High-flux nanofiltration membranes tailored by bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets for enhanced selectivity towards organics and salts†
Wenyuan
Ye
a,
Hongwei
Liu
a,
Fang
Lin
b,
Jiuyang
Lin
 *b,
Shuaifei
Zhao
*b,
Shuaifei
Zhao
 c,
Shishi
Yang
d,
Jingwei
Hou
e,
Shungui
Zhou
c,
Shishi
Yang
d,
Jingwei
Hou
e,
Shungui
Zhou
 *a and
Bart
Van der Bruggen
f
*a and
Bart
Van der Bruggen
f
aFujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou 350002, China. E-mail: sgzhou@soil.gd.cn
bFujian Provincial Engineering Research Center of Rural Waste Recycling Technology, School of Environment and Resources, Fuzhou University, Fuzhou 350116, China. E-mail: linjiuyang@126.com
cState Environmental Protection Key Laboratory of Integrated Surface Water-Groundwater Pollution Control, School of Environmental Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China
dKey Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
eSchool of Chemical Engineering, University of Queensland, St. Lucia, Brisbane, QLD 4072, Australia
fDepartment of Chemical Engineering, KU Leuven, Celestijnenlaan 200F, B-3001 Leuven, Belgium
First published on 1st August 2019
Abstract
Surface modification with advanced nanomaterials (i.e., 2D nanosheets) can be used to strategically tailor membrane properties, providing improved solute permselectivity to targeted molecules. In particular, 2D graphite-like carbon nitride (g-C3N4) nanosheets are a promising alternative for membrane modification, due to their exceptional physicochemical properties and facile synthesis. Herein, high-flux nanofiltration (NF) membranes were designed using bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets with a polydopamine (PDA)/polyethylenimine (PEI) layer onto porous ultrafiltration (UF) substrates. The g-C3N4 nanosheets created additional nanochannels in the PDA/PEI layer to facilitate water molecule transport, resulting in high permeability (28.4 ± 1.2 L m−2 h−1 bar−1). Particularly, the bio-inspired layer structure was tailored from the UF to the NF (592 Da) scale by incorporating g-C3N4 nanosheets, thereby breaking through the permeability–selectivity trade-off effect. The tailored NF membrane enabled ultrahigh retention of three reactive dyes (610–630 Da, >99.3%) and low salt rejection (2.9% for NaCl; 7.6% for Na2SO4), significantly promoting the fractionation of dyes and salts for dye desalination. Additionally, the hydrophilic g-C3N4 nanosheets with oxygen plasma treatment further enhanced the wettability of the membrane surfaces, resulting in a superior antifouling performance. This study indicates the promise of g-C3N4 nanosheets to engineer high-flux NF membranes with desirable fractionation performance for sustainable treatment of highly saline wastewater.
Environmental significanceIn sustainable management of highly saline wastewater, nanofiltration (NF) technology is increasingly applied in an emerging paradigm shift to separate and reuse organics/salt mixtures as resources rather than remove them completely as wastes. However, the commercial NF membranes with negative charges yield high rejections to both organics and inorganic salts, compromising the fractionation of organics/salt mixtures. This paper reports a strategy of fast bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets with a polydopamine/polyethylenimine layer onto porous ultrafiltration substrates for fabrication of high-flux NF membranes. Specifically, these bio-inspired NF membranes show enhanced dye retention, salt transmission, and antifouling properties, demonstrating a competitive and practical solution for sustainable management of highly saline wastewater, e.g., textile wastewater. |
1. Introduction
In the concept of advanced sustainability, wastewater treatment increasingly requires an emerging paradigm shift from removal-centered approaches to recovery processes.1–3 By using novel strategies and practices, the pollutants in industrial wastewater streams can be potentially extracted and transformed into high value-added resources, i.e., nutrients, bioplastics, energy and even products, thereby expediting the water and material loop.4 Specifically, industrial waste streams, e.g., textile wastewater, glyphosate neutralization liquor, soy sauce wastewater, and liquors containing heterocyclic drug derivatives or iminodiacetic acid, are usually highly loaded with valuable organics with elevated salinity. To achieve the sustainable management of these liquors, the key challenge is to effectively separate the organic and inorganic compounds.Nanofiltration (NF) has been emerging as a promising technology for fractionation of organics with molecular weights (MWs) of 200–1000 Da and inorganic salts through steric exclusion and the Donnan effect.5,6 To date, many attempts have been made to tailor the physicochemical properties of the NF membrane surface, e.g., pore sizes, surface charges, wettability, and roughness, to improve the filtration performance. Nevertheless, restricted by the chain stiffness of the polymer matrix and the interchain spacing, polymer-based membranes suffer from an inherent limitation of the selectivity–permeability trade-off.7–9 Recently, the incorporation of advanced nanomaterials into polymer matrices has attracted considerable interest as an alternative strategy to break through this trade-off effect.
Mixed matrix NF membranes blended with nanoparticles were developed through phase inversion to yield high water permeability and low salt rejection, showing potential for dye desalination and purification. Unfortunately, the poor miscibility of the polymer matrix with nanoparticles would result in nonselective interface voids and surface defects, deteriorating the solute permselectivity. Zhang et al. constructed loose NF membranes by blending nanoparticles, i.e., poly(ionic liquid) brush-grafted silica nanospheres and zwitterionic polymer-functionalized graphene oxide, with a polyethersulfone matrix which facilitated salt transport but yielded ca. 90% rejection of dyes.10,11 The moderate dye rejection induced a high loss of dyes when utilizing the diafiltration process to desalinate dye/salt mixtures.
Alternatively, the use of thin film composite (TFC) NF membranes has emerged as a pathway to enhance the solute selectivity. Generally, TFC NF membranes are coated with a thin and selective polyamide layer by crosslinking of trimesoyl chloride (TMC) and m-phenylenediamine (MPD) or piperazine (PIP).12 However, it is difficult to manipulate the crosslinking degree for polymerization between TMC and MPD or PIP monomers, inducing a tight surface structure of the resultant NF membranes to sufficiently retain both organic and inorganic solutes. This not only compromises the fractionation of organics and inorganic salts, but also significantly reduces the permeation flux of TFC NF membranes due to the enhanced osmotic pressure resulting from high salt rejection. Several approaches have been investigated to overcome this challenge. On the one hand, controllable interfacial polymerization kinetics are of great significance to construct a targeted selective layer for NF membranes. Tan et al. designed a polyamide NF membrane with a Turing structure by manipulating the reaction–diffusion kinetics within the aqueous–organic interface, thus breaking through the upper bound of the permeability–selectivity trade-off.13 In addition, the use of novel acyl chloride monomers (e.g., mm-biphenyl tetraacyl chloride,14 cyanuric chloride,15 and 5-chloroformyloxy-isophthaloyl chloride16) and amine monomers (e.g., dopamine,17 triethanolamine,18 sericin,19 and zwitterionic amine20) diversifies and tailors the surface structures of the resultant NF membranes, potentially facilitating the solute selectivity. On the other hand, creating a thin film nanocomposite (TFN) NF membrane by incorporating nanomaterials into the polyamide layer would yield more pathways or channels to enhance the transport of water.21 Graphene-based nanosheets (i.e., GO and rGO) as representative two dimensional structured materials are emerging as candidates to design the surface structure of TFN NF membranes and enhance the filtration performance,22,23 However, the complicated, high-cost synthesis procedure limits the practical applications of these graphene-based nanosheets, making it necessary to explore alternative two-dimensional (2D) nanosheets for membrane modification.
Recently, bulk carbon nitride (g-C3N4) with a graphite-like structure consisted of tri-s-triazine units bonded with tertiary amino groups, where the nanosheet layers interacted via delicate van der Waals forces.24 This allows the bulk g-C3N4 to be facilely exfoliated into 2D g-C3N4 nanosheets. Due to their exceptional physicochemical properties (i.e., thermal and chemical stability, optical and photoelectrochemical characteristics) and facile synthesis (calcination of cheap nitrogen-rich precursors, e.g., dicyandiamide, melamine, carbamide and thiocarbamide), 2D g-C3N4 nanosheets have been increasingly applied for H2 production, oxygen reduction, and photocatalysis.25–28 Furthermore, with regularly distributed triangular nanopores (ca. 3.11 Å) throughout the entire laminar structure, the assembly of g-C3N4 nanosheets into 3D stacks potentially enables the creation of more nanochannels for the free passage of water molecules.8 Stacked g-C3N4 nanosheet-based membranes fabricated by simple vacuum filtration were found to have a high flux, which was confirmed by molecular dynamics simulation.24 However, the unbound g-C3N4 nanosheet layers are too fragile to survive practical cross-flow testing conditions and cannot even tolerate water rinsing or a gentle finger touch, which is similar to the case of GO layers.29 Therefore, new strategies are strongly required to address the weak binding of g-C3N4 nanosheets in membrane synthesis.
In this study, we proposed the first attempt to apply hydrophilic 2D g-C3N4 nanosheets to design high-flux TFN NF membranes for efficient dye/salt fractionation. First, oxygen plasma was applied to treat the 2D g-C3N4 nanosheets to enhance their hydrophilicity; subsequently, the homogeneous dispersion of hydrophilic 2D g-C3N4 nanosheets in dopamine and polyethylenimine (PEI) mixed solutions was coated on the top of porous hydrolyzed polyacrylonitrile (HPAN) ultrafiltration (UF) substrates using bio-inspired co-deposition. Finally, the resultant membranes were systematically characterized to evaluate their applicability for separation of organics (i.e., dyes) and inorganic salts, in view of sustainable treatment of highly saline wastewater, e.g., textile wastewater.
2. Experimental
2.1 Chemicals and membranes
Porous PAN substrates with a molecular weight cut-off (MWCO) of 200 kDa (AmFor Membrane Technology Engineering Co. Ltd., China) were hydrolyzed in a 2 M NaOH solution for 20 h (denoted as HPAN) and then completely rinsed with distilled water to remove the residual NaOH agent. Dopamine hydrochloride, PEI (Mn: 600 Da), tris(hydroxymethyl)aminomethane (Tris, purity of 99.8%), and ammonium persulfate (APS, purity of 98.0%) were purchased from Sigma Aldrich. Melamine powder (Sinopharm Chemical Reagent, China) was applied to synthesize the g-C3N4 nanosheets.The model dyes, including reactive blue 19 (RB19), reactive orange 1 (RO1), and reactive orange 16 (RO16), were selected to test the separation performance of the as-prepared membranes. The molecule structures of these tested reactive dyes are shown in Fig. S1 of the ESI.†
2.2 Bio-inspired co-deposition of g-C3N4 nanosheets on HPAN membranes
Hydrophilic g-C3N4 nanosheets were obtained through an oxygen plasma treatment. Specifically, 0.5 g g-C3N4 bulk powder was put in a PC-6D microwave radical generator (Jiarun Wanfeng Technology Ltd., Beijing) with an operating power of 100 W and an O2 flow rate of 36 L h−1 for 0.5 h. Subsequently, the treated g-C3N4 powder was placed in 100 mL pure water for 12 h ultrasonication and then was centrifuged at 3000 rpm for 10 min to remove the unexfoliated layered g-C3N4.31 Ultimately, the hydrophilic 2D g-C3N4 nanosheet solution was prepared for further use.
2.3 Characterization
The roughness of the membrane surface was determined by AFM under ambient conditions using a Bruker Dimension Icon with a FastScan-B tip with a scan area of 5 × 5 μm2. The root-mean-squared roughness (Rrms) was used to evaluate the surface morphology.
The surface charges on the modified membranes were measured in terms of zeta potential using a SurPASS electrokinetic analyzer (Anton Paar GmbH); all the measurements were performed in 1 mM KCl solution at pH 6.0 ± 0.2 and 25 °C.
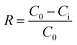 | (1) |
Aiming to quantitatively determine the pore size of these as-prepared membranes, the log-normal density function between rejections of polyethylene glycol polymers and their Stokes diameters (dp) was employed:32
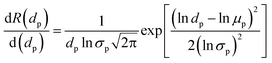 | (2) |
Based on this log-normal density function, the MWCO of the bio-inspired membranes, defined as the molecular weights (MW) of solutes at R of 90%, can be determined through the relationship between the Stokes diameter (ds) of solutes (i.e., polyethylene glycol) and their MW as shown below:32
| ds = 33.46 × 10−12 × MW0.557 | (3) |
2.4 Filtration behaviors
The separation behaviors of the as-prepared membranes were assessed in a home-made cross-flow filtration cell. After compaction of the membranes through the filtration of pure water, a stable permeation flux was obtained at 25 °C. Subsequently, the filtration of the salt solutions (1.0 g L−1 NaCl or Na2SO4) was conducted at 4 bar. Additionally, the rejection of three reactive dyes (i.e., RO16, RO1 and RB19) at 500 ppm was measured at different feed pH values ranging from 3 to 11 and salt concentrations (i.e., up to 10.0 g L−1) to evaluate the applicability of the modified membranes for separating reactive dyes and inorganic salts. The permeability (J) and rejection (Rs) of dyes and salts for the as-prepared membranes are determined as follows: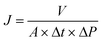 | (4) |
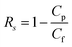 | (5) |
2.5 Fouling propensity
The fouling propensity of the modified membranes was evaluated with a 100 ppm humic substance solution (mainly humic acid and fulvic acid) extracted from landfill leachate. In a typical process, pure water was filtered at 4 bar for 1 hour to render a steady initial permeation flux (Jw,0). Subsequently, the humic substance solution was filtered for another hour and the final permeation flux was recorded as Js. Ultimately, the fouled membranes were thoroughly rinsed with pure water for 1 hour, which provided the recovered permeation flux (Jw,1). The antifouling behaviors of the as-prepared membranes were determined in 3 cycles. The flux recovery ratio (η) was determined to analyze the fouling propensity of the membranes, which is expressed as: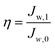 | (6) |
To describe the flux decline during the fouling experiments, the total fouling ratio (Rt) was used; it is defined as the degree of the flux decline caused by reversible and irreversible fouling. The reversible fouling (Rr) refers to the permeation flux reduction due to a gel layer deposited on the membrane surface and the irreversible fouling (Rir) is ascribed to the blocking of the foulants into the membrane pores or adsorption onto the surface. These three fouling ratios are calculated as follows:
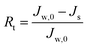 | (7) |
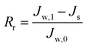 | (8) |
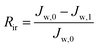 | (9) |
3. Results and discussion
3.1 Characterization of hydrophilic g-C3N4 nanosheets
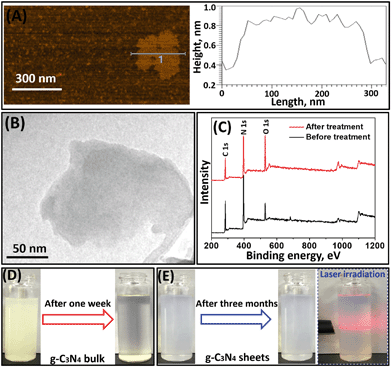
The intrinsic hydrophobicity of the bulk g-C3N4 not only renders it difficult to well disperse in the aqueous solution but also does not improve the membrane wettability during membrane modification. Therefore, it is important to improve its hydrophilicity. After oxygen plasma treatment, the g-C3N4 can be well exfoliated into a layer structure with dimensions of ca. 200 nm × 200 nm (Fig. 2A and B). The AFM image (Fig. 2A) shows that the bulk g-C3N4 was successfully treated and exfoliated into one-layered nanosheets with a thickness of ca. 0.5 nm.33XPS analysis was conducted to investigate the elemental composition of pristine and treated g-C3N4 nanosheets (Fig. 2C). The characteristic peaks for C 1s, N 1s and O 1s species can be clearly observed in the XPS spectra of g-C3N4 nanosheets before or after oxygen plasma treatment. The O element in pristine g-C3N4 nanosheets is derived from intermediates of the melamine after thermal polymerization.34 After oxygen plasma treatment, an increasing intensity of the O 1s peak but a decreasing intensity of both C 1s and N 1s peaks in g-C3N4 nanosheets were observed. Specifically, the atomic percentage of the O element increased from 11.3% to 25.7% (Table S1†). These results indicate that the oxygen plasma treatment resulted in successful incorporation of the O element into the g-C3N4 nanosheets, thereby potentially improving their wettability.35
3.2 Surface morphologies of the membranes
Fig. S2† shows the digital images of the pristine and modified HPAN-based membranes treated with polydopamine (PDA)/PEI coating. As shown in Fig. S2A and B,† the surface of the HPAN substrate changed from white to homogeneously brown after the exposure to the PDA/PEI solution, suggesting that APS can trigger the coating of the PDA/PEI layer on the HPAN substrate through the Schiff base reaction and Michael addition. However, the color of bio-inspired coated membrane surfaces was slightly lighter when the g-C3N4 nanosheets were present in the PDA/PEI solutions during co-deposition (Fig. S2C–F†), suggesting the successful co-deposition of g-C3N4 nanosheets onto the membrane surfaces.In order to study the effect of incorporation of hydrophilic g-C3N4 nanosheets on the surface morphology of the PDA/PEI layer, surface SEM and AFM images of the modified membranes were visualized (Fig. 3). The pristine HPAN membrane had a smooth surface. Specifically, the valleys in the AFM image (Fig. 3A), which denote the pore structure of the HPAN substrate, resulted in a surface roughness of 39.4 nm. After coating with the PDA/PEI layer, the pristine HPAN substrate with a porous structure was fully covered with a smooth and tight surface (Fig. 3B). However, some nanoparticles appeared and made the surface of the M0 membrane rougher, which was confirmed by the increase in the roughness (Rrms = 50.7 nm). This is attributed to the aggregation of PDA through covalent polymerization of dopamine. Furthermore, the surface morphologies of the membranes incorporated with g-C3N4 nanosheets were significantly altered. After the co-deposition of g-C3N4 nanosheets, the membrane surfaces became more compact (Fig. 3C–F). The g-C3N4 nanosheets present in the PDA/PEI solution may stimulate the generation of sulfate radicals from APS, allowing for fast polymerization of dopamine and PEI near the vicinity of g-C3N4 nanosheets. This allows the bio-inspired coating to firmly adhere to the surface of the g-C3N4 nanosheets, providing robust linkages between the g-C3N4 nanosheets and membrane surface and consequently enhancing the solute selectivity. Simultaneously, the covalent polymerization of dopamine was limited, minimizing the aggregation of PDA nanoparticles. On the other hand, the g-C3N4 nanosheets were stacked with more nodules observed on the membrane surfaces as the concentrations of nanosheets increased, exhibiting scale-like structures and rougher morphologies. Noticeably, the stacking of the g-C3N4 nanosheets would create more nanochannels, which is likely beneficial for enhanced permeation flux.
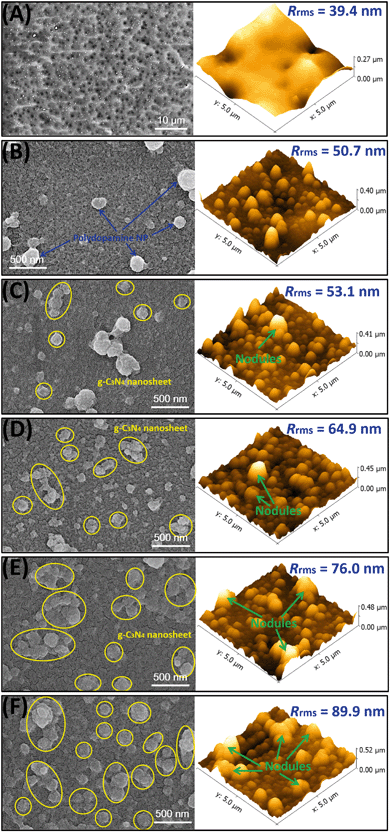 | ||
| Fig. 3 Surface SEM and AFM images of the pristine HPAN substrate and as-prepared membranes. (A) Pristine HPAN; (B) M0; (C) M1; (D) M2; (E) M3; (F) M4. | ||
3.3 Hydrophilicity and surface charge
Generally, the incorporation of nanoparticles into the polymer matrix is a key strategy to enhance the permeability and alleviate membrane fouling during filtration. Fig. 4A shows the dynamic contact angles of the pristine and bio-inspired HPAN-based membranes. Specifically, the initial contact angle of the HPAN-based membranes slightly dropped from 44.9° to 40.6° after coating with the PDA/PEI composite layer (i.e., M0 membrane), attributed to the presence of amino or hydroxyl groups derived from dopamine and PEI on the membrane surface for enhanced hydrophilicity. The wettability of the PDA/PEI coating was further boosted with co-deposition of hydrophilic g-C3N4 nanosheets. For example, the initial contact angle of the bio-inspired membranes was reduced from 40.6° to 37.1° as a result of the bio-inspired co-deposition of 0.005% hydrophilic g-C3N4 nanosheets. When more g-C3N4 nanosheets (i.e., 0.04%) were deposited onto the PDA/PEI layer, the initial contact angle of the membrane surface further dropped to 28.7°. This was mainly due to the greater stack of hydrophilic g-C3N4 nanosheets (SEM images in Fig. 3). Fig. 4B highlights the stable static contact angles of the membranes resulting from the bio-inspired co-deposition of g-C3N4 nanosheets. The static contact angles of the bio-inspired membranes exhibited a negative dependence on the amount of the co-deposited g-C3N4 nanosheets. However, the correlation between the membrane hydrophilicity and the amount of g-C3N4 nanosheets was slightly non-linear at higher concentrations of these nanosheets (i.e., 0.04%). This is mainly restricted by the intrinsic hydrophilicity of the g-C3N4 nanosheets after oxygen plasma treatment. In order to obtain membranes with higher wettability, a longer oxygen plasma treatment is recommended to further improve the hydrophilicity of the g-C3N4 nanosheets.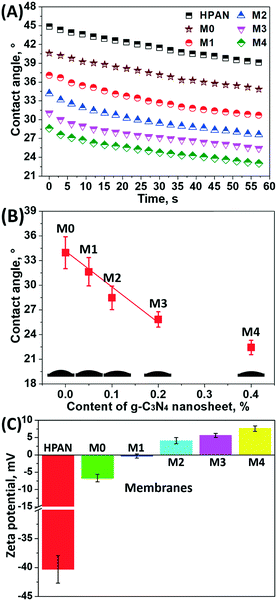 | ||
| Fig. 4 (A) Time-dependent dynamic water contact angles, (B) stable static water contact angles, and (C) zeta potentials of the pristine HPAN substrate and as-prepared membranes. | ||
In view of resource recovery from highly saline wastewater by membrane technology, a high salt permeation is favorable to obtain effective fractionation of organics and salts. Generally, the charge of the membrane surface critically dominates the separation behavior of the charged species through the Donnan effect, especially for multivalent ions. Therefore, the surface charges of the bio-inspired membranes were determined through zeta potential measurements (Fig. 4C). After the deposition of the PDA/PEI layer, the zeta potential of the coated HPAN substrate (i.e., M0 membrane) increased from −40.3 ± 2.4 to −6.7 ± 1.1 mV. On the one hand, the carboxyl groups with negative charges on the pristine HPAN substrate were completely covered by the bio-inspired coating; on the other hand, the binding of the positively charged PEI onto the membrane surface elevated the zeta potential to some extent. However, the result in this study deviated slightly from a previously reported positive zeta potential (4.3 mV) of a PDA/PEI coating with an H2O2/CuSO4 trigger.36 The plausible explanation for this difference is the aggregation of PDA nanoparticles with negative charges on the membrane surfaces, which resulted in a lower zeta potential in this study, as observed in the SEM images in Fig. 3B. Moreover, the zeta potential of the bio-inspired membranes positively increased with the concentration of the co-deposited g-C3N4 nanosheets. For example, the zeta potential of the M4 membrane surface was 7.6 ± 0.8 mV with the co-deposition of 0.04% g-C3N4 nanosheets onto the PDA/PEI layer. As previously mentioned, the presence of the g-C3N4 nanosheets triggered more sulfate radicals and thus facilitated the binding of more PEI molecules with dopamine, confining the self-polymerization of dopamine and thus giving rise to a highly positive charge density on the membrane surface. This elevated zeta potential may enable high salt permeation, especially for Na2SO4, thereby providing a higher organic/salt selectivity.
3.4 Membrane filtration performance
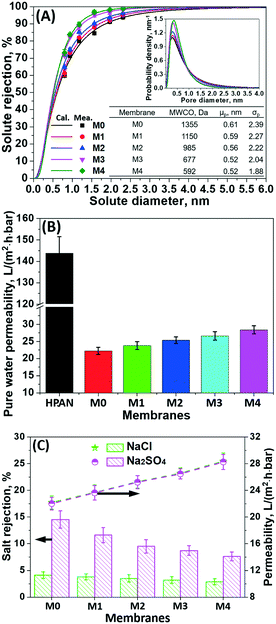 | ||
| Fig. 5 (A) Pore size distribution, (B) pure water permeability, and (C) filtration performance of the bio-inspired membranes in 1.0 g L−1 NaCl and Na2SO4 solutions. | ||
As a substrate, the pristine hydrolyzed PAN membrane with a porous structure exhibited a pure water permeability of 143.9 ± 7.6 L m−2 h−1 bar−1. After 4 h bio-inspired coating of the dopamine/PEI solution, the bio-inspired membrane (i.e., M0) yielded an effective mean pore size (μp) of 0.61 nm (i.e., 1355 Da MWCO), presenting the effective formation of the PDA/PEI layer. However, the pure water permeability decreased to 22.3 ± 1.0 L m−2 h−1 bar−1, due to the enhanced hydraulic membrane resistance. After the incorporation of g-C3N4 nanosheets onto the PDA/PEI layer, the pore sizes of these membranes become more narrow and uniform, as reflected from the steeper slopes of the solute rejection curves and the decreasing geometric standard deviations (σp). In particular, when the concentration of the g-C3N4 nanosheets reached 0.01%, the modified membrane (i.e., M2) had an MWCO of less than 1000 Da (i.e., 985 Da). This suggests the successful tailoring of the pore sizes from the UF to the NF scale. When increasing the concentration of g-C3N4 nanosheets to 0.04%, the pore size (i.e., 0.52 nm for μp) of the modified membrane further decreased with 592 Da MWCO. This was mainly attributed to the fact that the increasing concentration of g-C3N4 nanosheets present in dopamine/PEI solution induced the generation of sulfate radicals originating from APS to rapidly trigger the polymerization of dopamine and PEI through the Michael addition and Schiff base reactions, yielding a denser and more compact structure.
Noticeably, the permeability of the membranes after the bio-inspired co-deposition of g-C3N4 nanosheets increased monotonically with the concentration of g-C3N4 nanosheets, even though the membrane surface became much denser (Fig. 5B). Specifically, the pure water permeability of the bio-inspired membrane (M4 with 592 Da MWCO) was significantly boosted to 28.4 ± 1.2 L m−2 h−1 bar−1 as the concentration of incorporated g-C3N4 nanosheets reached 0.04%. Such improvement in permeation flux for these bio-inspired membranes with co-deposition of g-C3N4 nanosheets is mainly attributed to (1) the greater hydrophilicity of the membrane surfaces functionalized with hydrophilic g-C3N4 nanosheets and (2) the stacking of g-C3N4 nanosheets in the bio-inspired PDA/PEI layer creating more nanochannels for faster transport of water molecules. Compared to previously reported NF membranes, the co-deposition of g-C3N4 nanosheets into the PDA/PEI layer leads to the most advantageous water permeability and enhanced solute selectivity, which breaks through the permeability–selectivity trade-off effect (Fig. S3†).
The filtration performance of the as-prepared membranes through bio-inspired co-deposition of g-C3N4 nanosheets in the salt solutions was investigated (Fig. 5C). The PDA/PEI coating onto the HPAN-based membrane provided a fairly low rejection of Na2SO4 (i.e., 14.5 ± 1.6%) and NaCl (i.e., 4.7 ± 0.6%), although this bio-inspired coating was negatively charged. However, the reduced steric effect caused by the large pore size dominated the salt retention behavior, which facilitated the salt permeation. With the co-deposition of hydrophilic g-C3N4 nanosheets, the membranes had a decreasing pore size, but an increasing permeation of NaCl and Na2SO4 was observed. Specifically, the rejection of Na2SO4 for the modified membranes decreased from 14.5 ± 1.6% to 7.6 ± 0.8% as the concentration of the g-C3N4 nanosheets increased from 0 to 0.04%. This was mainly due to the increasing number of nanochannels created by stacking of g-C3N4 nanosheets onto the membrane surface. Additionally, the increased zeta potential on the membrane surfaces partially contributed to enhanced salt transport (Fig. 4C).
To recover the renewable resources from highly saline wastewater, conventional dense NF membranes typically have a moderate or high salt rejection, compromising the permeation flux due to the enhanced osmotic pressure; this limits the sufficient fractionation of organics and salts. In this study, the higher salt permeation of the bio-inspired membranes functionalized with g-C3N4 nanosheets would suppress the reduction in permeation flux.
The PDA/PEI layer (i.e., M0 with 1355 Da MWCO) provided a moderate retention to three dyes (i.e., >96%) on the HPAN membrane, due to synergistic effects of steric exclusion and Donnan repulsion (Fig. 6A). Unfortunately, this moderate dye rejection may be insufficient to effectively extract these reactive dyes during diafiltration for dye desalination, since these reactive dyes with small molecular sizes would penetrate through the membrane. Furthermore, the bio-inspired coating functionalized with the hydrophilic g-C3N4 nanosheets enabled an increasing tendency in the retention of these reactive dyes because of the reduced pore size for enhancing steric exclusion. In particular, an increase in the concentration of the g-C3N4 nanosheets to 0.04% achieved nearly complete retention (i.e., >99.3%) of the reactive dyes. Simultaneously, the permeation flux of the dye solutions for the modified membranes was also elevated with incorporation of the g-C3N4 nanosheets. Since the fouling of dyes occurred on the membrane surface, the permeation flux of these bio-inspired membranes in all dye solutions was lower than that in pure water (Fig. 5B). Nevertheless, a sufficiently high permeation flux can be successfully achieved for practical applications. For instance, the permeability of the bio-inspired membrane (i.e., M4) functionalized with 0.04% hydrophilic g-C3N4 nanosheets in dye solutions was observed to be around 23.7 L m−2 h−1 bar−1.
Fig. 6B shows the effect of the feed pH on the dye rejection of the bio-inspired membranes. The dye rejection of the M0 and M4 membranes was boosted at higher pH, due to the enhanced Donnan effect. Although the electrostatic attraction between the dye molecules and membrane surface reduced the rejection of the charged dyes at low pH, the M4 membrane with a smaller pore size still had a considerably higher dye rejection (i.e., 98.7 ± 0.3% for RO16 at pH 3) than the M0 membrane (94.8 ± 0.6% for RO16) at the same pH.
In textile wastewater derived from dye production or dyeing processes, inorganic salts (i.e., NaCl or Na2SO4) are either additives or by-products, which have a tremendous impact on the filtration performance of the membranes. Fig. 6C and D show the effect of NaCl or Na2SO4 on the fractionation of dyes and salts for these bio-inspired membranes. The rejection of the RO16 dye for the M0 membrane decreased from ca. 96.3% to ca. 92.8% with increasing concentration of inorganic salts from 0 to 10.0 g L−1. This is due to the fact that the presence of inorganic salts in the dye solutions can generally intensify the “shielding effect” and thus weaken the electrostatic repulsion of the charges in the membrane pores, which facilitates the transport of dyes through the membranes.37–40 Nevertheless, the size exclusion mechanism dominated the dye retention behavior over the “shielding effect” for the M4 membrane with a smaller pore size. Therefore, only a slight reduction (i.e., 0.3%) in the dye rejection was observed in the considered salt concentration range. To highlight the advantage of these bio-inspired membranes, Table S2† summarizes the filtration performance of the synthesized NF membranes previously reported in terms of water permeability and salt/dye retention. Combining the extremely low rejection of inorganic salts (i.e., NaCl or Na2SO4) and high retention of dyes, the NF membranes functionalized with the g-C3N4 nanosheets through bio-inspired co-deposition exhibited a superior performance for dye and salt fractionation in the dye/salt binary solutions.
3.5 Fouling propensity
NF membranes may suffer from the fouling caused by organic foulants, e.g., humic substances, which are commonly present in the aqueous environment. In this case, humic substances (mainly humic acid and fulvic acid) extracted from landfill leachate were employed as model foulants. Fig. 7 shows the fouling propensity of the bio-inspired membranes functionalized with g-C3N4 nanosheets for the humic substance foulant solutions.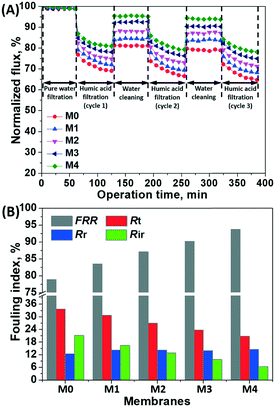 | ||
| Fig. 7 (A) Normalized flux of the bio-inspired membranes before and after the filtration of humic substance solution; (B) flux recovery and fouling resistance ratio of the membranes. | ||
Initially, a stable flux was obtained during the filtration of pure water, indicating that no membrane compaction occurred at 4 bar. When the pure water was replaced with the humic substance solution for filtration, the permeation flux of the bio-inspired membranes was markedly decreased, due to the membrane pore blocking and foulant cake layer. The permeation flux of these membranes was recovered to varying degrees after rinsing with pure water for foulant removal during the 3-cycle filtration (Fig. 7A).
The flux recovery rate and fouling resistance were calculated for a quantitative evaluation of the antifouling performance of the bio-inspired membranes (Fig. 7B). The pristine PDA/PEI coating (i.e., M0) had the lowest flux recovery rate (78.8%) but the highest Rt value (33.7%), presenting a high fouling propensity. When the hydrophilic g-C3N4 nanosheets were incorporated into the PDA/PEI layer, all bio-inspired membranes exhibited higher flux recovery (>83.0%) while maintaining a low Rt value. Specifically, the flux recovery of the M4 membrane functionalized with the highest concentration of the g-C3N4 nanosheets (i.e., 0.04%) reached 93.7%, and the Rt value was only 20.8%. This is due to the increased hydrophilicity of the membrane surfaces functionalized with hydrophilic g-C3N4 nanosheets, which significantly improved the antifouling performance. On the other hand, the increasing roughness of the membrane surfaces functionalized with the g-C3N4 nanosheets potentially facilitated the deposition of organic foulants, resulting in a slight increase in the reversible fouling resistance (Rr); these accumulated foulants can be eventually removed by water rinsing. However, the M0 membrane with the pristine PDA/PEI coating suffered from the most severe irreversible fouling, likely attributed to the pore blocking. With an increase in the concentration of g-C3N4 nanosheets, a decrease in the irreversible fouling ratio was yielded, as the reduction in pore sizes of these bio-inspired membranes prevented the organic foulants with larger sizes from entering the pore structure. Therefore, the g-C3N4 nanosheets can tailor the PDA/PEI layer from the UF to the NF scale, providing an excellent antifouling and filtration performance to the modified membranes.
Conclusions
In this study, a bio-inspired co-deposition of PDA/PEI with hydrophilic g-C3N4 nanosheets triggered by APS was proposed to construct high-flux NF membranes through bridging UF membrane surface cavities. SEM and AFM measurements demonstrated the incorporation of g-C3N4 nanosheets onto the PDA/PEI layer, which provided an excellent antifouling and filtration performance to the bio-inspired NF membranes. This is plausibly related to the fact that g-C3N4 nanosheets can act as catalysts in inducing the generation of sulfate radicals from APS, which accelerates the polymerization behavior of dopamine and PEI, and thus alters the structure of the membrane surface. In particular, the incorporation of g-C3N4 nanosheets can tailor the PDA/PEI layer from the UF to the NF scale in order to boost the retention of various reactive dyes (610–630 Da, >99.3%) with enhanced pure water permeability (28.4 ± 1.2 L m−2 h−1 bar−1). Low rejections of inorganic salts (i.e., 2.9% for NaCl; 7.6% for Na2SO4) can still be achieved, establishing these bio-inspired membranes as competitive and practical alternatives in dye desalination and purification of highly saline textile wastewater. Given the outstanding photocatalytic properties of the incorporated g-C3N4 nanosheets under visible light exposure, the photodegradation performance of these bio-inspired membranes will be investigated in future work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the funding support from the National Natural Science Foundation of China (Grant No: 21707018 and 21706035), the Natural Science Foundation of Fujian Province (Grant No: 2019Y0006 and 2017J01413) and the Fujian Agriculture and Forestry University Program for Distinguished Young Scholar (Grant No.: xjq201704) for this work.References
- J. Lin, C. Y. Tang, W. Ye, S. Sun, S. H. Hamdan, A. Volodin, C. V. Haesendonck, A. Sotto, P. Luis and B. Van der Bruggen, J. Membr. Sci., 2015, 493, 690–702 CrossRef CAS.
- J. Lin, W. Ye, J. Huang, B. Ricard, M. Baltaru, B. Greydanus, S. Balta, J. Shen, M. Vlad, A. Sotto, P. Luis and B. Van der Bruggen, ACS Sustainable Chem. Eng., 2015, 3, 1993–2001 CrossRef CAS.
- S. Wu, S. Zou, G. Liang, G. Qian and Z. He, Sci. Total Environ., 2018, 610–611, 137–146 CrossRef CAS PubMed.
- P. L. McCarty, J. Bae and J. Kim, Environ. Sci. Technol., 2011, 45, 7100–7106 CrossRef CAS PubMed.
- Z. F. Gao, Y. Feng, D. Ma and T. S. Chung, J. Membr. Sci., 2019, 574, 124–135 CrossRef CAS.
- Y. Zhang, S. Japip and T. S. Chung, Carbon, 2017, 123, 193–204 CrossRef CAS.
- G. M. Geise, H. B. Park, A. C. Sagle, B. D. Freeman and J. E. McGrath, J. Membr. Sci., 2011, 369, 130–138 CrossRef CAS.
- K. Cao, Z. Jiang, X. Zhang, Y. Zhang, J. Zhao, R. Xing, S. Yang, C. Gao and F. Pan, J. Membr. Sci., 2015, 490, 72–83 CrossRef CAS.
- B. D. Freeman, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- L. Yu, Y. Zhang, H. Zhang and J. Liu, Desalination, 2015, 359, 176–185 CrossRef CAS.
- J. Zhu, M. Tian, J. Hou, J. Wang, J. Lin, Y. Zhang, J. Liu and B. Van der Bruggen, J. Mater. Chem. A, 2016, 4, 1980–1990 RSC.
- D. Hu, Z. Xu and C. Chen, Desalination, 2012, 301, 75–81 CrossRef CAS.
- Z. Tan, S. Chen, X. Peng, L. Zhang and C. Gao, Science, 2018, 360, 518 CrossRef CAS PubMed.
- L. Li, S. Zhang and X. Zhang, J. Membr. Sci., 2009, 335, 133–139 CrossRef CAS.
- K. P. Lee, G. Bargeman, R. de Rooij, A. J. B. Kemperman and N. E. Benes, J. Membr. Sci., 2017, 523, 487–496 CrossRef CAS.
- M. Liu, D. Wu, S. Yu and C. Gao, J. Membr. Sci., 2009, 326, 205–214 CrossRef CAS.
- J. Zhao, Y. Su, X. He, X. Zhao, Y. Li, R. Zhang and Z. Jiang, J. Membr. Sci., 2014, 465, 41–48 CrossRef CAS.
- B. Tang, Z. Huo and P. Wu, J. Membr. Sci., 2008, 320, 198–205 CrossRef CAS.
- C. Zhou, Y. Shi, C. Sun, S. Yu, M. Liu and C. Gao, J. Membr. Sci., 2014, 471, 381–391 CrossRef CAS.
- Q. An, W. Sun, Q. Zhao, Y. Ji and C. Gao, J. Membr. Sci., 2013, 431, 171–179 CrossRef CAS.
- W. J. Lau, S. Gray, T. Matsuura, D. Emadzadeh, J. Paul Chen and A. F. Ismail, Water Res., 2015, 80, 306–324 CrossRef CAS PubMed.
- J. Yin, G. Zhu and B. Deng, Desalination, 2016, 379, 93–101 CrossRef CAS.
- A. Anand, B. Unnikrishnan, J. Mao, H. Lin and C. Huang, Desalination, 2018, 429, 119–133 CrossRef CAS.
- Y. Wang, L. Li, Y. Wei, J. Xue, H. Chen, L. Ding, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2017, 56, 8974–8980 CrossRef CAS PubMed.
- J. Zhang, M. Zhang, R. Sun and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 10145–10149 CrossRef CAS PubMed.
- Z. Lin and X. Wang, Angew. Chem., Int. Ed., 2013, 52, 1735–1738 CrossRef CAS PubMed.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- Q. Han, B. Wang, J. Gao, Z. Cheng, Y. Zhao, Z. Zhang and L. Qu, ACS Nano, 2016, 10, 2745–2751 CrossRef CAS PubMed.
- M. Hu and B. Mi, Environ. Sci. Technol., 2013, 47, 3715–3723 CrossRef CAS PubMed.
- X. Wang, S. Blechert and M. Antonietti, ACS Catal., 2012, 2, 1596–1606 CrossRef CAS.
- Q. Lin, L. Li, S. Liang, M. Liu, J. Bi and L. Wu, Appl. Catal., B, 2015, 163, 135–142 CrossRef CAS.
- M. Jiang, K. Ye, J. Deng, J. Lin, W. Ye, S. Zhao and B. Van der Bruggen, Environ. Sci. Technol., 2018, 52, 10698–10708 CrossRef CAS PubMed.
- J. Xu, L. Zhang, R. Shi and Y. Zhu, J. Mater. Chem. A, 2013, 1, 14766–14772 RSC.
- Z. Huang, J. Song, L. Pan, Z. Wang, X. Zhang, J. Zou, W. Mi, X. Zhang and L. Wang, Nano Energy, 2015, 12, 646–656 CrossRef CAS.
- X. Bu, J. Li, S. Yang, J. Sun, Y. Deng, Y. Yang, G. Wang, Z. Peng, P. He, X. Wang, G. Ding, J. Yang and X. Xie, ACS Appl. Mater. Interfaces, 2016, 8, 31419–31425 CrossRef CAS PubMed.
- J. Wang, J. Zhu, M. T. Tsehaye, J. Li, G. Dong, S. Yuan, X. Li, Y. Zhang, J. Liu and B. Van der Bruggen, J. Mater. Chem. A, 2017, 5, 14847–14857 RSC.
- J. Lin, W. Ye, H. Zeng, H. Yang, J. Shen, S. Darvishmanesh, P. Luis, A. Sotto and B. Van der Bruggen, J. Membr. Sci., 2015, 477, 183–193 CrossRef CAS.
- J. Lin, W. Ye, M. Baltaru, Y. P. Tang, N. J. Bernstein, P. Gao, S. Balta, M. Vlad, A. Volodin, A. Sotto, P. Luis, A. L. Zydney and B. Van der Bruggen, J. Membr. Sci., 2016, 514, 217–228 CrossRef CAS.
- W. Ye, J. Lin, R. Borrego, D. Chen, A. Sotto, P. Luis, M. Liu, S. Zhao, C. Y. Tang and B. Van der Bruggen, Sep. Purif. Technol., 2018, 197, 27–35 CrossRef CAS.
- J. Qin, X. Dai, Y. Zhou, L. Zhang, H. Chen and C. Gao, J. Membr. Sci., 2014, 468, 242–249 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9en00692c |
| This journal is © The Royal Society of Chemistry 2019 |