 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photon upconversion sensitized by earth-abundant transition metal complexes
Pengyue
Jin
 and
Cui
Wang†
and
Cui
Wang†
 *
*
Department of Biology and Chemistry, Osnabrück University, Barbararstraße 7, Osnabrück 49076, Germany. E-mail: cui.wang@uni-konstanz.de
First published on 20th June 2025
Abstract
Sensitized triplet–triplet annihilation upconversion (sTTA-UC) converts two lower-energy absorbed photons into one emitting photon of higher-energy, and has become a popular approach for a wide range of applications. Current photosensitizers rely mostly on transition metal complexes made of expensive platinum group elements, such as palladium, platinum, and osmium, due to their strong absorption in the visible range, unity intersystem crossing, and long-lived triplet excited lifetimes. In recent years, fundamental breakthroughs have been made with photoactive complexes based on earth-abundant 3d metals including chromium, manganese, iron, cobalt, copper, and zinc, and 4d elements like zirconium and molybdenum. These novel complexes offer advantages, such as cost-effectiveness, sustainability, low toxicity, scalability for industrial use, and potential for innovative research in areas including catalysis and energy conversion, making them promising alternatives to noble metal-based photosensitizers in sTTA-UC and other fields. In this review, we delineate the recent advancements in sTTA-UC utilizing photoactive earth-abundant transition metal complexes. We explore their energy transfer mechanisms, evaluate their upconversion performance, discuss their applications, and outline the challenges and perspectives, aiming to offer insights for the development of novel photosensitizers based on earth-abundant metals for future research and applications.
Introduction
Sensitized triplet–triplet annihilation upconversion (sTTA-UC) converts two low-energy absorbed photons into one higher-energy photon under non-coherent light excitation.1–4 This biphotonic process has attracted significant interest in many fields, including biosensing/imaging,5–12 photovoltaics,13–17 organic light-emitting diodes (OLEDs),18–20 photocatalysis,15,21–36 among others.37 Molecular sTTA-UC relies on the bimolecular interplay between a photosensitizer and an annihilator.10,16,22,38 As shown in Fig. 1a, triplet photosensitizers are often used for triplet–triplet energy transfer (TTET) to the energetically lowest triplet state (T1) of an annihilator, from which triplet–triplet annihilation (TTA) subsequently occurs. This leads to one annihilator released to the ground state and another one populated in the singlet state (S1), from which a photon of higher energy than the absorbed ones is emitted with a delayed nature of μs-scaled lifetimes.2–4,21,39 Key performance parameters for sTTA-UC include the pseudo anti-Stokes shift ΔE, the upconversion quantum yield ΦUC, and the excitation power density threshold Ith. The ΔE refers to the energy gap between the wavelengths of the excitation and the upconversion luminescence maximum (eqn (1)).| ΔE (eV) = EUC − Eexc | (1) |
The upconversion quantum yield (ΦUC) describes the overall efficiency of converting the input photons absorbed by the photosensitizer to the upconverted emission photons from the annihilator. ΦUC depends on the quantum efficiencies of the involved processes, i.e. intersystem crossing (ISC) of the photosensitizer, TTET, TTA, and the fluorescence (ΦFL) of the annihilator, as expressed by eqn (2).53,54
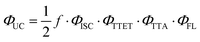 | (2) |
In this equation, the factor ½ indicates the theoretical maximum of 50% for biphotonic sTTA-UC.54f is the spin-statistical factor for generating one singlet spin-state via TTA. Annihilation of two triplet excited chromophores yields nine spin states, including one singlet, three triplet, and five quintet states.53 This leads to a maximum spin-statistical factor fmax of 1/9 (11.1%) for forming the singlet state that gives the upconversion luminescence and therefore a maximal ΦUC of 5.5%. Recent studies found that the f factor depends on the energy levels of the T1, T2, and S1 states for the annihilator, and fmax ∼ 40% (ΦUC,max ∼ 20%) becomes achievable when both the singlet and triplet channels are open.46,53,55,56
Another essential performance parameter for sTTA-UC is the excitation power density threshold (Ith), which indicates the transition of the quadratic dependence of the upconversion luminescence on the excitation power density to a linear regime.4,57,58Ith marks the point when TTA becomes the dominant reaction pathway for the triplet excited state of the annihilator.57,59Ith is affected by the inherent triplet excited state decay (kT) of the annihilator, the TTA rate constant (kTTA), the absorption cross-section (α) at the excitation wavelength as well the concentration [PS] of the photosensitizer, and the TTET efficiency (ΦTTET), as shown in eqn (3).46 Common strategies for lowering the Ith value include extension of the triplet excited state lifetime of the photosensitizer for efficient TTET28 and enhancement of the photosensitizer absorbance at the excitation wavelength.35,57
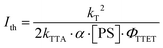 | (3) |
In comparison with the well-known lanthanide-based upconversion (UC) systems, molecular sTTA-UC is often beneficial due to the relatively lower excitation power densities,15,60 the substantially larger absorption coefficients of the photosensitizers than lanthanide absorbers like Yb(III) or Nd(III),61,62 the relatively high achievable ΦUC, and the tunable wavelengths of the excitation source and the delayed fluorescence by the choice of photosensitizer/annihilator combinations.4,21,63 Oxygen quenching of the involved triplet states in sTTA-UC dramatically abates the ΦUC, but this issue can now be addressed by encapsulating the chromophores into nanoconfinements64,65 or employing deoxygenating solvents.35,36,66
To date, triplet photosensitizers featuring excited state properties favorable for efficient sTTA-UC are mostly based on precious platinum metal complexes (Fig. 1b), such as Pd(II), Pt(II),66–74 Ru(II),35,75–78 Ir(III),28,79,80 and Os(II).22,47,48,81–83 However, the use of expensive heavy metals makes these photosensitizers less attractive, due to the high cost, scarcity, environmental damage, toxicity, and over-reliance risks. This leads to the desire for more sustainable and cost-effective alternatives. There has recently been enormous interest in the development of organic triplet photosensitizers for sTTA-UC, such as doublet organic radicals,84–86 TADF fluorophores with small S1–T1 energy gaps,40–45 and organic triplet photosensitizers based on the spin–orbit charge transfer intersystem crossing,87–89 as summarized in recent reviews.38,90 Photosensitizers based on earth-abundant transition metals are furthermore seen as attractive alternatives for sTTA-UC (Fig. 1b).
In recent years, remarkable achievements have been made with photoactive coordination compounds based on earth-abundant transition metals,91–99 including vanadium,100–104 chromium,29,105–114 manganese,115–119 iron,117,120–130 cobalt,131–134 nickel,135–140 copper,141–151 zinc,152–154 zirconium,155,156 and molybdenum.157–162 These fundamentally new metal complexes are considered as more sustainable substitutes for the well-known photosensitizers made of precious metals mentioned above.163–165 Using photoactive earth-abundant metal complexes for sTTA-UC is raising increasing interest, although in many cases their performance in upconversion is inferior to precious metal-based photosensitizers. This is mainly attributed to the fundamental challenges associated with the intrinsic photophysical properties of these complexes and the underexplored energy transfer mechanisms.
This review summarizes the sTTA-UC systems sensitized by earth-abundant metal complexes with focus on their unusual energy transfer mechanisms, the upconversion performance, and the applications based on these sTTA-UC systems. Our objective is to provide a comprehensive analysis that not only underscores the potential of using photoactive earth-abundant metal complexes for the sTTA-UC field, but also underlines the underexplored energy transfer mechanisms that are fundamentally important for photochemistry.
Photon upconversion sensitized by earth-abundant metal complexes and its applications
Herein, we provide an overview of the recent developments of using earth-abundant transition metal complexes as photosensitizers for sTTA-UC, including several photoactive first-row transition metal complexes based on 3d3 Cr(III),112,166 3d5 Fe(III),31,36,130 3d6 Cr(0)27,167 and Mn(I),116 3d10 Cu(I)141,144 and Zn(II),152,168–174 and two second-row 4d0 Zr(IV)175 and 4d6 Mo(0)157,158 metal complexes. The key relevant photophysical parameters of these photosensitizers are summarized in Table 1, and their performance parameters for sTTA-UC are given in Table 2. Additionally, further applications based on these sTTA-UC, such as photocatalysis, are discussed.| Photosensitizer | λ abs [nm] | λ em [nm] | E D/T [eV] | Φ PL [%] | τ PL [ns] | τ T,dark [μs] | Literature |
|---|---|---|---|---|---|---|---|
| a λ abs and λem are the wavelengths of the energetic lowest absorption band maximum and the luminescence peak, respectively. b E D/T stands for the doublet or triplet excited state energy level of the given photosensitizer for energy transfer to the annihilator. c Φ PL is the photoluminescence quantum yield. d τ PL is the photoluminescence lifetime. e τ T,dark is the dark triplet state lifetime. The solvent used for determining the above photophysical parameters is consistent with the solvent used for photon upconversion studies. | |||||||
| [Cr(bpmp)2](PF6)3 | 465 | 709 | 1.75 | — | 890![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 166 |
| [Cr2(dNinp)2(μ-OH)2(μ-O2CMe)](OTf)3 | 500 | 728 | 1.70 | 0.01 | 450 | — | 112 |
| [Fe(ImPP)2](PF6) | 635 | 725 | 1.71 | 0.07 | 0.267 | — | 31 |
| [Fe(CNAnt2)2](Br) | 635 | 725 | 1.82 | 0.08 | 98 | — | 130 |
| [Fe(phtmeimb)2](PF6) | 502 | 670 | 2.13 | 1.82 | 1.60 | — | 36 |
| [Cr(CNtBuAr3NC)3] | 500 | 630 | 2.05 | 0.001 | 2.2 | — | 167 |
| [Cr(Lpyr)3] | 550 | 740 | 1.67 | 0.66 | 24 | — | 27 |
| [Mn(Ltri)2](PF6) | 400 | 525 | 2.3 | 0.03 | 1.73 | — | 116 |
| [Cu(dsbtmp)2](PF6) | ∼450 | 630 | 1.97 | — | 2800 | — | 141 |
| [Cu(dsbp)2](PF6) | ∼460 | 690 | 1.80 | — | 400 | — | 141 |
| ZnTPTBP | 655 | 729 | — | 0.2 | 1.4 | 155.7 | 170 |
| ZnTPPOH | 421 | 660 | — | — | — | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
172 |
| F16ZnPc | — | — | 1.40 | — | — | — | 173 |
| ZnTPP | 549 | 646 | 1.59 | 3.3 | — | 1900 | 23, 168 and 171 |
| [Zn(m-L)2] | 419 | 504 | — | 50 | 25 | 38 | 152 |
| ZnBDP-An | 491 | 518 | — | 0.7 | 1.6 | 295 | 174 |
| ZnBDP-Pyr | 489 | 517 | — | 0.4 | 0.9 | 146 | 174 |
| Zr(MesPDPPh)2 | 525 | 581 | 1.93 | — | 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 175 |
| [Mo(LDMB)2] | 550 | 720 | 1.94 | 1.5 | 54 | — | 157 |
| [Mo(CO)3(tpe)] | 473 | 715 | 1.72 | 0.66 | 355 | — | 158 |
| Photosensitizer | Annihilator (mediator) | Φ DTET/TTET [%] | Φ UC [%] | I th [W cm−2] | λ exc/λUCL [nm] | ΔE [eV] | Ref. |
|---|---|---|---|---|---|---|---|
| a Theoretical maximal ΦUC is set to 50%.54 | |||||||
| [Cr(bpmp)2](PF6)3 | DPA | >90 | 12 | 1.56 | 532/432 | 0.54 | 166 |
| [Cr2(dNinp)2(μ-OH)2(μ-O2CMe)](OTf)3 | AnTIPS | — | — | — | 532/470 | 0.31 | 112 |
| [Fe(ImPP)2](PF6) | BPEA | 4 | 0.019 | 46 | 635/508 | 0.52 | 31 |
| [Fe(CNAnt2)2](Br) | BPEA | 94 | 1.30 | 1.4 | 635/508 | 0.52 | 130 |
| [Fe(phtmeimb)2](PF6) | An | 12.3 | 0.003 | — | 532/407 | 0.72 | 36 |
| PhAn | 14.5 | 0.06 | 52.3 | 532/420 | 0.62 | 36 | |
| PhAn(An) | — | 0.04 | — | 532/420 | 0.62 | 36 | |
| DPA | 22.5(22.9) | 0.03 | — | 532/435 | 0.52 | 36 | |
| DPA(An) | — | 0.19 | — | 532/435 | 0.52 | 36 | |
| DPA(PhAn) | — | 0.16 | — | 532/435 | 0.52 | 36 | |
| [Cr(CNtBuAr3NC)3] | An | — | — | — | 530/405 | 0.72 | 167 |
| [Cr(Lpyr)3] | AnTIPS | ∼40 | 1.8 | 5.9 | 705/470 | 0.88 | 27 |
| [Mn(Ltri)2](PF6) | An | 12.1 | — | — | 420/∼410 | ∼0.07 | 116 |
| [Cu(dsbtmp)2](PF6) | An | >90 | 0.46 | — | 488/384 | 0.69 | 141 |
| DMA | >90 | 4.6 | — | 488/410 | 0.48 | 141 | |
| DPA | >90 | 8.9 | — | 488/414 | 0.45 | 141 | |
| PAC | — | — | 7.73 | 488/∼410 | ∼0.48 | 144 | |
| [Cu(dsbp)2](PF6) | An | >90 | 0.34 | — | 488/404 | 0.53 | 141 |
| ZnTPTBP | Per-Bodipy | — | 0.38 | — | 654/545 | 0.38 | 170 |
| Perylene | — | 0.16 | — | 654/450 | 0.86 | 170 | |
| ZnTPPOH | TBPer | >90 | 12 | 0.359 | 532/∼470 | 0.31 | 172 |
| F16ZnPc | PDI-CH3 | — | — | — | 700/— | — | 173 |
| ZnTPP | ZnTPP | — | — | — | 532/∼430 | 0.55 | 23, 168 and 171 |
| [Zn(m-L)2] | Naph-Tips | — | 0.73 | — | 430/∼370 | 0.47 | 152 |
| ZnBDP-An | Perylene | — | 3.05 | — | 510/∼450 | 0.33 | 174 |
| ZnBDP-Pyr | Perylene | — | 1.65 | — | 510/∼450 | 0.33 | 174 |
| Zr(MesPDPPh)2 | DPA | 95 | 21.35 | — | 514.5/∼404 | 0.66 | 175 |
| CzPA | 95 | 18.7 | — | 514.5/∼413 | 0.59 | 175 | |
| F-CzPA | 95 | 18.9 | — | 514.5/∼413 | 0.59 | 175 | |
| CN-CzPA | 95 | 15.85 | — | 514.5/∼426 | 0.50 | 175 | |
| [Mo(LDMB)3] | DPA | 36 | 1.8 | — | 635/430 | 0.93 | 157 |
| [Mo(CO)3(tpe)] | DPA | 85 | 12 | 0.09 | 514/435 | 0.44 | 158 |
3d3 Cr(III) complexes
Important processes have recently been made with mononuclear luminescent 3d3 Cr(III) complexes with tridentate polypyridyl ligands, the so-called molecular rubies. Many of the spin–flip doublet excited states of these novel Cr(III) complexes show high luminescence quantum yields and extremely long lifetimes up to the ms-scale,107–109,176,177 due to the large ligand field splitting with strongly σ-donating and π-accepting ligands and almost perfectly octahedral geometry,105–109,176,178,179 and some of them exhibit high excited state redox potentials.180–182 These photophysical and photochemical properties make the photoactive Cr(III) complexes attractive for applications relying on energy- and electron-transfer processes,180–188 such as energy transfer or photoredox catalysis.Among the highly luminescent molecular rubies, [Cr(bpmp)2]3+ (bpmp = 2,6-bis(2-pyridylmethyl)pyridine) exhibits at room temperature bright phosphorescence maximized at 709 nm with a near millisecond lifetime from the 2E/2T1 spin–flip excited states (Table 1).166 In the presence of 9,10-diphenylanthracene (DPA, Fig. 2a), it was found that an unusual doublet-triplet energy transfer (DTET) occurs from the 2E/2T1 excited states of the Cr(III) complex to the T1 state of DPA with similar energy levels (Fig. 2c). The small energy gap between these excited states makes the DTET thermodynamically feasible, but also reversible to some extent. Importantly, the DTET is a spin-allowed process according to the Wigner spin conservation rule,189,190 because the total spin of the [Cr(bpmp)2](PF6)3/DPA pair remains unchanged (Fig. 2b). The DTET process was experimentally evidenced with steady-state and time-resolved Stern–Volmer studies and quantified with near unity efficiency using transient absorption spectroscopy.166
 | ||
| Fig. 2 Green-to-blue upconversion sensitized by a 3d3 Cr(III) complex. (a) Photon upconversion luminescence spectrum of the [Cr(bpmp)2](PF6)3/DPA pair under 532 nm laser excitation and their molecular structures. Inset: Image of the [Cr(bpmp)2](PF6)3/DPA pair under green light irradiation. (b) Reaction scheme of the excited states of [Cr(bpmp)2]3+ with the ground state DPA (DTET) and the reverse process TDET, accompanied with their relevant microstates. (c) Energy level diagram of sTTA-UC for the [Cr(bpmp)2](PF6)3/DPA pair. ISC: intersystem crossing, IC: internal conversion, VR: vibrational relaxation, DTET: doublet–triplet energy transfer, TDET: triplet–doublet energy transfer, TTA: triplet–triplet annihilation. (d) [4+4] cycloaddition of anthracene catalyzed by [Cr(bpmp)2](PF6)3via sTTA-UC.166 Reproduced under terms of the CC-BY license from ref. 166. Copyright 2022, Wiley-VCH. | ||
Selective excitation of the [Cr(bpmp)2](PF6)3/DPA pair in deaerated acidified dimethylformamide (DMF) by a 532 nm laser gives blue fluorescence centered at 432 nm from DPA. The upconversion pair was quantified with a high upconversion quantum yield (ΦUC) of 12% (relative to a theoretical limit of 50%54) (Fig. 2a and Table 2), together with an upconversion luminescence lifetime of 162 μs.166 Excitation power density dependence study showed a moderate threshold Ith value of 1.56 W cm−2, which is attributed to the weak absorption of the Cr(III) complex at the excitation wavelength.166 Sterically less hindered anthracenes are known to form a dimer from the singlet excited state.191,192 Using anthracene as the annihilator, [4+4] cycloaddition of anthracene was achieved via sTTA-UC with [Cr(bpmp)2](PF6)3 and green light (Fig. 2d).166 This study provides the proof-of-principle example of using a Cr(III)-based spin–flip emitter for sTTA-UC and UC-driving photocatalysis.
We recently reported another sTTA-UC example utilizing a novel dinuclear Cr(III) complex ([Cr2(dNinp)2(μ-OH)2(μ-O2CMe)](OTf)3) (dNinp = 2,6-di(N-7-azaindol-1-yl)pyridine) as the photosensitizer.112,193 Bridged by two hydroxo- and one carboxylato groups, the ferromagnetically coupled Cr(III) dimer with two dNinp ligands exhibits room temperature phosphorescence maximized at 728 nm with a lifetime of ∼450 ns in acetonitrile (Fig. 3 and Table 1).112 With 9,10-bis((triisopropylsilyl)ethynyl)anthracene (AnTIPS), a well-known blue-emissive annihilator,26,194 DTET from the emissive spin–flip excited states of the Cr(III) dimer to the T1 state of AnTIPS occurs upon selective excitation of the complex at 532 nm, which leads to green-to-blue upconversion with delayed fluorescence (Fig. 3).112 Although the weak photon upconversion makes the quantitative analysis challenging, our proof-of-principle study demonstrates that luminescent Cr(III) dimers are amenable to photon upconversion and energy transfer-based applications.
 | ||
Fig. 3 Photon upconversion luminescence spectra of the Cr(III) dimer/AnTIPS pair in deaerated acetonitrile/toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 20 °C with 50 ns (red traces) and 50 μs (blue traces) time delays after excitation at 532 nm (pulse energy of ∼50 mJ), together with the molecular structures of the upconversion pair.112 Reproduced under terms of the CC-BY license from ref. 112. Copyright 2024, Wiley-VCH. 1) at 20 °C with 50 ns (red traces) and 50 μs (blue traces) time delays after excitation at 532 nm (pulse energy of ∼50 mJ), together with the molecular structures of the upconversion pair.112 Reproduced under terms of the CC-BY license from ref. 112. Copyright 2024, Wiley-VCH. | ||
For sTTA-UC sensitized by Cr(III)-based spin–flip emitters, nearly unity DTET can be achieved with the extremely long excited state lifetime of the Cr(III) complexes, which leads to high upconversion efficiency.166 These sTTA-UC systems are however restricted to small pseudo anti-Stokes shifts, due to the large energy gap between the absorption and the phosphorescence bands of the Cr(III)-based photosensitizers. In contrast to the strong MLCT absorption available in platinum group metal complexes, the weak metal-centered (MC) transitions of Cr(III) complexes account primarily for the comparatively high excitation power density threshold value.
3d5 Fe(III) complexes
Photosensitizers based on iron could be highly attractive for sTTA-UC, due to their high natural abundance, low cost, and low-energy visible light absorption. However, this still remains a significant challenge due to the extremely short excited state lifetimes for, e.g. 3d6 Fe(II) and 3d5 Fe(III) complexes, as a consequence of the excited state deactivation by the energetically low-lying MC states.125,165,195–197Over the past few years, important processes have been achieved with 3d5 low-spin Fe(III) complexes bearing N-heterocyclic carbenes ligands, which show photoluminescence from the doublet ligand-to-metal charge transfer (2LMCT) excited state with a time constant up to multiple nanoseconds.31,120,122,124,130,198 The strong σ-donating character of the carbenes destabilizes the eg orbital and consequently the MC state,199 leading to substantially improved photophysical properties of these Fe(III) complexes,31,120,122,124,198 and their investigations in photoredox catalysis are gaining substantial interest.31,124,129,200–206
Among the luminescent Fe(III) complexes, [Fe(ImP)2]+ (HImP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,1′-(1,3-phenylene)bis(3-methyl-1-imidazol-2-ylidene)) exhibits room temperature luminescence centered at 735 nm from the 2LMCT excited state with a lifetime of 240 ps.122 Decoration of this complex with the different chemical substituents and improved synthesis207 led to [Fe(ImPP)2]+ and [Fe(ImPAr2)2]+ with recognized changes in the 2LMCT excited state properties, including the excited state energies and lifetimes (Table 1), which allow oxidative and reductive photoredox catalysis.31 By covalently linking multiple anthracene-based chromophores to the meta-positions of the outer phenyl group in [Fe(ImPP)2]+ (Fig. 4a), the lifetime of the 2LMCT excited state is extended by 350 fold up to ∼100 ns for [Fe(CNAnt2)2]+, due to the established doublet-triplet energy reservoir between the excited states of the Fe(III) center and the anthracene moieties (Table 1).130
1,1′-(1,3-phenylene)bis(3-methyl-1-imidazol-2-ylidene)) exhibits room temperature luminescence centered at 735 nm from the 2LMCT excited state with a lifetime of 240 ps.122 Decoration of this complex with the different chemical substituents and improved synthesis207 led to [Fe(ImPP)2]+ and [Fe(ImPAr2)2]+ with recognized changes in the 2LMCT excited state properties, including the excited state energies and lifetimes (Table 1), which allow oxidative and reductive photoredox catalysis.31 By covalently linking multiple anthracene-based chromophores to the meta-positions of the outer phenyl group in [Fe(ImPP)2]+ (Fig. 4a), the lifetime of the 2LMCT excited state is extended by 350 fold up to ∼100 ns for [Fe(CNAnt2)2]+, due to the established doublet-triplet energy reservoir between the excited states of the Fe(III) center and the anthracene moieties (Table 1).130
 | ||
| Fig. 4 Red-to-green upconversion sensitized by [Fe(ImPP)2]+ and [Fe(CNAnt2)2]+ with 9,10-bis(phenylethynyl)anthracene (BPEA) as the annihilator. (a) Molecular structures of [Fe(ImPP)2]+, [Fe(CNAnt2)2]+ and BPEA. (b) Energy-level diagram of sTTA-UC with [Fe(ImPP)2]+ and [Fe(CNAnt2)2]+ as the photosensitizers and BPEA as the annihilator. Inset: Photographs of the samples containing only [Fe(CNAnt2)2]+ without BPEA (left) and the upconverting pairs [Fe(CNAnt2)2]+/BPEA (middle) as well as [Fe(ImPP)2]+/BPEA (right) under 635 nm CW-laser excitation (laser power = 1.0 W). (c) Normalized luminescence spectra recorded from a [Fe(CNAnt2)2]+ solution (orange) and a solution containing the [Fe(CNAnt2)2]+/BPEA pair (blue) in deaerated 1,2-dichloroethane at 20 °C following CW-laser excitation at 635 nm. (d) Upconversion luminescence quantum yield ΦUC obtained from the upconversion pairs [Fe(CNAnt2)2]+/BPEA (orange) and [Fe(ImPP)2]+/BPEA (black) as a function of the excitation power density at 635 nm. (e) Log–log plots of the upconversion luminescence intensity as a function of the excitation power density, giving a threshold Ith value of ∼ 1.4 W cm−2 for the [Fe(CNAnt2)2]+/BPEA pair (orange) and ∼46 W cm−2 for the [Fe(ImPP)2]+/BPEA pair (black).31,130 (b)–(e) Reproduced under terms of the CC-BY-NC-ND license from ref. 166. Copyright 2025, American Chemical Society. | ||
Both [Fe(ImPP)2]+ and [Fe(CNAnt2)2]+ permit red-to-green upconversion with 9,10-bis(phenylethynyl)anthracene (BPEA) via an underexplored DTET from the 2LMCT excited state of the Fe(III) complex to the T1 state of BPEA (Fig. 4b).31 Selective excitation of the [Fe(ImPP)2]+/BPEA and the [Fe(CNAnt2)2]+/BPEA pairs with a 635 nm CW-laser leads to green fluorescence from BPEA maximized at 508 nm (Fig. 4c), which shows a delayed nature with microsecond lifetimes.31,130 This corresponds to a pseudo anti-Stokes shift of 0.52 eV. For the [Fe(CNAnt2)2]+/BPEA pair, the maximal achievable ΦUC is determined to be 1.30% (theoretical limit set to 50%54) and the Ith is found at ∼1.4 W cm−2 under their conditions (Fig. 4d, e and Table 2).130 These upconversion performance parameters (ΦUC, Ith) are more than one order of magnitude improved in comparison with [Fe(ImPP)2]+ under identical conditions (ΦUC = 0.019%, Ith = 46 W cm−2).31,130 These significantly enhanced upconversion performances are predominantly attributed to the much longer 2LMCT excited state lifetime for [Fe(CNAnt2)2]+ than [Fe(ImPP)2]+, which increases the DTET efficiency to 94% for [Fe(CNAnt2)2]+ assuming the bimolecular quenching at the diffusion limit of 1,2-DCE at 20 °C (Table 2).31,130 These studies provide the very few examples of utilizing Fe(III) complexes as photosensitizers for sTTA-UC, which paves the way of using Fe(III) complexes for energy transfer-based applications.
[Fe(phtmeimb)2]+ is a well-known 3d5 Fe(III) complex with a photoluminescence quantum yield of 2.1% and a lifetime of ∼2.0 ns from the 2LMCT excited state in acetonitrile at room temperature (Table 1), which enables symmetry-breaking charge separation and photoredox catalysis.120,200,201,203–206,208,209 In a recent study, two anthracene chromophores were covalently attached to the ligands of [Fe(phtmeimb)2]+, giving a Fe(III) dyad complex with μs-scaled lifetime from the dark T1 state of the anthracenes.129 This leads to a ten-fold enhancement of the cage escape quantum yield for the dyad with respect to the parent Fe(III) complex.129
Recently, we used [Fe(phtmeimb)2]+ as the photosensitizer and DPA annihilator for sTTA-UC (Fig. 5a). With a 2LMCT excited state energy of 2.13 eV,120 DTET from the 2LMCT state of [Fe(phtmeimb)2]+ to the T1 state of DPA is thermodynamically feasible with a large driving force of ∼0.4 eV (Fig. 5b),166,210 inhibiting the reversed process. Steady-state luminescence study, time-resolved transient absorption study, and NMR titration synergistically found that [Fe(phtmeimb)2]+ and DPA are preassociated in their ground states via π–π stacking, which is beneficial for distance-dependent Dexter-type energy transfer with a short-lived 2LMCT excited state.36 This leads to enhanced DTET with an efficiency of 22.5%, which exceeds the estimated DTET efficiency (∼9.6%) at the diffusion limit of DMSO at 20 °C.36 Notably, this DTET differs from that for the above-mentioned 3d3 Cr(III) complexes and anthracenes, because the spin of the Fe(III) complex remains unchanged, whereas the spin of DPA changes from singlet to triplet during DTET (Fig. 5c).36 Despite this unusual behavior, the DTET for the [Fe(phtmeimb)2]+/DPA pair is a spin-allowed process with conserved total spin according to the Wigner spin rule (Fig. 5c).189,190,211
 | ||
| Fig. 5 Green-to-blue upconversion and photopolymerization sensitized by [Fe(phtmeimb)2](PF6) with 9,10-diphenylanthracene (DPA) as the annihilator.36 (a) Molecular structures of [Fe(phtmeimb)2](PF6) and DPA. (b) Energy-level diagram of sTTA-UC with the [Fe(phtmeimb)2](PF6)/DPA pair with anthracene (An) as a mediator. DTET: doublet–triplet energy transfer; TTET: triplet–triplet energy transfer; and TTA: triplet–triplet annihilation. (c) Reaction scheme of DTET for the [Fe(phtmeimb)2](PF6)/DPA pair with their corresponding electronic microstates. (d) Upconversion luminescence spectra of [Fe(phtmeimb)2](PF6) (40 μM)/DPA (10 mM) in aerated DMSO at 20 °C, excited with a green 532 nm CW-laser at different powers (1 to 200 mW). Inset: Excitation power density dependence of the upconversion luminescence integral from 410 to 510 nm as a log–log plot. (e) Normalized upconversion luminescence decay at 430 nm recorded from solutions containing [Fe(phtmeimb)2](PF6) (40 μM) with different concentrations of DPA (0.07–7 mM) in aerated DMSO at 20 °C. Excitation occurred with the 532 nm laser (200 mW) with a pulse width of 250 μs. (f) Upconversion luminescence quantum yield (ΦUC) of the [Fe(phtmeimb)2](PF6) (40 μM)/DPA (10 mM) pair in the absence and presence of An (10 mM) as the mediator in aerated DMSO at 20 °C as a function of the excitation power density (532 nm CW-laser).36 (g) Photopolymerization reaction scheme of trimethylolpropane triacrylate (TMPTA) with its respective polymerization images at the indicated irradiation time with a 532 nm CW-laser.36 Reproduced under terms of the CC-BY license from ref. 36. Copyright 2024, American Chemical Society. | ||
Selective excitation of the [Fe(phtmeimb)2]+/DPA pair with a 532 nm CW-laser in aerated DMSO leads to upconverted blue emission from the S1 state of DPA maximized at 435 nm, giving a pseudo anti-Stokes shift ΔE of 0.52 eV (Fig. 5c).36 The integrated upconversion luminescence I410–510 displays a quadratic dependence on the excitation power density (slope of 1.92), indicating the biphotonic nature of TTA (second-order reaction) (Fig. 5d).59 The upconversion luminescence lifetime τUC shows a delayed nature, which ranges from 304 to 110 μs with increasing DPA concentration, due to the more frequent encounters among the T1-excited DPA (Fig. 5e). The reachable upconversion quantum yield ΦUC was determined to be 0.03% (relative to a theoretical limit of 50%)54 for the [Fe(phtmeimb)2]+/DPA pair, which can be enhanced by a factor of six to 0.19% by adding anthracene (An) as a triplet mediator (Fig. 5b, f and Table 2).36
We employed the Fe(III)/DPA upconversion pair to drive the catalytic polymerization of acrylate monomers like trimethylolpropane triacrylate (TMPTA) with low-energy green light (Fig. 5g).36 The S1-excited DPA accessed via sTTA-UC is reductively quenched by triethanolamine (TEOA), giving a strongly reducing DPA radical anion that acts as the initiator for radical polymerization (Fig. 5g).36 However, photoinduced electron transfer with TEOA is kinetically hindered for prompt DPA, due to the substantially shorter lifetime. This study provides a new strategy for photopolymerization by leveraging low-energy light, long upconversion luminescence lifetime, and the photoredox properties of sTTA-UC.
3d6 Cr(0) complexes
In recent years, significant progress has been made with photoactive coordination compounds with a 3d6 valence electronic configuration including Cr(0),29,111,127,162,167,212–214 Mn(I),116,117,214,215 Fe(II),117,121,123,125,126,128 and Co(III) complexes.131–133 Differing from the 4d6 and 5d6 electronic configurations based on platinum group metals, complexes with 3d6 configurations have fundamentally different excited state electronic structures.216 Important understandings of these novel first-row transition metal complexes have been gained, and their promising excited state properties make them emerging photocatalysts.125,126,134,165,217 However, only extremely few examples have employed these complexes for sTTA-UC,27,116,167 mainly due to their very short excited state lifetimes caused by the deactivation from the MC states.The first attempt of using a photoactive 3d6 metal complex for sTTA-UC was made with a Cr(0) complex with chelating diisocyanide ligands (Cr(CNtBuAr3NC)3) as the photosensitizer and anthracene annihilator in deaerated toluene (Fig. 6a).167 Upon excitation of the MLCT absorption centered at ∼500 nm, the Cr(0) complex shows luminescence maximized at 630 nm from the 3MLCT excited state with time constants of 0.64 ns (83%) and 4.33 ns (17%) in toluene at 20 °C (Fig. 6b and Table 1). Despite these short 3MLCT excited state lifetimes, TTET to the T1 state of anthracene (10 mM) occurs, as evidenced by transient absorption signatures of the triplet anthracene with a time constant of ∼170 μs (Fig. 6c and d).167
 | ||
| Fig. 6 Green-to-purple upconversion sensitized by Cr(CNtBuAr3NC)3 with an anthracene annihilator. (a) Molecular structures of Cr(CNtBuAr3NC)3 and anthracene. (b) UV-vis absorption (black trace) and luminescence (red) spectra (upper panel) and UV-vis transient absorption spectrum (green, bottom panel) of Cr(CNtBuAr3NC)3 in deaerated THF at 20 °C. Excitation at 450 nm was used for the luminescence measurement and at 532 nm with a ps-pulsed laser for the transient absorption measurement. (c) Transient absorption spectrum of the Cr(CNtBuAr3NC)3 (20 μM)/anthracene (10 mM) pair in deaerated toluene at 20 °C, and (d) the decay kinetics of the transient absorption signal at 430 nm. Excitation occurred at 532 nm with a ns-pulsed laser. (e) Upconversion luminescence spectra of the sample from (c) at different excitation power densities at 530 nm and the dependence of the relative upconversion luminescence intensity at 405 nm on the relative excitation intensity at 530 nm (inset), and (f) upconversion luminescence decay of the sample from (c) at 403 nm under 532 nm excitation.167 Reproduced with permission from ref. 167. Copyright 2017, American Chemical Society. | ||
For the Cr(CNtBuAr3NC)3/anthracene pair, selective excitation at 530 nm leads to purple fluorescence from anthracene maximized at 405 nm, which decays with a time constant of ∼65 μs (Fig. 6e and f).167 This gives a pseudo anti-Stokes shift of 0.72 eV. The upconversion luminescence exhibits a nonlinear dependence on the excitation power, due to the biphotonic upconversion process.57,59 Notably, the transient absorption study of the upconversion pair reveals a bleaching of the 1MLCT absorption of the Cr(0) complex, which recovers with a time constant of ∼30 μs, predominately due to the Förster-type energy transfer from the upconverted S1 state of anthracene to the 1MLCT state of the Cr(0) complex.167 This proof-of-principle study of using the Cr(0) complex for sTTA-UC promises the potential of using photoactive 3d6 metal complexes for applications based on energy transfer and photon upconversion.
A recent report of a pyrene-decorated Cr(0) isocyanide complex Cr(Lpyr)3 (Fig. 7a) shows a luminescent 3MLCT excited state centered at 740 nm with a lifetime of 24 ns in deaerated toluene at 20 °C (Table 1).29 Using AnTIPS (5 mM) as the annihilator, TTET from the 3MLCT excited state of Cr(Lpyr)3 to the T1 state of AnTIPS occurs, as evidenced with the finger-like transient absorption spectral features of AnTIPS with a decay time constant of 140 μs (Fig. 7b).27 The TTET efficiency was determined to be 40% for the Cr(Lpyr)3 (20 μM)/AnTIPS (5 mM) pair in deaerated toluene at 20 °C, which is mainly restricted by the relatively short lifetime of the photosensitizer.
 | ||
Fig. 7 Red-to-blue upconversion sensitized by Cr(Lpyr)3 with AnTIPS annihilator.27 (a) Molecular structures of Cr(Lpyr)3. (b) Transient absorption spectra of the [Cr(LPyr)3] (20 μM)/AnTIPS (5 mM) pair recorded after different time delays in deaerated toluene at 20 °C. Inset: Transient absorption decay of the upconversion pair recorded at 508 nm under 532 nm excitation. (c) Upconversion luminescence spectrum of the sample from (b) at 20 °C under 705 nm CW-laser excitation (45 mW) (blue trace) and the prompt emission of [Cr(LPyr)3] (20 μM) under 550 nm excitation (red trace). Inset: Decay kinetics of the upconversion luminescence at 470 nm under 532 nm ns-pulsed laser excitation. (d) Excitation power density dependent upconversion luminescence spectra of the sample from (b) at 20 °C and inset: The derived log–log plot of the integrated upconversion luminescence as a function of the excitation power density. Excitation occurred with a 705 nm CW-laser with various power densities (P) (1.3 to 18.6 W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2). (e) ΦUC of the upconversion pair from (b) at 20 °C as a function of the excitation power density (705 nm CW-laser), determined independently with two different references, ZnPc (1 μM) and [Cr(LPyr)3] (20 μM), respectively. Inset: Photograph of the employed reference ZnPc solution (left) and the upconversion pair (right) under 705 nm laser irradiation. (f) Reaction scheme of the photopolymerization of acrylamide using the [Cr(Lpyr)3]/AnTIPS upconversion pair at 705 nm irradiation, and photographs of the aqueous reaction mixture for polymerization before (left), during (middle), and after (right) the 705 nm laser irradiation for several hours. CQ: camphorquinone; TEOA: triethanolamine; DPI: diphenyliodonium chloride.27 Reproduced under terms of the CC-BY-NC-ND license from ref. 27. Copyright 2023, Wiley-VCH. cm−2). (e) ΦUC of the upconversion pair from (b) at 20 °C as a function of the excitation power density (705 nm CW-laser), determined independently with two different references, ZnPc (1 μM) and [Cr(LPyr)3] (20 μM), respectively. Inset: Photograph of the employed reference ZnPc solution (left) and the upconversion pair (right) under 705 nm laser irradiation. (f) Reaction scheme of the photopolymerization of acrylamide using the [Cr(Lpyr)3]/AnTIPS upconversion pair at 705 nm irradiation, and photographs of the aqueous reaction mixture for polymerization before (left), during (middle), and after (right) the 705 nm laser irradiation for several hours. CQ: camphorquinone; TEOA: triethanolamine; DPI: diphenyliodonium chloride.27 Reproduced under terms of the CC-BY-NC-ND license from ref. 27. Copyright 2023, Wiley-VCH. | ||
Selective excitation of the Cr(Lpyr)3/AnTIPS pair at 705 nm yields blue fluorescence from AnTIPS maximized at 470 nm, corresponding to a large pseudo anti-Stokes shift of 0.88 eV.27 The upconversion luminescence decays with a time constant of 72 μs (Fig. 7c), which accounts for roughly half of the observed triplet lifetime of AnTIPS (Fig. 7b), in line with literature reports.28,35,58 Excitation power density dependent study of the upconversion luminescence shows a quadratic to linear dependence with a threshold Ith found at 5.9 W cm−2, above which the upconversion reaches saturation (Fig. 7d).59 In comparison to the red-to-blue sTTA-UC sensitized by Os(II) or Pt(II) complexes, in which the Ith values are often below 1 W cm−2 in solution,51,72,82,83 the relatively high Ith for the Cr(Lpyr)3/AnTIPS pair is attributed to the significantly short 3MLCT lifetime and the weak absorption at the irradiation wavelength.55,57 The maximal achievable ΦUC was determined to be ∼2% for the Cr(Lpyr)3/AnTIPS pair under the chosen conditions (Fig. 7e and Table 2).27 This is a competitive value for red-to-blue upconversion, even in comparison with photosensitizers based on Os(II) complexes.47,50,51,82,83,218,219 In particular, the Cr(Lpyr)3/AnTIPS pair exhibits remarkable photostability under continuous laser irradiation at a high power density (18.6 W cm−2). The high photorobustness of the upconversion luminescence makes it suitable as a blue lamp for initiating the radical polymerization of acrylamide in an aqueous solution with 705 nm irradiation, yielding a free-standing hydrogel of polyacrylamide (Fig. 7f).27 This expands the very few examples of red light induced polymerization via sTTA-UC.220–222
3d6 Mn(I) complexes
Recent breakthroughs with coordination compounds based on 3d6 metals have led to a few photoactive Mn(I) complexes,116,117,223,224 among which two Mn(I) complexes with chelating isocyanide ligands are amenable to sensitizing photon upconversion.116 The Mn(I) complex with tridentate isocyanide ligands (Fig. 8a) exhibits luminescence from the MLCT excited state (2.9 eV) with an average lifetime of 1.73 ns in deaerated acetonitrile at 20 °C and shows a ligand-centered 3π–π* state at ∼2.3 eV that becomes emissive at low temperature (Fig. 8b and Table 1).116The long-lived triplet state of anthracene is sensitized by [Mn(Ltri)2]+via TTET, as evidenced by the characteristic transient absorption signatures of triplet anthracene upon selective excitation of the Mn(I) complex at 420 nm.116 The TTET efficiency was determined with relative actinometry experiments to 12.1% under optimized conditions (Table 2). Notably, the TTET occurs from the 3π–π* excited state to anthracene instead of from the emissive MLCT state, despite the larger driving force for the latter (Fig. 8b). Likely, the emissive MLCT state has a substantial singlet character, which makes the energy transfer to triplet anthracene a spin-forbidden process. Nevertheless, upconversion luminescence from anthracene was observed for the [Mn(Ltri)2]+ (25 μM)/anthracene (40 μM) pair with selective excitation at 420 nm, and no fluorescence was detected in the absence of the Mn(I) complex under identical conditions.116 Analogue energy transfer and sTTA-UC studies were made with another Mn(I) complex with bidentate isocyanide ligands.116 These proof-of-concept studies open the door to using photoactive Mn(I) for sTTA-UC.
3d10 Cu(I) complexes
Photoactive Cu(I) coordination compounds have been extensively explored for decades,142–149,225,226 primarily because the completely filled 3d10 subshell excludes the energetically low-lying MC states that often depopulate the charge transfer (CT) excited states significantly.163,227,228 Recent advances with Cu(I) bis-1,10-phenanthroline complexes provide important guidelines for stabilizing the 3MLCT excited states, leading to a series of Cu(I) complexes with long lifetimes.146,147,149,164,229Among the phenanthroline-based Cu(I) complexes, [Cu(dsbtmp)2](PF6) (dsbtmp = 2,9-di(sec-butyl)-3,4,7,8-tetramethyl-1,10-phenanthroline) (Fig. 9a) exhibits bright luminescence at ∼630 nm with an impressively long lifetime (τ = 1.2–2.8 μs) from the 3MLCT excited state (Table 1), due to the suppressed ground- and excited-state distortion achieved with cooperative steric effects.230 This long lifetime leads to high TTET efficiencies of >90% with millimolar concentrations of the annihilators, i.e., anthracene (An), 9,10-dimethylanthracene (DMA), or DPA (Fig. 9a).141 In the presence of the anthracene derivatives, selective excitation of [Cu(dsbtmp)2](PF6) at 488 nm leads to near-UV fluorescence from the anthracenes, which follows quadratic to linear dependence on the excitation power density in deaerated dichloromethane at room temperature (Fig. 9b and c). This gives Ith values of near 1 W cm−2 for the investigated [Cu(dsbtmp)2](PF6) (0.78 mM)/anthracene pairs (Fig. 9c). These Ith values, which can be tuned by monitoring the absorbance of the photosensitizer,55,231 are above the solar irradiance at this excitation wavelength, but remain lower than many sTTA-UC sensitized by earth-abundant metal complexes.27,31,166 For the [Cu(dsbtmp)2](PF6) (0.12 mM)/DPA (8.9 mM) pair in deaerated dichloromethane, a ΦUC of 8.9% (theoretical maximum of 50%54) was reached (Fig. 9b and Table 2), which is twice higher than the ΦUC obtained with DMA and roughly 10 times higher than that with An, due to the exciplex formation and the low fluorescence quantum yield of the latter.141
 | ||
| Fig. 9 Cyan-to-blue/purple upconversion sensitized by [Cu(dsbtmp)2](PF6) with anthracene derivatives as the annihilator. (a) Molecular structures of [Cu(dsbtmp)2](PF6) and the employed anthracenes. (b) Luminescence spectra of the [Cu(dsbtmp)2](PF6) (0.12 mM)/DPA (8.9 mM) pair in deaerated dichloromethane (green to orange traces) and the luminescence spectra of a [Ru(bpy)3](PF6)2 reference solution in acetonitrile (purple to blue traces) under various excitation powers at 488 nm. Inset: Upconversion quantum yield ΦUC plotted as a function of the excitation power density P.141 (c) Double logarithmic plot of the upconversion luminescence monitored at 385 nm for An as a function of the excitation power density at 488 nm obtained for the [Cu(dsbtmp)2](PF6) (0.76 mM)/An (5 mM) pair. (b) and (c) Reproduced with permission from ref. 141. Copyright 2015, American Chemical Society. (d) Schematic illustration of photon upconversion micelles made of cetyltrimethylammonium bromide surfactant and the [Cu(dsbtmp)2](PF6)/PAC (10-phenylanthracene-9-carboxylate) upconversion pair in water, together with their molecular structures. (e) Excitation power density-dependent upconversion luminescence spectra of the Cu-PS-PAC assembly from (d) at 488 nm excitation and (f) their corresponding double logarithmic plot.144 (d)–(f) Reproduced with permission from ref. 144. Copyright 2020, American Chemical Society. | ||
Changing the annihilator to negatively charged 10-phenylanthracene-9-carboxylate (PAC), the authors later immobilized the [Cu(dsbtmp)2]+/PAC upconversion pair to a micellar assembly made of cetyltrimethylammonium bromide surfactant (Fig. 9d).144 The hydrophobic Cu(I) complex is encapsulated within the micelle, while the anionic PAC binds electrostatically to the cationic assembly surface. Selective excitation of the aqueous upconversion assembly at 488 nm gives the characteristic fluorescence of PAC, and the excitation power density dependence study reveals a Ith value of 7.7 W cm−2 (Fig. 9e and f). This value is substantially higher than the Ith values for the [Cu(dsbtmp)2]+/anthracene pairs in organic solvents,141 which is likely attributed to the relatively lower TTET efficiency and the hindered TTA event in the micellar confinement.55,231 Attempts to increase the photosensitizer concentration failed to give a lower Ith value, because the assembly became turbid at a high loading concentration.144 The [Cu(dsbtmp)2]+/PAC upconversion assembly was further investigated for electron transfer with an electron acceptor methyl viologen (MV2+), yielding the transient absorption features of MV˙+.144 This combination of micellar architecture and photon upconversion system makes photochemistry viable in water with low irradiation energy.
3d10 Zn(II) complexes
Zn(II) porphyrin complexes with ligand-based photophysics have often been used as photosensitizers for sTTA-UC (Fig. 10), due to the low-energy of the Q absorption band, the relatively high intersystem crossing (ISC) efficiency,232 and the long-lived T1 dark state.233 Using perylene derivatives as an annihilator (Fig. 10 and Table 1), several red-to-green or green-to-blue upconversion sensitized by Zn(II) complexes have been reported, which exhibit mostly relatively small pseudo anti-Stokes shifts, as shown in Table 2. Notably, the ZnTPPOH/TBPer upconversion pair shows a high ΦUC of 12% at remarkably low concentrations of the TBPer annihilator (Table 2).172 Solid-state near infrared upconversion was achieved with the F16ZnPc/PDI-CH3 pair (Fig. 10), from which one electron is injected from the upconverted S1-state of PDI-CH3 into TiO2, and this is highly attractive for photovoltaics.173 | ||
| Fig. 10 Molecular structures of the Zn(II)-based photosensitizers and organic annihilators used for sTTA-UC. | ||
Recent fundamental developments for Zn(II) complexes have received substantial attention with triplet charge-transfer states152,234,235 or TADF characteristics.153,236,237 Among these new types of Zn(II) complexes with long-lived triplet excited states, fluorescent [Zn(m-L)2] (Fig. 10) shows a long-lived dark state from the triplet intraligand charge-transfer (3ILCT) (Table 1). This long-lived 3ILCT allows blue-to-UV upconversion with naphthalene substituted with triisopropylsilyl (Naph-Tips, Fig. 10), giving a ΦUC of 0.73%.152
For the ZnTPP derivatives, unusual homomolecular sTTA-UC from the S2 excited state has been frequently observed.23,168,171 Selective excitation of these Zn(II) complexes at 532 nm populates the emissive S2 state at ∼430 nm via TTA between the two ZnTPP molecules.23,168,171 Interestingly, the upconverted S2 state of ZnTPP undergoes electron transfer to acrylate monomers, allowing direct initiation of polymerization reactions with green light.23
4d0 Zr(IV)-based photosensitizer
Zirconium, as the fourth most abundant transition metal, seems attractive for developing low-cost luminophores or photosensitizers. The electron-deficient 4d0 configuration of Zr(IV) allows coordination with electron-rich ligands, e.g. pyridinedipyrrolide (PDP), which elevate Zr(IV) complexes with long phosphorescence lifetimes from the triplet ligand-to-metal charge transfer (3LMCT) state.155,156,238 Zr(MesPDPPh)2 (Fig. 11a) exhibits strong photoluminescence maximized at 581 nm with a high quantum yield (ΦPL) of 45% and a long lifetime of 350 μs in solution at room temperature (Table 1).156 This long-lived 3LMCT excited state promotes near unity TTET to DPA or carbazole-based DPA derivatives (CzPA) (Fig. 11a) at a remarkably low concentration of 0.25 mM. With this annihilator concentration, only linear excitation power density dependence was observed from the upconversion luminescence for the Zr(MesPDPPh)2/DPA pair upon 514 nm excitation (Fig. 11b). At an excitation power density of ∼13 mW cm−2, which is substantially lower than the solar irradiance of 26.7 mW cm−2 in this absorption regime, all investigated photosensitizer/annihilator pairs reach upconversion saturation, giving high ΦUC values ranging from 15.9 to 21.4% (theoretical maximum set to 50%54) (Fig. 11c and Table 2).175 These high ΦUC values, the low required excitation power densities, and the high abundance of zirconium make Zr(MesPDPPh)2 very promising for future solar energy-based applications. | ||
| Fig. 11 Green-to-blue upconversion sensitized by Zr(MesPDPPh)2 with DPA and carbazole-based DPA derivatives (CzPA) as the annihilator.175 (a) Molecular structures of Zr(MesPDPPh)2 and the CzPA annihilators. (b) Double logarithmic plot of integrated upconversion luminescence intensity for the Zr(MesPDPPh)2/DPA (0.25 mM) pair in deaerated THF as a function of the excitation power density at 514 nm, and the corresponding upconversion luminescence spectra (upper inset). Bottom inset: Image of the upconversion sample under 514 nm irradiation. (c) Upconversion quantum yields (theoretical maximum set to 50%54) determined with a Zr(MesPDPPh)2 photosensitizer and DPA or CzPAs annihilator in deaerated THF, plotted as a function of the excitation power density at 514 nm.175 Reproduced under terms of the CC-BY license from ref. 175. Copyright 2021, Royal Society of Chemistry. | ||
4d6 Mo(0)-based complexes
Earth-abundant Mo(0) shares the 4d6 electronic configuration with Ru(II), which makes it an attractive substitute for the precious metal ions.161,162,239 The luminescent Mo(0) complex with chelating diisocyanide ligands ([Mo(LDMB)3]) exhibits strong MLCT absorptions tailing to 550 nm and bright 3MLCT luminescence at ∼720 nm with an excited-state lifetime of 54 ns in toluene at 20 °C (Fig. 12a and Table 1).157 These photophysical properties make [Mo(LDMB)3] a suitable photosensitizer for sTTA-UC with e.g. DPA. Photoexcitation of the [Mo(LDMB)3]/DPA pair with a CW-laser at 635 nm across various excitation power densities leads to delayed fluorescence from DPA, giving a pseudo anti-Stokes shift of 0.93 eV (Fig. 12b and Table 2).157 The achievable ΦUC for the [Mo(LDMB)3]/DPA pair in deaerated toluene was determined to be 1.8% (theoretical maximum of 50%)54 under optimized conditions (Fig. 12c). This ΦUC value approaches that of many sTTA-UC systems with precious metal-based photosensitizers,22 including some of the recently reported systems with a record pseudo anti-Stokes shifts of around 1 eV.71,72,83,218,240 Based on this upconversion performance, the [Mo(LDMB)2]/DPA pair has been used to drive a blue-light-dependent sensitized photoisomerization reaction in a two-vessel setup.157 The upconverted blue light from the NMR tube was reabsorbed by [Ru(bpy)3]2+ (bpy = 2,2′-bipyridine) in the surrounding cuvette, which catalyzes the isomerization of trans-stilbene to cis-stilbene with a reaction yield of 50% after 17 hours of irradiation (Fig. 12d).157 | ||
| Fig. 12 Red-to-blue upconversion and photoisomerization sensitized by [Mo(LDMB)3] with 9,10-diphenylanthracene (DPA) as the annihilator. (a) Molecular structures of [Mo(LDMB)3] as the photosensitizer. (b) Luminescence spectra recorded from a [Mo(LDMB)3] solution (red traces) and a solution containing the [Mo(LDMB)3]/DPA pair (blue traces) in deaerated toluene at 20 °C following CW-laser excitation at 635 nm with different power densities. Inset: Photographs of the upconverting sample with the [Mo(LDMB)3]/DPA pair (left) and the sample containing only [Mo(LDMB)3] but no DPA (right) under 635 nm laser excitation. (c) Upconversion luminescence quantum yield ΦUC obtained with [Mo(L3)3] (12 μM) and DPA (50 mM) (grey triangles) as a function of the excitation power density at 635 nm. (d) Photochemical isomerization reaction in separated vessels via photon upconversion with red irradiation light. A sealed NMR tube containing 13 μM [Mo(LDMB)3] and 60 mM DPA in deaerated toluene is immersed into the cuvette containing 0.3 mM [Ru(bpy)3]2+ and 17 mM trans-stilbene in deaerated CD3CN. Red-to-blue upconversion inside the NMR tube is visible by the naked eye (right).157 Reproduced under terms of the CC-BY-NC-ND license from ref. 157. Copyright 2021, American Chemical Society. | ||
Another sTTA-UC example sensitized by the Mo(0) complex was recently made with [Mo(CO)3(tpe)] (tpe = 1,1,1-tris(pyrid-2-yl)ethane), which was prepared with a high yield over 78% following relatively simple synthetic routes (Fig. 13a).158 This photorobust Mo(0) complex exhibits deep-red 3MLCT luminescence with a lifetime of several hundred nanoseconds (Table 1).158 This long-lived 3MLCT excited state permits efficient TTET to DPA with a ΦTTET of 85%,158 which consequently leads to upconversion luminescence of DPA centered at 435 nm (Fig. 13b). Excitation power density dependent study of the upconversion luminescence reveals the quadratic to linear dependence with a low threshold Ith value found at 90 mW cm−2 and a maximal ΦUC of 12% (theoretical maximum of 50%54) (Fig. 13c and Table 2). This study underscores the potential of tripodal ligand-based carbonyl complexes in photochemical applications, offering a pathway to bypass the use of precious metals and complex ligand synthesis.
 | ||
| Fig. 13 Green-to-blue upconversion sensitized by [Mo(CO)3(tpe)] with DPA as the annihilator. (a) Molecular structure of [Mo(CO)3(tpe)]. (b) Upconversion luminescence spectra recorded from a [Mo(CO)3(tpe)] solution containing 10 mM DPA in deaerated THF following CW-laser excitation at 514 nm with different power densities. Inset: Photographs of the green excitation light (right) and blue upconverted emission (left). (c) Doubly logarithmic plot of the integrated upconverted fluorescence intensity IUCvs. laser power density P. Ith is the threshold excitation power density. (d) Upconversion quantum yield ΦUC (theoretical maximal ΦUC is set to 50%54) as a function of the excitation power density P.158 (b)–(d) Reproduced with permission from ref. 158. Copyright 2023, American Chemical Society. | ||
Conclusion
Substituting precious elements in photoactive complexes with abundant transition metals remains a significant challenge in the fields of coordination chemistry and photochemistry. Unlike the luminescent platinum group metal complexes with commonly long-lived triplet metal-to-ligand charge transfer (3MLCT) excited states, photoactive earth-abundant metal complexes often exhibit more labile and sophisticated excited state landscapes with distinct electronic structures and uncommon spin states. This often leads to short excited state lifetimes that kinetically hinder the diffusional encounter with an annihilator and therefore undermine their performance in photon upconversion, which is particularly true for 3d5 Fe(III)31,36 as well as 3d6 Cr(0)27,167 and Mn(I)116 complexes.To address these fundamental challenges, rationalized molecular design guidelines are needed for these earth-abundant transition metals, as currently being explored by many coordination chemists.91–98 In parallel, deliberate sTTA-UC design concepts have been shown to boost the reactivity of these novel metal complexes and improve their upconversion performance, such as preassociation of the photosensitizer/annihilator pair to circumvent diffusional encounter or introducing a mediator with a long excited state lifetime to enhance the dynamic energy transfer.36,241,242 Driven by the photon upconversion sensitized by earth-abundant metal complexes, energy transfer- and photoredox catalysis, such as photodimerization,166 photopolymerizations23,27,36 and photoisomerization,157 are achieved with low-energy visible light.
Our recent sTTA-UC studies with doublet photosensitizers based on 3d3 Cr(III) and 3d5 low-spin Fe(III) reported unusual doublet-triplet energy transfer (DTET) mechanisms,36,112,166,193 similar to the DTET explored with doublet organic radicals for sTTA-UC.84–86 Differing from the conventional understanding of triplet–triplet energy transfer dynamics, these findings open the door of utilizing metal complexes featuring doublet excited states for photon upconversion and energy transfer-based applications. Collectively, these studies offer an important fundamental understanding of the photophysical behavior for earth-abundant metal complexes and indicate their bright future as photosensitizers in the fields of photon upconversion, light harvesting, and photocatalysis.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
P. J. and C. W. thank the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) for the grants with the project number 535142873.Notes and references
- C. A. Parker and C. G. Hatchard, Proc. Chem. Soc., 1962, 386–387 CAS.
- C. A. Parker, C. G. Hatchard and T. A. Joyce, Nature, 1965, 205, 1282–1284 CrossRef CAS.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- V. Gray, K. Moth-Poulsen, B. Albinsson and M. Abrahamsson, Coord. Chem. Rev., 2018, 362, 54–71 CrossRef CAS.
- Q. Dou, L. Jiang, D. Kai, C. Owh and X. J. Loh, Drug Discovery Today, 2017, 22, 1400–1411 CrossRef CAS PubMed.
- Q. Liu, M. Xu, T. Yang, B. Tian, X. Zhang and F. Li, ACS Appl. Mater. Interfaces, 2018, 10, 9883–9888 CrossRef CAS PubMed.
- Z.-S. Yang, Y. Ning, H.-Y. Yin and J.-L. Zhang, Inorg. Chem. Front., 2018, 5, 2291–2299 RSC.
- L. Huang, E. Kakadiaris, T. Vaneckova, K. Huang, M. Vaculovicova and G. Han, Biomaterials, 2019, 201, 77–86 CrossRef CAS.
- L. Huang, T. Le, K. Huang and G. Han, Nat. Commun., 2021, 12, 1898 CrossRef PubMed.
- T. Schloemer, P. Narayanan, Q. Zhou, E. Belliveau, M. Seitz and D. N. Congreve, ACS Nano, 2023, 17, 3259–3288 CrossRef CAS.
- W. Lin, J. Li, H. Feng, F. Qi and L. Huang, J. Anal. Test., 2023, 7, 327–344 CrossRef.
- A. Prabhakaran, K. K. Jha, R. C. E. Sia, R. A. Arellano Reyes, N. K. Sarangi, M. Kogut, J. Guthmuller, J. Czub, B. Dietzek-Ivanšić and T. E. Keyes, ACS Appl. Mater. Interfaces, 2024, 16, 29324–29337 CrossRef CAS.
- T. F. Schulze and T. W. Schmidt, Energy Environ. Sci., 2015, 8, 103–125 RSC.
- T. Dilbeck and K. Hanson, J. Phys. Chem. Lett., 2018, 9, 5810–5821 CrossRef CAS PubMed.
- B. S. Richards, D. Hudry, D. Busko, A. Turshatov and I. A. Howard, Chem. Rev., 2021, 121, 9165–9195 CrossRef CAS.
- S. E. Seo, H.-S. Choe, H. Cho, H.-I. Kim, J.-H. Kim and O. S. Kwon, J. Mater. Chem. C, 2022, 10, 4483–4496 RSC.
- A. J. Carrod, V. Gray and K. Börjesson, Energy Environ. Sci., 2022, 15, 4982–5016 RSC.
- D. Y. Kondakov, Philos. Trans. R. Soc., A, 2015, 373, 20140321 CrossRef PubMed.
- C. Gao, W. W. H. Wong, Z. Qin, S.-C. Lo, E. B. Namdas, H. Dong and W. Hu, Adv. Mater., 2021, 33, 2100704 CrossRef CAS.
- X. Cao, K. Pan, J. Miao, X. Lv, Z. Huang, F. Ni, X. Yin, Y. Wei and C. Yang, J. Am. Chem. Soc., 2022, 144, 22976–22984 CrossRef CAS.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS.
- P. Bharmoria, H. Bildirir and K. Moth-Poulsen, Chem. Soc. Rev., 2020, 49, 6529–6554 RSC.
- N. Awwad, A. T. Bui, E. O. Danilov and F. N. Castellano, Chem, 2020, 6, 3071–3085 CAS.
- F. Glaser, C. Kerzig and O. S. Wenger, Chem. Sci., 2021, 12, 9922–9933 RSC.
- R. Pérez-Ruiz, Top. Curr. Chem., 2022, 380, 23 CrossRef.
- S. N. Sanders, T. H. Schloemer, M. K. Gangishetty, D. Anderson, M. Seitz, A. O. Gallegos, R. C. Stokes and D. N. Congreve, Nature, 2022, 604, 474–478 CrossRef CAS PubMed.
- C. Wang, C. Wegeberg and O. S. Wenger, Angew. Chem., Int. Ed., 2023, 62, e202311470 CrossRef CAS PubMed.
- H. Li, C. Wang, F. Glaser, N. Sinha and O. S. Wenger, J. Am. Chem. Soc., 2023, 145, 11402–11414 CrossRef CAS PubMed.
- N. Sinha, C. Wegeberg, D. Häussinger, A. Prescimone and O. S. Wenger, Nat. Chem., 2023, 15, 1730–1736 CrossRef CAS PubMed.
- M. Uji, T. J. B. Zähringer, C. Kerzig and N. Yanai, Angew. Chem., Int. Ed., 2023, 62, e202301506 CrossRef CAS PubMed.
- J. Wellauer, F. Ziereisen, N. Sinha, A. Prescimone, A. Velić, F. Meyer and O. S. Wenger, J. Am. Chem. Soc., 2024, 146, 11299–11318 CAS.
- L. Huang and G. Han, Nat. Rev. Chem., 2024, 8, 238–255 CrossRef CAS.
- W. Yao, X. Song, L. Xue, S. Liu, L. Tang, Y. Chen, H. Liu and X. Li, ChemPhotoChem, 2024, 8, e202400184 CrossRef CAS.
- L. R. Beck, K. A. Xie, B. C. Lainhart, T. C. Sherwood, E. R. Welin, C. L. Joe and T. Rovis, ACS Catal., 2024, 14, 18515–18522 CrossRef CAS.
- H. Hammecke, D. Fritzler, N. Vashistha, P. Jin, B. Dietzek-Ivanšić and C. Wang, Chem. - Eur. J., 2024, 30, e202402679 CrossRef CAS.
- P. Jin, X. Xu, Y. Yan, H. Hammecke and C. Wang, J. Am. Chem. Soc., 2024, 146, 35390–35401 CrossRef CAS PubMed.
- D. Yildiz, C. Baumann, A. Mikosch, A. J. C. Kuehne, A. Herrmann and R. Göstl, Angew. Chem., Int. Ed., 2019, 58, 12919–12923 CrossRef CAS.
- K. Ye, M. Imran, X. Chen and J. Zhao, ACS Appl. Opt. Mater., 2024, 2, 1803–1824 CrossRef CAS.
- C. A. Parker and C. G. Hatchard, Proc. Math. Phys. Eng. Sci., 1962, 269, 574–584 Search PubMed.
- T. C. Wu, D. N. Congreve and M. A. Baldo, Appl. Phys. Lett., 2015, 107, 031103 CrossRef.
- J. Peng, X. Guo, X. Jiang, D. Zhao and Y. Ma, Chem. Sci., 2016, 7, 1233–1237 RSC.
- N. Yanai, M. Kozue, S. Amemori, R. Kabe, C. Adachi and N. Kimizuka, J. Mater. Chem. C, 2016, 4, 6447–6451 RSC.
- N. Yanai and N. Kimizuka, Acc. Chem. Res., 2017, 50, 2487–2495 CrossRef CAS PubMed.
- T. J. B. Zähringer, J. A. Moghtader, M. S. Bertrams, B. Roy, M. Uji, N. Yanai and C. Kerzig, Angew. Chem., Int. Ed., 2023, 62, e202215340 CrossRef.
- M. Zheng, Y. Li, Y. Wei, L. Chen, S. Liu and X. Zhou, J. Phys. Chem. C, 2023, 127, 2846–2854 CrossRef CAS.
- A. Olesund, J. Johnsson, F. Edhborg, S. Ghasemi, K. Moth-Poulsen and B. Albinsson, J. Am. Chem. Soc., 2022, 144, 3706–3716 CrossRef CAS PubMed.
- D. Liu, Y. Zhao, Z. Wang, K. Xu and J. Zhao, Dalton Trans., 2018, 47, 8619–8628 RSC.
- Y. Wei, M. Zheng, L. Chen, X. Zhou and S. Liu, Dalton Trans., 2019, 48, 11763–11771 RSC.
- D. Beery, A. Arcidiacono, J. P. Wheeler, J. Chen and K. Hanson, J. Mater. Chem. C, 2022, 10, 4947–4954 RSC.
- Z. Yuan, J. He, Z. Mahmood, L. Xing, S. Ji, Y. Huo and H.-L. Zhang, Dyes Pigm., 2022, 199, 110049 CrossRef CAS.
- Y. Sasaki, M. Oshikawa, P. Bharmoria, H. Kouno, A. Hayashi-Takagi, M. Sato, I. Ajioka, N. Yanai and N. Kimizuka, Angew. Chem., Int. Ed., 2019, 58, 17827–17833 CrossRef CAS PubMed.
- Y. Sasaki, N. Yanai and N. Kimizuka, Inorg. Chem., 2022, 61, 5982–5990 CrossRef CAS PubMed.
- D. G. Bossanyi, Y. Sasaki, S. Wang, D. Chekulaev, N. Kimizuka, N. Yanai and J. Clark, JACS Au, 2021, 1, 2188–2201 CrossRef CAS PubMed.
- Y. Zhou, F. N. Castellano, T. W. Schmidt and K. Hanson, ACS Energy Lett., 2020, 5, 2322–2326 CrossRef CAS.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campione and F. Meinardi, Phys. Chem. Chem. Phys., 2012, 14, 4322–4332 RSC.
- V. Gray, A. Dreos, P. Erhart, B. Albinsson, K. Moth-Poulsen and M. Abrahamsson, Phys. Chem. Chem. Phys., 2017, 19, 10931–10939 RSC.
- A. Haefele, J. Blumhoff, R. S. Khnayzer and F. N. Castellano, J. Phys. Chem. Lett., 2012, 3, 299–303 CrossRef CAS.
- F. Edhborg, A. Olesund and B. Albinsson, Photochem. Photobiol. Sci., 2022, 21, 1143–1158 CrossRef CAS.
- A. Monguzzi, J. Mezyk, F. Scotognella, R. Tubino and F. Meinardi, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 195112 CrossRef.
- U. Resch-Genger and H. H. Gorris, Anal. Bioanal. Chem., 2017, 409, 5855–5874 CrossRef CAS PubMed.
- L. M. Wiesholler, F. Frenzel, B. Grauel, C. Wurth, U. Resch-Genger and T. Hirsch, Nanoscale, 2019, 11, 13440–13449 RSC.
- R. Marin, D. Jaque and A. Benayas, Nanoscale Horiz., 2021, 6, 209–230 RSC.
- T. J. B. Zähringer, M.-S. Bertrams and C. Kerzig, J. Mater. Chem. C, 2022, 10, 4568–4573 RSC.
- S. Baluschev, K. Katta, Y. Avlasevich and K. Landfester, Mater. Horiz., 2016, 3, 478–486 RSC.
- S. H. C. Askes and S. Bonnet, Nat. Rev. Chem., 2018, 2, 437–452 CrossRef.
- H. Zhou, J. Lin, S. Wan and W. Lu, Phys. Chem. Chem. Phys., 2022, 24, 29151–29158 RSC.
- P. Duan, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2013, 135, 19056–19059 CrossRef CAS PubMed.
- T. Ogawa, N. Yanai, A. Monguzzi and N. Kimizuka, Sci. Rep., 2015, 5, 10882 CrossRef CAS PubMed.
- P. W. Zach, S. A. Freunberger, I. Klimant and S. M. Borisov, ACS Appl. Mater. Interfaces, 2017, 9, 38008–38023 CrossRef CAS PubMed.
- D. Dzebo, K. Moth-Poulsen and B. Albinsson, Photochem. Photobiol. Sci., 2017, 16, 1327–1334 CrossRef CAS PubMed.
- N. Nishimura, V. Gray, J. R. Allardice, Z. Zhang, A. Pershin, D. Beljonne and A. Rao, ACS Mater. Lett., 2019, 1, 660–664 CrossRef CAS.
- C. Fan, L. Wei, T. Niu, M. Rao, G. Cheng, J. J. Chruma, W. Wu and C. Yang, J. Am. Chem. Soc., 2019, 141, 15070–15077 CrossRef CAS PubMed.
- S. Gharaati, C. Wang, C. Förster, F. Weigert, U. Resch-Genger and K. Heinze, Chem. - Eur. J., 2020, 26, 1003–1007 CrossRef CAS PubMed.
- M. Kanoh, Y. Matsui, K. Honda, Y. Kokita, T. Ogaki, E. Ohta and H. Ikeda, J. Phys. Chem. B, 2021, 125, 4831–4837 CrossRef CAS PubMed.
- W. Wu, S. Ji, W. Wu, J. Shao, H. Guo, T. D. James and J. Zhao, Chem. - Eur. J., 2012, 18, 4953–4964 CrossRef CAS PubMed.
- P. C. Boutin, K. P. Ghiggino, T. L. Kelly and R. P. Steer, J. Phys. Chem. Lett., 2013, 4, 4113–4118 CrossRef CAS.
- C. Kerzig and O. S. Wenger, Chem. Sci., 2018, 9, 6670–6678 RSC.
- A. C. Sell, J. C. Wetzel, M. Schmitz, A. W. Maijenburg, G. Woltersdorf, R. Naumann and C. Kerzig, Dalton Trans., 2022, 51, 10799–10808 RSC.
- J. Sun, F. Zhong, X. Yi and J. Zhao, Inorg. Chem., 2013, 52, 6299–6310 CrossRef CAS PubMed.
- J. Peng, X. Jiang, X. Guo, D. Zhao and Y. Ma, Chem. Commun., 2014, 50, 7828–7830 RSC.
- S. Amemori, Y. Sasaki, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2016, 138, 8702–8705 CrossRef CAS PubMed.
- Y. Sasaki, S. Amemori, H. Kouno, N. Yanai and N. Kimizuka, J. Mater. Chem. C, 2017, 5, 5063–5067 RSC.
- Y. Wei, Y. Li, M. Zheng, X. Zhou, Y. Zou and C. Yang, Adv. Opt. Mater., 2020, 8, 1902157 CrossRef CAS.
- J. M. O'Shea, Y. J. Yun, A. M. Jamhawi, F. Peccati, G. Jimenez-Oses and A. J. Ayitou, J. Am. Chem. Soc., 2025, 147, 1017–1027 CrossRef PubMed.
- J. Han, Y. Jiang, A. Obolda, P. Duan, F. Li and M. Liu, J. Phys. Chem. Lett., 2017, 8, 5865–5870 CrossRef CAS PubMed.
- Y. Wei, K. An, X. Xu, Z. Ye, X. Yin, X. Cao and C. Yang, Adv. Opt. Mater., 2024, 12, 2301134 CrossRef CAS.
- Z. Wang and J. Zhao, Org. Lett., 2017, 19, 4492–4495 CrossRef CAS PubMed.
- R. A. Arellano-Reyes, A. Prabhakaran, R. C. E. Sia, J. Guthmuller, K. K. Jha, T. Yang, B. Dietzek-Ivanšić, V. McKee and T. E. Keyes, Chem. - Eur. J., 2023, 29, e202300239 CrossRef CAS PubMed.
- Y. Li, J. Zhang, S. E. Zhu, Y. Wei, F. Zhang, L. Chen, X. Zhou and S. Liu, J. Phys. Chem. B, 2023, 127, 8476–8486 CrossRef CAS PubMed.
- X. Zhang, Z. Wang, Y. Hou, Y. Yan, J. Zhao and B. Dick, J. Mater. Chem. C, 2021, 9, 11944–11973 RSC.
- V. W.-W. Yam and W.-K. Kwok, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 1–31 Search PubMed.
- M. Kato, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 33–62 Search PubMed.
- P. S. Wagenknecht, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 63–109 Search PubMed.
- C. Förster and K. Heinze, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 111–159 Search PubMed.
- B. Thomas and A. J. Morris, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 161–187 Search PubMed.
- J. Schaab, P. I. Djurovich and M. E. Thompson, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 189–221 Search PubMed.
- M. V. Appleby, R. A. Cowin and J. A. Weinstein, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 223–267 Search PubMed.
- P. C. Ford, in Advances in Inorganic Chemistry, ed. R. van Eldik and P. C. Ford, Academic Press, 2024, vol. 83, pp. 269–303 Search PubMed.
- V. Ferraro, C. Bizzarri and S. Bräse, Adv. Sci., 2024, 11, 2404866 CrossRef CAS PubMed.
- R. D. Dill, R. I. Portillo, S. G. Shepard, M. P. Shores, A. K. Rappé and N. H. Damrauer, Inorg. Chem., 2020, 59, 14706–14715 CrossRef CAS PubMed.
- M. Dorn, J. Kalmbach, P. Boden, A. Papcke, S. Gomez, C. Förster, F. Kuczelinis, L. M. Carrella, L. A. Büldt, N. H. Bings, E. Rentschler, S. Lochbrunner, L. Gonzalez, M. Gerhards, M. Seitz and K. Heinze, J. Am. Chem. Soc., 2020, 142, 7947–7955 CrossRef CAS PubMed.
- M. Dorn, J. Kalmbach, P. Boden, A. Kruse, C. Dab, C. Reber, G. Niedner-Schatteburg, S. Lochbrunner, M. Gerhards, M. Seitz and K. Heinze, Chem. Sci., 2021, 12, 10780–10790 RSC.
- J. P. Zobel, T. Knoll and L. González, Chem. Sci., 2021, 12, 10791–10801 RSC.
- M. Dorn, D. Hunger, C. Förster, R. Naumann, J. van Slageren and K. Heinze, Chem. - Eur. J., 2023, 29, e202202898 CrossRef CAS PubMed.
- S. Otto, M. Grabolle, C. Förster, C. Kreitner, U. Resch-Genger and K. Heinze, Angew. Chem., Int. Ed., 2015, 54, 11572–11576 CrossRef CAS PubMed.
- C. Wang, S. Otto, M. Dorn, E. Kreidt, J. Lebon, L. Srsan, P. Di Martino-Fumo, M. Gerhards, U. Resch-Genger, M. Seitz and K. Heinze, Angew. Chem., Int. Ed., 2018, 57, 1112–1116 CrossRef CAS PubMed.
- S. Treiling, C. Wang, C. Förster, F. Reichenauer, J. Kalmbach, P. Boden, J. P. Harris, L. M. Carrella, E. Rentschler, U. Resch-Genger, C. Reber, M. Seitz, M. Gerhards and K. Heinze, Angew. Chem., Int. Ed., 2019, 58, 18075–18085 CrossRef CAS PubMed.
- J.-R. Jiménez, B. Doistau, C. M. Cruz, C. Besnard, J. M. Cuerva, A. G. Campaña and C. Piguet, J. Am. Chem. Soc., 2019, 141, 13244–13252 CrossRef PubMed.
- F. Reichenauer, C. Wang, C. Förster, P. Boden, N. Ugur, R. Báez-Cruz, J. Kalmbach, L. M. Carrella, E. Rentschler, C. Ramanan, G. Niedner-Schatteburg, M. Gerhards, M. Seitz, U. Resch-Genger and K. Heinze, J. Am. Chem. Soc., 2021, 143, 11843–11855 CrossRef CAS PubMed.
- N. Sinha, J. R. Jimenez, B. Pfund, A. Prescimone, C. Piguet and O. S. Wenger, Angew. Chem., Int. Ed., 2021, 60, 23722–23728 CrossRef CAS PubMed.
- C. Wegeberg, D. Häussinger and O. S. Wenger, J. Am. Chem. Soc., 2021, 143, 15800–15811 CrossRef CAS PubMed.
- S. Trippmacher, S. Demeshko, A. Prescimone, F. Meyer, O. S. Wenger and C. Wang, Chem. - Eur. J., 2024, 30, e202400856 CrossRef CAS PubMed.
- F. Reichenauer, R. Naumann, C. Förster, W. R. Kitzmann, A.-P. M. Reponen, S. Feldmann and K. Heinze, Chem. Sci., 2024, 15, 20251–20262 RSC.
- F. Reichenauer, D. Zorn, R. Naumann, C. Förster and K. Heinze, Inorg. Chem., 2024, 63, 23487–23496 Search PubMed.
- N. R. East, R. Naumann, C. Förster, C. Ramanan, G. Diezemann and K. Heinze, Nat. Chem., 2024, 16, 827–834 CrossRef CAS PubMed.
- P. Herr, C. Kerzig, C. B. Larsen, D. Häussinger and O. S. Wenger, Nat. Chem., 2021, 13, 956–962 CrossRef CAS PubMed.
- C. Wegeberg, D. Häussinger, S. Kupfer and O. S. Wenger, J. Am. Chem. Soc., 2024, 146, 4605–4619 CrossRef CAS PubMed.
- T. Huang, P. Du, X. Cheng and Y. M. Lin, J. Am. Chem. Soc., 2024, 146, 24515–24525 CrossRef CAS PubMed.
- N. Kaul, E. Asempa, J. A. Valdez-Moreira, J. M. Smith, E. Jakubikova and L. Hammarström, J. Am. Chem. Soc., 2024, 146, 24619–24629 CrossRef CAS PubMed.
- K. S. Kjær, N. Kaul, O. Prakash, P. Chábera, N. W. Rosemann, A. Honarfar, O. Gordivska, L. A. Fredin, K.-E. Bergquist, L. Häggström, T. Ericsson, L. Lindh, A. Yartsev, S. Styring, P. Huang, J. Uhlig, J. Bendix, D. Strand, V. Sundström, P. Persson, R. Lomoth and K. Wärnmark, Science, 2019, 363, 249–253 CrossRef PubMed.
- J. D. Braun, I. B. Lozada, C. Kolodziej, C. Burda, K. M. E. Newman, J. van Lierop, R. L. Davis and D. E. Herbert, Nat. Chem., 2019, 11, 1144–1150 CrossRef CAS PubMed.
- J. Steube, A. Kruse, O. S. Bokareva, T. Reuter, S. Demeshko, R. Schoch, M. A. Arguello Cordero, A. Krishna, S. Hohloch, F. Meyer, K. Heinze, O. Kühn, S. Lochbrunner and M. Bauer, Nat. Chem., 2023, 15, 468–474 CrossRef CAS PubMed.
- J. T. Malme, R. A. Clendening, R. Ash, T. Curry, T. Ren and J. Vura-Weis, J. Am. Chem. Soc., 2023, 145, 6029–6034 CrossRef CAS PubMed.
- Y. Ye, P. Garrido-Barros, J. Wellauer, C. M. Cruz, R. Lescouëzec, O. S. Wenger, J. M. Herrera and J. R. Jiménez, J. Am. Chem. Soc., 2024, 146, 954–960 CrossRef CAS PubMed.
- T. Reuter, D. Zorn, R. Naumann, J. Klett, C. Förster and K. Heinze, Angew. Chem., Int. Ed., 2024, 63, e202406438 CrossRef CAS PubMed.
- L. Lindh, N. W. Rosemann, I. B. Losada, S. Persson, Y. Goriya, H. Fan, O. Gordivska, K. Wärnmark, J. Uhlig, P. Chábera, A. Yartsev and P. Persson, Coord. Chem. Rev., 2024, 506, 215709 CrossRef CAS.
- N. Sinha, J. Wellauer, T. Maisuradze, A. Prescimone, S. Kupfer and O. S. Wenger, J. Am. Chem. Soc., 2024, 146, 10418–10431 CrossRef CAS PubMed.
- R. J. Ortiz, R. Mondal, J. K. McCusker and D. E. Herbert, J. Am. Chem. Soc., 2025, 147, 1694–1708 CrossRef CAS PubMed.
- F. Glaser, S. De Kreijger and L. Troian-Gautier, J. Am. Chem. Soc., 2025, 147, 8559–8567 CrossRef CAS PubMed.
- J. Wellauer, B. Pfund, I. Becker, F. Meyer, A. Prescimone and O. S. Wenger, J. Am. Chem. Soc., 2025, 147, 8760–8768 CrossRef CAS PubMed.
- A. K. Pal, C. Li, G. S. Hanan and E. Zysman-Colman, Angew. Chem., Int. Ed., 2018, 57, 8027–8031 CrossRef CAS PubMed.
- N. Sinha, B. Pfund, C. Wegeberg, A. Prescimone and O. S. Wenger, J. Am. Chem. Soc., 2022, 144, 9859–9873 CrossRef CAS PubMed.
- M. M. Alowakennu, A. Ghosh and J. K. McCusker, J. Am. Chem. Soc., 2023, 145, 20786–20791 CrossRef CAS PubMed.
- A. Y. Chan, A. Ghosh, J. T. Yarranton, J. Twilton, J. Jin, D. M. Arias-Rotondo, H. A. Sakai, J. K. McCusker and D. W. C. MacMillan, Science, 2023, 382, 191–197 CrossRef CAS PubMed.
- Y. S. Wong, M. C. Tang, M. Ng and V. W. Yam, J. Am. Chem. Soc., 2020, 142, 7638–7646 CrossRef CAS PubMed.
- S. I. Ting, S. Garakyaraghi, C. M. Taliaferro, B. J. Shields, G. D. Scholes, F. N. Castellano and A. G. Doyle, J. Am. Chem. Soc., 2020, 142, 5800–5810 CrossRef CAS PubMed.
- T. Ogawa, N. Sinha, B. Pfund, A. Prescimone and O. S. Wenger, J. Am. Chem. Soc., 2022, 144, 21948–21960 CrossRef CAS PubMed.
- T. Ogawa and O. S. Wenger, Angew. Chem., Int. Ed., 2023, 62, e202312851 CrossRef CAS PubMed.
- E. Sutcliffe, D. A. Cagan and R. G. Hadt, J. Am. Chem. Soc., 2024, 146, 15506–15514 CrossRef CAS PubMed.
- M. Gao, W.-P. To, G. S. M. Tong, L. Du, K.-H. Low, Z. Tang, W. Lu and C.-M. Che, Angew. Chem., Int. Ed., 2025, 64, e202414411 CrossRef CAS PubMed.
- C. E. McCusker and F. N. Castellano, Inorg. Chem., 2015, 54, 6035–6042 CrossRef CAS PubMed.
- A. Hossain, A. Bhattacharyya and O. Reiser, Science, 2019, 364, eaav9713 CrossRef PubMed.
- R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich, G. Bertrand and M. E. Thompson, Science, 2019, 363, 601–606 CrossRef CAS PubMed.
- R. Fayad, A. T. Bui, S. G. Shepard and F. N. Castellano, ACS Appl. Energy Mater., 2020, 3, 12557–12564 CrossRef CAS.
- M. Gernert, L. Balles-Wolf, F. Kerner, U. Müller, A. Schmiedel, M. Holzapfel, C. M. Marian, J. Pflaum, C. Lambert and A. Steffen, J. Am. Chem. Soc., 2020, 142, 8897–8909 CrossRef CAS PubMed.
- Y. Yang, F. Doettinger, C. Kleeberg, W. Frey, M. Karnahl and S. Tschierlei, Front. Chem., 2022, 10, 936863 CrossRef CAS PubMed.
- F. Doettinger, Y. Yang, M. Karnahl and S. Tschierlei, Inorg. Chem., 2023, 62, 8166–8178 CrossRef CAS PubMed.
- D. Kim, M. C. Rosko, F. N. Castellano, T. G. Gray and T. S. Teets, J. Am. Chem. Soc., 2024, 146, 19193–19204 CrossRef CAS PubMed.
- F. N. Castellano and M. C. Rosko, Acc. Chem. Res., 2024, 57, 2872–2886 CrossRef CAS PubMed.
- C. Bizzarri, Eur. J. Org. Chem., 2022, e202200185 CrossRef CAS.
- C. Bruschi, X. Gui, O. Fuhr, W. Klopper and C. Bizzarri, Dalton Trans., 2023, 52, 7809–7818 RSC.
- J. A. Kübler, B. Pfund and O. S. Wenger, JACS Au, 2022, 2, 2367–2380 CrossRef PubMed.
- O. Mrózek, M. Mitra, B. Hupp, A. Belyaev, N. Lüdtke, D. Wagner, C. Wang, O. S. Wenger, C. M. Marian and A. Steffen, Chem. - Eur. J., 2023, 29, e202203980 CrossRef PubMed.
- M. Mitra, O. Mrózek, M. Putscher, J. Guhl, B. Hupp, A. Belyaev, C. M. Marian and A. Steffen, Angew. Chem., Int. Ed., 2024, 63, e202316300 CrossRef CAS PubMed.
- Y. Zhang, T. S. Lee, J. L. Petersen and C. Milsmann, J. Am. Chem. Soc., 2018, 140, 5934–5947 CrossRef CAS PubMed.
- Y. Zhang, T. S. Lee, J. M. Favale, D. C. Leary, J. L. Petersen, G. D. Scholes, F. N. Castellano and C. Milsmann, Nat. Chem., 2020, 12, 345–352 CrossRef CAS PubMed.
- J. B. Bilger, C. Kerzig, C. B. Larsen and O. S. Wenger, J. Am. Chem. Soc., 2021, 143, 1651–1663 CrossRef CAS PubMed.
- W. R. Kitzmann, M. S. Bertrams, P. Boden, A. C. Fischer, R. Klauer, J. Sutter, R. Naumann, C. Förster, G. Niedner-Schatteburg, N. H. Bings, J. Hunger, C. Kerzig and K. Heinze, J. Am. Chem. Soc., 2023, 145, 16597–16609 CrossRef CAS PubMed.
- W. R. Kitzmann, D. Hunger, A. M. Reponen, C. Förster, R. Schoch, M. Bauer, S. Feldmann, J. van Slageren and K. Heinze, Inorg. Chem., 2023, 62, 15797–15808 CrossRef CAS PubMed.
- T. Jin, D. Wagner and O. S. Wenger, Angew. Chem., Int. Ed., 2024, 63, e202314475 CrossRef CAS PubMed.
- T. Jin, N. Sinha, D. S. Wagner, A. Prescimone, D. Häussinger and O. S. Wenger, J. Am. Chem. Soc., 2025, 147, 4587–4594 CrossRef CAS PubMed.
- A. C. Fischer, C. Förster, W. R. Kitzmann and K. Heinze, Inorg. Chem., 2025, 64, 6100–6114 CrossRef CAS PubMed.
- C. Förster and K. Heinze, Chem. Soc. Rev., 2020, 49, 1057–1070 RSC.
- C. E. Housecroft and E. C. Constable, Chem. Sci., 2022, 13, 1225–1262 RSC.
- N. Sinha and O. S. Wenger, J. Am. Chem. Soc., 2023, 145, 4903–4920 CrossRef CAS PubMed.
- C. Wang, F. Reichenauer, W. R. Kitzmann, C. Kerzig, K. Heinze and U. Resch-Genger, Angew. Chem., Int. Ed., 2022, 61, e202202238 CrossRef CAS PubMed.
- L. A. Büldt, X. Guo, R. Vogel, A. Prescimone and O. S. Wenger, J. Am. Chem. Soc., 2017, 139, 985–992 CrossRef PubMed.
- J. A. O’Brien, S. Rallabandi, U. Tripathy, M. F. Paige and R. P. Steer, Chem. Phys. Lett., 2009, 475, 220–222 CrossRef.
- S. K. Sugunan, U. Tripathy, S. M. K. Brunet, M. F. Paige and R. P. Steer, J. Phys. Chem. A, 2009, 113, 8548–8556 CrossRef CAS PubMed.
- X. Cui, J. Zhao, P. Yang and J. Sun, Chem. Commun., 2013, 49, 10221–10223 RSC.
- R. Rautela, N. K. Joshi, S. Novakovic, W. W. H. Wong, J. M. White, K. P. Ghiggino, M. F. Paige and R. P. Steer, Phys. Chem. Chem. Phys., 2017, 19, 23471–23482 RSC.
- N. A. Durandin, J. Isokuortti, A. Efimov, E. Vuorimaa-Laukkanen, N. V. Tkachenko and T. Laaksonen, Chem. Commun., 2018, 54, 14029–14032 RSC.
- K. M. Felter, M. C. Fravventura, E. Koster, R. D. Abellon, T. J. Savenije and F. C. Grozema, ACS Energy Lett., 2020, 5, 124–129 CrossRef CAS PubMed.
- Z. Mahmood, N. Rehmat, S. Ji, J. Zhao, S. Sun, M. Di Donato, M. Li, M. Teddei and Y. Huo, Chemistry, 2020, 26, 14912–14918 CrossRef CAS PubMed.
- M. Yang, S. Sheykhi, Y. Zhang, C. Milsmann and F. N. Castellano, Chem. Sci., 2021, 12, 9069–9077 RSC.
- S. Otto, M. Dorn, C. Förster, M. Bauer, M. Seitz and K. Heinze, Coord. Chem. Rev., 2018, 359, 102–111 CrossRef CAS.
- W. R. Kitzmann, J. Moll and K. Heinze, Photochem. Photobiol. Sci., 2022, 21, 1309–1331 CrossRef CAS PubMed.
- J. R. Jiménez, M. Poncet, S. Miguez-Lago, S. Grass, J. Lacour, C. Besnard, J. M. Cuerva, A. G. Campaña and C. Piguet, Angew. Chem., Int. Ed., 2021, 60, 10095–10102 CrossRef PubMed.
- Y. Cheng, Q. Yang, J. He, W. Zou, K. Liao, X. Chang, C. Zou and W. Lu, Dalton Trans., 2023, 52, 2561–2565 RSC.
- T. H. Bürgin, F. Glaser and O. S. Wenger, J. Am. Chem. Soc., 2022, 144, 14181–14194 Search PubMed.
- S. Sittel, R. Naumann and K. Heinze, Front. Chem., 2022, 10, 887439 Search PubMed.
- S. Sittel, A. C. Sell, K. Hofman, C. Wiedemann, J. P. Nau, C. Kerzig, G. Manolikakes and K. Heinze, ChemCatChem, 2023, 15, e202201562 CrossRef CAS.
- J. Kalmbach, C. Wang, Y. You, C. Förster, H. Schubert, K. Heinze, U. Resch-Genger and M. Seitz, Angew. Chem., Int. Ed., 2020, 59, 18804–18808 CrossRef CAS PubMed.
- F. A. Baptista, D. Krizsan, M. Stitch, I. V. Sazanovich, I. P. Clark, M. Towrie, C. Long, L. Martinez-Fernandez, R. Improta, N. A. P. Kane-Maguire, J. M. Kelly and S. J. Quinn, J. Am. Chem. Soc., 2021, 143, 14766–14779 CrossRef CAS PubMed.
- C. Förster and K. Heinze, Chem. Phys. Rev., 2022, 3, 041302 CrossRef.
- W. R. Kitzmann and K. Heinze, Angew. Chem., Int. Ed., 2022, 62, e202213207 CrossRef PubMed.
- C. Wang, K. Ebel, K. Heinze, U. Resch-Genger and I. Bald, Chem. - Eur. J., 2023, 29, e202203719 CrossRef CAS PubMed.
- C. Wang, H. Li, T. H. Bürgin and O. S. Wenger, Nat. Chem., 2024, 16, 1151–1159 CrossRef CAS PubMed.
- E. Wigner, Math Physik, Kl, IIa, 1927, 375–381 Search PubMed.
- A. R. Lee, C. S. Enos and A. G. Brenton, Int. J. Mass Spectrom. Ion Processes, 1991, 104, 49–62 CrossRef CAS.
- K. S. Wei and R. Livingston, Photochem. Photobiol., 1967, 6, 229–232 CrossRef CAS.
- G. W. Breton and X. Vang, J. Chem. Educ., 1998, 75, 81 CrossRef CAS.
- D. Fritzler and C. Wang, ChemPhotoChem, 2025, e202500094 Search PubMed.
- J. K. Li, M. Y. Zhang, L. Zeng, L. Huang and X. Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202303093 CrossRef CAS PubMed.
- S. G. Shepard, S. M. Fatur, A. K. Rappé and N. H. Damrauer, J. Am. Chem. Soc., 2016, 138, 2949–2952 CrossRef CAS PubMed.
- P. Dierks, Y. Vukadinovic and M. Bauer, Inorg. Chem. Front., 2022, 9, 206–220 RSC.
- K. Witas, S. S. Nair, T. Maisuradze, L. Zedler, H. Schmidt, P. Garcia-Porta, A. S. J. Rein, T. Bolter, S. Rau, S. Kupfer, B. Dietzek-Ivanšić and D. U. Sorsche, J. Am. Chem. Soc., 2024, 146, 19710–19719 CrossRef CAS PubMed.
- P. Chábera, Y. Liu, O. Prakash, E. Thyrhaug, A. E. Nahhas, A. Honarfar, S. Essén, L. A. Fredin, T. C. Harlang, K. S. Kjær, K. Handrup, F. Ericson, H. Tatsuno, K. Morgan, J. Schnadt, L. Häggström, T. Ericsson, A. Sobkowiak, S. Lidin, P. Huang, S. Styring, J. Uhlig, J. Bendix, R. Lomoth, V. Sundström, P. Persson and K. Wärnmark, Nature, 2017, 543, 695–699 CrossRef PubMed.
- P. Chábera, L. Lindh, N. W. Rosemann, O. Prakash, J. Uhlig, A. Yartsev, K. Wärnmark, V. Sundström and P. Persson, Coord. Chem. Rev., 2021, 426, 213517 CrossRef.
- A. Aydogan, R. E. Bangle, A. Cadranel, M. D. Turlington, D. T. Conroy, E. Cauët, M. L. Singleton, G. J. Meyer, R. N. Sampaio, B. Elias and L. Troian-Gautier, J. Am. Chem. Soc., 2021, 143, 15661–15673 CrossRef CAS PubMed.
- A. Ilic, J. Schwarz, C. Johnson, L. H. M. de Groot, S. Kaufhold, R. Lomoth and K. Wärnmark, Chem. Sci., 2022, 13, 9165–9175 RSC.
- J. Schwarz, A. Ilic, C. Johnson, R. Lomoth and K. Wärnmark, Chem. Commun., 2022, 58, 5351–5354 RSC.
- L. H. M. de Groot, A. Ilic, J. Schwarz and K. Wärnmark, J. Am. Chem. Soc., 2023, 145, 9369–9388 Search PubMed.
- S. De Kreijger, A. Ripak, B. Elias and L. Troian-Gautier, J. Am. Chem. Soc., 2024, 146, 10286–10292 CrossRef CAS PubMed.
- A. Ripak, A. K. Vega Salgado, D. Valverde, S. Cristofaro, A. de Gary, Y. Olivier, B. Elias and L. Troian-Gautier, J. Am. Chem. Soc., 2024, 146, 22818–22828 CrossRef CAS PubMed.
- A. Ilic, B. R. Strücker, C. E. Johnson, S. Hainz, R. Lomoth and K. Wärnmark, Chem. Sci., 2024, 15, 12077–12085 RSC.
- C. E. Johnson, J. Schwarz, M. Deegbey, O. Prakash, K. Sharma, P. Huang, T. Ericsson, L. Häggström, J. Bendix, A. K. Gupta, E. Jakubikova, K. Wärnmark and R. Lomoth, Chem. Sci., 2023, 14, 10129–10139 RSC.
- N. Kaul and R. Lomoth, J. Am. Chem. Soc., 2021, 143, 10816–10821 CrossRef CAS PubMed.
- M. Zhang, C. E. Johnson, A. Ilic, J. Schwarz, M. B. Johansson and R. Lomoth, J. Am. Chem. Soc., 2023, 145, 19171–19176 CrossRef CAS PubMed.
- A. Farrán and K. D. Deshayes, J. Phys. Chem., 1996, 100, 3305–3307 Search PubMed.
- D. Guo, T. E. Knight and J. K. McCusker, Science, 2011, 334, 1684–1687 Search PubMed.
- L. A. Büldt and O. S. Wenger, Angew. Chem., Int. Ed., 2017, 56, 5676–5682 CrossRef PubMed.
- P. Boden, P. Di Martino-Fumo, T. Bens, S. Steiger, U. Albold, G. Niedner-Schatteburg, M. Gerhards and B. Sarkar, Chemistry, 2021, 27, 12959–12964 Search PubMed.
- C. Wegeberg and O. S. Wenger, Dalton Trans., 2022, 51, 1297–1302 Search PubMed.
- M. Scaccaglia, M. P. Birbaumer, S. Pinelli, G. Pelosi and A. Frei, Chem. Sci., 2024, 15, 3907–3919 Search PubMed.
- J. K. McCusker, Science, 2019, 363, 484–488 Search PubMed.
- C. Wegeberg and O. S. Wenger, JACS Au, 2021, 1, 1860–1876 Search PubMed.
- R. Haruki, Y. Sasaki, K. Masutani, N. Yanai and N. Kimizuka, Chem. Commun., 2020, 56, 7017–7020 Search PubMed.
- F. Glaser and O. S. Wenger, Chem. Sci., 2023, 14, 149–161 Search PubMed.
- B. D. Ravetz, A. B. Pun, E. M. Churchill, D. N. Congreve, T. Rovis and L. M. Campos, Nature, 2019, 565, 343–346 CrossRef CAS PubMed.
- A. Caron, G. Noirbent, D. Gigmes, F. Dumur and J. Lalevée, Macromol. Rapid Commun., 2021, 42, 2100047 Search PubMed.
- W. Liang, C. Nie, J. Du, Y. Han, G. Zhao, F. Yang, G. Liang and K. Wu, Nat. Photonics, 2023, 17, 346–353 Search PubMed.
- S. Ossinger, A. Prescimone, D. Häussinger and O. S. Wenger, Inorg. Chem., 2022, 61, 10533–10547 CrossRef CAS PubMed.
- S. Kronenberger, R. Naumann, C. Förster, N. East, J. Klett and K. Heinze, ChemRxiv, 2024preprint DOI:10.26434/chemrxiv-2024-gzvbj.
- A. Lavie-Cambot, M. Cantuel, Y. Leydet, G. Jonusauskas, D. M. Bassani and N. D. McClenaghan, Coord. Chem. Rev., 2008, 252, 2572–2584 CrossRef CAS.
- M. S. Lazorski and F. N. Castellano, Polyhedron, 2014, 82, 57–70 CrossRef CAS.
- O. S. Wenger, J. Am. Chem. Soc., 2018, 140, 13522–13533 CrossRef CAS PubMed.
- J. Beaudelot, S. Oger, S. Peruško, T. A. Phan, T. Teunens, C. Moucheron and G. Evano, Chem. Rev., 2022, 122, 16365–16609 CrossRef CAS PubMed.
- L. Gimeno, B. T. Phelan, E. A. Sprague-Klein, T. Roisnel, E. Blart, C. Gourlaouen, L. X. Chen and Y. Pellegrin, Inorg. Chem., 2022, 61, 7296–7307 Search PubMed.
- C. E. McCusker and F. N. Castellano, Inorg. Chem., 2013, 52, 8114–8120 Search PubMed.
- N. A. Durandin, J. Isokuortti, A. Efimov, E. Vuorimaa-Laukkanen, N. V. Tkachenko and T. Laaksonen, J. Phys. Chem. C, 2019, 123, 22865–22872 CrossRef CAS.
- J. E. Rogers, K. A. Nguyen, D. C. Hufnagle, D. G. McLean, W. Su, K. M. Gossett, A. R. Burke, S. A. Vinogradov, R. Pachter and P. A. Fleitz, J. Phys. Chem. A, 2003, 107, 11331 Search PubMed.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of Photochemistry, CRC Press, Boca Raton, 3rd edn, 2006 Search PubMed.
- D. Temerova, K. S. Kisel, T. Eskelinen, A. S. Melnikov, N. Kinnunen, P. Hirva, J. R. Shakirova, S. P. Tunik, E. V. Grachova and I. O. Koshevoy, Inorg. Chem. Front., 2021, 8, 2549–2560 Search PubMed.
- O. Mrózek, M. Gernert, A. Belyaev, M. Mitra, L. Janiak, C. M. Marian and A. Steffen, Chem. - Eur. J., 2022, 28, e202201114 Search PubMed.
- Y. Sakai, Y. Sagara, H. Nomura, N. Nakamura, Y. Suzuki, H. Miyazaki and C. Adachi, Chem. Commun., 2015, 51, 3181–3184 Search PubMed.
- A. S. Berezin, K. A. Vinogradova, V. P. Krivopalov, E. B. Nikolaenkova, V. F. Plyusnin, A. S. Kupryakov, N. V. Pervukhina, D. Y. Naumov and M. B. Bushuev, Chem. - Eur. J., 2018, 24, 12790–12795 Search PubMed.
- Y. Zhang, J. L. Petersen and C. Milsmann, J. Am. Chem. Soc., 2016, 138, 13115–13118 Search PubMed.
- L. A. Büldt, X. Guo, A. Prescimone and O. S. Wenger, Angew. Chem., Int. Ed., 2016, 55, 11247–11250 Search PubMed.
- N. Kiseleva, P. Nazari, C. Dee, D. Busko, B. S. Richards, M. Seitz, I. A. Howard and A. Turshatov, J. Phys. Chem. Lett., 2020, 11, 2477–2481 Search PubMed.
- L. Hou, A. Olesund, S. Thurakkal, X. Zhang and B. Albinsson, Adv. Funct. Mater., 2021, 31, 2106198 Search PubMed.
- F. Glaser, M. Schmitz and C. Kerzig, Nanoscale, 2024, 16, 123–137 RSC.
Footnote |
| † Current address: Department of Chemistry, University of Konstanz, Universitätsstraße 10, Konstanz 78464, Germany. |
| This journal is © the Owner Societies 2025 |




