Palladium-catalyzed arylation of β-methylene C(sp3)–H bonds at room temperature: desymmetrization of simple cycloalkyl carboxylic acids†
W. A.
Nack
b,
B.
Wang
b,
X.
Wu
b,
R.
Jiao
b,
G.
He
a and
G.
Chen
*ab
aState Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, China. E-mail: gongchen@nankai.edu.cn; guc11@psu.edu
bDepartment of Chemistry, The Pennsylvania State University, University Park, Pennsylvania 16802, USA
First published on 1st March 2016
Abstract
A new protocol for Pd-catalyzed β methylene C–H arylation of N-quinolyl cycloalkylcarboxamides with aryl iodides at room temperature is reported. The β methylene C–H bonds of symmetrical cycloalkylcarboxamides of varied ring size can be arylated in moderate to good yields and monoselectivity with excellent diastereoselectivity. This mono-selective β methylene C–H arylation reaction enabled the rapid synthesis of complex carbocycle products from easily accessible symmetrical cycloalkyl carboxylic acids via sequential C–H functionalization.
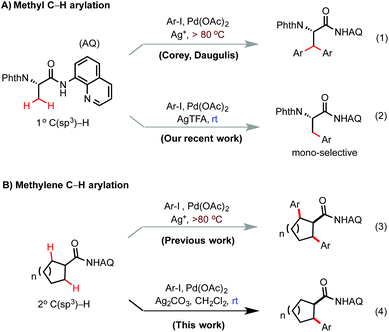
Reactions based on palladium-catalyzed sp3 C–H functionalization of aliphatic carboxamides bearing the aminoquinoline (AQ) auxiliary have quickly advanced over the past ten years.1 Among these reactions, sp3 C–H arylation with aryl halides has been shown to be particularly useful for constructing various alkylarene motifs from readily available precursors.2 Compared with other amide-linked directing groups, the AQ group is unique for its effectiveness at functionalizing β methylene C–H bonds of carboxamide substrates.3–10 However, the high efficiency of methylene C–H functionalization in these AQ-directed reaction systems sometimes presents unique challenges. For instance, arylation of the Phth-protected AQ-coupled alanine substrate with aryl iodides under typical reaction conditions at elevated temperatures does not give mono-arylated β-aryl α-amino acids, but instead forms symmetrically β-di-arylated α-amino acids as the major products (Scheme 1A).4a Recently, we demonstrated that mono-selective β C–H arylation of alanine substrates with aryl iodides could be achieved with high efficiency at room temperature via kinetic control.6a Cycloalkyl carboxamides represent another class of useful substrates for Pd-catalyzed C–H arylation reactions.11 Several groups have demonstrated that N-quinolyl cycloalkylcarboxamides can readily undergo β methylene C–H arylation, however β,β′-diarylated products are typically obtained (Scheme 1B).3a,b,4d,7,8 Herein, we report a new protocol for Pd-catalyzed β methylene C–H arylation of N-quinolyl cycloalkylcarboxamides with aryl iodides at room temperature. The β methylene C–H bonds of symmetrical cycloalkylcarboxamides of varied ring size can be arylated in moderate to good yields and monoselectivity with excellent diastereoselectivity.
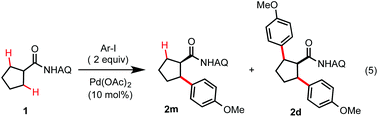
As shown in Table 1, we commenced our study with arylation of cyclopentyl carboxamide 1 with 2 equiv. of 4-iodoanisole under various Pd-catalyzed conditions. Reaction of 1 under previously reported conditions at elevated temperatures gave the di-arylated product 2d as the major product (entries 1 and 2). Reaction of 1 under our room temperature conditions for C–H arylation of alanine with AgTFA in dioxane gave little product (entries 3–6).6 To our delight, further optimization revealed that the use of 1 equiv. of Ag2CO3 in dichloromethane (DCM) or 1,1,2,2-tetrachloroethane (TCE) at room temperature for 2 days gave the desired mono-arylated product 2m in excellent yield and selectivity (entry 7). Various carboxylic acid additives have no useful promoting effect (entries 12–14). DCM is used as a solvent in the subsequent experiments due to its lower cost and toxicity than TCE.
| Entry | Reagents (equiv.) | Solvents | t (°C)/time (h) | Yielda (%) | |
|---|---|---|---|---|---|
| 2m | 2d | ||||
| a Yields are based on 1H-NMR analysis on a 0.2 mmol scale, under an ambient atmosphere. b Isolated yield. | |||||
| 1 | AgOAc | Neat | 70/24 | <5% | 73% |
| 2 | AgOAc (2) | Toluene | 110/24 | 38% | 62% |
| 3 | AgTFA (2) | Dioxane | rt/48 | <5% | <5% |
| 4 | AgOAc (2), TFA (2) | Dioxane | rt/48 | <5% | <5% |
| 5 | AgTFA (2) | tAmylOH | rt/48 | <5% | <5% |
| 6 | AgTFA (2) | DCM | rt/48 | <5% | <5% |
| 7 | Ag2CO3 (1) | DCM | rt/48 | 85 (80b) | <5% |
| 8 | Ag2CO3 (1) | Toluene | rt/48 | 21 | <5% |
| 9 | Ag2CO3 (1) | Dioxane | rt/48 | 11 | <5% |
| 10 | Ag2CO3 (1) | tAmylOH | rt/48 | 15 | <5% |
| 11 | Ag2CO3 (1) | TCE | rt/48 | 85 | <5% |
| 12 | Ag2CO3 (1), TFA (1) | DCM | rt/48 | <5% | <5% |
| 13 | Ag2CO3 (1), AcOH (1) | DCM | rt/48 | 71% | <5% |
| 14 | Ag2CO3 (1), PivOH (1) | DCM | rt/48 | 68% | <5% |
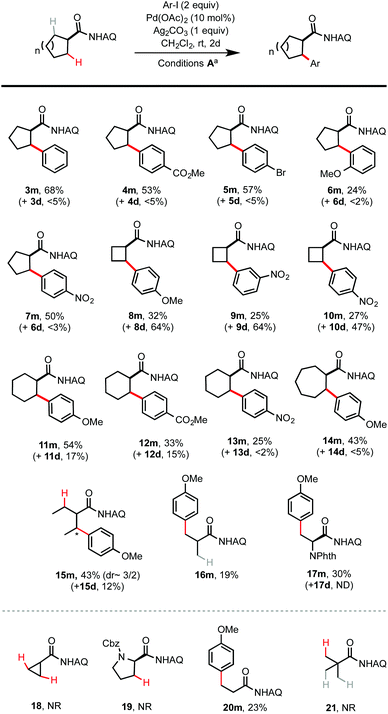
Next, we proceeded to evaluate the substrate scope of the Pd-catalyzed β C–H arylation of symmetric cycloalkyl carboxamides of varied ring size and other related structures under the optimized conditions at rt (Scheme 2). All C–H arylation reactions of symmetric cycloalkyl carboxamides provided exclusive cis diastereoselectivity under rt conditions. Electron rich aryl iodides gave higher arylation yield than electron deficient aryl iodides (e.g.2mvs.7m). Sterically more hindered aryl iodides gave lower yields (e.g.6m). The ring size has a notable effect on reactivity. Reactions of cyclopentyl carboxamide 1 gave moderate to good yields with excellent monoselectivity. Arylation of cyclobutyl carboxamides proceeded with higher yield but decreased mono-selectivity (see 8m and 8d). Reactions of cyclohexyl and cycloheptyl carboxamides had reactivity similar to cyclopentyl carboxamides, and proceeded with moderate yield and selectivity (see 11m and 14m). Cyclopropyl carboxamide 18 was completely unreactive. 2-Ethylbutyramide, the ring-opened analog of 1, gave comparable arylation yield but reduced mono- and diastereo-selectivity (see 15m). Interestingly, pivalamide 21 was completely unreactive under rt conditions.
As shown in Scheme 3, several mono-arylated products were subjected to a sequential Pd-catalyzed, AQ-directed C–H functionalization including C–H arylation, alkylation, and alkenylation at the remaining β C–H position to give cycloalkyl products with three contiguous stereocenters with cis stereochemistry. Interestingly, the attempted acetoxylation of 11m resulted in the formation of the β-lactam product 26via intramolecular dehydrogenative C–H amination (IDCA) along with a small amount of the acetoxylated product 27.12 Similarly, 9m can undergo IDCA to give a highly strained β-lactam 28 in high yield. As shown in Scheme 3E, the AQ group of the mono-arylated product 8m can be easily removed in two steps under mild conditions to give the carboxylic acid product 29.
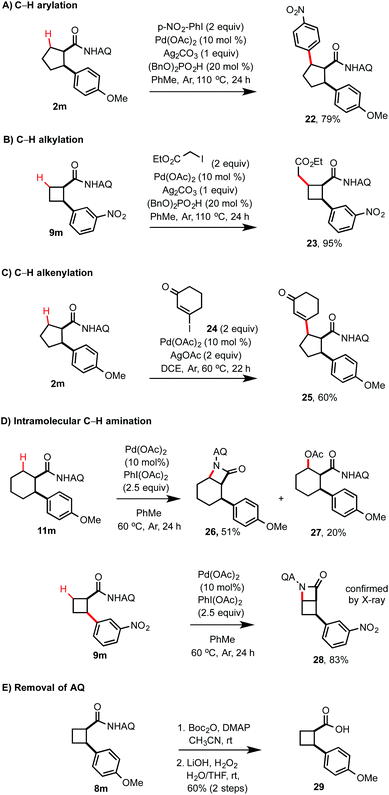 | ||
| Scheme 3 Pd-catalyzed sequential C–H functionalization of cycloalkyl carboxamides. (a) Isolated yields on a 0.2 mmol scale. | ||
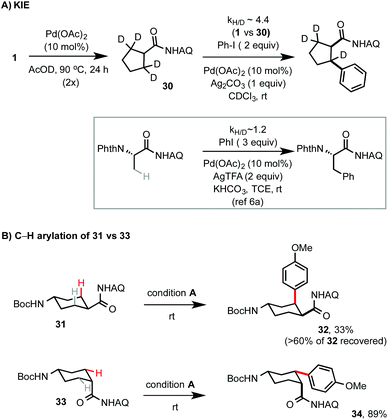
Previous work has suggested that AQ-directed, Pd-catalyzed C–H arylations with aryl iodides proceed through a Pd(II)/Pd(IV) catalytic cycle.3b A primary KIE of ∼4.4 was observed from the parallel arylation of 30 and 1 with PhI under our rt conditions, suggesting that C–H palladation is the rate-limiting step (Scheme 4). This is in sharp contrast to a small KIE observed in our previously reported Pd-catalyzed C–H arylation of Phth-protected AQ-coupled alanine at rt.6a As exemplified by the markedly different arylation performance of Boc protected trans- and cis-4-amino cyclohexanecarboxamides 31 and 33, the stereochemical configuration of cycloalkane rings strongly influences their reactivity towards C–H arylation. However, the details of this stereochemical influence are not clearly understood.
In summary, we have developed a new protocol for Pd-catalyzed β methylene C–H arylation of N-quinolyl cycloalkylcarboxamides with aryl iodides that proceeds at room temperature. Symmetrical cycloalkylcarboxamides of varied ring size can be arylated at the β methylene C–H position in good yield and with good mono- and diastereo-selectivity. This β mono-selective methylene C–H arylation enabled the rapid synthesis of complex carbocycle products from easily accessible symmetrical cycloalkyl carboxylic acids via sequential C–H functionalization. Further experiments concerning other C–H functionalization reactions of cycloalkylcarboxamides, as well as mechanistic studies, are underway.
We gratefully thank Pennsylvania State University and Nankai University for financial support of this work.
Notes and references
- For selected reviews of Pd-catalyzed C(sp3)–H arylation: (a) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (b) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (c) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (d) O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902 RSC; (e) Z. Huang and G. Dong, Tetrahedron Lett., 2014, 55, 5869 CrossRef CAS; (f) Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107 RSC.
- Selected examples of auxiliary-directed Pd-catalyzed C(sp3)–H arylation with Ar–X: (a) N. Rodríguez, J. A. Romero-Revilla, M. Á. Fernández-Ibáñez and J. C. Carretero, Chem. Sci., 2013, 4, 175 RSC; (b) M. Fan and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 12152 CrossRef CAS PubMed; (c) Q. Zhang, K. Chen, W. Rao, Y. Zhang, F.-J. Chen; and B.-F. Shi, Angew. Chem., Int. Ed., 2013, 52, 13588 CrossRef CAS PubMed; (d) J. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homes and J.-Q. Yu, Science, 2014, 343, 1216 CrossRef CAS PubMed; (e) C. Wang, C. Chen, J. Zhang, J. Han, Q. Wang, K. Guo, P. Liu, M. Guan, Y. Yao and Y.-S. Zhao, Angew. Chem., Int. Ed., 2014, 53, 9884 CrossRef CAS PubMed; (f) Y.-F. Zhang, H.-W. Zhao, H. Wang, J.-B. Wei and Z.-J. Shi, Angew. Chem., Int. Ed., 2015, 54, 13686 CrossRef CAS PubMed; (g) K. Chen, Z.-W. Li, P.-X. Shen, H.-W. Zhao and Z.-J. Shi, Chem. – Eur. J., 2015, 21, 7389 CrossRef CAS PubMed.
- (a) V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154 CrossRef CAS PubMed; (b) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965 CrossRef CAS PubMed; (c) L. D. Tran and O. Daugulis, Angew. Chem., Int. Ed., 2012, 51, 5188 CrossRef CAS PubMed; (d) E. T. Nadres, G. I. F. Santos, D. Shabashov and O. Daugulis, J. Org. Chem., 2013, 78, 9689 CrossRef CAS PubMed; (e) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed.
- For selected examples of AQ-directed Pd-catalyzed C(sp3)–H arylation: (a) B. V. S. Reddy, L. R. Reddy and E. J. Corey, Org. Lett., 2006, 8, 3391 CrossRef CAS PubMed; (b) F. Pan, P.-X. Shen, L.-S. Zhang, X. Wang and Z.-J. Shi, Org. Lett., 2013, 15, 4758 CrossRef CAS PubMed; (c) N. Hoshiya, T. Kobayashi, M. Arisawa and S. Shuto, Org. Lett., 2013, 15, 6202 CrossRef CAS PubMed; (d) Y. Wei, H. Tang, X. Cong, B. Rao, C. Wu and X. Zeng, Org. Lett., 2014, 16, 2248 CrossRef CAS PubMed; (e) D. P. Affron, O. A. Davis and J. A. Bull, Org. Lett., 2014, 16, 4956 CrossRef CAS PubMed; (f) C. P. Ting and T. J. Maimone, Angew. Chem., Int. Ed., 2014, 53, 3115 CrossRef CAS PubMed; (g) K. Chen, S.-Q. Zhang, J.-W. Xu, F. Hu and B.-F. Shi, Chem. Commun., 2014, 50, 13924 RSC; (h) K. Chen, S.-Q. Zhang, H.-Z. Jiang, J.-W. Xu and B.-F. Shi, Chem. – Eur. J., 2015, 21, 3264 CrossRef CAS PubMed; (i) S.-B. Yan, S. Zhang and W.-L. Duan, Org. Lett., 2015, 17, 2458 CrossRef CAS PubMed; (j) R. Feng, B. Wang, Y. Liu, Z. Liu and Y. Zhang, Eur. J. Org. Chem., 2015, 142 CrossRef CAS; (k) Q. Guo, Z.-F. Zhang, Z.-C. Liu and J. Qin, J. Org. Chem., 2015, 80, 3176 CrossRef PubMed.
- (a) Y. Feng and G. Chen, Angew. Chem., Int. Ed., 2010, 49, 958 CrossRef CAS PubMed; (b) Y. Feng, Y. Wang, B. Landgraf, S. Liu and G. Chen, Org. Lett., 2010, 12, 3414 CrossRef CAS PubMed; (c) S.-Y. Zhang, Q. Li, G. He, W. A. Nack and G. Chen, J. Am. Chem. Soc., 2013, 135, 12135 CrossRef CAS PubMed; (d) G. He, S.-Y. Zhang, W. A. Nack, Q. Li and G. Chen, Angew. Chem., Int. Ed., 2013, 52, 11124 CrossRef CAS PubMed; (e) G. He, S.-Y. Zhang, W. A. Nack, R. Pearson, J. Rabb-Lynch and G. Chen, Org. Lett., 2014, 16, 6488 CrossRef CAS PubMed.
- Recent examples of Pd-catalyzed AQ-directed C(sp3)–H functionalization reactions at room temperature: (a) B. Wang, W. A. Nack, G. He, S.-Y. Zhang and G. Chen, Chem. Sci., 2014, 5, 3952 RSC; (b) B. Wang, C. Lu, S.-Y. Zhang, G. He, W. A. Nack and G. Chen, Org. Lett., 2014, 16, 6260 CrossRef CAS PubMed; (c) B. Wang, X. Wu, R. Jiao, S.-Y. Zhang, W. A. Nack, G. He and G. Chen, Org. Chem. Front., 2015, 2, 1318 RSC.
- (a) W. R. Gutekunst and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 19076 CrossRef CAS PubMed; (b) W. R. Gutekunst, R. Gianatassio and P. S. Baran, Angew. Chem., Int. Ed., 2012, 51, 7507 CrossRef CAS PubMed; (c) W. R. Gutekunst and P. S. Baran, J. Org. Chem., 2014, 79, 2430 CrossRef CAS PubMed.
- (a) R. Parella, B. Gopalakrishnan and S. A. Babu, J. Org. Chem., 2013, 78, 11911 CrossRef CAS PubMed; (b) R. Parella, B. Gopalakrishnan and S. A. Babu, Org. Lett., 2013, 15, 3238 CrossRef CAS PubMed; (c) R. Parella and S. A. Babu, J. Org. Chem., 2015, 80, 2339 CrossRef CAS PubMed.
- For selected reports on AQ-directed C(sp3)–H arylation catalyzed by other metals: (a) R. Shang, L. Ilies, A. Matsumoto and E. Nakamura, J. Am. Chem. Soc., 2013, 135, 6030 CrossRef CAS PubMed; (b) Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2014, 136, 898 CrossRef CAS PubMed; (c) M. Iyanaga, Y. Aihara and N. Chatani, J. Org. Chem., 2014, 79, 11933 CrossRef CAS PubMed; (d) M. Li, J. Dong, X. Huang, K. Li, Q. Wu, F. Song and J. You, Chem. Commun., 2014, 50, 3944 RSC.
- G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed.
- For selected examples of C(sp3)–H arylation with cycloalkyl carboxamides: (a) M. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 19598 CrossRef CAS PubMed; (b) M. Wasa, K. S. L. Chan, X.-G. Zhang, J. He, M. Miura and J.-Q. Yu, J. Am. Chem. Soc., 2012, 134, 18570 CrossRef CAS PubMed; (c) K.-J. Xiao, D. W. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 8138 CrossRef CAS PubMed; (d) Q. Zhang, X.-S. Yin, S. Zhao, S.-L. Fang and B.-F. Shi, Chem. Commun., 2014, 50, 8353 RSC; (e) J. Kim, M. Sim, N. Kim and S. Hong, Chem. Sci., 2015, 6, 3611 RSC; (f) J. Liu, Y. Xie, W. Zeng, D. Lin, Y. Deng and X. Lu, J. Org. Chem., 2015, 80, 4618 CrossRef CAS PubMed; (g) S.-K. Zhang, X.-Y. Yang, X.-M. Zhao, P.-X. Li, J.-L. Niu and M.-P. Song, Organometallics, 2015, 34, 4331 CrossRef CAS.
- (a) T. S. Mei, L. Kou, S. Ma, K. M. Engle and J.-Q. Yu, Synthesis, 2012, 1778 CAS; (b) Q. Zhang, K. Chen and B.-F. Shi, Synlett, 2014, 1941 CAS; (c) W. A. Nack and G. Chen, Synlett, 2015, 2505 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1451082. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00421g |
| This journal is © the Partner Organisations 2016 |