, S. Guo, X. Zuo, R. Zeng and J. Nan, J. Electroanal. Chem., 2019, 846, 1131412 CrossRef.
- L. Hamenu, H. S. Lee, M. Latifatu, K. M. Kim, J. Park, Y. G. Baek, J. M. Ko and R. B. Kaner, Curr. Appl. Phys., 2016, 16, 611–617 CrossRef.
- J. H. Won, H. S. Lee, L. Hamenu, M. Latifatu, Y. M. Lee, K. M. Kim, J. Oh, W. Il Cho and J. M. Ko, J. Ind. Eng. Chem., 2016, 37, 325–329 CrossRef CAS.
- H. Xiang, D. Mei, P. Yan, P. Bhattacharya, S. D. Burton, A. Von Wald Cresce, R. Cao, M. H. Engelhard, M. E. Bowden, Z. Zhu, B. J. Polzin, C. M. Wang, K. Xu, J. G. Zhang and W. Xu, ACS Appl. Mater. Interfaces, 2015, 7, 20687–20695 CrossRef CAS PubMed.
- J. Zheng, P. Yan, R. Cao, H. Xiang, M. H. Engelhard, B. J. Polzin, C. Wang, J. G. Zhang and W. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 5715–5722 CrossRef CAS PubMed.
- Q. Li, S. Jiao, L. Luo, M. S. Ding, J. Zheng, S. S. Cartmell, C. M. Wang, K. Xu, J. G. Zhang and W. Xu, ACS Appl. Mater. Interfaces, 2017, 9, 18826–18835 CrossRef CAS PubMed.
- H.-J. Buysch, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000, pp. 565–582 Search PubMed.
- R. Petibon, J. Harlow, D. B. Le and J. R. Dahn, Electrochim. Acta, 2015, 154, 227–234 CrossRef CAS.
- L. Ma, S. L. Glazier, R. Petibon, J. Xia, J. M. Peters, Q. Liu, J. Allen, R. N. C. Doig and J. R. Dahn, J. Electrochem. Soc., 2017, 164, A5008–A5018 CrossRef CAS.
- M. Haruta, T. Okubo, Y. Masuo, S. Yoshida, A. Tomita, T. Takenaka, T. Doi and M. Inaba, Electrochim. Acta, 2017, 224, 186–193 CrossRef CAS.
- X. Fan, X. Ji, L. Chen, J. Chen, T. Deng, F. Han, J. Yue, N. Piao, R. Wang, X. Zhou, X. Xiao, L. Chen and C. Wang, Nat. Energy, 2019, 4, 882–890 CrossRef CAS.
- M. J. Piernas-Muñoz, S. E. Trask, A. R. Dunlop, E. Lee and I. Bloom, J. Power Sources, 2019, 441, 227080 CrossRef.
- M. C. Smart, B. V. Ratnakumar, S. Surampudi, Y. Wang, X. Zhang, S. G. Greenbaum, A. Hightower, C. C. Ahn and B. Fultz, J. Electrochem. Soc., 1999, 146, 3963 CrossRef CAS.
- M. C. Smart, B. V. Ratnakumar and S. Surampudi, J. Electrochem. Soc., 2002, 149, A361 CrossRef CAS.
- Y. Ein-Eli, S. R. Thomas, R. Chadha, T. J. Blakley and V. R. Koch, J. Electrochem. Soc., 1997, 144, 823 CrossRef CAS.
- M. C. Smart, B. V. Ratnakumar, K. B. Chin and L. D. Whitcanack, J. Electrochem. Soc., 2010, 157, A1361 CrossRef CAS.
- K. A. Smith, M. C. Smart, G. K. S. Prakash and B. V. Ratnakumar, ECS Trans., 2008, 11, 91–98 CrossRef CAS.
- W. Lu, K. Xie, Z. Chen, S. Xiong, Y. Pan and C. Zheng, J. Power Sources, 2015, 274, 676–684 CrossRef CAS.
- X. Ma, R. S. Arumugam, L. Ma, E. Logan, E. Tonita, J. Xia, R. Petibon, S. Kohn and J. R. Dahn, J. Electrochem. Soc., 2017, 164, A3556–A3562 CrossRef CAS.
- X. Ma, J. Li, S. L. Glazier, L. Ma, K. L. Gering and J. R. Dahn, Electrochim. Acta, 2018, 270, 215–223 CrossRef CAS.
- J.-P. Jones, M. C. Smart, F. C. Krause and R. V. Bugga, J. Electrochem. Soc., 2020, 167, 020536 CrossRef CAS.
- E. R. Logan, E. M. Tonita, K. L. Gering, J. Li, X. Ma, L. Y. Beaulieu and J. R. Dahn, J. Electrochem. Soc., 2018, 165, A21–A30 CrossRef CAS.
- K. Chen, Z. Yu, S. Deng, Q. Wu, J. Zou and X. Zeng, J. Power Sources, 2015, 278, 411–419 CrossRef CAS.
- X. Dong, Z. Guo, Z. Guo, Y. Wang and Y. Xia, Joule, 2018, 2, 902–913 CrossRef CAS.
- J. F. Côté, D. Brouillette, J. E. Desnoyers, J. F. Rouleau, J. M. St-Arnaud and G. Perron, J. Solution Chem., 1996, 25, 1163–1173 CrossRef.
- M. L. Lazar and B. L. Lucht, J. Electrochem. Soc., 2015, 162, A928–A934 CrossRef CAS.
- P. Shi, S. Fang, J. Huang, D. Luo, L. Yang and S. I. Hirano, J. Mater. Chem. A, 2017, 5, 19982–19990 RSC.
- Y. Gu, S. Fang, L. Yang and S. Ichi Hirano, Electrochim. Acta, 2021, 394, 139120 CrossRef CAS.
- H. F. Xiang, B. Yin, H. Wang, H. W. Lin, X. W. Ge, S. Xie and C. H. Chen, Electrochim. Acta, 2010, 55, 5204–5209 CrossRef CAS.
- I. A. Shkrob, T. W. Marin, Y. Zhu and D. P. Abraham, J. Phys. Chem. C, 2014, 118, 19661–19671 CrossRef CAS.
- M. Kunze, S. Jeong, G. B. Appetecchi, M. Schönhoff, M. Winter and S. Passerini, Electrochim. Acta, 2012, 82, 69–74 CrossRef CAS.
- F. Wohde, M. Balabajew and B. Roling, J. Electrochem. Soc., 2016, 163, A714–A721 CrossRef CAS.
- M. Yamagata, Y. Matsui, T. Sugimoto, M. Kikuta, T. Higashizaki, M. Kono and M. Ishikawa, J. Power Sources, 2013, 227, 60–64 CrossRef CAS.
- W. Wang, T. Yang, S. Li, W. Fan, X. Zhao, C. Fan, L. Yu, S. Zhou, X. Zuo, R. Zeng and J. Nan, Electrochim. Acta, 2019, 317, 146–154 CrossRef CAS.
- K. Oldiges, J. Michalowsky, M. Grünebaum, N. von Aspern, I. Cekic-Laskovic, J. Smiatek, M. Winter and G. Brunklaus, J. Power Sources, 2019, 437, 226881 CrossRef CAS.
- C. S. Rustomji, Y. Yang, T. K. Kim, J. Mac, Y. J. Kim, E. Caldwell, H. Chung and Y. S. Meng, Science, 2017, 356, eaal4263 CrossRef PubMed.
- Y. Yang, M. Daniel, Y. Yin, C. S. Rustomji, Y. Yang, D. M. Davies, Y. Yin, O. Borodin, J. Z. Lee, C. S. Rustomji and Y. S. Meng, Joule, 2019, 3, 1986–2000 CrossRef CAS.
- Y. Yang, Y. Yin, D. M. Davies, M. Zhang, M. Mayer, Y. Zhang, E. S. Sablina, S. Wang, J. Z. Lee, O. Borodin, C. S. Rustomji and Y. S. Meng, Energy Environ. Sci., 2020, 13, 2209–2219 RSC.
- S. Zhang, K. Xu and T. Jow, Electrochem. Commun., 2002, 4, 928–932 CrossRef CAS.
- S. S. Zhang, K. Xu and T. R. Jow, J. Solid State Electrochem., 2003, 7, 147–151 CrossRef CAS.
- K. Xu, S. S. Zhang, U. Lee, J. L. Allen and T. R. Jow, J. Power Sources, 2005, 146, 79–85 CrossRef CAS.
- T. R. Jow, M. S. Ding, K. Xu, S. S. Zhang, J. L. Allen, K. Amine and G. L. Henriksen, J. Power Sources, 2003, 119–121, 343–348 CrossRef CAS.
- K. Xu, S. Zhang and R. Jow, US Pat., US8632918B2, 2014 Search PubMed.
- K. Xu, J. Electrochem. Soc., 2008, 155, A733 CrossRef CAS.
- S. Shui Zhang, Electrochem. Commun., 2006, 8, 1423–1428 CrossRef.
- H. Zhou, K. Xiao and J. Li, J. Power Sources, 2016, 302, 274–282 CrossRef CAS.
- B. K. Mandal, A. K. Padhi, Z. Shi, S. Chakraborty and R. Filler, J. Power Sources, 2006, 162, 690–695 CrossRef CAS.
- J. Chen, J. Vatamanu, L. Xing, O. Borodin, H. Chen, X. Guan, X. Liu, K. Xu and W. Li, Adv. Energy Mater., 2020, 10, 1902654 CrossRef CAS.
- M. Becker, R. S. Kühnel and C. Battaglia, Chem. Commun., 2019, 55, 12032–12035 RSC.
- H. I. Kim, E. Shin, S. H. Kim, K. M. Lee, J. Park, S. J. Kang, S. So, K. C. Roh, S. K. Kwak and S. Y. Lee, Energy Storage Mater., 2021, 36, 222–228 CrossRef.
- S.-T. Myung, Y. Hitoshi and Y.-K. Sun, J. Mater. Chem., 2011, 21, 9891 RSC.
- X. Zuo, J. Zhu, P. Müller-Buschbaum and Y.-J. Cheng, Nano Energy, 2017, 31, 113–143 CrossRef CAS.
- G. G. Eshetu and E. Figgemeier, ChemSusChem, 2019, 12, 2515–2539 CrossRef CAS PubMed.
- H. Zhao, W. Yuan and G. Liu, Nano Today, 2015, 10, 193–212 CrossRef CAS.
- U. S. Kasavajjula and C. Wang, Indian J. Chem. - Sect. A Inorganic, Phys. Theor. Anal. Chem., 2005, 44, 975–982 Search PubMed.
- E. Markevich, G. Salitra and D. Aurbach, J. Electrochem. Soc., 2016, 163, A2407–A2412 CrossRef CAS.
- T. Subburaj, W. Brevet, F. Farmakis, D. Tsiplakides, S. Balomenou, N. Strataki, C. Elmasides, B. Samaniego and M. Nestoridi, Electrochim. Acta, 2020, 354, 0–8 CrossRef CAS.
- K. Richter, T. Waldmann, N. Paul, N. Jobst, R. G. Scurtu, M. Hofmann, R. Gilles and M. Wohlfahrt-Mehrens, ChemSusChem, 2020, 13, 529–538 CrossRef CAS PubMed.
- Y. Yan, L. Ben, Y. Zhan and X. Huang, Electrochim. Acta, 2016, 187, 186–192 CrossRef CAS.
- D. H. C. Wong, J. L. Thelen, Y. Fu, D. Devaux, A. A. Pandya, V. S. Battaglia, N. P. Balsara and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3327–3331 CrossRef CAS PubMed.
- X. Ren, S. Chen, H. Lee, D. Mei, M. H. Engelhard, S. D. Burton, W. Zhao, J. Zheng, Q. Li, M. S. Ding, M. Schroeder, J. Alvarado, K. Xu, Y. S. Meng, J. Liu, J.-G. Zhang and W. Xu, Chem, 2018, 4, 1877–1892 CAS.
- Y. Zhao, C. Fang, G. Zhang, D. Hubble, A. Nallapaneni, C. Zhu, Z. Zhao, Z. Liu, J. Lau, Y. Fu and G. Liu, Front. Chem., 2020, 8, 1–9 CrossRef PubMed.
Footnote |
| † An important exception may be found in large-format cells at high discharge rates, where poor Li+ diffusivity can cause localized high- or low-concentration hotspots which dominate behavior (see ref. 60). |
| This journal is © The Royal Society of Chemistry 2022 |