Jorge Juan Cabrera-Trujillo and Israel Fernández
Org. Biomol. Chem., 2019,17, 2985-2991
DOI:
10.1039/C9OB00132H,
Paper
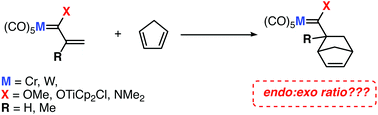
The factors controlling the selectivity of the Diels–Alder cycloaddition reactions involving Fischer-type carbene complexes and cyclopentadiene have been explored computationally by means of density functional theory calculations. To this end, the influence of the substituents directly attached to the carbene ligand on the endo : exo ratio has been compared to the available experimental data and quantitatively analysed in detail by means of the combination of the activation strain model of reactivity and energy decomposition analysis methods. The insight gained in this computational study may be important for the rational design of exo-selective Diels–Alder reactions.