Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fatty acids as biomimetic replication agents for luminescent metal–organic framework patterns†
Michael R.
Hafner
a,
Francesco
Carraro
 a,
Lea A.
Brandner
a,
Sivakumar
Maniam
b,
Gianluca
Grenci
a,
Lea A.
Brandner
a,
Sivakumar
Maniam
b,
Gianluca
Grenci
 bc,
Senka
Ljubojevic-Holzer
bc,
Senka
Ljubojevic-Holzer
 d,
Helmut
Bischof
e,
Roland
Malli
d,
Helmut
Bischof
e,
Roland
Malli
 e,
Sergey M.
Borisov
e,
Sergey M.
Borisov
 f,
Christian
Doonan
f,
Christian
Doonan
 *g and
Paolo
Falcaro
*g and
Paolo
Falcaro
 *ag
*ag
aInstitute of Physical and Theoretical Chemistry, Graz University of Technology, Graz 8010, Austria. E-mail: paolo.falcaro@tugraz.at
bMechanobiology Institute, National University of Singapore, 117411, Singapore
cBiomedical Engineering Department, National University of, Singapore, 117583, Singapore
dDepartment of Cardiology, Medical University of Graz, Graz 8036, Austria
eGottfried Schatz Research Center, Molecular Biology and Biochemistry, Medical University of Graz, Graz 8010, Austria
fInstitute of Analytical Chemistry and Food Chemistry, Graz University of Technology, Graz 8010, Austria
gDepartment of Chemistry, The University of Adelaide, Adelaide 5005, South Australia, Australia
First published on 11th September 2020
Abstract
Luminescent metal–organic frameworks (MOFs) are known to spontaneously self-assemble on human fingerprints. Here, we investigate the different chemical components of fingerprints and determine that MOF growth is predominantly induced by insoluble fatty acids. This finding shows that these simple biomolecules can be employed for the precise positioning of luminescent MOFs.
Spatially controlled self-assembly processes on substrates underpin MOF patterning protocols that are necessary for the fabrication of MOF-based devices (e.g. sensors).1 Biomimetic replication is an emerging, bottom-up, water-based patterning strategy that capitalizes on the affinity of MOF precursors for certain biomolecules.1,2 For example, proteins and carbohydrates that have a negative surface charge (isoelectric point, p. I. < 7)3 are efficient heterogeneous nucleation agents for MOFs.4,5 To this end, the water-soluble protein bovine serum albumin (BSA, p. I. = 5.3)3 has been microcontact printed on silicon wafers and immersed in solutions containing MOF precursors to yield patterns of zeolitic imidazolate framework-8 (ZIF-8) and different luminescent MOFs (i.e., Ln2(BDC)3*(H2O)4, BDC = 1,4-benzene dicarboxylic acid, Ln = lanthanide metal).2 By assuming the presence of proteins and amino acids in fingerprint residues, biomimetic replication was tested on sebaceous secretions.2,6,7 In minutes, MOF crystals could be selectively grown on fingerprint residues thus producing a fluorescent pattern (Fig. 1a and Fig. S1, ESI†). The potential of MOFs for forensic applications was investigated by Moret et al. and de Jong et al.6,7 However, sebaceous secretions are composed of numerous molecular species that could potentially promote MOF formation and only a minor fraction is made of water-soluble proteins and amino acids (vide infra). Thus, we were motivated to systematically investigate which of the chemical species present in fingerprints engendered MOF crystallization. Our study revealed two significant findings relevant to both the application of MOFs to forensics and for applications that require precise positioning on substrates: (1) proteins do not contribute to the growth of Tb2(BDC)3*(H2O)4 MOFs on fingerprints, rather crystallisation is triggered by the fatty acid component (Fig. 1a); and (2) with respect to the biomimetic replication process, biomolecules with low solubility prevent the seeding of, uncontrolled, crystal growth in solution (Fig. 1b), which is crucial for the fabrication of MOF films and patterns.1,2,4,8–13 Both aspects are demonstrated here for the first time. Chemical fingerprint analyses revealed that their composition is similar from person to person; however, the relative amount of the chemical components can vary depending on factors including age, gender, and habits.14–19 Fingerprint residues on surfaces are typically a mixture of specific biomolecules including triglycerides, fatty acids (e.g. palmitic, oleic, and myristic acids), wax esters and squalene (Table 1).16,20–22
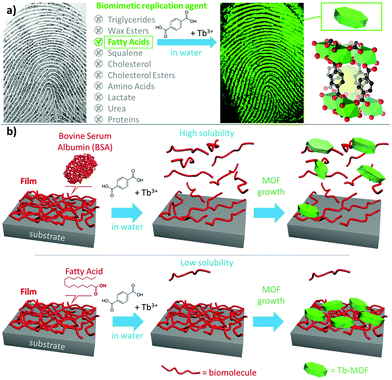 | ||
| Fig. 1 (a) Human fingerprint and major biomolecules present in sebaceous and eccrine secretions. Tb-MOF can grow on fingerprints residues (see Fig. S1, ESI†). By investigating one biomolecule at the time, we found that fatty acids are the most effective biomimetic replication agents. (b) Film of biomolecules on a substrate and influence of its water solubility on the fabrication of a MOF film via biomimetic replication. | ||
| Sebaceous secretion16,22 | Eccrine secretion16,20,22 | ||
|---|---|---|---|
| Compound | Content [w%] | Compound | Content [w%] |
| Triglycerides | 30–40 | Amino Acids | 0.002–0.02 |
| Free fatty acids | 15–25 | Lactate | 0.1–0.2 |
| Wax esters | 20–25 | Urea | 0.004–0.04 |
| Squalene | 10–12 | Proteins | 0.002–1 |
| Cholesterol | 1–3 | Inorganics | 0.02–0.6 |
| Cholesterol esters | 2–3 | Water | 98–99 |
Proteins and amino acids are only minor components of the biomolecule mixture: the weight percentage (w%) in eccrine secretions ranges from 0.002% to 1%. The main amino acids in fingerprints have been identified as L-Serine, Glycine and L-Alanine and the protein content consists of a broad variety of mostly unknown macromolecules.14,16,20–22 Given the assumption that the MOF growth was induced by this low amount of amino acids and proteins of unknown p. I. in sebaceous secretions and that the molecular components have not been investigated yet, here, we examine the crystallization of Tb2(BDC)3*(H2O)4, a green fluorescent MOF (for details on absorption and emission of Tb-MOF, see Fig. S2, ESI†), on each of the main molecular components present in fingerprint residues.
We examined the molecular components of sebaceous and eccrine secretions (Table 1) along with BSA (the model protein previously used to simulate fingerprints)2,8,11,23 as the biomimetic replication agent. For each class of molecule, we selected a representative compound: for triglycerides, trimyristin was selected;16,19 for fatty acids, we selected palmitic acid;15,16 for wax esters, we chose palmityl palmitate;16,19 squalane was used instead of squalene due to its chemical stability towards oxidation;16,19,24 and for amino acids, we selected L-serine.16,25 Finally, cholesterol, lactate and urea were used as themselves. Each molecule was dissolved in water (2 mg mL−1) and 2 μL drops of the respective solutions were cast on a glass slide. After drying, the samples were immersed for 30 s in a freshly prepared aqueous solution of MOF precursors (see ESI,† for details). Then, the substrates were washed with water and the glass slides were illuminated using a UV lamp (254 nm) to qualitatively verify the presence of the luminescent MOF.
Surprisingly, the fatty acid was the only sample that showed a homogenous luminescent spot (Fig. 2a and Fig. S3, ESI†), which is indicative of Tb-MOF growth via biomimetic replication. To observe the luminescent signal from drop-cast BSA solution, 30 mg mL−1 solution (15 times more concentrated than the fatty acid solution) was required. The MOF grown from BSA forms a luminescent ring (Fig. 2a and Fig. S3, ESI†). This inhomogeneity can be attributed to the Marangoni effect (i.e., during the solvent evaporation the solvated molecule concentrates at the edge of the spot).26 Thus the higher local concentration of BSA at the perimeter of the ring promotes biomimetic replication. On exposing the samples to longer reaction times (1 min, 5 min and 15 min), a qualitative increase in luminescence intensity under UV illumination was observed only in the case of the fatty acid spot (Fig. S3, ESI†). For BSA, the sharp ring that was initially observed appears to decompose over time (Fig. S3, ESI†). This may be caused by the high solubility of BSA in water,2,27 as the protein would dissolve in solution and initiate the detachment of the MOF crystals grown on the BSA film. For other samples (e.g. wax and triglyceride), the increased luminescence from longer reaction time was qualitatively comparable to the background (uncoated SiO2). Thus, the increase in luminescence intensity is likely due to the spontaneous formation of the Tb-MOF. It is noteworthy that without any seeding agents the Tb-MOF can be detected with the naked eye after 5 min. We quantitatively examined biomimetic replication as a function of the reaction time (1, 5 and 15 min), by measuring the optical response of the different films of biomolecules using a confocal fluorescence microscope (ESI,† for details, Fig. S15–S20). Intense luminescent signals with increasing intensity were measured only in the case of fatty acid and BSA (Fig. 2b and Fig. S15, S20, ESI†). The other biomolecules displayed only a few random luminescent spots after 5 minutes of reaction. The box-plot representation (Fig. 2b and Fig. S22, ESI†) shows the narrowest distribution of intensities and the most intense luminescence signal collected from the MOF grown on fatty acid films, suggesting the formation of a homogeneous MOF film. To investigate the formation of MOF crystals both on the substrate surface and in bulk solution, we monitored the luminescence intensity over time (15 minutes in total) using a camera. In each vial, a different biomolecule was deposited via drop-cast (see ESI,† for details) and dried to obtain a film. Then, the MOF precursor solution was added, and the formation of the luminescent material was detected only in the vial with the palmitic acid film (Fig. S21 and Video V1, ESI†). Optical and electron microscopy analyses were also performed to examine the growth of Tb-MOF on surfaces. The results are entirely consistent with previous observations and confirm that Tb-MOF grows homogeneously on fatty acids, that the concentrated ring of BSA decomposes with time and that the other biomolecules do not significantly promote MOF crystallization on surfaces (Fig. 2c–e and Fig. S4–S10, S23–S25, ESI†). In addition, complete dissolution of the biomacromolecule film was observed when water-soluble molecule films (e.g. BSA 2 mg mL−1, urea, lactate and amino acid) were immersed in the MOF precursor solution.
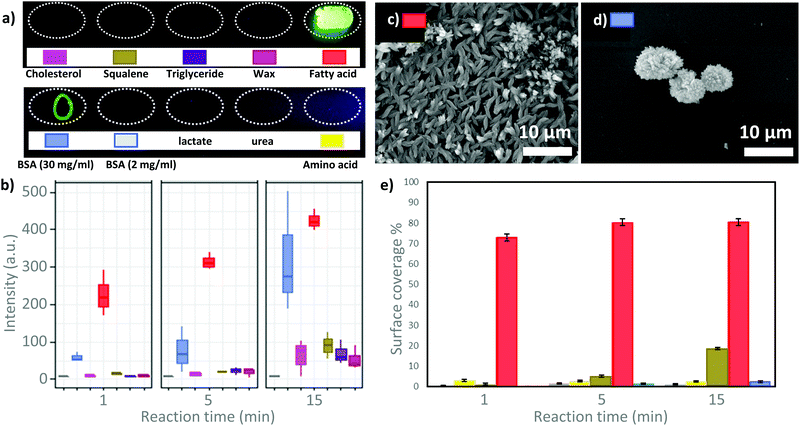 | ||
| Fig. 2 (a) Glass slide with spots of biomolecules after 30 seconds of exposure to Tb-MOF precursors (upper row, f.l.t.r.: cholesterol, squalene, triglyceride, wax, fatty acid; lower row: f.l.t.r.: BSA (30 mg mL−1), BSA, lactate, urea, amino acid). (b) Box-plot representation of luminescence intensity calculated from fluorescence micrographs (UV source: 340 nm, investigated area 250 by 150 μm; see Fig. S2, ESI,† for absorption and emission spectra) acquired at different MOF growth times for the different biomolecule spots (Fig. S15–S20, ESI†). For details about the interpretation of box-plots, see Fig. S22 (ESI†). (c) SEM micrograph of the Tb-MOF grown on drop-cast fatty acid (15 minutes of growth). (d) SEM micrograph of the Tb-MOF grown on drop-cast BSA (15 minutes of growth). (e) Growth-time dependent Tb-MOF surface coverage calculated from SEM micrographs (100× magnification, Fig. S23–S25, ESI†). | ||
Fourier-transform infrared spectroscopy (FT-IR) was used to evaluate the chemical composition of the biomolecule films after exposure to the MOF precursor solution. Close inspection of the data from the fatty acid (2 mg mL−1) and BSA (30 mg mL−1) films confirmed the presence of vibrational modes that could be assigned to Tb-MOF (e.g., 1540 and 1400 cm−1, de-protonated and coordinated 1,4-benzene dicarboxylic acid; 510 and 440 cm−1, Tb–O stretching modes,28,29 Fig. S11, ESI†). Furthermore, FT-IR confirmed that all the other molecules were ineffective biomimetic replication agents as vibrational modes of the Tb-MOF were not detected (Fig. S12 and S13, ESI†).
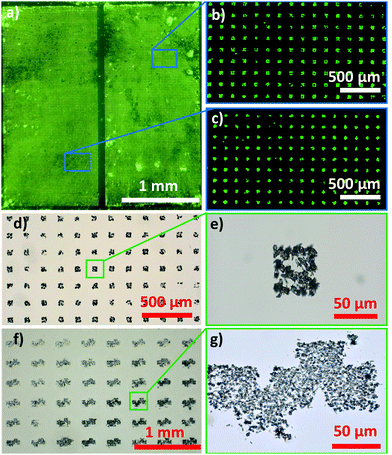
Next, XRD was performed to examine the crystallinity of the MOF films. The diffractograms of the control Tb-MOF (formed in solution without biomolecules), and the Tb-MOF on BSA were identical to those of previously reported Tb-MOF (Fig. S14, ESI†).2,28–30 However, the XRD pattern of the Tb-MOF grown on a fatty acid substrate showed additional peaks (Fig. S14, ESI†): in addition to the (010) peak assigned to Tb2(BDC)3*(H2O)4, we measured peaks attributed to the crystallisation of the fatty acid molecules (4.9°, 7.4°, 12.3° and 17.3°)31,32 and to the presence of Tb-palmitate (6°, 6.6°, 8° and 10°).33 The absence of the Tb-MOF peaks at 11.3°, 14.6° and 15.6° indicates that the MOF crystals grown on fatty acids have a preferential direction of growth ((010)).34 The morphology of these crystals, as determined using SEM, supports this hypothesis (Fig. 2c). The formation of Tb-palminate indicates a strong affinity between the fatty acid (carboxylic group) and the Tb cation.3,4,35 Thus, we were motivated to explore the fabrication of microscopic MOF patterns from palmitic acid. A micropattern of palmitic acid was fabricated using micro-contact printing (μCP)36,37 (see ESI,† for details, Fig. 3 and Fig. S24–S26), and the pattern was used for the controlled growth of Tb-MOF. This soft lithographic method allows for the facile preparation of complex patterns of fatty acids on several substrates including glass, silicon, polystyrene, and flexible polypropylene foils (Fig. 3 and Fig. S26–S28, ESI†). After exposing the patterned substrate to the MOF precursor solution, the MOF crystals were found to selectively grow on the fatty acid pattern (Fig. 3 and Fig. S27, ESI†).
In summary, we established that it is the presence of fatty acids that promotes the selective growth of luminescent MOF films on fingerprint residues. Indeed, fatty acids are significantly more efficient as biomimetic replication agents than BSA, a model protein used in previous studies. For example, after 1 minute of reaction, the drop-casting of a low concentration fatty acid solution (2 mg mL−1, 20 μL) afforded a MOF film with a 300% more intense luminesce signal compared to that of the drop-cast high concentration BSA solution (30 mg mL−1, 20 μL). Due to both the affinity of palmitic acid for cations and the low solubility in water of fatty acids, these biomolecules are ideal candidates for the water-based synthesis of MOF patterns and films.
The authors acknowledge the European Union's Horizon 2020 Programme (FP/2014–2020)/ERC Grant Agreement no. 771834 POPCRYSTAL. The authors thank TU Graz for the Lead Project (LP-03) and Varta Microtechnologies GmbH for the use of SEM.
Conflicts of interest
There are no conflicts to declare.References
- P. Falcaro, R. Ricco, C. M. Doherty, K. Liang, A. J. Hill and M. J. Styles, Chem. Soc. Rev., 2014, 43, 5513–5560 Search PubMed.
- K. Liang, C. Carbonell, M. J. Styles, R. Ricco, J. Cui, J. J. Richardson, D. Maspoch, F. Caruso and P. Falcaro, Adv. Mater., 2015, 27, 7293–7298 Search PubMed.
- N. K. Maddigan, A. Tarzia, D. M. Huang, C. J. Sumby, S. G. Bell, P. Falcaro and C. J. Doonan, Chem. Sci., 2018, 9, 4217–4223 Search PubMed.
- E. Astria, M. Thonhofer, R. Ricco, W. Liang, A. Chemelli, A. Tarzia, K. Alt, C. E. Hagemeyer, J. Rattenberger, H. Schroettner, T. Wrodnigg, H. Amenitsch, D. M. Huang, C. J. Doonan and P. Falcaro, Mater. Horiz., 2019, 6, 969–977 Search PubMed.
- S. Li, M. Dharmarwardana, R. P. Welch, C. E. Benjamin, A. M. Shamir, S. O. Nielsen and J. J. Gassensmith, ACS Appl. Mater. Interfaces, 2018, 10, 18161–18169 Search PubMed.
- R. de Jong and M. de Puit, Forensic Sci. Int., 2018, 291, 12–16 Search PubMed.
- S. Moret, E. Scott, A. Barone, K. Liang, C. Lennard, C. Roux and X. Spindler, Forensic Sci. Int., 2018, 291, 83–93 Search PubMed.
- J. Zhuang, A. P. Young and C.-K. Tsung, Small, 2017, 13, 1700880 Search PubMed.
- F. Nudelman and N. A. J. M. Sommerdijk, Angew. Chem., Int. Ed., 2012, 51, 6582–6596 Search PubMed.
- K. Liang, R. Ricco, C. M. Doherty, M. J. Styles and P. Falcaro, CrystEngComm, 2016, 18, 4264–4267 Search PubMed.
- C. Doonan, R. Riccò, K. Liang, D. Bradshaw and P. Falcaro, Acc. Chem. Res., 2017, 50, 1423–1432 Search PubMed.
- A. Poddar, J. J. Conesa, K. Liang, S. Dhakal, P. Reineck, G. Bryant, E. Pereiro, R. Ricco, H. Amenitsch, C. Doonan, X. Mulet, C. M. Doherty, P. Falcaro and R. Shukla, Small, 2019, 1902268 Search PubMed.
- P. Falcaro, A. J. Hill, K. M. Nairn, J. Jasieniak, J. I. Mardel, T. J. Bastow, S. C. Mayo, M. Gimona, D. Gomez, H. J. Whitfield, R. Riccò, A. Patelli, B. Marmiroli, H. Amenitsch, T. Colson, L. Villanova and D. Buso, Nat. Commun., 2011, 2, 237 Search PubMed.
- L. S. Ferguson, F. Wulfert, R. Wolstenholme, J. M. Fonville, M. R. Clench, V. A. Carolan and S. Francese, Analyst, 2012, 137, 4686 Search PubMed.
- Y. Nunome, T. Tsuda and K. Kitagawa, Anal. Sci., 2010, 26, 917–919 Search PubMed.
- A. Girod, R. Ramotowski and C. Weyermann, Forensic Sci. Int., 2012, 223, 10–24 Search PubMed.
- K. M. Antoine, S. Mortazavi, A. D. Miller and L. M. Miller, J. Forensic Sci., 2010, 55, 513–518 Search PubMed.
- D. K. Williams, C. J. Brown and J. Bruker, Forensic Sci. Int., 2011, 206, 161–165 Search PubMed.
- A. Girod and C. Weyermann, Forensic Sci. Int., 2014, 238, 68–82 Search PubMed.
- S. Oonk, T. Schuurmans, M. Pabst, L. C. P. M. de Smet and M. de Puit, Sci. Rep., 2018, 8, 16425 Search PubMed.
- M. M. Houck, Forensic Fingerprints, Elsevier Science, San Diego, 2016 Search PubMed.
- S. M. Bleay, R. S. Croxton and M. D. Puit, Fingerprint development techniques: theory and application, 2018 Search PubMed.
- K. Liang, R. Ricco, C. M. Doherty, M. J. Styles, S. Bell, N. Kirby, S. Mudie, D. Haylock, A. J. Hill, C. J. Doonan and P. Falcaro, Nat. Commun., 2015, 6, 7240 Search PubMed.
- S. Cadd, M. Islam, P. Manson and S. Bleay, Sci. Justice, 2015, 55, 219–238 Search PubMed.
- S. Hong, I. Hong, A. Han, J. Y. Seo and J. Namgung, Forensic Sci. Int., 2015, 257, 403–408 Search PubMed.
- P. Innocenzi, L. Malfatti and P. Falcaro, Water droplets to nanotechnology: a journey through self-assembly, RSC Publ., Royal Soc. of Chemistry, Cambridge, 2013 Search PubMed.
- C. Nick Pace, S. Treviño, E. Prabhakaran and J. Martin Scholtz, Philos. Trans. R. Soc. London, Ser. B, 2004, 359, 1225–1235 Search PubMed.
- T. M. Reineke, M. Eddaoudi, M. Fehr, D. Kelley and O. M. Yaghi, J. Am. Chem. Soc., 1999, 121, 1651–1657 Search PubMed.
- C. Daiguebonne, N. Kerbellec, O. Guillou, J.-C. Bünzli, F. Gumy, L. Catala, T. Mallah, N. Audebrand, Y. Gérault, K. Bernot and G. Calvez, Inorg. Chem., 2008, 47, 3700–3708 Search PubMed.
- N. Kerbellec, D. Kustaryono, V. Haquin, M. Etienne, C. Daiguebonne and O. Guillou, Inorg. Chem., 2009, 48, 2837–2843 Search PubMed.
- F. F. de Sousa, C. E. S. Nogueira, P. T. C. Freire, S. G. C. Moreira, A. M. R. Teixeira, A. S. de Menezes, J. Mendes Filho and G. D. Saraiva, Spectrochim. Acta, Part A, 2016, 161, 162–169 Search PubMed.
- E. Moreno, R. Cordobilla, T. Calvet, F. J. Lahoz and A. I. Balana, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2006, 62, o129–o131 Search PubMed.
- H. Li, W. Bu, W. Qi and L. Wu, J. Phys. Chem. B, 2005, 109, 21669–21676 Search PubMed.
- O. Shekhah, Materials, 2010, 3, 1302–1315 Search PubMed.
- A.-W. Xu, Y. Ma and H. Cölfen, J. Mater. Chem., 2007, 17, 415–449 Search PubMed.
- C. Thibault, V. Le Berre, S. Casimirius, E. Trévisiol, J. François and C. Vieu, J. Nanobiotechnol., 2005, 3, 7 Search PubMed.
- S. Alom Ruiz and C. S. Chen, Soft Matter, 2007, 3, 168–177 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; XRD, FTIR, and optical, fluorescent and electron microscopy data. See DOI: 10.1039/d0cc03876h |
| This journal is © The Royal Society of Chemistry 2020 |