Brønsted acid-catalyzed synthesis of carbazoles from 2-substituted indoles†‡
Qingjiang
Li
a,
Xiao-Shui
Peng
ab and
Henry N. C.
Wong
*ab
aDepartment of Chemistry, Centre of Novel Functional Molecules and State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, China
bShenzhen Municipal Key Laboratory of Chemical Synthesis of Medicinal Organic Molecules and Shenzhen Center of Novel Functional Molecules, Shenzhen Research Institute, The Chinese University of Hong Kong, No. 10. Second Yuexing Road, Shenzhen 518507, China. E-mail: hncwong@cuhk.edu.hk; Fax: +852 26035315
First published on 6th October 2014
Abstract
A simple and efficient method for the synthesis of disubstituted carbazoles has been developed. In this approach, carbazoles are synthesized from o-haloanilines and terminal alkynes using a two-step strategy, namely, Sonogashira coupling and intramolecular cyclization to provide indoles, which are followed by a p-TSA·H2O-catalyzed carbazole formation.
The tricyclic carbazole nucleus is one of the most important heterocycles since it is not only found in many natural products and bioactive molecules1 but also broadly serves as a building block for potential electroluminescent materials2 due to its special thermal3 and electrical4 properties, as well as a host material for triplet emitters in organic light-emitting diodes.5 Accordingly, a variety of well-documented traditional and modern methods have been developed for the construction of carbazoles and their derivatives.6,7 These synthetic methods are mainly classified into two broad categories: one is the formation of the pyrrole core from biphenyls, and the other is the formation of a benzene ring from indoles. However, these literature methods have suffered from some drawbacks such as harsh reaction conditions, low yields, and poor atom economy. Therefore it is still of interest to uncover new synthetic methods for the realisation of these important molecules.
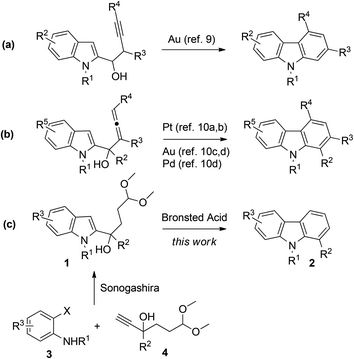
Synthetic pathways towards carbazoles from indoles have drawn a great deal of attention because indole substances abundantly exist.8 For example, Ma and coworkers reported a Au-catalyzed cyclization of 1-(indol-2-yl)-3-alkyn-1-ols for the synthesis of carbazoles (Scheme 1a).9 Cyclization reaction of 1-(indol-2-yl)-2,3-allenols involving Pt,10a,b Au,10c,d and Pd10d-catalysis was also achieved by Ma and Alcaide, respectively (Scheme 1b). Knölker also reported many applications of iron-mediated10k–q and palladium-catalysed10e–j approaches for the synthesis of carbazole derivatives. In 1995, Mahboobi reported carbazole synthesis from l-(N-benzylindol-2-y1)-3-(l,3-dioxolan-2-yl)-propan-1-ol in the presence of HCl, but only one example was recorded.11 In this paper, we report herein our preliminary results on the synthesis of disubstituted carbazoles using a two-step procedure from o-haloanilines 3 and terminal alkynes 4 (Scheme 1c).
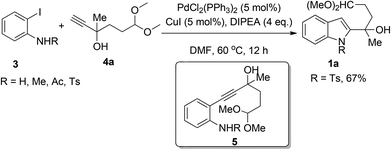
In order to introduce different substitutes on the indole ring, the starting materials, 1a–j, are prepared mainly through a Sonogashira12 coupling reaction and then with an intramolecular cyclization, starting from o-haloanilines 3 and terminal alkynes 4. We initiated our studies by examining different protecting groups of 3 with terminal alkyne 4a under classical Sonogashira conditions. When R was H, Me, or Ac, indole products were not obtained, but only the direct Sonogashira coupling product 5 was generated. However, a Ts protecting group was found to give the desired indole product 1a with an acceptable yield of 67% (Scheme 2).
After determining the optimal protecting group, we turned our attention to the optimization of the carbazole formation reaction conditions. 5,5-Dimethoxy-2-(1-tosyl-1H-indol-2-yl)pentan-2-ol 1a was employed as a model substrate. A variety of reaction conditions (catalyst, temperature) were examined and some of the representative results are shown in Table 1. It was found that p-TSA·H2O was more effective than the other acids, such as Amberlyst 15, albeit capable of promoting the reaction but with lower yields (entries 1 and 2). When the reaction was conducted at a higher temperature (40 °C), it rapidly gave the carbazole product in 95% yield within 1.5 h (entry 3). Decreasing the amount of catalyst to 20 mol% and prolonging the reaction time to 3 h led to the best result (entry 4).
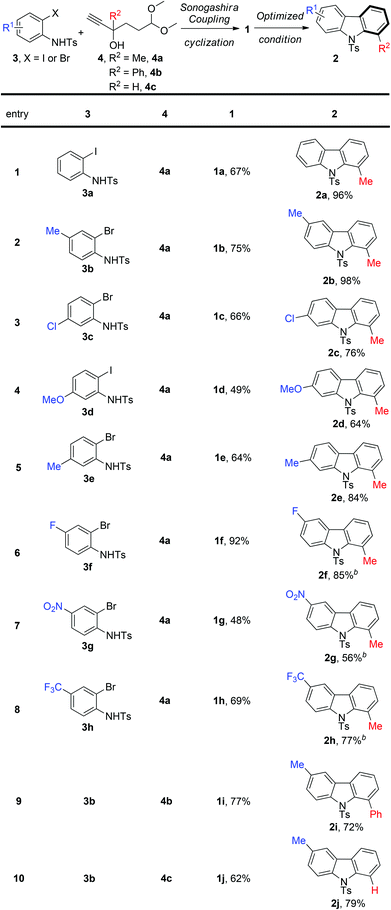
With the optimized conditions in hand, we next investigated the substrate scope of this reaction. As illustrated in Scheme 3, the transformation was found to be very general. Various substituted indoles can successfully give the corresponding carbazole derivatives (2a–2h). However, the electron-withdrawing groups (F, NO2, and CF3) on indoles slowed down the reaction, and needed a higher temperature (60 °C), but the products were still isolated in moderate to good yields (56%–85%) (2f–2h). Moreover, R2 could also be an aryl group (2i), or H (2j).
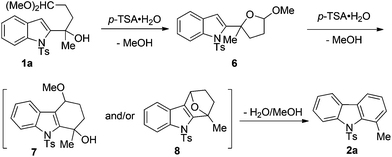
A plausible mechanism for the annulation of 5,5-dimethoxy-2-(1-tosyl-1H-indol-2-yl)pentan-2-ol 1a catalyzed by p-TSA·H2O is presented in Scheme 4. The intermediate acetal 6 is formed through the elimination of MeOH from indole 1a in the presence of p-TSA·H2O, which was also isolated as diastereoisomers in a nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Then the successive elimination of water/MeOH from intermediates 7 and/or 8 gave carbazole product 2a.13
1 ratio. Then the successive elimination of water/MeOH from intermediates 7 and/or 8 gave carbazole product 2a.13
Conclusions
In summary, we have developed a simple and efficient two-step approach (Sonogashira/intramolecular cyclization for indole synthesis and p-TSA·H2O-catalyzed carbazole synthesis) for the synthesis of diversified substituted carbazoles from o-haloanilines 3 and terminal alkynes 4 in good isolated yields under mild conditions. Because of the ready availability of the starting materials and the potential of the products, this method should be useful in organic synthesis and medicinal chemistry. Further studies on the synthetic applications of this reaction are in progress in our laboratory.Acknowledgements
This work was supported by the National Natural Science Foundation of China/Research Grants Council Joint Research Scheme (N_CUHK451/13), the Research Grants Council of the Hong Kong SAR, China (GRF Project 403012) and a grant to the State Key Laboratory of Synthetic Chemistry from the Innovation and Technology Commission, and The Chinese Academy of Sciences–Croucher Foundation Funding Scheme for Joint Laboratories, and the National Natural Science Foundation of China (NSFC no. 21272199). The Shenzhen Science and Technology Innovation Committee for the Municipal Key Laboratory Scheme (ZDSY20130401150914965), and the Shenzhen Basic Research Program (JCYJ20120619151721025) are also gratefully acknowledged.Notes and references
- (a) R. S. Kapil, in The Alkaloids, ed. R. H. F. Manske, Academic Press, New York, 1971, vol. 13, p. 273 Search PubMed; (b) H. P. Husson, in The Alkaloids, ed. A. Brossi, Academic Press, New York, 1985, vol. 26, p. 1 Search PubMed; (c) P. Bhattacharyya and D. P. Chakraborty, in Progress in the Chemistry of Organic Natural Products, ed. W. Herz and H. Grisebach and G. W. Kirby, Springer, Wien, 1987, Vol. 52, p. 159 Search PubMed; (d) D. P. Chakraborty, in The Alkaloids, ed. A. Brossi, Academic Press, New York, 1993, vol. 44, p. 257 Search PubMed; (e) H.-J. Knölker, Advances in Nitrogen Heterocycles, ed. C. J. Moody, JAI, Greenwich, 1995, vol. 1, p. 173 Search PubMed; (f) C. Ito, M. Itoigawa, A. Sato, C. M. Hasan, M. A. Rashid, H. Tokuda, T. Mukainaka, H. Nishino and H. Furukawa, J. Nat. Prod., 2004, 67, 1488 CrossRef CAS PubMed; (g) H.-J. Knölker, Chem. Lett., 2009, 38, 8 CrossRef; (h) I. Bauer and H.-J. Knölker, Top. Curr. Chem., 2012, 309, 203 CrossRef CAS; (i) Y. Qiu, D. Ma, C. Fu and S. Ma, Org. Biomol. Chem., 2013, 11, 1666 RSC.
- (a) J. K. R. Thomas, J. T. Lin, Y.-T. Tao and C.-W. Ko, J. Am. Chem. Soc., 2001, 123, 9404 CrossRef PubMed; (b) J. Huang, Y. Niu, W. Yang, Y. Mo, M. Yuan and Y. Cao, Macromolecules, 2002, 35, 6080 CrossRef CAS.
- M. Haussler, J. Liu, R. Zheng, J. W. Y. Lam, A. Qin and B. Z. Tang, Macromolecules, 2007, 40, 1914 CrossRef.
- F. Sanda, T. Nakai, N. Kobayashi and T. Masuda, Macromolecules, 2004, 37, 2703 CrossRef CAS.
- (a) A. Dijken, J. J. A. M. Bastiaansen, N. M. M. Kiggen, B. M. W. Langeveld, C. Rothe, A. Monkman, I. Bach, P. Stössel and K. Brunner, J. Am. Chem. Soc., 2004, 126, 7718 CrossRef PubMed; (b) W.-Y. Wong, L. Liu, D. Cui, L. M. Leung, C.-F. Kwong, T.-H. Lee and H.-F. Ng, Macromolecules, 2005, 38, 4970 CrossRef CAS; (c) R. M. Adhikari and D. C. Neckers, J. Org. Chem., 2009, 74, 3341 CrossRef CAS PubMed.
- For reviews on carbazole synthesis, see: (a) H.-J. Knölker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef PubMed; (b) A. W. Schmidt, K. R. Reddy and H.-J. Knölker, Chem. Rev., 2012, 112, 3193 CrossRef CAS PubMed.
- For select recent examples for carbazole syntheses, see: (a) W. C. P. Tsang, N. Zheng and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 14560 CrossRef CAS PubMed; (b) B. Witulski and C. Alayrac, Angew. Chem., Int. Ed., 2002, 41, 3281 CrossRef CAS; (c) L.-C. Campeau, P. Thansandote and K. Fagnou, Org. Lett., 2005, 7, 1857 CrossRef CAS PubMed; (d) S. H. Cho, J. Yoon and S. Chang, J. Am. Chem. Soc., 2011, 133, 5996 CrossRef CAS PubMed; (e) V. P. Kumar, K. K. Gruner, O. Kataeva and H.-J. Knölker, Angew. Chem., Int. Ed., 2013, 52, 11073 CrossRef CAS PubMed; (f) A. C. Hernandez-Perez and S. K. Collins, Angew. Chem., Int. Ed., 2013, 52, 12696 CrossRef CAS PubMed; (g) L. Ackermann and A. Althammer, Angew. Chem., Int. Ed., 2007, 46, 1627 CrossRef CAS PubMed; (h) Z. Liu and R. C. Larock, Org. Lett., 2004, 6, 3739 CrossRef CAS PubMed; (i) B.-Y. Lim, M.-K. Choi and C.-G. Cho, Tetrahedron Lett., 2011, 52, 6015 CrossRef CAS PubMed; (j) R. B. Bedford and M. Betham, J. Org. Chem., 2006, 71, 9403 CrossRef CAS PubMed; (k) R. B. Bedford and C. S. J. Cazin, Chem. Commun., 2002, 2310 RSC; (l) R. Forke, M. P. Krahl, T. Krause, G. Schlechtingen and H.-J. Knölker, Synlett, 2007, 268 CAS; (m) Y. Ou and N. Jiao, Chem. Commun., 2013, 49, 3473 RSC; (n) H. Hagelin, J. D. Oslob and B. Åkermark, Chem. – Eur. J., 1999, 5, 2413 CrossRef CAS; (o) D. J. S. Jean Jr., S. F. Poon and J. L. Schwarzbach, Org. Lett., 2007, 9, 4893 CrossRef PubMed; (p) K. Nozaki, K. Takahashi, K. Nakano, T. Hiyama, H.-Z. Tang, M. Fujiki, S. Yamaguchi and K. Tamao, Angew. Chem., Int. Ed., 2003, 42, 2051 CrossRef CAS PubMed; (q) T. Kitawaki, Y. Hayashi, A. Ueno and N. Chida, Tetrahedron, 2006, 62, 6792 CrossRef CAS PubMed; (r) A. Kuwahara, K. Nakano and K. Nozaki, J. Org. Chem., 2005, 70, 413 CrossRef CAS PubMed; (s) A. Kumar, A. Yadav, A. Verma, S. Jana, M. Sattar, S. Kumar, C. D. Prasad and S. Kumar, Chem. Commun., 2014, 50, 9481 RSC.
- (a) D. Shu, G. N. Winston-McPherson, W. Song and W. Tang, Org. Lett., 2013, 15, 4162 CrossRef CAS PubMed; (b) S. Tu, C. Ding, W. Hu, F. Li, Q. Yao and A. Zhang, Mol. Diversity, 2011, 15, 91 CrossRef CAS PubMed; (c) K. Ozaki, H. Zhang, H. Ito, A. Lei and K. Itami, Chem. Sci., 2013, 4, 3416 RSC; (d) C. Zhu and S. Ma, Org. Lett., 2014, 16, 1542 CrossRef CAS PubMed.
- Y. Qiu, W. Kong, C. Fu and S. Ma, Org. Lett., 2012, 14, 6198 CrossRef CAS PubMed.
- For Pt-catalyzed: (a) W. Kong, C. Fu and S. Ma, Chem. Commun., 2009, 4572 RSC; (b) W. Kong, C. Fu and S. Ma, Org. Biomol. Chem., 2012, 10, 2164 RSC. For Au-catalyzed: (c) W. Kong, C. Fu and S. Ma, Chem. – Eur. J., 2011, 17, 13134 CrossRef CAS PubMed; (d) B. Alcaide, P. Almendros, J. Alonso, M. T. Quirós and P. Gadziński, Adv. Synth. Catal., 2011, 353, 1871 CrossRef CAS. For palladium-catalyzed route to carbazoles: ref. 10d and (e) R. Forke, A. Jäger and H.-J. Knölker, Org. Biomol. Chem., 2008, 6, 2481 RSC; (f) R. Forke, M. P. Krahl, F. Däbritz, A. Jäger and H.-J. Knölker, Synlett, 2008, 1870 CAS; (g) M. Schmidt and H.-J. Knölker, Synlett, 2009, 2421 CAS; (h) C. Börger, O. KataevA and H.-J. Knölker, Org. Biomol. Chem., 2012, 10, 7269 RSC; (i) R. Hesse, K. K. Gruner, O. Kataeva, A. W. Schmidt and H.-J. Knölker, Chem. – Eur. J., 2013, 19, 14098 CrossRef CAS PubMed; (j) R. Hesse, A. Jäger, A. W. Schmidt and H.-J. Knölker, Org. Biomol. Chem., 2014, 12, 3866 RSC. For iron-mediated synthesis of carbazoles: (k) H.-J. Knölker and M. Bauermeister, J. Chem. Soc., Chem. Commun., 1989, 1468 RSC; (l) H.-J. Knölker, M. Bauermeister and J.-B. Pannek, Chem. Ber., 1992, 125, 2783 CrossRef; (m) H.-J. Knölker and M. Wolpert, Tetrahedron, 2003, 59, 5317 CrossRef; (n) O. Kataeva, M. P. Krahl and H.-J. Knölker, Org. Biomol. Chem., 2005, 3, 3099 RSC; (o) R. Czerwonka, K. R. Reddy, E. Baum and H.-J. Knölker, Chem. Commun., 2006, 711 RSC; (p) K. E. Knott, S. Auschill, A. Jäger and H.-J. Knölker, Chem. Commun., 2009, 1467 RSC; (q) K. K. Gruner, T. Hopfmann, K. Matsumoto, A. Jäger, T. Katsuki and H.-J. Knölker, Org. Biomol. Chem., 2011, 9, 2057 RSC.
- S. Mahboobi and S. Kuhr, Arch. Pharm., 1995, 328, 45 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef.
- S. M. Rafiq, R. Sivasakthikumaran and A. K. Mohanakrishnan, Org. Lett., 2014, 16, 2720 CrossRef CAS PubMed.
Footnotes |
| † This paper is dedicated to Professor Ei-ichi Negishi on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures and the characterization data of new compounds. See DOI: 10.1039/c4qo00242c |
| This journal is © the Partner Organisations 2014 |