A challenging Heck reaction of maleimides†
Li Hui
Lim
and
Jianrong (Steve)
Zhou
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371. E-mail: jrzhou@ntu.edu.sg; Fax: (+65) 67911961
First published on 16th April 2015
Abstract
Maleimides are extremely susceptible to basic hydrolysis and gave poor yields under Heck conditions. The longstanding problem is solved with the use of a weak base KOAc in a carbonate solvent.
A palladium-catalyzed Heck reaction enables arylation and vinylation of olefins and is commonly used to prepare drugs, fragrance, agrochemicals and advanced materials.1 Electron-deficient olefins such as enones, acrylates and acrylonitriles are commonly used and they react efficiently with aryl halides and sulfonates.2 However, maleimides typically give very poor yields due to fast hydrolysis of the cyclic imides under basic conditions. Pd-catalyzed couplings of halomaleimides were often used as alternative methods.3 In synthesis of drug candidates, copper-catalyzed Meerwein reaction of maleimides and aryldiazonium salts is also used, although yields are often low-to-moderate.4 Recently, other metal-catalyzed methods were developed to prepare substituted maleimides, for example carbonylative couplings of alkynes and amines5 or isocyanates.6
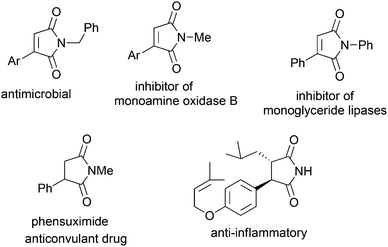
Herein, we report an efficient general Heck reaction of problematic substrates, maleimides using aryl iodides. A weak acetate base KOAc was used to prevent hydrolysis of maleimides and arylated products. The motifs of 3-arylmaleimide and the related arylsuccinimide are present in many bioactive compounds and some drugs (Fig. 1).7 For example, phensuximide is an anticonvulsant drug8 and another succinimide exhibited both immunostimulatory and anti-inflammatory activities.9
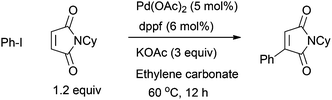
In a model reaction of the parent maleimide (MI), after many experiments we found that a combination of a weak base KOAc in ethylene carbonate was crucial to obtain good yield at 60 °C after 12 hours (Table 1, entry 3). Notably, a longer reaction time led to diminished yields due to increased hydrolysis of the products. This testified to the challenge associated with this particular olefin. We noticed that many bases including K2CO3, Cs2CO3, CsF, KF and K3PO4 caused hydrolysis of both maleimide and Heck products and led to poor yields (entries 6–11). In addition, trialkylamines also caused fast hydrolysis of the imide linkage (entries 12 and 13).
The choice of solvents was also important (Table 2). For example, in THF, dioxane, toluene and DCE, the yield was only poor-to-moderate (entries 1–5). Notably, in highly polar solvents such as DMF and DMSO (entries 7 and 8), base hydrolysis of the cyclic imide became much faster and gave rise to poor yields. Better solvation of the metal ion in these solvents leaves the acetate ion more exposed and more nucleophilic. However, in ethylene carbonate, good yield was obtained (entry 9).10 It is a highly polar aprotic solvent with an exceptionally high dielectric constant of 95 at 25 °C.
| Entry | Solvent | Conv (% PhI) | Conv (% MI) | Yield (%) |
|---|---|---|---|---|
| 1 | THF | 85 | 92 | 62 |
| 2 | 1,4-Dioxane | 82 | 85 | 69 |
| 3 | Diglyme | 97 | 102 | 64 |
| 4 | Toluene | 26 | 35 | 15 |
| 5 | DCE | 55 | 56 | 46 |
| 6 | DMA | 96 | 108 | 64 |
| 7 | DMF | 66 | 120 | 38 |
| 8 | DMSO | 8 | 120 | 0 |
| 9 | Ethylene carbonate | 100 | 112 | 88 |
Many phosphines can support the Pd catalysis well (Table 3). Good examples of ligands include SPhos, MePhos, Q-Phos, PCy3, dppe and DPEphos. A ferrocene bisphosphine dppf also afforded good yield and was used for isolations, due to its substrate generality and low cost (entry 10). In the absence of a phosphine, the ligand-free procedure also gave 64% yield, probably via an anionic pathway of [(aryl)PdX2]− or [(aryl)PdX3]2− (entry 17).11
| Entry | Ligand | Conv (% PhI) | Conv (% MI) | Yield (%) |
|---|---|---|---|---|
| 1 | Pt-Bu3 | 69 | 114 | 45 |
| 2 | PCy3 | 100 | 120 | 90 |
| 3 | PPh3 | 100 | 117 | 60 |
| 4 | P(2-furyl)3 | 95 | 107 | 73 |
| 5 | XPhos | 99 | 116 | 61 |
| 6 | SPhos | 100 | 111 | 92 |
| 7 | DavePhos | 50 | 117 | 33 |
| 8 | MePhos | 100 | 112 | 93 |
| 9 | Q-Phos | 98 | 106 | 97 |
| 10 | dppf | 100 | 112 | 88 |
| 11 | dppe | 100 | 116 | 89 |
| 12 | dppp | 57 | 119 | 39 |
| 13 | dppb | 87 | 116 | 63 |
| 14 | Xantphos | 96 | 108 | 78 |
| 15 | BINAP | 5 | 80 | <5 |
| 16 | DPEphos | 100 | 105 | 96 |
| 17 | No added ligand | 85 | 100 | 64 |
Next, we explored the scope of aryl iodides (Fig. 2a). In a typical procedure, Pd(OAc)2, dppf, KOAc, ArI and maleimides were mixed in ethylene carbonate under argon (or nitrogen) and heated with stirring at 60 °C for 12 hours. Notably, higher temperatures or a longer reaction time led to decreased yields due to partial hydrolysis of both maleimide and arylated products. The aryl rings can have electron-withdrawing and donating groups. The ortho-substituted aryl iodides led to slightly higher yields than the para ones, because of slower hydrolysis of products. One indolyl iodide also reacted smoothly and the yield was much higher in THF than that in ethylene carbonate. Common aryl bromides remained unreactive under similar conditions. The N-groups can be both alkyl and aryl groups (Fig. 2b). When N-arylmaleimides were used, another bisphosphine DPEphos proved to be a better catalyst. The N-anisylmethyl group in Heck products can be cleaved by treatment of AlCl3 or CF3CO2H.3b In addition, the Heck reaction of 3-methylmaleimide afforded a product containing an exo-benzylidene unit. This is probably formed via base-induced isomerization of the endocyclic olefin in maleimide (Fig. 2c).12
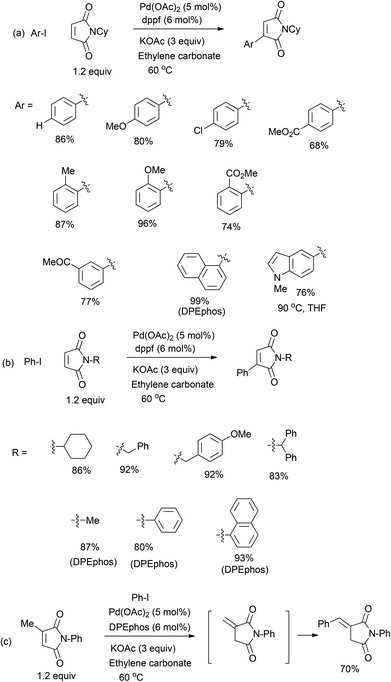 | ||
| Fig. 2 Examples of the Heck reaction of maleimides (isolated yield on the 0.5 mmol scale unless stated otherwise). | ||
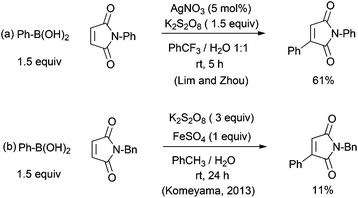
As a side note, we attempted radical arylation by modifying Baran's procedure using PhB(OH)2 and persulfate.13 Unfortunately, the yield was only 61% even after extensive optimization (Fig. 3a). Other arylboronic acids led to very poor yields. In another procedure for radical arylation using FeSO4 and oxone, only 11% was reported previously by Komeyama et al. (Fig. 3b).14 These unsatisfactory results confirmed that maleimides are very sensitive to hydrolysis even under very mild conditions.
In summary, we report an efficient Heck method for arylation of challenging maleimides, which are prone to undergo facile hydrolysis. A specific combination of KOAc and a carbonate solvent allowed the formation of Heck products in good yields with generality.
We thank the Singapore Ministry of Education Academic Research Fund (MOE2013-T2-2-057 and MOE2014-T1-001-021) and Nanyang Technological University for financial support and Johnson Matthey for palladium salts.
Notes and references
- Reviews: (a) R. F. Heck, Acc. Chem. Res., 1979, 12, 146 CrossRef CAS; (b) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed; (c) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (d) S. Bräse and A. de Meijere, in Metal-Catalyzed Cross-coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2004, p. 217 Search PubMed; (e) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644 CrossRef CAS PubMed; (f) The Mizoroki-Heck Reaction, ed. M. Oestreich, Wiley, New York, 2009 Search PubMed.
- A. F. Littke and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 6989 CrossRef CAS PubMed.
- (a) A. I. Roshchin and E. V. Polunin, Mendeleev Commun., 2008, 18, 332 CrossRef CAS PubMed; (b) E. Awuah and A. Capretta, J. Org. Chem., 2011, 76, 3122 CrossRef CAS PubMed; (c) A review of synthesis: P. S. Deore and N. P. Argade, Synthesis, 2014, 281 CrossRef PubMed.
- (a) C. S. Rondestvedt and O. Vogl, J. Am. Chem. Soc., 1955, 77, 2313 CrossRef CAS; (b) P. T. Izzo, J. Org. Chem., 1963, 28, 1713 CrossRef CAS; (c) C. S. Rondestvedt, M. J. Kalm and O. Vogl, J. Am. Chem. Soc., 1956, 78, 6115 CrossRef CAS.
- K. M. Driller, H. Klein, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 6041 CrossRef CAS PubMed.
- T. Kondo, M. Nomura, Y. Ura, K. Wada and T.-a. Mitsudo, J. Am. Chem. Soc., 2006, 128, 14816 CrossRef CAS PubMed.
- (a) N. Matuszak, G. G. Muccioli, G. Labar and D. M. Lambert, J. Med. Chem., 2009, 52, 7410 CrossRef CAS PubMed; (b) C. I. Manley-King, G. Terre'Blanche, N. Castagnoli, J. J. Bergh and J. P. Petzer, Bioorg. Med. Chem., 2009, 17 Search PubMed; (c) H. Yuki, T. Yamamoto, Y. Tohira, B. Aoki, T. Kano and T. Yamazaki, Chem. Pharm. Bull., 1967, 15, 1101 CrossRef CAS; (d) A. Kar and N. P. Argade, Synthesis, 2005, 2284 CAS.
- (a) C. A. Miller and L. M. Long, J. Am. Chem. Soc., 1951, 73, 4895 CrossRef CAS; (b) T. C. McKenzie, J. W. Epstein, W. J. Fanshawe, J. S. Dixon, A. C. Osterberg, L. P. Wennogle, B. A. Regan, M. S. Abel and L. R. Meyerson, J. Med. Chem., 1984, 27, 628 CrossRef CAS.
- S.-C. Chien, M.-L. Chen, H.-T. Kuo, Y.-C. Tsai, B.-F. Lin and Y.-H. Kuo, J. Agric. Food Chem., 2008, 56, 7017 CrossRef CAS PubMed.
- (a) Y. Chernyak, J. Chem. Eng. Data, 2006, 51, 416 CrossRef CAS; (b) R. P. Seward and E. C. Vieira, J. Phys. Chem., 1958, 62, 127 CrossRef CAS.
- B. P. Carrow and J. F. Hartwig, J. Am. Chem. Soc., 2009, 132, 79 CrossRef PubMed.
- Y. Liu and W. Zhang, Angew. Chem., Int. Ed., 2013, 52, 2203 CrossRef CAS PubMed.
- I. B. Seiple, S. Su, R. A. Rodriguez, R. Gianatassio, Y. Fujiwara, A. L. Sobel and P. S. Baran, J. Am. Chem. Soc., 2010, 132, 13194 CrossRef CAS PubMed.
- K. Komeyama, T. Kashihara and K. Takaki, Tetrahedron Lett., 2013, 54, 1084 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental procedure of the Heck reaction and characterization of compounds. See DOI: 10.1039/c5qo00015g |
| This journal is © the Partner Organisations 2015 |