Organic–inorganic hybrid photocatalyst for carbon dioxide reduction†
Abstract
Efficient hybrid photocatalysts for carbon dioxide reduction were developed from dye-sensitized TiO2 nanoparticles and their catalytic performance was optimized by ternary organic/inorganic components. Thus, the hybrid system consists of (E)-2-cyano-3-(5′-(5′′-(p-(diphenylamino)phenyl)thiophen-2′′-yl)thiophen-2′-yl)-acrylic acid as a sensitizer and fac-[Re(4,4′-bis(diethoxyphosphorylmethyl)-2,2′-bipyridine)(CO)3Cl] as a reduction catalyst (ReP), both of which have been fixed onto TiO2 semiconductors (s-TiO2, h-TiO2, d-TiO2). Mott–Schottky analysis on flat-band potential (Efb) of TiO2 mesoporous films has verified that Efb can be finely modulated by volume variation of water (0 to 20 vol%). The increase of added water resulted in substantial positive shifts of Efb from −1.93 V at 0 vol% H2O, to −1.74 V (3 vol% H2O), to −1.56 V (10 vol% H2O), and to −1.47 V (20 vol% H2O). As a result, with addition of 3–10 vol% water in the photocatalytic reaction, conversion efficiency of CO2 to CO increased significantly reaching a TON value of ∼350 for 30 h. Catalytic activity enhancement is mainly attributed to (1) the optimum alignment of Efb by 3–10 vol% water with respect to the 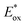 of the dye and Ered of ReP for smooth electron transfer from photo-excited dye to RePvia the TiO2 semiconductor and (2) the water-induced acceleration of chemical processes on the fixed ReP. In addition, the energy level was further tuned by variation of the dye and ReP amounts. We also found that the intrinsic properties of TiO2 sources (morphology, size, agglomeration) exert a great influence on the overall photocatalytic activity of this hybrid system. Implications of the present observations and reaction mechanisms are discussed in detail.
of the dye and Ered of ReP for smooth electron transfer from photo-excited dye to RePvia the TiO2 semiconductor and (2) the water-induced acceleration of chemical processes on the fixed ReP. In addition, the energy level was further tuned by variation of the dye and ReP amounts. We also found that the intrinsic properties of TiO2 sources (morphology, size, agglomeration) exert a great influence on the overall photocatalytic activity of this hybrid system. Implications of the present observations and reaction mechanisms are discussed in detail.
- This article is part of the themed collection: Artificial Photosynthesis

 Please wait while we load your content...
Please wait while we load your content...