Production of chemicals from marine biomass catalysed by acidic ionic liquids
Li
Liu
 ab
ab
aCollege of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot 010020, China. E-mail: lliu@imu.edu.cn
bFujian Provincial Key Lab of Coastal Basin Environment, Fujian Polytechnic Normal University, Fuqing 350300, China
First published on 2nd November 2021
Abstract
The past decade has witnessed the rapid development of shell biorefining. Among the green methodologies, the application of ionic liquids (ILs) to catalyze the conversion of marine biomass including chitosan, chitin, and crustacean shells, has attracted increasing attention. However, in comparison with the significant achievements of ILs in the conversion of lignocellulosic biomass, the methodological developments of ILs in marine biomass have been rather limited due to the greater structural complexity of marine biomass. Herein, the conversion of marine biomass to a variety of value-added chemicals (chitosan oligomers, sugars, 3-acetamido-5-acetylfuran, 5-hydroxyfurfural, levulinic acid, etc.) using acidic ILs as catalysts, has been reviewed according to the order of feedstock from simple to complex (chitosan, chitin, and crustacean shells). The different characteristics of ILs for each type of marine biomass have been summarized and compared with lignocellulosic biomass for the first time, with respect to acidity, hydrogen bonding ability and recyclability, demonstrating the structural effect of marine biomass on their conversion.
1. Introduction
Relative to the remarkable achievements in lignocellulosic biorefinery,1–19 the conversion of marine biomass, especially crustacean shells, has a rather short history. Globally, 6–8 million tons of crab and shrimp shells are discarded every year,20 causing serious resource waste and environmental pollution. Over the long term, converting the shell resources efficiently into high value-added chemicals can not only promote the circular economy but also help to protect the environment.21–33 However, less than 2.5% of about 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 publications on renewable biomass concentrated on crustacean shells suggests great potential to utilize crustacean shells for chemical production.
000 publications on renewable biomass concentrated on crustacean shells suggests great potential to utilize crustacean shells for chemical production.
Crustacean shells are mainly composed of chitin (15–40%), protein (20–40%), and calcium carbonate (20–50%), of which chitin is the second largest biomass in the world next to cellulose.33 Chitin is structurally similar to cellulose and has an acetamide group at the C-2 position instead of the hydroxyl group as in cellulose. Chitosan is a deacetylative derivative of chitin and has more similarities with cellulose. The only difference also depends on the functional group at the C-2 position, i.e., amino group for chitosan and hydroxyl group for cellulose.
Due to a lack of mechanistic understanding, the methodologies to convert chitin and chitosan have lagged far behind in comparison with that of cellulose. Certainly, whether the feedstock contains nitrogen or not can make a big difference. On the one hand, owing to the advantage of containing nitrogen in chitin and chitosan feedstocks, the nitrogen could be passed on to the chemical product in the absence of any other nitrogen source, which is impossible for cellulose.34 On the other hand, the existence of the amino group in chitosan or the acetamide group in chitin results in different mechanisms than in cellulose, which have not been thoroughly elucidated in the previous studies.
Ionic liquids (ILs) have been widely used in green chemistry, due to their characteristics of designability and recyclability.35–37 Application of ILs has greatly contributed to the development of lignocellulosic biorefining,5,38–48 since Rogers’ group first reported dissolving cellulose in [C4mim]Cl in 2002,38 which profoundly inspired their applications in the field of marine biomass. In 2006, Xie and Zhang49 found that [C4mim]Cl could also be utilized to dissolve chitosan and chitin. Thereafter, Wu et al.50 proposed [C4mim]OAc to be a better solvent for chitosan and chitin. Rogers et al.51 adopted [C2mim]OAc to dissolve raw crustacean shells, which exhibited higher solubility than [C4mim]Cl and [C2mim]Cl. Likewise, applications of ILs to dissolve chitosan, chitin, and crustacean shells have led to some progress in derivatization and functionalization.52,53
Early interest in utilizing ILs for the production of chemicals from marine biomass was focused on the solvent role. For example, Zhao et al. reported the conversion of chitosan and chitin in [C4mim]Cl/Br using mineral acid catalysts, leading to higher total reducing sugar (TRS) yields when compared with water-involving processes.54 These methods illustrated that IL solvents are oftentimes advantageous to facilitate the hydrolysis of chitosan and chitin. On the other hand, the IL solvent systems have some shortcomings, such as the lack of control of the strength of the acidic catalysts, leading to low selectivity due to further degradation of the products into unwanted chemicals. Correspondingly, ILs can provide a solution to this problem by incorporating the acid functionality into either the cation or anion, whereby the strength of the acidic ILs can be modulated by the design of the IL structures.62 At present, the production of chemicals from marine biomass, including chitosan, chitin, and crustacean shells using acidic ILs as catalysts has just started.
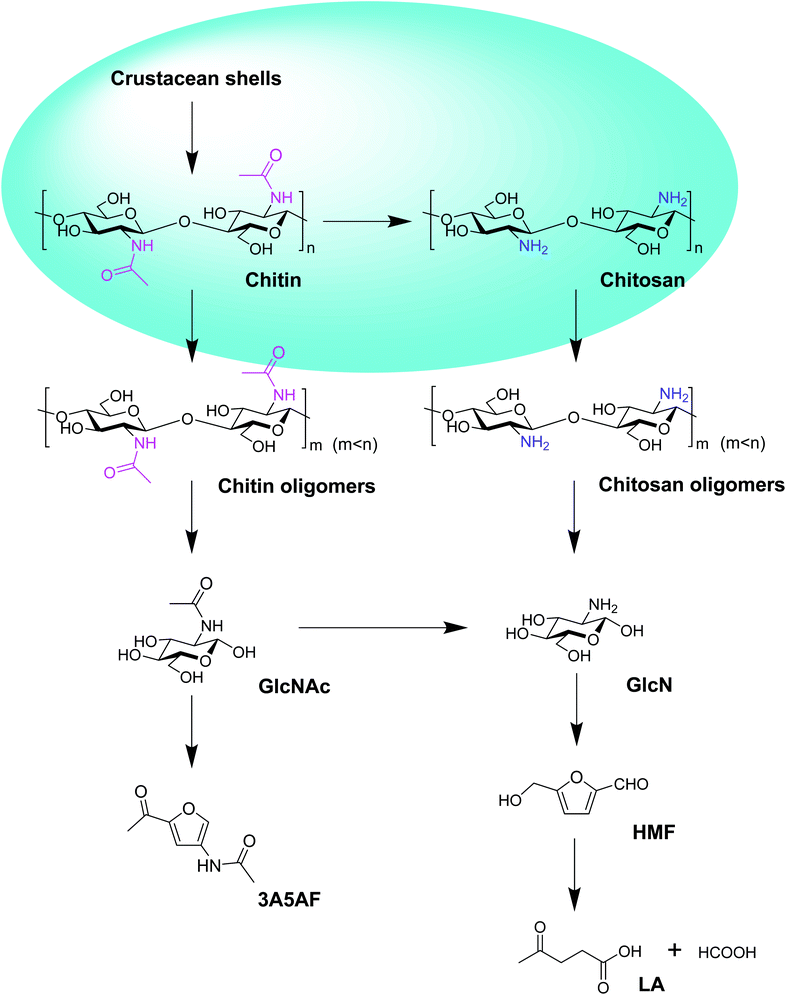
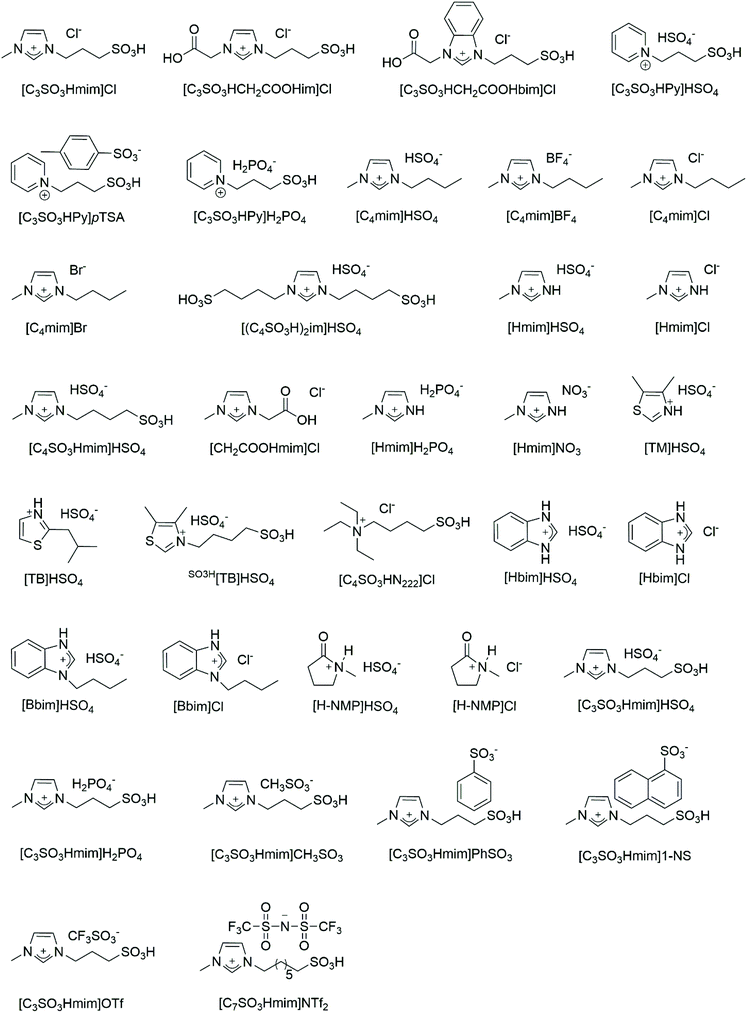
In this review, the chemical routes from crustacean shells to chitin, chitosan, oligomers, sugars, 3-acetamido-5-acetylfuran (3A5AF), 5-hydroxyfurfural (HMF), and levulinic acid (LA) have been outlined (Fig. 1). The relevant literature using acidic ILs as catalysts to convert chitosan, chitin and crustacean shells to oligomers, sugars, 3A5AF, HMF and LA have been listed in Table 1. Firstly, according to the order of feedstock from simple to complex, the eminent research using acidic ILs to convert chitosan, chitin, and crustacean shells to downstream chemicals will be summarized in turn. The IL structures reported in the literature will be discussed in detail as well (Fig. 2). Secondly, the recyclability of ILs for these three types of marine biomass, including chitosan, chitin, and crustacean shells will be reviewed.
| Entry | Feedstock | IL catalyst (wt%) | Solvent | Role of IL | T (°C) | Time | Product (yield) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Chitosan | [C3SO3HCH2COOHbim]Cl (17 wt%) | H2O | Cat. | 110 | 12 h | Chitosan oligomers (−) | 55 |
| 2 | Chitosan | [C3SO3HPy]HSO4 (14 wt%) | [Amim]Cl–[Hmim]Cl | Cat./sol. | MW 640 W | 2 min | TRS (93.2%) | 56 |
| 3 | Chitosan | [Hmim]HSO4 (4 wt%) | H2O | Cat. | 180 | 5 h | HMF (29.5%) | 57 |
| 4 | Chitosan | [Hmim]HSO4 (10 wt%) | DMSO–H2O | Cat. | 180 | 6 h | HMF (34.7%) | 58 |
| 5 | Chitosan | [Hmim]HSO4–0.5FeCl2 (1.25 wt%) | H2O | Cat. | 180 | 4 h | HMF (44.1%) | 59 |
| 6 | Chitosan | [Hbim]Cl (2.5 wt%) | DMSO–H2O | Cat. | 180 | 3 h | HMF (34.9%) | 60 |
| 7 | Chitosan | [C3SO3Hmim]HSO4 (20 wt%) | H2O | Cat. | 170 | 5 h | LA (64.0%) | 61 |
| 8 | Chitin | [C3SO3Hmim]OTf (6 wt%) | [C4mim]Cl | Cat./sol. | 120 | 5 h | GlcNAc (15%) | 62 |
| 9 | Chitin | [Hmim]HSO4 (4 wt%) | H2O | Cat. | 180 | 5 h | HMF (19.3%) | 57 |
| 10 | Chitin | [Hmim]HSO4 (10 wt%) | DMSO–H2O | Cat. | 180 | 6 h | HMF (25.7%) | 58 |
| 11 | Chitin | [C3SO3Hmim]HSO4 (14 wt%) | H2O | Cat. | 180 | 5 h | LA (67.0%) | 63 |
| 12 | Crab shells | [C3SO3Hmim]HSO4 (20wt%) | H2O | Cat. | 180 | 5 h | LA (77.9%) | 64 |
2. Conversion of chitosan to chemicals catalysed by ILs
By virtue of the amino groups, the hydrogen bonding in chitosan is stronger than in cellulose, resulting in its notorious resistance to dissolution. Applications of ILs in chitosan research started from early interest in chitosan dissolution,27,49,50,65 to chitosan conversion in IL solvents.54 Zhao and co-workers54 used [C4mim]Cl or [C4mim]Br as the solvent and mineral acids (HCl, HNO3, and H2SO4) as the catalyst to hydrolyze chitosan into TRS of 63%. Recently, Zang and co-workers66 reported that 44.1 mol% HMF could be obtained from chitosan using Brønsted–Lewis acidic ILs ([Hmim]HSO4–0.5FeCl2) as catalysts. We explored the selective conversion of chitosan to LA (64%) catalysed by ILs,61 on the basis of our previous research on IL-catalysed conversions of cellulose45,67 and lignocellulose,68 thus revealing the effect of the –NH2 group on chitosan conversion.2.1 Chitosan oligomers
Chitosan with high molecular weight (Mw) has some shortcomings that hinder its practical usage, such as poor solubility and low bulk density. Hence, it is necessary to reduce the Mw of chitosan to enhance the solubility, so as to enlarge its application scope. Moreover, good water solubility could endow chitosan oligomers with some special physiological properties towards applications in cosmetics and health, e.g., antifungal, antibacterial, and antitumor effects. Chitosan oligomers can be prepared by different methods including enzymatic hydrolysis, oxidative hydrolysis and acid hydrolysis.56Shan and co-workers69 established oxidative hydrolysis of chitosan with molecular oxygen as the oxidant catalyzed by iron(II) phthalocyanine (FePc) in [C4mim]NTf2 (1-methyl-3-butylimidazolium bis((trifluoromethyl)sulfonyl)imide) to form chitosan oligomers in a biphasic system. When the temperature was increased from 40 °C to 120 °C for 6 h, the intrinsic viscosity kept decreasing. The optimal temperature was determined to be 110 °C, beyond which more complicated by-products were formed. Later, Yu et al.70 came up with another oxidative hydrolysis system of H2O2/[Gly]Cl (glycine chloride), forming chitosan oligomers homogeneously at 80 °C for 2 h.
Conventionally, acid hydrolysis using mineral acids such as HCl and HNO3 leads to the rapid and stochastic breakdown of chitosan. In order to control the Mw of the chitosan oligomer products, new techniques are required. Dandekar and co-workers55 applied three SO3H-functionalized ILs (SFILs) to hydrolyze chitosan homogeneously (Table 1, entry 1). Besides [C3SO3Hmim]Cl, they designed two new ILs with Cl− as the anion, introducing the carboxymethyl group into SO3H-functionalized imiadazole and benzimidazole based cationic structures, whereas the carboxymethyl groups serve as proton donors together with SO3H moieties. It has been reported that the imidazole and benzimidazole ring structures cause some differences in acidity. Therefore, three ILs with different acidities were employed for chitosan hydrolysis.
After heating at 110 °C for 12 h, HCl and HOAc reduced the Mw of chitosan from 410 kDa to 14 kDa and 32 kDa, respectively. The Mw reduction of chitosan was achieved in the sequence HCl > [C3SO3HCH2COOHbim]Cl > [C3SO3HCH2COOHim]Cl > [C3SO3Hmim]Cl > HOAc, which was consistent with their acidity. Among the ILs, [C3SO3HCH2COOHbim]Cl exhibited higher efficiency and reduced the Mw of chitosan to 20 kDa. It was proposed that due to the bulky character, the ILs are unable to interact with chitosan as efficiently as with mineral acids, resulting in a controlled degradation rate. Thus, ILs provided an alternative technique to produce chitosan oligomers with specific Mw.
2.2 TRS
Wu and co-workers56 dissolved chitosan in mixed ILs of [Amim]Cl/[Hmim]Cl, and homogeneous hydrolysis quickly occurred under microwave (MW) irradiation using pyridinium-type SFILs at 640 W for 2 min (Table 1, entry 2). Noticeably, the TRS yield was improved sharply after the addition of DMSO, due to the decreased viscosity, which in turn increased the contact between H+ and the 1,4-β-glycosidic bonds. The Brønsted acidities of the SFILs depend on the anions and decrease in the order: [C3SO3HPy]HSO4 > [C3SO3HPy]pTSA > [C3SO3HPy]H2PO4, which is consistent with the activity order of the SFILs to hydrolyse chitosan. Furthermore, the effect of reaction time was examined. With [C3SO3HPy]HSO4 as catalyst, the viscosity-average molecular weight (Mv) of chitosan dropped continuously from the beginning 560![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 24
000 to 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 (30 s), 882 (90 s), and 450 (120 s). Meanwhile, the TRS yield increased from 15% (30 s) to 84% (90 s) and 92% (120 s). They attributed the high yield of TRS to the combination of ILs, SFILs and MW. Wu et al.'s homogeneous depolymerization of chitosan to TRS56 suggested a depolymerization mechanism: first, complete dissolution of chitosan in mixed ILs enables 1,4-β-glycosidic bonds to be more accessible to the catalytic species H+. Secondly, ILs with high polarity absorb MW emission and markedly accelerate the reaction rate compared to conventional heating in an oil bath.
167 (30 s), 882 (90 s), and 450 (120 s). Meanwhile, the TRS yield increased from 15% (30 s) to 84% (90 s) and 92% (120 s). They attributed the high yield of TRS to the combination of ILs, SFILs and MW. Wu et al.'s homogeneous depolymerization of chitosan to TRS56 suggested a depolymerization mechanism: first, complete dissolution of chitosan in mixed ILs enables 1,4-β-glycosidic bonds to be more accessible to the catalytic species H+. Secondly, ILs with high polarity absorb MW emission and markedly accelerate the reaction rate compared to conventional heating in an oil bath.
2.3 HMF
Owing to the bifunctional aldehyde and hydroxyl groups, HMF has been selected by the US Department of Energy as one of the top 12 platform chemicals from biomass for the production of various valuable chemicals, e.g., 2,5-furandicarboxylic acid (monomer of terephthalate alternatives),72,73 2,5-dimethylfuran (fuel),74,75 furan-2,5-dicarbaldehyde (chemical intermediate),76,77 and levulinic acid (platform chemical).78 In comparison with a large number of research on HMF preparation from lignocellulosic biomass,5,44,46,79–81 there are only a few reports on the conversion of chitosan to HMF. Kerton and co-workers71 hydrolyzed chitosan to HMF in 10.0% yield with SnCl4·5H2O as a catalyst under MW irradiation, whereas they found higher yield of HMF was obtained under dilute conditions versus more concentrated conditions. The proposed mechanism of HMF formation from chitosan monomer by deamination is shown in Fig. 3. Meanwhile, Qiao and Hou25 obtained HMF (12.8%) from chitosan using concentrated ZnCl2 aqueous solution. Lee et al.82 used H2SO4 to hydrolyze chitosan to HMF in 12.1% yield at 174 °C.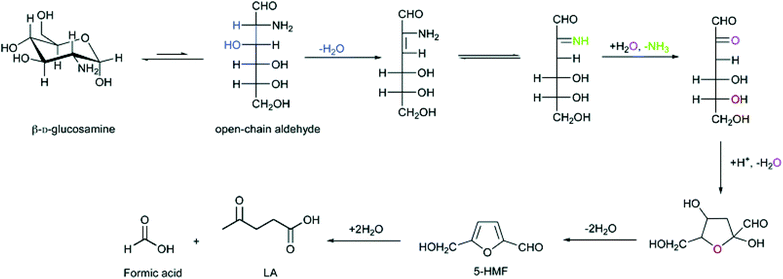 | ||
| Fig. 3 Proposed mechanism for HMF and LA from chitosan monomer. Figure reproduced from ref. 71 with kind permission from The Royal Society of Chemistry. | ||
Zang and co-workers57 screened nine ILs to catalyze chitosan conversion into HMF, including [C4mim]HSO4, [C4mim]BF4, [C4mim]Cl, [C4mim]Br, [(C4SO3H)2im]HSO4, [Hmim]HSO4, [Hmim]Cl, [C4SO3Hmim]HSO4, and [CH2COOHmim]Cl (Table 1, entry 3). The effect of IL structure on the yield of HMF was investigated. The acidic ILs ([C4mim]HSO4, [(C4SO3H)2im]HSO4, [Hmim]HSO4, [C4SO3Hmim]HSO4, [CH2COOHmim]Cl) showed stronger catalytic activity than some neutral ILs ([C4mim]Cl, [C4mim]Br), which failed to catalyze chitosan conversion. It was proposed that the intramolecular H-bonds of chitosan from –OH and –NH2 groups were broken by the interaction with H+ leading to new intermolecular H-bonds, so that the breakage of glycosidic bonds was accelerated and chitosan hydrolysis was promoted. With the same cationic structure, ILs with acidic anions ([C4mim]HSO4, [Hmim]HSO4) showed stronger catalytic activity than those with neutral anions ([C4mim]BF4, [C4mim]Cl, [C4mim]Br, [Hmim]Cl). When Cl− was fixed as the anion, the IL with an acidic cation ([CH2COOHmim]Cl) showed stronger catalytic activity than those with neutral cations ([C4mim]Cl, [Hmim]Cl). When HSO4− was used as the anion, likewise, ILs with more acidic cations should show stronger catalytic activity. However, [(C4SO3H)2im]HSO4 and [C4SO3Hmim]HSO4, which are more acidic, showed lower catalytic activity than [C4mim]HSO4 and [Hmim]HSO4. This could be ascribed to the steric hindrance of the longer SO3H group, thus enabling the ILs difficult to enter chitosan and decreasing the catalytic activity accordingly. Hence, it was suggested that the acidity and structure of ILs play key roles in the catalysis of chitosan conversion. Among the ILs, [Hmim]HSO4 led to the highest HMF yield of 21.7 mol%, due to the dual effects of the cationic and anionic structures. As the amount of IL increased from 2 wt% to 4 wt%, the yield of HMF reached a maximum of 29.5 mol% at 180 °C for 5 h (Table 1, entry 3). Another possible mechanism of deamination proposed is shown in Fig. 4, wherein an open-chain form was initially formed, followed by isomerization to the enol-intermediate and then the formation of a five-membered ring with the –NH2 group removed from GlcN. It was assumed that the so-formed five-membered compounds are key intermediates towards the formation of HMF.
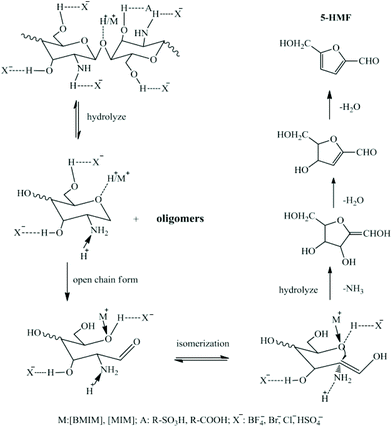 | ||
| Fig. 4 Proposed mechanism of chitosan conversion to HMF. Figure reproduced from ref. 57 with kind permission from Elsevier. | ||
When the DMSO/H2O mixture solvent was used instead of a single water solvent,58,83 the conversion yield of chitosan to HMF could be improved to 34.7% with [Hmim]HSO4 as a catalyst at 180 °C for 6 h, probably due to the suppression of humin byproducts (Table 1, entry 4).
Furthermore, by combining the advantages of a Brønsted acid and a Lewis acid, the mixed-type Brønsted–Lewis acidic IL catalysts59 were employed in the conversion of chitosan, and the HMF yield was further improved to 44.1% by catalysis of [Hmim]HSO4–0.5FeCl2 at 180 °C for 4 h (Table 1, entry 5).
In addition to [Hmim]HSO4, benzimidazole-based ILs that can be synthesized from environmentally friendly and biocompatible benzimidazole, were used to catalyze the conversion of chitosan at 180 °C for 3 h, leading to a higher HMF yield of 34.9% in the DMSO/H2O mixed solvent than 30.8% in pure water for [Hbim]Cl.60 In comparison, [Bbim]-type ILs were less effective probably due to the increased steric hindrance after substitution of H at the 1-position by a butyl group (Table 1, entry 6).
2.4 LA
Due to the bifunctional carboxylic and keto groups, levulinic acid (LA) has also been widely recognized as one of the top 12 platform chemicals to produce a variety of downstream chemicals, for example, 5-bromolevulinic acid (pharmaceutical agent), ethyl levulinate (flavor compound), 5-nonanone (fuel), δ-aminolevulinic acid (herbicide), γ-valerolactone (solvent), succinic acid (plasticiser), and nylon-6,6 (polymer).84–90 Biomass conversion to produce LA was reported to be able to bridge with petroleum processing, thus becoming critical in biorefining.15,67,68,71,78,91–97 Chitosan conversion to LA has recently attracted increasing attention. Kerton and co-workers71 hydrolysed chitosan by the catalysis of SnCl4·5H2O under MW irradiation at 200 °C for 30 min, leading to a LA yield of 23.9 wt% (33.2 mol%). Mika and co-workers97 degraded chitosan in the presence of H2SO4 and HCl catalysts under MW irradiation at 190 °C for 20 min, resulting in a LA yield of 19.3–37.0 mol%. To date, efficiently converting chitosan into LA still remains challenging.Based on our previous research on the production of LA by IL-catalysed conversion of cellulose45,67 and lignocellulose,68 we further expanded to convert chitosan to LA.61 By the catalysis of [C3SO3Hmim]HSO4 at 170 °C for 5 h, the yield of LA was significantly increased to 64% at a lower chitosan intake of 50 mg. It was inferred that lower reactive intermediate concentration could inhibit the intermolecular polymerization to humin byproducts,98–105 thus promoting the intramolecular conversion of chitosan to the LA target product (Table 1, entry 7).
The relationship between acidic IL structure and the yield of LA was studied next. The Brønsted acidity of six acidic ILs was determined by the Hammett method.67 For [C3SO3Hmim]-type ILs, the acidities of the ILs decreased in the sequence HSO4− > PhSO3− ∼ CH3SO3− > Cl− > 1-NS > H2PO4−. Consequently, a stronger acidity of IL led to a higher LA yield obtained from chitosan, thus suggesting the critical role of acidity for IL catalysts during chitosan conversion, presumably through protonation of the glycosidic bond.
Noticeably, for cellulose feedstock, [C3SO3Hmim]Cl led to higher LA yield than [C3SO3Hmim]HSO4, although [C3SO3Hmim]Cl is less acidic than [C3SO3Hmim]HSO4.67 This could be ascribed to the stronger H-bonding acceptors of Cl− that break the H-bonding network in cellulose,38,106–108 thus enhancing the accessibility of the IL catalyst to the catalytic sites in cellulose and improving the catalytic activity of IL.
In comparison, for chitosan feedstock, the H-bonding ability of IL had no prominent effect on chitosan conversion as in the case of cellulose. Even though due to the stronger H-bonding ability of Cl−, the solubility of chitosan could indeed be improved from 0% ([C3SO3Hmim]HSO4) to ca. 5% ([C3SO3Hmim]Cl) in the presence of water (3.31 mmol of IL and 4.000 g of water), the yield of LA catalyzed by [C3SO3Hmim]Cl was still lower than that by [C3SO3Hmim]HSO4. This could be explained that the quaternization interactions between the –NH2 groups of chitosan and acidic IL dominate over the H-bonding interactions. As a result, the yield of LA from chitosan conversion was solely dictated by the acidity of IL, rather than the H-bonding ability of IL. It can be seen that the IL structure has different effects on the yield of LA due to the presence of –NH2 group in chitosan feedstock versus cellulose feedstock.
3. Conversion of chitin to chemicals catalysed by ILs
The acetamide groups account for the huge differences between chitin and cellulose, which contribute to an even stronger H-bonding network in chitin compared to chitosan.109 The earlier applications of ILs in chitin research were mostly concentrated on chitin dissolution,49–52,54,107,110–112 which has been thoroughly reviewed recently. Dissolution of chitin in ILs is based on the principle that ILs could form new hydrogen bonds with chitin to disrupt the original inter- and intramolecular H-bonds in the chitin polymer.52 Zhao and co-workers converted chitin to TRS at 25% using HCl as the catalyst and [C4mim]Cl as the solvent.54 Reports of ILs as catalysts for chitin conversion have been rather limited. One example is that of [Hmim]HSO4 adopted to convert chitin into HMF at 19.3%.57 Recently, we explored the selective conversion of chitin to LA (67.0%) catalysed by acidic IL,113 in comparison with our aforementioned research on IL-catalysed conversion of chitosan,61 demonstrating the effect of the acetamido group on chitin conversion.3.1 TRS
Zhao and co-workers54 hydrolysed chitin using HCl as the catalyst and [C4mim]Cl as the solvent at 100 °C for 7 h, leading to a yield of 25% for TRS, which was much lower than that of chitosan (63%). They noticed the increased viscosity of the reaction mixture for chitin and suggesed that other complex products were formed under these conditions. In comparison with chitosan, more efforts are needed for chitin feedstock to achieve efficient hydrolysis.Reichert and Davis62 also chose [C4mim]Cl to prepare a 1 wt% chitin solution, whereas the IL with OAc− was found to deactivate the catalyst causing chitin unable to hydrolyze. Afterwards, alkyl chain SFILs were used to catalyze chitin hydrolysis. With [C3SO3Hmim]OTf as the catalyst, the yield of N-acetyl-D-glucosamine (GlcNAc) increased from 6% to 15% as the reaction temperature increased from 100 °C to 120 °C. Changing the catalyst to [C7SO3Hmim]NTf2 made the product GlcNAc undetectable, probably due to the degradation of GlcNAc (Table 1, entry 8).
3.2 3A5AF
Chitin is made up of GlcNAc monomers, which are nitrogenous monosaccharides. The unique structure of chitin holds great potential in the production of nitrogen-containing compounds that cannot be obtained from lignocellulosic biomass.Kerton and co-workers71 initially obtained LA from chitosan and GlcN in water medium. Nevertheless, when the transformation of GlcNAc was conducted in dipolar aprotic solvents or imidazolium ILs in the presence of Cl− and boric acid, 3A5AF was obtained.24,114 Under both circumstances, 60% yield of 3A5AF could be achieved, which was 30 times higher than the pyrolysis route. Later, Kerton and Yan115 reported that chitin could be directly converted into 7.5% yield of 3A5AF in N-methyl-2-pyrrolidone (NMP) solvent with alkaline chlorides and boric acid as additives at 215 °C for 1 h.
Furthermore, Kerton and Yan116 tested 10 ILs as the solvent and screened 25 additives, suggesting that solubility was not the crucial factor and Cl− may participate in the reaction since 3A5AF could not be produced without Cl−. The yield of 3A5AF was improved from less than 1% to 6.2% in [C4mim]Cl with HCl and boric acid as additives at 180 °C for 1 h, whereas the reaction temperature was lower than in organic solvents (215 °C). The enhanced yield was ascribed to the synergistic effects of the two additives, whereas the acids catalyzed chitin hydrolysis to the monomers and boric acid promoted the subsequent dehydration step. In addition, the concept whether acidic ILs could replace the neutral ILs with acidic additives was tested on GlcNAc. It was found that the addition of 1 and 2 equivalents of NaCl to the reaction in [C4mim]HSO4 could increase the 3A5AF yield from 10.7% (in the absence of NaCl) to 15.4% and 19.7%, respectively. These results confirmed the crucial role of Cl− in chitin conversion towards 3A5AF, indicating that acidic ILs were less effective for the production of 3A5AF.
3.3 HMF
Kerton and co-workers71 hydrolyzed chitin using SnCl4·5H2O as the catalyst under MW irradiation and no HMF was produced with only LA detected. In comparison, Qiao and Hou25 obtained HMF in 9.0% yield from chitin using concentrated ZnCl2 aqueous solution. Zang and co-workers57 hydrolyzed chitin in [Hmim]HSO4 at 180 °C for 5 h, leading to a higher HMF yield of 19.3% (Table 1, entry 9). When DMSO/H2O mixture solvent was used instead of a single water solvent,58 the conversion yield of chitin to HMF could be improved to 25.7% with [Hmim]HSO4 as the catalyst, probably due to the suppression of humin by-product (Table 1, entry 10).3.4 LA
Kerton and co-workers71 hydrolyzed chitin to LA in 12.7% yield using SnCl4·5H2O as the catalyst under MW irradiation. Due to the stronger hydrogen bonding network in chitin, highly efficient conversion of chitin is more difficult.On the basis of our research on selective chitosan conversion to LA, we proceeded to investigate the conversion of chitin into LA by the catalysis of ILs, whereas 67.0% yield of LA was achieved at a lower feedstock intake with [C3SO3Hmim]HSO4 as the catalyst at 180 °C for 5 h (Table 1, entry 11).63 The effect of IL structure on conversion efficiency of chitin was also examined. The Brønsted acidity of six acidic ILs was quantified and followed the sequence HSO4− > PhSO3− ∼ CH3SO3− > Cl− > 1-NS > H2PO4−. According to the catalytic outcome, the IL with stronger acidity led to a higher LA yield, thus demonstrating the crucial role of acidity during the catalytic conversion of chitin.
One exception is [C3SO3Hmim]Cl resulting in 54.0% yield of LA, which is particularly high but does not overtake [C3SO3Hmim]HSO4 (56.5%). As learnt from our previous research,67 [C3SO3Hmim]Cl could result in a higher yield of LA as compared to [C3SO3Hmim]HSO4 for cellulose feedstock. Therefore, it was proposed that the LA yield in the presence of 3.31 mmol of [C3SO3Hmim]Cl might not reach the maximum yield for chitin feedstock, since deamination causes a decrease of IL acidity. When the dosage of [C3SO3Hmim]Cl was increased to 4.97 mmol, the LA yield from chitin conversion indeed improved to 61.5% and levelled off, which verified our hypothesis. Hence, after ruling out the factor of acidity loss, the strong H-bonding ability of Cl− predominated and surpassed [C3SO3Hmim]HSO4, although the acidity of [C3SO3Hmim]Cl is lower than that of [C3SO3Hmim]HSO4. It can be seen that the effect of IL structure on the LA yield for chitin feedstock not only resembles that for cellulose feedstock wherein the stronger H-bonding acceptor of Cl− helps to break the original H-bonding network38,106 and enhances the accessibility of the IL catalyst to the catalytic sites, but also resembles that for chitosan feedstock wherein deamination occurs causing acidity loss.
4. Conversion of crustacean shells to chemicals catalysed by ILs
The main components in raw crustacean shells are chitin, protein, and calcium carbonate. Chitin functions as the skeleton, with calcium carbonate imparting the necessary strength and proteins endowing a living tissue.117 The traditional process to separate the chitin component from crustacean shells includes demineralization by acidic treatment and then deproteinization by alkaline treatment.118,119 Lately, in order to upgrade to green technologies, ILs have been employed in chitin production.52,120,121Rogers’ group first adopted [C2mim]OAc to extract chitin from shrimp shells,51 from which the resultant chitin solution in [C2mim]OAc can be further used to fabricate nanomats,122 fibers,123 hydrogels,124 and films.125 Through comparison, only chitin which is extracted by IL can produce strong films,125 while the commercially available chitin cannot. In addition, ILs can be designed toward deproteinization, whereas chitin separation could be realized alternatively not through chitin extraction. Rogers’ group used [NH3OH][OAc] to deproteinize and demineralize from shrimp shells, leading to chitin separation with over 80% purity.126 Zhang and co-workers adopted phosphate IL [C2mim]DMP for deproteinization, followed by Zn(OAc)2 for demineralization, offering a green method to produce chitin/Zn composite directly from shrimp shells.127
Compared with chitin separation from crustacean shells, the conversion of crustacean shells to chemicals has just started. Yan and Jin converted shrimp shells by oxidation of CuO and O2 in 2 M NaOH solution, leading to HOAc at a yield of 47.9%.26 Yan's group transformed shrimp shells into low Mw chitosan by NaOH catalysed mechanochemistry.32 Recently, they came up with an integrated process whereas the shell waste was converted into tyrosine and L-DOPA by a microbial engineering method after pretreatment.128 The economic advantage will be greatly enhanced if the raw crustacean shells could be efficiently converted to high value-added chemicals, thus combining chitin separation and conversion of chitin into one step. Application of ILs as the catalyst to convert raw crustacean shells has not yet been reported. Following our systematic research on IL-catalysed conversion of chitosan61 and chitin,63 we continued to investigate the conversion of raw crustacean shells into LA by the catalysis of acidic ILs, unveiling the difference between chitin and crustacean shells.64
4.1 LA
For 250 mg of crab shells, 1.5 g of [C3SO3Hmim]HSO4 was required to reach the maximum yield of LA, but only 1.0 g of [C3SO3Hmim]HSO4 was needed for 250 mg of chitin feedstock. This could be ascribed to higher acid consumption by both deamination and the CaCO3 component. By catalysis of [C3SO3Hmim]HSO4, the yield of LA could be improved to 77.9% at a lower intake of crab shells at 180 °C for 5 h (Table 1, entry 12).64The relationship between the structure of ILs and conversion efficiency of crab shells was investigated next. The Hammett acidity function (H0) of six acidic ILs was measured to evaluate the Brønsted acidity, and the acidities follow the sequence HSO4− > PhSO3− ∼ CH3SO3− > Cl− > 1-NS > H2PO4−. The yields of LA from the conversion of crab shells mostly agree with the acidity sequence of ILs, highlighting the essential role of acidity for IL catalysts.
The only exception is [C3SO3Hmim]Cl, whose acidity is lower than [C3SO3Hmim]HSO4 but leads to higher LA yield (77.6%) surpassing [C3SO3Hmim]HSO4 (62.6%). It can be seen that the effect of IL structure on the LA yield for crab shells resembles that for cellulose feedstock. During the conversion of crab shells, the Cl− anion can destroy the original H-bonding network to form new strong hydrogen bonds with the chitin fraction38,106 in the crab shells, thus enabling chitin greater access to the cationic structure of the IL through the ion–dipole interactions between the anions and cations of the IL. Therefore, it becomes easier for the SO3H groups on the cation to protonate the β-glycosidic linkages within the chitin fraction and promote the hydrolysis reaction to produce LA. By the analogy with an enzyme comprising binding domain and catalytic domain,46 the mechanism underlying how IL catalysts work could be understood intuitively, whereas Cl− and SO3H groups function as the binding domain and the catalytic domain, respectively. Hence, the stronger H-bonding ability of Cl− helps to improve access of the SO3H group to the chitin component and enhances the catalytic efficiency of IL towards the LA product.
The reason that the effect of the IL structure on the LA yield for crab shells does not resemble that for chitin feedstock was attributed to the use of 1.5 g of IL, which makes up for the acidity loss caused by deamination and the CaCO3 component in crab shells. Thus, the effect of the IL structure on the yield of LA for crustacean shells has been unraveled, which is different from chitin feedstock. Furthermore, the self-healing phenomenon of the chitin fraction in crab shells was discovered after the removal of CaCO3 and protein as driven by the strong hydrogen bonds (Fig. 5).
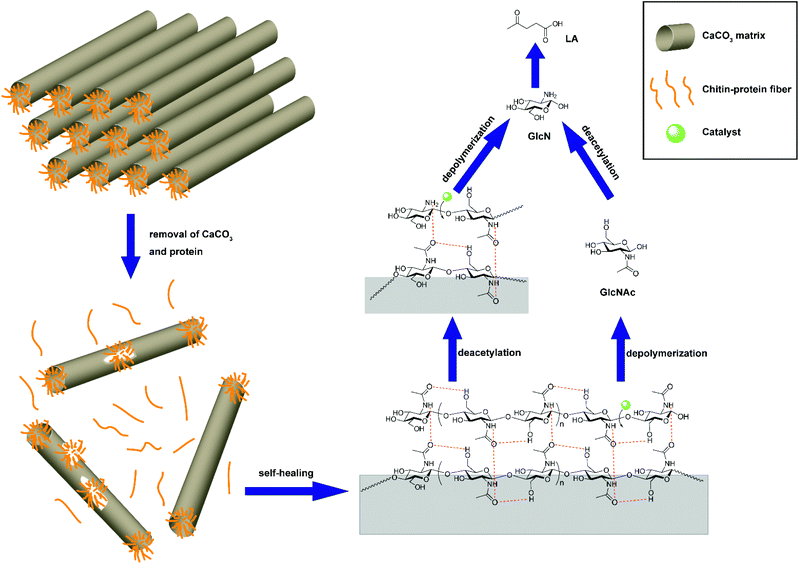 | ||
| Fig. 5 Self-healing of chitin fraction during conversion of crab shells. Figure reproduced from ref. 64 with kind permission from the American Chemical Society. | ||
5. Separation of products and recyclability of ILs
For practical applications of industrial processes, it is necessary to separate the products and recycle the ILs efficiently. So far, a big portion of the research work on conversion of marine biomass is still at the preliminary stage of methodology development. Only a few have managed to realize the separation of the products and reuse of the ILs.5.1 Separation of products
For production of chitosan oligomers from chitosan conversion, Dandekar et al.55 used acetone as the anti-solvent to precipitate chitosan oligomers from the IL reaction mixture and the chitosan oligomers could be separated by cold centrifugation. Alternatively, Na2CO3 was added to precipitate the chitosan oligomers which could be separated after centrifugation and washing till neutral.For HMF product, some organic solvents (e.g. tetrahydrofuran, toluene, ether, ethyl acetate) were traditionally employed to separate HMF by extraction.129–131 Recently, efficient extraction of HMF from glucose conversion in [C4mim]Cl has been reported by charging compressed CO2 into the reaction mixtures.132 Zang et al. found ethyl acetate was the best solvent to extract HMF from chitosan conversion by catalysis of [Hmim][HSO4], [Hmim]HSO4–0.5FeCl2 or [Hbim]Cl.57–60
For LA product, as early as 2013, we have demonstrated good recyclability of the acidic IL [C3SO3Hmim]HSO4 for the conversion of cellulose feedstock, after esterification of the LA product followed by a facile phase separation.45 Two years later, we found out that the LA product can be directly separated by methyl isobutyl ketone (MIBK) extraction, and the efficiency of extracting LA can reach 98%.67,133 Meanwhile, the LA product can be simply purified from MIBK by distillation and MIBK is also reusable. Afterwards, we found that the separation of LA product by MIBK extraction could be applied for feedstocks of chitosan,61 chitin63 and crustacean shells.64
5.2 Recyclability of ILs
As discussed above, one of the advantages of IL is their structural designability, whereas the desired performance can be achieved by modulating cationic and anionic structures of ILs. Beyond that, another advantage of ILs is their recyclability,35–37 which will be discussed taking LA as an example. For cellulose feedstock, the recycling performance of ILs was measured after phase separation from MIBK extractant followed by vacuum drying. The LA yield did not decrease, but rose slightly from 58.5% to 65.7% after five cycles (Fig. 6), showing that the acidic IL maintained stable catalytic activity. The slight increase in LA yield was ascribed to the residual oligomers that can transform into LA in the next cycle.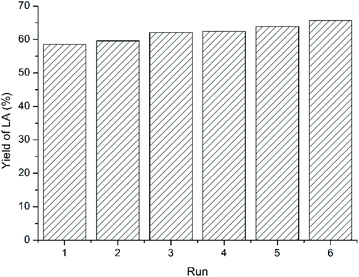 | ||
| Fig. 6 The reuse of IL for cellulose feedstock. Figure reproduced from ref. 67 with kind permission from Elsevier. | ||
In comparison with cellulose feedstock, the IL recyclability of acidic IL differs significantly for marine biomass including chitosan, chitin, and crustacean shells, which will be discussed in turn according to the aforementioned order of feedstock from simple to complex.
For chitosan feedstock (Fig. 7), the LA yield increased slightly from 49.1% (first cycle) to 53.3% (second cycle), since the residual oligomers could transform to LA in the latter cycle. Surprisingly, during the following cycles, the LA yield declined to 27.4%, 10.7% and 6.4%, respectively. It was assumed that the catalytic activity was reduced due to deamination that led to a decrease in IL acidity. To prove this hypothesis, 1 equivalent of H2SO4 was supplemented to the recovered IL to make up for the acidity loss. Thereafter, the recovered IL could be recycled over five times without appreciable decrease in LA yield.61
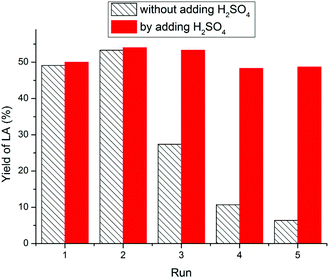 | ||
| Fig. 7 The reuse of IL for chitosan feedstock. Figure reproduced from ref. 61 with kind permission from Elsevier. | ||
Though deamination from chitosan has been proposed previously,26,66,71,97 efforts to detect NH3 have been unsuccessful. In 2018, the typical triplet peaks were accidently observed on the 1H NMR spectra of the reaction mixture and they could be assigned to NH4+ by comparison with standard NH4HSO4 (Fig. 8). In addition, 15.3 mg of NH4+ was quantified by ion chromatography, indicating that 54.6% of the –NH2 group on chitosan has been converted to NH4+. It is reasonably higher than LA yield (49.0%) because NH3 elimination occurs prior to LA formation. To the best of our knowledge, this is the first report of clear evidence for deamination during chitosan conversion.61
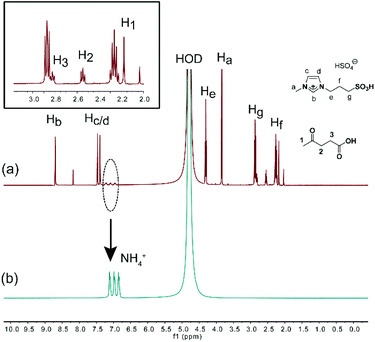 | ||
| Fig. 8 1H NMR spectra (in D2O) of (a) reaction mixture of chitosan by [C3SO3Hmim]HSO4 catalysis and (b) standard NH4HSO4. Figure reproduced from ref. 61 with kind permission from Elsevier. | ||
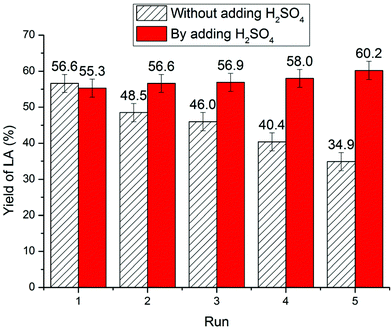
For chitin feedstock (Fig. 9), the yield of LA decreased from 56.5% (first cycle) to 48.5% (second cycle), 46.0% (third cycle), 40.4% (fourth cycle), and 34.9% (fifth cycle). The reduced catalytic activity could be also attributed to NH3 elimination during conversion of chitin resulting in a decrease in IL acidity. On analysis of the reaction mixture, characteristic triplet peaks corresponding to NH4+ appeared on the 1H NMR spectra confirming deamination during chitin conversion.26,71,83,97 Moreover, to compensate for the acidity loss, 1 equivalent of H2SO4 was added to the reused IL and the LA yield did not decrease noticeably over five times.63
 | ||
| Fig. 9 The reuse of IL for chitin feedstock. Figure reproduced from ref. 63 with kind permission from The Royal Society of Chemistry. | ||
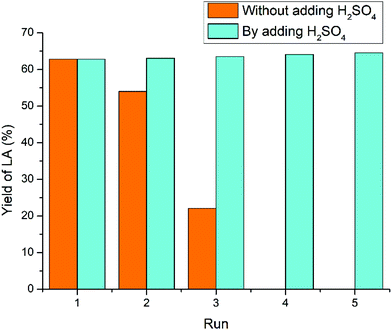
For the raw crab shells (Fig. 10), the yield of LA dropped down from 62.8% (first cycle) to 54.0% (second cycle), 22.1% (third cycle), 0% (fourth cycle), and 0% (fifth cycle). This dramatic decline in catalytic activity could be partly ascribed to NH3 elimination during the conversion of raw crab shells, whereas NH4+ was also detectable similar to our previous research on chitosan and chitin.61,63 Besides, the CaCO3 component in the raw crab shells also consumes 1 equivalent of acidic IL, as CO2 bubbles were observed immediately once acidic IL was added to the crab shells. Hence, both deamination and the CaCO3 component accounted for the more dramatic decline in the acidity during recycling of raw crab shells compared with chitin feedstock. To offset this acidity loss, 1 equivalent of H2SO4 corresponding to chitin and CaCO3 components was added to the reused IL and then the LA yield remained stable over five times.64
 | ||
| Fig. 10 The reuse of IL for raw crab shells. Figure reproduced from ref. 64 with kind permission from the American Chemical Society. | ||
6. Comparison of different marine biomass
In order to demonstrate the different performance of various marine biomass (i.e., chitosan, chitin, and crustacean shells), the same product model should be adopted. To date, there is only limited research on HMF and LA available to discuss.6.1 HMF
With HMF product as the model, different performance of chitosan and chitin could be compared. Zang and co-workers used [Hmim]HSO4 to hydrolyze chitosan and chitin at 180 °C for 5 h, leading to a HMF yield of 29.5 and 19.3%, respectively (Table 1, entries 3 and 9).57With DMSO/H2O mixture as the solvent instead of water itself,58 owing to the suppression of humin by-products, the conversion yields of chitosan and chitin to HMF were improved to 34.7% and 25.7%, respectively, by [Hmim]HSO4 catalysis at 180 °C for 6 h (Table 1, entries 4 and 10). Both the aforementioned trends of higher HMF yields from chitosan than chitin indicated that efficient conversion of chitin is more difficult than that of chitosan under the same reaction conditions. Certainly, the optimum reaction conditions for chitosan and chitin should vary, which need to be optimized independently.
6.2 LA
Furthermore, with LA product as the model, the different performance of chitosan, chitin, and crustacean shells could be compared, whereas the reaction conditions have been optimized independently. The conversion yield of chitosan feedstock to LA was significantly increased to 64% by [C3SO3Hmim]HSO4 catalysis at 170 °C for 5 h,61 whereas higher temperature at 180 °C for 5 h promotes the formation of humins and causes the LA yield to decrease. This could be understood since the activation energy of humins is relatively higher than that of LA.For chitin feedstock, through optimization of reaction conditions, 67.0% yield of LA was achieved with [C3SO3Hmim]HSO4 as the catalyst at 180 °C for 5 h.63 At 170 °C which is the optimum temperature for chitosan, chitin conversion to LA was incomplete, verifying that chitin conversion is more difficult than that of chitosan. This could be ascribed to the stronger hydrogen bonding network in chitin than chitosan.
For feedstock of crustacean shells such as crab shells, the yield of LA could be improved to 77.9% at 180 °C for 5 h by [C3SO3Hmim]HSO4 catalysis.64 1.5 g of [C3SO3Hmim]HSO4 was required for 250 mg of crab shells due to higher acid consumption by both deamination and the CaCO3 component, while only 1.0 g of [C3SO3Hmim]HSO4 was needed for 250 mg of chitin feedstock. Except the amount of [C3SO3Hmim]HSO4, the conversion difficulty of crab shells and chitin is similar (Table 1, entries 7, 11 and 12).
In addition to the effect of reaction conditions discussed as above, the different structural effects of ILs for each type of marine biomass have been summarized, with respect to acidity and hydrogen bonding ability, as described in Sections 2.4, 3.4, and 4.1. Moreover, the different recyclability of ILs for each type of marine biomass have been discussed in Section 5. Therefore, it can be seen that different marine biomass perform differently to achieve the same product, with respect to reaction conditions, and structural effect and recyclability of ILs.
7. Conclusions and outlook
In comparison with the significant achievements of ILs in the conversion of lignocellulosic biomass, the methodological developments of ILs in marine biomass have been rather limited due to the higher structural complexity of marine biomass. In this review, the early applications of acidic ILs as catalysts in the conversion of marine biomass, including chitosan, chitin, and crustacean shells have been reviewed according to the order of feedstock from simple to complex. A variety of high value-added chemicals, e.g., chitosan oligomers, sugars, 3A5AF, HMF and LA, have been produced. The different characteristics of ILs for each type of marine biomass have been summarized and compared with lignocellulosic biomass, with respect to acidity, hydrogen bonding ability and recyclability, demonstrating the structural effect of marine biomass on their conversion. In addition, as derived through comparison of different marine biomass, the conversion difficulty of crab shells and chitin is similar, which is much higher than that of chitosan. The review is deemed to provide in-depth insights into how to improve the conversion efficiency of marine biomass towards the target product by the catalysis of ILs. It can be foreseen that more exciting applications of ILs in the conversion of marine biomass are on the way to advance the marine-based green chemistry. There are many challenges and opportunities, and we would like to highlight some as follows:(1) According to the special structural features of different marine biomass, comprehensive research on rational design of the IL structures should be conducted to efficiently convert marine biomass into specific chemical products.
(2) The network of downstream chemicals starting from marine biomass catalysed by ILs should be enlarged. More brand-new chemical routes await to be established, such as those of nitrogen-containing chemical products.
(3) In-depth mechanistic understanding on the conversion of marine biomass is required, i.e., the mechanism of deamination, the formation of humin by-products, etc. which could in turn help to design the IL catalyst structures and develop new chemical routes.
(4) Highly efficient post-treatment after the conversion is necessary for practical applications. More innovative and environmentally benign technologies need to be developed to separate the chemical products and reuse the ILs simultaneously.
(5) Conversion of the raw crustacean shells, e.g., crab and shrimp shells, requires more attention. The high-value utilization of the other components of CaCO3 and protein needs to be explored. The integrated route of all components could be developed by taking advantage of the natural structures of the crustacean shells.
(6) Industrial applications of producing chemicals from marine biomass are the longstanding goals in this new but promising field. The final utility of a chemical route will mainly depend on the feedstock and processing cost. This involves tremendous efforts from fundamental research to process integration in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Program of Higher-level Talents of IMU (10000-21311201/145), and the Fujian Provincial Key Lab of Coastal Basin Environment (S1-KF2008).Notes and references
- G. W. Huber, J. N. Chheda, C. J. Barrett and J. A. Dumesic, Science, 2005, 308, 1446–1450 CrossRef CAS PubMed.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- J. C. Serrano-Ruiz, R. Luque and A. Sepulveda-Escribano, Chem. Soc. Rev., 2011, 40, 5266–5281 RSC.
- C.-H. Zhou, X. Xia, C.-X. Lin, D.-S. Tong and J. Beltramini, Chem. Soc. Rev., 2011, 40, 5588–5617 RSC.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Chem. Soc. Rev., 2012, 41, 8075–8098 RSC.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- D. M. Alonso, S. G. Wettstein, M. A. Mellmer, E. I. Gurbuz and J. A. Dumesic, Energy Environ. Sci., 2013, 6, 76–80 RSC.
- E. I. Gurbuz, J. M. R. Gallo, D. M. Alonso, S. G. Wettstein, W. Y. Lim and J. A. Dumesic, Angew. Chem., Int. Ed., 2013, 52, 1270–1274 CrossRef PubMed.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed.
- K. Barta and P. C. Ford, Acc. Chem. Res., 2014, 47, 1503–1512 CrossRef CAS PubMed.
- J. Q. Bond, A. A. Upadhye, H. Olcay, G. A. Tompsett, J. Jae, R. Xing, D. M. Alonso, D. Wang, T. Y. Zhang, R. Kumar, A. Foster, S. M. Sen, C. T. Maravelias, R. Malina, S. R. H. Barrett, R. Lobo, C. E. Wyman, J. A. Dumesic and G. W. Huber, Energy Environ. Sci., 2014, 7, 1500–1523 RSC.
- L. T. Mika, E. Cséfalvay and A. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr., J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- J. B. Binder and R. T. Raines, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4516–4521 CrossRef CAS PubMed.
- J. S. Luterbacher, J. M. Rand, D. M. Alonso, J. Han, J. T. Youngquist, C. T. Maravelias, B. F. Pfleger and J. A. Dumesic, Science, 2014, 343, 277–280 CrossRef CAS PubMed.
- Q. Xia, Z. Chen, Y. Shao, X. Gong, H. Wang, X. Liu, S. F. Parker, X. Han, S. Yang and Y. Wang, Nat. Commun., 2016, 7, 11162 CrossRef PubMed.
- N. Yan and X. Chen, Nature, 2015, 524, 155–157 CrossRef CAS PubMed.
- T. Maschmeyer, R. Luque and M. Selva, Chem. Soc. Rev., 2020, 49, 4527–4563 RSC.
- F. M. Kerton, Y. Liu, K. W. Omari and K. Hawboldt, Green Chem., 2013, 15, 860–871 RSC.
- M. Mascal and E. B. Nikitin, ChemSusChem, 2009, 2, 859–861 CrossRef CAS PubMed.
- M. W. Drover, K. W. Omari, J. N. Murphy and F. M. Kerton, RSC Adv., 2012, 2, 4642–4644 RSC.
- Y. Wang, C. M. Pedersen, T. Deng, Y. Qiao and X. Hou, Bioresour. Technol., 2013, 143, 384–390 CrossRef CAS PubMed.
- X. Gao, X. Chen, J. Zhang, W. Guo, F. Jin and N. Yan, ACS Sustainable Chem. Eng., 2016, 4, 3912–3920 CrossRef CAS.
- X. Chen, Y. Gao, L. Wang, H. Chen and N. Yan, ChemPlusChem, 2015, 80, 1565–1572 CrossRef CAS PubMed.
- M. Yabushita, H. Kobayashi, K. Kuroki, S. Ito and A. Fukuoka, ChemSusChem, 2015, 8, 3760–3763 CrossRef CAS PubMed.
- H. Kobayashi, K. Techikawara and A. Fukuoka, Green Chem., 2017, 19, 3350–3356 RSC.
- G. Margoutidis, V. H. Parsons, C. S. Bottaro, N. Yan and F. M. Kerton, ACS Sustainable Chem. Eng., 2018, 6, 1662–1669 CrossRef CAS.
- E. Husson, C. Hadad, G. Huet, S. Laclef, D. Lesur, V. Lambertyn, A. Jamali, S. Gottis, C. Sarazin and A. N. V. Nhien, Green Chem., 2017, 19, 4122–4131 RSC.
- X. Chen, H. Yang, Z. Zhong and N. Yan, Green Chem., 2017, 19, 2783–2792 RSC.
- X. Chen, H. Yang and N. Yan, Chem. – Eur. J., 2016, 22, 13402–13421 CrossRef CAS PubMed.
- M. J. Hulsey, H. Yang and N. Yan, ACS Sustainable Chem. Eng., 2018, 6, 5694–5707 CrossRef CAS.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- A. Brandt, J. Grasvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- C. Li and Z. K. Zhao, Adv. Synth. Catal., 2007, 349, 1847–1850 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- R. Rinaldi, R. Palkovits and F. Schueth, Angew. Chem., Int. Ed., 2008, 47, 8047–8050 CrossRef CAS PubMed.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS PubMed.
- H. Ren, Y. Zhou and L. Liu, Bioresour. Technol., 2013, 129, 616–619 CrossRef CAS PubMed.
- A. M. da Costa Lopes and R. Bogel-Łukasik, ChemSusChem, 2015, 8, 947–965 CrossRef CAS PubMed.
- A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed.
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS PubMed.
- H. Xie, S. Zhang and S. Li, Green Chem., 2006, 8, 630–633 RSC.
- Y. Wu, T. Sasaki, S. Irie and K. Sakurai, Polymer, 2008, 49, 2321–2327 CrossRef CAS.
- Y. Qin, X. Lu, N. Sun and R. D. Rogers, Green Chem., 2010, 12, 968–971 RSC.
- J. L. Shamshina, Green Chem., 2019, 21, 3974–3993 RSC.
- J. L. Sharnshina, P. Berton and R. D. Rogers, ACS Sustainable Chem. Eng., 2019, 7, 6444–6457 CrossRef.
- Z. Zhang, C. Li, Q. Wang and Z. K. Zhao, Carbohydr. Polym., 2009, 78, 685–689 CrossRef CAS.
- A. Pandit, L. Khare, P. Ganatra, R. Jain and P. Dandekar, Carbohydr. Polym., 2021, 260, 117828 CrossRef CAS PubMed.
- Q. Chen, W. J. Xiao, L. L. Zhou, T. H. Wu and Y. Wu, Polym. Degrad. Stab., 2012, 97, 49–53 CrossRef CAS.
- M. Li, H. Zang, J. Feng, Q. Yan, N. Yu, X. Shi and B. Cheng, Polym. Degrad. Stab., 2015, 121, 331–339 CrossRef CAS.
- H. J. Zang, S. B. Yu, P. F. Yu, H. Y. Ding, Y. N. Du, Y. C. Yang and Y. W. Zhang, Carbohydr. Res., 2017, 442, 1–8 CrossRef CAS PubMed.
- Y. Jiang, H. J. Zang, S. Han, B. Yan, S. B. Yu and B. W. Cheng, RSC Adv., 2016, 6, 103774–103781 RSC.
- M. C. Zhang, H. J. Zang, B. Ma, X. L. Zhang, R. R. Xie and B. W. Cheng, ChemistrySelect, 2017, 2, 10323–10328 CrossRef CAS.
- W. Hou, L. Liu and H. Shen, Carbohydr. Polym., 2018, 195, 267–274 CrossRef CAS PubMed.
- W. M. Reichert, A. Mirjafari, J. H. Davis Jr., T. Goodie, N. G. Williams, V. Ho, M. Yoder and M. La, in Ionic Liquids: Science and Applications, ed. A. E. Visser, N. J. Bridges and R. D. Rogers, 2012, vol. 1117, pp. 189–198 Search PubMed.
- W. Hou, Q. Zhao and L. Liu, Green Chem., 2020, 22, 62–70 RSC.
- Q. Zhao and L. Liu, ACS Sustainable Chem. Eng., 2021, 9, 1762–1771 CrossRef CAS.
- Q. Chen, A. Xu, Z. Li, J. Wang and S. Zhang, Green Chem., 2011, 13, 3446–3452 RSC.
- Y. Jiang, H. Zang, S. Han, B. Yan, S. Yu and B. Cheng, RSC Adv., 2016, 6, 103774–103781 RSC.
- H. Ren, B. Girisuta, Y. Zhou and L. Liu, Carbohydr. Polym., 2015, 117, 569–576 CrossRef CAS PubMed.
- L. Liu, Z. Li, W. Hou and H. Shen, Carbohydr. Polym., 2018, 181, 778–784 CrossRef CAS PubMed.
- X. Zhao, A. Kong, Y. Hou, C. Shan, H. Ding and Y. Shan, Carbohydr. Res., 2009, 344, 2010–2013 CrossRef CAS PubMed.
- L. Li, B. Yuan, S. W. Liu, S. T. Yu, C. X. Xie, F. S. Liu and L. J. Shan, J. Polym. Environ., 2012, 20, 388–394 CrossRef CAS.
- K. W. Omari, J. E. Besaw and F. M. Kerton, Green Chem., 2012, 14, 1480–1487 RSC.
- S. E. Davis, B. N. Zope and R. J. Davis, Green Chem., 2012, 14, 143–147 RSC.
- E. Hayashi, Y. Yamaguchi, K. Kamata, N. Tsunoda, Y. Kumagai, F. Oba and M. Hara, J. Am. Chem. Soc., 2019, 141, 890–900 CrossRef CAS PubMed.
- B. Saha, C. M. Bohn and M. M. Abu-Omar, ChemSusChem, 2014, 7, 3095–3101 CrossRef CAS PubMed.
- W. Guo, H. Liu, S. Zhang, H. Han, H. Liu, T. Jiang, B. Han and T. Wu, Green Chem., 2016, 18, 6222–6228 RSC.
- R. Fang, R. Luque and Y. Li, Green Chem., 2016, 18, 3152–3157 RSC.
- Z. Zhang and G. W. Huber, Chem. Soc. Rev., 2018, 47, 1351–1390 RSC.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Green Chem., 2006, 8, 701–709 RSC.
- R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- J. B. Binder, A. V. Cefali, J. J. Blank and R. T. Raines, Energy Environ. Sci., 2010, 3, 765–771 RSC.
- B. Kim, J. Jeong, D. Lee, S. Kim, H. J. Yoon, Y. S. Lee and J. K. Cho, Green Chem., 2011, 13, 1503–1506 RSC.
- S.-B. Lee and G.-T. Jeong, Appl. Biochem. Biotechnol., 2015, 176, 1151–1161 CrossRef CAS PubMed.
- S. Yu, H. Zang, S. Chen, Y. Jiang, B. Yan and B. Cheng, Polym. Degrad. Stab., 2016, 134, 105–114 CrossRef CAS.
- D. W. Rackemann and W. O. S. Doherty, Biofuels, Bioprod. Biorefin., 2011, 5, 198–214 CrossRef CAS.
- S. M. Sen, D. M. Alonso, S. G. Wettstein, E. I. Gurbuz, C. A. Henao, J. A. Dumesic and C. T. Maravelias, Energy Environ. Sci., 2012, 5, 9690–9697 RSC.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC.
- A. Morone, M. Apte and R. A. Pandey, Renewable Sustainable Energy Rev., 2015, 51, 548–565 CrossRef CAS.
- F. D. Pileidis and M.-M. Titirici, ChemSusChem, 2016, 9, 562–582 CrossRef CAS PubMed.
- Y. Shao, K. Sun, Q. Li, Q. Liu, S. Zhang, Q. Liu, G. Hu and X. Hu, Green Chem., 2019, 21, 4499–4511 RSC.
- H.-J. Feng, X.-C. Li, H. Qian, Y.-F. Zhang, D.-H. Zhang, D. Zhao, S.-G. Hong and N. Zhang, Green Chem., 2019, 21, 1743–1756 RSC.
- R. Weingarten, W. C. Conner and G. W. Huber, Energy Environ. Sci., 2012, 5, 7559–7574 RSC.
- S. G. Wettstein, D. M. Alonso, Y. X. Chong and J. A. Dumesic, Energy Environ. Sci., 2012, 5, 8199–8203 RSC.
- J. J. Bozell, Science, 2010, 329, 522–523 CrossRef CAS PubMed.
- S. Van de Vyver, J. Thomas, J. Geboers, S. Keyzer, M. Smet, W. Dehaen, P. A. Jacobs and B. F. Sels, Energy Environ. Sci., 2011, 4, 3601–3610 RSC.
- J. Q. Bond, D. M. Alonso, D. Wang, R. M. West and J. A. Dumesic, Science, 2010, 327, 1110–1114 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- Á. Szabolcs, M. Molnár, G. Dibó and L. T. Mika, Green Chem., 2013, 15, 439–445 RSC.
- S. J. Dee and A. T. Bell, ChemSusChem, 2011, 4, 1166–1173 CrossRef CAS.
- I. van Zandvoort, Y. H. Wang, C. B. Rasrendra, E. R. H. van Eck, P. C. A. Bruijnincx, H. J. Heeres and B. M. Weckhuysen, ChemSusChem, 2013, 6, 1745–1758 CrossRef CAS PubMed.
- X. Fu, J. Dai, X. Guo, J. Tang, L. Zhu and C. Hu, Green Chem., 2017, 19, 3334–3343 RSC.
- Z. Cheng, J. L. Everhart, G. Tsilomelekis, V. Nikolakis, B. Saha and D. G. Vlachos, Green Chem., 2018, 20, 997–1006 RSC.
- V. Maruani, S. Narayanin-Richenapin, E. Framery and B. Andrioletti, ACS Sustainable Chem. Eng., 2018, 6, 13487–13493 CrossRef CAS.
- Z. Cheng, K. A. Goulas, N. Q. Rodriguez, B. Saha and D. G. Vlachos, Green Chem., 2020, 22, 2301–2309 RSC.
- A. Sangregorio, A. Muralidhara, N. Guigo, L. G. Thygesen, G. Marlair, C. Angelici, E. de Jong and N. Sbirrazzuoli, Green Chem., 2020, 22, 2786–2798 RSC.
- H. Shen, H. Shan and L. Liu, ChemSusChem, 2020, 13, 513–519 CrossRef CAS PubMed.
- R. C. Remsing, R. P. Swatloski, R. D. Rogers and G. Moyna, Chem. Commun., 2006, 1271–1273 RSC.
- A. Xu, J. Wang and H. Wang, Green Chem., 2010, 12, 268–275 RSC.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- P. Sikorski, R. Hori and M. Wada, Biomacromolecules, 2009, 10, 1100–1105 CrossRef CAS PubMed.
- P. S. Barber, C. S. Griggs, G. Gurau, Z. Liu, S. Li, Z. Li, X. Lu, S. Zhang and R. D. Rogers, Angew. Chem., Int. Ed., 2013, 52, 12350–12353 CrossRef CAS PubMed.
- M. Shimo, M. Abe and H. Ohno, ACS Sustainable Chem. Eng., 2016, 4, 3722–3727 CrossRef CAS.
- T. Uto, S. Idenoue, K. Yamamoto and J.-i. Kadokawa, Phys. Chem. Chem. Phys., 2018, 20, 20669–20677 RSC.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS.
- K. W. Omari, L. Dodot and F. M. Kerton, ChemSusChem, 2012, 5, 1767–1772 CrossRef CAS PubMed.
- X. Chen, S. L. Chew, F. M. Kerton and N. Yan, Green Chem., 2014, 16, 2204–2212 RSC.
- X. Chen, Y. Liu, F. M. Kerton and N. Yan, RSC Adv., 2015, 5, 20073–20080 RSC.
- T. Setoguchi, T. Kato, K. Yamamoto and J.-i. Kadokawa, Int. J. Biol. Macromol., 2012, 50, 861–864 CrossRef CAS PubMed.
- H. K. No, S. P. Meyers and K. S. Lee, J. Agric. Food Chem., 1989, 37, 575–579 CrossRef CAS.
- F. Shahidi and J. Synowiecki, J. Agric. Food Chem., 1991, 39, 1527–1532 CrossRef CAS.
- M. Boric, H. Puliyalil, U. Novak and B. Likozar, Green Chem., 2018, 20, 1199–1204 RSC.
- P. Beaney, J. Lizardi-Mendoza and M. Healy, J. Chem. Technol. Biotechnol., 2005, 80, 145–150 CrossRef CAS.
- P. S. Barber, C. S. Griggs, J. R. Bonner and R. D. Rogers, Green Chem., 2013, 15, 601–607 RSC.
- J. L. Shamshina, G. Gurau, L. E. Block, L. K. Hansen, C. Dingee, A. Walters and R. D. Rogers, J. Mater. Chem. B, 2014, 2, 3924–3936 RSC.
- X. Shen, J. L. Shamshina, P. Berton, J. Bandomir, H. Wang, G. Gurau and R. D. Rogers, ACS Sustainable Chem. Eng., 2016, 4, 471–480 CrossRef CAS.
- C. King, J. L. Shamshina, G. Gurau, P. Berton, N. F. A. F. Khan and R. D. Rogers, Green Chem., 2017, 19, 117–126 RSC.
- J. L. Shamshina, P. S. Barber, G. Gurau, C. S. Griggs and R. D. Rogers, ACS Sustainable Chem. Eng., 2016, 4, 6072–6081 CrossRef CAS.
- M. Feng, X. Lu, L. Wang, J. Zhang, S. Yang, C. Shi, Q. Zhou and S. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 11990–11998 CAS.
- X. Ma, G. Gozaydin, H. Yang, W. Ning, X. Han, N. Y. Poon, H. Liang, N. Yan and K. Zhou, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 7719–7728 CrossRef CAS PubMed.
- S. Hu, Z. Zhang, Y. Zhou, J. Song, H. Fan and B. Han, Green Chem., 2009, 11, 873–877 RSC.
- J. Song, H. Fan, J. Ma and B. Han, Green Chem., 2013, 15, 2619–2635 RSC.
- L. Wu, J. Song, B. Zhang, B. Zhou, H. Zhou, H. Fan, Y. Yang and B. Han, Green Chem., 2014, 16, 3935–3941 RSC.
- X. Sun, Z. Liu, Z. Xue, Y. Zhang and T. Mu, Green Chem., 2015, 17, 2719–2722 RSC.
- L. C. Nhien, L. Nguyen Van Duc, S. Kim and M. Leet, Ind. Eng. Chem. Res., 2016, 55, 5180–5189 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |