Color-tunable luminescent materials via a CB[8]-based supramolecular assembly strategy†
Wei-Hang
Jin‡
,
Qian
Wang‡
,
Meng
Chen
,
Qi
Zhang
and
Da-Hui
Qu
 *
*
Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: dahui_qu@ecust.edu.cn
First published on 21st January 2021
Abstract
Fabricating color-tunable organic luminescent materials through a supramolecular host–guest strategy has attracted considerable attention recently. Herein, a series of color-tunable emissive materials consisting of small organic molecules, cucurbit[8]uril (CB[8]), and their host–guest complexes were developed. Fluorescent wavelength can be reversibly regulated by controlling the concentration of CB[8] in aqueous solution. Such properties exhibited promising applications for smart luminescent materials.
Color-tunable luminescent materials based on small organic molecules have attracted much attention due to their potential applications in sensors, probes, and electronic devices.1–3 Traditional strategies to design multi-color fluorescence materials mainly involve (i) chemical covalent modifications of the fluorophores to extend π systems, and introduce donor–acceptor (D–A) groups;4 (ii) strictly mixing multiple components with energy matching based on the principle of fluorescence resonance energy transfer (FRET) processes, which usually require a time-consuming synthesis and purification.2,5 In addition, fluorescence properties of such materials are difficult to regulate owing to their static structures, which are unfavorable to fabricate smart luminescent materials with wavelength tenability, stimuli-responsive and adaptive properties as their potential applications in light emitting devices as well as encryption materials.3,6 Therefore, developing intelligent multi-color luminescent materials in a controllable way remains a challenge.
Recently, supramolecular assembly based on non-covalent interactions has been proven to efficiently regulate fluorescence properties.7–11 In particular, supramolecular host–guest interactions based on macrocycles such as cyclodextrins,11 cucurbit[n]uril (CB[n], n = 5–8, 10),10,12 crown ethers,8 box,13 and other molecular motifs14 have been widely used to fabricate intelligent multi-color fluorescent materials due to their specific recognition and dynamic properties. In particular, cucurbit[n]uril (CB[n]) with high selectivity and affinity towards positively charged units have been incorporated to develop various host–guest systems.10,12,15 Cucurbit[8]uril (CB[8]), a promising molecule in the cucurbit[n]uril family, have been utilized to fabricate diverse emissive host–guest systems owing to its large cavity that can combine multiple guest molecules to regulate various intermolecular processes (e.g. charge transfer, excimer formation and so on), resulting in changes of the fluorescence.16–21 For example,21 Ni et al. fabricated color-tunable luminescent materials in aqueous solution through the CB[8]-based host–guest strategy. However, fabricating intelligent multi-color fluorescent materials with simple molecules through supramolecular host–guest interaction strategies remains a challenge.
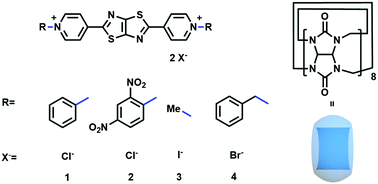
Herein, we designed color-tunable fluorescent materials, based on simple small molecules via a CB[8]-based supramolecular assembly strategy that can be dynamically controlled. As shown in Fig. 1, small and rigid fluorescent molecules were constructed by the incorporation of fluorescent core thiazolothiazole between two positively charged pyridinium units. The positive charges could provide water solubility as well as the binding sites of cucurbit[8]uril, allowing the formation of dynamic host–guest complexes that can couple the fluorophores together and prevent unpredictable aggregation. Resultantly, the fluorescence wavelength and intensity could be reversibly regulated. Significantly, the fluorescence of these complexes showed a response to humidity, displaying reversible emission color changes under both dry and wet conditions. Such humidity-dependent emissive properties exhibited great potential of applications for organic sensing materials with light-emitting and humidity properties.
 | ||
| Fig. 1 A schematic presentation of the chemical structures for four compounds and macrocyclic cucurbit[8]uril. | ||
The synthesis procedure of the four compounds (1, 2, 3 and 4) could be found in the supplementary material and the compound structures were confirmed by 1H and 13C NMR spectroscopies and mass spectrometry (Fig. S1–S16, ESI†). Compounds 1 and 2 were synthesized in an accessible route with different substitutions. Meanwhile, the control molecules, compounds 3 and 4, bearing a methyl (compound 3) and a benzyl substituent respectively (compound 4), where their molecular structures are similar to 1 and 2, were also synthesized for comparison.
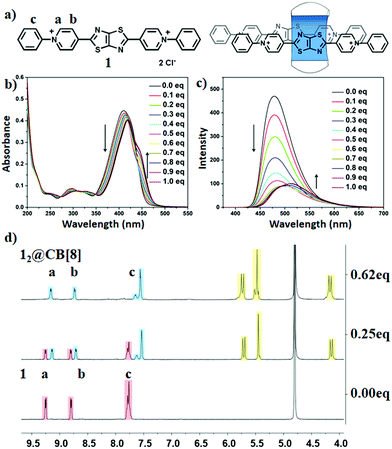
To confirm the water solubility of compounds 1 and 2, powders of the two compounds were directly dissolve in distilled water. Transparent solutions (>1 mM) of phenyl substituted (compound 1) and 2,4-dinitrophenyl substituted pyridinium units (compound 2) were observed (Fig. S17, ESI†), indicating the good solubility of the two compounds. Next, a macrocycle, cucurbit[8]uril, was introduced to the aqueous solutions to further regulate the fluorescence properties. Firstly, an optical spectrum study of compound 1 and CB[8] was performed (Fig. 2b, c and Fig. S18, ESI†). The maximum absorption peak at 410 nm gradually decreased and showed a considerable red-shift (Δλm = 10 nm) (Fig. 2b) owing to the addition of CB[8] to the solution of compound 1. Four distinctive isosbestic points at 273, 325, 348 and 445 nm suggested the guest, compound 1, was included into CB[8]. In addition, changes in the fluorescence emission peak at 478 nm (Fig. 2c) also suggested the supramolecular assembly of compound 1 and CB[8]. Two changes were observed when the addition of CB[8] was more than 0.5 equivalent: (i): shoulder absorption peaks at 445 nm increased; and (ii): a 34 nm red-shift of the fluorescence emission peaks (512 nm), suggesting a possible appearance of the multistep host–guest assembly states within this system (Fig. 2b, c and Fig. S19, S20, ESI†). Therefore, upon the addition of CB[8] solutions with an increasing concentration (0–1.0 eq.), multi-color fluorescence was observed (Fig. S21 and S22, ESI†). Based on these results, we hypothesized that two possible binding motifs between CB[8] and compound 1 could exist, i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes. To further confirm this, isothermal titration calorimetry (ITC) measurement was performed (Fig. S23, ESI†). A second transition was observed at a molar ratio of 2, indicating the formation of 1
2 complexes. To further confirm this, isothermal titration calorimetry (ITC) measurement was performed (Fig. S23, ESI†). A second transition was observed at a molar ratio of 2, indicating the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes (12@CB[8]). A transition point with a large enthalpy change of approximately 100 kJ mol−1 was also observed at around 0.6, which may be attributed to the formation of the 12@CB[8]3 complex.16 In addition, 1H NMR spectroscopy has proven to be an accessible tool to probe the way of combining CB[8] with the guest, owing to the upfield (downfield) shifts of the proton signals when a guest molecule was located inside (outside) the CB cavity (Fig. 2d). Therefore, 1H NMR analysis was carried out to further investigate the supramolecular assembly of compound 1 and CB[8]. Upon the addition of CB[8], significant upfield shifts for the Ha,b,c suggested that the pyridinium and phenyl moieties resided inside of the host cavity and therefore formed stacking complexes. Moreover, the conformations of the host–guest complexes were confirmed by 2D NOESY spectrum (Fig. S24 and S25, ESI†). Such stacking conformations resulted in the red-shifts of the absorption and fluorescence spectra.
2 complexes (12@CB[8]). A transition point with a large enthalpy change of approximately 100 kJ mol−1 was also observed at around 0.6, which may be attributed to the formation of the 12@CB[8]3 complex.16 In addition, 1H NMR spectroscopy has proven to be an accessible tool to probe the way of combining CB[8] with the guest, owing to the upfield (downfield) shifts of the proton signals when a guest molecule was located inside (outside) the CB cavity (Fig. 2d). Therefore, 1H NMR analysis was carried out to further investigate the supramolecular assembly of compound 1 and CB[8]. Upon the addition of CB[8], significant upfield shifts for the Ha,b,c suggested that the pyridinium and phenyl moieties resided inside of the host cavity and therefore formed stacking complexes. Moreover, the conformations of the host–guest complexes were confirmed by 2D NOESY spectrum (Fig. S24 and S25, ESI†). Such stacking conformations resulted in the red-shifts of the absorption and fluorescence spectra.
To understand the role for the incorporation of the phenyl substituent, two reference compounds, 3 and 4, were utilized to replace the phenyl substituent with a methyl (compound 3) and a benzyl substituent (compound 4). When 0.5 equivalent of CB[8] solution was added to the solution of 3, the fluorescence emission was close to quantitatively quenched, and no obvious change was observed when more CB[8] solution was added according to the UV/Vis and fluorescence spectra (Fig. S26 and S27, ESI†). These results showed the formation of host–guest complex induced fluorescence intensity quenching without any shift of the fluorescence wavelength (Fig. S28, ESI†). The host–guest combination between 3 and CB[8] was studied by Zhang, et al.19 The induced fluorescence quenching of 3 may be due to the π–π stacking between the electron-deficient pyridinium module and the thiazolothiazole module.19 Similar optical changes were observed upon the addition of CB[8] to the compound 4 solution. Both the absorption and fluorescence intensity decreased when the amount of CB[8] was <0.5 eq (Fig. S29 and S30, ESI†). However, the increasing shoulder absorption peak at 425 nm and the rising fluorescence emission peak at 460 nm indicated that compound 4 and CB[8] possibly formed other types of host–guest complexes upon the addition of the CB[8] at >0.5 eq. Thus, ITC experiments and 1H NMR spectra were performed (Fig. S31 and S32, ESI†). Two transition points at a molar ratio of 2.0 and 0.5 were observed in ITC experiments, suggesting two types of host–guest complexes. When a solution of compound 4 (1.0 mM) was added dropwise into the solution of CB[8] (0.1 mM), an abrupt change was observed at a molar ratio of 0.5 (Fig. S31, ESI†), suggesting the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes (4@CB[8]2)2+ at the beginning of the titration when an excess of CB[8] appeared in the system (Fig. S31, ESI†). With continuing the addition of 4 into the CB[8] solution, a second transition appeared indicating that a 2
2 complexes (4@CB[8]2)2+ at the beginning of the titration when an excess of CB[8] appeared in the system (Fig. S31, ESI†). With continuing the addition of 4 into the CB[8] solution, a second transition appeared indicating that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (42@CB[8])4+ complex was formed. Such combination was also confirmed by 1H NMR spectra (Fig. S32, ESI†).
1 (42@CB[8])4+ complex was formed. Such combination was also confirmed by 1H NMR spectra (Fig. S32, ESI†).
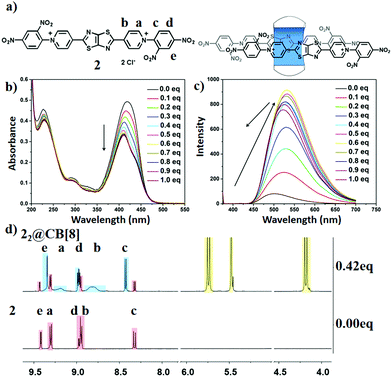
Considering the connection of the molecular structure and material function, i.e., the fluorescence emission wavelength could be influenced by the guest structure and the conformation of the host–guest complexes, another electron poor substituent group, 2,4-dinitrophenyl, was introduced to investigate whether it will cause a red-shift of the fluorescence emission. The optical study was thus performed. A yellow solution was observed for the 10 μM aqueous solution while other solutions were colorless (Fig. S33, ESI†). Detailed absorption spectral analysis indicated that the molar extinction coefficient (ε2) was larger than other compounds (Fig. S34, ESI†), and the maximum absorption peak red shifted about 9 nm (relative to compound 1). In addition, a fluorescence emission peak at 500 nm was observed and the 1931 CIE chromaticity coordinate was located at 0.23, 0.47 (Fig. 3c and Fig. S35, S36, ESI†). To further regulate its fluorescence emission, CB[8] was introduced. Upon the addition of CB[8], the absorption gradually decreased with the appearance of the shoulder absorption peak at 434 nm, suggesting the formation of the supramolecular host–guest complexes. In addition, as the amount of CB[8] was >0.5 eq, the absorption spectra reached equilibrium, suggesting that the binding mode of the host–guest system between compound 2 and CB[8] was the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 type. Meanwhile, remarkable changes were observed in fluorescence spectra upon the titration of the CB[8]: (i) the red-shift of the fluorescence emission peak (from 500 nm to 532 nm, Fig. S37, ESI†); (ii) the appearance of the maximum fluorescence intensity at 0.6 eq, the quantum yield of 2 increased significantly (Fig. 3c and Fig. S38, ESI†). These results also suggested that compound 2 and CB[8] formed 1
2 type. Meanwhile, remarkable changes were observed in fluorescence spectra upon the titration of the CB[8]: (i) the red-shift of the fluorescence emission peak (from 500 nm to 532 nm, Fig. S37, ESI†); (ii) the appearance of the maximum fluorescence intensity at 0.6 eq, the quantum yield of 2 increased significantly (Fig. 3c and Fig. S38, ESI†). These results also suggested that compound 2 and CB[8] formed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 type supramolucular host–guest complexes. Moreover, the fluorescence wavelength blue shifted when the amount of CB[8] was >0.6 eq. (Fig. 3c and Fig. S37, ESI†) and the emission peak blue shifted to 504 nm with a continuous addition of the CB[8] (form 0.6 to 5 eq.), suggesting other host–guest complexes were formed. Therefore, we hypothesized that compound 2 and CB[8] formed 2
2 type supramolucular host–guest complexes. Moreover, the fluorescence wavelength blue shifted when the amount of CB[8] was >0.6 eq. (Fig. 3c and Fig. S37, ESI†) and the emission peak blue shifted to 504 nm with a continuous addition of the CB[8] (form 0.6 to 5 eq.), suggesting other host–guest complexes were formed. Therefore, we hypothesized that compound 2 and CB[8] formed 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type of host–guest complexes due to the occurrence of two binding sites of compound 2. Such a binding molar ratio was also confirmed by the ITC titration (Fig. S39, ESI†).
1 type of host–guest complexes due to the occurrence of two binding sites of compound 2. Such a binding molar ratio was also confirmed by the ITC titration (Fig. S39, ESI†).
The conformations were also analysed by 1H NMR spectra. According to the 1H NMR spectra, after adding CB[8] into the D2O solution of 2 (Fig. 3d), the changes in proton signals for the pyridine (Ha,b) suggested a host–guest combination between 2 and CB[8]. The binding mode was well demonstrated by carefully analyzing the 1H NMR spectra. The proton Hc shifted downfield (8.3 to 8.4 ppm), indicating that the 2,4-dinitrophenyl groups were outside the CB[8] cavity.16 As shown in the NOESY spectrum (Fig. S40, ESI†) of 2 (2.6 mM) with CB[8] (1.1 mM), Ha was positioned close to proton Hd and Hb was close to Hc,d,e, indicating the stacking between the pyridinium and 2,4-dinitrophenyl moieties. On the other hand (Fig. S40, ESI†), this also suggested that the other two pyridinium moieties were stacked with the fluorescent core, thiazolothiazole, which is strongly related with the red-shift that appeared in the fluorescence spectra leading to an emission change from blue to yellow (Fig. S41, ESI†).
According to the previous demonstrations, the multi-color fluorescence could be generated by a simple small compound through the supramolecular assembly strategy. Such assembly behaviours were influenced by the structure of the substituted components, i.e., different substituent molecules (phenyl, 2,4-dinitrophenyl, methyl, benzyl) led to different supramolecular complexes being formed in water with the increasing concentrations of CB[8], resulting in an intrinsic change of the fluorescence emission. In particular, compounds 1 and 2 solutions exhibited multi-colour luminescence with the addition of CB[8]. Such property allows us to raise an important question here: whether it is possible to transfer multi-color luminescence from the solution phase to the solid phase. It is well known that the later is more efficient for constructing intelligent fluorescent materials with diverse applications of smart materials with stimuli-responsive, adaptive and dynamic properties in recent years.22 Our strategy was to support 1 or 2 on silica gel by simply daubing the solution, dried by airing or heating. Compounds 1 and 2 exhibited green and blue luminescence on dry silica gel, respectively (Fig. 4b and c). As shown in Fig. 4, the blue luminescence could turn into green luminescence after dropping water onto it, reversed backed after evaporation. Moreover, introducing CB[8] resulted in the red-shift of the fluorescence, i.e., green luminescence transferred into green-yellow luminescence after adding CB[8] solution on the silica gel. Such change may be attributed to the formation of the host–guest complexes and such complexes could be reversibly regulated by Amantadine hydrochloride (ADA) (Fig. S42–S46, ESI†). Similar changes were observed in compound 2 (Fig. 4c). Fluorescence was reversibly changed between green (dry) and yellow (wet) when the wetness of the materials was altered. Further red-shift could be observed by adding CB[8] solutions and reversed with the addition of ADA solutions. Such water-dependent fluorescence properties have provided the studied molecular system with great practical potential in developing organic light-emitting and humidity sensing materials.
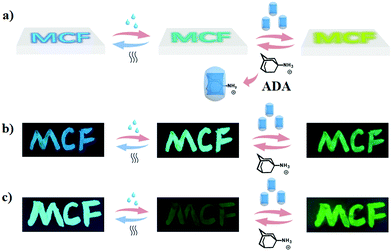 | ||
| Fig. 4 (a) A schematic presentation of the intelligent fluorescent materials. Images of 1 (b) and 2 (c) supported on silica gel under UV light at 365 nm. | ||
Conclusions
A color-tunable molecular system based on host–guest recognition has been successfully developed. Different substituent groups were discovered to influence the emission of the fluorescent compounds remarkably when interacting with CB[8]. The results could be potentially utilized to develop intelligent fluorescent materials with response to humidity. This study has given a clear demonstration of designing smart luminescent materials based on supramolecular fluorescent systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 22025503, 21790361, 21871084, and 21672060), Shanghai Municipal Science and Technology Major Project (grant 2018SHZDZX03), the Fundamental Research Funds for the Central Universities, the Programme of Introducing Talents of Discipline to Universities (grant B16017), the Program of Shanghai Academic/Technology Research Leader (19XD1421100), and the Shanghai Science and Technology Committee (grant 17520750100).References
- (a) M. Zuo, W. Qian, T. Li, X. Hu, J. Jiang and L. Wang, Full-Color Tunable Fluorescent and Chemiluminescent Supramolecular Nanoparticles for Anti-counterfeiting Inks, ACS Appl. Mater. Interfaces, 2018, 10, 39214–39221 CrossRef CAS; (b) H. Ding, J. Li, G. Xie, G. Lin, R. Chen, Z. Peng, C. Yang, B. Wang, J. Sun and C. Wang, An AIEgen-based 3D covalent organic framework for white light-emitting diodes, Nat. Commun., 2018, 9, 5234 CrossRef CAS; (c) Z. Wang, Z. Wang, B. Lin, X. Hu, Y. Wei, C. Zhang, B. An, C. Wang and W. Lin, Warm-White-Light-Emitting Diode Based on a Dye-Loaded Metal-Organic Framework for Fast White-Light Communication, ACS Appl. Mater. Interfaces, 2017, 9, 35253–35259 CrossRef CAS; (d) S. Guo, X. Yan, Z. Luo, M. Ding, H. Liu and X. Yang, Tunable WLED with UV chip based on all-inorganic perovskite quantum dots, Chem. Phys. Lett., 2019, 720, 124–127 CrossRef CAS.
- C. Giansante, C. Schäfer, G. Raffy and A. D. Guerzo, Exploiting Direct and Cascade Energy Transfer for Color-Tunable and White-Light Emission in Three-Component Self-Assembled Nanofibers, J. Phys. Chem. C, 2012, 116, 21706–21716 CrossRef CAS.
- D. Lim, M. Kang, S. Jang, K. Hwang, I. Kim, E. Jung, Y. Jo, Y. Kim, J. Kim, H. Choi, T. Kim, S. Mathur, B. Kim and D. Kim, Unsymmetrical Small Molecules for Broad-Band Photoresponse and Efficient Charge Transport in Organic Phototransistors, ACS Appl. Mater. Interfaces, 2020, 12, 25066–25074 CrossRef CAS.
- (a) V. Piradi, G. Zhang, T. Li, M. Zhang, Q. Peng, X. Zhan and X. Zhu, Side-Chain Engineering of Benzodithiophene-Bridged Dimeric Porphyrin Donors for All-Small-Molecule Organic Solar Cells, ACS Appl. Mater. Interfaces, 2020, 12, 41506–41514 CrossRef; (b) X. Zhang, S. Rehm, M. M. Safont-Sempere and F. Würthner, Vesicular perylene dye nanocapsules as supramolecular fluorescent pH sensor systems, Nat. Chem., 2009, 1, 623–629 CrossRef CAS; (c) Z. Xie, C. Chen, S. Xu, J. Li, Y. Zhang, S. Liu, J. Xu and Z. Chi, White-Light Emission Strategy of a Single Organic Compound with Aggregation-Induced Emission and Delayed Fluorescence Properties, Angew. Chem., Int. Ed., 2015, 54, 7181–7184 CrossRef CAS.
- (a) C. Giansante, G. Raffy, C. Schäfer, H. Rahma, M. Kao, A. G. L. Olive and A. Guerzo, White-Light-Emitting Self-Assembled NanoFibers and Their Evidence by Microspectroscopy of Individual Objects, J. Am. Chem. Soc., 2011, 133, 316–325 CrossRef CAS; (b) D. K. Maiti, R. Bhattacharjee, A. Datta and A. Banerjee, Modulation of Fluorescence Resonance Energy Transfer Efficiency for White Light Emission from a Series of Stilbene-Perylene Based Donor-Acceptor Pair, J. Phys. Chem. C, 2013, 117, 23178–23189 CrossRef CAS.
- (a) L. Chen, Y. Chen, H. Fu and Y. Liu, Reversible Emitting Anti-Counterfeiting Ink Prepared by Anthraquinone-Modified β-Cyclodextrin Supramolecular Polymer, Adv. Sci., 2020, 7, 2000803 CrossRef CAS; (b) Y. Hou, Z. Zhang, S. Lu, J. Yuan, Q. Zhu, W. Chen, S. Ling, X. Li, Y. Zheng, K. Zhu and M. Zhang, Highly Emissive Perylene Diimide-Based Metallacages and Their Host-Guest Chemistry for Information Encryption, J. Am. Chem. Soc., 2020, 142, 18763–18768 CrossRef CAS; (c) H. Wang, X. Ji, Z. Li, C. N. Zhu, X. Yang, T. Li, Z. L. Wu and F. Huang, Preparation of a white-light-emitting fluorescent supramolecular polymer gel with a single chromophore and use of the gel to fabricate a protected quick response code, Mater. Chem. Front., 2017, 1, 167–171 RSC.
- (a) F. Biedermann, E. Elmalem, I. Ghosh, W. M. Nau and O. A. Scherman, Strongly Fluorescent, Switchable Perylene Bis(diimide) Host-Guest Complexes with Cucurbit[8]uril In Water, Angew. Chem., Int. Ed., 2012, 51, 7739–7743 CrossRef CAS; (b) J. Mohanty, S. D. Choudhury, H. P. Upadhyaya, A. C. Bhasikuttan and H. Pal, Control of the Supramolecular Excimer Formation of Thioflavin T within a Cucurbit[8]uril Host: A Fluorescence On/Off Mechanism, Chem. Eur. J., 2009, 15, 5215–5219 CrossRef CAS; (c) K. Kotturi and E. Masson, Directional Self-Sorting with Cucurbit[8]uril Controlled by Allosteric π–π and Metal-Metal Interactions, Chem. Eur. J., 2018, 24, 8670–8678 CrossRef CAS; (d) J. Mohanty, N. Thakur, S. D. Choudhury, N. Barooah, H. Pal and A. C. Bhasikuttan, Recognition-Mediated Light-Up of Thiazole Orange with Cucurbit[8]uril: Exchange and Release by Chemical Stimuli, J. Phys. Chem. B, 2012, 116, 130–135 CrossRef CAS; (e) X. Ma, J. Wang and H. Tian, Assembling-Induced Emission: An Efficient Approach for Amorphous Metal-Free Organic Emitting Materials with Room-Temperature Phosphorescence, Acc. Chem. Res., 2019, 52, 738–748 CrossRef CAS; (f) H. Wu, Y. Chen, X. Dai, P. Li, J. F. Stoddart and Y. Liu, In Situ Photoconversion of Multicolor Luminescence and Pure White Light Emission Based on Carbon Dot-Supported Supramolecular Assembly, J. Am. Chem. Soc., 2019, 141(16), 6583–6591 CrossRef CAS.
- Y. Liu, Q. Zhang, W. Jin, T. Xu, D. Qu and H. Tian, Bistable [2]rotaxane encoding an orthogonally tunable fluorescent molecular system including white-light emission, Chem. Commun., 2018, 54, 10642–10645 RSC.
- (a) M. R. Molla, D. Gehrig, L. Roy, V. Kamm, A. Paul, F. Laquai and S. Ghosh, Self-Assembly of Carboxylic Acid Appended Naphthalene Diimide Derivatives with Tunable Luminescent Color and Electrical Conductivity, Chem. Eur. J., 2014, 20, 760–771 CrossRef CAS; (b) X. Yan, T. R. Cook, P. Wang, F. Huang and P. J. Stang, Highly emissive platinum(II) metallacages, Nat. Chem., 2015, 7, 342–348 CrossRef CAS; (c) M. Zhang, S. Yin, J. Zhang, Z. Zhou, M. L. Saha, C. Lu and P. J. Stang, Metallacycle-cored supramolecular assemblies with tunable fluorescence including white-light emission, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 3044–3049 CrossRef CAS.
- W. Jin, H. Lu, Q. Zhang and D. Qu, A dual-mode orthogonally tunable fluorescent system covering the whole white light region, Mater. Chem. Front., 2020, 4, 532–536 RSC.
- (a) Q. Wang, Q. Zhang, Q. Zhang, X. Li, C. Zhao, T. Xu, D. Qu and H. Tian, Color-tunable single-fluorophore supramolecular system with assembly-encoded emission, Nat. Commun., 2020, 11, 158 CrossRef; (b) Q. Zhang, D. Li, X. Li, P. B. White, J. Mecinovic, X. Ma, H. Ågren, R. J. M. Nolte and H. Tian, Multicolor Photoluminescence Including White-Light Emission by a Single Host-Guest Complex, J. Am. Chem. Soc., 2016, 138, 13541–13550 CrossRef CAS; (c) X. Chen, Y. Chen and Y. Liu, Multiple-Stimuli Responsive and Tunable Luminescent Supramolecular Assembly by Oligo(p-phenylvinylene) and Surfactant, Chin. J. Chem., 2018, 36, 526–530 CrossRef CAS.
- (a) Y. Jiao, K. Liu, G. Wang, Y. Wang and X. Zhang, Supramolecular free radicals: near-infrared organic materials with enhanced photothermal conversion, Chem. Sci., 2015, 6, 3975–3980 RSC; (b) M. Freitag, L. Gundlach, P. Piotrowiak and E. Galoppini, Fluorescence Enhancement of Di-p-tolyl Viologen by Complexation in Cucurbit[7]uril, J. Am. Chem. Soc., 2012, 134, 3358–3366 CrossRef CAS.
- (a) E. J. Dale, N. A. Vermeulen, M. Jurícek, J. C. Barnes, R. M. Young, M. R. Wasielewski and J. F. Stoddart, Supramolecular Explorations: Exhibiting the Extent of Extended Cationic Cyclophanes, Acc. Chem. Res., 2016, 49, 262–273 CrossRef CAS; (b) J. C. Barnes, M. Jurícek, N. L. Strutt, M. Frasconi, S. Sampath, M. A. Giesener, P. L. McGrier, C. J. Bruns, C. L. Stern, A. A. Sarjeant and J. F. Stoddart, ExBox: A Polycyclic Aromatic Hydrocarbon Scavenger, J. Am. Chem. Soc., 2013, 135, 183–192 CrossRef CAS; (c) I. Roy, S. Bobbala, J. Zhou, M. T. Nguyen, S. K. M. Nalluri, Y. Wu, D. P. Ferris, E. A. Scott, M. R. Wasielewski and J. F. Stoddart, ExTzBox: A Glowing Cyclophane for Live-Cell Imaging, J. Am. Chem. Soc., 2018, 140, 7206–7212 CrossRef CAS.
- (a) P. Spenst and F. Würthner, A Perylene Bisimide Cyclophane as a “Turn-On” and “Turn-Off” Fluorescence Probe, Angew. Chem., Int. Ed., 2015, 54, 10165–10168 CrossRef CAS; (b) B. Gui, X. Liu, G. Yu, W. Zeng, A. Mal, S. Gong, C. Yang and C. Wang, Tuning of Förster Resonance Energy Transfer in Metal-Organic Frameworks: Toward Amplified Fluorescence Sensing, CCS Chem., 2020, 2, 2054–2062 CrossRef; (c) B. Gui, G. Lin, H. Ding, C. Gao, A. Mal and C. Wang, Three-Dimensional Covalent Organic Frameworks: From Topology Design to Applications, Acc. Chem. Res., 2020, 53, 2225–2234 CrossRef CAS.
- (a) D. Jiao, J. Geng, X. J. Loh, D. Das, T. Lee and O. A. Scherman, Supramolecular Peptide Amphiphile Vesicles through Host-Guest Complexation, Angew. Chem., Int. Ed., 2012, 51, 9633–9637 CrossRef CAS; (b) Z. Huang, L. Yang, Y. Liu, Z. Wang, O. A. Scherman and X. Zhang, Supramolecular Polymerization Promoted and Controlled through Self-Sorting, Angew. Chem., Int. Ed., 2014, 53, 5351–5355 CrossRef CAS; (c) M. Olesińska, G. Wu, S. Gómez-Coca, D. Antón-García, I. Szabó, E. Rostab and O. A. Scherman, Modular supramolecular dimerization of optically tunable extended aryl viologens, Chem. Sci., 2019, 10, 8806–8811 RSC; (d) Q. Zhang, R. Xing, W. Wang, Y. Deng, D. Qu and H. Tian, Dynamic Adaptive Two-Dimensional Supramolecular Assemblies for On-Demand Filtration, iScience, 2019, 19, 14–24 CrossRef.
- G. Wu, Y. J. Bae, M. Olesińska, D. Antón-García, I. Szabó, E. Rosta, M. R. Wasielewski and O. A. Scherman, Controlling the structure and photophysics of fluorophore dimers using multiple cucurbit[8]uril clampings, Chem. Sci., 2020, 11, 812–825 RSC.
- (a) H. Kim, P. C. Nandajan, J. Gierschner and S. Y. Park, Light-Harvesting Fluorescent Supramolecular Block Copolymers Based on Cyanostilbene Derivatives and Cucurbit[8]urils in Aqueous Solution, Adv. Funct. Mater., 2018, 28, 1705141 CrossRef; (b) Y. Li, C. Qin, Q. Li, P. Wang, X. Miao, H. Jin, W. Ao and L. Cao, Supramolecular Organic Frameworks with Controllable Shape and Aggregation-Induced Emission for Tunable Luminescent Materials through Aqueous Host-Guest Complexation, Adv. Opt. Mater., 2020, 8, 1902154 CrossRef CAS; (c) H. Kim, D. R. Whang, J. Gierschner and S. Y. Park, Cover Picture: Structurally Defined Molecular Hypervalent Iodine Catalysts for Intermolecular Enantioselective Reactions, Angew. Chem., Int. Ed., 2016, 55, 1–6 CrossRef; (d) J. Wang, Z. Huang, X. Ma and H. Tian, Visible-Light-Excited Room-Temperature Phosphorescence in Water by Cucurbit[8]uril-Mediated Supramolecular Assembly, Angew. Chem., Int. Ed., 2020, 59, 9928–9933 CrossRef CAS; (e) G. Wu, Z. Huang and O. A. Scherman, Quantitative Supramolecular Heterodimerization for Efficient Energy Transfer, Angew. Chem., Int. Ed., 2020, 59, 15963–15967 CrossRef CAS; (f) H. Yang, Y. Liu, K. Liu, L. Yang and Z. Wang, and Xi Zhang, Rational Adjustment of Multicolor Emissions by Cucurbiturils-Based Host-Guest Chemistry and Photochemistry, Langmuir, 2013, 29, 12909–12914 CrossRef CAS.
- G. Wu, I. Szabó, E. Rosta and O. A. Scherman, Cucurbit[8]uril-mediated pseudo[2,3]rotaxanes, Chem. Commun., 2019, 55, 13227–13230 RSC.
- B. Tang, W. Li, Y. Chang, B. Yuan, Y. Wu, M. Zhang, J. Xu, J. Li and X. Zhang, A Supramolecular Radical Dimer: High-Efficiency NIR-II Photothermal Conversion and Therapy, Angew. Chem., Int. Ed., 2019, 58, 15526–15531 CrossRef CAS.
- T. Jiang, X. Wang, J. Wang, G. Hu and X. Ma, Humidity- and Temperature-Tunable Multicolor Luminescence of Cucurbit[8]uril-Based Supramolecular Assembly, ACS Appl. Mater. Interfaces, 2019, 11, 14399–14407 CrossRef CAS.
- X. Ni, S. Chen, Y. Yang and Z. Tao, Facile Cucurbit[8]uril-Based Supramolecular Approach To Fabricate Tunable Luminescent Materials in Aqueous Solution, J. Am. Chem. Soc., 2016, 138, 6177–6183 CrossRef CAS.
- (a) J. Hou, W. X. Ren, K. Li, J. Seo, A. Sharma, X. Yu and J. S. Kim, Fluorescent bioimaging of pH: from design to applications, Chem. Soc. Rev., 2017, 46, 2076–2090 RSC; (b) J. Du, L. Sheng, Q. Chen, Y. Xu, W. Li, X. Wang, M. Li and S. X. Zhang, Simple and general platform for highly adjustable thermochromic fluorescent materials and multi-feasible applications, Mater. Horiz., 2019, 6, 1654–1662 RSC; (c) W. Luo and G. Wang, Photo-Responsive Fluorescent Materials with Aggregation-Induced Emission Characteristics, Adv. Opt. Mater., 2020, 2001362 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qm01029d |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2021 |