Open Access Article
Open Access ArticleLuminescent core-isolated solvent-free liquids as a soft material platform for optical gas sensing
Shinsuke Ishihara†
 *a,
Avijit Ghosh†
*a,
Avijit Ghosh† ab,
Tatsuya Mori‡
a,
Mandeep K. Chahalac,
Daniel T. Payne
ab,
Tatsuya Mori‡
a,
Mandeep K. Chahalac,
Daniel T. Payne ad,
Akinori Saeki
ad,
Akinori Saeki e,
Tsuyoshi Hyakutakef and
Takashi Nakanishi
e,
Tsuyoshi Hyakutakef and
Takashi Nakanishi *a
*a
aResearch Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: ISHIHARA.Shinsuke@nims.go.jp; NAKANISHI.Takashi@nims.go.jp
bSchool of Applied Science & Technology, Department of Forensic Science & Technology, Maulana Abul Kalam Azad University of Technology, Haringhata 741249, West Bengal, India
cSchool of Chemistry and Forensic Science, University of Kent, Canterbury, CT2 7NH, UK
dSchool of Life, Health & Chemical Sciences, The Open University, Milton Keynes, MK7 6AA, UK
eDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
fInnovative Materials and Resources Research Center, Public Works Research Institute, 1-6 Minamihara, Tsukuba, Ibaraki 305-8516, Japan
First published on 22nd January 2026
Abstract
Solvent-free functional molecular liquids have attracted great interest as a new class of stimuli-responsive soft materials, yet their potential as optical gas sensors remains unexplored. Conventionally, luminescent organic molecules are employed in combination with a solid support or matrix. However, their performance in chemical sensing and optoelectronic devices is often hindered by adverse phenomena such as aggregation, concentration quenching, and photodegradation. In this study, we employ a strategy to isolate and wrap a phosphorescent Pt(II)-porphyrin core with bulky yet flexible branched alkyl chains, resulting in a solvent-free liquid at room temperature that demonstrates excellent properties for sensing oxygen (O2) gas. Compared to reference material composed of Pt(II)-tetraphenylporphyrin and a highly gas-permeable polymer matrix, our Pt(II)-porphyrin liquid shows comparable sensitivity (I0/I100 = 75 ∼ 90), better linearity, and greater photostability in its O2-responsive phosphorescence. This is attributed to the high homogeneity and gas solubility of the liquids, as well as to the shielding of luminescent-core units by bulky alkyl chains. The liquid nature of the materials allows for ratiometric sensing, where the compatibility of a phosphorescent Pt(II)-porphyrin liquid (O2-sensitive) and a fluorescent alkyl-pyrene liquid (O2-insensitive) enables reproducible monitoring of O2 concentration without specific calibration. Indeed, these results highlight the significant benefits of core-isolated luminescent liquids in diverse sensing applications.
Introduction
Functional molecular liquids (FMLs) have recently become a transformative category in soft functional materials.1 Within this group, alkyl-π liquids—solvent-free systems with π-conjugated molecular units isolated and wrapped by bulky yet flexible branched alkyl chains—provide tunable optoelectronic properties and liquid-phase behaviors that differ from traditional solid-state frameworks.2–4 These alkyl-π liquids have unique physical properties: molecular uniformity, fluidity, deformability, miscibility, and guest solubility, etc. Owing to their abundant designability for functional core units, various types of FMLs have been developed to date (e.g., tunable luminescence including phosphorescence,5–9 triplet-mediated photochemical functions,10,11 optoelectronic- and energy-related functions,12–14 permanent porosity and gas adsorption,15,16 and guest- and mechano-responsiveness17–21). Among those intriguing FMLs, although alkyl-π liquids have been developed as stimuli-responsive liquid materials, to the best of our knowledge, no reports have demonstrated the utility of their liquid properties for optical gas sensing. In related works, Isoda et al. reported alkylated N-heteroacene liquids that change their fluorescence color upon exposure to HCl vapor, where the vapor responsiveness is accompanied by protonation-induced solidification of the liquids.20,21 Unique aspects of alkyl-π liquids include their ability to provide a distinct mode of operation as stable liquid media that retain responsiveness and miscibility. According to Henry's law, the dissolution of gas molecules into liquids is proportional to their partial pressure.22 This motivated us to elucidate the potential of alkyl-π luminescent liquids as optical gas sensors and unveil any fundamental aspects distinct from conventional solid support or matrix systems.Luminescent organic molecules (LOMs) have been utilized for optical sensing of physical, chemical, and biological events.23–26 In particular, oxygen (O2) is a vital target analyte27–29 due to strong connections with the atmospheric environment, energy, and life, as exemplified by the spatiotemporal visualization of aerodynamics,30 fuel cell operation,31 and hypoxia in cancer cells.32,33 Among the various optical detection modes (e.g., wavelength, intensity, ratiometric, frequency, upconversion, lifetime, etc.), monitoring of luminescence intensity is widely utilized in O2 sensing due to the low cost and simplicity of the devices.24,29 Triplet photo-excited states of LOMs can be effectively quenched by O2, which makes their phosphorescence intensity sensitive to O2 levels. The interaction dynamics and correlation between luminescence intensity and a quencher's concentration are described by the Stern–Volmer equation (eqn (1)).27
| I0/Ix − 1 = Ksv[Q] | (1) |
Since the luminescence of LOMs is generally maximized in their discrete (i.e., non-aggregated) states except for rare cases where molecular motion is restricted within the aggregate or confinement,34 optical sensing is often performed in a solution (dissolved in water or organic solvent) or a composite with solid support or matrix (e.g., polymers,35–37 oxides,38,39 porous materials,40–42 and nanoparticles43–45). Consequently, the performance of optical O2 sensors is influenced not only by LOMs but also by the compatibility and O2 permeability of the solid support or matrix. Among various phosphorophores (e.g., polycyclic aromatic carbons, transition metal complexes, and fullerenes), Pt(II) and Pd(II)-porphyrins are extensively studied for optical O2 sensing because of their intense phosphorescence at room temperature.27,46–48 For example, Amao et al. found that Pt(II)-octaethylporphyrin (PtOEP) embedded in a highly gas-permeable poly(1-trimethylsilyl-1-propyne) (PTMSP)49 shows considerable sensitivity to O2 (I0/I100 = 225).50 In contrast, the same Pt(II)-porphyrin embedded in polystyrene or poly-(dimethylsiloxane) exhibits only moderate sensitivity (I0/I100 = ∼5).
Here, we present the first demonstration of an optical oxygen (O2) sensing based on a phosphorescent core-isolated solvent-free liquid, utilizing a Pt(II)-porphyrin core ([5,10,15,20-tetrakis(3,5-bis((2-hexyldecyl)oxy)phenyl)porphyrinato]platinum(II) PtPL, Fig. 1). The liquid exhibits benchmark-level sensitivity (I0/I100 = 75 ∼ 90), superior linearity, and improved photostability compared to conventional reference materials composed of Pt(II)-tetraphenylporphyrin (PtTPP)51 and PTMSP. Additionally, by mixing PtPL with a fluorescent alkyl-pyrene liquid, we develop a robust ratiometric sensing that operates without specific calibration. This work provides novel insights into optical gas sensing, establishing luminescent solvent-free liquids not only as responsive FMLs but also as active media, opening a versatile pathway toward a future soft sensing platform.
Results and discussion
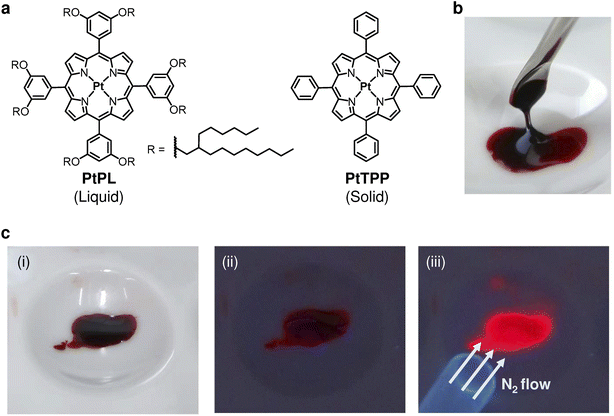
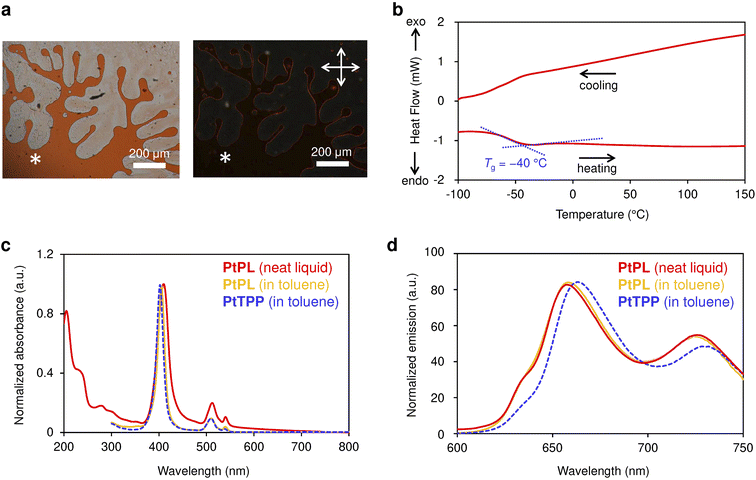
To obtain Pt(II)-porphyrin liquid PtPL, a free-base liquid porphyrin with 2-hexyldecyl branched alkyl chains13 was reacted with Pt(II)Cl2 in refluxing benzonitrile for 4–5 h under argon (Ar) (Fig. 1a).51 After purification and drying under vacuum, PtPL was obtained as a viscous red-orange liquid (Fig. 1b). Disappearance of the inner pyrrolic protons in the 1H NMR spectrum of PtPL indicates the successful insertion of a Pt(II) ion into the porphyrin core, and the high-resolution mass spectrum of PtPL is in agreement with its chemical formula, [C172H285O8N4194Pt]+ (Fig. S2–S5). Under ultraviolet (UV) irradiation, an intense red emission was observed from PtPL when under an N2 or Ar atmosphere (Fig. 1c). The emission was largely quenched in air due to energy transfer from photo-excited PtPL to O2. Thus, PtPL exhibits the expected phosphorescent properties for a long-lived triplet excited state. Even though branched alkyl chains surround the Pt(II) porphyrin core, small gas molecules can access the core through a mechanism akin to the facilitation of pyridine vapor into Zn(II) liquid porphyrin,13 which is structurally similar to PtPL (see Fig. S6–9 and 21).It is revealed that PtPL is a stable liquid at room temperature and shows optical properties in neat state almost identical to PtTPP in a diluted toluene solution (Fig. 2). A sample of PtPL sandwiched between glass plates is fluidic, and its cross-polarized optical microscopy (POM) image shows no birefringence, supporting the absence of long-range ordered domains (Fig. 2a). Differential scanning calorimetry (DSC) thermogram of PtPL shows only a reversible glass transition temperature (Tg) at around −40 °C; thus, PtPL maintains a liquid state above that temperature (Fig. 2b). Absorption and emission spectra of PtPL in neat liquid are similar to those of PtPL and PtTPP in toluene due to the bulky alkyl chains isolating the Pt(II)-porphyrin core from the surrounding environment (Fig. 2c and d). Note that the luminescent lifetime and quantum yield of PtPL were slightly longer and larger than those of PtTPP in toluene (Fig. S11 and Table S1).
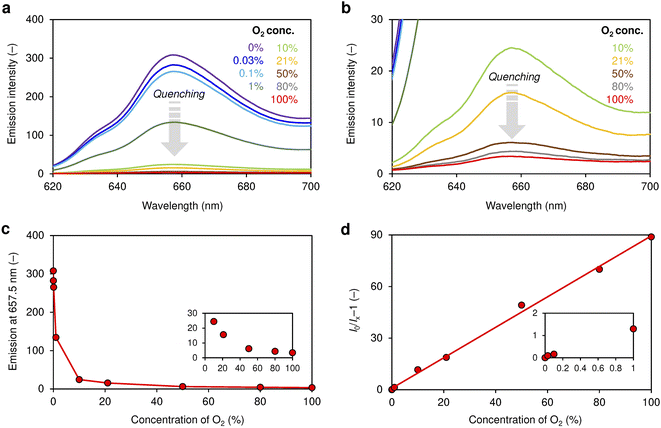
As shown in Fig. 3a–c, the emission from neat film PtPL is quenched (signal intensity is reduced) as the concentration of O2 in the atmosphere increases from 0% to 100%. There is a certain response to 0.03% O2, and the emission intensity halved at an O2 concentration of 1% (Fig. 3a). The Stern–Volmer plot of neat film PtPL shows linear correlations between O2 concentration (x-axis) and I0/Ix − 1 (y-axis). The value of I0/I100 is often used in the literature to represent O2 sensitivity, and I0/I100 = ∼90 is much greater than most phosphorescent O2 sensing materials (I0/I100 = 5 ∼ 10).27,35,42 As described above, Amao et al. reported that PtOEP embedded in polystyrene or poly-(dimethylsiloxane) shows modest sensitivity (I0/I100 = ∼5).50 Whereas, significant sensitivity to O2 (I0/I100 = 225) was obtained when PtOEP was embedded in a highly gas-permeable PTMSP polymer. Thus, our solvent-free liquid PtPL is a suitable medium for accommodating O2 molecules from the gas phase. The higher sensitivity of Amao's film could be ascribed to the excellent gas permeability of PTMSP as well as minimum substituents around the Pt(II)-porphyrin unit, enabling efficient energy transfer to proximal O2. However, it should be noted that the porphyrin concentration in the Amao's film was very dilute (ca. 2.9 × 10−5 mol dm−3, estimated as ca. 0.003 wt% based on molecular weight of the PtOEP (727.8 g mol−1) and density of PTMSP (0.7 g cm−3)52), presumably for preventing undesirable aggregation of porphyrins in the polymer matrix. Therefore, compared to the neat liquid PtPL, the brightness of the PtOEP–PTMSP film should be modest.
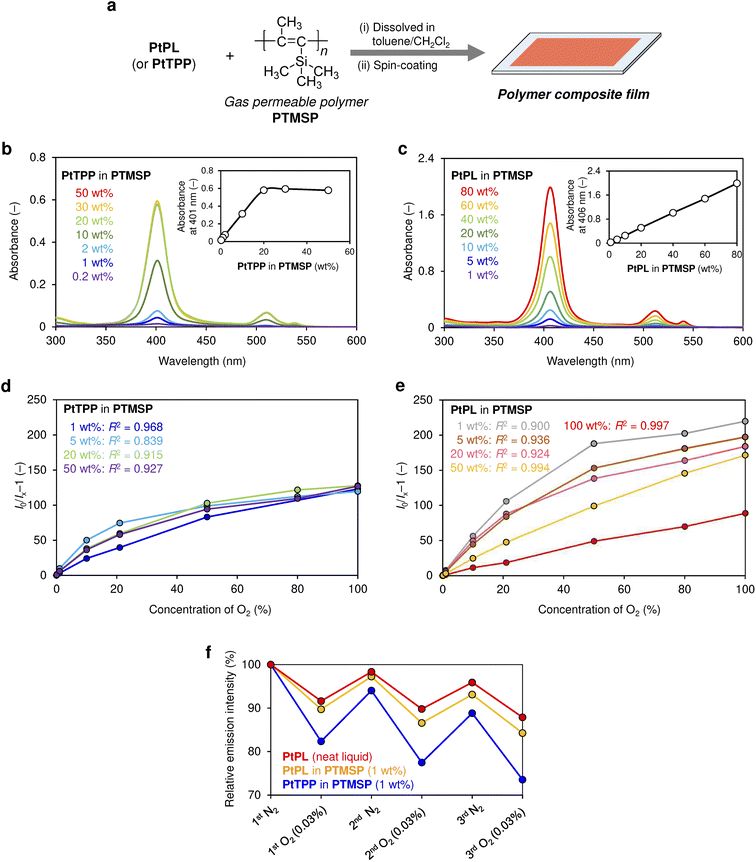
To investigate the effect of the bulky alkyl side chains compared to a porous polymer matrix, the O2 sensing performance of the neat liquid PtPL was compared with PtTPP–PTMSP and PtPL–PTMSP composites. A solution of PtTPP and PTMSP was spin-coated on a quartz substrate, and the optical properties of the thin films were investigated (Fig. 4a). Absorption signals corresponding to the Soret-band (λmax = 401 nm) of PtTPP linearly increased when the amount of PtTPP was increased from 0.2 to 20 wt% (Fig. 4b). However, the absorption signals did not grow beyond 20 wt%, suggesting aggregation or precipitation of PtTPP either within or outside the polymer matrix. In contrast, absorption signals of PtPL blended into PTMSP (λmax = 406 nm) did not saturate even at 80 wt% owing to the absence of aggregation of PtPL in the PtPL–PTMSP composite (Fig. 4c). Composite films of PtTPP–PTMSP (1, 5, 20, and 50 wt%) exhibited slightly better sensitivity to O2 (I0/I100 = ∼120) than a neat liquid film of PtPL (I0/I100 = ∼90), which can be attributed to the higher gas permeability of PTMSP compared to PtPL (Fig. 4d). The linearity of each plot was quantitatively assessed using the coefficient of determination (R2) obtained from linear fitting, showing moderate linearity (R2 = 0.84–0.97). In contrast, PtPL–PTMSP displays clear composition-dependent behavior (Fig. 4e). At low loadings (1, 5, and 20 wt%), a nonlinear response with high sensitivity is observed. This high sensitivity can be attributed to the high gas permeability of the PTMSP matrix as well as the increased number of O2 molecules available per PtPL molecule. Another possible interpretation is that PtPL, bearing branched alkyl chains reminiscent of those typically present in plasticizers, may slightly modify the local polymer environment, potentially facilitating O2 diffusion. By contrast, at higher loadings (50 and 100 wt%), the Stern–Volmer plots exhibit excellent linearity (R2 > 0.99), while maintaining a sufficiently high level of sensitivity compared with other materials, despite some reduction. Such excellent linearity is advantageous for the quantification of a wide O2 range based on two-point calibration. Downward-curved Stern–Volmer plots are commonly observed in sensor films and are often attributed to the presence of multiple emissive states with different luminescence lifetimes and/or quenching efficiencies within heterogeneous matrices.27,53–56 Thus, the improvement of linearity is likely due to the increased homogeneity of the sensing phase upon reducing the influence of the polymer matrix, which suppresses microenvironmental heterogeneity. Although neat liquid PtPL shows the lowest sensitivity among the samples in Fig. 4e, this limitation is addressed in the blended liquid system discussed in a later section, where both high sensitivity and good linearity are simultaneously achieved.
Notably, neat liquid PtPL shows better photostability than PtTPP–PTMSP upon repeated measurements, which can be ascribed to protecting the Pt(II)-porphyrin unit by the bulky alkyl chains (Fig. 4f). Although fluorinated porphyrins are known to show improved photostability,57 fluorinated organic compounds potentially cause environmental concerns due to poor biodegradability. The core-shielding effect of phosphorescent liquids (e.g., PtPL) by hydrocarbon alkyl chains is advantageous in this regard. Toward practical implementation, photostability could be further improved by elongating or densifying the branched alkyl chains; however, this may adversely affect O2 sensitivity because of reduced energy transfer efficiency. Therefore, photostability and sensitivity should be balanced depending on the aim of the application. We note that the present study focused on the equilibrium response to O2, and response time was not evaluated due to the unavailability of appropriate equipment. Overall, these studies elucidated, for the first time, that phosphorescent solvent-free liquids can be a promising platform for creating advanced optical gas sensors with high dye-loading amounts, excellent sensitivity, linearity, and photostability.
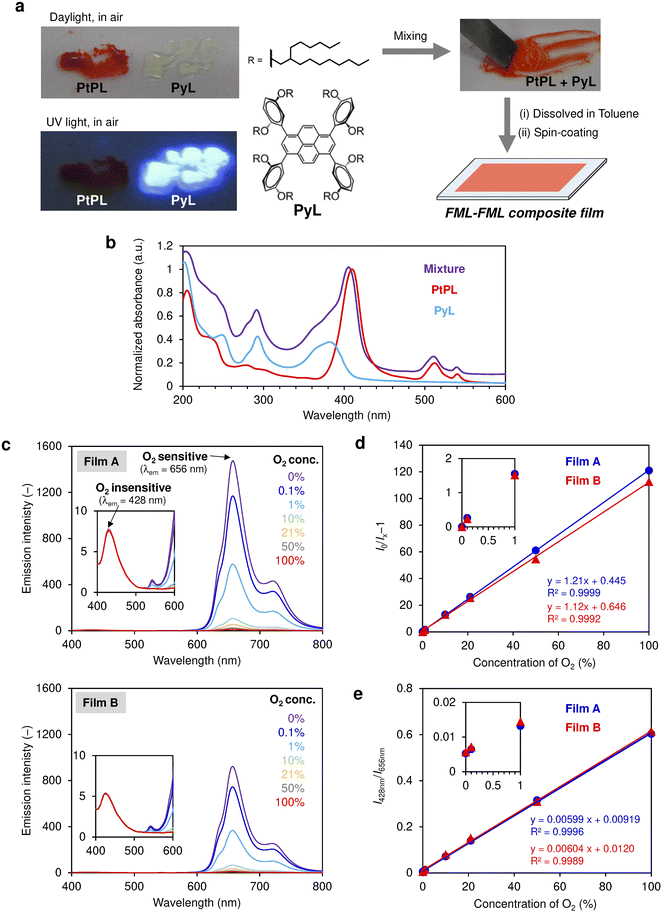
Finally, ratiometric optical O2 sensing was performed simply by blending PtPL with an alkylated pyrene fluorescent (O2-insensitive) liquid PyL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 (Fig. 5a and S18). The emission intensity of dye-loaded polymeric films can be influenced by various factors such as beam intensity, film thickness, and homogeneity of LOMs in the polymer matrix, and the accurate determination of I0 (i.e., emission intensity in the absence of O2) is indispensable for reliable quantification of O2.24,27,59 To avoid frequent calibrations, ratiometric O2 detection based on phosphorescent (O2 sensitive) and fluorescent (O2 insensitive) dyes is a promising approach.60–62 In the present study, ratiometric O2 sensing was achieved simply by blending two types of liquids. Since both PtPL and PyL are hydrophobic and have similar liquid physical properties due to the same 2-hexyldecyl branched alkyl chains, the two liquids are miscible with each other,63 and the blended liquid contains absorption profiles from both individual components (Fig. 5a and b). Two films (A and B) with different loadings were prepared from the blended liquid of PtPL+PyL (1
58 (Fig. 5a and S18). The emission intensity of dye-loaded polymeric films can be influenced by various factors such as beam intensity, film thickness, and homogeneity of LOMs in the polymer matrix, and the accurate determination of I0 (i.e., emission intensity in the absence of O2) is indispensable for reliable quantification of O2.24,27,59 To avoid frequent calibrations, ratiometric O2 detection based on phosphorescent (O2 sensitive) and fluorescent (O2 insensitive) dyes is a promising approach.60–62 In the present study, ratiometric O2 sensing was achieved simply by blending two types of liquids. Since both PtPL and PyL are hydrophobic and have similar liquid physical properties due to the same 2-hexyldecyl branched alkyl chains, the two liquids are miscible with each other,63 and the blended liquid contains absorption profiles from both individual components (Fig. 5a and b). Two films (A and B) with different loadings were prepared from the blended liquid of PtPL+PyL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, by weight) and investigated for ratiometric O2 sensing (Fig. 5c). Upon excitation at 360 nm, the fluorescence (λem = 428 nm) from PyL is insensitive to O2, while the phosphorescence from PtPL (λem = 656 nm) is sensitive to O2. Stern–Volmer plots of the two films are highly linear (R2 > 0.999), and the value of I0/I100 in film A reaches ∼120 (comparable to that of PtTPP–PTMSP in Fig. 4d). A increase in O2 sensitivity in the liquid blend system (compared with a neat liquid film of PtPL) may originate from enhanced O2 solubility and/or diffusion in the liquid upon blending with the relatively smaller-sized molecule PyL. Stern–Volmer plots of films A and B are slightly different, presumably due to differences in the loading amount of liquid or experimental errors. Nevertheless, when ratios of phosphorescence and fluorescence are plotted against O2 levels, films A and B demonstrate almost identical linear lines despite nearly double the difference in their emission intensity. Thus, the miscibility of liquids offers reliable ratiometric O2 sensing without the need for elaborate synthesis, fine-tuning of film loading, and frequent calibrations.
2, by weight) and investigated for ratiometric O2 sensing (Fig. 5c). Upon excitation at 360 nm, the fluorescence (λem = 428 nm) from PyL is insensitive to O2, while the phosphorescence from PtPL (λem = 656 nm) is sensitive to O2. Stern–Volmer plots of the two films are highly linear (R2 > 0.999), and the value of I0/I100 in film A reaches ∼120 (comparable to that of PtTPP–PTMSP in Fig. 4d). A increase in O2 sensitivity in the liquid blend system (compared with a neat liquid film of PtPL) may originate from enhanced O2 solubility and/or diffusion in the liquid upon blending with the relatively smaller-sized molecule PyL. Stern–Volmer plots of films A and B are slightly different, presumably due to differences in the loading amount of liquid or experimental errors. Nevertheless, when ratios of phosphorescence and fluorescence are plotted against O2 levels, films A and B demonstrate almost identical linear lines despite nearly double the difference in their emission intensity. Thus, the miscibility of liquids offers reliable ratiometric O2 sensing without the need for elaborate synthesis, fine-tuning of film loading, and frequent calibrations.
To confirm the enhanced sensitivity in the blended system, the sensitivity (I0/I100) of six independently prepared PtPL and PtPL+PyL films was statistically analyzed (Tables S2 and S3). As a result, the average sensitivity of PtPL+PyL (I0/I100 = 113.2, σ = 3.3) was reproducibly higher than that of PtPL (I0/I100 = 75.3, σ = 1.8). The sensitivity of PtPL in Fig. 3d (I0/I100 ≈ 90) is slightly higher than that shown in Table S2 (I0/I100 = 75.3 on average). This moderate difference can be attributed to cumulative decay of the emission intensity upon repeated exposure to excitation light (see Fig. 4f). The data in Fig. 3 were obtained at multiple O2 levels, with 100% O2 measured at the final stage of the experiment, which likely led to a reduction in the I100 value compared to its actual value. In contrast, the data in Table S2 were obtained from only two measurements, namely under N2 for I0 and under O2 for I100. Therefore, the values reported in Table S2 are considered to be more reliable.
Conclusions
This work reveals numerous benefits of luminescent core-isolated solvent-free liquids for optical gas sensing applications. Due to the liquid characteristics (e.g., homogeneity, gas solubility, diffusion, and miscibility) and the shielding effects of the phosphorescent-core units by the bulky yet flexible alkyl chains, the Pt(II) porphyrin liquid demonstrates exceptional sensitivity, linearity, photostability, and calibration-free ratiometric operations in phosphorescent O2 sensing. The concept presented in this study is broadly applicable to other functional π-chromophores and gaseous species, paving the way for a new platform for optical sensing materials.Methods
Synthesis of PtPL
A previously reported liquid free-base porphyrin13 was used to prepare a liquid Pt(II) porphyrin (PtPL). Typically, the alkylated free-base porphyrin (140 mg, 0.055 mmol) and Pt(II)Cl2 (147 mg, 0.55 mmol) were refluxed in dry benzonitrile (15 ml) under an Ar atmosphere,51 and the progress of metalation was monitored by thin-layer chromatography (TLC) and variation in the Q-bands in the UV-vis spectrum. After the reaction (ca. 4–5 h) was completed, the solvent was removed under reduced pressure, and the crude product was purified by column chromatography on silica gel (eluent: 10–20% CH2Cl2 in n-hexane). After drying under vacuum at 40 °C, a red-orange liquid (PtPL) was obtained (yield: 80%). 1H NMR (400 MHz, CDCl3) in ppm: 8.86 (s, 8H, pyrrole β-H), 7.29 (d, J = 2.4 Hz, 8H, Ar–H), 6.86 (t, J = 2.0 Hz, 4H, Ar–H), 3.96 (d, J = 5.6 Hz, 16H, OCH2), 1.83 (m, 8H, CH), 1.35–1.23 (m, 192H, CH2), 0.82 (m, 48H, CH3). 13C NMR (100 MHz, CDCl3) in ppm: 158.68, 143.04, 140.62, 130.64, 122.18, 113.44, 101.21, 71.27, 38.10, 31.89, 31.86, 31.42, 30.03, 29.71, 29.59, 29.33, 26.87, 22.66, 14.11. HR-ESI-MS (m/z): calculated for [C172H285O8N4194Pt]+ = 2729.1639 m/z, found 2729.1736 m/z.Preparation of liquid films
Liquid films for O2 sensing were obtained by spin-coating a toluene solution of liquid materials onto a quartz substrate. Typically, it took 5 seconds to reach 3000 rpm, and the film was dried at 3000 rpm for 60 seconds. Thus, homogeneous liquid films were obtained. The loading amount of the liquid film was adjusted by changing the concentration of the toluene solution or repeating the spin-coating process. A blended liquid film of PtPL+PyL was prepared from a solution of PtPL and PyL in toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, by weight). Liquid films were dried in air for more than 12 h before spectroscopic measurements. See Fig. S20 for a discussion of residual solvent in a liquid film.
2, by weight). Liquid films were dried in air for more than 12 h before spectroscopic measurements. See Fig. S20 for a discussion of residual solvent in a liquid film.
Preparation of polymer films
Stock solutions of PtPL in dichloromethane (5.00 mg ml−1) and PTMSP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49,64 in toluene (10.0 mg ml−1) were mixed at various ratios. The mixed solutions were spin-coated on a quartz substrate, as described above. In the case of PtTPP, a more diluted stock solution (1.67 mg ml−1) in dichloromethane was used due to limited solubility. See Fig. S19 for a discussion on the homogeneity of PtPL in polymer films.
49,64 in toluene (10.0 mg ml−1) were mixed at various ratios. The mixed solutions were spin-coated on a quartz substrate, as described above. In the case of PtTPP, a more diluted stock solution (1.67 mg ml−1) in dichloromethane was used due to limited solubility. See Fig. S19 for a discussion on the homogeneity of PtPL in polymer films.
O2 sensing
The porphyrin film containing quartz substrate was placed in a quartz cell (1 cm × 1 cm), as illustrated in Fig. S1. The quartz cell was capped with a rubber septum, and then dry N2 or Ar containing various concentrations of O2 was flowed through the cell using inlet and outlet needles to measure emission spectra (FP-8300 spectrophotometer, JASCO) under controlled O2 levels. The typical flow rate was 100 ml min−1, and 5 min flow was sufficient to replace the interior gases of the small cell (∼3 ml). The flow rate was adjusted and monitored using a float-ball-type flow meter (KOFLOCK) and a digital flow meter (7000 flowmeter, Ellutia). Dry N2 and O2 from laboratory lines were used as 0% and 100% O2, respectively. Ambient air supplied by a battery-powered pump (GSP-400FT, GASTEC) was regarded as 21% O2. For 0.1%, 1%, and 10% O2, standard gases supplied from high-pressure gas cylinders were directly used. For 50% and 80% O2, dry N2 and O2 from laboratory lines were mixed at appropriate flow rates (monitored by digital flow meters). Similarly, 0.1% O2 with dry N2 dilution yielded 0.03% O2.Author contributions
A. G. and T. M. synthesized porphyrins. A. G., S. I., and T. M. performed sensing experiments. M. K. C. and D. T. P. conducted material characterization. A. S. measured transient emission properties. T. H. provided PTMSP and discussed the results of O2 sensing. S. I. wrote the manuscript with input from all other authors. All authors read and approved the final version of the manuscript. S. I. and T. N. designed and directed the research.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data presented in this study are available upon reasonable request from the corresponding authors. Supplementary information (SI) is available for materials, methods, characterizations, photophysical and statistical analyses, and miscellaneous data. See DOI: https://doi.org/10.1039/d5sc08398b.Acknowledgements
This work was supported by JSPS KAKENHI (JP18H03922, JP24H01733, JP25H01264). This work was supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan. Ms. Reiko Takano is acknowledged for assisting with sensing measurements. Dr Zhenfeng Guo and Mr Mina Fahmy are acknowledged for supporting the spectroscopic measurements.References
- Functional Organic Liquids, ed. T. Nakanishi, Wiley-VCH, Weinheim 2019 Search PubMed.
- A. Ghosh and T. Nakanishi, Frontiers of Solvent-Free Functional Molecular Liquids, Chem. Commun., 2017, 53, 10344–10357 RSC.
- F. Lu and T. Nakanishi, Solvent-Free Luminous Molecular Liquids, Adv. Opt. Mater., 2019, 7, 1900176 CrossRef.
- A. Tateyama and T. Nakanishi, Responsive Molecular Liquid Materials, Responsive Mater., 2023, 1, e20230001 CrossRef.
- S. S. Babu, J. Aimi, H. Ozawa, N. Shirahata, A. Saeki, S. Seki, A. Ajayaghosh, H. Möhwald and T. Nakanishi, Solvent-Free Luminescent Organic Liquids, Angew. Chem., Int. Ed., 2012, 51, 3391–3395 CrossRef PubMed.
- A. M. Goudappagouda, V. C. Wakchaure, K. C. Ranjeesh, T. Das, K. Vanka, T. Nakanishi and S. S. Babu, Paintable Room-Temperature Phosphorescent Liquid Formulations of Alkylated Bromonaphthalimide, Angew. Chem., Int. Ed., 2019, 58, 2284–2288 CrossRef CAS PubMed.
- M. Komura, T. Ogawa and Y. Tani, Room-Temperature Phosphorescence of a Supercooled Liquid: Kinetic Stabilisation by Desymmetrisation, Chem. Sci., 2021, 12, 14363–14368 RSC.
- A. Ikenaga, Y. Akiyama, T. Ishiyama, M. Gon, K. Tanaka, Y. Chujo and K. Isoda, Stimuli-Responsive Self-Assembly of π-Conjugated Liquids Triggers Circularly Polarized Luminescence, ACS Appl. Mater. Interfaces, 2021, 13, 47127–47133 CrossRef CAS PubMed.
- Y. Tani, Y. Oshima, R. Okada, J. Fujimura, Y. Miyazaki, M. Nakano, O. Urakawa, T. Inoue, T. Ehara, K. Miyata, K. Onda and T. Ogawa, Fast and Efficient Room-Temperature Phosphorescence from Metal-Free Organic Molecular Liquids, Chem. Sci., 2025, 16, 17480–17486 RSC.
- P. Duan, N. Yanai and N. Kimizuka, Photon Upconverting Liquids: Matrix-Free Molecular Upconversion Systems Functioning in Air, J. Am. Chem. Soc., 2013, 135, 19056–19059 CrossRef CAS PubMed.
- R. K. Gupta, T. Nakanishi and D. T. Payne, Alkyl-π Liquids as Condensed-State Singlet Oxygen Photosensitizers, Chem. – Eur. J., 2025, 31, e202500739 CrossRef CAS PubMed.
- S. Hirata, K. Kubota, H. H. Jung, O. Hirata, K. Goushi, M. Yahiro and C. Adachi, Improvement of Electroluminescence Performance of Organic Light-Emitting Diodes with a Liquid-Emitting Layer by Introduction of Electrolyte and a Hole-Blocking Layer, Adv. Mater., 2011, 23, 889–893 CrossRef CAS PubMed.
- A. Ghosh, M. Yoshida, K. Suemori, H. Isago, N. Kobayashi, Y. Mizutani, Y. Kurashige, I. Kawamura, M. Nirei, O. Yamamuro, T. Takaya, K. Iwata, A. Saeki, K. Nagura, S. Ishihara and T. Nakanishi, Soft Chromophore Featured Liquid Porphyrins and their Utilization toward Liquid Electret Applications, Nat. Commun., 2019, 10, 4210 CrossRef PubMed.
- Y. Shi, M. A. Gerkman, Q. Qiu, S. Zhang and G. D. D. Han, Sunlight-Activated Phase Change Materials for Controlled Heat Storage and Triggered Release, J. Mater. Chem. A, 2021, 9, 9798–9808 RSC.
- N. Giri, M. G. Del Pópolo, G. Melaugh, R. L. Greenaway, K. Rätzke, T. Koschine, L. Pison, M. F. Costa Gomes, A. I. Cooper and S. L. James, Liquids with Permanent Porosity, Nature, 2015, 527, 216–220 CrossRef CAS PubMed.
- Y.-H. Zou, Y.-B. Huang, D.-H. Si, Q. Yin, Q.-J. Wu, Z. Weng and R. Cao, Porous Metal–Organic Framework Liquids for Enhanced CO2 Adsorption and Catalytic Conversion, Angew. Chem., Int. Ed., 2021, 60, 20915–20920 CrossRef CAS PubMed.
- M. J. Hollamby, M. Karny, P. H. H. Bomans, N. A. J. M. Sommerdjik, A. Saeki, S. Seki, H. Minamikawa, I. Grillo, B. R. Pauw, P. Brown, J. Eastoe, H. Möhwald and T. Nakanishi, Directed Assembly of Optoelectronically Active Alkyl-π-Conjugated Molecules by Adding n-Alkanes or π-Conjugated Species, Nat. Chem., 2014, 6, 690–696 CrossRef CAS PubMed.
- T. Ogoshi, K. Maruyama, Y. Sakatsume, T. Kakuta, T. Yamagishi, T. Ichikawa and M. Mizuno, Guest Vapor-Induced State Change of Structural Liquid Pillar[6]arene, J. Am. Chem. Soc., 2019, 141, 785–789 CrossRef CAS PubMed.
- A. Shinohara, C. Pan, Z. Guo, L. Zhou, Z. Liu, L. Du, Z. Yan, F. J. Stadler, L. Wang and T. Nakanishi, Viscoelastic Conjugated Polymer Fluids, Angew. Chem., Int. Ed., 2019, 58, 9581–9585 CrossRef CAS PubMed.
- K. Isoda, M. Matsubara, A. Ikenaga, Y. Akiyama and Y. Mutoh, Reversibly/Irreversibly Stimuli-Responsive Inks Based on N-Heteroacene Liquids, J. Mater. Chem. C, 2019, 7, 14075–14079 RSC.
- K. Isoda, T. Ishiyama, Y. Mutoh and D. Matsukuma, Stimuli-Responsive Room-Temperature N-Heteroacene Liquid: In Situ Observation of the Self-Assembling Process and Its Multiple Properties, ACS Appl. Mater. Interfaces, 2019, 11, 12053–12062 CrossRef CAS PubMed.
- 'Henry's law' in IUPAC Compendium of Chemical Terminology, edn. 5th edn, International Union of Pure and Applied Chemistry, 2025, Online version 5.0.0, 2025, DOI:10.1351/goldbook.H02783.
- O. S. Wolfbeis, Materials for Fluorescence-Based Optical Chemical Sensors, J. Mater. Chem., 2005, 15, 2657–2669 RSC.
- C. McDonagh, C. S. Burke and B. D. MacCraith, Optical Chemical Sensors, Chem. Rev., 2008, 108, 400–422 CrossRef CAS PubMed.
- S. W. Thomas, G. D. Joly and T. M. Swager, Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers, Chem. Rev., 2007, 107, 1339–1386 CrossRef CAS PubMed.
- J. Kramer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids, Chem. Rev., 2022, 122, 3459–3636 CrossRef PubMed.
- X.-D. Wang and O. S. Wolfbeis, Optical Methods for Sensing and Imaging Oxygen: Materials, Spectroscopies and Applications, Chem. Soc. Rev., 2014, 43, 3666–3761 RSC.
- R. Ramamoorthy, P. K. Dutta and S. A. Akbar, Oxygen Sensors: Materials, Methods, Designs and Applications, J. Mater. Sci., 2003, 38, 4271–4282 CrossRef CAS.
- A. A. Mendonsa and K. J. Cash, Oxygen-Sensitive Optical Nanosensors: Current Advances and Future Perspectives, ACS Sens., 2025, 10, 3194–3206 CrossRef CAS PubMed.
- J. W. Gregory, H. Sakaue, T. Liu and J. P. Sullivan, Fast Pressure-Sensitive Paint for Flow and Acoustic Diagnostics, Annu. Rev. Fluid. Mech., 2014, 46, 303–330 Search PubMed.
- J. Inukai, K. Miyatake, K. Takada, M. Watanabe, T. Hyakutake, H. Nishide, Y. Nagumo, M. Watanabe, M. Aoki and H. Takano, Direct Visualization of Oxygen Distribution in Operating Fuel Cells, Angew. Chem., Int. Ed., 2008, 47, 2792–2795 Search PubMed.
- Y. E. Koo Lee, E. E. Ulbrich, G. Kim, H. Hah, C. Strollo, W. Fan, R. Gurjar, S. Koo and R. Kopelman, Near Infrared Luminescent Oxygen Nanosensors with Nanoparticle Matrix Tailored Sensitivity, Anal. Chem., 2010, 82, 8446–8455 CrossRef CAS PubMed.
- X. Zheng, H. Mao, D. Huo, W. Wu, B. Liu and X. Jiang, Successively Activatable Ultrasensitive Probe for Imaging Tumour Acidity and Hypoxia, Nat. Biomed. Eng., 2017, 1, 0057 Search PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar, Chem. Rev., 2015, 115, 11718–11940 Search PubMed.
- Y. Amao, Probes and Polymers for Optical Sensing of Oxygen, Microchim. Acta, 2003, 143, 1–12 Search PubMed.
- S. M. Borisov, A. S. Vasylevska, C. Krause and O. S. Wolfbeis, Composite Luminescent Material for Dual Sensing of Oxygen and Temperature, Adv. Funct. Mater., 2006, 16, 1536–1542 CrossRef CAS.
- H. Xiang, L. Zhou, Y. Feng, J. Cheng, D. Wu and X. Zhou, Tunable Fluorescent/Phosphorescent Platinum(II) Porphyrin–Fluorene Copolymers for Ratiometric Dual Emissive Oxygen Sensing, Inorg. Chem., 2012, 51, 5208–5212 CrossRef CAS PubMed.
- S.-K. Lee and I. Okura, Porphyrin-Doped Sol-Gel Glass as a Probe for Oxygen Sensing, Anal. Chim. Acta, 1997, 342, 181–188 CrossRef CAS.
- S. M. Borisov, P. Lehner and I. Klimant, Novel Optical Trace Oxygen Sensors Based on Platinum(II) and Palladium(II) Complexes with 5,10,15,20-meso-Tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin Covalently Immobilized on Silica-Gel Particles, Anal. Chim. Acta, 2011, 690, 108–115 Search PubMed.
- C.-S. Chu and Y.-L. Lo, High-Performance Fiber-Optic Oxygen Sensors Based on Fluorinated Xerogels Doped with Pt(II) Complexes, Sens. Actuators, B, 2007, 124, 376–382 Search PubMed.
- B.-H. Han, I. Manners and M. A. Winnik, Oxygen Sensors Based on Mesoporous Silica Particles on Layer-by-Layer Self-Assembled Films, Chem. Mater., 2005, 17, 3160–3171 CrossRef CAS.
- T. Burger, C. Winkler, I. Dalfen, C. Slugovc and S. M. Borisov, Porphyrin Based Metal–Organic Frameworks: Highly Sensitive Materials for Optical Sensing of Oxygen in Gas Phase, J. Mater. Chem. C, 2021, 9, 17099–17112 RSC.
- Y. E. Koo, Y. Cao, R. Kopelman, S. M. Koo, M. Brasuel and M. A. Philbert, Real-Time Measurements of Dissolved Oxygen Inside Live Cells by Organically Modified Silicate Fluorescent Nanosensors, Anal. Chem., 2004, 76, 2498–2505 CrossRef CAS PubMed.
- X.-D. Wang, J. A. Stolwijk, T. Lang, M. Sperber, R. J. Meier, J. Wegener and O. S. Wolfbeis, Ultra-Small, Highly Stable, and Sensitive Dual Nanosensors for Imaging Intracellular Oxygen and pH in Cytosol, J. Am. Chem. Soc., 2012, 134, 17011–17014 CrossRef CAS PubMed.
- C. M. Lemon, E. Karnas, M. G. Bawendi and D. G. Nocera, Two-Photon Oxygen Sensing with Quantum Dot-Porphyrin Conjugates, Inorg. Chem., 2013, 52, 10394–10406 Search PubMed.
- Y. Amao and I. Okura, Optical Oxygen Sensor Devices Using Metalloporphyrins, J. Porphyrins Phthalocyanines, 2009, 13, 1111–1122 Search PubMed.
- I. Okura and T. Kamachi, Applications of Porphyrins and Related Compounds as Optical Oxygen Sensors, in Handbook of Porphyrin Science, ed. K. M. Kadish, K. M. Smith and R. Guilard, World Scientific Publishing, Singapore, 2011, vol. 12, pp. 297–348 Search PubMed.
- S. Ishihara, J. Labuta, W. V. Rossom, D. Ishikawa, K. Minami, J. P. Hill and K. Ariga, Porphyrin-Based Sensor Nanoarchitectonics in Diverse Physical Detection Modes, Phys. Chem. Chem. Phys., 2014, 16, 9713–9746 RSC.
- T. Masuda, E. Isobe, T. Higashimura and K. Takada, Poly[1-(trimethylsilyl)-1-propyne]: A New High Polymer Synthesized with Transition-Metal Catalysts and Characterized by Extremely High Gas Permeability, J. Am. Chem. Soc., 1983, 105, 7473–7474 Search PubMed.
- Y. Amao, K. Asai, I. Okura, H. Shinohara and H. Nishide, Platinum Porphyrin Embedded in Poly(1-trimethylsilyl-1-propyne) Film as an Optical Sensor for Trace Analysis of Oxygen, Analyst, 2000, 125, 1911–1914 RSC.
- W. Wu, W. Wu, S. Ji, H. Guo, X. Wang and J. Zhao, The Synthesis of 5, 10, 15, 20-Tetraarylporphyrins and their Platinum (II) Complexes as Luminescent Oxygen Sensing Materials, Dyes Pigm., 2011, 89, 199–211 Search PubMed.
- A. Gugliuzza, A. Iulianelli and A. Basile, Membranes for Hydrocarbon Fuel Processing and Separation, in Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications, Elsevier, 2011, pp. 295–338 Search PubMed.
- E. R. Carraway, J. N. Demas, B. A. DeGraff and J. R. Bacon, Photophysics and Photochemistry of Oxygen Sensors Based on Luminescent Transition-Metal Complexes, Anal. Chem., 1991, 63, 337–342 CrossRef CAS.
- J. N. Demas, B. A. DeGraff and W. Xu, Modeling of Luminescence Quenching-Based Sensors: Comparison of Multisite and Nonlinear Gas Solubility Models, Anal. Chem., 1995, 67, 1377–1380 CrossRef CAS.
- P. Hartmann, M. J. P. Leiner and M. E. Lippitsch, Luminescence Quenching Behavior of an Oxygen Sensor Based on a Ru(II) Complex Dissolved in Polystyrene, Anal. Chem., 1995, 67, 88–93 CrossRef CAS.
- W. Xu, R. C. McDonough, B. Langsdorf, J. N. Demas and B. A. DeGraff, Oxygen Sensors Based on Luminescence Quenching: Interactions of Metal Complexes with the Polymer Supports, Anal. Chem., 1994, 66, 4133–4141 Search PubMed.
- W. W. S. Lee, K. Y. Wong, X. M. Li, Y. B. Leung, C. S. Chan and K. S. Chan, Halogenated Platinum Porphyrins as Sensing Materials for Luminescence-Based Oxygen Sensors, J. Mater. Chem., 1993, 3, 1031–1035 RSC.
- F. Lu, T. Takaya, K. Iwata, I. Kawamura, A. Saeki, M. Ishii, K. Nagura and T. Nakanishi, A Guide to Design Functional Molecular Liquids with Tailorable Properties using Pyrene-Fluorescence as a Probe, Sci. Rep., 2017, 7, 3416 Search PubMed.
- P. Lehner, C. Staudinger, S. M. Borisov, J. Regensburger and I. Klimant, Intrinsic Artifacts in Optical Oxygen Sensors—How Reliable are our Measurements?, Chem. – Eur. J., 2015, 21, 3978–3986 Search PubMed.
- Y. Feng, J. Cheng, L. Zhou, X. Zhou and H. Xiang, Ratiometric Optical Oxygen Sensing: A Review in Respect of Material Design, Analyst, 2012, 137, 4885–4901 Search PubMed.
- T. Hyakutake, I. Okura, K. Asai and H. Nishide, Dual-Mode Oxygen-Sensing Based on Oxygen-Adduct Formation at Cobaltporphyrin–Polymer and Luminescence Quenching of Pyrene: An Optical Oxygen Sensor for a Practical Atmospheric Pressure, J. Mater. Chem., 2008, 18, 917–922 Search PubMed.
- H. Zhao, L. Zang, L. Wang, F. Qin, Z. Zhang and W. Cao, Luminescence Ratiometric Oxygen Sensor Based on Gadolinium Labeled Porphyrin and Filter Paper, Sens. Actuators, B, 2015, 215, 405–411 CrossRef CAS.
- Z. Guo, C. Pan, A. Shinohara and T. Nakanishi, Merging π-Molecular Functions Achieved through Homogeneous Liquid-Liquid Blending of Solvent-Free Alkyl-π Liquids, Sci. Technol. Adv. Mater., 2025, 26, 2515007 CrossRef PubMed.
- W. Waskitoaji, T. Hyakutake, J. Kato, M. Watanabe and H. Nishide, Biplanar Visualization of Oxygen Pressure by Sensory Coatings of Luminescent Pt-Porpholactone and -Porphyrin Polymers, Chem. Lett., 2009, 38, 1164–1165 CrossRef CAS.
Footnotes |
| † These authors contributed equally to this work. |
| ‡ Present address: Department of Chemistry, Graduate School of Science and Integrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Furo, Chikusa, Nagoya, 464-8602, Japan. |
| This journal is © The Royal Society of Chemistry 2026 |