Open Access Article
Open Access ArticleFluorine-mediated single-step ethylene purification in face-transitive metal–organic frameworks from binary to ternary gas mixtures
Wei-Hong
Zhang
,
Ya-Nan
Ma
,
Guo-Tong
Du
,
Ping
Wang
and
Dong-Xu
Xue
 *
*
Shaanxi Provincial Key Laboratory of New Concept Sensors and Molecular Materials, Key Laboratory of Applied Surface and Colloid Chemistry of Ministry of Education, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China. E-mail: xuedx@snnu.edu.cn
First published on 6th October 2025
Abstract
Ethylene is a pivotal feedstock for the chemical industry. Obtaining polymer-grade ethylene in a single step from either binary ethane/ethylene or ternary acetylene/ethane/ethylene mixtures via porous adsorbents is highly energy-efficient yet remains a formidable challenge. Face-transitive topologies, a particular class of nets in reticular chemistry, possess only one window type and thus hold exceptional promise for discriminating between closely related C2 hydrocarbons. Guided by the nia-d topology, we synthesized two isoreticular, trinuclear-manganese-cluster-based, ternary metal–organic frameworks (MOFs), namely nia-d-TZB and nia-d-FTZB, under solvothermal conditions using MnCl2, the tritopic linker 2,4,6-tri(4-pyridyl)-1,3,5-triazine (TPT), and the heterofunctional linear linkers 4-(1H-tetrazol-5-yl)benzoic acid (H2TZB) or 2-fluoro-4-(1H-tetrazol-5-yl)benzoic acid (H2FTZB). Although the resultant trigonal-bipyramidal cages remain dimensionally invariant, the introduction of fluorine in the latter linker subtly reduces the size of the antiprismatic cages and the sole triangular window in nia-d-FTZB. Single-component adsorption isotherms reveal that nia-d-TZB preferentially adsorbs ethane, whereas nia-d-FTZB preferentially adsorbs both acetylene and ethane. Consequently, nia-d-TZB enables one-step purification of ethylene from an ethane/ethylene mixture, while nia-d-FTZB achieves simultaneous removal of acetylene and ethane from an acetylene/ethane/ethylene ternary stream, again delivering polymer-grade ethylene in a single pass. These findings are corroborated by ideal adsorbed solution theory (IAST), breakthrough experiments with both binary and ternary gas mixtures, and detailed theoretical simulations. This study furnishes compelling evidence for the rational design of face-transitive MOFs to tackle complex gas-separation tasks.
Introduction
Ethylene (C2H4), a cornerstone of the modern petrochemical industry with an annual global production exceeding 200 million tons, serves as the primary feedstock for polyethylene and other commodity chemicals. Currently, industrial C2H4 production relies on the processes such as naphtha steam cracking and ethane dehydrogenation, which inevitably generate by-products like acetylene (C2H2) and ethane (C2H6). Achieving polymer-grade ethylene (purity > 99.9%) demands complete removal of these impurities. Conventional purification employs a multi-step approach: selective hydrogenation of C2H2 using noble-metal catalysts (e.g., Pd) followed by cryogenic distillation to separate C2H4/C2H6.1–4 However, this process suffers from excessive energy consumption (distillation operates at high pressure and subzero temperatures) and environmental burdens. The challenge is further exacerbated by the overlapping boiling points (184.6 K, 188.4 K, and 169.4 K for C2H6, C2H2, and C2H4, respectively), rendering traditional separation methods inefficient. Adsorptive separation employing porous materials offers a highly promising, energy-efficient alternative to conventional methods.5Metal–organic frameworks (MOFs), as a class of emerging crystalline porous materials, have attracted intensive attention from the scientific community to industry over the past two decades.6–10 These materials combine facile synthesis, well-defined structures, tunable functionalities, and rich chemical diversity, endowing them with broad potential across gas storage,11–18 separation,19–24 catalysis,25–29 sensing,30–36 pharmaceutical delivery37,38 and so on. In particular, MOFs have achieved notable progress in ethylene purification. Current reports on ethylene purification primarily address binary gas separations, namely ethylene/acetylene and ethylene/ethane mixtures, as well as trinary gas separations, i.e., ethylene/acetylene/ethane mixtures.39–44 In binary ethylene/ethane separations, ethane-selective adsorbents have garnered considerable interest in recent years because they enable direct, single-step ethylene purification with reduced energy expenditure by avoiding secondary desorption steps.45,46 Given that the kinetic diameters, the quadrupole moment and polarizability of ethylene (C2H4: 4.16 Å, approximately 1.5 × 10−26 esu cm2 and 42.5 × 10−25 cm3, respectively) lie between those of acetylene (C2H2: 3.33 Å, 7.2 × 10−26 esu cm2 and 39.3 × 10−25 cm3, respectively) and ethane (C2H6: 4.44 Å, 0.65 × 10−26 esu cm2 and 44.7 × 10−25 cm3, respectively), achieving simultaneous removal of acetylene and ethane from a C2 mixture to yield one-step ethylene purification from the three-component ethylene/acetylene/ethane feed remains exceptionally challenging.47 In recent years, numerous MOF materials have been reported that enable one-step purification of ethylene from both two-component48–56 and three-component C2 mixtures.57–64 Nevertheless, the design and synthesis of new MOFs that combine high selectivity with large uptake remains highly anticipated within the community.
Topology-guided reticular chemistry plays a crucial role in the design and synthesis of MOFs.65–68 In particular, MOF networks with a single window are referred to as face-transitive.69 The window serves as the sole gateway for gas molecules to enter and exit the internal cavities. Consequently, structures featuring only a single window are more readily tunable during bottom-up assembly. As a result, the regulated synthesis of face-transitive MOFs has emerged as an effective strategy to address efficient gas separations. Among them, nia-d, also known as pacs, is a (3,9)-c topology with a transitivity of 2212.70 This notation indicates that the network contains two kinds of vertices, two kinds of edges, one kind of face (window), and two kinds of tiles (cavities), thereby constituting a face-transitive network. In recent years, nia-d/pacs-type network has attracted considerable attention from numerous research groups for its potential in guiding high-performance gas separation MOFs.71–77
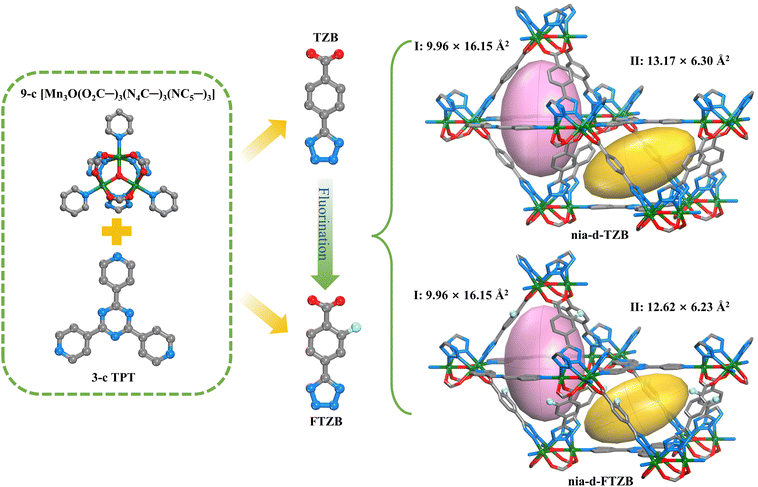
Building on the above findings and the isoreticular chemistry strategy, we report the synthesis of two nia-d-type trinuclear manganese-based MOFs using the same metal source and a tridentate triazine ligand, as well as a hetero-functional linear linker bearing carboxylic acid and tetrazole motifs. One linker is fluorine-free, while the side arm of the carboxylate in the other linker is fluorinated. Notably, these two materials exhibit distinct adsorption behaviours toward C2 hydrocarbons, leading to different separation performances. The synthesis and structure, as well as the adsorption and separation properties, breakthrough measurements with mixed C2 feeds, and mechanistic interpretations, are described in detail below.
Results and discussion
Synthesis and structure
Indeed, solvothermal reactions at 135 °C in DMF solvent combining MnCl2 with 2,4,6-tri(4-pyridyl)-1,3,5-triazine (TPT) and either 4-(1H-tetrazol-5-yl)benzoic acid (H2TZB) or 2-fluoro-4-(1H-tetrazol-5-yl)benzoic acid (H2FTZB) with trifluoroacetic acid as additive yielded yellow hexagonal-prismatic single crystals. Single-crystal X-ray diffraction (SCXRD), together with dye exchange experiments and X-ray photoelectron spectroscopy (XPS) (Fig. S3–S6), established the molecular formulae as [MnIIMnIII2(μ3-O)(TZB)3TPT]·x(solvent) for nia-d-TZB and [MnIIMnIII2(μ3-O)(FTZB)3TPT]·x(solvent) for nia-d-FTZB. SCXRD reveals that nia-d-TZB and nia-d-FTZB are isoreticular (Tables S4 and S5), both crystallizing in the hexagonal space group P63/mmc. In both structures, linear linkers yield the well-known 6-c acs network. The hexagonal channels are then sequentially occupied by triangular TPT linkers that cap the exposed [Mn3(μ3-O)] clusters, leading to the intended (3,9)-c nia-d topological 3-periodic framework.Two distinct cage motifs are present in both compounds, i.e., a triangular bipyramidal cage (cage-I) and an anti-prismatic cage (cage-II). The dimensions of cage-I are 9.96 × 16.15 Å2 in both structures. The presence of fluorine in the linear linker slightly perturbs cage-II, changing its dimensions from 13.17 × 6.30 Å2 in nia-d-TZB to 12.62 × 6.23 Å2 in nia-d-FTZB (Fig. 1). At the same time, the triangular windows, defined by portions of the TPT linker and two linear linkers, serve as the sole entrances/exits for the framework. The aperture size modestly decreases from 5.16 Å in nia-d-TZB to 5.0 Å in nia-d-FTZB (Fig. S2). Nevertheless, these window dimensions remain sufficient to permit the passage of C2 gas molecules.
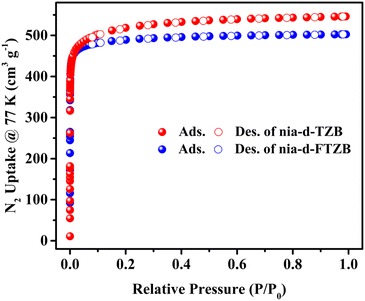
Porosity, adsorption and separation
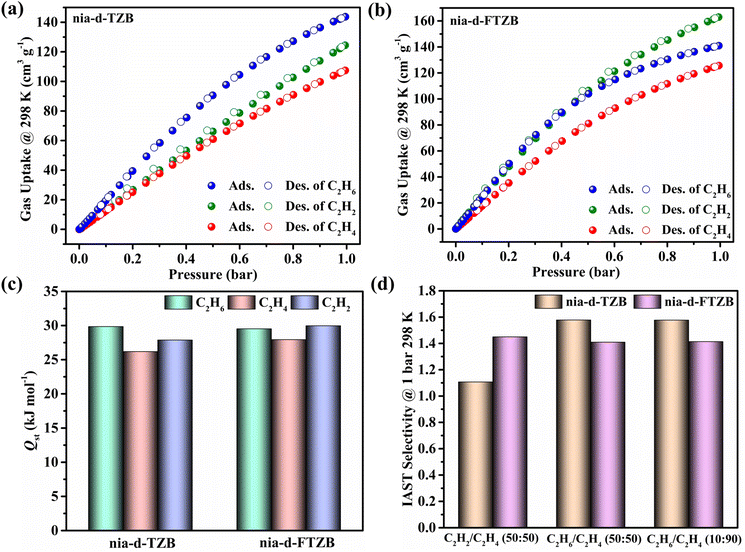
The solvent-accessible pore volumes for nia-d-TZB and nia-d-FTZB are 57.9% and 54.2%, respectively. The excellent agreement between bulk powder X-ray diffraction (PXRD) patterns and single-crystal simulations confirms the phase purity of the synthesized materials (Fig. S12 and S13). To assess porosity, the two samples were subjected to three days of consecutive exchanges with acetonitrile or acetone, followed by evacuation at 120 °C under vacuum prior to adsorption measurements. Nitrogen sorption isotherms measured at 77 K display Type-I behaviour for both samples, consistent with their microporous character. At 1 bar, nia-d-TZB (546 cm3 g−1) exhibits a higher nitrogen uptake than nia-d-FTZB (503 cm3 g−1) (Fig. 2 and S7). Correspondingly, the BET surface areas and pore volumes are 2029 m2 g−1 and 0.85 cm3 g—1, respectively, for nia-d-TZB, and 1987 m2 g−1 and 0.78 cm3 g—1, respectively, for nia-d-FTZB, which are consistent with their respective theoretical pore volumes of 0.85 cm3 g−1 and 0.77 cm3 g−1, thereby confirming that the materials were fully activated. The pore size distributions are centered at 11.5 Å and 11.0 Å, respectively (Fig. S8). These results are in good agreement with the pore architectures inferred from the single-crystal structures, indicating that both materials were successfully activated and preserved their structural integrity.To probe the C2 gas adsorption behaviour, we collected a series of static sorption isotherms for nia-d-TZB and nia-d-FTZB. As shown in Fig. 3a, both materials exhibit higher uptake for ethane and acetylene than for ethylene. Specifically, at 298 K and 1 bar, the uptakes are 143.72 cm3 g−1 for C2H6, 124.46 cm3 g−1 for C2H2, and 107.42 cm3 g−1 for C2H4 in nia-d-TZB. These uptakes are consistent with the zero-coverage isosteric heats of adsorption (Qst), i.e., 29.85 kJ mol−1 (C2H6), 27.88 kJ mol−1 (C2H2), and 26.19 kJ mol−1 (C2H4) (Fig. 3c and S16). Notably, the ethane uptake for nia-d-TZB reaches 6.42 mmol g−1, a value higher than those of many benchmark ethane-selective adsorbents, such as PCN-250 (5.21 mmol g−1),78 Ni-MOF-2 (5.94 mmol g−1),55 MOF-303 (5.01 mmol g−1),79 and UiO-67-(NH2)2 (5.32 mmol g−1).80 The C2H6/C2H4 and C2H2/C2H4 uptake ratios are 1.34 and 1.16, respectively. It is noteworthy that the adsorption isotherms for C2H2 and C2H4 are very close at low pressure, and the C2H2/C2H4 uptake ratio is relatively low. Collectively, nia-d-TZB demonstrates a pronounced ethane-selective adsorption behaviour toward C2 gas mixtures, which is advantageous for the removal of ethane from binary C2H6/C2H4 feeds and enables one-step purification of ethylene.
As for nia-d-FTZB, the introduction of fluorine markedly alters its adsorption profile, yielding the highest uptake for acetylene among the C2 molecules studied. Specifically, at 298 K and 1 bar, the uptakes are 162.98 cm3 g−1 for C2H2, 140.94 cm3 g−1 for C2H6, and 125.69 cm3 g−1 for C2H4 (Fig. 3b). The corresponding zero-coverage Qst is 29.98 kJ mol−1 (C2H2), 29.53 kJ mol−1 (C2H6), and 27.95 kJ mol−1 (C2H4) (Fig. 3c and S17). The ethane uptake remains notably high, and although the ethylene uptake also increases, the C2H2 isotherm lies above the C2H4 isotherm within 0–1.0 bar. The C2H2/C2H4 uptake ratio increases to 1.30. These data indicate that nia-d-FTZB exhibits concurrent selective adsorption of both acetylene and ethane, suggesting its potential for simultaneous removal of C2H2 and C2H6 from ternary C2 feeds and enabling one-step purification of ethylene.
To assess the gas separation performance of nia-d-TZB and nia-d-FTZB, we calculated the selectivities for binary mixtures of C2H2/C2H4 and C2H6/C2H4 using ideal adsorbed solution theory (IAST) (Fig. S20–S22). At 298 K and 1 bar, the selectivities for the C2H2/C2H4 (50/50, v/v) mixtures are 1.11 for nia-d-TZB and 1.45 for nia-d-FTZB (Fig. 3d). Under the same conditions, the selectivities for the C2H6/C2H4 (50/50, 10/90, v/v) mixture are 1.58 for nia-d-TZB and 1.41 for nia-d-FTZB (Fig. 3d). These values are comparable to those of many benchmark ethane-selective MOFs, such as Azole-Th-1 (1.46),57 Zn-atz-oba (1.27),81 JNU-2 (1.6),82 and BF-108-Zn (1.37).83 The results align with the observation that nia-d-TZB favors ethane removal from binary ethane/ethylene feeds, while nia-d-FTZB shows promise for simultaneous removal of acetylene and ethane from ternary C2 mixtures, enabling one-step ethylene purification.
Breakthrough experiment
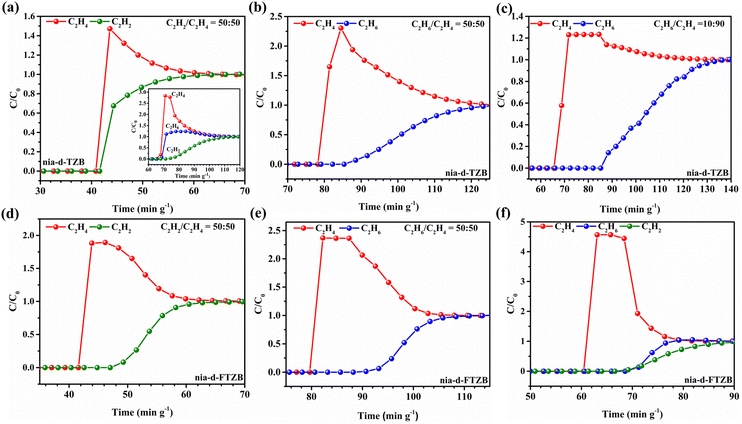
To evaluate the practical separation performance, fixed-bed breakthrough experiments were performed with the samples activated at 120 °C. For nia-d-TZB, we first tested the binary C2H2/C2H4 (50/50, v/v) and ternary C2H2/C2H6/C2H4 (1/1/1, v/v/v) mixtures at 298 K and 1 bar with a total flow rate of 1.0 mL min−1. In two configurations, both C2H2 and C2H4 were observed at the outlet, indicating that no effective separation of the two components could be achieved under these conditions (Fig. 4a and S24). This result is consistent with the similar low-pressure isotherms for C2H2 and C2H4 and the modest difference in their adsorption affinities. In contrast, when the binary C2H6/C2H4 mixtures (50/50, v/v) at the total flow-rates of 1.0 mL min−1 were introduced into the fixed-bed, nia-d-TZB demonstrated a meaningful separation of C2H6 from C2H4 (Fig. 4b and S23). The breakthrough behaviour yielded a C2H6/C2H4 separation interval of about 6.4 min g−1. Notably, with a 10/90 (v/v) C2H6/C2H4 feed and a flow rate of 1.0 mL min−1, C2H4 began to emerge at the outlet around 78 min g−1, followed by a slower C2H6 elution after ∼19.1 min g−1 (Fig. 4c and S23). The observed performance rivals several established ethane-selective materials reported in the literature, e.g., JNU-2 (16 min g−1),82 TKL-106 (15 min g−1),84 UPC-612 (11.5 min g−1),85 Zn-BPZ-TATB (14 min g−1),86 NUM-9 (16 min g−1),87 JNU-74 (22 min g−1),88 and MOF-801 (20 min g−1).89 The material demonstrated a C2H4 production capacity of 17.19 L kg−1. These results demonstrate that nia-d-TZB can effectively separate C2H6 from C2H4 in binary feeds, enabling practical single-step ethylene purification from ethane-containing streams.For nia-d-FTZB, breakthrough experiments were conducted similarly. In the binary C2H2/C2H4 (50/50, v/v) test with a 1.0 mL min−1 flow rate, C2H4 eluted prior to C2H2, with C2H4 breakthrough occurring at ∼42 min g−1 and C2H2 subsequently reaching equilibrium after ∼4.6 min g−1 (Fig. 4d and S26). This demonstrates effective separation of acetylene from ethylene in this binary gas pair. We also tested the binary C2H6/C2H4 (50/50, 10/90, v/v) feeds at 1.0 and 2.0 mL min−1. nia-d-FTZB showed a measurable separation performance for these two gases, with a separation interval of roughly 7.9 min g−1 and 13.2 min g−1 observed. With a 10/90 (v/v) C2H6/C2H4 feed and a flow rate of 1.0 mL min−1, the C2H4 productivity reached 11.88 L kg−1, confirming its ability to discriminate between C2H6 and C2H4 under practical flow conditions (Fig. 4e, S25 and S26).
To probe the full potential for ternary gas mixture separation, fixed-bed breakthrough experiments were performed with a C2H2/C2H6/C2H4 (1/1/1, v/v/v) feed at 298 K and 1.0 mL min−1. In this mixed-gas test, C2H4 was detected at the outlet first (i.e., 61 min g−1), followed by C2H6 (after 5.3 min g−1), and finally C2H2, validating the potential for one-step ethylene purification in the presence of acetylene and ethane (Fig. 4f). The productivity of C2H4 separation from the ternary mixture after the elution of C2H2 and C2H6 can be 1.77 L kg−1.
The materials demonstrate outstanding regenerability, retaining high separation performance over multiple breakthrough cycles. Regeneration does not require heating. It only needs to be purged with 10.0 mL min−1 helium gas at 298 K for 30 minutes. Both nia-d-TZB and nia-d-FTZB exhibit exceptional cycling stability without noticeable degradation in adsorption capacity or selectivity across five times repeated runs (Fig. S26).
GCMC simulation
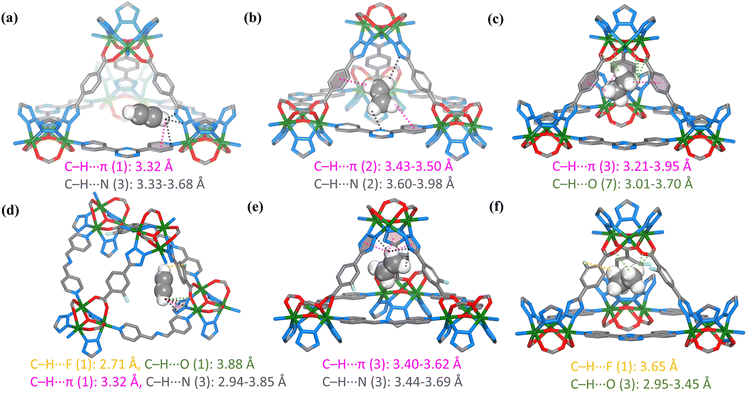
To gain a deeper understanding of the selective adsorption and separation mechanism, modelling studies were conducted on nia-d-TZB and nia-d-FTZB using grand canonical Monte Carlo (GCMC) simulation. As illustrated in Fig. 5, the C2 gases are located at different positions within the framework of nia-d-TZB. C2H2 and C2H4 molecules exhibit similar adsorption positions and interaction features. Both gases are predominantly distributed around the triangular windows, where C2H2 molecules form C–H⋯N interactions with nitrogen atoms in the tetrazole rings or TPT, with distances ranging from 3.33 to 3.68 Å. Additionally, these molecules engage in C–H⋯π interactions with pyridine rings on the TPT ligands, with an average distance of 3.32 Å (Fig. 5a). Similarly, C2H4 molecules occupy comparable binding sites, primarily interacting with tetrazole nitrogen atoms, benzene ring and TPT via C–H⋯π and C–H⋯N interactions, with distances ranging from 3.43 to 3.50 Å and 3.60–3.98 Å, respectively (Fig. 5b). In contrast, the distribution of C2H6 is distinct. These molecules are mainly localized at the metal cluster vertices, where they are surrounded by the framework, leading to stronger interactions. The primary binding sites for C2H6 involve interactions with carboxylate oxygen atoms through C–H⋯O hydrogen bonds (distances of 3.01–3.70 Å) and with benzene rings via C–H⋯π interactions (distances of 3.21–3.95 Å) (Fig. 5c). The computational results indicate that nia-d-TZB exhibits stronger interactions with C2H6 than with C2H4 or C2H2, which aligns well with the experimental adsorption isotherms and the dynamic breakthrough results.In contrast, all C2 molecules are located at the metal cluster vertices of nia-d-FTZB. C2H2 and C2H6 display similar adsorption behaviours and binding sites. Specifically, C2H2 molecules form strong C–H⋯F interactions with fluorine atoms on FTZB (distance: 2.71 Å), C–H⋯O interactions with carboxylate oxygen (distance: 3.88 Å), C–H⋯N interactions with tetrazole nitrogen atoms (distances: 2.94–3.85 Å), and C–H⋯π interactions with the tetrazole ring (distance: 3.32 Å) (Fig. 5d). C2H6 molecules also form multiple hydrogen bonds with fluorine atoms and carboxylate oxygen atoms, with interaction distances of C–H⋯F at 3.65 Å and C–H⋯O at 2.95–3.45 Å (Fig. 5f). The binding sites for C2H4 differ significantly. These molecules primarily interact with the surrounding three tetrazole rings, forming relatively weak C–H⋯π interactions with the tetrazole (distances: 3.40–3.62 Å) and multiple C–H⋯N interactions with tetrazole nitrogen atoms (distances: 3.44–3.69 Å) (Fig. 5e). The calculated binding energies follow the order: C2H2 (33.74 kJ mol−1) > C2H6 (27.58 kJ mol−1) > C2H4 (21.52 kJ mol−1). These results indicate that nia-d-FTZB exhibits strong and comparable adsorption capacities for C2H2 and C2H6, with the strongest interaction observed for C2H2 and the weakest for C2H4, consistent with the adsorption results.
Overall, computational findings confirm that both nia-d-TZB and nia-d-FTZB possess the capability for reverse separation of C2H6/C2H4. The introduction of fluorine atoms on the linear linkers modifies the chemical microenvironment as well as the interaction sites, subsequently strengthening the interactions between guest molecules and the framework. Therefore, nia-d-FTZB demonstrates the potential not only for the reverse separation of the binary C2H6/C2H4 mixture, but also the one-step purification of C2H4 from ternary C2 hydrocarbon mixtures.
Conclusions
Leveraging reticular chemistry and a mixed-ligand strategy, two isoreticular, trinuclear-manganese-cluster-based, ternary MOFs, designated as nia-d-TZB and nia-d-FTZB, have been successfully synthesized. Both frameworks adopt the nia-d topology, a face-transitive net that endows the structures with a single window type. The strategic installation of fluorine atoms along the linear linker transforms TZB into FTZB, which in turn elicits markedly divergent C2-hydrocarbon sorption behaviours. In nia-d-TZB, acetylene and ethylene are taken up in comparable amounts, whereas ethane is adsorbed most strongly, enabling one-step ethylene purification from an ethane/ethylene binary mixture. In stark contrast, nia-d-FTZB exhibits the highest uptake for acetylene, followed by ethane, with ethylene being the least adsorbed. This hierarchy allows simultaneous removal of both acetylene and ethane from an acetylene/ethane/ethylene ternary stream, again delivering polymer-grade ethylene in a single step. Collectively, this work establishes a critical paradigm for the deliberate design of face-transitive MOFs that achieve advanced gas-separation performance, underscoring the potential of crystalline porous materials to contribute to energy savings and emission reductions.Author contributions
Wei-Hong Zhang: writing – original draft, supervision; Ya-Nan Ma: analysis of the single crystal structure; Guo-Tong Du: computational analysis; Ping Wang: performed a part of characterization of MOF materials; Dong-Xu Xue: writing – review & editing, supervision, data curation.Conflicts of interest
There are no conflicts to declare.Data availability
CCDC 2482490 and 2482491 contain the supplementary crystallographic data for this paper.90a,bAll experimental supporting data and procedures are available in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc06836c.
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (No. 22471148 and 21871170), the National Key Research and Development Program of China (No. 2022YFA1205502), the Fundamental Research Funds for the Central Universities (No. GK202505027), and the Thousand Talents Program of Shaanxi Province and the Youth Innovation Team of Shaanxi Universities (2023).Notes and references
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2016, 16, 16–22 CrossRef PubMed.
- S. M. Sadrameli, Fuel, 2016, 173, 285–297 CrossRef CAS.
- I. Amghizar, L. A. Vandewalle, K. M. Van Geem and G. B. Marin, Engineering, 2017, 3, 171–178 CrossRef CAS.
- S. A. Chernyak, M. Corda, J. P. Dath, V. V. Ordomsky and A. Y. Khodakov, Chem. Soc. Rev., 2022, 51, 7994–8044 RSC.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- B. Li, H. M. Wen, Y. Cui, W. Zhou, G. Qian and B. Chen, Adv. Mater., 2016, 28, 8819–8860 CrossRef CAS PubMed.
- H. C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- Y. He, W. Zhou, T. Yildirim and B. Chen, Energy Environ. Sci., 2013, 6, 2735–2744 RSC.
- K. G. Froudas, M. Vassaki, K. Papadopoulos, C. Tsangarakis, X. Chen, W. Shepard, D. Fairen-Jimenez, C. Tampaxis, G. Charalambopoulou, T. A. Steriotis and P. N. Trikalitis, J. Am. Chem. Soc., 2024, 146, 8961–8970 CrossRef CAS PubMed.
- L. Liang, C. Liu, F. Jiang, Q. Chen, L. Zhang, H. Xue, H. L. Jiang, J. Qian, D. Yuan and M. Hong, Nat. Commun., 2017, 8, 1233 CrossRef PubMed.
- D. Sengupta, P. Melix, S. Bose, J. Duncan, X. Wang, M. R. Mian, K. O. Kirlikovali, F. Joodaki, T. Islamoglu, T. Yildirim, R. Q. Snurr and O. K. Farha, J. Am. Chem. Soc., 2023, 145, 20492–20502 CrossRef CAS PubMed.
- J. Zhou, Y. N. Ma, Y. F. Zhang, B. Zheng, K. Zheng, S. Liu, X. A. Guo, Y. B. Zhang and D. X. Xue, J. Am. Chem. Soc., 2025, 147, 21811–21817 CrossRef CAS PubMed.
- Y. Yabuuchi, H. Furukawa, K. M. Carsch, R. A. Klein, N. V. Tkachenko, A. J. Huang, Y. Cheng, K. M. Taddei, E. Novak, C. M. Brown, M. Head-Gordon and J. R. Long, J. Am. Chem. Soc., 2024, 146, 22759–22776 CrossRef CAS PubMed.
- R. C. Rohde, K. M. Carsch, M. N. Dods, H. Z. H. Jiang, A. R. McIsaac, R. A. Klein, H. Kwon, S. L. Karstens, Y. Wang, A. J. Huang, J. W. Taylor, Y. Yabuuchi, N. V. Tkachenko, K. R. Meihaus, H. Furukawa, D. R. Yahne, K. E. Engler, K. C. Bustillo, A. M. Minor, J. A. Reimer, M. Head-Gordon, C. M. Brown and J. R. Long, Science, 2024, 386, 814–819 CrossRef CAS PubMed.
- S. Lu, Y.-J. Zhang, Y.-J. Cheng, Z.-H. Qin, G.-D. Wang, Y. Bai, Y. Lin, H. Wang, Y. Sui, L. Hou and Y.-Z. Li, J. Mater. Chem. A, 2025, 13, 10581–10589 RSC.
- Y. Ding, A. Dey, L. O. Alimi, P. M. Bhatt, J. Du, C. Maaliki, M. Eddaoudi, J. Jacquemin and N. M. Khashab, Chem. Mater., 2021, 34, 197–202 CrossRef.
- M. Chang, F. Wang, Y. Wei, Q. Yang, J. X. Wang, D. Liu and J. F. Chen, AIChE J., 2022, 68, e17794 CrossRef CAS.
- K. B. Idrees, Z. Li, H. Xie, K. O. Kirlikovali, M. Kazem-Rostami, X. Wang, X. Wang, T. Y. Tai, T. Islamoglu, J. F. Stoddart, R. Q. Snurr and O. K. Farha, J. Am. Chem. Soc., 2022, 144, 12212–12218 CrossRef CAS PubMed.
- S. Capelo-Aviles, M. de Fez-Febre, S. R. G. Balestra, J. Cabezas-Gimenez, R. Tomazini de Oliveira, S. Gallo II, A. Vidal-Ferran, J. Gonzalez-Cobos, V. Lillo, O. Fabelo, E. C. Escudero-Adan, L. R. Falvello, J. B. Parra, P. Rumori, G. Turnes Palomino, C. Palomino Cabello, S. Giancola, S. Calero and J. R. Galan-Mascaros, Nat. Commun., 2025, 16, 3243–3258 CrossRef CAS PubMed.
- Y. Qi, C. Xue, Y. Zhang, Y. Huang, H. Huang, L. Gan and H. Yang, ACS Mater. Lett., 2025, 7, 1488–1495 CrossRef CAS.
- N. Sun, X. Zhou, J. Liu, H. Yu, X. Si, F. Ding and Y. Sun, J. Mater. Chem. A, 2025, 13, 9665–9672 RSC.
- D.-H. Nam, O. Shekhah, A. Ozden, C. McCallum, F. Li, X. Wang, Y. Lum, T. Lee, J. Li, J. Wicks, A. Johnston, D. Sinton, M. Eddaoudi and E. H. Sargent, Adv. Mater., 2022, 34, 2207088 CrossRef CAS PubMed.
- Y. L. Li, N. Li, Z. B. Mei, J. R. Li, S. J. Yao, F. Yu, S. L. Li, J. M. Lin, J. Liu and Y. Q. Lan, Chem. Sci., 2025, 16, 11939–11948 RSC.
- Y. Liang, H. Zhou, Y. Zhao, X. Liang, Z. Chen, M. Ji and M. Wang, Chem. Sci., 2025, 16, 15223–15230 RSC.
- A. Pal, S. Suresh, A. Khan, L. H. Kuo, L. T. Chi, A. Ganguly, C. Y. Kao, M. K. Sharma, T. S. A. Wang, D. Y. Kang and Z. H. Lin, Sci. Adv., 2025, 11, eads4711 CrossRef CAS PubMed.
- Y.-Y. Tang, X. Luo, R.-Q. Xia, J. Luo, S.-K. Peng, Z.-N. Liu, Q. Gao, M. Xie, R.-J. Wei, G.-H. Ning and D. Li, Angew. Chem., Int. Ed., 2024, 63, e202408186 CrossRef CAS PubMed.
- K. Gupta and A. K. Patra, ACS Sens., 2020, 5, 1268–1272 CrossRef CAS PubMed.
- J. Wang, Y. Cheng, J. Zhou and W. Tang, J. Mater. Chem. C, 2021, 9, 12086–12093 RSC.
- S. Ghosh, R. Lipin, A. Ngoipala, N. Ruser, D. M. Venturi, A. Rana, M. Vandichel and S. Biswas, Inorg. Chem., 2023, 62, 14632–14646 CrossRef CAS PubMed.
- J. Y. M. Chan, E. O. Shehayeb, D. L. Pennington, C. H. Hendon and K. A. Mirica, J. Am. Chem. Soc., 2025, 147, 29003–29012 CrossRef CAS.
- Y. Shen, A. Tissot and C. Serre, Chem. Sci., 2022, 13, 13978–14007 RSC.
- Z. Han, K. Y. Wang, R. R. Liang, Y. Guo, Y. Yang, M. Wang, Y. Mao, J. Huo, W. Shi and H. C. Zhou, J. Am. Chem. Soc., 2025, 147, 3866–3873 CrossRef CAS PubMed.
- M. Ran, Y. Liu, L. Feng, J. Ning, J. Li and X. Dou, ACS Appl. Nano Mater., 2025, 8, 8231–8240 CrossRef CAS.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- X.-X. Zeng, J.-S. Lu, D.-W. Ma, Y.-T. Huang, L. Chen, G. Wang, Q. Chen and N. Lin, Rare Met., 2024, 43, 4867–4883 CrossRef CAS.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- Z. Bao, G. Chang, H. Xing, R. Krishna, Q. Ren and B. Chen, Energy Environ. Sci., 2016, 9, 3612–3641 RSC.
- H. Li, K. Wang, Y. Sun, C. T. Lollar, J. Li and H.-C. Zhou, Mater. Today, 2018, 21, 108–121 CrossRef CAS.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS.
- L. Yang, S. Qian, X. Wang, X. Cui, B. Chen and H. Xing, Chem. Soc. Rev., 2020, 49, 5359–5406 RSC.
- L. Yang, P. Zhang, J. Cui, X. Cui and H. Xing, Angew. Chem., Int. Ed., 2024, 63, e202414503 CrossRef CAS PubMed.
- C. Gücüyener, J. van den Bergh, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed.
- P. Q. Liao, W. X. Zhang, J. P. Zhang and X. M. Chen, Nat. Commun., 2015, 6, 8697 CrossRef PubMed.
- H. G. Hao, Y. F. Zhao, D. M. Chen, J. M. Yu, K. Tan, S. Ma, Y. Chabal, Z. M. Zhang, J. M. Dou, Z. H. Xiao, G. Day, H. C. Zhou and T. B. Lu, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS PubMed.
- Y. Chen, Z. Qiao, H. Wu, D. Lv, R. Shi, Q. Xia, J. Zhou and Z. Li, Chem. Eng. Sci., 2018, 175, 110–117 CrossRef CAS.
- Y. Han, L. Meng, Y. Liu, H. Li, Z. Ji, Y. Zhou, M. Wu and Z. Han, Sep. Purif. Technol., 2023, 315, 123642 CrossRef CAS.
- L. Li, R. B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 CrossRef CAS PubMed.
- M. Li, L. Yin, S. Li, J. Miao, Z. Wang and H. Wang, Inorg. Chem., 2025, 64, 12440–12445 CrossRef CAS.
- S. M. Wang, M. Shivanna, S. T. Zheng, T. Pham, K. A. Forrest, Q. Y. Yang, Q. Guan, B. Space, S. Kitagawa and M. J. Zaworotko, J. Am. Chem. Soc., 2024, 146, 4153–4161 CrossRef CAS PubMed.
- Y. Wang, F. Zhang, Y. Yang, X. Wang, L. Li, J. Li and J. Yang, J. Colloid Interface Sci., 2025, 687, 439–448 CrossRef CAS PubMed.
- F. Xie, J. Liu, W. Graham, S. Ullah, E. M. Cedeño Morales, K. Tan, T. Thonhauser, H. Wang and J. Li, Chem. Eng. J., 2023, 473, 145096 CrossRef CAS.
- Y. Ye, Y. Xie, Y. Shi, L. Gong, J. Phipps, A. M. Al-Enizi, A. Nafady, B. Chen and S. Ma, Angew. Chem., Int. Ed., 2023, 62, e202302564 CrossRef CAS PubMed.
- L. P. Zhang, G. W. Guan, Y. T. Li, H. R. Liu, S. T. Zheng, Y. Jiang, R. Bai and Q. Y. Yang, Small, 2024, 20, e2402382 CrossRef.
- Z. Xu, X. Xiong, J. Xiong, R. Krishna, L. Li, Y. Fan, F. Luo and B. Chen, Nat. Commun., 2020, 11, 3163 CrossRef CAS PubMed.
- B. Zhu, J. W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest, M. J. Zaworotko and K. J. Chen, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS PubMed.
- G. D. Wang, Y. Z. Li, W. J. Shi, L. Hou, Y. Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202205427 CrossRef CAS PubMed.
- J. Liu, H. Wang and J. Li, Chem. Sci., 2023, 14, 5912–5917 RSC.
- Z. Ji, Q. Li, Y. Zhou, R. Krishna, M. Hong and M. Wu, Angew. Chem., Int. Ed., 2024, 63, e202411175 CrossRef CAS PubMed.
- X. Jiang, Y. Wang, H. Wang, L. Cheng, J. W. Cao, J. B. Wang, R. Yang, D. H. Zhang, R. Y. Zhang, X. B. Yang, S. H. Wang, Q. Y. Zhang and K. J. Chen, Nat. Commun., 2025, 16, 694–704 CrossRef CAS PubMed.
- Q. Ding, Z. Zhang, Y. Liu, K. Chai, R. Krishna and S. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208134 CrossRef CAS PubMed.
- Y. N. Ma, T. L. Liu, W. H. Zhang, C. H. He and D. X. Xue, Angew. Chem., Int. Ed., 2025, 64, e202513208 CrossRef CAS PubMed.
- O. Delgado Friedrichs, M. O’Keeffe and O. M. Yaghi, Acta Crystallogr., Sect. A: Found. Adv., 2003, 59, 515–525 CrossRef PubMed.
- N. W. Ockwig, O. Delgado-Friedrichs, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2005, 38, 176–182 CrossRef CAS PubMed.
- O. Delgado-Friedrichs, M. O'Keeffe and O. M. Yaghi, Acta Crystallogr., Sect. A: Found. Adv., 2006, 62, 350–355 CrossRef PubMed.
- J. Kim and W. Choe, Chem, 2022, 8, 617–631 CAS.
- P. M. Bhatt, V. Guillerm, S. J. Datta, A. Shkurenko and M. Eddaoudi, Chem, 2020, 6, 1613–1633 CAS.
- M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef PubMed.
- Y. Ye, Z. Ma, R. B. Lin, R. Krishna, W. Zhou, Q. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2019, 141, 4130–4136 CrossRef CAS PubMed.
- L. Liu, Z. Yao, Y. Ye, Y. Yang, Q. Lin, Z. Zhang, M. O'Keeffe and S. Xiang, J. Am. Chem. Soc., 2020, 142, 9258–9266 CrossRef CAS PubMed.
- A. N. Hong, H. Yang, X. Bu and P. Feng, EnergyChem, 2022, 4, 100080 CrossRef CAS.
- X. Mu, Y. Xue, M. Hu, P. Zhang, Y. Wang, H. Li, S. Li and Q. Zhai, Chin. Chem. Lett., 2023, 34, 107296 CrossRef CAS.
- Y. Zhang, Y. Han, B. Luan, L. Wang, W. Yang, Y. Jiang, T. Ben, Y. He and B. Chen, J. Am. Chem. Soc., 2024, 146, 17220–17229 CrossRef CAS PubMed.
- Y. Z. Hao, K. Shao, X. Zhang, Y. H. Yu, D. Liu, H. M. Wen, Y. Cui, B. Li, B. Chen and G. Qian, J. Am. Chem. Soc., 2025, 147, 11257–11266 CrossRef CAS PubMed.
- W. Wang, Y. Chen, K. X. Phan, Z. Jia, Y. Xiao, X. Bu and P. Feng, J. Am. Chem. Soc., 2025, 147, 31239–31248 CrossRef CAS PubMed.
- Y. W. Chen, Z. W. Qiao, H. X. Wu, D. F. Lv, R. F. Shi, Q. B. Xia, J. Zhou and Z. Li, Chem. Eng. Sci., 2018, 175, 110–117 CrossRef CAS.
- H. M. Wen, C. Yu, M. Liu, C. Lin, B. Zhao, H. Wu, W. Zhou, B. Chen and J. Hu, Angew. Chem., Int. Ed., 2023, 62, e202309108 CrossRef CAS PubMed.
- X. W. Gu, J. X. Wang, E. Wu, H. Wu, W. Zhou, G. Qian, B. Chen and B. Li, J. Am. Chem. Soc., 2022, 144, 2614–2623 CrossRef CAS PubMed.
- J. W. Cao, S. Mukherjee, T. Pham, Y. Wang, T. Wang, T. Zhang, X. Jiang, H. J. Tang, K. A. Forrest, B. Space, M. J. Zaworotko and K. J. Chen, Nat. Commun., 2021, 12, 6507–6515 CrossRef CAS PubMed.
- H. Zeng, X. J. Xie, M. Xie, Y. L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS PubMed.
- Q. L. Hong, W. J. Wang, S. M. Chen, K. Chen, M. Liu, H. X. Zhang and J. Zhang, Chem. Mater., 2022, 34, 307–313 CrossRef CAS.
- M. H. Yu, H. Fang, H. L. Huang, M. Zhao, Z. Y. Su, H. X. Nie, Z. Chang and T. L. Hu, Small, 2023, 19, 2300821 CrossRef CAS.
- Y. Wang, C. Hao, W. Fan, M. Fu, X. Wang, Z. Wang, L. Zhu, Y. Li, X. Lu, F. Dai, Z. Kang, R. Wang, W. Guo, S. Hu and D. Sun, Angew. Chem., Int. Ed., 2021, 60, 11350–11358 CrossRef CAS PubMed.
- G. D. Wang, Y. Z. Li, W. J. Shi, L. Hou, Y. Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2023, 62, e202311654 CrossRef CAS PubMed.
- S. Q. Yang, F. Z. Sun, P. Liu, L. Li, R. Krishna, Y. H. Zhang, Q. Li, L. Zhou and T. L. Hu, ACS Appl. Mater. Interfaces, 2021, 13, 962–969 CrossRef CAS.
- G. D. Wang, Y. Z. Li, H. Zeng, L. Hou, W. G. Lu and D. Li, Adv. Funct. Mater., 2025, 35, 2504916 CrossRef CAS.
- F. Xie, J. Q. Liu, W. Graham, S. Ullah, E. M. C. Morale, K. Tan, T. Thonhauser, H. Wang and J. Li, Chem. Eng. J., 2023, 473, 145096 CrossRef CAS.
- (a) CCDC 2482490: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pb7bm; (b) CCDC 2482491: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pb7cn.
| This journal is © The Royal Society of Chemistry 2025 |