Homogeneous and heterogeneous catalysts for multicomponent reactions
Maria José Climent, Avelino Corma* and Sara Iborra
Instituto de Tecnología Química, UPV-CSIC; Universitat Politécnica de Valencia, Avda. de los Naranjos s/n, 46022, Valencia, Spain. E-mail: acorma@itq.upv.es; Fax: +34 963877809; Tel: + 34 96 3877800
First published on 29th November 2011
Abstract
Organic synthesis performed through multicomponent reactions is an attractive area of research in organic chemistry. Multicomponent reactions involve more than two starting reagents that couple in an exclusive ordered mode under the same reaction conditions to form a single product which contains the essential parts of the starting materials. Multicomponent reactions are powerful tools in modern drug discovery processes, because they are an important source of molecular diversity, allowing rapid, automated and high throughput generation of organic compounds. This review aims to illustrate progress in a large variety of catalyzed multicomponent reactions performed with acid, base and metal heterogeneous and homogeneous catalysts. Within each type of multicomponent approach, relevant products that can be obtained and their interest for industrial applications are presented.
 Maria José Climent | Maria Jose Climent Olmedo was born in Alginet (Spain). After studying Pharmacy at the Universidad de Valencia (Spain) she obtained her Ph. D. in 1991, working on the application of heterogeneous catalysts in organic synthesis. From 1988 to 1991 she held a position as Associate Professor in the Chemistry Department at the Universidad Politécnica de Valencia and in 1992 she obtained a permanent position as Professor in Organic Chemistry. Since 1991 she is a member of the Institute of Chemical Technology (UPV-CSIC), working in the group of Prof. Avelino Corma. Her current research involves the application of heterogeneous catalysts to the synthesis of fine chemicals and catalytic biomass transformation. |
 Avelino Corma | Avelino Corma was born in Moncófar, Spain in 1951. He studied Chemistry at the Universidad de Valencia (1967–1973), and received his Ph.D. at the Universidad Complutense de Madrid in 1976. He did his postdoctoral studies at the Department of Chemical Engineering at Queen’s University (Canada, 1977–79). He is director of the Instituto de Tecnología Química (UPV-CSIC) at the Universidad Politécnica de Valencia since 1990. His current research field is catalysis, covering aspects of synthesis, characterization and reactivity in acid–base and redox catalysis. Avelino Corma is co-author of more than 700 articles and 100 patents on these subjects. |
 Sara Iborra | Sara Iborra was born in Carlet (Spain). She received her Ph.D. in 1987 at the Universidad de Valencia and in the same year she joined the Chemistry Department of the Technical University of Valencia as Assistant Professor. In 1992, she obtained her current position as Professor in Organic Chemistry. She is member of the Institute of Chemical Technology (ITQ) at the Technical University of Valencia since 1991. The main focus of her work is the application of heterogeneous catalysts to the synthesis of fine chemicals, green chemistry, and biomass transformation. |
1. Introduction
Organic-chemical synthesis performed through one-pot, tandem, domino or cascade reactions1,2 have become a significant area of research in organic chemistry3–10 since such processes improve atom economy. The one-pot transformations can be carried out through multi-step sequential processes where the consecutive steps take place under the same reaction conditions or, when this is not possible, they can be performed in two or more stages under different reaction conditions, with the correct addition sequence of reactants. There are cases however, in where the desired product can be prepared in a one-pot mode throughout a multicomponent reaction. Multicomponent reactions (MCRs) are defined as reactions that occur in one reaction vessel and involve more than two starting reagents that form a single product which contains the essential parts of the starting materials.11,12 Thus, an ideal multicomponent reaction involves the simultaneous addition of reactants, reagents and catalyst at the beginning of the reaction and requires that all reactants couple in an exclusive ordered mode under the same reaction conditions. The success of multi-step sequential or multicomponent one-pot transformations, requires a balance of equilibria and a suitable sequence of reversible and irreversible steps. Thus, in the case of MCRs three types of reactions are known:13 (a) Type I MCRs in which there is an equilibrium between reactants, intermediates and final products; (b) Type II MCRs in where an equilibrium exists between reactants and intermediates with the final product being irreversibly formed; (c) Type III MCRs which involve a sequence of practically irreversible steps that proceed from the reactants to the products. Type III MCRs are usual in biochemical transformations, but rarely occur in preparative chemistry.13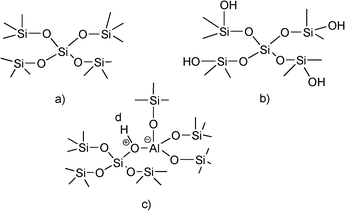 | ||
| Fig. 1 Structure of silicates. | ||
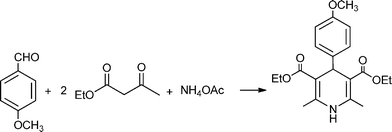
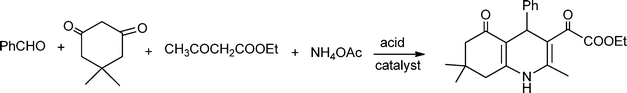
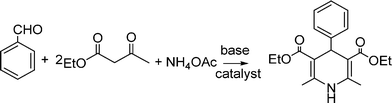
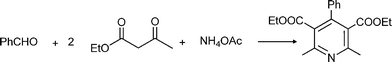
MCRs have been known for over 150 years and it is generally considered that this chemistry began in 1850 when Strecker14 reported the general formation of α-aminocyanides from ammonia, carbonyl compounds and hydrogen cyanide. Since then, many multicomponent reactions have been developed, some of the first examples are the Hantzsch dihydropyridine synthesis (1882)15 and the Biginelli16 3CR (1893) (Scheme 1). The first isocyanide-based 3CRs was introduced by Passerini in 1921, while in 1959 Ugi introduced the four component reaction of the isocyanides17 which involves the one-pot reaction of amines, carbonyl compounds, acid and isocyanides. The Ugi reaction has been the most extensively studied and applied MCR in the drug discovery process.
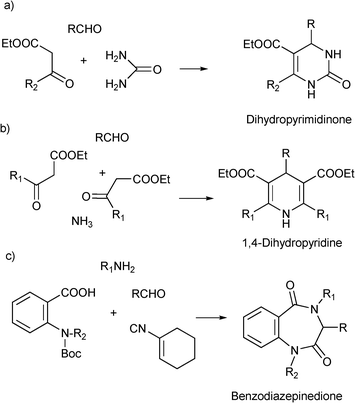 | ||
| Scheme 1 Preparation of privileged scaffolds by (a) Biginelli reaction, (b) Hantzsch synthesis and (c) Ugi deBoc/cyclize methodology. | ||
One key aspect of multicomponent reactions is that they are an important source of molecular diversity.18 For instance, a three component coupling reaction will provide 1000 compounds when 10 variants of each component are employed. This aspect together with its inherent simple experimental procedures and its one-pot character, make MCRs highly suitable for automated synthesis. They are powerful tools in modern drug discovery processes allowing rapid, automated and high throughput generation of organic compounds.19–22 Additionally, the one-pot character delivers fewer by-products compared to classical stepwise synthetic routes, with lower costs, time and energy.
Although most of the established MCRs do not require a catalyst, the search for new MCR products has resulted in an intensified effort to find catalysts and new catalyzed MCRs. We will show that while a variety of homogeneous and heterogeneous catalysts have been reported to perform MCRs, the advantages inherent to the use of heterogeneous catalysts undoubtedly would reinforce the environmental benefits of these interesting reactions.
In this work we will review a large variety of MCRs, particularly three-component coupling reactions (A3 coupling) performed with acid, base and metal heterogeneous catalysts as well as with bifunctional catalytic systems. We will present within each type of MCR, relevant products that can be obtained and their interest for industrial applications.
2. Solid catalysts of interest for MCR
The simplest approximation to heterogeneous catalysis starting from homogeneous mineral and organic acids has been to support them on porous solids. For instance, perchloric, sulphuric and phosphoric acids are normally supported on silica either by simple pore filling and/or by interacting with the surface of the solid. In the case of the sulfonic acids an heterogenization procedure involves the synthesis of organic polymers bearing sulphonic groups. In this case organic resins can be excellent catalysts, especially when their pore structure is adapted to the nature and dimensions of reactants.23,24Inorganic solid acids can be prepared with acidity that ranges from weak to strong, going through solids with controlled intermediate acidities. One type of inorganic solid acid is the family of silicates. In high surface area silica, the silicon atoms are tetrahedrally coordinated and the system is charge neutral (Fig. 1a). However the silicananoparticles terminate at the surface with silanolgroups (Fig. 1b). In this silanolgroup the density of positive charge on the hydrogen of the hydroxylgroup is very small and it can be considered as a very weak Brønsted acid site. Nevertheless they could be used for acid catalyzed reactions that require weak acidity, provided that the silica has a relatively high surface area. With this type of catalyst the reactants become activated by surface adsorption, being the heat of adsorption the additive effect of the small van der Waals and hydrogen bridging type of interactions.
Larger O–H polarizations are achieved when an isomorphic substitution of Al by Si occurs. In this case, the tetrahedrally coordinated Al generates a negative charge that is compensated by the positive charge associated with the hydrogen of the bridging hydroxylgroups (Fig. 1c). These Brønsted acid sites are clearly stronger than the silanolgroups and they exist in well prepared amorphous and long range structured silica aluminas and in crystalline aluminosilicates.25–27 When the T–O–T’ bond in aluminosilicates is not constrained, as it occurs in amorphous silica alumina, the tendency to release the proton and to relax the structure is lower and consequently the Brønsted acidity is mild. However, in the case of crystalline aluminosilicates such as zeolites the bridging T–O–T’ bond is constrained and the Brønsted acidity of these materials is higher than in amorphous silica alumina. If one takes into account that it is possible to synthesize zeolites with different Al contents and with pores within a wide range of diameters,28–30 it is not surprising that zeolites have found and still find a large number of applications as solid acid catalysts.31 Their applications can be even enlarged through the synthesis of acid zeolites with pores of different dimensions within the same structure. Thus, structures with pores formed by 12 and 10 ring,32 18 × 10,33 14 × 12,34 17 × 1230,35 and the recently discovered ITQ-43 with 24 × 1236 rings, with pores in the mesoporous range have been presented.
If one takes into account that other metal atoms, such as Ti, Sn, Fe and Cr with catalytic activity for oxidations, can be incorporated in the structure of the crystalline microporous silicates or aluminosilicates37–39 enlarging the reactivity of the zeolites and allowing the preparation of bifunctional acid-oxidationscatalysts. When metal nanoparticles are formed on the internal and/or external surface of acid zeolites, bifunctional hydrogenation/dehydrogenation solid acid catalysts are obtained40–43 allowing zeolites to catalyze multistep reactions.44,45
There are reactions that require sites with an acid strength stronger than that of zeolites. Then, solid catalysts containing sulfonic groups can be used. For instance, acidic resins with sulfonic acidgroups are strong solid acid catalysts that can be useful for acid catalysis, provided that the reaction temperature does not surpass their thermal stability limit.46 Along this line, Nafion is a strong solid acid catalyst but its surface area is too low. To avoid this limitation, Harmer et al. have shown that it is possible to partially depolymerize Nafion and to disperse it in silica.47,48 The resultant high surface solid catalysts can be used in a relatively larger number of acid catalyzed reactions.49–52 Nevertheless, the acidity of this hybrid material is somewhat lower than Nafion, owing to the interaction of sulfonic groups with the silanols of the silica.53–54 In any case it should be considered that polymer derived catalysts may be difficult to regenerate if poisoned by deposition of organic compounds. Indeed, regeneration by calcination with air will be limited because of thermal stability, and washing out the adsorbed products with solvents can not always restore the initial activity.
Looking for strong acid catalysts, heteropolyacids such as H3PW12O40 (H3PW) are able to catalyze at low temperatures a wide range of homogeneous catalytic processes.55Heteropolyacids can be heterogeneized by either supporting them on a high surface area carrier such a silica56 or by forming their cesium or potassium salts (Cs2.5H0.5PW or K2.5H0.5PW) that are solids with micro and mesoporosity and are insoluble for organic reactions.57
Other solid acids such as metal organic frameworks bearing sulfonic groups or metal Lewis acids,58 sulfonated zirconia59,60 and metal phosphates have also been used as catalysts.61,62
With respect to solid bases, basic resins, amines and alkyl ammonium hydroxides grafted on silicas, or amines bearing part of MOF structures, KF on Al2O3, alkaline metal oxides on alumina and zeolites, zeolites exchanged with alkaline cations, alkaline earth oxides and anionic clays such as hydrotalcites and their corresponding mixed oxides are useful catalysts and their basic properties and catalytic activity have been very well described in a series of reviews.31,63–68
The solid acid and base catalysts named above account for the majority of the catalysts required for MCRs presented in this review. In those cases, in which significantly different catalyst will be required, we will briefly describe their nature.
3. Heterogeneous catalyzed multicomponent reactions
3.1 Synthesis of propargylamines
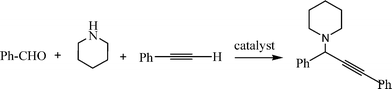
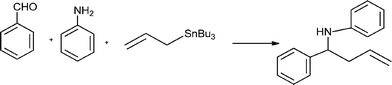
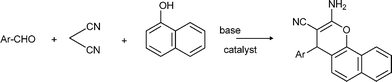
The Mannich reaction is a classic example of a three component condensation (A3 coupling). In general, an aldehyde, an amine and an active hydrogen compound such as an enolyzable ketone or terminal alkyne, react affording the corresponding β-aminoketone or β-aminoalkyne (propargylamine) (Scheme 2).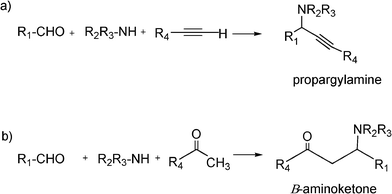 | ||
| Scheme 2 Mannich type reactions. | ||
Propargylamines are important synthetic intermediates for potential therapeutic agents and polyfunctional amino derivatives.69–71 Traditionally these compounds have been synthesized by nucleophilic attack of lithium acetylides or Grignard reagents to imines or their derivatives. However these reagents must be used in stoichiometric amounts, are highly moisture sensitive, and sensitive functionalities such as esters are not tolerated. Therefore, the most convenient synthetic method for preparing propargylamines has been the Mannich one-pot three component coupling reaction of an aldehyde, a secondary amine and a terminal alkyne. The reactions are usually performed in polar solvents (mostly dioxane) and in the presence of a catalytic amount of a copper salt (CuCl, Cu(OAc)2)72 which increases the nucleophilicity of the acetylenic substrate towards the Mannich reaction. Mechanistic studies indicate that the reaction involves the formation of an iminium intermediate from the starting aldehyde and amine. The C–H bond of the alkyne is activated by the metal to form a metal acetylide intermediate which subsequently reacts with the iminium ion leading to the corresponding propargylamine (Scheme 3).
 | ||
| Scheme 3 Proposed mechanism for the Mannich reaction. | ||
A variety of transition metals such as AgI salts,73AuI/AuIII salts,74,75AuIIIsalen complexes,76 Cu1 salts,77–78Ir complexes,79InCl3,80Hg2Cl281 and Cu/RuII bimetallic system82 have been employed as catalysts under homogeneous conditions. In addition, alternative energy sources like microwave83 and ultrasonic84 radiations have been used in the presence of CuI salts. Considering that chiral propargylamines are widely present in many important bioactive compounds, enantioselective synthesis of propargylamines throughout this protocol have been recently developed using chiral Cu(I) complexes.85,86
However, operating under homogenous media two main drawbacks must be considered: the difficulty to recover and reuse the catalyst and the possible absorption of some of the metal catalyst on the final product (fine chemical). Currently the upper tolerance limit for the contamination of drugs or other compounds set aside for human consumption by transition metals is 5 ppm and future regulations are expected to lower this threshold to the ppb range.
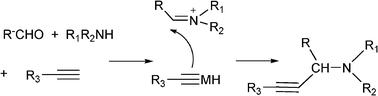
In order to achieve the recyclability of transition metal catalysts, gold, silver and copper salts in ionic liquids, [Bmim]PF687,72 as well as heterogeneous catalysts have been used to obtain propargylamines. Thus, different metal exchanged hydroxyapatites (metal–HAP) are able to catalyze the condensation of benzaldehyde, piperidine and phenylacetylene in acetonitrile under reflux temperature.88 The results showed that the order of efficiency was Cu–HAP > Cu(OAc)2 > Ru–HAP > Fe–HAP achieving yields of the corresponding propargylamine of 85%, 80%, 60%, and 25% respectively. A variety of structurally different aldehydes, amines and acetylenes in the presence of Cu–HAP were converted into the corresponding propargylamines with 55–92% yield. Cu–HAP was reused several times showing consistent activity even after the fourth cycle. Silica gel anchored copper chloride has been described by Sreedhar et al.89 as an efficient catalyst for the synthesis of propargylamines via C–H activation. Both aromatic and aliphatic aldehydes and amines and phenylacetylene have been used to generate a diverse range of acetylenic amines in good to moderate yields (52–98%) using water as a solvent and without any organic solvent or co-catalyst. A stable and efficient catalyst for the three component coupling Mannich reaction of aldehydes, amines and alkynes was prepared by Liet al.90 by immobilizing Cu(I) on organic–inorganic hybrid materials. Thus, a silica-CHDA-CuI catalyst was prepared from benzylchloride functionalized silica gel which was subsequently reacted with 1,2-diaminecyclohexane. This organic–inorganic hybrid material was reacted with couprous iodide to generate a silica-CHDA-CuI catalyst with 1.6 wt% of Cu. Reactions performed in the absence of solvent afforded the corresponding propargylamines in excellent yields (82–96%). No catalystleaching was observed in the reaction media, and the catalyst remained active through at least 15 consecutive runs. Others immobilized metals such a Ag(I) and Au(I) exhibited lower activity than Cucatalysts while silica supported Pd(II) failed in this reaction.
Recently Wang et al.91 have reported a novel silica-immobilized N-heterocyclic carbene metal complex (Si–NHC–CuI) as an efficient and reusable catalyst for the synthesis of propargylamines. Reacting different combinations of aldehydes, amines and alkynes at room temperature under solvent free conditions, produces the corresponding propargylamines in moderate to good yields (43–96%) after 24 h reaction time (see Table 1). Higher yields were obtained when the MCR was carried out at 70 °C in 4 h, while no metal leaching in the liquid media was observed.
| Alkyne | Aldehyde | Amine | Yield (%)b |
|---|---|---|---|
| a Reaction conditions: aldehyde (1.0 mmol), amine (1.1 mmol), alkyne (1.2 mmol), SiO2–NHC–CuI (2 mol%), nitrogen, room temperature, 24 h.b Isolated yield.c 70 °C for 4 h. | |||
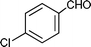
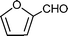
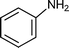
C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | CH2O | 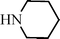 | 95 |
C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | CH2O | 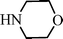 | 94 |
C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | CH2O | 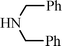 | 94 |
C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | CH2O | 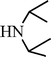 | 96 |
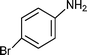
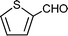
C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | C6H5CHO | 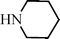 | 79 |
| 91c | |||
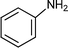
C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | C6H5CHO | 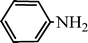 | 71 |
| 88c | |||
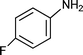
C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | m–ClC6H4CHO | 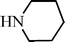 | 93c |
p–CH3C6H5C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | CH2O | 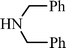 | 96 |
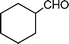
n–C8H17C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH CH | CH2O | 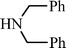 | 78 |
| 92c | |||
EtOOCC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH3 CCH3 | CH2O | 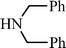 | 75c |
Cu(0)nanoparticles have also been used by Kidway and co-workers92 as active and recyclable catalysts in the synthesis of propargylamines following the Mannich protocol. The Cunanoparticles prepared in a reverse micellar system (with size of 18±2 nm) gave a diverse range of propargylamines in excellent yields (65–98%).
Different metal-supported zeolites such as Cu-modified zeolites (H–USY, HY, H–Beta, Mordenite and ZSM-5), have been successfully used for the synthesis of propargylamines.93 The MCR between piperidine, benzaldehyde and phenylacetylene performed at 80 °C in the absence of solvent, showed that the order of activity was: CuI–USY > CuI–Y > CuI–Beta > CuI–ZSM–5 > CuI–Mordenite, indicating that the pore topology of the zeolites has a marked influence on the reaction efficiency (Table 2). The authors suggest that the formation of the iminium intermediate assisted by the zeolite is combined with the formation of the acetylide within the micropores leading to an efficient reaction. The scope of the CuI–USY catalyst was examined using different reagents, including bulky amines, and in all cases propargylamines were obtained in good to moderate yields (55–90%), though long reaction times (15 h) were required. Furthermore, CuI–USY could be recycled up to four times without loss of activity. Cu exchanged NaYzeolite has also been used as a catalyst in the synthesis of substituted propargylamines.94 Different metal exchanged zeolites (Cu–NaY, Ag–NaY and Au–NaY) were prepared by conventional ion exchange method by treating NaYzeolite with an aqueous solution of different salts. When an equimolar mixture of benzaldehyde, piperidine and phenylacetylene were reacted at 100 °C for 5 h in the presence of Au–NaYzeolite the corresponding propargylamine was obtained in low yield (32%) whereas both Cu–NaY and Ag–NaYcatalysts gave the propargylamine in moderate yields (43% and 52% respectively). Metal leaching of Cu–NaY and Au–NaYcatalysts was observed in the reaction media, but Ag–NaY was stable and metal leaching was not detected. Several substituted propargylamines were prepared with Ag–NaY affording the corresponding propargylamines in moderate to good yields (42–97%) and high selectivities (90–99%) after 15 h. Very recently Namitharan et al.95 have reported that Ni-exchanged Y zeolite (Ni–Y) exhibits excellent activity for the A3 coupling of cyclohexanecarbaldehyde, morpholine and phenylacetylene giving the corresponding propargylamine in 97% yield under solvent free conditions at 80 °C. No leaching of metal ions provides strong support for the heterogeneous nature of the catalyst. In Table 3 the activity of Ni–Y zeolite is compared with other homogeneous and heterogeneous based catalysts. The Ni–Y zeolite could be recycled at least four times retaining yield and selectivity. A variety of aliphatic, aromatic, cyclic and heterocyclic alkynes were coupled using this catalyst, and yields between 84–97% were achieved. The authors claim that NiII is the catalytic site for the coupling reaction. In the mechanism proposed, the NiII species in NiII–Y zeolite react with the terminal alkyne and the subsequent cleavage of one of the oxo bridges in the zeolite generates the nickel(II) acetylide intermediate. Then, the acetylide reacts with the iminium ion generated in situ from aldehyde and amine to give the propargylamine while the NiII–Y zeolite is ready for a subsequent reaction cycle (Scheme 4).
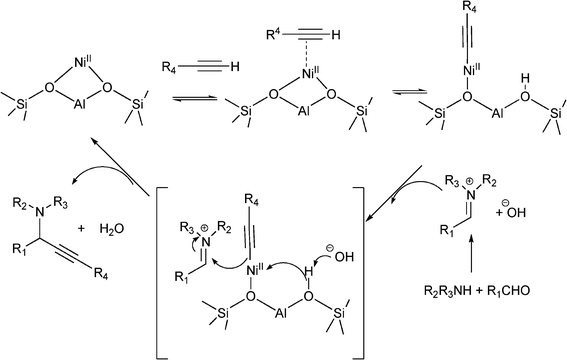 | ||
| Scheme 4 Possible mechanism of NiII–Y-catalyzed three component coupling. | ||
| Catalyst | Solvent | T/°C | Yieldb (%) | Acid sites (mmol g−1) |
|---|---|---|---|---|
| a Reaction conditions: 1 mmol of each component for 15 h with a zeolite loading of 20 mg.b Yields were evaluated by H–NMR analysis of the crude mixture.c Upon mixing without solvent, intense heat was evolved leading to decomposition, whereas in solvent good yields were obtained.d No transformation was observed. | ||||
| Cu1–USY | DMF | 60 | 40 | 4.39 |
| Cu1–USY | Toluene | 80 | 72 | 4.39 |
| Cu1–USY | THF | 80 | 79 | 4.39 |
| Cu1–USY | MeCN | 60 | 35 | 4.39 |
| Cu1–USY | none | 80 | 95 | 4.39 |
| Cu1–Y | none | 80 | 92 | 6.67 |
| Cu1–Beta | none | 80 | 90 | 0.91–1.23 |
| Cu1–ZSM-5 | none | 80 | 80 | 1.04 |
| Cu1–Mor | none | 80 | 71 | 1.48 |
| H–USY | none | 80 | —c | 6.67 |
| CuCl | none | 80 | 40–90c | — |
| none | none | 80 | —d | — |
| Nickel source | Solvent | Yield(%)b |
|---|---|---|
| a Cyclohexanecarbaldehyde (1.0 mmol), morpholine (1.2 mmol), phenylacetylene (1.2 mmol), Ni–Y (20 mg), 80 °C, 4 h.b Yield of isolated product.c 100 °C, for 15 h. | ||
| Ni(OAc)2·4H2O | DMF | 25c |
| Ni(OAc)2·4H2O | toluene | 21c |
| Ni(OAc)2·4H2O | THF | 20c |
| Ni(OAc)2·4H2O | MeCN | 26c |
| Ni(OAc)2·4H2O | none | 63 |
| NiCl2·6H2O | none | 53 |
| Ni (SO4)2·6H2O | none | 55 |
| Ni(NO3)2·6H2O | none | 36 |
| Ni/Al–HT | none | 31 |
| Ni–K10 clay | none | 64 |
| Ni–Al–MCM-41 | none | 73 |
| Ni–Y zeolite | none | 97 |
| H–Y zeolite | none | 0 |
A silver salt of 12-tungstophosphoric acid (AgTPA) has been reported by Reddy and coworkers96 as a heterogeneous catalyst to prepare different propargylamines via a three component coupling reaction in very good yields (70–98%).
We have recently found that Cu–MOFs97 were active and selective solid catalysts for the A3 coupling of a large variety of aldehydes, amines and alkynes. The Cu–MOF catalyst deactivates because of a loss of crystallinity, but the original activity was fully restored by treating with DMF at reflux and regenerating the initial MOF structure.
While homogeneous gold complexes were reasonable active catalysts for the three component reaction, it has now been shown that gold supported catalysts can also catalyze the A3 coupling for preparation of propargylamines with excellent success. For instance, Kantam et al.98 reported the use of a gold support onto a layered double hydroxide (LDH–AuCl4) for the Mannich reaction. A range of propargylamines was obtained in excellent yields (89–93%) at reflux of THF. However a significant deactivation of LDH–AuCl4 after the second and third reuse was detected, which was attributed to the formation of Au(0) and Au(I) as revealed by XPS studies. Aunanoparticles (Au–np), prepared in a reverse micellar system, has also been used as a catalyst for the MCR of aldehydes, amines and alkynes.99 It was found that the nature of reaction media has an important role in the MCR reaction. Among the different solvents investigated, the acetonitrile was the best for carrying out the coupling reaction. This was attributed to its high polarity that may result in the stabilization of the acetylide–Au intermediate which reacted with the iminium ion generated in situ, to give the corresponding propargylamine. A variety of structural aldehydes and amines with a wide range of functional groups were coupled affording the propargylamines in excellent yields (67–96%). The maximum reaction rate was observed for an average particle size of about 20 nm. In addition, the Au–np catalyst was reused without further purification for seven runs with only a slight drop in activity. Recently, Datta et al.100 have reported that Aunanoparticles (7 nm) encapsulated with highly ordered mesoporous carbon nitride (Au/MCN) are an active and recyclable catalyst for coupling benzaldehyde, piperidine and phenylacetylene. However, better yields (>95%) were obtained by Corma et al. for the same reaction using Aunanoparticles supported on nanocrystalline ZrO2 and CeO2101 (Table 4). Different studies and theoretical calculations have evidenced that cationic gold species can be stabilized on ZrO2 and CeO2 but not on other supports as shown in Table 4. The proposed mechanism involves the activation of the Csp–H bond by Au(III) species stabilised by nanocrystalline ZrO2 or CeO2 to give a gold acetylide intermediate (A), which reacts with the immonium ion formed in situ (B) to give the corresponding propargylamine (Scheme 5). The catalytic activity TON (turn-over number) and TOF (turn-over frequency) is the highest reported up to now (see Table 4). The reaction was extended to different combinations of aldehydes, amines and alkynes giving excellent yields to the corresponding propargylamines. Besides, when a chiral amine was used in the presence of Au/ZrO2 and Au/CeO2 the corresponding chiral propargylamine was obtained with high yields (>97%) and excellent diasteroselectivities (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Table 5 summarizes the results obtained in the MCR of benzaldehyde, piperidine, and phenylacetylene using different solid catalysts.
1). Table 5 summarizes the results obtained in the MCR of benzaldehyde, piperidine, and phenylacetylene using different solid catalysts.
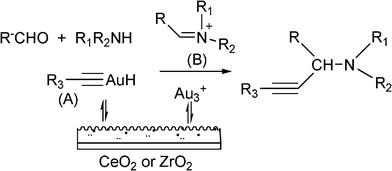 | ||
| Scheme 5 Mechanism proposed in the presence of gold supported on CeO2 or ZrO2. | ||
| ||||
|---|---|---|---|---|
| Catalystb | Gold (mol%) | Conv. (%)c | Yield propargylamine(%)d | TONe |
| a Reaction conditions: benzaldehyde (1.0 mmol), piperidine (1.2 mmol), phenylacetilene (1.3 mmol), H2O (MiliQ, 1.0 mL), 6 h, 100 °C.b The number (e.g. 0.2%) is the mass weight of gold loaded on the support (e.g.SiO2).c Determined by GC analysis based on aldehyde.d Yields of isolated propargylamine based on benzaldehyde; n.d. not determined.e Calculated on the basis of total weight of gold. | ||||
| 0.2% Au/SiO2 | 0.013 | < 5 | — | — |
| 3.0% Au/C | 0.081 | 13 | nd | 161 |
| 1.5% Au/TiO2 | 0.075 | 35 | nd | 464 |
| 4.5% Au/Fe2O3 | 0.247 | 40 | nd | 162 |
| 2.8% Au/ZrO2 | 0.142 | 95 | 93 | 668 |
| 2.5% Au/CeO2 | 0.127 | 100 | >99 | 788 |
| Catalyst | Yield (%) | Solvent | T °C | t(h) | Catalyst (mol%) | Molar ratioa | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
a mmol of benzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) piperidine piperidine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenylacetylene, under N2.b Catalyst was recovered and reused.c At 70 °C for 4 h.d Grams of catalyst.e mmol of benzaldehyde phenylacetylene, under N2.b Catalyst was recovered and reused.c At 70 °C for 4 h.d Grams of catalyst.e mmol of benzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) morpholine morpholine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenylacetylene. phenylacetylene. | |||||||||
| CuI–[bmim]PF6b | 85 | [bmim]PF6 | 120 | 2 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | 72 | ||
| Cu-npb | 94 | CH3CN | 100 | 6 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5e 1.5e | 92 | ||
| Cu–HAPb | 85 | CH3CN | reflux | 6 | 0.10d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 | 88 | ||
| Silica gel CuClb | 86 | H2O | reflux | 10 | 0.05 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | 89 | ||
| SiNHC–Cu1b | 79–91c | — | rt | 24 | 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 | 91 | ||
| SiCHDA–Cu1 | 92 | — | 80 | 12 | 0.04d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 102 | ||
| USY–Cu1 | 95 | — | 80 | 15 | 0.02d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 | 93 | ||
| AgTPAb | 92 | CH3CN | 80 | 6 | 0.03d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 | 96 | ||
| Au–npb | 94 | CH3CN | 80 | 5 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | 99 | ||
| Ag–NaYb | 81 | — | 100 | 15 | 5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | 94 | ||
| Zn dustb | 90 | CH3CN | reflux | 9 | 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 | 103 | ||
| Au/MCNb | 61 | Toluene | 100 | 24 | 0.05 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 | 100 | ||
| Au/CeO2b | >99 | H2O | 100 | 6 | 0.127 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 | 101 | ||
| LDH–AuCl4 | 92 | THF | reflux | 5 | 0.025d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | 98 | ||
| Fe3O4 npb | 45 | THF | 80 | 24 | 5 | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 | 104 | ||
| Ni–HY | 85 | — | 80 | 10 | 0.02d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2e 1.2e | 95 | ||
3.2 Synthesis of indole derivatives
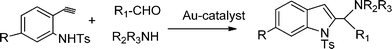
Functionalized indoles are biologically active compounds102 that can be obtained using a variety of approaches.103 Recently, following the Mannich approach functionalized indols have been obtained by three component coupling and cyclization of N-tosyl protected ethynylaniline, paraformaldehyde and piperidine in the presence of Au/ZrO2101 (Scheme 6).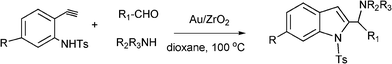 | ||
| Scheme 6 Three component coupling and cyclization of an aldehyde, amine, and N-protected ethynylaniline. | ||
It was found that only a fraction of the total gold species i.e. only the Au(III) are active for this reaction. Thus, the MCR of paraformaldehyde, piperidine and N-protected ethynylaniline in the presence of Au/ZrO2 in dioxane at 100 °C and after 5 h, yielded 95% of 2-(aminomethyl) indole. No propargylamines were detected in the reaction media. The reaction was also extended to different combinations of aldehydes and amines giving the corresponding indoles in good yields.
More recently the same authors104 have prepared metal organic frameworks (IRMOF-3-Si–Au) containing a Au(III)Schiff base complex lining the pore walls. This material was obtained by reacting the –NH2groups of IRMOF-3 with salicylaldehyde to form the corresponding imine. The final step consists of reacting a gold precursor (NaAuCl4) with the imine. A maximum functionalization of about 3% of the total amino groups was produced which allowed the introduction of up to 2 wt% of gold using this method. IRMOF-3-Si–Aucatalyst was tested for the condensation reaction of N–tosyl protected ethynylaniline, piperidine and paraformaldehyde in dioxane at 40 °C (see Table 6). It was found that IRMOF-3-Si-Au gives much higher catalytic performance than other goldcatalysts such as Au/ZrO2 and homogeneous gold/salt complexes (Au(III)Schiff base complex) and AuCl3. The results showed that the cationic goldAu(III) species are the active sites in this reaction which are more stable in heterogeneous media than in homogeneous media, while the higher activity of IRMOF-3-Si-Aucatalyst should be attributed to the existence of well defined, stable and accessible isolated Au(III) active sites on this material. Good yields of indole derivatives (70–95%) were obtained from N-protected ethynylanilines, and different aldehydes and amines (Table 6). The IRMOF-3-Si-Aucatalyst can be successfully reused and no goldleaching from the solid to the liquid media was detected. However, the reaction should be carried out with freshly prepared catalyst since catalyst amorphization occurs upon catalyststorage for long time.
| |||
|---|---|---|---|
| Catalyst | R1–CHO | R2R3NH | Yield (%) |
| a Aldehyde (0.2 mmol), aniline (0.24 mmol) and N-protected ethynylaniline (0.26 mmol), gold (0.0007 mmol) dioxane (1.0 mL), h ; Ts: toluene-4-sulfonyl.b Aldehyde (0.40 mmol), aniline (0.24 mmol) and N-protected ethynylaniline (0.20 mmol), gold (14 mg, 0.001 mmol) dioxane (1.0 mL), 40 °C.c At 80 °C.d Chiral amines: CA1 (S)-(+)-2-(metoxymethyl)-pyrrolidine and CA2 (S)-(+)-2-methylpiperidine. | |||
| Au/ZrO2a | (HCOH)n R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H | piperidine | 95 |
| Heptyl | piperidine | 97 | |
| Cyclohexyl | piperidine | 75 | |
(HCOH)n R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H | pyrrolidine | 87 | |
(HCOH)n R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H | morpholine | 70 | |
(HCOH)n R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H | diethylamine | 90 | |
| IRMOF–3–Si–Aub | (HCOH)n R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H | piperidine | 90(16 h) |
| Heptyl | piperidine | 95(6 h) | |
| Cyclohexylc | piperidine | 80(4 h) | |
(HCOH)n R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H | CA1d | 83(12 h) | |
(HCOH)n R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H | CA2d | 91(6 h) | |
3.3 Substituted benzo[b]furans
Benzo[b]furan derivatives are compounds of relevance because of their natural occurrence associated with their biological properties.70,105 The most general methods to prepare benzo[b]furans involve reductive cyclization of ketoesters by low valent titanium,106 photochemical rearrangement of phosphateesters,107 Suzuki coupling of boronic acids with organic halides or triflates catalyzed by palladium,108 Sonogashira cross coupling reaction of o-halophenols with terminal alkynes in the presence of palladium and/or copper as catalysts.109Recently, following the Mannich protocol, Kabalka et al.110 have reported the synthesis of a variety of propargylamines in good yields from different alkynes, primary or secondary amines and paraformaldehyde using cuprous iodide doped alumina as the catalyst under microwave irradiation. The reaction was extended to the synthesis of 2-substituted benzo[b]furan derivatives when ethynylphenol was condensed with secondary amines (such as piperidine, morpholine, 1-phenylpiperazineetc.) and paraformaldehyde (see Table 7). In this case the Mannich adduct resulting from the A3 coupling undergoes a subsequent cyclization into the benzofuran ring (Scheme 7). The reaction is highly efficient and moderated to good yields of 2-substituted benzo[b]furans (52–70%) were obtained in a short reaction time, but high amounts of catalyst were required.
![Synthesis of substituted benzo[b]furans through a MC Mannich reaction followed by cyclization.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s7.gif) | ||
| Scheme 7 Synthesis of substituted benzo[b]furans through a MC Mannich reaction followed by cyclization. | ||
| R | Amine | Yield (%)b |
|---|---|---|
| a Reaction conditions: paraformaldehyde (3 mmol), o-ethynylphenol (1 mmol), secondary amine (1 mmol), cuprous iodide (3 mmol) Al2O3 (1 g) irradiated at 300 W for 10 min.b Isolated yields. | ||
| H |  | 65 |
| H |  | 68 |
| H | 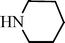 | 65 |
| H | 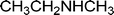 | 62 |
| H | 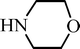 | 55 |
| H | 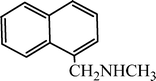 | 70 |
| H | 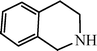 | 59 |
| H | 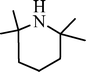 | 52 |
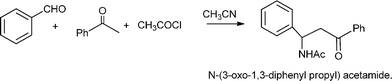
3.4 Synthesis of β-aminocarbonyl compounds
N-Substituted aminocarbonyl compounds can be synthesised by the versatile Mannich type reaction (see Scheme 2). The MCR between an aldehyde, amine and ketones using Lewis111or Brønsted acids112 and Lewis bases113 as catalysts produces β-aminocarbonyl compounds (Scheme 8). β-Aminocarbonyl compounds are important building blocks for the synthesis of biologically active nitrogen-containing compounds such as β-aminoalcohols, βamino acids and β-lactams and pharmaceuticals.114,115 | ||
| Scheme 8 Mannich reaction of aromatic aldehyde, ketones and amines. | ||
Mechanistically the reaction proceeds typically viaimine formation through the condensation of aldehyde and amine followed by the attack of the enol form of ketone on imine to afford the desired product.
Recently, the synthesis of β-aminoketones by a three component Mannich reaction in liquid phase under solvent free and at room temperature, have been carried out using tungstated zirconia (WOx-ZrO2).116 WOx from ammonium metatungstate was incorporated into hydrous zirconia and calcined at 923 K to give a solid, which exhibits strong acidity. Different aromatic aldehydes, anilines and cyclohexanone give the corresponding β-aminoketones in good yields (66–90%) as a mixture of syn and anti stereoisomers. In most of the examples studied the syn selectivity was higher as compared to anti selectivity (Scheme 9).
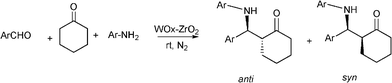 | ||
| Scheme 9 Three component reaction between aromatic aldehyde, aniline and cyclohexanone catalyzed by tungstated zirconia. | ||
Also, the sulfated ceria-zirconia (SO42−/CexZr1-xO2) reported by Reddy et al.117 was an efficient catalyst for the synthesis of β-aminoketonesvia a Mannich reaction. The reaction between benzaldehyde, aniline and cyclohexanone proceeded smoothly to afford 82% of 2-[1-phenyl-1-N-phenylamino]methylcyclohexanone, with an anti/syn ratio of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82. The catalyst could be recycled and no appreciable change in activity was observed for 2–3 runs.
82. The catalyst could be recycled and no appreciable change in activity was observed for 2–3 runs.
It is known that fluorine containing compounds produce an enhancement of biological activity as well as a decrease in toxicity.118 Xia and co-workers119 reported that fluorinated β-aminobutanones can be obtained through a one-pot three-component Mannich type reaction of unmodified acetone with aldehydes and fluorinated anilines in good to excellent yields (81–96%) catalysed by sulfamic acid (H2NSO3H, SA) at room temperature (Scheme 10). Due to its zwitter-ionic property this heterogeneous catalyst can be recycled and reused through simple filtration and washing.
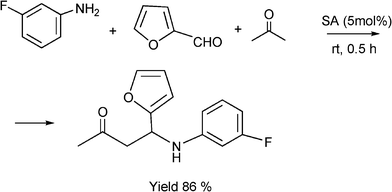 | ||
| Scheme 10 Synthesis 4-(3-fluorophenylamino)-4-(furan-2yl)butan-2-one. | ||
SA is also an efficient and recyclable heterogeneous catalyst for the ultrasound assisted one-pot reaction of aldehydes with amines and ketones. Different aromatic aldehydes, anilines and acetophenone in ethanol at room temperature gave the corresponding β-amino ketone in 88–95% yield after 90–120 min. Also, β-aminocarbonyl compounds with ortho substituted aromatic amines are obtained in acceptable to good yields (53–95%) after 2–8 h in the presence of sulfamic acidcatalyst under ultrasound irradiation (600 W).120 The authors claimed that the accelerating effect of ultrasound can be an important tool for the one-pot Mannich reaction of sterically hindered arylamines. Recyclable Cunanoparticles for the one-pot reaction to obtain β-aminoketones have been proposed by Kidwai and co-workers.121 For comparative purposes various metal nanoparticles such as Au and Ni were selected for the Mannich reaction. The authors found that Cu-np (particle diameter of about 20 nm), was the most active catalyst. The catalyst was recovered and reused in four consecutive runs showing a gradual loss of activity. A variety of aromatic aldehydes, aromatic amines and acetophenone or cyclohexanone were coupled giving the corresponding β-aminoketones in good yields (Table 8). Optimum yields of β-aminoketones were achieved using a concentration of 10 mol% of Cu-np while increasing the Cu-np concentration, their oxidation to form CuO occurs, producing agglomeration and reducing the surface area of the nanoparticles and hence decreasing the catalytic activity.
| Ketone | R–CHO | R–NH2 | Time (h) | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: acetophenone or cyclohexanone (1 mmol), aromatic aldehyde (1 mmol), aromatic amine (1 mmol), 10 mol% Cu-np(18± 2 nm) methanol as solvent, room temperature, nitrogen atmosphere.b Isolated yields. | ||||
| Acetophenone | Ph | Ph | 4 | 93 |
| Acetophenone | Ph | 4–CH3C6H4 | 9 | 97 |
| Acetophenone | Ph | 3,4–(CH3)2C6H3 | 10.5 | 92 |
| Acetophenone | Ph | 4–ClC6H4 | 9 | 95 |
| Acetophenone | 4–CH3C6H4 | Ph | 8 | 97 |
| Acetophenone | Ph | 4–OCH3C6H4 | 10 | 91 |
| Acetophenone | Ph | 4–NO2C6H4 | 12 | 73 |
| Acetophenone | 4–OCH3C6H4 | Ph | 9 | 91 |
| Acetophenone | 4–NO2C6H4 | Ph | 12 | 74 |
| p-methylacetophenone | Ph | Ph | 10 | 85 |
| p-nitroacetophenone | Ph | Ph | 10 | 87 |
| cyclohexanone | Ph | Ph | 9 | 88 |
| cyclohexanone | Ph | 4–CH3C6H4 | 11 | 90 |
| cyclohexanone | Ph | 4–ClC6H4 | 12 | 91 |
| cyclohexanone | 4–OCH3C6H4 | Ph | 12 | 83 |
3.5 Synthesis of dihydropyrimidinones
The synthesis of functionalized dihydropyrimidinones (DHPM) represents an excellent example of the utility of one-pot multiple component condensation reactions.Aryl substituted 3,4-dihydropyrimidinones are important heterocyclic compounds in organic synthesis and medicinal chemistry due to their therapeutic and pharmacological properties. The DHPM and their derivatives exhibit a broad spectrum of biological effects such as antitumor, antiviral, antibacterial and antiinflammatory activities and antioxidative properties.122 Furthermore, appropriately functionalized 3,4-dihydropyridimidones can act as calcium channel modulators, antihypertensive agents, α1a-adrenergic antagonists and neuropeptide antagonists.123
Apart from non natural DHPM, several marine alkaloids isolated from the Sponge Batzella as batzelladine compounds are potential new leads for drug development for AIDS therapy.124 More recently Monastrol, a 3,4-dihydropyrimidin-2(1H)-thione derivative has been identified and inhibits the mitotic kinesinEg5motorprotein and can be considered as a new lead for the development of anticancer drugs.125Scheme 11 shows different 3,4-dihydropyrimidinones, (Monastrol, SQ 32926 and SQ 32547) reported to be effective as orally active antihypertensive agents.126
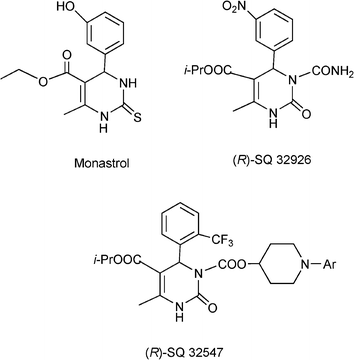 | ||
| Scheme 11 Different 3,4-dihydropyrimidinones with pharmaceutical interest. | ||
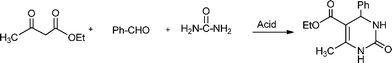
The simplest method for synthesising 3,4-dihydropyrimidin-2(1H)-one was reported first by Biginelli16 and involves a three component one-pot cyclocondensation reaction of an aldehyde, an open chain β-ketoester and urea or thiourea in presence of acid catalysts such as hydrochloric acid in ethanol at reflux temperature127,128 (Scheme 12).
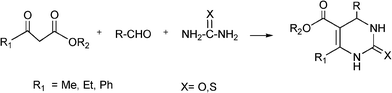 | ||
| Scheme 12 Three component Biginelli reaction. | ||
The plausible mechanism (Scheme 13) of the acid catalyzed Biginelli condensation postulated by Kappe,129 involves the formation of an N-acyliminium ion intermediate from the aldehyde and urea precursors. Interception of the iminium ion by ethyl acetoacetate, presumably trough its enol tautomer, produces an open chain ureide, which undergoes cyclization and subsequent dehydratation to yield the dihydropyrimidinones.
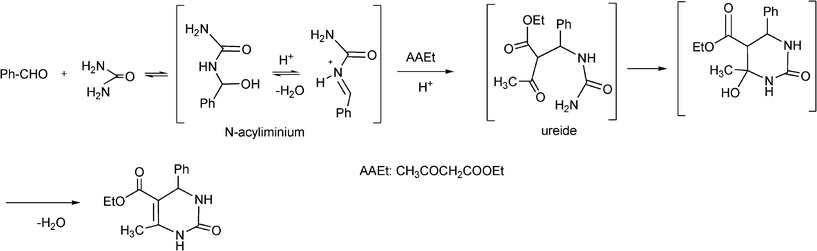 | ||
| Scheme 13 Proposed mechanism of the acid catalyzed Biginelli reaction. | ||
In recent years, many synthetic methods for preparing DHPM based on the Biginelli reaction have been reported which include classical conditions and microwave and ultrasound irradiation in the presence of Brønsted130 and Lewis acids as catalysts. For instance, lanthanide triflates,131 phase transfer catalysts (tetra-n-butyl ammonium bromide),132 and NaCl in DMF.133 However some of the reported methods suffer from drawbacks derived from the product isolation procedure and environmental pollution. Moreover, in the case of substituted aromatic and aliphatic aldehydes bearing sensitive functional groups the original Biginelli reaction is unsuitable and affords DHPM compounds in low yields (20–40%) due to the strongly acidic conditions and prolonged time of heating required.
In the last years, replacement of conventional toxic and polluting Brønsted and Lewis acid catalysts by eco-friendly reusable solid acid heterogeneous catalysts, has achieved considerable importance in the synthesis of 3,4-dihydropyrimidinones. Thus, a wide variety of solid acid catalysts including supported Brønsted and Lewis acids, heteropolyacids, zeolites and metal complexes have been reported in the literature for performing the Biginelli reaction with variable success.134–157 As an example, Table 9 summarizes results corresponding to the Biginelli reaction between benzaldehyde, ethyl acetoacetate and urea to synthesize 5-(ethoxycarbonyl)-6-methyl-4-phenyl-3,4-dihydropyridin-2(1H)-one over different heterogeneous catalysts using both conventional heating or microwaves. For instance, excellent yields of DHPM derivatives (85–98%) were reported using ZrO2-pillared clay (Zr–Pilc) under microwave irradiation.158 The efficacy of the procedure was exemplified by the synthesis of a biologically active racemic mixture of monastrol (a potent anticancer drug) and nitractin (an antibacterial and antiviral drug) which were obtained in high yields (90% and 87% respectively). The catalyst was recycled three times without any loss of activity. Also ion exchange resins such as the perfluorinated resin sulfonic acidNafion NR-50, resulted in a very active and reusable catalyst143 for the synthesis of DHPM derivatives with acceptable yields (74–96%). While the catalytic activity of metallophthalocyanine complexes142 strongly depends on the metal, showing the order of activity: Co(II)-phthalocyanine > tetraphenoxyvanadyl(II)-phthalocyanine > Fe(II)phthalocyanine > Cu(II)-phthalocyanine > Ru(II)-phthalocyanine. Particularly, the Co(II) phthalocyanine complex was an efficient and recyclable heterogeneous catalyst giving good yields of different DHPM (82–98%).
| |||||||
|---|---|---|---|---|---|---|---|
| Catalysts | B:E:Ua | Solvent | T (°C) | Catalyst (g) | t (h) | Yield DHPM | Ref. |
a B:E:U mmol of benzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetoacetate ethyl acetoacetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea.b mol%.c 0.2 mmol of iodine adsorbed on 0.5 g of neutral alumina.d wt% based on total weight.e wt% based on β-ketoester.f wt% based on aldehyde.g Alum–SiO2: KAl(SO4)2·12H2O supported on silica gel.h PVP-Cu complex: Poly(4-vinylpyridine-divinylbenzene)–Cu(II) complex. urea.b mol%.c 0.2 mmol of iodine adsorbed on 0.5 g of neutral alumina.d wt% based on total weight.e wt% based on β-ketoester.f wt% based on aldehyde.g Alum–SiO2: KAl(SO4)2·12H2O supported on silica gel.h PVP-Cu complex: Poly(4-vinylpyridine-divinylbenzene)–Cu(II) complex. | |||||||
| I2–Al2O3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 | — | MW | 0.2c | 0.02 | 90 | 145 |
| SiO2–NaHSO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 | CH3CN | reflux | 10b | 1.5 | 93 | 135 |
| Alum–SiO2g | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 | — | 80 | 0.16 | 4 | 92 | 134 |
| Ferrihydrite in a silica aerogel | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 | EtOH | reflux | 1.77(4 mmol of Fe) | 84 | 65 | 138 |
| Silica sulphuric acid | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 | EtOH | reflux | 0.23 | 6 | 91 | 144 |
| FeCl3–SiMCM-41 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 | — | (MW) | 10d | 0.08 | 89 | 141 |
| FeCl3–Nanopore Silica | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 | — | (MW) | 10d | 0.25 | 55 | 139 |
| Montmorillonite | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 | — | 130 | 0.5 | 48 | 82 | 140 |
| ZrO2–pillared clay | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7 3.7 | — | (MW) | 0.25 | 0.08 | 92 | 158 |
| Nafion-NR-50 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | CH3CN | reflux | 0.25 | 3 | 96 | 143 |
| Amberlyst-15 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | CH3CN | reflux | 0.25 | 5.5 | 85 | 143 |
| Yb(III)-resin and Polymer-supported scavengers | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | — | 120 | 0.17 | 48 | 80 | 147 |
| Ag3PW12O40 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 | H2O | 80 | 10d | 4 | 92 | 155 |
| (PVP)-Cu complexh | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 | MeOH | reflux | 20f | 24 | 70 | 156 |
| Scolecite | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 | CH3CN | reflux | 2d | 0.5 | 83 | 153 |
| ZrO2/SO42− | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 | — | (MW) | 0.1 | 0.5 | 98 | 148 |
| Heulandite | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 7.2 | Acetic acid | 100 | 0.2 | 5 | 75 | 154 |
| HY | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 | Toluene | reflux | 0.5 | 12 | 21 | 151 |
| HZSM-5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 | Toluene | reflux | 0.5 | 12 | 80 | 151 |
| Ersorb-4 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 | EtOH | 80 | 1 | 8 | 93 | 137 |
| Co(II)phthalocyanine | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | CH3CN | reflux | 2b | 1 | 98 | 142 |
| TS-1 | 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 | — | 50 | 0.01 | 0.16 | 98 | 150 |
| HBF4–SiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | Ethanol | r.t. | 5b | 2 | 94 | 157 |
Recently Shaabani et al.152 have reported a Biginelli-like reaction which combines an aldehyde, a cyclic β-dicarbonyl compound (5,5-dimethyl-1,3-cyclohexanedione) and a urea derivative such as N-methylurea or thiourea, using silica supported sulfuric acid (SSA) as a solid acid catalyst and in the presence of an ionic liquid (1-butyl-1,3-methylimidazonium bromide ([bmim]Br)). The reactions performed at 100 °C, yielded 4-aryl-7,7-dimethyl-1,7,7-trimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione derivatives (46–86% yield) (Scheme 14) in less than two hours. The authors found that the combination of the ionic liquid with SSA decreases the reaction time and produces an increase of the yield of the target compound with respect to the classical conditions using HCl and conventional solvents. The acceleration of the multicomponent reaction was associated with the existence of solvophobic interactions in the ionic liquid media that generate an internal pressure which promotes the association of the reactants in a solvent cavity during the activation process.
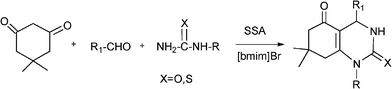 | ||
| Scheme 14 Synthesis of 4-aryl-7,7-dimethyl-1,7,7-trimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione derivatives. | ||
Finally, it is interesting to note that DHPMs obtained from the Biginelli type reaction are inherently asymmetric molecules and the influence of the absolute configuration at the sterogenic centre at C4 on biological activity is well documented.159 Thus for instance, the 1,4-DHPM known as SQ32926 (see Scheme 11) is exclusively the (R)-enantiomer that carries the therapeutical desired antihypertensive effect. However no general asymmetric synthesis for this heterocyclic system has been reported up to now and resolution strategies have been so far the method of choice to obtain enantiomerically pure DHPM.
3.6 Synthesis of tetrahydroquinoline derivatives
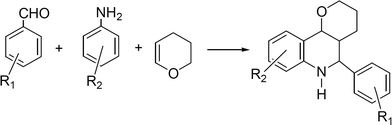
Quinolines and their derivatives are achieving increasing importance due to their wide range of biological activity. Tetrahydroquinolines are an important class of natural product and exhibit diverse biological properties such as antiallergic, antiinflammatory, estrogenic and psychotropic activity.160–161 The classical method for the synthesis of tetrahydroquinolines involves the aza Diels–Alder reaction between N-aryl-imines and nucleophilic olefins in the presence of Lewis acids, such as FeCl3 in Et2O/t-BuOH, BF3·Et2O, AlCl3/Et3N162 which are frequently used in stoichiometric amounts. Moreover, many imines are unstable, hygroscopic and difficult to purify, and so the one-pot approach that involves the condensation of aldehydes with anilines and alkenes in the presence of Lewis acid catalysts where the imine is in situ formed, is much more efficient and economic process to produce this type of compounds. We will describe here some examples of dihydroquinoline derivatives synthesis through MCR using heterogeneous catalysts.![Synthesis of aryl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline derivatives through a three component reaction.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s15.gif) | ||
| Scheme 15 Synthesis of aryl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline derivatives through a three component reaction. | ||
The authors propose that the aza-Diels–Alder cycloaddition may proceed through a concerted polar [4π+ + 2π] cycloaddition with subsequent tautomerization (via a, Scheme 16) or by an intermolecular 1,2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ addition-intramolecular cationic cyclisation sequence (via b, Scheme 16). The active species are produced by H-bond activation or by nitrogen protonation of the imine by the strong acid sites of the catalyst.
N+ addition-intramolecular cationic cyclisation sequence (via b, Scheme 16). The active species are produced by H-bond activation or by nitrogen protonation of the imine by the strong acid sites of the catalyst.
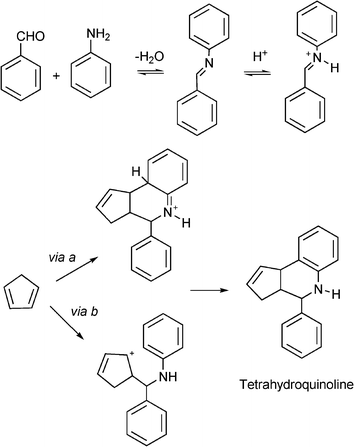 | ||
| Scheme 16 Proposed mechanism of formation of cyclopentatetrahydroquinolines. | ||
Kobayashi et al.164 have prepared diverse tetrahydroquinoline derivatives (Scheme 17) using a polymer supported scandium ((polyallyl)scandium trifylamide ditriflate, (PA-Sc-TAD)) as a catalyst. Thus, diverse quinoline derivatives have been efficiently obtained (99–65%) from aldehydes (aromatic, aliphatic, heterocycic, and glyoxals and glyoxylates), aromatic amines and different olefins, at 40 °C in CH2Cl2:CH3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent and 15 h reaction time. The method is especially useful for the construction of a quinoline library due to the efficiency and simplicity of the process.
1) as the solvent and 15 h reaction time. The method is especially useful for the construction of a quinoline library due to the efficiency and simplicity of the process.
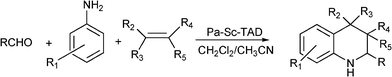 | ||
| Scheme 17 MC synthesis of tetrahydroquinoline derivatives. | ||
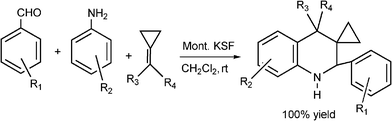 | ||
| Scheme 18 Synthesis of aryl 1,2,3,4-tetrahydrospiro(3,1’-cyclopropyl)quinolines derivatives through MCR. | ||
 | ||
| Scheme 19 Multicomponent synthesis of pyran- and furandihydroquinolines (n = 2 and 1 respectively). | ||
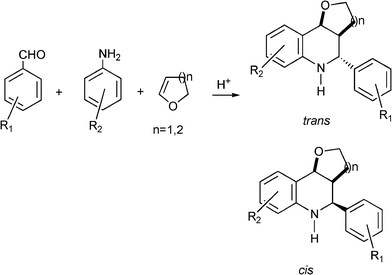
As in the case of spyrocyclopropyldihydroquinolines, the imines formed in situ by the condensation of benzaldehyde and aniline derivatives act as heterodienes which subsequently undergo the aza-Diels–Alder reaction with 3,4-dihydro-2H-pyran or 3,4-dihydro-2H-furan to form pyran and furanquinolines.
Different benzaldehyde derivatives and anilines were reacted with 3,4-dihydro-2H-pyran or 3,4-dihydro-2H-furan giving the corresponding pyran and furanquinolines in good yields. In all cases a mixture of trans and cis isomers were detected being the trans isomer the major product (Scheme 19). The Fe3+–K10 Montmorillonite clay and HYcatalysts were recovered and recycled in three consecutive reactions without lost of activity. In Table 10 the results obtained with different homogeneous and heterogeneous acid catalysts in the synthesis of 5-phenyl-3,4,4a,5,6,10b-hexahydro-2H-pyran-[3,2-c]quinoline are compared.
| ||||||
|---|---|---|---|---|---|---|
| Catalyst | Catalyst (mol%) | T (°C) | Solvent | Time (h) | Yield (%) | Ref. |
| a IL= [Bmin]PF6.b Only the trans-pyran[3,2-c]quinoline was obtained.c Under nitrogen atmosphere.d Grams. | ||||||
| ZrCl4 | 10 | rt | MeCN | 0.60 | 88 | 170 |
| Bi(OTf)3 | 10 | rt | ILa | 2 | 90 | 175 |
| KHSO4 | 40 | rt | MeCN | 1 | 64 | 171 |
| Fe+3-K10 | 0.1d | rt | MeCNc | 3.5 | 86 | 174 |
| HY | 0.1d | reflux | MeCNc | 5 | 82 | 174 |
| SbCl3-HAP | 1.6 | reflux | MeCNc | 2 | 85b | 172 |
| HClO4-SiO2 | 5 | rt | MeCN | 0.25 | 95 | 173 |
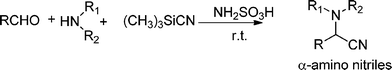
3.7 Synthesis of α-amino nitrile derivatives
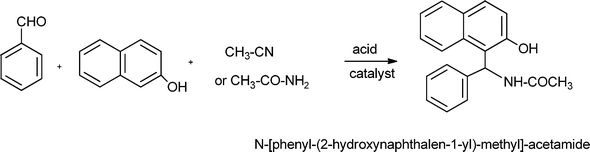
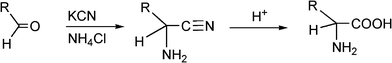
α-Aminonitriles are a very useful intermediate compounds for the synthesis of versatile α-amino acids, various nitrogen-containing heterocyclic compounds (imidazoles, thiadiazolesetc.) and biologically useful molecules (such as for instance Saframycin A, a highly potent antitumor drug from Streptomyces lavendulae).173,174The most important route for the synthesis of α-amino acids via the formation of α-aminonitriles is the well known Strecker reaction (1850).14 The classical Strecker reaction involves a direct multi-component reaction of an aldehyde or a ketone, an ammonium salt and alkaline cyanides in aqueous solution to form α-aminonitriles, which can be subsequently converted to α-amino acids (Scheme 20).
 | ||
| Scheme 20 3MC Strecker reaction. | ||
The reaction involving aldehydes is typically catalyzed by Lewis acids such as BiCl3, NiCl2, InCl3, LiClO4, RuCl3.175 However, in the case of ketones, the reaction is more difficult and other acid catalysts such as gallium triflate [Ga(SO3CF3)] or the related metal triflates, trimethylsilyltriflate and Fe(Cp)2PF6 are required.176 Some of these Lewis acid catalysts are strong and expensive and their use involves harsh conditions, long reaction times and tedious aqueous work-up, leading to the generation of large amounts of toxic metal-containing waste.
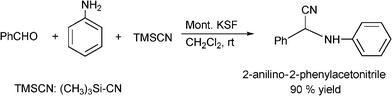
Several modifications of the Strecker reaction have been reported using a variety of cyanating agents in the presence of solid or supported acids as heterogeneous catalysts. For instance, polyoxometalate salts (K5CoW12O40·3H2O) have been used as efficient and recyclable heterogeneous catalysts in the three-component condensation of aldehydes, amines, and KCN. The reaction is performed at ambient temperature in acetonitrile giving the corresponding α-aminonitriles in good to moderate yields (51-98%) and excellent selectivity.177 Yadav and co-workers178 prepared 2-anilino-2-phenylacetonitrile in 90% yield by treatment of benzaldehyde, aniline and trimethylsilyl cyanide (TMSCN) in dichloromethane at room temperature with Montmorillonite KSF clay as the catalyst (Scheme 21). No cyanohydrin trimethylsilyl ester (an adduct obtained from the aldehyde and TMSCN) was obtained under these reaction conditions. A variety of aldehydes were reacted with a range of amines and TMSCN in a one-pot procedure to produce aminonitriles in 85–94% yields.
 | ||
| Scheme 21 Three component Strecker reaction of aldehyde, amine and TMSCN. | ||
The mechanism of the process involves the formation of imines or iminium ions and the subsequent nucleophilic attack of the cyanide ion of TMSCN to provide the final product.
Using the same approach, Heydari et al.179 performed the synthesis of several α-aminonitriles using sulfamic acid (NH2SO3H, SA), a stable, non corrosive acid, as a heterogeneous catalyst. The three-component coupling reaction involving an aldehyde (aliphatic, aromatic, heterocyclic and conjugated aldehyde), an amine (aliphatic and aromatic) and TMSCN in the presence of 5 mol% of sulfamic acid at room temperature under solvent free conditions afforded the corresponding α-aminonitriles in excellent yields (82–98%) and selectivities in short reaction times (see Table 11). No undesired side products such as cyanohydrins were obtained under these conditions due to the rapid formation and activation of the imine intermediates catalyzed by sulfamic acid. The catalyst was recovered by simple filtration and recycled in subsequent three cycles giving similar yields.
| |||
|---|---|---|---|
| R | R1 | R2 | Yield (%) |
| a Reaction conditions: aldehyde (3 mmol), amine (3.2 mmol), trimethylsilyl cyanide (3 mmol) and sulfamic acid (5 mol%) at room temperature. | |||
| Phenyl | H | Phenyl | 98 |
| n-Butyl | H | Phenyl | 90 |
| 4-CH3phenyl | H | Phenyl | 97 |
| 2-Furyl | H | Phenyl | 95 |
| Cinnamyl | H | Phenyl | 98 |
| n-butyl | Ethyl | Ethyl | 82 |
| Phenyl | Ethyl | Ethyl | 90 |
| 4-CH3phenyl | Ethyl | Ethyl | 85 |
| 2-Furyl | Ethyl | Ethyl | 80 |
| Cinnamyl | Ethyl | Ethyl | 88 |
| 4-CH3Ophenyl | Ethyl | Ethyl | 91 |
| Phenyl | Benzyl | Benzyl | 94 |
| i-Propyl | Benzyl | Benzyl | 94 |
| 4-CH3phenyl | tert-Butyl | H | 84 |
| Cinnamyl | tert-Butyl | H | 88 |
A polymer, poly(4-vinylpyridine) (PVP)–SO2 complex with mild acidity, has been prepared by Olah et al.180 and used in the multicomponent synthesis of α-aminonitriles. The catalyst was prepared by passing SO2 gas through 2% cross linked poly(4-vynilpyridine) at −78 °C. The Strecker reaction of aromatic and conjugated aldehydes, aliphatic, benzylic and aromatic amines and TMSCN was performed in dichloromethane at 50 °C giving excellent yields (81–98%) of the corresponding α-aminonitriles. No differences in yield were found using aromatic aldehydes with electron donating or electron withdrawing groups, but attempts to perform the reaction with ketones failed. The PVP could be recycled to form the PVP–SO2 complex.
A bio-supported catalyst, cellulose sulphuric acid,181 (CSA) has been also used as a highly efficient, selective and recyclable catalyst for performing the MC condensation of aldehydes, amines and TMSCN. Reactions performed at room temperature in acetonitrile as a solvent, gave excellent yields (85–97%) in rather short reaction times (45–80 min). Lower yields were achieved with other solvents such as water, methanol, ethanol, dichloromethane, toluene or under solvent-free conditions. No undesired side products, such as cyanohydrin trimethylsilyl ether, were observed owing to the rapid formation of the imine intermediate.
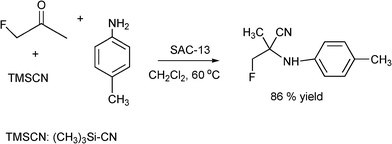
Fluorinated amino acids are important building blocks in pharmaceuticals for anticancer drugs for the control of tumor growth, antihypertensive and anti allergic applications.173 Following the Strecker route, efficient synthesis of α-aminonitriles using aldehydes, ketones and fluorinated ketones has been achieved with Nafion-H, Nafion SAC-13 (10-20% Nafion-H polymer on amorphous silica porous nanocomposite) silica gel and fumed silica.182 When the reaction was carried out with aldehydes or ketones, primary amines and TMSCN at 60 °C in dichloromethane as a solvent in the presence of Nafion, yields between 75–97% of the corresponding α-aminonitriles were obtained after 6 h reaction time. A similar result was obtained using Nafion SAC-13. When the Strecker reaction was performed with monofluoroacetone, p-toluidine and TMSCN, it was found that though silica and fumed silica showed catalytic activity, Nafion and Nafion SAC-13 gave the best yields (86%) (Scheme 22). It is worth mentioning that these catalysts gave comparable results to those obtained in the case of metal triflates (Ga(OTf)3) and trimethylsilyl triflate (TMSOTf) with 96 and 74% yield respectively. The Nafioncatalyst was reused for five consecutive runs and the catalytic activity remains practically unchanged. It is interesting to note that when ketones are involved in the reaction the nature of the solvent plays an important role. Acetonitrile, THF, and toluene are not suitable for the direct Strecker reaction of ketones, since they are more basic and interact with the acidic sites, thus reducing the catalytic activity.183 However, dichloromethane minimizes such interactions enhancing the catalytic activity.
 | ||
| Scheme 22 Strecker reaction of monofluoroacetone, p-toluidine and TMSCN. | ||
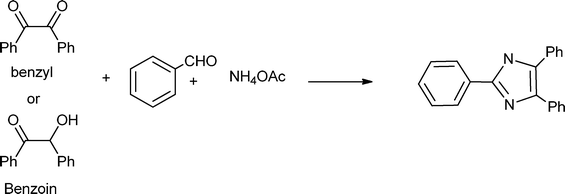
3.8 Synthesis of multi-substituted imidazole derivatives
Multi-substituted imidazole derivatives are an important class of compounds which exhibit a wide spectrum of biological activities as for instance antiinflammatory and antithrombotic activities.184 The well known microtubule stabilizing agents such as Eleutherobin and Sarcodictyn, among other marine and plant derived products contain imidazole.185 Also, Trifenagrel, a potent arachidonate cyclooxygenasa inhibitor that reduces platelet aggregation is structurally a 2,4,5-triarylimidazole186 (Scheme 23). Furthermore 2,4,5-trisubstituted imidazole moieties are common structures in numerous synthetic compounds used in agriculture, for plant growth regulators, herbicides and fungicides.187 In addition 2,4,5-triarylimidazole have received great attention for the development of fluorescence labelling agents for biological imaging applications188 or chromophores for non linear optics systems.189 Among them Lophine is one of the few long-lasting chemiluminescent molecules and its dimers have piezochromic and photochromic properties.190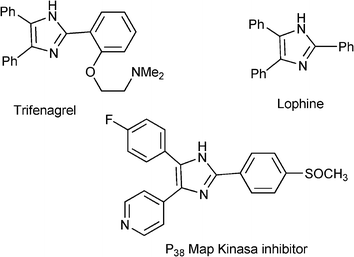 | ||
| Scheme 23 Different 2,4,5-trisubstituted imidazole derivatives with pharmaceutical interest. | ||
Numerous classical methods for the synthesis of multisubstituted imidazoles have been developed. Among these methods a typical procedure is the multicomponent reaction approach involving the cyclocondensation of a 1,2-diketone (or α-hydroxyketones), an aldehyde and ammonia or ammonium acetate in the presence of a homogeneous strong protic acid catalysts (such as phosphoric acid, sulphuric acid, acetic acid),191 Lewis acids192 or oxidant agents such as ceric ammonium nitrate.193 The reactions are usually performed under reflux of a polar organic solvent (acetic acid, methanol, ethanol, DMF and DMSO) under inert atmosphere. More recently, microwave irradiation in the absence of any catalyst194 has been used to produce multisubstituted imidazole derivatives with good success, however high reaction temperatures (180–210 °C) are required.
Some research groups have reported the one-pot condensation of 1,2-diketone, (or α-hydroxy ketone or α-keto-oxime), aldehydes and ammonium acetate under microwave irradiation using acetic acid as a solvent195,196 or solid supports impregnated with ammonium acetate. Thus, Xu et al.197 have reported the condensation of α-hydroxy ketone (benzoin) (instead of benzyl) with an aldehyde over silica gel or alumina impregnated with ammonium acetate. Reactions performed under solvent free conditions and microwave irradiation gave the corresponding trisubstituted imidazoles in good yields. Contrarily to conventional condensation of α-hydroxyketones, no oxidizing reagents such as Cu(II) were required and an air oxidation mechanism of the hydroxyl to carbonyl group was proposed. In Scheme 24 the proposed mechanism starting from an aldehyde, an α-hydroxyketone, and ammonium acetate is displayed. The process involves the formation of an imine intermediate from the aldehyde and ammonia which undergoes the nucleophilic addition of the imine intermediate coming from a α-hydroxyketone and ammonia. Subsequent cyclocondensation and oxidation steps lead to the substituted pyrazole derivative.
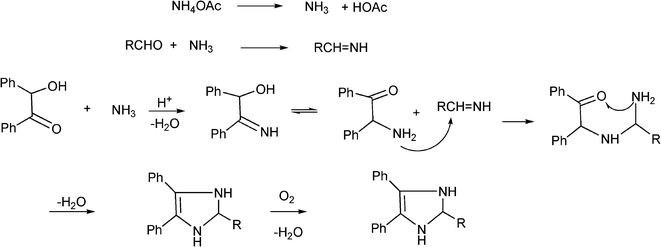 | ||
| Scheme 24 Proposed mechanism for the formation of multi-substituted imidazole derivatives. | ||
Bentonite, Montmorillonite K-10 and KSF, and acid alumina impregnated with ammonium acetate have been used as solid acid catalysts to prepare 2,4,5-trisubtituted imidazole derivatives from 1,2-dicarbonyl compounds and aldehydes under microwave irradiation. Also 1,2,4,5-substituted imidazoles from 1,2-dicarbonyl compounds, aldehydes and primary amines were also obtained in good yields (Scheme 25). Comparison of the different supports show that acidic alumina was the most suitable support yielding imidazoles in 75–85% yield.198
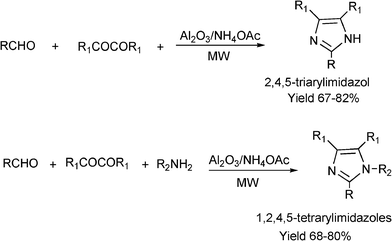 | ||
| Scheme 25 MC synthesis of 2,4,5-trisubtituted and 1,2,4,5- tetrasubstituted imidazole derivatives. | ||
HYzeolite and silica gel199 have also been used as heterogeneous acid catalysts for the synthesis of triarylimidazoles by condensation of benzyl, benzaldehyde derivatives and ammonium acetate under solvent free conditions and microwave irradiation (Scheme 26). The corresponding triarylimidazoles were obtained in good yields (80–90%) after six minute reaction times.
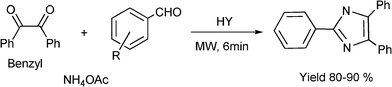 | ||
| Scheme 26 MC synthesis of 2,4,5-triarylimidazoles. | ||
Shaabani et al.200 have reported that silica supported sulfuric acid (SSA) is an excellent and recyclable catalyst for the synthesis of trisubstituted imidazoles under reflux of water or solvent free conditions. When the reactions were performed with different aldehydes, 1,2-diketone, (or α-hydroxy ketone or α-ketooxime), ammonium acetate at reflux of water, the corresponding imidazoles were obtained in yields between 59–81% for 45–90 min. Under microwave irradiation similar yields were achieved after 10 min (Scheme 27). Sulfuric acid on silicacatalyst could be reused for four consecutive runs maintaining its catalytic activity.
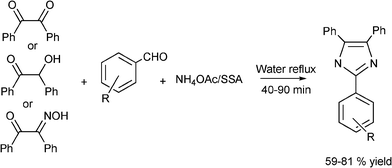 | ||
| Scheme 27 Synthesis of 2,4,5-triarylimidazol from benzil or benzoin or benzylmonoxime, aldehyde and ammonium acetate in the presence of silica sulphuric acid (SSA) catalyst. | ||
Recently Wang et al.201 have prepared a polymer supported zinc chloride which was found to be extremely efficient as a Lewis acid catalyst for the preparation of 2,4,5-trisubstituted imidazoles. The polymer supported zinc was prepared from chloroacetylated polystyrene resin which reacted with diethanolamine and then zinc chloride was anchored to the polymer matrix (PSZC). The condensation of benzyl, benzaldehyde and ammonium acetate at reflux of ethanol in the presence of 15 mol% of catalyst give 96% yield of 2,4,5-triphenylimidazole after 1.5 h. For comparison purposes the reaction was carried out using different conventional Lewis acids such as AlCl3, FeCl3·6H2O, NiCl2·6H2O, and ZnCl2 in 20 mol% of catalyst which afforded lower yields of the 2,4,5-triphenylimidazole that the ZnCl2 supported catalyst (52, 47, 71, and 83% respectively after 3 h). The condensation was extended to different substituted benzaldehydes achieving excellent yields of the corresponding imidazoles (85–95%). The immobilized catalyst was very stable and could be reused at least four times without further purification. Shelke et al.202 have been prepared cellulose sulphuric acid (CSA) as a bio-supported and recyclable solid acid catalyst for the one-pot synthesis of 2,4,5-triarylimidazoles. The condensation reaction of benzil or benzoin, aldehydes and ammonium acetate under microwave irradiation gave excellent yields (90–95%) with rather short reaction times (1–3 min). In Table 12 comparative results obtained with different catalyst in the coupling of benzyl (or benzoin), benzaldehyde and ammonium acetate are summarized.
| |||||||
|---|---|---|---|---|---|---|---|
| Catalyst | B:PhCHO:AAa | Solvent | Catal. (g) | T/°C | Time (min) | Yield (%) | Ref. |
a mmol of benzyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde benzaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ammonium acetate.b 9.3 g of alumina impregnated with 4.4 g of ammonium acetate.c Benzoin was used instead of benzyl.d mol%. ammonium acetate.b 9.3 g of alumina impregnated with 4.4 g of ammonium acetate.c Benzoin was used instead of benzyl.d mol%. | |||||||
| Al2O3 | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 | Et2O | 2.5b | MW(130W) | 20 | 76 | 198 |
| SiO2c | 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 | CH2Cl2 | 15.4 | MW(160W) | 20 | 70 | 197 |
| Al2O3c | 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 | CH2Cl2 | 17 | MW(160W) | 20 | 67 | 197 |
| SSA | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 6.0 | — | 0.2 | MW(160W) | 10 | 85 | 200 |
| SSA | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 6.0 | — | 0.2 | 130 | 50 | 83 | 200 |
| HY | 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 8.0 | — | 4 | MW | 6 | 81 | 199 |
| PSZC | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 | EtOH | 15d | MW | 90 | 96 | 201 |
| CSA | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 | — | 0.1 | MW(180) | 1 | 98 | 202 |
3.9 Synthesis of quinazolin-4-(3H)-one derivatives
4-(3H)-Quinazolinone derivatives were reported to possess analgesical, antibacterial, antifungical, antihelmentics, antiparkinson, anticancer, anti-HIV, MAO inhibitory, central nervous system and antiaggregating activity203–205 (some examples are displayed in Scheme 28). | ||
| Scheme 28 4-(3H)-Quinazolinones with different pharmacological activities. | ||
The most simple procedure for the synthesis of 4-(3H)-quinazolinones was reported by Niementowski in 1895206 and involves the decondensation of 2-aminobenzoic acid (anthranilic acid) or aminobenzoic acid derivatives with amides (Scheme 29). Other methods include cycloaddition reactions of anthranilic acid derivatives with a diverse range of substrates including imidates and iminohalides.
 | ||
| Scheme 29 Niementowski reaction. | ||
Recently, it has been reported that silica gel-supported ferric chloride207 catalyzes efficiently the three component reaction of anthralinic acid, orthoesters and amines to afford 4-(3H)-quinazolinones in one-pot reaction (Scheme 30).
 | ||
| Scheme 30 MC synthesis of 2,3-disusbtituted-4-(3H)-quinazolinones from anthranilic acid or isatoic anhydride, orthoesters and amines. | ||
Total conversion and good yields (84–98%) in short reaction times (5–10 min) were obtained when the reaction was performed at reflux temperature under solvent free conditions. The silica gel-supported ferric chloridecatalyst could be recovered and recycled without loss of activity. Nafion has also been used as an efficient catalyst in this multicomponent reaction to obtain 2,3-disubstituted 4-(3H)-quinazolinones under solvent free microwave irradiation.208 An equimolar mixture of isatoic anhydride or anthranilic acid, triethyl orthoester, aromatic aniline and a catalytic amount of Nafion was subjected to microwave irradiation (2–6 min) affording the corresponding quinazolin-4-(3H)-ones in good yields (71–94%). Anilines having an electron donating group (methyl) gave higher yields (89%) than anilines with electron withdrawing groups (CF3 and NO2) (77%). Substituents on the orthoester did not result in much variation in yield. On the other hand, the recovered catalysts can be reused and did not show any reduced activity after six consecutive runs.
3.10 Synthesis of 4-arylaminoquinazoline derivatives
Natural and synthetic compounds possessing the quinazoline structural motif, particularly 4-arylaminoquinazoline derivatives, display a wide range of biological activities. For instance, 6,7-dimethoxy-4-(3-bromophenylamino) quinazoline (PD 153035) (Scheme 31) and its analogues exhibit high tyrosine kinase inhibitor activity.209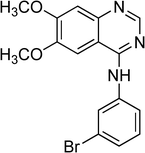 | ||
| Scheme 31 PD 153035. | ||
4-Arylaminoquinazolines can be obtained by reactions of 4-(3H)-quinazolones with aromatic aminehydrochlorides and dimethylcyclohexylamine in the presence of phosphorous pentoxide.210 Other methods to obtain 4-arylaminoquinazolines involve the reaction of 2-aminobenzonitrile and different anilines in the presence of AlCl3 and subsequent condensation of the products with formic acid.211 A new multi-component synthesis of 4-arylaminoquinazolines has been reported by Heravi et al.212 The protocol involves the reaction of 2-aminobenzamide, orthoesters, and substituted anilines in the presence of acid catalysts such as different Keggin-type heteropolyacids (Scheme 32).
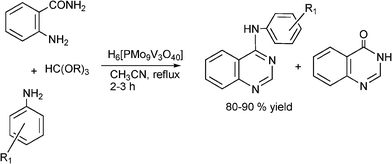 | ||
| Scheme 32 Multicomponent synthesis of 4-arylaminoquinazolines from reaction of 2-aminobenzamide, aniline derivative and orthoesters. | ||
Various anilines and orthoesters were reacted with 2-aminobenzamide in the presence of different heteropolyacids (H6[PMo9V3O40], H5[PMo10V2O40], H4[PMo11VO40], H3[PMo12O40]) in acetonitrile under refluxing conditions. The order of activity of different heteropolyacids was H6[PMo9V3O40] > H5[PMo10V2O40] > H4[PMo11VO40] > H3[PMo12O40]. Using H6[PMo9V3O40] as a catalyst, different 4-benzylaminoquinazolines were obtained in good yields (80–90%) within 2–3 h. In all cases, 3-quinazolin-4-one was also obtained as a by-product in low yield. Studies on the recyclability of the catalyst showed that when using the catalyst over three runs only a slight loss of activity was observed.
3.11 Synthesis of Homoallylic amine derivatives
Homoallylic amines are excellent building blocks in the synthesis of β-amino acids, γ-aminoalcohols, β-lactams antibiotics, aziridines, amino sugars HIV-protease inhibitors and other compounds (Scheme 33).213 | ||
| Scheme 33 Different transformations of homoallylic amines. | ||
The homoallylic amine moiety is not widely present in natural products, however compounds like Eponemycin,214 which exhibits strong activity against B16 melanoma cells, or a depsipeptideCryptophycin 337, which is analog of a potent antitumor compound Criptophycin,215 contain this subunits (Scheme 34).
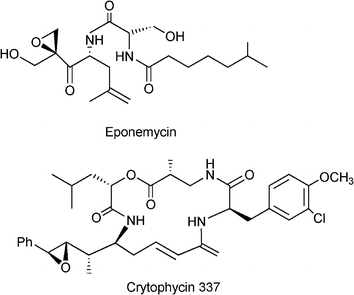 | ||
| Scheme 34 Natural products containing homoallylic amines moiety. | ||
Generally, homoallylic amines are prepared either by addition of organometallic reagents to imines or by nucleophilic addition of allylstannane, allylsilane, allyltin, allylboron or allylgermanium reagents to imines in the presence of Lewis acid catalysts216 such as BF3–OEt2, TiCl4 and PdCl2(Ph3P)2 or PtCl2(Ph3P)2, lithium perchlorate.217 The first addition of allylstannane with imine was catalyzed by Ln(OTf)3 affording moderate yields in 24 h.218 One of the main disadvantages of using Lewis acid catalysts is that the catalysts are deactivated or sometimes decomposed by the amine and water that is formed during the imine formation.
In order to circumvent some of the problems derived from the use of homogeneous catalysts, a one-pot A3 protocol involving aldehydes, aromatic amines and allyltributylstannane has been developed recently (Scheme 35). The one-pot process involves the in situ formation of imine followed by the nucleophilic addition of the organometallic reagent.
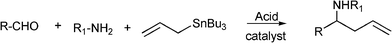 | ||
| Scheme 35 Three component coupling condensation to obtain homoallylic amines. | ||
Ionic liquids such as 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4), have been used as solvents and catalysts with success in this transformation.219 However, similar yields were achieved with heterogeneous catalysts such as Montmorillonite KSF220 clay. Using this catalyst, different aldehydes, anilines and allyltributylstannane were coupled at room temperature in acetonitrile producing the corresponding homoallylic amines in high yields (73–90%) and in short times (3–5.5 h) (see Table 13).220 The Montmorillonite catalyst was reused showing a gradual decrease in activity. Thus when benzaldehyde, aniline and allyltributylstannane was reacted it afforded 90%, 85% and 80% yields over three cycles.
In all cases, the homoallylic alcohol coming from the reaction between aldehyde and allyltributylstannane as well as decomposition or polymerization of sensitive aldehydes was not observed. On the other hand, ketones did not react under similar reaction conditions.
Under the same reaction conditions, analogous selective preparation of homoallylic amines have also been performed using silica supported sodium hydrogen sulfate (NaHSO4 SiO2) as an acid catalyst.221 The one-pot coupling between aldehydes (aromatic, heteroaromatic or aliphatic), aniline derivatives and allyltributylstannane in the presence of NaHSO4–SiO2 in acetonitrile at room temperature afforded the corresponding homoallylic amines in high yields (82–93%) within 1.5–3 h. Recently Yadav et al.222 have introduced a common organic acid with mild acidity (sulfamic acid , NH2SO3H) (SA) as a recyclable solid catalyst for the three component synthesis of homoallylic amines. When the reaction was carried out with aldehydes and anilines with different substituents a room temperature and in absence of solvent, the corresponding homoallylic amines (82–90% yield) were obtained. HClO4 supported on silica gel (HClO4–SiO2)223 with low loading (0.01 mmol) is also an efficient catalyst for the synthesis of homoallylic amines through a 3CR of various aldehydes, aniline derivatives and allyltributylstannane in acetonitrile at room temperature (yields 82–90%).
Yin et al.224 have synthesised polystyrene-bound super Brønsted acids and their ytterbium salts for the synthesis of homoallylic amines. The polystyrene-bound perfluoroalkyl sulfonic ytterbium (Yb–PS2–RF6) was the most efficient and recyclable catalyst in the coupling of aldehydes, anilines and allyltributylstannane achieving 86–95% yield of the corresponding homoallylic amines when benzoic acid is added as a promoter in the reaction media. It has been suggested225 that the Brønsted acidity of benzoic acid acts not only regenerating the catalyst, but also that Brønsted and the Lewis acid sites are working as a combined catalyst to produce a double activation of the substrate. Using Yb–PS2–RF6 and benzoic acid as the co-catalyst, the authors designed a one-pot four-component coupling reaction involving benzaldehyde, aniline, allyltributylstannane and acrylic chloride in order to obtain homoallylic amides. A reaction performed at room temperature in acetonitrile give N-phenyl-N-(1-phenylbut-3-enyl) acryl amide in good yield (78%) (Scheme 36).
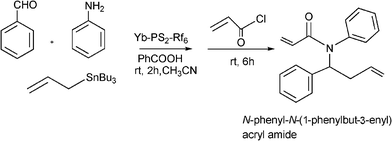 | ||
| Scheme 36 Synthesis of homoallylic acrylic amide by a one-pot four component coupling reaction. | ||
To examine the scope of the A4 reaction a number of acid chlorides were reacted under the above conditons achieving different homoallylic amides (benzamidescarboxamides, acryl amides, acetamides, and cinnamamides) in good to moderate yields (32–88%). Zhengfeng et al.226 have proposed the synthesis of homoallylic amines from aromatic aldehydes, aromatic amines and allyltributylstannane in the presence of phosphomolybdic acid (PMA). A variety of different homoallylic amines were obtained in good to excellent yields (83–99%) at room temperature using 10 mol% of PMA and water as a solvent.
Table 14 summarizes the results and experimental conditions for the synthesis of N-(1-phenyl-3-butenyl)aniline from benzaldehyde, aniline and allyltributylstannane using different acid catalysts.
| ||||||
|---|---|---|---|---|---|---|
| Catalyst | Yield (%) | t(h) | B:A:AS | Catalyst amount (g) | Solvent | Ref. |
| a B:A:AS mmol of benzaldehyde (B), aniline (A) and allyltributylstannane (AS).b mmol.c 10 mmol benzoic acid, 0.1 mol% of catalyst.d 2 mL. | ||||||
| [bmim]BF4 | 92 | 4.5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 | 2d | — | |
| Mont KSF | 90 | 4 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.1 5.1 | 1.00 | CH3CN | 220 |
| NaHSO4 SiO2 | 90 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 | 0.20 | CH3CN | 221 |
| NH2SO3H | 90 | 1 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | 5,00b | — | 222 |
| HClO4–SiO2 | 89 | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4 | 0.01b | CH3CN | 223 |
| Yb-PS2-Rf6 | 95 | 5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 3.3 | 0.1c | CH3CN | 224 |
| PMA | 97 | 24 | 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 | 0.04 | H2O | 226 |
3.12 Synthesis of amidoalkyl naphthol derivatives
Compounds bearing 1,3-amino-oxygenated functional motifs are common in a variety of natural products and drugs including nucleoside, antibiotics and HIV protease inhibitors (such as ritonavir and lipinavir).2271-Amidomethyl-2-naphthol is an important precursor of biological active 1-aminomethyl-2-naphthol derivatives. These compounds present hypotensive and bradycardiac effects.227 Also it is noteworthy that aminotetralin derivatives presents several biological activities such as antidepressant, immunomodulator, and antitumor.227Generally, 1-amidoalkyl-2-naphthol derivatives can be prepared through MCR (via a Ritter type reaction) of aryl aldehydes, 2-naphthol and acetonitrile or amides in the presence of Lewis or Brønsted acid catalysts (Scheme 37).
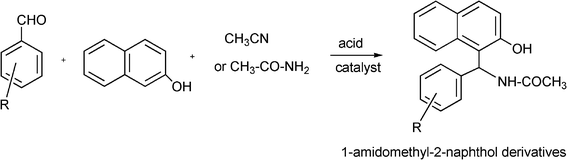 | ||
| Scheme 37 MC synthesis of 1-Amidomethyl-2-naphthol derivatives. | ||
The main preparation methods involve the use of acetonitrile as a reactant and solvent (Method A), or acetamide under thermal (or microwave irradiation) and solvent free conditions (Method B). The reaction involves first the alkylation of 2-naphthol with benzaldehyde in the presence of an acid catalyst to give ortho-quinone methydes (o-QMs, I). Intermediate I reacts with acetonitrile (Method A) to obtain the intermediate II through a Ritter type reaction that after hydrolysis gives the desired product. Following method B the o-QMs generated in situ react with acetamide, which acts as a nucleophile, viaconjugate addition to form 1-amidoalkyl-2-naphthol derivatives (Scheme 38).
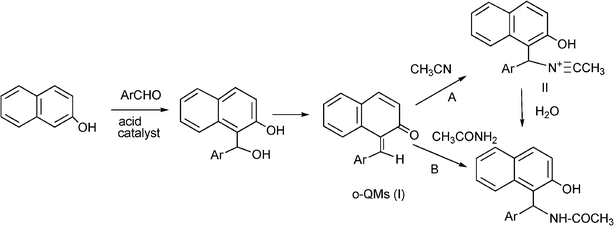 | ||
| Scheme 38 Reaction phatways in the formation of 1-Amidomethyl-2-naphthol derivatives. | ||
A variety of homogeneous (such as iodine,228Ce(SO4)2,229p-TSA230) and heterogeneous catalysts have been reported in the literature231–237 to perform this MCR. Thus, Montmorillonite K-10 clay, Amberlyst-15, K5CoW12O40·3H2O, H3PW12O40, FeCl3–SiO2, Al2O3–SO3H, HClO4–SiO2, Al2O3–HClO4catalysts have been used with different success for the preparation of 1-amidoalkyl-2-naphthol derivatives. It was found that aromatic aldehydes with electron-withdrawing groups reacted faster than those bearing electron donating groups. Table 15 summarizes the yields and experimental conditions for the formation of N-[phenyl-(2-hydroxynaphthalen-1-yl)-methyl]-acetamide using different acid catalysts reported in the literature.
| |||||
|---|---|---|---|---|---|
| Catalyst | Catalyst amount mol% or (g) | T/°C | Time (h) | Yield (%) | Ref. |
| a Benzaldehyde: 2-Naphthol molar ratio= 1, Method A: acetonitrile (Ritter type reaction) Method B: acetamide (thermal and solvent free conditions). Method C: acetamide (microwave and solvent free conditions).b Tetraethyl ammonium chloride (1 mmol) was also added. | |||||
| Iodine | 5 | 125, Meth B | 5.5 | 86 | 228 |
| Ce(SO4)2 | 100 | Reflux | 36 | 72 | 229 |
| p-TSA | 10 | 125, Meth B | 0.6 | 89 | 230 |
| Montm K-10 | (0.1) | 125, Meth B | 1.5 | 89 | 233 |
| Amberlyst-15 | (0.25) | 110, Meth B | 0.2 | 86 | 235 |
| K5CoW12O40·3H2O | 1 | 125, Meth B | 2 | 90 | 234 |
| H3PW12O40 | 2 | 100, Meth Bb | 1.4 | 90 | 232 |
| FeCl3-SiO2 | (0.025) | reflux, Meth A | 20 | 80 | 236 |
| FeCl3-SiO2 | (0.025) | 125, Meth B | 0.18 | 86 | 236 |
| Al2O3–SO3H | 20 | reflux, Meth A | 20 | 85 | 231 |
| Al2O3–SO3H | 20 | 125, Meth B | 0.06 | 83 | 231 |
| Al2O3–SO3H | 20 | MW, Meth C | 0.06 | 87 | 231 |
| HClO4–SiO2 | 0.6 | reflux, Meth A | 20 | 74 | 238 |
| HClO4–SiO2 | 0.6 | 110, Meth B | 0.66 | 89 | 238 |
| HClO4–SiO2 | 0.6 | MW, Meth C | 0.25 | 86 | 238 |
| Al2O3–HClO4 | 5 | 125, Meth B | 0.5 | 90 | 237 |
Recently Shaterian et al.239 have introduced the synthesis of 1-carbamate-alkyl-2-naphthol in the presence of silica-supported sodiumhydrogen sulphate (SiO2–NaHSO4) as a catalyst. The benefit of using carbamates instead of amides is that the carbamates can be deprotected more easily than amides for the preparation of 1-aminomethyl-2-naphthol derivatives (Scheme 39). The three component condensation reaction between aldehydes, 2-naphthol and carbamates in the presence of SiO2–NaHSO4 was carried out under thermal and solvent free conditions (100 °C). A wide variety of substituted 1-carbamato-alkyl-2-naphthol using various aryl aldehydes, 2-naphthol, and methyl/benzyl carbamates were obtained in good to moderate yields (60–92%) within 20–30 min.
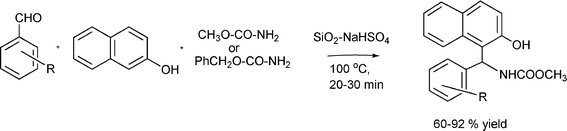 | ||
| Scheme 39 Synthesis of 1-carbamato-alkyl-2-naphthol derivatives. | ||
Das et al.240 have found that perchloric acid supported on silica (HClO4–SiO2) is an efficient catalyst for the synthesis of N-[(2-hydroxynaphthalen-1-yl)methyl]amides through the condensation of 2-naphthol, aromatic aldehydes and urea (or an amide) (Scheme 40). The reaction was performed by heating the corresponding mixture at 125 °C during 3–7 h giving the corresponding target products in good yields (71–93%). The reaction proceeded similarly with amides such as acetamide, benzamide, and acrylamide. In this case, the corresponding N-[(2-hydroxynaphthalen-1-yl)methyl]amides were obtained in yields of 68–82% in 5.5–9 h. Interestingly, when the reaction was carried out using aliphatic aldehydes the selectivity of the target compound was very low.
![Synthesis of N-[(2-hydroxynaphthalen-1- yl)methyl]amides derivatives.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s40.gif) | ||
| Scheme 40 Synthesis of N-[(2-hydroxynaphthalen-1- yl)methyl]amides derivatives. | ||
3.13 Synthesis of dihydropyridine derivatives
Dihydropyridines (DHPs) are an important class of compounds which cover a variety of pharmaceutical and agrochemical activities such as insecticidal, herbicidal and acaricidal.241 Some of them have used as cardiovascular agents for the treatment of hypertension and angina pectoris242 (nifedipine, nicardipine, and amlodipine) (Scheme 41), platelet antiaggregatory bactericidal agents, and bronchodilators.243 In addition, they have been used as cerebral antischemic agents in the treatment of Alzeimer's disease244 and also as a chemosensitizer in tumour therapy.241 In addition, DHP presents applications in stereospecific hydrogen transfer reactions.245 | ||
| Scheme 41 1,4-Dihydropyridines of pharmaceutical interest. | ||
The classical method to obtain DHPs is the MC Hantzsch reaction involving the condensation of and aldehyde, a β-ketoester and ammonia either in acetic acid or by refluxing in alcohol for long reaction times15 (Scheme 42).
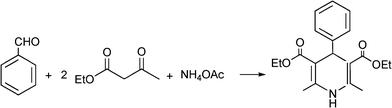 | ||
| Scheme 42 Synthesis of DHPs through the MC Hantzsch reaction. | ||
Numerous synthetic methods have been reported for the preparation of 1,4-dihydropyridine derivatives246 under classical or modified conditions. For instance using microwave irradiation in the absence of a catalyst,247,248 ionic liquids249 or metal triflates as acid catalyst.250 However, some of them suffer from drawbacks such as long reaction times, and low yields particularly when unsaturated and aliphatic aldehydes are involved.
Recently, heterogeneous acid and acid–base catalysts have been used for the preparation of DHPs. Thus, Gupta et al.251 have reported that sulfonic acid covalently anchored onto the surface of silica gel (SiO2–SO3H) is an efficient and recyclable catalyst to synthesize 1,4-dihydropyridines (1,4-DHPs). Various aldehydes (aromatic, heterocyclic and unsaturated) and β-ketoesters (ethyl and methyl acetoacetate) in the presence of ammonium acetate at 60 °C under solvent free conditions afforded the corresponding 1,4-DHPs in good yield (83–90%). For comparative purposes different supported sulfonic acidcatalysts such as polystyrene and polyethylene glycol supported sulfonic acid, were also used in the coupling of 4-methoxybenzaldehyde, ethyl acetoacetate and ammonium acetate for the synthesis of diethyl 4-(4-methoxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (see Table 16). In Table 16 it is shown that SiO2-SO3H was the most active catalyst followed by polystyrene–SO3H and PEG–SO3H. In addition it was found that SiO2–SO3H is a stable catalyst, and no significant change in the activity was found after eight consecutive runs.
| ||
|---|---|---|
| Catalyst | Time (h) | Yield (%) |
| a Reaction conditions: 4-methoxybenzaldehyde (1 mmol), ethyl acetoacetate (2 mmol), ammonium acetate (1.5 mmol) catalyst (0.2 g), at 60 °C. | ||
| SiO2–SO3H | 5.00 | 95 |
| Polystyrene–SO3H | 6.50 | 82 |
| PEG-SO3H | 6.25 | 85 |
The mechanism proposed by the authors for the acid catalyzed synthesis of 1,4-DHPs is presented in Scheme 43. The first step is the formation of the Knoevenagel adduct from one equivalent of ethyl acetoacetate and benzaldehyde (intermediate A). On the other hand, a second equivalent of ethyl acetoacetate reacts with the ammonia generated by the ammonium acetate. The N-addition of ammonia to a protonated carbonyl group which suffers dehydration gives ethyl-3-aminobut-2-enoate (intermediate B). Subsequent cyclocondensation of both intermediates and dehydration give the DHP.
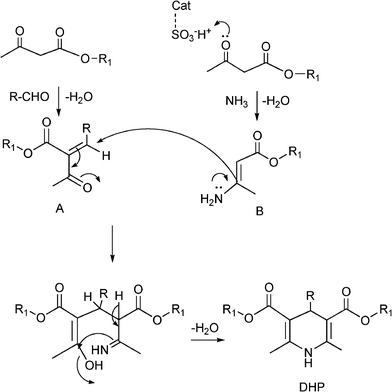 | ||
| Scheme 43 Proposed mechanism for the formation of DHP in the presence of acid catalyst. | ||
Recently Nikpassan et al.252 have developed the synthesis of fused 1,4-DHPs starting from dimedone (5,5-dimethyl-1,3-cyclohexadienone), different aldehydes and ammonium acetate in the presence of HYzeolite. The reactions were carried out at reflux temperature of ethanol giving the corresponding 1,4-DHPs in good yields (70–90%) and in short reaction times (2.5–3.5 h) (Scheme 44). The catalyst was recovered and its activity was maintained after three consecutive runs.
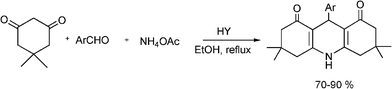 | ||
| Scheme 44 Synthesis of fused 1,4-DHP. | ||
N-Aryl-1,4-dihydropyridines and other related analogues are valuable compounds since they have applications as pharmaceuticals and agrochemicals. However, it is known that the classical Hantzsch reaction is not a suitable method for the preparation of N-aryl-1,4-dihydropyridines. For that reason, a complementary route to the Hantzsch synthesis has been developed to obtain N-aryl-1,4-DHP. This involves the coupling of aromatic amines, α,β-unsaturated aldehydes and ketoesters. Using sulfonic functionalized silica (SiO2–SO3H) 253 as a recyclable heterogeneous acid catalyst (Scheme 45) a series of N-aryl-1,4-DHPs were successfully obtained (80–89% yields) starting from cinnamaldehyde, different aromatic amines and methyl or ethyl acetoacetate, at room temperature within a short time (5–30 min). However, low selectivity was obtained using aliphatic amines and cinnamaldehyde derivatives containing nitro groups in the aromatic ring.
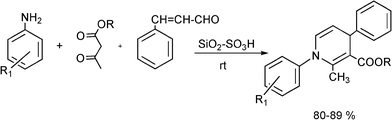 | ||
| Scheme 45 Synthesis of N-aryl-1,4-dihydropyridines. | ||
Polyhydroquinoline derivatives, compounds containing a 1,4-DHP moiety, are a source of valuable drugs, which have been prepared efficiently through an A4 Hantzsch type coupling condensation involving 1,3-cyclohexanediones (5,5-dimethyl-1,3-cyclohexadione or dimedone), ethyl acetoacetate, aldehydes and ammonium acetate in the presence of acid catalysts (Scheme 46).
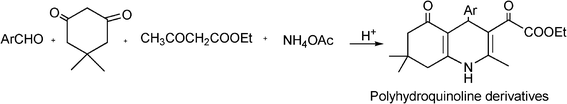 | ||
| Scheme 46 Synthesis of polyhydroquinoline derivatives through an A4 coupling Hantzsch condensation. | ||
Various methods for the preparation have been reported using conventional heating, microwave and ultrasound irradiation in the presence of a wide variety of homogeneous and heterogeneous catalysts. They include trimethylsilyl chloride (TMSCl),254iron(III) trifluoroacetate,255 metal triflates,250PTSA acid,256 Brønsted ionic liquid,249glycine,257silica supported perchloric acid (HClO4–SiO2),258 Montmorillonite K10,259heteropolyacid (K7[PW11CoO40]),260HYzeolite261 and nickelnanoparticles.262 Using the Hantzsch protocol and catalysts referenced above, a series of polyhydroquinoline derivatives have been prepared in good yields. For comparison purposes Table 17 summarizes some results of the synthesis of ethyl 2,7,7-trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-ethylcarboxylate from the 4CR of benzaldehyde, dimedone, ethyl acetoacetate and ammonium acetate using different heterogeneous catalysts reported in the literature.
| ||||||
|---|---|---|---|---|---|---|
| Catalyst | Catalyst amount mol% (g) | Solvent/T/°C | Time (min) | Yield (%) | B:D:E:Aa (mmol) | Ref. |
| a B:D:E:A: Molar ratio Benzaldehyde:Dimedone:Ethyl acetoacetate:Ammonium acetate. | ||||||
| Montmorillonite | (0.2) | CH3CH2OH/80 | 50 | 98 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 | 259 |
| HY | (0.1) | CH3CN/rt | 120 | 93 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | 261 |
| K7[PW11CoO40] | 1 | CH3CN/reflux | 30 | 85 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 260 |
| Glycine | 10 | MW | 1 | 95 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 | 263 |
| Ni np | 10 | MW | 1 | 95 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 | 262 |
Besides heterogeneous acid catalysts, solid base catalysts have also been used to perform the MC synthesis of 1,4-DHP. Thus, Antonyraj et al.264 have reported the coupling of benzaldehyde, ethyl acetoacetate and ammonium acetate using hydrotalcites (HT) and hydrotalcite-like materials as solid base catalysts. Various Al/Mg hydrotalcites with different Mg/Al ratios were tested in the synthesis of 1,4-DHP (Table 18). The authors found that the activity decreased when increasing the Mg/Al atomic ratio, with MgAl2-HT (with a Mg/Al = 2.1) being the most active catalyst. This catalyst possesses maximum aluminium content even though it possesses a lower Brønsted basicity than the other studied materials. In order to study the importance of the Brønsted basic OHgroups present in HT-like lattice, the MgAl2HT sample was subjected to calcination and the resulting Al/Mg mixed oxide (MgAl2-CHT), which presents basic Lewis sites associated at O2– centres, was tested in the synthesis of 1,4-DHP. A low yield of the 1,4-DHP was obtained in this case, which suggests that the presence OHgroups in the hydrotalcitecatalyst is required for the reaction. It is known that the original lamellar structure of hydrotalcites can be restored by hydration of the calcined mixed oxide (memory effect) while the carbonate anions are exchanged by hydroxyl anions resulting in a material with strong Brønsted basic character.265,266 When the Hantzsch reactions were carried out over a hydrated sample (MgAl4-RHT) and MgAl4-HT, similar results were obtained (Table 18). From these results, the authors conclude that the high activity of MgAl2-HT is due to the appropriate cooperative behaviour of acid–base sites existing in this catalyst. With this optimized catalyst, different aliphatic, cyclic and aromatic aldehydes were reacted affording the corresponding 1,4-DHP in moderate to good yields (57–75%). When different nitrogen sources such as liquid ammonia, ammonium carbonate and ammonium acetate were used to synthesize 1,4-DHP, the maximum yield was obtained using ammonium acetate followed of ammonia and ammonium carbonate.
| ||||
|---|---|---|---|---|
| Catalyst | M(II)/Al | Surface area (m2 g−1) | Yield (%)b | Yield (%)c |
| a Experimental conditions: benzaldehyde (0.0039 M), ethyl acetoacetate (0.0078 M) ammonium acetate (0.0039 M), room temperature.b 25 mg of catalyst, time 1 h, 10 mL EtOH.c 50 mg catalyst time 6.5 h, 10 mL MeCN.d Calcined at 450 °C for 5 h.e Nd: Not determined | ||||
| MgAl2-HT | 2.10 | 118 | 45 | 61 |
| MgAl3-HT | 2.87 | 100 | 29 | 35 |
| MgAl4-HT | 4.36 | 92 | 25 | 30 |
| NiAl3-HT | 2.95 | 145 | 20 | 22 |
| CoAl3-HT | 2.53 | 10 | 20 | 35 |
| MgAl2-CHTd | 2.10 | Nde | — | 15 |
| MgAl4-RHT | 4.36 | Nd | — | 32 |
| Blank | — | — | 18 | 9 |
Finally, the synthesis of 1,4-DHP was also carried out using HT with different M(II) metals such as NiAl3-HT and CoAl3-HT, however the catalytic activity was considerably lower than those obtained with MgAl2-HTcatalyst.
The mechanism involving three reactants at different stoichiometry is complex. The first step is the proton abstraction from the active methylene group of ethyl acetoacetate by the base catalyst, followed by N-addition to a protonated carbonyl group which suffers a dehydration giving the ethyl-3-aminobut-2-enoate intermediate (A). Subsequent condensation with another molecule of ethyl acetoacetate results in the imine B (ethyl 3-(4-ethoxy-4-oxobutan-2-ylideneamino)but-2-enoate). A tautomerisation reaction of this imine in the presence of base catalyst will form the enamine (C) which finally condensed with benzaldehyde to give the 1,4-DHP (Scheme 47).
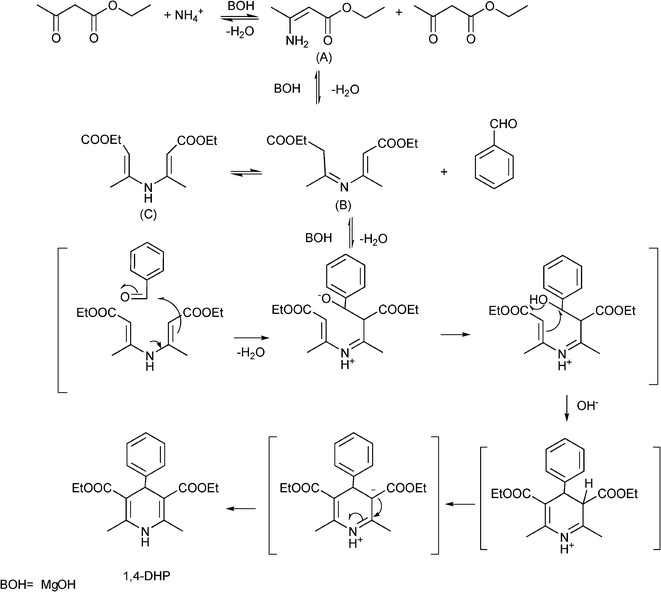 | ||
| Scheme 47 Reaction mechanism catalyzed by bases in the synthesis of 1,4-DHP. | ||
3.14 Synthesis of pyridine derivatives
Pyridines are interesting compounds because their saturated and partially saturated derivatives are present in many biologically active and natural products such as for instance pyridoxol (vitamin B6), NADnucleotide (nicotin adenosin) and pyridine alkaloids.267 The precursor compounds for the synthesis of pyridines are often 1,4-DHP, which can be prepared by the Hantzsch reaction mentioned above. Nowadays the synthesis of highly substituted pyridines by one-pot three component reactions has recently attracted much attention since this methodology allows one to obtain important medicinal pyridine derivatives that can act as ligands for a number of structurally diverse biological receptors. Some examples are presented in Scheme 48.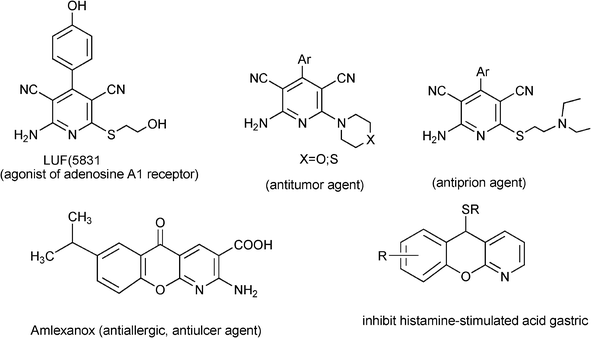 | ||
| Scheme 48 Some examples of pyridine derivatives with pharmacological interest. | ||
The traditional synthesis of pyridines involves the synthesis of 1,4-DHP followed by an additional step consisting in the oxidative aromatization to obtain the corresponding pyridine (Scheme 49). Some years ago Bocker et al.268 demonstrated that the metabolism of 1,4-DHPdrugs involves its catalyzed oxidation in the liver by cytochrome P-450. Due to the biological importance of the oxidation step of 1,4-DHPs, a large number of studies and reagents have been utilized to mimic the “in vivo” transformations. Many efficient oxidative aromatization processes using conventional oxidants, such as potassium permanganate,269 ceric ammonic nitrate,270ruthenium trichloride,271 as well as supported oxidants such as clayfen (iron(III) nitrate on clay),272 or Mn(pbdo)2Cl2/MCM-41 in acetic acid273 and a variety of other catalyst and reagents have been reported. However the use of liquid acids in the cyclization step along with the stoichometric amount of oxidant agent required for the oxidative aromatization produce significant drawbacks. In this section we will show different 3CRs (based mainly on Hantzsch type reaction) leading to a variety of pyridine derivatives.
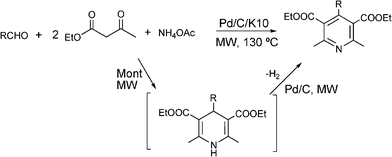 | ||
| Scheme 49 Pyridine derivative synthesis. | ||
The authors claimed that the presence of an acidic surface favors the dehydrogenation due to the anchoring of the intermediate (1,4-DHP) via its basic nitrogen. When the reaction was performed under conventional heating, a high yield was also achieved, however a longer reaction time was required (Table 19). Comparatively, triflic acid and acetic acid in the presence of Pd/C give lower yields than those obtained with Montmorillonite (Table 19). The scope of the reaction was shown by reacting a variety of aliphatic and aromatic aldehydes with ethyl acetoacetate and ammonium acetate affording the corresponding pyridines in good to moderate yields (45–95%) at 130 °C in short reaction time.
| ||||
|---|---|---|---|---|
| Catalysts | Method | T/°C | Time(h) | Yield (%) |
| a Reaction conditions: benzaldehyde (1 mmol), methyl acetoacetate (2 mmol) and ammonium acetate (1 mmol). Toluene, pressure tube for conventional heating (CH). | ||||
| Pt/Al2O3/K10 | MW | 130 | 1.5 | 15 |
| Pd/Al2O3/K10 | MW | 130 | 1.5 | 20 |
| Pd/C/K10 | MW | 130 | 1.5 | 78 |
| Pd/C | MW | 130 | 1.0 | 51 |
| K10 | MW | 130 | 1.5 | 0 |
| Pd/C/K10 | CH | 140 | 29 | 88 |
| Pd/C/K10 | CH | 100 | 28 | 11 |
| Pd/C/TfOH | CH | 130 | 14 | 50 |
| Pd/C/HOAc | CH | 110 | 24 | 10 |
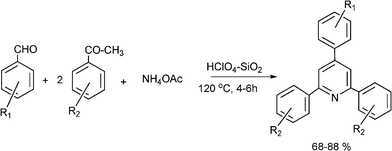 | ||
| Scheme 50 MC synthesis of 2,4,6-triarylpyridine derivatives. | ||
Then, a variety of symmetric 2,4,6-triarylpyridines were synthesized in 68–88% yield, by heating a mixture of aromatic aldehydes, aromatic ketones and ammonium acetate in the presence of HClO4–SiO2catalyst at 120 °C. In addition the catalyst could be recovered after reaction and reused seven times without significant decrease in activity.
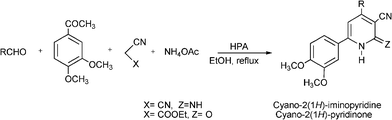 | ||
| Scheme 51 MC synthesis of 3-cyanopyridine derivatives. | ||
Then, condensation of different aldehydes, 3,4-dimethoxyacetophenone, malononitrile and ammonium acetate in ethanol in the presence of H14[NaP5W30O110] at 78 °C gave after 3 h the corresponding cyano-2-(1H)-iminopyridines in good yields (90–93%). When ethyl cyanoacetate was used instead of malononitrile the corresponding cyano-2(1H)-pyridinones were also obtained in excellent yields 91–94%.
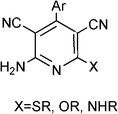 | ||
| Scheme 52 3,5-Dicyanopyridine derivatives. | ||
Due to their medicinal utility, various methods to prepare these compounds have been reported.279 Some of them include Vilsmeier reactions of tertiary alcohols,280 Diels–Alder reactions of 3-siloxy-1-aza-1,3-butadienes and 6-alkyl-3,5-dichloro-2H-1,4-oxazin-2-ones with different types of acetylenic compounds,281 reaction of imines with enamines or carbonyl compounds282 and [4 + 2] cycloadditions of oximinosulfonates.283 However these methods involve multistep sequences, give low yields, use expensive and toxic catalysts and lack generality.
Recently, a new MCR strategy for the preparation of 3,5-dicyanopyridinepyridines has been reported by Evdokimov et al.278,284 that involves the base catalyzed coupling of aldehydes, malononitrile and thiols. Homogeneous bases such as triethylamine or 1,4-diazabicyclo[2,2,2]octane (DABCO)278,284 afford 3,5-dicyanopyridines in low yields (20–48%) due to the formation of appreciable enaminonitrile as a by-product. However, the use of other basic catalysts such as piperidine on microwave irradiation,285 and Lewis acids such as (ZnCl2) have considerably improved the yields of 3,5-dicyanopyridines.286 Also, a basic ionic liquid such as 1-methyl-3-butylimidazolium hydroxide ([bmim]OH)287 has been described as recyclable catalyst to produce highly substituted pyridines in high yields (65–95%) a room temperature. As an alternative to homogeneous base or acid catalysts, nanoparticles have attracted much attention in catalysis because their improved efficiency (high surface area) under mild an environmentally benign conditions. Recently, silicananoparticles (silica NP) have been used as catalysts for the preparation of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines, via a single step multicomponent reaction of aldehydes, malononitrile and thiols288 (Scheme 53).
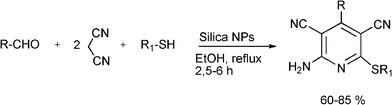 | ||
| Scheme 53 One-pot three components synthesis of substituted 2-amino-3,5-dicyanopyridines. | ||
A series of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine derivatives was prepared in good yields (60–85%) starting from different aliphatic and aromatic aldehydes and thiophenol or alkyl thiols. The significant improvement in the yield of pyridines derivatives obtained using the silica NP compared to other catalysts such as ZnCl2 (yield 45–67) Et3N or DABCO (20–48%), or piperidine under microwave irradiation (60–81%) was attributed to the presence of hydroxylgroups on the surface of the catalyst. In accordance with the mechanism proposed by Evdokimov278 the reaction starts by the formation of the Knoevenagel adduct (A) from the aldehyde and malononitrile. Subsequently a second molecule of malononitrile reacts with a Knoevenagel adduct through base catalyzed Michael addition followed by simultaneous thiolate addition to the nitrilegroup. Then, the cyclization process gives the dihydropyridine intermediate (B) after which posterior oxidation in the presence of air leads to the pyridine (Scheme 54).
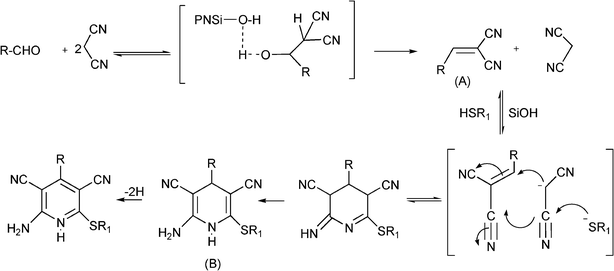 | ||
| Scheme 54 Possible mechanism for the formation of substituted 3,5-dicyanopyridines. | ||
The authors suggest that the polar amphoteric surface hydroxylgroups of the silicananoparticles facilitate the interaction of adsorbed weak acidic and basic components due to stabilization of the corresponding transition states and intermediates by hydrogen boding. In addition, the participation of two proximate silanolsgroups (one acting as a hydrogen bond donor and another as an acceptor) in the reaction mechanism is also speculated.
Singh et al.263 have recently reported the synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines (Scheme 53) using FK-alumina as the catalyst under microwave irradiation and conventional heating. Condensation of different aromatic aldehydes, malononitrile and thiophenols under microwave conditions give 62–93% yield of the corresponding pyridines in 5–10 min, whereas when refluxing in ethanol the reaction afforded the corresponding pyridines in 56–82% yield within 30–70 min.
Very recently Kantam et al.289 have reported the one-pot three component synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines from diverse aldehydes with various thiols and malononitrile in the presence of nanocrystalline magnesium oxide (NAP-MgO, with a surface area of 590 m2 g−1). Moderate to good yields (41–69%) of the pyridine derivative were obtained when the reaction was carried out under ethanol reflux. A possible rational clarification for the higher activity of NAP–MgO compared with other MgO samples [CM–MgO (30 m2 g−1), NA–MgO (250 m2 g−1)] is the presence of more surface Lewis acid sites (20%) along with the OHgroups present on the edge and corner sites on the NAP–MgO. The authors suggest a dual activation of the substrate by the catalyst, thus Lewis base sites (O−2/O−) activate the malononitrile while Lewis acid sites (Mg+2/Mg+) activate the aldehyde and thiol. The catalyst can be recovered and reused at least up to four cycles without appreciable lost of activity.
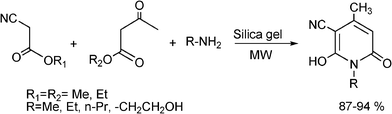 | ||
| Scheme 55 One-pot three component synthesis of 3-cyano-6-hydroxy-2(1H)-pyridinone derivatives. | ||
3.15 Synthesis of β-acetamido ketone derivatives
β-Acetamidoketones are considered versatile intermediates since their basic skeleton exists in a number of pharmacologically or biologically active compounds.293–294 Moreover they are important synthons for a variety of speciality chemicals295 and pharmaceuticals such as nikkomycine and neopolyxine antibiotics.296 The main route for the synthesis of these compounds is the Dakin–West reaction297 which involves the condensation of an α-aminoacid with acetic anhydride in the presence of a base via an intermediate azalactone. Recently Bathia et al.298 have proposed another general route for the synthesis of β-acetamidoketones that involves the condensation of an aryl aldehyde, an enolizable ketone or ketoester, acetyl chloride and acetonitrile in the presence of Lewis acid catalysts such as CoCl2 (Scheme 56).The same author performed this MCR using Montmorillonite K10 as the acid catalyst.299,300 The reactions were carried out at 70 °C using acetonitrile as reactant and as a solvent. Particularly for the coupling of substituted benzaldehydes, acetophenone, acetyl chloride and acetonitrile yields between 64–88% were achieved.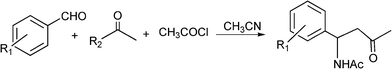 | ||
| Scheme 56 MC synthesis of β-acetamido ketone derivatives. | ||
When α-substituted ketones were used, for instance, ethyl methyl ketone or propiophenone, a diastereomeric mixture of the β-acetamidoketones were obtained, with the antidiasteroisomer being the most abundant (Scheme 57). In addition, the catalyst could be reused without considerable variation in yield and stereoselectivity.
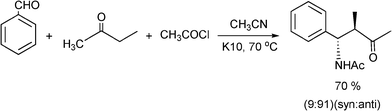 | ||
| Scheme 57 MC synthesis of β-acetamido ketone using α-substituted ketones. | ||
Besides Montmorillonite K10, a variety of solid acid catalysts promoting this MCR have been reported. For instance, HBeta zeolite has been used as an active and reusable catalyst to perform this reaction at room temperature.301 Using different substituted benzaldehydes and enolizable ketones 63–90% yields of the corresponding β-acetamidoketones can be achieved in 8–12 h. Also heteropolyacids,302–304 acid resins,305,306 sulfated zirconia,307sulfuric acid supported on silica308 or phosphomolybdic acid supported on silica (PMA/SiO2)309 have been used to perform this MCR using a wide variety of aromatic aldehydes and ketones or ketoesters and giving in general excellent yields of the desired product. As an example, a summary of the different catalysts ad their activity performing the coupling of benzaldehyde, acetophenone, acetyl chloride and acetonitrile is presented in Table 20.
| ||||
|---|---|---|---|---|
| Catalyst (amount) | T (°C) | Time (h) | Yield (%)a | Ref. |
| a Reactions are performed using 1 mmol benzaldehyde and 1 mmol acetophenone.b No data about the amount of catalyst are reported. | ||||
| K5CoW12O40·3H2O (0.01% mol) | rt | 1 | 86 | 304 |
| H6P2W18O62 (0.7% mol) | 80 | 0.4 | 86 | 303 |
| H3PW12O40 (0.05% mol) | rt | 0.8 | 95 | 302 |
| H3PMo12O40 (0.08% mol) | rt | 0.5 | 90 | 302 |
| H3SiW12O40 (0.08% mol) | rt | 0.5 | 92 | 302 |
| Amberlyst-15 (200 mg) | rt | 6 | 89 | 306 |
| HBeta | rt | 8 | 89 | 301 |
| Nafion (500 mg) | rt | 4 | 96 | 305 |
| Sulfated zirconiab | rt | 1–3 | 95 | 307 |
| Silica sulfuric acid (300 mg, 0.78 mmol H+) | 80 | 1–2 | 91 | 308 |
| PMA/SiO2(0.005 mol based on PMA) | rt | 6 | 94 | 309 |
A plausible mechanism for this transformation is presented in Scheme 58. The acid catalyst activates the aldehyde which reacts with acetyl chloride and acetonitrile giving an intermediate (A) which subsequently reacts with the enolisable ketone giving the intermediate (B) which hydrolysis gives the β-acetamido ketone.
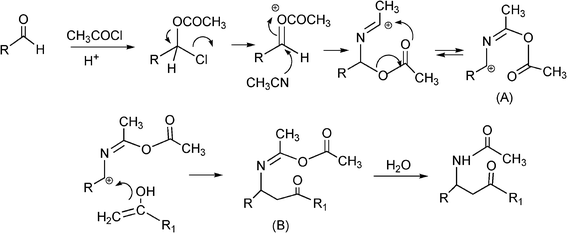 | ||
| Scheme 58 Mechanism in the one-pot formation of β-acetamidoketones. | ||
3.16 Synthesis of imidazo[1,2-a]pyridine derivatives
Imidazo[1,2-a]pyridines (Impy) is an important pharmacophore and is widely found in many natural and synthetic biologically active compounds310 Moreover, they exhibit antiviral (antivaricella-zoster and anticytomegalo-zoster virus) anti-inflammatory, antipyretic, antifungal activities and calcium channel blockers.311–313 They have also been found to be α-amyloid formation inhibitors, GABA and benzodiazepinesreceptoragonists.314,315Drug formulations containing imidazo[1,2-a]pyridines currently available on the market include Zolimidine (used for peptic ulcer and gastroesophageal disease), Zolpidem (hypnotic drug), and Alpidem (anxyolytic) (Scheme 59).316 | ||
| Scheme 59 Imidazopyridines of pharmacological activity. | ||
The classical route for the synthesis of Impy involves the coupling of 2-aminopyridines with lachrymatory α-haloketones.317 However this approach does not readily lend itself to diversity oriented synthesis. In 1998 three research groups simultaneously published a new version of the Ugi reaction in which 2-aminopyridine, aldehydes and isocyanides react in the presence of an acid catalyst to give imidazo[1,2-a]pyridines in one step (Scheme 60).318–320
![MC synthesis of imidazo[1,2-a]pyridines by Ugi reaction.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s60.gif) | ||
| Scheme 60 MC synthesis of imidazo[1,2-a]pyridines by Ugi reaction. | ||
Reactions were performed at room temperature by combining all three reagents in methanolic solution in the presence of homogeneous acids such as Sc(OTf)3,319perchloric acid318 or glacial acetic acid.320
This robust approach allows for the preparation of a diverse range of substituted imidazo[1,2-a] annulated nitrogen heterocycles. However this synthesis suffers from several drawbacks such as the acid catalyzed polymerization of isocyanides and the competitive Passerine reaction, leading to moderate yields of the target compound, with relatively long reaction times (Scheme 61). In order to overcome those drawbacks, a variety of catalytic systems have been reported to perform this multi-component reaction under classical conditions and under microwave irradiation. For instance, Lewis acids such as ZnCl2,321ammonium chloride,322,323 protic acids324,325 or ionic liquids,326 have been used as catalysts, however in most cases the catalyst is required in stoichiometric amounts in order to achieve a high yield of the target compound. Concerning to the use of heterogeneous catalysts, there are few studies. For instance, a variety of imidazo[1,2-a]pyridines were prepared starting from 2-aminopyridine, aldehydes and isocyanides using Montmorillonite K10 clay as the catalyst in a microwave reactor or by conventional heating at reflux of 1,4-dioxane,321 although moderate yields (61–72%). Improved results were achieved with Montmorillonite K10 in the condensation of aminopyridines, pyrazines and pyrimidines with different aldehydes and isocyanides under microwave irradiation and in absence of any solvent (Scheme 62).327 In this case the corresponding imidazo[1,2-a] annulated nitrogen heterocycles were obtained within 3–5 min in 56–88% yield.
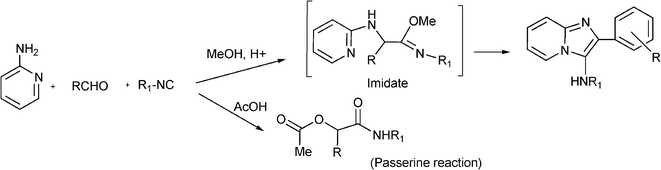 | ||
| Scheme 61 Ugi and Passerine reactions. | ||
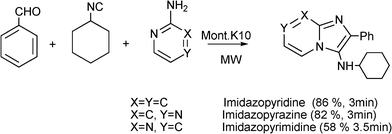 | ||
| Scheme 62 MC synthesis of imidazo-pyridine, -pyrazine and -pyrimidine using Montmorillonite K10 as the catalyst. | ||
The mechanism of formation of these heterocycles can be rationalized by the initial formation of iminium ion from the condensation of the amine with the aldehyde which is followed by the nucleophilic attack of isocyanide. Subsequently, internal nucleophilic attack of ring nitrogen leads to the bicycle adduct which upon aromatization and 1,3-shift of hydrogen atom results in the formation of the imidazole ring (Scheme 63).
![Proposed mechanism for the MC synthesis of imidazo[1,2-a]pyrimidines.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s63.gif) | ||
| Scheme 63 Proposed mechanism for the MC synthesis of imidazo[1,2-a]pyrimidines. | ||
Sulfuric acid supported on silica has also been used recently as a reusable acid catalyst328 to perform the synthesis of 3-aminoimidazo[1,2-a]pyridines and -pyrazines by condensation of an aldehyde, 2-amino-5-substitutedpyridines or 2-aminopyrazine and alkyl or aryl isocyanides. Reactions performed at room temperature in methanol gave good yields (77–99%) of the corresponding 3-aminoimidazo[1,2-a]pyridines and -pyrazines.
3.17 Synthesis of 1,2,4,5-tetrazinan-3-one derivatives
1,2,4,5-Tetrazines are heterocyclic compounds with numerous biological activities such as bronchodilating, bactericidal, antiallergical, antiulcer, antinflammatory, pesticidal and antineoplasic activities.329–331 Moreover, some tetrazoles have recently been introduced for the treatment of type 2 diabetes.332The formation of N–N bonds is not easy and 1,2,4,5-tetrazines have generally been prepared from hydrazine derivatives or from nitrilimines.331 Recently Gopalakrishnan and co-workers333 have reported the synthesis of 6-aryl-1,2,4,5-tetrazin-3-ones or thiones through a MC reaction involving urea, various substituted benzaldehydes, and ammonium acetate in the presence of NaHSO4 supported on silica gel (NaHSO4–SiO2) as an acid catalyst (Scheme 64). Reactions performed under microwave irradiation afforded 6-aryl-1,2,4,5-tetrazin-3-ones in 68–75% yield within 2 or 3 min, while under thermal conditions (heating at 75 °C) lower yield was achieved (30–38%) in 35–43 min. The catalyst also resulted active when using thiourea, affording the corresponding 6-aryl-1,2,4,5-tetrazin-3-thiones in similar yields.
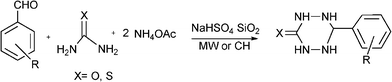 | ||
Scheme 64 MC synthesis of 6-aryl-1,2,4,5-tetrazinane-3-one (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 6-aryl-1,2,4,5-tetrazinane-3-thione (X O) and 6-aryl-1,2,4,5-tetrazinane-3-thione (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) derivatives. S) derivatives. | ||
The proposed mechanism involves the nucleophilic addition of the aminoalcohol adduct (formed by reaction of benzaldehyde with ammonia) to the urea, followed by cyclization and dehydrogenation to the target compound (Scheme 65).
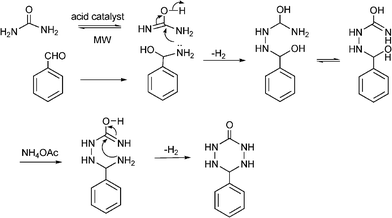 | ||
| Scheme 65 Proposed mechanism for the formation of 1,2,4,5-tetrazinane-3-ones. | ||
3.18 Synthesis of α-aminophosphonates
α-Aminophosphonates are an important class of biologically active compounds. Some of them act as peptide mimics,334antibiotics, enzyme inhibitors,335haptens of catalyticantibodies, pharmacological agents (antithrombotic, antibacterial, antiHIV, anticancer)336 and plant-growth regulators.337 Recently the syntheses of this type of organophosphorous compounds have attracted a lot of interest due to structural analogy to the corresponding α-amino acids and besides, they are key substrates in the synthesis of phosphonopeptides.Several methods to obtain α-aminophosphonates have been developed in the last decades. One conventional method is the nucleophilic addition of dialkylphosphites to imines in the presence of base or Lewis acid catalysts338 (Pudovik reaction).
However, recently the most common method to obtain α-aminophosphonates involves a three component coupling reaction of an aldehyde, an amine, and a di- or trialkyl phosphite in the presence of acid or base catalysts (Kabachnik–Fields reaction)339 (Scheme 66). A variety of homogeneous Lewis acids catalysts such as metal triflates,340 ZrOCl2·8H2O,341SbCl3/Al2O3,342indium in aqueous HCl,343 Brønsted acids as such as p-toluenesulfonic acid, CF3CO2H, and ionic liquids,344 as well as microwave irradiation itself345 and ultrasonic irradiation itself or in the presence of AlCl3346 techniques have been used to promote this reaction.
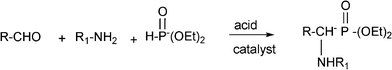 | ||
| Scheme 66 Synthesis of α-aminophosphonates. | ||
Following this approach, recently, different heterogeneous catalysts have been used as a green alternative for the synthesis of α-aminophosphonates by coupling carbonyl compounds, amines and diethyl phosphate.
Thus, silica supported sulfuric acid (SSA), sodiumhydrogen sulphate supported on silica gel and sulfamic acid (SA) have been selected to prepare α-aminophosphonates under solvent free conditions and at room temperature.347 The results demonstrate the superiority of sulfamic acid compared with other catalysts. Complete conversions and good yields (76–94%) were obtained in the reaction between aldehydes (aromatic and heteroaromatic) and a range of amines (aliphatic, aromatic and cycloalkyl) and dimethyl phosphonate in a short reaction time (1–4 h). It is noteworthy to mention, that when the reaction was carried out using diethyl amine, different benzaldehydes (4-chlorobenzaldehyde, 4-methylbenzaldehyde and 4-isopropilbenzaldehyde) and diethyl phosphite the condensation failed to form the corresponding α-aminophosphonate. In these cases, only the α-hydroxyphosphonates were obtained in excellent yields after 15 min, as a result of the competing nucleophilic addition of diethyl phosphite to the carbonyl compound.
The mechanism proposed for the synthesis of α-aminophosphonate involves the formation of an imine promoted by the acid catalyst which is subsequently converted to an iminium ion, a more electrophilic intermediate, to facilitate the attack of dialkyl or diaryl phosphite nucleophile (Scheme 67). The authors explain that in the case of diethyl amine, the imine intermediate either being unstable or its formation being difficult, the base catalyzes the addition of diethyl phosphite to aldehyde that results in the formation of α-hydroxyphosphonate.
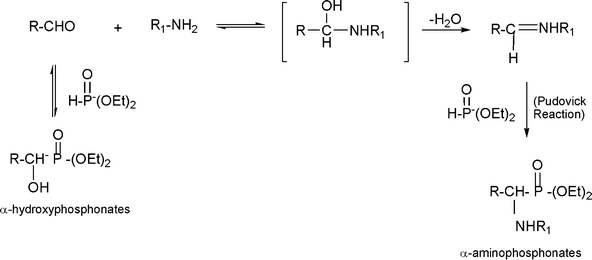 | ||
| Scheme 67 Proposed mechanism for the preparation of aminophosphonates. | ||
Acidic alumina has also been used as an acid catalyst in the synthesis of α-aminophosphonates under microwave irradiation.348 Different aldehydes, amines and diethyl phosphite reacted on alumina under solvent free conditions using microwave irradiation producing high yields of the corresponding α-aminophosphonate (70–95%) in 6 min. No α-hydroxyphosphonate was detected in the reaction mixture. The MCR was also carried out in acidic alumina supported ammonium formate at room temperature yielding the desired compounds in good yields (59–76%) after 4–8 h. Neutral and basic alumina and magnesium oxide were not as effective as acidic alumina, giving α-hydroxyphosphonate as the major product. Montmorillonite KSF clay has also been used for the synthesis of α-aminophosphonate by three component condensation of aldehydesamines and diethylphosphite under microwave irradiation under solvent free conditions.349 Various carbonyl compounds (aldehydes or ketones) and amines reacted with diethylphosphite to afford the corresponding α-aminophosphonate. In the case of aldehydes, the target compounds were obtained in excellent yields (80–92%) in short reaction times, whereas ketones gave phosphonates in lower yields (65–80%) after longer reaction times (6–8 min). When reactions were carried out by conventional heating in refluxing toluene, longer reaction times (5–10 h) were required to achieve good yields of the corresponding α-aminophosphonate (70–80%). Other solid acid catalysts such as Amberlyst-IR 120, h6p2w18o62, ALkIT-5 (a mesoporous metalosilicate) and SbCl3/Al2O3 also promote this MCR. In Table 21 the results obtained in the coupling reaction of benzaldehyde aniline and diethyl phosphonate using different acid catalysts are compared.
3.19 Tetrahydroisoquinolonic acid derivatives
Tetrahydroisoquinolonic acid derivatives have attracted the attention of synthetic organic chemists due to their potential activity in the field of pharmaceuticals. This family of compounds exhibit a wide spectrum of biological activities including antiinflammatory, antiallergenic, anti-tumor activity, psychotropic and estrogenic behaviour.354,355Tetrahydroisoquinolonic acid derivatives have been reported as starting materials for the synthesis of natural phenanthridinealkaloids such as corolyne derivatives, decumbenine B.356 and indenoisoquinolines357 possessing significant antitumor activity. Tetrahydroisoquinolonic acid derivatives can be synthesised by cycloaddition reaction of homophthalic anhydrides with imines in the presence of conventional Brønsted and Lewis acids (such as acetic acid, HCl, AlCl3, FeCl3) and bases (Et3N, Et2NH).358 When the reaction is performed under classical conditions, the reaction product resulting from this cycloaddition possesses two asymmetric centres and is therefore capable of existing as a mixture of cis- and trans-diastereoisomers, the cis compound being the main product. BF3-Et2O359 and titanium(IV)chloride-N,N-diisopropylethylamine360 have been used for the preparation of trans-isoquinolonic acid, meanwhile trimethyl orthoformate,361 ionic liquids,362rare earth metal triflates (ytterbium(III) triflate),363 and KAl(SO4)2·12.H2O364 have been employed for the synthesis of cis-isomers.Using this approach Azizian et al.365 have reported the synthesis of cis-isoquinolonic acid derivatives by coupling homophthalic anhydride, aldehydes and amines in the presence of KAl(SO4)2·12H2O (Alum) and silica sulphuric acid as heterogeneous catalysts (Scheme 68).
 | ||
| Scheme 68 MC synthesis of tetrahydroisoquinolonic acid derivatives. | ||
When a mixture of equimolar amounts of homophthalic anhydride, benzaldehyde and aniline in acetonitrile is allowed to react in the presence of Alumcatalyst at room temperature, 1-oxo-2,3-diphenyl-1,2,3,4-tetrahydro-isoquinoline-4-carboxylic acid was obtained with yield of 88% after 7 h. The reaction was extended to a range of different aldehydes and amines giving the corresponding cis-isoquinolonic acid in good yields (81–91%). Similar results were obtained using silica sulphuric acid. It is noteworthy that in all cases the reaction is stereoselective in the preparation of cis-isoquinolonic acid derivatives.
Recently, Karimi et al.366 have reported the use of sulfonic acid functionalized silica (SAFS) as a recyclable heterogeneous catalyst for the synthesis of isoquinolonic acids by a three component condensation of homophthalic anhydride, aldehydes and amines. When the reaction was carried out at room temperature in the presence of acetonitrile, good yields of the isoquinolonic acid derivatives were obtained (78–98%) in 1–5 h. The reaction was highly diastereoselective and only the cis diasteromer was obtained in all cases.
The mechanism proposed proceeded via the initial formation of an intermediate from homophthalic anhydride and an amine followed by (path i) initial methylene attack on to the aldehyde followed by Michael reaction or (path ii) nitrogen attack on to the aldehyde followed by methylene attack to give the final product (Scheme 69).
 | ||
| Scheme 69 Plausible reaction mechanism for the formation of cis-isoquinolonic acids. | ||
3.20 Synthesis of 4-amidotetrahydropyran derivatives
The 4-amidotetrahydropyran ring system is a core structure in a variety of natural products, among them ambrucitins VS, glycamino acid, and others.367 The most general method to obtain tetrahydropyran derivatives is via Prins cyclization reaction using acid catalysts.368 The Ritter amidation after Prins cyclisation (Prins–Ritter reaction) is a very useful methodology for natural product synthesis. Recently 4-amidotetrahydropyrans have been prepared by a three component coupling of carbonyl compounds, homoallylic alcohols and nitriles using phosphomolybdic acid (H3PMo12O40, PMA) as catalystvia Prins–Ritter reaction (Scheme 70).369 Various homoallylic alcohols and nitriles reacted at ambient temperature to produce the corresponding 4-acetoamidotetrahydropyrans in high yields (82–92%). In all cases the cis isomer was exclusively obtained.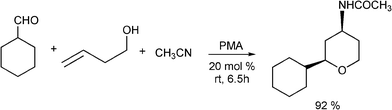 | ||
| Scheme 70 Synthesis of N-(2-cyclohexyltetrahydro-2H-4-pyranyl)-acetamide. | ||
For comparison purposes other solid acid catalysts such as Montmorillonite KSF and Amberlyst-15 were tested, however the PMA catalyst was more efficient in terms of conversion. Spirocyclic-4-amidotetrahydropyrans were also obtained in good yields (84–88%) from cycloketones, homoallylic alcohols and nitriles (Scheme 71).
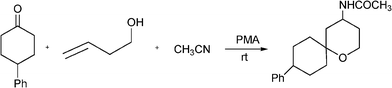 | ||
| Scheme 71 Spirocyclic-4-amidotetrahydropyrans. | ||
The formation of 4-amidotetrahydropyran could be explained by hemiacetal formation followed by Prins cyclization and subsequent Ritter amidation (Scheme 72).
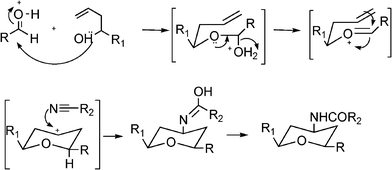 | ||
| Scheme 72 Reaction mechanism for the formation of cis-amidotetrahydropyrans via Prins–Ritter reaction sequence. | ||
3.21 Synthesis of DL-5-(4-hydroxyphenyl)hydantoin
DL-5-(4-Hydroxyphenyl)hydantoin is an important intermediate for the enzymatic production of (R)-2-(4-hydroxyphenyl)glycine, a compound widely used in the preparation of semi-synthetic penicillins and cephalosporines.370Various methods have been described for synthesising DL-5-(4-hydroxyphenyl)hydantoin in homogeneous media. However the most common method is by amidoalkylation of phenol with urea and glyoxylic acid in the presence of a larger excess of concentrated mineral acid (such as 35% hydrochloric acid)371 (Scheme 73). In this reaction a mixture of the DL-5-(4-hydroxyphenyl)hydantoin (para-isomer) and DL-5-(2-hydroxyphenyl)hydantoin (ortho-isomer) is obtained the para-isomer being the major product.
 | ||
| Scheme 73 Synthesis of DL-5-(4-hydroxyphenyl)hydantoin from amidoalkylation of phenol with urea and glyoxylic acid. | ||
The reaction proceeds possibly through the coupling of glyoxylic acid with urea leading allantoin which subsequently reacts with the phenolvia a Friedel–Crafts alkylation in the presence of the acid catalyst giving the ortho and para isomers (Scheme 74).
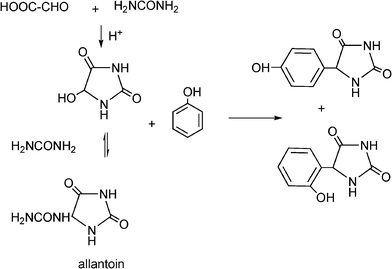 | ||
| Scheme 74 Proposed mechanism in the formation of DL-5-(4-hydroxyphenyl)hydantoin. | ||
Cativiela et al.372 have reported the synthesis of DL-5-(4-hydroxyphenyl)hydantoin following this approach using solid acids catalysts such as clays (KSF and K10 Montmorillonite), beta zeolite, and sulfonic organic polymers. The condensation reaction of phenol, urea and glyoxylic acid performed in water at 70 °C in the presence of clay or beta zeolite afforded the target product in low yields (7.5% and 4.2% respectively, after 21 h) even when they were used as a co-catalyst with hydrochloric acid. The reaction was also tested with Dowex and Duolite, two sulfonic acid resins of different particle size. It was found that Dowex promotes the synthesis of the hydantoin more efficiently than Duolite due probably to the diffusion restrictions imposed by the larger particle size of the later. With Dowex, a yield of 74% of hydantoin was achieved, while with Duolite and Nafion resins the yield was somewhat lower (47%).
3.22 Synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives
Among the large variety of nitrogen-containing heterocyclic compounds, heterocycles containing the phthalazine moiety are of interest because they show important pharmaceutical and biological activities.373 It has been reported that this type of compound possesses anticonvulsant,374 cardiotonic375 and vasorelaxant376 activities. Various methods have been reported for the synthesis of phthalazine derivatives.377 Recently, the multicomponent reaction of dimedone (5,5-dimethylcyclohexane-1,3-dione), phthalhydrazide (2,3-dihydro-1,4-phthalazinedione) and aromatic aldehydes in the presence of p-TSA to give 3,4-dihydro-3,3-dimethyl-13-aryl-2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives has been reported378 (Scheme 75). Good yields (83–93%) were obtained when the reaction was performed at 80 °C under solvent free conditions for several minutes.![Synthesis of 3,4-dihydro-3,3-dimethyl-13-phenyl-2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s75.gif) | ||
| Scheme 75 Synthesis of 3,4-dihydro-3,3-dimethyl-13-phenyl-2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione. | ||
Following this protocol, Shaterian et al.379 have reported the use of silica supported sulfuric acid as an efficient heterogeneous catalyst for the preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives. Good yields of the corresponding products (84–93%) were obtained using different aromatic aldehydes, when the condensation reaction was carried out under solvent free conditions, at 100 °C within short reaction time (7–35 min). The catalyst could be successfully recovered and recycled at least for five runs without significant loss in activity.
A possible mechanism for the formation of 2H-indazolo[2,1-b]phthalazine-triones is presented in Scheme 76 Initially a Knovenagel condensation between dimedone and benzaldehyde occurs to form a heterodyne intermediate (A). Then the subsequent Michael-type addition of the phthalhydrazide to the heterodyne (A) followed by cyclization affords the 2H-indazolo[2,1-b]phthalazine-trione product.
![Possible mechanism for the formation of 2H-indazolo[2,1-b]phthalazine-triones.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s76.gif) | ||
| Scheme 76 Possible mechanism for the formation of 2H-indazolo[2,1-b]phthalazine-triones. | ||
3.23 Synthesis of polyfunctionalized pyran, pyranodipirimidine and chromene derivatives
Compounds bearing 4H-pyran units present important biological and pharmacological activities. Its activity depends mainly on the presence of different heterocyclic ring systems. Among the different pharmacological activities exhibited by these compounds are anticancer, anticoagulants, antianaphylactics, and spasmolytics agents.380,381 They are usually prepared by reaction between arylidenemalononitriles and activated methylene compounds in the presence of organic bases.382 However, 4H-pyrans rings can be also obtained through a A3 coupling reaction of an aldehyde, malononitrile and an active methylenic diketo compound. Initially the reaction proceeds by abstracting a proton from the malononitrile which subsequently reacts with the benzaldehyde forming an arylidenemalononitrile intermediate (Knoevenagel condensation). Subsequently, the arylidenemalononitrile intermediate reacts with methylenic diketo compound giving the 4H-pyran unit (Scheme 81). Depending on the structure of the diketocompound 4H-pyran rings bearing different heterocyclic ring systems can be obtained. In this section some examples involving this MCR performed under heterogeneous catalysis is presented.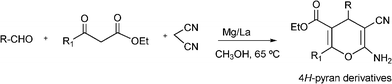 | ||
| Scheme 77 A3 coupling process for the synthesis of 4H-pyran derivatives. | ||
Compared to other solid basic catalysts such as MgO, KF-Alumina, Mg/Al hydrotalcite, and Mg-Al-CO3, the Mg/La mixed oxidecatalyst was the most active promoting the coupling of benzaldehyde, ethyl acetoacetate and malononitrile in high yield (92%) (Table 22). This result was attributed to the presence of La2O3 in proximity to MgO and leads to an increased basicity. The condensation of various aromatic and aliphatic aldehydes, malononitrile and several active methylene diketo-compounds afforded the corresponding 4H -pyran derivatives in variable yields (15–92%). The proposed mechanism is presented in Scheme 78. Recycling experiments showed that the Mg/La mixed oxide catalyzes the reaction with consistent activity even after four cycles.
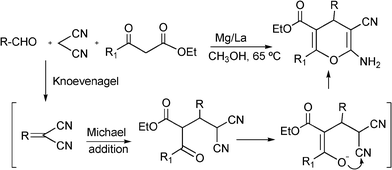 | ||
| Scheme 78 Proposed mechanism for the A3 coupling synthesis of 4H-pyran derivatives. | ||
| ||
|---|---|---|
| Catalyst | Time (h) | Yield (%) |
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol), malononitrile (1.1 mmol), ethyl acetoacetate (1.1 mmol) in MeOH at 65 °C. | ||
| MgO | 5 | 67 |
| KF–Alumina | 5 | 74 |
| Mg/Al hydrotalcite | 3 | 82 |
| Mg–Al–CO3 | 4 | 64 |
| Mg/La mixed oxide | 1 | 92 |
Conventionally the synthesis of 2-amino-3-alkyl-4-aryl-5-oxo-4,5-dihydropyran[3,2-c]chromene involves the MC condensation of 4-hydroxycoumarin, aldehydes and alkylnitriles (Scheme 79) in the presence of organic bases (piperidine or pyridine in organic solvents). However, recently Heravi et al.389 have reported this 3CR using heterogeneous acid catalysts such as H6P2W18O62·18H2O as a Wells–Dawson type heteropolyacidcatalyst.
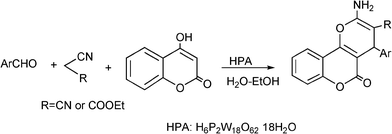 | ||
| Scheme 79 MCR of 4-hydroxycoumarin, aldehydes and alkylnitriles. | ||
A study of the synthesis of 2-amino-4-(4-bromophenyl)-3-cyano-4,5-dihydropyran[3,2-c]chromene-5-one from the condensation of 4-hydroxycoumarin, 4-bromobenzaldehyde and malononitrile in the presence of a variety of solvents and catalysts was performed. As can be seen in Table 23, the best results were obtained with H6P2W18O62·18H2O using a mixture of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (50
water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). The authors suggest that the reaction, which occurs in a pseudoliquid, is accelerated due to the participation of all bulk protons of the heteropolyanion.
50). The authors suggest that the reaction, which occurs in a pseudoliquid, is accelerated due to the participation of all bulk protons of the heteropolyanion.
Coupling different aromatic aldehydes, 4-hydroxycoumarin and malononitrile in the presence of H6P2W18O62·18H2O, a wide variety of substituted 2-aminochromenes were obtained in high yields (80–90%) and selectivity (85–90%) in 35–80 min. When ethyl cyanoacetate was used as the reactant a longer reaction time (16 h) was required in order to obtain the corresponding 2-aminochromene in good yield (90%) and selectivity (90%). The catalyst was recyclable and could be reused without significant loss of activity during five consecutive runs.
Following the same protocol as above, tetrahydro-4H-benzo-[b]-pyran derivatives can be obtained by coupling aromatic aldehydes, malononitrile and cyclic β-diketones such as dimedone. Conventionally this A3 coupling is performed under reflux in acetic acid,390 although other homogeneous catalysts such as diammoniumhydrogen phosphate,391 and ionic liquids392 (1,1,3,3-N,N,N′,N′-tetramethylguanidinium trifluoro acetate) have been reported to perform this MCR with variable success. Recently, Seifi et al.393 presented a highly efficient method for the synthesis of a pyrano annulated heterocyclic system via a three component reaction of an aldehyde, malononitrile and a α-hydroxy or an α-amino activated C–H acid in the presence of MgO as the catalyst. The study of the effect of the solvent in the reaction between benzaldehyde, malononitrile and dimedone in the presence of MgO reveals that polar solvents such as ethanol and acetonitrile afford better yields (85% and 74% after 0.6 and 1.5 h respectively) than nonpolar solvents such as toluene (63% after 2.5 h), being the most effective solvent a mixture of water and ethanol (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) yielding 94% after 0.5 h. A variety of tetrahydrobenzo[b]pyran-, [2,3-d]pyrano- and pyrido[2,3-d]pyrimidine derivatives were synthesized with this protocol in excellent yields in the presence of MgOcatalyst from aryl aldehyde, malononitrile and cyclic β-diketones (A: 1,3-cyclohexanedione or dimedone, B: 4-hydroxy-6-methylpyrone, 4-hydroxycoumarin, C: 1,3-dimethylbarbituric acid and D: 1,3-dimethyl-6-amino uracil) (Scheme 80).
10) yielding 94% after 0.5 h. A variety of tetrahydrobenzo[b]pyran-, [2,3-d]pyrano- and pyrido[2,3-d]pyrimidine derivatives were synthesized with this protocol in excellent yields in the presence of MgOcatalyst from aryl aldehyde, malononitrile and cyclic β-diketones (A: 1,3-cyclohexanedione or dimedone, B: 4-hydroxy-6-methylpyrone, 4-hydroxycoumarin, C: 1,3-dimethylbarbituric acid and D: 1,3-dimethyl-6-amino uracil) (Scheme 80).
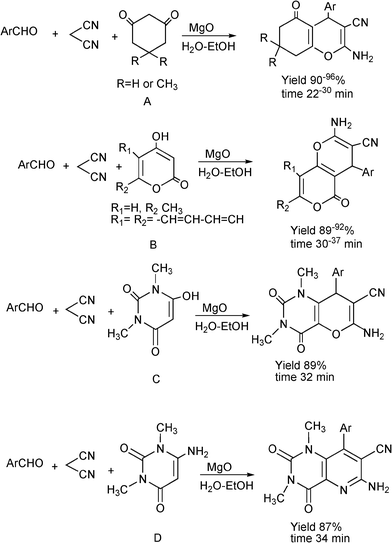 | ||
| Scheme 80 Synthesis of pyran annulated heterocyclic systems via three component reaction. | ||
The mechanism for the formation of these products involves the Knoevenagel condensation between the aromatic aldehyde and malononitrile as first step giving the α-cyanocinnamonitrile. Subsequently the methylene of the diketone is activated by MgO and reacts with the electrophilic carbon–carbon double bond (Michael addition) giving the intermediate (A) which cyclizes by nucleophilic attack of the hydroxylgroup on the cyano moiety, to form the intermediate B. Finally a tautomerization process affords the desired product (Scheme 81).
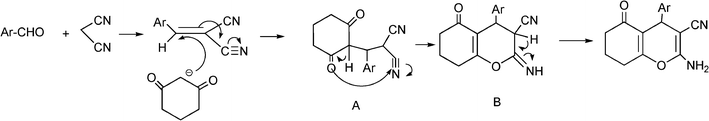 | ||
| Scheme 81 Proposed mechanism for the one-pot synthesis of tetrahydrobenzopyran derivatives. | ||
The MCR involving benzaldehyde, malononitrile and barbituric acid or its thio analogue was performed using neutral alumina as the catalyst under microwave irradiation, and yields 7-amino-6-cyano-5-aryl-5H-pyrano[2,3-d]pyrimidine-2,4(1H,3H)-diones, an intermediate in the synthesis of pyranodipyrimidines395 (Scheme 82). This intermediate compound was allowed to react with different aromatic carboxylic acids adsorbed on Montmorillonite under microwave irradiation to give the desired product. Under these reaction conditions, a mixture of the target compound (yield 62–70%) along with the N-acylated pyranopyrimidines (yield 20–28%) was obtained. However, surprisingly, using acidic alumina, the pyranodipyrimidines were obtained in good yields (55–65%) and 100% selectivity in 4–5 min.
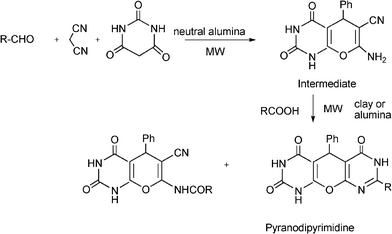 | ||
| Scheme 82 One-pot synthesis of pyranodipyrimidine derivatives. | ||
For comparison purposes the reaction was performed using a conventional procedure with hydrochloride acid at reflux temperature during 6.5–8 h. The yield of the pyranodipyrimidines was lower (35–48% yield) and the N-alcylated pyranopyrimidines were also obtained in a considerable amount. Although the N-acylated product is obtained in smaller quantities and is considered as a by-product this compound possesses various biological activities.395
The most straightforward synthesis for 2-aminobenzochromene derivatives involves a three-component coupling of aromatic aldehyde, malononitrile and an activated phenol in the presence of organic bases (such as piperidine), which is frequently used in stoichiometric amounts using ethanol or acetonitrile as solvents402 (Scheme 83).
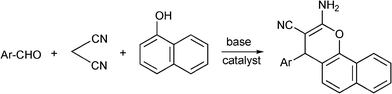 | ||
| Scheme 83 3MC synthesis of 2-aminochromene derivatives. | ||
As in the previous reactions presented above, benzylidenemalononitrile is formed fast and in quantitative yield by the Knoevenagel condensation between benzaldehyde and malononitrile. Subsequent ortho C–alkylation of α-naphthol by reaction with the electrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and the nucleophilic addition of the hydroxyl moiety to the nitrile produces the final 2-aminochromenes (Scheme 84).
C double bond and the nucleophilic addition of the hydroxyl moiety to the nitrile produces the final 2-aminochromenes (Scheme 84).
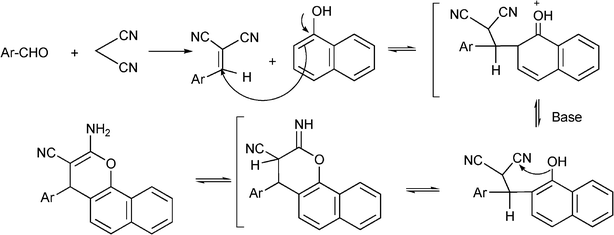 | ||
| Scheme 84 Mechanism of the one-pot synthesis of 2-aminochromene derivatives. | ||
Nevertheless, diverse heterogeneous catalysts have been employed for this multicomponent reaction. Wang et al.403 synthesized a series of 2-aminochromene derivatives from aryl aldehydes, malononitrile or ethyl cyanoacetate with 1-naphtol or 1,5-naphthalenediol, in the presence of alumina coated with potassium fluoride (KF-Alumina). When aryl aldehydes, malononitrile or ethyl cyanoacetate and 1-naphthol react in the presence of FK-Alumina in refluxing ethanol for 5–6 h, the 2-amino-4-aryl-4H-benzo[h]chromene derivatives were obtained in slightly high yields (72–90%). When 1,5-naphthalenediol was used instead of 1-naphthol, naphthol[1,2-b;6,5-b']dipyrans derivatives were isolated in good yields (83–94%) (Scheme 85).
![Synthesis of the naphthol[1,2-b;6,5-b']dipyrans derivatives from aryl aldehydes, malononitrile or ethyl cyanoacetate and 1,5-naphthalenediol.](/image/article/2012/RA/c1ra00807b/c1ra00807b-s85.gif) | ||
| Scheme 85 Synthesis of the naphthol[1,2-b;6,5-b']dipyrans derivatives from aryl aldehydes, malononitrile or ethyl cyanoacetate and 1,5-naphthalenediol. | ||
Basic alumina was proposed by Maggi et al.404 as a catalyst in the synthesis of substituted 2-amino-2-chromenes by coupling benzaldehyde, malononitrile and α-naphthol using water as a solvent. Basic alumina exhibited better activity and selectivity to 2-amino-2-chromenes than Montmorillonite KSF, hydrotalcite and silica gel. In all cases mixtures of Knoevenagel adduct (KA) and the desired product were obtained (Table 24).
| ||
|---|---|---|
| Catalyst | Yield (%) BM | Yield(%)chromene |
| a BM: benzylidenemalononitrile. Experimental conditions: benzaldehyde (10 mmol), malononitrile (10 mmol),1-naphthol (10 mmol), catalysts (0.50 g), 10 mL water at refluxing conditions after 2 h of reaction. | ||
| γ-Alumina | 13 | 84 |
| Silica gel | 35 | 61 |
| Montmorillonite KSF | 48 | 50 |
| Hydrotalcite Pural MG30 | 72 | 24 |
Excellent yields (83–98%) and selectivity to 2-aminochromene derivatives (85–99%) were obtained on basic γ-alumina when different aldehydes, malononitrile and α-naphthol, were reacted in water for 3 h. In addition, the reaction showed high regioselectivity affording only one of the two possible isomers that can be formed. The catalyst could be reused four times giving the same yield and excellent selectivity to chromene.
Nanosized magnesium oxide has been reported405 as an efficient catalyst for the three component condensation of aldehyde, malononitrile and α-naphthol in methanol, water or PEG-water as the reaction medium. Thus, when a mixture of equimolar amounts of benzaldehyde, malononitrile and α-naphthol, in methanol or water was refluxed for 1 h in the presence of MgO, the corresponding 2-aminochromene was obtained with yields of 96% and 86% respectively. Meanwhile, using PEG–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature, a 96% yield of aminochromene was obtained after 15 min reaction time. The catalytic activity of the nanosized MgO particles (100–200 nm) was superior to those exhibited by a commercially available MgO sample. The nanosized magnesium oxide was employed for the synthesis of a diverse set of 2-aminochromenes achieving yields of 70–98% within 15–90 min using PEG–water as a solvent. After the reaction the catalyst was reused in subsequent reactions with consistent activity.
1) at room temperature, a 96% yield of aminochromene was obtained after 15 min reaction time. The catalytic activity of the nanosized MgO particles (100–200 nm) was superior to those exhibited by a commercially available MgO sample. The nanosized magnesium oxide was employed for the synthesis of a diverse set of 2-aminochromenes achieving yields of 70–98% within 15–90 min using PEG–water as a solvent. After the reaction the catalyst was reused in subsequent reactions with consistent activity.
More recently, Mg/Alhydrotalcite was found to be a highly effective catalyst for the synthesis of 2-aminochromenes via a multicomponent reaction in a dry state under single-mode microwave irradiation.406 Thus, under optimal conditions (molar ratio aromatic aldehyde:malononitrile: α-naphthol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mmol, HT (Mg/Al = 3) at 140 °C), it is possible to obtain 2-aminochromenes in good yields (71–90%) in an interval of 5–34 min reaction time. When the reaction was carried out on calcined hydrotalcite the catalytic activity was lower than that the corresponding uncalcinated HT. On calcination at a high temperature the hydroxylgroups in the brucite layers are eliminated as water. Thus, the Brønsted basicity decreases, meanwhile the Lewis basicity increases, which indicates that Brønsted basicity is required for this reaction. The authors found that the catalyst was reusable however there was a reduction in the yield of the product after reuse (Table 25).
1 mmol, HT (Mg/Al = 3) at 140 °C), it is possible to obtain 2-aminochromenes in good yields (71–90%) in an interval of 5–34 min reaction time. When the reaction was carried out on calcined hydrotalcite the catalytic activity was lower than that the corresponding uncalcinated HT. On calcination at a high temperature the hydroxylgroups in the brucite layers are eliminated as water. Thus, the Brønsted basicity decreases, meanwhile the Lewis basicity increases, which indicates that Brønsted basicity is required for this reaction. The authors found that the catalyst was reusable however there was a reduction in the yield of the product after reuse (Table 25).
| Catalyst | B:M:N | Time (h) | Solvent/T (°C) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a B:M:N: mmol of benzaldehyde:malononitrile:1-naphthol.b 50 wt% respect naphthol.c 0.5 mmol. | |||||
| KF–Al2O3(0.5 g) | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | 5 | EtOH/80 | 83 | 403 |
| Al2O3(0.5 g) | 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 | 3 | H2O/reflux | 96 | 404 |
| MgO(0.05 g) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 | 1 | H2O/reflux | 86 | 405 |
| MgO(0.05 g) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 | 0.25 | H2O:PEG/rt | 96 | 405 |
| Mg/Al HTb | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 0.12 | MW/140 | 84 | 406 |
| [Bmim]BF4c | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | 1 | H2O/reflux | 81 | 407 |
| [Bmim]OHc | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | 0.16 | H2O/reflux | 91 | 407 |
4. Conclusions and catalytic opportunities
A large number of MCRs have been presented all of which show quite good conversion and selectivity for preparing products of industrial interest. It was seen that most of the work has been performed using homogeneous catalysts ranging from Brønsted and Lewis acids to base catalyzed reactions. Mineral and organic Brønsted acids and basic amines were the most commonly used homogeneous catalysts together with organic and inorganic transition metal salts. The number of catalytic studies with solid catalysts is still limited and less sophisticated materials have been used. For instance, mineral acids impregnated on inorganic porous carriers were extensively used. With these catalysts one should expect acid leaching to occur. Then, while they can be useful for laboratory preparative uses they will be limited for industrial use. This is more so if sustainable processes are going to be implemented.Supported heteropolyacids on silica and supported Lewis acids such as AlCl3 and SbCl5 on alumina give excellent results for various acid catalyzed MCRs. However, polar reactants and solvents will make these catalysts leach, while the presence of even small amounts of water could hydrolyze the supported Lewis acids. True heterogeneous catalysts such as silica and activated silica, activated alumina, montmorillonites, zeolites and organic resins with sulfonic groups have also been used successfully for Biginelli type reactions, and synthesis of imidazole and quinazoline derivatives. Even hybrid materials where organic molecules with sulfonic groups are grafted on inorganic solids (amorphous and ordered silicas) show reasonable to good activities for Hantzsch type reactions. However, it should be considered that if catalyst deactivation occurs it will be difficult in many cases to regenerate the hybrid organic–inorganic material. Metal substituted zeolites and zeolites with exchanged metal cations in where unusual metal valences are stabilized (for instance Cu(I)-zeolite) are also excellent catalysts for the A3 coupling of aldehydes, amines and alkynes.
In the case of basic and bifunctional metal-base solid catalysts such as MgO and mixed oxides of Al, Mg and transition metals derived from hydrotalcites good results for the synthesis of pyran and aminochromene derivatives have been obtained. These catalysts are stable and regenerable.
New possibilities are open for the preparation of catalysts containing acid and basic sites, if possible, with controlled distance and orientation408 that can work in homogeneous phase but which can be recycled, or integrated in solid catalysts.
In conclusion MCRs present clear advantages for process intensification, avoiding costly and energy consuming intermediate separation and purification steps, and there are open possibilities for the use of mono and bifunctional catalysts for achieving fully green processes.
Acknowledgements
The authors wish to gratefully acknowledge the Generalitat Valenciana for the financial support in the project CONSOLIDER-INGENIO 2010 (CSD2009-00050)References
- D. E. Fogg and E. N. dos Santos, Coor. Chem. Rev., 2004, 248, 9456 CrossRef.
- G. Poli and G. Giambastiani, J. Org. Chem., 2002, 67, 9456 CrossRef CAS.
- G. Balme, E. Bossharth and N. Monteiro, Eur. J. Org. Chem., 2003, 4101 CrossRef CAS.
- M. Malacria, Chem. Rev., 1996, 96, 289 CrossRef CAS.
- P. J. Parsons, C. S. Penkett and A. J. Shell, Chem. Rev., 1996, 96, 195 CrossRef CAS.
- L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS.
- J. M. Lee, Y. Na, H. Han and S. Chang, Chem. Soc. Rev., 2004, 33, 302 RSC.
- J. C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem.Rev., 2005, 105, 1001 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, Chemsuschem, 2009, 2, 500 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072–1072 CrossRef CAS.
- A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef.
- C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS.
- I. Ugi, Journal für Praktische Chemie, 1997, 339, 499 CrossRef CAS.
- A. Strecker, Liebigs Ann. Chem., 1850, 75, 27 CrossRef.
- A. Hantzsch, Liebigs Ann. Chem., 1882, 215, 1 CrossRef.
- P. Biginelli, Chim. Ital., 1893, 23, 360 Search PubMed.
- I. Ugi, R. Meyr, R. Fetzer and C. Steinbruckner, Angew. Chem., 1959, 71, 386 Search PubMed.
- P. Eilbracht, Chem. Rev., 1999, 99, 3329 CrossRef CAS.
- L. Weber, Curr. Med. Chem., 2002, 9, 1241 CrossRef.
- R. E. Dolle and K. H. Nelson, J. Com. Chem., 1996, 1, 235 Search PubMed.
- L. A. Thompson and J. A. Ellman, Chem. Rev., 1996, 96, 555 CrossRef CAS.
- I. Ugi, B. Werner and A. D”mling, Molecules, 2003, 8, 53 CrossRef.
- G. Gelbard, Ind. Eng. Chem. Res., 2005, 44, 8468 CrossRef CAS.
- A. Guyot, Pure Appl. Chem., 1988, 60, 365 CrossRef CAS.
- A. Corma, Chem. Rev., 1995, 95, 559 CrossRef CAS.
- R. A. van Santen and G. J. Kramer, Chem. Rev., 1995, 95, 637 CrossRef CAS.
- W. E. Farneth and R. J. Gorte, Chem. Rev., 1995, 95, 615 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS.
- J. Jiang, J. L. Jorda, M. J. Diaz-Cabanas, J. Yu and A. Corma, Angewandte Chemie, International Edition, 2010, 49, 4986 CrossRef CAS.
- J. Jiang, J. Yu and A. Corma, Angew. Chem. Int. Ed., 2010, 49, 3120 CrossRef CAS.
- E. G. Derouaneed., Catalysts for Fine Chemical Synthesis. Microporous and Mesoporous Solid Catalysts, vol 4, John Wiley and Sons, Chichester, 2006 Search PubMed.
- R. Simancas, D. Dari, N. Velamazan, M. T. Navarro, A. Cantin, J. L. Jorda, G. Sastre, A. Corma and F. Rey, Science, 2010, 330, 1219 CrossRef CAS.
- A. Corma, M. J. Diaz-Cabanas, J. L. Jorda, C. Martinez and M. Moliner, Nature, 2006, 443, 842 CrossRef CAS.
- A. Corma, M. J. Diaz-Cabanas, F. Rey, S. Nicolopoulus and K. Boulahya, Chem. Commun., 2004, 1356 RSC.
- A. Corma, M. J. Diaz-Cabanas, J. Jiang, M. Afeworki, D. L. Dorset, S. L. Soled and K. G. Strohmaierb, Proc. Natl. Acad. Sci., 2010, 107, 13997 CrossRef CAS.
- J. Jiang, J. L. Jorda, J. Yu, L. A. Baumes, E. Mugnaioli, M. J. Diaz-Cabanas, U. Kolb and A. Corma, Science, 2011, 333, 1131 CrossRef CAS.
- P. Ratnasamy, D. Srinivas and H. Knoezinger, Adv. Catal., 2004, 48, 1 CrossRef CAS.
- M. Bejblova and J. Cejka, Zeolites, 2008, 263 CAS.
- M. Boronat, P. Concepcion, A. Corma and M. Renz, Catal. Today, 2007, 121, 39 CrossRef CAS.
- F. Alvarez, A. I. Silva, F. R. Ribeiro, G. Giannetto and M. Guisnet, Stud. Surf. Sci. Catal., 1997, 108, 609 CrossRef CAS.
- J. Chupin, N. S. Gnep, S. Lacombe and M. Guisnet, Appl. Catal. A Gen, 2001, 206, 43 CrossRef CAS.
- M. Guisnet, F. Alvarez, G. Giannetto and G. Perot, Catal. Today, 1987, 1, 415 CrossRef CAS.
- A. I. Silva, F. Alvarez, F. Ramoa Ribeiro and M. Guisnet, Catal. Today, 2000, 60, 311 CrossRef CAS.
- F. Iosif, S. Coman, V. Parvulescu, P. Grange, S. Delsarte, D. De Vos and P. Jacobs, Chem. Commun., 2004, 1292 RSC.
- F. Neatu, S. Coman, V. I. Parvulescu, G. Poncelet, D. Vos and P. Jacobs, Top. Catal., 2009, 52, 1292 CrossRef CAS.
- B. R. Jermy and A. Pandurangan, Appl. Catal. A Gen, 2005, 288, 25 CrossRef CAS.
- M. A. Harmer, W. E. Farneth and Q. Sun, J. Am. Chem. Soc., 1996, 118, 7708 CrossRef CAS.
- M. A. Harmer, Q. Sun, A. J. Vega, W. E. Farneth, A. Heidekum and W. F. Hoelderich, Green Chem., 2000, 2, 7 RSC.
- M. C. Laufer, H. Hausmann and W. F. Hoelderich, J. Catal., 2003, 218, 315 CrossRef CAS.
- T. C. Wabnitz, J. Q. Yu and J. B. Spencer, Synlett, 2003, 1070 CAS.
- P. Beltrame and G. Zuretti, Appl. Catal. A Gen, 2003, 248, 75 CrossRef CAS.
- I. Ledneczki, M. Daranyi, F. Fülöp and A. Molnar, Catal. Today, 2005, 100, 437 CrossRef CAS.
- M. Alvaro, A. Corma, D. Das, V. Fornes and H. Garcia, J. Catal., 2005, 231, 48 CrossRef CAS.
- P. Botella, A. Corma and J. M. Lopez-Nieto, J. Catal., 1999, 185, 371 CrossRef CAS.
- T. Okuhara, N. Mizuno and N. Mison, Adv. Catal., 1996, 41, 113 CrossRef CAS.
- I. V. Kozhevnikov, Catal. Rev. - Sci. Eng., 1995, 37, 311 CAS.
- Y. Izumi, M. Ogawa and K. Urabe, Appl. Catal. A Gen, 1995, 132, 127 CrossRef CAS.
- A. Corma, H. Garcia and F. X. Llabres i Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS.
- R. L. White, E. C. Sikabwe, M. A. Coelho and D. E. Resasco, J.Catal., 1995, 157, 755 CrossRef CAS.
- D. A. Ward and E. I. Ko, J. Catal., 1995, 157, 321 CrossRef CAS.
- J. M. Campelo, J. M. Marinas, S. Mendioroz and J. A. Pajares, J. Catal., 1986, 101, 484 CrossRef CAS.
- M. J. Climent, A. Corma, H. Garcia, R. Guil-Lopez, S. Iborra and V. Fornes, J. Catal., 2001, 197, 385 CrossRef CAS.
- Y. Ono and T. Baba, Catal. Today, 1997, 38, 321 CrossRef CAS.
- H. Hattori, Chem. Rev., 1995, 95, 537 CrossRef CAS.
- J. Gascon, U. Aktay, M. D. Hernandez-Alonso, G. P. M. van Klink and F. Kapteijn, J. Catal., 2009, 261, 75 CrossRef CAS.
- J. Weitkamp, M. Hunger and U. Rymsa, Micropor. Mesopor. Mat., 2001, 48, 255 CrossRef CAS.
- B. F. Sels, D. E. De Vos and P. A. Jacobs, Catal. Rev. - Sci. Eng., 2001, 43, 443 CAS.
- A. Corma and S. Iborra, Adv. Catal., 2006, 49, 239 CrossRef CAS.
- B. Ringdahl, in The Muscarinic Receptors, Humana Press, Clifton, New Jersy 1989 Search PubMed.
- K. B. Sanders, A. J. Thomas, M. R. Pavia, R. E. Davis, L. L. Coughenour, S. L. Myers, S. Fisher and W. H. Moos, Bioorg. Med. Chem. Lett., 1992, 2, 803 CrossRef CAS.
- P. Matyus, B. Dajka-Halasz, A. Foldi, F. Haider, D. Barlocco and K. Magyar, Curr Med Chem, 2004, 11, 1285 CrossRef CAS.
- S. B. Park and H. Alper, Chem. Commun., 2005, 1315 RSC.
- C. Wei, Z. Li and C. J. Li, Org. Lett., 2003, 5, 4473 CrossRef CAS.
- C. Wei, L. Zhang and C. J. Li, Synlett., 2004, 1472 CAS.
- C. Wei and C. J. Li, J. Am. Chem. Soc., 2003, 125, 9584 CrossRef CAS.
- V. K. Y. Lo, Y. Liu, M. K. Wong and C. M. Che, Org. Lett., 2006, 8, 1529 CrossRef CAS.
- N. Gommermann and P. Knochel, Chem-Eur J, 2006, 12, 4380 CrossRef CAS.
- A. Bisai and V. K. Singh, Org. Lett., 2006, 8, 2405 CrossRef CAS.
- S. Sakaguchi, T. Mizuta, M. Furuwan, T. Kubo and Y. Ishii, Chem. Commum., 2004, 1638 RSC.
- Y. L. Zhang, P. Wang and M. Wan, J. Org. Chem., 2009, 74, 4364 CrossRef CAS.
- P. Li and L. Wang, Chin. J. Chem., 2005, 23, 1076 CrossRef CAS.
- C. J. Li and C. Wei, Chem. Commum., 2002, 3, 268 RSC.
- L. Shi, Y. Q. Tu, M. Wang, F. M. Zhang and C. A. Fan, Organic Letters, 2004, 6, 1001 CrossRef CAS.
- B. Sreedhar, P. S. Reddy, B. V. Prakash and A. Ravindra, Tetrahedron Lett, 2005, 46, 7019 CrossRef CAS.
- A. Bisai and V. K. Singh, Organic Letters, 2006, 8, 2405 CrossRef CAS.
- N. Gommermann and P. Knochel, Chem-Eur J, 2006, 12, 4380 CrossRef CAS.
- Z. Li, C. C. Wei, L. Chen, R. S. Varma and C.-J. Li, Tetrahedron Lett., 2004, 45, 2443 CrossRef CAS.
- B. M. Choudary, C. Sridhar, M. L. Kantam and B. Sreedhar, Tetrahedron Lett., 2004, 45, 7319 CrossRef CAS.
- B. Sreedhar, P. S. Reddy, C. S. V. Krishna and P. V. Babu, Tetrahedron Lett., 2007, 48, 7882 CrossRef CAS.
- P. Li and L. Wang, Tetrahedron, 2007, 63, 5455 CrossRef CAS.
- M. Wang, P. H. Li and L. Wang, Eur J Org Chem, 2008, 2255 CrossRef CAS.
- M. Kidwai, V. Bansal, N. K. Mishra, A. Kumar and S. Mazumdar, Synlett, 2007, 1581 CrossRef CAS.
- M. K. Patil, M. Keller, B. M. Reddy, P. Pale and J. Sommer, Eur.J.Org.Chem., 2008, 4440 CrossRef CAS.
- R. Maggi, A. Bello, C. Oro, G. Sartori and L. Soldi, Tetrahedron, 2008, 64, 1435 CrossRef CAS.
- K. Namitharan and K. Pitchumani, Eur. J. Med. Chem., 2010, 411 CAS.
- K. M. Reddy, N. S. Babu, I. Suryanarayana, P. S. S. Prasad and N. Lingaiah, Tetrahedron Lett., 2006, 47, 7563 CrossRef CAS.
- I. Luz, F. X. Llabrés i Xamena and A. Corma, J. Catal. DOI:doi:10.1016/j.jcat.2011.10.001.
- M. L. Kantam, B. V. Prakash, C. R. V. Reddy and B. Sreedhar, Synlett, 2005, 2329 CrossRef CAS.
- M. Kidwai, V. Bansal, A. Kumar and S. Mozumdar, Green Chem., 2007, 9, 742 RSC.
- K. K. R. Datta, B. V. Subba Reddy, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961 CrossRef CAS.
- X. Zhang and A. Corma, Angew Chem. Int. Edit., 2008, 47, 4358 CrossRef CAS.
- S. Rivara, S. Lorenzi, M. Mor, P. V. Plazzi, G. Spadoni, A. Bedini and G. Tarzia, Journal of Medicinal Chemistry, 2005, 48, 4049 CrossRef CAS.
- G. R. Humphrey and J. T. Kuethe, Chem Rev, 2006, 106, 2875 CrossRef CAS.
- X. Zhang, F. X. L. I. Xamena and A. Corma, Journal of Catalysis, 2009, 265, 155 CrossRef CAS.
- J. Y. Chang, C. Y. Chang, C. C. Kuo, L. T. Chen, Y. S. Wein and Y. H. Kuo, Mol Pharmacol, 2004, 65, 77 CrossRef CAS.
- J. Bosch, T. Roca, J. L. Catena, C. Farrerons and I. Miquel, Synth., 2000, 721 CrossRef CAS.
- R. S. Givens, P. S. Athey, B. Matuszewski, L. W. Kueper, J.-K. Xue and T. Fister, J. Am. Chem. Soc., 1993, 115, 6001 CrossRef CAS.
- S. Jinno, T. Okita and K. Inouye, Tetrahedron Lett., 1999, 9, 1029 CAS.
- K. M. Dawood, W. Solodenko and A. Kirschning, Arkivoc, 2007, 104 CAS.
- G. W. Kabalka, L. L. Zhou, L. Wang and R. M. Pagni, Tetrahedron, 2006, 62, 857 CrossRef CAS.
- D. Prukala, Tetrahedron Lett., 2006, 47, 9045 CrossRef CAS.
- S. Sahoo, T. Joseph and S. B. Halligudi, J. Mol. Catal. A. Chem, 2006, 244, 179 CrossRef CAS.
- E. Takahashi, H. Fujisawa and T. Mukaiyama, Chem. Lett., 2004, 33, 936 CrossRef CAS.
- E. F. Kleinmann, Comprehensive Organic Synthesis, Pergamon Press, New York, 1991 Search PubMed.
- R. Muller, H. Waldmann and H. Goesmann, Angew. Chem. Int. Ed., 1999, 38, 184 CrossRef CAS.
- B. M. Reddy, M. K. Patil and B. T. Reddy, Catal Lett, 2008, 125, 97 CrossRef CAS.
- B. M. Reddy, P. M. Sreekanth, P. Lakshmanan and A. Khan, J Mol Catal a-Chem, 2006, 244, 1 CrossRef CAS.
- B. Song, S. Yang, H. Zhong, L. Jin, D. Hu and G. Liu, J Fluorine Chem, 2005, 126, 87 CrossRef CAS.
- M. Xia and Y. D. Lu, J Fluorine Chem, 2006, 127, 1119 CrossRef CAS.
- H. Zeng, H. L.i and H. Shao, Ultrason. Sonochem., 2009, 16, 758 CrossRef CAS.
- M. Kidwai, N. K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Tetrahedron Lett., 2009, 50, 1355 CrossRef CAS.
- M. Ashok, B. S. Holla and N. S. Kumari, Eur. J. Med. Chem., 2007, 42, 380 CrossRef CAS.
- K. S. Atwai, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg and B. C. ÖReilly, J Med Chem, 1991, 34, 806 CrossRef.
- A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. De Brosse, S. Mai, A. Truneh, D. J. Faulkner, B. Carte, A. L. Breen, R. P. Hertzberg, R. K. Johnson, J. W. Westley and B. C. M. Potts, J. Org. Chem., 1995, 60, 1182 CrossRef CAS.
- T. U. Mayer, T. U. Kapoor, S. J. Haggarty, R. V. S. S. L. King and T. J. Mitchison, Science (Washington, D.C., 1883-) 1999, 286, 971 Search PubMed.
- J. V. Grover, S. Bzwoezyk, D. E. McMullen, P. G. Normandin, S. Selph and J. Moreland, J.Cardiovasc.Pharma Col, 1995, 26, 289 CrossRef.
- C. O. Kappe, Tetrahedron, 1993, 49, 6937 CrossRef CAS.
- C. O. Kappe, Acc. Chem. Res., 2000, 33, 879 CrossRef CAS.
- C. O. Kappe, J. Org. Chem., 1997, 62, 7201 CrossRef CAS.
- J. Lu and M. H. R. Lankhorst, Synlett, 2000, 63 CAS.
- Y. Ma, C. T. Qian, L. M. Wang and M. Yang, J Org Chem, 2000, 65, 3864 CrossRef CAS.
- B. Ahmed, R. A. Khan, H. Habibullah and M. Keshari, Tetrahedron Lett., 2009, 50, 2889 CrossRef CAS.
- M. A. Kolosov, V. D. Orlov, D. A. Beloborodov and V. V. Dotsenko, Mol Divers, 2009, 13, 5 CrossRef CAS.
- J. Azizian, A. A. Mohammadi, A. R. Karimi and M. R. Mohammadizadeh, Appl.Catal., A, 2006, 300, 85 CrossRef CAS.
- M. A. S. Chari and K. Syamasudar, J. Mol. Cat. A Chem., 2004, 221, 137 CrossRef CAS.
- R. Fazaeli, S. Tangestaninejad, H. Aliyan and M. Moghadam, Appl. Catal. A:Gen., 2006, 309, 44 CrossRef CAS.
- A. Hagedus, Z. Hell and I. Vigh, Synth. Comm., 2006, 129 CrossRef.
- S. Martinez, M. Meseguer, L. Casas, E. Rodriguez, E. Molins, M. Moreno-Mañas, A. Roig, R. M. Sebastian and A. Vallribera, Tetrahedron, 2003, 59, 1553 CrossRef CAS.
- B. J. Ahn, M. S. Gang, K. Chae, Y. Oh, J. Shin and W. Chang, J. Ind. Eng. Chem., 2008, 14, 401 CrossRef CAS.
- F. Bigi, S. Carloni, B. Frullanti, R. Maggi and G. Sartori, Tetrahedron Lett., 1999, 40, 3465 CrossRef CAS.
- V. R. Choudhary, V. H. Tillu, V. S. Narkhede, H. B. Borate and R. D. Wakharkar, Catal.Commun., 2003, 4, 449 CrossRef CAS.
- S. L. Jain, J. K. Joseph, S. Singhal and B. Sain, J. Mol. Catal., 2007, 268, 134 CrossRef CAS.
- J. K. Joseph, S. L. Jain and B. Sain, J. Mol. Catal.A: Chem., 2006, 247, 99 CrossRef CAS.
- P. Salehi, M. Daviri, M. A. Zolfigol and M. A. B. Fard, Tetrahedron Lett., 2003, 44, 2889 CrossRef CAS.
- I. Saxena, D. C. Borah and J. C. Sarna, Tetrahedron Lett., 2005, 46, 1159 CrossRef CAS.
- V. Singh, V. Sapehiyia, V. Srivastava and S. Kaur, Catal Commun, 2006, 7, 571 CrossRef CAS.
- A. Dondoni and A. Massi, Tetrahedron Lett., 2001, 42, 7975 CrossRef CAS.
- M. S. y. m. Gopalakrishnan, Lett. Org.Chem., 2006, 3, 484 CrossRef CAS.
- M. Kidwai, P. Mothsra, V. Bansal, R. K. Somvanshi, A. S. Ethayathulla, S. Dey and T. P. Singh, Journal of Molecular Catalysis a-Chemical, 2007, 265, 177 CrossRef CAS.
- M. G. Kulkarni, S. W. Chavhan, M. P. Shinde, D. D. Gaikwad, A. S. Borhade, A. P. Dhondge, Y. B. Shaikh, V. B. Ningdale, M. P. Desai and D. R. Birhade, Beilstein J Org Chem, 2009, 5 Search PubMed.
- V. R. Rani, N. Srinivas, M. R. Kishan, S. J. Kulkarni and K. V. Raghavan, Green Chem., 2001, 3, 305 RSC.
- A. Shaabani, A. Sarvary, A. Rahmati and A. H. Rezayan, Lett. Org. Chem., 2007 Search PubMed.
- S. V. Sinde, W. N. Jadhav, M. K. Lande, L. S. Gadekar, B. R. Arbad, J. M. Kondre and N. N. Karade, Catalysis Lett., 2008, 125, 57 CrossRef.
- M. Tajbakhsh, B. Mohajerani, M. M. Heravi and A. N. Ahmadi, J Mol Catal A Chem, 2005, 236, 216 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, P. Sridhar, J. S. S. Reddy, K. Nagaiah, N. Lingaiah and P. S. Saiprasad, Eur.J.Org.Chem., 2004, 552 CrossRef CAS.
- R. V. Yarapathi, S. Kurva and S. Tammishetti, Catal.Commun., 2004, 5, 511 CrossRef CAS.
- V. T. Kamble, D. B. Muley, S. T. Atkore and S. D. Dakore, Chin. J. Chem., 2010, 28, 388 CrossRef CAS.
- V. Singh, V. Sapehiyia, V. Srivastava and S. Kaur, Catal.Commun., 2006, 7, 571 CrossRef CAS.
- L. Z. Gong, X. H. Chen and X. Y. Xu, Chem-Eur J, 2007, 13, 8920 CrossRef CAS.
- N. Yamada, S. Kadowaki, K. Takhashi and K. Umeza, Biochem. Pharmacol., 1992, 44, 1211 CrossRef CAS.
- R. W. Carling, P. D. Leeson, A. M. Moseley, J. D. Smith, K. Saywell, M. D. Trickelbank, J. A. Kemp, G. R. Marsahall, A. C. Foster and S. Grimwood, Biorg. Med. Chem. Lett., 1993, 3, 65 CrossRef CAS.
- T. P. Loh, K. S. V. Koh, K. Y. Sim and W. K. Leong, Tetrahedron Lett, 1999, 40, 8447 CrossRef CAS.
- G. Sartori, F. Bigi, R. Maggi, A. Mazzacani and G. Oppici, Eur J Org Chem, 2001, 2513 CrossRef CAS.
- S. Kobayashi and S. Nagayama, J. Am. Chem. Soc., 1996, 118, 8977 CrossRef CAS.
- L. X. Shao and M. Shi, Adv Synth Catal, 2003, 345, 963 CrossRef CAS.
- Y. Ma, C. T. Qian, M. H. Xie and J. Sun, J Org Chem, 1999, 64, 6462 CrossRef CAS.
- B. R. Das, M. R. Reddy and V. S. Reddy, Chem. Lett., 2004, 33, 1526 CrossRef CAS.
- R. S. N. Kumar, R. Nagarajan and P. T. Perumal, Synth., 2004, 949 CAS.
- D. Mahajan, B. A. Ganai, R. L. Sharma and K. K. Kapoor, Tetrahedron Lett., 2006, 47, 7919 CrossRef CAS.
- V. T. Kamble, B. S. Davane, S. A. Chavan, D. B. Muley and S. T. Atkore, Chinese Chem. Lett., 2010, 21, 265 CrossRef CAS.
- K. V. N. S. Srinivas and B. Das, Synlett, 2004, 10, 1715 Search PubMed.
- J. S. R. Yadav and B.V.S. Reddy, Chem. Lett., 2004, 33, 1436 CrossRef CAS.
- R. Filler, Y. Kobayashi and L. M. Yagupolskii, Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications, Elsevier, Amsterdam, 1993 Search PubMed.
- G. Dyker, Angew Chem Int Edit, 1999, 38, 1699 CrossRef CAS.
- S. K. De, Synth. Commun., 2005, 35, 653 CrossRef CAS.
- N. H. Khan, S. Agrawal, R. I. Kureshi, S. H. R. Abdi, S. Singh, E. Suresh and R. V. Jasra, Tetrahedron Lett., 2008, 49, 640 CrossRef CAS.
- E. Rafiee and A. Azad, Synth. Commun., 2007, 37, 1127 CrossRef CAS.
- J. S. R. Yadav, B.V.S. Reddy, N. B. V. Subba Reddy, B. Eeshwaraiah and M. Srinivas, Tetrahedron, 2004, 60, 1767 CrossRef CAS.
- A. Heydari, M. Pourayoubi and A. R. Mahjoub, Tetrahedron Lett., 2007, 48, 4059 CrossRef CAS.
- G. A. Olah, T. Mathew, C. Panja, K. Smidth and G. S. Surya Prakash, Catal Lett, 2007, 114, 2007 CrossRef.
- A. Shaabani and A. Maleki, Appl. Catal. A Gen., 2007, 331, 149 CrossRef CAS.
- G. K. S. Prakash, T. E. Thomas, I. Bychinskaya and A. G. Prakash, Green Chem., 2008, 10, 1105 RSC.
- s. kawahara, S. Tsuzuki and T. Uchimaru, Chem. Eur. J., 2005, 11, 4458 CrossRef CAS.
- L. T. Vassilev, B. T. Vu, B. Graves, D. Carvajal, F. Podlaski, Z. Filipovic, N. Kong, U. Kammlott, C. Lukacs, C. Klein, N. Fotouhi and E. A. Liu, Science, 2004, 303, 844 CrossRef CAS.
- T. Lindel, P. R. Jensen, W. Fenical, B. H. Long, A. M. Casazza, J. Carboni and C. R. Fairchild, J. Am. Chem. Soc., 1997, 119, 8744 CrossRef CAS.
- S. L. Abrahams, R. J. Hazen, A. G. Batson and A. P. Phillips, J Pharmacol Exp Ther, 1989, 249, 359 CAS.
- T. Maier, R. Schmierer, K. Bauer, H. .Bieringer, H. Buerstell and B. Sachse, US Pat., 1989 Search PubMed.
- Y. F. Sun, W. Huang, C. G. Lu and Y. P. Cui, Dyes Pigments, 2009, 81, 10 CrossRef CAS.
- M. Stahelin, D. M. Burland, M. Ebert, R. D. Miller, B. A. Smith, R. J. Twieg, W. Volksen and C. A. Walsh, Appl. Phys. Lett., 1992, 61, 1626 CrossRef.
- N. Fridman, M. Kaftory and S. Speiser, Sensor Actuat. B-Chem., 2007, 126, 107 CrossRef CAS.
- J. Liu, J. Chem, J. Zhao, Y. Zhao, L. Li and H. Zhang, Synth., 2003, 2661 CAS.
- M. M. Heravi, K. Bakhtiari, H. A. Oskooie and S. Taheri, J. Mol. Catal. A Chem., 2007, 263, 279 CrossRef CAS.
- A. Parveen, A. Ahmed and S. K. Ahmed, J. Pharm. Biol. Chem. Sci., 2010, 1, 943 CAS.
- E. Chauveau, C. Marestin, F. Schiets and R. Mercier, Green Chem., 2010, 12, 1018 RSC.
- R. B. Sparks and A. P. Combs, Org. Lett., 2004, 6, 2473 CrossRef CAS.
- S. E. Wolkenberg, D. D. Wisnoski, W. H. Leister, Y. Wang, Z. J. Zhao and C. W. Lindsley, Org. Lett., 2004, 6, 1453 CrossRef CAS.
- Y. Xu, L. F. Wan, H. Salchi, W. Deng and Q. X. Guo, Heterocycles, 2004, 63, 1613 CrossRef CAS.
- A. Y. Usyatinsky and Y. L. Khmelnitsky, Tetrahedron Lett., 2000, 41, 5031 CrossRef CAS.
- S. Balalaie, A. Arabanian and M. S. Hashtroudi, Monatsh. Chem., 2000, 131, 945 CrossRef CAS.
- A. Shaabani and A. Rahmati, J. Mol. Catal. A Chem., 2006, 249, 246 CrossRef CAS.
- L. Wang and C. Cai, Monatsh. Chem., 2009, 140, 541 CrossRef CAS.
- K. F. Shelke, S. B. Sapkal, G. K. Kakade, B. B. Shingate and M. S. Shingare, Green Chem. Lett. Rev., 2010, 3, 27–27 CrossRef CAS.
- M. Dinakaran, P. Selvam, E. DeClercq and S. K. Sridhar, Biol Pharm Bull, 2003, 26, 1278 CAS.
- A. R. Desai and K. R. Desai, J. Heterocyclic Chem., 2005, 42, 995 CrossRef CAS.
- S. Kobayashi, M. Ueno, R. Suzuki and H. Ishitani, Tetrahedron Lett., 1999, 40, 2175 CrossRef CAS.
- V. Niementowski, Beilstein, 1895, 24, 143 Search PubMed.
- M. A. Chari, D. Shobha and K. Mukkanti, Catal Commun, 2006, 7, 787 CrossRef CAS.
- B. V. Lingaiah, G. Ezikiel, T. Yakaiah, G. V. Reddy and P. S. Rao, Synlett., 2006, 2507 CAS.
- D. W. Fry, A. J. Kraker, A. McMichael, L. A. Ambroso, J. M. Nelson, W. R. Leopold, R. W. Connors and A. J. Bridges, Science, 1994, 265, 1093 CAS.
- N. S. Gifts, J. Moiler and E. B. Pedersen, Chem. Scripta, 1986, 26, 617 Search PubMed.
- M. Szczepankiewicz and J. Suwinski, Tetrahedron Lett., 1998, 369, 1785 CrossRef.
- M. M. Heravi, S. Sadjadi, N. M. Haj, H. A. Oskooie, R. H. Shoar and F. F. Bamoharram, Tetrahedron Lett., 2009, 50, 943 CrossRef CAS.
- M. Brands, Y. C. Grande, R. Endermann, R. Gahlmann, J. Kruger and S. Raddatz, Bioorg. Med. Chem. Lett., 2003, 13, 2641 CrossRef CAS.
- U. Schmidt and J. Schmidt, Synthesis-Stuttgart, 1994, 300 CrossRef CAS.
- R. A. Barrow, R. E. Moore, L. H. Li and M. A. Tius, Tetrahedron, 2000, 56, 3339 CrossRef CAS.
- R. Bloch, Chem. Rev., 1998, 98, 1407 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, P. S. R. Reddy and M. S. Rao, Tetrahedron Lett., 2002, 43, 6245 CrossRef CAS.
- C. Bellucci, P. G. Cozzi and A. Umanironchi, Tetrahedron Lett., 1995, 36, 7289 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy and A. K. Raju, Synthesis-Stuttgart, 2003, 883 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, A. K. Raju and D. Gnaneshwar, Adv Synth Catal, 2002, 344, 938 CrossRef CAS.
- B. Das, B. Ravikanth, K. Laxminarayana and B. V. Rao, J Mol Catal a-Chem, 2006, 253, 92 CrossRef CAS.
- J. S. Yadav, H. Ather, P. P. Rao, R. S. Rao, K. Nagaiah and A. R. Prasad, Catal Commun, 2006, 7, 797 CrossRef CAS.
- L. Nagarapu, V. Paparaju, G. Pathuri, S. Kantevari, R. R. Pakkiru and R. Kamalla, J Mol Catal a-Chem, 2007, 267, 53 CrossRef CAS.
- Y. Yin, G. Zhao and G. L. Li, Tetrahedron, 2005, 61, 12042 CrossRef CAS.
- H. C. Aspinall, J. S. Bissett, N. Greeves and D. Levin, Tetrahedron Lett., 2002, 43, 323 CrossRef CAS.
- X. Zhengfeng, L. Guilong, Z. Gang and W. Jide, Chinese J. Chem., 2009, 27, 925 CrossRef.
- T. Dingermann, D. Stinhilber and G. Folkers, Molecular Biology in Medicinal Chemistry, Wiley-VCH, Weinheim, 2004 Search PubMed.
- B. Das, K. Laxminarayana, B. Ravikanth and B. R. Rao, J. Mol. Catal. A Chem., 2007, 261, 180 CrossRef CAS.
- N. P. Selvam and P. T. Perumal, Tetrahedron Lett., 2006, 47, 7481 CrossRef CAS.
- M. M. Khodaei, A. R. Khosropour and H. Moghanian, Synlett, 2006, 916 CrossRef CAS.
- H. R. Shaterian, A. Hosseinian, H. Yarahmadi and M. Ghashang, Lett. Org. Chem., 2008, 5, 290 CrossRef CAS.
- A. Dorehgiraee, H. Khabazzadeh and K. Saidi, Arkivoc, 2009, 303 CrossRef CAS.
- S. Kantevari, S. V. N. Vuppalapati and L. Nagarapu, Catal Commun, 2007, 8, 1857 CrossRef CAS.
- L. Nagarapu, M. Baseeruddin, S. Apuri and S. Kantevari, Catal Commun, 2007, 8, 1729 CrossRef CAS.
- J. C. Jun and O. S. Park, Synthetic Commun, 2007, 37, 1665 CrossRef.
- H. R. Shaterian and H. Yarahmadi, Tetrahedron Lett., 2008, 49, 1297 CrossRef CAS.
- H. R. Shaterian, F. Khorami, A. Amirzadeh and M. Ghashang, Chinese J Chem, 2009, 27, 815 CrossRef CAS.
- H. R. Shaterian, H. Yarahmadi and M. Ghashang, Tetrahedron, 2008, 64, 1263 CrossRef CAS.
- H. R. Shaterian, A. Hosseinian and M. Ghashang, Tetrahedron Lett., 2008, 49, 5804 CrossRef CAS.
- B. Das, D. N. Kumar, K. Laxminarayana and B. Ravikanth, Helv. Chim. Acta, 2007, 90, 1330 CrossRef CAS.
- M. Kawase, A. Shah, H. Gaveriya, N. Motohashi, H. Sakagami, A. Varga and J. Molnar, Bioorgan. Med. Chem., 2002, 10, 1051 CrossRef CAS.
- A. C. Gaudio, A. Korolkovas and Y. Takahata, J. Pharm. Sci., 1994, 83, 1110 CrossRef CAS.
- R. Shan, C. Velazquez and E. Knaus, J Med Chem, 2004, 47, 254 CrossRef CAS.
- R. G. Bretzel, C. C. Bollen, E. Maeser and K. F. Federlin, Drugs Future, 1992, 17, 465 Search PubMed.
- T. Itoh, K. Nagata, M. Miyazaki, A. Ishikawa, A. Kurihara and A. Ohsawa, Tetrahedron, 2004, 60, 6649 CrossRef CAS.
- D. M. Stout and A. I. Meyers, Chem.Rev., 1982, 82, 223 CrossRef CAS.
- L. Ohberg and J. Westman, Synlett, 2001, 1296 CrossRef CAS.
- R. Alajarin, J. J. Vaquero, J. L. G. Navio and J. Alvarezbuilla, Synlett, 1992, 297 CrossRef CAS.
- S. J. Ji, Z. Q. Jiang, J. Lu and T. P. Loh, Synlett, 2004, 831 CrossRef CAS.
- L. M. Wang, J. Sheng, L. Zhang, J. W. Han, Z. Y. Fan, H. Tian and C. T. Qian, Tetrahedron, 2005, 61, 1539 CrossRef CAS.
- R. Gupta, R. Gupta, S. Paul and A. Loupy, Synthesis-Stuttgart, 2007, 2835 CAS.
- M. Nikpassand, M. Mamaghani and K. Tabatabaeian, Molecules, 2009, 14, 1468 CrossRef CAS.
- B. Das, K. Suneel, K. Venkateswarlu and B. Ravikanth, Chem. Pharm. Bull., 2008, 56, 366 CrossRef CAS.
- G. Sabitha, G. S. K. Reddy, C. S. Reddy and J. S. Yadav, Tetrahedron Lett., 2003, 44, 4129 CrossRef CAS.
- H. Adibi, H. A. Samimi and M. Beygzadeh, Catal Commun, 2007, 8, 2119 CrossRef CAS.
- S. R. Cherkupalli and R. Mekalan, Chem. Pharm. Bull., 2008, 56, 1002 CrossRef.
- S. K. Singh and K. N. Singh, J. Heterocyclic. Chem., 2010, 47, 194 CAS.
- M. Maheswara, V. Siddaiah, G. Damu, L. V. Guri and C. V. Rao, Arkivoc, 2006, 2, 201 CrossRef.
- G. Song, B. Wang, X. Wu, Y. L. Kang and L. Yang, Synth. Commun., 2005, 35, 2875 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, N. M. Javadi, F. F. Bamoharram, M. Saeedi and H. A. Oskooie, J Mol Catal a-Chem, 2007, 264, 50 CrossRef CAS.
- B. Das, B. Ravikanth, R. Ramu and B. V. Rao, Chem. Pharm. Bull., 2006, 54, 1044 CrossRef CAS.
- S. B. Sapkal, K. F. Shelke, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 1754 CrossRef CAS.
- K. N. Singh and S. K. Singh, Arkivoc, 2009, xiii Search PubMed.
- C. A. Antonyraj and S. Kannan, Appl. Catal. A-Gen., 2008, 338, 121 CrossRef CAS.
- M. J. Climent, A. Corma, S. Iborra and A. Velty, J. Catal., 2004, 221, 474 CrossRef CAS.
- M. J. Climent, A. Corma, S. Iborra, K. Epping and A. Velty, J. Catal., 2004, 225, 316 CrossRef CAS.
- J. G. K. Balasubramanian, Comprehensive Heterocyclic Chemistry II, Pergamon Press, London, 1996 Search PubMed.
- R. H. Bocker and F. P. Guengerich, J Med Chem, 1986, 29, 1596 CrossRef CAS.
- J.-J. Vanden Eynde, R. D'oracio and H. Yves Van, Tetrahedron, 1994, 50, 2479 CrossRef CAS.
- J. R. Pfister, Synthesis, 1990, 689 CrossRef CAS.
- S. H. Mashraqui and M. A. Karnik, Tetrahedron Lett., 1998, 39, 12657 CrossRef.
- M. Balogh, I. Hermecz, Z. Meszaros and P. Laszo, Helv. Chim. Acta, 1984, 2270 CrossRef CAS.
- M. M. Heravi, H. A. Oskooie, R. Malakooti, B. Alimadadi, H. Alinejad and F. K. Behbahani, Catal Commun, 2009, 10, 819 CrossRef CAS.
- O. De Paolis, J. Baffoe, S. M. Landge and B. Torok, Synthesis, 2008, 3423 CAS.
- M. M. Heravi, Y. S. Beheshtia, M. Khorshidi, B. Baghernejad and F. F. Bamoharram, Chinese J Chem, 2009, 27, 569 CrossRef CAS.
- M. T. Cocco, C. Congiu, V. Lilliu and V. Onnis, Eur. J. Med. Chem., 2005, 40, 1365 CrossRef CAS.
- H. Chen, W. Zhang, R. Tam and A. K. Raney, wo Pat., 2005058315, 2005 Search PubMed.
- N. M. Evdokimov, A. S. Kireev, A. A. Yakovenko, M. Y. Antipin, I. V. Magedov and A. Kornienko, J. Org. Chem., 2007, 72, 3443 CrossRef CAS.
- M. Movassaghi and M. D. Hill, J. Am. Chem. Soc., 2006, 128, 4592 CrossRef CAS.
- A. D. Thomas and C. V. Asokan, Tetrahedron Lett, 2002, 43, 2273 CrossRef CAS.
- M. D. Fletcher, T. E. Hurst, T. J. Miles and C. J. Moody, Tetrahedron, 2006, 62, 5454 CrossRef CAS.
- M. Komatsu, H. Ohgishi, S. Takamatsu, Y. Ohshiro and T. Agawa, Angewandte Chemie-International Edition in English, 1982, 21, 213 CrossRef.
- A. R. Renslo and R. L. Danheiser, J Org Chem, 1998, 63, 7840 CrossRef CAS.
- N. M. Evdokimov, I. V. Magedov, A. S. Kireev and A. Kornienko, Org. Lett., 2006, 8, 899 CrossRef CAS.
- K. Guo, M. J. Thompson, T. R. K. Reddy, R. Mutter and B. Chen, Tetrahedron, 2007, 63, 5300 CrossRef CAS.
- M. Sridhar, B. C. Ramanaiah, C. Narsaiah, B. Mahesh, M. Kumaraswamy, K. K. R. Mallu, V. M. Ankathi and P. S. Rao, Tetrahedron Lett., 2009, 50, 3897 CrossRef CAS.
- B. C. Ranu, R. Jana and S. Sowmiah, J Org Chem, 2007, 72, 3152 CrossRef CAS.
- S. Banerjee and G. Sereda, Tetrahedron Lett., 2009, 50, 6959 CrossRef CAS.
- M. L. Kantam, K. Mahendar and S. Bhargava, J Chem Sci, 2010, 122, 63 CrossRef CAS.
- R. Sens, G. Lamm and K. H. Etzbach, EP 420036 (1991).
- A. R. Katritzky, S. Rachwal and T. P. Smith, J. Heterocyclic. Chem., 1995, 32, 1007 CrossRef CAS.
- S. Balalaie, E. Kowsari and M. S. Hashtroudi, Monatsh. Chem., 2003, 134, 453 CrossRef CAS.
- J. R. Casimir, C. Turetta, L. Ettouati and J. Paris, Tetrahedron Lett., 1995, 36, 4797 CAS.
- A. G. Godfrey, D. A Brooks, L. A. Hay, M. Peters, J. R. McCarthy and D. Mitchell, J. Org. Chem., 2003, 68, 2623 CrossRef CAS.
- J. Barluenga, A. L. Viado, E. Aguilar, S. Fustero and B. Olano, J. Org. Chem., 1993, 58, 5972 CrossRef CAS.
- K. Kobinata, M. Uramoto, M. Nishii, H. Kusakabe, G. Nakamura and K. Isono, Agricultural and Biological Chemistry, 1980, 44, 1709 CrossRef CAS.
- H. D. Dakin and R. West, J. Biol. Chem., 1928, 78, 745 CAS.
- B. Bhatia, M. M. Reddy and J. Iqbal, J. Chem. Soc, Chem. Commun., 1994, 713 RSC.
- D. Bahulayan, S. K. Das and M. M. Iqbal, J. Org. Chem., 2003, 68, 5735 CrossRef CAS.
- I. F. Rao, E. N. Prabhakaran, S. K. Das and J. Iqbal, J. Org. Chem., 2003, 68, 4079 CrossRef CAS.
- R. P. Bhat, V. P. Raje, V. M. Alexander, S. B. Patil and S. D. Samant, Tetrahedron Lett., 2005, 46, 4801 CrossRef CAS.
- E. Rafiee, F. Tork and M. Joshaghani, Bioorg. Med. Chem. Lett., 2006, 16, 1221 CrossRef CAS.
- M. M. Heravi, L. Ranjbar, F. Derikvand and F. F. Bamoharram, Catal.Commun., 2007, 8, 289 CrossRef CAS.
- L. Nagarapu, S. Kantevari, V. N. Cheemalapati, S. Apuri and N. V. Kumari, J. Mol. Catal., 2007, 264, 22 CrossRef CAS.
- T. Yakaiah, B. P. V. Lingaiah, G. V. Reddy, B. Narsaiah and P. S. Rao, ARKIVOC, 2007, 227 CrossRef CAS.
- B. Das and K. R. Reddy, Helv. Chim. Act., 2006, 89, 3109 CrossRef CAS.
- B. Das, M. Krishnaiah, K. Laxminarayana and K. R. Reddy, J. Mol. Catal., 2007, 270, 284 CrossRef CAS.
- M. M. Khodaei, A. R. Khosropour and P. Fattahpour, Tetrahedron Lett., 2005, 46, 2105 CrossRef CAS.
- B. Das, R. A. Kumar, P. Thirupathi and Y. Srinivas, Synthetic Commun, 2009, 39, 3305 CrossRef CAS.
- F. Couty and G. Evano, in Comprensive Heterocyclic Chemistry III, Elsevier, Oxford, 2008 Search PubMed.
- Y. Rival, G. Grassy, A. Taudaou and R. Ecalle, Eur. J. Med. Chem., 1991, 26, 13 CrossRef CAS.
- N. Hsua, S. K. Jha, T. Coleman and M. G. Frank, Behav. Brain Res., 2009, 223 Search PubMed.
- M. H. Wiegand, Drugs, 2008, 68, 2411 CrossRef CAS.
- A. Gueiffier, S. Mavel, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chavignon, J. C. Teulade, M. Witvrouw, J. Balzarini, E. De Clercq and J. P. Chapat, J Med Chem, 1998, 41, 5108 CrossRef CAS.
- R. W. Tully and C. R. Gardner, J. Med. Chem., 1991, 34, 2060 CrossRef.
- S. Z. Langer, S. Arbilla, J. Benavides and B. Scatton, Adv. Biochem. Psychopharmacol., 1990, 46, 61 CAS.
- A. R. Katritzky, X.-J. Xu and H. Tu, J. Org. Chem., 2003, 68, 4935 CrossRef CAS.
- H. Bienayme and K. Bouzid, Angew Chem Int Edit, 1998, 37, 2234 CrossRef CAS.
- C. Blackburn, Tetrahedron Lett., 1998, 39, 5469 CrossRef CAS.
- K. Groebke, L. Weber and F. Mehlin, Synlett, 1998, 661 CrossRef CAS.
- A. L. Rousseau, P. Matlaba and C. J. Parkinson, Tetrahedron Lett., 2007, 48, 4079 CrossRef CAS.
- A. Shaabani, F. Rezazadeh and E. Soleimani, Monatsh. Chem., 2008, 139, 931 CrossRef CAS.
- V. Z. Parchinsky, O. Shuvalova, O. Ushakova, D. V. Kravchenko and M. Krasavin, Tetrahedron Lett., 2006, 47, 947 CrossRef CAS.
- A. Shaabani, E. Soleimani, A. Maleki and J. Moghimi-Rad, Mol Divers, 2009, 13, 269 CrossRef CAS.
- M. A. Mironov, M. I. Tokareva, M. N. Ivantsova and V. S. Mokrushin, Russian Chem. Bull. Int. Ed., 2006, 55, 1835 CrossRef CAS.
- A. Shaabani, E. Soleimani and A. Maleki, Tetrahedron Lett., 2006, 47, 3031 CrossRef CAS.
- R. S. Varma and D. Kumar, Tetrahedron Lett., 1999, 40, 7665 CrossRef CAS.
- A. Shaabani, E. Soleimani and A. Maleki, Monatsh. Chem., 2007, 138, 73 CrossRef CAS.
- M. Gopalakrishnan, P. Sureshkumar, J. Thanusu and V. Kanagarajan, J Enzym Inhib Med Ch, 2008, 23, 87 CrossRef CAS.
- J. Xu, Bioorg. Med. Chem. Lett., 2005, 15, 2533 CrossRef CAS.
- R. W. Lamon, J. Org. Chem, 1969, 34, 756 CrossRef CAS.
- N. B. Das, N. Ravindranath, B. Venkataiah and P. Madhusudhan, J. Chem. Res., 2000, 482 CrossRef.
- V. Kanagarajan, P. Sureshkumar, J. Thanusu and G. Gopalakrishnan, Russian J. Org. Chem., 2009, 45(11), 1707 CrossRef CAS.
- P. Kafarski and B. Lejczak, Phosphorous Sulfur Silicon Relat. Elem., 1991, 63, 193 CrossRef CAS.
- E. Alonso, E. Alonso, A. Solis and C. del Pozo, Synlett., 2000, 698 CAS.
- S. K. Chung and D. H. Kang, Tetrahedron Asymmetry, 1996, 7, 21 CrossRef CAS.
- D. Miliszkiewicz, P. Wieczorek, B. Lejczak, E. Kowalik and P. Kafarski, Pestic. Sci., 1992, 34, 349 CrossRef CAS.
- R. Gallardo-Macias and K. Nakayama, Synth., 2010, 1, 57 Search PubMed.
- N. S. Zefirov and E. D. Matveeva, Arkivoc, 2008, 1 CrossRef CAS.
- H. Firouzabadi, N. Iranpoor and S. Sobhani, Synth., 2004, 16, 2692 CrossRef.
- S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2008, 73, 6029 CrossRef CAS.
- K. S. Ambica, S. C. Taneja, M. S. Hundal and K. K. Kapoor, Tetrahedron Lett., 2008, 49, 2208 CrossRef.
- B. Das, G. Satyalakshmi, K. Suneel and K. Damodar, J. Org. Chem., 2009, 74, 8400 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy and P. Sreedhar, Green Chem., 2002, 436 RSC.
- A. K. Battacharya and K. C. Rana, Tetrahedron Lett., 2008, 2598 CrossRef.
- M. Xia and Y. L.u, Ultrason. Sonochem., 2007, 14, 235 CrossRef CAS.
- S. D. Mitragotri, D. M. Pore, U. V. Desai and P. P. Wadgaonkar, Catal Commun, 2008, 9, 1822 CrossRef CAS.
- B. Kaboudin and R. Nazari, Tetrahedron Lett., 2001, 42, 8211 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy and C. Mandan, Synlett, 2001, 7, 1131 CrossRef.
- A. S. Kumar, S. C. Taneja, M. S. Hundal and K. K. Kapoor, Tetrahedron Lett., 2008, 49, 2208 CrossRef.
- A. Vinu, P. Kalita, V. V. Balasubramanian, H. Oveisi, T. Selvan, A. Mano, M. A. Chari and B. V. S. Reddy, Tetrahedron Lett, 2009, 50, 7132 CrossRef CAS.
- A. Heydari, H. Hamadi and M. Pourayoubi, Catal. Commun., 2007, 8, 1224 CrossRef CAS.
- M. T. Maghsoodiou, S. M. Habibi-Khorassani, R. H. Heydari, S. Nourollah and S. R. M. Seyed, Chinese J. Chem., 2010, 28, 285 CrossRef.
- J. V. Johnson, B. S. Rauckman, D. P. Baccanari and B. Roth, J Med Chem, 1989, 32, 1942 CrossRef CAS.
- N. Yamada, S. Kadowaki, K. Takhashi and K. Umeza, Biochem. Pharmacol., 1992, 44, 1211 CrossRef CAS.
- X. Y. Xu, G. W. Qin, R. S. Xu and X. Z. Zhu, Tetrahedron, 1998, 54, 14179 CrossRef CAS.
- M. Cushman and L. G. Chen, J. Org. Chem., 1987, 52, 1517 CrossRef CAS.
- M. Cushman, J. Gentry and F. W. Dekow, J. Org. Chem., 1977, 42, 1111 CrossRef CAS.
- N. F. Yu, L. Bourel, B. Deprez and J. C. Gesquiere, Tetrahedron Lett., 1998, 39, 829 CrossRef CAS.
- I. Vara, T. Bello, E. Aldaba, A. Arrieta, J. L. Pizarro, M. I. Arriortua, X. Lopez and F. P. Cossio, Org. Lett., 2008, 10, 4759 CrossRef.
- N. F. Yu, R. Poulain and J. C. Gesquiere, Synlett, 2000, 355 CAS.
- J. S. Sadav, B. V. S. Reddy, K. S. Raj and A. R. Prasad, Tetrahedron, 2003, 59, 1805 CrossRef.
- L. Wang, J. Liu, H. Tian, C. T. Qian and J. Sun, Adv Synth Catal, 2005, 347, 689 CrossRef CAS.
- J. Azizian, A. A. Mohammadi, A. R. Karimi and M. R. Mohammadizadeh, J. Org. Chem., 2005, 70, 350 CrossRef CAS.
- J. Azizian, A. A. Mohammadi, E. Soleimani, A. R. Karimi and M. R. Mohammadizadeh, J. Heterocyclic Chem., 2006, 43, 187 CrossRef CAS.
- A. R. Karimi and R. Pashazadeh, Synth., 2010, 3, 437 CrossRef.
- V. Michelet and J. P. Genett, Curr. Org. Chem., 2005, 9, 405 CrossRef CAS.
- P. O. Miranda, D. D. Diaz, J. I. Padron, M. A. Ramirez and V. S. Martin, J. Org. Chem., 2005, 70, 57 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, S. Aravind, G. G. K. S. N. Kumar, C. Madhavi and A. C. Kunwar, Tetrahedron, 2008, 64, 3025 CrossRef CAS.
- A. A. W. Long, J. H. C. Nayler, H. Smith, T. Taylor and N. Ward, J. Chem. Soc. C, 1971, 1920 RSC.
- T. Ohashi, S. Takahashi, T. Nagamachi, K. yoneda and H. Yamada, Agric. Biol. Chem., 1981, 45, 831 CrossRef CAS.
- C. Cativiela, J. M. Fraile, J. I. Garcia, B. Lazaro, J. A. Mayoral and A. Pallares, Appl. Catal. A-Gen, 2002, 224, 153 CrossRef CAS.
- R. P. Jain and J. C. Vederas, Bioorg. Med. Chem. Lett., 2004, 4, 1807 Search PubMed.
- S. Grasso, G. Desarro, N. Micale, N. Zappala, G. Puia, M. Beraldi and C. Demicheli, J Med Chem, 2000, 43, 2851 CrossRef CAS.
- Y. Nomoto, H. Obase, H. Takai, M. Teranishi, J. Nakamura and K. Kubo, Chem. Pharm. Bull., 1990, 38, 2179 CrossRef CAS.
- N. Watanabe, Y. Kabasawa, Y. Takase, M. Matsukura, K. Miyazaki, H. Ishihara, K. Kodama and H. Adachi, J Med Chem, 1998, 41, 3367 CrossRef CAS.
- L. P. Liu, J. M. Lu and M. Shi, Org. Lett., 2007, 9, 1303 CrossRef CAS.
- M. Sayyafi, M. Seyyedhamzeh, H. R. Khavasi and A. Bazgir, Tetrahedron, 2008, 64, 2375 CrossRef CAS.
- H. R. Shaterian, M. Ghashang and M. Feyzi, Appl. Catal. A Gen, 2008, 345, 128 CrossRef CAS.
- L. Loy, G. Bonsignore, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517 CrossRef.
- L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517 CrossRef CAS.
- J. M. Quintela, C. Peinador and M. J. Moreira, Tetrahedron, 1995, 51, 5901 CrossRef CAS.
- J. F. Zhou, S. J. Tu, Y. Gao and M. Ji, J. Org. Chem., 2001, 21, 742 CAS.
- Y. Peng and G. Song, Catal Commun, 2007, 8, 111 CrossRef CAS.
- L. Fotouhi, M. M. Heravi, A. Fatehi and K. Bakhtiari, Tetrahedron Lett., 2007, 48, 5379 CrossRef CAS.
- T. S. Jin, J. C. Xiao, S. J. Wang, T. S. Li and X. R. Song, Synlett, 2003, 2001 CrossRef CAS.
- S. Balalaie, M. Bararjanian, A. M. Amani and B. Movassagh, Synlett, 2006, 263 CrossRef CAS.
- N. S. Babu, N. Pasha, K. T. V. Rao, P. S. S. Prasad and N. Lingaiah, Tetrahedron Lett., 2008, 49, 2730 CrossRef CAS.
- M. M. Heravi, S. Sadjadi, H. A. Oskoole, R. H. Shoar and F. F. Bamoharram, Catal Commun, 2008, 9, 470 CrossRef CAS.
- S. Kamaljit, S. Jasbir and S. Harjit, Tetrahedron, 1996, 52, 14273 CrossRef.
- S. Abdolmohammadi and S. Balalaie, Tetrahedron Lett., 2007, 48, 3299 CrossRef CAS.
- A. Shaabani, S. Samadi, Z. Badri and A. Rahmati, Catalysis Lett., 2005, 104, 39 CrossRef CAS.
- M. Seifi and H. Sheibani, Catalysis Lett., 2008, 126, 275 CrossRef CAS.
- M. Witvrouw, B. Van Maele, J. Vercammen, A. Hantson, Y. Engelborghs, E. De Clercq, C. Pannecouque and Z. Debyser, Curr. Drug Metab., 2004, 5, 291 CrossRef CAS.
- M. Kidwai, R. Goyal and K. Singhal, Indian J. Chem. B, 2007, 46, 1159 Search PubMed.
- M. M. Khafagy, A. H. F. Abd El-Wahab, F. A. Eid and A. M. El-Agrody, Farmaco, 2002, 57, 715 CrossRef CAS.
- S. J. Mohr, M. A. Chirigos, F. S. Fuhrman and J. W. Pryor, Cancer Res, 1975, 35, 3750 CAS.
- D. R. Anderson, S. Hegde, E. Reinhard, L. Gomez, W. F. Vernier, L. Lee, S. Liu, A. Sambandam, P. A. Snider and L. Masih, Bioorg. Med. Chem. Lett., 2005, 15, 1587 CrossRef CAS.
- G. Bianchi and A. Tava, Agr Biol Chem Tokyo, 1987, 51, 2001 CrossRef CAS.
- F. Eiden and F. Denk, Arch Pharm, 1991, 324, 353 CrossRef CAS.
- M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295 CrossRef CAS.
- J. Bloxham, C. P. Dell and C. W. Smith, Heterocycles, 1994, 38, 399 CrossRef CAS.
- X. S. Wang, D. Q. Shi, H. Z. Yu, G. F. Wang and S. J. Tu, Synthetic Commun., 2004, 34, 509 CrossRef CAS.
- R. Maggi, R. Ballini, G. Sartori and R. Sartorio, Tetrahedron Lett., 2004, 45, 2297 CrossRef CAS.
- D. Kumar, V. B. Reddy, B. G. Mishra, R. K. Rana, M. N. Nadagouda and R. S. Varma, Tetrahedron, 2007, 63, 3093 CrossRef CAS.
- M. P. Surpur, S. Kshirsagar and S. D. Samant, Tetrahedron Lett., 2009, 50, 719 CrossRef CAS.
- K. Gong, H. L. Wang, D. Fang and Z. L. Liu, Catal. Commun., 2008, 9, 650 CrossRef CAS.
- M. Boronat, M. J. Climent, A. Corma, S. Iborra, R. Monton and M. J. Sabater, Chem. Eur. J., 2010, 16, 1221 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |