Synthesis of o-carborane-functionalized metal–organic frameworks through ligand exchanges for aggregation-induced emission in the solid state†
Sangdon
Choi‡
a,
Ha-Eun
Lee‡
a,
Chan Hee
Ryu‡
b,
Jooyeon
Lee
a,
Jihyun
Lee
c,
Minyoung
Yoon
 d,
Youngjo
Kim
d,
Youngjo
Kim
 a,
Myung Hwan
Park
a,
Myung Hwan
Park
 *e,
Kang Mun
Lee
*e,
Kang Mun
Lee
 *b and
Min
Kim
*b and
Min
Kim
 *a
*a
aDepartment of Chemistry and BK21Plus Research Team, Chungbuk National University, Cheongju, 28644, Korea. E-mail: minkim@chungbuk.ac.kr
bDepartment of Chemistry, Institute of Molecular Science and Fusion Technology, Kangwon National University, Chuncheon 24341, Korea. E-mail: kangmunlee@kangwon.ac.kr
cDepartment of Nanochemistry, Gachon University, Sungnam 13120, Korea
dDepartment of Chemistry, Kyungpook National University, Daegu 41566, Korea
eDepartment of Chemistry Education, Chungbuk National University, Cheongju, 28644, Korea. E-mail: mhpark98@chungbuk.ac.kr
First published on 9th September 2019
Abstract
The carborane (CB)-functionalized ligand was installed in a variety of MOFs through postsynthetic ligand exchange processes. This methodology is a general method for preparing o-CB-functionalized MOFs with known frameworks. Furthermore, the photoluminescence (PL) spectra revealed intriguing aggregation-induced emission (AIE) features following the systematic incorporation of o-CB functionalities into framework-type materials.
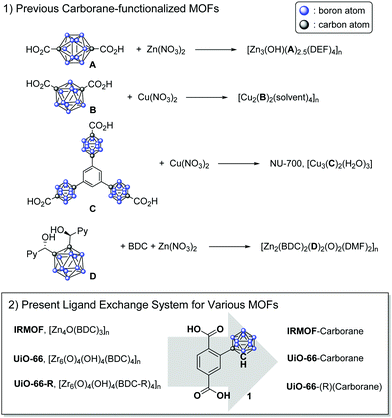
Icosahedral carboranes are inorganic cage molecules that consist of two carbon atoms and ten boron atoms and are utilized in the organometallic and materials fields because of their hydrophobicity and chemical/physical stability. In particular, o-carborane (o-CB) cages are considered a benzene substituent because they have three-dimensional aromaticity.1,2 In addition, various organometallic species have been shown to have unique photophysical properties, and as a result, they have been extensively studied for use in energy transfer materials.3–11 Recently, the incorporation of carborane cages into solid-state materials, such as in polymers and porous metal–organic frameworks (MOFs) along with optoelectronics, gas separation devices and hydrophobicity sensors, has been reported.12–14
MOFs are porous polymeric materials that consist of metal clusters and multitopic organic ligands. Their controllable porosity, relatively low density, and broad structural diversity as well as the chemical tunability of their ligands make these organic/inorganic hybrid materials of great importance for various applications, such as molecular storage, separation, and catalysis.15 The ligand tunability and/or installation of functional groups on MOFs are directly related to the performance of MOFs in the target applications. Since both MOFs and o-CB are tunable inorganic-based materials, the combination of these two materials is receiving considerable attention from the inorganic and materials chemistry fields. The o-CB unit is generally considered a benzene substituent in MOF ligands, serving as a strut in the framework; however, each ligand is designed for a specific MOF, and the tunability of the MOFs/o-CB is very limited in this system (Scheme 1).16–19 In addition, studies on carborane-MOFs have only focused on their hydrogen storage properties, stability and structural features, while their unique photophysical properties have not yet been investigated.
Herein, we demonstrate a postsynthetic ligand exchange (PSE) process for the installation of o-CB functionalities into MOFs and the photoluminescence (PL) of o-CB-functionalized MOFs. For this protocol, o-CB-functionalized benzene-1,4-dicarboxylic acid (BDC-CB) was prepared as the main ligand, and two representative BDC-based MOFs, IRMOF (IsoReticular MOFs) and UiO-66 (UiO = University of Oslo), were investigated (Scheme 1).20,21 Both IRMOF and UiO-66 were synthesized under simple solvothermal conditions with the appropriate metal salt (i.e., Zn(NO3)2 for IRMOF and ZrCl4 for UiO-66) and BDC ligand. IRMOF is a Zn(II)-based, landmark MOF that was reported in 1999 by Yaghi and co-workers, and various ligand functionalization processes involving this MOF have been studied.22 Since the practical uses of IRMOF are limited due to its low stability to humidity and water, CB installation into Zr(IV)-based UiO-66 as well as the practicality of the resulting material were also studied. UiO-66 shows superior chemical and physical stability due to the high oxophilicity of the zirconium cation, and a variety of organic functional groups were successfully installed in UiO-66 frameworks with functionalized BDCs. The solid-state PL of carborane-bearing MOFs with different functionalities in the framework was studied, and unprecedented aggregation-induced emission (AIE) was clearly visible from the MOFs.
The o-CB-functionalized BDC (BDC-CB, 1) was prepared through multiple organic reactions (Scheme S1, ESI†). All reactions were performed with the methyl ester form to facilitate separation by column chromatography. The key intermediate is an alkyne-functionalized BDCE, which can be synthesized by a Sonogashira cross-coupling with palladium and copper catalysts. Then, the o-CB cage was generated using decaborane (B10H14). After hydrolysis under basic conditions, the target BDC-CB was obtained.
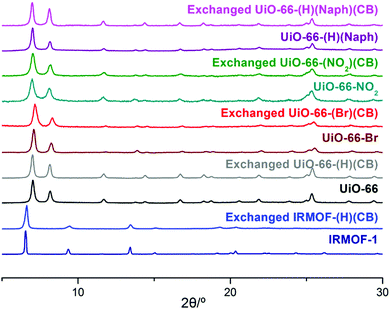
As an initial study, the direct synthesis of UiO-66-CB using ZrCl4 with BDC-CB was attempted; however, the o-CB-functionalized UiO-66-CB was not observed from the screening of various solvothermal conditions. Since the carborane cage is a relatively larger functional group than known functional groups in the UiO-66 pores, we also tried direct synthesis using a mixed ligand strategy. Several ratios of non-functionalized BDC and BDC-CB were tested, and carborane-functionalized UiO-66 was not obtained; only non-functionalized UiO-66 was synthesized. Therefore, a PSE strategy with the pre-synthesized UiO-66 was explored. Using PSE, the functionalized ligand could be incorporated into the MOF pores by exposing the parent MOF to the target ligand solution.23,24 Dry, non-functionalized UiO-66 was immersed in an aqueous solution of BDC-CB for 18 h, and UiO-66-CB was obtained by simple centrifugation. The crystallinity was completely retained during the PSE process, as evidenced by PXRD (powder X-ray diffraction, Fig. 1 and Fig. S1, ESI†). Infrared (IR) spectra displayed signals characteristic of B–H bonds after PSE, and the 1H NMR spectra after acid digestion showed that 14% BDC-CB was incorporated into the UiO-66 framework (Fig. S2, ESI†). This ratio did not change after extensive washing, which confirmed that the BDC-CB is not trapped in the pore but is exchanged into the framework. At the same time, PSE for IRMOF was also tested in an organic solvent (e.g., THF) due to its low stability in water. A smaller exchange ratio was achieved relative to that of UiO-66; thus, the exchange time and temperature were increased to 1 day and 40 °C. The retention of the bulk crystallinity of IRMOF was confirmed by PXRD, and a total of 4% BDC-CB was incorporated into the IRMOF structures, as evidenced by the IR and 1H NMR spectra of IRMOF after acid digestion (Fig. 1 and Fig. S3, S4, ESI†). Due to its chemical stability, UiO-66 is much easier to handle, better suited to carborane installation through ligand exchanges, and more practically applicable. Therefore, additional functionalizations were focused on UiO-66 with chemical tags.
Functionalized UiO-66-R (R = Br, NO2, and Naph) species were prepared using the solvothermal reaction between ZrCl4 and BDC-R (or NDC = 1,4-naphthalene dicarboxylic acid). Amino-functionalized UiO-66-NH2 and hydroxy-functionalized UiO-66-OH were not employed for this methodology because they have unique and relatively weak photoluminescence. The retention of the crystallinity of the UiO-66-Rs during the PSE process was confirmed by PXRD (Fig. 1 and Fig. S5–S7, ESI†). Notably, UiO-66-Naph has the smallest pore and window sizes among the functionalized UiO-66-Rs, and this prevented it from undergoing PSE. Therefore, a ligand mixing strategy between NDC and BDC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, see the ESI† for details) was employed to obtain more empty space for ligand exchange. The exchange ratio was determined by 1H NMR spectroscopy after acid digestion of thoroughly washed materials, and IR spectroscopy was employed to confirm the presence of B–H bonds in the ortho-carborane cages (Fig. S8–S10, ESI†). The remaining porosity in exchanged UiO-66-(R)(CB) was confirmed by N2 adsorption at 77 K, and the BET (Brunauer–Emmett–Teller) surface area was calculated (Fig. S11–S15, ESI†). Generally, the surface area decreased upon the introduction of carborane functionalities; however, the main pores of the MOFs were retained after installation of the bulky carborane cages. Thermogravimetric analysis (TGA) confirmed the thermal stability of the carborane-functionalized UiO-66s. The exchanged MOFs were generally stable up to 300 °C under N2 (Fig. S16, ESI†).
3 ratio, see the ESI† for details) was employed to obtain more empty space for ligand exchange. The exchange ratio was determined by 1H NMR spectroscopy after acid digestion of thoroughly washed materials, and IR spectroscopy was employed to confirm the presence of B–H bonds in the ortho-carborane cages (Fig. S8–S10, ESI†). The remaining porosity in exchanged UiO-66-(R)(CB) was confirmed by N2 adsorption at 77 K, and the BET (Brunauer–Emmett–Teller) surface area was calculated (Fig. S11–S15, ESI†). Generally, the surface area decreased upon the introduction of carborane functionalities; however, the main pores of the MOFs were retained after installation of the bulky carborane cages. Thermogravimetric analysis (TGA) confirmed the thermal stability of the carborane-functionalized UiO-66s. The exchanged MOFs were generally stable up to 300 °C under N2 (Fig. S16, ESI†).
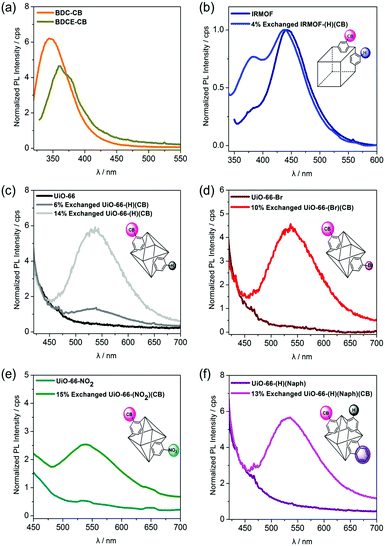
Prior to investigating the luminescent properties of the o-CB-appended MOFs, the PL spectra of the o-CB-functionalized ligands (BDCE-CB and BDC-CB (1)) were acquired. The ligands showed virtually no emission in solution (DMSO), which is typical of o-carborane derivatives;25,26 however, both BDCE-CB and BDC-CB in the solid state displayed intense emission in the high-energy region over λem = 400 nm (λem,max = 345 nm for BDC-CB and 360 nm for BDC-CB, Fig. 2(a)). This finding strongly indicates that the intense emission bands could be attributed to o-CB-based AIE induced by the restricted rotation of the carborane moiety in the rigid structure.27,28 To further check the AIE phenomenon observed in the solid state of BDC-CB ligand, the additional PL experiments were performed in DMSO–water mixed solvents.10,29 Unfortunately, no aggregation occurred upon increasing volumetric fractions of water in the DMSO–water mixed solvents and no emission peak was observed. Next, the emission spectra of o-CB-functionalized MOFs in the solid state were also investigated. A similar AIE was observed in the o-CB-functionalized IRMOFs in the region of 350–400 nm (Fig. 2(b), blue line). However, the only AIE from o-CB-bearing MOFs could not be precisely measured due to the strong emission at 442 nm originating from the IRMOF core (Fig. 2(b), dark blue line).30 To clarify the AIE characteristic of o-CB within MOF structures, UiO-66 MOFs, which are non-emissive, were utilized as a basic framework. Interestingly, all the o-CB-functionalized UiO-MOFs clearly exhibited broad emission in the range of 450 nm to 600 nm (Fig. 2(c)–(f)), which is significantly redshifted compared to the emission of o-CB-functionalized ligands. Furthermore, the PL spectra of UiO-66-(H)(CB) gradually intensified with increasing content of the carborane, verifying that the signals resulted from AIE caused by the presence of o-CBs (Fig. 2(c)). However, the non-emissive nature of o-CB-functionalized MOFs in the high-energy regions was also observed. The changes in the emissive behaviors of o-CB-appended MOFs could be attributed to the desired intermolecular interactions between the carborane cages, which are regularly incorporated into the building blocks of the framework. In contrast, the physical mixture of BDC-CB and UiO-66 (10 mol% of BDC-CB to UiO-66) did not show significant redshift in the PL spectra (Fig. S17, ESI†). These findings further suggest that such framework materials containing carborane moieties could play an important role in controlling the aggregation effects favored by various o-carborane-based molecules.
In conclusion, we have successfully prepared o-CB-functionalized BDC ligands for the installation of carborane cages into various BDC-based MOFs. Two representative frameworks, Zn(II)-based IRMOF and Zr(IV)-based UiO-66, were subjected to o-CB functionalization with a postsynthetic ligand exchange strategy, and approximately 10% carborane functionalities were incorporated into MOF structures. The good functional group tolerance of the carborane installation was confirmed for UiO-66 since it has superior chemical stability, which is an important advantage for practical applications. The maxima in the PL spectra were drastically shifted upon installation into MOFs, and these changes mainly originate from AIE. This methodology provides a general approach for the formation of systematic carborane-functionalized MOFs, which show intriguing photophysical properties as a novel class of carborane-bearing framework materials.
This research was supported by the Basic Science Research Program (2019R1A2C4070584) and the Science Research Center (2016R1A5A1009405) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. E. Williams, Chem. Rev., 1992, 92, 177–207 CrossRef CAS.
- V. I. Bregadze, Chem. Rev., 1992, 92, 209–223 CrossRef CAS.
- A. V. Marsh, N. J. Cheetham, M. Little, M. Dyson, A. J. P. White, P. Beavis, C. N. Warriner, A. C. Swain, P. N. Stavrinou and M. Heeney, Angew. Chem., Int. Ed., 2018, 57, 10640–10645 CrossRef CAS PubMed.
- N. Van Nghia, S. Park, Y. An, J. Lee, J. Jung, S. Yoo and M. H. Lee, J. Mater. Chem. C, 2017, 5, 3024–3034 RSC.
- H. Naito, Y. Morisaki and Y. Chujo, Angew. Chem., Int. Ed., 2015, 54, 5084–5087 CrossRef CAS PubMed.
- Y. H. Lee, J. Park, J. Lee, S. U. Lee and M. H. Lee, J. Am. Chem. Soc., 2015, 137, 8018–8021 CrossRef CAS PubMed.
- A. M. Prokhorov, T. Hofbeck, R. Czerwieniec, A. F. Suleymanova, D. N. Kozhevnikov and H. Yersin, J. Am. Chem. Soc., 2014, 136, 9637–9642 CrossRef CAS PubMed.
- R. Visbal, I. Ospino, J. M. López-De-Luzuriaga, A. Laguna and M. C. Gimeno, J. Am. Chem. Soc., 2013, 135, 4712–4715 CrossRef CAS PubMed.
- K. R. Wee, W. S. Han, D. W. Cho, S. Kwon, C. Pac and S. O. Kang, Angew. Chem., Int. Ed., 2012, 51, 2677–2680 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- J. Liu, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799–5967 CrossRef CAS PubMed.
- K. Kokado and Y. Chujo, Macromolecules, 2009, 42, 1418–1420 CrossRef CAS.
- M. H. Park, K. M. Lee, T. Kim, Y. Do and M. H. Lee, Chem. – Asian J., 2011, 6, 1362–1366 CrossRef CAS PubMed.
- M. Eo, M. H. Park, T. Kim, Y. Do and M. H. Lee, Polymer, 2013, 54, 6321–6328 CrossRef CAS.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- O. K. Farha, A. M. Spokoyny, K. L. Mulfort, M. F. Hawthorne, C. A. Mirkin and J. T. Hupp, J. Am. Chem. Soc., 2007, 129, 12680–12681 CrossRef CAS PubMed.
- S. L. Huang, L. H. Weng and G. X. Jin, Dalton Trans., 2012, 41, 11657–11662 RSC.
- D. J. Clingerman, W. Morris, J. E. Mondloch, R. D. Kennedy, A. A. Sarjeant, C. Stern, J. T. Hupp, K. Farha and C. A. Mirkin, Chem. Commun., 2015, 51, 6521–6523 RSC.
- S. Rodríguez-Hermida, M. Y. Tsang, C. Vignatti, K. C. Stylianou, V. Guillerm, J. Pérez-Carvajal, F. Teixidor, C. Viñas, D. Choquesillo-Lazarte, C. Verdugo-Escamilla, I. Peral, J. Juanhuix, A. Verdaguer, I. Imaz, D. Maspoch and J. Giner Planas, Angew. Chem., Int. Ed., 2016, 55, 16049–16053 CrossRef PubMed.
- H. Li, M. Eddaoudi, M. O’Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- M. Kim, J. F. Cahill, Y. Su, K. A. Prather and S. M. Cohen, Chem. Sci., 2012, 3, 126–130 RSC.
- M. Kim, J. F. Cahill, H. Fei, K. A. Prather and S. M. Cohen, J. Am. Chem. Soc., 2012, 134, 18082–18088 CrossRef CAS PubMed.
- A. Ferrer-Ugalde, E. J. Juárez-Pérez, F. Teixidor, C. Viñas, R. Sillanpää, E. Pérez-Inestrosa and R. Núñez, Chem. – Eur. J., 2012, 18, 544–553 CrossRef CAS PubMed.
- K. Kokado and Y. Chujo, J. Org. Chem., 2011, 76, 316–319 CrossRef CAS PubMed.
- L. Weber, J. Kahlert, R. Brockhinke, L. Böhling, A. Brockhinke, H.-G. Stammler, B. Neumann, R. A. Harder and M. A. Fox, Chem. – Eur. J., 2012, 18, 8347–8357 CrossRef CAS PubMed.
- B. H. Choi, J. H. Lee, H. Hwang, K. M. Lee and M. H. Park, Organometallics, 2016, 35, 1771–1777 CrossRef CAS.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC.
- M. Ji, X. Lan, Z. Han, C. Hao and J. Qiu, Inorg. Chem., 2012, 51, 12389–12394 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental detail and spectroscopic data for 1H and 13C NMR PDF. See DOI: 10.1039/c9cc06386b |
| ‡ S. Choi, H.-E. Lee, C. H. Ryu are equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2019 |