 Open Access Article
Open Access ArticleTwo-dimensional metal–organic frameworks and their derivatives for electrochemical energy storage and electrocatalysis
Kuangmin
Zhao†
,
Weiwei
Zhu†
,
Suqin
Liu
*,
Xianli
Wei
,
Guanying
Ye
,
Yuke
Su
and
Zhen
He
 *
*
College of Chemistry and Chemical Engineering, Hunan Provincial Key Laboratory of Chemical Power Sources, Central South University, Changsha, Hunan 410083, P. R. China. E-mail: zhenhe@csu.edu.cn; sqliu2003@126.com
First published on 6th January 2020
Abstract
Two-dimensional (2D) metal–organic frameworks (MOFs) and their derivatives with excellent dimension-related properties, e.g. high surface areas, abundantly accessible metal nodes, and tailorable structures, have attracted intensive attention as energy storage materials and electrocatalysts. A major challenge on the road toward the commercialization of 2D MOFs and their derivatives is to achieve the facile and controllable synthesis of 2D MOFs with high quality and at low cost. Significant developments have been made in the synthesis and applications of 2D MOFs and their derivatives in recent years. In this review, we first discuss the state-of-the-art synthetic strategies (including both top-down and bottom-up approaches) for 2D MOFs. Subsequently, we review the most recent application progress of 2D MOFs and their derivatives in the fields of electrochemical energy storage (e.g., batteries and supercapacitors) and electrocatalysis (of classical reactions such as the HER, OER, ORR, and CO2RR). Finally, the challenges and promising strategies for the synthesis and applications of 2D MOFs and their derivatives are addressed for future development.
1. Introduction
Advanced electrochemical energy storage and conversion technologies such as rechargeable batteries, supercapacitors, fuel cells, and electrolytic cells play crucial roles in the current energy systems.1–3 The exploration of advanced electrode materials (i.e. electrochemical energy storage materials and electrocatalysts) with sufficient ion storage sites, excellent ion diffusion ability, high conductivity, and abundant active catalytic sites is vital but challenging.4–6Metal–organic frameworks (MOFs), a series of crystalline materials consisting of metal ions and organic ligands,7,8 have been extensively investigated for electrochemical energy storage9–11 and electrocatalysis12–15 due to their abundant active sites, tunable pore distribution, and controllable morphologies7,16–23 Benefiting from the ordered arrangement of the metal ions and organic linkers in the MOFs, the MOF derivatives tend to show a homogeneous distribution of the metal atoms or/and metal nanoparticles,24,25 which could enhance the metal utilization efficiency26 and further leads to a great improvement for their applications in energy storage and electrocatalysis. To date, carbon materials,27 metals,28 transition metal oxides,25,29,30 transition metal carbides,31 transition metal dichalcogenides,32,33 transition metal phosphides,34–36 and their composites37 have been successfully fabricated from MOF precursors. It is well-known that the morphology of the electrode materials shows a correlation with the ion transport distance, which is a key factor that determines the ion transport resistance and then affects the ion diffusion ability.38,39 However, MOFs with a bulk morphology usually possess a low metal utilization and accessible surface area as well as poor ion diffusion ability. To further enhance such properties of MOFs and MOF derivatives, great efforts have been devoted to fabricating low-dimensional MOFs with a controllable morphology and size towards a larger specific surface area and better ion diffusion properties.40–42
Since the discovery of graphene in 2004,43–45 many two-dimensional (2D) materials, such as the nanosheets of porous carbon,46–48 transition metal disulfides,49,50 black phosphorus,51,52 BN,53 and Mxenes,54 have been extensively investigated due to their unique structures and electronic properties. Ascribing to the distinctive dimension-dependent properties, such as high aspect ratios, abundant accessible active sites, and short ion transport distances, 2D MOFs,55 as a new class of 2D materials, have fascinated researchers in various fields.55–57 In addition, the inorganic metal compounds/C composites derived from 2D MOFs with a better conductivity are extensively investigated as energy storage materials and electrocatalysts.58,59 Similar to other 2D materials, the properties of 2D MOFs and their derivatives greatly depend on the thickness.60 Exploring efficient methods to prepare high-quality 2D MOFs with a desirable thickness is a main concern for the practical application of 2D MOFs.61 To date, numerous synthetic methods have been developed to controllably synthesize 2D MOFs, which can be classified into two approaches, i.e., top-down and bottom-up.62 Generally, the top-down approach includes the physical and chemical/electrochemical exfoliation methods and the bottom-up approach includes the two-phase interfacial or intermediate layer growth methods, surfactant-assisted methods, molecule-precoordinated methods, template-assisted methods, and competitive coordination methods, etc.33,63,64 2D MOFs with excellent dimension-dependent properties have been widely employed as energy storage and electrocatalytic materials. More importantly, 2D MOF derivatives with better conductivity employed in energy storage and electrocatalysis are currently at a stage of rapid development.65,66 Thus, a critical review focusing on the synthesis and application of 2D MOFs and their derivatives for electrochemical energy storage and electrocatalysis is highly demanded.
In this review, the synthetic strategies of 2D MOFs in recent years as well as the applications of 2D MOFs and their derivatives in energy storage and electrocatalysis are summarized. We start with the discussion on the representative synthesis of 2D MOFs and strategies of regulating the 2D MOFs' surface compositions and structural properties. Then, to better help the readers to understand the advantages of the 2D MOFs and their derivatives in energy storage and electrocatalysis, we thoroughly summarize suitable 2D MOFs and their derivatives for specific applications in batteries, supercapacitors (SCs), the oxygen evolution reaction (OER), hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and CO2 reduction reaction (CO2RR). Finally, the opportunities and challenges of the synthesis and application of the 2D MOFs are discussed.
2. Strategies for the synthesis of 2D MOFs
To date, 2D MOFs have been synthesized by various synthesis strategies. In this section, these reported synthesis strategies for the 2D MOFs will be reviewed as two categories: top-down and bottom-up.622.1. Top-down approach
The top-down approach refers to a series of methods that synthesize the 2D materials from the corresponding bulk materials by breaking the interactions between the adjacent layers, which are commonly used for the fabrication of most of the 2D materials, such as graphene, transition metal dichalcogenides, and black phosphorus.67 Layered bulk MOFs, in which the adjacent MOF layers are combined by the weak van der Waals forces and chemical bonds, can also be separated into 2D MOF nanosheets via the top-down approach including physical exfoliation and chemical/electrochemical exfoliation.Previously, the layered bulk Zn(TPA)(H2O)DMF and [Cu2Br(IN)2]n MOFs (TPA = terephthalic acid, DMF = N,N-dimethylformamide, and IN = isonicotinato) were exfoliated by ultrasonication. As shown in Fig. 1a, a single layer of Zn(TPA)(H2O)DMF is constructed by the paddle-wheel Zn2 clusters and terephthalates. In the bulk crystal, the Zn(TPA)(H2O)DMF layers are held together by the hydrogen bonds between the H2O and DMF intercalated in the interlayer (Fig. 1b).69,71 The [Cu2Br(IN)2]n layer is constructed by bridging a copper dimer with a bromine ligand and isonicotinato linkers (Fig. 1d).70 The four coordination bonds of Cu2+ are two Cu–O bonds and two Cu–N bonds. The π-stacking interactions between the isonicotinato aromatic rings in the adjacent layers lead to the stacking of the [Cu2Br(IN)2]n layers along the a axis in the bulk (Fig. 1e). After a simple ultrasonic treatment, 2D Zn(TPA)(H2O)DMF and [Cu2Br(IN)2]n nanosheets with a thickness of about 2 and 0.5 nm, respectively, were successfully obtained (Fig. 1c and f). In addition, the ultrasonication method combined with suitable solvent interaction and substitution could be extended to synthesize other 2D MOFs. For example, the 3D cubic Zn4O(A)3 (A = 2,5-bis(3-methoxypropoxy)-1,4-benzenedicarboxylate) framework structure could be directly converted to the Zn4O(A)3 (DMF) nanosheets with a thickness of 0.94 nm by ultrasonication in a DMF solution due to the DMF being able to break the weak coordination between the Zn2+ and side-chain of the A.72
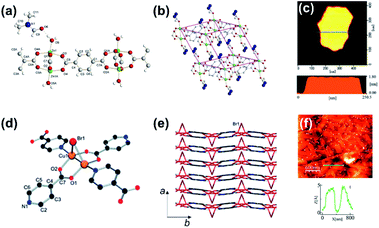 | ||
| Fig. 1 (a) 2D layer structure of Zn(TPA)(H2O)DMF with atom numbering. Note the distortion of the Zn2O8 units from the local D4h point symmetry. (b) Layer structure of Zn(TPA)(H2O)DMF with blue atoms depicting the DMF disorder. (c) AFM images and height profile of Zn(TPA)(H2O)DMF nanosheets. Reproduced with permission.69 Copyright©2011 The Royal Society of Chemistry. (d) Detailed view of the copper environment and (e) superposition of layers along the a axis in [Cu2Br(IN)2]n. (f) AFM images and height profile of [Cu2Br(IN)2]n. Reproduced with permission.70 Copyright©2010 The Royal Society of Chemistry. | ||
Compared to direct ultrasonication, wet ball-milling pretreatment combined with ultrasonication is a more efficient physical method for the exfoliation of layered bulk MOFs. Peng and co-workers employed a ball-milling pretreatment to ultrasonically exfoliate bulk Zn2(BIM)4 (Fig. 2a and b),22 a layered MOF constructed by Zn(BIM)2 chains cross-connected by Zn2+ in the ab plane and the weak van der Waals forces between the adjacent layers.22 As shown in Fig. 2b, the interlayer distance of the adjacent Zn2(BIM)4 layers is about 0.988 nm, which is large enough for the insertion of small molecules for further exfoliation. The wet ball milling pretreatment provides a shear force to enlarge the interlayer distance and intercalate more small molecules between the layers of Zn2(BIM)4. The low-magnification TEM image in Fig. 2c shows the Zn2(BIM)4 nanosheets obtained from the layered bulk Zn2(BIM)4 in a small-molecule solvent (i.e., a mixture of methanol and propanol) under ultrasonication. The selected-area electron diffraction (SAED) pattern of a few-layer Zn2BIM4 nanosheet collected along the c axis (inset in Fig. 2c) matches with the diffraction pattern of the (110) planes of Zn2BIM4, indicating the well preserved crystallinity of the Zn2BIM4 nanosheets after the exfoliation. The thickness of the resulting Zn2BIM4 nanosheets measured by AFM is about 1.12 nm (Fig. 2d). The ball-milling pretreatment combined with ultrasonication could significantly improve the yield and quality of the 2D MOFs, therefore it has gradually replaced the sole ultrasonic exfoliation. Since the invention of this method, a number of layered MOFs constructed by the weak van der Waals forces between the layers have been exfoliated to the corresponding MOF nanosheets, e.g. Zn2BIM3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 and [Cu(μ-pym2S2)(μ-Cl)]n·nMeOH (pymS2 = dipyrimidindisulfide).73
65 and [Cu(μ-pym2S2)(μ-Cl)]n·nMeOH (pymS2 = dipyrimidindisulfide).73
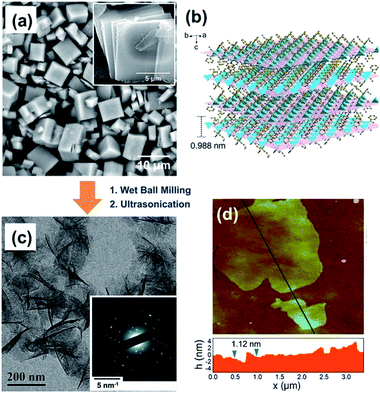 | ||
| Fig. 2 (a) SEM image of as-synthesized Zn2(BIM)4 crystals. The inset image shows the typical flake-like morphology of Zn2(BIM)4 crystals. (b) Architecture of the layered MOF precursor. The ab planes are highlighted in purple to better illustrate the layered structure. (c) TEM image of Zn2(BIM)4 nanosheets. The inset shows the SAED image of Zn2(BIM)4. (d) Tapping-mode AFM topographical image of Zn2(BIM)4 nanosheets on silicon wafer. The height profile of the nanosheets along the black lines is marked in the image. Reproduced with permission.22 Copyright© 2014 American Association for the Advancement of Science. | ||
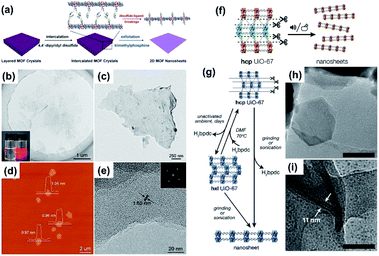 | ||
| Fig. 3 (a) Schematic illustration of the process developed to produce 2D MOF nanosheets via an intercalation and chemical exfoliation approach. (b) TEM image of the exfoliated MOF nanosheets. Inset: Tyndall effect (left) before and (right) after exfoliation. (c) TEM image of an individual exfoliated MOF nanosheet. (d) AFM image of the exfoliated MOF nanosheets with corresponding height profiles. (e) High-resolution TEM image of an exfoliated multilayer MOF nanosheet. The corresponding FFT pattern is shown in the inset. (f) Illustration of the preparation of hxl UiO-67 nanosheets. (g) Schematic illustration of the chemical transformation of hcp UiO-67 to hxl UIO-67 and UIO-67 nanosheets. (h) TEM and (i) HRTEM images of UIO-67 nanosheets. Reproduced with permission.74,75 Copyright©2017 American Chemistry Society. | ||
Since most of the pillared-layer type MOFs are stable enough under various reaction conditions, selective breaking of the coordination bonds between the layers is still challenging. More recently, the electrochemical oxidation method was also employed to exfoliate the pillared-layer type MOFs. By selecting the pillar ligand with a redox active backbone that can be selectively oxidized and removed, the pillared-layer type MOFs can be easily exfoliated to 2D MOF nanosheets. As shown in Fig. 4a, Zhang reported an electrochemical exfoliation strategy relying on the covalent modification of the pillar ligands.77 In detail, H2[Co6O(dhbdc)2(H2dhbdc)2(EtOH)4]2(EtOH–H2O) (denoted as MCF-13 or 3D-Co) was synthesized by treating Co(CH3COO)2 and 2,3-dihydroxy-1,4-benzenedicarboxylic acid (H4dhbdc) mixed solution in an autoclave at 140 °C for 72 h. The electrochemical exfoliation strategy could directly remove the pillar ligands (H4dhbdc) in the 3D-Co and afford the 2D Co6O(dhbdc)2(OH)2(H2O)10 nanosheets (2D-Co-NS) without additional complex processes. Fig. 4b illustrates the possible oxidative transformation route from the H4dhbdc pillar ligand (m/z 198) to a pillar ligand with m/z 168, which could significantly lower the coordination ability of –COO− in the pillar ligands and facilitate the removal of the pillar ligands by the solvent. Fig. 4c–e show the morphology evolution from 3D-Co to 2D-Co-NS. The TEM image (Fig. 4f) shows that 2D-Co-NS has a wrinkled nanosheet morphology and the AFM image (Fig. 4g) and height profile (Fig. 4h) show that the thickness of the 2D-Co-NS is only about 2 nm.
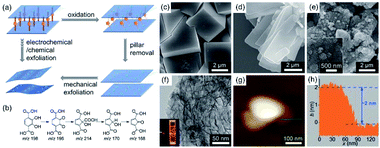 | ||
| Fig. 4 (a) Illustration of the synthetic process of 2D MOFs by using the electrochemical/chemical exfoliation method and (b) a possible oxidative transformation route from H4dhbdc to a species with m/z 168. SEM images of (c) 3D-Co, (d) 2D-Co, and (e) 2D-Co-NS. (f) TEM image of 2D-Co-NS (inset: Tyndall effect of a colloidal solution). (g) AFM image and (h) the corresponding height profile of 2D-Co-NS. Reproduced with permission.77 Copyright©2018 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
The top-down approach, with the advantages of simplicity and low-cost, has been widely used for the synthesis of 2D MOF nanosheets from the layered bulk MOFs with relatively large interlayer distances and/or weak coordination ability. The yield of the 2D MOFs in the top-down approach greatly depends on the structural properties of the bulk MOF precursors. The relatively low yield of the 2D MOF nanosheets derived from the bulk MOFs by the top-down approach gives rise to inhomogeneous products, which requires troublesome separation processes and therefore has hindered the practical application of the top-down approach.78 Thus, the development of the top-down approach is much slower than that of the bottom-up approach, which will be discussed in the next section.
2.2. Bottom-up approach
The bottom-up approach, which fabricates the 2D MOF nanosheets by directly assembling the organic linkers and metal ions via coordination interactions, has been intensively investigated in the past few years because it can facilely control the growth of the 2D MOF nanosheets with various functional metal nodes and thicknesses. A key point of the bottom-up approach for the fabrication of 2D MOFs is to control the growth rate of the MOF crystals along certain directions. Compared to the top-down approach, the bottom-up approach is considered to be more efficient to fabricate the 2D MOFs with better homogeneity.79 Based on the synthesis mechanism, the bottom-up approach can be classified into four categories: template-assisted method, interface or intermediate layer growth method, surfactant-assisted method, and molecule-precoordinated method.The hard-template method is a conventional method to controllably deposit the target materials on an unreacted substrate, in which the morphology of the target materials could be regulated by the surface properties of the substrate. A great advantage of the hard-template method for the synthesis of 2D MOFs is that by selecting suitable substrates the growth of the 2D MOFs can be controlled as nanosheet arrays,84,85 which is significant for their application in gas separation. Besides, the introduction of the conductive substrates could also overcome some shortcomings of the 2D MOFs such as low conductivity and agglomeration. For instance, Li and coworkers chemically deposited 2D leaf-like ZIF (ZIF-L) arrays on a halloysite nanotube (HNT) coated PAN membrane.84 As shown in Fig. 5a, in order to controllably grow the ZIF-L nanosheets, a uniformly oriented poly(sodium-p-styrenesulfonate)–HNT (PSS–HNT) layer was coated on the PAN membrane with PVA as the binder, then the ZIF-L was selectively deposited on the PSS–HNT-coated PAN membrane. More importantly, the authors found that the good arrangement of the HNTs was a vital factor for obtaining the ZIF-L nanosheet arrays, which can regulate the growth direction of the ZIF-L nanosheets due to the abundant surface functional groups and large surface area of the HNTs. The SEM (Fig. 5b) and EDS mapping (Fig. 5c) images show that the ZIF-L nanosheets are vertically grown on the HNT-coated PAN membrane. However, the thickness of the ZIF-L nanosheets prepared by this method is about 80 nm due to that there is no sufficient force to restrict the growth along the out-of-plane orientation of the nanosheets, which is a common shortcoming for the hard-template method to prepare the 2D materials.86
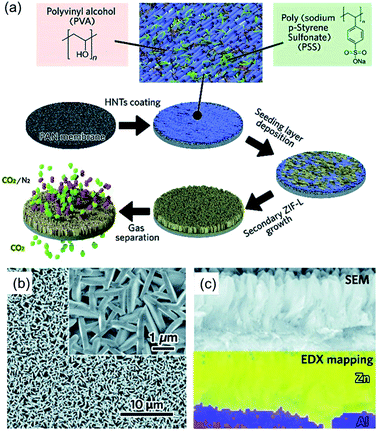 | ||
| Fig. 5 (a) Schematic illustration for the preparation of the PAN–HNTs–ZIF-L membrane. (b) SEM image of the top-surface of the PAN–HNTs–ZIF-L membrane, and (c) EDS mapping and reference SEM image of the PAN–HNTs–ZIF-L membrane cross-sectional region. Reproduced with permission.84 Copyright©2019 The Royal Society of Chemistry. | ||
The sacrificial-template method, such as the in situ conversion of 2D metal oxides or hydroxides to 2D MOF nanosheets, has also been intensively investigated in recent years. Wang and coworkers developed an in situ transformation strategy to synthesize ultrathin bimetal-MOF nanosheet (BMNS) arrays from layered double hydroxide nanosheets (LDH NSs) on conductive substrates (Fig. 6a).87 Specifically, the LDH NSs were pre-deposited on the carbon clothes (Fig. 6b) and worked as a self-sacrificial template providing metal ions for the growth of BMNSs. The as-prepared LDH NSs were placed in 4 mL of DMF/H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixed solvent containing a certain amount of H2BDC as the ligand. The deprotonated H+ from H2BDC could slowly react with the LDH NSs to release the metal ions which were in situ coordinated with the BDC linkers to form the ultrathin NiCo-BDC BMNSs (Fig. 6c). The XRD pattern (Fig. 6d) and TEM images (Fig. 6e and f) reveal the successful preparation of the BMNSs. The thickness of the BMNSs measured by AFM is about 5 nm (Fig. 6g). Moreover, the authors also found that the water content in the solution in which the conversion occurred was vital to the thickness of the BMNSs (Fig. 6g–i). This sacrificial-template method is considered as a general method for the conversion of metal LDH or metal oxide NSs to the M-MOF nanosheets (M refers to the metal in the precursors), which has been used in the fabrication of other MOF nanosheets from the corresponding metal LDH and oxide nanosheets.88
1, v/v) mixed solvent containing a certain amount of H2BDC as the ligand. The deprotonated H+ from H2BDC could slowly react with the LDH NSs to release the metal ions which were in situ coordinated with the BDC linkers to form the ultrathin NiCo-BDC BMNSs (Fig. 6c). The XRD pattern (Fig. 6d) and TEM images (Fig. 6e and f) reveal the successful preparation of the BMNSs. The thickness of the BMNSs measured by AFM is about 5 nm (Fig. 6g). Moreover, the authors also found that the water content in the solution in which the conversion occurred was vital to the thickness of the BMNSs (Fig. 6g–i). This sacrificial-template method is considered as a general method for the conversion of metal LDH or metal oxide NSs to the M-MOF nanosheets (M refers to the metal in the precursors), which has been used in the fabrication of other MOF nanosheets from the corresponding metal LDH and oxide nanosheets.88
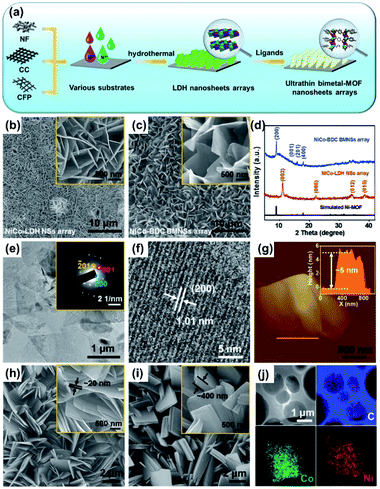 | ||
Fig. 6 (a) Schematic illustration showing the fabrication of ultrathin BMNS arrays on the 3D conductive matrix. SEM images of (b) NiCo–LDH NSs and (c) NiCo-BDC BMNS arrays (the insets are the corresponding high-magnification SEM images). (d) XRD patterns of NiCo-BDC BMNS arrays and pristine NiCo–LDH NS array. (e) TEM, (f) HRTEM, (g) AFM images and (j) EDS mapping of NiCo-BDC BMNS. (Inset of (e): the SAED pattern of a single BMNS; inset of (g): the corresponding height profile measured along the yellow line). SEM images of NiCo-BDC BMNSs with different thicknesses synthesized at (h) DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 6 H2O = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (i) DMF 1 and (i) DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 4 H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (the insets are the high-magnification SEM images). Reproduced with permission.87 Copyright©2019 WILEY-VCH Verlag GmbH & Co. KGaA. 1 (the insets are the high-magnification SEM images). Reproduced with permission.87 Copyright©2019 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
In 2010, Makiura invented a liquid–liquid interfacial growth method and prepared a preferentially oriented MOF nanofilm on a solid surface.90 As shown in Fig. 7, the synthesis procedure involved spreading a solution of the molecular building units, 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrinato-cobalt(II) (CoTCPP) and pyridine (py), in chloroform/methanol onto an aqueous solution of CuCl2·2H2O, leading to the formation of a 2D copper-mediated CoTCPP array (CoTCPP-py-Cu) at the liquid–liquid interface.90 Then, the single-layer CoTCPP-py-Cu was layer-by-layer transferred to the surface of a Si(001) substrate, resulting in a nanofilm constructed with highly oriented 2D CoTCPP-py-Cu MOF nanosheets. Later, the Kitagawa group developed a variety of interfacial synthetic methods combining the Langmuir–Blodgett method.91–93 The key point of these methods is to well disperse the oil layer containing organic linkers on the surface of an aqueous solution containing the metal ions, while the 2D MOFs could form as the metal ions diffuse to the oil layer and coordinate with the organic linkers.
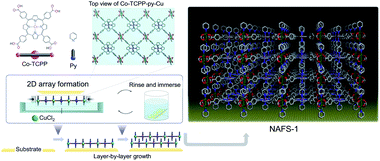 | ||
| Fig. 7 Schematic illustration of fabrication method of NAFS-1. Reproduced with permission.90 Copyright©2010 Nature Publishing Group. | ||
Nowadays, the liquid–liquid interfacial growth strategy is the most useful method among all the reported two-phase interfacial growth methods for the 2D MOFs. Huang and co-workers demonstrated that a 2D π–d conjugated MOF, Cu-BHT (BHT = benzenehexathiol), could be assembled at the interface of the BHT/dichloromethane and aqueous copper(II) nitrate solutions, as shown in Fig. 8a and b.94 The SEM images (Fig. 8c–e) show that the Cu-BHT film with a thickness of about 200 nm has been constructed by the stacking of the Cu-BHT nanosheets. More attractively, the conductivity of this as-assembled Cu-BHT film at room temperature could reach up to 1580 S cm−1, which was the highest value ever reported for the conductive coordination polymers. These highly conductive 2D Cu-BHT nanosheets delivered excellent performance in electrochemical energy storage and electrocatalysis. However, this liquid–liquid method has an obvious shortcoming, that is, the later-formed Cu-BHT nanosheets are less ordered, leading to a rougher surface and lower conductivity (Fig. 8d and e). The authors mainly attributed this issue to the weaker interface-confining effect when the film grew thicker (Fig. 8f). To minimize the possible negative impact of the disordered arrangement of the Cu-BHT nanosheets, the reaction time should be carefully controlled to obtain the Cu-BHT film with an ordered surface. As discussed above, the liquid–liquid interfacial growth is a handy and concise method for directly assembling the 2D MOF nanosheets by only using the metal ions and organic linkers in suitable solvents. Unfortunately, the yield of the 2D MOF nanosheets by using the liquid–liquid interfacial growth method is usually quite low due to the small reaction area, which hinders their further applications.
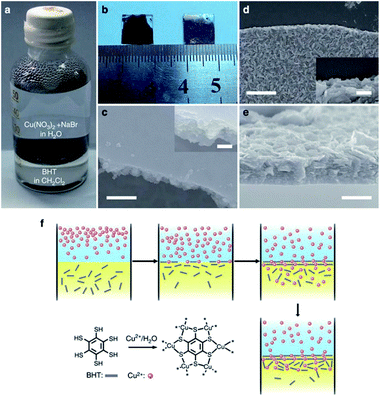 | ||
| Fig. 8 (a) Photograph of the Cu-BHT film forming at the interface between an aqueous solution and CH2Cl2. (b) Photograph of upside-up (right) and upside-down (left) films transferred on glass substrates. The SEM image of (c) upside, (d) downside surface and (e) cross-section of a 200 nm-thick film. The insets in (c and d) show the details of the film edge at an enlarged scale. Scare bar, 200 nm in (c), 400 nm in (d and e) and 100 nm in the insets. (f) Scheme of the formation of Cu-BHT film. Reproduced with permission.94 Copyright©2015 Nature Publishing Group. | ||
To overcome the low yield of the 2D MOF nanosheets due to the small reaction area in the liquid–liquid interfacial growth method,95,96 Rodenas and co-workers presented an intermediate layer growth method, in which the reaction solution consisted of three different liquid layers vertically stacked according to their densities, to generate 2D MOF nanosheets in the intermediate miscible co-solvent layer (Fig. 9). To emphasize this approach, the synthesis of the Cu-based 1,4-benzenedicarboxylate (CuBDC) MOF is selected as a showcase.97 In this case, the upper solution layer containing Cu(NO3)2 (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 1
CH3CN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) and the lower solution layer containing BDC (DMF
2, v/v) and the lower solution layer containing BDC (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 2
CH3CN = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were separated by an intermediate solution layer (DMF
1, v/v) were separated by an intermediate solution layer (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN = 1
CH3CN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 9a). Under static conditions, the Cu2+ ions and BDC linker molecules slowly diffused into the intermediate solution layer, where the CuBDC MOF crystals formed locally in a highly diluted medium. This intermediate solution layer provided a sufficient reaction zone to form 2D CuBDC MOFs and the nascent 2D CuBDC crystals were naturally removed from the reaction zone by sedimentation, after which further growth of the CuBDC crystals was inhibited in the Cu2+-depleted underlying organic phase (Fig. 9b). The SEM (Fig. 9c) and AFM (Fig. 9d) results show that the as-fabricated Cu-BDC nanosheets have lateral dimensions of 0.5–4 μm and thicknesses in the range of 5–25 nm. Besides, this intermediate layer growth method could also be extended to synthesize other ultrathin 2D MOFs, such as nanosheets of Zn-BDC, Co-BDC, copper 1,4-naphthalenedicarboxylate (Cu(1,4-NDC)), and copper 2,6-naphthalenedicarboxylate (Cu(2,6-NDC)).97
1, v/v) (Fig. 9a). Under static conditions, the Cu2+ ions and BDC linker molecules slowly diffused into the intermediate solution layer, where the CuBDC MOF crystals formed locally in a highly diluted medium. This intermediate solution layer provided a sufficient reaction zone to form 2D CuBDC MOFs and the nascent 2D CuBDC crystals were naturally removed from the reaction zone by sedimentation, after which further growth of the CuBDC crystals was inhibited in the Cu2+-depleted underlying organic phase (Fig. 9b). The SEM (Fig. 9c) and AFM (Fig. 9d) results show that the as-fabricated Cu-BDC nanosheets have lateral dimensions of 0.5–4 μm and thicknesses in the range of 5–25 nm. Besides, this intermediate layer growth method could also be extended to synthesize other ultrathin 2D MOFs, such as nanosheets of Zn-BDC, Co-BDC, copper 1,4-naphthalenedicarboxylate (Cu(1,4-NDC)), and copper 2,6-naphthalenedicarboxylate (Cu(2,6-NDC)).97
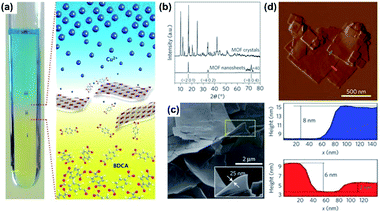 | ||
| Fig. 9 (a) Picture showing the spatial arrangement of different liquid layers during the synthesis of CuBDC MOF nanosheets. Layers labelled (i), (ii) and (iii) correspond to a benzene 1,4-dicarboxylic acid (BDCA) solution, the solvent spacer layer and the solution of Cu2+ ions, respectively. (b) X-ray diffractograms (Cu K radiation) for the bulk-type and nanosheet CuBDC MOF. (c and d) Scanning electron micrograph and atomic-force micrograph (with corresponding height profiles), respectively, for CuBDC MOF nanosheets synthesized as illustrated in (c). The height profiles, color-coded red and blue, are measured along the corresponding tracks shown in the atomic-force micrograph. Reproduced with permission.97 Copyright©2010 Nature Publishing Group. | ||
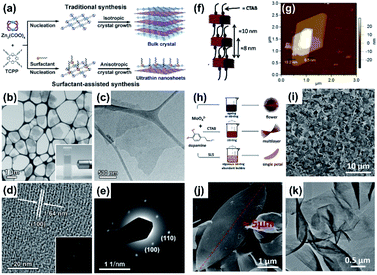 | ||
| Fig. 10 PVP-assisted synthesis of Zn-TCPP nanosheets. (a) Schematic illustration of the traditional synthesis and surfactant-assisted synthesis of the Zn-TCPP MOF. (b) STEM image of Zn-TCPP nanosheets obtained by SEM with a transmission electron detector. Inset: Tyndall effect of colloidal Zn-TCPP nanosheets in ethanol. (c) TEM image of a single Zn-TCPP nanosheet. (d) HRTEM image of Zn-TCPP nanosheets and corresponding FFT pattern (inset). (e) SAED pattern of Zn-TCPP nanosheets in (c). CTAB-assisted synthesis of Zn-MOF nanosheets. Reproduced with permission.86 Copyright©2015 WILEY-VCH Verlag GmbH & Co. KGaA. (f) Schematic illustration and (g) AFM images of the Zn-MOF nanosheets. Reproduced with permission.66 Copyright©2013 American Chemical Society. CTAB and SLS assisted preparation of Mo–polydopamine nanosheets. (h) Schematic illustration, (i and j) SEM images, and (k) TEM image of Mo–polydopamine nanopetals. Reproduced with permission.98 Copyright©2018 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
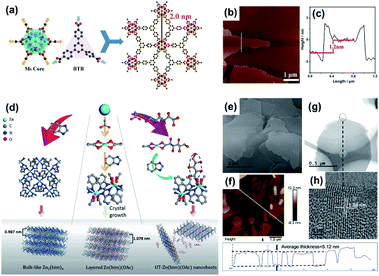 | ||
| Fig. 11 (a) Schematic illustration, (b) AFM, and (c) height profile of Hf6(μ3-O)4(μ3-OH)4-(HCO2)6(BTB)2 MOF nanosheets. Reproduced with permission.100 Copyright©2016 WILEY-VCH Verlag GmbH & Co. KGaA. (d) Schematic illustrations of the synthesis of Zn2(bim)4, Zn(bim)(OAc), and UT-Zn(bim)(OAc). (e) SEM, (f) AFM and height profile, (g) TEM, and (h) HRTEM images of UT-Zn(bim)(OAc). Reproduced with permission.101 Copyright©2018 The Royal Society of Chemistry. | ||
Recently, our group developed a series of small molecule-precoordinated methods for the synthesis of 2D MOF nanosheets.101–103 The key to this method is to pre-coordinate the metal ions with certain small molecules, consequently slowing down the reaction rate between the metal ions and organic linkers. Besides, the small molecules attached on the surface of the as-prepared MOF nanosheets could decrease the surface energy and then promote the formation of individual MOF nanosheets. For example, gluconate, a hydrophilic short-chain carboxylic acid that could pre-coordinate with Zn2+, was used as the crystal growth inhibitor to synthesize ultrathin Zn(bim)(OAc) in a water/ethanol mixed solution. As shown in Fig. 11d, each layer of the 2D Zn(bim)(OAc) MOFs is constructed by two kinds of chains (i.e., Zn2(bim)4 chains along the b axis and Zn2(OAc)4 chains along the c axis) cross-connected by Zn2+.101 During the reaction, gluconate partially replaced OAc−, which could effectively break the interaction between the adjacent Zn(bim)(OAc) layers. The SEM (Fig. 11e), AFM (Fig. 11f), and TEM (Fig. 11g and h) images demonstrate the formation of the individual Zn(bim)(OAc) MOF nanosheets. The height profile measured from the AFM image implies that the Zn(bim)(OAc) nanosheets have an average thickness of about 5.12 nm. In addition, the yield of the 2D Zn(bim)(OAc) nanosheets is as high as 65% without forming any bulk MOF crystals, which provides a new approach to producing high-quality 2D MOFs. In addition to the gluconate, sucrose and pyridine105 are also effective pre-coordination reagents for the molecule-precoordinated synthesis of 2D MOFs.
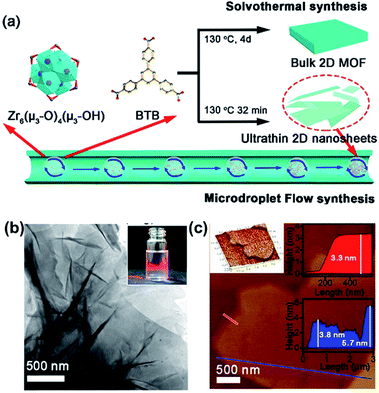 | ||
| Fig. 12 (a) Schematic illustrations of the overall process developed to produce 2D MF-ZrBTB and ST-ZrBTB via microdroplet flow reaction and conventional solvothermal methods. (b) TEM image of MF-ZrBTB. The inset shows the Tyndall light scattering effect of MF-ZrBTB colloidal suspension. (c) AFM image and corresponding height profiles of MF-ZrBTB nanosheets along the red and blue lines. Reproduced with permission.104 Copyright©2018 American Chemical Society. | ||
| Method | Top-down approach | Bottom-up approach | |||||
|---|---|---|---|---|---|---|---|
| Physical exfoliation | Chemical/electrochemical exfoliation | Template-assisted | Two-phase interfacial growth | Intermediate layer growth | Surfactant-assisted | Molecule-precoordination | |
| Thickness | Thin | Thin | Medium | Thin | Medium | Ultrathin | Ultrathin |
| Uniformity | Low | Low | Good | Good | Good | Excellent | Excellent |
| Lateral size | Medium | Small | Medium | Large | Large | Small | Small |
| Condition | Mild | Mild | Harsh | Harsh | Harsh/medium | Medium | Medium |
| Yield | Medium | Medium | Low | Low | Medium | High | High |
| Defect | Abundant | Abundant | Low | Low | Low | Medium | Medium |
| Application | Gas separation | Catalysis | Gas adsorption, catalysis | Gas separation | Gas separation, photocatalysis | DNA detection, LIBs, catalysis, derivatives | Derivatives |
| Reference | 22, 65, 69, 71 and 73 | 74, 75 and 77 | 84 and 87 | 76, 90, 91 and 94 | 96 and 97 | 86, 98 and 99 | 100 and 101 |
3. 2D MOFs for electrochemical energy storage and electrocatalysis
3.1. Application of 2D MOFs for energy storage
2D MOF nanosheets with a large content of Li+ storage sites have been directly used as the anode materials in LIBs. For instance, porous [Mn(tfbdc)(4,4′-bpy)(H2O)2] (Mn-LCP) nanosheets were fabricated by mixing 2,3,5,6-tetrafluoroterephthalatic acid (H2tfbdc), 4,4′-bipyridine (4,4′-bpy), and manganese(II) acetate tetrahydrate in D.I. water for five days. Based on the FT-IR, XPS, and magnetic studies, the lithium storage in the Mn-LCP nanosheets could be attributed to the substitution of the Mn(II) ions for Li+.112 However, the structure destruction of the Mn-LCP nanosheets caused by the Li+ substitution led to a quite poor cycling performance (with a capacity retention of 76.6% after 50 cycles) and low first-cycle coulombic efficiency (31.5%). Similar to the Mn-LCP, a hierarchical tremella-like 2D Al-MOF (aluminum fumarate), fabricated using Al(NO3)3·9H2O and AlCl3·6H2O, exhibited a specific capacity of 392 mA h g−1 after 100 cycles at 37.5 mA g−1.113 Obviously, the capacity of these MOF nanosheets as the LIB anodes arises from the redox reaction of the metal sites, which causes serious structural destruction and low stability of these MOF anodes. Thus, employing more nonstructural sites for Li+ storage should enhance the stability and capacity of the MOF anodes. To date, two simple methods have been employed to modify 2D MOF-based LIB anodes. One is the intercalation of the metal ions (e.g., FeIII ions) with variable valences into the MOFs, which could greatly enhance the specific capacity of the MOF-based electrode materials.114 Besides these metal centers as the active sites, some of the uncoordinated ligands could also undergo redox reactions to store the lithium ions. Li and coworkers synthesized manganese-based ultrathin MOF nanosheets (Mn-UMOFNs) from BDC and MnCl2via an ingenious ultrasonication route (Fig. 13a).115 Mn-UMOFNs with thicknesses of 1.8–7.0 nm (mostly <3 nm) (Fig. 13b) showed a high capacity (1187 mA h g−1 at 100 mA g−1 for 100 cycles) and ultrahigh rate performance (701 mA h g−1 at 2 A g−1) (Fig. 13c). The excellent rate performance of the Mn-UMOFNs could be attributed to their ultrathin morphology which could greatly enhance the diffusion of Li+. The high capacity was attributed to the two-step lithium intercalation on the Mn-UMOFNs, i.e. the first step involved a reduction process of Mn2+ to Mn0 (at above 0.5 V) and the second step involved a process at a low voltage (below 0.5 V) in which the coordinated oxygen atoms with high electronic density as well as the benzene rings with delocalized π electrons work as the active sites for lithium intercalation (Fig. 13d). However, most of the synthesized 2D MOFs have a relatively low conductivity and therefore cannot meet the requirements of the high-rate charge–discharge. Coupling the 2D MOFs with conductive substrates could alleviate this problem. Jin and co-workers reported a hybrid material of 2D NiCo-MOFs and black phosphorus (BP).116 The bulk BP was firstly exfoliated to few-layer BP nanosheets. Then the few-layer BP nanosheets were directly added into the mixed solution of Co2+, Ni2+, and BDC (Fig. 13e). After continuous sonication in an ice bath for 8 h, BP/NiCo-MOFs were successfully obtained. The SEM (Fig. 13f) and TEM images (Fig. 13g and h) demonstrate that this BP/NiCo MOF hybrid has a nanosheet morphology with a thickness of ∼6 nm. The EDS mapping analysis (Fig. 13i) of the BP/NiCo-MOF hybrid nanosheets evidences that the NiCo-MOFs uniformly form a cover on the BP nanosheets. The synthesized BP/NiCo-MOF nanosheets exhibited a high discharge capacity of about 2483 mA h g−1 at 0.1 mA g−1 in the first cycle and a high reversible capacity (853 mA h g−1) at 0.5 A g−1 (Fig. 13j). Moreover, the hybrid 2D MOFs also exhibited excellent rate performance (with a high reversible capacity of 436 mA h g−1 at 5 A g−1) and high capacity retention of 398 mA h g−1 after 1000 cycles at the charge–discharge current density of 5 A g−1 (Fig. 13k). The superior electrochemical performance of the BP/NiCo-MOF hybrid nanosheets can be ascribed to the synergistic effects of the few-layer BP and NiCo-MOF nanosheets, i.e., BP and NiCo-MOF both provide sufficient redox-active sites, while the few-layer structure and high conductivity of the BP provide short diffusion paths and fast electron-charge transfer.
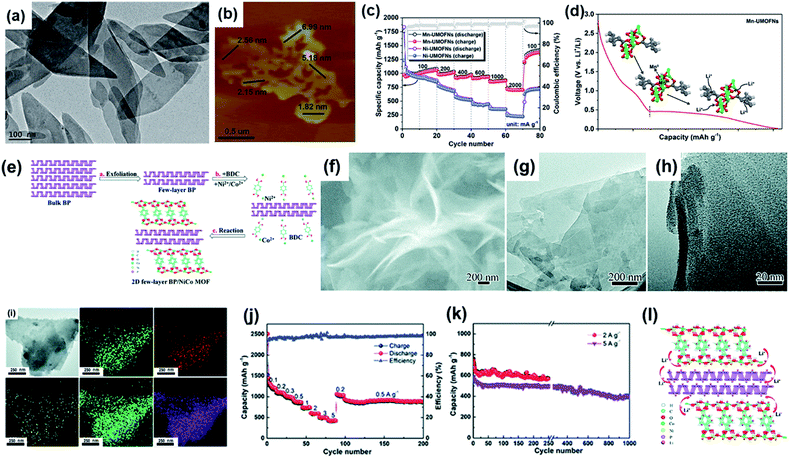 | ||
| Fig. 13 (a) TEM and (b) AFM images of Mn-UMOFNs. (c) Rate performance of Mn-UMOFNs and Ni-UMOFNs at various current densities from 100 to 2000 mA g−1. (d) Proposed electrochemical redox action mechanism of Mn-UMOFNs. Reproduced with permission.115 Copyright©2017 American Chemical Society. (e) Illustration of the preparation process of the 2D BP/NiCo MOF hybrid. (f) SEM image, (g and h) TEM images, (i) EDS mapping, (j) rate performance, (k) cycle performance, and (l) illustration of the Li+ insertion of the BP/NiCo MOF NSs. Reproduced with permission.116 Copyright©2019 The Royal Society of Chemistry. | ||
Co(II)–L + OH−![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Co(III)(OH)–L + e− Co(III)(OH)–L + e− | (1) |
Co(III)(OH)–L + OH−![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Co(IV)–O–L + H2O + e− Co(IV)–O–L + H2O + e− | (2) |
In addition, Abazari and coauthors reported a fast and simple technique to synthesize S2−-modified layered MOF nanosheets ([Co(4,4′-oxybis(benzoic acid))2(N,N′-bis-(4-pyridylformamide)-1,4-benzenediamine)4](DMF)2, S-TMU-61) (Fig. 14a),118 which could also be employed as the SC electrode material and exhibited an ultrahigh capacitance of 636.6 F g−1 at 5 A g−1 (Fig. 14b–d). The asymmetric SC (ASC) assembled by using S-TMU-61 as the electrode material showed a high energy density (25.73 W h kg−1) and power density (2549.3 W kg−1). The capacitance of these kinds of MOFs was mainly attributed to the pseudo-capacitance of the redox reaction of the metal centers. Therefore, the number of active sites for the charge storage and the conductivity of the 2D MOFs are essential for their capacitance and rate performance, which are still challenges in the development of 2D MOF-based SCs.
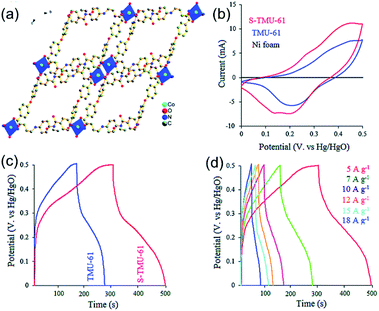 | ||
| Fig. 14 (a) Topological diagram of TMU-61. (b) CV curves of TMU-61, S-TMU-61, and Ni foam at a scan rate of 25 mV s−1, (c) GCD curves of TMU-61 and S-TMU-61 electrode materials at a current density of 5 A g−1 and (d) GCD curves of S-TMU-61 at different current densities. Reproduced with permission.118 Copyright©2019 The Royal Society of Chemistry. | ||
Conductive MOFs, a series of conjugated planar polymers, are considered as the next-generation energy storage materials due to the combined advantages of MOFs and conductive materials. Their active sites and structural properties could be flexibly adjusted to match the requirements of different types of energy storage devices. The Bao group designed two kinds of conductive 2D MOFs (Fig. 15a), i.e., M-HAB (M = Cu, Ni, and Co; HAB = hexaaminobenzene) and Cu-HHB (HHB = hexahydroxybenzene).119,121,122 Among them, conductive M-HABs with redox-active sites were investigated as the electrode materials of SCs. The HRTEM and HAADF-STEM images of the as-prepared Cu-HAB clearly show pores smaller than 1 nm with a honeycomb arrangement along the [001] direction with d100 = 1.15 nm (Fig. 15b–g). Since the HAB linkers prefer to coordinate with d8 and d9 metal species with a square-planar coordination geometry, which could well maintain the highly ordered pore channels along the (001) direction, M-HABs display excellent diffusion ability as the SC electrode. Ni-HAB and Cu-HAB exhibited high gravimetric capacitances of 420 F g−1 and 215 F g−1 at the scan rate of 0.2 mV s−1, respectively (Fig. 15h). The higher capacitance of Ni-HAB could be attributed to the oxidation of Ni(II) to a higher valence state (Ni(IV)), which possessed a larger pseudocapacitance compared to Cu-HAB. Another important advantage of the Ni-HAB electrode was that the assembled 2D conductive MOF sample had a high volumetric density of 1.8 g cm−3, which led to a volumetric capacitance as high as 760 F cm−3 (Fig. 15h) and an areal capacitance of 22.5 F cm−2 at 0.2 mV s−1. This volumetric capacitance is abnormally high for the electrode with a thickness of 50 μm and almost surpasses the best values of the carbon electrodes reported for SCs (Fig. 15k).1,5,123,124 Ni-HAB also possessed high stability with capacitance retention of 90% after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at the charge–discharge current density of 10 A g−1 (Fig. 15j). Besides these hexasubstituted benzene based conductive MOFs, 2D Ni3(HITP)2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene) with a remarkable bulk conductivity (5000 S m−1) has also exhibited excellent capacitive performance with a capacitance of 111 F g−1 at 0.05 A g−1 and 90% capacitance retention after 10
000 cycles at the charge–discharge current density of 10 A g−1 (Fig. 15j). Besides these hexasubstituted benzene based conductive MOFs, 2D Ni3(HITP)2 (HITP = 2,3,6,7,10,11-hexaiminotriphenylene) with a remarkable bulk conductivity (5000 S m−1) has also exhibited excellent capacitive performance with a capacitance of 111 F g−1 at 0.05 A g−1 and 90% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 2 A g−1.125
000 cycles at a current density of 2 A g−1.125
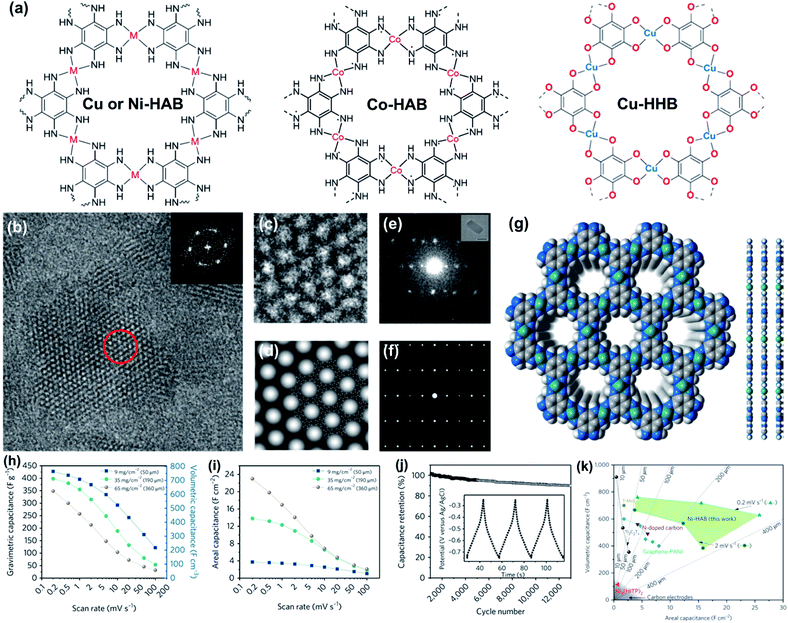 | ||
| Fig. 15 (a) 2D MOFs in M3(C6X6)2 (M = Cu, Co, and Ni. X = NH, S, and O) family. (b) HR-TEM image of Cu-HAB along [001] that shows a hexagonal pore packing with d 100 = 1.15 nm (a = 1.33 nm). Scale bar, 10 nm. Inset: Fourier transform of the image. (c) HR-TEM image of the region within the red circle in (b). (d) Symmetry-imposed and lattice-averaged image calculated from the HR-TEM image. Embedded is the eclipsed structure model of Cu-HAB. Scale bar, 2 nm. Experimental (e) and simulated (f) electron-diffraction pattern (eclipsed model) of Cu-HAB viewed along [−110]. Scale bar 5 nm−1. Inset (e): the crystal from which the electron-diffraction pattern was taken. Scale bar, 50 nm. (g) A space-filling model of the Cu-HAB model. Blue, grey, green and white spheres represent N, C, Ni and H atoms, respectively; a.u.: arbitrary units. (h) Gravimetric and volumetric rate performance for Ni-HAB pellet electrodes with different areal densities of 9, 35, and 65 mg cm−2. (i) Areal rate performance for the Ni-HAB electrodes with different weight loadings. (j) Capacitance retention data collected by galvanostatic charge–discharge at 10 A g−1. Inset: galvanostatic charge–discharge profiles. (k) Comparison of the volumetric and areal capacitances of Ni-HAB additive-free electrodes (green area) with the performance of other materials. Reproduced with permission.119 Copyright©2018 Nature Publishing Group. | ||
3.2. Applications of 2D MOFs in electrocatalysis
The electrochemical catalytic reactions are types of surface reactions, in which the catalytic performance of the catalysts greatly depends on their surface properties. 2D MOFs, with the advantages of high active surface areas and flexible surface chemical compositions, are regarded as promising catalysts. Although the poor stability and low conductivity of some easily prepared 2D MOFs have limited their applications in electrocatalysis, recent work has demonstrated that the applications of the 2D MOFs as the electrocatalysts for the OER, ORR, HER, and CO2RR could be realized by constructing 2D MOFs/conductive substrate composites or by using conductive 2D MOFs.| Material/substrate | Application | Loading (mg cm−2) | Electrolyte | η (mV) @ 10 mA cm−2 | Tafel slope (mV dec−1) | Catalytic stability | Ref. |
|---|---|---|---|---|---|---|---|
| Co2(OH)2BDC/GC | OER | 0.25 | 1 M KOH | 263 | 74 | ∼5% degradation for 4 h at 1.5 V | 131 |
| NiCo-UMOFNs/GC | OER | 0.2 | 1 M KOH | 250 | 42 | Almost unchanged for 3 h at 1.5 V | 134 |
| Ni-MOF@Fe-MOF/GC | OER | 0.2 | 1 M KOH | 265 | 54 | N/A | 138 |
| NiFe-UMNs/GC | OER | 0.4 | 1 M KOH | 260 | 30 | Almost unchanged for 3 h at 1.5 V | 139 |
| Co-ZIF9/GC | OER | 0.21 | 1 M KOH | 380 | 55 | ∼6% degradation for 10 h at 1.7 V | 140 |
| MOF/OM-NFH/GC | OER | 0.4 | 1 M KOH | 270 | 123 | ∼18% degradation for 11 h at 1.5 V | 132 |
| MIL-53(FeNi)/NF | OER | N/A | 1 M KOH | 233 @ 50 mA cm−2 | 31.3 | Almost unchanged for 4 h at 1.45 V | 133 |
| NiFe0.2Co0.3-ZIF/NF | OER | N/A | 1 M KOH | 216 @ 100 mA cm−2 | 23.25 | Almost unchanged for 20 h at 1.52 V | 141 |
| Co-BTH/GC | HER | N/A | H2SO4 (pH = 1.3) | 185 | 88 | N/A | 142 |
| Ni-THT/GC | HER | N/A | 0.5 M H2SO4 | 330 | 80.5 | N/A | 143 |
| Co-THTA | HER | N/A | 0.5 M H2SO4 | 283 | 74 | Unchanged for 4 h at 10 mA cm−2 | 144 |
| UiO-66-NH2-Mo/GC | HER | N/A | 0.5 M H2SO4 | 125 | 59 | Almost unchanged for 7 h at −0.2 V | 145 |
| Ni0.8Fe0.2-MOF/NF | OER | N/A | 0.1 M KOH | 240 | 34 | ∼3% degradation for 5.5 h at 1.42 V | 146 |
| HER | 134 | N/A | Almost unchanged for 5.5 h at −0.2 V | ||||
| NiFeZn-MNS/NF | OER | N/A | 1 M KOH | 308 @ 50 mA cm−2 | 49 | ∼5% degradation for 120 h at 1.62 V | 147 |
| HER | 180 | N/A | N/A | ||||
| IrO2 | OER | N/A | 0.5 M KOH | 411 | 91 | N/A | 148 |
| RuO2 | OER | N/A | 0.5 M KOH | 358 | 55 | N/A | 148 |
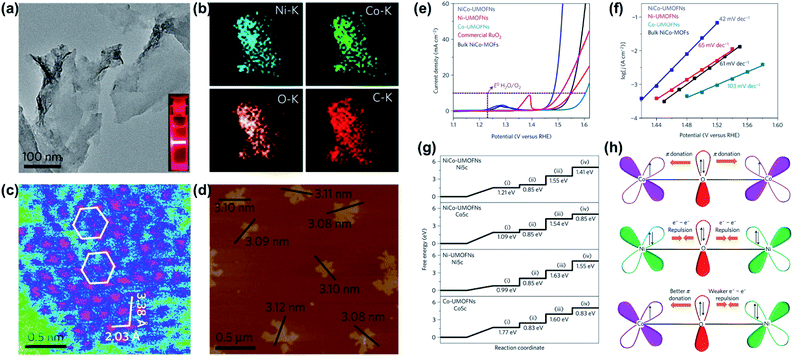 | ||
| Fig. 16 (a) TEM image of NiCo-UMOFNs. The inset shows the Tyndall light scattering of NiCo-UMOFNs in an aqueous solution. (b) TEM-EDS mapping images of NiCo-UMOFNs. (c) HAADF-STEM image of the (200) plane for NiCo-UMOFNs showing the hexagonal arrangement of the metal atoms. The pink colour represents metal atoms, blue is for light elements (carbon and oxygen), and green is for the background. (d) AFM image of as-prepared NiCo-UMOFNs, showing measured dimensions of individual flakes. (e) Polarization curves of NiCo-UMOFNs, Ni-UMOFNs, Co-UMOFNs, RuO2 and bulk NiCo-MOFs in O2-saturated 1 M KOH solution at a scan rate of 5 mV s−1. (f) Tafel plots of NiCo-UMOFNs, Ni-UMOFNs, Co-UMOFNs and bulk NiCo-MOFs derived from Koutecky–Levich plots in O2-saturated 1 M KOH solution. (g) Standard free energy diagram of the OER process on UMOFNs surfaces. (h) Schematic representation of the electronic coupling between Co and Ni in UMOFNs. Reproduced with permission.134 Copyright©2016 Nature Publishing Group. | ||
Recently, several highly stable conductive 2D MOFs were also employed to catalyze the ORR. As shown in Fig. 17a–c, 2D Ni3(HITP)2 with a highly conjugated framework and a striking conductivity was used as the electrocatalyst for the ORR and exhibited an onset potential of 0.82 VRHE.159 More recently, Feng and coworkers prepared a series of conjugated MOFs and employed them as the ORR electrocatalysts. For example, a phthalocyanine-based layered 2D conjugated MOF was constructed using copper phthalocyanine (PcCu) and square-planar cobalt bis(dihydroxy) complexes (Co–O4) as the linkages (PcCu–O8–Co).160Fig. 17d displays the structure of PcCu–O8–Co, which was periodically assembled using Co–O4 and PcCu–O4 coordination units. In this kind of MOFs, the square-planar Co–O4 could not only efficiently transfer the electrons between the two conjugated PcCu to construct a large 2D conjugated plane but also work as highly active sites for the ORR due to its specific electronic configuration (with single-electron occupied eg orbitals). Then, the PcCu–O8–Co was composited with CNTs and exhibited further enhanced catalytic performance for the ORR with a E1/2 of 0.83 VRHE and a limiting current density of 5.3 mA cm−2 (Fig. 17e). This work for the first time highlighted 2D MOFs as ORR electrocatalysts and verified that tuning the electronic structures of the 2D MOFs was an effective way to optimize their catalytic activities toward the ORR (Fig. 17f).
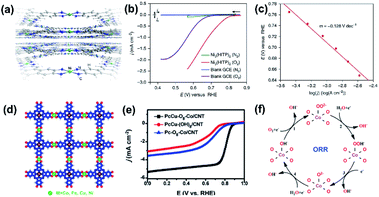 | ||
| Fig. 17 (a) Perspective view of the 2D layered structure of Ni3(HITP)2. (b) Polarization curves of Ni3(HITP)2 in a N2 (green) versus O2 atmosphere (red) as well as of the blank glassy carbon electrode in a N2versus O2 atmosphere (blue and purple, respectively). (c) Activation-controlled Tafel plot for the Ni3(HITP)2-electrocatalyzed ORR. Reproduced with permission.159 Copyright©2016 Nature Publishing Group. (d) Structure of PcM-O8-Co. (e) LSV curves for the as-prepared PcCu–O8–Co/CNTs. (f) Possible reaction route for the ORR in CoO4 sites. Reproduced with permission.160 Copyright©2019 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
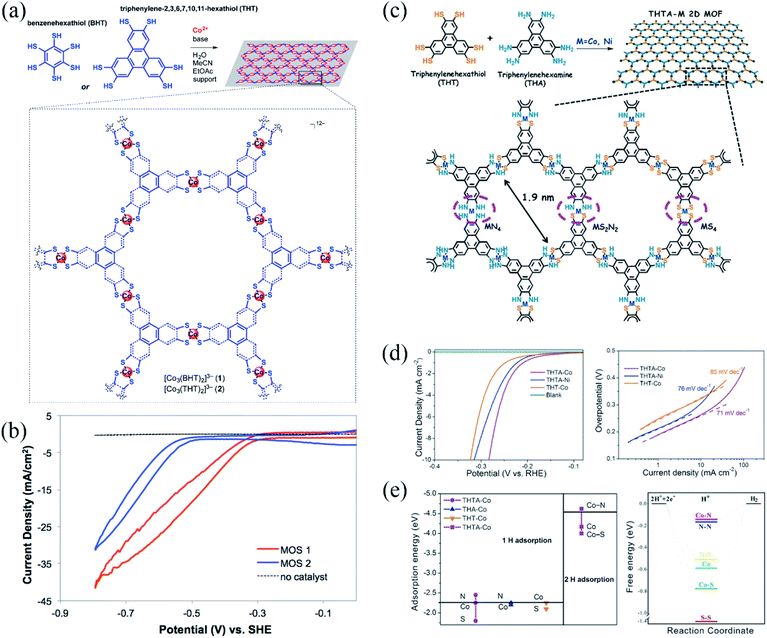 | ||
| Fig. 18 (a) Synthesis of cobalt dithiolene films, Co-BHT and Co-THT, the films are deposited onto the desired supports, generating MOS1 and MOS2; (b) polarization curves of MOS1 (red) and MOS2 (blue) at pH 1.3, scan rate, 100 mV s−1; reproduced with permission.162 Copyright©2015 American Chemical Society. (c) The synthesis of single-layer 2D MOFs, in which MS2N2 complexes along with MN4 and MS4 moieties were incorporated into the hexagonal networks through metal dithiolene–diamine coordination (THTAM) (M = Co and Ni); (d) HER polarization plots (left) of three types of 2D MOFs: THTA-Co (purple), THTA-Ni (blue), and THT-Co (yellow) and a blank glassy carbon disk electrode in 0.5 M H2SO4 and the Tafel plots (right) obtained from the polarization curves; (e) the adsorption energy of one H and two H radicals bonding to the proposed active sites on the surface of THTA-Co, THA-Co, and THT-Co 2D MOFs (left) and the free-energy diagram of the HER at the equilibrium potential for the THTA-Co 2D MOFs with the possible active sites (right). Reproduced with permission.144 Copyright©2017 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
4. 2D MOF derivatives for electrochemical energy storage and electrocatalysis
Although a number of 2D MOFs have been employed as electrochemical energy storage materials and electrocatalysts in the past few years, the unsatisfactory conductivity and stability still hinder their further applications in these fields. Nevertheless, the 2D MOF derivatives (which can be easily obtained from the 2D MOF precursors through a simple thermal treatment in different atmospheres), such as carbon materials, metal, metal compounds, and their composites, with improved physical and chemical properties as well as new functions have attracted increasing attention for electrochemical energy storage and electrocatalysis. In this section, we highlight some promising applications of 2D MOF derivatives in batteries, SCs, OER, HER, ORR, and CO2RR.4.1. 2D MOF derivatives for electrochemical energy storage
2D MOF derivatives with an ultrahigh specific area and well-defined pore distribution demonstrate a large number of active sites for ion storage, exhibiting a great potential for energy storage devices (Table 3). In this section, we highlight some of the 2D MOF derivatives, such as carbon nanosheets, inorganic metal compound nanosheets, and their composites, as the electrode materials in batteries and SCs.| Materials | Application | Thickness/nm | Specific capacity | Rate performance | Cycles | Ref. |
|---|---|---|---|---|---|---|
| NCH | LIB | 3.7 | 609 mA h g−1 at 1 A g−1 | 510 mA h g−1 at 2 A g−1 | 500 | 171 |
| UHCS-900 | LIB | 1–2 | 750 mA h g−1 at 0.5C | 700 mA h g−1 at 1C | 250 | 172 |
| NCW@Fe3O4/NC | LIB | N/A | 1741 mA h g−1 at 1 A g−1 | 723 mA h g−1 at 10 A g−1 | 600 | 25 |
| ZnFe2O4/ZnO NSs | LIB | 2–10 | 537 mA h g−1 at 0.5 A g−1 | 266 mA h g−1 at 1.5 A g−1 | 500 | 173 |
| Co3O4/ZnO | LIB | 109 | 716 mA h g−1 at 1 A g−1 | 404 mA h g−1 at 4 A g−1 | 1000 | 174 |
| CoO@C | LIB | N/A | 991 mA h g−1 at 0.2 A g−1 | 587 mA h g−1 at 2 A g−1 | 300 | 29 |
| NPCNs | SIB | 2.7–6 | 322 mA h g−1 at 0.1 A g−1 | 194 mA h g−1 at 10 A g−1 | 1000 | 175 |
| h-Co3O4 NSs | SIB | 10–30 | 571 mA h g−1 at 0.1 A g−1 | 257 mA h g−1 at 12 A g−1 | 400 | 176 |
| NiSe2/NC | SIB | 6 | 410 mA h g−1 at 1 A g−1 | 255 mA h g−1 at 10 A g−1 | 1000 | 58 |
| NiFe2@NCNs | SIB | 10 | 289 mA h g−1 at 0.1 A g−1 | 268 mA h g−1 at 10 A g−1 | 5000 | 177 |
| Ni(OH)2@ZnCoS NSs | SC | 120 | 2730 F g−1 at 3 mA cm−2 | 390 mF cm−2 at 15 mA cm−2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
33 |
| CoSNC | SC | 42 | 360 F g−1 at 1.5 A g−1 | 205 F g−1 at 30 A g−1 | 2000 | 178 |
| APC | SC | 3.5 | 260 F g−1 at 0.5 A g−1 | 165 F g−1 at 10 A g−1 | 5000 | 47 |
| CC@Co3O4 | SC | 100–150 | 322 F g−1 at 6.25 A g−1 | 260 F g−1 at 100 A g−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
179 |
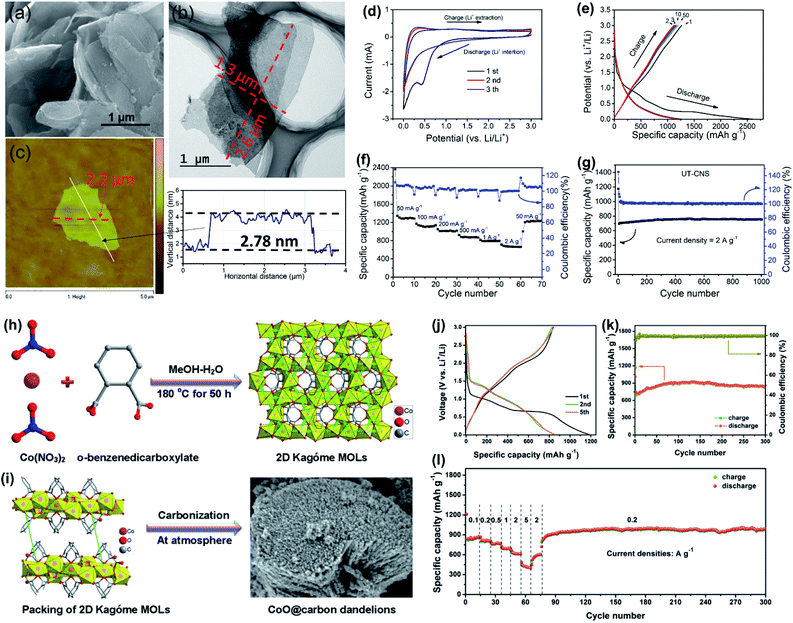 | ||
Fig. 19 Morphologies of UT-CNS and performance of UT-CNS as the anode material for LIBs. (a) SEM, (b) TEM, (c) AFM images with measured dimensions, (d) CV curves at the scan rate of 0.5 mV s−1, (e) charge–discharge profiles at the current density of 100 mA g−1, (f) rate performance at current densities from 100 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA g−1, and (g) cycling performance at the current density of 2 A g−1 for 1000 cycles. Reproduced with permission.101 Copyright©2018 The Royal Society of Chemistry. (h) Schematic illustration of the synthesis process of CoO@carbon dandelions. (i) Stacking of two 2D MOLs in one-unit cell as revealed by X-ray single crystal analysis and FESEM image of the as-synthesized CoO@carbon dandelions. (j) Charge–discharge curves, (k) cycling performance and (l) rate performance of CoO@carbon dandelions. Reproduced with permission.29 Copyright©2018 WILEY-VCH Verlag GmbH & Co. KGaA. 000 mA g−1, and (g) cycling performance at the current density of 2 A g−1 for 1000 cycles. Reproduced with permission.101 Copyright©2018 The Royal Society of Chemistry. (h) Schematic illustration of the synthesis process of CoO@carbon dandelions. (i) Stacking of two 2D MOLs in one-unit cell as revealed by X-ray single crystal analysis and FESEM image of the as-synthesized CoO@carbon dandelions. (j) Charge–discharge curves, (k) cycling performance and (l) rate performance of CoO@carbon dandelions. Reproduced with permission.29 Copyright©2018 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
In addition, porous inorganic metal compounds and their carbon-based composites derived from 2D MOFs have also attracted intensive attention as the anode materials for LIBs in recent years. For instance, 2D mesoporous hetero-ZnFe2O4/ZnO nanosheets (ZFOZ NSs) derived from Zn3[Fe(CN)6] were employed as the anode for LIBs and exhibited long-life cycling behaviors and large reversible capacities at high rates.173 Other 2D MOF-derived metal oxides and their inorganic composites, such as Co3O4,181 Co3O4–NiO,182 Fe3O4@NC,25 and CoO@C,29 also exhibited excellent lithium-ion storage ability. Especially, CoO@C, with the advantages of both metal oxides and carbon materials, showed excellent LIB performance. As shown in Fig. 19h–i, the CoO nanoarrays with a thin carbon layer on the surface (CoO@C) were directly derived from 2D Kagóme MOF nanoarrays. Benefiting from the well-organized core–shell structure, CoO@C exhibited a highly reversible capacity of 1003 mA h g−1 for over 200 cycles at the current density of 0.2 A g−1, superior rate capability (620 mA h g−1 at 2 A g−1), and excellent cycling stability (Fig. 19j–l).
2D MOF derivatives also show great potential for other batteries, such as SIBs, Li–S batteries, and Zn–air batteries.48,183–185 It is well known that the diffusion of Na+ in the electrodes is more difficult than that of Li+ due to the larger radius of Na+.186 Thus, the electrode materials for SIBs require more mesopores and larger interlayer distances for the Na+ intercalation.109,111,187 2D MOF derivatives with an ultrathin morphology and hierarchical pore structure were widely employed as the suitable electrode candidates for SIBs.188 For example, Fang and co-workers fabricated a leaf-like 2D CoZn-MOF, which was then directly converted to a Co3O4/ZnO hybrid.174 Due to the homogeneous distributions of Co3O4 and ZnO, the Co3O4/ZnO hybrid exhibited a high rate property of 242 mA h g−1 at 2 A g−1. Moreover, the harmonious multi-step conversion reaction of Co3O4 and ZnO was helpful for volume buffering, leading to outstanding cycling stability (with capacity retention of 91% after 1000 cycles at 2 A g−1). In addition, this kind of leaf-like 2D Co-MOFs could also be converted to other inorganic metal compounds, which could further boost the performance of SIBs.174 The Wu group developed a versatile and scalable protocol for the synthesis of 2D holey cobalt sulfide (h-Co4S3) from leaf-like 2D Co-MOFs (Fig. 20a–c).176 Benefiting from the 2D nanosheet nature with in-plane mesopores (with a diameter of 2–30 nm), ultrathin morphology (<30 nm), and microscale lateral size providing more active sites and enhanced sodiation/desodiation kinetics, h-Co4S3 exhibited a high reversible capacity of 571 mA h g−1 at 0.1 A g−1 (Fig. 20d). Moreover, the h-Co3S4 had the highest rate capacity of 254 mA h g−1 at a charge–discharge current density of 12 A g−1 among the reported cobalt sulfide-based SIB anodes (Fig. 20e).
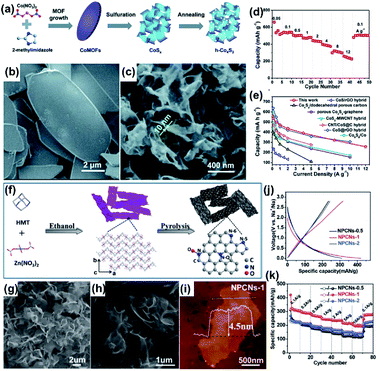 | ||
| Fig. 20 2D MOF derivatives for SIBs. (a) Schematic illustration of the fabrication of h-Co4S3 and SEM images of (b) CoMOFs and (c) h-Co4S3. (d) Rate performance of h-Co4S3 as SIB anodes. (e) Rate performance comparison studies of h-Co4S3 with other CoSx composites. Reproduced with permission.176 Copyright©2018 The Royal Society of Chemistry. (f) Schematic illustration of the preparation of Zn-HMTs and their derivatives (NPCNs). (g) SEM images of Zn-HMT nanosheets. (h) SEM and (i) AFM images of NPCNs. (j) Charge–discharge and (k) rate performance curves of NPCNs as the SIB anodes. Reproduced with permission.175 Copyright©2018 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
Carbon materials and inorganic metal compound/carbon composites with better conductivity are regarded as more suitable candidates for SIBs. Liu and co-workers reported a simple method to prepare ultrathin nitrogen-doped porous carbon nanosheets (NPCNs) from 2D layered MOFs (Zn-hexamine, Zn-HMT) (Fig. 20f).175 The SEM characterizations reveal the successful fabrication of NPCNs (Fig. 20h) from Zn-HMT nanosheets (Fig. 20g) and the AFM result shows that the thickness of the as-prepared NPCNs is about 4.5 nm (Fig. 20i). Due to the high N content in the HMT, an ultrahigh N-doping level (16.64 at%) was realized in the NPCNs. The N-doping in the ultrathin carbon nanosheets could significantly enhance the interlayer distance of the carbon lattice and provide abundant active sites, which therefore significantly improves the sodium storage performance of the carbon-based materials. In addition, the mesopores originating from the removal of the Zn-based contents could enhance the ion-diffusion property of the NPCNs as the anode materials for SIBs. As a proof-of-concept application, the NPCNs exhibited a high reversible capacity of 318 mA h g−1 at 0.1 A g−1 and maintained a high rate performance with a capacity of 194 mA h g−1 even at a high current density of 10 A g−1 for SIBs (Fig. 20j and k). In addition, the HMT, as a universal organic linker, could easily coordinate with the transition metal ions by using different coordination modes, which were used to synthesize the metal-HMT and its derived inorganic metal compounds/C composites toward superior SIB anodes. Later on, the same group reported a series of Ni-based inorganic metal compounds/C (e.g., NiSe2/NC,58 NiTe2/NC,177 and Ni/NC189) by using Ni-HMT nanosheets as the precursors, which displayed much better performance for SIBs than the NPCNs.
Besides, 2D MOF derivatives can also be used as the electrode materials in Li–S batteries due to their variable surface elemental compositions and suitable pore distribution for S storage. For instance, the Zhang group reported a thermal method to in situ thermo-exfoliate layered 2D MOFs into ultrahydrophilic graphene stacks (UHCS) with a highly polar carbon surface, hierarchically porous structure, and defined uniform nanosheet morphology.172 This kind of porous carbon could well cage-confine the polysulfides and provide a fine electron and ion accessible environment. Therefore, a reversible manner of the coupled redox reaction was achieved in UHCS as the electrode of Li–S batteries.
The aforementioned UT-CNSs derived from UT-Zn(bim)(OAc) developed by our group could not only be used in LIBs, but also exhibited excellent performance while employed as electrode materials in SCs.101 Benefiting from their high electrical conductivity (due to the high degree of graphitization), low contact resistance of the 2D materials, and large ultrathin nanosheet morphology with a high-porosity structure, the UT-CNSs showed a high capacitance of 347 F g−1 at the current density of 0.5 A g−1. Even at a high current density of 10 A g−1, the UT-CNSs still presented a high gravimetric capacitance of 283 F g−1. In addition to the 2D MOF-derived carbon nanosheets, the inorganic metal compounds/C materials derived from the 2D MOFs with pseudocapacitive behaviors were extensively investigated as the possible electrode materials for SCs. For instance, Cao and co-workers reported 2D MOF-derived 2D CoS1.097/nitrogen-doped carbon nanocomposites (CoSNCs).178 As shown in Fig. 21a, 2D porphyrin paddlewheel framework-3 (PPF-3) MOF nanosheets (Fig. 21b) with a thickness of ca. 12–43 nm (Fig. 21c) were synthesized by using Co2(COO)4 and TCPP as the reactants and 4,4′-bipyridine (BPY) as the intercalation agent. Then, the PPF-3 nanosheets were directly carbonized to the CoSNCs. As shown in Fig. 21d, the nanosheet morphology was retained after the carbonization. The top inset in Fig. 21d shows that the CoS1.097 nanodots are uniformly distributed in the carbon matrix. All CV curves (Fig. 21e) of the 2D CoSNC nanocomposite electrode exhibit rectangular shapes with distinct redox peaks, which indicates that the capacitance characteristics of this nanocomposite are governed by both the faradaic redox reactions and EDLC behaviors. The 2D CoSNCs also delivered a much higher specific capacitance than that of the bulk CoSNC electrode (Fig. 21f). Besides, the 2D carbon nanosheets and 2D inorganic metal compounds/C derived from the 2D MOFs could also be employed as the positive and negative electrodes for the asymmetric SCs, respectively. As shown in Fig. 21g, the Co-MIM nanosheets were firstly grown on carbon cloth, which were then directly converted to the carbon nanosheets (CC@NCN) and Co3O4 nanosheets (CC@Co3O4) in different atmospheres.179 CC@NCN (Fig. 21h) and CC@Co3O4 (Fig. 21i) exhibited highly porous nanosheet morphologies. The asymmetric SCs assembled with CC@NCN and CC@Co3O4 exhibited a maximum energy density of 41.5 W h kg−1 with a power density of 6.2 kW kg−1 (Fig. 21j), and delivered excellent durability with the capacitance retention of 90.8% and 85.5% (compared to the initial capacitance) after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 20
000 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively (Fig. 21k). CC@NCN could also be employed as a superior substrate to load different inorganic metal compounds (e.g., MnO2 (ref. 27) and ZnCoS33) to enhance the pseudocapacitive performance.
000 cycles, respectively (Fig. 21k). CC@NCN could also be employed as a superior substrate to load different inorganic metal compounds (e.g., MnO2 (ref. 27) and ZnCoS33) to enhance the pseudocapacitive performance.
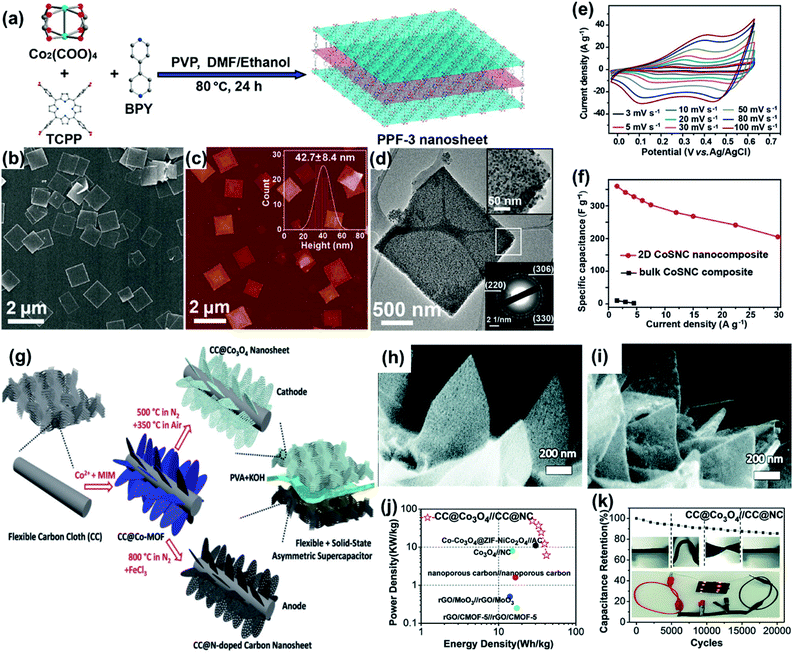 | ||
| Fig. 21 (a) Schematic illustration of the synthesis process of PPF-3 nanosheets. (b) SEM image and (c) AFM image of PPF-3 nanosheets. Inset: Statistical analysis of the thickness of 75 PPF-3 nanosheets measured in AFM images. (d) TEM images of CoSNC. (e) CV and (f) galvanostatic charge/discharge curves of the 2D CoSNC nanocomposite electrode measured in 2.0 M KOH. Reproduced with permission.178 Copyright©2016 American Chemical Society. (g) Schematic illustration of a flexible asymmetric supercapacitor. SEM image of (h) CC@NCN and (i) CC@Co3O4. (j) Ragone plots and (k) cycling test result of the asymmetric supercapacitor with PVA–KOH gel electrolyte. Reproduced with permission.179 Copyright©2017 The Royal Society of Chemistry. | ||
4.2. 2D MOF derivatives used as electrocatalysts
Facile approaches to synthesize 2D MOFs provide an effective way to obtain MOF derivatives with structural complexity and tunable compositions.190–192 Owing to the ordered arrangement of the metal ions and organic ligands in the MOF crystals, the obtained MOF derivatives usually have nanoporous structures and uniform distribution of different components (e.g., metal nanoparticles and carbon).25,193 Moreover, the organic ligands in MOFs enable the formation of carbon-based nanomaterials after carbonization, leading to the improvement of the electrical conductivity and electrochemical activity for the MOF derivatives.194 In this section, we highlight some promising applications of 2D MOF-derived materials for the electrocatalysis of the OER, HER, and ORR.The derivatives of the Fe-, Co-, and Ni-based 2D MOF nanosheets are considered as high-performance electrocatalysts for the OER due to the high concentration of Fe, Co, and Ni atoms/ions distributed on the surface of the electrocatalysts, which can form catalytically active sites during the OER process, such as the Co(IV)–OH and Ni(IV)–OH intermediates. Fan et al. template-grew Ni-doped ZIF-67 nanosheets on Mxenes (Ti3C2Tx) and then directly converted them to NiCoS/Ti3C2Tx composites through a heat treatment in the presence of sufficient S powder. NiCoS/Ti3C2Tx exhibited a low OER overpotential of 365 mV at 10 mA cm−2 and Tafel slope of 58.2 mV dec−1.196 Wang fabricated a series of Co1−xFexP nanoparticles embedded in 2D MOF-derived carbon nanosheets and investigated their electrocatalytic activities toward the OER.197 As shown in Fig. 22a and b, Co0.7Fe0.3-MOF nanosheets and Co0.7Fe0.3P/C nanosheets are successfully obtained. The HRTEM image of Co0.7Fe0.3P/C indicates that the Co1−xFexP nanoparticles are embedded in the carbon nanosheets (Fig. 22c). The experimental results demonstrated that moderate iron doping could preserve the catalytically active sites. Hence, the Co0.7Fe0.3P/C had the best activity with an overpotential of about 270 mV to reach an OER current density of 10 mA cm−2 (Fig. 22d) and a low Tafel slope of 27 mV dec−1 (Fig. 22e). Furthermore, Co0.7Fe0.3P/C also barely delivered overpotential increase after 1000 cycles as the electrocatalyst for the OER in 1 M KOH (Fig. 22f). Attributed to the excellent spatial confinement of 2D materials, the sizes of metals or metal inorganic compounds derived from the 2D MOF nanosheets could be flexibly regulated by the thickness of the 2D MOF precursors. For instance, sub-10 nm uniformly distributed nickel nanoparticles encapsulated in few-layer nitrogen-doped graphene (Ni@NC) could be facilely prepared by using ultrathin Ni-BDC nanosheets as the precursor through a high-temperature annealing treatment (Fig. 22g and h).198 Owing to its ultrasmall particle size and complete carbon coating (Fig. 22i), Ni@NC displayed an anodic current density of 10 mA cm−2 at an overpotential of 280 mV (Fig. 22j) with a Tafel slope as low as 45 mV dec−1 (Fig. 22k) as well as excellent stability (Fig. 22l).
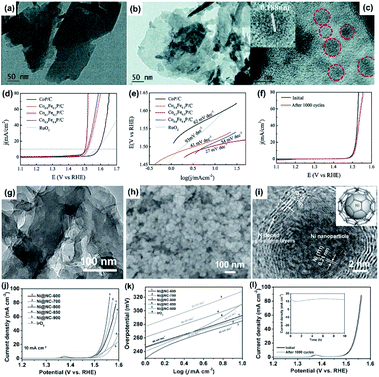 | ||
| Fig. 22 TEM images of (a) Co0.7Fe0.3-MOF nanosheets and (b) Co0.7Fe0.3P/C nanosheets. (c) HRTEM image of Co0.7Fe0.3P/C nanosheets. (d) LSV curves and (e) the corresponding Tafel plots of Co1−xFexP/C nanosheets and commercial RuO2 catalyst in 1 M KOH solution. (f) Polarization curves of the Co0.7Fe0.3P/C catalyst before and after 1000 potential cycles. Reproduced with permission.197 Copyright ©2018 The Royal Society of Chemistry. (g) TEM image of Ni-BDC nanosheets. (h) SEM and (i) HRTEM images of Ni@NC. (j) The OER polarization curves and (k) the corresponding Tafel plots of various Ni@NC samples and commercial IrO2 catalyst in 1.0 M KOH solution. (l) Polarization curves of the Ni@NC-800 catalyst before and after 1000 potential cycles. The inset of (l) is the chronoamperometric curve at 1.52 V (vs. RHE).198 Copyright©2017 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
2D MOF derivatives with a high accessible surface area and inhomogeneous plane electron distribution were also used as catalysts for the HER. For instance, Lee reported a 2D MOF-derived Co/N-doped carbon electrocatalyst for the HER.199 Owing to its low H-atom binding energy on metallic cobalt and high conductivity and stability of the carbon-encapsulated Co nanoparticles, Co/N–carbon exhibited an outstanding activity with a small overpotential of 103 mV at 10 mA cm−2. Luo synthesized CoP nanoparticles encapsulated in an ultrathin nitrogen-doped porous carbon shell (CoP@NC) using ZIF-9 as the precursor via a two-step MOF-derivation route.200 Compared to CoP@C (η10 = 130 mV) and CoP (η10 = 128 mV), CoP@NC presented excellent performance with an overpotential of 78 mV at 10 mA cm−2 and Tafel slope of 49 mV dec−1. The DFT calculation demonstrated that the dopants, CoP and N, were beneficial to optimizing the binding energy of the H species on the active sites and therefore led to better HER activity. Moreover, a number of 2D MOF derivatives can also work as bifunctional catalysts for both the OER and HER. Du fabricated phosphide–carbon nanosheet composites from 2D cobalt porphyrinic MOF nanosheets, which consisted of cobalt phosphide nanoparticles embedded in mesoporous N-doped graphitic carbon materials.36 This catalyst exhibited good electrocatalytic activities for the HER in 0.5 M H2SO4 and the OER in 1 M KOH with overpotentials of 98 and 370 mV at 10 mA cm−2 and the Tafel slopes of 74 and 79 mV dec−1, respectively.
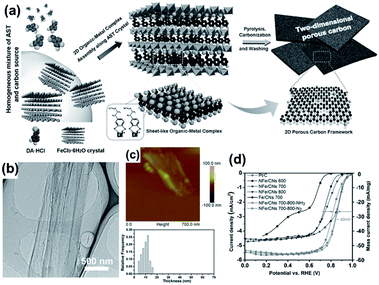 | ||
| Fig. 23 (a) Schematic illustration of the overall synthetic procedure of 2D microporous carbon nanosheets (CNs). Representative (b) TEM, (c) AFM images, and corresponding thickness distribution based on 50 height section measurements of NFe/CNs-700. (d) RDE polarization curves of Pt/C, Fe/CNs-700, and NFe/CN catalysts prepared at different temperatures. The catalyst loading amount is 0.1 mg cm−2 for all samples. Reproduced with permission.206 Copyright ©2017 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
5. Conclusions
In this review, some recent significant research progress on the synthesis and applications of 2D MOFs and their derivatives is summarized. First, we thoroughly reviewed the synthetic strategies that have been developed for the 2D MOFs, including the representative methods in both top-down and bottom-up approaches. The mechanisms and the applicability of these synthetic strategies are discussed. Then, the typical applications of 2D MOFs and their derivatives for electrochemical energy storage and electrocatalysis are reviewed. The advantages and drawbacks of the 2D MOFs and their derivatives in these applications are discussed.Generally, the synthesis of high-quality 2D MOFs with a high yield and at a low cost is one of the most critical challenges in the development and application of 2D MOFs and their derivatives. Although much effort has been made, some significant challenges toward the practical application of the 2D MOFs and their derivatives for energy storage and electrocatalysis still exist.
(1) High-quality and scalable fabrication of the 2D MOFs still needs continuous efforts. Both modifications/upgrades of the currently existing synthetic strategies and the development of novel synthetic methods to achieve better quality, higher yield, and lower cost of the 2D MOFs are welcome. Regarding the novel synthetic methods, electrochemical deposition, as a kind of facile and economical synthesis method, might be a possible solution since it could directly fabricate target materials on conducting or semiconducting substrates (i.e., the current collectors of the electrodes in energy storage and electrocatalysis) with better conductivity compared to the powdery material-based electrodes. To the best of our knowledge, facile electrodeposition of 2D MOFs with a controllable morphology and composition has not been achieved.
(2) The poor stability of the 2D MOFs hinders their wide applications. Thus, constructing highly stable 2D MOFs with strong coordination bonds is highly demanded in the future for their applications under critical conditions such as for the OER. The MOF structures with strong coordination bonds or significant steric hindrance could possibly prevent the metal–ligand coordination bonds from hydrolysis. Such strong metal–ligand bonds could be probably designed based on the hard/soft acid/base rule (i.e. the hard-acid metal ions coordinate with hard-base ligands such as CH3COO−, and the soft-acid metal ions coordinate with the soft-base ligands such as CN−).
(3) The currently reported conductive MOFs are all based on high-cost organic ligands, which are far from practical application. Thus, exploration of low-cost ligands for the construction of conductive MOF nanosheets is urgently needed. For example, square-planar Cu(NH)4 and Ni(NH)4 nodes with a d–π conjugation could probably be used to design and fabricate highly conductive MOF nanosheets.
(4) The high tendency to agglomerate is another issue for 2D MOFs. Although some strategies have been developed to prevent 2D MOFs from agglomeration, more efficient and economical methods are still desired.
(5) The destruction of the pore structure and ordered surface metal node arrangement during the transformation from the 2D MOFs to their derivatives is still one of the biggest issues for the application of the 2D MOF derivatives. Thus, the development of strategies to convert 2D MOFs to their 2D derivatives while better maintaining the structural properties is highly demanded in future research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 51772332), the Hunan Provincial Science and Technology Plan Project (grant no. 2018RS3008 and 2017TP1001), and the Natural Science Foundation of Hunan Province (grant no. 2018JJ2485).Notes and references
- X. Yang, C. Cheng, Y. Wang, L. Qiu and D. Li, Science, 2013, 341, 534–537 CrossRef CAS PubMed.
- T.-S. Kim, H. J. Song, M. A. Dar, H.-W. Shim and D.-W. Kim, J. Am. Ceram. Soc., 2018, 101, 3749–3754 CrossRef CAS.
- Y. Wang, Y. Song and Y. Xia, Chem. Soc. Rev., 2016, 45, 5925–5950 RSC.
- X. Yang, A.-Y. Lu, Y. Zhu, M. N. Hedhili, S. Min, K.-W. Huang, Y. Han and L.-J. Li, Nano Energy, 2015, 15, 634–641 CrossRef CAS.
- Q. Wang, J. Yan and Z. Fan, Energy Environ. Sci., 2016, 9, 729–762 RSC.
- L. Wang and X. Hu, Chem.–Asian J., 2018, 13, 1518–1529 CrossRef CAS PubMed.
- X. Cao, C. Tan, M. Sindoro and H. Zhang, Chem. Soc. Rev., 2017, 46, 2660–2677 RSC.
- C. H. Hendon, A. J. Rieth, M. D. Korzynski and M. Dinca, ACS Cent. Sci., 2017, 3, 554–563 CrossRef CAS PubMed.
- H. Wang, Q.-L. Zhu, R. Zou and Q. Xu, Chem, 2017, 2, 52–80 CAS.
- S. Dutta, Z. Liu, H. Han, A. Indra and T. Song, ChemElectroChem, 2018, 5, 3571–3588 CrossRef CAS.
- Z. Jiang, T. Liu, L. Yan, J. Liu, F. Dong, M. Ling, C. Liang and Z. Lin, Energy Storage Materials, 2018, 11, 267–273 CrossRef.
- G. Ye, Q. He, S. Liu, K. Zhao, Y. Su, W. Zhu, R. Huang and Z. He, J. Mater. Chem. A, 2019, 7, 16508–16515 RSC.
- X.-L. Wang, L.-Z. Dong, M. Qiao, Y.-J. Tang, J. Liu, Y. Li, S.-L. Li, J.-X. Su and Y.-Q. Lan, Angew. Chem., Int. Ed., 2018, 57, 9660–9664 CrossRef CAS PubMed.
- Y. Wang and C. Woell, Catal. Lett., 2018, 148, 2201–2222 CrossRef CAS.
- Q. Yang, Q. Xu and H.-L. Jiang, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- M. G. Campbell, S. F. Liu, T. M. Swager and M. Dinca, J. Am. Chem. Soc., 2015, 137, 13780–13783 CrossRef CAS PubMed.
- S. Homayoonnia and S. Zeinali, Sens. Actuators, B, 2016, 237, 776–786 CrossRef CAS.
- D. Ning, Q. Liu, Q. Wang, X.-M. Du, W.-J. Ruan and Y. Li, Sens. Actuators, B, 2019, 282, 443–448 CrossRef CAS.
- R. Xu, Y. Wang, X. Duan, K. Lu, D. Micheroni, A. Hu and W. Lin, J. Am. Chem. Soc., 2016, 138, 2158–2161 CrossRef CAS PubMed.
- X. Zhao, Y. Wang, D.-S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189 CrossRef PubMed.
- Y. Hua, H. Wang, Q. Li, G. Chen, G. Liu, J. Duan and W. Jin, J. Mater. Chem. A, 2018, 6, 599–606 RSC.
- Y. Peng, Y. Li, Y. Ban, H. Jin, W. Jiao, X. Liu and W. Yang, Science, 2014, 346, 1356–1359 CrossRef CAS PubMed.
- H. Li, K. Wang, Y. Sun, C. T. Lollar, J. Li and H.-C. Zhou, Mater. Today, 2018, 21, 108–121 CrossRef CAS.
- M. A. N. Khan, P. K. Klu, C. Wang, W. Zhang, R. Luo, M. Zhang, J. Qi, X. Sun, L. Wang and J. Li, Chem. Eng. J., 2019, 363, 234–246 CrossRef.
- Y. Wang, Y. Gao, J. Shao, R. Holze, Z. Chen, Y. Yun, Q. Qu and H. Zheng, J. Mater. Chem. A, 2018, 6, 3659–3666 RSC.
- J. Cong, H. Xu, M. Lu, Y. Wu, Y. Li, P. He, J. Gao, J. Yao and S. Xu, Chem.–Asian J., 2018, 13, 1485–1491 CrossRef CAS PubMed.
- X. Liu, C. Guan, Y. Hu, L. Zhang, A. M. Elshahawy and J. Wang, Small, 2018, 14, 1702641 CrossRef PubMed.
- W. Shuang, L. Kong, M. Zhong, D. Wang, J. Liu and X.-H. Bu, Dalton Trans., 2018, 47, 12385–12392 RSC.
- F. Wu, S. Zhang, B. Xi, Z. Feng, D. Sun, X. Ma, J. Zhang, J. Feng and S. Xiong, Adv. Energy Mater., 2018, 8, 1703242 CrossRef.
- S. Wang, J. Teng, Y. Xie, Z.-W. Wei, Y. Fan, J.-J. Jiang, H.-P. Wang, H. Liu, D. Wang and C.-Y. Su, J. Mater. Chem. A, 2019, 7, 4036–4046 RSC.
- K. Wang, H. Chen, Y. Hua, Y. Tong, Y. Wang and S. Song, Mater. Today Energy, 2018, 10, 343–351 CrossRef.
- Q. Lu, Y. Yu, Q. Ma, B. Chen and H. Zhang, Adv. Mater., 2016, 28, 1917–1933 CrossRef CAS PubMed.
- W. Sun, Y. Du, G. Wu, G. Gao, H. Zhu, J. Shen, K. Zhang and G. Cao, J. Mater. Chem. A, 2019, 7, 7138–7150 RSC.
- R. Wang, X.-Y. Dong, J. Du, J.-Y. Zhao and S.-Q. Zang, Adv. Mater., 2018, 30, 1703711 CrossRef PubMed.
- K. Wang, C. Wu, F. Wang and G. Jiang, ACS Appl. Nano Mater., 2018, 1, 5843–5853 CrossRef CAS.
- M. Zhai, F. Wang and H. Du, ACS Appl. Mater. Interfaces, 2017, 9, 40171–40179 CrossRef CAS PubMed.
- J.-S. Li, S. Zhang, J.-Q. Sha, H. Wang, M. Z. Liu, L.-X. Kong and G.-D. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 17140–17146 CrossRef CAS PubMed.
- X. Fan, C. Yu, J. Yang, Z. Ling, C. Hu, M. Zhang and J. Qiu, Adv. Energy Mater., 2015, 5, 1401761 CrossRef.
- T. He, B. Ni, Y. Ou, H. Lin, S. Zhang, C. Li, J. Zhuang, W. Hu and X. Wang, Small Methods, 2018, 2, 1800068 CrossRef.
- L. Cao, T. Wang and C. Wang, Chin. J. Chem., 2018, 36, 754–764 CrossRef CAS.
- S. Stepanow, N. Lin, D. Payer, U. Schlickum, F. Klappenberger, G. Zoppellaro, M. Ruben, H. Brune, J. V. Barth and K. Kern, Angew. Chem., Int. Ed., 2007, 46, 710–713 CrossRef CAS PubMed.
- W. Liu, R. Yin, X. Xu, L. Zhang, W. Shi and X. Cao, Adv. Sci., 2019, 6, 1802373 CrossRef PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- J. Hou, K. Jiang, M. Tahir, X. Wu, F. Idrees, M. Shen and C. Cao, J. Power Sources, 2017, 371, 148–155 CrossRef CAS.
- Z.-X. Li, B.-L. Yang, K.-Y. Zou, L. Kong, M.-L. Yue and H.-H. Duan, Carbon, 2019, 144, 540–548 CrossRef CAS.
- C. Li, H. Liu and Z. Yu, Appl. Catal., B, 2019, 241, 95–103 CrossRef CAS.
- F. Tu, Y. Han, Y. Du, X. Ge, W. Weng, X. Zhou and J. Bao, ACS Appl. Mater. Interfaces, 2019, 11, 2112–2119 CrossRef CAS PubMed.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- L. Li, Y. Yu, G. J. Ye, Q. Ge, X. Ou, H. Wu, D. Feng, X. H. Chen and Y. Zhang, Nat. Nanotechnol., 2014, 9, 372–377 CrossRef CAS PubMed.
- M. Buscema, D. J. Groenendijk, S. I. Blanter, G. A. Steele, H. S. J. van der Zant and A. Castellanos-Gomez, Nano Lett., 2014, 14, 3347–3352 CrossRef CAS PubMed.
- Q. Weng, X. Wang, X. Wang, Y. Bando and D. Golberg, Chem. Soc. Rev., 2016, 45, 3989–4012 RSC.
- Y. Xia, T. S. Mathis, M.-Q. Zhao, B. Anasori, A. Dang, Z. Zhou, H. Cho, Y. Gogotsi and S. Yang, Nature, 2018, 557, 409–412 CrossRef CAS PubMed.
- M. Zhao, Y. Huang, Y. Peng, Z. Huang, Q. Ma and H. Zhang, Chem. Soc. Rev., 2018, 47, 6267–6295 RSC.
- H. Liu, X. Li, L. Chen, X. Wang, H. Pan, X. Zhang and M. Zhao, J. Phys. Chem. C, 2016, 120, 3846–3852 CrossRef CAS.
- M. Zhang, G. Feng, Z. Song, Y.-P. Zhou, H.-Y. Chao, D. Yuan, T. T. Y. Tan, Z. Guo, Z. Hu, B. Z. Tang, B. Liu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 7241–7244 CrossRef CAS PubMed.
- S. Liu, D. Li, G. Zhang, D. Sun, J. Zhou and H. Song, ACS Appl. Mater. Interfaces, 2018, 10, 34193–34201 CrossRef CAS PubMed.
- W. Zhao, J. Peng, W. Wang, S. Liu, Q. Zhao and W. Huang, Coord. Chem. Rev., 2018, 377, 44–63 CrossRef CAS.
- L. Huang, X. Zhang, Y. Han, Q. Wang, Y. Fang and S. Dong, J. Mater. Chem. A, 2017, 5, 18610–18617 RSC.
- J. Liu, H. Yu, L. Wang, Z. Deng, K.-u.-R. Naveed, A. Nazir and F. Haq, Inorg. Chim. Acta, 2018, 483, 550–564 CrossRef CAS.
- M. Zhao, Q. Lu, Q. Ma and H. Zhang, Small Methods, 2017, 1, 1600030 CrossRef.
- Y.-z. Li, Z.-h. Fu and G. Xu, Coord. Chem. Rev., 2019, 388, 79–106 CrossRef CAS.
- J. Wang, S. Li, R. Lin, G. Tu, J. Wang and Z. Li, Electrochim. Acta, 2019, 301, 258–266 CrossRef CAS.
- Y. Peng, Y. Li, Y. Ban and W. Yang, Angew. Chem., Int. Ed., 2017, 56, 9757–9761 CrossRef CAS PubMed.
- S. C. Junggeburth, L. Diehl, S. Werner, V. Duppel, W. Sigle and B. V. Lotsch, J. Am. Chem. Soc., 2013, 135, 6157–6164 CrossRef CAS PubMed.
- X. M. Li, L. Tao, Z. F. Chen, H. Fang, X. S. Li, X. R. Wang, J. B. Xu and H. W. Zhu, Appl. Phys. Rev., 2017, 4, 021306 Search PubMed.
- Z. Liang, C. Qu, D. Xia, R. Zou and Q. Xu, Angew. Chem., Int. Ed., 2018, 57, 9604–9633 CrossRef CAS PubMed.
- P.-Z. Li, Y. Maeda and Q. Xu, Chem. Commun., 2011, 47, 8436–8438 RSC.
- P. Amo-Ochoa, L. Welte, R. Gonzalez-Prieto, P. J. Sanz Miguel, C. J. Gomez-Garcia, E. Mateo-Marti, S. Delgado, J. Gomez-Herrero and F. Zamora, Chem. Commun., 2010, 46, 3262–3264 RSC.
- H. F. Clausen, R. D. Poulsen, A. D. Bond, M.-A. S. Chevallier and B. B. Iversen, J. Solid State Chem., 2005, 178, 3342–3351 CrossRef CAS.
- J. A. Foster, S. Henke, A. Schneemann, R. A. Fischer and A. K. Cheetham, Chem. Commun., 2016, 52, 10474–10477 RSC.
- C. Hermosa, B. R. Horrocks, J. I. Martínez, F. Liscio, J. Gómez-Herrero and F. Zamora, Chem. Sci., 2015, 6, 2553–2558 RSC.
- Y. Ding, Y.-P. Chen, X. Zhang, L. Chen, Z. Dong, H.-L. Jiang, H. Xu and H.-C. Zhou, J. Am. Chem. Soc., 2017, 139, 9136–9139 CrossRef CAS PubMed.
- M. J. Cliffe, E. Castillo-Martinez, Y. Wu, J. Lee, A. C. Forse, F. C. N. Firth, P. Z. Moghadam, D. Fairen-Jimenez, M. W. Gaultois, J. A. Hill, O. V. Magdysyuk, B. Slater, A. L. Goodwin and C. P. Grey, J. Am. Chem. Soc., 2017, 139, 5397–5404 CrossRef CAS PubMed.
- A. Dmitriev, H. Spillmann, N. Lin, J. V. Barth and K. Kern, Angew. Chem., Int. Ed., 2003, 42, 2670–2673 CrossRef CAS PubMed.
- J. Huang, Y. Li, R.-K. Huang, C.-T. He, L. Gong, Q. Hu, L. Wang, Y.-T. Xu, X.-Y. Tian, S.-Y. Liu, Z.-M. Ye, F. Wang, D.-D. Zhou, W.-X. Zhang and J.-P. Zhang, Angew. Chem., Int. Ed., 2018, 130, 4722–4726 CrossRef.
- L.-J. Han, D. Zheng, S.-G. Chen, H.-G. Zheng and J. Ma, Small, 2018, 14, 1703873 CrossRef PubMed.
- R. A. Vilá, K. Momeni, Q. Wang, B. M. Bersch, N. Lu, M. J. Kim, L. Q. Chen and J. A. Robinson, 2D Mater., 2016, 3, 041003 CrossRef.
- D. Wei, Y. Liu, H. Zhang, L. Huang, B. Wu, J. Chen and G. Yu, J. Am. Chem. Soc., 2009, 131, 11147–11154 CrossRef CAS PubMed.
- Z. Fan, Y. Liu, J. Yan, G. Ning, Q. Wang, T. Wei, L. Zhi and F. Wei, Adv. Energy Mater., 2012, 2, 419–424 CrossRef CAS.
- L. Wan, W. Sun, J. Shen and X. Li, Electrochim. Acta, 2016, 211, 962–971 CrossRef CAS.
- K. Zhu, J. Sun, H. Zhang, J. Liu and Y. Wang, J. Nat. Gas Chem., 2012, 21, 215–232 CrossRef CAS.
- H. Li, J. Hou, T. D. Bennett, J. Liu and Y. Zhang, J. Mater. Chem. A, 2019, 7, 5811–5818 RSC.
- S. J. Limmer, S. Seraji, Y. Wu, T. P. Chou, C. Nguyen and G. Z. Cao, Adv. Funct. Mater., 2002, 12, 59–64 CrossRef CAS.
- M. Zhao, Y. Wang, Q. Ma, Y. Huang, X. Zhang, J. Ping, Z. Zhang, Q. Lu, Y. Yu, H. Xu, Y. Zhao and H. Zhang, Adv. Mater., 2015, 27, 7372–7378 CrossRef CAS PubMed.
- B. Wang, J. Shang, C. Guo, J. Zhang, F. Zhu, A. Han and J. Liu, Small, 2019, 15, 1804761 CrossRef PubMed.
- X. Bai, J. Liu, Q. Liu, R. Chen, X. Jing, B. Li and J. Wang, Chem.–Eur. J., 2017, 23, 14839–14847 CrossRef CAS PubMed.
- H. Gu, Z. Yang, J. Gao, C. K. Chang and B. Xu, J. Am. Chem. Soc., 2005, 127, 34–35 CrossRef CAS PubMed.
- R. Makiura, S. Motoyama, Y. Umemura, H. Yamanaka, O. Sakata and H. Kitagawa, Nat. Mater., 2010, 9, 565–571 CrossRef CAS PubMed.
- R. Makiura and O. Konovalov, Sci. Rep., 2013, 3, 2506 CrossRef PubMed.
- G. Xu, T. Yamada, K. Otsubo, S. Sakaida and H. Kitagawa, J. Am. Chem. Soc., 2012, 134, 16524–16527 CrossRef CAS PubMed.
- S. Motoyama, R. Makiura, O. Sakata and H. Kitagawa, J. Am. Chem. Soc., 2011, 133, 5640–5643 CrossRef CAS PubMed.
- X. Huang, P. Sheng, Z. Tu, F. Zhang, J. Wang, H. Geng, Y. Zou, C.-a. Di, Y. Yi, Y. Sun, W. Xu and D. Zhu, Nat. Commun., 2015, 6, 7408 CrossRef CAS PubMed.
- D. J. Ashworth and J. A. Foster, J. Mater. Chem. A, 2018, 6, 16292–16307 RSC.
- R. Sakamoto, K. Hoshiko, Q. Liu, T. Yagi, T. Nagayama, S. Kusaka, M. Tsuchiya, Y. Kitagawa, W.-Y. Wong and H. Nishihara, Nat. Commun., 2015, 6, 6713 CrossRef CAS PubMed.
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. X. Llabrés i Xamena and J. Gascon, Nat. Mater., 2014, 14, 48–55 CrossRef PubMed.
- L. Sun, C. Wang, X. Wang and L. Wang, Small, 2018, 14, 1800090 CrossRef PubMed.
- X. Zhang, P. Zhang, C. Chen, J. Zhang, G. Yang, L. Zheng, J. Zhang and B. Han, Green Chem., 2019, 21, 54–58 RSC.
- L. Cao, Z. Lin, F. Peng, W. Wang, R. Huang, C. Wang, J. Yan, J. Liang, Z. Zhang, T. Zhang, L. Long, J. Sun and W. Lin, Angew. Chem., Int. Ed., 2016, 55, 4962–4966 CrossRef CAS PubMed.
- K. Zhao, S. Liu, G. Ye, Q. Gan, Z. Zhou and Z. He, J. Mater. Chem. A, 2018, 6, 2166–2175 RSC.
- K. Zhao, Z. Xu, Z. He, G. Ye, Q. Gan, Z. Zhou and S. Liu, J. Mater. Sci., 2018, 53, 13111–13125 CrossRef CAS.
- K. Zhao, S. Liu, G. Ye, X. Wei, Y. Su, W. Zhu, Z. Zhou and Z. He, ChemSusChem, 2020 DOI:10.1002/cssc.201902776.
- Y. Wang, L. Li, L. Yan, X. Gu, P. Dai, D. Liu, J. G. Bell, G. Zhao, X. Zhao and K. M. Thomas, Chem. Mater., 2018, 30, 3048–3059 CrossRef CAS.
- J. Zha and X. Zhang, Cryst. Growth Des., 2018, 18, 3209–3214 CrossRef CAS.
- S. He, Y. Chen, Z. Zhang, B. Ni, W. He and X. Wang, Chem. Sci., 2016, 7, 7101–7105 RSC.
- M. Ko, L. Mendecki and K. A. Mirica, Chem. Commun., 2018, 54, 7873–7891 RSC.
- G. Jia, S. Liu, G. Yang, F. Li, K. Wu, Z. He and X. Shangguan, Ionics, 2019, 25, 399–410 CrossRef CAS.
- Q. Gan, J. Xie, Y. Zhu, F. Zhang, P. Zhang, Z. He and S. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 930–939 CrossRef CAS PubMed.
- Q. Gan, K. Zhao, Z. He, S. Liu and A. Li, J. Power Sources, 2018, 384, 187–195 CrossRef CAS.
- Q. Gan, H. He, K. Zhao, Z. He, S. Liu and S. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 7031–7042 CrossRef CAS PubMed.
- Q. Liu, L. Yu, Y. Wang, Y. Ji, J. Horvat, M.-L. Cheng, X. Jia and G. Wang, Inorg. Chem., 2013, 52, 2817–2822 CrossRef CAS PubMed.
- Y. Wang, Q. Qu, G. Liu, V. S. Battaglia and H. Zheng, Nano Energy, 2017, 39, 200–210 CrossRef CAS.
- G. Ferey, F. Millange, M. Morcrette, C. Serre, M. L. Doublet, J. M. Greneche and J. M. Tarascon, Angew. Chem., Int. Ed., 2007, 46, 3259–3263 CrossRef CAS PubMed.
- C. Li, X. Hu, W. Tong, W. Yan, X. Lou, M. Shen and B. Hu, ACS Appl. Mater. Interfaces, 2017, 9, 29829–29838 CrossRef CAS PubMed.
- J. Jin, Y. Zheng, S.-z. Huang, P.-p. Sun, N. Srikanth, L. B. Kong, Q. Yan and K. Zhou, J. Mater. Chem. A, 2019, 7, 783–790 RSC.
- K. Zhao, S. Liu, Y. Wu, K. Lv, H. Yuan and Z. He, Electrochim. Acta, 2015, 174, 1234–1243 CrossRef CAS.
- R. Abazari, S. Sanati, A. Morsali, A. M. Z. Slawin, C. L. Carpenter-Warren, W. Chen and A. Zheng, J. Mater. Chem. A, 2019, 7, 11953–11966 RSC.
- D. Feng, T. Lei, M. R. Lukatskaya, J. Park, Z. Huang, M. Lee, L. Shaw, S. Chen, A. A. Yakovenko, A. Kulkarni, J. Xiao, K. Fredrickson, J. B. Tok, X. Zou, Y. Cui and Z. Bao, Nat. Energy, 2018, 3, 30–36 CrossRef CAS.
- Y. Zheng, S. Zheng, Y. Xu, H. Xue, C. Liu and H. Pang, Chem. Eng. J., 2019, 373, 1319–1328 CrossRef CAS.
- J. Park, A. C. Hinckley, Z. Huang, D. Feng, A. A. Yakovenko, M. Lee, S. Chen, X. Zou and Z. Bao, J. Am. Chem. Soc., 2018, 140, 14533–14537 CrossRef CAS PubMed.
- J. Park, M. Lee, D. Feng, Z. Huang, A. C. Hinckley, A. Yakovenko, X. Zou, Y. Cui and Z. Bao, J. Am. Chem. Soc., 2018, 140, 10315–10323 CrossRef CAS PubMed.
- Y. Xu, Z. Lin, X. Zhong, X. Huang, N. O. Weiss, Y. Huang and X. Duan, Nat. Commun., 2014, 5, 4554 CrossRef CAS PubMed.
- T. Lin, I.-W. Chen, F. Liu, C. Yang, H. Bi, F. Xu and F. Huang, Science, 2015, 350, 1508–1513 CrossRef CAS PubMed.
- D. Sheberla, J. C. Bachman, J. S. Elias, C.-J. Sun, Y. Shao-Horn and M. Dinca, Nat. Mater., 2017, 16, 220–224 CrossRef CAS PubMed.
- J. A. Koza, Z. He, A. S. Miller and J. A. Switzer, Chem. Mater., 2012, 24, 3567–3573 CrossRef CAS.
- S. Han, S. Liu, R. Wang, X. Liu, L. Bai and Z. He, ACS Appl. Mater. Interfaces, 2017, 9, 17186–17194 CrossRef CAS PubMed.
- M. K. Bates, Q. Jia, H. Doan, W. Liang and S. Mukerjee, ACS Catal., 2016, 6, 155–161 CrossRef CAS.
- Y. Zhang, H. Zhang, J. Yang, Y. Bai, H. Qiu and Y. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 11396–11402 CrossRef CAS PubMed.
- G. Jia, S. Liu, G. Yang, F. Li, K. Wu, Z. He and X. Shangguan, Ionics, 2018, 24, 3705–3715 CrossRef CAS.
- Y. Xu, B. Li, S. Zheng, P. Wu, J. Zhan, H. Xue, Q. Xu and H. Pang, J. Mater. Chem. A, 2018, 6, 22070–22076 RSC.
- X. Li, D. D. Ma, C. Cao, R. Zou, Q. Xu, X. T. Wu and Q. L. Zhu, Small, 2019, 15, 1902218 CrossRef PubMed.
- F. Sun, G. Wang, Y. Ding, C. Wang, B. Yuan and Y. Lin, Adv. Energy Mater., 2018, 8, 1800584 CrossRef.
- S. Zhao, Y. Wang, J. Dong, C.-T. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. M. Khattak, N. A. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao and Z. Tang, Nat. Energy, 2016, 1, 1–10 Search PubMed.
- R. Yang, Y. M. Zhou, Y. Y. Xing, D. Li, D. L. Jiang, M. Chen, W. D. Shi and S. Q. Yuan, Appl. Catal., B, 2019, 253, 131–139 CrossRef CAS.
- W. Zhou, D. D. Huang, Y. P. Wu, J. Zhao, T. Wu, J. Zhang, D. S. Li, C. H. Sun, P. Y. Feng and X. H. Bu, Angew. Chem., Int. Ed., 2019, 58, 4227–4231 CrossRef CAS PubMed.
- M. T. Lu, Y. W. Li, P. P. He, J. K. Cong, D. N. Chen, J. W. Wang, Y. H. Wu, H. Xu, J. K. Gao and J. M. Yao, J. Solid State Chem., 2019, 272, 32–37 CrossRef CAS.
- K. Rui, G. Zhao, Y. Chen, Y. Lin, Q. Zhou, J. Chen, J. Zhu, W. Sun, W. Huang and S. X. Dou, Adv. Funct. Mater., 2018, 28, 1801554 CrossRef.
- G. Hai, X. Jia, K. Zhang, X. Liu, Z. Wu and G. Wang, Nano Energy, 2018, 44, 345–352 CrossRef CAS.
- K. Jayaramulu, J. Masa, D. M. Morales, O. Tomanec, V. Ranc, M. Petr, P. Wilde, Y.-T. Chen, R. Zboril, W. Schuhmann and R. A. Fischer, Adv. Sci., 2018, 5, 1801029 CrossRef PubMed.
- M. Ding, J. Chen, M. Jiang, X. Zhang and G. Wang, J. Mater. Chem. A, 2019, 7, 14163–14168 RSC.
- C. A. Downes, A. J. Clough, K. Chen, J. W. Yoo and S. C. Marinescu, ACS Appl. Mater. Interfaces, 2018, 10, 1719–1727 CrossRef CAS PubMed.
- R. Dong, M. Pfeffermann, H. Liang, Z. Zheng, X. Zhu, J. Zhang and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 12058–12063 CrossRef CAS PubMed.
- R. Dong, Z. Zheng, D. C. Tranca, J. Zhang, N. Chandrasekhar, S. Liu, X. Zhuang, G. Seifert and X. Feng, Chem.–Eur. J., 2017, 23, 2255–2260 CrossRef CAS PubMed.
- X. Dai, M. Liu, Z. Li, A. Jin, Y. Ma, X. Huang, H. Sun, H. Wang and X. Zhang, J. Phys. Chem. C, 2016, 120, 12539–12548 CrossRef CAS.
- J. Duan, S. Chen and C. Zhao, Nat. Commun., 2017, 8, 15341 CrossRef CAS PubMed.
- X. Wei, N. Li and N. Liu, Electrochim. Acta, 2019, 318, 957–965 CrossRef CAS.
- C.-W. Tung, Y.-Y. Hsu, Y.-P. Shen, Y. Zheng, T.-S. Chan, H.-S. Sheu, Y.-C. Cheng and H. M. Chen, Nat. Commun., 2015, 6, 8106 CrossRef CAS PubMed.
- X. L. Xing, R. J. Liu, K. C. Cao, U. Kaiser and C. Streb, Chem.–Eur. J., 2019, 25, 11098 CrossRef CAS PubMed.
- J. Y. Liu, H. Xu, H. P. Li, Y. H. Song, J. J. Wu, Y. J. Gong, L. Xu, S. Q. Yuan, H. M. Li and P. M. Ajayan, Appl. Catal., B, 2019, 243, 151–160 CrossRef CAS.
- Y. Li, Z. Qiao, Y. Cao, H. Wang, H. Liang, H. Yu and F. Peng, ChemSusChem, 2019, 12, 1133–1138 CrossRef CAS PubMed.
- R. Z. Gao, Y. H. Yin, F. Q. Niu, A. L. Wang, S. Y. Li, H. Y. Dong and S. T. Yang, ChemElectroChem, 2019, 6, 1824–1830 CrossRef CAS.
- G. Li, S. Zhao, Y. Zhang and Z. Tang, Adv. Mater., 2018, 30, 1800702 CrossRef PubMed.
- M. Jahan, Q. Bao and K. P. Loh, J. Am. Chem. Soc., 2012, 134, 6707–6713 CrossRef CAS PubMed.
- J. Mao, L. Yang, P. Yu, X. Wei and L. Mao, Electrochem. Commun., 2012, 19, 29–31 CrossRef CAS.
- K. Cho, S.-H. Han and M. P. Suh, Angew. Chem., Int. Ed., 2016, 55, 15301–15305 CrossRef CAS PubMed.
- H. Wang, F.-X. Yin, B.-H. Chen, X.-B. He, P.-L. Lv, C.-Y. Ye and D.-J. Liu, Appl. Catal., B, 2017, 205, 55–67 CrossRef CAS.
- M. Jahan, Z. Liu and K. P. Loh, Adv. Funct. Mater., 2013, 23, 5363–5372 CrossRef CAS.
- E. M. Miner, T. Fukushima, D. Sheberla, L. Sun, Y. Surendranath and M. Dincă, Nat. Commun., 2016, 7, 10942 CrossRef CAS PubMed.
- H. X. Zhong, K. H. Ly, M. C. Wang, Y. Krupskaya, X. C. Han, J. C. Zhang, J. Zhang, V. Kataev, B. Buchner, I. M. Weidinger, S. Kaskel, P. Liu, M. W. Chen, R. H. Dong and X. L. Feng, Angew. Chem., Int. Ed., 2019, 58, 10677–10682 CrossRef CAS PubMed.
- W. Lu, X. Li, F. Wei, K. Cheng, W. Li, Y. Zhou, W. Zheng, L. Pan and G. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 12501–12509 CAS.
- A. J. Clough, J. W. Yoo, M. H. Mecklenburg and S. C. Marinescu, J. Am. Chem. Soc., 2015, 137, 118–121 CrossRef CAS PubMed.
- X. Sun, K.-H. Wu, R. Sakamoto, T. Kusamoto, H. Maeda, X. Ni, W. Jiang, F. Liu, S. Sasaki, H. Masunaga and H. Nishihara, Chem. Sci., 2017, 8, 8078–8085 RSC.
- B. Qin, Y. Li, H. Wang, G. Yang, Y. Cao, H. Yu, Q. Zhang, H. Liang and F. Peng, Nano Energy, 2019, 60, 43–51 CrossRef CAS.
- W. Zheng, C.-S. Tsang, L. Y. S. Lee and K.-Y. Wong, Mater. Today Chem., 2019, 12, 34–60 CrossRef CAS.
- R. Wang, X. Sun, S. Ould-Chikh, D. Osadchii, F. Bai, F. Kapteijn and J. Gascon, ACS Appl. Mater. Interfaces, 2018, 10, 14751–14758 CrossRef CAS PubMed.
- W. Zhu, K. Zhao, S. Liu, M. Liu, F. Peng, P. An, B. Qin, H. Zhou, H. Li and Z. He, J. Energy Chem., 2019, 37, 176–182 CrossRef.
- F. N. Al-Rowaili, A. Jamal, M. S. Ba Shammakh and A. Rana, ACS Sustainable Chem. Eng., 2018, 6, 15895–15914 CrossRef CAS.
- N. Kornienko, Y. Zhao, C. S. Kley, C. Zhu, D. Kim, S. Lin, C. J. Chang, O. M. Yaghi and P. Yang, J. Am. Chem. Soc., 2015, 137, 14129–14135 CrossRef CAS PubMed.
- J. Shen, R. Kortlever, R. Kas, Y. Y. Birdja, O. Diaz-Morales, Y. Kwon, I. Ledezma-Yanez, K. J. P. Schouten, G. Mul and M. T. M. Koper, Nat. Commun., 2015, 6, 8177 CrossRef PubMed.
- X. Han, L. Sun, F. Wang and D. Sun, J. Mater. Chem. A, 2018, 6, 18891–18897 RSC.
- G. P. Hao, C. Tang, E. Zhang, P. Zhai, J. Yin, W. Zhu, Q. Zhang and S. Kaskel, Adv. Mater., 2017, 29, 1702829 CrossRef PubMed.
- H. Cao, S. Zhu, C. Yang, R. Bao, L. Tong, L. Hou, X. Zhang and C. Yuan, Nanotechnology, 2016, 27, 465402 CrossRef PubMed.
- G. Fang, J. Zhou, Y. Cai, S. Liu, X. Tan, A. Pan and S. Liang, J. Mater. Chem. A, 2017, 5, 13983–13993 RSC.
- S. Liu, J. Zhou and H. Song, Adv. Energy Mater., 2018, 8, 1800569 CrossRef.
- Y. Dong, W. Shi, P. Lu, J. Qin, S. Zheng, B. Zhang, X. Bao and Z.-S. Wu, J. Mater. Chem. A, 2018, 6, 14324–14329 RSC.
- D. Sun, S. Liu, G. Zhang and J. Zhou, Chem. Eng. J., 2019, 359, 1659–1667 CrossRef CAS.
- F. Cao, M. Zhao, Y. Yu, B. Chen, Y. Huang, J. Yang, X. Cao, Q. Lu, X. Zhang, Z. Zhang, C. Tan and H. Zhang, J. Am. Chem. Soc., 2016, 138, 6924–6927 CrossRef CAS PubMed.
- C. Guan, W. Zhao, Y. Hu, Z. Lai, X. Li, S. Sun, H. Zhang, A. K. Cheetham and J. Wang, Nanoscale Horiz., 2017, 2, 99–105 RSC.
- M. Nagalakshmi and N. Kalaiselvi, Electrochim. Acta, 2019, 304, 175–183 CrossRef CAS.
- H. Wen, C. Shi, Y. Gao, H. Rong, Y. Sha, H. Liu and Q. Liu, Nano, 2019, 13, 1850139 CrossRef.
- B.-W. Hu, Y.-J. Zhu, L. Du, T.-S. Mu, W.-Q. Zhu, G.-P. Yin, P. Chen and Q.-W. Li, Inorg. Chim. Acta, 2019, 494, 1–7 CrossRef CAS.
- T. Wang, Z. Kou, S. Mu, J. Liu, D. He, I. S. Amiinu, W. Meng, K. Zhou, Z. Luo, S. Chaemchuen and F. Verpoort, Adv. Funct. Mater., 2018, 28, 1705048 CrossRef.
- S. Liao, Y. Sun, J. Wang, H. Cui and C. Wang, Electrochim. Acta, 2016, 211, 11–17 CrossRef CAS.
- W. Bao, Z. Zhang, Y. Qu, C. Zhou, X. Wang and J. Li, J. Alloys Compd., 2014, 582, 334–340 CrossRef CAS.
- J. Zhao, X. Yang, Y. Yao, Y. Gao, Y. Sui, B. Zou, H. Ehrenberg, G. Chen and F. Du, Adv. Sci., 2018, 5, 1700768 CrossRef PubMed.
- R. Bi, C. Zeng, H. Huang, X. Wang and L. Zhang, J. Mater. Chem. A, 2018, 6, 14077–14082 RSC.
- Y. Cai, G. Fang, J. Zhou, S. Liu, Z. Luo, A. Pan, G. Cao and S. Liang, Nano Res., 2018, 11, 449–463 CrossRef CAS.
- S. Liu, J. Zhou and H. Song, Chem. Commun., 2018, 54, 9825–9828 RSC.
- M. Huang, K. Mi, J. Zhang, H. Liu, T. Yu, A. Yuan, Q. Kong and S. Xiong, J. Mater. Chem. A, 2017, 5, 266–274 RSC.
- R. Wu, D. P. Wang, V. Kumar, K. Zhou, A. W. K. Law, P. S. Lee, J. Lou and Z. Chen, Chem. Commun., 2015, 51, 3109–3112 RSC.
- K. Chen, Z. Sun, R. Fang, Y. Shi, H.-M. Cheng and F. Li, Adv. Funct. Mater., 2018, 28, 1707592 CrossRef.
- S. Liu, M. Tong, G. Liu, X. Zhang, Z. Wang, G. Wang, W. Cai, H. Zhang and H. Zhao, Inorg. Chem. Front., 2017, 4, 491–498 RSC.
- H. Han, Z. Bai, X. Wang, S. Chao, J. Liu, Q. Kong, X. Yang and L. Yang, Catal. Today, 2018, 318, 126–131 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- H. Zou, B. He, P. Kuang, J. Yu and K. Fan, ACS Appl. Mater. Interfaces, 2018, 10, 22311–22319 CrossRef CAS PubMed.
- M. Jiang, J. Li, X. Cai, Y. Zhao, L. Pan, Q. Cao, D. Wang and Y. Du, Nanoscale, 2018, 10, 19774–19780 RSC.
- Y. Xu, W. Tu, B. Zhang, S. Yin, Y. Huang, M. Kraft and R. Xu, Adv. Mater., 2017, 29, 1605957 CrossRef PubMed.
- T. Huang, Y. Chen and J.-M. Lee, ACS Sustainable Chem. Eng., 2017, 5, 5646–5650 CrossRef CAS.
- F. Yang, Y. Chen, G. Cheng, S. Chen and W. Luo, ACS Catal., 2017, 7, 3824–3831 CrossRef CAS.
- J.-S. Li, S.-L. Li, Y.-J. Tang, M. Han, Z.-H. Dai, J.-C. Bao and Y.-Q. Lan, Chem. Commun., 2015, 51, 2710–2713 RSC.
- W. Xia, J. Li, T. Wang, L. Song, H. Guo, H. Gong, C. Jiang, B. Gao and J. He, Chem. Commun., 2018, 54, 1623–1626 RSC.
- L. Liu, G. Zeng, J. Chen, L. Bi, L. Dai and Z. Wen, Nano Energy, 2018, 49, 393–402 CrossRef CAS.
- J. Cao, C. Lei, J. Yang, X. Cheng, Z. Li, B. Yang, X. Zhang, L. Lei, Y. Hou and K. Ostrikov, J. Mater. Chem. A, 2018, 6, 18877–18883 RSC.
- Y. Jiang, H. Liu, X. Tan, L. Guo, J. Zhang, S. Liu, Y. Guo, J. Zhang, H. Wang and W. Chu, ACS Appl. Mater. Interfaces, 2017, 9, 25239–25249 CrossRef CAS PubMed.
- S. Li, C. Cheng, H.-W. Liang, X. Feng and A. Thomas, Adv. Mater., 2017, 29, 1700707 CrossRef PubMed.
- Z. Li, M. Shao, L. Zhou, R. Zhang, C. Zhang, M. Wei, D. G. Evans and X. Duan, Adv. Mater., 2016, 28, 2337–2344 CrossRef CAS PubMed.
- Q. Zuo, T. Liu, C. Chen, Y. Ji, X. Gong, Y. Mai and Y. Zhou, Angew. Chem., Int. Ed., 2019, 58, 10198–10203 CrossRef CAS PubMed.
- X. Wang, P. Li, Z. Li, W. Chen, H. Zhou, Y. Zhao, X. Wang, L. Zheng, J. Dong, Y. Lin, X. Zheng, W. Yan, J. Yang, Z. Yang, Y. Qu, T. Yuan, Y. Wu and Y. Li, Chem. Commun., 2019, 55, 6563–6566 RSC.
Footnote |
| † Kuangmin Zhao and Weiwei Zhu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |




