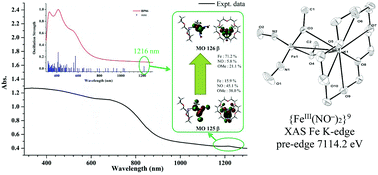
The synthesis, characterization and transformation of the thermally unstable {Fe(NO)2}9 dinitrosyl iron complex (DNIC) [(OMe)2Fe(NO)2]− (2) were investigated. The {Fe(NO)2}9 DNIC 2 characterized by single-crystal X-ray diffraction is exclusively stabilized by the weak intermolecular [Fe(OMe)2⋯(K+)] interactions (O(3)⋯K(1) and O(4)⋯K(1) distances of 2.818(3) and 2.810(3) Å, respectively). The binding affinity of chalcogenolate-containing ligands toward the {Fe(NO)2}9 motif follows the series [SEt]− > [SPh]− > [OPh]− > [OMe]−, which is dictated by the synergistic cooperation of the electron-donating order ([SEt]− > [SPh]− > [OPh]−) and the soft–hard order (from soft to hard, [SEt]− ∼ [SPh]− > [OPh]− > [OMe]−). In comparison with the XAS Fe K-edge pre-edge energy of {Fe(NO)2}9 [(RS)2Fe(NO)2]− (R = Ph (4), Et (5)) and [(PhO)2Fe(NO)2]− (6) DNICs falling within the reported range of 7113.4–7113.9 eV, the distinctive pre-edge energy of 7114.2 eV exhibited by complex 2 suggests that the electronic structure of {Fe(NO)2}9 DNIC 2 may be qualitatively described as a {FeIII(NO−)2}9 electronic structure induced by the dominant ionic character of Fe–OMe bonds, instead of the resonance hybrids of {FeII(NO−)(˙NO)}9 and {FeIII(NO−)2}9 electronic structures induced by the dominant metal–ligand covalency of {Fe(NO)2}9 DNICs 4–6. As shown in TD-DFT computation, the increased population of NO ligands in MO 125β (45.1% NO) attenuating the OMe-induced polarization imposed on the Fe center through the delocalized covalent nature of Fe–NO bonds supports the lower/synergistic NO/OMe → Fe charge transfer energy (1216 nm) observed in the solid-state UV-vis spectrum of complex 2 compared to those (1140 nm) of complexes 4–6.