Naoyuki Shimada, Kenji Fukuhara, Sari Urata and Kazuishi Makino
Org. Biomol. Chem., 2019,17, 7325-7329
DOI:
10.1039/C9OB01445D,
Communication
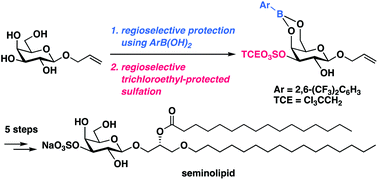
A concise total synthesis of seminolipid, a sulfoglycolipid, has been achieved; key features include regioselective, tin-free sulfation of allyl β-D-galactopyranoside using 2,6-bis(trifluoromethyl)phenylboronic acid as protective reagent, stereoselective epoxidation, and site-selective acylation. The utility of this divergent synthetic approach to introduce 2,2,2-trichloroethyl-protected sulfate group at an early stage without toxic and environmentally unfavorable tin reagents was demonstrated by the syntheses of three seminolipid analogues with different side-chains from the common intermediate.