Open Access Article
Open Access ArticleBoronic acid-modified poly(amidoamine) dendrimers as sugar-sensing materials in water†
X.
Liang
and
M.
Bonizzoni
*
Department of Chemistry, The University of Alabama, Box 870336, Tuscaloosa, AL 35487-0336, USA. E-mail: marco.bonizzoni@ua.edu
First published on 1st March 2016
Abstract
Boronic acids can be used as receptors in chemical sensors for sugars, but their binding affinity and solubility are usually very poor in water. We improved these parameters by covalently connecting boronic acid moieties to the surface of third-generation poly(amido)amine (PAMAM) dendrimers to form a family of PAMAM–boronic acid receptors that display multivalent behavior. We confirmed the increased interaction strength of these modified boronic acid receptors using diol-containing dyes such as 4-methylesculetin and alizarin red S as probes in optical spectroscopy experiments. We then translated these results to sugar sensing using the self-assembled [PAMAM-ba·(dye)n] complexes as sensors in an indicator displacement assay. Our approach successfully detected simple sugars (e.g. fructose, glucose, galactose and ribose) in water, traditionally a very challenging medium for carbohydrate detection. Finally, we demonstrated the use of these polymer-based sensors in a multivariate array sensing platform for the discrimination of simple sugars in water as a proof of principle towards their broader applicability under physiologically and environmentally relevant conditions.
Introduction
An ongoing quest of sensing science is to create an efficient sensing method to detect and discriminate sugars with good sensitivity and selectivity.1–6 Sugars play crucial roles as carbon and energy sources to build and fuel cells in living creatures; carbohydrate conjugates are also crucially involved in cell signalling and recognition in living systems.7–9 They relate to most of the important functioning processes in our bodies such as controlling metabolism, stress resistance, growth and development.10 In biological systems, enzymes are used to sense and recognize different sugars with a high selectivity due to their unique geometric shapes.Although sugars have also long been established as important targets for which efficient artificial sensors are required,11 sugar sensing is still challenging using synthetic receptors. First, many synthetic receptors do not dissolve well in aqueous solution, in which sugars dissolve. Second, sugars have high solvation enthalpies in water that are difficult for synthetic sensors to overcome. Third, selectivity has been generally difficult to achieve as most simple sugars show very little structural difference except configurations of certain stereocenters.12
The covalent reversible interaction of boronic acids with diols (shown in Scheme 1) has long generated great interest in this field as one of the few chemical interactions that are somewhat selective for sugars under highly competitive conditions; this property has allowed the development of boronic acid-based chemosensors to detect sugars functioning in organic solvents, but the relatively low affinity associated with this process in water has made direct sensing in this crucial medium extremely challenging.13
Two classical methods, direct sensing and indicator displacement assays (IDA), have been used to design sugar sensing systems (Scheme 2). In a direct sensing approach, a signalling unit is covalently and permanently connected to a recognition unit; binding of the analyte of interest to the recognition unit results in a signal change. In sugar sensing, the recognition unit often contains a boronic acid moiety; signalling is commonly accomplished by exploiting a change in the absorption or emission properties of an appropriately chosen chromophore. Successful boronic acid sugar receptors used in direct sensing are often multivalent in order to overcome the high solvation enthalpies of sugars in water.14–16 To increase the solubility of such receptors in water, the boronic acids have been covalently linked to water-soluble polymers,17 or bound to a charged moiety.18 Selectivity has been typically difficult to achieve.18,19
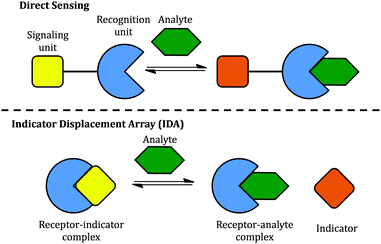 | ||
| Scheme 2 Two approaches to the construction of chemical sensors: (top) direct sensing; (bottom) an indicator displacement assay (IDA). | ||
However, one disadvantage of direct sensing is that the selectivity of the sensing system is difficult to tune since the signalling unit and the recognition unit are covalently linked. Besides, higher synthetic effort goes into creating a receptor compared to IDA. In the latter case, the recognition and signalling units are separate molecules, held together by non-covalent interactions to form a supramolecular sensor; upon exposure to analytes, the signalling unit is then displaced by the analyte, leading to a detectable signal change (Scheme 2). The properties of an IDA sensing system can be tuned more easily because of the increased flexibility afforded by the non-covalent linkage. IDA systems have been proposed for sugar sensing as well: for instance, a complex between phenylboronic acid and dye alizarin red S (ARS) has been used to sense sugars, but the relatively low interaction energy limited the applicability of this system to organic solvents.20 However, there is no single system able to sense different sugars effectively.
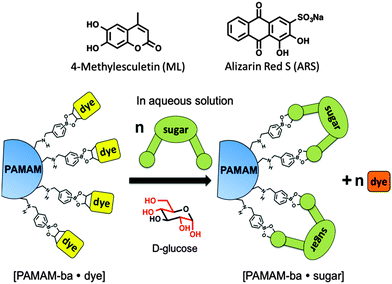
Here we report on our efforts towards the creation of a sugar-sensitive boronic acid-modified water-soluble polymeric material capable of sugar detection and discrimination in neat water solution. Sensing is achieved using an IDA approach based on two synthetically modified boronic acid–PAMAM receptors (PAMAM-m-ba and PAMAM-o-ba) and two commercially available dyes (ML and ARS), at two pH levels (7.4 and 10.0). As further proof of the functionality of these receptors, we also report on the development of an array-sensing system based on these receptors capable of discriminating between four common sugars (fructose, glucose, galactose, and ribose) in neat water.
We selected poly(amidoamine) (PAMAM) dendrimers21,22 as polymeric scaffolds with which to build our multivalent sugar-reactive sensors because of their excellent water solubility over a wide range of pH values,23,24 their highly branched, globular and symmetrical structure,25 the large number of surface groups available for covalent surface modification, and their wide commercial availability in high purity. By decorating the surface of a PAMAM dendrimer with boronic acids, we aimed to significantly increase the solubility of the boronic acid recognition moieties and to introduce beneficial multivalency in the receptors: multiple interactions of the receptor with each analyte will afford higher overall binding affinity and a much better chance for these sensors to work even in neat water, being able to overcome energetic barrier posed by the high cost of desolvation of these sugar analytes in aqueous media.
We then selected dyes 4-methylesculetin (ML) and alizarin red S (ARS) (see Scheme 3) for the construction of an indicator displacement assay. The Anslyn26–28 and Wang groups20,29 have previously reported on the binding of these dyes to boronic acid moieties accompanied by changes in the absorbance spectrum and enhanced fluorescence emission.
We constructed sugar sensors for use in an IDA scheme by forming complexes of these two dyes with our surface-modified PAMAM dendrimers ([PAMAM-ba·dye], Scheme 3). When an analyte (sugar) is added to this complex, dye molecules are displaced by the sugar; because the dye displays characteristically different spectroscopic signatures when free or bound, the dye's optical signal reports on the binding of the sugar guest (Scheme 3, bottom).
Experimental
The following numbering scheme will be used for the assignment of 1H-NMR spectra in these polymers: (aCH2aCH2)[N(bCH2cCH2CONHdCH2eCH2N(fCH2gCH2CONHhCH2iCH2N(jCH2kCH2CONHlCH2mCH2N(nCH2oCH2CONHpCH2qCH2NHrCH2C6H4B(OH)2)2)2)2)2]2. Also see the ESI† for full spectra, assignments, and further details.PAMAM-m-ba
To a 25 mL round bottom flask were added a 20 wt% MeOH solution of the ethylenediamine core (1.00 g), amine terminated third-generation PAMAM dendrimers (0.0289 mmol), 0.174 g (1.16 mmol) of 3-formylphenylboronic acid, and 1.30 g of freshly regenerated 4 Å molecular sieves. The mixture was stirred at 70 °C in an oil bath for 3 days. The molecular sieves were then removed by vacuum filtration and washed 3× with MeOH. The washing solvent was returned to the reacting mixture. To this mixture were added 0.665 g (17.6 mmol) of NaBH4 and 3.5 mL of MeOH. The resulting mixture was stirred at r.t. for 24 h. Solvent was then removed on a rotary evaporator, yielding a solid, which was re-dissolved in 6.0 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O and MeOH. The solution was transferred to a dialysis membrane (6–8 kDa MW cut-off), which was sealed and suspended in 800 mL of a 1
1 mixture of H2O and MeOH. The solution was transferred to a dialysis membrane (6–8 kDa MW cut-off), which was sealed and suspended in 800 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH
1 MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture for 3 h. The membrane was then removed from this mixture and suspended in 800 mL of neat DI water for 24 h, followed by 800 mL of neat MeOH for another 24
H2O mixture for 3 h. The membrane was then removed from this mixture and suspended in 800 mL of neat DI water for 24 h, followed by 800 mL of neat MeOH for another 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. The solution in the membrane was collected in a tared 50 mL round bottom flask, and the volatiles were removed on a rotary evaporator, yielding 0.221 g of a colourless solid (68.3%).
h. The solution in the membrane was collected in a tared 50 mL round bottom flask, and the volatiles were removed on a rotary evaporator, yielding 0.221 g of a colourless solid (68.3%).
1H-NMR (360 MHz, CD3OD, ppm) δH: 7.40–7.60 (50H, o-PhB(OH)2), 7.22–7.32 (25H, p-PhB(OH)2), 7.05–7.20 (25H, m-PhB(OH)2), 4.58 (50H, r), 3.51–3.77 (120H, d, h, l, p), 2.63–3.00 (184H, b, f, j, n, q), 2.49–2.63 (58H, a, e, i, m), 2.04–2.49 (120H, c, g, k, o). MALDI-TOF: calcd for C477H783N122O110B25Na = [M + Na]+: 10279.2. Found: 10279.0.
PAMAM-o-ba
To a 25 mL round bottom flask were added a 20 wt% MeOH solution of the en-core (1.00 g), amine terminated G3 PAMAM dendrimers (0.0289 mmol), 0.174 g (1.16 mmol) of 2-formylphenylboronic acid, and 1.45 g of freshly regenerated 4 Å molecular sieves. The mixture was stirred at a 70 °C in an oil bath for 3 days. The molecular sieves were then removed by vacuum filtration and washed 3× with MeOH. The washing solvent was returned to the reaction mixture, to which 1.34 g (35.42 mmol) of NaBH4 and 6.0 mL of MeOH were added. The resulting mixture was left to stir at r.t. for 24 h. The solvent was removed on a rotary evaporator to yield a solid, which was re-dissolved in 6.0 mL of MeOH. This solution was transferred to a rehydrated dialysis membrane (6–8 kDa MW cut-off), which was suspended in 800 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH
1 MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture for 24 h. The membrane was then transferred to 800 mL of neat DI water for another 24
H2O mixture for 24 h. The membrane was then transferred to 800 mL of neat DI water for another 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h, and finally to 800 mL of neat MeOH for 24
h, and finally to 800 mL of neat MeOH for 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. The solution was then collected in a 25 mL tared round bottom flask and the volatiles were evaporated to yield 0.0973 g (30.2%) of a colourless solid.
h. The solution was then collected in a 25 mL tared round bottom flask and the volatiles were evaporated to yield 0.0973 g (30.2%) of a colourless solid.
1H-NMR (360 MHz, CD3OD, ppm) δH: 7.33–7.50 (26H, o-PhB(OH)2), 7.16–7.25 (52H, m-PhB(OH)2), 7.09–7.16 (26H, p-PhB(OH)2), 4.04 (52H, r), 3.45–3.59, (56H, d, h, l), 3.15–3.25 (64H, p), 2.90–3.13 (64H, q), 2.72–2.81 (120H, b, f, j, n), 2.49–2.53 (58H, a, e, i, m), 2.27–2.41 (120H, c, g, k, o). MALDI-TOF: calcd for C484H790N122O112B26K = [M + K]+: 10429.2. Found: 10429.0.
Titration conditions and multivariate analysis
See ESI.†Results and discussion
Design and synthesis of the polymeric receptors
Commercially available amine-terminated PAMAM dendrimers are ideal starting scaffolds to construct large yet compact and globular multivalent structures in which multiple binding sites are held in close proximity to each other, facilitating multiple interactions with their intended guest, and ultimately opening the way for a significant affinity boost. The fact that these polymers are commercially available in high purity,30 and that they possess reactive amine surface groups that can be selectively functionalized with a host of standard techniques significantly adds to their value in this application.31–33 James and Shinkai previously reported on the modification with boronic acids of a branched polymer equivalent in size to a G1 PAMAM dendrimer. In that case they fully converted the primary amine termini, which were also decorated with a fluorophore for detection. The much smaller receptor they obtained did display increased affinity towards simple sugars over a plain boronic acid, but unfortunately it only worked in neat MeOH, as it was insoluble in water.34In the approach presented here, we used the PAMAM dendrimers' high water solubility across a wide pH range to improve the solubility and water-compatibility of the boronic acid sensing moieties. To do so, we specifically sought incomplete conversion of the dendrimer's terminal groups into boronic derivatives, so that some of the unreacted surface amine groups in these receptors can be protonated in water solution, and the resulting positive charges will improve the solubility of our receptor under these conditions. In this context, a larger third generation (G3) PAMAM dendrimer provides an excellent compromise between achieving a large enough size to obtain a significant affinity boost and yet to be affordable and practical enough for other researchers to adopt our method.
We synthesized two receptors, one with the boronic acid group in the ortho position to the PAMAM attachment point (PAMAM-o-ba), and the other with attachment at the meta position (PAMAM-m-ba). Previous studies on (p-methylamino)phenylboronic acids have shown that the substitution pattern is ineffective in binding to diol moieties, so we did not pursue it here.35,36 Covalent modification of the dendrimers was achieved in a two-step, one-pot reaction involving the formation of an imine by reaction of the primary amines on the dendrimer's surface with formyl groups of commercially available formyl phenylboronic acids, followed by in situ reduction to the corresponding secondary amine. This pathway (Scheme 4) afforded the final products in good yields and purity.
 | ||
| Scheme 4 Synthesis of receptors PAMAM-m-ba and PAMAM-o-ba. Isolated yields after purification by membrane dialysis were 68.3% and 30.2% respectively. | ||
The reaction was conveniently monitored by 1H-NMR to follow the degree of conversion of the starting aldehyde (signal at 10.0–10.1 ppm) into the corresponding imine (signal at 8.0–9.5 ppm). When sufficient conversion was achieved as judged by NMR spectroscopy, the condensation reaction was interrupted by introduction of a large excess of NaBH4, which both reduced the newly formed imine to the desired secondary amines, and inactivated the excess of formyl phenylboronic acid by reduction of its carbonyl moiety.37–39
The polymeric product was then purified by repeated equilibrium dialysis in alternating water and methanol to remove the excess small-molecule reagents and inorganic by-products. After a final solvent exchange to MeOH, the each PAMAM-ba product was obtained as a solid after evaporation of the organic solvent. The purity of the product was confirmed by 1H NMR and MALDI-TOF spectra, which allowed us to calculate an average extent of conversion of the surface amines to boronic acid groups; conversion was found to be around 80% for both receptors, in accordance with our partial conversion goal stated above. Since G3 PAMAM dendrimers has totally 32 branches, around 25 of those ended up carrying boronic acid moieties.
We will show below that these water-soluble multivalent receptors are able to bind to the diol functional group efficiently, and that they can be used in the construction of effective IDA sensors for sugars in neat water.
PAMAM-ba binding to the 4-methylesculetin (ML) dye
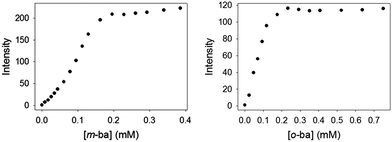
The quenching observed initially may be due to two possible effects. On the one hand, at the beginning of the titration a large excess of ML dye is present with respect to the polymer, so high molecularity dye–polymer complexes will be formed, which may lead to self-quenching of the dye as its molecules are forced into close proximity. However, this is expected to be a negligible effect for a dye like ML, which displays the typical large Stokes shift associated with coumarin-type fluorophores. Instead, it is more likely that two processes are at work simultaneously in this interaction: inefficient dynamic quenching of ML by random collision of excited ML molecules with boronic acids on the surface of the dendrimer, and more efficient fluorescence reviving upon formation of the ground-state complex between ML and a boronic acid moiety. As more polymer is added to the solution during the course of the titration the extent of binding increases, making the latter process more important and causing an overall increase in the observed fluorescence intensity.
This value was compared to that obtained from the binding of isolated 2-formylphenylboronic acid to the ML dye, for which we calculated a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1
K1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
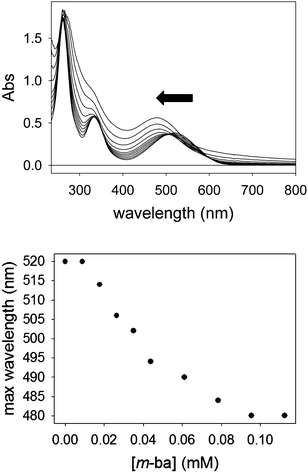
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 3.61(3) (see ESI†), which is in accordance with literature reporting that boronic acids decorated in their ortho position display significant affinity for diols.28 Indeed, 2-formylphenylboronic acid was the only isomeric boronic acid that formed complexes with this dye (see ESI†). Nevertheless, the much higher binding constant reported for the boronic acid site in the dendritic structure vs. the isolated one strongly supports our hypothesis that inclusion of the boronic acid moiety in the PAMAM structure significantly increases its affinity for diols.
1 = 3.61(3) (see ESI†), which is in accordance with literature reporting that boronic acids decorated in their ortho position display significant affinity for diols.28 Indeed, 2-formylphenylboronic acid was the only isomeric boronic acid that formed complexes with this dye (see ESI†). Nevertheless, the much higher binding constant reported for the boronic acid site in the dendritic structure vs. the isolated one strongly supports our hypothesis that inclusion of the boronic acid moiety in the PAMAM structure significantly increases its affinity for diols.
The binding affinity of PAMAM-m-ba for the ML dye was also determined in the same way (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 5.0(3)); unfortunately, however, the corresponding 3-formylphenylboronic acid did not display any binding interaction with ML (see discussion below), so a direct comparison was not possible in that case.
Ka = 5.0(3)); unfortunately, however, the corresponding 3-formylphenylboronic acid did not display any binding interaction with ML (see discussion below), so a direct comparison was not possible in that case.
It is also noteworthy that no binding was observed between ML and this simple isolated boronic acid, although binding of ML to the polymeric PAMAM-ba receptors was clearly observed under comparable conditions (see Fig. 1). This further highlights the large increase in affinity.
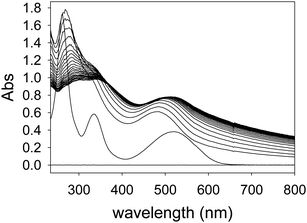
PAMAM-ba binding to alizarin red S (ARS) dye
The formation of nanoscopic aggregates has been previously observed for these polyelectrolytes in water solution. Indeed, PAMAM dendrimers and other similar polyelectrolytes have been shown to form nanoparticles of definite and controllable size and shape, and assembly triggering was demonstrated with polyhydroxylated compounds such as cyclodextrins.41,42 Both electrostatic effects and solvation changes have been deemed responsible for the formation of aggregates displaying decreased solubility.43 These reports strongly support our hypothesis that the dendrimers themselves are soluble under the conditions of our work, but the dendrimer–ARS complexes exhibit decreased solubility.
Sugar detection
Once binding of these dyes to the PAMAM-ba receptors had been confirmed, and conditions to induce these binding events were known, we were in a position to use these receptors and dyes to set up an indicator displacement assay to detect the binding of sugars to these receptors. In fact, since most common sugars do not have a significant intrinsic chromophore or fluorophore, even the direct binding would be spectroscopically silent. Use of an indicator dye in an IDA assay allows us to circumvent that problem, and to continue the use of optical spectroscopy, one of the cheapest, fastest, and easiest to automate instrumental techniques in the analytical chemist's toolbox.We first determined favourable conditions to prepare each [PAMAM-ba·dye] self-assembled sensor. Previous experience in our group with the construction of IDA sensors suggests using conditions in which the dye is ca. 80% bound to its receptor to optimize responsiveness and sensitivity of the assay.44–46
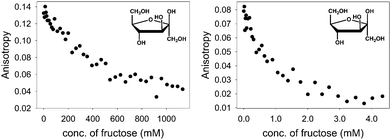
With the above binding data in hand, such conditions were easily determined. D-(−)-Fructose was the first sugar studied: as aliquots of a fructose solution were added to an aqueous solution containing [PAMAM-m-ba·ML] at pH 7.4, the anisotropy decreased gradually (Fig. 7, left), showing a reversal of the trend observed in the corresponding binding titration (Fig. 1) and indicating that fructose was successfully displacing the ML dye from its complex with PAMAM-m-ba. Although this provided a clearly successful proof of principle, the sensitivity of this assay was not satisfactory: the concentration of fructose had to reach 1 M to induce complete displacement of the dye, suggesting that the [PAMAM-m-ba·ML] complex was not very sensitive to fructose under these conditions.
Comparable results were obtained when trying to displace the ML dye from its complex with the PAMAM-o-ba receptor using fructose at pH 7.4: full dye displacement could be attained at a comparable or slightly improved concentration of the sugar (ca. 0.5 M fructose, see ESI†). Additionally, an attempt to sense galactose using the [PAMAM-m-ba·ML] complex was unsuccessful under these conditions: very little dye displacement was observed even at high concentrations of this sugar. In short, the sensitivity of either receptor to sugars was found to be regrettably low under these conditions.
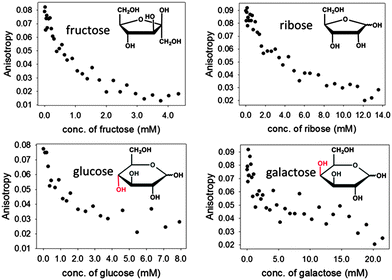
Encouraged by these results, we expanded the method to other sugars, including D-ribose, D-glucose and D-galactose. The relevant results are shown in Fig. 8 below. It is particularly promising to see that even sugars such as galactose, to which our sensors did not respond well at neutral pH, could be readily sensed in these improved conditions.
The results in Fig. 8 also show that structurally different sugars display varying affinities towards the PAMAM-ba system. We attribute these differences to the structural features of these sugars: for instance, fructose and ribose are known to form a five-membered furanose ring in solutions, which contain a rigid cis-diol moiety that is known to bind to boronic acid very efficiently.48 On the other hand, the pyranose rings prevalent in glucose and galactose in aqueous solution do not contain an equally rigid interaction site; the energetic cost that must be paid to freeze their higher conformational freedom upon binding therefore results in markedly lower affinities observed here.
Similar displacement experiments were also performed on sensing complexes containing the ARS dye (Fig. 9, right). As already mentioned above, anisotropy measurements with ARS complexes were unfortunately hampered by the formation of a small quantity of highly scattering aggregates and were not informative. We monitored the fluorescence emission intensity signal instead. The emission signal was normalized to that of the bound dye: a decrease in the normalized emission indicates dye displacement and therefore sugar binding. Although the use of an ARS complex is complicated by the aggregation process described above, it provides an improved dynamic range to the sensing process, since the spectroscopic difference between the dye's free and bound states is more marked.
Discrimination of sugars through array sensing
The experiments shown above have established that the PAMAM-ba modified dendrimers can act as ligands for carbohydrate moieties; in combination with appropriate dyes, the sugar concentration stimulus can be translated into an optical response by the system.However, the comparison of sugar responses summarized in Fig. 9 for [PAMAM-m-ba·ML] and [PAMAM-m-ba·ARS] clearly indicates that neither of these sensors is very selective for any one of the sugars under study: although each sensor responds to the sugar analytes, different analytes cause similar responses and discrimination based on any single analytical signal (i.e. a univariate approach) would be unsuccessful.
For instance, the largest difference between all the sugars in the [PAMAM-m-ba·ML] system was observed at around a 2 mM analyte concentration; however, fructose could not be differentiated from glucose. On the other hand, the sensor using the ARS dye was able to discriminate between glucose and fructose under those conditions, but not between ribose and galactose. In short, the combined results obtained from both sensors were found to contain sufficient information to effect differentiation. In order to harvest this information effectively, we use these two sensing systems to construct a cross-reactive array sensing system to sense and discriminate different sugars.49,50 Using array sensing techniques our group has recently reported on the recognition of e.g. physiologically relevant phosphate esters,46 organophosphates including pesticides and nerve gas agents,45 and divalent metal ions.44
Since each one of the four sugars tested so far has a slightly different interaction with each [PAMAM-ba·dye] complex, a unique response pattern for each certain sugar can be generated from which that sugar can be recognized (Scheme 5).
In the present case we constructed a sensing array by using the following building blocks: 2× receptors, PAMAM-m-ba and PAMAM-o-ba; 2× dyes (ARS and ML); 2× pH conditions (pH 7.4 and 10.0). Each receptor-dye-condition combination represented one component of the sensing array. Each component was exposed to the four sugars in turn ([sugar] = 2.00 mM; [ARS] = 0.0514 mM; [ML] = 0.0103 mM; [PAMAM-m-ba] = 0.0174 mM; [PAMAM-o-ba] = 0.0102 mM).
Array responses were measured by monitoring absorption, and fluorescence intensity for both ML and ARS complexes, and fluorescence anisotropy for the ML sensor (anisotropy for the ARS sensor was not available as discussed above); a total of 9 experimental measurements were acquired per each sample replicate. Twelve replicates were measured for each analyte. Samples were laid out on 384-well plates together with relevant blanks and references. All measurements were then carried out on a multimode microwell plate reader capable of acquiring absorbance, fluorescence emission intensity and polarization; the use of a microwell plate reader was crucial to the development of the assay, as it allowed for automated and highly repeatable multivariate data acquisition. Plate layouts and further experimental details are reported in the ESI.†
Once raw experimental measurements were acquired, we used linear discriminant analysis (LDA) to analyse the data set. LDA has long been used for complex data reduction in applications such as face and voice recognition;51 more recently, it has also been introduced to the chemometrics community.52,53 The purpose of the multivariate LDA algorithm is to generate linear combinations of the original raw measurements with the purpose of minimizing the dispersion between replicate measurements of each analyte, while at the same time maximizing the distance between analyte clusters. In doing so, LDA generates a new set of variables (factors), returned in the order of decreasing information content. By choosing the two most information-rich factors and discarding others that contributed only marginally to the differentiation, the dimensions of the data set were drastically reduced; data points were then plotted on a two dimensional graph according to their scores along each LDA factor.
The ultimate results of data processing on the samples obtained from our sensing array are shown in Fig. 10 as an LDA score plot. Replicate samples corresponding to the same sugar were found to cluster close together while at the same time clusters of samples corresponding to different sugars were found to be well separated from each other. The score plot reported here represents 97.9% of the total information content that was available in the raw data set, indicating that data reduction could be carried out with only minimal information loss.
In short, the LDA plot in Fig. 10 demonstrated that we were able to use a series of poorly selective self-assembled sensors based on PAMAM-m-ba and PAMAM-o-ba and two dyes to construct a sensing array capable of qualitative differentiation of simple sugars in neat water solution. Of particular interest is the ability of this sensor to discriminate between very similar structures such as glucose and galactose, which differ only by the configuration of a single chiral centre in their structure.
Conclusions
Two new sugar receptors, PAMAM-m-ba and PAMAM-o-ba, were synthesized. The binding properties of these receptors and dyes 4-methylesculetin (ML) and alizarin red S (ARS) were studied by fluorescence and UV-Vis spectroscopy in neutral aqueous solution. As desired, PAMAM-m-ba and PAMAM-o-ba displayed excellent solubility in water, much improved from that of the corresponding isolated boronic acid. Both PAMAM-m-ba and PAMAM-o-ba bound to ML and ARS dyes with high affinity in aqueous solution, which opened the door for the construction of indicator displacement assays for the sensing of sugars in neat water.Displacement experiments with sugars were performed successfully at pH 7.4 and at pH 10.0. These sensors displayed greatly improved affinity for simple sugars even in neat water, typically a very challenging medium for sugar recognition and sensing. The highest affinity was observed at around pH 10.0: under the latter conditions, this sensing system was also sensitive to glucose, galactose and ribose. In conclusion, our initial hypothesis that dendritic boronic acid derivatives would display much improved affinity towards sugars when compared to their isolated counterparts was proved correct.
As a proof of principle of possible applications of these receptors and because the selectivity of the individual receptors was found to be poor under these conditions, we constructed a pattern-based sensing array using multiple combinations of the two receptors (PAMAM-m-ba and PAMAM-o-ba), two dyes (ARS and ML), and two pH conditions (7.4 and 10.0) outlined above. The high-dimensionality data obtained from this array were analysed using multivariate techniques, resulting in excellent clustering results: replicate samples for the same sugar were clustered tightly, while data clusters from different sugars were well-separated. As a proof of principle, the system we propose here is therefore capable of qualitative recognition and differentiation of fructose, ribose, glucose, and galactose, the latter two being particularly impressive feats given their great structural similarity.
Acknowledgements
We gratefully acknowledge the help of Dr Yuping Bao and her group in the Department of Chemical Engineering at The University of Alabama for use of their instrument and their help in carrying out the dynamic light scattering experiments. We also acknowledge the financial support from the Department of Chemistry at The University of Alabama (XL), and from the University of Alabama Faculty Start-up Funds (MB).References
- X. Sun, W. Zhai, J. S. Fossey and T. D. James, Chem. Commun., 2016, 52, 3456–3469 RSC.
- K. Ariga, H. Ito, J. P. Hill and H. Tsukube, Chem. Soc. Rev., 2012, 41, 5800–5835 RSC.
- N. Y. Edwards, T. W. Sager, J. T. McDevitt and E. V. Anslyn, J. Am. Chem. Soc., 2007, 129, 13575–13583 CrossRef CAS PubMed.
- M. Mazik, Chem. Soc. Rev., 2009, 38, 935–956 RSC.
- C. D. Geddes and J. R. Lakowicz, Glucose sensing, Springer, New York, NY, 2006 Search PubMed.
- T. E. Curey, A. Goodey, A. Tsao, J. Lavigne, Y. Sohn, J. T. McDevitt, E. V. Anslyn, D. Neikirk and J. B. Shear, Anal. Biochem., 2001, 293, 178–184 CrossRef CAS PubMed.
- C. C. Chen, V. Llado, K. Eurich, H. T. Tran and E. Mizoguchi, Clin. Immunol., 2011, 140, 268–275 CrossRef CAS PubMed.
- N. Kawashima, S. J. Yoon, S. Hakomori and K. Nakayama, Glycobiology, 2006, 16, 1117–1118 Search PubMed.
- L. D. Carrillo, L. Krishnamoorthy and L. K. Mahal, J. Am. Chem. Soc., 2006, 128, 14768–14769 CrossRef CAS PubMed.
- M. Ramon, F. Rolland and J. Sheen, The Arabidopsis Book, American Society of Plant Biologists, 2008, vol. 6, p. e0117 Search PubMed.
- S. Shinkai and M. Takeuchi, Trends Anal. Chem., 1996, 15, 188–194 CrossRef CAS.
- X. Wu, Z. Li, X. X. Chen, J. S. Fossey, T. D. James and Y. B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048 RSC.
- G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, Angew. Chem., Int. Ed., 2007, 46, 2366–2393 CrossRef CAS PubMed.
- S. Arimori, M. L. Bell, C. S. Oh and T. D. James, Org. Lett., 2002, 4, 4249–4251 CrossRef CAS PubMed.
- T. D. James, K. R. A. Samankumara Sandanayake and S. Shinkai, Nature, 1995, 374, 345–347 CrossRef CAS.
- Y. Egawa, R. Miki and T. Seki, Materials, 2014, 7, 1201–1220 CrossRef.
- Y. Egawa, R. Gotoh, T. Seki and J.-i. Anzai, Mater. Sci. Eng., C, 2009, 29, 115–118 CrossRef CAS.
- H. Eggert, J. Frederiksen, C. Morin and J. C. Norrild, J. Org. Chem., 1999, 64, 3846–3852 CrossRef CAS.
- W. Yang, H. He and D. G. Drueckhammer, Angew. Chem., Int. Ed., 2001, 40, 1714–1718 CrossRef CAS.
- G. Springsteen and B. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef CAS.
- R. M. Crooks and A. J. Ricco, Acc. Chem. Res., 1998, 31, 219 CrossRef CAS.
- R. Esfand and D. A. Tomalia, Drug Discovery Today, 2001, 6, 427 CrossRef CAS PubMed.
- D. Cakara, J. Kleimann and M. Borkovec, Macromolecules, 2003, 36, 4201 CrossRef CAS.
- Y. Niu, L. Sun and R. M. Crooks, Macromolecules, 2003, 36, 5725 CrossRef CAS.
- T. J. Prosa, B. J. Bauer and E. J. Amis, Macromolecules, 2001, 34, 4897–4906 CrossRef CAS.
- X. Zhang, L. You, E. V. Anslyn and X. Qian, Chemistry, 2012, 18, 1102–1110 CrossRef CAS PubMed.
- S. H. Shabbir, C. J. Regan and E. V. Anslyn, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10487–10492 CrossRef CAS PubMed.
- L. Zhu, Z. L. Zhong and E. V. Anslyn, J. Am. Chem. Soc., 2005, 127, 4260–4269 CrossRef CAS PubMed.
- G. Springsteen and B. Wang, Chem. Commun., 2001, 1608–1609 RSC.
- For instance, see Dendritech Inc., http://www.dendritech.com whose products are also distributed by Sigma-Aldrich.
- F. Chen, K. Wu, Y. Liu, H. Huang and K. He, J. Polym. Eng., 2014, 34, 733–738 CAS.
- B. Menot, J. Stopinski, A. Martinez, J.-B. Oudart, F.-X. Maquart and S. Bouquillon, Tetrahedron, 2015, 71, 3439–3446 CrossRef CAS.
- Z. Wang, C. Chen, R. Liu, A. Fan, D. Kong and Y. Zhao, Chem. Commun., 2014, 50, 14025–14028 RSC.
- T. D. James, H. Shinmori, M. Takeuchi and S. Shinkai, Chem. Commun., 1996, 705–706 RSC.
- S. Shinkai and A. Robertson, The design of molecular artificial sugar sensing systems, Springer-Verlag, 2001 Search PubMed.
- T. D. James, K. R. A. Sandanayake Samankumara and S. Shinkai, Angew. Chem., Int. Ed., 1996, 35, 1911–1922 CrossRef CAS.
- S. W. Chaikin and W. G. Brown, J. Am. Chem. Soc., 1949, 71, 122–125 CrossRef CAS.
- M. Hudlicky, Reductions in organic chemistry, American Chemical Society, Washington, DC, 2nd edn, 1996 Search PubMed.
- M. R. Naimi-Jamal, J. Mokhtari, M. G. Dekamin and G. Kaupp, Eur. J. Org. Chem., 2009, 3567–3572 CrossRef CAS.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- I. Willerich, T. Schindler, H. Ritter and F. Groehn, Soft Matter, 2011, 7, 5444–5450 RSC.
- I. Willerich, Y. Li and F. Gröhn, J. Phys. Chem. B, 2010, 114, 15466 CrossRef CAS PubMed.
- I. Willerich and F. Groehn, J. Am. Chem. Soc., 2011, 133, 20341–20356 CrossRef CAS PubMed.
- A. M. Mallet, A. B. Davis, D. R. Davis, J. Panella, K. J. Wallace and M. Bonizzoni, Chem. Commun., 2015, 51, 16948–16951 RSC.
- Y. L. Liu and M. Bonizzoni, J. Am. Chem. Soc., 2014, 136, 14223–14229 CrossRef CAS PubMed.
- A. M. Mallet, Y. Liu and M. Bonizzoni, Chem. Commun., 2014, 50, 5003–5006 RSC.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205–11209 CrossRef CAS.
- S. Jin, Y. Cheng, S. Reid, M. Li and B. Wang, Med. Res. Rev., 2010, 30, 171–257 CAS.
- K. L. Diehl and E. V. Anslyn, Chem. Soc. Rev., 2013, 42, 8596–8611 RSC.
- J. J. Lavigne and E. V. Anslyn, Angew. Chem., Int. Ed., 2001, 40, 3118–3130 CrossRef CAS.
- J. Lu and K. N. Plataniotis, in IEEE Transactions on Neural Networks, The Institute of Electrical and Electronics Engineers (IEEE), 2003, vol. 14, pp. 195–201.
- M. Otto, Chemometrics: statistics and computer application in analytical chemistry, Wiley-VCH, Weinheim, New York, 1999 Search PubMed.
- R. G. Brereton, Chemometrics for pattern recognition, Wiley Interscience, Chichester, U.K., 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5tb02530c |
| This journal is © The Royal Society of Chemistry 2016 |