Copper-mediated annulation of 2-(1-arylvinyl) anilines and aryl nitrosos towards 2,3-diaryl-2H-indazoles†
Weiming
Hu
,
Jin-Tao
Yu
,
Suqin
Liu
,
Yan
Jiang
and
Jiang
Cheng
*
School of Petrochemical Engineering, Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Jiangsu Province Key Laboratory of Fine Petrochemical Engineering, Changzhou University, Changzhou 213164, P. R. China. E-mail: jiangcheng@cczu.edu.cn
First published on 20th October 2016
Abstract
A copper-mediated annulation of 2-(1-substituted vinyl) anilines and aryl nitrosos is developed for the synthesis of 2,3-diaryl pyrazoles. Compared with previously reported sequential azobenzene C–H ortho-acylation, reduction and cyclization procedures towards such frameworks, no external reductant was required during this cyclization, where the vinyl served as a formal innate reductant. Moreover, in this procedure, no selectivity was involved in the case of Ar1 ≠ Ar2.
2H-Indazoles are remarkable potential medicines against a variety of diseases.1 However, compared with thermodynamically favored 1H-analogues,2 the development of methodologies to access 2H-indazole was far below expectations. Coarctate cyclization reactions required a high reaction temperature (160–200 °C) towards 2H-indazoles.3 To circumvent it, Lindenschmidt developed a palladium-catalyzed cascade reaction of 1-chloro-2-phenylethynylbenzene and phenylhydrazine leading to 2H-pyrazoles.4 Almost at the same time, Knochel disclosed the reaction of 2-chloromethylarylzinc and aryldiazonium to generate polyfunctional 2H-indazoles.5 Afterwards, Driver demonstrated an iron(II)-catalyzed intramolecular transformation of azides with methyl oximes,6 and Genung achieved a mild, one-pot condensation–cadogan reductive cyclization to access 2H-indazoles.7 Nevertheless, the development of both new reaction substrates and methodologies towards such frameworks remained highly desirable in organic chemistry.
Besides, Shi has reported the [3 + 2] cycloaddition of arynes and sydnones in 2010 to access 2H-indazoles.8 And Lee achieved the construction of this motif via the reaction of diazos with 2-iodoazobenzenes.9 Recently, Wang developed the synthesis of 2H-indazoles by sequential acylation of azo compounds (or their derivatives), followed by cyclization at the cost of stoichiometric reductants such as NaBH4 and Zn.10 Subsequently, similar procedures were independently achieved by Ellman11 and Wang,12 wherein an external reductant was not required since the intermediate before cyclization was a low oxidation state alcohol rather than a ketone via ortho C–H acylation of azobenzene. Since a vinyl group could serve as a precursor of diol, we expect a similar intermediate whereby the appropriate oxidation of 2-vinyl in azobenzene could furnish the ring closure to construct 2H-indazole frameworks, where nitroso compounds were employed.13 Such a procedure shows several advantages: (1) the potential starting material 2-(1-arylvinyl) aniline is readily available by either the reaction of terminal alkyne and aniline,14 or the reaction between 2-iodoaniline and phenacylamine N-Ts hydrazone;15 (2) no additional external reductant is required; and (3) in comparison with the procedure starting with benzyne and azobenzene, no regional selectivity is involved.
Initially, we tested the reaction of 2-(1-phenylvinyl) aniline 1a with nitrosobenzene 2a in the presence of Cu(acac)2 and di-tert-butyl peroxide (DTBP) in DMSO under O2 at 130 °C (Table 1, entry 1). To our delight, 2,3-diphenyl-2H-indazoles 3aa were isolated in 12% yield. On replacing Cu(acac)2 with Cu(OAc)2, the yield dramatically increased to 60% (Table 1, entry 2), while CuCl2 did not work at all (Table 1, entry 3). Other cooxidants, such as K2S2O8 and p-benzoquinone (BQ) were much less effective than DTBP (Table 1, entries 4 and 5). 3aa could be obtained in 45% without any cooxidant (Table 1, entry 6). This result encouraged us to employ O2 as the terminal cooxidant. In this case, the yield increased substantially with the increasing loading of Cu(OAc)2 (Table 1, entries 7–9), and the yield increased to 75% when 2.0 equivalents of Cu(OAc)2 were added (Table 1, entry 9). However, a further increase of the loading of the catalyst didn't give higher yield (Table 1, entry 10). To investigate the solvent effects on reaction efficiency, DMF, DMAc, xylene and PhCF3 were employed. However, they were all inferior to DMSO, generating 3aa in 25%, 10%, 46% and 40% yields, respectively (Table 1, entry 9). On varying either the atmosphere (air or N2) or the reaction temperature (80, 100 or 120 °C) all the efficiencies decreased (Table 1, entry 11). No reaction took place in the absence of Cu(OAc)2 (Table 1, entry 12). No 3aa was detected when Cu(OAc)2 was replaced with OsO4 (2.0 equiv.), (NH4)2S2O8 (2.0 equiv.) and H2O2 (2.0 equiv.) (Table 1, entries 13–15). Interestingly, NaIO4 worked well, giving 3aa in 62% yield (Table 1, entry 16).
| Entry | [Cu](equiv.) | Cooxidant | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 2-(1-phenylvinyl)aniline 1a (0.1 mmol), nitrosobenzene 2a (0.25 mmol), copper, cooxidant (2.0 equiv.), in DMSO (2 mL), under O2, 130 °C, 15 h. b Isolated yield. c DMF (2 mL). d DMAc (2 mL). e Xylene (2 mL). f PhCF3 (2 mL). g In air. h Under N2. i At 80 °C. j At 100 °C. k At 120 °C. | |||
| 1 | Cu(acac)2(0.2) | DTBP | 12 |
| 2 | Cu(OAc)2(0.2) | DTBP | 60 |
| 3 | CuCl2(0.2) | DTBP | <5 |
| 4 | Cu(OAc)2(0.2) | K2S2O8 | <5 |
| 5 | Cu(OAc)2(0.2) | BQ | 15 |
| 6 | Cu(OAc)2(0.2) | — | 45 |
| 7 | Cu(OAc)2(0.1) | — | 37 |
| 8 | Cu(OAc)2(1.0) | — | 63 |
| 9 | Cu(OAc)2(2.0) | — | 75, 25c, 10d, 46e, 40f |
| 10 | Cu(OAc)2(2.5) | — | 73 |
| 11 | Cu(OAc)2(2.0) | — | 67g, 38h, 49i, 61j, 68k |
| 12 | — | — | <5 |
| 13 | — | OsO4 | <1 |
| 14 | — | (NH4)2S2O8 | <1 |
| 15 | — | H2O2 | <1 |
| 16 | — | NaIO4 | 62 |
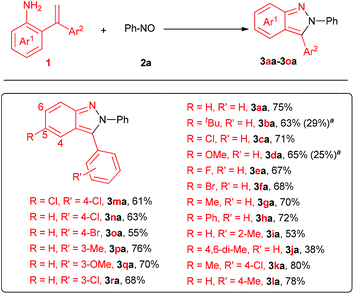
Once the optimized conditions were established, the scope of 2-(1-arylvinyl) anilines were studied (Fig. 1). As expected, this procedure was applicable to a series of 2,3-diaryl-2H-pyrazoles, providing the corresponding products in moderate to good yields (3aa–3ja, 38%–80%). All the products except 3aa were hard to access via the previously reported procedures starting from azobenzenes and aryne owing to their regioselectivity. Thus, this method represents an exceedingly practical complement to access 2,3-diaryl-2H-pyrazoles. Pleasingly, functional groups, such as methyl, methoxy, bromo, chloro and fluoro were all well tolerated under the standard conditions, providing facile handles for potentially further functionalization. Notably, R′ of Ar2 on ortho-, meta- and para-positions were all studied, providing corresponding 2H-pyrazole derivatives in 53%–80% yields (3ia and 3ka–3ra). However, the hindrance on the 2-phenyl and 4-position of the product had some effect on the reaction efficiency, as 3ia and 3ja were isolated in 53% and 38% yields, respectively. Unfortunately, the attempt to access 2-phenyl-3-methyl-2H-pyrazoles was failed.
Meanwhile, the scope of nitrosobenzenes was studied, as shown in Fig. 2. Once again, the diversity of the product further increased as the procedure produced 2,3-diaryl-2H-pyrazoles with varied groups on 3-phenyl (methyl, tert-butyl, chloro, bromo) in moderate to good yields (45%–65%, 3ab–3ag). In the cases of 3ia, 3ja and 3ab, the formation of 3-aryl indole byproducts via aza-Wacker-type cyclization may account for the slightly low yields.
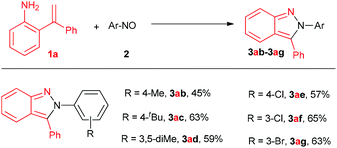 | ||
| Fig. 2 Substrate scope of nitrosos. Reaction conditions: 2-(1-phenylvinyl)aniline 1a (0.1 mmol), aryl nitroso 2 (0.25 mmol) and Cu(OAc)2 (2.0 equiv.) in DMSO (2 mL) under O2, 130 °C, 15 h. | ||
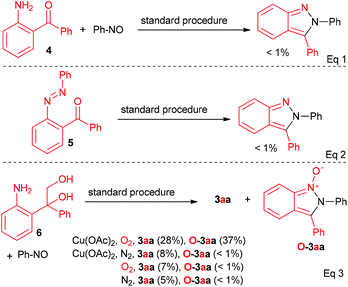
More experiments were conducted to gain some insights into this transformation. First, some potential intermediates (4–6) were prepared and subjected to the reaction, respectively (Scheme 1). The reaction of 4 and nitrosobenzene, as well as the reaction of 5 under a standard procedure did not lead to the desired product (Scheme 1, eqn (1) and (2)). Thus, 4 and 5 were unlikely the intermediates in this transformation. However, 3aa was obtained in 28% yield when diol 6 was employed under a standard procedure, along with a byproduct (Fw = 286) in 37% yield (Scheme 1, eqn (3)). The MS, 1H NMR and 13C NMR were all consistent with the structure of O-3aa (Scheme 1, eqn (3)), which may be derived from the oxidation of 3aa by O2. In most cases, trace byproducts with one more oxygen formula weight were detected by GC-MS (see the ESI† for details). Further studies revealed that both Cu(OAc)2 and O2 were required in this reaction to ensure the transformation towards the final product.
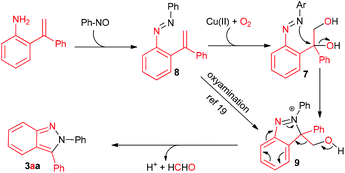
Based on these experimental results, a proposed mechanism was outlined in Scheme 2. Firstly, the addition of amino to nitroso followed by the elimination of H2O produces aryl azo 8.16,17 Then, the oxidation of vinyl by Cu(OAc)2 and/or O2 provides the diol intermediate 7. Secondly, similar to Ellman's procedure,11 the intra- molecular SN reaction furnishes the ring closure and leads to intermediate 9. Finally, 9 undergoes the retro-Friedel–Crafts reaction to deliver 2,3-diphenyl-2H-indazoles along with formaldehyde.18 Copper may also take part in this step. Alternatively, an intermolecular oxyamination of 8 to 9 is also reasonable.19
Conclusions
In conclusion, we have developed a copper-mediated annulation of 2-(1-arylvinyl) anilines with aryl nitrosos towards 2,3-aryl-2H-Indazoles. The reaction involves: (1) the reaction of amine and nitroso towards azo; (2) the key step whereby the appropriate oxidation of vinyl to diol as a low oxidation state intermediate rather than a ketone; and (3) the intramolecular SN reaction of hydroxyl attacked by azo furnishing C–N bond formation with ring closure to construct the framework of 2,3-aryl-2H-indazoles. In comparison with the previously reported sequential azobenzene C–H ortho-acylation, reduction and cyclization procedures towards such frameworks, no regional selectivity was involved and no external reductant was required during the cyclization step, where the vinyl served as a formal innate reductant.Acknowledgements
We thank the National Natural Science Foundation of China (no. 21272028, 21572025 and 21672028), “Innovation & Entrepreneurship Talents” Introduction Plan of Jiangsu Province, the Natural Science Foundation for Colleges and Universities of Jiangsu Province (no. 16KJB150002 and 15KJA150001), the Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology (BM2012110) and the Advanced Catalysis and Green Manufacturing Collaborative Innovation Center, Changzhou University for financial supports.Notes and references
- For reviews: (a) M. J. Haddadin, W. E. Conrad and M. J. Kurth, Mini-Rev. Med. Chem., 2012, 12, 1293 CrossRef CAS PubMed; (b) A. Schmidt, A. Beutler and B. Snovydovych, Eur. J. Org. Chem., 2008, 4073 CrossRef CAS; (c) D. D. Gaikwad, A. D. Chapolikar, C. G. Devkate, K. D. Warad, A. P. Tayade, R. P. Pawar and A. J. Domb, Eur. J. Med. Chem., 2015, 90, 707 CrossRef CAS PubMed; (d) A. Thangadurai, M. Minu, S. Wakode, S. Agrawal and B. Narasimhan, Med. Chem. Res., 2012, 21, 1509 CrossRef CAS.
- For selected recent examples: (a) C.-Y. Chen, G. Tang, F. He, Z. Wang, H. Jing and R. Faessler, Org. Lett., 2016, 18, 1690 CrossRef CAS PubMed; (b) B. C. Wray and J. P. Stambuli, Org. Lett., 2010, 12, 4576 CrossRef CAS PubMed; (c) X. Xiong, Y. Jiang and D. Ma, Org. Lett., 2012, 14, 2552 CrossRef CAS PubMed; (d) H.-J. Liu, S.-F. Hung, C.-L. Chen and M.-H. Lin, Tetrahedron, 2013, 69, 3907 CrossRef CAS; (e) J. Yu, J. W. Lim, S. Y. Kim, J. Kim and J. N. Kim, Tetrahedron Lett., 2015, 56, 1432 CrossRef CAS; (f) X. Tang, H. Gao, J. Yang, W. Wu and H. Jiang, Org. Chem. Front., 2014, 1, 1295 RSC; (g) T. Zhang and W. Bao, J. Org. Chem., 2013, 78, 1317 CrossRef CAS PubMed; (h) R. C. Wheeler, E. Baxter, I. B. Campbell and S. J. F. MacDonald, Org. Process Res. Dev., 2011, 15, 565 CrossRef CAS; (i) Q. Wang and X. Li, Org. Lett., 2016, 18, 2102 CrossRef CAS PubMed; (j) L. Li, H. Wang, S. Yu, F. Yang and X. Li, Org. Lett., 2016, 18, 3662 CrossRef CAS PubMed.
- (a) B. S. Young, R. Herges and M. M. Haley, Chem. Commun., 2012, 48, 9441 RSC; (b) D. B. Kimball, T. J. R. Weakley and M. M. Haley, J. Org. Chem., 2002, 67, 6395 CrossRef CAS PubMed; (c) D. B. Kimball, A. G. Hayes and M. M. Haley, Org. Lett., 2000, 2, 382 CrossRef.
- N. Halland, M. Nazaré, O. R'Kyek, J. Alonso, M. Urmann and A. Lindenschmidt, Angew. Chem., Int. Ed., 2009, 48, 6879 CrossRef CAS PubMed.
- B. Haag, Z. Peng and P. Knochel, Org. Lett., 2009, 11, 4270 CrossRef CAS PubMed.
- B. J. Stokes, C. V. Vogel, L. K. Urnezis, M. Pan and T. G. Driver, Org. Lett., 2010, 12, 2884 CrossRef CAS PubMed.
- N. E. Genung, L. Wei and G. E. Aspnes, Org. Lett., 2014, 16, 3114 CrossRef CAS PubMed.
- C. Wu, Y. Fang, R. C. Larock and F. Shi, Org. Lett., 2010, 12, 2234 CrossRef CAS PubMed.
- W.-S. Yong, S. Park, H. Yun and P. H. Lee, Adv. Synth. Catal., 2016, 358, 1958 CrossRef CAS.
- (a) H. Li, P. Li, H. Tan and L. Wang, Chem. – Eur. J., 2013, 19, 14432 CrossRef CAS PubMed; (b) H. Li, P. Li, Q. Zhao and L. Wang, Chem. Commun., 2013, 49, 9170 RSC; (c) H. Li, P. Li and L. Wang, Org. Lett., 2013, 15, 620 CrossRef CAS PubMed.
- J. R. Hummel and J. A. Ellman, J. Am. Chem. Soc., 2015, 137, 490 CrossRef CAS PubMed.
- X. Geng and C. Wang, Org. Lett., 2015, 17, 2434 CrossRef CAS PubMed.
- For the transformations of unsaturated compounds with nitroso compounds, please see: (a) X.-H. Yang, R.-J. Song and J.-H. Li, Adv. Synth. Catal., 2015, 357, 3849 CrossRef CAS; (b) T. Shen, Y. Yuan and N. Jiao, Chem. Commun., 2014, 50, 554 RSC; (c) F. Chen, X. Huang, X. Li, T. Shen, M. Zou and N. Jiao, Angew. Chem., Int. Ed., 2014, 53, 10495 CrossRef CAS PubMed; (d) Y. Liu, J.-L. Zhang, R.-J. Song, P.-C. Qian and J.-H. Li, Angew. Chem., Int. Ed., 2014, 53, 9017 CrossRef CAS PubMed; (e) U. Dutta, S. Maity, R. Kancherla and D. Maiti, Org. Lett., 2014, 16, 6302 CrossRef CAS PubMed; (f) P. Gao, H.-X. Li, X.-H. Hao, D.-P. Jin, D.-Q. Chen, X.-B. Yan, X.-X. Wu, X.-R. Song, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2014, 16, 6298 CrossRef CAS PubMed; (g) Y. Lin, W. Kong and Q. Song, Org. Lett., 2016, 18, 3702 CrossRef CAS PubMed; (h) G. C. Senadi, B. S. Gore, W.-P. Hu and J.-J. Wang, Org. Lett., 2016, 18, 2890 CrossRef CAS PubMed.
- (a) Z. Zhang, L.-L. Liao, S.-S. Yan, L. Wang, Y.-Q. He, J.-H. Ye, J. Li, Y.-G. Zhi and D.-G. Yu, Angew. Chem., Int. Ed., 2016, 55, 7068 CrossRef CAS PubMed; (b) K. Sasano, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2013, 135, 10954 CrossRef CAS PubMed; (c) L. Wang, J. Ferguson and F. Zeng, Org. Biomol. Chem., 2015, 13, 11486 RSC; (d) A. Arienti, F. Bigi, R. Maggi, E. Marzi, P. Moggi, M. Rastelli, G. Sartori and F. Tarantola, Tetrahedron, 1997, 53, 3795 CrossRef CAS; (e) J. Ferguson, F. L. Zeng, N. Alwis and H. Alper, Org. Lett., 2013, 15, 1998 CrossRef CAS PubMed.
- (a) D. P. Ojha and K. R. Prabhu, J. Org. Chem., 2012, 77, 11027 CrossRef CAS PubMed; (b) D. Ganapathy and G. Sekar, Org. Lett., 2014, 16, 3856 CrossRef CAS PubMed. For reviews on the N-tosyl hydrazine, see: (c) Y. Zhang and J. Wang, Top. Curr. Chem., 2012, 327, 239 CrossRef CAS PubMed; (d) A. P. Jadhav, D. Ray, V. U. B. Rao and R. P. Singh, Eur. J. Org. Chem., 2016, 2369 CrossRef CAS; (e) Z. Liu and J. J. Wang, Org. Chem., 2013, 78, 10024 CrossRef CAS PubMed; (f) Z. Liu, Y. Zhang and J. Wang, Chin. J. Org. Chem., 2013, 33, 687 CrossRef CAS; (g) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef CAS PubMed; (h) Z. Shao and H. Zhang, Chem. Soc. Rev., 2012, 41, 560 RSC.
- For reviews on the synthetic application of nitroso, please see: (a) D. B. Huple, S. Ghorpade and R.-S. Liu, Adv. Synth. Catal., 2016, 358, 1348 CrossRef CAS; (b) P. Merino, T. Tejero, I. Delso and R. Matute, Synthesis, 2016, 653 CrossRef CAS; (c) B. Maji and H. Yamamoto, Bull. Chem. Soc. Jpn., 2015, 88, 753 CrossRef CAS; (d) S. Huang, H. Huo, W. Li and R. Hong, Chin. J. Org. Chem., 2012, 32, 1776 CrossRef CAS; (e) A. V. Malkov, Chem. Heterocycl. Compd., 2012, 48, 39 CrossRef CAS; (f) M. Baidya and H. Yamamoto, Synthesis, 2013, 1931 CrossRef CAS.
- (a) R. Zhao, C. Tan, Y. Xie, C. Gao, H. Liu and Y. Jiang, Tetrahedron Lett., 2011, 52, 3805 CrossRef CAS; (b) T. H. L. Nguyen, N. Gigant, S. Delarue-Cochin and D. Joseph, J. Org. Chem., 2016, 81, 1850 CrossRef CAS PubMed.
- For the Friedel–Crafts reactions of indoles, please see: (a) C. W. Downey, C. D. Poff and A. N. Nizinski, J. Org. Chem., 2015, 80, 10364 CrossRef CAS PubMed; (b) J. A. Joule, Sci. Synth., 2001, 10, 361 CAS.
- For copper-promoted oxyamination of alkenes, please see: (a) F. C. Sequeira and S. R. Chemler, Org. Lett., 2012, 14, 4482 CrossRef CAS PubMed; (b) S. Ren, S. Song, L. Ye, C. Feng and T.-P. Loh, Chem. Commun., 2016, 52, 10373 RSC; (c) M. Noack and R. Göttlich, Chem. Commun., 2002, 536 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00540c |
| This journal is © the Partner Organisations 2017 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(OAc)2 (0.2 equiv.).
Cu(OAc)2 (0.2 equiv.).