Open Access Article
Open Access ArticleMembrane technologies in toilet urine treatment for toilet urine resource utilization: a review†
Chengzhi Yu‡
a,
Wenjun Yin‡a,
Zhenjiang Yua,
Jiabin Chena,
Rui Huangb and
Xuefei Zhou *ac
*ac
aState Key Laboratory of Pollution Control and Resource Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China. E-mail: zhouxuefei@tongji.edu.cn; Tel: +86-21-6598-2693
bThe Third Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou 310053, China
cShanghai Institute of Pollution Control and Ecological Security, Tongji University, Shanghai 200092, China
First published on 3rd November 2021
Abstract
Membrane technologies have broad potential in methods for separating, collecting, storing, and utilizing urine collected from toilets. Recovering urine from toilets for resource utilization instead of treating it in a sewage treatment plant not only reduces extra energy consumption for the degradation of N and P but also saves energy in chemical fertilizer production, which will contribute to carbon emission reduction of 12.19–17.82 kg kgN−1 in terms of N alone. Due to its high efficiency in terms of volume reduction, water recycling, nutrient recovery, and pollutant removal, membrane technology is a promising technology for resource utilization from urine collected from toilets. In this review, we divide membrane technologies for resource utilization from urine collected from toilets into four categories based on the driving force: external pressure-driven membrane technology, vapor pressure-driven membrane technology, chemical potential-driven membrane technology, and electric field-driven membrane technology. These technologies influence factors such as: recovery targets and mechanisms, reaction condition optimization, and process efficiency, and these are all discussed in this review. Finally, a toilet with source-separation is suggested. In the future, membrane technology research should focus on the practical application of source-separation toilets, membrane fouling prevention, and energy consumption evaluation. This review may provide theoretical support for the resource utilization of urine collected from toilets that is based on membrane technology.
1. Introduction
For a long time, urine from toilets was collected with other domestic sewage and then discharged into the wastewater treatment plant (WWTP). Urine collected from toilets contributes about 80% of N, 50% of P, 10% of chemical oxygen demand (COD) to domestic sewage, but is only responsible for 1% of the total volume of municipal wastewater.1 A large gas![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio is required in the traditional activated sludge process to achieve satisfactory N and P removal, which brings with it a high energy requirement. Furthermore, excessive COD degradation was caused by high aeration, and consequently, an external carbon source is needed. According to previous studies, the energy demand for N and P removal in the traditional activated sludge process is 45 MJ kg−1 and 49 MJ kg−1, respectively.2 Although an anammox-based process could reduce oxygen demand by up to 63%,3 low-cost P removal processes have not yet been found. However, in light of the dire situation of fertilizer depletion, N and P in human urine should be reclassified as resources. According to the Food and Agriculture Organization of the United Nations (FAO),4 the demand of N, P and K were 111.575, 38.372, and 33.149 tonnes, respectively, in 2016, whereas the demand increased by 1.4–1.5% every year. By 2020, the requirement of N, P and K may rise to 118.763, 42.133 and 37.042 tonnes, respectively. Currently, the N fertilizers are mainly synthesized via the Haber–Bosch process,5 and the P, K fertilizers are mostly produced from the exploitation of phosphate rock and potassium minerals.6 However, the energy consumption of the Haber–Bosch process is 8.9–19.3 kW h kgN−1, which accounts for about 1–2% of the world's energy use.7 On the one hand, phosphate rock and potassium minerals are limited in nature; on the other hand, fertilizer processing introduces heavy metals into the environment.8 Therefore, sustainable, heavy metal-free sources of fertilizer will be indispensable in the future. Human urine is rich in N, P, and K. In addition to N, P and K, the secondary nutrients found in human urine such as sulfur, calcium, magnesium and micronutrients such as boron, copper, and zinc are all that plants need to grow.9 The heavy metal content in urine is far below than found in chemical fertilizers.10 To sum up, there are several benefits of separately collecting human urine from toilets and utilizing it as a resource: (1) producing fertilizer with a low heavy metal content, (2) saving energy consumption in sewage treatment and N fertilizer production, (3) solving the phosphate rock and potassium mineral deficiency problem.
water ratio is required in the traditional activated sludge process to achieve satisfactory N and P removal, which brings with it a high energy requirement. Furthermore, excessive COD degradation was caused by high aeration, and consequently, an external carbon source is needed. According to previous studies, the energy demand for N and P removal in the traditional activated sludge process is 45 MJ kg−1 and 49 MJ kg−1, respectively.2 Although an anammox-based process could reduce oxygen demand by up to 63%,3 low-cost P removal processes have not yet been found. However, in light of the dire situation of fertilizer depletion, N and P in human urine should be reclassified as resources. According to the Food and Agriculture Organization of the United Nations (FAO),4 the demand of N, P and K were 111.575, 38.372, and 33.149 tonnes, respectively, in 2016, whereas the demand increased by 1.4–1.5% every year. By 2020, the requirement of N, P and K may rise to 118.763, 42.133 and 37.042 tonnes, respectively. Currently, the N fertilizers are mainly synthesized via the Haber–Bosch process,5 and the P, K fertilizers are mostly produced from the exploitation of phosphate rock and potassium minerals.6 However, the energy consumption of the Haber–Bosch process is 8.9–19.3 kW h kgN−1, which accounts for about 1–2% of the world's energy use.7 On the one hand, phosphate rock and potassium minerals are limited in nature; on the other hand, fertilizer processing introduces heavy metals into the environment.8 Therefore, sustainable, heavy metal-free sources of fertilizer will be indispensable in the future. Human urine is rich in N, P, and K. In addition to N, P and K, the secondary nutrients found in human urine such as sulfur, calcium, magnesium and micronutrients such as boron, copper, and zinc are all that plants need to grow.9 The heavy metal content in urine is far below than found in chemical fertilizers.10 To sum up, there are several benefits of separately collecting human urine from toilets and utilizing it as a resource: (1) producing fertilizer with a low heavy metal content, (2) saving energy consumption in sewage treatment and N fertilizer production, (3) solving the phosphate rock and potassium mineral deficiency problem.
According to the CO2 emission coefficients of electricity and fossil fuels,11 carbon reduction from recycling N before treatment in the WWTP and using it for N fertilizer production is about 12.19–17.82 kg kgN−1.
Source-separation technology provides a new idea to replace the treatment of human urine in a WWTP and further realize the resource utilization. Separated urine has a higher resource value than mixed manure and urine. Urine source-separation, in other words, urine-diversion, was first proposed in 1996,12 after which research on urine-derived fertilizer,13 urine diversion systems installation and operation,14 microbiological and physical–chemical process for urine treatment,15 user attitudes towards urine diversion16 commenced. In recent years, source-separation toilets have developed gradually all over the world,17 including Sweden, South Africa and so on. However, source-separated urine was commonly diluted 5–20 times during source-separation due to water flushing.18 Because of the large volume, transportation costs would be extremely high, so in-person use is advised. If transportation is unavoidable, a volume reduction of 80% is recommended.19
Maurer et al.20 proposed several objectives for source-separated urine treatment: disinfection, stabilization, volume reduction, P-recovery, N-recovery, organic compounds' removal and micropollutant handling. There have been many technologies for source-separated urine resource utilization, such as the struvite method,21 an adsorption method,22 ion exchange,23 freezing–thawing,24 drying,25 and bio-electrochemical technologies.26,27 Membrane technologies, in comparison to other urine resource utilization technologies, can address a broader range of objectives. Membrane technologies, for example, provide unrivalled benefits in urine treatment, particularly in urine reduction and water recovery. Furthermore, membrane technologies are crucial in N and P recovery, organic compound removal and micropollutant treatment. As far as is known, reviews on urine recycling by membrane technologies are still limited.
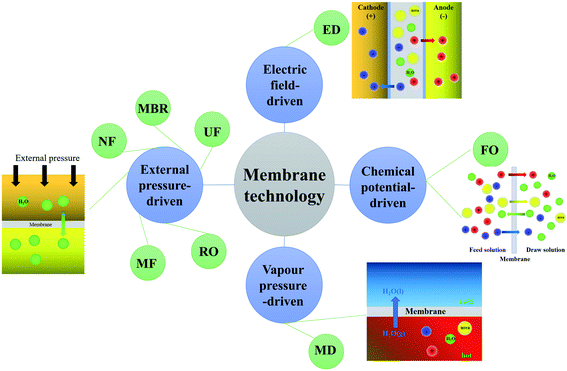
Herein, the properties of urine collected from toilets are summarized, based on an extensive literature review to provide a comprehensive understanding of human urine composition, utilization value and chemical properties, so that the selection of a membrane process according to different recovery purposes and the nature of the human urine is clearer. More importantly, membrane technology treatments for urine collected from toilets are classified as external pressure-driven membrane technology, vapor pressure-driven membrane technology, chemical potential-driven membrane technology, and electric field-driven membrane technology, according to the driving force, as shown in Fig. 1. These membrane technologies' process applications and influencing factors, including combination processes, are described. The research hotspots of membrane technology are summarized and the directions of membrane technology development have been explored. These studies provide preliminary theoretical support for the flexible selection of membrane processes and the establishment of a source-separation toilet system incorporating membrane technology.
2. Human urine properties
2.1 Yield and composition
Urine is a sterile, with amber colored fluid, excreted by the kidney filtration process.28 The daily urine output of an adult is 1.2–1.5 L, and the annual discharge is about 400–500 L.29 The composition of urine is complicated, water makes up more than 90% of the urine, and the other 10% is composed of urea, dissolved ions, creatinine, organic and inorganic compounds and salts.30,31 Different living conditions, such as age, gender, eating habits, geographic location, income and local culture, can affect the specific components and characteristics.32 Table 1 shows the main composition and properties of human urine.31,33–36| Component | Concentration | Component | Concentration |
|---|---|---|---|
| pH | 4.88–9.3 | Mg (mg L−1) | 11–121 |
| TN (mg L−1) | 254–7109 | TDS (mg L−1) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700–24 700–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 380 |
| TP (mg L−1) | 210–740 | Alkalinity (mg CaCO3 per L) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230–16 230–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890 |
| COD (mg L−1) | 3600–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906 906 |
NH3 (mg L−1) | 254–7100 |
| K (mg L−1) | 863–2250 | Conductivity (mS cm−1) | 13.08–43.7 |
| S (mg L−1) | 505–1500 | PO4 (mg L−1) | 180–740 |
| Na (mg L−1) | 508–3730 | NO3 (mg L−1) | 9.74–10.26 |
| Cl (mg L−1) | 3000–5346 | NO2 (mg L−1) | 44.18–45.22 |
| Ca (mg L−1) | (17.7–32) | SO4 (mg L−1) | 681–1500 |
2.2 Hydrolysis of human urine
In fresh urine, urea accounts for approximately 90% of the total nitrogen (TN), other organic nitrogen accounts for 5%, and ammonium accounts for the remainder.37 Once exposed to air, urine will decompose to produce ammonia (NH3), NH4+ and HCO3− (eqn (1)), a process catalyzed by urease, and an increase of the urine pH occurs. Enzymatic urea hydrolysis can cause a number of problems, for example, deposition of the products formed. According to the composition, these scaling substances are classified as struvite (magnesium ammonium phosphate, MgNH4PO4·6H2O),4 hydroxyapatite (HAP, Ca10(PO4)6(OH)2),38 and potassium struvite (struvite-(K), KMgPO4·6H2O).39 In particular, the reaction between Mg2+, PO43−, NH4+ and H2O produces struvite (eqn (2)), the reaction between PO43−, Ca2+ and OH− produces HAP (eqn (3)), and the reaction between K+, Mg2+, PO43− and H2O produces potassium struvite (eqn (4)). Struvite deposition often occurs spontaneously at a pH of 7–8, and then HAP sediment occurs at a higher pH.38,40 This may be related to the difference between supersaturation,41 because the saturation of struvite is 4.33 × 10−14, whereas the saturation of HAP is 2.91 × 10−58. These precipitates are found attached to urinal traps, drain lines and storage tanks during the excretion, collection and storage of urine, causing a significant inconvenience during urine transportation. Another disadvantage of urea hydrolysis is the unpleasant odor. There is an equilibrium between NH4+ and NH3, and an equilibrium between NH3 solution and NH3 gas, whose main driving force is pH.4 When the pH is between 4 and 7, ammonia nitrogen transforms between NH4+ and NH3 (eqn (5)). When the pH is higher than 7, the NH3 dissolved in the liquid will convert to NH3 gas and escape into the air (eqn (6)), which continuously causes an unpleasant smell. The urine decay process is another name for these processes. The smell of urine grows stronger as the process progresses, and more scaling is produced. After that, fresh urine is hydrolyzed.
 | (1) |
| Mg2+ + PO43− + NH4+ + H2O → MgNH4PO4·6H2O | (2) |
| 3PO43− + 5Ca2+ + OH− → Ca5(OH)(PO4)3 | (3) |
| K+ + Mg2+ + PO43− + H2O → KMgPO4·6H2O | (4) |
| NH3 (aq) + H+ ⇌ NH4+ (4 < pH < 7) | (5) |
| NH3 (aq) ⇌ NH3 (g) (7 < pH < 9.5) | (6) |
In urine collection and storage, stabilizing the urine is the most important process. The optimum pH for urease is 6.8–8.7.42 As a result, inactivating urease by pH regulation is a viable strategy. To prevent urine hydrolysis, solid Ca(OH)2 can be placed in the urine tank to increase the pH to above 12.5.43 In addition, adding enzyme inhibitors and using electrochemical treatment can help to stabilize the urine.44 Another solution is to accelerate urine hydrolysis on-site. Installation of a urea hydrolysis reactor in the toilet can speed up urea hydrolysis, and allows for the controlled collection of phosphate and NH3.45
3. Membrane technologies for urine resource utilization
Fig. 2 shows the evolution of membrane technologies in the utilization of human urine resources after 2016, and membrane research has increased every year since then. Forward osmosis (FO), membrane distillation (MD), electrodialysis (ED) have always been research hotspots, and FO has received the most attention. There has been less research on reverse osmosis (RO) and nanofiltration (NF). The membrane bioreactor (MBR) has only been used in combined processes in recent years. Furthermore, hybrid processes combining various membrane technologies are gradually gaining traction, with the FO-MD process gaining the most attention. The specific process will be discussed in greater depth later, and combined processes in the form of the main process and the auxiliary process will be introduced.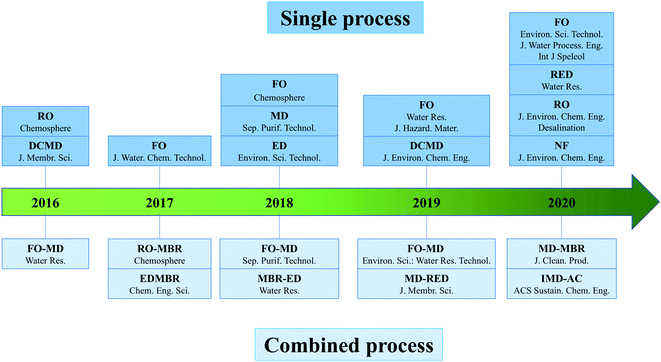 | ||
| Fig. 2 A timeline of the development of membrane processes (specific references to the literature are shown in the text of S1, ESI†). | ||
3.1 External pressure-driven membrane technology
External pressure-driven membrane related technology includes microfiltration (MF), ultrafiltration (UF), NF, RO and MBR. The MF and UF are low-pressure processes, which can retain bacteria and large particles, but N, P and soluble organic matter can pass through the membrane.46 High-pressure membranes, such as NF and RO, have better interception effects but require a lot of energy. Activated sludge degrades organic matter and converts ammonia nitrogen into nitrogenous nitrogen in the MBR process, while a membrane is used for solid–liquid separation. Only a combined process of MF, UF and MBR can recycle urine. Table 2 shows recent research on external pressure-driven technologies for urine resource utilization.| Process | RC | Target | Performance | Reference |
|---|---|---|---|---|
| a Note: FU – fresh urine, HU – hydrolyzed urine, RC – reaction conditions, TOC – total organic carbon. | ||||
| RO | HU | Urea and ammonia retention | 64% unionized ammonia, 93% TOC retention | 48 |
| RO | FU | 57% urea retention, ≥92% TOC retention, 86% conductivity decrease | 48 | |
| RO | FU and HU pH 9 | P recovery | 2.58 kg and 1.24 kg of precipitates from 1 m3 HU and FU, precipitated solids contain 8.1–19.0% P, 10.3–15.2% Ca, 3.7–5.0% Mg, and 0.1–3.5% ammonium nitrogen | 49 |
| RO | Mixed water | Water recovery | 87 ± 5% water recovery | 51 |
| NF | HU, pH 11.5 | 90% unionized ammonia recovery, 98% TOC retention | 48 | |
| NF | FU, pH 5 | Urea retention | 56% urea retention, ≥92% TOC retention, 96–97% conductivity decrease | 48 |
| RO-MBR | FU | N removal and P recovery | 90% phosphorus recovery, 45% nitrogen removal | 50 |
Urine hydrolysis and the pH value will affect the nutrient recovery efficiency due to the existence of acidic functional groups in the membrane. On the one hand, hydrolysis of urine produces NH4+, the anion was first rejected by the membrane, and then NH4+ was more easily retained due to the electroneutrality principles.47 On the other hand, a high pH not only converted NH4+ into NH3 but also lead to electrostatic repulsion of these acidic functional groups, resulting in pore expansion.48,49 Ray et al.47 investigated urea and NH3 rejection in hydrolyzed and fresh urine by RO and NF. For hydrolyzed urine, 64% of unionized NH3 was recovered by RO, and 90% of unionized NH3 was recovered by NF. At a pH of 11.5, the NF membrane would achieve 90% unionized NH3 recovery, 86% conductivity reduction and 98% TOC rejection. For fresh urine, NF rejected 42–56% of urea and the base addition would decrease the rejection. The RO could reject 57% of urea and was not affected by the pH because the RO membranes have tighter pores.
In addition to the RO membrane, RO brine can be used to recover P from source-separated urine. One of the main sources of RO brine is cooling water from thermal power plants.50 When the RO brine-to-urine ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the pH was 9.0, more than 90% of the phosphorus could be removed from both fresh urine and hydrolysis urine. From 1 m3 of fresh urine, approximately 1.24 kg of precipitates could be obtained, whereas 2.58 kg of precipitates could be obtained from the same volume of hydrolyzed urine. The precipitates contained 0.1–3.5% of ammonium nitrogen, 3.7–5.0% of Mg, 10.3–15.2% of Ca, and 8.1–19.0% of P. Furthermore, using RO brine to flush urine-diverting toilets can achieve on-site phosphorus recovery from human urine.51 Nitrogen can be removed in an MBR process after phosphorus precipitation via a short-cut nitrification–denitrification. When the pH was greater than 9, 90% of the phosphorus in the precipitation process was recovered, with recovered precipitates containing 10–15% of phosphorus. Without using an external carbon source, the MBR process removed 45% of the TN. The COD and nitrogen removal was 90% when 3 g L−1 of methanol was added.
1 and the pH was 9.0, more than 90% of the phosphorus could be removed from both fresh urine and hydrolysis urine. From 1 m3 of fresh urine, approximately 1.24 kg of precipitates could be obtained, whereas 2.58 kg of precipitates could be obtained from the same volume of hydrolyzed urine. The precipitates contained 0.1–3.5% of ammonium nitrogen, 3.7–5.0% of Mg, 10.3–15.2% of Ca, and 8.1–19.0% of P. Furthermore, using RO brine to flush urine-diverting toilets can achieve on-site phosphorus recovery from human urine.51 Nitrogen can be removed in an MBR process after phosphorus precipitation via a short-cut nitrification–denitrification. When the pH was greater than 9, 90% of the phosphorus in the precipitation process was recovered, with recovered precipitates containing 10–15% of phosphorus. Without using an external carbon source, the MBR process removed 45% of the TN. The COD and nitrogen removal was 90% when 3 g L−1 of methanol was added.
In the field of manned space flight, external pressure-driven membrane technologies play an important role. In long-term human space missions,52 electrodialysis is integrated with crystallization, COD-removal, ammonification, and nitrification in long-term human space missions to treat human urine (1.2 L d−1) before it was mixed with shower water. Electrodialysis was used specifically to recover NO3−, and RO was the final step to recover clean water from the mixture of shower water and treated urine.
3.2 Vapor pressure-driven membrane technology
The MD is a non-isothermal separation membrane technology driven by a vapor-pressure gradient,53,54 which is widely used in various fields and has four configurations: (1) air gap membrane distillation (AGMD), (2) direct contact membrane distillation (DCMD), (3) sweep gas membrane distillation (SGMD), (4) vacuum membrane distillation (VMD).55–57 The MD can completely reject non-volatile matter, and the salt concentration in the feed solution has little influence on its efficiency.58,59 The research on MD for urine resource utilization in recent years are shown in Table 3.| Process | RC | Target | Performance | Reference |
|---|---|---|---|---|
| a Note: RC – reaction conditions, FU – fresh urine, HU – hydrolyzed urine. | ||||
| DCMD | FU | Volume reduction and nutrient concentration | Urine concentrated 17.8 times, 97% P and K rejection | 18 |
| DCMD | Specific ammonia transfer inhibition | SAT was reduced to 6.91 × 10−5 g-N per g-H2O | 59 | |
| MD | HU, pH 10, water vapor gradient 30 °C | Water recovery | 80% water recovery, 98% of TOC, 98% of Na+, and 89% of K+ rejected | 62 |
| IMD-AC | HU | Ammonia recovery | 60% ammonia recovery, 95% energy saving | 63 |
| FO-MD | FU | Water recovery | 98% TOC, TN, and NH4+ removal | 34 |
| FO-MD | FU and HU | Water recovery | Water flux of 31.5 (FU) to 28.7 (HU) L m−2 h−1 | 64 |
| MD-MBR | HU | Non-odorous high-concentration liquid fertilizer production | Total dissolved solid concentration of 280 g L−1 | 65 |
In terms of volume reduction and nutrient reconcentration, use of urine collected from toilets for DCMD performs admirably.18 More than 97% of P and K rejection was achieved when hydrolyzed urine was concentrated 17.8 times. Nevertheless, the NH3 concentration was increased to 11.0 gN L−1, so the water generation quality was affected. An NH3 concentration in urine is frequently high in the process of recovering water from urine, and nutrients' concentration, and this resulted in free NH3 transfer through the MD membrane to the permeate.
Two important factors in MD are temperature and pH. Except for pretreatment, pH control, temperature regulation and utilization of new membrane materials are also solutions. The water flux was more affected by temperature than the NH3 flux, and NH3 transfer can be effectively inhibited by a low pH. Taking DCMD as an example, increasing the feed solution temperature from 40 °C to 70 °C, the specific ammonia transfer (SAT) value would decrease from 8 × 10−3 to 1.62 × 10−3 g-N per g-H2O. By reducing the pH from 9 to 5, the SAT value decreased from 2.05 × 10−3 to 6.91 × 10−5 g-N per g-H2O.60 In addition, water permeate flux and NH3 transfer were also determined by the membrane material.61,62 In particular, a thin structure and high porosity help to improve the water flux. Khumalo et al.63 applied microporous hydrophobic composite membranes in membrane distillation. The membrane was made of poly(vinylidene fluoride)/poly(tetrafluoroethylene) (PVDF/PTFE), which were modified with methyl functionalized silica nanoparticles (MfSNPs). Under the conditions of a pH of 10.5 and a water vapor gradient of 30 °C, 80% of water was recovered from hydrolyzed human urine, and 95% of the NH3, 98% of the TOC, 98% of the Na+, 89% of the K+ were rejected.
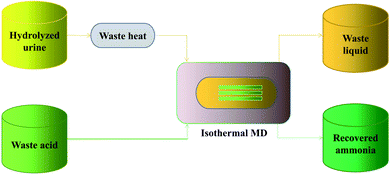
A temperature difference cannot be maintained without heating, which has a great energy demand. Another way of thinking, inhibiting the transfer of water and collecting NH3 provides an energy saving solution, as shown in Fig. 3. A novel isothermal membrane distillation with an acidic collector (IMD-AC) was devised to improve selectivity for NH3 transport.64 Water vapor permeation was suppressed 68 times by keeping the feed and collector temperature equal, and NH3 (g) was collected by acidic solutions to enhance the NH3 vapor. Compared with conventional MD, the IMD-AC showed an increase of 46.5% NH3 vapor, reaching 60% NH3 recovery. Furthermore, when compared to the traditional nitrogen fixation process, approximately 95% of the energy consumed was saved, with the final energy requirement being 2.2 kW h kgN−1. In other words, compared to traditional MD, IMD can selectively capture volatile matter other than water and the process requires less energy.
In addition, a combined process can also achieve nitrogen retention. For example, the FO process, which has received extensive attention, can achieve effective interception of NH3. In a forward osmosis-MD (FO-MD) for real human urine treatment, more than 98% of the TOC, TN, and NH4+ were rejected by the FO process.34 Volpin et al.65 combined FO and MD for extracting distilled water from fresh urine and stored urine. To prevent membrane wetting and improve the overall nitrogen rejection, FO was chosen as a pretreatment for MD. The combination of the FO and MD processes provided a new treatment idea for water regeneration in the space station and resource recovery in urban applications. The MBR process can convert NH3 into nitrate, which cannot pass through the MD membrane in the form of steam, but at the same time also degrades a large amount of TOC. An MBR-DCMD process has been investigated, which produces an odorless and high-concentration liquid fertilizer.66 At first, the MBR removed 95% of the TOC and converted 50% of the NH4+–N to NO3−–N. Then the DCMD recovered 80% of the water and the final total dissolved solids concentration reached 280 g L−1. These results showed that DCMD could concentrate the urine 20-fold.
3.3 Chemical potential-driven membrane technology
The FO is a low-pressure or non-pressure membrane technology in which water is transferred from a high-concentration solution to a low-concentration solution until a thermodynamic equilibrium is achieved, with the chemical potential as the driving force.67,68 The advantages of FO include: (1) it is a low-pressure operation, which derives other advantages such as a lower propensity for fouling, lower energy demand, lower membrane strength requirement, and fouling reversibility,69 (2) a high rejection of pollutants,70,71 and (3) flexible use. Depending on the application, the draw solution (DS) composition can be manipulated to obtain different recovery targets.72 The FO process has been widely used in food processing, nuclear wastewater treatment, desalination, and drinking water and landfill leachate treatment because of these advantages.71,73,74 In resource utilization of urine, FO has also been widely studied, as shown in Table 4.| Process | RC | Target | Performance | Reference |
|---|---|---|---|---|
| a Note: FU – fresh urine, HU – hydrolyzed urine, RC – reaction conditions. | ||||
| FO | HU | Volume reduction | The urine volumes were reduced to 1/2–1/5 | 19 |
| FO | FU | Ammonia recovery | 86% recovery of ammonia | 74 |
| DS pH < 6.5 | ||||
| FS pH > 11 | ||||
| FO | FS | N, P recovery | 40% N recovery, 50% P recovery | 75 |
| FO | FS | Urine concentration | 50% N recovery, 93% P recovery, economic benefits are 5.3 times the running cost | 76 |
| FO | HU | Water recovery | 89% TN rejection with 75% water recovery using 5 M NaCl as the DS, 97% TN rejection with 50% water recovery using 5 M glucose as the DS | 77 |
| FO | Cave exploration | Urine volume reduction | 86% TN rejection with 75% water recovery | 78 |
| FO | FU, HU | Chlorella vulgaris culture dewatering | Algal concentration was increased four-fold | 79 |
| FO-MD | FU | Urea recovery | 45–68% urea concentration with 90% TOC rejection | 81 |
The volumes of both real human urine and synthetic human urine were reduced by 1/2–1/5 using a cellulose triacetate membrane in FO, but NH3 and inorganic carbon passed through the membrane easily, about 35–40% and 30%, respectively. As a result, measures to improve nutrient reduction should be taken.19
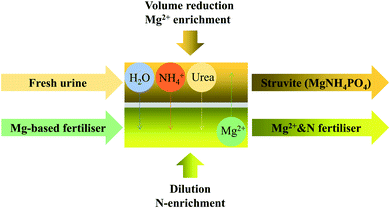
The original potential difference between the two sides of the membrane can be changed by manipulating the DS composition to prevent water or NH3 transmission. Adding electrolyte solutions or changing the pH of DS are two examples of specific regulatory methods. By adjusting the feed solution so that it has a high pH and the DS so it has a low pH,75 NH3 is transformed into NH4+ upon crossing over the FO membrane. By keeping the DS at pH < 6.5 and the feed solution pH > 11, the NH3 recovery rate achieved was up to 86%. Magnesium salts are an ideal electrolyte additive because Mg2+ is one of the plant nutrients, and it can precipitate with P to produce struvite. In terms of agricultural utilization, Volpin et al.76 employed fertilizer driven FO to recover N and P from human urine, as shown in Fig. 4. The MgSO4 and Mg(NO3)2 were chosen as the DSs for dewatering synthetic non-hydrolyzed urine, and Mg2+ reverse salt flux was selected to precipitate the P as struvite. At the same time, urea was concentrated in the DS because the FO membrane had a poor interception effect on it. The fertilizer-drawn forward osmosis (FDFO) process recovered 40% of N and 50% of P while reducing the volume of urine by more than 60%. Following a preliminary investigation, use of a commercial fertilizer as a FO draw solution was developed.77 With 50% concentrated urine, 93% of the P was recovered as struvite, and 50% of the N was recovered in the diluted DS. When the downstream nutrient load is reduced, the economic benefits would be 5.3 times the operating cost.
Urine hydrolysis has a significant impact on the FO process, which is influenced by pH and the enzyme urease. Engelhardt et al.78 used hollow fiber, aquaporin-based membranes for NH3 rejection and water recovery to improve the nitrogenous compound rejection, and pH control was also used. The results showed that the best pH for urea hydrolysis was 7.4, and by using urease-processing and pH adjustment, the TN rejection could reach 89% (with 75% water recovery) to 98% (with 25% water recovery), using 5 M NaCl as the DS. When using 5 M glucose as the DS, the NH3 recovery ranged from 97% (with 50% water recovery) to 99% (with 25% water recovery). Following that, the performance of an Aquaporin Inside hollow fiber FO module (Sterlitech) used for urine volume reduction without DS for long-duration cave expeditions was tested, and a portable FO prototype was introduced, which was able to reduce the urine volume by approximately 75% and reject approximately 86% of the TN.79
In addition to the nutrient and water recovery, the urine-FO combination can be used in other fields, such as microalgae culture.80 The concentration of algae increased by four times with a water flux of 14.2 L m−2 h−1 using hydrolyzed urine as the DS. The diluted urine could be used as a nutrition source and pharmaceuticals could be removed via biodegradation and photolysis.81
A combined process to improve FO performance did not appear to be required because the single FO process has a satisfactory effect on urine treatment. The FO process, on the other hand, is occasionally used as a pretreatment for the MD process. To recover urea from fresh human urine, for example, an FO-MD method has been developed.82 Urea separation was accomplished with FO, and urea concentration reduction was accomplished with MD. After five pretreatment methods used for urine stabilization, the FO process recovered 11–21% of the urea in the DS, then the draw solutions were concentrated 1.9–3.3 times via the MD process. The product solution contained 45–68% of the urea concentration of fresh urine and 90% of the TOC was rejected.
3.4 Electric field-driven membrane technology
The ED is an electrochemical membrane separation technology driven by an applied electric field,83 which consists of a anion exchange membrane (AEM), a cation exchange membrane (CEM) and a direct current electric field. Due to its ability to generate high-quality nutrient products successfully,84 ED has been extensively used, especially in the demineralization of industrial processes and desalination of brackish water, with a treatment capacity of more than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 d−1.85 In urine treatment (Table 5), the purposes of ED include: (1) water or nutrient recovery, (2) urine desalination, and (3) micropollutant removal.86
000 m3 d−1.85 In urine treatment (Table 5), the purposes of ED include: (1) water or nutrient recovery, (2) urine desalination, and (3) micropollutant removal.86
| Process | RC | Target | Performance | Reference |
|---|---|---|---|---|
| a Note: FU – fresh urine, HU – hydrolyzed urine, RC – reaction conditions. | ||||
| ED | Nitrogen recovery | 95.6% nitrogen recovery | 86 | |
| EDMBR | HU | Phosphate and sulfate recovery | 65% phosphate recovery, 54.9% sulfate recovery | 87 |
| MBR-ED | FU | Urine treatment | 80% ion collection | 85 |
| RED | FU and HU | Energy recovery | A maximum ENet of 0.053–0.039 kW h m−3 of real urine | 89 {90} |
| MD-RED | Water and energy recovery | 47% Gibbs free energy recovery | 90 | |
Tarpeh et al.87 used an electrochemical stripping setup that included ED and membrane stripping to recover nitrogen from source-separated urine. In batch experiments, 93% of the nitrogen was recovered selectively. In continuous-flow experiments, when the influent concentration was 7490 mgN L−1, the nitrogen concentration was 2960 mgN L−1 in the anode chamber, 1950 mgN L−1 in the cathode chamber, 2250 mgN L−1 in the trapped chamber after 3–5 hydraulic residence times (HRT), and the energy demand was 30.6 MJ kgN−1. In addition, in the ammonium sulfate fertilizer product, there were no trace organics or elements detected.
However, because it is difficult to consider both pollutant removal and nutrient recovery in a single ED process, combined processes or a pretreatment for the ED are required. The combination of NH3 stripping, ED and MBR works well for source-separated urine treatment.88 Ammonia stripping was performed as a pretreatment to decrease the NH3 concentration from 1292.2 ± 47.5 mg L−1 to 235.1 ± 5.7 mg L−1, which was about an 81.8% removal rate. Then phosphate and sulfate were recovered whereas the NH3 and COD were removed in situ in the EDMBR, with a power density of 23.5 W m−3. Finally, 94.5% of the SO42−, 76.7% of the PO43−, and 97.4% of the NH4+ was removed, whereas the phosphate and sulfate were recovered as a concentrated solution, with recovery rates of 65% and 54.9%, respectively. To avoid precipitation and remove organics, researchers86 combined precipitation, MBR and ED in a pilot installation for the treatment of human urine. The process was continuously run for seven months with a treatment capacity of 1.2 L d−1 (one person equivalent). More than 95% of the urea was converted into nitrate under salinities of 10–20 mS cm−1, and 70% of the ions were collected in 15% of the initial volume using a 20% urine solution (1.2 L of urine and 4.6 L of demineralized water), and 80% of the ions were collected in 20% of the initial volume using a 40% urine solution (1.3 L of urine and 2.2 L of demineralized water).
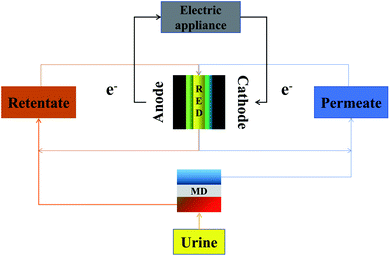
Using electric energy, traditional ED can be used to recover nutrients. The introduction of reverse electrodialysis (RED) in recent years has made it possible to convert potential energy into electrical energy.89 According to Volpin et al.,90 there is a large salinity gradient between urine and flushing water that could be used as a source of potential energy. When homogenous redox couples were used as an electrolyte solution, the RED device could achieve a maximum ENet of 0.053–0.039 kW h m−3 of real urine, with 13%, 6%, 4.4% removal of TOC, NH3 and urea, respectively. Nutrient and energy recovery cannot be realized at the same time by RED, and combined processes are still needed. The MD was an ideal pre-process for RED to generate electrical power and clean water from waste heat and human urine, as shown in Fig. 5.91 Using waste heat, MD was used to produce high-quality water from urine, and the concentrated urine with a high nutrient concentration was used as the retentate in RED. The RED, on the other hand, was used to generate electrical power. In that process, 47% of the available Gibbs free energy was recovered, and low power fluidic devices with 100% water recovery in MD can be used.
4. Conclusions and future prospects
Membrane technology has good prospects for the utilization of resources in urine collected from toilets. The energy consumption of low external pressure membrane technologies is low, but the interception effect is poor. A high external pressure membrane has a good interception effect, but its energy consumption is high, so it is not suitable for urine resource treatment alone. Vapor pressure-driven membrane technology has a high rejection rate for non-volatile substances, but volatile substances easily pass through the membrane and pollute the product. When using urine for water recovery, precautions should be taken to prevent NH3 volatilization. Ammonia can also be recovered under isothermal conditions. The advantages of chemical potential gradient driven membrane technology include low energy consumption and ease of use. Chemical potential gradient driven membrane technology can be used in a variety of situations because the composition of the draw solution can be adjusted flexibly to accommodate different components of urine or different recovery goals. It can also be combined with other technologies to improve nutrient recovery and pollutant removal. There is little research on electric field-driven membrane technology, but reverse electrodialysis technology is the only one that can recover energy, making it very promising technology.The membrane process used should be chosen based on the specific situation and for the particular construction of the source-separation toilet itself. For example, in water-stressed areas, the MD process is an excellent way to recover clean water. The IMD, FO, and RED processes can all have more effective roles in energy conservation. A combination process, on the other hand, may be the best option for achieving more comprehensive nutrient recovery. At the same time, MBR is still the most effective way to deal with the remaining waste after resource utilization. From current process development, the FO-MBR combined process shows a good recovery effect, low energy consumption, and the good removal of pollutants. Therefore, the FO-MBR combined process is recommended as a preliminary system for resource utilization and as a harmless treatment for urine.
At present, the application of membrane technologies in the utilization of resources from urine collected from toilets is mostly done at the laboratory scale. For existing technologies, larger-scale trials need to be conducted in the future to achieve the purpose of their final application with the help of engineering. At the same time, operational parameters should be optimized constantly to enhance pollution interception efficiency and nutrient recovery productivity, as well as to reduce membrane fouling and energy consumption. Additionally, the disposal of the final waste after urine recycling needs to be taken into consideration, which was not reported in previous research. For future research, the combination of FO and commercial fertilizer shows great potential. Facing the problem of energy shortages, the application of RED for energy recovery would be a good choice. In addition, integrated technologies bring many opportunities. For example, the application of solar energy technology on MD, and the combination of a high concentration of microalgae and FO. There are more unknown processes waiting to be exploited. Finally, a set of evaluation systems based on membrane pollution and economic benefits should be established as these membrane technologies and processes mature.
Author contributions
The manuscript was written through with contributions from all the authors. All the authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 51878465). The authors gratefully acknowledge support from the National Key R&D Programme of China (2018YFD1100500).References
- G. L. Zang, G. P. Sheng, W. W. Li, Z. H. Tong, R. J. Zeng and C. Shi, Phys. Chem. Chem. Phys., 2012, 14, 1978–1984 RSC.
- M. Maurer, P. Schwegler and T. A. Larsen, Water Sci. Technol., 2003, 48, 37–46 CrossRef CAS PubMed.
- T. Khin and A. P. Annachhatre, Biotechnol. Adv., 2004, 22, 519–532 CrossRef CAS PubMed.
- A. Patel, A. A. Mungray and A. K. Mungray, Chemosphere, 2020, 259, 127372 CrossRef CAS PubMed.
- J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik and A. K. Jones, Sci. Total Environ., 2018, 360, 6391 Search PubMed.
- P. Ledezma, P. Kuntke, C. Buisman, J. Keller and S. Freguia, Trends Biotechnol., 2015, 33, 214–220 CrossRef CAS PubMed.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- B. VinnerS and H. J Nsson, Bioresour. Technol., 2002, 84, 275–282 CrossRef.
- A. Yaa, B. Sla and B. Tat, Sustain. Prod. Consum., 2020, 24, 219–231 CrossRef.
- M. Ronteltap, M. Maurer and W. Gujer, Water Res., 2007, 41, 1859–1868 CrossRef CAS PubMed.
- G. Houillon and O. Jolliet, J. Cleaner Prod., 2005, 13, 287–299 CrossRef.
- T. A. Larsen and W. Gujer, Water Sci. Technol., 1996, 34, 87–94 CrossRef CAS.
- C. Bonvin and B. Etter, Ambio, 2015, 44, 217–227 CrossRef CAS PubMed.
- L. Rossi, J. Lienert and T. A. Larsen, J. Environ. Manage., 2009, 90, 1909–1917 CrossRef CAS PubMed.
- M. U. Kai, C. A. Buckley, M. Wächter, C. S. Mcardell, T. Kohn, L. Strande, H. Zöllig, A. Fumasoli, A. Oberson and B. Etter, Water SA, 2016, 41, 212–221 CrossRef.
- K. M. Lamichhane and R. W. Ba Bcock, Sci. Total Environ., 2013, 443, 749–756 CrossRef CAS PubMed.
- P. Simha and M. Ganesapillai, Sustainable Environ. Res., 2016, 27, 107–116 CrossRef.
- K. Xu, D. Qu, M. Zheng, X. Guo and C. Wang, J. Environ. Eng., 2019, 145, 04018144 CrossRef.
- W.-Y. N. Benedicte Carolle, I. Ryusei and G. Mokhtar, J. Water Environ. Technol., 2017, 15, 163–173 CrossRef.
- M. Maurer, W. Pronk and T. A. Larsen, Water Res., 2006, 40, 3151–3166 CrossRef CAS PubMed.
- S. K. L. Ishii and T. H. Boyer, Water Res., 2015, 79, 88–103 CrossRef CAS PubMed.
- M. Dimitris, P. Maria, T. Nikolaos, P. Dimitrios and K. Efstratios, Chem. Eng. J., 2018, 347, 618–630 CrossRef.
- P. Simha, J. Senecal, A. Nordin, C. Lalander and B. Vinneras, Water Res., 2018, 142, 325–336 CrossRef CAS PubMed.
- B. B. Lind, Z. Ban and S. Bydén, Ecol. Eng., 2001, 16, 561–566 CrossRef.
- S. Antonini, P. T. Nguyen, U. Arnold, T. Eichert and J. Clemens, Sci. Total Environ., 2012, 414, 592–599 CrossRef CAS PubMed.
- S. G. Barbosa, L. Peixoto, O. Soares, M. Pereira, A. T. Heijne, P. Kuntke, M. M. Alves and M. A. Pereira, Electrochim. Acta, 2018, 26, 122–132 CrossRef.
- A. Tremouli, J. Greenman and I. Ieropoulos, Bioelectrochemistry, 2018, 123, 19–25 CrossRef CAS PubMed.
- S. Bouatra, F. Aziat, R. Mandal, C. G. An, M. R. Wilson, C. Knox, T. C. Bjorndahl, R. Krishnamurthy, F. Saleem and P. Liu, PLoS One, 2013, 8, e73076 CrossRef CAS PubMed.
- E. J. Saude and B. D. Sykes, Metabolomics, 2007, 3, 19–27 CrossRef CAS.
- T. Karak and P. Bhattacharyya, Resour., Conserv. Recycl., 2011, 55, 400–408 CrossRef.
- K. M. Udert, T. A. Larsen and W. Gujer, Water Sci. Technol., 2006, 54, 413–420 CrossRef CAS PubMed.
- R. G. Feachem, D. J. Bradley and H. Garelick, Water Res., 1985, 19, 131 Search PubMed.
- S. Hassan, H. I. Abdel-Shafy and M. Mansour, Arabian J. Chem., 2016, 12, 4074–4083 CrossRef.
- Q. Liu, C. Liu, L. Zhao, W. Ma, H. Liu and J. Ma, Water Res., 2016, 91, 45–54 CrossRef CAS PubMed.
- W. Pronk, M. Biebow and M. Boller, Environ. Sci. Technol., 2006, 40, 2414–2420 CrossRef CAS PubMed.
- W. Pronk, H. Palmquist, M. Biebow and M. Boller, Water Res., 2006, 40, 1405–1412 CrossRef CAS PubMed.
- C. Höglund, B. Vinnerås, T. A. Stenström and H. Jönsson, J. Environ. Sci. Health, 2000, 35, 1463–1475 CrossRef.
- M. U. Kai, T. A. Larsen, M. Biebow and W. Gujer, Water Res., 2003, 37, 2571–2582 CrossRef.
- J. A. Wilsenach, C. Schuurbiers and M. Loosdrecht, Water Res., 2007, 41, 458–466 CrossRef CAS PubMed.
- E. Tilley, J. Atwater and D. Mavinic, Environ. Technol., 2008, 29, 807–816 CrossRef CAS PubMed.
- L. C. Bell, H. Mika and B. J. Kruger, Arch. Oral Biol., 1978, 23, 329–336 CrossRef CAS PubMed.
- H. L. T. Mobley and R. P. Hausinger, Microbiol. Rev., 1989, 53, 85–108 CrossRef CAS PubMed.
- C. P. Flanagan and D. G. Randal, J. Environ. Chem. Eng., 2018, 6, 6344–6350 CrossRef CAS.
- M. Ikematsu, K. Kaneda, M. Iseki and M. Yasuda, Sci. Total Environ., 2007, 382, 159–164 CrossRef CAS PubMed.
- E. R. Marlies, J. V. De, L. Clinckemaillie, R. Ganigué and K. Rabaey, Water Res., 2018, 148, 97–105 Search PubMed.
- F. Li, J. Behrendt, K. Wichmann and R. Otterpohl, Water Sci. Technol., 2008, 57, 1901–1907 CrossRef CAS PubMed.
- H. Ray, F. Perreault and T. H. Boyer, J. Environ. Chem. Eng., 2020, 8, 103993 CrossRef CAS.
- C. Bellona, J. E. Drewes, P. Xu and G. Amy, Water Res., 2004, 38, 2795–2809 CrossRef CAS PubMed.
- P. Berg, G. Hagmeyer and R. Gimbel, Desalination, 1997, 113, 205–208 CrossRef CAS.
- X. Tian, G. Wang, D. Guan, J. Li, A. Wang, J. Li, Z. Yu, Y. Chen and Z. Zhang, Chemosphere, 2016, 165, 202–210 CrossRef CAS PubMed.
- S. Yao, L. Chen, D. Guan, Z. Zhang, X. Tian, A. Wang, G. Wang, Q. Yao and D. Peng, Chemosphere, 2017, 175, 210–218 CrossRef CAS PubMed.
- B. Refla, A. Jdp, C. Mv, D. Afa, A. Wc, H. Aaa, A. Merc, E. Cd, E. Hb and F. Bl, Desalination, 2020, 495(114634) Search PubMed.
- T. S. Chung and S. Y. Yan, Chem. Eng. J., 2011, 171, 684–691 CrossRef.
- A. Alkhudhiri, N. Darwish and N. Hilal, Desalination, 2012, 287, 2–18 CrossRef CAS.
- M. Shirazi, A. Kargari and M. Tabatabaei, Chem. Eng. Process., 2014, 76, 16–25 CrossRef CAS.
- M. S. El-Bourawi, Z. Ding, R. Ma and M. Khayet, J. Membr. Sci., 2006, 285, 4–29 CrossRef CAS.
- M. Khayet, Adv. Colloid Interface Sci., 2011, 164, 56–88 CrossRef CAS PubMed.
- D. L. Shaffer, L. Chavez, M. Ben-Sasson, R. V. Castrillon, N. Y. Yip and M. Elimelech, Environ. Sci. Technol., 2013, 47, 9569–9583 CrossRef CAS PubMed.
- D. Adams and A. E. Childress, J. Membr. Sci., 2005, 257, 111–119 CrossRef.
- L. L. Tun, D. Jeong, S. Jeong, K. Cho, S. Lee and H. Bae, J. Membr. Sci., 2016, 512, 13–20 CrossRef CAS.
- T. Duong, Z. Xie, D. Ng and M. Hoang, Water Environ. J., 2013, 27, 425–434 CAS.
- Y. M. Manawi, M. Khraisheh, A. K. Fard, F. Benyahia and S. Adham, Desalination, 2014, 336, 110–120 CrossRef CAS.
- N. Khumalo, L. Nthunya, S. Derese, M. Motsa and D. S. Dlamini, Sep. Purif. Technol., 2018, 211, 610–617 CrossRef.
- S. N. McCartney, N. A. Williams, C. Boo, X. Chen and N. Y. Yip, ACS Sustainable Chem. Eng., 2020, 8, 7324–7334 CrossRef CAS.
- F. Volpin, L. Chekli, S. Phuntsho, N. Ghaffour and H. K. Shon, Sep. Purif. Technol., 2018, 212, 368–375 CrossRef.
- F. Volpin, J. Jiang, I. E. Saliby, M. Preire and H. K. Shon, J. Cleaner Prod., 2020, 270, 122390 CrossRef CAS.
- S. Phuntsho, H. K. Shon, S. Hong, S. Lee and S. Vigneswaran, Rev. Environ. Sci. Biotechnol., 2012, 11, 147–168 CrossRef CAS.
- J. R. Mccutcheon and M. Elimelech, J. Membr. Sci., 2017, 284, 237–247 CrossRef.
- T. Y. Cath, A. E. Childress and M. Elimelech, J. Membr. Sci., 2006, 281, 70–87 CrossRef CAS.
- N. T. Hancock, X. Pei, D. M. Heil, C. Bellona and T. Y. Cath, Environ. Sci. Technol., 2011, 45, 8483–8490 CrossRef CAS PubMed.
- A. Sz, A. Lz, B. Cyt and A. Dm, J. Membr. Sci., 2012, 396, 1–21 CrossRef.
- H. T. Madsen, N. Bajraktari, C. Hélix-Nielsen, V. Bart and E. G. S. Gaard, J. Membr. Sci., 2015, 476, 469–474 CrossRef CAS.
- K. Lutchmiah, A. Verliefde, K. Roest, L. C. Rietveld and E. R. Cornclissen, Water Res., 2014, 58, 179–197 CrossRef CAS PubMed.
- J. Zhang, Q. She, V. Chang, C. Y. Tang and R. D. Webster, Environ. Sci. Technol., 2014, 48, 3386–3394 CrossRef CAS PubMed.
- H. Ray, F. Perreault and T. H. Boyer, Environ. Sci. Technol., 2020, 54, 11556–11565 CrossRef CAS PubMed.
- F. Volpin, L. Chekli, S. Phuntsho, J. Cho, N. Ghaffour, J. S. Vrouwenvelder and H. K. Shon, Chemosphere, 2018, 203, 482–489 CrossRef CAS PubMed.
- F. Volpin, H. Heo, M. Johir, J. Cho, S. Phuntsho and H. K. Shon, Water Res., 2019, 150, 47–55 CrossRef CAS PubMed.
- S. Engelhardt, J. Vogel, S. E. Duirk, F. B. Moore and H. A. Barton, J. Water Process. Eng., 2020, 34, 101135 CrossRef.
- S. Engelhardt, K. Bender, J. Vogel, S. Duirk and H. Barton, Int. J. Speleol., 2020, 49, 229–234 CrossRef.
- F. Volpin, H. Yu, J. Cho, C. Lee and H. K. Shon, J. Hazard. Mater., 2019, 378, 120724 CrossRef CAS PubMed.
- A. D. Wilt, A. Butkovskyi, K. Tuantet, L. H. Leal and G. Zeeman, J. Hazard. Mater., 2015, 304, 84–92 CrossRef PubMed.
- H. Ray, F. Perreault and T. H. Boyer, Environ. Sci.: Water Res. Technol., 2019, 5, 1993–2003 RSC.
- A. H. Galama, G. Daubaras, O. S. Burheim, H. Rijn Aa Rts and J. W. Post, J. Membr. Sci., 2014, 452, 219–228 CrossRef CAS.
- M. Xie, H. K. Shon, S. R. Gray and M. Elimelech, Water Res., 2016, 89, 210–221 CrossRef CAS PubMed.
- H. Strathmann, Desalination, 2010, 264, 268–288 CrossRef CAS.
- J. De Paepe, R. Lindeboom, M. Vanoppen, K. De Paepe, D. Demey, W. Coessens, B. Lamaze, A. Verliefde, P. Clauwaert and S. E. Vlaeminck, Water Res., 2018, 144, 76–86 CrossRef CAS PubMed.
- W. A. Tarpeh, J. M. Barazesh, T. Y. Cath and K. L. Nelson, Environ. Sci. Technol., 2018, 52, 1453–1460 CrossRef CAS PubMed.
- Y. K. Wang, Y. K. Geng, X. R. Pan and G. P. Sheng, Chem. Eng. Sci., 2017, 171, 451–458 CrossRef CAS.
- M. Ying and C. Y. Tang, Desalination, 2018, 425, 156–174 CrossRef.
- V. Federico, C. Yun, K. Hanki, F. Stefano and J. Namjo, Water Res., 2020, 186, 116320 CrossRef PubMed.
- C. J. Davey, D. Azzini, A. L. Eusebi, R. Tierney, L. Williams, Y. Jiang, A. Kolios, S. Tyrrel and M. Pidou, J. Membr. Sci., 2019, 584, 343–352 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05816a |
| ‡ These authors contributed equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2021 |