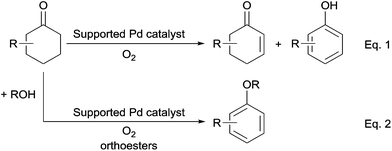
Aerobic oxidation of cyclohexanones to phenols and aryl ethers over supported Pd catalysts†‡
Zhenzhong
Zhang
a,
Taishin
Hashiguchi
a,
Tamao
Ishida
*b,
Akiyuki
Hamasaki
a,
Tetsuo
Honma
c,
Hironori
Ohashi
d,
Takushi
Yokoyama
a and
Makoto
Tokunaga
*a
aDepartment of Chemistry, Graduate School of Sciences, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan. E-mail: mtok@chem.kyushu-univ.jp; Fax: +81-92-642-7528; Tel: +81-92-642-7528
bResearch Center for Gold Chemistry, Graduate School of Urban Environmental Sciences, Tokyo Metropolitan University, 1-1 Minami-Osawa, Hachioji, Tokyo 192-0397, Japan. E-mail: tamao@tmu.ac.jp; Fax: +81-42-677-2360; Tel: +81-42-677-2360
cJapan Synchrotron Radiation Research Institute (JASRI/SPring-8), 1-1-1 Kouto, Sayo, Hyogo 679-5198, Japan
dFaculty of Arts and Science, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
First published on 12th February 2015
Abstract
Transformation of cyclohexanones to phenols and aryl ethers over supported Pd catalysts using molecular oxygen as the sole oxidant is developed. Several metal oxide supported Pd catalysts were used to activate the C–H bond in cyclohexanones to produce cyclohexenones and phenols through oxidation. Although the selectivity of cyclohexenones was difficult to control, phenols were obtained in excellent yield with a broad substrate scope. A novel catalytic system, using ZrO2 supported Pd(OH)2, was proposed for the synthesis of aryl ethers, and the products were obtained in moderate to excellent yields. Orthoesters, such as trimethyl orthoformate (TMOF), triethyl orthoformate (TEOF), and triisopropyl orthoformate (TIPOF), enabled nucleophilic addition and elimination after activation of cyclohexanones over a Pd catalyst to produce the corresponding aryl ethers. TIPOF was also used as the dehydrating reagent to promote the reaction of cyclohexanones with alcohols for the preparation of versatile aryl ethers.
Introduction
Over the past decades, Pd-catalyzed aerobic oxidation reactions have been widely developed.1 Dehydrogenation of ketones to synthesize enones is one of the important reactions, since enones are regarded as useful building blocks in organic synthesis.2 Li and Wang reported a direct aerobic dehydrogenation of aldehydes to the corresponding enones catalyzed by Pd(OAc)2/amine, but only moderate product yields were obtained.3 On the other hand, synthesis of phenols from cyclohexanones has also been performed via enone formation by Pd-catalyzed aerobic oxidation of cyclohexanones.4,5 One of our authors developed a Pd(TFA)2/bipy catalytic system for synthesizing cyclohexenones from cyclohexanones with a yield of up to 84%.4a In that report, phenol was partly formed as a by-product. Stahl and co-workers reported the oxidative dehydrogenation of cyclohexanones over Pd(DMSO)2(TFA)2 and Pd(TFA)2/2-dimethylaminopyridine (2-Me2Npy) catalytic systems to produce cyclohexenones4c and phenols,5b respectively. In general, cyclic enones and phenols were formed by PdII-catalyzed α-hydrogen activation of cyclohexanones followed by β-hydride elimination or further dehydrogenation, and oxygen was used to re-oxidize the Pd0 species (Scheme 1). Stahl concluded that DMSO inhibited the formation of Pd black, and soluble PdII species was highly active for the oxidation of cyclohexanones to cyclohexenones.4d,6 In contrast, soluble Pd nanoparticles (NPs) were formed in the Pd(TFA)2/2-Me2Npy system, and they were more active for the oxidation of cyclohexenones to phenols than PdII species.5c Although Pd NP-mediated oxidation of cyclohexanones to phenols has been proposed, active catalysts are limited to pseudo-homogeneous Pd NPs. Heterogeneous catalysts, such as Pd/C, have been scarcely reported for this reaction in spite of their advantages, such as easy catalyst separation.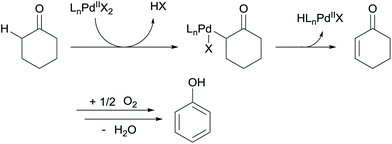 | ||
| Scheme 1 Proposed mechanism for the Pd-catalyzed dehydrogenation of cyclohexanones by Stahl and co-workers. | ||
Aryl ethers are also important compounds for organic synthesis7 and are usually obtained via Williamson ether synthesis8 or Ullmann coupling reactions.9 However, both of these methods generally use halogen-containing substrates and need a stoichiometric amount of base to promote the reaction. Li and co-workers reported a method to prepare aryl ethers from cyclohexenones catalyzed by homogeneous Cu catalysts in the presence of N-hydroxyphthalimide (NHPI) as a co-catalyst.10 Since cyclohexenones can be obtained by Pd-catalyzed aerobic oxidation of cyclohexanones, direct transformation of aryl ethers from cyclohexanones is more simple and straightforward. Recently, Lemaire and co-workers reported the synthesis of aryl ethers from cyclohexanones over Pd/C.11 The proposed mechanism involved activation of the carbonyl group in cyclohexanone by Pd, followed by reaction with alcohols to form the hemiacetal intermediate. Elimination of hemiacetal to enol ethers and subsequent dehydrogenation yielded aryl ethers. In the previous reports,10,11 alcohols were limited to those having high boiling points (>100 °C), and simple alcohols, such as methanol and ethanol, were not described.
In this work, using metal oxide supported Pd catalysts, we studied the aerobic oxidation of cyclohexanones to synthesize cyclic enones and phenols (Scheme 2, eqn (1)). Moreover, preparation of aryl ethers from cyclohexanones in simple alcohols is developed over supported Pd catalysts under an oxygen atmosphere in the presence of orthoesters, such as trimethyl orthoformate (TMOF) and triisopropyl orthoformate (TIPOF) (eqn (2)).
Results and discussion
Preparation and characterization of catalysts
Metal oxide, such as TiO2, Al2O3, CeO2, and ZrO2, supported Pd(OH)2 catalysts were prepared by the deposition–precipitation (DP) method using PdCl2 as the precursor according to the literature with modifications.12 Metal oxide supported PdO and Pd catalysts were prepared from supported Pd(OH)2 catalysts by calcination in air and reduction with pure H2, respectively (also see ESI‡). The high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image of 10 wt% Pd(OH)2/ZrO2 revealed that Pd NPs were well dispersed on the surface of ZrO2, and the mean diameter was calculated to be 1.8 ± 0.9 nm (Fig. S1‡). On the other hand, the HAADF-STEM image of 10 wt% Pd/ZrO2 showed that Pd NPs were aggregated to large particles during H2 reduction at 200 °C (Fig. S2‡).Characterization of supported Pd catalysts was performed by X-ray absorption fine structure (XAFS) analysis at BL14B2 of SPring-8 (Hyogo, Japan).13 To identify the chemical state of Pd in supported Pd(OH)2 and Pd catalysts, Pd K-edge X-ray absorption near edge structure (XANES) spectra were recorded (Fig. S3‡). As we have previously reported, the chemical state of Pd in Pd(OH)2/ZrO2 and Pd/ZrO2 was confirmed to be PdII and Pd0, respectively. In the radial structure functions (RSF) (Fig. S4‡), two peaks were observed at 1.5 and 2.9 Å (phase-uncorrected) in PdO, and these peaks correspond to the Pd–O interaction of the first coordination shell and both Pd–Pd and Pd–O interactions of the second one, respectively. Due to the fact that both Pd–Pd and Pd–O bonds at 2.9 Å were small and correspond to Pd(OH)2, Pd(OH)2 might be present in Pd(OH)2/ZrO2 as major species, although the formation of PdO could not be fully excluded by XAFS.14
Synthesis of cyclohexenones and phenols
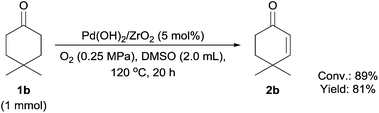
The catalytic performance of supported Pd(OH)2 catalysts was evaluated by the transformation of cyclohexanones into cyclohexenones and phenols (Table 1). Initially, several metal oxide supported Pd(OH)2 catalysts were screened in DMSO (Table 1, entries 1–4). Among these catalysts, the basic metal oxide (CeO2) and the relatively acidic metal oxide (TiO2) supported Pd(OH)2 showed a lower catalytic activity than amphoteric metal oxides (Al2O3 and ZrO2) as the support. However, the selectivity to enones and phenols over Pd(OH)2/Al2O3 was lower than Pd(OH)2/ZrO2. Thus, ZrO2 was selected as a suitable support. Other solvents such as 1,4-dioxane, EtOAc, toluene, and PhCl were also investigated for the reaction with supported Pd(OH)2 catalysts, whereas the selectivity to cyclohexenones or phenols was found to be unsatisfactory (Table S1, entries 1–6‡). When the catalytic amount of Pd decreased from 5 mol% to 2 mol%, the selectivity to cyclohexenones was increased to 26% yield (Table 1, entry 5). When Pd(OH)2/ZrO2 was reduced to Pd/ZrO2 using H2, the selectivity for phenol was improved (Table 1, entry 6). Decreasing the reaction temperature improved the cyclohexenone yield to 34% (Table 1, entry 7). Although we tried to further improve the selectivity of cyclohexenones, subsequent oxidation of cyclohexanones to phenols could not be avoided. The only exception is 4,4-dimethylcyclohexenone (2b) which was obtained in 81% yield, due to the absence of the hydrogen atom at the 4-position (Scheme 3). In this case, 4,4-dimethylcyclohexa-2,5-dien-1-one was not formed, suggesting that the second oxidation to the dienone did not proceed as the substrate cannot be aromatized.| Entry | Catalyst | Temp. (°C) | Time (h) | Conv.b (%) | Yield of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
Yield of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: cyclohexanone (1 mmol), catalyst (Pd 5 mol%), DMSO (2.0 mL), O2 (0.25 MPa). b Calculated on the basis of GC analysis using tridecane as an internal standard. c Catalyst (Pd 8 mol%). d Catalyst (Pd 2 mol%). e O2 (0.5 MPa). | ||||||
| 1 | Pd(OH)2/Al2O3 | 120 | 8 | 99 | 8 | 18 |
| 2 | Pd(OH)2/CeO2 | 120 | 15 | 44 | 7 | 22 |
| 3c | Pd(OH)2/TiO2 | 120 | 20 | 70 | 7 | 25 |
| 4 | Pd(OH)2/ZrO2 | 120 | 15 | 90 | 15 | 38 |
| 5d | Pd(OH)2/ZrO2 | 120 | 15 | 73 | 26 | 40 |
| 6 | Pd/ZrO2 | 120 | 15 | 86 | 12 | 54 |
| 7 | Pd/ZrO2 | 100 | 24 | 81 | 34 | 36 |
| 8e | Pd/ZrO2 | 100 | 24 | 100 | <1 | 99 |
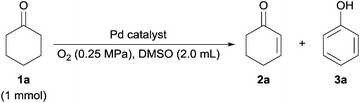
Then we focused on the synthesis of phenols. Selectivity of phenols was significantly affected by the pressure of O2. Neither cyclohexenones nor phenols were obtained under an atmospheric pressure of O2 (Table S1, entry 7‡), an increase in O2 pressure to 0.5 MPa gave phenols in excellent yield of 99% (Table 1, entry 8).
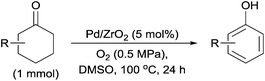
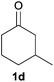
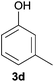
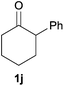
Next, we examined the scope of substrates for phenol synthesis (Table 2). For methyl substituted cyclohexanones, the corresponding phenols were obtained in good to excellent yields (Table 2, entries 1–3); the reactivity order was 4- > 3- > 2-substituted cyclohexanones, and this might be ascribed to the steric effect on 3- and 2-substituted cyclohexenones for further oxidation to cyclohexa-2,5-dien-1-ones, which were considered to be formed faster than cyclohexa-2,4-dien-1-ones as the intermediate before phenol formation. Other 4-substituted cyclohexanones were also tested (Table 2, entries 4–6). 4-Ethylcyclohexanone (1f) and 4-t-butylcyclohexanone (1g) exhibited excellent reactivity to form the corresponding phenols. 4-Phenylcyclohexanone (1h) was transformed to 4-phenylphenol (3h) in 82% yield by using a highly loaded Pd catalyst. Low reactivity was observed in 3,5-dimethylcyclohexanone (1i) (Table 2, entry 7). 2-Phenyl substituted cyclohexanone (1j) was examined (Table 2, entry 8), but this compound showed poor reactivity.
| Entry | Substrate | Product | Conv.b (%) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: substrate (1 mmol), 10 wt% Pd/ZrO2 (Pd 5 mol%), DMSO (2.0 mL), O2 (0.5 MPa), 100 °C, 24 h. b Calculated on the basis of GC analysis using tridecane as an internal standard. c Catalyst (Pd 8 mol%). | ||||
| 1 |
|
|
87 | 63 |
| 2 |
|
|
89 | 78 |
| 3 |
|
|
100 | 99 |
| 4 |
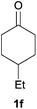
|
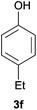
|
100 | 99 |
| 5 |
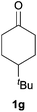
|
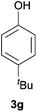
|
100 | 99 |
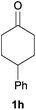
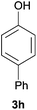
| 6c |
|
|
98 | 82 |
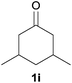
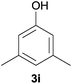
| 7 |
|
|
19 | 13 |
| 8 |
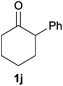
|
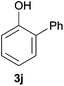
|
66 | 46 |
In order to elucidate the chemical state of Pd for the synthesis of phenols, the Pd/ZrO2 catalyst was tested by Pd K-edge XANES before and after the reaction, and the chemical state of Pd both on the fresh and recycled catalysts was confirmed to be Pd0 (Fig. S5a‡). In RSF of Pd/ZrO2 after use, the magnitude of the peak at 2.5 Å, which corresponds to the Pd–Pd interaction as is seen in Pd foil, decreased as compared to the fresh catalyst (Fig. S5b‡). In the k3-weighted EXAFS oscillations, the amplitude of the catalyst after reaction was also lower than the fresh catalyst (Fig. S5c‡). This result indicated that the Pd NP size decreased during the reaction. In addition, in the X-ray diffraction (XRD) patterns of fresh Pd/ZrO2, the diffraction peaks at 40, 46 and 68° were assigned to (111), (200), and (220) crystalline planes of the face-centered cubic (fcc) lattice of Pd (PDF-2 Database, no. 01-087-0645) (Fig. S6a‡). In contrast, these diffraction peaks were not present in the XRD patterns of the used Pd/ZrO2 (Fig. S6b‡). From these results, we suggest that Pd was dissolved into the solution during the reaction and was re-deposited on ZrO2 as small Pd NPs. The microwave plasma-atomic emission spectrometry (MP-AES) analysis result demonstrated that only 0.6 wt% Pd was lost from the fresh catalyst (Table S2‡). Although the reaction would proceed via quasi-homogeneous catalysis, most of Pd could be recovered after the reaction as supported catalysts. In addition, the recyclability of Pd/ZrO2 for synthesis of phenols was studied. Although the yields of phenols were slightly decreased at the fourth run, the catalyst showed good recyclability (Fig. 1).
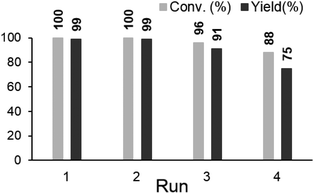 | ||
| Fig. 1 Recyclability of Pd/ZrO2 in phenol synthesis. Reaction conditions: cyclohexanone (1 mmol), 10 wt% Pd/ZrO2 (Pd 5 mol%), DMSO (2.0 mL), O2 (0.5 MPa), 100 °C, 24 h. | ||
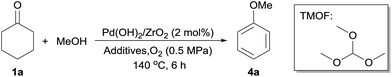
Synthesis of aryl ethers
The reaction of cyclohexanone and methanol into anisole was explored under oxidative conditions. In the absence of additives, the reaction proceeded over 2 mol% Pd(OH)2/ZrO2 at 140 °C to afford anisole in 37% yield (Table 3, entry 1). Several additives such as Na2SO4 and molecular sieves of 4 Å were added as dehydrating reagents, but the conversion decreased markedly (Table 3, entries 2 and 3). However, the reaction proceeded very well, yielding anisole in 66% yield, when using trimethyl orthoformate (TMOF) as the additive (Table 3, entry 4). The reaction also proceeded in the absence of methanol to form anisole (Table 3, entry 5). We suggest that TMOF, which is commonly used for introduction of a protecting group in aldehydes, worked as both dehydrating and nucleophilic reagents during the reaction. After the optimization of reaction conditions, 96% yield of anisole was achieved under the following reaction conditions: 0.5 MPa of O2, at 140 °C for 6 h (Table 3, entry 6). ZrO2 supported PdO and Pd catalysts also promoted this reaction to give anisole in good yields (Table 3, entries 7 and 8).| Entry | Catalyst | MeOH (eq.) | Additive (eq.) | Conv.b (%) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: cyclohexanone (1 mmol), 10 wt% Pd(OH)2/ZrO2 (Pd 2 mol%), MeOH, additives, O2 (0.5 MPa). b Calculated on the basis of GC analysis using tridecane as an internal standard. c Molecular sieves of 4 Å (100 mg) were added. d The catalyst was calcined at 300 °C for 4 h from Pd(OH)2/ZrO2. e The catalyst was reduced using H2 (20 mL min−1) at 200 °C for 2 h from Pd(OH)2/ZrO2. | |||||
| 1 | Pd(OH)2/ZrO2 | 40 | — | 100 | 37 |
| 2 | Pd(OH)2/ZrO2 | 40 | Na2SO4 | 19 | 0 |
| 3 | Pd(OH)2/ZrO2 | 40 | MS 4 Åc | 23 | 2 |
| 4 | Pd(OH)2/ZrO2 | 40 | TMOF | 100 | 66 |
| 5 | Pd(OH)2/ZrO2 | — | TMOF | 91 | 70 |
| 6 | Pd(OH)2/ZrO2 | — | TMOF | 100 | 96 |
| 7 | PdO/ZrO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
— | TMOF | 100 | 92 |
| 8 | Pd/ZrO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
— | TMOF | 100 | 77 |
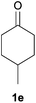
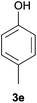
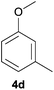
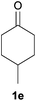
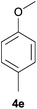
With the optimized reaction conditions in hand, several substituted cyclohexanones were investigated. Methyl (1d and 1e) and phenyl (1j) substituted cyclohexanones were transformed into their corresponding aryl ethers in moderate yields (Table 4, entries 1–3). 1-Tetralone (1k), which contains a benzocyclohexanone structure, exhibited better reactivity than other substrates and gave 1-methoxynaphthalene (4k) in 78% yield (Table 4, entry 4). Moreover, other orthoester reagents, including triethyl orthoformate (TEOF) and triisopropyl orthoformate (TIPOF), could be used instead of TMOF. Ethoxybenzene and isopropoxybenzene were obtained as products in 49 and 11% yield, respectively (Table 4, entries 5 and 6). Relatively low product yields compared to high conversions were due to the formation of phenols as by-products.
| Entry | Substrate | R′ | Product | Conv.b (%) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: substrate (1 mmol), 10 wt% Pd(OH)2/ZrO2 (Pd 2 mol%), orthoester reagent (1.5 mL), O2 (0.5 MPa), 140 °C, 6 h. b Calculated on the basis of GC analysis using tridecane as an internal standard. | |||||
| 1 |
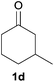
|
Me |
|
91 | 66 |
| 2 |
|
Me |
|
85 | 45 |
| 3 |
|
Me |
|
100 | 22 |
| 4 |
|
Me |
|
95 | 78 |
| 5 |
|
Et |
|
92 | 49 |
| 6 |
|
iPr |
|
79 | 11 |
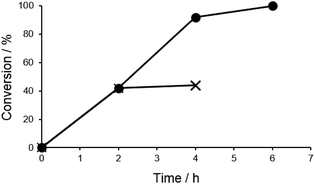
Next, we tested the recyclability of the catalyst in anisole synthesis (Fig. 2). Although the catalytic activity was gradually decreased with consecutive runs, 80% yield was maintained for the fourth run. The reasons for the decrease in the catalytic activity were probably due to the leaching of Pd and/or aggregation of Pd species. Consequently, the reaction solution was analysed by MP-AES, and the concentration of residual Pd in the solution after filtration was determined to be 1.33 ppm (in 1.5 mL TMOF). Moreover, after the removal of the catalyst by filtration at 42% conversion, the reaction of the filtrate stopped (Fig. 3). These results proved that the loss of Pd could not be the cause of the decrease in catalytic activity.
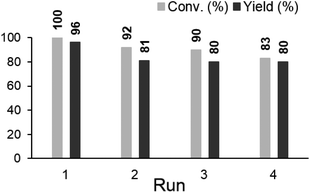 | ||
| Fig. 2 Recyclability of Pd(OH)2/ZrO2 in anisole synthesis. Reaction conditions: cyclohexanone (1 mmol), 10 wt% Pd(OH)2/ZrO2 (Pd 2 mol%), TMOF (1.5 mL), O2 (0.5 MPa), 140 °C, 6 h. | ||
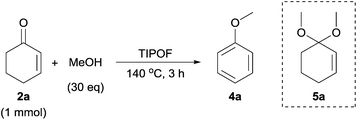
Since TIPOF possesses lower reactivity to produce isopropoxybenzene than TMOF and TEOF, we considered that TIPOF could be used as a dehydrating reagent in the presence of alcohols to synthesize aryl ethers without the formation of isopropoxybenzene (Table 5). The ratio of alcohols and TIPOF was optimized to be 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In entry 1, anisole was obtained in 74% yield, which was lower than the yield obtained using only TMOF. For other primary alcohols, we evaluated ethanol, propanol, and butanol (Table 5, entries 2–4). The corresponding products, ethoxybenzene, propoxybenzene, and butoxybenzene, were obtained in good yields, and only small amounts of isopropoxybenzene and phenol (4–11% yield) were detected as by-products. This reaction also proceeded with 2-propanol to produce isopropoxybenzene (Table 5, entry 5), although the reaction only gave 4o in 23% yield due to the steric hindrance in the formation of the acetal intermediate. Furthermore, higher alcohols, such as n-heptanol and n-octanol, were also employed, and the corresponding aryl ethers were afforded in good yields (Table 5, entries 6 and 7).
1. In entry 1, anisole was obtained in 74% yield, which was lower than the yield obtained using only TMOF. For other primary alcohols, we evaluated ethanol, propanol, and butanol (Table 5, entries 2–4). The corresponding products, ethoxybenzene, propoxybenzene, and butoxybenzene, were obtained in good yields, and only small amounts of isopropoxybenzene and phenol (4–11% yield) were detected as by-products. This reaction also proceeded with 2-propanol to produce isopropoxybenzene (Table 5, entry 5), although the reaction only gave 4o in 23% yield due to the steric hindrance in the formation of the acetal intermediate. Furthermore, higher alcohols, such as n-heptanol and n-octanol, were also employed, and the corresponding aryl ethers were afforded in good yields (Table 5, entries 6 and 7).
| Entry | ROH | Product | Conv.b (%) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: cyclohexanone (1 mmol), 10 wt% Pd(OH)2/ZrO2 (Pd 2 mol%), ROH (30 eq.), TIOPF (5 eq.), O2 (0.5 MPa), 140 °C, 6 h. b Calculated on the basis of GC analysis using tridecane as an internal standard. c 8 h. d Alcohol (15 eq.). | ||||
| 1 | MeOH |
|
100 | 74 |
| 2c | EtOH |
|
100 | 83 |
| 3 | n PrOH |
|
100 | 76 |
| 4 | n BuOH |
|
91 | 73 |
| 5 | iPrOH |
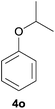
|
51 | 23 |
| 6d | n Heptanol |
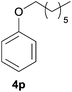
|
95 | 78 |
| 7d | n Octanol |
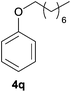
|
98 | 79 |
Possible reaction pathway
In order to investigate the reaction pathway for the synthesis of aryl ethers, several experiments were performed by using cyclohexenone (2a) as the starting material in the absence of Pd (Table 6). When both TIPOF and ZrO2 were used in this process, the reaction proceeded efficiently, affording the anisole (4a) with a good yield in 2 h (Table 6, entry 1). As orthoesters can facilitate the formation of acetals from aldehydes and ketones, a comparative test was carried out (Table 6, entry 2) using the reaction conditions as entry 1 in the absence of ZrO2. The anisole yield was slightly decreased, indicating that ZrO2 is required for this reaction. Since ZrO2 has both Lewis acid and base sites, we assumed that ZrO2 acted as a Lewis acid to promote the formation of the acetal intermediate and/or as a Lewis base for the production of anisole by elimination. In addition, cyclohexenones did not react with methanol in the absence of ZrO2 and TIPOF (Table 6, entry 3). Also, molecular oxygen appeared to be essential to produce aryl ethers, because only dimethylacetal (5a) was detected under a N2 atmosphere (Table 6, entry 4).The proposed reaction pathway for the synthesis of cyclohexenones, phenols, and aryl ethers is presented in Scheme 4. The mechanism for the synthesis of cyclohexenone (2a) from cyclohexanone (1a) has been studied in previous reports.4c,5b Similarly, a cyclohexanone is activated by Pd(OH)2 on the metal oxide surface to produce Pd-enolate intermediate (A), and a cyclic enone is formed, followed by β-hydride elimination. Pd0 is regenerated to PdII by oxidation with molecular oxygen. In the second step, the cyclic enone reacts with an alcohol to form acetal (5), and an aryl ether is obtained by further elimination and oxidation. These steps are promoted by ZrO2 as the Lewis acid–base catalyst and by orthoesters as efficient dehydrating reagents. Alternatively, cyclic enones could be oxidized to dienones when the six-membered ring could be aromatized, and dienones were tautomerized to phenols.
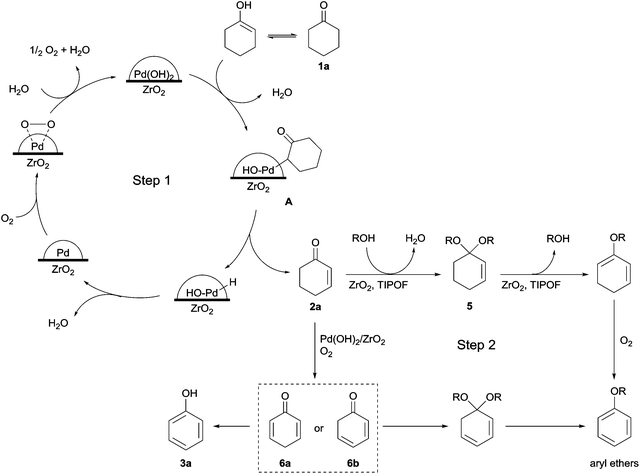 | ||
| Scheme 4 Suggested reaction pathway for synthesis of cyclohexenones, phenols, and aryl ethers from cyclohexanones. | ||
Conclusions
In conclusion, we have developed a method for the direct aerobic oxidation of cyclohexanones to cyclic enones, phenols, and aryl ethers over supported Pd0 or PdII catalysts using molecular oxygen as the sole oxidant. Although the reaction exhibited low selectivity for the transformation of cyclohexanones to cyclohexenones, 4,4-dimethylcyclohexenone could be obtained in 81% yield from the corresponding cyclohexanone. For the synthesis of phenols, the ZrO2 supported Pd0 catalyst showed better catalytic activity than other supported PdII catalysts, and the corresponding phenols were obtained in moderate to excellent yields. Notably, two novel catalytic protocols were proposed for the preparation of aryl ethers from cyclohexanones with ZrO2 supported Pd(OH)2 as the catalyst. Cyclohexanones could be converted to aryl ethers in the presence of the corresponding orthoesters, which act as nucleophilic and dehydrating reagents. In the direct reaction with alcohols, the orthoesters (TIPOF) only acted as efficient dehydrating reagents. In contrast to previous work, both these processes provide synthetically useful and convenient methods to prepare simple aryl ethers, such as anisole, ethoxybenzene, and propoxybenzene.Acknowledgements
This work was supported by JST (Japan Science and Technology Agency), ALCA program. The synchrotron radiation experiments were performed at the BL14B2 of SPring-8 with the approval of JASRI (2012A1454, 2012B1075, 2014B1832). HAADF-STEM was performed at the Ultramicroscopy Research Center, Kyushu University.Notes and references
- (a) S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400 CrossRef CAS PubMed; (b) S. S. Stahl, Science, 2005, 309, 1824 CrossRef CAS PubMed; (c) T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329 CrossRef CAS PubMed; (d) Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381 RSC; (e) K. M. Gligorich and M. S. Sigman, Chem. Commun., 2009, 3854 RSC; (f) A. N. Campbell and S. S. Stahl, Acc. Chem. Rev., 2002, 102, 4303 CrossRef PubMed; (g) B. A. Steinhoff, S. R. Fix and S. S. Stahl, J. Am. Chem. Soc., 2002, 124, 766 CrossRef CAS PubMed; (h) S. Gowrisankar, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 5139 CrossRef CAS PubMed; (i) T. A. Dwight, N. R. Rue, D. Charyk, R. Josselyn and B. DeBoef, Org. Lett., 2007, 9, 3137 CrossRef CAS PubMed; (j) D. R. Stuart and K. Fagnou, Science, 2007, 316, 1172 CrossRef CAS PubMed; (k) T. Mitsudome, T. Umetani, N. Nosaka, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, Angew. Chem., Int. Ed., 2006, 45, 481 CrossRef CAS PubMed; (l) X. Wang, N. S. Venkataramanan, H. Kawanami and Y. Ikushima, Green Chem., 2007, 9, 1352 RSC; (m) Z. K. Wickens, B. Morandi and R. H. Grubbs, Angew. Chem., Int. Ed., 2013, 52, 11257 CrossRef CAS PubMed.
- (a) S. B. Herzon, L. Lu, C. M. Woo and S. L. Gholap, J. Am. Chem. Soc., 2011, 133, 7260 CrossRef CAS PubMed; (b) H. Yokoe, C. Mitsuhashi, Y. Matsuoka, T. Yoshimura, M. Yoshida and K. Shishido, J. Am. Chem. Soc., 2011, 133, 8854 CrossRef CAS PubMed.
- J. Zhu, J. Liu, R. Ma, H. Xie, J. Li, H. Jiang and W. Wang, Adv. Synth. Catal., 2009, 351, 1229 CrossRef CAS.
- (a) M. Tokunaga, S. Harada, T. Iwasawa, T. Obora and Y. Tsuji, Tetrahedron Lett., 2007, 48, 6860 CrossRef CAS PubMed; (b) X. Zhang, D. Y. Wang, T. J. Emge and A. S. Goldman, Inorg. Chim. Acta, 2011, 369, 253 CrossRef CAS PubMed; (c) T. Diao and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 14566 CrossRef CAS PubMed; (d) T. Diao, D. Pun and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 8205 CrossRef CAS PubMed.
- (a) Y. Shov and A. H. I. Arisha, J. Org. Chem., 1998, 63, 5640 CrossRef; (b) Y. Izawa, D. Pun and S. S. Stahl, Science, 2011, 333, 209 CrossRef CAS PubMed; (c) D. Pun, T. Diao and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 8213 CrossRef CAS PubMed.
- (a) B. A. Steinhoff, S. R. Fix and S. S. Stahl, J. Am. Chem. Soc., 2002, 124, 766 CrossRef CAS PubMed; (b) R. I. McDonald and S. S. Stahl, Angew. Chem., Int. Ed., 2010, 49, 5529 CrossRef CAS PubMed.
- G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed.
- K. P. C. Volhardt and N. E. Schore, Organic Chemistry: Structure and Function, W. H. Freeman &. Co. through Japan UNI Agency, Inc., Tokyo, 4th edn, 2004, p. 378 Search PubMed.
- R. A. Altman, A. Shafir, A. Choi, P. A. Lichtor and S. L. Buchwald, J. Org. Chem., 2008, 73, 284 CrossRef CAS PubMed.
- M. O. Simon, S. A. Girard and C. J. Li, Angew. Chem., Int. Ed., 2012, 51, 7537–7540 CrossRef CAS PubMed.
- (a) M. Sutter, R. Lafon, Y. Raoul, E. Métay and M. Lemaire, Eur. J. Org. Chem., 2013, 5902 CrossRef CAS; (b) M. Sutter, N. Sotto, Y. Raoul, E. Métay and M. Lemaire, Green Chem., 2013, 15, 347 RSC.
- S. S. Soomro, F. L. Ansari, K. Chatziapostolou and K. Köhler, J. Catal., 2010, 273, 138 CrossRef CAS PubMed.
- (a) T. Honma, H. Oji, S. Hirayama, Y. Taniguchi, H. Ofuchi and M. Takagaki, AIP Conf. Proc., 2010, 1234, 13 CrossRef CAS PubMed; (b) H. Oji, Y. Taniguchi, S. Hirayama, H. Ofuchi, M. Takagaki and T. Honma, J. Synchrotron Radiat., 2012, 19, 54 CrossRef PubMed.
- T. Ishida, R. Tsunoda, Z. Zhang, A. Hmasaki, T. Honma, H. Ohashi, T. Yokoyama and M. Tokunaga, Appl. Catal., B, 2014, 150–151, 523 CrossRef CAS PubMed.
Footnotes |
| † Celebrating the 80th Birthday of Professor Ei-ichi Negishi. |
| ‡ Electronic supplementary information (ESI) available: Catalyst preparation method, experimental procedures, reaction conditions for synthesis of phenols and cyclohexenones, characterization of catalysts by HAADF-STEM, XAFS, XRD, and MP-AES, characterization of isolated compounds, and spectra of 1H and 13C NMR. See DOI: 10.1039/c4qo00354c |
| This journal is © the Partner Organisations 2015 |