DOI:
10.1039/C4RA16992A
(Paper)
RSC Adv., 2015,
5, 24126-24131
Highly selective zirconia-based propene sensor attached with sol–gel derived NiO nanospheres
Received
24th December 2014
, Accepted 25th February 2015
First published on 25th February 2015
Abstract
Nanospheres of nickel oxide (NiO) were synthesized by a simple modified sol–gel route using nickel nitrate as a precursor and citric acid as a gelling agent. An yttria-stabilized zirconia (YSZ)-based sensor was fabricated using the sol–gel derived NiO nanospheres as the sensing electrode (SE) and Pt as the reference electrode (RE), and its sensing characteristics were examined in the temperature range of 650–800 °C under various O2 concentrations (5, 10, 15 and 21 vol%). The fabricated NiO-SE was characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). It turned out that the sensor attached with the sol–gel derived NiO-SE exhibited a selective and sensitive response to propene (C3H6) at 700 °C in all measured O2 concentrations. The sensor showed the highest sensitivity and selectivity to propene in 21 vol% O2. In addition, the sensor exhibited a stable response to C3H6 for about two months. The sensing mechanism was confirmed to be mixed-potential-type. It is believed that the nanofeatures associated with the sol–gel derived NiO seemed to be responsible for the high sensitivity and selectivity to C3H6.
1. Introduction
In recent decades, humans have gained a notable rise in their living standards due to increased and modernized industrialization, including in the automobile industry. Although industrialization and modernization have led to a lot of positive impacts, they have also led to severe environmental issues, especially air pollution, caused by the expanding usage of automobiles. Among the various types of pollution, air pollution poses a challenging factor in living environments. In fact, various toxic pollutants such as NOx (NO + NO2), CO, SOx and unburnt hydrocarbons (HCs) including propane, propene etc., are released from industry and automobiles, leading to adverse effects such as acid rain, ozone depletion, the greenhouse effect, photochemical smog, etc.1–3 Among the various sources, combustion of fossil fuels in industry and automobiles is the major source of air pollution. One way to control the toxic emissions from automobiles is to make sure of the presence of the required air to fuel ratio (A/F) for efficient fuel combustion with the help of feedback control units equipped with emission monitoring systems. Another way to reduce toxic emissions is to use post cylinder de-emission technologies. Currently, stringent emission legislations such as Euro VI (Europe), Tier-3 (USA) and BS-IV (India) should be enforced in automobiles to monitor toxic emissions. For example, Euro VI and BS-IV set the NOx, CO and HC emission limits to be 0.06, 0.5 and 0.1 g km−1, respectively.4–6 Thus, the automobile manufacturers are forced to adopt de-emission technology equipped with feedback control units which have emission monitoring systems. To meet such stringent norms, it is important to develop high performance NOx, CO and HC sensors which would work well at elevated temperatures of above 500 °C under harsh conditions such as varying O2, CO2 and H2O concentrations.6,7
Recently, electrochemical sensors using a high temperature solid electrolyte, namely yttria-stabilized zirconia (YSZ), have received great attention for detecting various analytes as they can work well even in the aforesaid harsh environments. Currently, several reports on YSZ-based sensors operating in different modes such as mixed potential, impedancemetric and amperometric are reported.6–8 Among them, mixed-potential-type sensors are preferred due to their attractive features such as high sensitivity, portability and low cost.9,10
For sensing electrode (SE) materials, metals, metal oxides and composites have all been utilized and their sensing performances have been reported.11–14 For example, Dutta et al., reported the use of WO3 to detect NO2 and CO gases.15 Diao et al., utilized MnCr2O4 as a NO2 sensing material. The sintering temperature of MnCr2O4 was modulated and it was reported that the MnCr2O4 sample sintered at 1000 °C exhibited a higher response to NO2 gas.16 Quite recently, Zhou et al., optimized the sintering temperature of (La0.8Sr0.2)2FeMnO6−δ and the sensor sintered at 1200 °C showed the high sensitivity to NO2 at 550 °C.17 Park et al., used NiO, CuO and NiO (+YSZ) as sensing electrodes for sensing total NOx concentration.18 Liang et al., utilized NiO as a sensing material and reported an improvement in the NO2 sensitivity by tuning the triple-phase boundary using hydrofluoric acid (HF), and the sensor showed higher sensitivity to NO2 using YSZ treated with 40% HF.19 The use of metal/metal oxide composites as SEs showed higher sensitivity than individual metals and metal oxides. For example, Westphal et al., utilized a composite consisting of Ga2O3 and Au for the sensing of hydrocarbons.20 Wang et al. used a composite consisting of NiO and Rh for the sensing of NOx gas. NiO with 3 wt% added Rh showed a higher response to NO2 compared with pure NiO.21 A NiO–Au composite was found to exhibit a selective response to propene, while the pure NiO and Au are not so selective.22 In most of the above cases, a commercial oxide was utilized. To the best of our knowledge, the influence of the synthetic process on the sensing performance was not explored for NiO. Thus, in the present work, NiO nanospheres were synthesized by using a simple modified sol–gel route and characterized by powder X-ray diffraction (XRD) and scanning electron microscopy (SEM). The obtained NiO was used for the fabrication of an YSZ-based mixed-potential-type sensor and its gas sensing properties are reported here.
2. Experimental
2.1 Sol–gel synthesis of NiO
Nickel oxide nanospheres were synthesized by a simple citrate-based sol–gel method by using nickel nitrate as a precursor and citric acid as a gelling agent. The detailed synthesis and influence of citric acid concentration on the formation of sol–gel end products have been reported by us recently elsewhere.23 However, the sol–gel process for the synthesis of NiO nanospheres is described briefly here. Nickel nitrate and citric acid in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 molar ratio were used for the synthesis of NiO. The required quantity of nickel nitrate was dissolved in distilled water and kept at 80 °C on a magnetic stirrer. The required amount of citric acid was also dissolved in distilled water separately. To the warm nickel nitrate solution, citric acid solution was added in dropwise. After the complete addition of all citric acid, the contents were heated to 130 °C to form sol and continued heating to get a gel. The formed gel was allowed to dry at 130 °C overnight. The calcination of the gel at 500 °C for 2 h in air was carried out to get the final product.
8 molar ratio were used for the synthesis of NiO. The required quantity of nickel nitrate was dissolved in distilled water and kept at 80 °C on a magnetic stirrer. The required amount of citric acid was also dissolved in distilled water separately. To the warm nickel nitrate solution, citric acid solution was added in dropwise. After the complete addition of all citric acid, the contents were heated to 130 °C to form sol and continued heating to get a gel. The formed gel was allowed to dry at 130 °C overnight. The calcination of the gel at 500 °C for 2 h in air was carried out to get the final product.
2.2 Fabrication of the sensor device
The YSZ-based sensor was made up of a one-end-opened commercially available YSZ tube (8 mol% Y2O3-doped zirconia) as a solid electrolyte (oxide-ion conductor), the above-synthesised NiO as the sensing electrode (SE) and Pt as the reference electrode (RE). The YSZ tube was 300 mm in length and had an inner diameter of 9 mm. For fabrication of the NiO-SE and Pt-RE, NiO and Pt powders (Ants Ceramics, India) were each mixed with α-terpineol solvent in an agate mortar to make a uniform paste. The obtained pastes were applied to the outer surface of the YSZ tube and dried at 110 °C for 6 h in air and subsequently sintered at 1300 °C for 2 h in air. After the sintering, a 0.4 mm thick Pt wire was wound on each of the NiO and Pt layers for the purpose of a current collector. Then, the sensor was housed in a quartz tube. Fig. 1 shows a photograph of the exterior of the fabricated YSZ-based sensor device. The crystal structure of the sintered NiO layer formed on the YSZ was determined by means of X-ray diffraction analysis (XRD Diffractometer, Bruker D8 Advanced) using a CuKα source (λ = 1.5418 Å). The surface morphology of the NiO layer was observed by means of a scanning electron microscope (SEM/EDX – Hitachi S3400N).
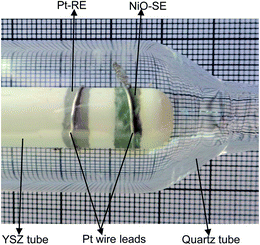 |
| | Fig. 1 Photograph of the exterior of the assembled YSZ-based sensor attached with the sol–gel derived NiO-SE and Pt-RE. | |
2.3 Evaluation of gas sensing properties
The evaluation of the gas sensing characteristics of the fabricated sensor was carried out in a homemade gas-flow apparatus equipped with digital mass-flow controllers and a furnace operating in the temperature range of 650–800 °C. The required concentration of propene sample gas was prepared by diluting commercially-available propene (1000 ppm propene gas + N2 balance) with nitrogen and this was allowed to flow over the sensor surface at a constant flow rate of 100 cm3 min−1. The ppm refers to the volume concentration of the gaseous mixture. The potential difference (emf) between the NiO-SE and the Pt-RE of the sensor was measured with a digital electrometer (GWINSTEK, GDM-8261). Cross sensitivities to each of 400 ppm NO2, NO, CO, C3H8 and C3H6 were measured at 700 °C. All the measurements were performed under dry conditions. Base gas refers to the mixture of O2 and N2 gas in a desired proportion (5, 10, 15 and 21 vol% O2 + N2 balance). Sample gas refers to the mixture of target gas (in a desired concentration) and the base gas.
The current–voltage (polarization) curves of the sensor were measured by using a source meter (Agilent technologies, model: B2900A) based on a potential-sweep method at a scan rate of 2 mV s−1 in the base gas (5 or 21 vol% O2 + N2 balance) and in the sample gas (400 ppm C3H6 + base gas). The current of the cathodic polarization curve (measured in the base gas) was subtracted from that of the anodic polarization curve (measured in the sample gas) at each potential, so as to obtain the modified-anodic polarization curve in which the current axis is expressed in an absolute scale.
3. Results and discussion
3.1 Crystal structure and morphology of the SE layer
Fig. 2 shows the XRD pattern of the NiO layer on the YSZ substrate sintered at 1300 °C. For comparison, the XRD pattern obtained from the pure YSZ substrate is also given. The set of Bragg peaks obtained for the YSZ substrate could be conveniently indexed to the cubic phase of YSZ as per the JCPDS # 772112. In the case of the oxide on YSZ, the set of Bragg peaks appearing at 30.09, 34.94, 50.19, 59.59, 62.39, 73.57, 81.71, and 84.25° correspond to the XRD pattern of cubic YSZ, while the set of Bragg peaks appearing at 37.04, 43.04, 75.28 and 79.29° could be indexed to face centered cubic NiO as per the JCPDS # 731519. No other impurity peaks were observed. Thus, the XRD results confirmed that even after sintering at 1300 °C, nickel oxide is stable and could exist on the YSZ substrate. It is known that the melting point of NiO is above 1900 °C, which is well away from the sintering temperature used in the present fabrication process. The crystallite size of NiO estimated from the Bragg peak (43.04°) was about 142 nm using the Scherrer formula given below.
Lhkl = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
where Lhkl denotes crystallite size, λ is X-ray wavelength (1.5418 Å), β is the full width at half maximum (FWHM) of the Bragg peak (in radians), and θ is the Bragg angle. Fig. 3 shows the surface morphology of the 1300 °C-sintered NiO layer. The surface consists of uniform spherical shaped NiO grains with interconnections throughout the matrix (Fig. 3(b)). The surface also consists of uniform pores with the pore size varying from 80 to 350 nm. The NiO grain size is about 180 nm. It is expected that these nano-grains and pores would influence the sensing performance of the sensor.
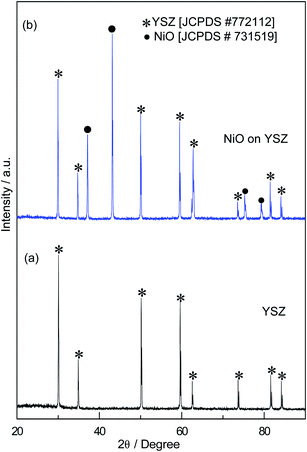 |
| | Fig. 2 XRD patterns of (a) the YSZ substrate and (b) the sol–gel derived 1300 °C-sintered NiO-SE layer on the YSZ substrate. | |
 |
| | Fig. 3 SEM images at (a) low magnification and (b) high magnification of the surface of the 1300 °C-sintered NiO-SE layer. | |
3.2 Sensing performance of the sensor
To examine the sensing performance of the sensor attached with 1300 °C-sintered NiO-SE, its cross sensitivities to various gases such as NO2, NO, CO, C3H8 (propane) and C3H6 (propene) were measured in 5, 10, 15 and 21 vol% base gas (O2 + N2 balance) at 700 °C. Fig. 4 shows a comparison of the obtained cross sensitivities of the sensor. The cross sensitivity (Δemf) is defined as the difference between the emf value in the sample gas and that in the base gas, as shown below,
| Δemf = emfsample − emfbase gas |
 |
| | Fig. 4 Cross sensitivities to various gases for the YSZ-based sensor attached with the sol–gel derived NiO-SE at 700 °C under dry conditions. | |
It is seen that the sensor showed high sensitivity and selectivity to propene in each of the examined oxygen concentrations. It is noted that the cross sensitivities to all other gases are much lower than that of propene, irrespective of oxygen concentration. As the oxygen concentration is increased the sensitivity to propene also increases, and the highest sensitivity and selectivity was observed in 21 vol% O2. Thus, among the various oxygen concentrations examined, 21 vol% O2 is the optimum to obtain high sensitivity and selectivity. It is noteworthy that the oxygen concentration in the air is 21 vol% and that in the automobile exhaust is almost 5 vol%. Thus, the present sensor can be used in practical exhausts with a control unit for oxygen concentration. Even under 5 vol% base gas, the sensitivity and selectivity are quite good.
Since the sensor attached with the sol–gel derived NiO-SE exhibited a selective response to C3H6, further C3H6 sensing characteristics were examined in detail. Fig. 5 shows response transients to C3H6 in the concentration range of 10–400 ppm at 700 °C for the sensor attached with the sol–gel derived NiO-SE. The emf of the sensor in the base gas was almost constant and changed quickly from the base value, and then almost reached a steady-state value in due course. The sensor returned to the original base value upon changing the sample gas at each concentration back to the base gas. It can be seen that the steady-state emf of the sensor increased with increasing C3H6 concentration, showing the excellent sensing ability of the sensor to varying concentrations. It is worth noting that the sensor showed a good response to even very low concentrations of C3H6 (10 ppm). The response time is the time taken to reach the saturation emf while changing from base gas to sample gas, whereas recovery time refers to the time taken to return to the original base value while changing from the sample gas to the base gas. For a practical sensor, response/recovery rates are important key factors. Thus, the estimated 80% response/recovery times of the sensor were about 60 s/120 s. It was observed that the response and recovery times were the same for all the propene concentrations except for 10 ppm, which was quicker. It can be seen that the sensor would have taken a few more seconds to reach saturation emf. However, a constant duration was kept for registering sensing signals in each of the propene concentrations. It is noted that the flow rate in our experiments was only 100 cm3 min−1. But, in actual exhaust, the flow rate is much higher. Thus, the present sensor is expected to show much faster response/recovery rates in actual exhaust.
 |
| | Fig. 5 Response transients to various concentrations of C3H6 for the YSZ-based sensor attached with the sol–gel derived NiO-SE at 700 °C. | |
The temperature of the automobile exhaust is dynamic, it even reaches 800 °C, and will play a significant role in deciding the sensitivity of the sensor. Thus, it is important to examine the sensitivity of the sensor at various operating temperatures. To examine the influence of operating temperature on the C3H6 sensitivity, the response transients to various concentrations of C3H6 were recorded at 650 °C, 700 °C, 750 °C and 800 °C. From the obtained response transients, the sensitivity to each C3H6 concentration was calculated by using the following equation.
| Δemf = emfpropene − emfbase gas |
Fig. 6 shows the dependence of Δemf on the concentration of C3H6 at 650 °C, 700 °C, 750 °C and 800 °C. It can be seen that the Δemf varies linearly with C3H6 concentrations on a logarithmic scale at each temperature although with slight change in the slope. Such a linear dependence of Δemf on a logarithmic scale is characteristic of a mixed-potential-type sensor, as has been reported elsewhere.6–10 It is noteworthy that, as the operating temperature is increased, the sensitivity to C3H6 decreases significantly (note: sign of Δemf). The more negative Δemf could be due to the increased rate of the C3H6 anodic reaction at elevated temperature. It is noted that even at 800 °C, the sensitivity to 400 ppm C3H6 is about −98 mV, which is highly desirable.
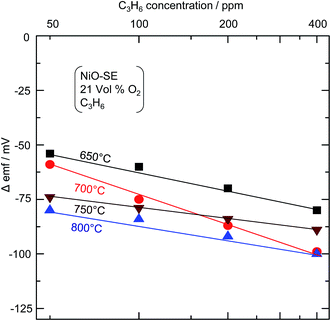 |
| | Fig. 6 Dependence of Δemf on the concentration of C3H6 at various operating temperatures for the YSZ-based sensor attached with the sol–gel derived NiO-SE. | |
The long-term stability of the sensor is one of the vital parameters for a practical sensor. Thus, to examine its stability and sensitivity to 400 ppm C3H6, the sensor attached with the sol–gel derived NiO-SE was monitored for about two months and the obtained data are given in Fig. 7. On the first day of sensor operation, the sensor exhibited a sensitivity of about −98 mV and at the end of two months, the sensor retained as high as 90% (−88 mV) of its original (−98 mV) sensitivity. The response/recovery rates were also almost invariant as depicted in the inset of Fig. 7. Thus, the present sensor using the sol–gel derived NiO-SE exhibits excellent stability even for about two months and could be a potential candidate for practical applications in automobile exhausts.
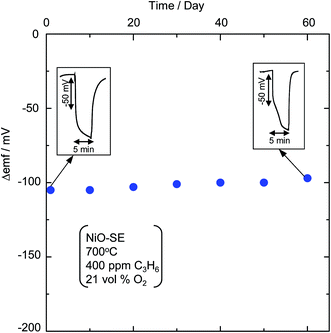 |
| | Fig. 7 Time course of Δemf to 400 ppm C3H6 at 700 °C for the YSZ-based sensor attached with the sol–gel derived NiO-SE (inset: response transients of the sensor on the first and sixtieth day of operation). | |
3.3 Sensing mechanism and estimation of mixed potential
Though the YSZ tube was used, the sensor configuration of the present work can be best described as a planar sensor and its electrochemical cell configuration is shown below:
| 21 vol% O2, NiO |YSZ| Pt, 21 vol% O2 (in base gas) |
| C3H6 + 21 vol% O2, NiO |YSZ| Pt, 21 vol% O2 + C3H6 (in sample gas) |
Based on the aforementioned results and the available literature reports, the sensing mechanism operating in the present sensor seems to be a mixed potential mechanism. In the base gas, the following electrochemical reaction will be equilibrating at both the electrodes.
On the other hand, in the sample gas, the following two competitive electrochemical reactions would be occurring simultaneously at the interface of NiO/YSZ.
| | |
1/2O2 + 2e− → O2− (cathodic)
| (2) |
| | |
C3H6 + 9O2− → 3CO2 + 3H2O + 18e− (anodic)
| (3) |
As the rate of these two electrochemical reactions are different at dissimilar electrodes (NiO-SE and Pt-RE), mixed potential appears when the rate of the anodic reaction is equal to that of the cathodic reaction, which is recorded in the response transients. The appearance of the mixed potential can be estimated from the polarization curves. In fact, the mixed potential can be defined as the potential where the cathodic and anodic polarization-curves intersect in an absolute current scale. Thus, the polarization (current–voltage, I–V) curves for the sensor attached with the sol–gel derived NiO-SE were measured in the base gas (5 vol% O2 + N2 balance or 21 vol% O2 + N2 balance) and in the sample gas (400 ppm C3H6 + base gas) at 700 °C. Fig. 8 shows the obtained I–V curves. It can be seen that in the 5 vol% O2 base gas, the estimated mixed potential (−43 mV) is close to that of the observed emf (−40 mV). Similarly, in the 21 vol% O2 base gas, the estimated mixed potential is −94 mV which is close to the observed emf (−98 mV). Such close coincidence of the observed and the estimated emf values confirms that the mechanism of the present sensor is based on mixed potential.
 |
| | Fig. 8 Anodic polarization curves of C3H6 and modified cathodic polarization-curves of 5 and 21 vol% O2 at 700 °C for the sensor attached with the 1300 °C-sintered NiO-SE. | |
The change in sensitivity of the sensor with varying base gas was examined with the help of I–V curves. In fact, from the I–V curves, it can be inferred that the higher the polarization current, the higher the reaction rate and vice versa. Thus, as is evident, the polarization current of 400 ppm C3H6 obtained in 21 vol% O2 is higher than that obtained in 5 vol% O2. This means that the rate of anodic reaction of C3H6 is higher in 21 vol% O2 than that in 5 vol% O2. Thus, accordingly the anodic polarization curve of C3H6 shifts upward when the base gas is changed from 5 vol% O2 to 21 vol% O2, leading to a drastic shift in the mixed potential. The high C3H6 sensitivity and selectivity of the sensor using the sol–gel derived NiO-SE is believed to be due to the improved kinetics of C3H6 anodic reaction because of nanofeatures associated with the sol–gel derived NiO-SE. It is assumed that on the NiO layer, the other gases NO, CO, C3H8 and NO2 are decomposed to the maximum extent before reaching the NiO/YSZ interface, leading to high C3H6 selectivity. The commercial NiO (with a different size, shape and size distribution, and hence different sensing properties) is not selective to propene.24,25 Interestingly, the present sensor attached with sol–gel derived NiO nanospheres exhibited excellent selectivity, sensitivity and stability to propene. There are other materials showing a response to propene, but these are composites, not a single material, consisting of an oxide and expensive gold,22 and besides, NiO is a thermally stable material which is non-toxic and has a high electrocatalytic activity toward propene. Therefore, the present sensor is highly significant.
4. Conclusions
An YSZ-based sensor was fabricated using the sol–gel derived NiO nanospheres. The sensor attached with the sol–gel derived NiO-SE showed high sensitivity and selectivity to C3H6 at 700 °C in 21 vol% O2. The Δemf of the sensor varied linearly with C3H6 concentrations on the logarithmic scale. The sensor exhibited an excellent response to even very low concentrations of C3H6 (10 ppm). The sensor retained good stability even for about two months. Thus, the sensor attached with the sol–gel derived NiO-SE can be a reliable propene sensor, although its sensing characteristics under wet conditions and varying flow rates are still to be examined.
Acknowledgements
Authors thank Department of Science and Technology (DST), Govt. of India, New Delhi for financial support under Fast-Track Scheme (DST/FT/CS-64/2010). CSIR-CECRI (Karaikudi and Chennai) and the Central Instrumentation Facility – Pondicherry University are also acknowledged for characterization studies.
References
- T. Watson, Nature, 2014, 513, S14–S15 CrossRef CAS PubMed.
- M. Kampa and E. Castanas, Environ. Pollut., 2008, 15, 362–367 CrossRef PubMed.
- B. M. Graver, H. C. Frey and H. W. Choi, Environ. Sci. Technol., 2011, 45, 9044–9051 CrossRef CAS PubMed.
- S. Zhuiykov and N. Miura, Sens. Actuators, B, 2007, 121, 639–651 CrossRef CAS PubMed.
- P. K. Sekhar, E. L. Brosha, R. Mukundan, W. Li, M. A. Nelson, P. Palanisamy and F. H. Garzon, Sens. Actuators, B, 2010, 144, 112–119 CrossRef CAS PubMed.
- N. Miura, T. Sato, S. A. Anggraini, H. Ikeda and S. Zhuiykov, Ionics, 2014, 20, 901–925 CrossRef CAS PubMed.
- Y. Liu, J. Parisi, X. Sun and Y. Lei, J. Mater. Chem. A, 2014, 2, 9919–9943 CAS.
- J. W. Fergus, Sens. Actuators, B, 2007, 122, 683–693 CrossRef CAS PubMed.
- G. Lu, Q. Diao, C. Yin, S. Yang, Y. Guan, X. Cheng and X. Liang, Solid State Ionics, 2014, 262, 292–297 CrossRef CAS PubMed.
- X. Xu, X. Li, W. Wang, B. Wang, P. Sun, Y. Sun and G. Lu, RSC Adv., 2014, 4, 4831–4835 RSC.
- J. S. Narayanan, M. Bhuvana and V. Dharuman, Biosens. Bioelectron., 2014, 58, 326–332 CrossRef PubMed.
- Y. Fujio, T. Sato and N. Miura, Solid State Ionics, 2014, 262, 266–269 CrossRef CAS PubMed.
- J. W. Fergus, Sens. Actuators, B, 2007, 121, 652–663 CrossRef CAS PubMed.
- K. Mahendraprabhu, N. Miura and P. Elumalai, Ionics, 2013, 19, 1681–1686 CrossRef CAS.
- A. Dutta, N. Kaabbuathong, M. L. Grilli, E. D. Bartolomeo and E. Traversa, J. Electrochem. Soc., 2003, 150, H33–H37 CrossRef CAS PubMed.
- Q. Diao, C. Yin, Y. Guan, X. Liang, S. Wang, Y. Liu, Y. Hu, H. Chen and G. Lu, Sens. Actuators, B, 2013, 177, 397–403 CrossRef CAS PubMed.
- L. Zhou, Q. Yuan, X. Li, J. Xu, F. Xia and J. Xiao, Sens. Actuators, B, 2015, 206, 311–318 CrossRef CAS PubMed.
- J. Park, B. Y. Yoon, C. O. Park, W. J. Lee and C. B. Lee, Sens. Actuators, B, 2009, 135, 516–523 CrossRef CAS PubMed.
- X. Liang, S. Yang, J. Li, H. Zhang, Q. Diao, W. Zhao and G. Lu, Sens. Actuators, B, 2011, 158, 1–8 CrossRef CAS PubMed.
- D. Westphal, S. Jakobs and U. Guth, Ionics, 2001, 7, 182–186 CrossRef CAS.
- J. Wang, P. Elumalai, D. Terada, M. Hasei and N. Miura, Solid State Ionics, 2006, 177, 2305–2311 CrossRef CAS PubMed.
- P. Elumalai, V. V. Plashnitsa, Y. Fujio and N. Miura, Sens. Actuators, B, 2010, 144, 215–219 CrossRef CAS PubMed.
- K. Mahendraprabhu and P. Elumalai, J. Sol-Gel Sci. Technol., 2015, 73, 428–433 CrossRef CAS.
- P. Elumalai, J. Wang, S. Zhuiykov, D. Terada, M. Hasei and N. Miura, J. Electrochem. Soc., 2005, 152, H95–H101 CrossRef CAS PubMed.
- P. Elumalai and N. Miura, Solid State Ionics, 2005, 176, 2517–2522 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 molar ratio were used for the synthesis of NiO. The required quantity of nickel nitrate was dissolved in distilled water and kept at 80 °C on a magnetic stirrer. The required amount of citric acid was also dissolved in distilled water separately. To the warm nickel nitrate solution, citric acid solution was added in dropwise. After the complete addition of all citric acid, the contents were heated to 130 °C to form sol and continued heating to get a gel. The formed gel was allowed to dry at 130 °C overnight. The calcination of the gel at 500 °C for 2 h in air was carried out to get the final product.
8 molar ratio were used for the synthesis of NiO. The required quantity of nickel nitrate was dissolved in distilled water and kept at 80 °C on a magnetic stirrer. The required amount of citric acid was also dissolved in distilled water separately. To the warm nickel nitrate solution, citric acid solution was added in dropwise. After the complete addition of all citric acid, the contents were heated to 130 °C to form sol and continued heating to get a gel. The formed gel was allowed to dry at 130 °C overnight. The calcination of the gel at 500 °C for 2 h in air was carried out to get the final product.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ
θ






