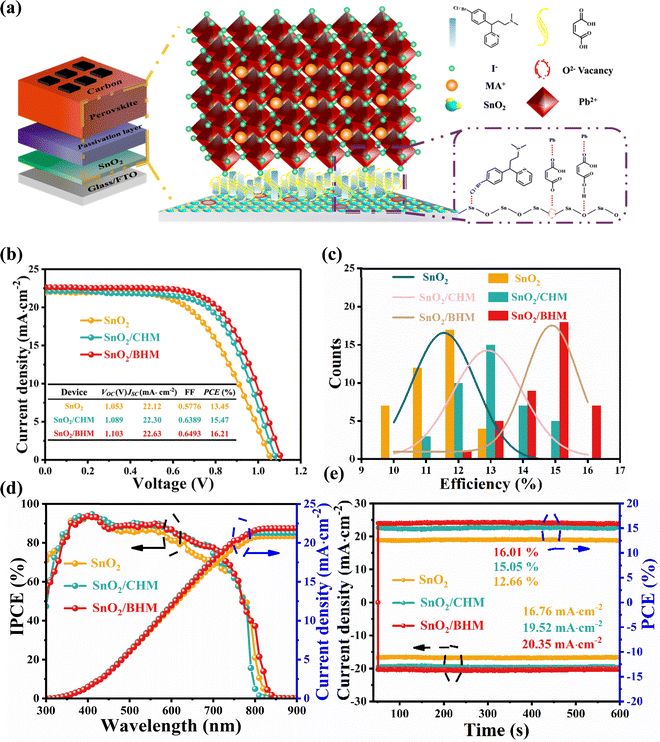
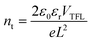Interfacial engineering between SnO2/MAPbI3 by maleate pheniramine halides toward carbon counter electrode-based perovskite solar cells with 16.21% efficiency†
Duoling
Cao‡
a,
Zuhong
Li‡
a,
Wenbo
Li
a,
Ke
Pei
a,
Xu
Zhang
*a,
Li
Wan
 *a,
Li
Zhao
*a,
Li
Zhao
 a,
Alexey
Cherevan
a,
Alexey
Cherevan
 b,
Dominik
Eder
b,
Dominik
Eder
 b and
Shimin
Wang
*a
b and
Shimin
Wang
*a
aKey Laboratory for the Green Preparation and Application of Functional Materials, Ministry of Education, Hubei Key Laboratory of Polymer Materials, Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials, Faculty of Materials Science and Engineering, Hubei University, Wuhan 430062, P. R. China. E-mail: xuzhang@hubu.edu.cn; wanli@hubu.edu.cn; wanli1983_3@aliyun.com; shiminwang@126.com
bInstitute of Materials Chemistry, Technische Universität Wien, Getreidemarkt 9/165, 1060 Vienna, Austria
First published on 18th January 2023
Abstract
Carbon counter electrode-based perovskite solar cells (C-PSCs) are considered among the most promising solar cells due to their excellent stability and low cost. However, the device performance is still unsatisfactory because of numerous defects at the interface between the electron transport layer (ETL) and perovskite (PVK) layer. In this work, we used two novel pheniramine halides containing maleic acid (i.e., chlorphenamine maleate (CHM) and brompheniramine maleate (BHM)) to modify the SnO2/MAPbI3 interface. All modifiers can passivate interface defects, reduce interface strain, and enhance device performance. As a result, the champion power conversion efficiency (PCE) values of HTL-free C-PSCs modified with CHM and BHM are 15.47% and 16.21%, respectively, which are much higher than the 13.45% of the control device. Moreover, the unencapsulated BHM-modified device maintains approximately 82.7% of its initial PCE under ambient conditions with 35% relative humidity at room temperature for 800 h. This study provides a new idea for the application of multifunctional complex molecules in high-efficiency and stable C-PSCs.
1. Introduction
In the past decade, organic–inorganic hybrid perovskite solar cells (PSCs) have received extensive attention from scholars, with the power conversion efficiency (PCE) significantly improving from 3.8% to 25.7%.1–4 Unfortunately, high-efficiency devices contain an organic hole transporting layer (HTL) and precious metal electrodes, which increase the cost and energy consumption and complicate the manufacturing steps.5,6 Recent studies have shown that metal corrosion adversely affects the performance and stability of the PSCs.7 Therefore, HTL-free carbon counter electrode-based PSCs (C-PSCs) have become one of the most promising alternatives owing to their low raw material cost and simple manufacturing process.8–10 Recently, Ye et al. obtained the highest PCE of 18.9% in C-PSCs without a HTL.11 Despite all the progress, C-PSCs suffer from serious energy losses caused by the high resistance of the carbon counter electrode (CE), poor interface contact between the carbon CEs, and quality of the electron transport layer (ETL) and the perovskite layer.12–14The ETL and perovskite film are also crucial to the performance and stability of PSCs, except for the influence of conductive carbon paste.4,15,16 Generally, tin oxide (SnO2) is commonly used as an ETL in PSCs due to the advantages of low-temperature processing, high transmittance, excellent chemical stability and low photocatalytic activity.17,18 However, the interfacial energy between SnO2 and the perovskite can hinder the charge extraction and transfer, leading to severe charge recombination.19–21 In particular, the undercoordinated Sn4+ and –OH groups on the SnO2 surface could seriously affect the contact between SnO2 and the perovskite, resulting in the degradation of the quality of the perovskite film and the performance of the PSCs.22
Various functional materials have been developed to regulate the energy alignment of the SnO2 and passivate the surface defects, mainly including inorganic or organic salts,21,23,24 Lewis acid and base molecules,25 polymers, and quantum dots.19,26,27 Among them, carboxyl (–O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)28–30 and halide (–Cl,31,32 –Br,33etc.) groups can coordinate with the undercoordinated interfacial metal atoms, such as Sn and Pb, to regulate the energy levels of SnO2, passivate the defect density at the SnO2/perovskite interface, and ultimately improve the device performance.
O)28–30 and halide (–Cl,31,32 –Br,33etc.) groups can coordinate with the undercoordinated interfacial metal atoms, such as Sn and Pb, to regulate the energy levels of SnO2, passivate the defect density at the SnO2/perovskite interface, and ultimately improve the device performance.
For example, Chen et al. found that 4-imidazoleacetic acid hydrochloride (ImAcHCl) can be used as a passivation layer at the SnO2/perovskite interface in planar PSCs because it can passivate the interface defects by reactions with the hydroxyl group (–OH) on the SnO2 surface.28 Diao et al. used maleic anhydride to improve the efficiency and stability of PSCs, and its hydrolysate (maleic acid) can also act as a passivator for perovskites. The perovskite film would become more compact and smooth due to the interaction between carboxyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the undercoordinated Pb2+.34 In addition, Dong et al. used 2,4,5-trichlorobenzenesulfonic acid potassium salt (3Cl-BSAK) as a functional ETL, and the undercoordinated Sn on the surface of the SnO2 film was efficiently passivated with the Cl in 3Cl-BSAK.35 Xiong et al. reported that biguanide hydrochloride (BGCl) was chemically linked to the SnO2 film via Lewis coordination or electrostatic coupling to enhance electron extraction.36 Zhang et al. used the strong interaction between F− ions or C
O) and the undercoordinated Pb2+.34 In addition, Dong et al. used 2,4,5-trichlorobenzenesulfonic acid potassium salt (3Cl-BSAK) as a functional ETL, and the undercoordinated Sn on the surface of the SnO2 film was efficiently passivated with the Cl in 3Cl-BSAK.35 Xiong et al. reported that biguanide hydrochloride (BGCl) was chemically linked to the SnO2 film via Lewis coordination or electrostatic coupling to enhance electron extraction.36 Zhang et al. used the strong interaction between F− ions or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in potassium trifluoroacetate (KTFA) and Sn2+ or Pb2+ to improve the quality of the SnO2 and perovskite films, passivate the interface defects, and suppress ion migration.37 These works demonstrate that adding appropriate multifunctional modification molecules to the SnO2/perovskite interface can improve the quality of the SnO2 films and passivate the interface defects. Consequently, designing and developing more efficient multifunctional interface modifiers are greatly important to further improve the PCE and stability of C-PSCs.
O groups in potassium trifluoroacetate (KTFA) and Sn2+ or Pb2+ to improve the quality of the SnO2 and perovskite films, passivate the interface defects, and suppress ion migration.37 These works demonstrate that adding appropriate multifunctional modification molecules to the SnO2/perovskite interface can improve the quality of the SnO2 films and passivate the interface defects. Consequently, designing and developing more efficient multifunctional interface modifiers are greatly important to further improve the PCE and stability of C-PSCs.
Herein, we demonstrate a multifunctional interfacial modification strategy using two novel maleate pheniramine halides (CHM and BHM) to modify the interface between SnO2 and the perovskite. The two maleate pheniramine halides can interact with the –OH in SnO2 and undercoordinated Pb2+ in the perovskite; thus, the interface between them could be stabilized. Moreover, the Cl– or Br– groups in the benzene ring can form Sn–X bonds with the Sn atoms in the SnO2 ETL, thereby effectively reducing dangling bonds on the surface. Furthermore, the device based on BHM exhibits a lower trap density and a higher PCE than the device based on CHM. Finally, the device based on BHM attains a PCE as high as 16.21%, with much improved stability under ambient conditions.
2. Results and discussion
Fig. S1 (ESI†) shows the 2D molecular structures and 3D conformer schematics of CHM and BHM. The PSCs are fabricated in the FTO/SnO2/MAPbI3/carbon architecture. The CHM and BHM are used as interface passivation layers to modify the SnO2/MAPbI3 interface (Fig. 1a). On the one hand, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in CHM or BHM can be esterified with –OH on the surface of the SnO2 film to passivate the defects and avoid the oxidative decomposition of SnO2, thereby improving the device stability.38 On the other hand, the electron-donating C
O in CHM or BHM can be esterified with –OH on the surface of the SnO2 film to passivate the defects and avoid the oxidative decomposition of SnO2, thereby improving the device stability.38 On the other hand, the electron-donating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on CHM or BHM can interact with the undercoordinated Pb2+ ions in the MAPbI3 perovskite, promoting the crystallization of the perovskite and passivating the film defects.25,33,36 In addition, the halogen atoms on the benzene ring act as a Lewis base to passivate the undercoordinated Sn on the surface of the SnO2 film, which can enhance the carrier transport at the interface of the ETL and the perovskite layer.
O on CHM or BHM can interact with the undercoordinated Pb2+ ions in the MAPbI3 perovskite, promoting the crystallization of the perovskite and passivating the film defects.25,33,36 In addition, the halogen atoms on the benzene ring act as a Lewis base to passivate the undercoordinated Sn on the surface of the SnO2 film, which can enhance the carrier transport at the interface of the ETL and the perovskite layer.
We initially systematically compare the effects of two modifier on the photovoltaic performance of the device, as shown in Fig. S2 and S3 (ESI†) with the statistics listed in Table S1 and S2 (ESI†). The devices exhibit the best performance when the amount of CHM and BHM is 18 and 20 mg mL−1, respectively. Fig. 1b shows the photoinduced photocurrent density–voltage (J–V) curves of PSCs using different maleate pheniramine halide modifier, including key parameters such as the short-circuit current density (JSC), open-circuit voltage (VOC), fill factor (FF), and PCE summarized in Table S3 (ESI†) (the record PCE of the state-of-the-art HTL-free CE-based MAPbI3 PSCs is shown in Table S4, ESI†). The PSCs based on CHM (18 mg mL−1) show a PCE of 15.47%, with a VOC of 1.089 V, a JSC of 22.30 mA cm−1, and a FF of 0.639. With the introduction of BHM (20 mg mL−1) in the ETL, the PCE is increased to 16.21%, with a VOC of 1.103 V, a JSC of 22.63 mA cm−1, and a FF of 0.649. Compared with CHM based devices, the VOC of the BHM based device is significantly increased from 1.087 ± 0.01 V to 1.104 ± 0.01 V. Interestingly, the significant increase in efficiency is mainly attributed to the improvement in VOC, which may be related to the decrease in non-radiative recombination of defects at the interface between the ETL and the perovskite. As shown in Fig. 1c and Fig. S4 (ESI†), 40 individual devices were subsequently fabricated to characterize consistency and reproducibility. The incident photon-to-electron conversion efficiency (IPCE) and the integrated JSC for the champion devices are plotted in Fig. 1d. The corresponding integrated JSC values of SnO2, SnO2/CHM, and SnO2/BHM ETL-based devices were 20.85, 21.27, and 21.88 mA cm−1, respectively, which were consistent with the JSC values from the J–V results. Subsequently, we measured the steady-state power output (SPO) of the best performing devices of each substrate for 100 seconds at a fixed voltage near the maximum power point (MPP) obtained from the peak J–V curves (Fig. 1e).39 The device based on untreated SnO2 possessed an output current density of 16.76 mA cm−1 and an output PCE of 12.66%, whereas the CHM-modified device (19.52 mA cm−1 and 15.05%) and BHM-modified device (20.35 mA cm−1 and 16.01%) produced a higher current density and PCE at the same time.
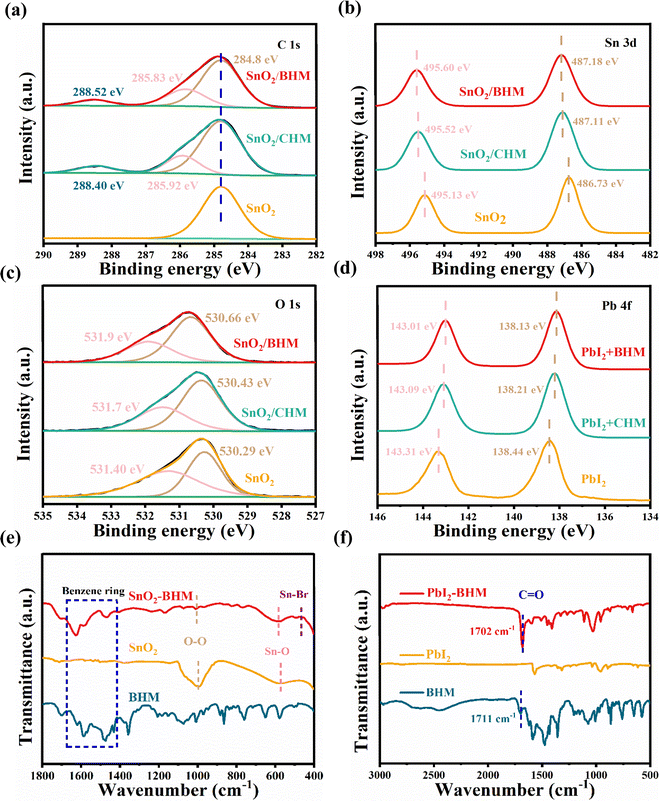
X-ray photoelectron spectroscopy (XPS) measurement is used to analyze the surface chemical properties of SnO2, SnO2/CHM, and SnO2/BHM films.40 The full scan XPS spectra are presented in Fig. S5a (ESI†). The CHM/SnO2 and BHM/SnO2 films show several additional peaks located at ca. 401.5 eV and 398.8 eV, respectively, ascribed to the N 1s peak (Fig. S5b, ESI†), C–N, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. 2a) contrary to the pristine SnO2, indicating the combination of CHM/BHM and the SnO2. Meanwhile, the Sn 3d3/2 and 3d5/2 peaks from BHM/SnO2 and CHM/SnO2 are shifted to higher binding energies than the pristine SnO2 in Fig. 2b. This finding is mainly attributed to the electrostatic coupling interaction of halogen atoms in pheniramine halides with Sn atoms in SnO2.35,41 This result is further supported by the formation of Sn–X bonds (Fig. S5c and d, ESI†), leading to the enhanced electron extraction capability of ETLs. Additionally, it can be determined that BHM bound more strongly to SnO2 than CHM by calculating the CHM and BHM complexation energies with SnO2 (Fig. S6, ESI†). The O 1s for pure SnO2 film displays two distinct peaks at 530.29 and 531.40 eV, corresponding to the Sn–O bond (lattice oxygen, OL) in SnO2 and the oxygen vacancies (OV) or surface absorbed hydroxyl groups (OOH), respectively (Fig. 2c).35,38 Both groups moved to a higher binding energy after CHM (530.43 eV for OL and 531.70 eV for OV or OOH) and BHM (530.66 eV for OL and 531.90 eV for OV or OOH) treatments, indicating the esterification reaction between –OH at the surface of the SnO2 film and –COOH of CHM or BHM. Fig. 2d evidently shows that the two distinct peaks of 143.31 and 138.44 eV that belong to Pb 4f5/2 and Pb 4f7/2 in the PbI2 film, respectively, are also shifted to lower binding energies after CHM (143.09 eV and 138.21 eV) and BHM (143.01 eV and 138.13 eV) modification. The peak shifts of C
O (Fig. 2a) contrary to the pristine SnO2, indicating the combination of CHM/BHM and the SnO2. Meanwhile, the Sn 3d3/2 and 3d5/2 peaks from BHM/SnO2 and CHM/SnO2 are shifted to higher binding energies than the pristine SnO2 in Fig. 2b. This finding is mainly attributed to the electrostatic coupling interaction of halogen atoms in pheniramine halides with Sn atoms in SnO2.35,41 This result is further supported by the formation of Sn–X bonds (Fig. S5c and d, ESI†), leading to the enhanced electron extraction capability of ETLs. Additionally, it can be determined that BHM bound more strongly to SnO2 than CHM by calculating the CHM and BHM complexation energies with SnO2 (Fig. S6, ESI†). The O 1s for pure SnO2 film displays two distinct peaks at 530.29 and 531.40 eV, corresponding to the Sn–O bond (lattice oxygen, OL) in SnO2 and the oxygen vacancies (OV) or surface absorbed hydroxyl groups (OOH), respectively (Fig. 2c).35,38 Both groups moved to a higher binding energy after CHM (530.43 eV for OL and 531.70 eV for OV or OOH) and BHM (530.66 eV for OL and 531.90 eV for OV or OOH) treatments, indicating the esterification reaction between –OH at the surface of the SnO2 film and –COOH of CHM or BHM. Fig. 2d evidently shows that the two distinct peaks of 143.31 and 138.44 eV that belong to Pb 4f5/2 and Pb 4f7/2 in the PbI2 film, respectively, are also shifted to lower binding energies after CHM (143.09 eV and 138.21 eV) and BHM (143.01 eV and 138.13 eV) modification. The peak shifts of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the appearance of the new peak of the Pb–O bond in modified films, further confirm the chemical interaction between O–C
O, and the appearance of the new peak of the Pb–O bond in modified films, further confirm the chemical interaction between O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the CHM or BHM and undercoordinated Pb2+, as expected in the O1s XPS spectra (Fig. S7, ESI†).37
O in the CHM or BHM and undercoordinated Pb2+, as expected in the O1s XPS spectra (Fig. S7, ESI†).37
The FTIR results in Fig. 2e show that in contrast to unmodified SnO2, three characteristic peaks at ca. 1596, 1481, and 1465 cm−1 in the modified films are due to benzene rings in the BHM, indicating the existence of BHM in the SnO2 films (Fig. S8, ESI†). For the BHM/SnO2 film, the peaks belonging to the Sn–O vibration shift from 562 to 579 cm−1, suggesting the interaction between the –OH in SnO2 and –COOH in BHM. Furthermore, the peak at 1046 cm−1 in the SnO2 film can be ascribed to the stretching vibrations of the O–O in the ion-radical forms of adsorbed oxygen of type O2− on the SnO2 surface.42 A weak absorption peak at 1072 cm−1 is observed after the BHM treatment, which may also be due to the esterification. The peaks at 447 and 459 cm−1 belong to Sn–Cl and Sn–Br vibrations in the modified film (Fig. S9a, ESI†), respectively. These results can further demonstrate the existence of CHM and BHM, consistent with the XPS results. Fig. 2f and Fig. S9b (ESI†) show the FTIR spectra of PbI2, BHM (CHM), and PbI2-BHM (PbI2-CHM) films. The results indicate that the interaction between BHM and the perovskite is ascribed to the chemical affinity of Pb2+ with the maleate acid group. An additional peak at around 1700 cm−1 is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the PbI2-BHM or PbI2-CHM film.23,30 Meanwhile, the characteristic peaks of the C
O stretching vibration in the PbI2-BHM or PbI2-CHM film.23,30 Meanwhile, the characteristic peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in CHM (1706 cm−1) and BHM (1702 cm−1) shift to 1710 cm−1 (CHM) and 1711 cm−1 (BHM), further demonstrating the chemical interaction between the maleate acid group in the maleate pheniramine halides and undercoordinated Pb2+ or Pb clusters in the perovskite.
O stretching vibration in CHM (1706 cm−1) and BHM (1702 cm−1) shift to 1710 cm−1 (CHM) and 1711 cm−1 (BHM), further demonstrating the chemical interaction between the maleate acid group in the maleate pheniramine halides and undercoordinated Pb2+ or Pb clusters in the perovskite.
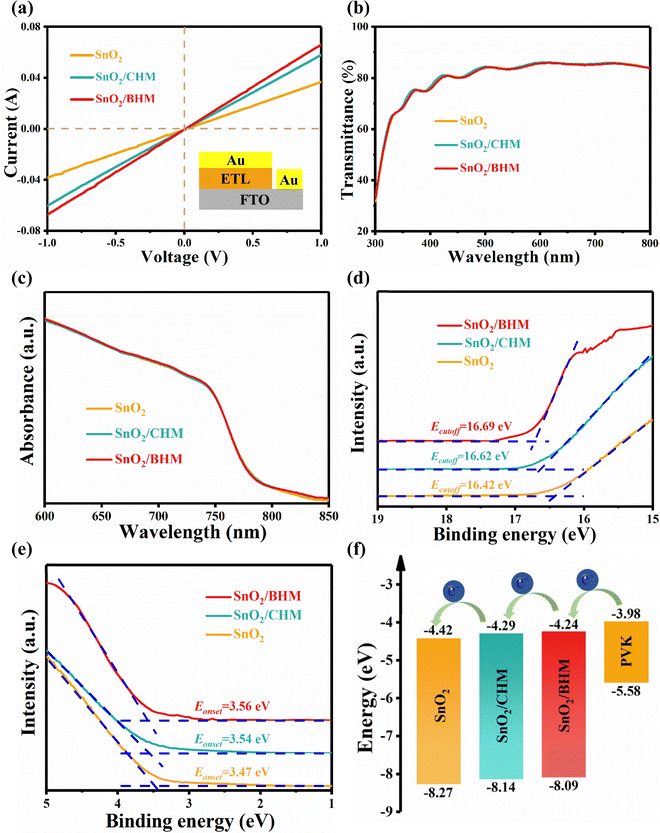
We measured the conductivity of various ETLs with the device structure of FTO/ETL/Au. The conductivity of the ETL is related to the slope of the current–voltage (I–V) curve. As shown in Fig. 3a, the SnO2/CHM and SnO2/BHM show a larger slope than the pure SnO2, suggesting that the film conductivity was slightly improved after CHM and BHM treatments, which is likely to be beneficial for electron transfer.43 The optical transmission spectra of SnO2, SnO2/CHM, and SnO2/BHM are shown in Fig. 3b. These films show high transmittance in the visible region, indicating excellent optical quality. Acquired from the UV visible spectra (Fig. S10, ESI†), the bandgap (Eg) of the SnO2 and SnO2 modified films is the same at 3.85 eV. The absorption spectra in Fig. 3c also show that the CHM or BHM treatment has a negligible effect on the perovskite films. The films without and with modification by CHM and BHM exhibit the same bandgap of 1.60 eV in Fig. S11 (ESI†).
Ultraviolet photoelectron spectroscopy (UPS) measurement is performed to investigate the energy band structure of ETLs.22,27 The conduction band minimum (EC) and valence band maximum (EV) without and with CHM and BHM modification are calculated using the energy cutoff (Ecutoff) region (Fig. 3d) and the energy onset (Eonset) region (Fig. 3e). Fig. 3f illustrates the energy band alignment between the ETLs and perovskite. In the equation of EV = Ecutoff − Eonset − h, where hν is the energy of the He α ultraviolet photon source. We calculated the EV to be −8.27, −8.14, and −8.09 eV for SnO2, SnO2/CHM and SnO2/BHM, respectively. We then calculated the EC of SnO2, SnO2/CHM, and SnO2/BHM samples at −4.42, −4.29, and −4.24 eV from EC = EV + Eg, respectively. After modification, the VBM and CBM of SnO2 are closer to the conduction band and valence band of the perovskite. As a result, the interface energy barrier is reduced, and interface recombination is inhibited to improve the VOC of the devices. Furthermore, in contrast to the CHM/SnO2, the energy band alignment of BHM/SnO2 is closer to the perovskite, which is one of the reasons for the better photovoltaic performance of the corresponding devices.
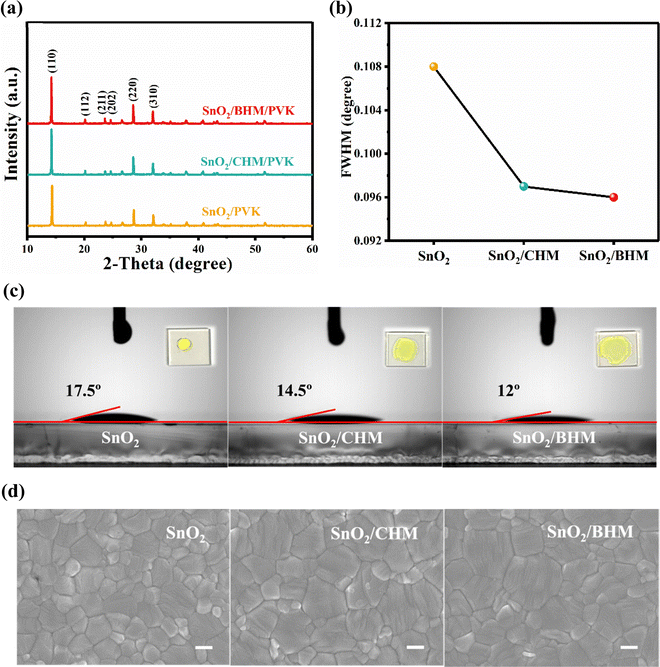
The effect of different ETLs on the quality of perovskite films was investigated by X-ray diffraction (XRD). In Fig. 4a, the relatively sharp peaks at 14.263°, 28.584° and 32.031° correspond to the (110) (220) and (310) planes of the perovskite for all samples.44 This finding indicates that the addition of BHM and CHM in SnO2 has slight effects on the structure of the perovskite. Fig. 4b demonstrates the full width at half maximum (FWHM) for the (110) peak of perovskite films with various ETLs. Therefore, the modified ETLs have a favorable effect on the crystallinity of the perovskite film due to the significant decrease in the FWHM. The contact angles of SnO2, SnO2/CHM, and SnO2/BHM shown in Fig. 4c are 17.5°, 14.5°, and 12.0°, respectively. The smaller contact angle results in the wettability interface for the perovskite.45 Therefore, the small contact angle of the ETLs could provide a low surface energy,46 which is probably responsible for the improved crystallinity of the perovskite coated on the BHM/SnO2 ETL.21,47Fig. 4d shows the top-view scanning electron microscopy (SEM) images of the perovskite films deposited on different ETLs, including SnO2, SnO2/CHM, and SnO2/BHM. The images clearly show that compact and dense surface morphologies were obtained. Compared with the perovskite film deposited on the pure SnO2 substrates, the CHM or BHM modified perovskite films have a more uniform morphology, larger grains, and fewer grain boundaries. Fig. S12 (ESI†) shows the distribution diagram with an average grain size of the perovskite spin-coated on ETLs. The grain size is increased from 289 nm to 473 nm for the BHM-modified sample. Fig. S13 (ESI†) shows the cross-sectional SEM images of the complete solar cell structure. The perovskite film is approximately 650 nm. The grains of the perovskite film coated on the BHM/SnO2 substrate are sufficiently large enough to penetrate through the film thickness, without any pinhole. The result is consistent with the top-view SEM images.
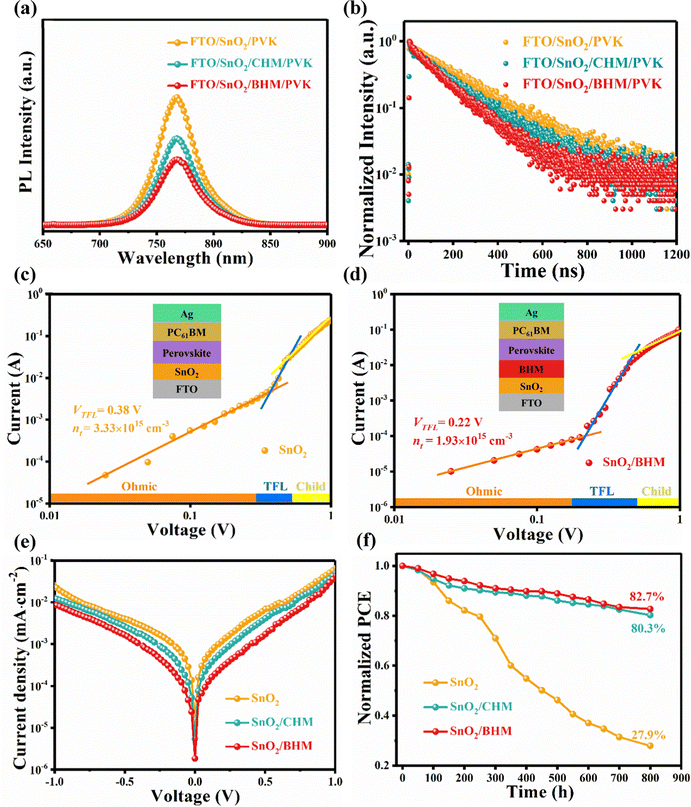
We estimated the recombination dynamics in different ETLs by photoluminescence (PL) and time-resolved photoluminescence (TRPL) spectroscopy.48 The prominent PL in the FTO/SnO2/BHM or CHM/perovskite in Fig. 5a indicates more efficient charge mobility after CHM or BHM treatment. Fig. 5b and Table S5 (ESI†) show the lifetime and average carrier lifetime (τave) of the TRPL spectra calculated by the equation 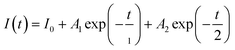 , and
, and 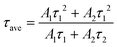 , where A1 and A2 are the amplitude of τ1 and τ2, I0 is a constant, and τ1 and τ2 are the fast and slow decay component, respectively.49 The τave of the modified ETLs is only one third (90.23 ns for BHM/SnO2) or one half (178.48 ns for CHM/SnO2) of the original film (273.62 ns for SnO2). In addition, to illustrate that the modification of BHM and CHM inhibits the generation of perovskite defects, we constructed glass/BHM or CHM /perovskite film structure models. According to Table S6 and Fig. S14 (ESI†), compared with the τave of control samples (84.71 ns), the ones of the BHM (156.23 ns) and CHM (142.95 ns) modified samples show an obvious increase. These results suggest that the interface modification of CHM and BHM can effectively reduce the defects and improve the efficiency of electron extraction and transport between ETLs and the perovskite.
, where A1 and A2 are the amplitude of τ1 and τ2, I0 is a constant, and τ1 and τ2 are the fast and slow decay component, respectively.49 The τave of the modified ETLs is only one third (90.23 ns for BHM/SnO2) or one half (178.48 ns for CHM/SnO2) of the original film (273.62 ns for SnO2). In addition, to illustrate that the modification of BHM and CHM inhibits the generation of perovskite defects, we constructed glass/BHM or CHM /perovskite film structure models. According to Table S6 and Fig. S14 (ESI†), compared with the τave of control samples (84.71 ns), the ones of the BHM (156.23 ns) and CHM (142.95 ns) modified samples show an obvious increase. These results suggest that the interface modification of CHM and BHM can effectively reduce the defects and improve the efficiency of electron extraction and transport between ETLs and the perovskite.
The space charge-limited current (SCLC) measurement was conducted to quantify the trap densities of the perovskite films coated on different ETLs. Fig. 5c, d and Fig. S15 (ESI†) show the dark I–V curves of the electron-only devices with a structure of FTO/ETL/perovskite/PCBM/Ag. The I–V curve has linear correlation at low bias voltage, showing an ohmic-type response. When the bias voltage exceeds the trap-filled limit voltage (VTFL), the current begins to increase nonlinearly, indicating that all the available trap states would be filled. The equation of the trap density (nt) is obtained as follows:
Electrochemical impedance spectroscopy (EIS) is employed to gain deep insight into charge transfer and recombination.51 Fig. S16 and Table S7 (ESI†) show the Nyquist plots of the devices with different ETLs. The series resistance (Rs) of the SnO2 is slightly increased after CHM and BHM treatment. In the EIS analysis, the high-frequency and low-frequency regions represent the charge transfer resistance (Rtr) and recombination resistance (Rrec), respectively. Compared with SnO2 and SnO2/CHM-based PSCs, the SnO2/BHM-based device shows the smallest Rtr of 233.3 Ω and the largest Rrec of 2500 Ω, indicating the best film morphology, most efficient carrier transport and lowest recombination. Fig. 5e shows the dark current densities of the solar cells with different ETLs. It shows a decreasing tendency with the CHM and BHM modification, which may be related to the improvement of carrier transport efficiency and the reduction of leakage current induced by charge recombination.52
Stability is one of the most important characteristics of PSCs. Fig. 5f shows the normalized PCE of the unencapsulated devices with different ETLs stored under ambient conditions with 35% relative humidity and room temperature. The CHM and BHM modified devices retain over 80% of their initial PCE after 800 h, but the device with SnO2 only maintains ca. 30% of its initial PCE. This large gap indicates that the long-term stability of the device has been greatly improved after interfacial modification. This may be ascribed to the large grain size of the perovskite film, so the perovskite degradation at the grain boundary is inhibited, which ultimately leads to the improvement of device stability.
3. Conclusions
We developed an effective maleate pheniramine halide modified SnO2 ETL for the MAPbI3 perovskite solar cell and achieved an increased PCE of 16.21%. The excellent device performance is related to the larger grain size, better crystallinity, lower trap density and interface energy barrier of the MAPbI3 film deposited on BHM/SnO2. Therefore, improved charge transfer efficiency and inhibited interface recombination are realized in the highly efficient devices. Meanwhile, the long-term stability is significantly improved with the modified SnO2 ETL. We believe that this work has made great strides in developing high-quality electron transport layers, which will play an important role in promoting the progress of perovskite photovoltaics.Author contributions
Duoling Cao and Zuhong Li carried out experimental research, wrote the draft of manuscript, and participated in writing the theoretical part of the manuscript. Wenbo Li provided help for the problems encountered in the experiment. Li Wan supervised the experimental research of the manuscript, revised the draft of manuscript. Li Zhao, Ke Pei and Alexey Cherevan helped revising the manuscript. Xu Zhang, Li Wan, Dominik Eder and Shimin Wang provided funding support.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (21402045), the Higher Education Discipline Innovation Project (“111 Project”, D18025), the Key Program for Intergovernmental S&T Innovation Cooperation Projects of National Key R&D Program of China (2019YFE0107100), and Austria/China Scientific & Technological Cooperation (WTZ-OeAD CN03/2020). This work was also financially supported by the Hubei Provincial Department of Education (D20181005), and the Department of Science & Technology of Hubei Province of China (2014CFB167 and 2022CFB981).References
- NREL. Best Research-Cell Efficiency Chart, (accessed: August 2022), https://www.nrel.gov/pv/cell-efficiency.html.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal halide perovskites asvisible-light sensitizers for photovoltaic cells, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- Y. Chen, S. Tan, N. Li, B. Huang, X. Niu, L. Li, M. Sun, Y. Zhang, X. Zhang, C. Zhu, N. Yang, H. Zai, Y. Wu, S. Ma, Y. Bai, Q. Chen, F. Xiao, K. Sun and H. Zhou, Self-elimination of intrinsic defects improves the low-temperature performance of perovskite photovoltaics, Joule, 2020, 4, 1961–1976 CrossRef CAS.
- H. Min, D. Y. Lee, J. Kim, G. Kim, K. S. Lee, J. Kim, M. J. Paik, Y. K. Kim, K. S. Kim, M. G. Kim, T. J. Shin and S. Il Seok, Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes, Nature, 2021, 598, 444–450 CrossRef CAS PubMed.
- W. Zhu, W. Chai, D. Chen, J. Ma, D. Chen, H. Xi, J. Zhang, C. Zhang and Y. Hao, High-efficiency (>14%) and air-stable carbon-based, all-inorganic CsPbI2Br perovskite solar cells through a top-seeded growth strategy, ACS Energy Lett., 2021, 6, 1500–1510 CrossRef CAS.
- M. Jeong, I. W. Choi, E. M. Go, Y. Cho, M. Kim, B. Lee, S. Jeong, Y. Jo, H. W. Choi, J. Lee, J.-H. Bae, S. K. Kwak, D. S. Kim and C. Yang, Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3 V voltage loss, Science, 2020, 369, 1615–1620 CrossRef CAS PubMed.
- N. Arora, M. I. Dar, A. Hinderhofer, N. Pellet, F. Schreiber, S. M. Zakeeruddin and M. Grätzel, Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%, Science, 2017, 358, 768–771 CrossRef CAS PubMed.
- H. Zhang, J. Xiao, J. Shi, H. Su, Y. Luo, D. Li, H. Wu, Y.-B. Cheng and Q. Meng, Self-adhesive macroporous carbon electrodes for efficient and stable perovskite solar cells, Adv. Funct. Mater., 2018, 28, 1802985 CrossRef.
- Y. Rong, Y. Hu, A. Mei, H. Tan, M. I. Saidaminov, S. Il Seok, M. D. McGehee, E. H. Sargent and H. Han, Challenges for commercializing perovskite solar cells, Science, 2018, 361, 6408 CrossRef PubMed.
- C. Zhang, S. Liang, W. Liu, F. T. Eickemeyer, X. Cai, K. Zhou, J. Bian, H. Zhu, C. Zhu, N. Wang, Z. Wang, J. Zhang, Y. Wang, J. Hu, H. Ma, C. Xin, S. M. Zakeeruddin, M. Grätzel and Y. Shi, Ti1-graphene single-atom material for improved energy level alignment in perovskite solar cells, Nat. Energy., 2021, 6, 1154–1163 CrossRef CAS.
- T. Ye, Y. Hou, A. Nozariasbmarz, D. Yang, J. Yoon, L. Zheng, K. Wang, K. Wang, S. Ramakrishna and S. Priya, Cost-effective high-performance charge-carrier-transport-layer-free perovskite solar cells achieved by suppressing ion migration, ACS Energy Lett., 2021, 6, 3044–3052 CrossRef CAS.
- C. Zhang, S. Wang, H. Zhang, Y. Feng, W. Tian, Y. Yan, J. Bian, Y. Wang, S. Jin, S. M. Zakeeruddin, M. Grätzel and Y. Shi, Efficient stable graphene-based perovskite solar cells with high flexibility in device assembling via modular architecture design, Energy Environ. Sci., 2019, 12, 3585–3594 RSC.
- Y. Zhu, S. Jia, J. Zheng, Y. Lin, Y. Wu and J. Wang, Facile synthesis of nitrogen-doped graphene frameworks for enhanced performance of hole transport material-free perovskite solar cells, J. Mater. Chem. C, 2018, 6, 3097–3103 RSC.
- Z. Wu, Z. Liu, Z. Hu, Z. Hawash, L. Qiu, Y. Jiang, L. K. Ono and Y. Qi, Highly efficient and stable perovskite solar cells via modification of energy levels at the perovskite/carbon electrode interface, Adv. Mater., 2019, 31, 1804284 CrossRef PubMed.
- Y. Wu, H. Zhu, B.-B. Yu, S. Akin, Y. Liu, Z. Shen, L. Pan and H. Cai, Interface modification to achieve high-efficiency and stable perovskite solar cells, Chem. Eng. J., 2022, 433, 134613 CrossRef CAS.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Surface passivation of perovskite film for efficient solar cells, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS.
- S. You, H. Zeng, Z. Ku, X. Wang, Z. Wang, Y. Rong, Y. Zhao, X. Zheng, L. Luo, L. Li, S. Zhang, M. Li, X. Gao and X. Li, Multifunctional polymer-regulated SnO2 nanocrystals enhance interface contact for efficient and stable planar perovskite solar cells, Adv. Mater., 2020, 32, 2003990 CrossRef CAS PubMed.
- C. Zhang, H. Wang, H. Li, Q. Zhuang, C. Gong, X. Hu, W. Cai, S. Zhao, J. Chen and Z. Zang, Simultaneous passivation of bulk and interface defects through synergistic effect of anion and cation toward efficient and stable planar perovskite solar cells, J. Energy Chem., 2021, 63, 452–460 CrossRef CAS.
- Z.-W. Gao, Y. Wang, H. Liu, J. Sun, J. Kim, Y. Li, B. Xu and W. C. H. Choy, Tailoring the interface in FAPbI3 planar perovskite solar cells by imidazole-graphene-quantum-dots, Adv. Funct. Mater., 2021, 31, 2101438 CrossRef CAS.
- Z. Liu, K. Deng, J. Hu and L. Li, Coagulated SnO2 colloids for high-performance planar perovskite solar cells with negligible hysteresis and improved stability, Angew. Chem., Int. Ed., 2019, 131, 11621–11628 Search PubMed.
- L. Yang, J. Feng, Z. Liu, Y. Duan, S. Zhan, S. Yang, K. He, Y. Li, Y. Zhou, N. Yuan, J. Ding and S. Liu, Record-efficiency flexible perovskite solar cells enabled by multifunctional organic ions interface passivation, Adv. Mater., 2022, 34, 2201681 CrossRef CAS PubMed.
- Q. Lou, Y. Han, C. Liu, K. Zheng, J. Zhang, X. Chen, Q. Du, C. Chen and Z. Ge, π-conjugated small molecules modified SnO2 layer for perovskite solar cells with over 23% efficiency, Adv. Energy Mater., 2021, 11, 2101416 CrossRef CAS.
- Y. Huang, H. Zhong, W. Li, D. Cao, Y. Xu, L. Wan, X. Zhang, X. Zhang, Y. Li, X. Ren, Z. Guo, X. Wang, D. Eder and S. Wang, Bifunctional ionic liquid for enhancing efficiency and stability of carbon counter electrode-based MAPbI3 perovskites solar cells, Sol. Energy, 2022, 231, 1048–1060 CrossRef CAS.
- P.-Y. Gu, N. Wang, A. Wu, Z. Wang, M. Tian, Z. Fu, X. Sun and Q. Zhang, An azaacene derivative as promising electron-transport layer for inverted perovskite solar cells, Chem. – Asian J., 2016, 11, 2135–2138 CrossRef CAS PubMed.
- G. Xu, C. Xu, L. Chen, J. Ye, J. Dong, Y. Zhong, F. Li, X. He, Y. Yao, J. You and Q. Song, Interface barrier strategy for perovskite solar cells realized by In-situ synthesized polyionic layer, Chem. Eng. J., 2022, 439, 135704 CrossRef CAS.
- X. Gong, L. Guan, Q. Li, Y. Li, T. Zhang, H. Pan, Q. Sun, Y. Shen, C. Grätzel, S. M. Zakeeruddin, M. Grätzel and M. Wang, Black phosphorus quantum dots in inorganic perovskite thin films for efficient photovoltaic application, Sci. Adv., 2020, 6, eaay5661 CrossRef CAS PubMed.
- M. Kim, J. Jeong, H. Lu, T. K. Lee, F. T. Eickemeyer, Y. Liu, I. W. Choi, S. J. Choi, Y. Jo, H.-B. Kim, S.-I. Mo, Y.-K. Kim, H. Lee, N. G. An, S. Cho, W. R. Tress, S. M. Zakeeruddin, A. Hagfeldt, J. Y. Kim, M. Grätzel and D. S. Kim, Conformal quantum dot–SnO2 layers as electron transporters for efficient perovskite solar cells, Science, 2022, 375, 302–306 CrossRef CAS PubMed.
- J. Chen, X. Zhao, S.-G. Kim and N.-G. Park, Multifunctional chemical linker imidazoleacetic acid hydrochloride for 21% efficient and stable planar perovskite solar cells, Adv. Mater., 2019, 31, 1902902 CrossRef PubMed.
- L. Zhu, X. Zhang, M. Li, X. Shang, K. Lei, B. Zhang, C. Chen, S. Zheng, H. Song and J. Chen, Trap state passivation by rational ligand molecule engineering toward efficient and stable perovskite solar cells exceeding 23% efficiency, Adv. Energy Mater., 2021, 11, 2100529 CrossRef CAS.
- Q. Zhou, D. He, Q. Zhuang, B. Liu, R. Li, H. Li, Z. Zhang, H. Yang, P. Zhao, Y. He, Z. Zang and J. Chen, Revealing steric-hindrance-dependent buried interface defect passivation mechanism in efficient and stable perovskite solar cells with mitigated tensile stress, Adv. Funct. Mater., 2022, 32, 2205507 CrossRef CAS.
- Z. Xing, S. Lin, X. Meng, T. Hu, D. Li, B. Fan, Y. Cui, F. Li, X. Hu and Y. Chen, A highly tolerant printing for scalable and flexible perovskite solar cells, Adv. Funct. Mater., 2021, 31, 2107726 CrossRef CAS.
- H. Wang, F. Li, P. Wang, R. Sun, W. Ma, M. Chen, W. Miao, D. Liu and T. Wang, Chlorinated fullerene dimers for interfacial engineering toward stable planar perovskite solar cells with 22.3% efficiency, Adv. Energy Mater., 2020, 10, 2000615 CrossRef CAS.
- D. He, T. Zhou, B. Liu, L. Bai, W. Wang, H. Yuan, C. Xu, Q. Song, D. Lee, Z. Zang, L. Ding and J. Chen, Interfacial defect passivation by novel phosphonium salts yields 22% efficiency perovskite solar cells: Experimental and theoretical evidence, EcoMat, 2022, 4, e12158 CrossRef CAS.
- Z.-L. Diao, Y.-W. Zhang, J.-Y. Chen, W.-Y. Tan, Y.-N. Qian, L.-G. Xiao and Y. Min, High-performance blue perovskite light-emitting diodes enabled by a sacrificial agent maleic anhydride, J. Phys. Chem. C, 2022, 126, 6153–6160 CrossRef CAS.
- Y. Dong, W. Shen, W. Dong, C. Bai, J. Zhao, Y. Zhou, F. Huang, Y.-B. Cheng and J. Zhong, Chlorobenzenesulfonic potassium salts as the efficient multifunctional passivator for the buried interface in regular perovskite solar cells, Adv. Energy Mater., 2022, 12, 2200417 CrossRef CAS.
- Z. Xiong, X. Chen, B. Zhang, G. O. Odunmbaku, Z. Ou, B. Guo, K. Yang, Z. Kan, S. Lu, S. Chen, N. A. N. Ouedraogo, Y. Cho, C. Yang, J. Chen and K. Sun, Simultaneous interfacial modification and crystallization control by biguanide hydrochloride for stable perovskite solar cells with PCE of 24.4%, Adv. Funct. Mater., 2022, 34, 2106118 CAS.
- X. Zhang, D. Zhang, Y. Zhou, Y. Du, J. Jin, Z. Zhu, Z. Wang, X. Cui, J. Li, S. Wu, J. Zhang and Q. Tai, Fluorinated interfaces for efficient and stable low-temperature carbon-based CsPbI2Br perovskite solar cells, Adv. Funct. Mater., 2022, 32, 2205478 CrossRef CAS.
- H. Bi, B. Liu, D. He, L. Bai, W. Wang, Z. Zang and J. Chen, Interfacial defect passivation and stress release by multifunctional KPF6 modification for planar perovskite solar cells with enhanced efficiency and stability, Chem. Eng. J., 2021, 418, 129375 CrossRef CAS.
- Y. Liu, W. Xiang, S. Mou, H. Zhang and S. Liu, Synergetic surface defect passivation towards efficient and stable inorganic perovskite solar cells, Chem. Eng. J., 2022, 447, 137515 CrossRef CAS.
- D. Cao, W. Li, X. Zhang, L. Wan, Z. Guo, X. Wang, D. Eder and S. Wang, Current state-of-the-art characterization methods for probing defect passivation towards efficient perovskite solar cells, J. Mater. Chem. A, 2022, 10, 19278–19303 RSC.
- H. Bi, X. Zuo, B. Liu, D. He, L. Bai, W. Wang, X. Li, Z. Xiao, K. Sun, Q. Song, Z. Zang and J. Chen, Multifunctional organic ammonium salt-modified SnO2 nanoparticles toward efficient and stable planar perovskite solar cells, J. Mater. Chem. A, 2021, 9, 3940–3951 RSC.
- T. A. Gundrizer and A. A. Davydov, IR spectra of oxygen adsorbed on SnO2, React. Kinet. Catal. Lett., 1975, 3, 63–70 CrossRef CAS.
- H. Zhong, W. Li, Y. Huang, D. Cao, C. Zhang, H. Bao, Z. Guo, L. Wan, X. Zhang, X. Zhang, Y. Li, X. Ren, X. Wang, D. Eder, K. Wang, S. Liu and S. Wang, All-inorganic perovskite solar cells with tetrabutylammonium acetate as the buffer layer between the SnO2 electron transport film and CsPbI3, ACS Appl. Mater. Interfaces, 2022, 14, 5183–5193 CrossRef CAS PubMed.
- Z. Li, J. Feng, J. Cao, J. Jin, Y. Zhou, D. Cao, Z. Liang, B. Zhu, M. Li, L. Zhao and S. Wang, New carbon nitride C3N3 additive for improving cationic defects of perovskite solar cells, Energy Environ. Mater., 2022, 6, e12283 Search PubMed.
- P. Li, C. Liang, Y. Zhang, F. Li, Y. Song and G. Shao, Polyethyleneimine high-energy hydrophilic surface interfacial treatment toward efficient and stable perovskite solar cells, ACS Appl. Mater. Interfaces, 2016, 8, 32574–32580 CrossRef CAS PubMed.
- P. Fu, L. Huang, W. Yu, D. Yang, G. Liu, L. Zhou, J. Zhang and C. Li, Efficiency improved for inverted polymer solar cells with electrostatically self-assembled BenMeIm-Cl ionic liquid layer as cathode interface layer, Nano Energy, 2015, 13, 275–282 CrossRef CAS.
- Y. Han, H. Zhao, C. Duan, S. Yang, Z. Yang, Z. Liu and S. Liu, Controlled n-doping in air-stable CsPbI2Br perovskite solar cells with a record efficiency of 16.79%, Adv. Funct. Mater., 2020, 30, 1909972 CrossRef CAS.
- J. Zhuang, P. Mao, Y. Luan, N. Chen, X. Cao, G. Niu, F. Jia, F. Wang, S. Cao and J. Wang, Rubidium fluoride modified SnO2 for planar n-i-p perovskite solar cells, Adv. Funct. Mater., 2021, 31, 2010385 CrossRef CAS.
- Y. Li, L. Meng, Y. Yang, G. Xu, Z. Hong, Q. Chen, J. You, G. Li, Y. Yang and Y. Li, High-efficiency robust perovskite solar cells on ultrathin flexible substrates, Nat. Commun., 2016, 7, 10214 CrossRef CAS PubMed.
- M. Sendner, P. K. Nayak, D. A. Egger, S. Beck, C. Müller, B. Epding, W. Kowalsky, L. Kronik, H. J. Snaith, A. Pucci and R. Lovrinčić, Optical phonons in methylammonium lead halide perovskites and implications for charge transport, Mater. Horiz., 2016, 3, 613–620 RSC.
- D. Yang, R. Yang, K. Wang, C. Wu, X. Zhu, J. Feng, X. Ren, G. Fang, S. Priya and S. Liu, High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2, Nat. Commun., 2018, 9, 3239 CrossRef PubMed.
- W. Zhao, P. Guo, J. Su, Z. Fang, N. Jia, C. Liu, L. Ye, Q. Ye, J. Chang and H. Wang, Synchronous passivation of defects with low formation energies via terdentate anchoring enabling high performance perovskite solar cells with efficiency over 24%, Adv. Funct. Mater., 2022, 32, 2200534 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of materials, device fabrications, and characterization. See DOI: https://doi.org/10.1039/d2qm01149b |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |