Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemosensors based on N-heterocyclic dyes: advances in sensing highly toxic ions such as CN− and Hg2+
María-Camila Ríos
,
Néstor-Fabián Bravo
,
Christian-Camilo Sánchez
and
Jaime Portilla
 *
*
Bioorganic Compounds Research Group, Department of Chemistry, Universidad de los Andes, Carrera 1 No. 18A-10, Bogotá 111711, Colombia. E-mail: jportill@uniandes.edu.co
First published on 21st October 2021
Abstract
CN− and Hg2+ ions are harmful to both the environment and human health, even at trace levels. Thus, alternative methods for their detection and quantification are highly desirable given that the traditional monitoring systems are expensive and require qualified personnel. Optical chemosensors (probes) have revolutionized the sensing of different species due to their high specificity and sensitivity, corresponding with their modular design. They have also been used in aqueous media and different pH ranges, facilitating their applications in various samples. The design of molecular probes is based on organic dyes, where the key species are N-heterocyclic compounds (NHCs) due to their proven photophysical properties, biocompatibility, and synthetic versatility, which favor diverse applications. Accordingly, this review aims to provide an overview of the reports from 2016 to 2021, in which fluorescent probes based on five- and six-membered N-heterocycles are used for the detection of CN− and Hg2+ ions.
1. Introduction
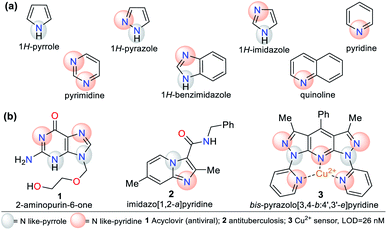
N-Heterocyclic compounds (NHCs) are molecules in which nitrogen atoms (N) replace one or more carbon atoms in their ring. These compounds have a nucleophilic or basic character due to the unbound electrons pair in the nitrogen atom-like-pyrrole (–N) or -pyridine (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), respectively.1,2 In saturated NHCs, the heteroatom behaves similarly to that in alkylamines; however, in heteroaromatic rings, the N-like-pyrrole contributes to the π-conjugation and allows special electronic properties, which depend on the substituents and fused or rigid structural nature of the rings.1–4 Various NHCs are present in natural products such as nucleic acids, coenzymes, amino acids, and alkaloids.1–6 NHCs are classified based on the number of bonds and nitrogen atoms in their ring, from three members onwards. Five- and six-membered rings such as pyrroles, imidazoles, pyrazoles, pyridines, and pyrimidines are more common due to their high stability and applicability. These compounds can also be classified based on their saturation or fusion with other rings, e.g., benzimidazole and quinoline are benzo-fused cores of five- and six-membered rings, respectively (Fig. 1a).1–8
N), respectively.1,2 In saturated NHCs, the heteroatom behaves similarly to that in alkylamines; however, in heteroaromatic rings, the N-like-pyrrole contributes to the π-conjugation and allows special electronic properties, which depend on the substituents and fused or rigid structural nature of the rings.1–4 Various NHCs are present in natural products such as nucleic acids, coenzymes, amino acids, and alkaloids.1–6 NHCs are classified based on the number of bonds and nitrogen atoms in their ring, from three members onwards. Five- and six-membered rings such as pyrroles, imidazoles, pyrazoles, pyridines, and pyrimidines are more common due to their high stability and applicability. These compounds can also be classified based on their saturation or fusion with other rings, e.g., benzimidazole and quinoline are benzo-fused cores of five- and six-membered rings, respectively (Fig. 1a).1–8
N-Heterocyclic systems have many applications due to their high synthetic versatility and significant structural diversity obtained by varying their substituent or functional groups. These applications include relevant biological effects such as anticoagulant,9 anti-inflammatory,10 antimicrobial,11,12 and anticancer13 agents. Additionally, they can be applied in photophysical and materials sciences. N-Heterocycles have been applied for the design and synthesis of new luminescent compounds such as organic light-emitting diodes (OLEDs),14 technological devices,15 hybrid-phosphorescent materials,16 and more frequently, fluorescent probes for the detection of different species17–20 (Fig. 1b).
1.1. Photophysical features of N-heterocycles
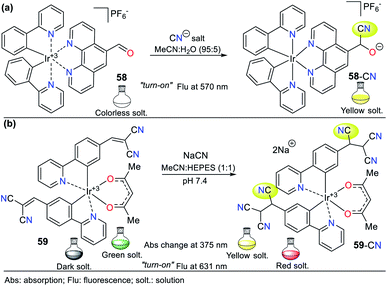
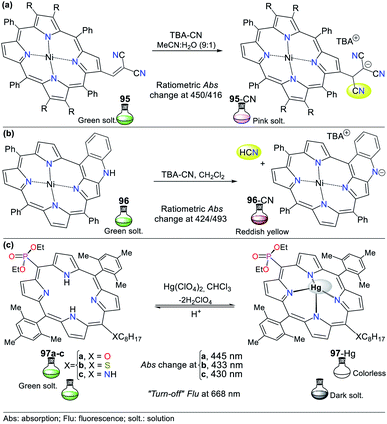
The physicochemical properties of N-heteroaromatic systems are unique given that saturated derivatives behave analogous to open-chain compounds.1,2 Thus, NHCs tend to be conjugated molecules with unique and intrinsic photophysical properties, which can be employed for the development of sensors and organic materials. For instance, porphyrins (pyrrole tetramer) have high absorption in the visible spectrum red region and are potential dyes for sensitizers in photodynamic therapy. This feature promotes studies on the synthesis of porphyrins bearing aromatic substituents on their rings to extend their π-conjugation. The inclusion of chromophore groups in the porphyrinic ring enhances its photophysical properties and versatility, resulting in a shift in absorption wavelength. Attractive examples of π-extended conjugation porphyrins are systems in which aryl and acenaphthylene groups are adequately incorporated.1,21Regarding probes bearing an N-heterocyclic core that distinguish cyanide (CN−) and mercury(II) (Hg2+) ions, obviously they include those possessing other heteroatoms besides nitrogen. Indeed, the thiazole ring has nitrogen and sulfur atoms, and its derivatives have been used for this purpose. The oligothiophene–benzothiazole 4 is a colorimetric and fluorimetric probe for sensing CN− with a limit of detection (LOD) of 4.60 × 10−7 M in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which acts via a nucleophilic addition reaction on the electrophilic cyanovinylidene group of 4. Colorimetric studies showed that 4 has an absorption band at 349 nm attributed to the π–π* transitions and another at 485 nm due to the internal charge transfer (ICT) process (Fig. 2a).22
1), which acts via a nucleophilic addition reaction on the electrophilic cyanovinylidene group of 4. Colorimetric studies showed that 4 has an absorption band at 349 nm attributed to the π–π* transitions and another at 485 nm due to the internal charge transfer (ICT) process (Fig. 2a).22
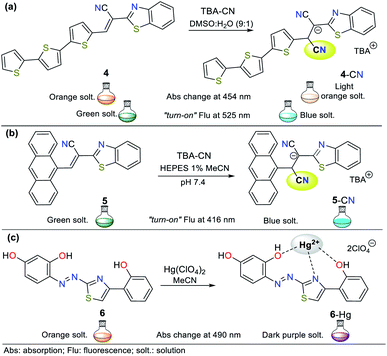 | ||
Fig. 2 Probes based on thiazoles for CN− sensing in (a) DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and (b) in HEPES solution (1% MeCN as a co-solvent), and (c) for Hg2+ recognition. H2O and (b) in HEPES solution (1% MeCN as a co-solvent), and (c) for Hg2+ recognition. | ||
Upon the addition of CN− to a solution of 4, the band at 485 nm decreases with the appearance of a new band at 353 nm and an isosbestic point at 411 nm accompanied with a color change from orange to colorless. Besides, emission studies showed a color change from pale green to fluorescent blue with the addition of CN−. The spectra for the titration showed a blue-shift in its fluorescence band from 560 nm to 525 nm (λexc = 353 nm). The color changes occur due to the interruption of both the π-conjugation and ICT process (Fig. 2a).22 Similarly, Vidya et al.23 developed chemodosimeter 5 for sensing CN− (LOD = 5.52 × 10−8 M), working via “turn-on” fluorescence in a ratiometric manner (Fig. 2b). This probe also acts via the disruption of the ICT process, where in this case, it occurs between the anthracene and benzothiazole moieties.
Alternatively, Yeap and co-workers24 synthesized the resorcinol derivative 6 bearing the 2-thiazolyldiazenyl group at position 4, which works for the colorimetric detection of Hg2+ (LOD = 1.20 × 10−7 M in MeCN). This probe shows two absorption bands at 350 and 465 nm, which are assigned to the n–π* and π–π* transitions of the aryl-azo group and the thiazole ring, respectively (Fig. 2c). Upon the addition of Hg2+ to the solution of 6 in acetonitrile, a new band appears at 490 nm, changing from orange to purple. The redshifted band (465 nm) decreased with an increase in the cation concentration, while a new band appeared and increased, accompanied by an isosbestic point at 476 nm.
Although probes 4 to 6 work well for the detection of CN− and Hg2+, their N-heterocyclic cores also possess a sulfur heteroatom. Thus, these probes use a signaling unit based on different heteroatoms (N and S); however, the probes discussed throughout this review have a signaling unit based mainly on rings bearing only one type of heteroatom, i.e., one or two nitrogen atoms. Additionally, both five- and six-membered rings considered herein are not only found as chromophores or fluorophores but as fused systems or high π-conjugation molecules. This is due to the synthetic and photophysical versatility of NHCs, which allow their good functionalization and utility in ion detection, respectively. Ultimately, because of this, we managed to focus our discussion on the strategic rings investigated for the recognition of different species.
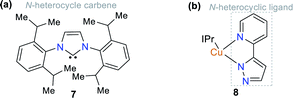
Some NHCs have been used as ligands of organometallic and coordination compounds. Their combination with metals can modify their charge transfer features and some photophysical properties in the resulting complex.18,25 For example, complexes with carbene derivatives such as 1,3-bis(2,6-diisopropylphenyl)imidazol-2-yl-idene (IPr, structure 7) and coordinating metals such as copper(I) (complex 8) show significant photophysical properties (Fig. 3).25,26 Regarding coordination complexes, some derivatives with N,N-donor ligands are notable optical materials.27 Thus, complexes based on N-heterocyclic compounds represent a good option for the development of luminescent materials and applications related to fluorescence properties such as molecular organic electronics and monitoring applications in both environmental and biological systems.28–31
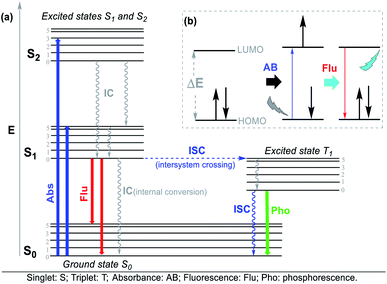
The Jablonski diagram (Fig. 4a) is suitable to interpret the molecular photophysical routes (Fig. 4b) given that it shows the energies of the electronic states and the interconversion processes. This diagram displays two photophysical pathways, i.e., the radiative (indicated by straight lines) and non-radiative (denoted by wavy lines) pathways. The former occurs via the absorption or emission of light, whereas the latter occurs without these routes.32 Absorbance (Abs), fluorescence (Flu), and phosphorescence (Pho) are the typical photophysical processes occurring on NHCs. Abs (blue line) involves one-electron excitation from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) in a dye molecule, that is, a transition from the ground state to the excited state. In the Flu process (red line), photon emission is accompanied by a transition from the excited state to the ground state without a change in multiplicity. The Pho processes (green line) are the emission from photons accompanied by a transition from the excited state to the ground state, where a change in multiplicity usually occurs from the triplet excited state to singlet ground state (Fig. 4).32
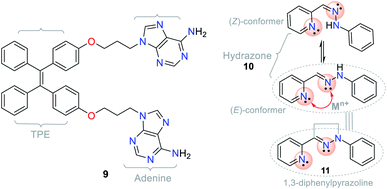
The different photophysical pathways allow NHCs to be employed as strategic molecules for sensing studies via mechanisms such as ICT, twisted intramolecular charge transfer (TICT), and photoinduced electron transfer (PET). The synthetic versatility provided by these compounds for the modification of their photophysical properties makes them a good option for sensing systems, and accordingly, for the detection of analytes. For instance, the bis-adenine–tetraphenylethylene (TPE) hybrid system (probe 9) showed fluorescence “turn-on” in the presence of Ag+ via the aggregation-induced emission (AIE) phenomenon (Fig. 5),17,18,20,33,34 in which the adenine-mediated coordination of the silver ions induced the aggregation of TPE, resulting in a fluorescent system.34
Another example involving NHCs is the isomerization of the hydrazone (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N) moiety, where the signaling route originates from the conformationally restricted molecules, as shown in the photophysical study. For instance, hydrazone 10 has a freely rotating C
N–N) moiety, where the signaling route originates from the conformationally restricted molecules, as shown in the photophysical study. For instance, hydrazone 10 has a freely rotating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, while pyrazoline (cyclic hydrazone) 11 has restricted rotation. When hydrazones are isomerized, their fluorescence is low or non-existent. Nevertheless, several pyrazoline derivatives show high fluorescence intensities, as evidenced in PET “turn-on” processes (PET-ON) when the isomerization of some hydrazones is restricted in the presence of metal ions (Fig. 5, red arrow).35 In hydrazone-free derivatives, this conformational phenomenon occurs because the isomerization is the principal decay process of the excited states, making these molecules non-fluorescent.33
N bond, while pyrazoline (cyclic hydrazone) 11 has restricted rotation. When hydrazones are isomerized, their fluorescence is low or non-existent. Nevertheless, several pyrazoline derivatives show high fluorescence intensities, as evidenced in PET “turn-on” processes (PET-ON) when the isomerization of some hydrazones is restricted in the presence of metal ions (Fig. 5, red arrow).35 In hydrazone-free derivatives, this conformational phenomenon occurs because the isomerization is the principal decay process of the excited states, making these molecules non-fluorescent.33
Exited state intramolecular proton transfer (ESIPT) is a notable process that also involves the above-mentioned phenomenon, in which the protons of the system involved in the excited state depart or join a molecule at different rates to that in the ground state, for instance, the fluorescent properties incorporate the ground and excited states of two different tautomers. This process usually involves the transfer of a proton from a hydroxyl (OH) or amino (NH2) group to a carbonyl oxygen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) or imine nitrogen (C
O) or imine nitrogen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) that are no more than 2 Å apart. Thus, it can be expected that molecular probes for “turn-on” or “turn-off” fluorescence33 based on NHCs will be useful.
N) that are no more than 2 Å apart. Thus, it can be expected that molecular probes for “turn-on” or “turn-off” fluorescence33 based on NHCs will be useful.
1.2. Chemosensors for the detection of CN− and Hg2+
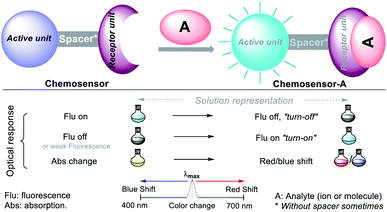
Chemical detection via colorimetry or fluorimetry is a promising qualitative and quantitative method for ion or molecule sensing in the environment and medicine. The advantages of this method include the use of simple equipment, short-term detection, high selectivity and sensitivity, and avoiding sample pretreatment.17,18,20,24,36–39 Numerous screening tests are performed with the standard atomic absorption (AA) methods and inductively coupled plasma emission spectroscopy (ICP-AES) to quantify different ions with high efficiency and at trace levels.36–39 The rapidly developed molecular sensor chemistry strongly relates to photochemistry given that the probe interaction with the analyte results in variations in their photophysical properties and spectral changes in the chemosensor–analyte complexes.40A chemosensor is a chemical species capable of transforming a molecular change into a measurable analytical signal (color or fluorescence). This variation generates electronic changes, and thus photophysical changes in the host molecule, promoting the signal in the chromophore or fluorophore unit of the chemosensor. Precisely, the probe consists of a receptor moiety, which is responsible for the selective binding of the analyte (ion or molecule). The properties of this photoactive unit change upon binding, and in some cases, a spacer, due to its flexibility, can modify the system geometry and tune the interaction between the receiver and photoactive unit (Fig. 6).36–39 This recognition allows numerous electronic phenomena to occur; consequently, fluorescence “turn-off” or “turn-on” occurs.
Among the various analytes, many metals are considered harmful to tissues and organs after prolonged exposure. Diverse human health and environmental effects have been described in the literature; thus, the detection of heavy and transition metals is an essential goal in both the biological and ecological fields.41 Similarly, the synthesis of molecular sensors for the detection of anions is relevant due to their toxic effects in nature.42 For instance, CN− is known for its extreme toxicity, and thus many conventional recognition approaches (i.e., electrochemical, potentiometric, titrimetric, and voltametric methods) have been developed for its quantitative analysis.43 However, several of these methods use robust and expensive equipment, complex procedures and require a long analysis time.
1.3. Aims of this review
This contribution seeks to highlight some progress in the recent works (from 2016 to 2021) on fluorescent and colorimetric chemosensors (probes) based on NHCs for the recognition of highly toxic ions such as CN− and Hg2+. Additionally, we present and evaluate the recognition units and fluorescent and sensing processes of these probes, providing valuable tools for designing new probes for the detection of hazardous ions. For the analysis and detection of ions, there is a plethora of information on sensors for different toxic species; however, the two above-mentioned species have a more significant environmental and human impact given that they can cause death in high concentrations. Likewise, it is vital to analyze and control the maximum limits of CN− and Hg2+ to comply with current legislation to prevent some foods or residues in industry from harming human health.This review focuses only on examples of NHCs without other heteroatoms (i.e., O or S), increasing the importance of this group of compounds; in this case, up to two nitrogens are included in each ring. This review discusses the most recent advances in optical chemosensors for the detection of cyanide, and subsequently for mercury recognition. For both species, data will be presented on the LODs, absorption and emission bands, key further details, and association constants for complexes formed with Hg2+ ions. To the best of our knowledge, this is the first review that collects information regarding the application of N-heterocyclic compounds in the detection of highly toxic ions such as CN− and Hg2+. Therefore, we hope that this review will be a helpful contribution to both applied heterocyclic synthesis and ion recognition, considering the high versatility of N-heterocycles.
2. Chemosensors for CN− sensing
Cyanide is a highly toxic ion in nature and to humans. Its exposure at low concentrations (1.15 × 10−4 M) to humans via different routes such as inhalation, ingestion, and skin contact can cause chronic diseases and even death.44 In mammals, CN− absorption causes cell death given that it inactivates mitochondrial cytochrome C oxidase, impeding the mitochondrial electron transfer cycle. Thus, CN− inhibits oxidative phosphorylation and ATP production, leading to blockage of the cellular respiration process.44–46 Although cyanide is a deadly agent, many industries use it in mineral extraction, electroplating, and the manufacture of synthetic fibers. For example, illegal gold mining uses cyanide and mercury salts to extract the metal, poisoning the environment and the population that uses nearby water sources.Due to the toxic scope of CN−, the World Health Organization (WHO) has established a limit for the concentration of this anion in drinking water of 1.90 × 10−6 M.35,47 Besides, cyanide has been used in different terrorist attacks worldwide,48 limiting the use of this raw material and establishing security measures to control its use. Therefore, in recent years, many researchers have focused their effort into developing economic and fast analytic methodologies where low LODs, high sensibility, and selectivity can be achieved. In addition, these methods must have an easy analytical preparation that can replace the conventional techniques such as gas chromatography (GC), GC-mass spectrometry (GC-MS),49 high-performance liquid chromatography (HPLC), and HPLC-MS.50 In this context, fluorescent and colorimetric probes have aroused the interest of many organic and analytical chemists given that they offer advantages in the above-mentioned situations.
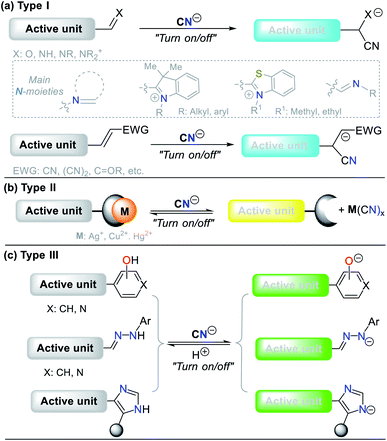
The design of probes for sensing cyanide involves three different strategies. (I) Nucleophilic addition reaction of CN− on an electrophilic carbon of a carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), azomethine (C
O), azomethine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), or iminium (C
N), or iminium (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+) group, and a carbon Cβ of a vinyl moiety conjugated with electron withdrawing groups (EWG) (Fig. 7a). (II) Displacement of a metal ion (often Cu2+) from the coordination sphere of a complex to yield a cyano-metallated compound (M(CN)x); in this case, the coordination sphere of the complex has a fluorophore, which being free from Cu2+, turns on the fluorescence (Fig. 7b). (III) The reaction of CN− as a base to deprotonate the acidic hydrogen present in phenol derivatives (ArOH), hydrazones (R1HN–N
N+) group, and a carbon Cβ of a vinyl moiety conjugated with electron withdrawing groups (EWG) (Fig. 7a). (II) Displacement of a metal ion (often Cu2+) from the coordination sphere of a complex to yield a cyano-metallated compound (M(CN)x); in this case, the coordination sphere of the complex has a fluorophore, which being free from Cu2+, turns on the fluorescence (Fig. 7b). (III) The reaction of CN− as a base to deprotonate the acidic hydrogen present in phenol derivatives (ArOH), hydrazones (R1HN–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–R2), or NH-heterocyclic compounds (Fig. 7c).
C–R2), or NH-heterocyclic compounds (Fig. 7c).
2.1. Pyrrole derivatives
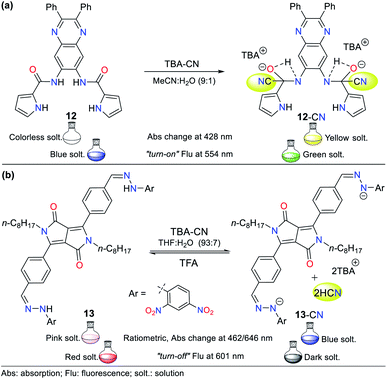
Pyrrole derivatives are one of the most significant N-heterocyclic systems due to their excellent properties in different fields such as materials science, pharmacology, and polymers. Many pyrrole derivatives exist, among which some pyrroles, indoles, carbazoles, BODIPYs, and porphyrins stand out due to the high π-conjugation in these heteroaromatic–polycyclic systems.50–56 The pyrrole ring itself has rarely been applied as a molecular probe core in detection chemistry.Therefore, herein, we present only one example of a probe bearing a free pyrrole ring linked to a quinoxaline ring attached by an amide fragment (probe 12, Fig. 8a), which reported in 2006.51 This probe exhibits absorption bands at 291 and 378 nm and shows a weak emission band at 425 nm (λexc = 363 nm). After the addition of CN− to 12 in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the absorption bands decreased while three new bands appeared (at 299, 372, and 428 nm) accompanied with three isosbestic points (at 293, 360, and 391 nm), and a color change from colorless to yellow was observed. Likewise, the fluorescence studies showed that the emission band had a redshift with strong intensity at 554 nm, and the color of the solution changed from blue to green. This probe follows detection strategy I via the nucleophilic addition of CN− on the carbonyl groups (C
1), the absorption bands decreased while three new bands appeared (at 299, 372, and 428 nm) accompanied with three isosbestic points (at 293, 360, and 391 nm), and a color change from colorless to yellow was observed. Likewise, the fluorescence studies showed that the emission band had a redshift with strong intensity at 554 nm, and the color of the solution changed from blue to green. This probe follows detection strategy I via the nucleophilic addition of CN− on the carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the amide moieties in 12 (Fig. 8a).
O) of the amide moieties in 12 (Fig. 8a).
In 2016, Wang et al.52 reported the synthesis of probe 13 based on a diketopyrrolopyrrole (DPP) bearing a hydrazone moiety as a selective colorimetric and “turn-off” fluorescent probe for the detection of CN− (LOD = 2.30 × 10−7 M in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O). This probe works by detection strategy III with the DPP moiety as the fluorophore. The absorption spectrum of 13 showed two peaks at 396 and 516 nm. Upon the addition of CN− to the solution, these two peaks shifted to 462 and 646 nm and were enhanced with an increase in the concentration of CN−, leading to the appearance of two isosbestic points at 315 and 439 nm. This variation was accompanied by a color change from pink to indigo. The emission studies showed that 13 was strongly fluorescent (λexc/em = 516/601 nm), which was quenched by up to 92% with the progressive addition of CN−, and a minor blue shift in its the emission band was observed. These results are attributed to the deprotonation of hydrazone, which enhances the electron-donating ability of the nitrogen atom, facilitating the PET process with fluorescence “turn-off” (Fig. 8b). Under a UV lamp, the probe solution appeared to fluoresce red and turn dark upon the addition of CN−.
H2O). This probe works by detection strategy III with the DPP moiety as the fluorophore. The absorption spectrum of 13 showed two peaks at 396 and 516 nm. Upon the addition of CN− to the solution, these two peaks shifted to 462 and 646 nm and were enhanced with an increase in the concentration of CN−, leading to the appearance of two isosbestic points at 315 and 439 nm. This variation was accompanied by a color change from pink to indigo. The emission studies showed that 13 was strongly fluorescent (λexc/em = 516/601 nm), which was quenched by up to 92% with the progressive addition of CN−, and a minor blue shift in its the emission band was observed. These results are attributed to the deprotonation of hydrazone, which enhances the electron-donating ability of the nitrogen atom, facilitating the PET process with fluorescence “turn-off” (Fig. 8b). Under a UV lamp, the probe solution appeared to fluoresce red and turn dark upon the addition of CN−.
 | ||
| Fig. 9 Probes based on indoles with (a) thiazolium salt 14/15, (b) pyridinium salt 16/17, and (c) spirochromene moiety 18. | ||
Alternatively, titration experiments with CN− were carried out for compounds 15 and 17, showing absorption bands at 471 nm and 425 nm, respectively, which decreased with the addition CN−, and no new absorption bands were observed. Colorimetric changes from yellow to colorless and a gradual decrease in fluorescence were observed for both probes. In the sensing reaction for all the probes (14 to 17), the classic CN− addition to the iminium group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+) occurs; however, for 14 and 16, a hydrogen bond is also formed with the analyte followed by deprotonation of the indole ring NH group. Therefore, both strategies I and III are followed. After the addition of cyanide on the double bond, the intramolecular charge transfer is broken, thus quenching the fluorescence (Fig. 9a and b).
N+) occurs; however, for 14 and 16, a hydrogen bond is also formed with the analyte followed by deprotonation of the indole ring NH group. Therefore, both strategies I and III are followed. After the addition of cyanide on the double bond, the intramolecular charge transfer is broken, thus quenching the fluorescence (Fig. 9a and b).
As the final example of indole derivatives, Erdemir and co-workers developed probe 18 this year.54 This probe showed a ratiometric absorbance change with bands at 415 and 542 nm. Once CN− was added to 18 in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a new band emerged at 425 nm, and the color of the solution changed from purple to colorless. In its spectrum, the formation of an isosbestic point at 460 nm indicated the presence of an 18-CN adduct. Accordingly, the authors explored the emission properties of 18, revealing that the compound alone exhibit a weak emission band at 475 nm given that its ICT process is deficient. However, after the CN− addition reaction on the spiro carbon atom of 18 (analogous to strategy I), an enhancement in fluorescence was observed due to the ring-opening of spiropyrane, making this probe a “turn-on” sensor for CN− with an LOD of 2.08 × 10−7 M (Fig. 9c).
1), a new band emerged at 425 nm, and the color of the solution changed from purple to colorless. In its spectrum, the formation of an isosbestic point at 460 nm indicated the presence of an 18-CN adduct. Accordingly, the authors explored the emission properties of 18, revealing that the compound alone exhibit a weak emission band at 475 nm given that its ICT process is deficient. However, after the CN− addition reaction on the spiro carbon atom of 18 (analogous to strategy I), an enhancement in fluorescence was observed due to the ring-opening of spiropyrane, making this probe a “turn-on” sensor for CN− with an LOD of 2.08 × 10−7 M (Fig. 9c).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), the absorption band at 472 nm gradually decreased, while the band at 320 nm increased with an isosbestic point at 385 nm. A significant color change from orange-red to colorless was accompanied by this. This chemosensor follows strategy I via the nucleophilic addition of CN− to the C
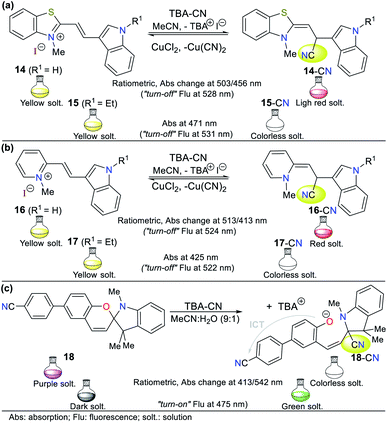
9), the absorption band at 472 nm gradually decreased, while the band at 320 nm increased with an isosbestic point at 385 nm. A significant color change from orange-red to colorless was accompanied by this. This chemosensor follows strategy I via the nucleophilic addition of CN− to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ group in benzothiazole salt 19, inhibiting the π-conjugation and the ICT process. Similarly, two emission peaks were observed when probe 19 was excited at 330 nm (424 and 589 nm). The progressive addition of CN− caused gradual fluorescence quenching of the band at 589 nm with an enhancement of the peak at 424 nm, generating an isoemissive point at 538 nm, which led to bright blue emission in the probe (LOD = 9.00 × 10−8 M).
N+ group in benzothiazole salt 19, inhibiting the π-conjugation and the ICT process. Similarly, two emission peaks were observed when probe 19 was excited at 330 nm (424 and 589 nm). The progressive addition of CN− caused gradual fluorescence quenching of the band at 589 nm with an enhancement of the peak at 424 nm, generating an isoemissive point at 538 nm, which led to bright blue emission in the probe (LOD = 9.00 × 10−8 M).
 | ||
| Fig. 10 Probes based on carbazoles bearing (a) benzothiazole 19 and (b) barbituric acid 20 moieties. | ||
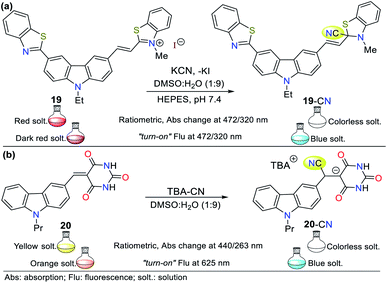
Zou and co-workers reported another example in 2019,56 involving the synthesis of a carbazole-based colorimetric and fluorescent probe (compound 20) with AIE phenomena and LOD of 6.74 × 10−8 M. This probe exhibited absorption bands between 270 and 350 nm attributed to the π–π* transitions of the barbituric acid and the carbazole unit. Additionally, a sharp peak assigned to the ICT process from the carbazole ring to the barbituric acid moiety was observed at 434 nm. Upon the addition of CN−, the solution turned from yellow to colorless. Alternatively, fluorescence spectra were recorded in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) given that water promotes the aggregation of the probe, leading to an enhanced emission. The studies showed that an increase in the content of water in the solution led to an AIE-active molecule with a strong fluorescence. When the mixture was H2O
9) given that water promotes the aggregation of the probe, leading to an enhanced emission. The studies showed that an increase in the content of water in the solution led to an AIE-active molecule with a strong fluorescence. When the mixture was H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (99
DMSO (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the emission peak appeared at 623 nm (λexc = 440 nm). Upon the addition of CN−, the fluorescence of the solution turned from orange to blue (Fig. 10b). In this case, probe 20 follows strategy I, meaning that cyanide acted as a nucleophile.
1), the emission peak appeared at 623 nm (λexc = 440 nm). Upon the addition of CN−, the fluorescence of the solution turned from orange to blue (Fig. 10b). In this case, probe 20 follows strategy I, meaning that cyanide acted as a nucleophile.
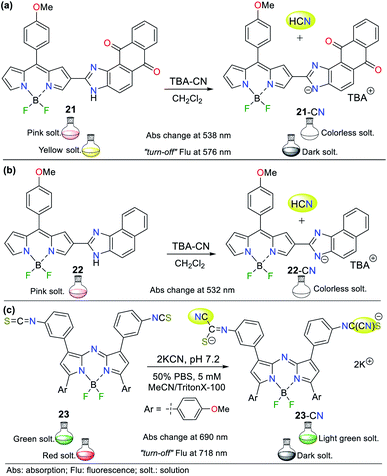
In 2019, Piyanuch et al. reported a fluorescent isothiocyanate-aza-BODIPY NIR (near infrared) probe for sensing CN− with an LOD of 7.52 × 10−7 M (compound 23, Fig. 11c).50 This probe was tested in aqueous PBS buffer (pH 7.2) in acetonitrile (with TritonX-100), which showed fluorescence “turn-off” with the addition of the CN− anion. Additionally, this molecule exhibited selectivity towards CN− and discriminated other anions such as HPO4−, HSO4−, Br−, and NO3−. In this case, 23 works following design strategy I via the nucleophilic addition of CN− to the isothiocyanate moiety of 23, providing a more water-soluble dianion. The colorimetric studies showed that the absorption band at 690 nm decreased with the addition of CN− accompanied by a color change from deep green to light green. Similarly, its emission spectrum showed a decrease in the band at 718 nm (λexc = 680 nm) with the addition of CN−, and a change in color from red to a dark solution under a UV lamp.
2.2. Pyrazole derivates
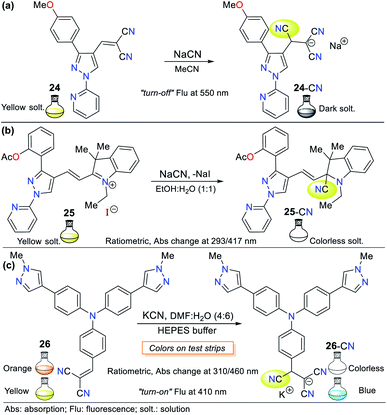
Pyrazole is a 5-membered heteroaromatic ring having two vicinal nitrogen atoms. Although few pyrazole derivates have been reported for the recognition of cyanide, our research group has interestingly advanced this matter. Among them, the most relevant compounds are those with a signaling unit based on fused systems such as pyrazolo[1,5-a]pyrimidines, and pyrazolo[3,4-b]pyridines. For example, our group previously studied probe 3, as shown in Fig. 1, which exhibited fluorescence “turn-on” by sensing mechanism II.Regarding the pyrazole ring itself, Orrego-Hernández and Portilla57 reported the synthesis of 3-aryl-4-dicyanovinyl-1-(2-pyridinyl)pyrazoles following design strategy I. Among the different aryl groups studied (4-O2NPh, Ph, and 4-MeOPh) in these pyrazoles, 4-methoxyphenyl derivative 24 is a fluorescent donor–π–acceptor (D–π–A) system that acts via the ICT phenomenon. This probe exhibited fluorescence quenching upon the addition of CN− and presented a bigger absorption band (∼360 nm) compared to the other pyrazoles tested due to the donor character of its aryl group (Fig. 12a). Furthermore, this probe displayed high selectivity, appreciable quantum yields and a short CN− sensing time with an LOD of 6.80 × 10−6 M. For 24, the ICT process is responsible for the high Stokes shift and bathochromic effect on its emission spectra with an increase in the solvent polarity.
Another pyrazole example based on probe 24 reported by us is the hemicyanine–arylpyrazole hybrid system, where the aryl group electron-donor character was modulated (i.e., Ph, 4-MeOPh, 3,4,5-(MeO)3Ph, 4-AcOPh, and 2-AcOPh) to achieve a better design.58 3-(2-Acetoxyphenyl)pyrazole 25 exhibited the best sensing properties but only as a colorimetric probe given that all the hybrid systems tested showed low fluorescence quantum yields in various solvents. This probe works through the nucleophilic attack of CN− on its iminium group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+), interrupting the D–π–A system. Consequently, a color change from deep yellow to colorless was observed in a solution of 25 (EtOH
N+), interrupting the D–π–A system. Consequently, a color change from deep yellow to colorless was observed in a solution of 25 (EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with an LOD of 9.90 × 10−7 M (Fig. 12b).
1) with an LOD of 9.90 × 10−7 M (Fig. 12b).
The UV-vis spectra of probe 25 in solvents with different polarity exhibited two absorption bands (293/417 nm) attributed to the π–π* transitions and an S0 → ICT process from the pyrrole-like nitrogen (N1) atom in the pyrazole ring to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ group. The band at 417 nm disappeared upon the addition of CN−, indicating that the ICT process was broken, while the intensity of the other band increased for this colorimetric and ratiometric probe. Importantly, the steric effect occurring between the acetyl group and the azolic nitrogen (N2) in 25 prevents the π-conjugation from the aryl group towards the acceptor moiety; thus, ICT occurs only from N1, which possibly is the reason for its better performance compared to the other pyrazoles tested.58
N+ group. The band at 417 nm disappeared upon the addition of CN−, indicating that the ICT process was broken, while the intensity of the other band increased for this colorimetric and ratiometric probe. Importantly, the steric effect occurring between the acetyl group and the azolic nitrogen (N2) in 25 prevents the π-conjugation from the aryl group towards the acceptor moiety; thus, ICT occurs only from N1, which possibly is the reason for its better performance compared to the other pyrazoles tested.58
Siva and Beneto reported a similar method using triphenylamine (TPA) derivative 26, having a dicyanovinylidene group as the receptor unit (Fig. 12c).59 Probe 26 also acts by design strategy I, where the addition of CN− to the acceptor moiety interrupts the ICT process of the molecule, thus resulting in a suitable colorimetric and fluorimetric sensor. This probe exhibited two absorption bands at 310 and 460 nm, where the former increased with the addition of CN−, whereas the latter decreased, creating an isosbestic point and a change in color from red to colorless (LOD = 4.40 × 10−8 M). Similarly, fluorescence studies showed that 26 exhibits moderate blue fluorescence at 400 nm (λexc = 320 nm). The fluorescence intensity increased with the addition of CN−, turning the fluorescence of the solution to a stronger blue. Unfortunately, these findings were not quantified. Notably, the presence of a pyrazolic ring in 26 induces a bathochromic shift from around 440 to 460 nm in the absorption band in comparison to similar triphenylamine derivatives, which allows this compound to act as a ratiometric and colorimetric probe.
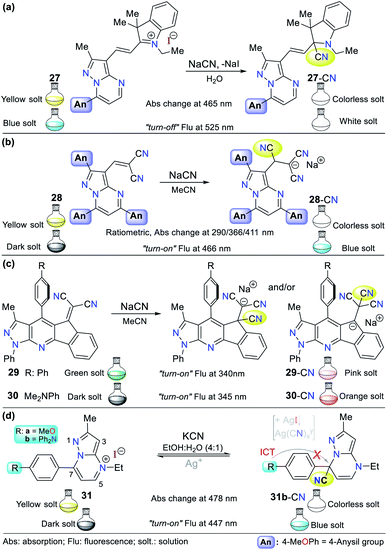
Alternatively, our group continued research on CN− sensing, this time using fused pyrazoles, which evidently possess higher π-conjugation and structural rigidity than single pyrazoles, and thus enhanced photophysical properties. The first case is hybrid system 27 (a pyrazolo[1,5-a]pyrimidine–hemicyanine), which works as a colorimetric and fluorometric probe in 100% aqueous solution (Fig. 13a).60 The probe acts via the nucleophilic attack of CN− on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ bond of its indolium moiety, which blocks the ICT process. This probe was tested on three different solvents (DCM, EtOH, and water) and showed a good quantum yield (φ = 0.42) in aqueous medium with an emission band at 525 nm (λexc = 464 nm). Upon the addition of CN−, color change was observed in solution from yellow to colorless (LOD = 6.00 × 10−7 M at 464 nm, ε = 41
N+ bond of its indolium moiety, which blocks the ICT process. This probe was tested on three different solvents (DCM, EtOH, and water) and showed a good quantum yield (φ = 0.42) in aqueous medium with an emission band at 525 nm (λexc = 464 nm). Upon the addition of CN−, color change was observed in solution from yellow to colorless (LOD = 6.00 × 10−7 M at 464 nm, ε = 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1 cm−1), while the fluorimetric studies showed that the emission band was reduced accompanied by a color change from green-cyan to white (LOD = 8.60 × 10−8 M).
400 M−1 cm−1), while the fluorimetric studies showed that the emission band was reduced accompanied by a color change from green-cyan to white (LOD = 8.60 × 10−8 M).
Inspired by the results obtained in the development of probes bearing pyrazole derivatives for cyanide sensing, we recently reported a second case based on the pyrazolo[1,5-a]pyrimidine (PP) core, but now connected to a dicyanovinylidene group.61 The effect of the peripheral 4-anisyl (4-MeOPh) substituents at positions 2, 5, and 7 on the fused ring was studied to achieve a better-designed probe. According to the results, probe 28 bearing three 4-anisyl groups exhibited the highest selectivity towards CN− (Fig. 13b). Upon the addition of CN− to 28 in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the three absorption bands (at 290/366/411 nm) generated varied, resulting in a change in the solution color from light yellow to white with an LOD of up to 6.50 × 10−7 M considering the three detection channels. The probe showed fluorescence “turn-on”, changing the color of the solution from yellow to light blue (λexc/em = 300/466 nm) with an LOD of 1.70 × 10−7 M. Both probes mentioned above (27 and 28) follow design strategy I via the addition of CN− on an electrophilic carbon. Notably, the PP core has been successfully used as a fluorochromophore linked with two types of recognition units (i.e., dicyanovinylidene group and indolium salt) in the development of CN− sensing probes.
1), the three absorption bands (at 290/366/411 nm) generated varied, resulting in a change in the solution color from light yellow to white with an LOD of up to 6.50 × 10−7 M considering the three detection channels. The probe showed fluorescence “turn-on”, changing the color of the solution from yellow to light blue (λexc/em = 300/466 nm) with an LOD of 1.70 × 10−7 M. Both probes mentioned above (27 and 28) follow design strategy I via the addition of CN− on an electrophilic carbon. Notably, the PP core has been successfully used as a fluorochromophore linked with two types of recognition units (i.e., dicyanovinylidene group and indolium salt) in the development of CN− sensing probes.
A different fused pyrazole also reported by our group is based on the pyrazolo[3,4-b]pyridine core.62 In this case, some strategic products of an indeno[1,2-b]pyrazolo[4,3-e]pyridine-5-one library having aryl groups at position 4 of the fused-ring were used as precursors of the novel dicyanovinyl substituted systems. The products were found to be tunable ICT fluorophores and could be used as chemodosimeters for the detection of CN−. Two of the compounds tested, where the aryl substituents were benzene and 4-dimethylaniline (4-DMA) rings (29 and 30 in Fig. 13c, respectively), showed the best results.
The absorption spectra for probes 29 and 30 exhibited two characteristic bands at around 300 and 430 nm, which are attributed to the π–π* transitions and the So → ICT process from the pyrrole-like nitrogen of the pyrazole moiety to the nitrile groups. After the addition of CN− to the probe solution in acetonitrile, the band at around 430 nm decreased, showing that the nucleophilic attack to the dicyanovinylidene moiety occurred. Thus, the sensor works by sensing strategy I. Curiously, the emission spectra of both compounds showed opposite results. Upon the addition of CN−, 29 (4-Ph, λexc/em = 340/490 nm) exhibited a new band at around 620 nm, whereas for 30 (4-DMA, λexc/em = 345/550 nm), its emission band decreased, which was accompanied by a color change from green to light pink and from dark to bright pink, respectively.
The last offered example about fused pyrazoles involves an ideal probe (compound 31b) also bearing a pyrazolo[1,5-a]pyrimidine core, in which Portilla and collaborators synthesized the novel pyrazolo[1,5-a]pyrimidinium salts 31a and 31b substituted at position 7 with anisyl (31a) or TPA (31b) as strong electron donor groups (EDGs), respectively.63 In this case, nucleophilic attack of the cyanide occurs on the N-heterocyclic core, which interrupts the π-conjugation, blocking the ICT process inside the probe molecule (strategy I). The absorbance experiments for 31b in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4
H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed two bands at 327 (decreases) and 478 (increases) nm, which changed gradually with the successive addition of CN− to the solution. The changes proceeded with the appearance of an isosbestic point at 370 nm and a color change in the solution from yellow to colorless. However, the fluorescence studies showed that the probe has a weak emission band at 447 nm, which increased with the addition of CN−, making this a “turn-on” probe.
1) showed two bands at 327 (decreases) and 478 (increases) nm, which changed gradually with the successive addition of CN− to the solution. The changes proceeded with the appearance of an isosbestic point at 370 nm and a color change in the solution from yellow to colorless. However, the fluorescence studies showed that the probe has a weak emission band at 447 nm, which increased with the addition of CN−, making this a “turn-on” probe.
In short, 31b detects CN− via notable selectivity/sensitivity (34 different species were tested) and three spectroscopic channels (LOD(Abs) of 1.50 nM at 327/478 nm and LOD(Flu) = 2.50 nM at 447 nm). Remarkably, 31b can act as a reversible probe by adding silver ions (from AgNO3). The sensing mechanism was confirmed using DFT calculations and 1H NMR and HRMS experiments, suggesting that the reaction proceeds via nucleophilic attack at position 7 of 31. Ultimately, the practical applicability of 31b was proven using test strips, studies in the solid state (with silica and almonds), and tap water.63
2.3. Imidazole derivates
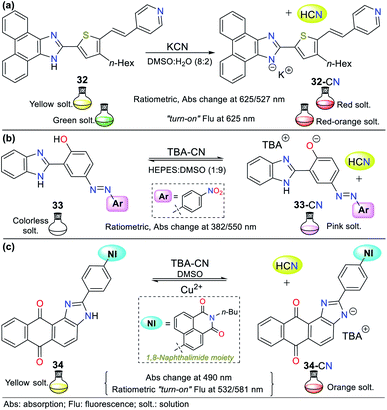
Although the imidazole ring is usually found in natural molecules, few examples for sensing CN− have been reported. Most probes are based on benzimidazole and other fused analogs. Accordingly, Siva and Beneto,59 in addition to pyrazole derivative 26 (see Fig. 12), synthesized phenanthro[9,10-d]imidazole 32 for the selective detection of CN− via design strategy III (Fig. 14a). This probe showed absorption (400 nm) and emission bands (527 nm) attributed to an ICT process from the fused-imidazole moiety to the pyridine ring of the substituent at position 2. After the addition of CN− in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8
H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), deprotonation of the NH-azolic occurs, improving the donor moiety electron density, and thus favoring the ICT process. The absorption spectrum of 32 showed three bands (at 300/400/460 nm), and upon the addition of CN−, the band at 400 nm was reduced while the two other peaks increased, producing two isosbestic points at 420 and 350 nm. The fluorescence studies showed that the emission bands at 527 and 625 nm gradually decreased and increased, respectively, with an increase in the concentration of CN−, generating an isoemissive point at 600 nm. The sensor changed its color from light yellow to red, which could be observed both by the naked eye and under a UV lamp, and the LOD was 3.30 × 10−8 M.
2), deprotonation of the NH-azolic occurs, improving the donor moiety electron density, and thus favoring the ICT process. The absorption spectrum of 32 showed three bands (at 300/400/460 nm), and upon the addition of CN−, the band at 400 nm was reduced while the two other peaks increased, producing two isosbestic points at 420 and 350 nm. The fluorescence studies showed that the emission bands at 527 and 625 nm gradually decreased and increased, respectively, with an increase in the concentration of CN−, generating an isoemissive point at 600 nm. The sensor changed its color from light yellow to red, which could be observed both by the naked eye and under a UV lamp, and the LOD was 3.30 × 10−8 M.
In an interesting example developed by Wang's research group, the authors synthesized and applied benzimidazole derivative 33 for the colorimetric recognition of CN− with an LOD of 1.18 × 10−9 M in HEPES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1
DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, buffer solution).64 The absorption spectrum of 33 showed a moderate-intensity band at 382 nm due to the π–π* transitions and another band with weak intensity at 550 nm attributed to n–π* transitions. Firstly, competition studies were performed, demonstrating that with ratios lower than 1
9, buffer solution).64 The absorption spectrum of 33 showed a moderate-intensity band at 382 nm due to the π–π* transitions and another band with weak intensity at 550 nm attributed to n–π* transitions. Firstly, competition studies were performed, demonstrating that with ratios lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 of buffer solution, the probe was not only sensitive to CN− but also to other anions such as F−, AcO− and H2PO4−, although 33 showed the highest selectivity to CN− in a 4
9 of buffer solution, the probe was not only sensitive to CN− but also to other anions such as F−, AcO− and H2PO4−, although 33 showed the highest selectivity to CN− in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio of this solution. The addition of CN− to the probe solution changed its color from colorless to pink, whereas no color change was observed with other anions (Fig. 14b).
6 ratio of this solution. The addition of CN− to the probe solution changed its color from colorless to pink, whereas no color change was observed with other anions (Fig. 14b).
Titration with CN− revealed that the band at 382 nm in 33 decreased, while that at 550 nm increased, displaying ratiometric behavior with two isosbestic points at 366 and 448 nm. This probe works by sensing strategy III, where cyanide deprotonates the phenolic hydrogen of 33 and yields an anion that fortifies the ICT process from the oxygen to its nitro group. The imidazole–NH and phenolic–OH deprotonation were confirmed by 1H NMR and HRMS experiments, concluding that the reactions are not simultaneous given that OH acts first due to its higher acidity; therefore, an intramolecular hydrogen bond [Nδ−⋯Hδ+⋯Oδ−] in compound 33 could be established (Fig. 14b).64
In 2018, Son and co-workers65 reported the synthesis of a sensor bearing anthra[1,2-d]imidazole-6,11-dione with a 1,8-naphthalimide moiety (34, Fig. 14c). In this case, the anion interacts with the N–H azolic group by deprotonation (strategy III for CN− sensing). The colorimetric studies (in both solution and test strips) confirmed the CN− selectivity of 34, showing a change in color from yellow to deep orange (under natural and UV light), which is attributed to the ICT process from the imidazole ring to the quinone fragment. Unfortunately, the solution also changed color to light orange in the presence of F−; thus, this sensor is not suitable for the detection of CN− when fluoride ions are present as interferents in the sample to be analyzed. Emission spectra were recorded at two different excitation wavelengths (430 and 520 nm). Excitation at 430 nm showed an emission band at around 532 nm, and excitation at 520 nm exhibited a new intense peak at around 581 nm, in addition to the original peak at 532 nm. The fluorescence titration studies with CN− showed ratiometric behavior at both excitation wavelengths. Additionally, the sensor was reversible in the presence of Cu2+ and suitable for working in the pH range of 2 to 10.
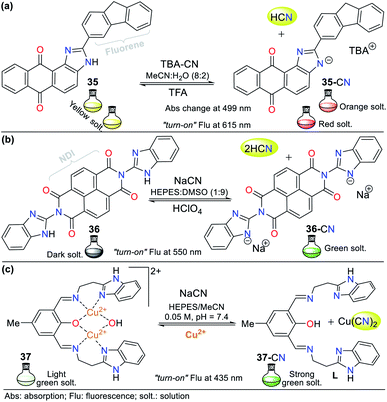
Recently, Bhaskar et al.66 obtained another anthra[1,2-d]imidazole-6,11-dione, but now conjugated to a fluorene moiety (35, Fig. 15a), which is capable of detecting CN− in semi-aqueous media by both colorimetric and fluorimetric methods. The fluorescence studies showed that 35 itself exhibited a band at 585 nm (λexc = 415 nm), while the addition of CN− to the solution led to quenching of the emission band together with a redshift (615 nm). Moreover, only in the presence of CN−, the solution turned from yellow to orange. Finally, the studies on pH within the range of 3 to 10 confirmed the suitability of the sensor for the analysis of real samples. This probe follows the fluorescence mechanism via the ESIPT process between the carbonyl group of the quinone moiety and the N–H azolic group. The addition of CN− leads to the deprotonation of 35, interrupting the ESIPT process and quenching the fluorescence. Additionally, the absorbance studies showed two peaks at 415 and 499 nm, which decreased and increased, respectively, causing two isosbestic points at 455 and 359 nm. The absorbance and fluorescence LODs were found to be 5.30 × 10−6 M and 4.11 × 10−8 M, respectively.
Similarly, Lin and co-workers67 synthesized and studied the bis-benzimidazole 36 as a fluorescent probe for sensing cyanide in HEPES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO buffer solution (1
DMSO buffer solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), which also has a naphthalene-tetracarboxylic diimide (NDI) fluorophore (Fig. 15b). The emission spectrum for 36 alone showed a maximum emission band at 510 nm (λexc = 455 nm), and when CN− was added to the solution, the emission band shifted to 550 nm, with an enhancement in intensity. The color change in the sensor was from dark to green, and the LOD was found to be 8.32 × 10−7 M. In this case, CN− deprotonates the benzimidazole hydrogen (N–H); therefore, the sensing strategy for probe 36 is III.
9), which also has a naphthalene-tetracarboxylic diimide (NDI) fluorophore (Fig. 15b). The emission spectrum for 36 alone showed a maximum emission band at 510 nm (λexc = 455 nm), and when CN− was added to the solution, the emission band shifted to 550 nm, with an enhancement in intensity. The color change in the sensor was from dark to green, and the LOD was found to be 8.32 × 10−7 M. In this case, CN− deprotonates the benzimidazole hydrogen (N–H); therefore, the sensing strategy for probe 36 is III.
Recently, Anbu and co-workers68 reported an unusual probe (complex 37) compared to those described above where, the CN− sensing mechanism occurs via addition or acid–base reactions. This new probe follows recognizing strategy type II and has the ability to act as a fluorogenic differential/sequential probe to identify Cu2+, Zn2+, CN−, P2O74− and DNA. The ligand (L) of complex 37 was obtained by reacting 2-(1H-benzo[d]imidazol-2-yl)ethanamine, 2,6-diformyl-p-cresol, and K2CO3 in ethanol at 40 °C. This ligand can chelate Cu2+ (37) and Zn2+ ions with a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (L: Cu2+, LOD = 2.44 × 10−8 M) and 1
2 (L: Cu2+, LOD = 2.44 × 10−8 M) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (L: Zn2+, LOD = 2.19 × 10−9 M), respectively. The presence of cations caused a decrease in the maximum absorption of L at 440 nm with a blue shift of around 18 nm due to the ligand–metal interactions. Similarly, the maximum absorption at 360 nm decreased markedly, and a new absorption band appeared at around 308 nm as a product of the charge transfer inside 37 and L: Zn2+ (Fig. 15c).
1 (L: Zn2+, LOD = 2.19 × 10−9 M), respectively. The presence of cations caused a decrease in the maximum absorption of L at 440 nm with a blue shift of around 18 nm due to the ligand–metal interactions. Similarly, the maximum absorption at 360 nm decreased markedly, and a new absorption band appeared at around 308 nm as a product of the charge transfer inside 37 and L: Zn2+ (Fig. 15c).
Complex 37 showed a broad band centered at 376 nm and three bands of lower intensity at 360, 385, and 405 nm. The fluorescence spectra of L showed an intense band at 515 nm, which decreased gradually with the addition of Cu2+ (2.50 × 10−5 M) and became red-shifted, resulting in a weak band at 530 nm. The addition of 5 equiv. of NaCN to 37 in MeCN/5.00 × 10−4 M HEPES buffer medium (pH = 7.4) generated a change in its emission spectrum. The intensity of the band at 530 nm increased and it was red-shifted by 5 nm, suggesting competition between L and CN− by the Cu2+ ions. This competition restored up to 99.6% of the initial fluorescence of L and allows the use of 37 as a CN− chemosensor with an LOD of 9.43 × 10−9 M. However, the mechanism by which L does not exhibit the same fluorescence spectrum once it has been released from Cu2+ is not clear. Nevertheless, this probe has high potential for the detection of CN− in biological samples and other matrices such as different water sources.68
2.4. Pyridine derivates
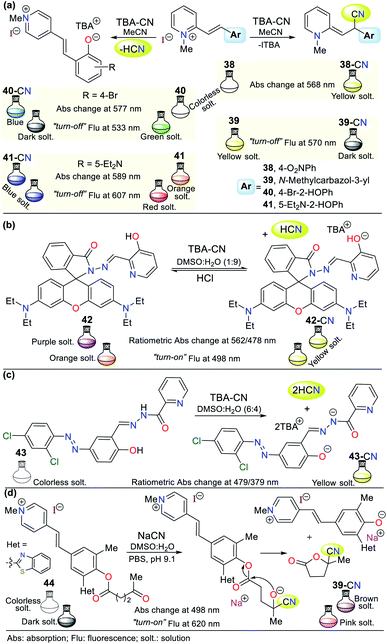
Similar to the imidazole ring, the pyridine ring is very prevalent in the biological environment and studied in the development of probes for the detection of CN−. Accordingly, there are various pyridine derivates such as fused systems and organometallic or coordination complexes. Some crucial characteristics of pyridine derivatives, such as their electronic nature, basicity, synthetic versatility, high stability, and good optical properties, are the reason why these compounds are currently used in the design of chemosensors.1–4,69,70![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) activated with a pyridinium electron-withdrawing group (EWG). This mechanism was confirmed by 1H NMR studies, in which the typical signals of the ethylene group hydrogens disappeared. However, given that 40 and 41 have phenol rings, the acid–base reaction with the anion was confirmed via the 1H NMR signals of the ethylene group hydrogens; thus, the recognition unit for these probes follows strategy II, which acts by diminishing the fluorescence intensity during the sensing process.
C) activated with a pyridinium electron-withdrawing group (EWG). This mechanism was confirmed by 1H NMR studies, in which the typical signals of the ethylene group hydrogens disappeared. However, given that 40 and 41 have phenol rings, the acid–base reaction with the anion was confirmed via the 1H NMR signals of the ethylene group hydrogens; thus, the recognition unit for these probes follows strategy II, which acts by diminishing the fluorescence intensity during the sensing process.
In the absorption spectra of 38, the band at 336 nm decreased gradually with an increase in the concentration of CN− and new peaks appeared at 425 nm and 568 nm. Additionally, an isosbestic point at 373 nm was formed. Similarly, the fluorescence intensity of 39 diminished by 11% with a blue shift of 6 nm from 570 nm to 564 nm. For probe 40, a fluorescence quenching was observed upon the addition of 1.4 equiv. of CN− (band at 533 nm), while for analogous phenol derivative 41, only a 7% reduction in the intensity of the emission band was observed at 607 nm with a redshift of 21 nm. Possibly, the diethylamino group of 41 decreases its reactivity, and thus its sensitivity towards the cyanide addition reaction (Fig. 16a).71
Alternatively, Jing-Han Hu and co-workers synthesized a fluorometric sensor bearing 3-hydroxipyridine and rhodamine B moieties (probe 42), which follows strategy II of cyanide sensing by deprotonation of the OH group in 42.72 This was confirmed via 1H NMR studies; in contrast, the OH group signal at 11.08 ppm disappeared after the successive CN− addition. Similarly, the intensity of the aromatic signals weakened, which appeared at a high field. The color changes were evident to the naked eye, changing from colorless to yellow due to the addition of CN−. Furthermore, a variation in fluorescence from dark to shinny yellow for 42-CN was observed under a UV lamp, following the ICT fluorescence mechanism. Notably, compound 42 could be recovered upon the addition of HCl for up to 8 cycles without significant loss in its effectiveness (Fig. 16b).71
Around the same time, Zheng Li et al.73 synthesized a chromogenic probe (compound 43), in which the sensing route indicated that the cyanide deprotonates not only the OH group from the phenol ring but also the hydrazide hydrogen due to the highly basic character of this anion (Fig. 16c). The absorption spectra of 43 showed a band at 321 nm, which disappeared with an increase in the concentration of CN− and exhibited new bands at 398 and 479 nm, allowing a color change from colorless to yellow to be observed in the presence of cyanide ions. The 1H NMR studies showed the disappearance of the signals at 12.64 (OH) and 12.07 (NH) ppm with the addition of 0.5 equiv. of CN− and the Job method confirmed that the reaction stoichiometry was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (43-CN).
2 (43-CN).
In another recent example, a hemicyanine pyridinium-based salt was used for CN− sensing, which consists of probe 44 reported by Tang and co-workers.74 This probe has a levulinate moiety, and besides CN−, can detect hydrazine (N2H4) due to its unusual sensing mechanism (Fig. 16d). Moreover, 44 can obtained in a single step at room temperature, making it very attractive from an operational point of view; however, the access and cost of some of its precursors and poor reaction performance (31%) are considered significant drawbacks. The absorption spectrum of this compound does not exhibit any band between 400 to 600 nm. However, the addition of cyanide or hydrazine (N2H4) to a solution of 44 in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3
H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) resulted in substantial changes in its absorption spectrum, with an intense band at 498 nm and a color change from colorless to brown, as shown in Fig. 16d.
7) resulted in substantial changes in its absorption spectrum, with an intense band at 498 nm and a color change from colorless to brown, as shown in Fig. 16d.
Alternatively, its fluorescence spectrum shows a band at 620 nm, which increased with the gradually addition of CN− or N2H4 with and LOD of 1.38 × 10−6 M and 5.47 × 10−6 M, respectively. These findings suggest a similar sensing process for both analytes via nucleophilic attack on the carbonyl of the ester group in 44 (strategy I). Then, intramolecular attack occurs, leading to fluorescent phenol derivative 44a and 3-carbonitrile-γ-lactone 44. The 1H NMR studies confirmed this sensing mechanism given that 44 has three signals located at high field assigned to the levulinate portion (CH2 × 2 and CH3), which disappeared upon the addition of CN−. In contrast, a new signal appeared at low field (∼12.60 ppm), which was assigned to the phenolic hydrogen. This probe was tested with real water samples, resulting in recovery rates of the probe for CN− or N2H4 of 92–104% and 94–104%, respectively. Moreover, 44 was shown to be stable in the pH range of 9 to 11.74
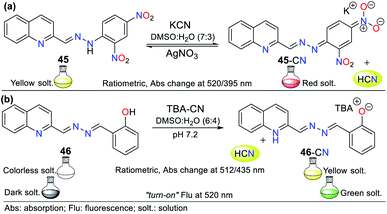
This is the case of probe 45 designed by Wang et al.,75 which allowed the colorimetric detection of CN− in semi-aqueous media. This probe works by strategy III, where the deprotonation of the hydrazone moiety produced an anionic species, leading to a redshift from 395 to 520 nm. This redshift happens given that the elimination of hydrogen in the hydrazone fragment increases the electronic density of nitrogen, reorganizing the charge of the molecule into canonic structures to finally obtain a stable delocalized structure. Thus, the proposed fluorescence mechanism for sensing CN− with 45 is via the ICT process, which is accompanied by a color change from yellow to red. The UV-vis studies exhibited a decrease in the absorption band at 395 nm and an increase in the band at 520 nm upon the successive addition of CN−. The 1H NMR studies confirmed the presence of the anion, wherein the signal at 11.98 ppm is attributed to the disappearance of the hydrazone hydrogen. The addition of Ag+ to the solution of 45-CN reversed the sensing process (up to 4 reuse cycles), which was confirmed by 1H NMR studies with a similar spectrum to that of probe 45, with the signal at 11.98 ppm restored (Fig. 17a).
Another example following strategy III is that reported by Hu and co-workers76 in which the hydrazone of salicylaldehyde and quinoline 46 was synthesized. This probe showed a colorimetric response from colorless to yellow and a fluorescent response in the presence of cyanide (Fig. 17b). The colorimetric changes were studied via UV-vis spectroscopy and titration with 0 to 34.5 equiv. of CN−. Probe 46 showed a band at 320 nm, the intensity of which decreased with the addition of CN−, while the band at 435 nm increased, generating an isosbestic point at 385 nm. Similarly, the probe turned from dark to green under UV light, and the band in its fluorescence emission spectrum shifted from 440 to 516 nm with an increase in intensity upon the addition of 50 equiv. of CN−.
Both the colorimetric and fluorometric changes in 46 are attributed to the deprotonation of its OH group. This mechanism was confirmed via 1H NMR studies, in which the OH group signal at 11.15 ppm disappeared when 2 equiv. of CN− was added. Moreover, the aromatic signals exhibited high-field displacement. The action of CN− on probe 46 resulted in delocalization of the charge in the conjugated system, resulting in the ICT process. Similar to the example mentioned before, this probe was reversible and could be used up to seven cycles in the presence of a protic acid. Finally, both probes (45 and 46) could be used in paper strips for the in situ detection of CN− in aqueous samples.76
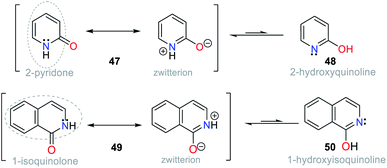
Accordingly, keto–enol tautomerism can result in two resonance structures for 2-hydroxypyridine (48). The keto form 47 corresponds to an amide and resonates with the enol structure (Fig. 18). Similarly, 1-hydroxyisoquinoline (50) is found as amide tautomer 49. This type of N-heterocycle system is used to promote the ICT process, where the formation of an anion produced by deprotonation in the presence of CN− leads to charge displacement, and consequently different canonical structures are formed. In addition, strategically adding an electron donor group at specific positions of the ring can help compensate the electron deficiency, and thus favor the formation of a tautomer and stabilize the system.1–4,69,70
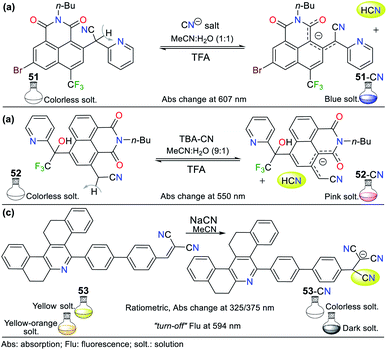
Some fused pyridines for the selective detection of CN− and exhibiting a resonance like 49 were studied by Liu and co-workers77,78 using the 1,8-naphthamide ring as the fluorophore for ICT processes. Initially, the authors introduced a 2-(pyridin-2-yl)acetonitrile group at position 2, obtaining probe 51. For this, they blocked position 6, avoiding the formation of isomers.77 This probe was evaluated against different ions, proving to be active for CN−. Upon the addition of the anion in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the absorbance band at 607 nm increased, causing a change in the color of the solution from colorless to blue. This optical result is due to the deprotonation of one of the Hα adjacent to the nitrile group. The stability of the carbanion formed is favored by the resonant effects, similar to that mentioned above. However, some ions such as HSO4−, F−, H2PO4−, and AcO− were interferents given that they also caused color changes (Fig. 19a).
1), the absorbance band at 607 nm increased, causing a change in the color of the solution from colorless to blue. This optical result is due to the deprotonation of one of the Hα adjacent to the nitrile group. The stability of the carbanion formed is favored by the resonant effects, similar to that mentioned above. However, some ions such as HSO4−, F−, H2PO4−, and AcO− were interferents given that they also caused color changes (Fig. 19a).
Next, the authors reported the synthesis of another 1,8-naphthamide derivative (probe 52). It was not necessary to block position 6 of the N-heterocyclic moiety because the substituent at position 4 favors cyanomethylation at position 2 (Fig. 19b).78 The solution of 52 in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was colorless. However, an increase in the absorbance band at 545 nm occurred upon the addition of CN− to the solution, resulting in a change in color to a pink hue. Unfortunately, the presence of S2−, HSO4−, F−, and AcO− was shown to be interfering ions. Unlike the other probes that follow design strategy II, this probe offers an advantage by deprotonating a Cα instead of groups such as phenols or amines, and thus notable changes in stock displacements are obtained.
1) was colorless. However, an increase in the absorbance band at 545 nm occurred upon the addition of CN− to the solution, resulting in a change in color to a pink hue. Unfortunately, the presence of S2−, HSO4−, F−, and AcO− was shown to be interfering ions. Unlike the other probes that follow design strategy II, this probe offers an advantage by deprotonating a Cα instead of groups such as phenols or amines, and thus notable changes in stock displacements are obtained.
Notably, the reversibility of probes 51 and 52 was evidenced by using a proton source such as trifluoroacetic acid (TFA), allowing these probes to be used for several cycles. Probes 51 and 52 achieved LODs of 1.22 × 10−5 M and 5.53 × 10−7 M, respectively; thus, only probe 52 can detect lower levels of CN− than that allowed by the WHO. Despite this, the works carried out by Liu and co-workers are a clear example of chemosensor design for the detection of anions.78
In this case, Manickam and Iyer79 developed probe 53 for sensing CN−, having tetrahydrodibenzo[a,i]phenanthridine. This probe showed a color change from yellow to colorless with the addition of CN− in acetonitrile according to sensing strategy I (Fig. 19c). The UV-vis studies showed a hypsochromic effect in the absorption band at 375 nm, changing to 325 nm, with an isosbestic point at 338 nm. Similarly, when evaluating the fluorescence of 53 under a UV lamp at 365 nm, the probe changed from yellow-orange to dark. The fluorescence spectrum of 53 showed a band at 594 nm, which decreased in intensity upon the addition of CN−. The sensing mechanism of 53 involves the resonance effect from the pyridine ring through biphenyls.
The absorption spectrum of probe 53 shows three bands, an intense at 375 nm and two lower intensity bands at 270 nm and 298 nm, evidencing ICT processes due to its high π-conjugation.79 This probe also was evaluated by cyclic voltammetry, proving that the addition of CN− causes the reduction potential of 53 at −1.41 eV to shift towards more negative potentials, reaching up to −1.07 eV for the adduct 53-CN. These results show that the sensing mechanism involves a shutdown by the ICT process from the N-heterocyclic core to the dicyanovinylidene moiety in 53. Finally, this probe achieved a better LOD than that reported by Liu et al.,77,78 reaching 3.93 × 10−8 M with a rapid response of around 20 s in a wide pH range of 3 to 11.
2.5. Metal complexes
Various NHCs have been proven to be functional scaffolds for developing chemosensors. For example, some pyridine derivatives are part of coordination compounds with ruthenium(II) and iridium(III), which have the following photophysical advantages with respect to organic fluorophores: large Stokes displacements,80 high stability,81 relatively long emission lifetimes, and emission wavelengths in the visible region.82 In these probes, the coordination complex is the signaling unit, and the recognition moiety is generally an electron-deficient carbon; thus, various probes have been applied. For example, Zheng et al. developed probes 54 and 55, where an Ru(II) complex moiety is the signaling unit, and another complex of Cu(II) is the recognition site (Fig. 20a).83 These probes have very weak emission bands at around 590 nm, but without Cu2+, the intensity of their bands increase dramatically. Consequently, these probes were used for sensing CN− following strategy II, evidencing high sensibility even in the presence of chelating agents such as EDTA due to the high affinity of CN− for Cu2+. The LOD for the probes was 3.60 × 10−7 M for 54 and 8.70 × 10−7 M for 55. Besides, they were not selective towards other anions that can interact with Cu2+.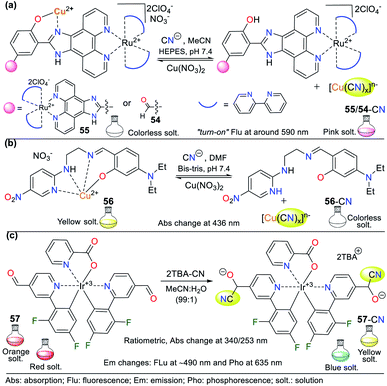 | ||
| Fig. 20 Probes for sensing CN− based on coordination compounds bearing (a) ruthenium, (b) copper, and (c) iridium. | ||
Pyridine derivatives are not only used to facilitate the resonance effects in ICT phenomena or as EWG to increase the electrophilic character of vicinal carbons to the ring. They have also been used to create coordination bonds with metallic ions, taking advantage of the free electron pair on the nitrogen atom. In particular, copper complexes are used to detect CN− due to the high affinity of this metal for the CN− anion. In the detection process, a stable complex of [Cu(CN)x]n− is formed together with a free ligand, which was evidenced due to the variations in the photophysical properties of the sensor solution.83,84
For example, Kang et al.84 studied the Cu2+ complex 56, which was formed in situ from a ligand bearing two aromatic rings (the EWG 5-nitropyridin-2-yl and the electron-releasing group (ERG) dimethylaminophenol), both connected by an imine bridge with ethylenediamine. Regarding the probe design, the ligand molecule was colorless in solution and allowed the colorimetric detection of Cu2+ (LOD = 8.80 × 10−7 M) by a color change to intense yellow and the formation of 56. The ligand molecule became free again by adding CN− to a solution of complex 56, as evidenced by the color change from yellow to colorless. The absorption spectrum of 56 showed a band at 436 nm, which diminished after the gradual addition of CN− (LOD = 2.72 × 10−5 M) (Fig. 20b).
Organometallic compounds of 2-phenylpyridine derivatives have also been used as signaling units in probes to detect cyanide, in which the emission is due to phosphorescence. Accordingly, Reddy and co-workers synthesized chemodosimeter 57, a complex bis[[2′,6′-difluorophenyl-4-formylpyridinate]-N,C4′]iridium(III). The detection occurs with the formation of cyanohydrin following strategy I of the probe design and via the nucleophilic attack of CN− on the formyl group of 57 (Fig. 20c).85 The emission spectrum of 57 in acetonitrile showed a band at 635 nm, while in the presence of 2 equiv. of CN−, it shifted to 480 nm with a color change from red to blue. Moreover, the intensity was enhanced by 536 times, giving a quantum yield of 11%.
The formed adduct 57-CN was confirmed to have a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry with high selectivity towards the analyte and an LOD of 2.16 × 10−8 M. The 1H NMR experiments revealed that 57 has a signal at 10.17 ppm (s, 2H), which is assigned to its formyl groups. This signal decreased with an increase in the concentration of CN−, while a signal appeared at 7.57 ppm, evidencing the formation of cyanohydrin. The disappearance of the aldehyde signal was evidenced when the reaction was completed upon the addition of two equiv. of the analyte. The FTIR spectrum of the probe showed the disappearance of the C
2 stoichiometry with high selectivity towards the analyte and an LOD of 2.16 × 10−8 M. The 1H NMR experiments revealed that 57 has a signal at 10.17 ppm (s, 2H), which is assigned to its formyl groups. This signal decreased with an increase in the concentration of CN−, while a signal appeared at 7.57 ppm, evidencing the formation of cyanohydrin. The disappearance of the aldehyde signal was evidenced when the reaction was completed upon the addition of two equiv. of the analyte. The FTIR spectrum of the probe showed the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 1706 cm−1 and the appearance of the C
O stretching band at 1706 cm−1 and the appearance of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N band at 2248 cm−1 upon the addition of cyanide.85
N band at 2248 cm−1 upon the addition of cyanide.85
Recently, Lin and colleagues obtained probe 58, which also consists of an iridium(III) complex formed with 2-phenylpyridine ligands, and in this case, they used 5-formyl-1,10-phenantroline as an auxiliary ligand (Fig. 21a).86 Similar to probe 57 designed by the Reddy group,85 the formyl group of 58 acts as the recognition unit through the cyanohydrin 58-CN. The solvents used in this type of probe play a crucial role in the analytical signal intensity, where the most widely used mixture is MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. In this case, a 95
H2O. In this case, a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio was used, which showed no variation in the intensity of the emission bands. Upon the addition of cyanide, the absorption spectrum of 58 exhibited two bands at 267 and 380 nm, which are attributed to the metal–ligand charge transfer (MLCT) process. This behavior is typical in metalorganic complexes using carbonyl groups or Michael acceptors as recognition units. The color of probe 58 changed from light yellow to shiny orange under a UV lamp (365 nm) upon the addition of CN−. Even though its recognition unit has only 1 equiv. to detect CN−, its emission spectrum showed an enhancement of up to 15 times in the intensity of the band at 570 nm, giving a quantum yield of 28%.
5 ratio was used, which showed no variation in the intensity of the emission bands. Upon the addition of cyanide, the absorption spectrum of 58 exhibited two bands at 267 and 380 nm, which are attributed to the metal–ligand charge transfer (MLCT) process. This behavior is typical in metalorganic complexes using carbonyl groups or Michael acceptors as recognition units. The color of probe 58 changed from light yellow to shiny orange under a UV lamp (365 nm) upon the addition of CN−. Even though its recognition unit has only 1 equiv. to detect CN−, its emission spectrum showed an enhancement of up to 15 times in the intensity of the band at 570 nm, giving a quantum yield of 28%.
Alternatively, the fluorescence intensity of 58 became stable at 130 s when it reacted with 1 equiv. of CN−. Similar to the above-mentioned example, the high specificity towards the formation of cyanohydrin makes this probe selective to CN− in the presence of 17 other anions. This process was studied via 1H NMR, applying the same method as that for 57, where CN− was successively added and analyzed. The spectra showed a diminution in the signal at 10.56 ppm, corresponding to the formyl group, and a progressive increase in the signal at 8.6 ppm, which is assigned to the formed cyanohydrin. In these types of complexes, density functional theory (DFT) studies display that the HOMO is localized mainly on the metal center, while the LUMO is localized in the zones in which there is a lower electronic density. For 57 and 58, the LUMO is localized in the aldehyde.85
A different approach regarding the recognizing unit for this type of probe was developed by Kim et al., who developed probe 59, which combines electrochemiluminescence with sensing CN−.87 For the sensing process, 59 has a dicyanovinylidene group, working via strategy I. This probe exhibited poor CN− selectivity because sulfide ions (S2−) and thiols such as cysteine are interferent species, which could form similar adducts to 59-CN (Fig. 21b). However, under an oxidation potential of 1.2 V, 59-CN showed unique photophysical properties including luminescence at 631 nm, which could be observed by the naked eye; in contrast, the adducts formed with the interferents only had weak luminescence at 1.5 V. Consequently, the electrochemical manipulation towards the positive potentials of the probe allowed it to have a detection limit of 4.00 × 10−8 M for cyanide. Additionally, DFT calculations demonstrated that the formed adduct 59-CN has a higher HOMO than that formed with S2− and cysteine, making it more susceptible to oxidation at a lower potential, thus discriminating between these interferents.
3. Chemosensors for Hg2+ sensing
Similar to cyanide, mercury is a hazardous toxicant raw material that impacts human health and the environment. The concentration and speciation of mercury in the atmosphere depend on the proximity to its sources, the availability of oxidants, the concentrations and properties of aerosols, regional and global-scale meteorology, and surface conditions.88 Mercury deposited in the terrestrial environment can cause environmental harm given that it is transported to aquatic systems, where it can be methylated and then bio-accumulated in the aquatic food chain. Human or animal consumption of high trophic-level fish or other foods that have been contaminated with mercury can lead to toxic effects.89 The United States Environmental Protection Agency (EPA) has permitted a maximum limit of 2 ppb Hg2+ in potable water.90 According to the WHO data, the mercury level in the ground and surface water is about ∼5.00 × 10−7 M (μg L−1), and in the case of daily intake, in food it is in the range of 2 to 20 μg per day.91 Moreover, this metal ion generally shows fluorescence quenching due to its paramagnetic nature and strong spin–orbit coupling.92Consequently, the design and synthesis of probes for Hg2+ have increased in importance in recent times. The development of new chemosensors for d-block metal ions or transition metals, where heavy metal ions such as Hg2+ are found, is of great interest given that these species play a crucial role in different environmental areas and biological systems.93 This section of this review focuses on the design, development, and evaluation of probes based on N-heterocycles for sensing Hg2+, which is one of the most toxic cations considering environmental pollution.94 In general, the probes analyzed below show structural variations to favor the sensing process via the chelation of Hg2+. In this case, the introduction of oxygen and sulfur atoms generates specific changes in sensitivity and selectivity in an appreciable manner. Similarly, as in Section 2, probes having 5- and 6-membered N-heterocycles are divided in this section. In these chemosensors, the N-heterocycle acts as a recognition site, in some it acts as a linker, and others it has fluorophore functions.
3.1. Pyrrole derivates
Although there are many probes to detect Hg2+ based on pyrrole derivatives, the examples using the monocyclic ring are very scarce in recent studies. Consequently, the studied pyrroles in this contribution have fused structures such as indoles, carbazoles, and BODIPYs. These pyrrole derivatives are characterized by their exceptional synthetic and functional versatility, high quantum fluorescence yields, and the low limits of detection they can achieve.95–103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with HEPES buffer solution (pH 7.4). This probe exhibited an absorption spectrum with the typical oxindole and rhodanine absorption bands, including a strong band at 272 nm and a weak band at 390 nm. Upon the addition of Hg2+ to 60, the band at 272 nm increased to 275 nm with a blue/redshift and that at 390 nm redshifted to 415 nm with a substantial increase in absorbance. A significant color change from colorless to orange was observed with the naked eye. Likewise, 60 showed weak fluorescence at 515 nm (λexc = 390 nm), and after the successive addition of Hg2+, this emission band was enhanced. Furthermore, studies related to the Job's plot showed that the stoichiometry of complex 60-Hg is 1
1) with HEPES buffer solution (pH 7.4). This probe exhibited an absorption spectrum with the typical oxindole and rhodanine absorption bands, including a strong band at 272 nm and a weak band at 390 nm. Upon the addition of Hg2+ to 60, the band at 272 nm increased to 275 nm with a blue/redshift and that at 390 nm redshifted to 415 nm with a substantial increase in absorbance. A significant color change from colorless to orange was observed with the naked eye. Likewise, 60 showed weak fluorescence at 515 nm (λexc = 390 nm), and after the successive addition of Hg2+, this emission band was enhanced. Furthermore, studies related to the Job's plot showed that the stoichiometry of complex 60-Hg is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a binding constant of 2.15 × 104 M−1. The LOD value of 60 was also calculated to be 3.36 × 10−6 M for the Hg2+ recognition.
1 with a binding constant of 2.15 × 104 M−1. The LOD value of 60 was also calculated to be 3.36 × 10−6 M for the Hg2+ recognition.
Similarly, Trivedi et al.96 synthesized and characterized probes 61, 62, and 63 having an isatin core as the chromophoric unit and a 3,3′-dihydroxybenzidine moiety, possessing hydroxy, imine, and amide groups as binding units for the detection of both Hg2+ and AsO2− (Fig. 22b). Chemosensor 61 showed a color change from orange to colorless for Hg2+ and orange to aqua-blue for AsO2−, 62 changed from yellow to pink for Hg2+ and 63 revealed selectivity towards Hg2+, changing from orange to colorless and orange to aqua blue for AsO2−. All experiments were carried out in DMSO, where the chemosensors displayed negligible absorption changes for 60 min.
Probes 61 (8.00 × 10−5 M), 62 (5.00 × 10−5 M), and 63 (4.00 × 10−5 M) showed two absorption bands at 308/480 nm, 274/340 nm, and 315/477 nm, respectively, which match the π–π* and n–π* transitions. The stoichiometry, LODs, and binding constants for the probe–Hg2+ complexes were determined. The analysis of the probes indicated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (61 and 62) and 1
1 (61 and 62) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (63) binding stoichiometry; and LODs of 4.13 × 10−6 M, 4.65 × 10−6 M, and 3.93 × 10−6 M for; and binding constants of 2.51 × 103 M−1, 1.34 × 104 M−1 and 4.94 × 109 M−2, respectively, for 61 to 63. DFT calculations were carried out, which showed that the charge transfer occurs from the molecule to mercury atom in 61-Hg, that is, ligand–metal charge transfer (LMCT) was observed, whereas the other sensor molecules, 62 and 63, exhibited MLCT.96
2 (63) binding stoichiometry; and LODs of 4.13 × 10−6 M, 4.65 × 10−6 M, and 3.93 × 10−6 M for; and binding constants of 2.51 × 103 M−1, 1.34 × 104 M−1 and 4.94 × 109 M−2, respectively, for 61 to 63. DFT calculations were carried out, which showed that the charge transfer occurs from the molecule to mercury atom in 61-Hg, that is, ligand–metal charge transfer (LMCT) was observed, whereas the other sensor molecules, 62 and 63, exhibited MLCT.96
Similar to the last example, Joshi et al.97 synthesized the fluorescent probe 64 (Fig. 22c). UV-vis/fluorescence studies were carried in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3
H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) with different metal ions (5 equiv.) and a probe concentration of 10−4 M. This probe exhibited a strong absorption band at 294 nm with a weak hump at 344 nm and an emission band at 465 nm (φ = 22%, λexc = 340 nm). In addition, the fluorescence intensity change versus pH value was evaluated, showing that the band at 465 nm remained unperturbed in the pH range of 4.5 to 7.5, but its intensity increased at a higher pH value. These results indicate that the pH range of 4.5 to 7.5 is suitable for exploring sensing ability in ambient conditions. Upon the addition of Hg2+ (5 equiv.), a redshift of 3 to 4 nm occurred in the absorption band at 294 nm, but no change was observed with the addition of other metals; similarly, fluorescence quenching (φ = 5) due to ICT phenomena occurred via the complexation of 64 and Hg2+. This fluorescence quenching could be due to the excitation energy transfer from the probe to the metal d-orbital and/or metal to probe charge transfer.
7) with different metal ions (5 equiv.) and a probe concentration of 10−4 M. This probe exhibited a strong absorption band at 294 nm with a weak hump at 344 nm and an emission band at 465 nm (φ = 22%, λexc = 340 nm). In addition, the fluorescence intensity change versus pH value was evaluated, showing that the band at 465 nm remained unperturbed in the pH range of 4.5 to 7.5, but its intensity increased at a higher pH value. These results indicate that the pH range of 4.5 to 7.5 is suitable for exploring sensing ability in ambient conditions. Upon the addition of Hg2+ (5 equiv.), a redshift of 3 to 4 nm occurred in the absorption band at 294 nm, but no change was observed with the addition of other metals; similarly, fluorescence quenching (φ = 5) due to ICT phenomena occurred via the complexation of 64 and Hg2+. This fluorescence quenching could be due to the excitation energy transfer from the probe to the metal d-orbital and/or metal to probe charge transfer.
For probe 64, a decrease in fluorescence intensity was observed at pH 4 because the N-indolic protonation generates a hindrance for binding to Hg2+ ions. The fluorescence intensity of 64 at higher pH increased with Hg2+ in alkaline solution. The authors explained that it also showed quenching, which could be due to decomplexation, and later the formation of mercury hydroxide. Based on the fluorescence titration curve using 3σ/m, the LOD was calculated to be 1.43 × 10−7 M in the Hg2+ concentration range of 0 to 3.00 × 10−5 M. In addition, the linearity of the Benesi–Hildebrand plot indicates a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between 64 and Hg2+, as confirmed by the ESI-MS analysis of the complex 64-Hg, which presented an association constant of 6.4 × 103 M−1. Using 1H NMR studies and DFT calculations, the complexation mechanism was proposed, which could be the probable balance between the NH-amidic and NH-indolic donor centers given that Hg2+ shows greater affinity.97
1 stoichiometry between 64 and Hg2+, as confirmed by the ESI-MS analysis of the complex 64-Hg, which presented an association constant of 6.4 × 103 M−1. Using 1H NMR studies and DFT calculations, the complexation mechanism was proposed, which could be the probable balance between the NH-amidic and NH-indolic donor centers given that Hg2+ shows greater affinity.97
By adding alkaline-earth and heavy/transition metal ions to 65, no major changes its absorption intensity were noticed, but with the treatment of 1 equiv. of Hg2+, a notable fluorescence “turn-off” response was observed; furthermore, 65 exhibited partial quenching upon the addition of Cu2+. In addition, excellent selectivity was observed towards different Hg2+ salts irrespective of the counterions and in the vicinity of other competing metal ions. The fluorescence intensity of 65 with Hg2+ remained unperturbed in the pH range of 5.4 to 8.5, establishing its capacity to be used under physiological conditions. The stoichiometric ratio of 65-Hg was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as determined by the Job's plot and confirmed by MALDI-TOF, with a binding constant of 4.75 × 105 M−1 and LOD of 1.35 × 10−7 M. The plausible sensing process could be via chelating quenched fluorescence (CHQF), with reversibility by adding S2−.101
1, as determined by the Job's plot and confirmed by MALDI-TOF, with a binding constant of 4.75 × 105 M−1 and LOD of 1.35 × 10−7 M. The plausible sensing process could be via chelating quenched fluorescence (CHQF), with reversibility by adding S2−.101
Furthermore, in 2018, Kala et al.100 developed the selective “turn-off” probe 66 for sensing Hg2+ in acetonitrile and THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) based on a carbazole–thiobarbituric acid conjugate system (Fig. 23b). This probe has characteristics of a π-conjugated donor–acceptor system with an ICT absorption band. In addition, 66 has a carbazole-based locally-excited (LE) state and an ICT state due to its pre-twisted form, having a twisted excited state giving rise to a carbazole-type emission at 425 and 600 nm, respectively. In acetonitrile, the orange solution turned dark brown with a redshift due to the change in the electron affinity of the pyrimidine moiety on complexation with Hg2+ ions.
1) based on a carbazole–thiobarbituric acid conjugate system (Fig. 23b). This probe has characteristics of a π-conjugated donor–acceptor system with an ICT absorption band. In addition, 66 has a carbazole-based locally-excited (LE) state and an ICT state due to its pre-twisted form, having a twisted excited state giving rise to a carbazole-type emission at 425 and 600 nm, respectively. In acetonitrile, the orange solution turned dark brown with a redshift due to the change in the electron affinity of the pyrimidine moiety on complexation with Hg2+ ions.
Moreover, the orange-colored solution of 66 changed to light brown, which could be observed by the naked eye, and from orange to dark fluorescence in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF
1 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture. The results showed that the LOD was 13.35 × 10−9 M for fluorescence in MeCN (λexc = 404 nm) and 53.34 × 10−9 M in 9
H2O mixture. The results showed that the LOD was 13.35 × 10−9 M for fluorescence in MeCN (λexc = 404 nm) and 53.34 × 10−9 M in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF
1 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution (λexc = 360 nm), the Job's plot showed a 1
H2O solution (λexc = 360 nm), the Job's plot showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry, and binding constant was 1.49 × 109 M−2 by absorption spectroscopy and 0.58 × 109 M−2 by fluorescence spectroscopy, which indicate that 66 is very sensitive to Hg2+ ions. Moreover, the fluorescence was restored by adding cysteamine hydrochloride, indicating that 66 can serve as a reusable probe.100
2 binding stoichiometry, and binding constant was 1.49 × 109 M−2 by absorption spectroscopy and 0.58 × 109 M−2 by fluorescence spectroscopy, which indicate that 66 is very sensitive to Hg2+ ions. Moreover, the fluorescence was restored by adding cysteamine hydrochloride, indicating that 66 can serve as a reusable probe.100
Finally, Yin et al.99 reported the hydrazide–carbazole system 67, which showed AIE enhancement behavior for the selective sensing of Hg2+ and Al3+ in aqueous medium. This colorimetric/fluorimetric probe showed a weak fluorescence band at 438 nm in DMSO (10−5 M, φ = 0.84%), while in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v, HEPES buffer, pH 7.4), it emitted intense blue light under a UV lamp (365 nm) with a redshift from 438 to 458 nm (φ = 3.03%). Similarly, 67 (10−6 M) in ∼100% aqueous solution (HEPES buffer, pH 7.4) showed two absorption bands at 355 and 310 nm, which are related to π–π* and n–π* transitions, respectively. The color change from colorless to yellow was only observed in the presence of Hg2+ at 355 nm, indicating that 67 coordinates with Hg2+. In addition, the presence of 2 equiv. of Hg2+ led to complete fluorescence quenching at 458 nm, which could be related to the chelation-enhanced fluorescence quenching (CHEQ) effect (Fig. 23c). In contrast, upon the addition of Al3+ (2 equiv.) to 67, a remarkable “naked-eye” fluorescence color change from blue to green and emission enhancement with a redshift in emission (18 nm) were observed. This result is associated with C
99, v/v, HEPES buffer, pH 7.4), it emitted intense blue light under a UV lamp (365 nm) with a redshift from 438 to 458 nm (φ = 3.03%). Similarly, 67 (10−6 M) in ∼100% aqueous solution (HEPES buffer, pH 7.4) showed two absorption bands at 355 and 310 nm, which are related to π–π* and n–π* transitions, respectively. The color change from colorless to yellow was only observed in the presence of Hg2+ at 355 nm, indicating that 67 coordinates with Hg2+. In addition, the presence of 2 equiv. of Hg2+ led to complete fluorescence quenching at 458 nm, which could be related to the chelation-enhanced fluorescence quenching (CHEQ) effect (Fig. 23c). In contrast, upon the addition of Al3+ (2 equiv.) to 67, a remarkable “naked-eye” fluorescence color change from blue to green and emission enhancement with a redshift in emission (18 nm) were observed. This result is associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and inhibition of the PET process.
N isomerization and inhibition of the PET process.
The binding stoichiometry for 67-Hg2+ and 67-Al was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for both, as determined by Job's method, with association constants of 4.06 × 104 M−1 and 2.92 × 104 M−1, respectively. The LODs were calculated to be 1.47 × 10−8 M for Hg2+ and 4.72 × 10−8 M for Al3+, which are much lower than the WHO guidelines for drinking water. Probe 67 (10−5 M) showed excellent stability within a wide pH range (2 to 11), as well as its complexes (pH 3 to 10), which makes 67 suitable for application in biological systems, with good sensibility (15 s). The sensing process was sustained several times with the alternating addition of Al3+ and F−, and similarly after the addition of Hg2+ and EDTA systems, demonstrating the reversibility and regeneration of 67. Lastly, 67 was successfully applied in different samples such as soil, environmental water, food sample analysis, and live-cell imaging, demonstrating its great potential in environmental and biological applications.99
1 for both, as determined by Job's method, with association constants of 4.06 × 104 M−1 and 2.92 × 104 M−1, respectively. The LODs were calculated to be 1.47 × 10−8 M for Hg2+ and 4.72 × 10−8 M for Al3+, which are much lower than the WHO guidelines for drinking water. Probe 67 (10−5 M) showed excellent stability within a wide pH range (2 to 11), as well as its complexes (pH 3 to 10), which makes 67 suitable for application in biological systems, with good sensibility (15 s). The sensing process was sustained several times with the alternating addition of Al3+ and F−, and similarly after the addition of Hg2+ and EDTA systems, demonstrating the reversibility and regeneration of 67. Lastly, 67 was successfully applied in different samples such as soil, environmental water, food sample analysis, and live-cell imaging, demonstrating its great potential in environmental and biological applications.99
In 2019, Xue et al.102 reported that probe 68 was highly selective and sensitive for recognizing Hg2+, which is based on 4-hydroxystyryl-substituted BODIPYs (Fig. 24a). The photophysical properties of 68 were measured in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; HEPES 10−4 M, pH 7.2), exhibiting an intense absorption band at 653 nm with a molar absorption coefficient (ε) of 1.40 × 105 M−1 cm−1 due to the low energy S0 → S1 transition of the BODIPY core and two weaker bands at 322 nm and 377 nm with a molar absorption coefficient (ε) of 3.30 × 104 M−1 cm−1 and 7.50 × 104 M−1 cm−1, respectively. This probe showed an emission band at 681 nm with a quantum yield of 89%, indicating the high fluorescence of the dye (λexc = 650 nm).
1; HEPES 10−4 M, pH 7.2), exhibiting an intense absorption band at 653 nm with a molar absorption coefficient (ε) of 1.40 × 105 M−1 cm−1 due to the low energy S0 → S1 transition of the BODIPY core and two weaker bands at 322 nm and 377 nm with a molar absorption coefficient (ε) of 3.30 × 104 M−1 cm−1 and 7.50 × 104 M−1 cm−1, respectively. This probe showed an emission band at 681 nm with a quantum yield of 89%, indicating the high fluorescence of the dye (λexc = 650 nm).
With the addition of Hg2+ to a solution of 68, a redshift in its bands was observed with a color change in the solution from light green to orange. However, the addition of different metal ions to a solution of 68 did not show noticeable changes due to the low affinity of these ions, except Hg2+. When Hg2+ ions were added, the two intense peaks located at 433 nm and 801 nm were replaced by a new intense peak located at 515 nm, which was similar to absorption spectrum of BODIPY, indicating that the Hg2+ ions broke the ICT process from the styryl groups to the BODIPY core. Probe 68 in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; HEPES 10−4, pH = 7.2) showed bright red fluorescence with an intense emission band around the NIR region at 681 nm. Upon the addition of different ions, the emission intensity of the band at 681 nm remained the same, except in the presence of Hg2+. In this case, the fluorescence emission was almost quenched, with a change in color from red to green, which could be observed by the naked eye. The LOD of 68 was estimated to be 7.00 × 10−8 M, and the association constant was 4.30 × 103 M−1. Finally, the Job's plot indicates that Hg2+ is coordinated to 68 in a 2
1; HEPES 10−4, pH = 7.2) showed bright red fluorescence with an intense emission band around the NIR region at 681 nm. Upon the addition of different ions, the emission intensity of the band at 681 nm remained the same, except in the presence of Hg2+. In this case, the fluorescence emission was almost quenched, with a change in color from red to green, which could be observed by the naked eye. The LOD of 68 was estimated to be 7.00 × 10−8 M, and the association constant was 4.30 × 103 M−1. Finally, the Job's plot indicates that Hg2+ is coordinated to 68 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.102
1 ratio.102
Similarly, Huang et al.103 developed the NIR styryl-BODIPY probe 69 for the detection of Hg2+ and Cu2+ (Fig. 24b) in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (5
H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This probe showed a strong red-shift for the band at 663 nm due to the ICT process and the other peaks at 614 and 385 nm. In addition, an NIR fluorescence emission was observed at 730 nm (λexc = 620 nm), which was largely blue-shifted from 730 to 672 nm upon the addition of Hg2+, with a remarkable fluorescence enhancement by the naked eye, corresponding to the inhibition of ICT. The binding mode of 69-Hg was 1
1). This probe showed a strong red-shift for the band at 663 nm due to the ICT process and the other peaks at 614 and 385 nm. In addition, an NIR fluorescence emission was observed at 730 nm (λexc = 620 nm), which was largely blue-shifted from 730 to 672 nm upon the addition of Hg2+, with a remarkable fluorescence enhancement by the naked eye, corresponding to the inhibition of ICT. The binding mode of 69-Hg was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as determined by the Job's plot of the fluorescence at 673 nm, with a binding constant of 1.0 × 109 M−2 and LOD of 9.00 × 10−8 M. An advantage of this probe was that with its introduction in HeLa cells for living cell imaging, it could discriminate Hg2+ and Cu2+ ions via two NIR fluorescence emission channels.
2, as determined by the Job's plot of the fluorescence at 673 nm, with a binding constant of 1.0 × 109 M−2 and LOD of 9.00 × 10−8 M. An advantage of this probe was that with its introduction in HeLa cells for living cell imaging, it could discriminate Hg2+ and Cu2+ ions via two NIR fluorescence emission channels.
Compound 70 obtained by Zhou and co-workers104 is very similar to the previous examples. These authors developed the fluorescent probe “turn-on” 70, having a benzo-BODIPY fluorophore with a carboxyl-thiol metal bonding receptor for Hg2+ (Fig. 24c). This probe showed a visible absorption band at 512 nm and was almost nonfluorescent at 581 nm (φ = 0.2%) due to the efficient PET process quenching of the excited benzo-BODIPY fluorophore by the electron-donating nitrogen atom from the carboxyl-thiol metal bonding receptor. After the addition of Hg2+, the emission band rapidly increased with a marked color change from dark to orange due to the PET-OFF pathway between BODIPY and carboxyl-thiol (φ = 12% with 100 equiv. of Hg2+). By titration with Hg2+, the binding constant was determined to be 1.16 × 104 M−1 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for 70-Hg in the pH range of 4.5 to 9.1, indicating that 70 can be used in neutral natural systems. The LOD was calculated to be 5.70 × 10−9 M. The results of this study may be considered in the future to use 70 for the detection of Hg2+ in real samples and other biological uses.
1 stoichiometry for 70-Hg in the pH range of 4.5 to 9.1, indicating that 70 can be used in neutral natural systems. The LOD was calculated to be 5.70 × 10−9 M. The results of this study may be considered in the future to use 70 for the detection of Hg2+ in real samples and other biological uses.
3.2. Imidazole derivatives
A large number of imidazole derivatives has been developed for different uses in medicine. Imidazole derivatives have occupied a significant place in photophysical investigations in recent years.1–4,69,70 In this section, the aim is to present some of the works reported on the synthesis of probes for the recognition of Hg2+, bearing an imidazole core as a recognition site.105–108 For instance, Gao et al.105 obtained Schiff-base 71 to detect Cu2+ (“turn-off”) and Hg2+ (“turn-on”), bearing benzimidazole and coumarin fluorophores. This probe showed a weak fluorescent emission at 428 nm (φ = 0.9%) due to the possible C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization-rotation deactivation process and high selectivity for Hg2+ with a light-blue emission (φ = 15%). An increase in fluorescence was observed with a redshift of 20 nm to 448 nm upon the addition of 5 equiv. of Hg2+, monitoring for 30 min in 2.00 × 10−4 M HEPES buffer/DMSO solution (9
N isomerization-rotation deactivation process and high selectivity for Hg2+ with a light-blue emission (φ = 15%). An increase in fluorescence was observed with a redshift of 20 nm to 448 nm upon the addition of 5 equiv. of Hg2+, monitoring for 30 min in 2.00 × 10−4 M HEPES buffer/DMSO solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH 7.2).
1, pH 7.2).
The linear relationship of the fluorescence titration studies and Job's plot suggests a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding for 71-Hg with an association constant of 8.75 × 104 M−1 and LOD of 7.00 × 10−8 M. The fluorescence of 71 remained stable in the pH range of 6 to 12, which was greatly enhanced in the pH range of 6 to 8 with a maximum at pH 7, showing its promising application in biological systems. By 1H NMR and FTIR spectral analysis, the possible mechanism between Hg2+ and 71 was proposed (Fig. 25a), where Hg2+ may be first coordinated with 71. Then, the imine bond of the complex may be more easily attacked by a water molecule to produce compounds 71a and 71b. This probe was used in the imaging of U87 MG human primary glioblastoma cells, indicating its promising application in living cells.105
1 binding for 71-Hg with an association constant of 8.75 × 104 M−1 and LOD of 7.00 × 10−8 M. The fluorescence of 71 remained stable in the pH range of 6 to 12, which was greatly enhanced in the pH range of 6 to 8 with a maximum at pH 7, showing its promising application in biological systems. By 1H NMR and FTIR spectral analysis, the possible mechanism between Hg2+ and 71 was proposed (Fig. 25a), where Hg2+ may be first coordinated with 71. Then, the imine bond of the complex may be more easily attacked by a water molecule to produce compounds 71a and 71b. This probe was used in the imaging of U87 MG human primary glioblastoma cells, indicating its promising application in living cells.105
Motivated by the advantage of fluorescent protein derivatives for detecting pH, RNA, and human serum albumin, Ramanathan and Singh obtained 72, an analog of the red fluorescent protein (RFP) (Fig. 25b), which was recognized as a probe for Cu2+ and Hg2+.106 This probe has a fluorescent coumarin moiety to enhance the emission property and showed an absorption band at 426 nm (ε = 48.406 M−1 cm−1) and an emission band at 550 nm. Upon the addition of Hg2+ (10 equiv.) to 72 in acetonitrile (10−4 M), a color change from yellow to orange was perceived by the naked eye. Similarly, the emission band suffered a redshift from 550 (φ = 6.7%) to 610 (φ = 6.3%) nm with fluorescence “turn-off” due to the enhanced spin–orbit quenching, and a color change from bright orange to red. The formation of complex 72-Hg took place in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with a binding constant of 3.8 × 105 M−1 and LODFlu of 2.00 × 10−11 M. A static quenching pathway was observed given that the decay time was 7.6 ± 0.3 ns both in the presence and absence of Cu2+ and Hg2+ ions, showing that the formation of a ground state non-emissive complex occurs with both ions. Similarly, the coumarin moiety did not participate in the binding with the metal center but significantly favors the LOD of 72 as a colorimetric chemosensor.
1 ratio with a binding constant of 3.8 × 105 M−1 and LODFlu of 2.00 × 10−11 M. A static quenching pathway was observed given that the decay time was 7.6 ± 0.3 ns both in the presence and absence of Cu2+ and Hg2+ ions, showing that the formation of a ground state non-emissive complex occurs with both ions. Similarly, the coumarin moiety did not participate in the binding with the metal center but significantly favors the LOD of 72 as a colorimetric chemosensor.
In 2018, Emandi et al. described the synthesis of two fluorescent probes for the reversible detection of Hg2+ and CN− in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10−5 M).107 These dual probes are fused imidazole 73a and tetraarylimidazole 73b, which displayed absorption bands at 370 and 345 nm with strong fluorescence at 495 nm (φ = 19%) and 491 nm (φ = 12%), respectively. The recognition studies indicated the formation of cyanohydrins 73a-CN (LOD = 8.0 × 10−7 M) and 73b-CN (LOD = 1.2 × 10−6 M) upon the addition of CN− to the probe solution. Later, upon the addition of Hg2+, a reduction in the absorption band at 425 nm was observed with an increase in the band at 370 nm; equally, the fluorescence intensity markedly increased at 495 nm, turning the solution green. These results suggest the binding of Hg2+ to 73a-CN, which was confirmed by 1H NMR and supported by ESI-MS studies. Similar optical changes were observed for 73b. CN− induces notable fluorescence changes by “turn-off” behavior, while the subsequent addition Hg2+ results in fluorescence “turn-on”, suggesting a reversible and reusable probe for around ten cycles. Thus, the binding stoichiometry was probably a 1
1, 10−5 M).107 These dual probes are fused imidazole 73a and tetraarylimidazole 73b, which displayed absorption bands at 370 and 345 nm with strong fluorescence at 495 nm (φ = 19%) and 491 nm (φ = 12%), respectively. The recognition studies indicated the formation of cyanohydrins 73a-CN (LOD = 8.0 × 10−7 M) and 73b-CN (LOD = 1.2 × 10−6 M) upon the addition of CN− to the probe solution. Later, upon the addition of Hg2+, a reduction in the absorption band at 425 nm was observed with an increase in the band at 370 nm; equally, the fluorescence intensity markedly increased at 495 nm, turning the solution green. These results suggest the binding of Hg2+ to 73a-CN, which was confirmed by 1H NMR and supported by ESI-MS studies. Similar optical changes were observed for 73b. CN− induces notable fluorescence changes by “turn-off” behavior, while the subsequent addition Hg2+ results in fluorescence “turn-on”, suggesting a reversible and reusable probe for around ten cycles. Thus, the binding stoichiometry was probably a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 25c).
1 ratio (Fig. 25c).
Additionally, Rao et al.108 developed chemosensor 74, having an N-methylimidazole linked by a hydrazide linker to a rhodamine 6G fragment (Fig. 25d). This probe presented exceptional sensing ability in an acid or neutral medium, working by three chelation points, as confirmed by the 1H NMR and ESI-MS spectra (i.e., –O, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and
N, and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). The absorption spectra of 74 in DMF
N). The absorption spectra of 74 in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH 7.3) showed two peaks at 267 and 309 nm, while with an increase in the concentration of Hg2+, another band emerged at 538 nm due to the ring-opening spirolactam; equally, the emission spectra offered a new intense band at 562 nm. Upon the addition of Hg2+, the color of 74 changed from colorless to pink rapidly in daylight with yellow fluorescence “turn-on”. The binding constant was 5.12 × 105 M, indicating a strong interaction in 74-Hg with a binding stoichiometry of 1
1, pH 7.3) showed two peaks at 267 and 309 nm, while with an increase in the concentration of Hg2+, another band emerged at 538 nm due to the ring-opening spirolactam; equally, the emission spectra offered a new intense band at 562 nm. Upon the addition of Hg2+, the color of 74 changed from colorless to pink rapidly in daylight with yellow fluorescence “turn-on”. The binding constant was 5.12 × 105 M, indicating a strong interaction in 74-Hg with a binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by the Job's plot. The practical use of this molecular probe as electrospun nanofiber test strips to recognize Hg2+ in aqueous media was accomplished.
1 by the Job's plot. The practical use of this molecular probe as electrospun nanofiber test strips to recognize Hg2+ in aqueous media was accomplished.
3.3. Pyrazole derivatives
In recent years, pyrazole derivatives have attracted significant attention in organic and medicinal chemistry because of their usefulness in preparing bioactive compounds or ligands for the formation of coordination complexes with different metal centers. There are some examples of pyrazole-based probes for the detection of Hg2+, in which the ring acts as a signaling unit, being part of a fused system; however, in other cases, pyrazoles and pyrazolines also act as recognition sites. Remarkably, in all the examples presented in this section, the chemosensors have blue fluorescence, which is quenched when they bind to Hg2+ ions.109–113Taking advantage of our knowledge on pyrazole derivatives2,13,20 and considering their important electronic properties, our group synthesized a family of 4-styrylpyrazoles and applied 3-phenyl-1-(2-pyridyl)-4-styrylpyrazole (75) as a chemosensor to detect Hg2+.109 The 2-pyridyl acceptor group in 4-styrylpyrazoles favors the D–π–A system and their dipole moment, generating fluorophores with strong blue-light emission (quantum yields of up to 66%). Consequently, probe 75 (4.0 × 10−6 M in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) was selected to evaluate its metal ion sensing capacity, where only the presence of Hg2+ induced significant fluorescence quenching. In the emission studies, with an increase in the concentration of Hg2+, the fluorescence was completely turned off with the addition of 200 equiv. of Hg2+ to the solution. The binding mode of 75-Hg was 2
H2O) was selected to evaluate its metal ion sensing capacity, where only the presence of Hg2+ induced significant fluorescence quenching. In the emission studies, with an increase in the concentration of Hg2+, the fluorescence was completely turned off with the addition of 200 equiv. of Hg2+ to the solution. The binding mode of 75-Hg was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as determined by the Job's plot of the fluorescence at 390 nm, with an LOD of 3.10 × 10−7 M (R2 = 0.9950) and an LMCT process (Fig. 26a).
1, as determined by the Job's plot of the fluorescence at 390 nm, with an LOD of 3.10 × 10−7 M (R2 = 0.9950) and an LMCT process (Fig. 26a).
Pyrazolines can also be applied for sensing Hg2+, for example, Bozkurt and Gul reported the development of probe 76.110 This chemosensor was obtained with diverse substituents in the molecule such as 4-hydroxyphenyl at position 3, thiophene-2-yl at position 5, and benzenesulfonamide at position 1 (Fig. 26b). In addition, 76 showed an absorption band at 355 nm and a relatively high-intensity emission at 449 nm (φ = 68% in water), which remained unchanged upon the addition of various metals to the solution. However, both the quantum yield (φ = 16%) and the fluorescence lifetime of 76 decreased in the presence of Hg2+ (from 0 to 10−4 M), indicating that this analyte quenched the fluorescence. The results from the Job's plot indicated the interaction ratio of 76-Hg was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the calculated LOD was 1.45 × 10−5 M in the linear range of (2 to 20) × 10−5, and the binding constant was 8.06 × 104 M−1. The EDTA test confirmed the reversibility of this probe, showing that detecting Hg2+ is reversible several times. This probe was applied for the analysis of real water samples, providing excellent LOD results (4.70 × 10−7 M).
1, the calculated LOD was 1.45 × 10−5 M in the linear range of (2 to 20) × 10−5, and the binding constant was 8.06 × 104 M−1. The EDTA test confirmed the reversibility of this probe, showing that detecting Hg2+ is reversible several times. This probe was applied for the analysis of real water samples, providing excellent LOD results (4.70 × 10−7 M).
Naskar et al.111 were inspired by the photophysical benefits of pyrene derivatives to obtain pyrene–pyrazole probe 77 for sensing Hg2+ (Fig. 27a). The successive addition of Hg2+ to the probe solution from 0 to 5 × 10−5 M in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8
H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with HEPES buffer at pH 7.2 caused a gradual decrease in the absorption intensity and confirmed the existence of a new product due to the interaction of 77-Hg. Probe 77 showed intense green fluorescence when excited at 374 nm (φ = 21%). Typical emission bands at 418 and 438 nm were observed, which are related to the monomer and excimer emissions of the pyrene moiety, respectively. The addition of Hg2+ quenched the emission (φ = 2%), and fluorescence was tested as a function of pH, showing that in the pH range of 4 to 8, the solution of 77 exhibits negligible fluorescence in the presence of Hg2+, which is suitable for biological applications.
2) with HEPES buffer at pH 7.2 caused a gradual decrease in the absorption intensity and confirmed the existence of a new product due to the interaction of 77-Hg. Probe 77 showed intense green fluorescence when excited at 374 nm (φ = 21%). Typical emission bands at 418 and 438 nm were observed, which are related to the monomer and excimer emissions of the pyrene moiety, respectively. The addition of Hg2+ quenched the emission (φ = 2%), and fluorescence was tested as a function of pH, showing that in the pH range of 4 to 8, the solution of 77 exhibits negligible fluorescence in the presence of Hg2+, which is suitable for biological applications.
According to the Job's plot studies, it was determined that Hg2+ forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with 77 with a binding constant of 7.10 × 105 M−1, as confirmed by NMR experiments, ESI-MS spectrometry, and DFT calculations. This probe presented reversibility upon the alternating addition of Hg2+/Na2H2EDTA, making 77 a reversible Hg2+ sensor with an LOD of 9.20 × 10−9 M at 438 nm. The DFT calculations revealed that three rotamers are accessible to bind to Hg2+ ions by rotating the pyrazole ring and carbonyl groups, which validated the important role of the pyrazole ring in 77. By testing the utility of 77 in live-cell imaging, the studies established that the 77 could detect cellular cytoplasmic Hg2+ in HepG2 cells.111
1 complex with 77 with a binding constant of 7.10 × 105 M−1, as confirmed by NMR experiments, ESI-MS spectrometry, and DFT calculations. This probe presented reversibility upon the alternating addition of Hg2+/Na2H2EDTA, making 77 a reversible Hg2+ sensor with an LOD of 9.20 × 10−9 M at 438 nm. The DFT calculations revealed that three rotamers are accessible to bind to Hg2+ ions by rotating the pyrazole ring and carbonyl groups, which validated the important role of the pyrazole ring in 77. By testing the utility of 77 in live-cell imaging, the studies established that the 77 could detect cellular cytoplasmic Hg2+ in HepG2 cells.111
Several reported nanocomposites can be used for the removal of heavy metals in contaminated waters. In this case, Mir et al.112 studied the detection of Hg2+ using fluorescent probe 78 bearing a pyrazole derivative functionalized with Fe3O4@SiO2. Probe 78 has the advantage of easy separation by an external magnetic field because of its magnetic core (Fig. 27b). The solution of 78 (10−5 M) in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with HEPES buffer (10−4 M, pH 7) showed an emission band at 463 nm (λexc = 225 nm) and no change upon the addition of toxic metals except for a slight change in intensity for some cations. However, a notable variation for Hg2+ was observed with fluorescence quenching at 463 nm. The decrease in fluorescence intensity could be attributed to well–known processes such as enhanced spin–orbit coupling or energy/electron transfer of Hg2+. The coordination geometry, nitrogen affinity, and ion size and charge are vital for selectivity high Hg2+. A good linear correlation between the Hg2+ concentration and emission intensity in the range of 10 to 50 equiv. was observed with an LOD of 7.60 × 10−9 M. Computational calculations revealed nonparticipation of the pyrazole core in the coordination complex, acting as a fluorophore.
1) with HEPES buffer (10−4 M, pH 7) showed an emission band at 463 nm (λexc = 225 nm) and no change upon the addition of toxic metals except for a slight change in intensity for some cations. However, a notable variation for Hg2+ was observed with fluorescence quenching at 463 nm. The decrease in fluorescence intensity could be attributed to well–known processes such as enhanced spin–orbit coupling or energy/electron transfer of Hg2+. The coordination geometry, nitrogen affinity, and ion size and charge are vital for selectivity high Hg2+. A good linear correlation between the Hg2+ concentration and emission intensity in the range of 10 to 50 equiv. was observed with an LOD of 7.60 × 10−9 M. Computational calculations revealed nonparticipation of the pyrazole core in the coordination complex, acting as a fluorophore.
Finally, considering the advantages of pyrazolines as widely known fluorophores, Bozkurt and Gul synthesized fluorescent derivative 79 for the fluorometric detection of Hg2+ (Fig. 27c).113 Probe 79 showed two absorption bands at 278/363 nm and a relatively intense emission band at 464 nm in water. Notably, its absorption spectrum did not change in the presence of metal ions, while its fluorescence intensity decreased only with Hg2+ (φ = 5%). The LOD of Hg2+ was determined to be 1.60 × 10−7 M by a linear relationship, and the interaction ratio 79-Hg was found to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the Job's plot analysis with a binding constant of 4.69 × 105 M−2. The results revealed that 79 is a reversible probe with recycling capability by the successive addition of Hg2+/EDTA (turn-off/turn-on). The detection of Hg2+ was studied at different pH values, and the results showed that 79 could be used for the analysis of neutral water samples and biological applications at physiological pH. The spectral changes observed in the FTIR and 1H NMR studies indicated that the decrease in the emission intensity of 79 is due to the electrostatic interactions of probe–Hg2+. This probe was successfully used for the measurement of real water samples.
1 according to the Job's plot analysis with a binding constant of 4.69 × 105 M−2. The results revealed that 79 is a reversible probe with recycling capability by the successive addition of Hg2+/EDTA (turn-off/turn-on). The detection of Hg2+ was studied at different pH values, and the results showed that 79 could be used for the analysis of neutral water samples and biological applications at physiological pH. The spectral changes observed in the FTIR and 1H NMR studies indicated that the decrease in the emission intensity of 79 is due to the electrostatic interactions of probe–Hg2+. This probe was successfully used for the measurement of real water samples.
3.4. Pyridine derivatives
Pyridine derivatives have long been used primarily in the pesticide and pharmaceutical industries. The pyridine ring is a structural component of some natural products such as vitamins, and coenzymes, alkaloids. Pyridines have been used in supramolecular chemistry, mainly due to their properties such as basicity, water-solubility, and hydrogen bond formation, and its nitrogen atom acts as a receptor site in chemosensors. In a few cases, free pyridines act as Hg2+ recognition sites; however, many reversible probes are observed in fused systems, where nitrogen is part of the complex and yields greater stability. Good LOD results have been observed in other fused pyridines, which means that some probes can be applied in real and biological samples.114–121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4
H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were carried out by adding 4 equiv. of different metal ions (4.8 × 10−5 M) to the solution. The solution changed from yellow-orange to colorless with Hg2+, but in the presence of other metal ions, it remained unaffected when observed by the naked eye. Similarly, a change from orange to strong fluorescent yellow under UV light was observed only with Hg2+. Compound 80 is weakly fluorescent (φ = 0.9%) at 590 nm, and with the addition of 4 equiv. of Hg2+, a notable fluorescence enhancement was observed (φ = 4.4%) with a blue shift in the emission band at 566 nm. This fluorescence “turn-on” may be explained by the principle of chelation-enhanced fluorescence (CHEF) due to the inhibition of the PET process and increased conformational rigidity. The 1
1) were carried out by adding 4 equiv. of different metal ions (4.8 × 10−5 M) to the solution. The solution changed from yellow-orange to colorless with Hg2+, but in the presence of other metal ions, it remained unaffected when observed by the naked eye. Similarly, a change from orange to strong fluorescent yellow under UV light was observed only with Hg2+. Compound 80 is weakly fluorescent (φ = 0.9%) at 590 nm, and with the addition of 4 equiv. of Hg2+, a notable fluorescence enhancement was observed (φ = 4.4%) with a blue shift in the emission band at 566 nm. This fluorescence “turn-on” may be explained by the principle of chelation-enhanced fluorescence (CHEF) due to the inhibition of the PET process and increased conformational rigidity. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for 80-Hg was determined by the Job's method with an association constant of 1.13 × 105 M−1 in water and 1.46 × 105 M−1 in MeOH
1 binding stoichiometry for 80-Hg was determined by the Job's method with an association constant of 1.13 × 105 M−1 in water and 1.46 × 105 M−1 in MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and LOD of 7.50 × 10−6 M. These results suggest that 80 can be applied as a reversible fluorescent probe for the selective sensing of Hg2+ in an aqueous medium under physiological conditions.
H2O and LOD of 7.50 × 10−6 M. These results suggest that 80 can be applied as a reversible fluorescent probe for the selective sensing of Hg2+ in an aqueous medium under physiological conditions.
Alternatively, Zhang et al.115 developed probe 81, having a pyridine–organoboron complex for the detection of Hg2+ in THF (10−5 M) or gaseous acid/base upon aggregation (Fig. 28b). Probe 81 showed a strong absorption band at 415 nm due to the π–π* transition with a light-cyan fluorescence band at 480 nm, which was altered in different polar solvents together with its absorption spectrum. These results indicate that boron restricts the σ-bond rotation and isomerization process of the imine, resulting in a high fluorescence quantum yield.
Upon the addition of Hg2+ to a solution of 81, the emission band intensity at 480 nm was gradually reduced to generate a new band at 515 nm with a change from cyan to green, which could be observed by the naked eyes. This probe has high Hg2+ selectivity via interactions with the pyridinic N; thus, an ICT process occurs, as evidenced by the new emission band at 515 nm. The fluorescence intensity showed good linearity for Hg2+ in the range of (1 to 10) × 10−5 M with an LOD of 5.00 × 10−8 M. The chemical shifts in its 1H NMR spectrum remained unchanged with 1.0 equiv. of Hg2+, indicating that 81 reached saturation coordination with 1 equiv. of Hg2+ via a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. When S2− ions were added to the 81-Hg complex, HgS precipitated due to the stronger combining capacity of Hg2+ with S2−, confirming that the reaction mechanism is reversible and can be regenerated for three cycles (Fig. 28b).115
1 stoichiometry. When S2− ions were added to the 81-Hg complex, HgS precipitated due to the stronger combining capacity of Hg2+ with S2−, confirming that the reaction mechanism is reversible and can be regenerated for three cycles (Fig. 28b).115
Similarly, Eremina et al.116 developed a new application via bis(cyclometalated) iridium(III) species bearing an analyte. For all the complexes, their absorption spectra exhibited a strong band at 340 nm with high molar extinction coefficients (ε = 1.03/7.96 × 104 M−1 cm−1) due to the spin-allowed π–π* ligand-centered transitions. In addition, less intense bands appeared in the region of 340–400 nm (ε = 0.40/9.40 × 103 M−1 cm−1), which are attributed to the spin-allowed singlet MLCT transitions. These results agree with the DFT calculations for the complexes. All the complexes presented broad blue-green phosphorescence in acetonitrile after photoexcitation at different wavelengths. Complex 82 showed an 80% decrease in intensity emission at 472 nm (λexc = 375 nm) and weak residual luminescence upon the addition of Hg2+. The titration studies indicated 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometries for 82-Hg according to the Job's plot with an LOD of 2.63 × 10−7 M. The detection mechanism was explained by the conversion of cyanide 82 to methyl isocyanide 82-Hg via different Hg2+ complexes (i.e., 82′-Hg and 82′-Hg-82′), which resulted in nearly complete disappearance of its optical properties (Fig. 28c).
1 binding stoichiometries for 82-Hg according to the Job's plot with an LOD of 2.63 × 10−7 M. The detection mechanism was explained by the conversion of cyanide 82 to methyl isocyanide 82-Hg via different Hg2+ complexes (i.e., 82′-Hg and 82′-Hg-82′), which resulted in nearly complete disappearance of its optical properties (Fig. 28c).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by the Job's plot. Moreover, 83 was successfully applied for the detection of Hg2+ in human cells.
1 by the Job's plot. Moreover, 83 was successfully applied for the detection of Hg2+ in human cells.
Motivated by the versatility of quinolines, Wang et al.118 developed the “turn-on” probe 84 for sensing Hg2+ in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (∼99
DMSO (∼99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), bearing nitrobenzo-oxadiazole, piperazine, pyridine, and quinoline moieties (Fig. 29b). The solution of 84 is orange, and its absorption spectrum showed a strong band at 495 nm due to the ICT process from the piperazine ring to the nitro group. After the gradual addition of Hg2+, the ICT band at 482 showed an increase in absorption intensity with a blue shift nm due to the reduced electron-donating ability of the piperazine moiety after the coordination of Hg2+, which changed the solution color from orange to yellow. Similarly, the fluorescence quantum yield increased from 1.1% to 14% at 543 nm (λexc = 488 nm) due to the suppression of the PET process in the complex formed, given that the piperazine nitrogen donor was no longer available; thus, the solution emission was turned on from dark to yellow coloration (pH 6 to 7.5).
1), bearing nitrobenzo-oxadiazole, piperazine, pyridine, and quinoline moieties (Fig. 29b). The solution of 84 is orange, and its absorption spectrum showed a strong band at 495 nm due to the ICT process from the piperazine ring to the nitro group. After the gradual addition of Hg2+, the ICT band at 482 showed an increase in absorption intensity with a blue shift nm due to the reduced electron-donating ability of the piperazine moiety after the coordination of Hg2+, which changed the solution color from orange to yellow. Similarly, the fluorescence quantum yield increased from 1.1% to 14% at 543 nm (λexc = 488 nm) due to the suppression of the PET process in the complex formed, given that the piperazine nitrogen donor was no longer available; thus, the solution emission was turned on from dark to yellow coloration (pH 6 to 7.5).
The complexation mechanism of 84 with Hg2+ was confirmed by 1H NMR and DFT calculations. The LOD was found to be 1.92 × 10−8 M, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex according to the Job's plot analysis and the binding constant of 84-Hg was 1.15 × 104 M−1. The reversibility of the sensing process was established and quenched by NaI, implying the decomplexation of 84-Hg with repetitions for a minimum of four cycles.118 The in vivo detection of Hg2+ in living zebrafish was applied to evaluate the practical use of 84.
1 complex according to the Job's plot analysis and the binding constant of 84-Hg was 1.15 × 104 M−1. The reversibility of the sensing process was established and quenched by NaI, implying the decomplexation of 84-Hg with repetitions for a minimum of four cycles.118 The in vivo detection of Hg2+ in living zebrafish was applied to evaluate the practical use of 84.
Similarly, Zareh Jonaghani and Zali-Boeini119 synthesized the fluorimetric and colorimetric probe 85 (hybrid quinoline–naphthothiazole system) for sensing Hg2+ in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3.00 × 10−5 M). This probe showed an absorption maximum at 356 nm (ε = 18.100 M−1 cm−1) and a fluorescence emission at 433 nm (φ = 32%). Upon the addition of Hg2+ (2 × 10−6 M), compound 86 showed a redshift to 504 nm and a decline in its fluorescence intensity (φ = 26%) with a color change from colorless to yellow, which could be observed by the naked eye. This probe showed an excellent linear relationship between the fluorescence intensity and concentration of Hg2+ with an LOD of 3.42 × 10−8 M, while the association constant was 2.00 × 106 M−1 (pH 5 to 8). Finally, the Job's plot showed a 1
1, 3.00 × 10−5 M). This probe showed an absorption maximum at 356 nm (ε = 18.100 M−1 cm−1) and a fluorescence emission at 433 nm (φ = 32%). Upon the addition of Hg2+ (2 × 10−6 M), compound 86 showed a redshift to 504 nm and a decline in its fluorescence intensity (φ = 26%) with a color change from colorless to yellow, which could be observed by the naked eye. This probe showed an excellent linear relationship between the fluorescence intensity and concentration of Hg2+ with an LOD of 3.42 × 10−8 M, while the association constant was 2.00 × 106 M−1 (pH 5 to 8). Finally, the Job's plot showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for the 85-Hg complex (Fig. 29c).
1 stoichiometric ratio for the 85-Hg complex (Fig. 29c).
According to the widely reported properties in other sensor designs, Guo et al.120 developed the colorimetric and fluorescent probe 86, bearing a photochromic di-3-thienylethene with 2-formylquinoline (Fig. 29d). Probe 86 showed a sharp absorption band at 261 nm (ε = 4.9 × 104 mol−1 L cm−1 in MeCN), which is assigned to the π–π* transitions. Upon irradiation with light at 297 nm, 86 showed a photocyclization reaction. The solution color changed from colorless to purple under natural light, but from bright fluorescent cyan (φ = 24.5%) to dark (φ = 2.1%) under UV light. This probe showed strong fluorescence at 506 nm (λexc = 370 nm). Moreover, when its response to Hg2+ was examined, its absorption spectrum exhibited a redshift from 261 to 275 nm due to the LMCT process, while in fluorescence, the band at 506 nm showed quenching until 97%, and the solution changed from bright cyan to dark. The emission was restored with the addition of EDTA, confirming that the detection mechanism is reversible. The association constant of 84-Hg was 4.01 × 104 L with a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as confirmed by ESI-MS, and the LOD was 5.63 × 10−8 M. According to these results, an integrated logic circuit with multiple control switches was constructed.
1, as confirmed by ESI-MS, and the LOD was 5.63 × 10−8 M. According to these results, an integrated logic circuit with multiple control switches was constructed.
As the last example, Jiang et al.121 obtained the hybrid rhodamine B–hydrazonoquinoline system 87 for the dual sensing of Cu2+ and Hg2+ in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4
H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 29e). The solution of 87 (5.00 × 10−5 M) exhibited an absorption band at around 280 nm, but a new absorption band appeared at ∼560 nm upon the addition of Cu2+ or Hg2+ ions (2.00 × 10−4 M). The new band increased considerably with an increase in the concentration of these metals. The isoabsorptive point at 350 nm for Cu2+ and 350 nm for Hg2+ indicated that 87 could be used as a ratiometric probe. The probe solution (10−5 M) showed weak emission at 572 nm (λexc = 355 nm) without notable changes upon the addition of solutions of different metal ions. An increase in fluorescence was observed upon the addition of 4.00 × 10−5 M of Cu2+ or Hg2+, and the maximum emission shifted to 582 nm; however, a change in the pale and bright orange fluorescence could be easily observed by the naked eye under a 365 nm UV lamp.
1, v/v) (Fig. 29e). The solution of 87 (5.00 × 10−5 M) exhibited an absorption band at around 280 nm, but a new absorption band appeared at ∼560 nm upon the addition of Cu2+ or Hg2+ ions (2.00 × 10−4 M). The new band increased considerably with an increase in the concentration of these metals. The isoabsorptive point at 350 nm for Cu2+ and 350 nm for Hg2+ indicated that 87 could be used as a ratiometric probe. The probe solution (10−5 M) showed weak emission at 572 nm (λexc = 355 nm) without notable changes upon the addition of solutions of different metal ions. An increase in fluorescence was observed upon the addition of 4.00 × 10−5 M of Cu2+ or Hg2+, and the maximum emission shifted to 582 nm; however, a change in the pale and bright orange fluorescence could be easily observed by the naked eye under a 365 nm UV lamp.
The binding constant values were 1.45 × 104 M−1 for 87-Cu with an LOD of 1.20 × 10−7 M and 4.98 × 106 M−2 for 87-Hg with an LOD 3.20 × 10−8 M. The fluorescence quantum yields of 87, 87-Cu, and 87-Hg are 5.4%, 82%, and 98%, respectively. The Job's plot analysis indicated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for 87-Cu and 1
1 binding stoichiometry for 87-Cu and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for 87-Hg. The emissions at 582 nm and UV-vis absorption at 560 nm attributed to metals could induce the opening of the spirocycle of the rhodamine unit. In addition, the excited-state intramolecular proton transfer (ESIPT) of the OH group in the quinoline moiety could be inhibited by both Cu2+ and Hg2+. The emission of 87-Hg2+ was stronger than that with the same amount of Cu2+, probably due to the above-mentioned ring-opening, unique regenerative cycle of the quinoline unit in 87, and the inhibition of the PET process. Studies in live Chinese hamster lung cells suggested that 87 exhibited low cytotoxicity and could be utilized in live cells in vivo with minor damage to monitor Cu2+ and Hg2+.121
2 for 87-Hg. The emissions at 582 nm and UV-vis absorption at 560 nm attributed to metals could induce the opening of the spirocycle of the rhodamine unit. In addition, the excited-state intramolecular proton transfer (ESIPT) of the OH group in the quinoline moiety could be inhibited by both Cu2+ and Hg2+. The emission of 87-Hg2+ was stronger than that with the same amount of Cu2+, probably due to the above-mentioned ring-opening, unique regenerative cycle of the quinoline unit in 87, and the inhibition of the PET process. Studies in live Chinese hamster lung cells suggested that 87 exhibited low cytotoxicity and could be utilized in live cells in vivo with minor damage to monitor Cu2+ and Hg2+.121
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) with different metal ions indicated that the intensity of the bands was unchanged. With the addition of Hg2+, the intensity of the bands decreased with two isosbestic points at 311/364 nm for 88 and 306/362 nm for 89, supporting the formation of complexes with this highly toxic analyte.
99) with different metal ions indicated that the intensity of the bands was unchanged. With the addition of Hg2+, the intensity of the bands decreased with two isosbestic points at 311/364 nm for 88 and 306/362 nm for 89, supporting the formation of complexes with this highly toxic analyte.
Conversely, the emission intensity increased, observing notable changes upon the addition of 5 equiv. of Hg2+ to 88 (φ from 8.9% to 77% at 395 nm) and 3 equiv. to 89 (φ from 9.5% to 75% at 386 nm) due to the CHEF effect in both probes, which was favored by the better aggregation of the complexes. In addition, the fluorescence lifetimes of 88 and 89 were 2.5 and 2.3 ns in water, while upon the addition of Hg2+, they changed to 7.7 and 8.5 ns, respectively, with a stoichiometry 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for both complexes according to the Job's plot and HRMS analysis. The LODs were found to be 2.20 × 10−8 and 5.60 × 10−9 M with binding constants of 3.40 × 109 and 4.60 × 109 M−2 for 88 and 89, respectively. Notably, these probes exhibited reversibility with the addition of 10 equiv. of KI at pH 7 (HEPES-buffer solution).
1 for both complexes according to the Job's plot and HRMS analysis. The LODs were found to be 2.20 × 10−8 and 5.60 × 10−9 M with binding constants of 3.40 × 109 and 4.60 × 109 M−2 for 88 and 89, respectively. Notably, these probes exhibited reversibility with the addition of 10 equiv. of KI at pH 7 (HEPES-buffer solution).
Julolidine derivatives have essential photophysical properties as part of photoconductive, chemiluminescent, and chromogenic materials. Accordingly, Gupta and co-workers123 obtained a probe having a thiosemicarbazide moiety linked to this fluorophore (Fig. 30b), which works as a chemosensor multitarget for Hg2+ and Mn2+ in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8
H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). With the addition of Hg2+ (0 to 1.6 equiv.) to 90 (2.5 × 10−5 M), a redshift from 383 nm to around 400 nm was observed with a color change from colorless to yellow. These results were induced by Hg2+ binding to the probe, which favors LMCT from the nitrogen in the julolidine core towards the receptor site (push–pull effect).
2). With the addition of Hg2+ (0 to 1.6 equiv.) to 90 (2.5 × 10−5 M), a redshift from 383 nm to around 400 nm was observed with a color change from colorless to yellow. These results were induced by Hg2+ binding to the probe, which favors LMCT from the nitrogen in the julolidine core towards the receptor site (push–pull effect).
The emission spectrum of 90 showed a band at 442 nm (λexc = 397 nm) without significant changes upon the addition of various metal ions (10 equiv.) to the solution (6.0 × 10−5 M), except for Hg2+, which quenched the fluorescence due to the complexing based on the MLCT-heavy metal effect. The complex stoichiometry was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the Job's plot and confirmed by 1H NMR titration with a formation constant of 7.73 × 103 M−1 by the Benesi–Hildebrand method. The LOD by fluorescence was 1.50 × 10−5 M for Hg2+ and 2.00 × 10−7 M for Mn2+. This probe worked efficiently in the pH range of 5 to 10, which is within physiological limits. DFT calculations clarified the coordination and selectivity of 90 with Hg2+ and Mn2+ due to the decrease in the HOMO–LUMO energy band gap in the structures of the complexes formed. It should also be noted that 90 was used in the analysis of real water samples with satisfactory results.123
1 according to the Job's plot and confirmed by 1H NMR titration with a formation constant of 7.73 × 103 M−1 by the Benesi–Hildebrand method. The LOD by fluorescence was 1.50 × 10−5 M for Hg2+ and 2.00 × 10−7 M for Mn2+. This probe worked efficiently in the pH range of 5 to 10, which is within physiological limits. DFT calculations clarified the coordination and selectivity of 90 with Hg2+ and Mn2+ due to the decrease in the HOMO–LUMO energy band gap in the structures of the complexes formed. It should also be noted that 90 was used in the analysis of real water samples with satisfactory results.123
Alternatively, considering the particular affinity between Hg2+ and sulfur, Ponram et al.124 obtained the fluorescent mono-sulfur 91a and tetra-sulfur 91b probes having a tetrahydrodibenzo-phenanthridine core (Fig. 31a). The absorption spectrum of 91a showed two redshifted bands from 270/326 to 280/348 nm with the addition of Hg2+; similarly, 91b exhibited a redshift from 255 to 263 nm, but the band at 346 nm was blue-shifted to 330 nm. The observed changes in fluorescence colors showed that these probes detect Hg2+ via “turn-on” for 91a and “turn-off” for 91b. The nature of the TICT process can explain these results given that the detection state returns to the ground state through the redshifted emission or more efficient non-radiative relaxation. Upon the addition of Hg2+ (1 equiv.) to 91a, the emission band at 455 nm gradually increased, while for 91b, it gradually decreased at 456 nm in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (7
H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The LOD was calculated to be 9.10 × 10−10 M (MeCN) for 91a and 4.18 × 10−11 M (DMSO) for 91b with a 1
3). The LOD was calculated to be 9.10 × 10−10 M (MeCN) for 91a and 4.18 × 10−11 M (DMSO) for 91b with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for both according to the Job's method. DFT calculations confirmed the sensing mechanism, showing that the LMCT phenomenon occurs between chemosensor molecule 91 and Hg2+.
1 stoichiometry for both according to the Job's method. DFT calculations confirmed the sensing mechanism, showing that the LMCT phenomenon occurs between chemosensor molecule 91 and Hg2+.
Considering the high complexing capacity of phenanthroline with d-block metals, Tang et al.125 developed the AIE-active fluorescent probe 92, bearing a tetraphenylethene (TPE) unit, to detect Zn2+ (in THF) and Hg2+ (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O). The photophysical results proved the formation of nanoscopic aggregates in THF when the amount of water was increased, with a blue-green emission (φ from 0.8% to 80%) at 518 nm (λexc = 375 nm) caused by the restricted intramolecular rotation in 92-Hg (Fig. 31b). In addition, upon the addition of Hg2+ to the probe solution in THF
H2O). The photophysical results proved the formation of nanoscopic aggregates in THF when the amount of water was increased, with a blue-green emission (φ from 0.8% to 80%) at 518 nm (λexc = 375 nm) caused by the restricted intramolecular rotation in 92-Hg (Fig. 31b). In addition, upon the addition of Hg2+ to the probe solution in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the emission intensity decreased significantly with a slight redshift and changed from bright blue-green to dark. These results were supported by 1H NMR studies, concluding that the coordination of 92 with Hg2+ was achieved using the two nitrogen atoms in the phenanthroline ring. The stoichiometry of the 92-Hg complex was 1
1), the emission intensity decreased significantly with a slight redshift and changed from bright blue-green to dark. These results were supported by 1H NMR studies, concluding that the coordination of 92 with Hg2+ was achieved using the two nitrogen atoms in the phenanthroline ring. The stoichiometry of the 92-Hg complex was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as confirmed by MS analysis with a quenching constant and LOD of 5.58 × 107 M−1 and 2.55 × 10−9 M, respectively. Remarkably, hybrid system 92 also was used as a fluorescence “turn-on” probe for the detection of Zn2+ in THF with an LOD of 1.24 × 10−6 M. In addition, 92 was also studied in the solid-state, demonstrating reversible mechanochromic behavior with the fluorescent of colors blue–green–blue.
1, as confirmed by MS analysis with a quenching constant and LOD of 5.58 × 107 M−1 and 2.55 × 10−9 M, respectively. Remarkably, hybrid system 92 also was used as a fluorescence “turn-on” probe for the detection of Zn2+ in THF with an LOD of 1.24 × 10−6 M. In addition, 92 was also studied in the solid-state, demonstrating reversible mechanochromic behavior with the fluorescent of colors blue–green–blue.
Ultimately, Cuerva et al. described several Pt(II) metallomesogens by exploring the capacity of some metal ions to induce self-assembly via intermolecular Pt⋯Pt interactions, generating aggregates and emission changes in solution.126 Only Hg2+ caused a notable emission change in the unsymmetrical Pt(II) complex having pyridine 93. The emission band at 500 nm was quenched, and the minimum intensity was achieved by adding 1 equiv. of Hg2+ in dichloromethane (DCM). Hg2+ interacts with the axial position of 93 (93-Hg2+ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), but an interaction with the pyrazolic N of 93 is also suggested (Fig. 32a). In contrast, Hg2+ ions resulted in a notable increase in the emission intensity for isoquinoline derivative 94 in a 1
1), but an interaction with the pyrazolic N of 93 is also suggested (Fig. 32a). In contrast, Hg2+ ions resulted in a notable increase in the emission intensity for isoquinoline derivative 94 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 32b). In this case, the emission maximum exhibited a redshift from 527 to 644 nm (λexc = 365 nm), from a low greenish emission to bright red. This was attributed to the existence of metal-MLCT excited states and the intermolecular Pt⋯Pt interactions in a sandwich-type conformation.
1 ratio (Fig. 32b). In this case, the emission maximum exhibited a redshift from 527 to 644 nm (λexc = 365 nm), from a low greenish emission to bright red. This was attributed to the existence of metal-MLCT excited states and the intermolecular Pt⋯Pt interactions in a sandwich-type conformation.
It is important to note that other metal ions interact with probe 94, but a strong quenching effect in its natural greenish emission was observed. The result obtained in the recognition of Hg2+ using both probes is mainly because the coordination of Pt with the ligands prevents the formation of aggregates in the presence of the analyte. However, the additional intermolecular π⋯π interactions between the isoquinoline rings in 94 are an additional explanation for the stabilization of the Pt⋯Pt aggregates. The photophysical results revealed that these luminescent Pt(II) compounds are excellent candidates as dyes for the fabrication of several stimuli-responsive luminescent materials.126
4. Some porphyrin-based probes
The tetrameric structure of pyrrole, known as porphyrin, is a versatile structural motif; consequently, it has been extensively studies in different areas of science, even in the ion sensing field,127–131 and frequently in cation129–131 recognition. Accordingly, we mention this important macrocyclic NHC concerning its application for the detection of cyanide127,128 and mercury(II)129 considering that they are hazardous. In general, porphyrins have high absorption in the red visible spectrum region and are potential dyes for sensitizers in photodynamic therapy; similarly, the inclusion of chromophore groups in the porphyrinic ring increases its photophysical versatility, causing significant changes in the wavelength its respective derivatives can absorb.1,21,127–131Regarding the anion, porphyrin derivatives 95 as chemodosimeters for CN− were reported by Chahal and Sankar in 2015. They obtained dicyanovinylidene-appended β-substituted porphyrins via Vilsmeier–Haack formylation followed by a Knoevenagel condensation reaction between the aldehyde obtained and malononitrile to obtain the dicyanovinyl derivative. The β carbon is an electrophilic center, where cyanide anions can perform a nucleophilic attack via design strategy I.127 This probe has essential characteristics that favor the charge transfer process, for instance, the quasi-planar conformation confirmed by crystallographic studies. Simultaneously, this structure has extended pi conjugation between the pyrrole ring and the olefinic substituent. Upon the addition of CN− to a toluene solution, the color of the probe changed from green to light pink, as evidenced by the naked eye. The photophysical studies showed that the intensity bands of the bands at 385 nm, 450 nm, and 602 nm of the free sensor gradually decreased with the addition of CN−, while two new bands appeared at 416 nm and 534 nm. The stoichiometry of the nucleophilic addition reaction is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The LOD of this chemodosimeter is 0.023–0.082 ppm, which is lower than that of other reported porphyrins (Fig. 33a).
1. The LOD of this chemodosimeter is 0.023–0.082 ppm, which is lower than that of other reported porphyrins (Fig. 33a).
In 2020, Dar and Sankar obtained fused porphyrin 96 as a probe for the detection of cyanide.128 Upon adding the anion, it interacts with the acidic N–H proton, resulting in its deprotonation by strategy III for sensing CN−. This was confirmed via 1H-NMR titration experiments, showing that the N–H proton at 9.22 ppm disappeared during the titration, congruent with the probe structure. It has no other reactive points towards anions. In this case, the negative charge is stabilized via conjugation throughout the molecule. The absorption spectrum showed a band at around 424 nm, which decreased with the addition of CN− and blue-shifted to 410 nm. At this point, a new band appeared at 493 nm. The probe was tested against other anions, and it showed no interference except for fluoride. The naked eye evidenced these absorption changes due to the color change from green to reddish-yellow. Notably, this probe was shown to be reversible by washing it with distilled water. After cleaning the sensor, its color changed back to green and its absorption spectrum changed, given that water serves as a protonating agent and dissolves the anion. Finally, the calculated LOD was 2.13 ppm (Fig. 33b).
Regarding the use of porphyrin derivatives for the detection of Hg2+, Ermakova et al. synthesized three amphiphilic porphyrin derivatives 97a–c substituted at the meso-positions for detecting this analyte.129 Color changes and fluorescence quenching (λexc = 420 nm) were observed upon the addition of Hg2+ and other cations to these probes (Fig. 33c). The naked eye could observe the color change in the solutions from light green to colorless only with Hg2+. The redshift of the Soret band and the appearance of three or four Q-bands for the complexes can explain the metal complexation. This behavior is because the bulky metal ion is located above the macrocycle plane, and the ligand is coordinated to Hg2+, generating stable complexes with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In contrast, other metals (Cu2+, Zn2+, and Pb2+) were ligated to the peripheral donor sites. The formation of a neutral complex liberates two protons, but it is not possible to form porphyrin aggregates due to the presence of bulky substituents in the porphyrin macrocycle.
1. In contrast, other metals (Cu2+, Zn2+, and Pb2+) were ligated to the peripheral donor sites. The formation of a neutral complex liberates two protons, but it is not possible to form porphyrin aggregates due to the presence of bulky substituents in the porphyrin macrocycle.
Monolayers of porphyrinyl-phosphonate 97a–c were transferred to a PVC support using the Langmuir–Schaefer film method. According to the analysis of the probes, it was concluded that the films were suitable for the selective spectrophotometric recognition of Hg2+ in an aqueous environment. The sensor treatment by an acidic solution led to the removal of the Hg2+ ions, and these films could be regenerated for at least five times without loss in their efficiency. The heteroatom substituent in 97 (O, S, or NH) had a significant effect on the surface pressure-assisted supramolecular assembly of the porphyrins on solid surfaces. The LOD of these selective and reusable dual-channel probe (absorbance and fluorescence) thin-film sensors was about 10−8 M (2 ppb), which is lower that the recommended levels of Hg2+ ions in drinking water by the U.S. Environmental Protection Agency (EPA) (Fig. 33c).129
5. Conclusions and perspectives
Aza-heterocyclic compounds play a significant role in the design of probes to detect cyanide and mercury. In this case, 5- and 6-membered rings prevail in the design of probes due to their resonance effects and heteroaromaticity, allowing exceptional photophysical responses in the sensing process. Besides, the presence of N–H groups in the rings favors multiple structures with some strategic pathways such as ESIPT. Chemosensors allow the detection of ions, affording analytical methods with fewer limitations and lower costs than other approaches. With the probes considered herein, toxic and poisonous ions are quickly identified via colorimetric and/or fluorimetric “turn-on” or “turn-off”. Remarkably, in almost all the recently published works, tetrabutylammonium cyanide (TBACN) salt was used as the primary CN− source due to its practical benefits. In addition, the characteristic complexing ability of N-heterocycles makes them highly specific for the recognition of Hg2+ in nature.After analyzing the examples studied in this contribution, it was possible to qualitatively determine that Hg2+ tends to form stable complexes having 5- or 6-membered rings with N, O, and S atoms, mainly with a ligand–Hg 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Through this review, all the remarkable properties of a wide variety of probes could be compiled as promising tools for the detection of Hg2+ in the biological and environmental fields and real samples. The experimental results observed herein show a positive future for the design of N-heterocycle-based sensors with high sensitivity and selectivity towards Hg2+. Further, novel ways are revealed to more easily develop simple, selective, highly sensitive, low-cost, and reversible probes bearing different fused and non-fused NHCs conjugated with other fluorophores to have a greater diversity of coordination sites, and thus detect toxic ions such as Hg2+.
1 stoichiometry. Through this review, all the remarkable properties of a wide variety of probes could be compiled as promising tools for the detection of Hg2+ in the biological and environmental fields and real samples. The experimental results observed herein show a positive future for the design of N-heterocycle-based sensors with high sensitivity and selectivity towards Hg2+. Further, novel ways are revealed to more easily develop simple, selective, highly sensitive, low-cost, and reversible probes bearing different fused and non-fused NHCs conjugated with other fluorophores to have a greater diversity of coordination sites, and thus detect toxic ions such as Hg2+.
Ultimately, we showed that N-heterocycles are valuable molecules in the design of chemosensors due to their advantages such as synthetic versatility, use in wide pH range, the low detection limit, and the characteristic characterization of their sensing mechanism is straightforward. Nevertheless, the design of new probes with lower LOD, more selectivity towards some interferents (e.g., F−, SH−, and some metal ions), the possibility of using 100% aqueous medium in the detection, and reversibility are still crucial to give a closer approach to real samples. Additionally, although many published works on fluorescent and colorimetric chemosensors are small-molecule-based, several of them have been developed without a rational design; consequently, a good look at this review can significantly improve this issue.
Authors contributions
The four individuals listed as authors have contributed substantially to the development of this review, and no other person was involved with its development. The contribution of authors is as follows:Miss María C. Ríos. Papers analysis involving azole derivatives in cyanide detection and original draft composition.
Mr Néstor F. Bravo. Literature analysis involving all described probes in mercury(II) recognition and original draft composition.
Mr Christian C. Sánchez. Papers analysis on pyridine derivatives and metal complexes in cyanide sensing and original draft composition.
Prof Jaime Portilla. Literature investigation, supervision, writing, manuscript review and editing, conceptualization, and resources.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We wish to thank the Chemistry Department and Vicerrectoría de Investigaciones from Universidad de los Andes for financial support. JP and NB acknowledge support from the science faculty (projects INV-2019-84-1800 and INV-2019-86-1802). JP also wishes to credit all members of the Bioorganic Compounds Research Group for their valuable collaboration in some chemosensors development.Notes and references
- J. Alvarez-Builla Julio, J. J. Vaquero and J. Barluenga, Modern Heterocyclic Chemistry, Wiley-VCH Verlag & Co. KGaA, Weinheim, Germany, 2011, vol. 4 Search PubMed.
- J.-C. Castillo and J. Portilla, Targets Heterocycl. Syst., 2019, 22, 194–223 Search PubMed.
- J. Yin, Y. Hu and J. Yoon, Chem. Soc. Rev., 2015, 44, 4619–4644 RSC.
- A. Aliyan, N. P. Cook and A. A. Martí, Chem. Rev., 2019, 119, 11819–11856 CrossRef CAS PubMed.
- A. Kellett, Z. Molphy, C. Slator, V. McKee and N. P. Farrell, Chem. Soc. Rev., 2019, 48, 971–988 RSC.
- Z. Xu, Q. Wang and J. Zhu, Chem. Soc. Rev., 2018, 47, 7882–7898 RSC.
- D. Vargas-Oviedo, E. Butassi, S. Zacchino and J. Portilla, Monatshefte fur Chemie, 2020, 151, 575–588 CrossRef CAS.
- A. C. Reiersølmoen and A. Fiksdahl, Eur. J. Org. Chem., 2020, 2020, 2867–2877 CrossRef.
- M. De Candia, F. Fiorella, G. Lopopolo, A. Carotti, M. R. Romano, M. D. Lograno, S. Martel, P. A. Carrupt, B. D. Belviso, R. Caliandro and C. Altomare, J. Med. Chem., 2013, 56, 8696–8711 CrossRef CAS PubMed.
- B. Bano, K. M. Khan, A. Jabeen, A. Hameed, A. Faheem, M. Taha, S. Perveen and S. Iqbal, ChemistrySelect, 2017, 2, 10050–10054 CrossRef CAS.
- S. Roland, C. Jolivalt, T. Cresteil, L. Eloy, P. Bouhours, A. Hequet, V. Mansuy, C. Vanucci and J. M. Paris, Chem.–Eur. J., 2011, 17, 1442–1446 CrossRef CAS PubMed.
- N. R. Elejalde, E. Butassi, S. Zacchino, M. A. Macías and J. Portilla, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2019, 75, 1–12 Search PubMed.
- A. A. Gómez, A. Godoy and J. Portilla, Molecules, 2021, 26, 2708 CrossRef PubMed.
- C. Deng, S. Zheng, D. Wang, J. Yang, Y. Yue, M. Li, Y. Zhou, S. Niu, L. Tao, T. Tsuboi and Q. Zhang, J. Phys. Chem. C, 2019, 123, 29875–29883 CrossRef CAS.
- G. Chai, J. Zhang, M. Pan, Z. Wang, J. Yu, J. Liang, H. Yu, Y. Chen, A. Shang, X. Liu, F. Bai, R. Ma, Y. Chang, S. Luo, A. Zeng, H. Zhou, K. Chen, F. Gao, H. Ade and H. Yan, ACS Energy Lett., 2020, 5, 3415–3425 CrossRef CAS.
- H. Beucher, S. Kumar, R. Kumar, E. Merino, W. H. Hu, G. Stemmler, S. Cuesta-Galisteo, J. A. González, L. Bezinge, J. Jagielski, C. J. Shih and C. Nevado, Chem.–Eur. J., 2020, 26, 17604–17612 CrossRef CAS PubMed.
- W. Sun, S. Guo, C. Hu, J. Fan and X. Peng, Chem. Rev., 2016, 116, 7768–7817 CrossRef CAS PubMed.
- M. García, I. Romero and J. Portilla, ACS Omega, 2019, 4, 6757–6768 CrossRef PubMed.
- A. Tigreros, M. Macías and J. Portilla, Dyes Pigm., 2021, 184, 108730 CrossRef CAS.
- A. Tigreros and J. Portilla, RSC Adv., 2020, 10, 19693–19712 RSC.
- Y. M. Sung, J. Oh, W. Y. Cha, W. Kim, J. M. Lim, M. C. Yoon and D. Kim, Chem. Rev., 2017, 117, 2257–2312 CrossRef CAS PubMed.
- Q. Niu, L. Lan, T. Li, Z. Guo, T. Jiang, Z. Zhao, Z. Feng and J. Xi, Sens. Actuators, B, 2018, 276, 13–22 CrossRef CAS.
- B. Vidya, M. Iniya, G. Sivaraman, R. V. Sumesh and D. chellappa, Sens. Actuators, B, 2017, 242, 434–442 CrossRef CAS.
- G. Y. Yeap, E. Hrishikesan, Y. H. Chan and W. A. K. Mahmood, Tetrahedron, 2017, 73, 5517–5521 CrossRef CAS.
- V. A. Krylova, P. I. Djurovich, J. W. Aronson, R. Haiges, M. T. Whited and M. E. Thompson, Organometallics, 2012, 31, 7983–7993 CrossRef CAS.
- V. A. Krylova, P. I. Djurovich, M. T. Whited and M. E. Thompson, Chem. Commun., 2010, 46, 6696–6698 RSC.
- H. Shahroosvand, F. Nasouti, E. Mohajerani and A. Khabbazi, Opt. Mater., 2012, 35, 79–84 CrossRef CAS.
- C. S. Smith, C. W. Branham, B. J. Marquardt and K. R. Mann, J. Am. Chem. Soc., 2010, 132, 14079–14085 CrossRef CAS PubMed.
- M. Osawa, I. Kawata, R. Ishii, S. Igawa, M. Hashimoto and M. Hoshino, J. Mater. Chem. C, 2013, 1, 4375–4383 RSC.
- J. C. Deaton, S. C. Switalski, D. Y. Kondakov, R. H. Young, T. D. Pawlik, D. J. Giesen, S. B. Harkins, A. J. M. Miller, S. F. Mickenberg and J. C. Peters, J. Am. Chem. Soc., 2010, 132, 9499–9508 CrossRef CAS PubMed.
- F. Liu, K. A. Meadows and D. R. McMillin, J. Am. Chem. Soc., 1993, 115, 6699–6704 CrossRef CAS.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, 2006 Search PubMed.
- J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
- L. Liu, G. Zhang, J. Xiang, D. Zhang and D. Zhu, Org. Lett., 2008, 10, 4581–4584 CrossRef CAS PubMed.
- J. Orrego-Hernández, N. Nuñez-Dallos and J. Portilla, Talanta, 2016, 152, 432–437 CrossRef PubMed.
- B. Kaur, N. Kaur and S. Kumar, Coord. Chem. Rev., 2018, 358, 13–69 CrossRef CAS.
- Y. J. Na, Y. W. Choi, G. R. You and C. Kim, Sens. Actuators, B, 2016, 223, 234–240 CrossRef CAS.
- M. Y. Berezin and S. Achilefu, Chem. Rev., 2010, 110, 2641–2684 CrossRef CAS PubMed.
- G. S. Jiao, L. H. Thoresen and K. Burgess, J. Am. Chem. Soc., 2003, 125, 14668–14669 CrossRef CAS PubMed.
- G. Fukuhara, J. Photochem. Photobiol., C, 2020, 42, 100340 CrossRef CAS.
- A. P. De Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- S. Y. Lee, K. H. Bok, J. A. Kim, S. Y. Kim and C. Kim, Tetrahedron, 2016, 72, 5563–5570 CrossRef CAS.
- F. Wang, L. Wang, X. Chen and J. Yoon, Chem. Soc. Rev., 2014, 43, 4312–4324 RSC.
- A. H. Hall, G. E. Isom and G. A. Rockwood, Toxicology of Cyanides and Cyanogens, John Wiley & Sons, Ltd, Chichester, UK, 2015 Search PubMed.
- M. Panda and N. C. Robinson, Biochemistry, 1995, 34, 10009–10018 CrossRef CAS PubMed.
- H. B. Leavesley, L. Li, K. Prabhakaran, J. L. Borowitz and G. E. Isom, Toxicol. Sci., 2008, 101, 101–111 CrossRef CAS PubMed.
- D. Udhayakumari, Sens. Actuators, B, 2018, 259, 1022–1057 CrossRef CAS.
- M. E. Keim, Prehosp. Disast. Med., 2006, 21, s56–s60 CrossRef PubMed.
- S. Dixit and N. Agarwal, J. Photochem. Photobiol., A, 2017, 343, 66–71 CrossRef CAS.
- P. Piyanuch, J. Sirirak, A. Kamkaew, O. Weeranantanapan, V. Promarak, K. Burgess and N. Wanichacheva, Chempluschem, 2019, 84, 252–259 CrossRef CAS PubMed.
- C. L. Chen, Y. H. Chen, C. Y. Chen and S. S. Sun, Org. Lett., 2006, 8, 5053–5056 CrossRef CAS PubMed.
- L. Wang, X. Chen and D. Cao, RSC Adv., 2016, 6, 96676–96685 RSC.
- K. Wang, X. Yu, Y. Zhu, M. Xing, M. Liang, Y. Sun, D. Cao, R. Guan and Z. Liu, J. Mol. Struct., 2018, 1173, 647–652 CrossRef CAS.
- S. Erdemir and S. Malkondu, Talanta, 2021, 231, 122385 CrossRef CAS PubMed.
- X. Sun, Y. Wang, X. Deng, J. Zhang and Z. Zhang, RSC Adv., 2016, 6, 10266–10271 RSC.
- Q. Zou, F. Tao, H. Wu, W. W. Yu, T. Li and Y. Cui, Dyes Pigm., 2019, 164, 165–173 CrossRef CAS.
- J. Orrego-Hernández and J. Portilla, J. Org. Chem., 2017, 82, 13376–13385 CrossRef PubMed.
- L.-M. Garzón and J. Portilla, Eur. J. Org. Chem., 2019, 2019, 7079–7088 CrossRef.
- A. J. Beneto and A. Siva, Photochem. Photobiol. Sci., 2017, 16, 255–261 CrossRef CAS PubMed.
- A. Tigreros, H. A. Rosero, J. C. Castillo and J. Portilla, Talanta, 2019, 196, 395–401 CrossRef CAS PubMed.
- A. Tigreros, J. C. Castillo and J. Portilla, Talanta, 2020, 215, 120905 CrossRef CAS PubMed.
- J. Orrego-Hernández, C. Lizarazo, J. Cobo and J. Portilla, RSC Adv., 2019, 9, 27318–27323 RSC.
- A. Tigreros, J. Zapata-Rivera and J. Portilla, ACS Sustainable Chem. Eng., 2021, 9, 12058–12069 CrossRef CAS.
- J. Wang, J. He, J. Zhang, Z. Chen and X. Yin, Supramol. Chem., 2019, 31, 713–722 CrossRef CAS.
- M. Kim, N. Mergu and Y. A. Son, J. Lumin., 2018, 204, 244–252 CrossRef CAS.
- R. Bhaskar, V. Vijayakumar, V. Srinivasadesikan, S. L. Lee and S. Sarveswari, Spectrochim. Acta, Part A, 2020, 234, 118212 CrossRef CAS PubMed.
- Q. Lin, L. Liu, F. Zheng, P. P. Mao, J. Liu, Y. M. Zhang, H. Yao and T. B. Wei, RSC Adv., 2017, 7, 38458–38462 RSC.
- S. Anbu, A. Paul, K. Surendranath, N. S. Solaiman and A. J. L. Pombeiro, Sens. Actuators, B, 2021, 337, 129785 CrossRef CAS.
- L. E. S. Figueroa, M. E. Moragues, E. Climent, A. Agostini, R. Martínez-Máñez and F. Sancenón, Chem. Soc. Rev., 2013, 42, 3489–3613 RSC.
- X. X. Guo, D. W. Gu, Z. Wu and W. Zhang, Chem. Rev., 2015, 115, 1622–1651 CrossRef CAS PubMed.
- M. Liang, K. Wang, R. Guan, Z. Liu, D. Cao, Q. Wu, Y. Shan and Y. Xu, Spectrochim. Acta, Part A, 2016, 160, 34–38 CrossRef CAS PubMed.
- C. Long, J. H. Hu, Q. Q. Fu and P. W. Ni, Spectrochim. Acta, Part A, 2019, 219, 297–306 CrossRef CAS PubMed.
- Z. Li, C. Liu, S. Wang, L. Xiao and X. Jing, Spectrochim. Acta, Part A, 2019, 210, 321–328 CrossRef CAS PubMed.
- L. Tang, L. Zhou, A. Liu, X. Yan, K. Zhong, X. Liu, X. Gao and J. Li, Dyes Pigm., 2021, 186, 109034 CrossRef CAS.
- Z. M. Dong, W. Wang, Y. Bin Wang, J. N. Wang, L. Y. Qin and Y. Wang, Inorg. Chim. Acta, 2017, 461, 8–14 CrossRef CAS.
- P. X. Pei, J. H. Hu, C. Long and P. W. Ni, Spectrochim. Acta, Part A, 2018, 198, 182–187 CrossRef CAS PubMed.
- Z. Li, C. Rao, L. Chen, C. Fu, T. Zhu, X. Chen and C. Liu, J. Org. Chem., 2019, 84, 7518–7522 CrossRef CAS PubMed.
- Y. Chen, X. Hu, C. Rao, Z. Li, L. Chen, C. Fu and C. Liu, Analyst, 2018, 143, 4655–4661 RSC.
- S. Manickam and S. K. Iyer, RSC Adv., 2020, 10, 11791–11799 RSC.
- Z. W. Liu, M. Guan, Z. Q. Bian, D. B. Nie, Z. L. Gong, Z. B. Li and C. H. Huang, Adv. Funct. Mater., 2006, 16, 1441–1448 CrossRef CAS.
- K. P. S. Zanoni, R. L. Coppo, R. C. Amaral and N. Y. Murakami Iha, Dalton Trans., 2015, 44, 14559–14573 RSC.
- L. Murphy, A. Congreve, L. O. Pålsson and J. A. G. Williams, Chem. Commun., 2010, 46, 8743–8745 RSC.
- Z. B. Zheng, Q. Y. Huang, Y. F. Han, J. Zuo and Y. N. Ma, Sens. Actuators, B, 2017, 253, 203–212 CrossRef CAS.
- J. H. Kang, S. Y. Lee, H. M. Ahn and C. Kim, Inorg. Chem. Commun., 2016, 74, 62–65 CrossRef CAS.
- K. S. Bejoymohandas, A. Kumar, S. Sreenadh, E. Varathan, S. Varughese, V. Subramanian and M. L. P. Reddy, Inorg. Chem., 2016, 55, 3448–3461 CrossRef CAS PubMed.
- N. Lin, H. D. Ou, Q. Xu, Y. Jin, W. Deng and Z. J. Yao, ACS Omega, 2020, 5, 4636–4645 CrossRef CAS PubMed.
- T. Kim, H. J. Kim, I. S. Shin and J. I. Hong, Anal. Chem., 2020, 92, 6019–6025 CrossRef CAS PubMed.
- C. S. Eckley, M. Gustin, F. Marsik and M. B. Miller, Sci. Total Environ., 2011, 409, 514–522 CrossRef CAS PubMed.
- X. Faïn, D. Obrist, A. Pierce, C. Barth, M. S. Gustin and D. P. Boyle, Geochim. Cosmochim. Acta, 2011, 75, 2379–2392 CrossRef.
- Agency Environmental Protection (Office of Water), Mercury Update: Impact on Fish Advisories, Washington, United States, 2001 Search PubMed.
- WHO, Guidelines for drinking-water quality, , WHO, 2nd edn, 1996, vol. 2, pp. 285–298 Search PubMed.
- D. S. McClure, J. Chem. Phys., 1952, 20, 682–686 CrossRef CAS.
- S. Upadhyay, A. Singh, R. Sinha, S. Omer and K. Negi, J. Mol. Struct., 2019, 1193, 89–102 CrossRef CAS.
- F. Zahir, S. J. Rizwi, S. K. Haq and R. H. Khan, Environ. Toxicol. Pharmacol., 2005, 20, 351–360 CrossRef CAS PubMed.
- S. Bayindir, J. Photochem. Photobiol., A, 2019, 372, 235–244 CrossRef CAS.
- A. Krishna TG, V. Tekuri, M. Mohan and D. R. Trivedi, Sens. Actuators, B, 2019, 284, 271–280 CrossRef.
- S. Joshi, S. Kumari, A. Sarmah, D. D. Pant and R. Sakhuja, J. Mol. Liq., 2017, 248, 668–677 CrossRef CAS.
- Y. Wang, F. Pan, Y. Zhang, F. Peng, Z. Huang, W. Zhang and W. Zhao, Analyst, 2016, 141, 4789–4795 RSC.
- P. Yin, Q. Niu, J. Liu, T. Wei, T. Hu, T. Li, X. Qin and J. Chen, Sens. Actuators, B, 2021, 331, 129418 CrossRef CAS.
- K. Kala, P. K. Vineetha and N. Manoj, New J. Chem., 2017, 41, 5176–5181 RSC.
- D. Giri, A. Bankura and S. K. Patra, Polymer, 2018, 158, 338–353 CrossRef CAS.
- Z. Xue, T. Liu and H. Liu, Dyes Pigm., 2019, 165, 65–70 CrossRef CAS.
- Y. Huang, C. F. Li, W. J. Shi, H. Y. Tan, Z. Z. He, L. Zheng, F. Liu and J. W. Yan, Talanta, 2019, 198, 390–397 CrossRef CAS PubMed.
- B. Zhou, S. Qin, B. Chen and Y. Han, Tetrahedron Lett., 2018, 59, 4359–4363 CrossRef CAS.
- Y. Gao, C. Zhang, S. Peng and H. Chen, Sens. Actuators, B, 2017, 238, 455–461 CrossRef CAS.
- A. Singh and G. Ramanathan, J. Lumin., 2017, 182, 220–225 CrossRef CAS.
- G. Emandi, K. J. Flanagan and M. O. Senge, Photochem. Photobiol. Sci., 2018, 17, 1450–1461 CrossRef CAS PubMed.
- P. G. Rao, B. Saritha and T. Siva Rao, J. Fluoresc., 2019, 29, 353–360 CrossRef CAS PubMed.
- J. Orrego-Hernández, J. Cobo and J. Portilla, ACS Omega, 2019, 4, 16689–16700 CrossRef PubMed.
- E. Bozkurt and H. I. Gul, Sens. Actuators, B, 2018, 255, 814–825 CrossRef CAS.
- B. Naskar, A. Dhara, R. Modak, D. K. Maiti, C. Prodhan, K. Chaudhuri, A. Requena, J. P. Cerón-Carrasco and S. Goswami, ChemistrySelect, 2017, 2, 2512–2519 CrossRef CAS.
- N. Mir, A. Heidari, H. Beyzaei, S. Mirkazehi-Rigi and P. Karimi, Chem. Eng. J., 2017, 327, 648–655 CrossRef CAS.
- E. Bozkurt and H. I. Gul, Inorg. Chim. Acta, 2020, 502, 119288 CrossRef CAS.
- M. Tripathy, U. Subuddhi and S. Patel, Dyes Pigm., 2020, 174, 108054 CrossRef CAS.
- Y. Zhang, G. Zhang, R. Zhang, L. Yang, X. Xu, L. Kong, Y. Tian and J. Yang, Sens. Actuators, B, 2017, 243, 642–649 CrossRef CAS.
- A. A. Eremina, M. A. Kinzhalov, E. A. Katlenok, A. S. Smirnov, E. V. Andrusenko, E. A. Pidko, V. V. Suslonov and K. V. Luzyanin, Inorg. Chem., 2020, 59, 2209–2222 CrossRef CAS PubMed.
- B. Khan, A. Hameed, A. Minhaz and M. R. Shah, J. Hazard. Mater., 2018, 347, 349–358 CrossRef CAS PubMed.
- J. H. Wang, Y. M. Liu, Z. M. Dong, J. Bin Chao, H. Wang, Y. Wang and S. M. Shuang, J. Hazard. Mater., 2020, 382, 121056 CrossRef CAS PubMed.
- M. Zareh Jonaghani and H. Zali-Boeini, Spectrochim. Acta, Part A, 2017, 178, 66–70 CrossRef CAS PubMed.
- S. Guo, C. Fan, G. Liu and S. Pu, RSC Adv., 2018, 8, 39854–39864 RSC.
- H. Jiang, D. Tang, N. Li, J. Li, Z. Li, Q. Han, X. Liu and X. Zhu, Spectrochim. Acta, Part A, 2021, 250, 119365 CrossRef CAS PubMed.
- M. Bahta and N. Ahmed, J. Photochem. Photobiol., A, 2019, 378, 85–93 CrossRef CAS.
- N. Gupta, D. Singhal, A. K. Singh, N. Singh and U. P. Singh, Spectrochim. Acta, Part A, 2017, 176, 38–46 CrossRef CAS PubMed.
- M. Ponram, U. Balijapalli, B. Sambath, S. K. Iyer, B. Venkatachalapathy, R. Cingaram and K. Natesan Sundaramurthy, New J. Chem., 2018, 42, 8530–8536 RSC.
- A. Tang, Y. Yin, Z. Chen, C. Fan, G. Liu and S. Pu, Tetrahedron, 2019, 75, 130489 CrossRef CAS.
- C. Cuerva, J. A. Campo, M. Cano, M. Caño-García, J. M. Otón and C. Lodeiro, Dyes Pigm., 2020, 175, 108098 CrossRef CAS.
- M. K. Chahal and M. Sankar, RSC Adv., 2015, 5, 99028–99036 RSC.
- T. A. Dar and M. Sankar, Front. Chem., 2020, 8, 1–14 CrossRef PubMed.
- E. V. Ermakova, E. O. Koroleva, A. V. Shokurov, V. V. Arslanov and A. Bessmertnykh-Lemeune, Dyes Pigm., 2021, 186, 108967 CrossRef CAS.
- J. I. T. Costa, E. Oliveira, H. M. Santos, A. C. Tomé, M. G. P. M. S. Neves and C. Lodeiro, Chempluschem, 2016, 81, 143–153 CrossRef CAS PubMed.
- N. M. M. Moura, S. Valentini, V. Cheptene, A. Pucci, M. G. P. M. S. Neves, J. L. Capelo, C. Lodeiro and E. Oliveira, Dyes Pigm., 2021, 185, 108897 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |