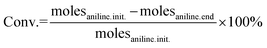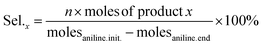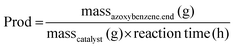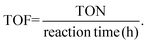Open Access Article
Open Access ArticleNiobium oxide prepared through a novel supercritical-CO2-assisted method as a highly active heterogeneous catalyst for the synthesis of azoxybenzene from aniline†
Yehan
Tao
,
Bhawan
Singh
,
Vanshika
Jindal
,
Zhenchen
Tang
and
Paolo P.
Pescarmona
 *
*
Chemical Engineering Group, Engineering and Technology Institute Groningen (ENTEG), Faculty of Science and Engineering, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: p.p.pescarmona@rug.nl
First published on 11th September 2019
Abstract
High-surface area Nb2O5 nanoparticles were synthesised by a novel supercritical-CO2-assisted method (Nb2O5-scCO2) and were applied for the first time as a heterogeneous catalyst in the oxidative coupling of aniline to azoxybenzene using the environmentally friendly H2O2 as the oxidant. The application of scCO2 in the synthesis of Nb2O5-scCO2 catalyst resulted in a significantly enhanced catalytic activity compared to a reference catalyst prepared without scCO2 (Nb2O5-Ref) or to commercial Nb2O5. Importantly, the Nb2O5-scCO2 catalyst achieved an aniline conversion of 86% (stoichiometric maximum of 93% with the employed aniline-to-H2O2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4) with an azoxybenzene selectivity of 92% and with 95% efficiency in H2O2 utilisation in 45 min without requiring external heating (the reaction is exothermic) and with an extremely low catalyst loading (weight ratio between the catalyst and substrate, Rc/s = 0.005). This performance largely surpasses that of any other heterogeneous catalyst previously reported for this reaction. Additionally, the Nb2O5 catalyst displayed high activity also for substituted anilines (e.g. methyl or ethyl-anilines and para-anisidine) and was reused in consecutive runs without any loss of activity. Characterisation by means of N2-physisorption, XRD, FTIR and TEM allowed the correlation of the remarkable catalytic performance of Nb2O5-scCO2 to its higher surface area and discrete nanoparticle morphology compared to the aggregated larger particles constituting the material prepared without scCO2. A catalytic test in the presence of a radical scavenger proved that the reaction follows a radical pathway.
1.4) with an azoxybenzene selectivity of 92% and with 95% efficiency in H2O2 utilisation in 45 min without requiring external heating (the reaction is exothermic) and with an extremely low catalyst loading (weight ratio between the catalyst and substrate, Rc/s = 0.005). This performance largely surpasses that of any other heterogeneous catalyst previously reported for this reaction. Additionally, the Nb2O5 catalyst displayed high activity also for substituted anilines (e.g. methyl or ethyl-anilines and para-anisidine) and was reused in consecutive runs without any loss of activity. Characterisation by means of N2-physisorption, XRD, FTIR and TEM allowed the correlation of the remarkable catalytic performance of Nb2O5-scCO2 to its higher surface area and discrete nanoparticle morphology compared to the aggregated larger particles constituting the material prepared without scCO2. A catalytic test in the presence of a radical scavenger proved that the reaction follows a radical pathway.
Introduction
Over the past few years, azoxybenzene and its derivatives have garnered interest due to their importance in organic synthesis as intermediates for producing dyes or medicines, as inhibitors or stabilisers for polymerisation, and as liquid crystals in electronic displays.1–4 For example, azoxybenzene is the precursor to prepare hydroxyazobenzene, a common azo dye, by Wallach rearrangement.5 Reductive cleavage of azoxybenzene or its derivatives gives indazole derivatives, which have pharmaceutical activity.6 Methoxy-substituted azoxybenzene, i.e. azoxyanisole, is one of the first known and most readily prepared liquid crystals.7 In addition, exploration of the functionalisation of azoxybenzene, such as acylation or alkenylation, has also been reported.8 Azoxybenzene can be synthesised either by oxidative coupling of aniline or by selective reduction of nitrobenzene or nitrosobenzene. The reduction route has been extensively studied, employing for example Ni–C–CeO2,9 Au–hydrotalcite,10 Ag–Cu–ZrO2,11 and Pd–CdS12 as catalysts. On the industrial scale, nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulphuric acid, nitric acid and water, whereas nitrosobenzene is prepared by reduction of nitrobenzene. Both of them are obtained from petroleum-based raw materials. On the other hand, the production of aniline from renewable resources (unrefined raw sugar) has been recently reported by Covestro,13 which makes the oxidation route preferable from a green chemistry point of view.The oxidation route comprises competitive oxidation and condensation reactions (Scheme 1). Therefore, the reaction can generate several products, including phenylhydroxylamine,14 nitrosobenzene,15 azobenzene,16 azoxybenzene17 and nitrobenzene.18 This represents a major challenge for achieving high selectivity towards a specific product, such as azo or azoxybenzene. Several metal-based heterogeneous catalysts have been applied in the oxidation of aniline and its substituted derivatives with the aim of selectively yielding azoxy products using H2O2 as the oxidant. H2O2 is an environmentally friendly oxidant as it gives water as the only side product, it can be operated at ambient pressure and more safely compared to molecular oxygen,15,19 and is significantly cheaper and greener compared to other oxidants that have been reported for this reaction, such as peracetic acid,20tert-butylhydroperoxide, dimethyldioxirane,21 sodium perborate etc.22 An overview of the state-of-the-art heterogeneous catalysts for the oxidative coupling of aniline with H2O2 as the oxidant is shown in Table S1.† Ti-Based materials such as TiO223–25 and Ti-silicates (TS-1,26,27 Ti-MCM-48,28 TAPSO-5, Ti-HMS, and Ti-Beta29) are the most common types of heterogeneous catalysts that have been reported for this reaction. They have been reported to activate H2O2 by forming hydroperoxo species.23,28 Most of these Ti-based catalysts typically operate in the 50–70 °C temperature range, while the TiO2 pillared montmorillonite clays (∼60 wt% TiO2) allowed the oxidation of aniline at room temperature (RT), reaching 50% aniline conversion with 99% azoxybenzene selectivity after 8 h with a weight ratio between the catalyst and substrate, Rc/s = 0.02.25 Other metal oxide catalysts such as Co–Si-oxide30 and CuCr2O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 were also reported to catalyse the oxidative coupling of aniline. Higher temperatures (70 or 80 °C) were used in both cases to obtain higher than 70% conversion (Table S1†). Supported metal nanoparticles were also reported as catalysts for aniline oxidation with H2O2. For example, an Ag-WO3 catalyst, in which the Ag nanoparticles were proposed to be the sites responsible for the activation of H2O2,32 exhibited an aniline conversion of 87% with an azoxybenzene selectively of 91% at RT after 24 h employing a Rc/s = 0.1. The non-noble metal catalyst Cu-CeO2 was reported to display higher aniline conversion and azoxybenzene selectivity compared to the Ag-WO3 catalyst after 6 h with the same Rc/s, although using 50 °C as the reaction temperature.33 Control tests showed that the Cu nanoparticles acted as catalytic sites while CeO2 performed as a support. The above-mentioned catalytic systems achieve good performance but still have some limitations, which can be related to the synthesis of the catalysts (e.g. synthesis requiring expensive templates26–33 or dangerous chemicals such as hexafluorosilicic acid24) and/or to the employed conditions in which the oxidation of aniline is carried out (e.g. relatively high reaction temperatures,23,24,26,28,29,31,33 problematic or hazardous solvents26,27,29–33 and/or high loading of the catalyst, e.g. Rc/s > 0.1, see Table S1†).23,27,29,31–33 On this backdrop, we report the novel synthesis of amorphous Nb2O5 nanoparticles in supercritical CO2 medium and their application as a heterogeneous catalyst with superior performance for the oxidative coupling of aniline and its derivatives to produce (substituted) azoxybenzene. The catalyst allowed performing the reaction without external heating and with an extremely low catalyst loading (Rc/s = 0.005) in a green solvent as ethanol,34 displaying very high aniline conversion and azoxybenzene yield in a short reaction time. All these achievements represent an important green advance as they would allow carrying out the conversion of (potentially) bio-based aniline into a useful compound under extremely mild and green conditions.
31 were also reported to catalyse the oxidative coupling of aniline. Higher temperatures (70 or 80 °C) were used in both cases to obtain higher than 70% conversion (Table S1†). Supported metal nanoparticles were also reported as catalysts for aniline oxidation with H2O2. For example, an Ag-WO3 catalyst, in which the Ag nanoparticles were proposed to be the sites responsible for the activation of H2O2,32 exhibited an aniline conversion of 87% with an azoxybenzene selectively of 91% at RT after 24 h employing a Rc/s = 0.1. The non-noble metal catalyst Cu-CeO2 was reported to display higher aniline conversion and azoxybenzene selectivity compared to the Ag-WO3 catalyst after 6 h with the same Rc/s, although using 50 °C as the reaction temperature.33 Control tests showed that the Cu nanoparticles acted as catalytic sites while CeO2 performed as a support. The above-mentioned catalytic systems achieve good performance but still have some limitations, which can be related to the synthesis of the catalysts (e.g. synthesis requiring expensive templates26–33 or dangerous chemicals such as hexafluorosilicic acid24) and/or to the employed conditions in which the oxidation of aniline is carried out (e.g. relatively high reaction temperatures,23,24,26,28,29,31,33 problematic or hazardous solvents26,27,29–33 and/or high loading of the catalyst, e.g. Rc/s > 0.1, see Table S1†).23,27,29,31–33 On this backdrop, we report the novel synthesis of amorphous Nb2O5 nanoparticles in supercritical CO2 medium and their application as a heterogeneous catalyst with superior performance for the oxidative coupling of aniline and its derivatives to produce (substituted) azoxybenzene. The catalyst allowed performing the reaction without external heating and with an extremely low catalyst loading (Rc/s = 0.005) in a green solvent as ethanol,34 displaying very high aniline conversion and azoxybenzene yield in a short reaction time. All these achievements represent an important green advance as they would allow carrying out the conversion of (potentially) bio-based aniline into a useful compound under extremely mild and green conditions.
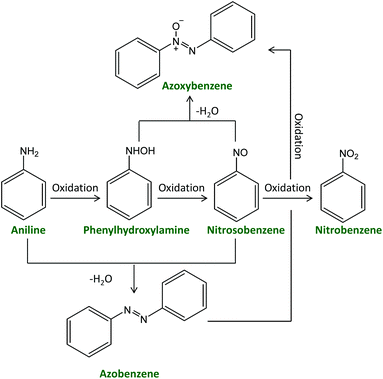 | ||
| Scheme 1 Generally-accepted reaction scheme for the synthesis of azoxybenzene via the oxidation of aniline. | ||
A crucial factor in achieving a catalyst with enhanced activity was the development of a novel supercritical-CO2-assisted precipitation method for the preparation of highly-dispersed Nb2O5 nanoparticles. CO2 in the supercritical state (scCO2) is an attractive reaction medium for preparing nanostructured oxide catalysts for the combination of its properties, which are intermediate between those of a liquid and a gas and can be easily tuned by changing the temperature and pressure. More specifically, scCO2 has good dissolving ability that can be exploited to promote the contact between compounds with different physicochemical features (e.g. polar and apolar; gas and liquid). In addition, scCO2 has high diffusivity and extremely low surface tension, which are very important for maximum preservation of the formed nanostructures when removing CO2 from the system, which can be achieved simply by depressurisation.35 It has been extensively reported that the formation of nanomaterials typically proceeds differently with the assistance of scCO2.36–38 Further advantages of the use of scCO2 as a reaction medium include the low toxicity and low cost of carbon dioxide and the relatively mild conditions needed to achieve its supercritical point (Tc = 31.1 °C, pc = 73.9 bar).
Experimental
Materials
Niobium chloride (NbCl5), deionised water and ethanol were used for the preparation of the Nb2O5 materials. Commercially available Nb2O5 (Nb2O5-Comm), TiO2-P25, and WO3 (WO3-Comm) were used as reference catalysts. For the catalytic tests, aniline, ortho-toluidine, meta-toluidine, para-toluidine, 2-ethylaniline, 3-ethylaniline, 4-ethylaniline, para-anisidine, and benzylamine were used as substrates, anisole was used as the internal standard for gas chromatography (GC) analysis, while aqueous H2O2 with different concentrations (10 wt%, 20 wt%, 30 wt% and 50 wt%) was employed as the oxidant. Ethanol, 1,4-dioxane, acetone, acetonitrile, 2-butanol, isopropanol and methanol were tested as reaction solvents. 2,2,6,6-Tetramethyl-1-piperidinyloxy (TEMPO) was used as a radical scavenger. The purity and supplier of all chemicals (except for 10 wt% and 20 wt% H2O2) used during this research are listed in Table S2.† All chemicals were used without any further purification. 10 wt% and 20 wt% H2O2 aqueous solutions were prepared by diluting 30 wt% H2O2 with deionised water.Catalyst preparation
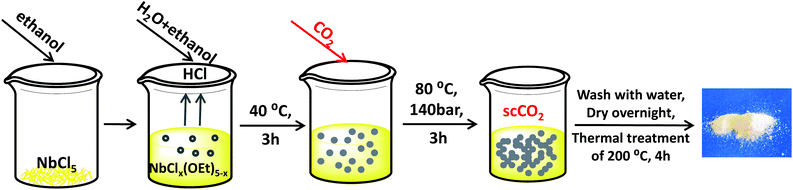
Nb2O5 was prepared using a scCO2-assisted precipitation method, with a novel protocol developed by adapting the synthesis methods of other metal oxides in scCO2 medium36,38,39 and by applying it to a precipitation method inspired by those used in the literature to prepare Nb2O5.40–42 The synthesis was carried out in a high-throughput scCO2 reactor unit manufactured by Integrated Lab Solutions (ILS),43 which consists of two modules: a batch reactor equipped with a borosilicate glass window to allow visualisation of the phase behaviour within the reactor, and a block with 10 batch reactors (Fig. S1†). The window reactor and the 10-reactor block can be used simultaneously with individual operation steps. Each reactor has a volume of 84 mL (30 mm internal diameter), can be stirred individually with a magnetic stirrer and is equipped with an automated closing valve that allows avoiding cross-contamination between the reactors. The reactors are heated with electric heating elements, pressurised with an ISCO pump and cooled with a water-circulation system. The high-throughput unit can operate at a temperature between 20 and 200 °C and at a CO2 pressure between 1 and 200 bar. An automated depressurisation protocol and rupture disks prevent the risks of overpressure. In a typical synthesis of the Nb2O5-scCO2 material, firstly a niobium precursor solution was prepared by dissolving 1.0 g NbCl5 in 2 mL ethanol with magnetic stirring for 5 min. HCl was formed as a gaseous product, indicating the formation of niobium ethoxide species. Then, 10 mL deionised water and 3 mL ethanol were added slowly to the stirred solution over 5 min. Upon the addition of water, the liquid became gradually opaque. Next, the mixture was transferred into the scCO2 reactor and stirred vigorously at 40 °C for 3 h. At this stage, the white reaction mixture behaved like a high-viscosity liquid. Then, the reactor was closed, heated up to 80 °C and pressurised with CO2 to 140 bar while stirring (this process took around 1.5 h). The reaction mixture was allowed to react under scCO2 conditions for 3 h. Then, the reactor was cooled down to 20 °C and CO2 was removed by slow depressurisation with an average rate of 1.5 bar min−1. The obtained white slurry was aged overnight and then washed thoroughly with deionised water over a Büchner filter until the pH of the filtered water became neutral. Next, the material was dried overnight at 100 °C and the resulting powder was thermally treated in a calcination oven at 200 °C for 4 h with a heating rate of 2 °C min−1. The catalyst prepared by this method was named Nb2O5-scCO2. The synthesis process is summarised in Fig. 1. Since no formation of a gel was observed during the reaction, but only a precipitate, the used synthesis method is referred to as a scCO2-assisted precipitation method. The mass of the calcined powders was always above 90% of the theoretical yield of Nb2O5 (around 0.45 g for each batch reactor). The as-prepared Nb2O5 powder was also thermally treated at 800 °C for 4 h with a heating rate of 2 °C min−1 and used as a reference catalyst (Nb2O5-800 °C). Another reference Nb2O5 catalyst (Nb2O5-Ref) was prepared in a round-bottom flask with a similar procedure but without the assistance of scCO2. Briefly, 1.0 g NbCl5 was dissolved in 2 mL ethanol by stirring for 5 min, after which 10 mL deionised water and 3 mL ethanol were added slowly in 5 min. Next, the solution was stirred at 40 °C for 3 h. Then, the mixture was heated up to 80 °C and stirred for another 3 h. After that, the obtained product was aged, dried, washed and thermally treated with the same procedure as that used for Nb2O5-scCO2.Characterisation
The Nb2O5 materials were characterised by a combination of techniques. N2-Physisorption isotherms were recorded on a Micromeritics ASAP 2420 apparatus at −196 °C. The surface area was evaluated with the BET method. Before N2 adsorption, the samples were degassed under reduced pressure at 200 °C for 5 h. X-ray diffraction patterns (XRD) were recorded on a Bruker D8 Phaser diffraction meter equipped with Cu Kα radiation (λ = 1.5406 Å). The XRD patterns were obtained in reflection geometry in the 2θ range between 10 and 80°. Fourier transform infrared spectroscopy (FTIR) measurements were performed with an IRTracer-100 spectrometer by averaging 64 scans with a spatial resolution of 4 cm−1. The background spectrum was recorded by using an empty cell. Transmission electron microscopy (TEM) images were recorded using an electron microscope CM12 (Philips) operating at 120 keV. The samples were ground, then dispersed in ethanol by sonication and deposited on a holey-carbon-coated copper grid for TEM analysis. Scanning electron microscopy (SEM) analysis was carried out on a Philips XL30 ESEM microscope operating at 20 keV. The SEM samples were prepared by grinding the powder, dispersing it on carbon tape and spraying it with gold. Elemental analysis was carried out by using an inductively coupled plasma optical emission spectroscopy (ICP-OES) instrument. The samples were digested using HF before the analysis.Catalytic experiments
The liquid-phase oxidation of aniline was carried out in a round-bottom flask equipped with a magnetic stirrer and water-cooled condenser. A soft rubber cap was placed on top of the condenser instead of a glass cap for safety reasons to prevent accidents in the event of blowing up of the cap caused by possible pressure build-up due to H2O2 decomposition (vide infra). In a typical experiment, 20 mmol aniline, 10 mmol anisole and the desired amount of H2O2 were added to 10 mL of solvent. Safety note: The reaction without any solvent under our optimum reaction conditions showed vigorous bubbling and using a solvent could avoid pressure build-up. Then, 10 mg catalyst was added and the reaction mixture was stirred for 45 min without external heating. After the reaction time was over, the stirring was stopped and ca. 30 mL of ethanol were added to ensure that the reaction mixture was in one phase. Then, the catalyst was separated by centrifugation at 4000 rpm for 3 min. The supernatant was analysed by means of an Agilent Technologies 7980B GC equipped with an Agilent DB-5#6 (5%-phenyl)-methylpolysiloxane column (15 m, 320 μm ID) and a flame ionisation detector. Safety note: Though the employed conditions did not pose any safety issue, care should be taken when using acetone and hydrogen peroxide together as they can form acetoneperoxide (explosive).For the catalyst recycling tests, the rest of the supernatant was carefully removed by using a pipette. Then, ca. 45 mL ethanol was added to the centrifuge tube and the tube was shaken vigorously. After that, the tube was centrifuged at 4500 rpm for 20 min. Next, the supernatant was removed by using a pipette and ca. 45 mL fresh ethanol was added. This washing procedure was repeated 5 times. Then, the catalyst was dried at 100 °C overnight and regenerated by thermal treatment in a calcination oven at 200 °C for 4 h with a heating rate of 2 °C min−1 before reuse. For the leaching test, after 10 min of reaction the Nb2O5-scCO2 catalyst was separated from the reaction mixture by centrifugation (4000 rpm, 3 min), followed by filtration with a filter connected to a syringe. A small aliquot of the filtrate was analysed by GC. The remaining filtrate was stirred for further 35 min at room temperature, after which the solution was analysed by GC.
The molar amounts of aniline and products (azoxybenzene, azobenzene, nitrobenzene and nitrosobenzene) were calculated using the following formula:
(n = 1 for nitrosobenzene and nitrobenzene, n = 2 for azobenzene and azoxybenzene)
| Yieldx = Conv. × Sel.x |
Results and discussion
In this work, we studied the aniline oxidative coupling to azoxybenzene using H2O2 as the oxidant under mild reaction conditions over a Nb2O5-scCO2 catalyst. The catalyst was prepared by a novel scCO2-assisted procedure; the ease, high yield and low cost of which are assets in the perspective of scale-up of the catalyst production. The choice of investigating Nb2O5 as the catalyst for the oxidation of aniline with H2O2 stems from the reported ability of this oxide to activate H2O2 for oxidation reactions through the formation of active oxygen species, including peroxo, superoxo and hydroxyl radical species.44,45 The exact form of active oxygen species depends on the active sites of the catalyst and the reaction conditions (e.g. pH, H2O2 concentration). Until now, Nb2O5 has been applied as a heterogeneous catalyst in many reactions employing H2O2 as the oxidant, such as the oxidation of organic sulfides,44,45 glycerol, and alkenes.46 Carreño et al. reported that Nb2O5 prepared by a microwave-assisted hydrothermal method catalysed the oxidation of aniline with H2O2 at 25 °C with phenylhydroxylamine and nitrosobenzene as the main products.14 To the best of our knowledge, the application of Nb2O5 in aniline oxidation with azoxybenzene as the product has not been reported yet. On the other hand, two heterogeneous catalysts containing Nb in their formulation have been recently reported in aniline oxidation with H2O2 to produce azoxybenzene. NbOOH supported on FeOOH was reported to reach full aniline conversion with 70% azoxybenzene selectivity after 25 h at RT, though this required a relatively high Rc/s of 0.1 (see Table S1†).47 A Nb–Zn–Al-oxide catalyst (∼4 wt% Nb loading, Rc/s = 0.1) was reported to reach 92% azoxybenzene yield in the presence of UV irradiation for 48 h, whereas the yield dropped to 0% without irradiation.48The initial test with Nb2O5-scCO2 as a heterogeneous catalyst for the oxidation of aniline to azoxybenzene with aqueous H2O2 as the oxidant was carried out without external heating and with a low catalyst loading (Rc/s = 0.005) compared to the values generally employed in the literature (Table S1†). Under these challenging conditions, an excellent aniline conversion of 86% was achieved in 45 min (stoichiometric maximum 93% with the employed H2O2 ratio of 1.4), with an azoxybenzene selectivity of 92% (Table 1, entry 1). The productivity of the Nb2O5-scCO2 catalyst is remarkably high (209 grams of azoxybenzene generated per gram of catalyst in 1 h), which is more than double compared to that of the previous optimum and around two orders of magnitude larger than most of the catalysts reported in the literature (Table S1†). Additionally, the TON and TOF values for the Nb2O5-scCO2 catalyst after 45 min of reaction were calculated to be 129 and 172 h−1, respectively, based on the Nb content (57 wt%) of Nb2O5-scCO2 obtained by ICP-OES analysis. The catalytic performance of Nb2O5-scCO2 was directly compared under the same reaction conditions to that of selected commercial catalysts (Nb2O5-Comm, TiO2-P25, and WO3-Comm), which were chosen since they have been extensively reported to be able to activate H2O2 towards oxidation reactions (Table 1, entries 2–4).23,46,49 Only minor amounts (<5%) of aniline could be converted and the only product was nitrosobenzene over these reference catalysts, and even TiO2-P25, which had been reported to catalyse this reaction at 60 °C,24 gave only slightly higher conversion at room temperature compared to a blank reaction (entries 3 and 5 in Table 1). A control experiment with Nb2O5-scCO2 as the catalyst but without the use of H2O2 gave almost no conversion of aniline (Table 1, entry 6), thus confirming the need of H2O2 as the oxidant and excluding a significant contribution of oxygen from air as the oxidant.
| Entry | Catalyst | Conversiona (%) | Yield (%) | Selectivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Azoxy. | Nitroso. | Nitro. | Azo. | Azoxy. | Nitroso. | Nitro. | Azo. | |||
Reaction conditions: 20 mmol aniline, 28 mmol of H2O2 (as 30 wt% aqueous solution), 10 mmol anisole, 10 mL ethanol, 10 mg of the selected catalyst (Rc/s = 0.005), no applied heating, 45 min.a Under the employed reaction conditions (aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1 H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4), the theoretical maximum conversion is 93%.b Without a catalyst.c Without H2O2.d In the presence of TEMPO (1 mol% to H2O2) as a radical scavenger. 1.4), the theoretical maximum conversion is 93%.b Without a catalyst.c Without H2O2.d In the presence of TEMPO (1 mol% to H2O2) as a radical scavenger. |
||||||||||
| 1 | Nb2O5-scCO2 | 86 | 79 | 4 | 2 | 1 | 92 | 5 | 2 | 1 |
| 2 | Nb2O5-Comm | 4 | 0 | 4 | 0 | 0 | 0 | >99 | 0 | 0 |
| 3 | TiO2-P25 | 5 | 0 | 5 | 0 | 0 | 0 | >99 | 0 | 0 |
| 4 | WO3-Comm | 3 | 0 | 3 | 0 | 0 | 0 | >99 | 0 | 0 |
| 5 | Blankb | 2 | 0 | 2 | 0 | 0 | 0 | >99 | 0 | 0 |
| 6 | Nb2O5-scCO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
<1 | 0 | <1 | 0 | 0 | 0 | >99 | 0 | 0 |
| 7 | Nb2O5-scCO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
5 | 0 | 5 | 0 | 0 | 0 | >99 | 0 | 0 |
| 8 | Nb2O5-800 °C | 4 | 0 | 4 | 0 | 0 | 0 | >99 | 0 | 0 |
A leaching test was performed to evaluate the heterogeneous nature of the Nb2O5-scCO2 catalyst. After 10 min of reaction, the Nb2O5-scCO2 catalyst was separated from the reaction mixture. At this stage, the aniline conversion was 4%. The filtrate was stirred for further 35 min at room temperature. No further increase in aniline conversion was observed (Fig. S2†), which indicates that no or negligible leaching of active species occurred, thus demonstrating that the Nb2O5-scCO2 catalyst is truly heterogeneous. Importantly, based on the results of the catalytic test with Nb2O5-scCO2, the H2O2 utilisation efficiency was calculated to be 95%, which indicates that the Nb2O5-scCO2 catalyst was highly selective in activating H2O2 towards the conversion of aniline against the competitive decomposition into H2O and O2. Further insight into the catalytic reaction was provided by a kinetic study during which the temperature of the reaction mixture was monitored (Fig. S2†). The exothermic nature of the reaction led to a notable increase of the temperature of the reaction mixture after 25 min reaction time, reaching 77 °C at around 37 min reaction time, which corresponds to the time at which the maximum in aniline conversion was reached. At that moment, we also observed a bubbling phenomenon which was most probably related to the decomposition of residual H2O2 by the catalyst. These data suggest that the heat from the exothermic reaction promoted the decomposition of H2O2 and that, in turn, the complete consumption of H2O2 caused by the decomposition reaction prevented further conversion of aniline. The increase of the temperature of the solution as the exothermic reaction proceeded, also explains the observed gradual increase in the reaction rate (compare the conversion rate between 15 and 30 min with that between 30 and 40 min in Fig. S2b†).
Different mechanisms have been proposed for the activation of hydrogen peroxide by Nb2O5 catalysts, involving the formation of active oxygen species, among which metal (hydroxy)peroxo species and hydroxyl radicals are the most common.46,50 In order to gain more insight into how Nb2O5-scCO2 activates H2O2 towards the oxidation of aniline, a control experiment was conducted under the optimum reaction conditions but with the addition of TEMPO (1 mol% relative to H2O2), which has been widely reported to act as a hydroxyl radical scavenger.51,52 A drastic decrease in the conversion of aniline to 5% was observed (Table 1, entry 7). In light of the above experimental findings, the aniline oxidative coupling with H2O2 is proposed to follow a free-radical mechanism. The catalytic cycle most likely starts with the formation of niobium hydroperoxo (Nb-OOH) species through the reaction between surface niobium-hydroxyls (Nb-OH) and H2O2.46,50 Then, the interaction between Nb-OOH and H2O2 can generate highly reactive hydroxyl radicals (˙OH) and hydroperoxo radicals (˙OOH) and/or niobium peroxo radicals (NbO2˙) (Scheme S1†). This mechanism is supported by previous spectrosocopic studies, which identified the formation of all these three radical species when metal-hydroperoxo species interact with H2O2.46,53 The formed radicals lead to the oxidation of aniline to form phenylhydroxylamine and nitrosobenzene (Scheme 1 and S1†), which in turn can react with each other through a condensation reaction yielding azoxybenzene and water as the final products (Scheme 1). The final condensation step can be catalysed by the Brønsted acid sites provided by the –OH groups on the surface of the Nb2O5-scCO2 catalyst, which have been reported to be able to catalyse other dehydration reactions.40–42
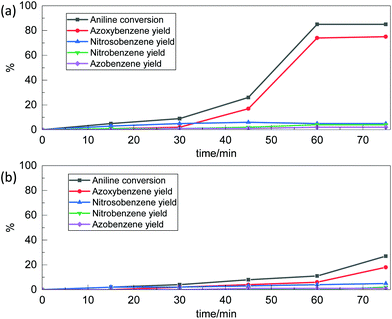
The results obtained with Nb2O5-scCO2 using a Rc/s = 0.005 suggested that our catalyst might perform well at an even lower catalyst loading. Therefore, we carried out a kinetic test with a Rc/s = 0.002 while maintaining the other reaction conditions. The aniline conversion reached 85% with an azoxybenzene selectivity of 88% after 60 min, which remained constant at longer reaction times (Fig. 2a). The side products were 5% of nitrosobenzene, 5% of nitrobenzene and 2% of azobenzene. The TOF value with this lower catalyst loading (calculated after 60 min of reaction) was even more notable, reaching 305 h−1. This result proves that the Nb2O5-scCO2 catalyst is able to reach very high aniline conversion also with this extremely low catalyst loading. These conditions were also employed for a comparison between Nb2O5-scCO2 and a catalyst prepared with the same procedure but without the involvement of scCO2 (Nb2O5-Ref). The comparison was based on a kinetic test in which we monitored the conversion of aniline and the selectivity of products as a function of reaction time by GC analysis (Fig. 2) and visually (Fig. S3†). These results indicate that Nb2O5-scCO2 was able to catalyse the reaction much faster than Nb2O5-Ref. The conversion of aniline over Nb2O5-scCO2 reached the maximum (85%) within 60 min, whereas only 11% conversion of aniline with 55% selectivity of azoxybenzene was achieved over Nb2O5-Ref at the same reaction time. This difference in activity was also reflected by the significantly lower TOF for the Nb2O5-Ref catalyst (24 h−1, calculated after 60 min of reaction based on the 59 wt% Nb content determined by ICP-OES) compared to that for Nb2O5-scCO2.
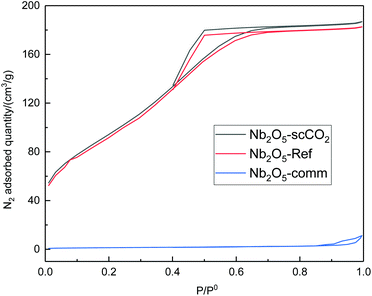
With the purpose of correlating the remarkable catalytic performance of Nb2O5-scCO2 to its physicochemical properties, we performed a characterisation study by means of N2-physisorption, XRD, FTIR and TEM. The textural properties of selected catalysts were investigated by N2-physisorption. The adsorption–desorption isotherms of Nb2O5-scCO2 and Nb2O5-Ref belong to type IV and have hysteresis loops at high p/p0 values (Fig. 3), which are attributed to the presence of interparticle void spaces at the mesopore scale. Nb2O5-scCO2 exhibits a slightly higher specific surface area compared to Nb2O5-Ref (340 m2 g−1vs. 305 m2 g−1), and both of them are significantly higher than those of the commercial materials used as reference catalysts (Nb2O5-Comm: 5 m2 g−1; TiO2-P25: 65 m2 g−1; WO3-Comm: 3 m2 g−1). The surface areas of Nb2O5-scCO2 and Nb2O5-Ref are also higher than those of Nb2O5 materials synthesised by different routes but with similar (200 °C)54 or even lower (60 or 80 °C)40,42 temperature of the thermal treatment, which range between 142 and 184 m2 g−1. The high surface area of Nb2O5-scCO2 can contribute to the explanation of its catalytic activity as it implies a higher number of exposed active sites that are easily accessible to the reactants per gram of material, particularly compared to the commercial catalysts displaying very low surface areas (Nb2O5-Comm, TiO2-P25, and WO3-Comm).
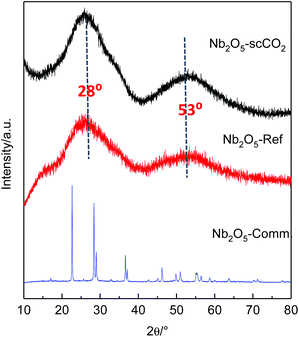
The crystallinity of the selected catalysts was examined by XRD measurements. The patterns of both Nb2O5-scCO2 and Nb2O5-Ref display two characteristic broad peaks centred at around 28° and 53°, corresponding to the amorphous Nb2O5 structure that consists of NbO4 tetrahedra and NbO6 octahedra (Fig. 4).14,55 The amorphous nature of these two materials contrasts with the crystalline structure of Nb2O5-Comm (Fig. 4), with the pattern corresponding to the orthorhombic phase mainly consisting of NbO6 octahedra (JCPDS No. 00-028-0317).14,56 Crystalline Nb2O5 is typically obtained through a thermal treatment of amorphous or low crystallinity materials, during which the –OH groups on the surface gradually condense. Therefore, the degree of crystallinity is generally reflected in the surface hydrophilicity, which can be estimated by FTIR analysis. The peaks centred at 1640 cm−1 in the FTIR spectra of Nb2O5-scCO2 and Nb2O5-Ref are assigned to the –OH bending mode of physisorbed water.40 The broad absorption peaks in the range of 2800–3600 cm−1 of these two spectra are assigned to –OH stretching vibrations of physisorbed water40 and, in analogy to other amorphous metal oxides,23,57 to surface hydroxyl groups. These signals indicate the hydrophilic nature of the surface of Nb2O5-scCO2 and Nb2O5-Ref. On the other hand, these peaks are virtually absent in the spectrum of Nb2O5-Comm (Fig. 5), indicating the much lower hydrophilicity of this material. The FTIR spectra of Nb2O5-scCO2 and Nb2O5-Ref are quite similar, referring to the amorphous Nb2O5 structure. The FTIR spectrum of Nb2O5-Comm showed a well-defined absorption peak at ∼800 cm−1 and two shoulders at ∼870 cm−1 and ∼715 cm−1, which are characteristic of the Nb2O5 orthorhombic crystalline structure,56 in agreement with the XRD results, whereas such peaks are absent in the spectra of the amorphous Nb2O5-scCO2 and Nb2O5-Ref (Fig. 5). Based on the XRD and FTIR analyses, the higher activity of Nb2O5-scCO2 and Nb2O5-Ref compared to Nb2O5-Comm is ascribed to the larger surface area of the two amorphous materials and the presence of surface –OH groups on their surfaces. The combination of these two features implies a much higher density of surface niobium-hydroxyls, which are the proposed catalytic sites for the activation of H2O2 (see Scheme S1†). This hypothesis is further supported by the drastic decrease in the specific surface area (2 m2 g−1) and aniline conversion (4%, see Table 1, entry 8) observed by converting Nb2O5-scCO2 into crystalline niobium oxide upon calcination at 800 °C (Fig. S4†).
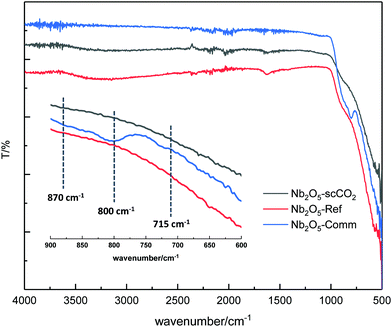 | ||
| Fig. 5 FTIR spectra of Nb2O5-scCO2, Nb2O5-Ref and Nb2O5-Comm. The small bands between 2000 and 2500 cm−1 present in all three spectra are due to the adsorption of CO2 from air. | ||
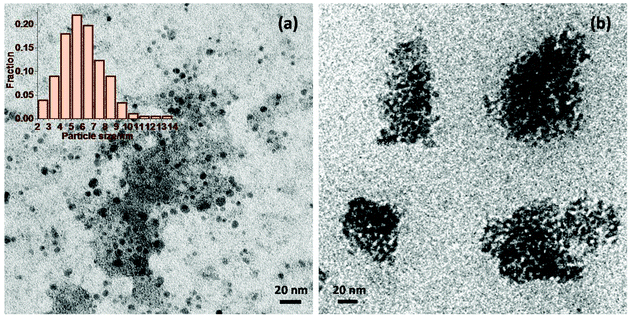
The above characterisation still does not explain the observed difference in catalytic activity between Nb2O5-scCO2 and Nb2O5-Ref (see Fig. 2). A clue in this sense is provided by the TEM analysis of the two materials. Nb2O5-scCO2 consists of discrete nanoparticles with small size and narrow particle size distribution in the 4 to 9 nm range (Fig. 6a and Fig. S5†). Some aggregated particles are also observed, though these might be caused simply by inadequate dispersion of the Nb2O5 nanoparticles in ethanol during the preparation of the TEM sample. On the other hand, the Nb2O5-Ref material prepared without scCO2 mainly consists of aggregated larger particles with size between 50 to 100 nm (Fig. 6b). Such aggregation is probably caused by condensation reactions that are prevented if the synthesis is carried out in scCO2 medium. The difference in morphology brought about by scCO2 synthesis is likely to enable a better contact between the catalyst particles and the reactants in the liquid-phase oxidation of aniline, and would thus account for the superior catalytic activity of Nb2O5-scCO2 compared to Nb2O5-Ref. The two materials were also characterised by SEM (Fig. S6†), though this analysis only indicates that for both catalysts the nanoparticles tend to aggregate into large secondary particles, which disaggregate once the materials are suspended in a liquid (as proven by the fact that such secondary particles are not observed by TEM).
Optimising the reaction conditions and catalyst reusability
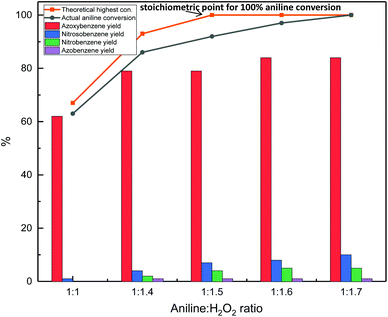
Once the best catalyst for the oxidation of aniline to azoxybenzene was identified (Nb2O5-scCO2), the reaction conditions were optimised with the aim of enhancing the aniline conversion and azoxybenzene selectivity. Firstly, the aniline-to-H2O2 ratio was optimised (Fig. 7). The theoretical stoichiometric ratio to achieve full conversion of aniline is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (see Scheme 1). When the reaction was performed with an under-stoichiometric amount of oxidant (1
1.5 (see Scheme 1). When the reaction was performed with an under-stoichiometric amount of oxidant (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio between aniline and H2O2), 63% of aniline conversion with an azoxybenzene selectivity of 98% was achieved over Nb2O5-scCO2, which is very close to the maximum theoretical conversion under these conditions (67%). Upon increasing the relative amount of H2O2 so that full conversion is theoretically achievable, 92% of aniline conversion was obtained. Although this conversion with aniline-to-H2O2 ratio of 1
1 ratio between aniline and H2O2), 63% of aniline conversion with an azoxybenzene selectivity of 98% was achieved over Nb2O5-scCO2, which is very close to the maximum theoretical conversion under these conditions (67%). Upon increasing the relative amount of H2O2 so that full conversion is theoretically achievable, 92% of aniline conversion was obtained. Although this conversion with aniline-to-H2O2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 is higher than with 1
1.5 is higher than with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4, full conversion was not achieved and the azoxybenzene selectivity slightly decreased. This is attributed to the consumption of H2O2 caused by the formation of other side products and to H2O2 decomposition. For the 1
1.4, full conversion was not achieved and the azoxybenzene selectivity slightly decreased. This is attributed to the consumption of H2O2 caused by the formation of other side products and to H2O2 decomposition. For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratio between aniline and H2O2, the reaction was also performed by adding H2O2 dropwise for the first 15 minutes, with the aim of decreasing the chance of its decomposition and thus enhancing its utilisation. However, this did not prove beneficial and the aniline conversion and azoxybenzene selectivity obtained (data not shown) were the same as those obtained for the experiment in which all H2O2 was added initially. A further increase of the relative amount of H2O2 led to higher aniline conversion, eventually reaching full conversion for a 1
1.5 ratio between aniline and H2O2, the reaction was also performed by adding H2O2 dropwise for the first 15 minutes, with the aim of decreasing the chance of its decomposition and thus enhancing its utilisation. However, this did not prove beneficial and the aniline conversion and azoxybenzene selectivity obtained (data not shown) were the same as those obtained for the experiment in which all H2O2 was added initially. A further increase of the relative amount of H2O2 led to higher aniline conversion, eventually reaching full conversion for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 ratio between aniline and H2O2. However, under these conditions the selectivity towards azoxybenzene dropped to 84% with an increase in the formation of side products (i.e. nitrosobenzene, nitrobenzene and a trace amount of azobenzene). Nitrosobenzene was detected as the only side product when an aniline-to-H2O2 ratio of 1
1.7 ratio between aniline and H2O2. However, under these conditions the selectivity towards azoxybenzene dropped to 84% with an increase in the formation of side products (i.e. nitrosobenzene, nitrobenzene and a trace amount of azobenzene). Nitrosobenzene was detected as the only side product when an aniline-to-H2O2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used. Nitrobenzene was also detected as a side product when an aniline-to-H2O2 ratio equal to 1
1 was used. Nitrobenzene was also detected as a side product when an aniline-to-H2O2 ratio equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 was used and with a higher relative amount of H2O2, with the yields of this compound increasing with the increasing of H2O2 amount as a consequence of over-oxidation of the reaction intermediates (Scheme 1). Since the yield of azoxybenzene achieved using a 1
1.4 was used and with a higher relative amount of H2O2, with the yields of this compound increasing with the increasing of H2O2 amount as a consequence of over-oxidation of the reaction intermediates (Scheme 1). Since the yield of azoxybenzene achieved using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 ratio between aniline and H2O2 (79%) increased only in a minor way by increasing the relative amount of hydrogen peroxide, while this caused a gradual drop in azoxybenzene selectivity (92%), this ratio between the substrate and oxidant was employed in the following steps of this study.
1.4 ratio between aniline and H2O2 (79%) increased only in a minor way by increasing the relative amount of hydrogen peroxide, while this caused a gradual drop in azoxybenzene selectivity (92%), this ratio between the substrate and oxidant was employed in the following steps of this study.
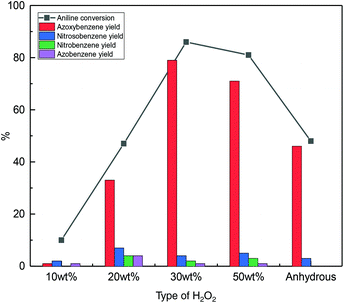
Aqueous hydrogen peroxide is commercially available in different concentrations. The presence of water could be detrimental as it can compete with hydrogen peroxide for adsorption on the catalyst surface and could hinder the dehydration step from phenyhydroxylamine and nitrosobenzene to azoxybenzene. To test these hypotheses, the effect of using different concentrations of H2O2 was investigated (Fig. 8 and Fig. S7†). Although visual observation of the colour of the reaction mixture clearly indicated that the rate of azoxybenzene formation in the first 25 min of reaction was higher with the higher concentration of hydrogen peroxide (Fig. S7†), at the end of the catalytic test (45 min), the highest aniline conversion and azoxybenzene selectivity were obtained with 30 wt% H2O2 rather than with 50 wt% H2O2 (Fig. 8). In the literature, a higher rate of azoxybenzene formation was observed over a TiO2 catalyst by employing hydrogen peroxide solutions with higher concentrations.23 Such a relation between the H2O2 concentration and reaction rate can be ascribed to the lower dilution effect with a higher concentration of H2O2 solution, which would grant higher accessibility of aniline and H2O2 to the catalyst and lead to faster heat transfer with a consequent faster rise of the temperature of the reaction mixture and thus a higher reaction rate.26,30,32,33 Additionally, the amount of water is the lowest with 50 wt% H2O2, thus probably facilitating the dehydration between phenylhydroxylamine and nitrosobenzene to form azoxybenzene. Although this trend is followed with our Nb2O5-scCO2 catalyst in the first 25 min of reaction (Fig. S7†), it is valid over the whole catalytic test only for the H2O2 concentration up to 30 wt% (Fig. 8). The observed lower yield of azoxybenzene with 50 wt% H2O2 compared to 30 wt% H2O2 at the end of the catalytic test can be ascribed to the higher extent of H2O2 decomposition in the former case, as a consequence of a faster and larger temperature increase in the more concentrated reaction mixture. This hypothesis was supported by performing a test with the Nb2O5-scCO2 catalyst using 1.7 times H2O2 (50 wt% aqueous solution) relative to aniline. Under these conditions, 94% aniline conversion with 87% azoxybenzene selectivity was obtained, compared to the full conversion of aniline achieved with 30 wt% H2O2 (Fig. 7), suggesting that a higher percentage of H2O2 was decomposed when 50 wt% H2O2 was used. In addition, according to the Global Harmonised System (GHS) for the classification and labelling of chemicals, 50 wt% H2O2 belongs to the GHS03 hazard category (oxidising liquid), which requires more rigid precautionary measures than 30 wt% H2O2. Therefore, the use of 30 wt% H2O2 is preferable not only because it gives higher utilisation efficiency of H2O2 with the Nb2O5-scCO2 catalyst, but also in terms of safety concerns and, therefore, in the context of the green chemistry principles.
The solvent in which the reaction takes place was also optimised by screening a set of solvents with different polarities and aprotic/protic nature (Table 2). These solvents were selected because they form a single phase with aniline and the aqueous H2O2 solution, thus averting the mass transfer problem associated with the presence of different liquid phases. According to the CHEM21 guide for ranking the greenness of solvents,34 the selected alcohols and acetone are recommended solvents, acetonitrile is recognised as a problematic solvent, whereas 1,4-dioxane is labelled as hazardous. The use of ethanol as a solvent led to the highest azoxybenzene yield (79%) and selectivity (92%), while isopropanol gave the highest aniline conversion (90%), which represents a slight improvement compared to that in ethanol (86%). Although there is no clear correlation between the catalytic activity and solvent polarity (Fig. S8†), in general the aniline conversion and azoxybenzene yield are higher in protic solvents compared to those in aprotic solvents. Such a positive effect of the protic solvents can be related to the radical mechanism over the Nb2O5-scCO2 catalyst. It has been reported that the polarisation of hydroxyl radicals in protic solvents is stronger than that in aprotic solvents,58 thus increasing the electrophilicity of the radical and promoting its reactivity. It should be noted that other solvent-related factors might play a role too, including the radical quenching ability of solvents,59 their interaction with the catalyst surface and their solvating and stabilising effects on reactants, intermediates and products.25,28,60,61
| Solvent | Aprotic (A) or protic (P) | Boiling point (°C) | Dipole moment (D) | Dielectric constant (at 25 °C) | Conversiona (%) | Yield (%) | Selectivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azoxy | Nitroso | Nitro | Azo | Azoxy | Nitroso | Nitro | Azo | ||||||
Reaction conditions: 20 mmol aniline, 28 mmol 30 wt% H2O2, 10 mmol anisole, 10 mL selected solvent, 10 mg Nb2O5-scCO2 catalyst, no applied heating, 45 min.a Under the employed reaction conditions (aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1 H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4), the theoretical maximum conversion is 93%. 1.4), the theoretical maximum conversion is 93%. |
|||||||||||||
| 1,4-Dioxane | A | 88 | 0.45 | 2.2 | 58 | 48 | 5 | 3 | 2 | 82 | 9 | 5 | 4 |
| Acetone | A | 56 | 2.85 | 20.7 | 54 | 45 | 4 | 3 | 3 | 83 | 7 | 5 | 5 |
| Acetonitrile | A | 82 | 3.92 | 37.5 | 78 | 52 | 17 | 6 | 3 | 66 | 22 | 8 | 4 |
| 2-Butanol | P | 98 | 1.66 | 16.6 | 82 | 67 | 7 | 4 | 3 | 83 | 9 | 5 | 3 |
| Isopropanol | P | 83 | 1.66 | 17.9 | 90 | 77 | 8 | 4 | 1 | 86 | 9 | 4 | 1 |
| Ethanol | P | 78 | 1.69 | 24.3 | 86 | 79 | 4 | 2 | 1 | 92 | 5 | 2 | 1 |
| Methanol | P | 65 | 1.69 | 32.7 | 81 | 69 | 8 | 4 | 0 | 86 | 10 | 4 | 0 |
Finally, the recyclability of the Nb2O5-scCO2 catalyst in consecutive runs under the optimum reaction conditions was tested (Fig. S9†), demonstrating that the catalyst fully retained its activity and selectivity in 4 consecutive runs (aniline conversion or azoxybenzene selectivity ±2%).
Substrate scope
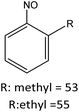
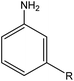
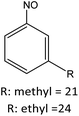
Having established the best reaction conditions, we completed our study by investigating the scope of substrates that can be converted over Nb2O5-scCO2 (Table 3). For this purpose, we selected substituted anilines with different types and positions of the substituting group. Some of these substituted anilines would afford azoxy products that can find application in pharmaceutical compounds (from alkyl-substituted anilines) and liquid crystals (from para-anisidine).6,7 Nb2O5-scCO2 efficiently catalysed the transformation of all investigated alkyl-substituted anilines with high conversion (≥78%), though the unsubstituted aniline affords the highest yield of the azoxy product in 45 min. Although the conversion of the alkyl substituted anilines is in the same range, the selectivity towards the azoxy product is strongly influenced by the presence and position of the alkyl group on the aromatic ring. Among the alkyl-substituted anilines, the highest selectivity towards the azoxy product was observed when the alkyl group was in the para-position, followed by the meta and ortho positions.28,48 The main side product was the corresponding alkyl-nitrosobenzene, which becomes the major product with the ortho-substituted anilines. These results indicate that the alkyl substituents do not play a major role in the initial oxidation of the amino group with hydrogen peroxide, but have a relevant steric effect in the condensation step that leads to the formation of the azoxy product (Scheme 1). The reaction with para-anisidine achieved 53% conversion, which is lower compared to the conversion obtained with aniline and alkyl-substituted anilines. This is ascribed to the presence of the methoxy substituent, which is expected to increase the electron density of the –NH2 group in para-anisidine as a consequence of the resonance effect of this substituent (dominating over its electron withdrawing inductive effect). The higher electron density on the amino group compared to that in aniline is detrimental to the radical-induced oxidation steps.58 Furthermore, no conversion was observed using benzylamine as the substrate. The difficulty in converting benzylamine under the employed mild conditions is also related to electronic effects. Compared to aniline, the α carbon between the amino group and the aromatic ring in benzylamine disrupts the resonance effect, thus decreasing the electrophilicity of –NH2 and thus negatively affecting its reactivity towards the hydroxyl radical species.| Anilines | Conversion (%)a | Selectivity (%) | |||
|---|---|---|---|---|---|
| Azoxy. | Nitroso. | Nitro. | Azo | ||
Reaction conditions: 20 mmol substituted aniline, 28 mmol 30 wt% H2O2, 10 mmol anisole, 10 mL ethanol, 10 mg Nb2O5-scCO2 catalyst, no applied heating, 45 min.a Under the employed reaction conditions (aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1 H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4), the maximum theoretical conversion is 93%. 1.4), the maximum theoretical conversion is 93%. |
|||||
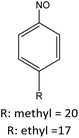
|
R: methyl = 78 |
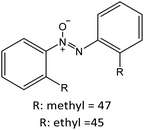
|
|
||
| R: ethyl = 81 | |||||
|
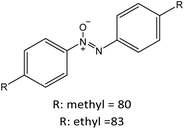
R: methyl = 82 |
|
|
|
|
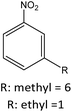
| R: ethyl = 84 | |||||

|
R: methyl = 81 |
|
|
||
| R: ethyl = 81 | |||||
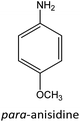
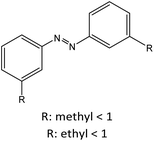
|
53 |
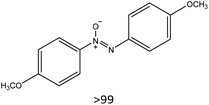
|
|||
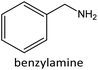
|
0 | ||||
Conclusions
In this work, Nb2O5 nanoparticles with a high specific surface area (340 m2 g−1) were prepared by a novel, supercritical-CO2-assisted method and were applied as a heterogeneous catalyst for the oxidative coupling of aniline with H2O2 to produce azoxybenzene. The catalyst achieved excellent activity and selectivity towards azoxybenzene in a short time (45 min) under mild conditions (no applied heating) and with a catalyst loading that was much lower compared to those reported in the literature for this reaction. Full aniline conversion could be achieved by employing only a small excess of H2O2 compared to the stoichiometric amount, demonstrating a high efficiency of the catalyst in activating hydrogen peroxide towards the desired oxidation. A control test in the presence of a radical scavenger suggested that the reaction follows a free-radical pathway. The Nb2O5-scCO2 catalyst does not suffer from leaching and can be reused in consecutive runs without losing activity. Notably, the catalyst is also versatile as it is active in the conversion of a variety of substituted anilines, though the selectivity towards the azoxy product is lower particularly for ortho-substituted substrates. The discrete nanoparticle morphology brought about by the utilisation of scCO2 in the synthesis accounts for the much superior catalytic performance of Nb2O5-scCO2 compared to the state-of-the-art catalysts for this reaction. The green aspects of this system include the use of an environmentally benign oxidant as H2O2, the extremely mild reaction conditions, the use of a green solvent as ethanol, the short reaction time, the extremely low catalyst loading and the high efficiency in the utilisation of H2O2. These features are also attractive from the perspective of an industrial application of this catalytic system. Additionally, the scCO2-assisted method that led to the synthesis of this excellent Nb2O5 catalyst has the potential to be applied to the preparation of other metal–oxide heterogeneous catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Yehan Tao acknowledges financial support from the China Scholarship Council (CSC) for her Ph.D. grant. The authors acknowledge Leon Rohrbach and Marcel de Vries for their analytical and technical support.References
- H. Takahashi, T. Ishioka, Y. Koiso, M. Sodeoka and Y. Hashimoto, Biol. Pharm. Bull., 2000, 23(11), 1387–1390 CrossRef CAS PubMed.
- A. B. Vix, P. Müller-Buschbaum, W. Stocker, M. Stamm and J. P. Rabe, Langmuir, 2000, 16(26), 10456–10462 CrossRef CAS.
- D. Aronzon, E. P. Levy, P. J. Collings, A. Chanishvili, G. Chilaya and G. Petriashvili, Liq. Cryst., 2007, 34(6), 707–718 CrossRef CAS.
- E. Voutyritsa, A. Theodorou, M. G. Kokotou and C. G. Kokotos, Green Chem., 2017, 19(5), 1291–1298 RSC.
- E. Buncel and B. T. Lawton, Can. J. Chem., 1965, 43(4), 862–875 CrossRef CAS.
- K. Selvam, S. Balachandran, R. Velmurugan and M. Swaminathan, Appl. Catal., A, 2012, 413, 213–222 CrossRef.
- I. W. Stewart, Book: The static and dynamic continuum theory of liquid crystals, 2004, p. 17 Search PubMed.
- H. Li, X. Xie and L. Wang, Chem. Commun., 2014, 50(32), 4218–4221 RSC.
- L. Liu, P. Concepción and A. Corma, J. Catal., 2019, 369, 312–323 CrossRef CAS.
- Q. Xiao, Z. Liu, F. Wang, S. Sarina and H. Zhu, Appl. Catal., B, 2017, 209, 69–79 CrossRef CAS.
- Z. Liu, Y. Huang, Q. Xiao and H. Zhu, Green Chem., 2016, 18(3), 817–825 RSC.
- B. Zhou, J. Song, T. Wu, H. Liu, C. Xie, G. Yang and B. Han, Green Chem., 2016, 18(13), 3852–3857 RSC.
- https://www.covestro.com/en/sustainability/lighthouse-projects/bio-aniline .
- W. M. Ventura, D. C. Batalha, H. V. Fajardo, J. G. Taylor, N. H. Marins, B. S. Noremberg, T. Tański and N. L. Carreño, Catal. Commun., 2017, 99, 135–140 CrossRef CAS.
- Y. Shiraishi, H. Sakamoto, K. Fujiwara, S. Ichikawa and T. Hirai, ACS Catal., 2014, 4(8), 2418–2425 CrossRef CAS.
- A. Grirrane, A. Corma and H. García, Science, 2008, 322(5908), 1661–1664 CrossRef CAS PubMed.
- F. Yang, Z. Wang, X. Zhang, L. Jiang, Y. Li and L. Wang, ChemCatChem, 2015, 7(21), 3450–3453 CrossRef CAS.
- K. Ju and R. E. Parales, Microbiol. Mol. Biol. Rev., 2010, 74(2), 250–272 CrossRef CAS PubMed.
- M. I. Qadir, J. D. Scholten and J. Dupont, Catal. Sci. Technol., 2015, 5(3), 1459–1462 RSC.
- W. D. Emmons, J. Am. Chem. Soc., 1957, 79(20), 5528–5530 CrossRef CAS.
- R. W. Murray, R. Jeyaraman and L. Mohan, Tetrahedron Lett., 1986, 27(21), 2335–2336 CrossRef CAS.
- A. Mckillop and J. A. Tarbin, Tetrahedron, 1987, 43(8), 1753–1758 CrossRef CAS.
- H. Tumma, N. Nagaraju and K. V. Reddy, Appl. Catal., A, 2009, 353(1), 54–60 CrossRef CAS.
- L. Yang, G. Shi, X. Ke, R. Shen and L. Zhang, CrystEngComm, 2014, 16(9), 1620–1624 RSC.
- N. Jagtap and V. Ramaswamy, Appl. Clay Sci., 2006, 33(2), 89–98 CrossRef CAS.
- S. Gontier and A. Tuel, Appl. Catal., A, 1994, 118(2), 173–186 CrossRef CAS.
- H. Sonawane, A. V. Pol, P. P. Moghe, S. S. Biswas and A. Sudalai, J. Chem. Soc., Chem. Commun., 1994, 10, 1215–1216 RSC.
- D. R. Das and A. K. Talukdar, ChemistrySelect, 2017, 2(28), 8983–8989 CrossRef CAS.
- S. Gontier and A. Tuel, J. Catal., 1995, 157(1), 124–132 CrossRef CAS.
- C.-F. Chang and S.-T. Liu, J. Mol. Catal. A: Chem., 2009, 299(1–2), 121–126 CrossRef CAS.
- S. S. Acharyya, S. Ghosh and R. Bal, ACS Sustainable Chem. Eng., 2014, 2(4), 584–589 CrossRef CAS.
- S. Ghosh, S. S. Acharyya, T. Sasaki and R. Bal, Green Chem., 2015, 17(3), 1867–1876 RSC.
- A. Shukla, R. K. Singha, L. S. Konathala, T. Sasaki and R. Bal, RSC Adv., 2016, 6(27), 22812–22820 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2015, 18(1), 288–296 RSC.
- Y. Tao and P. P. Pescarmona, Catalysts, 2018, 8(5), 212 CrossRef.
- M. B. Chowdhury, R. Sui, R. A. Lucky and P. A. Charpentier, Langmuir, 2009, 26(4), 2707–2713 CrossRef PubMed.
- J. Yoo, Y. Bang, S. J. Han, S. Park, J. H. Song and I. K. Song, J. Mol. Catal. A: Chem., 2015, 410, 74–80 CrossRef CAS.
- J. Jammaer, C. Aprile, S. W. Verbruggen, S. Lenaerts, P. P. Pescarmona and J. A. Martens, ChemSusChem, 2011, 4(10), 1457–1463 CrossRef CAS PubMed.
- N. Farhangi, R. R. Chowdhury, Y. Medina-Gonzalez, M. B. Ray and P. A. Charpentier, Appl. Catal., B, 2011, 110, 25–32 CrossRef CAS.
- K. Nakajima, Y. Baba, R. Noma, M. Kitano, J. N. Kondo, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2011, 133(12), 4224–4227 CrossRef CAS PubMed.
- M. T. Reche, A. Osatiashtiani, L. J. Durndell, M. A. Isaacs, Â. Silva, A. F. Lee and K. Wilson, Catal. Sci. Technol., 2016, 6(19), 7334–7341 RSC.
- C. Yue, G. Li, E. A. Pidko, J. J. Wiesfeld, M. Rigutto and E. J. Hensen, ChemSusChem, 2016, 9(17), 2421–2429 CrossRef CAS PubMed.
- A. J. Kamphuis, F. Milocco, L. Koiter, P. P. Pescarmona and E. Otten, ChemSusChem, 2019, 12, 1–8 CrossRef.
- J. V. Coelho, M. Guedes, G. Mayrink, P. P. Souza, M. C. Pereira and L. C. Oliveira, New J. Chem., 2015, 39(7), 5316–5321 RSC.
- A. Bozzi, R. Lavall, T. Souza, M. Pereira, P. De Souza, H. De Abreu, A. De Oliveira, P. Ortega, R. Paniago and L. C. A. Oliveira, Dalton Trans., 2015, 44(46), 19956–19965 RSC.
- M. Ziolek, I. Sobczak, P. Decyk, K. Sobańska, P. Pietrzyk and Z. Sojka, Appl. Catal., B, 2015, 164, 288–296 CrossRef CAS.
- A. L. Lima, D. C. Batalha, H. V. Fajardo, J. L. Rodrigues, M. C. Pereira and A. C. Silva, Catal. Today, 2018 DOI:10.1016/j.cattod.2018.10.035.
- G. S. de Carvalho, L. H. Chagas, C. G. Fonseca, P. P. De Castro, A. C. Sant, A. A. Leitão and G. W. Amarante, New J. Chem., 2019, 43, 5863 RSC.
- C. Hammond, J. Straus, M. Righettoni, S. E. Pratsinis and I. Hermans, ACS Catal., 2013, 3(3), 321–327 CrossRef CAS.
- I. D. Ivanchikova, I. Y. Skobelev, N. V. Maksimchuk, E. A. Paukshtis, M. V. Shashkov and O. A. Kholdeeva, J. Catal., 2017, 356, 85–99 CrossRef CAS.
- S. S. Acharyya, S. Ghosh and R. Bal, Chem. Commun., 2014, 50(87), 13311–13314 RSC.
- S. S. Acharyya, S. Ghosh and R. Bal, Chem. Commun., 2015, 51(27), 5998–6001 RSC.
- M. Ziolek, P. Decyk, I. Sobczak, M. Trejda, J. Florek, H. G. W. Klimas and A. Wojtaszek, Appl. Catal., A, 2011, 391(1–2), 194–204 CrossRef CAS.
- H. T. Kreissl, K. Nakagawa, Y.-K. Peng, Y. Koito, J. Zheng and S. C. E. Tsang, J. Catal., 2016, 338, 329–339 CrossRef CAS.
- M. N. Catrinck, E. S. Ribeiro, R. S. Monteiro, R. M. Ribas, M. H. Barbosa and R. F. Teófilo, Fuel, 2017, 210, 67–74 CrossRef CAS.
- M. Graça, A. Meireles, C. Nico and M. Valente, J. Alloys Compd., 2013, 553, 177–182 CrossRef.
- A. J. Bonon, Y. N. Kozlov, J. O. Bahú, R. Maciel Filho, D. Mandelli and G. B. Shul'pin, J. Catal., 2014, 319, 71–86 CrossRef CAS.
- S. Mitroka, S. Zimmeck, D. Troya and J. M. Tanko, J. Am. Chem. Soc., 2010, 132(9), 2907–2913 CrossRef CAS.
- A. Brizzolari, G. M. Campisi, E. Santaniello, N. Razzaghi-Asl, L. Saso and M. C. Foti, Biophys. Chem., 2017, 220, 1–6 CrossRef CAS PubMed.
- A. G. da Silva, D. C. Batalha, T. S. Rodrigues, E. G. Candido, S. C. Luz, I. C. de Freitas, F. C. Fonseca, D. C. de Oliveira, J. G. Taylor, S. I. C. de Torresi, P. H. C. Camargo and H. V. Fajardo, Catal. Sci. Technol., 2018, 8(7), 1828–1839 RSC.
- S. B. Waghmode, S. M. Sabne and S. Sivasanker, Green Chem., 2001, 3(6), 285–288 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Image of the high-throughput scCO2 reactor. Kinetic study of 20 mmol aniline conversion over the Nb2O5-scCO2 catalyst. Pictures of 50 mmol aniline conversion over Nb2O5-scCO2 and Nb2O5-Ref catalysts as a function of time. XRD pattern of Nb2O5-800 °C. TEM pictures of Nb2O5-scCO2. SEM images of Nb2O5-scCO2 and Nb2O5-Ref catalysts. Pictures of conversion of aniline with different concentrations of H2O2 after 25 min. Plots of aniline conversion and products yields in different solvents. Reusability test of the Nb2O5-scCO2 catalyst. Comparison with the previously reported heterogeneous catalysts. List of chemicals and their purity used in this work. Reaction pathway from the oxidative coupling of aniline with H2O2 to azoxybenzene over the Nb2O5-scCO2 catalyst. See DOI: 10.1039/c9gc02623a |
| This journal is © The Royal Society of Chemistry 2019 |